Abstract
Females are often underrepresented in the scientific literature, but awareness of the need for female-specific research is increasing. Review articles have been published on the effects of the menstrual cycle on aspects of exercise performance and physiology, yet to date no research has reviewed the effect of menstrual cycle phase on dietary energy intake. Fluctuations in endogenous sex hormones across the menstrual cycle influence a range of physiological processes, including those involved in nutritional status. Observational research typically quantifies female athletes’ nutritional intakes at a single time point; however, this may provide inaccurate information if dietary intake fluctuates across the menstrual cycle. Similarly, this may have implications for interventional research, where dietary intake is often poorly controlled or monitored. This review aimed to synthesize the published literature on dietary energy intakes of naturally menstruating females in various phases of the menstrual cycle. The review critiques the relevant literature in light of recent publications on good practice for female research, explores the impact of the menstrual cycle on energy intake, identifies gaps within the evidence base, and informs future research. Overall, energy intake appears to be lower in the follicular phase compared with the luteal phase, with a particular decrease in the days leading up to and including ovulation. The magnitude of these fluctuations is not yet clearly quantifiable and most likely varies, both between individuals, and from cycle to cycle. This review notes the lack of high-quality research investigating the energy intakes of females across the menstrual cycle, and the very limited data available for female athletes and others who undertake large amounts of physical activity. It also highlights the need for researchers to take into consideration anovulatory cycles and the potential effects of premenstrual disorders on dietary intake.
Keywords: energy, follicular, luteal, ovulation
INTRODUCTION
Females have long been underrepresented as research participants across a range of fields, hindering the progress in better understanding female physiology.1–4 For example, across more than 5200 papers published in 6 of the leading sports science journals between 2014 and 2020, only one third of all participants were female, with only 6% of studies conducted exclusively in females.4 Yet, important physiological differences exist between the sexes, such that research conducted in males may not always be directly applicable to females.5 One of these differences is the menstrual cycle, which involves dramatic fluctuations in endogenous sex hormones over a 21-day–35-day period, from menarche until menopause.6,7
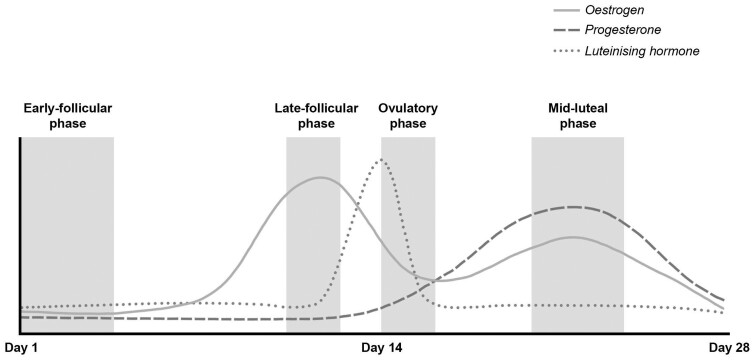
The menstrual cycle is commonly divided into 2 phases: the follicular phase (from day 1 of menstrual bleeding until ovulation) and the luteal phase (post ovulation).6,8 However, within 1 cycle there are 4 distinct hormonal environments: (1) the early-follicular phase, characterized by low estrogen and low progesterone; (2) the late-follicular phase, characterized by high estrogen and low progesterone; (3) the ovulatory phase, characterized by medium estrogen and low progesterone; and (4) the mid-luteal phase, characterized by medium estrogen and high progesterone7 (Figure 1).
Figure 1.
Schematic diagram of the relative rise and fall in estrogen, progesterone, and luteinizing hormone concentrations across an idealized 28-day menstrual cycle with ovulation occurring on day 14. Hormonal fluctuations are superimposed over the menstrual cycle phases representing 4 distinct hormonal environments: the early-follicular phase, the late-follicular phase, the ovulatory phase, and the mid-luteal phase. The solid line represents estrogen, the dashed line represents progesterone, and the dotted line represents luteinizing hormone. Figure 1 was created with Adobe Photoshop 2020 (Adobe Inc., San Jose, CA, USA).
Aside from their role in reproduction, these hormonal fluctuations also influence a range of physiological processes throughout the body, including those involved in nutritional status. Estrogen is hypothesized to suppress appetite, while progesterone, in the presence of estrogen, may have the opposite effect.9 Rates of carbohydrate and fat oxidation vary across the cycle, with greater glycogen storage at rest,10 and a stronger preference for fat utilization during exercise, in the luteal phase compared with the follicular phase.11 Protein catabolism also appears to increase in the luteal phase,12,13 potentially contributing to the greater resting metabolic rate observed during this time.14
There is growing awareness of the need to close the sex data gap and better understand female physiology, especially given increasing rates of female participation in both recreational and elite-level sport.15,16 Recent reviews have explored the effect of the menstrual cycle on exercise performance,17,18 adaptation to resistance training,19 resting metabolism,14 and thermoregulation during exercise in the heat20; yet, to date, no published paper has synthesized the literature on energy intake across the menstrual cycle. Dietary intake is the product of a complex interplay between social, cultural, environmental, psychological, and physiological factors, and there is no clear consensus on the role that the menstrual cycle might play in this whole system. A clear understanding of how hormonal fluctuations across the menstrual cycle influence energy intake is of clinical importance. Changes in energy intake could have important implications for energy availability (the amount of dietary energy available per kilogram of fat-free mass, after accounting for the energy expended during exercise), particularly in athletes for whom training volume may not be modified to account for phase-related changes in energy intake. Low energy availability can lead to severe health consequences, including menstrual disturbances, impaired bone health, endocrinological effects, decreased athletic performance and increased risk of injury and illness.21 Consequently, if energy intake is lower during certain periods of the menstrual cycle, additional nutritional support may be required to ensure adequate energy availability. Therefore, this review aims to: (1) explore the current observational research on dietary energy intakes of naturally menstruating females across the menstrual cycle, as well as intervention studies assessing phase-related ad-libitum food intake; (2) identify gaps in the literature; and (3) inform future research.
METHODS
To identify the published literature regarding energy intake across the menstrual cycle, PubMed and Scopus were searched with the search terms “energy intake” AND “menstrual cycle,” returning approximately 300 results (excluding duplicates). Titles and abstracts were screened to identify those that investigated the effect of menstrual cycle phase on energy intake or availability, rather than the effect of energy intake or availability on menstrual function. After full-test screening of the remaining manuscripts, papers were included if they were original research articles that assessed dietary energy intake or availability at more than 1 phase of the menstrual cycle in naturally menstruating adult human females and were published in the English language. The reference lists of included studies were additionally screened to locate further relevant literature.
ENERGY INTAKE IN THE FOLLICULAR VERSUS LUTEAL PHASE
Despite the presence of 4 unique hormonal environments, many studies simply compare the follicular phase with the luteal phase—the definitions of which vary widely. The early-follicular phase (or “menses” as it may also be called) typically refers to the days of menstrual bleeding. Occasionally studies will use a later time point, such as days 7–9 after the onset of menstruation, to represent the follicular phase (what one might call the “mid-follicular phase”, although there is no accepted definition of this). It is only recently that the late-follicular and ovulatory phases have been recognized and defined as 2 distinct phases; historically researchers used the “peri-ovulatory phase” to refer to the several days leading up to and including ovulation. The luteal phase is generally defined by a variable number of days relative to menstruation or ovulation. Earlier research often compared the 10-days pre-menstruation onset, with the 10-days post-menstruation onset, however, new guidelines propose stricter phase definitions to improve research quality,7 and are as follows:
Early-follicular phase: defined by the onset of bleeding (day 1) until day 5, when estrogen and progesterone are at their lowest levels.
Late-follicular phase: defined as the 14 hours–26 hours prior to ovulation, when estrogen levels are higher than in the other phases, and progesterone levels are higher than in the early-follicular phase, but lower than 6.36 nmol/L.
