ABSTRACT
Objective:
We aimed to evaluate the efficacy of an oral combined tablet of Glycyrrhiza glabra, Viola odorata, and Operculina turpethum (Anti-Asthma®) as an add-on therapy for the relief of the severity of symptoms in mild-to-moderate childhood asthma.
Methods:
This randomized placebo-controlled clinical trial was performed on 60 children and adolescents with chronic mild-to-moderate childhood asthma. Patients were randomly divided into cases who received Anti-Asthma® oral combined tablets 2 tablets twice dailt for 1 month and controls, received placebo tablets identically the same to Anti-Asthma® (2 tablets, twice daily, for 1 month) as add-ons to their standard therapy according to the guideline. The severity and frequency of cough attacks and shortness of breath, respiratory test indices (based on spirometry), and the extent of disease control and treatment adherence were measured clinically by validated questionnaires at the beginning and after the study.
Findings:
Respiratory test indices improved and the severity of activity restriction decreased significantly in the cases compared to the controls However, the mean difference before and after the study was significantly different between the cases and controls only for the number and severity of coughs and the severity of activity restriction. In the scores of the Asthma Control Questionnaire, the cases group had a significant improvement compared to the controls.
Conclusion:
Anti-Asthma® oral formulation may be effective as an adjunct add-on treatment in the maintenance therapy of mild-to-moderate childhood asthma.
KEYWORDS: Adolescents, asthma, children, clinical trial, Glycyrrhiza glabra, Operculina turpethum, Viola odorata
INTRODUCTION
Asthma affects approximately twenty percent of the world's population. Childhood asthma is a chronic inflammatory disease in children and adolescents. Like other atopic diseases such as allergic rhinitis, atopic dermatitis, and food allergies, its prevalence increased in recent decades.[1]
In children, asthma typically begins before the age of 3 years, and in most cases, the symptoms subside before school or early school.[2,3] Clinical forms of asthma are classified from mild to severe. In children, the most common form is mild-to-moderate intermittent asthma, which has daily and nocturnal symptoms.[4] The probability of a complete asthma cure is lower in adults than in children, and the disease is often more severe and progressive over time.[5]
Asthma treatment aims to minimize the patient's chronic manifestations, such as nocturnal symptoms, reduce asthma attacks, and eliminate the patient's need for emergency visits. Chemical drugs, especially inhaled and systemic corticosteroids, are the main treatments for asthma, and their use in children and adolescents is always associated with many concerns.[6] Although herbal remedies are believed to be safer than chemical drugs, the safety and effectiveness of these treatments are not clear enough due to a lack of clinical evidence.[7,8] In a study by Javadi et al. (2017), many antiasthma plants, including saffron, licorice, thyme, and violet, were identified from traditional medicine sources that have tracheal muscle relaxing, anti-inflammatory, anti-allergy, and anti-oxidative effects.[9]
Glycyrrhiza glabra, commonly known as licorice, is one of the most widely used plants in medical history. Licorice has been used as one of the main therapeutic components in medicinal oils to relieve the symptoms of seizures, paralysis, rheumatism, and coagulative diseases.[10,11,12] Licorice phytochemicals show mineral-corticoid and glucocorticoid activity by inhibiting hepatic metabolism of aldosterone, the enzyme 5-beta reductase, or having a structure similar to adrenal hormones.[13,14] On the other hand, licorice compounds, especially glycyrrhizin, are potent anti-inflammatories and antioxidants which neutralize-free radicals produced by neutrophils at the site of inflammation.[15] Some compounds in licorice can inhibit capsaicin, which causes coughing. Studies have also shown mucus's expectorant and lubricating effects on the respiratory tract and soothing sore throats.[16] Viola is a genus of the Violaceae family, which originates from Europe and Asia. It is found in the northern regions of Iran.[17] In traditional Iranian medicine, this plant has a long history and has been used in dry and chronic coughs, hoarseness, shortness of breath, and pneumonia. All parts of the plant are used to treat bronchitis, cough, and sneezing. Studies show that this plant significantly affects allergic rhinitis in adults and children's cough.[18] The roots of Viola have anti-inflammatory properties and are appropriate expectorants.[19] The effects of using this plant in pediatric asthma have been mentioned in Persian traditional medicine but lack recent clinically acceptable evidence.[20] Operculina turpethum is also a member of the Convolvulaceae family.[21] Animal studies have shown that it has anti-inflammatory, analgesic, and hepatoprotective activities.[22,23] This plant can also be used as an antispasmodic through its action on calcium channels and bronchodilators in asthma therapy.[24]
Various resources of Persian traditional medicine have mentioned the beneficial properties of these three plants in controlling allergic diseases such as asthma. But still, recent clinical studies in this field are limited. To the best of our knowledge, no study has been conducted to evaluate the efficacy of combining these three plants as an oral formulation for childhood asthma. Therefore, we aimed to clinically evaluate the effectiveness of the combined product of these three plants on the severity of symptoms and control of asthma in children and adolescents aged 6–18 years.
