Abstract
Middle childhood and early adolescence have received disproportionately low levels of scientific attention relative to other life stages, especially as related to nutrition and health. This is partly due to the justified emphasis on the first 1000 days of life, and the idea that early deficits and consequences may not be fully reversible. In addition, these stages of life may superficially appear less “eventful” than infancy or late adolescence. Finally, there has been historical ambiguity and inconsistency in terminology, depending on whether viewing “childhood” through physiologic, social, legal, or other lenses. Nevertheless, this age bracket, which encompasses most of the primary education and basic schooling years for most individuals, is marked by significant changes, inflection points, and sexually driven divergence in somatic and brain growth and development trajectories. These constitute transformative changes, and thus middle childhood and early adolescence represents a major and last opportunity to influence long-term health and productivity. This review highlights the specificities of growth and development in school age, with a focus on middle childhood and early adolescence (5 years–15 years of age, for the purposes of this review), the role of nutrition, the short- and long-term consequences of inadequate nutrition, and the current global status of nutrition in this age group. Adequate attention and emphasis on nutrition in the school-age years is critical: (a) for maintaining an adequate course of somatic and cognitive development, (b) for taking advantage of this last major opportunity to correct deficits of undernutrition and “catch-up” to normal life course development, and (c) for addressing the nutritional inadequacies and mitigating the longer-term consequences of overnutrition. This review summarizes and provides a rationale for prioritizing nutrition in school-age children, and for the need to revisit priorities and focus on this part of the life cycle to maximize individuals’ potential and their contribution to society.
Keywords: adolescence, children, growth, malnutrition, middle childhood, nutrition, obesity, school age, stunting
INTRODUCTION
The last three decades of academic and public health efforts have enthusiastically embraced the importance of early life nutrition as a foundational component of lifelong health. The gestational period through the first 2 years of age (the first 1000 d) and early childhood through 5 years of age have received justified attention over the last few decades. However, the ultimate realization of an individual’s potential requires a successful bridging from early childhood to adulthood. The subsequent periods in the life cycle—5 years to 9 years of age, referred to as “middle childhood,” and 10 years–15 years, “early adolescence”—are commonly encompassed in the “school years.”
Middle childhood and early adolescence bridge the period between the relatively steady growth occurring from 2 years to 5 years of age and the final maturation period of late adolescence to adulthood. This period is characterized by multiple dramatic inflection points in the course of growth and development, as well as behavioral and psychosocial events occurring around the arrival of puberty. These inflections represent transformational changes in the brain and cognitive processing, linear bone growth and bone mineralization, body composition, and other organ systems. It is also during this period that major sex-driven inflections and divergences occur in growth and development. The nutrition of children during this period is critical for supporting these changes. In addition, can help overcome early deficits, and may help correct dietary excesses that have been occurring since infancy. Thus, school age constitutes a final major window of opportunity to influence growth and development, and the associated health consequences in mature life.
Unfortunately, relative to other life stages, school-age nutrition has received a disproportionately low level of scientific attention, in part due to a misleading but widespread perception that early deficits in growth and development cannot be rectified. In the last few years, scientific, public health, and other academic voices have been calling attention to this life stage as a critical and potentially last major window of opportunity for intervention in maximizing the potential of individuals as productive members of society.1–5
This aim of this review was to highlight the critical growth, developmental, and nutritional aspects of these transformative school-age years, and the challenges and gaps in knowledge around these, and to provide arguments for why nutrition during school age deserves greater and more focused attention to maximize individuals’ growth, development, and ultimate productivity.
To achieve this aim, a comprehensive review of the literature was conducted, using PubMed to identify eligible and relevant publications through 2021. Papers were identified by combining the following Medical Subject Heading keywords: children, school age, middle childhood, adolescence, nutrition, nutrients, growth, development (multiple aspects/organ systems), malnutrition, stunting, overweight, and obesity. Literature was selected and prioritized that included information and data for the 5–15-year age group, primarily based on population, cohort, or epidemiologic studies and reviews, as well as literature addressing biologic aspects of specific areas of growth and development for this age group, with a focus on somatic growth, body composition, and neurologic development. Nutrition- and diet-related behavioral, psychologic, or social aspects were not included in the scope of this review.
SCHOOL-AGE HEALTH AND NUTRITION TERMINOLOGY AND KNOWLEDGE GAPS
Compared with the nutrition and health research literature for other life stages, there is a historical neglect of middle childhood and adolescence. Estimates of the published literature describing child health (PubMed sources 2005–2016) show 95.3% of this literature is dedicated to early childhood (<5 y), 3.5% to 5 years–9 years, 0.55% to 10 years–14 years, and 0.61% to 15 years–19 years.6 The health and nutritional status of school-age children, particularly that during middle childhood, remains the least studied of all life stages.
Public databases of most agencies track rates of malnutrition, stunting, and other health markers for children, but usually do so only until 5 years of age, and only pick up again during adolescence or adulthood.7 Regional or international databases of nutritional data for middle childhood (5 y–10 y of age) are extremely scarce. Many reviews for this age group rely on extrapolations, eg, using Demographic and Health Surveys (DHS) data for children 4 years–5 years of age8 or including data of 10-year-olds to 14-year-olds within child surveys.9 On the other end of school age, most research and data for children 10 years–15 years (early adolescence) is sometimes conflated with the data of adults, eg, Multiple Indicator Cluster Surveys (MICS) and DHS data for females 15 years–19 years. As discussed below, research, particularly concerning child growth and cognition, led to the notion that the consequences of nutritional and environmental insults in the first 2 years of age were irreversible. This may have resulted in reduced interest and research bias, due to underestimating the significant potential for growth and developmental catch-up possible during middle childhood and adolescence.
In part, inadequate research in this age group is also due to ambiguity, inconsistency, and overlapping terminology, resulting from viewing this age group through different lenses: physiologic, reproductive, social, legal, or school system, etc. Terms such as “early childhood,” “middle childhood,” “late childhood,” “school age”, “adolescence,” and “young adulthood” often overlap. From the general physiologic point of view, “middle childhood” (ages 5 y–9 y) is a period of growth and consolidation, followed by an adolescent growth spurt (ages 10 y–14 y), each associated with specific behavioral changes, before a final growth consolidation (ages 15 y to early 20s), and subsequent maturation into adulthood.1 More broadly speaking, the developmental stages in the life cycle have been classified into 3 main categories: physical growth, cognitive development, and socioemotional/psychosocial development.10 And while interdependent, the rate of progress for each of these life-stage categories can vary individually, making it hard to propose a purely chronological or age-based approach.
Many organizations and legal systems define “child” as an individual 0 years–18 years.8,11 WHO defines an “adolescent” as aged 10 years to 19 years, “youth” in general as 15 years–24 years, and “young people” covers 10 years–24 years.2,12 More recently, a broader definition for “adolescence” has been advocated as including the entire 10-year-old to 24-year-old group within the term. As explained by the 2016 Lancet Commission on Adolescent Health and elsewhere,9,12 this would support consideration of appropriate social and economic policies, service systems, and legal frameworks for this broad age group. While useful for certain objectives, this approach fails to distinguish the significant differences between the transitional aspects of development (and therefore the distinct difference in needs) of early adolescence versus late adolescence. Others support the use of “young people” (not “adolescents”) as a term for all 10–24-year-olds, distinguishing “adolescents” (10 y–19 y) from “young adults” or “emerging adults” (20 y–24 y). And some suggest that considering people in their early 20s as adolescents could lead to underestimating their competencies and capabilities.13 Clearly, no definition should be rigid. Approaches to defining “school age” and “adolescence” can vary by setting and should consider the cultural and societal context.
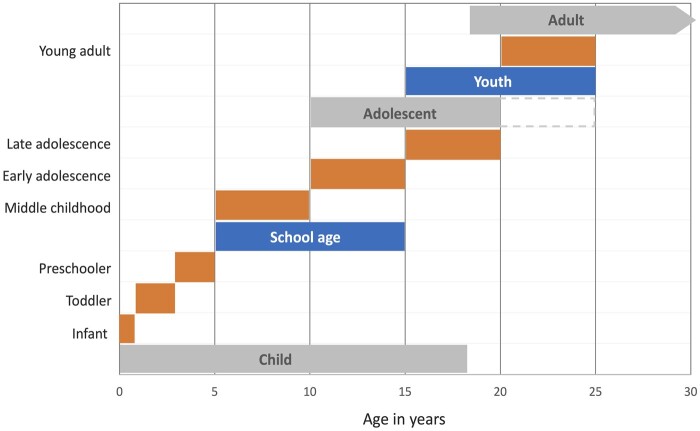
Schools are a significant platform, not always adequately utilized, for delivering nutrition as well as education in nutrition. “Schooling” plays a role as a defining factor in a person’s development, includes what is generally called “primary” and “secondary” schooling, and is quite variable from country to country. Globally, most children in primary school are between 5 years and 14 years of age, and there tends to be late entry into school in low- and middle-income countries. Many consider that “high school” and “preparatory school” fall into this category; others do not. Schooling provides opportunities for promoting nutrition and health. Research and interventions can leverage society’s investment in education and take advantage of the potential synergy between health and education investments.1Figure 1 summarizes the terminology most commonly used for childhood growth and developmental stages. Acknowledging there is no perfect life-stage categorization, given the physiologic and growth changes, most agree that data should be disaggregated for 5 years–9 years (middle childhood), 10 years–14 years (early adolescence), 15 years–20 years (late adolescence), and 20 years–24 years (older adolescents or young adults).1,9 ”School age” defined as comprising “middle childhood” (5 y–9 y) and “early adolescence” (10 y–15 y) will be the age group of focus and the terminology used in this paper.
Figure 1.
Major developmental life stages (in gray) and commonly used terminology for specific developmental stages as related to age. Modified and adapted from Bundy et al 20171 and Sawyer et al 20189
SPECIFICITIES OF GROWTH AND DEVELOPMENT IN SCHOOL AGE
What makes school age of particular significance nutritionally is that it encompasses numerous changes in trajectory from the relatively steady growth of the preschool child, through sex differentiation, and into the final consolidation period of late adolescence. These changes are driven by pubertal onset and course, with population variations primarily dependent on genetic, environmental, and nutritional factors. Pubertal sex hormone secretion will also determine changes in growth rate and growth termination. However, much work still remains to be done in understanding the underlying genetics, the timing of puberty (including early-life determining factors), growth variability during puberty, and adiposity and weight gain.14
Two specific processes contribute to the sex-differentiating physical developmental changes during this period: adrenarche and gonadarche. Adrenarche occurs between 6 years and 8 years of age, earlier in girls and later in boys, and refers to the maturation of the adrenal cortex and increased secretion of adrenal androgens, namely dehydroepiandrosterone. It is involved in the development of pubic hair (pubarche), body odor, skin oiliness, and axillary hair. Gonadarche is initiated by specialized neurons of the hypothalamus that secrete gonadotropin-releasing hormone (GnRH) in a cyclical pattern that regulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary, leading to gonadarche, starting at around 9 years–10 years of age in girls, and around 10 years–12 years in boys. In girls, FSH stimulates estrogen production, follicle formation, and eventually ovulation and menarche. In boys, LH stimulates testosterone production and eventually maturation of spermatozoa.