Ovulatory phase: defined by a positive urinary ovulation (luteinizing hormone) test and lasting 24 hours–36 hours, when estrogen levels are higher than in the early-follicular phase, but lower than in the other phases, and progesterone levels are higher than in the early-follicular phase, but <6.4 nmol/L.
Mid-luteal phase: defined as 7 days after confirmation of ovulation, when estrogen levels are higher than in the early-follicular and ovulatory phases, but lower than in the late-follicular phase, and progesterone levels are >16 nmol/L (Figure 1).7
However, of the research concerning energy intake across the menstrual cycle, adherence to these recommended menstrual phase definitions is limited, such that comparing energy intake between phases is often restricted to just the follicular and luteal phases: Tables 1 and 2 provide a summary of the literature comparing energy intake between the various phases of the menstrual cycle. With this in mind, there is evidence to suggest that daily energy intake is greater in the luteal phase of the menstrual cycle compared with the follicular phase in naturally menstruating females.7,20,22–36 A study in 30 Brazilian females found mean energy intake to be 1730 ± 254 kcal/day in the follicular phase, compared with 2259 ± 375 kcal/day in the luteal phase, representing a mean increase of 529 kcal/day.36 Similarly, Reimer et al reported a mean increase of 337 kcal/day from the follicular phase to the luteal phase,35 while Martini et al reported a mean increase of 159 kcal/day.27 Many others have observed a similar pattern, with mean increases from the follicular to luteal phase ranging from 90 kcal/day to 504 kcal/day.7,20,22–31,34–36 Yet this observation is not universal, with several studies finding no evidence of a difference in phase-related energy intake.37–42 Differences in methodology and study quality may in part explain these conflicting findings.
Table 1.
Studies assessing dietary energy intake of naturally menstruating females across the various phases of the menstrual cycle with phases verified by serum estrogen and progesterone concentrations
| Title | Reference | Country | Populationa | Menstrual cycle phases and quantification method | Number of cycles | Dietary assessment method | Findingsa |
|---|---|---|---|---|---|---|---|
| Energy regulation over the menstrual cycle26 | Johnson et al 1994 | United States |
|
|
1 | Daily estimated diet records over one cycle | EI was greater in the luteal vs ovulatory phase (1902 ± 452 kcal/d vs 1736 ± 427 kcal/d, mean difference: 166 kcal/dc, P < .05d) |
| Effect of the menstrual cycle on energy and nutrient intake27 | Martini et al 1994 | United States |
|
|
|
3-d estimated diet records | EI was greater in the mid-luteal vs mid-follicular phase (1908 ± 38 kcal/d vs 1749 ± 37 kcal/de, mean difference: 159 kcal/dc, P = .003) |
| Dietary and metabolic differences in pre- versus post-menopausal women taking or not taking hormonal replacement therapy35 | Reimer et al 2005 | Canada |
|
|
1 | 3-d estimated diet records. | EI was greater in the luteal vs follicular phase (2089 ± 178 kcal/d vs 1752 ± 158 kcal/d, mean difference: 337 kcal/dc, P < .05d) |
| Changes in mood, cognitive performance, and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD33 | Reed et al 2008 | United States |
|
|
2 | Intake based on single provided meal (weighed by researchers) | EI was 16% greater in the luteal vs follicular phase for the PMDD group (P = .04), but there was no evidence of a difference in EI for the control group (P > .05d) |
| Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women32 | Brennan et al 2009 | Australia |
|
|
1 | Intake based on single provided meal (weighed by researchers) | EI was greater in the luteal phase (914 ± 113 kcal) compared with the first and second follicular phase time points (771 ± 84 kcal and 728 ± 82 kcal, mean difference: 143 kcal and 186 kcal, respectivelyc, P < .05d) |
| Regulation of menstrual cycle and alimentary consumption in women36 | Gil et al 2009 | Brazil |
|
|
1 | 3-d estimated diet records (including one weekend day) | EI was greater in the luteal vs follicular phase (2259 ± 375 kcal/d vs 1730 ± 254 kcal/d, mean difference: 529 kcal/dc, P < .001) |
| Food intake changes across the menstrual cycle in Taiwanese women25 | Chung et al 2010 | Taiwan |
|
|
1 | 3-d estimated diet records (one weekend day included where possible) | EI was greater during the luteal vs follicular phase (1753 kcal/d vs 1593 kcal/db, mean difference: 160 kcal/dc, P = .03). For those with correctly identified cycle phases (n = 22), EI was greater in the luteal vs follicular phase by 180 kcal/dc (P = .04) |
| Greater overall olfactory performance, explicit wanting for high-fat food, and lipid intake during the mid-luteal phase of the menstrual cycle43 | McNeil et al 2013 | Canada |
|
|
1 | Intake based on single provided meal (weighed by researchers) | EI in the early-follicular, late-follicular, and mid-luteal phases were 670 ± 293 kcal, 525 ± 289 kcal, and 711 ± 334 kcal, respectively (P = .05 for trend) |
| Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women37 | Gorczyca et al 2016 | United States |
|
|
|
24-h recalls | No evidence of a difference in EI between phases for either ovulatory (P = .35) or anovulatory cycles (P = .36) |
| Influence of menstrual cycle of hormonal contraceptive phase on energy intake and metabolic hormone—a pilot study40 | Ihalainen et al 2021 | Finland |
|
|
1 | 3-d estimated diet records | No evidence of a difference in EI (P = .825) or energy availability (P = .465) between phases |
Values represent mean ± SD unless otherwise specified.
Standard deviation not reported.
Mean difference not reported and calculated from absolute values reported in each phase, or mean difference reported without SD of the difference.
Exact P-value not reported.
Values represent mean ± SE.
Represents age range of participants; mean ± SD not reported.
Abbreviations: BBT, basal body temperature; BMI, body–mass index; EI, energy intake; LH, luteinizing hormone; PMDD, premenstrual dysphoric disorder
Table 2.