METHODS
This study was designed as an add-on therapy and randomized placebo-controlled clinical trial that was conducted in the pediatric pulmonary diseases clinic of the University children's hospital affiliated with Isfahan University of Medical sciences from October 2020 to April 2021. The research protocol was reviewed and approved by the Institutional Ethics Committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.818), and also registered in the Iranian Registry of Clinical Trials (IRCT) with the code number IRCT20090808002306N6. Informed consent was obtained from all participants and their parents or legal guardians. They were free to leave the study whenever they wanted without any penalty or deprivation from their standard therapy.
All patients aged 6–18 years with a clinical diagnosis of mild-to-moderate childhood asthma by a pediatric pulmonologist who had not a history of cardiovascular, hepatic, or renal diseases were included in the study. Patients with a history of hypersensitivity to herbal drugs or children who took medications out of the study protocol or with poor compliance with the study protocol were excluded. The sample size of this study was 60 participants.
We randomly allocated the screened patients into the cases and control groups using their national Iranian code numbers if it was odds or even, respectively. Standard treatment was prescribed in both groups, and no patient was excluded from the usual treatment. The treatment regimen with or without herbal products was administered to the case or control groups.
Before starting the study, the study protocol was fully explained to the children participants and their parents in simple language, and the consent form was provided to the patients and their parents. Patients’ demographic information and details related to the severity and frequency of asthma attacks were recorded in data collection forms. Furthermore, the participants and their parents completed validated questionnaires (Asthma Control Questionnaire [ACQ][25] and Childhood Asthma Control Test [C-ACT][26]) inquiring about the quality of life, severity, and frequency of asthma symptoms control before and after the medical add-on intervention. Before taking the drugs, patients in both groups were tested for spirometry, and the relevant indices (first second of forced expiratory volume [FEV1], forced vital capacity [FVC], FEV1/FVC, and Forced expiratory flow [FEF25-75]) were recorded. Then, both groups received the standard pediatric asthma treatment based on the appropriate guideline of childhood asthma treatment, and the case group, in addition to the standard treatment, was given the herbal product (4 Anti-Asthma® pills, containing all three herbal remedies: 2 pills in the morning and day, 2 in the evening; each pill contains 650 mg of herbals) for 1 month. The control group also received placebo pills with identically the same shape, size, and color as the herbal pills.
To ensure the proper use of the drug, apart from the initial training, the pill count method, by asking for the number of pills remaining during the follow-up of patients, was used. Patients with more than a 40% difference in the expected number of remaining drugs were excluded from the study. The placebo, as well as the standardized herbal product of the three plants: licorice, Viola, and Operculina turpethum, were prepared in the form of tablets made by Sinafaravar® pharmaceutical company (Isfahan, Iran) and given to the patients accordingly. Patients in both groups did not pay for the add-on therapy with the herbal remedy or the placebo.
After 1 month of monitored use of the add-on drugs, the participants were recalled and referred to get a second spirometry test and the test results before and after the intervention were compared. Participants were also asked to complete the ACQ and C-ACT questionnaires and data collection sheets for the second time. FR (Faezeh Rabbani, Author) also completed a questionnaire (similar to the 8-item Morisky Questionnaire[27]) evaluating patients’ or their parent's compliance toward the regular usage of the herbal medicine, or the placebo was also completed. Suspectable side effects that the children or the parents reported were recorded.
All data were collected and analyzed for statistical analysis using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, N.Y., USA). We used an independent t-test, and Kolmogorov–Smirnov, Mann–Whitney, and Wilcoxon signed-rank tests for data analysis. P < 0.05 was considered statistically significant.