Finally, these changes during middle childhood and early adolescence are directly related to a differentiated phase of social learning and experimentation, heralding shifts in cognition, motivation, and social behavior, with significant implications for the ultimate development of each child’s personality. These shifts encompass major domains such as the development of independence and decision-making, acquisition of cultural norms, increase in complex moral reasoning, increase in understanding of social hierarchies, increase in sense of gender identity, gender segregation, and romantic attraction, as well as changes in food preferences and dietary habits, the expansion of which are beyond the scope of this paper.15
These growth and change phenomena and influential factors are interrelated, and nutrition plays a fundamental role. Protein-energy malnutrition is associated with delayed puberty, and subsequently poor growth and development. Secular trends have changed the timing of these processes in different populations, likely reflecting nutrition and health changes in the last century. In Europe and North America, from the early 19th century to the mid 20th century, age at menarche decreased from approximately 17 years to about 12 years–13 years.16 In China, in just the past 25 years, the mean age of menarche has decreased by 4.5 months per decade.17 As discussed below, changes in growth patterns, particularly in height and body–mass index (BMI), are interrelated with the onset of puberty. Increases in height and BMI are associated with an earlier onset of puberty, and earlier puberty is associated with an increased rate of later obesity.14 Obesity, which has risen dramatically in children, is associated with a shift towards earlier onset of puberty, particularly in girls; the situation is less clear in boys.16
The course of sex hormone secretion will determine the termination of growth during late adolescence into adulthood. The pubertal process is usually complete 2 years to 4 years after physical changes begin to occur. However, physical maturation will continue into the third decade of life. For instance, bone and brain development continues into the 20 s. Hormonal differences during puberty will also affect the size and function of organ systems related to aerobic and anaerobic physical fitness. Heart size and cardiac function, lung size, bone development, muscle volume and strength, erythropoiesis, and substrate utilization will diverge and determine different ultimate fitness and strength levels.18
Puberty happens in the middle of the school-age years and marks and determines the changes in trajectory and the switch in somatic and brain growth and development rates, which characterize this part of the life cycle. Two significant phenomena arise in this period:
Several inflection points and trajectory changes occur in somatic and brain growth and development, at different time points, for various measures of development (eg, height, adiposity, lean mass accretion, bone mineralization, brain growth and reorganization, with subsequent cognitive development, and secondary sexual characteristics) and social and behavioral changes
Sex divergences appear or become significantly more pronounced in these measures.
The dynamic somatic, cognitive, and behavioral changes that occur during school age underscore the importance of preparing children during middle childhood and facilitating their transition into adolescence during this period. Figure 2 shows the changes in trajectory for key anthropometric changes during the school years.
Figure 2.
Growth trajectories in school-age years (gray-shaded area). Fat and fat mass: estimates. All others are medians. Compiled and adapted from Tanner and Davies 198524 (height velocity), Weaver et al 201633 (BMC gain), Veldhuis et al 200521 (fat and fat mass), US CDC19 (BMI). BMC: bone mineral content; BMI: body–mass index
Body composition
Although height velocity decreases during the preschool years, height gain remains relatively steady, and the amount of body fat remains relatively constant; therefore, most children in middle school will appear slimmer than when they were toddlers. In fact, median BMI will be at its lowest in life at about 5.2 years in girls and at about 5.75 years in boys, ie, just as they enter school age.19 During this period, a child’s adiposity (corresponding to an increase in number of adipocytes) will rise, giving way to what is called the “adiposity rebound” or second rise in BMI during life. BMI, a reflection of adiposity, is the first anthropometric inflection point to appear in the school-age years.20
The age at which this inflection happens is inversely proportional to their BMI percentile (children with higher BMI will rebound earlier). Furthermore, an earlier adiposity rebound is associated with a higher risk of later obesity. As mentioned above, secular trends show an interplay between nutritional status and puberty. In addition, overweight and obesity in girls will lead to earlier puberty. Boys, however, show a less clear pattern: overweight boys seem to mature earlier, but obese boys mature later. The mechanisms are not yet clear, and there appears to be a bidirectional influence between puberty and weight gain.16
Body compositional changes during the school years also mark significant changes in trajectory and sex divergence. Large-scale normative data for body compositional changes for middle childhood and early adolescence are lacking, although some estimations and extrapolations have been done. Fat mass, fat-free mass, and percentage body fat have been estimated by aggregating data from several cross-sectional analyses from European and American populations.21 During the preschool years, actual fat mass in kilograms is similar in both sexes. Between 5 years and 10 years, girls will accumulate fat mass faster than boys, gaining approximately 6% (2 kg) more than boys. After this, with the onset of puberty, girls will gain about 1.14 kg of fat, while boys will maintain a relatively fixed fat mass. Throughout the school years, until 15 years, girls will have increased their fat mass almost 5-fold, while boys will have increased theirs by around 3-fold. Ultimately, fat mass will increase from 10%–12% of body weight at birth to approximately 15% in young men and approximately 25% in young women. In boys, after a prepubertal increase in percentage body fat, this percentage actually declines with puberty and stabilizes with maturation.21 The rate of accumulation of fat-free mass (including muscle mass) remains comparable by sex until the onset of puberty. With the onset of puberty, boys will accumulate lean mass significantly faster and for a longer period than girls. Gains in adipose tissue are primarily driven by an increase in the number and size of fat cells, while muscle development happens mainly by an increase in muscle cell mass (hypertrophy). The increase in body weight gain during puberty is mainly attained through an increase in lean tissue in boys.
Skeletal muscle mass, which represents about 25% of body weight at birth, will increase to 40%–45% body weight in late adolescence.18,20 Girls will reach adult stabilization by 15 years–16 years, while boys will do so by 18 years–20 years.21 Skeletal muscle mass has been estimated for children of school age using appendicular lean tissue mass extracted from dual photon absorptiometry measurements22 and bioelectrical impedance.23 Both approaches show that muscle mass and rates of gain are similar in both sexes until middle childhood. At around 5 years of age, boys maintain a slightly higher muscle mass, and girls a higher fat mass, after which trajectories increasingly diverge. This results in an approximately 3.5–5-fold increase in muscle mass in boys and a 3–4-fold increase in muscle mass in girls between 5 years and 15 years, in part depending on the methodology used. Conversely, fat mass will increase more in girls than in boys during this period.
Height and linear bone growth
The increase in height gain is the most noticeable change in trajectory in anthropometric measures of the school years. About 40% of an individual’s linear growth will occur during this time. Based on the United States CDC growth curve medians, by 5 years of age, boys and girls will, on average, be at 61% and 65%, respectively, of their ultimate height. By 15 years, they will be at 96% and 99%, respectively of ultimate height.19 There are major differences between the first 5 and second 5 years of school age.
During middle childhood, height velocity actually decreases, to the lowest levels of the entire life cycle, only to quickly increase in the middle of the school years to the highest rate of linear gain of all post-infancy years. In North America, CDC growth velocity charts19 show median height velocity will be at its lowest since birth just before 9 years of age in girls and at approximately 10.5 years in boys. At that point, before the puberty-related acceleration, both girls and boys will have reached ∼80% of their final height. Thus, height at that point will be a strong predictor of ultimate height in most individuals. This speaks to the importance of adequate nutrition and sustained growth between 5 years and 10 years of age.
With the onset of puberty, height velocity rapidly increases. In early adolescence, median peak height velocity in girls reaches its peak at around 11.5 years of age, with growth rates similar to those at 2 years of age (∼8.3 cm/y). In boys, a peak growth rate of about 9.5 cm/year happens at around 13.5 years of age, surpassing the 2 years of age rate of height attainment.24 In girls, this growth spurt starts earlier (∼9 y in girls vs ∼10.5 y in boys) and ends earlier (∼11.5 y vs ∼13.5 y), lasting at least 0.5 years less than in boys. The ultimate median height in males will be greater due to greater height at the onset of puberty (boys ∼9 cm–10 cm taller than girls), a more prolonged growth spurt period, and a greater increase following pubertal onset (boys gaining ∼3 cm more than girls between the onset and end of the growth spurt). The pubertal growth rate declines rapidly after their gender-specific peak in both sexes, to 1 cm/year or less after 14.5 years in girls and 17 years in boys.21,24
Height gains are dependent on longitudinal bone growth determined by epiphyseal growth plate function. Growth plate chondrocytes proliferate by mitosis, mature, become hypertrophic, lengthen the bone, and ultimately replace osteoblasts to form new calcified bone tissue. Growth hormone and insulin-like growth factor are the key hormonal drivers of this process. Adequate nutrition is critical for providing substrates for epiphysial growth, particularly energy, protein, and zinc. Calcium and vitamin D may play lesser roles in longitudinal bone growth. Very importantly, independent of nutrient provision, bone growth regulation can be blocked by corticosteroids and inflammatory cytokines. Chronic inflammation from infection, environmental factors, autoimmune disease, and the use of corticosteroids can all curtail linear bone growth. In addition, inflammation can lead to insulin and growth hormone resistance, which can further inhibit linear bone growth, thus compounding the effect of undernutrition, as often occurs in underserved populations.25–27 As discussed below, poor linear growth and stunting (two standard deviations beyond the normal curve median) remain the most prevalent clinical manifestation of undernutrition globally.28
Bone mineralization
Linear bone growth is followed by increases in bone mass and bone mineralization. Bone matrix becomes mineralized with the deposition of calcium phosphate nanocrystals (carbonated hydroxyapatite). The degree of deposition will determine bone mineral content (BMC), measured in grams, for a specific skeletal location or for the total body. This sequence of events will be similar, with variations by sex.