Studies assessing dietary energy intake of naturally menstruating females across the various phases of the menstrual cycle without verification of phases with serum estrogen and progesterone concentrations
| Title | Reference | Country | Populationa | Menstrual cycle phases and quantification method | Number of cycles | Dietary assessment method | Findingsa |
|---|---|---|---|---|---|---|---|
| The effect of the menstrual cycle on patterns of food intake29 | Dalvit 1981 | United States |
|
|
2 | Daily 24-h recalls for 60 d | EI was greater in the luteal vs follicular phase for both cycle 1 (1935 kcal/d vs 1431 kcal/dc, mean difference: 504 ± 219 kcal/d, P = .0004) and cycle 2 (1945 vs 1449 kcal/dc, mean difference: 496 ± 378 kcal/d, P = .008) |
| Food intake, body weight, and sweetness preferences over the menstrual cycle in humans28 | Pliner and Fleming 1983 | Canada |
|
|
1 | 24-h recalls | EI was greater in the luteal vs follicular phase (2013 ± 533 kcal/d vs 1790 ± 642 kcal/d, mean difference: 223 kcal/dd, P < .05e) |
| A study of dietary intake in pre- and post-menstrual period44 | Manocha et al 1986 | India |
|
|
2 | Daily estimated diet records for 60 d | EI was greater in the luteal vs follicular phase for both cycle 1 (1620 ± 275 kcal/d vs 1300 ± 290 kcal/d, mean difference: 320 kcal/dd, P < .05e) and cycle 2 (1605 ± 270 kcal/d vs 1300 ± 255 kcal/d, mean difference: 305 kcal/dd, P < .01e) |
| Menstrual cycle and voluntary food intake45 | Gong et al 1989 | United States |
|
|
1 | Daily weighed-food records for 1 cycle | EI was greater in the luteal vs follicular phases (2040 ± 156 kcal/d vs 1833 ± 146 kcal/d, mean difference: 207 kcal/dd, P < .05e), and in the luteal vs periovulatory phase (2040 ± 156 kcal/d vs 1766 ± 252 kcal/d, mean difference: 274 kcal/dd, P < .05e) |
| Reduction of food intake in the ovulatory phase of the menstrual cycle46 | Lyons et al 1989 | Australia |
|
1 | Daily weighed-diet records for 1 cycle | EI was lower in the ovulatory phase (1874 ± 81 kcal/d) vs post-ovulatory phase (2198 ± 86 kcal/d, mean difference: 324 kcal/dd, P < .05e), pre-menses (2150 ± 86 kcal/d, mean difference: 276 kcal/dd, P < .05e) and menses (2155 ± 100 kcal/d mean difference: 281 kcal/dd, P < .05e). There was no evidence of a difference in EI between 10 d pre- and post-menstruation (2133 kcal/d vs 2102 kcal/dc,e) | |
| Menstrual-cycle patterns in energy and macronutrient intake31 | Tarasuk and Beaton 1991 | United States |
|
|
|
Daily weighed-diet records | EI was greater in the luteal vs follicular phase (1912 kcal/d vs 1822 kcal/dc, mean difference: 90 ± 38 kcal/d, P = .03) |
| Changes in dietary intake, urinary nitrogen, and urinary volume across the menstrual cycle39 | Fong and Kretsch 1993 | United States |
|
|
1 | Daily weighed-diet records for 1 cycle (by researchers in metabolic ward study) | No evidence of a difference in EI between menses (2045 ± 468 kcal/d), follicular (2027 ± 443 kcal/d), periovulatory (1968 ± 516 kcal/d) and luteal phases (2204 ± 475 kcal/de) |
| Energy intakes are higher during the luteal phase of ovulatory menstrual cycle23 | Barr et al 1995 | Canada |
|
|
3 | 3-d weighed-diet records | EI was greater during the luteal vs follicular phase for ovulatory cycles (2248 ± 652 kcal/d vs 1942 ± 572 kcal/d, mean difference: 305 kcal/dd, P < .001), but there was no evidence of a difference for anovulatory cycles (1918 ± 529 kcal/d vs 1990 ± 359 kcal/de) |
| Menstrual cycle and voluntary food intake in young Chinese women30 | Li et al 1999 | China |
|
|
1 | 3-d estimated diet records (not necessarily consecutive, but including 1 weekend day) | EI was greater in the mid-luteal vs mid-follicular phase (1692 ± 448 kcal/d vs 1478 ± 285 kcal/d, mean difference: 214 kcal/dd, P = .02). There was a 23% increase in EI in the luteal vs follicular phase based on weekend records (1749 ± 574.4 kcal/d vs 1419 ± 417 kcal/d, mean difference: 330 kcal/dd, P = .018), but no evidence of a difference in EI for weekday records (1663 ± 507 kcal/d vs 1508 ± 357 kcal/d, mean difference: 155 kcal/dd, P = .149) |
| Changes in nutrient intake during the menstrual cycle of overweight women with premenstrual syndrome34 | Cross et al 2001 | Australia |
|
|
2 | 4-d estimated diet records | EI was greater in the luteal vs follicular phase for both groups, but the PMS group had a larger difference (1467 ± 307 kcal/d vs 2097 ± 419 kcal/d, mean difference: 603 kcal/dd, P < .001), compared with the control group (1802 ± 411 kcal/d vs 1914 ± 326 kcal/d, mean difference: 112 kcal/dd, P < .05e). There was a weak correlation between severity of PMS symptoms and energy intake changes (Spearman’s correlation = .29) |
| Modest changes in dietary intake across the menstrual cycle: implication for food intake research42 | Bryant et al 2006 | United States |
|
|
1 | 3-d estimated diet records | No evidence of a difference in EI between the follicular and luteal phases for either those with PMS (2032 kcal/d vs 2009 kcal/dc) or the control group (1981 kcal/d vs 2080 kcal/dc,e) |
| Energy and nutrient intakes during different phases of the menstrual cycle in the United Arab Emirates24 | Cheikh Ismail et al 2009 | United Arab Emirates |
|
|
1 | 2-d estimated diet records | EI was higher in the pre-menstrual vs menstrual phase (1363 ± 550 kcal/d vs 1126 ± 462 kcal/d, mean difference: 237 kcal/dd, P = .002) |
| The influence of the menstrual cycle on energy balance and taste preference in Asian Chinese women41 | Elliot et al 2015 | Singapore |
|
|
3-d estimated diet record (including 1 weekend day) | EI was greater in the menstrual vs luteal phase (1687 ± 419 kcal/d vs 1404 ± 311 kcal/d, mean difference: 283 kcal/dd, P < .05e). For those who completed 2 cycles (n = 13), there was no evidence of a difference in EI between phases (P = .07) | |
| Change in women’s eating habits during the menstrual cycle22 | Kammoun et al 2017 | Tunisia |
|
|
1 | Dietary surveys (no further specification, assumed to be retrospective recalls) | EI was greater during the ovulatory vs follicular phase (2175.2 ± 321.8 kcal/d vs 1688 ± 332 kcal/d, mean difference: 487 kcal/dd, P < .001) and for the luteal vs follicular phase (mean difference: 476 kcal/dd, P < .001) |
| Ovarian hormone fluctuations predict within-cycle shifts in women’s food intake48 | Roney and Simmons 2017 | United States |
|
Daily salivary estrogen and progesterone used to estimate date of ovulation | 1 | Daily survey on quantity eaten, meal size, intentional food restriction, and hunger relative to usual on 1–5 scale for 1 cycle | Decrease in reported food intake in the days approaching ovulation, with the lowest intake corresponding to peak salivary estrogen (P = .024). Reported food intake rose concomitant with increasing salivary progesterone in the luteal phase (P < .01e) |
| Do food intake and food cravings change during the menstrual cycle of young women?38 | Souza et al 2018 | Brazil |
|
|
1 | 24-h recalls | No evidence of a difference in EI between the luteal and follicular phases (1738 ± 414 kcal/d vs 1694 ± 437 kcal/d, mean difference: 44 kcal/dd, P = .383) |
Values represent mean ± SD unless otherwise specified.
Represents age range of participants; mean ± SD not reported.
Standard deviation not reported.
Mean difference not reported; calculated from absolute values reported in each phase, or mean difference reported without SD of the difference.
Exact P-value not reported.
Values for age and BMI only reported for those who completed 2 cycles.
Abbreviations: BBT, basal body temperature; BMI, body–mass index; EI, energy intake; LH, luteinizing hormone; PMS, premenstrual syndrome
IDENTIFICATION AND VERIFICATION OF MENSTRUAL CYCLE PHASE
Poor-quality methods of identifying menstrual cycle phase pervade this research field, and only recently have guidelines emerged.8,9 Verification of menstrual phase with serum estrogen and progesterone concentrations (with exclusion of those studies that fail to meet phase-specific hormonal thresholds) is now considered the gold standard protocol for research.7,8 Urinary luteinizing hormone tests may be used in conjunction with serum hormone measurements to detect ovulation and help identify the luteal phase.7,8Table 125–27,32,33,35–37,40,43 shows the studies that verified phases with serum estrogen and progesterone concentrations, while Table 222–24,28–31,34,38,39,41,42,44–47 shows the studies without such hormonal verification. Failure to adhere to these methods risks collecting data outside of target phases, compromising the validity of the results. For example, Chung et al observed a mean increase in energy intake from the follicular to the luteal phase of 160 kcal/day, but after excluding 17 of the 39 participants with incorrectly identified cycle phases (via retrospective analysis of serum hormones), the difference increased to 180 kcal/day.25 This not only highlights the large number of cycles that could be misclassified without hormonal verification (∼44%), but shows that when such cycles are included, the reported phase-related difference in energy intake may not reflect the actual difference.