RESULTS
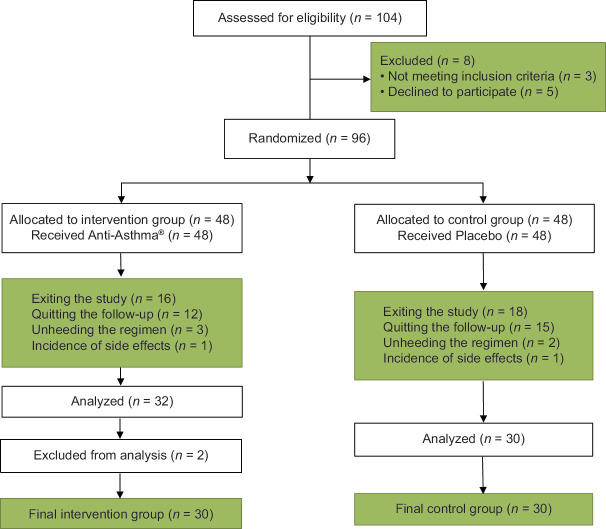
The eligibility assessment of 104 patients was carried out from October 2020 to April 2021; out of whom, 96 were included in the study using randomization, with only 62 eligible for further assessment. Furthermore, two patients were considered out-layers and excluded because of a more than 40% statistical difference with the median of the data for the rest of the patients. Finally, 30 patients were analyzed in each group of cases who had Anti-Asthma® and controls with an identical placebo as an add-on medication [Figure 1]. Based on the results of the Kolmogorov–Smirnov test, the numerical values of the measured variables in this study have no normal distribution (P < 0.05), so nonparametric tests were employed.
Figure 1.
CONSORT flowchart of the study
The basic and demographic details of the participants of the two groups of cases and controls are shown in Table 1. Based on these results at the beginning of the study, no statistically significant difference in age, gender, nutritional sensitivity, family history of asthma, and contact with asthma risk factors between the two groups was detected.
Table 1.
The basic demographic and clinical characteristics of participants
| Variables | Groups | P-value* | |
|---|---|---|---|
|
| |||
| Placebo | Anti-Asthma® | ||
| Age | |||
| Mean±SD | 10.97±2.0 | 11.7±3.0 | 0.340 |
| Gender (%) | |||
| Female | 14 (47.0) | 8 (27.0) | 0.108 |
| Male | 16 (53.0) | 22 (73.0) | |
| Nutritional sensitivity (%) | |||
| No | 16 (53.0) | 12 (40.0) | 0.301 |
| Yes | 14 (47.0) | 18 (60.0) | |
| Family history of asthma (%) | |||
| No | 17 (57.0) | 12 (40.0) | 0.196 |
| Yes | 13 (43.0) | 18 (60.0) | |
| Contact with asthma risk factors (%) | |||
| No | 8 (27.0) | 12 (40.0) | 0.273 |
| Yes | 22 (73.0) | 18 (60.0) | |
*P-value calculated by Chi-square and Mann–Whitney test at 95% levels of CI. SD=Standard deviation, CI=Confidence interval
The comparison of measured respiratory indices in this study between the two groups before and after the intervention is shown in Table 2. The comparison was implemented in each group before and after the intervention, and the results were also compared. These results indicate significant differences before and after intervention in terms of weekly breath shortness episodes, daily usage of salbutamol spray puffs, and cough frequency each day in both groups. There were significant differences in FEV1, FEV1/FVC, and FEF25-27 before and after the intervention in the cases group but not in the control group. Furthermore, the differences in frequency of breath shortness episodes and cough severity and frequency both before and after intervention were significant in both groups, with a better improvement in the cases (Anti-Asthma®) group than the control group. However, the difference in activity limitation severity before and after the intervention was significant only for the cases (Anti-Asthma®) group. The difference in breath shortness and activity limitation severity between the two groups after intervention and with change score methods was significant.
Table 2.