Total BMC will rapidly increase in early adolescence. Data derived from North American individuals show that by the end of middle childhood, prior to the onset of the growth spurt (2.5 y to 3 y before peak height), children will have achieved 37%–40% of ultimate total body BMC. In the short period between the 2 years before and 2 years after peak height is attained, another 39% of ultimate BMC is accrued. By the end of school age, more than 80% of total body BMC will have been attained, and final total body BMC appears to plateau at around 18 years of age in girls and 20 years in boys.29,30
Similar to height gain, the BMC accretion will increase rapidly with the onset of puberty, and the median peak rate of BMC accretion will occur at around 12.5 years in girls and 14 years in boys. Thus, the peak BMC accretion rate will lag compared with the peak height velocity, which occurs at around 11.5 years and around 13.5 years in girls and boys, respectively. Therefore, there is a transient decrease in bone density relative to height and bone elongation, and consequently an increase in bone fragility lasting about 12 months in girls and 6 months in boys (see Figure 2). This may partially explain the higher rate of forearm fractures reported in girls between 8 years and 10 years, and in boys between 10 years and 12 years.30,31
Ultimate bone mass, measured as bone mineral density (BMD) or mineral content divided by bone area, will depend on genetic and environmental factors. Studies in twins suggest that genetics can explain 50%–85% of the variance in peak bone mass, with multiple genes being involved, some of which may interact with environmental factors, including diet.32 Thus diet and “lifestyle” factors, including exercise, can still significantly influence BMC and BMD. As stated above, middle childhood and early adolescence are the periods of fastest mineral accrual, and more than 90% bone mass is achieved by 18 years–20 years. By the late 20s, bone mass will begin a gradual process of decline, leading to varying degrees of osteoporosis, which can only partially be modified by diet and other environmental factors. It follows that school age becomes a critical age of intervention and “investment” in bone health by maximizing peak bone mass and decreasing the risk of fractures in later life. This includes optimizing nutrition, particularly provision of protein, calcium, and vitamin D, as well as activity and exercise during this life stage.31,33
Brain and cognition
Brain size increases by 4-fold during the preschool years, reaching approximately 90% of adult volume by age 6.34 However, brain development will be a continuous process with age-specific phases until adulthood. The growth rate of cortical gray matter peaks during school age, by 10 years–12 years of age. Cerebral white matter volume increases through school age until mid-to-late adolescence, peaking by 18 years–20 years.35,36 Total brain size is about 9% greater in males than females, and the difference persists, even if controlling for height and weight. These differences should not be understood as conferring advantage or disadvantage, as they do not represent neuronal or synaptic connectivity or other components of brain architecture and function (see Figure 3).37,38
Figure 3.
Neuromaturational and cognitive development trajectories in school-age years (gray-shaded area). Compiled and adapted from Peterson et al 202138 (brain and gray matter growth), Tapert and Schweinsburg 2005114 (neuromaturation process rate), Lee et al 2014115 (brain region development), and Anderson 200246 (cognitive development executive domains)
During the school years, though at a slower rate than during the preschool years, total brain size increases, as does the sex-driven divergence, with boys being faster and peaking by 14.5 years, and girls peaking earlier by 11.5 years.37 During this period, brain development is also marked by a significant increase in 2 major neuro-maturational processes: continued myelination and an increase in synaptic refinement and pruning, both of which are important for the efficiency of neuronal networks. Dendritic synaptic pruning eliminates unused or weak connections, and a reduction in myelination rates improves connectivity. This fine-tuning within and between brain regions strengthens a number of particular pathways, which increases brain efficiency, critical to the development of cognitive abilities.39,40
School age will be marked by the highest rate of development of specific areas of the brain, particularly the posterior sensorimotor cortex, temporal association complex, and prefrontal cortex, and this development peaks at between 5 years and 15 years of age, in the middle of school age. All these areas are “associative cortices,” which process input from the sensory cortices and ultimately generate behaviors (see Figure 3). These structures are the key determinants of higher-order functions, particularly cognitive development (including language, mathematics, and executive function [EF]) and socio-emotional regulation, which among other things, allows for organization of information to serve goal-directed behaviors, decision-making, peer affiliation, and social behaviors.41,42 The parietal and temporal association cortex, responsible for language skills, also develops at a fast rate and this development peaks during school age. For example, language acquisition and proficiency, especially the ability to master a second language as a native speaker, decreases at the end of school age, by 15 years of age.43
The development of the prefrontal cortex peaks in the middle of the school years and continues to mature into the third decade. The gray matter volume in the frontal cortex peaks at 11 years of age in girls and at just after 12 years in boys.44 This part of the brain supports higher-level integration and processing, allowing for abstract thinking, problem-solving, understanding others’ thoughts and intentions, and the relating of thoughts temporally, allowing for goal setting. Thus, the prefrontal cortex is generally regarded as the “seat” of EF. EF is a broad term that incorporates a collection of mental processes that (a) enable individuals to hold and recall relevant information (working memory), (b) focus their attention, inhibiting automatic responses to stimuli (inhibitory control), and (c) shift the focus of attention to the managing of problems or multiple-aspect tasks successfully (cognitive flexibility), to yield purposeful, goal-directed behaviors. Ultimately, these processes influence behavior, emotional control, and social interaction. EF is associated with academic performance as well as intelligence quotient (IQ).42,45,46
One well-recognized model of EF conceptualizes and maps chronologically 4 distinct and interrelated developmental domains, each of which gathers and processes stimuli from multiple sources: attention control, information processing, cognitive flexibility, and goal setting. Attentional control matures relatively early, by the end of preschool age; information processing and cognitive flexibility are mostly developed by the end of middle childhood; and goal setting is well developed before the end of early adolescence (see Figure 3).46
In addition, the increased synaptic pruning and myelination that occurs during this period significantly reshapes and modifies circuitry and allows malleability and adaptation to environmental experiences, or brain plasticity. There is evidence, eg, that both synaptic pruning and myelination are driven or modified by an individual’s experiences.47 Thus, behaviors toward the environment are shaped by biologic changes in the brain, which in turn may be shaped by environmental, social, and cultural learning experiences. School age marks a major development of the associative brain regions and the resulting cognitive development and EFs, as well as the maturation of reward and emotional sensitivity areas, which interact with higher function control areas to develop emotional regulation, identity development, and longer-term planning and purpose. Adequate provision of nutrition, healthy social interactions, and cultural experiences, as well as adequate sleep, are all key to physical and psychosocial well-being.41
The onset of early adolescence will be marked by an increase in social and cultural interactions, and changes in the home, community, and school relationships, which influence behavior. The interaction between behavior resulting from brain functions and external influences appears to be bidirectional. At the onset of puberty, the prefrontal associative brain areas continue to develop, but at a slower pace than some subcortical brain areas. These limbic emotion- and reward-related regions, such as the amygdala, appear to mature earlier than the prefrontal areas, which are responsible for inhibitory control of impulses and regulation of gratification and other emotions. This “mismatch” may in part explain the increase in risk-taking, emotion-driven behaviors seen in adolescence.48
In summary, between the age of 5 years and 15 years, children go through major accelerations and inflections in their somatic growth, from a prepubertal state in the first 5 years to early adolescence changes in the next 5 years. By the end of school age, and before entering the final stages of “late adolescence,” healthy boys and girls, respectively, will have attained approximately 96% and 95% of their adult height,19 92% and 77% of their total BMC,49 89% and 85% of their fat mass,21 and 84% and 95% of their muscle mass.22 During this period, neuro-maturational processes will have also undergone significant changes. These include peaks in the development of specific areas of the brain and particularly the prefrontal cortex (implicated in complex cognitive behavior, planning, personality expression, decision-making, and moderation of social behavior) in the middle of the school years. Processes like synaptic pruning, which increase brain efficiency, will also peak by the end of this period, allowing for plasticity and increased brain efficiency. Delaying, altering, or blunting these accelerations and inflection points can significantly affect the ultimate attainment of physical growth, and cognitive, and socioemotional/psychosocial development. The necessity of preparing the child for these changes and supporting them through these accelerations and inflections, leading to the final maturational phase of late adolescence and ultimately adulthood, cannot be overstated.
ROLE OF NUTRITION IN SCHOOL AGE
The number of changes and dynamics of development mentioned above make the school years a particularly sensitive time, especially since most of the final growth and development is attained, which if not achieved will limit physical, cognitive, and social potential. These changes happen against a genetic backdrop expressed in, and dependent upon, multiple environmental and social scenarios that modulate physical and psychosocial development.
All these changes are underpinned by adequate nutrition in this period, as is true for all life cycle phases. Inadequate nutrition will slow or blunt physical and neurocognitive development trajectories during this last period of growth and development, with long-term consequences, inhibiting an individual’s ultimate potential. If environmental conditions, particularly nutrition, are favorable, the growth course and final height and overall body shape will be determined by an individual’s genes.27 The acceleration and change in growth trajectories discussed above increase the chances for curtailing growth and development if increased nutritional demands are not met. Because of the growth dynamics in this period, the school years become critical for the necessary nutrition (a) to maintain adequate growth trajectories until maturity, or (b) to correct inadequacies and imbalances (deficits and excesses) for a healthy transition to a productive adulthood.
WHO, the US Institute of Medicine, the European Food Safety Authority (EFSA), and other regional authoritative groups all distinguish and define specific energy, macro-, and micro-nutrient requirements for the school-age years (early childhood and early adolescence) distinct from those of other life stages.
Energy and protein
As the child grows, changes in metabolism are directly related to total energy requirements and indirectly to growth, and consist of basal metabolic rate, energy cost of growth, and activity energy expenditure.50 As a fraction of the total energy requirements, the energy cost of growth is highest in the newborn period, decreases to about 3% at 1 year of age, and goes up again between middle childhood and early adolescence to about 4%.51 Imbalances between energy intake and expenditure can result in deficits (leading to a decrease in body fat and a deceleration of growth) or excesses (in the form of fat accumulation, increased body weight, and its related consequences).
The factors that affect child energy requirements are growth and body composition (which are sex-dependent), as well as physical activity. Daily energy requirements diverge by sex at the start of school age, and will remain different throughout the life cycle. Sex differences in metabolic rate and energy expenditure are in part driven by differences in body fat and fat-free mass that emerge during school age.
Between 5 years and 15 years of age, physical activity is a particularly important factor in energy balance. In adults, the estimated difference in energy requirements between a sedentary individual and an active individual is below 20%, while in 5-year-olds to 15-year-olds it is around 35%, indicating the need for adequate energy provision—in proportion to the recommended “active” level of physical activity during this period of the life cycle.51 Energy provision throughout the day is also critical for brain activity, where increased neuronal activity drives increased energy consumption. In addition to all the neurodevelopmental changes occurring during school age mentioned above, the cerebral metabolic rate of glucose utilization is at its highest in middle childhood and early adolescence (apart from during the newborn period), then drops towards the end of adolescence. The mature brain is only approximately 2% of the body weight in adulthood, but is responsible for around 20% of energy consumption. Estimations for 12-year-old children suggest that brain energy consumption is as high as 30%.52–54 Lastly, this increase in energy requirement and utilization is also dependent on the presence of adequate quantities of several micronutrients. These include riboflavin (vitamin B2), niacin (nicotinamide; vitamin B3), pyridoxine (vitamin B6), cobalamin (vitamin B12), vitamin C, vitamin D, calcium, iron, and phosphorus. They all act as co-factors for key enzymes in the metabolic pathways that generate and use energy.