The various methods for quantifying the menstrual cycle phase exist within a hierarchy of accuracy. While serum hormone verification sits at the top,8 it is not necessary to completely disregard studies using other methods, but rather to exercise more caution when interpreting the results. For example, using urinary luteinizing hormone tests alone to discern the phase allows more confidence in the assessment of the phase than when it is based solely on menstruation onset,8 and the weight placed on the findings of studies using this method should therefore reflect that (see Table 2 for studies that did not verify menstrual phase with serum sex hormones). Tarasuk and Beaton reported one of the smaller mean differences in energy intake between the follicular and luteal phases (90 kcal/day greater energy intake in the luteal phase); however, phase determination was based on retrospective accounts of menses reported after the year-long study.31 At the other extreme, Kammoun et al observed a mean increase of 476 kcal/day energy intake in the luteal phase over that of the early-follicular, but in that study phase assessments were exclusively based on anticipated cycle length.22 Of those studies that adhered to recommended phase verification methods, 5 studies observed greater energy intakes in the luteal phase compared with the follicular phase of between 159 kcal/day and 529 kcal/day,25–27,35,36 while Gorczyca et al and Ihalainen et al found no evidence of a difference in energy intake between the early-follicular, mid-follicular, ovulatory, or luteal phases.37,40
Several studies that verified phases with serum hormones compared energy intake between cycle phases based on one meal alone: Brennan et al reported a greater mean energy intake during the luteal phase compared with the follicular phase32; Reed et al observed the same pattern, but only for those with premenstrual dysphoric disorder33; McNeil et al reported a trend for the menstrual cycle phase, with intakes in the early-follicular, late-follicular, and mid-luteal phases of 670 ± 293 kcal, 525 ± 289 kcal, and 711 ± 334 kcal, respectively.43 However, data from a single meal may be insufficient to capture overall patterns of energy intake, and extrapolating results to usual daily intake may be less appropriate. Similarly, the buffet or menu-style meals provided in these studies may not accurately reflect food choices made in free-living conditions (and their relationship to cycle phase).
THE LATE-FOLLICULAR AND OVULATORY PHASES
Simply comparing the follicular phase with the luteal phase fails to acknowledge potential changes in dietary intake within these broader umbrellas. Certainly, the high estrogen and low progesterone concentrations of the late-follicular phase, and the phenomenon of ovulation present unique environments.6,8 Given the large inter-individual variation in follicular phase duration,48 capturing these relatively transient phases is particularly unreliable without hormonal verification.8 Yet hormonal verification can be challenging and burdensome, such that it is often neglected,23,24,27–36,38,40–42,44 or merely approximated.22,39,45,46 While Kammoun et al reported a 487 kcal/day greater energy intake in the ovulatory phase compared with the early-follicular phase, the ovulatory phase was simply defined as days 12–16 of the cycle,22 so we cannot be confident that all participants were indeed in the target phase.
A more common observation is a decrease in energy intake during the days leading up to and including ovulation,36,43,46,47 supporting the argument of Fessler for the evolutionary advantage of prioritizing reproduction over food intake during this time.49 Roney and Simmons reported a decrease in food intake in the days approaching ovulation, with the lowest intake coinciding with the peak in salivary estrogen indicative of the late-follicular phase.47 Food intake then increased in the luteal phase, tracking closely with the rise in salivary progesterone.47 However, reported intake was based on a questionnaire about the previous day’s food consumption, meal size and hunger, relative to usual consumption, rather than quantitative dietary assessment.47 Therefore, these findings only provide evidence that hormonal fluctuations across the menstrual cycle may be associated with changes in perceptions of food consumed, rather than actual energy intake.
Johnson et al provided stronger evidence to support the hypothesis of Fessler26: when cycle phase was verified by serum hormones, the greatest increase in energy intake was recorded between the ovulatory phase (defined by increasing estrogen, low progesterone, peaks in follicle-stimulating hormone and luteinizing hormone, and a rise in basal body temperature) and the luteal phase (an increase of 166 kcal/day).26 Using the same hormonal and oral temperature profile to define the late-follicular/ovulatory phase, McNeil et al reported a similar pattern of energy intake from a lunch meal alone (the limitations of which have been previously discussed).43 This pattern was also observed by Lyons et al, who measured the dietary intakes of 18 Australian females over one complete menstrual cycle with daily weighed diet records.46 Using urinary luteinizing hormone tests to detect ovulation, the menstrual cycle was then divided into 5 phases: menses, post menses, ovulatory, post ovulatory, and pre-menses.46 Mean daily energy intake was lower during the ovulatory phase compared with menses and both luteal phases, with the maximum effect observed between the 4 days leading up to and including ovulation, and the 4 days immediately after (324 kcal/day mean difference).46 However, when comparing energy intake between the 10 days pre-menstruation and the 10 days post menstruation, there was no longer evidence of a difference.46 While Lyons et al did not verify phases with serum estrogen and progesterone,46 their results do draw attention to the possibility of more specific within-phase fluctuations in energy intake.
Inferences about energy intake in the follicular phase compared with the luteal phase are often reported as such; however, it may be more appropriate to compare part of the follicular phase with part of the luteal phase. This terminology would not carry the assumption that the specific days of data collection represent the entire phase (unless of course the whole phase has been measured). Should changes in energy intake be a secondary effect of sex hormone fluctuations, the fact that these hormones fluctuate within the follicular and luteal phases means that selection of different time points between studies (and potentially between participants of the same study) may explain some of the disagreement in the magnitude of the difference. However, overall, it does appear that dietary energy intake is lower in the early-follicular phase compared with the mid-luteal phase, with an additional decrease in the days leading up to and including ovulation. Despite the methodological and participant burden challenges, researchers should include the late-follicular and ovulatory phases as they appear to be potentially important periods for changes in energy intake.
MEASUREMENT ACROSS MULTIPLE CYCLES
Any phase-related differences in energy intake that may exist likely vary from cycle to cycle. To reduce the influence of such variability, recent guidelines now encourage collecting data across at least 2 menstrual cycles.7 Despite the evidence in favor of the aforementioned trend in energy intake across the cycle, several studies failed to observe any phase-related differences.37–42 Of particular interest are the findings of Gorczyca et al, who found no evidence of a difference in energy intake (as measured by 24-h recalls) between the early-follicular, mid-follicular, ovulatory, and luteal phases in a sample of 259 American females.37 These results are interesting, given not only the large sample size, but the fact that cycle phases were verified by serum estrogen and progesterone, and that all but 9 participants were followed for 2 cycles.37 Elliot et al also reported that tracking participants over 2 cycles failed to provide evidence of a difference in phase-related energy intake.41 While energy intake was found to be greater in the follicular phase compared with the luteal phase based on 1 cycle, when 13 of the 31 Chinese participants were followed for an additional cycle, there was no longer evidence of a difference in energy intake.41 However, several other studies that tracked more than 1 cycle, still found increases in energy intake from the follicular phase to the luteal phase of between 90 kcal/day and 605 kcal/day.23,27,29,31,33,34,44
It is possible that other factors, such as cultural or societal influences, could attenuate any potential dietary changes of endocrinological origin, and may play a more important role in energy intake than the menstrual cycle. Such factors may have varying degrees of influence across populations differing by age, lifestyle, environment, country, and culture. Alternatively, the large sample size and favorable methodology of Gorczyca et al37 could suggest that significant phase-related differences in energy intake may not exist, especially given the methodological biases frequently unaccounted for in the published literature, which are discussed in subsequent sections.
ANOVULATORY CYCLES
The presence of menstrual bleeding alone does not guarantee regular hormonal fluctuations, so without measuring sex hormone concentrations, one is unable to accurately identify anovulatory cycles or other menstrual irregularities.8 Barr et al found that, while energy intake was on average, 303 kcal/day greater during the luteal phase compared with the follicular phase for ovulatory cycles, there was no evidence of a difference in energy intake for anovulatory cycles.23 While these findings were based on basal body temperature (a less reliable method that may not always correlate well with hormone levels),8 they do highlight a potential discrepancy between ovulatory and anovulatory cycles that needs to be addressed in research. This is particularly important when considering underlying mechanisms: the rise in luteal progesterone hypothesized to influence energy intake requires ovulation to occur.9 It is not uncommon for those with apparently regular menstrual cycles to occasionally experience anovulation or luteal phase deficiency (a condition characterized by insufficient luteal progesterone and a shortened luteal phase),50,51 so confirming luteal progesterone has reached 16 nmol/L is important for understanding the true relationship between menstrual cycle phase and energy intake.8 Studies that included cycles failing to reach this threshold may have underestimated, or indeed failed to observe, a difference in energy intake between the luteal phase and other phases.