The comparison of parameters between two groups
| Parameters | Groups (mean±SD) | P** | |
|---|---|---|---|
|
| |||
| Placebo | Anti-Asthma® | ||
| FEV1 | |||
| Before | 90.6±16.86 | 85.07±24.65 | 0.113 |
| After | 96.73±15.82 | 91.53±22.32 | 0.098 |
| P* | 0.130 | 0.010 | |
| FEV1/FVC | |||
| Before | 98.3±9.51 | 90.67±12.87 | 0.023 |
| After | 100.83±8.61 | 92.2±11.79 | 0.089 |
| P* | 0.234 | 0.04 | |
| FEF25-27 | |||
| Before | 99.2±26.11 | 84.47±42.46 | 0.344 |
| After | 104.3±33.37 | 86.4±35.26 | 0.091 |
| P* | 0.580 | 0.04 | |
| Number of breath shortness | |||
| Before | 2.97±2.89 | 3.27±2.82 | 0.679 |
| After 1 week | 1.83±2.78 | 1.73±2.15 | 0.789 |
| P* | 0.006 | 0.001 | |
| Number of spray puff | |||
| Before | 3.73±3.47 | 2.47±2.26 | 0.286 |
| After 1 day | 1.93±2.9 | 1.53±2.16 | 0.189 |
| P* | 0.001 | 0.001 | |
| Number of coughs | |||
| Before | 9.7±7.34 | 14.67±11.92 | 0.099 |
| After 1 day | 7.9±6.53 | 6.27±8.2 | 0.096 |
| P* | 0.041 | 0.001 | |
| The severity of breath shortness | |||
| Before | 0.73±0.91 | 0.87±0.73 | 0.391 |
| After | 0.2±0.55 | 0.2±0.41 | 0.989 |
| P* | 0.003 | 0.001 | |
| Severity of cough | |||
| Before | 1.23±0.77 | 1.4±0.72 | 0.393 |
| After | 0.93±0.87 | 0.53±0.82 | 0.001 |
| P* | 0.003 | 0.001 | |
| The severity of activity limitation | |||
| Before | 1.07±0.45 | 1.87±0.35 | 0.001 |
| After | 0.9±0.66 | 0.33±0.48 | 0.001 |
| P* | 0.132 | 0.001 | |
**P-value calculated by *Mann–Whitney, and **Wilcoxon signed-rank tests, at 95% levels of CI. CI=Confidence interval, FEV=Forced expiratory volume, FVC=Forced vital capacity, FEF=Forced expiratory flow, SD=Standard deviation
Table 3 compares questionnaire scores between the two groups of cases (Anti-Asthma®) and controls (placebo). Based on these results, a significant difference in scores of ACQ and C-ACT questionnaires was seen before and after intervention in both groups (P < 0.05). Furthermore, the mean difference between the two groups in both questionnaires (score change, after-before) was statistically significant.
Table 3.
The comparison of questionnaire analysis between two groups
| Questionnaires | Groups (mean±SD) | P-value | |
|---|---|---|---|
|
| |||
| Placebo | Anti-Asthma® | ||
| The severity of Asthma attacks episodes (ACQ) | |||
| Before | 9.2±3.8 | 9.26±2.79 | 0.930 |
| After | 11.36±4.87 | 13.46±3.8 | 0.06 |
| P-value* | 0.001 | 0.001 | |
| Mean difference | 2.17±2.97 | 4.2±2.93 | 0.003 |
| Patients/parent’s opinions about disease severity (C-ACT) | |||
| Before | 14.53±5.67 | 12.66±3.43 | 0.120 |
| After | 17.51±6.05 | 19.6±4.83 | 0.100 |
| P-value* | 0.001 | 0.001 | |
| **Mean difference | 2.7±2.34 | 6.93±4.03 | 0.001 |
| Treatment compliance | |||
| After | 13.6±4.73 | 15.13±2.87 | 0.130 |
*P-value calculated by Mann–Whitney and Wilcoxon tests at 95% levels of CI, **Mean difference was calculated by change score method with subtraction after of before. CI=Confidence interval, SD=Standard deviation, ACQ=Asthma Control Questionnaire, C-ACT=Childhood Asthma Control Test
Asthma was not controlled in the participants of the cases group before the intervention, and this measure increased to 80% after the intervention (P = 0.001) with Anti-Asthma®. In the control group, asthma was controlled in 20% of participants before intervention, which only increased in a significantly lower ratio after intervention, 50% (P = 0.004). However, the adherence to the treatment was not significantly different among these two groups (P < 0.05).
In our study, two patients discontinued their treatment for various reasons, including allergic reactions. The first patient, belonging to the control group, complained of itching and redness of the eyes. The second patient, from the drug group, complained of worsening respiratory problems and shortness of breath. This is while he discontinued his former Anti-Asthma® sprays spontaneously. It should be noted that none of the above patients were referred to a physician to evaluate their problems. No complaints of side effects due to the drug (Anti-Asthma®) or placebo were detected or reported from the other patients. It is noteworthy that three parents of patients from the drug group reported that their children's digestive problems, including gastritis, anorexia, and indigestion, improved significantly after treatment.