Protein is the major functional and structural component of every cell in the body. The quality of a source of dietary protein depends on its ability to provide the nitrogen and amino acid requirements necessary for the body’s growth, maintenance, and repair. Through to the end of the growth years, enough protein is required to maintain the nitrogen equilibrium plus protein deposition in tissues. Low consumption of protein, often associated with low protein quality, is strongly associated with stunting, and if marked, other signs of undernutrition. As opposed to requirements for energy and some micronutrients, protein requirements do not change significantly by age or sex during the school years. The United States recommended dietary allowance is 0.95 g/kg/day, representing 10%–30% of total calories, from 4 years to 13 years of age. The recommended dietary allowance decreases slightly after adolescence. In general, proteins from animal sources such as meat, poultry, fish, eggs, milk, cheese, and yogurt provide all indispensable amino acids and are referred to as “complete proteins.” Proteins from plants, legumes, grains, nuts, seeds, and vegetables tend to be deficient in one or more of the indispensable amino acids.Thus, attention needs to be paid to children whose diets are low in animal protein sources to avoid essential amino acid deficiencies.51
Micronutrients
In the United States, the Estimated Adequate Requirements, and Dietary Reference Intakes for micronutrients are the same for all children up to age 8 years. For the first time, they diverge for boys and girls during the school years: there are small differences (eg, for iron) for 9 years–13 years, and larger differences in requirements for late adolescence, at 14 years–18 years.51 EFSA has slightly different and more specific age cut-offs for Population Reference Intakes (PRIs) for most vitamins and minerals.55 Recommendations are made for 4 years–6 years, 7 years–10 years, 11 years–14 years of age, and separately define PRIs for 4 years–10 years and 11 years–17 years for calcium, 1 year–6 years, 7 years–11 years, and 12 years–17 years for iron, and 3 years–9 years and 10 years–17 years for copper, with some differences for sex in these nutrients. EFSA also differentiates requirements in energy and protein for boys and girls starting at 4 years. While specific benefits have been well established in relation to deficiencies for many micronutrients, consensus on optimal doses and combinations of these nutrients for promotion of specific health benefits in otherwise healthy school-age children is not universal and would benefit from further clinical research and substantiation.
Linear growth appears particularly sensitive to restrictions in energy, protein (particularly essential amino acids), zinc, iodine, and phosphorus, as well as some electrolytes. Protein quantity and quality remain fundamental components of adequate growth and function at all ages. Yet, the minimum protein necessary for adequate linear growth remains to be ascertained. Animal proteins, including dairy protein sources, have a selective effect in promoting height gain in undernourished and well-nourished children. In populations with low consumption of foods from animal sources, protein and zinc deficiencies will be more common. While iron and vitamin A are essential for multiple other reasons, intervention studies suggest that their deficiency does not affect linear growth. As discussed further below, calcium is critical for bone mineralization but appears to be of less consequence regarding linear growth.27
Independent of nutrient provision, as mentioned above, bone growth regulation can be blocked by inflammation, such as recurrent childhood infections, which, if bi-directionally compounded by undernutrition, result in poor growth led to stunting. The term “environmental enteric dysfunction” has been used to refer to chronic and recurrent infections and infestations in areas with poor sanitation, where infection, inflammation, and malabsorption coalesce and perpetuate undernutrition. These conditions may explain why nutrition or dietary interventions alone may not be sufficient to address stunting in children. Current interventions to reduce stunting need to target sanitation and environmental factors as well as nutrition in low- and middle-income country settings.25,27
Calcium is essential for adequate mineralization of bones, and 99% of all calcium in humans is found in bones and teeth. Dietary calcium can be absorbed passively, but the active transport of calcium in the gut is mediated by vitamin D. Both nutrients are inextricably linked in determining BMC. Low calcium intake in young children is associated with low BMC, and sustained low intakes (below 200 mg daily) with radiographic signs of rickets.56
There is clear evidence suggesting that peak bone mass and risk of fractures in later life are influenced by bone mineral accretion throughout childhood, including school age. As discussed above, peak rates of bone mineralization are reached 6 months–12 months after the peak rate of bone elongation is reached. By the end of school age, median peak height has been achieved in girls (achieved a year later in boys), and more than 80% BMC has been accrued. The exact cessation of mineral accrual varies depending on the skeletal site, but appears to be complete by 18 years in the spine and femoral neck. After peak BMC is reached, the rate of bone mass and mineral content accretion gradually and continually decreases for the rest of an individual’s life.33,57 Therefore, the rate and amount of BMC attained in the school years will greatly determine peak bone mass and be a major contributor to the relative risk of low bone mass and eventual osteoporosis and bone fractures for the rest of an individual’s life. Recent estimates from the United States in adults older than 50 years show a prevalence of low bone mass of 51.5% in women and 33.5% in men, and frank osteoporosis of 19.6% in women and 4.4% in men.58 Estimates from the International Osteoporosis Foundation indicate that, worldwide, after 50 years of age, 30% of women and 20% of men will have hip, vertebral, or wrist fractures from osteoporosis in the remainder of their lives.59 Thus, meeting dietary protein and calcium requirements, maintaining adequate vitamin D through diet and sunlight exposure, and physical activity during school-age years constitute critical investments in achieving long-term bone health.31,60
Iron, zinc, polyunsaturated fatty acids (especially docosahexaenoic acid), vitamin B12, and folate have all been specifically identified as critical nutrients for adequate brain growth, cognition, and EF development. However, as for other aspects of life-stage nutrition, most data have been accumulated for these nutrients and their longer-term effects in relation to consumption in the first 5 years of life. They have often not been the primary focus of studies and are poorly investigated in healthy school-age populations.61
Deficiency in iron deserves highlighting in relation to nutritional consequences for later life, due to its prevalence in and coexistence with all forms of malnutrition. Iron deficiency is the most common nutritional deficit in people worldwide and the most common deficiency in children, whether suffering or not from acute or chronic malnutrition. Anemia, half of which is due to iron deficiency, affects around 33% of the global population. Globally in 2019, iron deficiency was the leading cause of years lived with disability in children and young adults (aged 10 y–24 y), with the highest prevalence in most African and many Asian countries.62 While its prevalence is higher in low- and middle-income countries, it persists in varying degrees in all socio-economic levels. Iron is a crucial nutrient in maintaining levels of neurotransmitters, including dopamine and serotonin. And its deficiency can decrease brain myelination, alter synaptogenesis, and decrease the functioning of basal ganglia. The consequences in childhood include deficits in motor function and impaired cognitive development, leading to lower cognitive skills, lower school achievement, impaired psychomotor and behavioral development, and ultimately lower work capacity and productivity in adulthood.63,64
The consequences of iron deficiency in childhood, some of which may be irreversible, have been recognized for a long time and have led to global efforts in improving iron status through supplementation in infancy and childhood.64,65 Given the significant changes in brain structure and function that happen during the school years, and the potential long-term consequences of deficiency, iron remains a major nutrient of interest, highlighting the need for adequate preventive interventions as well as treatment of deficiencies during this life stage. There is increasing and robust evidence that improving iron status, particularly in the presence of anemia, significantly affects cognitive performance in school-age children older than 5 years. Interestingly, the evidence for this effect of iron on older children appears stronger than for interventions in children under 2 years of age.66
Consequences of inadequate nutrition in school age on growth and development
Inadequate nutrition in school age ultimately results from inadequate diets, which in turn are a consequence of multiple factors. On the one hand, food security, availability, and provision are essential. On the other, a healthy diet requires appropriate food choices, which depend on many environmental influences—from home, the community, the school, and broader society. These influences will not only determine the delivery of adequate foods but shape the behavior of children and thus their food choices for the long term. Influences at this age are critical, when cognitive, socioemotional, and psychosocial development are associated with increasing independence, decision-making, and self-image and awareness. Addressing all these factors remains critical for increasing an individual’s chances for long-term health. Discussion of these is beyond the scope of this review.
The immediate and most visible consequences of not meeting nutritional requirements are loss of body weight and adipose tissue (thinness/wasting/low BMI). Prolonged marginal provision of macronutrients, most often accompanied by micronutrient deficits, leads to slowing of linear growth (low height for age). In both cases, infection, or other inflammatory states, coupled with enteropathies related to poor sanitation, can further increase requirement for an effective immune response, lead to a negative nitrogen balance, mobilization of protein from muscle tissues, as well as inhibition of linear bone growth.25,28 Wasting and stunting are often linked and can occur together in the same population, often in the same child.67 Longitudinal analyses show that wasting is a precursor to stunting.68 In addition, low BMI and wasting are related to delayed pubertal onset, which in school-age children affects growth trajectories. Globally, stunting (height-for-age greater than 2 standard deviations below the WHO reference) remains by far the most prevalent clinical manifestation of undernutrition, including micronutrient deficiencies.28
Stunting in early life is associated with poor cognitive development, lower development of EF, lower rates of schooling and school achievement, and ultimately decreased productivity and earning power. These associations, however, do not necessarily infer causality. Stunting is often accompanied by multiple nutrient deficiencies and their consequences, beyond iron deficiency anemia. And the occurrence of stunting due to poor nutrition, and its cognitive and other consequences, cannot be delinked from the effects of the physical and social environments where stunting occurs69–71; nonetheless, it is likely they are causally related. Beyond cognitive impact, not achieving an individual’s height potential can also be associated with higher psychologic dysfunction all the way to late adolescence, with increased chances of developing low self-esteem, anxiety, depressive symptoms, and anti-social behaviors.72,73
Stunting can occur at any age before adulthood, but for most school-age children, it is a continuation of poor growth in early infancy. WHO and World Bank global estimates show that globally, stunting in children under 5 years of age has decreased from 33% (203.6 million children) to 22% (149.2 million children) from 2000 to 2020,7 with the highest prevalence in Africa and parts of Asia and Latin America. Thus, a great number of children are stunted when entering school age. Stunting prevalence in school age will result from stunting occurring under 5 years, with some “new” cases being added or a decrease in cases from “catch-up” growth.74 Although most stunting may start in infancy, it can continue or worsen in the school years. As discussed below, given school age is the last and second fastest period of height attainment after infancy, this life stage may offer the last “window of opportunity” for correcting deficits and potentially achieving catch-up growth and catch-up cognitive development, ameliorating its negative consequences for individuals and society.
Lastly, children who remain stunted through school years may be at increased risk for obesity. There is growing evidence that stunted infants and children who gain weight rapidly in later childhood have an increased risk of overweight, obesity, and noncommunicable diseases as adults.75,76 However, stunting at 1 year alone does not seem to raise obesity risk consistently.77 This is becoming increasingly important, as the secular transition from undernutrition to obesity is accelerating in many populations, as discussed below. The peak incidence of obesity by age has been occurring earlier and earlier in life in many populations (see below). Its consequences (related to metabolic disease, diabetes, cardiovascular disease, and other noncommunicable conditions) constitute the greatest health challenges of this century.
Specific nutrient deficiencies, individually or in combination, and their syndromes are both present and prevalent in school age. The consequences of these in addition to those mentioned above are beyond this review. Suffice it to say that persistent deficiencies such as of iron (and its effects on long-term cognitive function) as well as calcium and vitamin D (and their potential in preventing osteoporosis and fractures well into adult and mature life) are prime examples of the need for maintaining adequacy or correcting deficiencies during school age.