PREMENSTRUAL SYNDROME AND PREMENSTRUAL DYSPHORIC DISORDER
Every individual’s experience of the menstrual cycle is unique, with some being more susceptible to certain secondary effects of sex hormone fluctuations. Premenstrual syndrome, and the more severe form termed premenstrual dysphoric disorder, are characterized by a range of distressing physical, emotional, and behavioral symptoms during the luteal phase,52 and they may also influence dietary intake. Reed et al compared energy intake of a lunch meal, self-selected from a menu, between those with premenstrual dysphoric disorder and those without, during the follicular and luteal phases.33 Those with premenstrual dysphoric disorder had a 16% greater energy intake during the luteal phase, while the control group experienced no change between phases.33 While hormonal verification of cycle phase revealed no significant differences in estrogen concentration between the follicular and luteal phases in either group,33 this likely reflects the time points used: days 6–10 of the cycle represented the follicular phase, when estrogen concentration is likely already rising (to peak in the late-follicular phase), and 1–5 days before menstruation represented the luteal phase, which possibly captured estrogen concentration as it was decreasing, to reach its nadir during menstruation). Therefore, the presence of a difference in energy intake between phases despite similar estrogen concentrations might suggest that progesterone plays a more important role in regulating dietary intake across the cycle, at least for those with premenstrual dysphoric disorder.
In a study of overweight females, Cross et al showed that the increase in energy intake from the follicular phase to the luteal phase was even more profound for those with premenstrual syndrome (605 kcal/day mean difference) compared with those without (112 kcal/day mean difference).34 They also observed a weak correlation between the severity of the symptoms and energy intake.34 In contrast, Bryant et al found no evidence of a difference in energy intake across the menstrual cycle, either for those with premenstrual syndrome, or those without premenstrual syndrome.42
Comparing these studies is difficult, as Reed et al studied females with the more severe condition of premenstrual dysphoric disorder,33 while Cross et al and Bryant et al used different diagnostic criteria for premenstrual syndrome.34,42 This could potentially contribute to the discrepancy present, especially if symptom severity is related to changes in energy intake, as the findings of Cross et al may suggest.34 Furthermore, Cross et al studied an overweight population,34 raising the question of whether body size affects phase-related changes in energy intake. Neither Bryant et al nor Cross et al confirmed cycle phase with serum hormones.34,42 While the underlying causes of premenstrual syndrome and premenstrual dysphoric disorder are not well understood,52 any potential influences on dietary intake may be of emotional or behavioral origin, rather than strictly endocrinological. Studies in these populations are lacking, and further research is required to investigate the effect of premenstrual conditions on energy intake across the menstrual cycle. As competitive sport may be associated with a greater incidence of premenstrual syndrome (with greater training volume and a longer sporting career being additional risk factors), it also seems prudent to explore the relationships between exercise physiology, premenstrual syndrome, and dietary energy intake.53
PHYSICAL ACTIVITY
Energy intake and energy expenditure are fundamentally linked, yet physical activity level is not often measured in the literature.22,24,28–32,35,36,38,44,46 Several studies collected information on exercise habits at baseline, but not during dietary assessment periods23,37,45,47 (with Barr et al excluding those who exercised over 7 hours/week),23 while others required abstinence from heavy exercise during the study.27,32 When Fong and Kretsch kept physical activity levels consistent across phases in their metabolic ward study,39 no differences in energy intake across the menstrual cycle were observed; however, the applicability of the data to free-living conditions makes inferences about these results difficult.
Levels of physical activity could plausibly fluctuate across the cycle for some individuals—such that the energy expenditure of exercise would become a mediator in the relationship between cycle phase and energy intake—particularly considering the recent swell of media commentary on modulating exercise across the menstrual cycle.54 Several studies that tested for phase-related changes in physical activity found no evidence of a difference across the cycle,25–27,42 but most of these findings were based on relatively crude estimations of exercise (such as the number of days per week,42 or hours per day of exercise without collecting information on intensity,25 or only asking participants to report daily physical activity above their subjective normal levels27), and for Chung et al this only included the 4 participants who regularly exercised.25
However, accounting for exercise in sedentary populations may not be as important as for athletes, or those who undertake large amounts of exercise, for whom daily energy intake may vary dramatically depending on training load. Energy availability (the dietary energy remaining after accounting for exercise energy expenditure, in relation to fat-free mass) may be more appropriate for comparing nutritional status than energy intake alone in these individuals.55 To date, only one pilot study has investigated the relationship between the menstrual cycle and energy intake in an active population.40 Ihalainen et al found no evidence of a difference in either energy intake or energy availability, between the early-follicular, mid-follicular, ovulatory, or mid-luteal phases of the cycle in recreationally active females (defined as strength training 3 times per week and endurance training 3 times per week).40 Although this is only one study, free-living athletic females may present a unique population: dietary patterns to meet the needs of exercise, which may not always correspond with hunger or desirability of food, and the potential impact of exercise on appetite must be considered. Among 15 endurance-trained female athletes who habitually exercised at least 5 days/week, single bouts of moderate- and high-intensity exercise were shown to transiently suppress subjective appetite and the orexigenic peptide acylated ghrelin, as well as increase anorexogenic peptides PYY3–36 and GLP-1.56 Furthermore, a recent study in male cyclists demonstrated an inability to sufficiently compensate for increases in exercise energy expenditure through changes in energy intake.57 However, the research on exercise, appetite, and energy intake is inconsistent in terms of exercise mode and intensity and study duration: for a full review see Dorling et al (2018).58 Briefly, it appears that a single exercise bout does not stimulate appetite or energy intake, whereas exercise training could impact appetite and enhance meal-induced satiety. Combined with the high prevalence of low energy availability and of menstrual disturbances in exercising populations (ranging from 14% to 63%59), these factors limit the applicability of research conducted in the general population, which warrants further investigation into understanding the nuances of energy intake and availability across the menstrual cycle in female exercisers.
WEEKDAY VERSUS WEEKEND DIETARY INTAKE
Li et al found that the increase in energy intake observed during the mid-luteal phase compared with the mid-follicular phase was primarily driven by weekend intake.30 Dietary energy intake was 23% greater in the luteal phase when comparing weekend intakes, but there was no evidence of a difference in phase-related energy intake based on weekday intakes alone.30 Weekends may offer more freedom to modulate intake based on appetite and preference, while routine, time constraints and food availability may leave weekday intakes less vulnerable to fluctuation. However, Li et al considered the weekend to be Thursday to Sunday, which represents over half the week and may not reflect what is typically considered the weekend. This phenomenon has not been replicated by any other studies: while Martini et al observed mean energy intake to be higher on the weekend (Saturday and Sunday) regardless of menstrual cycle phase, and energy intake to be higher in the mid-luteal phase compared with the mid-follicular phase, there was no interaction found between phase and day of the week.27
Given that patterns of dietary intake often differ between weekdays and weekends, while random, an imbalance between the number of weekend days in 2 phases being compared could theoretically influence findings of phase-related differences in energy intake. It is common practice to include both weekdays and weekends in multi-day diet records,60 and while several studies attempted to do so,25,30,36,41 the nature of the menstrual cycle certainly makes this difficult and often unrealistic. Furthermore, intentionally trying to capture a weekend day could potentially lead to inconsistent phase time points between participants. A more appropriate approach may be to track over multiple cycles, and ensure a large enough sample size to reach sufficient balance between phases.