DISCUSSION
Asthma treatment faces various safety challenges due to the nature of its primary therapy, especially inhaled corticosteroids. Some problems arise from asthma treatment drugs in children and adolescents, including systemic side effects (especially in long-term use with high doses), the possibility of relapse after discontinuation, lack of proper training on how to use respiratory sprays, and low acceptance of parents and children. In cases of poor control asthma, using adjuvant therapy in addition to corticosteroids makes it possible to lessen the increasing dosage of corticosteroids and even get better results in controlling symptoms.[28] Therefore, conducting the present study was essential to assess alternative therapies with minimal side effects, probable better effectiveness, and acceptability. Previous studies on the pharmacologically active ingredients of the three plants licorice, sweet violet, and Indian jalap – all used in Anti-Asthma® – converge on the fact that this formulation may show anti-inflammatory, bronchodilatory, and antioxidant effects. Hence, in the current study, we assessed the impact of add-on therapy with Anti-Asthma® drug on pediatric patients with mild-to-moderate chronic asthma.
Spirometry is one of the best tools to evaluate the severity of asthma and assess the effectiveness of treatment, so this study was performed before and after the intervention for both groups. The results of the Wilcoxon statistical test showed that all three parameters, i.e., FEV1, FEV1/FVC, and FEF25-75 after the intervention were higher than before in both groups, which was anticipated since both groups received their standard treatment. However, this difference was significant for the drug group (Anti-Asthma®). Therefore, it can be said that this observed difference between the two groups may be due to the effect of the Anti-Asthma® drug.
Furthermore, breath shortness and cough severity were significantly lower after intervention than before in both groups. However, the severity of activity limitation after the intervention was significantly lower than before for the drug group. The percentage of asthma control in the drug group after the intervention was 80% higher than before but in the control group was 30% higher than before the start of add-on therapy. This result showed the effectiveness of the Anti-Asthma® drug in the control of asthma. Furthermore, the mean questionnaire scores in the drug group were higher than in the control group. The ACQ questionnaire was about the severity of asthma before and after the intervention from the parenting view with a descending score range of 0–18 to the best level of disease control. The drug group showed a significant difference in improving the questionnaire score after the intervention compared to the control group (P = 0.003). However, both the groups had scores improvement at the end and homogeneity at the beginning of the study (P = 0.93). The C-ACT questionnaire looked for the patient's and parent's opinions about disease severity in divided sections with descending scores from 0 to 27. A score of 19 is denominated as controlled asthma. The results showed a higher score after intervention in both groups, with a significant difference in the drug group with a P = 0.000. Both groups were homogeneous before intervention from the second questionnaire aspect either. The control group also estimated improvement in disease control after the intervention, referring to the possible psychiatric aspects of using a placebo. The third questionnaire asked about treatment compliance in patients with the highest score of 20. The compliance was divided into four subgroups great (17–20), good (13–16), intermediate (9–12), and poor (0–9). The groups did not have significant distribution in subgroups of the questionnaire, and 65% of all participants scored as good-to-excellent compliance after the intervention reminds the accepted situation of herbal and traditional medicine in the general population.
Studies on herbal treatment generally include cellular and animal studies, and limited clinical trials are conducted in this area. Furthermore, the tendency to use complementary medicine has increased among different populations.[29] Hence, human randomized controlled studies in the herbal field are critical, especially in children and adolescents. Our study is the first to investigate the effectiveness of a combination of three plants (licorice, sweet violet, and Indian jalap) on asthma control.
Among the clinical studies performed on pediatric asthma, only one clinical trial is similar to ours and was conducted in Mashhad. It assessed the effect of an herbal formulation containing licorice, jujube, chamomile, persimmon, hyssop, and cheese plants. Results of this study showed that cough and intermitted asthma in children were improved after treatment with this formulation,[30] which confirms the present study's findings.
The effectiveness of licorice in controlling inflammation and its antioxidant and antimicrobial effects have been demonstrated in many preclinical and laboratory studies.[31] A clinical study of an herbal formulation containing licorice, Ganoderma, and bittersweet showed that this formulation was highly effective and safe in treating asthma in adults.[32] Furthermore, the effectiveness of this formulation has been demonstrated in mouse models of asthma.[33] The results of yet another clinical study conducted in Iraq to compare the effectiveness of frankincense and licorice in patients with asthma showed that in patients of the licorice group, FEV1 and FVC were higher, and the number of respiratory attacks was lower than in patients in frankincense group.[34] These results were congruent with those of several other studies.[35,36]
The effectiveness of licorice in gastrointestinal problems, such as gastritis and ulcers caused by Helicobacter pylori, has been demonstrated in some studies.[37] Similarly, in our study, the gastric pain and anorexia of a 13-year-old patient in Anti-Asthma® completely improved.