GLOBAL STATUS OF NUTRITION IN SCHOOL AGE
Compared with infant data, apart from some recent increase in data on the adolescent years, there is a serious lack of information on nutritional status and its consequences for middle childhood through adolescence. One analysis showed that, a literature search for 2004–2017 including the terms “health,” “mortality,” or “cause of death in the first 20 years of life” found that about 99% of the publications in Google Scholar and 95% of the publications in PubMed focused on children under age 5 years.8 Global School-based Student Surveys, a collaboration of WHO, CDC, UNICEF, UNESCO, and UNAIDS, have primarily included only 13-year-olds to 17-year-olds.78 In addition, data for this age group is often embedded and difficult to disaggregate from “childhood studies” that may include preschool and school-age children, or from studies on “adolescents” that include children of 10 years or 12 years and above. Overall, the ages 5 years–9 years and 10 years–14 years have the least number of research data sources for estimating morbidity and mortality risk factors compared with 0 years–5 years and 15 years–19 years. Another recent large population-based study showed that, within the relatively small amount of available data for height, weight, and BMI in school-age children, 78.9% of studies had data for 15 years–19 years, but only 50.3% had data for 10 years–14 years, and 39.9% for 5 years–9 years of age.79 In another larger analysis, less than half of the studies included data for middle childhood (5 y–9 y), compared with nearly 90% with data for adolescents (10 y–19 y). Overall, the quantity and quality of data vary significantly by country and region. Still, the relative lack of data for middle childhood is notable across the board, limiting the capacity to compare growth or nutrition outcomes of this age group with earlier or later life stages.80
A systematic review, one of the few studies focusing on school age (6 y–12 y) in low- and middle-income countries (LMICs), showed underweight and thinness were most prevalent (21%–36%) in South-East Asian and African countries, with lower prevalence in Latin America (8%–6%). The prevalence of overweight and obesity was highest in Latin America (∼26%), compared with 13% in Southeast Asia and 7% in Africa. The mean prevalence of iron deficiency ranged from 29% in Africa to 20% in Southeast Asia, and 14% in Latin America. Iodine, vitamin A, and zinc deficiencies are the most common. The prevalence of vitamin A deficiency was 9% in Latin America (on the lower end), to 54% zinc deficiency prevalence in Africa (on the highest end).81
Very recently, data from population-based studies supported by the Non-Communicable Disease Risk Factor Collaboration79,80 and the B&M Gates Foundation82 are shedding light on this global picture. These are the most comprehensive reviews on growth and temporal trends available to date for this age group, and the only available review addressing this age group at a global level. One analysis, NCD RisC 2017,79 included 31.5 million children from 200 countries aged 5 years–19 years, and estimated trends from 1975 to 2016. The other, NCD RisC 2020,80 pooled data from 2181 population-based studies, with height and weight measurements for 65 million participants in 200 countries, and estimated trends from 1985 to 2019 in height and BMI for children 5 years–19 years. In this study, data were reported without specific cut-off points for over- or under-nutrition. Data and trends from these studies are summarized immediately below.
Thinness and wasting
From 1975 to 2016, the overall global prevalence of moderate and severe underweight (thinness and wasting) in children 5 years–19 years decreased from 9.2% to 8.4% in girls and from 14.8% to 12.4% in boys, with the expected large variations regionally. The prevalence of moderate and severe underweight remained highest in south Asia, with 22.7% among girls and 30.7% among boys in India. Although the populations increased in most regions, the number of moderately and severely underweight school-age children actually decreased. And while prevalence declined, the relatively small change at the global level was partly due to greater population growth in countries where the prevalence of underweight is higher.79
Mean BMI trends showed increases in almost every country over the last 30 years, with the greatest increases seen in Sub-Saharan Africa. Low BMI (compared with the WHO reference median in 5-year-old children) persisted primarily in Southeast Asia and Sub-Saharan Africa. In most countries, it decreased as they entered adolescence, and in some countries it disappeared by age 19 years.80 The trends showed that, globally, the absolute number of underweight children peaked around the year 2000 and has since been decreasing, reaching levels in 2016 close to those in 1975.79
Stunting
Data from surveys in 57 LMICs between 2003 and 2013, comprising children 12 years–15 years, showed a global prevalence of stunting of 10%.75 However, the limited data available on stunting in this age group shows very wide variations. Stunting in adolescent girls (15 y–19 y) in LMICs range from 52% in Guatemala and 44% in Bangladesh to 6% in Brazil.83
The 2 most extensive global studies including children 5 years–19 years of age79,80 did not report height based on a particular cut-off for stunting. Nevertheless, in age-related trends, most countries showed that height was at or above the WHO median for children at 5 years of age, with girls doing better than boys. Still, in approximately 20% of countries for girls and 30% of countries for boys, the mean height during the school years was significantly below the WHO median. Today, the estimated difference in height of 19-year-olds between countries with the tallest populations (eg, the Netherlands, Denmark) and the shortest populations (eg, Timor-Leste, Laos, Guatemala, Bangladesh) was 20 cm. More concerning is that, in some countries, height adequacy in middle childhood may decrease as children grow older. Children who have optimal height at 5 years of age fall under the WHO median at 19 years by 2 cm or more, particularly in some middle-income countries.
In terms of temporal trends, with rare exceptions, the last 3 decades show significant gains in height in most countries and all regions for boys and girls, except for Sub-Saharan Africa (for both sexes) and Oceania (for boys). The greatest gains have been made in countries with emerging economies, including China and South Korea, and parts of Southeast Asia, the Middle East, and in some countries in North Africa, Latin America, and the Caribbean.80
Overnutrition
Over the last 40 years, obesity has increased in every country in the world. The NCD-RisC 2017 study79 showed that, from 1975 to 2016, the global prevalence of obesity in 5-year-olds to 29-year-olds increased from 0.7% to 5.6% in girls, and from 0.9% in 1975 to 7.8% in boys. The number of girls with obesity increased from about 5 million to 50 million in 2016, and the number of boys from about 6 million to 74 million. Trends in mean BMI have continued accelerating, particularly in east and south Asia. Southern African countries had the greatest rise in obesity (∼400% per decade), given the obesity prevalence was minimal 40 years ago.
On the other hand, since about 2000, the increase in prevalence has begun to plateau, and it recently flattened in northwestern Europe, in “high-income English-speaking” and Asia-Pacific regions for both sexes, in southwestern Europe for boys, and in central and Andean Latin America for girls. While not exactly comparable, these findings are consistent with another earlier large study82 that analyzed 1769 population-based surveys and studies in 19 244 children aged 2 years–19 years (not reporting disaggregated data for school age). These investigators found that, from 1980 to 2013, in developed countries, the prevalence of overweight and obesity for 2-year-old to 19-year-old children (as a group) increased from 16.9% to 23.8% in boys and 16.2% to 22.6% in girls. In the same period, in developing countries, the prevalence of overweight and obesity increased from 8.1% to 12.9% in boys and 8.4% to 13.4% in girls. All studies show that globally, the peak prevalence of obesity is shifting to younger ages. While the prevalence remains higher in developed countries, the great majority of overweight and obese girls and boys (in absolute numbers) are from LIMCs,79 thus representing a double burden of poor nutrition for the most populous countries in the world.
The NCD RisC 2020 study80 showed that the difference between the highest mean BMI (eg, Pacific Island countries, the United States, Chile, South Africa) and lowest mean BMI (eg, India, Bangladesh, Ethiopia, and Chad) was 9 kg/m2–10 kg/m2 in girls. Thus, the mean BMI difference, between these countries, was greater than 2 standard deviations in BMI for a 15-year-old girl. Trends by age varied significantly, and they worsened with age in many countries. In some countries (eg, Mexico, South Africa, New Zealand), 5-year-old children with healthy BMI progressively gained more in BMI than in height through the school-age years. Over time, some countries showed too little height gain, and/or too much weight gain for height (eg, Sub-Saharan Africa, New Zealand, the United States, Malaysia, some Pacific Island nations, and Mexico), with some differences by sex.
In summary, though the landscape has changed significantly in the last few decades, undernutrition (wasting/thinness/low BMI) and poor linear growth (low height for age/stunting), as well as overnutrition (elevated BMI with or without low height), remain major nutritional challenges globally. Today, still, despite the increase in overweight and obesity, more school-age children worldwide are moderately or severely underweight than overweight or obese. That said, in most countries, the prevalence increases in overweight and obesity are greater than the declines in prevalence of underweight. So, if current trends continue globally, the prevalence of obesity in school age will be higher than that of moderate and severe underweight before 2025.80
The considerable global differences in these markers of nutritional adequacy reflect the geographic and socio-economic gaps that also persist globally. While genetics and other factors play a role, a difference of 20 cm in height and 9 kg/m2–10 kg/m2 in BMI between extremes in populations is a partial reflection of the persistence of undernutrition and the large global nutritional and environmental gaps. Lastly, a rapid closing of those gaps may signal a “too rapid” transition from a mostly underweight population to a mostly overweight and obese population, as has occurred in parts of Asia and Latin America, accelerating and increasing the burden of nutrition-related conditions, particularly for LMIC populations.
While trajectories vary regionally, in many countries, based on height and BMI, nutritional status appears to be adequate at 5 years (which may reflect efforts over the last decades in improving early childhood health and nutrition) but deteriorates as children move through the school years. This heightens the relative neglect in attention to school-age nutritional focus, particularly for the 5–10 year-old population.
The global rise in obesity related to socio-economic and other environmental changes, including changes in nutrition, contributes to increases and exacerbations of type 2 diabetes, cardiovascular disease, and other noncommunicable diseases, exacerbating the double burden of disease and of cost to society. Prevention, starting prenatally, remains the most cost-effective and realistic approach.84 So far, however, no clear or strong regional or national success stories have been demonstrated in the last decades.82
Micronutrient deficiencies
Micronutrient deficiencies continue to be considered a major contributor to the global burden of disease. Despite this, a recent global report confirms a persistent and wide gap in data and information around micronutrient intake and nutrient status for all ages.85 Individual deficiencies rarely occur in isolation. As for other indicators of nutrition and health, some school-age micronutrient data is available for some nutrients, and some are available from studies in adolescents and young adults. Still, data in 5-year-old to 15-year-old children is the least available across the board.