DIETARY ASSESSMENT METHODS
The limitations of the various methods of dietary assessment are well documented, with multi-day weighed diet records considered the gold standard.60 A combination of (i) 24-hour recalls28,29,37,38, (ii) weighed,31–33,39,41,43,45 and estimated diet records,23–27,30,31,34–36,40–42,44 of varying duration, and (iii) other methods of dietary assessment22,47 were used throughout the literature. The accuracy of dietary intake estimates differs across these methods, and may contribute to the variation in differences observed. Furthermore, underreporting is a problem inherent to dietary assessment, and is particularly prevalent amongst females.61 While there is no evidence to suggest that this changes across the menstrual cycle, it is worth noting that reported phase-related differences in energy intake may be underestimating the actual difference.
INTER-INDIVIDUAL VARIATION
Inter-individual variation is important to consider, both in terms of the wide range of mean effects reported, as well as the practical application of menstrual cycle research. While Ihalainen et al observed no evidence of a difference in either mean energy intake or energy availability across the cycle, the authors noted the large inter-individual differences in their data.40 As the field of menstrual cycle research grows, the individual variability in responses to hormonal fluctuations across a variety of outcomes is becoming clearer. Yet this may be lost when studies report mean effects, with the range of individual responses, or potential “non-responders” being overlooked. Indeed, some females may be more likely to experience fluctuations in energy intake across the cycle than others, for example, those who concomitantly experience premenstrual syndrome.33,34 The infrequent reporting of variance measures of mean differences only perpetuates this.
POTENTIAL REASONS FOR THE DIFFERENCES IN ENERGY INTAKE
Mirroring the reported changes in energy intake across the menstrual cycle, changes in the controls of energy intake, such as subjective hunger, food cravings, transit time, and appetite hormones, have also been observed. Generally, it appears that estrogen inhibits appetite, whereas progesterone has a stimulatory effect, at least when provided in pharmacological ranges.9,62 The association between estrogen levels and energy intake across the menstrual cycle has been documented in rat models since the 1920s.63
Control of eating behavior and appetite regulation are closely linked to the functioning of the hypothalamic–pituitary–gonadal (HPG) axis. This central control of appetite is influenced by the sex hormones: there are estrogen receptors located in the hypothalamus, and they interact with gastrointestinal peptides (eg, cholecystokinin [CCK] and ghrelin), neurotransmitters, and adipocytes.64
Estrogen mediates the release of the satiating CCK from the small intestine,65 which acts to decrease meal sizes (rather than the frequency of eating)66 and attenuates the release of the appetite-stimulating hormone, ghrelin. In humans, hunger has been reported to be, on average, lower during the “fertile window” (ovulatory and early luteal phase) than on other days during the same cycle (γ = −.23, df = 533, P = .012), corresponding with lower energy intake.47 Other studies have shown that, in response to a preload glucose drink, hunger scores were lower during 2 follicular-phase time points compared with the luteal phase (as measured by a validated visual analogue scale questionnaire). In contrast to the findings of much of the published research, energy intake from a standardized meal was also lower at these time points (∼700 kJ), and there was a decreased rate of gastric emptying, potentially linked to progesterone.32 In rats, progesterone slows intestinal transit67 and inhibits gastric emptying,68 but whether the same effects are present in humans across the menstrual cycle is unclear: Jung et al reported a longer colonic transit time in 11 females in the luteal phase (coinciding with high progesterone levels), compared with that in 10 females who were in the follicular phase,69 while Degen et al found no evidence of a difference between phases in a group of 12 females.70 These conflicting results could reflect differences in study design, including control for physical activity, smoking history, body composition and dietary intakes, and neither study verified menstrual cycle phases with serum hormones. However, the slowing of transit time during the luteal phase (potentially related to increased progesterone) would be expected to increase satiety, and thus decrease energy intake.71,72 Yet this conflicts with the majority of studies, which show an increase in energy intake in the luteal phase compared with other phases in which progesterone is lower, highlighting the fact that several factors likely influence dietary intake across the menstrual cycle.
Indeed, it is not only the volume of food ingested that influences energy intake, but energy density. McNeil et al found that females displayed greater explicit wanting for high-fat foods during the mid-luteal phase compared with the late-follicular phase, manifesting as an increase in fat intake (from a single meal).43 While there was no evidence of a difference in explicit liking for high-fat foods, Robinson et al suggest wanting as opposed to liking may play a more important role in influencing ingestive behavior.73 Cohen et al showed that cravings for pastries, fried snacks, desserts and sweets, sandwiches and hot dogs, sausages, chocolate, and “brigadiero” (a typical Brazilian dessert made of chocolate and condensed milk) were greater in the luteal phase than in the follicular phase.74 Similarly, Gorczyca et al reported higher craving scores for chocolate, sweets, salty flavor and other cravings (as well as higher overall appetite) during the late-luteal phase compared with all other phases.37 Interestingly, this was not reflected in any changes in energy or macronutrient intakes, however, the 24-hour recalls were collected in the mid-luteal phase, while the cravings questionnaires were administered in the late-luteal phase, so the true relationship may not have been captured.37
Despite the number of aforementioned potential physiological mechanisms that may modify energy intake across the menstrual cycle via changes in hormonal profile, other factors such as societal pressures to look a certain way and the potential impact of estrogen on psychological state could also influence energy intake.
CONCLUSION
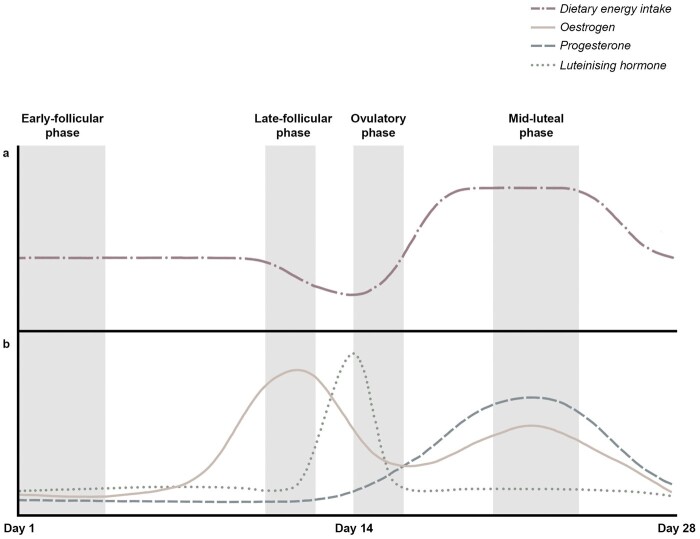
A host of methodological differences and poor compliance with menstrual research guidelines make confidently interpreting the existing literature difficult. Despite this, energy intake does appear to be greater in the luteal phase of the menstrual cycle compared with the follicular phase overall, with the lowest intake likely occurring during the late-follicular and ovulatory phases (however, the number of studies that have specifically researched these phases is limited) (Figure 2). Given the amount of methodological heterogeneity, the exact magnitude of change across the cycle is still unclear. While most studies reported differences of around 200 kcal/day–350 kcal/day between time points in the follicular and luteal phases, those that verified cycle phases with serum hormones observed an actual range of 159 kcal/day–529 kcal/day. At the upper end of the range for a 65 kg female with 15% body fat, the difference in energy intake between menstrual phases could equate to almost 10 kcal/kg fat-free mass/day, which could have significant clinical and research implications. However, phase-related differences in energy intake most likely vary both between individuals, and from cycle-to-cycle. This lack of generalizability means an individualized approach to interventions may be more appropriate where dietary intake across the menstrual cycle is concerned. It is worth acknowledging that these findings are not applicable to users of any form of hormonal contraception, or post-menopausal females; investigating the unique considerations of these populations is warranted, but is beyond the scope of this review.
Figure 2.