The effectiveness and safety of the sweet violet plant have been approved in several studies. Furthermore, in previous clinical trials, the efficacy of sweet violet has been demonstrated in allergic rhinitis,[38] pruritus in hemodialysis patients,[39] and eczema.[40] In our study, two patients with constipation from the Anti-Asthma® group improved after the intervention; this can be attributed to the laxative effects of sweet violet and Indian jalap, which are mentioned in many previous studies.[41,42] Moreover, the antispasmodic, anti-inflammatory, antioxidant, and bronchodilator effects of Indian jalap have also been approved in animal and pharmacological studies. Further, this plant's effectiveness and use in improving asthma symptoms are notable. In addition, this plant has a long history of treating respiratory diseases in traditional Greek and Iranian medicine.[9,24]
The lack of the identically same medication regimen as standard therapy for all patients may be considered a major limitation of this study that could have affected its conclusions. Furthermore, little documentation about the dosage of herbal medicine, the use of the minimum possible dose for children, and the limited duration of the study are other important limitations of the current study. In some cases, the COVID-19 pandemic makes stones in assessing patients by spirometry bearing the sample loss on the study. It was, moreover, the limited corporation of little children to do spirometry appropriately or heed the regimen. Hence, more studies are suggested to evaluate the possibility of reducing corticosteroid therapy in asthma, the long-term effects, and different dosages on populations’ variety. The results will help assess consumer attitudes toward using herbal medication in the treatment of asthma.
According to the results of this study, Anti-Asthma® is probably effective and safe for asthma and its complications, such as cough, breath shortness, and activity limitation. However, these results need further approval through the conduction of similar and better clinical trials.
AUTHORS’ CONTRIBUTION
A. Sabzghabaee conceptualized and designed the study. M. Raeisi and M. Keivanfar selected the eligible patients based on the inclusion criteria, prescribed the tablets, and evaluated the patients’ clinical response to the treatment.
F. Rabbani collected the patients’ data, monitored them during the intervention, and drafted the manuscript. A. Saffaei analyzed the data.
All authors revised the manuscript and confirmed the final version.
Financial support and sponsorship
In this project, Anti-Asthma® and placebo oral tablets were provided by Sinafaravar® Pharmaceutical Company
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the staff of the Asthma and Allergy Clinic of Children and Adolescents of Imam Hossein Children's Hospital for their kind support. Furthermore, we would like to appreciate the kind help of the CEO of Sinafaravar® Pharmaceutical Company Dr. Hossein Mahdavi M.D., and also their R&D scientific manager Dr. Mandana Ahmadi Ph.D., for providing Anti-Asthma® and placebo oral tablets.
REFERENCES
- 1.Dierick BJ, van der Molen T, Flokstra-de Blok BM, Muraro A, Postma MJ, Kocks JW, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437–53. doi: 10.1080/14737167.2020.1819793. [DOI] [PubMed] [Google Scholar]
- 2.Keçeci R, Reisli İ. Evaluation of children with infantile wheezing at the age of six: A new asthma predictive index. Dünya Insan Bilimleri Dergisi. 2018;1:84–95. [Google Scholar]
- 3.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life.The group health medical associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 4.Ahanchian H, Jafari S, Behmanesh H, Motavali HN, Khakshour A. Evaluation the clinical effects of short-course montelukast on asthma symptoms due to viral upper respiratory tract infection in children with intermittent asthma. North Khorasan Univ Med Sci. 2013;5:17–24. [Google Scholar]