Based on the high prevalence of their deficiencies, WHO considers iron, vitamin A, vitamin D, zinc, iodine, and folate the most critical micronutrients globally. It is estimated that 25% of school-age children (around 305 million children) have anemia and that 50% of it is primarily associated with iron deficiency.86 A 2004 report estimated the prevalence of vitamin A deficiency in school-age children in South Asia to be 23.4% or 83 million children, 9 million of whom had xerophthalmia.87 In low-income countries, vitamin A deficiency prevalence has been estimated at 20% among early adolescent (10–14-year-old) girls and 18% among late adolescent (15–19-year-old) girls.83 Worldwide, inadequate zinc intake is estimated at around 17%, with little data disaggregated for school children,88 90% of which is in Africa and Asia.89 The prevalence of inadequate iodine intake in 6-year-old to 12-year-old school-age children has been estimated at around 30% (241 million children), ranging from 13% in the Americas to 39% in Africa.90 Limited data on folate deficiency in females 12 years–49 years (reproductive age) indicate a prevalence of more than 20% in lower-income and less than 5% in higher-income countries. There are no good global estimates of folate deficiency for school-age children.88,91
In one report, vitamin D deficiency in 6-year-old to 12-year-old children ranged from 16% in North America, to 28% in Mexico, to 88% in China.92 For calcium, as opposed to most other nutrients where adequacy can be measured using biomarkers, there is no universally accepted definition for deficiency. So dietary intake is used as the best proxy for adequacy. In addition, recommended intakes vary significantly by regional or expert groups. For school-age children, EFSA has stated a PRI of 800 mg/day for 4-year-olds to 10-year-olds and 1150 mg/day for 11-year-olds to 17 year-olds. The Institute of Medicine in the United States has stated a recommended dietary allowance of 1000 mg/day for 4-year-olds to 8-year-olds and 1300 mg for 9-year-olds to 13-year-olds.55 While good estimates are lacking, average calcium intake in the United States for children 1 year to 14 years has been estimated at between 856 mg/day and 993 mg/day, depending on methodology, suggesting that many children fall below recommendations. There are no good global estimations, but from the little data available, it is evident that the great majority of children in developing countries fall far below any current recommendations.93 Micronutrient deficiencies further compound the total burden of poor nutrition, as they coexist with wasting and obesity.
RECOVERY FROM NUTRIENT DEFICIENCIES, GROWTH FALTERING, AND COGNITION IN SCHOOL-AGE CHILDREN
Studies and systematic reviews of micronutrient supplementation and fortification that include school-age children clearly show that micronutrient status can be improved. The demonstration of clinical effects, including growth and morbidity, varies significantly. The best documented is iron supplementation and fortification, which has been shown to improve iron status and reduce anemia in school-age children 5 years–12 years old.94 A positive effect of iron supplementation on cognitive development has been shown, and interestingly, effectiveness appears greater for children older than 7 years than for younger ages.95 Other effects of micronutrient supplementation, including growth and morbidity, are less clear.81,96
Early attention to the significant effect of nutrition until 2 years of age and the assertions, even until recently, that stunting and cognitive delays were irreversible97,98 may have contributed to the lower attention to middle childhood and adolescence as opportunities for recovery, particularly as related to stunting and cognitive deficits. Evidence today suggests this is not the case.
Catch-up growth through the school-age years is possible with the right interventions. Historical reports and observational studies of immigrant populations and adopted children document that in situations where environmental and nutritional conditions change positively, meaningful linear catch-up is possible.99 Longitudinal observational data from the COHORT multicounty study and longitudinal data from rural Gambia have shown that significant catch-up in height can be achieved between 2 years of age and the end of middle childhood (10 y of age), and between middle childhood and adulthood, even in the absence of any nutrition or health interventions.2 Catch-up growth is also possible in chronic conditions, including celiac disease and inflammatory bowel disease, with the right medical and nutritional interventions.100,101
Although results are not always consistent, some longitudinal studies that include school-age populations (6 y–11 y old) show linear catch-up is possible with multiple micronutrient supplementation.102,103 A recent systematic review and meta-analysis of the effectiveness of several nutrition-based interventions after 2 years of age (where more than half of the studies included children older than 5 years of age) showed including supplementation of protein, vitamin A, and/or multiple micronutrients, and particularly zinc supplementation, can improve linear growth, especially in children that have experienced early stunting. However, supplementation of other micronutrients, including iodine, iron, calcium, or food-based interventions, did not significantly affect growth, even if resolving anemia or other deficiencies.96
Although populations and methodologies vary, some studies have not found a correlation between linear growth recovery and cognitive measures in short-term studies over 6 months,104 while others have. Data from a longitudinal observational cohort in several LMICs found that children who had stunting by 1 year of age with linear growth catch-up by age 8 years had significantly better cognitive outcomes than those who remained stunted.105 Height catch-up in these children was positively associated with improvements in mathematics achievement, reading comprehension, and receptive vocabulary. Children who remained stunted performed less well, and children who were never stunted remained ahead of the other groups. A subsequent study of the same cohort74 analyzed catch-up growth between the ages of 8 years and 15 years and showed that more than one third of those stunted at age 8 years caught up to their peers by age 15 years, and also improved their cognitive scores compared with those who did not catch up in height. Notably, linear growth faltering was also accompanied by a decrease in cognitive outcomes in those children who became stunted between 8 years and 15 years. Thus, associations between linear growth and cognitive development vary from country to country and do not always persist from middle childhood through adolescence.74,106 The extent of linear catch-up effect in various studies will vary obviously due to the timing of the initial insult, the timing of the intervention, the duration of the intervention, and other environmental conditions beyond nutrition. Given the obvious genetic, epigenetic, and environmental carryover between young mothers and their offspring, it seems likely also that reversing the cycle of undernutrition will probably require cross-generational catch-up.107
Inadequate bone mass and mineral accretion in school-age children can have long-term consequences. While no good markers (except assessment of calcium intake and vitamin D) are available, adequate intakes remain important for reducing long-term risks. During the school-age years, a higher milk intake is associated with higher BMC, BMD, and reduced fracture risk in adulthood. Consuming less than one serving of milk a day in childhood was associated with a 2-fold increase in fracture risk as adults.108 Establishing healthy dietary behaviors with a well-balanced diet that includes adequate calcium and vitamin D, particularly with inclusion of dairy products and regular physical activity, can bring about long-term bone health.
Despite the limited knowledge we have, the nutritional objectives for school-age children appear quite clear: providing energy and protein adequacy (including avoiding excesses), decreasing deficiencies of iron, iodine, vitamin A, vitamin D, calcium, zinc, and folate, and avoiding excesses of simple sugars and sodium. Long-term studies are still lacking, and there appears to be no “magic bullet” for improving dietary intakes and avoiding excesses. However, there is increasing evidence to support school dietary and physical activity–based interventions in schoolchildren to prevent deficiencies and address overweight and obesity.109–111 The school setting has excellent potential for providing a significant part of daily intake to improve diet quality tailored to the local environment and educate children in nutrition and diet. The potential remains to be tapped.
This can only be accomplished with adequate individual, community, and population education, as well as policies that support these endeavours at multiple levels and by multiple stakeholders—a discussion that is beyond the scope of this review.
REVISITING PRIORITIES
Very recently, the last 4 years to 5 years have brought about an increasing level of attention and calls for action to address the health and nutrition of middle childhood and adolescence. In 2017, Bundy et al published a comprehensive volume as part of the World Bank’s Disease Control Priorities series, with support from the Bill and Melinda Gates Foundation, highlighting child and adolescent health, with a focus on ages 5 years–19 years, as “neglected potential” that needs to be realized.1 They noted an “asymmetry between the public investment in formal education versus health during the age range of 5 years–19 years, and a lack of recognition that the developmental returns from education are themselves dependent on concurrent good health and diet.” This “historical neglect of investments… [beyond the first 1000 d], including the next 7000 days of middle childhood and adolescence… is also reflected in investment in research into the older age-groups.”1
Around this time, collaborative efforts in population-based studies are finally presenting a more comprehensive and clearer global picture of the nutritional status of children above 5 years of age.79,80 Several research groups have reported on the significant potential that nutrition and health interventions have on improving outcomes during school age, thus constituting a true (and possibly last) major window of opportunity for supporting adequate nutrition, overcoming deficits from earlier life, shaping future dietary behaviors, and improving long-term health and well-being.2–5
Even more recently, UNICEF’s Nutrition Strategy for 2020–2030 Framework called for “strategic shifts” in upholding children’s right to nutrition and ending child malnutrition in all its forms (as part of the global Sustainable Development Goals). The Strategy includes a comprehensive life cycle approach to nutrition programming, and maternal and child nutrition during the first 1000 days as core to UNICEF programs, and also explicitly states that “nutrition during middle childhood and adolescence is both a right and a window of opportunity for growth, development and learning, particularly for girls, and for breaking the intergenerational cycle of malnutrition.” The 2 first measurable Results areas for the Strategy are 1. Early Childhood Nutrition and 2. Nutrition in Middle Childhood and Adolescence.112 UNICEF’s Programmatic Guidance for Nutrition in Middle Childhood and Adolescence includes specific priorities of nutritious foods, healthy food environments in schools and beyond, micronutrient supplementation and deworming, nutrition education in school curricula, and healthy dietary practices for school-age children and adolescents.113
While not neglecting early-life nutrition, a life-cycle approach to nutrition requires increased attention and a re-prioritization of middle childhood and early adolescence. The school-age years provide unique opportunities that will need to be embraced, even more so now, given the added challenges placed on the world by the recent COVID pandemic and climate change.
CONCLUSION
Nutrition during the formative years remains the foundation for long-term health and productivity of the individuals who make up society. Of these formative years, the first 5, with good reason, have received great attention over the last few decades. Decreasing infant mortality, including the vicious cycle of undernutrition and disease, and a better understanding of health and disease’s developmental origins, have improved our focus and understanding of the critical first few years of life. This, however, was coupled with the poorly documented notion that somatic and cognitive harm or delay in the first 2 years of life were irreversible, and hindered in part the attention given to the rest of childhood, particularly middle childhood and early adolescence. Middle childhood and early adolescence remain the most underrepresented of all life stages in health and nutrition research and clinical, nutritional, and epidemiologic data.
After the first 1000 days, the school-age years represent the most dynamic period of change in somatic and cognitive development before an individual reaches maturity, with multiple changes and inflection points in growth and development trajectories. Figure 4 summarizes key milestones in somatic and brain growth and development and shows how “eventful” this life-cycle period truly is. Deficits in growth, bone health, cognitive development, and alterations in body composition during this period have a life-long impact. It is possible and critical that we intervene during school age (a) to maintain an adequate course of somatic and cognitive development and a bridge to adult life, (b) to correct deficits of undernutrition and “catch-up” to the normal course of growth and development, and (c) to modulate or mitigate inadequacies of overnutrition and avoid longer-term consequences. Middle childhood and adolescence are thus a last major opportunity for investment, to affect growth, nutrition, and ultimate health and cognitive outcomes.
Figure 4.
The figure depicts key events in somatic and brain growth and development trajectories occurring in middle childhood and early adolescence. The timing is meant to show sequence, and the ages are best approximations. Divergence relates to differences between sexes. See text for related references. BMC: bone mineral content.
Childhood education, the basis for societal development, is not possible without adequate nutrition. In addition, child education and school systems themselves provide significant tangible opportunities for influencing dietary intake as well as for educating future generations on diet and nutrition. Therefore, it is imperative to improve our understanding of the opportunities presenting themselves during this period of life, and to develop policies and strategies to improve the current level of response to those opportunities. Only very recently has this understanding led to a revisiting of priorities in combating poor nutrition and its long-term consequences. The emphasis on intervention during the school-age years needs to be nurtured and reinforced.