Schematic diagram of the hypothesized changes in dietary energy intake across an idealized 28-day menstrual cycle with ovulation occurring on day 14 (a), and the corresponding relative estrogen, progesterone, and luteinizing hormone fluctuations (b). Both (a) and (b) are superimposed over the menstrual cycle phases representing 4 distinct hormonal environments: the early-follicular phase, the late-follicular phase, the ovulatory phase, and the mid-luteal phase. In (a) the dash-dotted line represents dietary energy intake, and in (b) the solid line represents estrogen, the dashed line represents progesterone, and the dotted line represents luteinizing hormone. Figure 2 was created with Adobe Photoshop 2020 (Adobe Inc., San Jose, CA, USA).
For future research, we highlight the importance of verifying menstrual cycle phase with serum estrogen and progesterone concentrations and including the late-follicular and ovulatory phases. The degree of inter-individual variability needs to be better reported, quantified, and used to ensure appropriate sample size in menstrual cycle research. Where dietary energy intake across the menstrual cycle may be relevant to, or is, the outcome of interest, weekday–weekend variation should be taken into account. While it may not be feasible to manipulate data collection days due to the nature of the menstrual cycle, tracking over multiple cycles, or increasing the sample size can help ensure balance between phases. Researchers should quantitatively measure and account for physical activity levels, and more studies are required in active populations, for whom comparing energy availability rather than energy intake may be more appropriate. Similarly, measuring premenstrual syndrome symptoms, as well as more specific research in those with premenstrual syndrome and premenstrual dysphoric disorder, would help to clarify any influence these conditions may have on changes in energy intake across the menstrual cycle. A discrepancy in dietary energy intake between menstrual cycle phases may have implications for research pertaining to dietary intake, energy availability, appetite, and eating behavior, as well as situations involving food provision, or diet and meal planning, whether that be in a research context or otherwise.
Acknowledgments
Author contributions. M.M.R. and K.E.B. both contributed significantly to the work’s conception, design, interpretation, and analysis; M.M.R. drafted the article and K.E.B. provided critical revision of the article; both M.M.R. and K.E.B. have read and approved the manuscript.
Funding. This study was funded by the Department of Human Nutrition of the University of Otago.
Declaration of interest. The authors have no relevant interests to declare.
Contributor Information
Michaela M Rogan, are with the Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
Katherine E Black, are with the Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
REFERENCES
- 1. Melloni C, Berger JS, Wang TY, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3:135–142. [DOI] [PubMed] [Google Scholar]
- 2. Kong BY, Haugh IM, Schlosser BJ, et al. Mind the gap: sex bias in basic skin research. J Invest Dermatol. 2016;136:12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beery AK, Zucker I.. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cowley ES, Olenick AA, McNulty KL, et al. “Invisible sportswomen”: the sex data gap in sport and exercise science research. Women Sport Phys Act J. 2021;29:146–151. [Google Scholar]
- 5. Lee SK. Sex as an important biological variable in biomedical research. BMB Rep. 2018;51:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Owen JA Jr. Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28:333–338. [DOI] [PubMed] [Google Scholar]
- 7. Elliott-Sale KJ, Minahan CL, de Jonge X, et al. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 2021;51:843–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janse DEJX, Thompson B, Han A.. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc. 2019;51:2610–2617. [DOI] [PubMed] [Google Scholar]
- 9. Hirschberg AL. Sex hormones, appetite and eating behaviour in women. Maturitas. 2012;71:248–256. [DOI] [PubMed] [Google Scholar]
- 10. McLay RT, Thomson CD, Williams SM, et al. Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int J Sport Nutr Exerc Metab. 2007;17:189–205. [DOI] [PubMed] [Google Scholar]
- 11. Devries MC, Hamadeh MJ, Phillips SM, et al. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1120–R1128. [DOI] [PubMed] [Google Scholar]
- 12. Lariviere F, Moussalli R, Garrel DR.. Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am J Physiol. 1994;267(3 Pt 1: ):E422–E428. [DOI] [PubMed] [Google Scholar]
- 13. Lamont LS, Lemon PW, Bruot BC.. Menstrual cycle and exercise effects on protein catabolism. Med Sci Sports Exerc. 1987;19:106–110. [PubMed] [Google Scholar]
- 14. Benton MJ, Hutchins AM, Dawes JJ.. Effect of menstrual cycle on resting metabolism: a systematic review and meta-analysis. PLoS One. 2020;15:e0236025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Olympic Committee. Gender equality and inclusion report 2021. 2021. Available at: https://olympics.com/ioc/gender-equality. Accessed March 11, 2022.
- 16. Women’s Sports Foundation. Chasing equity: the triumphs, challenges and opportunities in sports for girls and women.2020. Available at: https://www.womenssportsfoundation.org/articles_and_report/chasing-equity-the-triumphs-challenges-and-opportunities-in-sports-for-girls-and-women/. Accessed March 11, 2022.
- 17. McNulty KL, Elliott-Sale KJ, Dolan E, et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med. 2020;50:1813–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carmichael MA, Thomson RL, Moran LJ, et al. The impact of menstrual cycle phase on athletes’ performance: a narrative review. Int J Environ Res Public Health. 2021;18:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thompson B, Almarjawi A, Sculley D, et al. The effect of the menstrual cycle and oral contraceptives on acute responses and chronic adaptations to resistance training: a systematic review of the literature. Sports Med. 2020;50:171–185. [DOI] [PubMed] [Google Scholar]
- 20. Giersch GEW, Morrissey MC, Katch RK, et al. Menstrual cycle and thermoregulation during exercise in the heat: a systematic review and meta-analysis. J Sci Med Sport. 2020;23:1134–1140. [DOI] [PubMed] [Google Scholar]
- 21. Mountjoy M, Sundgot-Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 2018;52:687–697. [DOI] [PubMed] [Google Scholar]
- 22. Kammoun I, Ben Saada W, Sifaou A, et al. Change in women’s eating habits during the menstrual cycle. Ann Endocrinol (Paris). 2017;78:33–37. [DOI] [PubMed] [Google Scholar]
- 23. Barr SI, Janelle KC, Prior JC.. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr. 1995;61:39–43. [DOI] [PubMed] [Google Scholar]
- 24. Cheikh Ismail LI, Al-Hourani H, Lightowler HJ, et al. Energy and nutrient intakes during different phases of the menstrual cycle in females in the United Arab Emirates. Ann Nutr Metab. 2009;54:124–128. [DOI] [PubMed] [Google Scholar]
- 25. Chung S-C, Bond EF, Jarrett ME.. Food intake changes across the menstrual cycle in Taiwanese women. Biol Res Nurs. 2010;12:37–46. [DOI] [PubMed] [Google Scholar]
- 26. Johnson WG, Corrigan SA, Lemmon CR, et al. Energy regulation over the menstrual cycle. Physiol Behav. 1994;56:523–527. [DOI] [PubMed] [Google Scholar]
- 27. Martini MC, Lampe JW, Slavin JL, et al. Effect of the menstrual cycle on energy and nutrient intake. Am J Clin Nutr. 1994;60:895–899. [DOI] [PubMed] [Google Scholar]
- 28. Pliner P, Fleming AS.. Food intake, body weight, and sweetness preferences over the menstrual cycle in humans. Physiol Behav. 1983;30:663–666. [DOI] [PubMed] [Google Scholar]
- 29. Dalvit SP. The effect of the menstrual cycle on patterns of food intake. Am J Clin Nutr. 1981;34:1811–1815. [DOI] [PubMed] [Google Scholar]
- 30. Li ET, Tsang LB, Lui SS.. Menstrual cycle and voluntary food intake in young Chinese women. Appetite. 1999;33:109–118. [DOI] [PubMed] [Google Scholar]