- 5.Wenzel SE. Asthma: Defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 6.Jadad AR, Moher M, Browman GP, Booker L, Sigouin C, Fuentes M, et al. Systematic reviews and meta-analyses on treatment of asthma: Critical evaluation. BMJ. 2000;320:537–40. doi: 10.1136/bmj.320.7234.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark CE, Arnold E, Lasserson TJ, Wu T. Herbal interventions for chronic asthma in adults and children: A systematic review and meta-analysis. Prim Care Respir J. 2010;19:307–14. doi: 10.4104/pcrj.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park CS, Hong M, Ban JJ, Jeong HS, Choi JY. Review on herbal medications of asthma in domestic clinical research on traditional Korean medicine. J Physiol Pathol Korean Med. 2018;32:361–9. [Google Scholar]
- 9.Javadi B, Sahebkar A, Emami SA. Medicinal plants for the treatment of asthma: A traditional persian medicine perspective. Curr Pharm Des. 2017;23:1623–32. doi: 10.2174/1381612822666161021143332. [DOI] [PubMed] [Google Scholar]
- 10.El-Saber Batiha G, Magdy Beshbishy A, El-Mleeh A, Abdel-Daim MM, Prasad Devkota H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae) Biomolecules. 2020;10:352. doi: 10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang R, Wang LQ, Yuan BC, Liu Y. The pharmacological activities of licorice. Planta Med. 2015;81:1654–69. doi: 10.1055/s-0035-1557893. [DOI] [PubMed] [Google Scholar]
- 12.Anil K, Jyotsna D. Review on Glycyrrhiza glabra (Liquorice) Pharm Sci Innov. 2012;1:1–4. [Google Scholar]
- 13.Sil R, Chakraborti AS. Oxidative inactivation of liver mitochondria in high fructose diet-induced metabolic syndrome in rats: Effect of glycyrrhizin treatment. Phytother Res. 2016;30:1503–12. doi: 10.1002/ptr.5654. [DOI] [PubMed] [Google Scholar]
- 14.Armanini D, Fiore C, Mattarello MJ, Bielenberg J, Palermo M. History of the endocrine effects of licorice. Exp Clin Endocrinol Diabetes. 2002;110:257–61. doi: 10.1055/s-2002-34587. [DOI] [PubMed] [Google Scholar]
- 15.Frattaruolo L, Carullo G, Brindisi M, Mazzotta S, Bellissimo L, Rago V, et al. Antioxidant and anti-inflammatory activities of flavanones from glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants (Basel) 2019;8:186. doi: 10.3390/antiox8060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey S, Verma B, Arya P. A review on constituents, pharmacological activities and medicinal uses of Glycyrrhiza glabra. Pharm Res. 2017;2:26–31. [Google Scholar]
- 17.Feyzabadi Z, Ghorbani F, Vazani Y, Zarshenas MM. A critical review on phytochemistry, pharmacology of viola odorata L. And related multipotential products in traditional persian medicine. Phytother Res. 2017;31:1669–75. doi: 10.1002/ptr.5909. [DOI] [PubMed] [Google Scholar]
- 18.Fazeenah AA, Quamri MA. Banafsha (Viola Odorata)-AReview. World J Pharm Res. 2020;9:514–37. [Google Scholar]
- 19.Janbaz KH, Khan WU, Saqib F, Khalid M. Pharmacological basis for the medicinaluse of Viola odorata in diarrhea, bronchial asthma and hypertension. Bangladesh J Pharmacol. 2015;10:836–43. [Google Scholar]
- 20.Mahboubi M, Kashani LM. A narrative study about the role of viola odorata astraditional medicinal plant in management of respiratory problems. Adv Integr Med. 2018;5:112–8. [Google Scholar]
- 21.Gupta S, Ved A. Operculina turpethum (Linn.) Silva Manso as a medicinal plant species: A review on bioactive components and pharmacological properties. pharmacogn Rev. 2017;11:158–66. doi: 10.4103/phrev.phrev_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhande RM, Kumar P, Mahurkar NK, Setty SR. Pharmacological screening of root of operculina turpethum and its formulations. Acta Pharm Sci. 2006;48:11–7. [Google Scholar]
- 23.Ezeja MI, Onoja SO, Omeh YN, Chibiko CA. Analgesic and antioxidant activities of the methanolic extract of operculina turpethum leaves in mice. Int J Basic Clin Pharmacol. 2015;4:453–7. [Google Scholar]
- 24.Shareef H, Rizwani GH, Mandukhail SR, Watanabe N, Gilani AH. Studies on antidiarrhoeal, antispasmodic and bronchodilator activities of operculina turpethum linn. BMC Complement Altern Med. 2014;14:479. doi: 10.1186/1472-6882-14-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 26.Harris K, Mosler G, Williams SA, Whitehouse A, Raine R, Grigg J. Asthma controlin London secondary school children. J Asthma. 2017;54:1033–40. doi: 10.1080/02770903.2017.1299757. [DOI] [PubMed] [Google Scholar]