Acknowledgments
The authors would like to thank Dr Francois-Pierre Martin, Dr Laurence Donato-Capel, and Dr Marie-Claire Fichot for reviewing and providing valuable suggestions regarding the manuscript.
Author contributions. All authors participated in the planning, review, and final approval of the manuscript. The content is solely and entirely the work of the authors.
Funding. The collection of material for this manuscript was partially supported by the Société des Produits Nestlé.
Declaration of interest. J.M.S. is a consultant for Société des Produits Nestlé, and Scaled Microbiomics. A.P. is a board member of the Nestlé Nutrition Institute.
Contributor Information
Jose M Saavedra, is with the Division of Gastroenterology and Nutrition, Johns Hopkins University School of Medicine, Baltimore, USA.
Andrew M Prentice, is with the MRC Unit, The Gambia and MRC International Nutrition Group, London School of Hygiene & Tropical Medicine, London, UK.
REFERENCES
- 1. Bundy DAP, Silva ND, Horton AP, et al. Child and adolescent health and development: realizing neglected potential. In: Bundy DAP, Silva ND, Horton S, Jamison DT, Patton GC, eds. Child and Adolescent Health and Development. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/the World Bank; 2017:. 1–23. [PubMed] [Google Scholar]
- 2. Prentice AM, Ward KA, Goldberg GR, et al. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Best C, Neufingerl N, van Geel L, et al. The nutritional status of school-aged children: why should we care? Food Nutr Bull. 2010;31:400–417. [DOI] [PubMed] [Google Scholar]
- 4. Akseer N, Al-Gashm S, Mehta S, et al. Global and regional trends in the nutritional status of young people: a critical and neglected age group [published correction appears in Ann N Y Acad Sci. 2017;1396(1):236]. Ann NY Acad Sci. 2017;1393:3–20. [DOI] [PubMed] [Google Scholar]
- 5. Georgiadis A, Penny ME.. Child undernutrition: opportunities beyond the first 1000 days. Lancet Public Health. 2017;2:e399. [DOI] [PubMed] [Google Scholar]
- 6. Bundy DAP, de Silva N, Horton S, et al. Annex 1A. Supplemental material. In: Bundy DAP, Silva ND, Horton S, Jamison DT, Patton GC, eds. Child and Adolescent Health and Development. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/the World Bank; 2017:1. Available at: http://www.dcp-3.org/chapter/2754/annexes. Accessed May 11, 2022. [Google Scholar]
- 7. WHO. UNICEF, World Bank, and the Demographic and Health Surveys (DHS)/USAID database. 2021. Available at: https://dhsprogram.com/data/. Accessed May 11, 2022.
- 8. Galloway R. Global nutrition outcomes at ages 5 to 19. In: Bundy DAP, Silva ND, Horton S, Jamison DT, Patton GC, eds. Child and Adolescent Health and Development. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/the World Bank; 2017:37–45. [PubMed] [Google Scholar]
- 9. Sawyer SM, Azzopardi PS, Wickremarathne D, et al. The age of adolescence. Lancet Child Adolesc Health. 2018;2:223–228. [DOI] [PubMed] [Google Scholar]
- 10. Alderman H, Behrman JR, Glewwe P, et al. Evidence of impact of interventions on growth and development during early and middle childhood. In: Bundy DAP, Silva ND, Horton S, Jamison DT, Patton GC, eds. Child and Adolescent Health and Development. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/the World Bank; 2017:79–98. [PubMed] [Google Scholar]
- 11. United Nations Children’s Fund (UNICEF). Convention on the Rights of the Child. 1990. Available at: https://www.unicef.org/child-rights-convention/convention-text. Accessed May 11, 2022.
- 12. Patton GC, Sawyer SM, Santelli JS, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet. 2016;387:2423–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDonagh JE; European Training Effective Care and Health Faculty. The age of adolescence…and young adulthood. Lancet Child Adolesc Health. 2018;2:e6. [DOI] [PubMed] [Google Scholar]
- 14. Cousminer DL, Berry DJ, Timpson NJ, et al. ; The ReproGen Consortium. Genome-wide association and longitudinal analyses reveal genetic loci linking pubertal height growth, pubertal timing and childhood adiposity. Hum Mol Genet. 2013;22:2735–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DelGiudice M. Middle childhood: an evolutionary–developmental synthesis. In: Halfon N, Forrest CB, Lerner RM, Faustman EM, eds. Handbook of Life Course Health Development. Cham (CH: ): Springer; 2017:95–107. [Google Scholar]
- 16. Reinehr T, Roth CL.. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 2019;3:44–54. [DOI] [PubMed] [Google Scholar]
- 17. Song Y, Ma J, Wang HJ, et al. Trends of age at menarche and association with body mass index in Chinese school-aged girls, 1985–2010. J Pediatr. 2014;165:1172–1177.e1. [DOI] [PubMed] [Google Scholar]
- 18. Goswami B, Roy AS, Dalui R, et al. Impact of pubertal growth on physical fitness. Am J Sports Med. 2014;2:34–39. [Google Scholar]
- 19. USA Centers for Disease Control and Prevention (CDC): National Center for Health Statistics. CDC Growth Charts. Available at: https://www.cdc.gov/growthcharts/cdc_charts.htm. Accessed May 11, 2022.
- 20. Cole TJ. Children grow and horses race: is the adiposity rebound a critical period for later obesity? BMC Pediatr. 2004;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–146. [DOI] [PubMed] [Google Scholar]
- 22. Webber CE, Barr RD.. Age- and gender-dependent values of skeletal muscle mass in healthy children and adolescents. J Cachexia Sarcopenia Muscle. 2012;3:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCarthy HD, Samani-Radia D, Jebb SA, et al. Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes. 2014;9:249–259. [DOI] [PubMed] [Google Scholar]
- 24. Tanner JM, Davies PS.. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. [DOI] [PubMed] [Google Scholar]
- 25. Nijjar JK, Stafford D.. Undernutrition and growth in the developing world. Curr Opin Endocrinol Diabetes Obes. 2019;26:32–38. [DOI] [PubMed] [Google Scholar]
- 26. Owino V, Ahmed T, Freemark M, et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics. 2016;138:e20160641. [DOI] [PubMed] [Google Scholar]
- 27. Millward DJ. Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr Res Rev. 2017;30:50–72. [DOI] [PubMed] [Google Scholar]
- 28. Prendergast AJ, Humphrey JH.. The stunting syndrome in developing countries. Paediatr Int Child Health. 2014;34:250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baxter-Jones AD, Faulkner RA, Forwood MR, et al. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–1739. [DOI] [PubMed] [Google Scholar]
- 30. Maggioli C, Stagi S.. Bone modeling, remodeling, and skeletal health in children and adolescents: mineral accrual, assessment and treatment. Ann Pediatr Endocrinol Metab. 2017;22:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golden NH, Abrams SA; Committee on Nutrition. Optimizing bone health in children and adolescents. Pediatrics. 2014;134:e1229–e1243. [DOI] [PubMed] [Google Scholar]
- 32. Ralston SH, de Crombrugghe B.. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–2506. [DOI] [PubMed] [Google Scholar]
- 33. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations [published correction appears in Osteoporos Int. 2016;27(4):1387]. Osteoporos Int. 2016;27:1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stiles J, Jernigan TL.. The basics of brain development. Neuropsychol Rev. 2010;20:327–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Konrad K, Firk C, Uhlhaas PJ.. Brain development during adolescence: neuroscientific insights into this developmental period. Dtsch Arztebl Int. 2013;110:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mills KL, Goddings AL, Herting MM, et al. Structural brain development between childhood and adulthood: convergence across four longitudinal samples. Neuroimage. 2016;141:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lenroot RK, Giedd JN.. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. [DOI] [PubMed] [Google Scholar]
- 38. Peterson MR, Cherukuri V, Paulson JN, et al. Normal childhood brain growth and a universal sex and anthropomorphic relationship to cerebrospinal fluid. J Neurosurg Pediatr. 2021;28:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. [DOI] [PubMed] [Google Scholar]
- 40. Johnson SB, Blum RW, Giedd JN.. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Immordino-Yang MH, Darling-Hammond L, Krone C.. The Brain Basis for Integrated Social, Emotional, and Academic Development. How emotions and social relationships drive learning. Washington, DC: The Aspen Institute National Commission on Social, Emotional, and Academic Development. 2018. Available at: https://www.aspeninstitute.org/publications. Accessed May 11, 2022.
- 42. Dumontheil I. Development of abstract thinking during childhood and adolescence: the role of rostrolateral prefrontal cortex. Dev Cogn Neurosci. 2014;10:57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flege JE, Munro MJ, MacKay IR.. Factors affecting strength of perceived foreign accent in a second language. J Acoust Soc Am. 1995;97:3125–3134. [DOI] [PubMed] [Google Scholar]
- 44. Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. [DOI] [PubMed] [Google Scholar]
- 45. Diamond A. Executive functions. Handb Clin Neurol. 2020;173:225–240. [DOI] [PubMed] [Google Scholar]
- 46. Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. [DOI] [PubMed] [Google Scholar]
- 47. Spear LP. Adolescent neurodevelopment. J Adolesc Health. 2013;52:S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mills KL, Goddings AL, Clasen LS, et al. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–160. [DOI] [PubMed] [Google Scholar]
- 49. Kelley JC, Crabtree N, Zemel BS.. Bone density in the obese child: clinical considerations and diagnostic challenges. Calcif Tissue Int. 2017;100:514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Das JK, Salam RA, Thornburg KL, et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Ann N Y Acad Sci. 2017;1393:21–33. [DOI] [PubMed] [Google Scholar]
- 51. Trumbo P, Schlicker S, Yates AA, et al. ; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids [published correction appears in J Am Diet Assoc. 2003;103(5):563]. J Am Diet Assoc. 2002;102:1621–1630. [DOI] [PubMed] [Google Scholar]
- 52. Chugani HT. Positron emission tomography scanning: applications in newborns. Clin Perinatol. 1993;20:395–409. [PubMed] [Google Scholar]
- 53. Grande Covián F. El metabolismo energético del cerebro en la infancia [Energy metabolism of the brain in children (author’s transl)]. An Esp Pediatr. 1979;12:235–244. [PubMed] [Google Scholar]
- 54. Kalhan SC, Kiliç I.. Carbohydrate as nutrient in the infant and child: range of acceptable intake. Eur J Clin Nutr. 1999;53(suppl 1):S94–S100. [DOI] [PubMed] [Google Scholar]
- 55. European Food Safety Authority (EFSA). Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Summary of Dietary Reference Values – version 4. September 2017. Available at: https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values. Accessed May 11, 2022.