- 31. Tarasuk V, Beaton GH.. Menstrual-cycle patterns in energy and macronutrient intake. Am J Clin Nutr. 1991;53:442–447. [DOI] [PubMed] [Google Scholar]
- 32. Brennan IM, Feltrin KL, Nair NS, et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol. 2009;297:G602–G610. [DOI] [PubMed] [Google Scholar]
- 33. Reed SC, Levin FR, Evans SM.. Changes in mood, cognitive performance and appetite in the late luteal and follicular phases of the menstrual cycle in women with and without PMDD (premenstrual dysphoric disorder). Horm Behav. 2008;54:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cross GB, Marley J, Miles H, et al. Changes in nutrient intake during the menstrual cycle of overweight women with premenstrual syndrome. Br J Nutr. 2001;85:475–482. [DOI] [PubMed] [Google Scholar]
- 35. Reimer R, Debert C, House J, et al. Dietary and metabolic differences in pre- versus postmenopausal women taking or not taking hormone replacement therapy. Physiol Behav. 2005;84:303–312. [DOI] [PubMed] [Google Scholar]
- 36. Gil YRC, Fagundes RLM, Santos E, et al. Relation of menstrual cycle and alimentary consumption of women. Clin Nutr ESPEN. 2009;4:e257–e260. [Google Scholar]
- 37. Gorczyca AM, Sjaarda LA, Mitchell EM, et al. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur J Nutr. 2016;55:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Souza L. B d, Martins KA, Cordeiro MM, et al. Do food intake and food cravings change during the menstrual cycle of young women? Rev Bras Ginecol Obstet. 2018;40:686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fong AK, Kretsch MJ.. Changes in dietary intake, urinary nitrogen, and urinary volume across the menstrual cycle. Am J Clin Nutr. 1993;57:43–46. [DOI] [PubMed] [Google Scholar]
- 40. Ihalainen JK, Löfberg I, Kotkajuuri A, et al. Influence of menstrual cycle or hormonal contraceptive phase on energy intake and metabolic hormones—a pilot study. Endocrines. 2021;2:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Elliott SA, Ng J, Leow MK, et al. The influence of the menstrual cycle on energy balance and taste preference in Asian Chinese women. Eur J Nutr. 2015;54:1323–1332. [DOI] [PubMed] [Google Scholar]
- 42. Bryant M, Truesdale KP, Dye L.. Modest changes in dietary intake across the menstrual cycle: implications for food intake research. Br J Nutr. 2006;96:888–894. [DOI] [PubMed] [Google Scholar]
- 43. McNeil J, Cameron JD, Finlayson G, et al. Greater overall olfactory performance, explicit wanting for high fat foods and lipid intake during the mid-luteal phase of the menstrual cycle. Physiol Behav. 2013;112–113:84–89. [DOI] [PubMed] [Google Scholar]
- 44. Sherman BM, Korenman SG.. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manocha S, Choudhuri G, Tandon BN.. A study of dietary intake in pre- and post-menstrual period. Hum Nutr Appl Nutr. 1986;40:213–216. [PubMed] [Google Scholar]
- 46. Gong EJ, Garrel D, Calloway DH.. Menstrual cycle and voluntary food intake. Am J Clin Nutr. 1989;49:252–258. [DOI] [PubMed] [Google Scholar]
- 47. Lyons PM, Truswell AS, Mira M, et al. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am J Clin Nutr. 1989;49:1164–1168. [DOI] [PubMed] [Google Scholar]
- 48. Roney JR, Simmons ZL.. Ovarian hormone fluctuations predict within-cycle shifts in women’s food intake. Horm Behav. 2017;90:8–14. [DOI] [PubMed] [Google Scholar]
- 49. Fessler DM. No time to eat: an adaptationist account of periovulatory behavioral changes. Q Rev Biol. 2003;78:3–21. [DOI] [PubMed] [Google Scholar]
- 50. Prior JC, Naess M, Langhammer A, et al. Ovulation prevalence in women with spontaneous normal-length menstrual cycles – a population-based cohort from HUNT3, Norway. PLoS One. 2015;10:e0134473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schliep KC, Mumford SL, Hammoud AO, et al. Luteal phase deficiency in regularly menstruating women: prevalence and overlap in identification based on clinical and biochemical diagnostic criteria. J Clin Endocrinol Metab. 2014;99:E1007–E1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reid RL. Premenstrual dysphoric disorder (formerly premenstrual syndrome). In Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. NIH/NLM/NCBI Books; 2000. [Google Scholar]
- 53. Czajkowska M, Drosdzol-Cop A, Galazka I, et al. Menstrual cycle and the prevalence of premenstrual syndrome/premenstrual dysphoric disorder in adolescent athletes. J Pediatr Adolesc Gynecol. 2015;28:492–498. [DOI] [PubMed] [Google Scholar]
- 54. Saner E. The menstrual month: how to exercise effectively at every stage of your cycle. The Guardian. 2021. Available at: https://www.theguardian.com/lifeandstyle/2021/feb/02/the-menstrual-month-how-to-exercise-effectively-at-every-stage-of-your-cycle. Accessed February 25, 2022.
- 55. Loucks AB, Kiens B, Wright HH.. Energy availability in athletes. J Sports Sci. 2011;29(suppl 1):S7–S15. [DOI] [PubMed] [Google Scholar]
- 56. Howe SH, Larson-Meyer DE, Austin KJ, et al. No effect of exercise intensity on appetite in highly-trained endurance women. Nutrients. 2016;8:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor HL, Garabello G, Pugh J, et al. Patterns of energy availability of free-living athletes display day-to-day variability that is not reflected in laboratory-based protocols: insights from elite male road cyclists. J Sports Sci. 2022;40:1849–1856. [DOI] [PubMed] [Google Scholar]
- 58. Dorling J, Broom DR, Burns SF, et al. Acute and chronic effects of exercise on appetite, energy intake, and appetite-related hormones: the modulating effect of adiposity, sex, and habitual physical activity. Nutrients. 2018;10:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Logue DM, Madigan SM, Melin A, et al. Low energy availability in athletes 2020: an updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance. Nutrients. 2020;12:835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gibson G. Principles of Nutritional Assessment: Food Consumption of Individuals. 3rd ed. New York, NY: Oxford University Press; 2020. Available at: https://nutritionalassessment.org/individual/index.html. Accessed February 14, 2022.
- 61. Macdiarmid J, Blundell J.. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev. 1998;11:231–253. [DOI] [PubMed] [Google Scholar]
- 62. Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Slonaker JR. The effect of copulation, pregnancy, pseudopregnancy and lactation on the voluntary activity and food consumption of the albino rat. J Physiol. 1925;71:362–394. [Google Scholar]
- 64. Frank A, Brown LM, Clegg DJ.. The role of hypothalamic estrogen receptors in metabolic regulation. Front Neuroendocrinol. 2014;35:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eckel LA, Geary N.. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. [DOI] [PubMed] [Google Scholar]
- 66. Blaustein JD, Wade GN.. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. [DOI] [PubMed] [Google Scholar]
- 67. Scott LD, Deflora E.. Intestinal transit in female rats: effect of pregnancy, estrous cycle, and castration. Gastroenterology. 1983;84:1303. [Google Scholar]
- 68. Liu CY, Chen LB, Liu PY, et al. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J Gastroenterol. 2002;8:338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jung HK, Kim DY, Moon IH.. Effects of gender and menstrual cycle on colonic transit time in healthy subjects. Korean J Intern Med. 2003;18:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Degen LP, Phillips SF.. Variability of gastrointestinal transit in healthy women and men. Gut. 1996;39:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mizrahi M, Ben Ya’acov A, Ilan Y.. Gastric stimulation for weight loss. World J Gastroenterol. 2012;18:2309–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tremblay A, Bellisle F.. Nutrients, satiety, and control of energy intake. Appl Physiol Nutr Metab. 2015;40:971–979. [DOI] [PubMed] [Google Scholar]
- 73. Robinson TE, Berridge KC.. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:91–117. [DOI] [PubMed] [Google Scholar]
- 74. Cohen IT, Sherwin BB, Fleming AS.. Food cravings, mood, and the menstrual cycle. Horm Behav. 1987;21:457–470. [DOI] [PubMed] [Google Scholar]