- 27.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Tang Q, Lei H, You J, Wang J, Cao J. Evaluation of efficiency and safety of combined montelukast sodium and budesonide in children with cough variant asthma: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e26416. doi: 10.1097/MD.0000000000026416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welz AN, Emberger-Klein A, Menrad K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement Altern Med. 2018;18:92. doi: 10.1186/s12906-018-2160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javid A, Motevalli Haghi N, Emami SA, Ansari A, Zojaji SA, Khoshkhui M, et al. Short-course administration of a traditional herbal mixture ameliorates asthma symptoms of the common cold in children. Avicenna J Phytomed. 2019;9:126–33. [PMC free article] [PubMed] [Google Scholar]
- 31.Fouladi S, Masjedi M, Ganjalikhani Hakemi M, Eskandari N. The review of in Vitro and in Vivo Studies over the glycyrrhizic acid as natural remedy option for treatment of allergic asthma. Iran J Allergy Asthma Immunol. 2019;18:1–11. [PubMed] [Google Scholar]
- 32.Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Li XM, et al. Safety and tolerability of an antiasthma herbal formula (ASHMI) in adult subjects with asthma: A randomized, double-blinded, placebo-controlled, dose-escalation phase I study. J Altern Complement Med. 2009;15:735–43. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, et al. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann Allergy Asthma Immunol. 2010;104:236–46. doi: 10.1016/j.anai.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Jawad FH, Al-Razzuqi RA, Hashim HM, Al-Bayati NJ. Glycyrrhiza glabraversus Boswelliacarterii in chronic bronchial asthma: A comparative study of efficacy. Indian J Allergy Asthma Immunol. 2012;26:6–12. [Google Scholar]
- 35.Ghaemi H, Masoompour SM, Afsharypuor S, Mosaffa-Jahromi M, Pasalar M, Ahmadi F, et al. The effectiveness of a traditional Persian medicine preparation in the treatment of chronic cough: A randomized, double-blinded, placebo-controlled clinical trial. Complement Ther Med. 2020;49:102324. doi: 10.1016/j.ctim.2020.102324. [DOI] [PubMed] [Google Scholar]
- 36.Chang GH, Lin YS, Hsu KH, Cheng YC, Yang PR, Tsai MS, et al. Nasal irrigation with Glycyrrhiza glabra extract for treatment of allergic rhinitis – A study of in vitro, in vivo and clinical trial. J Ethnopharmacol. 2021;275:114116. doi: 10.1016/j.jep.2021.114116. [DOI] [PubMed] [Google Scholar]
- 37.Yoon JY, Cha JM, Hong SS, Kim HK, Kwak MS, Jeon JW, et al. Fermented milk containing Lactobacillus paracasei and Glycyrrhiza glabra has a beneficial effect in patients with Helicobacter pylori infection: A randomized, double-blind, placebo-controlled study. Medicine (Baltimore) 2019;98:e16601. doi: 10.1097/MD.0000000000016601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yazdi N, Kardooni M, Namjuyan F, Vardanjani HM, Tafazoli V, Jaladat AM. Efficacy of sweet violet (Viola odorata) flower oil on the symptoms of adults with allergic rhinitis: A double-blind randomized placebo-controlled clinical trial. Complement Ther Med. 2020;51:102408. doi: 10.1016/j.ctim.2020.102408. [DOI] [PubMed] [Google Scholar]
- 39.Khorsand A, Salari R, Noras MR, Saki A, Jamali J, Sharifipour F, et al. The effect of massage and topical violet oil on the severity of pruritus and dry skin in hemodialysis patients: A randomized controlled trial. Complement Ther Med. 2019;45:248–53. doi: 10.1016/j.ctim.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 40.Sari M, Hendel N, Boudjelal A, Sarri D. Inventory of medicinal plants used fortraditional treatment of eczema in the region of Hodna (M'sila-Algeria) Glob J Res Med Plants Indig Med. 2012;1:97–103. [Google Scholar]
- 41.Suboohi M, Qamar AK, Iqbal AQ. Clinical efficacy of Joshanda Mulayyan’in the treatment of constipation during pregnancy. Hamdard Medicus. 2008;51:42–5. [Google Scholar]
- 42.Masoomi F, Feyzabadi Z, Hamedi S, Jokar A, Sadeghpour O, Toliyat T, et al. Constipation and laxative herbs in iranian traditional medicine. Irani Red Crescent Med J. 2016;8:24–45. [Google Scholar]