- 56. Branca F, Vatueña S.. Calcium, physical activity and bone health–building bones for a stronger future. Public Health Nutr. 2001;4:117–123. [DOI] [PubMed] [Google Scholar]
- 57. Heaney RP, Abrams S, Dawson-Hughes B, et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. [DOI] [PubMed] [Google Scholar]
- 58. Sarafrazi N, Wambogo EA, Shepherd JA.. Osteoporosis or low bone mass in older adults: United States, 2017–2018. NCHS Data Brief. 2021;(405):1–8. [PubMed] [Google Scholar]
- 59. Fragility Fractures - Epidemiology. Nyon, Switzerland: International Osteoporosis Foundation; 2021. Available at: https://www.osteoporosis.foundation/health-professionals/fragility-fractures/epidemiology. Accessed May 11, 2022.
- 60. Prentice A, Schoenmakers I, Laskey MA, et al. Nutrition and bone growth and development. Proc Nutr Soc. 2006;65:348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Georgieff MK, Ramel SE, Cusick SE.. Nutritional influences on brain development. Acta Paediatr. 2018;107:1310–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Camaschella C. Iron deficiency. Blood 2019;133:30–39. [DOI] [PubMed] [Google Scholar]
- 64. Pivina L, Semenova Y, Doşa MD, et al. Iron deficiency, cognitive functions, and neurobehavioral disorders in children. J Mol Neurosci. 2019;68:1–10. [DOI] [PubMed] [Google Scholar]
- 65. Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:S560–S571. [DOI] [PubMed] [Google Scholar]
- 66. Larson LM, Phiri KS, Pasricha SR.. Iron and cognitive development: what is the evidence? Ann Nutr Metab. 2017;71(suppl 3):25–38. [DOI] [PubMed] [Google Scholar]
- 67. Briend A, Khara T, Dolan C.. Wasting and stunting—similarities and differences: policy and programmatic implications. Food Nutr Bull. 2015;36:S15–S23. [DOI] [PubMed] [Google Scholar]
- 68. Schoenbuchner SM, Dolan C, Mwangome M, et al. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am J Clin Nutr. 2019;110:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fernald LCG, Prado E, Kariger P, et al. A Toolkit for Measuring Early Childhood Development in Low and Middle-Income Countries. 2017 International Bank for Reconstruction and Development, The World Bank, Washington DC. License: CC BY 3.0 IGO. Available at: https://openknowledge.worldbank.org/handle/10986/29000. Accessed May 11, 2022.
- 70. Crookston BT, Penny ME, Alder SC, et al. Children who recover from early stunting and children who are not stunted demonstrate similar levels of cognition. J Nutr. 2010;140:1996–2001. [DOI] [PubMed] [Google Scholar]
- 71. Sudfeld CR, McCoy DC, Danaei G, et al. Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics. 2015;135:e1266–e1275. [DOI] [PubMed] [Google Scholar]
- 72. Walker SP, Chang SM, Powell CA, et al. Early childhood stunting is associated with poor psychological functioning in late adolescence and effects are reduced by psychosocial stimulation. J Nutr. 2007;137:2464–2469. [DOI] [PubMed] [Google Scholar]
- 73. Sánchez A. The structural relationship between early nutrition, cognitive skills and non-cognitive skills in four developing countries. Econ Hum Biol. 2017;27:33–54. [DOI] [PubMed] [Google Scholar]
- 74. Fink G, Rockers PC.. Childhood growth, schooling, and cognitive development: further evidence from the Young Lives study. Am J Clin Nutr. 2014;100:182–188. [DOI] [PubMed] [Google Scholar]
- 75. Caleyachetty R, Thomas GN, Kengne AP, et al. The double burden of malnutrition among adolescents: analysis of data from the Global School-Based Student Health and Health Behavior in School-Aged Children surveys in 57 low- and middle-income countries. Am J Clin Nutr. 2018;108:414–424. [DOI] [PubMed] [Google Scholar]
- 76. Schott W, Aurino E, Penny ME, et al. The double burden of malnutrition among youth: trajectories and inequalities in four emerging economies. Econ Hum Biol. 2019;34:80–91. [DOI] [PubMed] [Google Scholar]
- 77. Andersen CT, Stein AD, Reynolds SA, et al. Stunting in infancy is associated with decreased risk of high body mass index for age at 8 and 12 years of age. J Nutr. 2016;146:2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. World Health Organization. Noncommunicable Disease Surveillance, Monitoring and Reporting. Global School-based Student Health Survey. Available at: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/global-school-based-student-health-survey. Accessed May 11, 2022.
- 79. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body–mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. NCD Risk Factor Collaboration (NCD-RisC). Height and body–mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396:1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Best C, Neufingerl N, Del Rosso JM, et al. Can multi-micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review. Nutr Rev. 2011;69:186–204. [DOI] [PubMed] [Google Scholar]
- 82. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013 [published correction appears in Lancet. 2014;384(9945):746]. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Christian P, Smith ER.. Adolescent undernutrition: global burden, physiology, and nutritional risks. Ann Nutr Metab. 2018;72:316–328. [DOI] [PubMed] [Google Scholar]
- 84. Darnton-Hill I, Nishida C, James WP.. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2004;7:101–121. [DOI] [PubMed] [Google Scholar]
- 85. McLean E, Cogswell M, Egli I, et al. Global Nutrition Report. Chapter 3. Development Initiatives Poverty Research Ltd 2018. 2018. https://globalnutritionreport.org/reports/global-nutrition-report-2018/. Accessed May 11, 2022.
- 86. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. [DOI] [PubMed] [Google Scholar]
- 87. Singh V, West KP Jr. Vitamin A deficiency and xerophthalmia among school-aged children in Southeastern Asia. Eur J Clin Nutr. 2004;58:1342–1349. [DOI] [PubMed] [Google Scholar]
- 88. Bailey RL, West KP Jr, Black RE.. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33. [DOI] [PubMed] [Google Scholar]
- 89. Kumssa DB, Joy EJ, Ander EL, et al. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci Rep. 2015;5:10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Andersson M, Takkouche B, Egli I, et al. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83:518–525. [PMC free article] [PubMed] [Google Scholar]
- 91. Rogers LM, Cordero AM, Pfeiffer CM, et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann NY Acad Sci. 2018;1431:35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Palacios C, Gonzalez L.. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pettifor JM. Calcium and vitamin D metabolism in children in developing countries. Ann Nutr Metab. 2014;64(suppl 2):15–22. [DOI] [PubMed] [Google Scholar]
- 94. De-Regil LM, Jefferds ME, Sylvetsky AC, et al. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database Syst Rev 2011;2011:CD009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sachdev H, Gera T, Nestel P.. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005;8:117–132. [DOI] [PubMed] [Google Scholar]
- 96. Roberts JL, Stein AD.. The impact of nutritional interventions beyond the first 2 years of life on linear growth: a systematic review and meta-analysis. Adv Nutr. 2017;8:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martorell R, Khan LK, Schroeder DG.. Reversibility of stunting: epidemiological findings in children from developing countries. Eur J Clin Nutr. 1994;48(suppl 1):S45–S57. [PubMed] [Google Scholar]
- 98. Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital [published correction appears in Lancet. 2008;371(9609):302]. Lancet. 2008;371:340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Watkins KL, Bundy DAP, Jamison DT, et al. Evidence of impact of interventions on health and development during middle childhood and school age. In: Bundy DAP, Silva ND, Horton S, Jamison DT, Patton GC, eds. Child and Adolescent Health and Development. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/the World Bank; 2017:99–105. [PubMed] [Google Scholar]
- 100. Gasparetto M, Guariso G.. Crohn’s disease and growth deficiency in children and adolescents. World J Gastroenterol. 2014;20:13219–13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Boersma B, Houwen RH, Blum WF, et al. Catch-up growth and endocrine changes in childhood celiac disease. Endocrine changes during catch-up growth. Horm Res. 2002;58(suppl 1):57–65. [DOI] [PubMed] [Google Scholar]
- 102. Abrams SA, Mushi A, Hilmers DC, et al. A multinutrient-fortified beverage enhances the nutritional status of children in Botswana. J Nutr. 2003;133:1834–1840. [DOI] [PubMed] [Google Scholar]
- 103. Ash DM, Tatala SR, Frongillo EA Jr, et al. Randomized efficacy trial of a micronutrient-fortified beverage in primary school children in Tanzania. Am J Clin Nutr. 2003;77:891–898. [DOI] [PubMed] [Google Scholar]
- 104. Sokolovic N, Selvam S, Srinivasan K, et al. Catch-up growth does not associate with cognitive development in Indian school-age children. Eur J Clin Nutr. 2014;68:14–18. [DOI] [PubMed] [Google Scholar]
- 105. Crookston BT, Schott W, Cueto S, et al. Postinfancy growth, schooling, and cognitive achievement: young lives. Am J Clin Nutr. 2013;98:1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Georgiadis A, Benny L, Duc LT, et al. Growth recovery and faltering through early adolescence in low- and middle-income countries: determinants and implications for cognitive development. Soc Sci Med. 2017;179:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Golden MH. Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr. 1994;48(suppl 1):S58–S70; discussion S71. [PubMed] [Google Scholar]
- 108. Kalkwarf HJ, Khoury JC, Lanphear BP.. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr. 2003;77:257–265. [DOI] [PubMed] [Google Scholar]
- 109. Jacob CM, Hardy-Johnson PL, Inskip HM, et al. A systematic review and meta-analysis of school-based interventions with health education to reduce body mass index in adolescents aged 10 to 19 years. Int J Behav Nutr Phys Act. 2021;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Singhal J, Herd C, Adab P, et al. Effectiveness of school-based interventions to prevent obesity among children aged 4 to 12 years old in middle-income countries: a systematic review and meta-analysis. Obes Rev. 2021;22:e13105. [DOI] [PubMed] [Google Scholar]
- 111. Kyere P, Veerman JL, Lee P, et al. Effectiveness of school-based nutrition interventions in sub-Saharan Africa: a systematic review. Public Health Nutr. 2020;23:2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. United Nations Children’s Fund (UNICEF). Nutrition, for Every Child: UNICEF Nutrition Strategy 2020–2030. New York: UNICEF. 2020. Available at: https://www.unicef.org/media/92031/file/UNICEF%20Nutrition%20Strategy%202020-2030.pdf. Accessed May 11, 2022. [Google Scholar]
- 113. United Nations Children’s Fund (UNICEF). Programming Guidance: Nutrition in Middle Childhood and Adolescence. New York, NY: UNICEF. 2021. Available at: https://www.unicef.org/media/106406/file. Accessed May 11, 2022. [Google Scholar]
- 114. Tapert SF, Schweinsburg AD.. The human adolescent brain and alcohol use disorders. Recent Dev Alcohol. 2005;17:177–197. [DOI] [PubMed] [Google Scholar]
- 115. Lee FS, Heimer H, Giedd JN, et al. Mental health. Adolescent mental health—opportunity and obligation. Science. 2014;346:547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]