Abstract
Background/Aims:
Cells adapt to chronic extracellular hypotonicity by altering metabolism. Corresponding effects of sustained hypotonic exposure at the whole-person level remain to be confirmed and characterized in clinical and population-based studies. This analysis aimed to 1) describe changes in urine and serum metabolomic profiles associated with four weeks of sustained > +1 L/d drinking water in healthy, normal weight, young men, 2) identify metabolic pathways potentially impacted by chronic hypotonicity, and 3) explore if effects of chronic hypotonicity differ by type of specimen and/or acute hydration condition.
Materials:
Untargeted metabolomic assays were completed for specimen stored from Week 1 and Week 6 of the Adapt Study for four men (20-25 years) who changed hydration classification during that period. Each week, first-morning urine was collected after overnight food and water restriction, and urine (t+60 min) and serum (t+90 min) were collected after a 750 mL bolus of drinking water. Metaboanalyst 5.0 was used to compare metabolomic profiles.
Results:
In association with four weeks of > + 1 L/d drinking water, urine osmolality decreased below 800 mOsm/kg H2O and saliva osmolality decreased below 100 mOsm/kg H2O. Between Week 1 and Week 6, 325 of 562 metabolic features in serum changed by 2-fold or more relative to creatinine. Based on hypergeometric test p-value <0.05 or Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway impact factor >0.2, the sustained > + 1 L/d of drinking water was associated with concurrent changes in carbohydrate, protein, lipid, and micronutrient metabolism, a metabolomic pattern of carbohydrate oxidation via the tricarboxylic acid (TCA) cycle, instead of glycolysis to lactate, and a reduction of chronic disease risk factors in Week 6. Similar metabolic pathways appeared potentially impacted in urine, but the directions of impact differed by specimen type.
Conclusion:
In healthy, normal weight, young men with initial total water intake below 2 L/d, sustained > + 1 L/d drinking water was associated with profound changes in serum and urine metabolomic profile, which suggested normalization of an aestivation-like metabolic pattern and a switch away from a Warburg-like pattern. Further research is warranted to pursue whole-body effects of chronic hypotonicity that reflect cell-level effects and potential beneficial effects of drinking water on chronic disease risk.
Keywords: Drinking water, Hydration assessment, Chronic hydration, Biomarker, Metabolomics, Aestivation, Warburg effect
Introduction
Whole-body hydration translates to cell hydration and vice versa. Drinking water equilibrates throughout the body water pool within two hours [1]. Cell hydration, which is under tight homeostatic control, determines if body water is retained, lost, or produced by metabolism [2-10]. Although cell hydration is well known to strongly modify cell metabolism [4, 5, 11], corresponding relationships at the whole-body level are less well understood. Causal effects of chronic whole-body hydration on metabolic syndrome-related disorders and mortality are hypothesized based on cell hydration effects [12-16], but remain to be confirmed.
Cell hydration and metabolism
Cell hydration is perturbed by manifold osmotic stressors because cells’ plasma membranes are semipermeable to water and solutes. Cells respond to any deviation of the osmotic equilibrium between the intracellular and extracellular space with an obligated flux of water towards the compartment with the higher osmotic activity. The resulting alterations of cell volume, tension of the plasma and organellar membranes, ionic strength, and molecule concentrations elicit mechanotransduction and osmotic sensing which affect a wide range of cell processes and functions, including gene expression [17, 18], cell proliferation, transport, metabolism, autophagy, redox-balance, fluid homeostasis, solute uptake, cell motility and migration, immune function, and programmed cell death [11, 12, 19-34]. Sophisticated regulatory mechanisms precisely adjust cell volume, hydration, and metabolism [11, 29, 35-38].
Hypotonic swelling of hepatocytes promotes anabolic metabolism, including protein and glycogen synthesis. The hypoosmotic inhibition of proteolysis involves integrins as osmosensors and downstream signaling mediated by focal adhesion kinase, the tyrosine kinase c-Src, and the epidermal growth factor receptor and mitogen-activated protein kinases [35, 39, 40].
Hypertonic shrinkage favors catabolic metabolism. Chronic hyperosmotic stress causes cells to adapt in ways “so numerous as to suggest a major alteration in the state of cells” [15]. Adaptive responses favor intracellular accumulation of low molecular weight osmolytes, such as free amino acids, urea, polyols, and methylamines [41]. Adaptive responses are coordinated by tonicity-responsive-enhancer-binding-protein (TonEBP)/nuclear factor of activated T cells 5 (NFAT5)[15, 42-47]. Hyperosmotic phase separations mediate widespread cellular effects, including regulation of transcription [15, 48, 49]. Adapted metabolism remains altered for days after correction of extracellular tonicity [11, 50] and primes cells to respond differently to acute hypoosmotic shock. Cells that have accumulated osmolytes following chronic hyperosmolality release excess osmolytes in response to acute hypoosmotic shock [31].
Hypertonicity, cell metabolism and chronic disease risk
Many cellular or subcellular compensations for chronic extracellular hypertonicity are recognized risk factors for chronic disorders. As shown in various cell types, tissues, organs, and species, effects of prolonged hypertonicity include increased protein breakdown [9, 51], catabolism of branched chain amino acids (BCAA; i.e. valine, leucine and isoleucine) [52], insulin resistance [53-58], sorbitol production [11, 15, 31, 59], serum and glucocorticoid inducible kinase (SGK1) expression [14], increased intracellular accumulation of serine [60, 61], secretion of pro-inflammatory cytokines, including IL-6 and TNF-alpha, and T-cell proliferation [62-64], viral replication [65], as well as vascular and tissue calcification [66]. Hypertonicity decreases flux through the pentose phosphate pathway [67], glycine oxidation [68], and oxidative phosphorylation efficiency [69]. It alters the glutamine-glutamate ratio, upregulates enzymes of the ornithine-urea cycle [70], and generates reactive oxygen species that increase oxidative stress [71], DNA damage [26], and programmed cell death [72-76].
Drought-related aestivation switches metabolism to suppress ATP-expensive pathways, including protein synthesis, and accumulate organic osmolytes of low-molecular weight, including urea, at the expense of muscle protein breakdown [4, 5, 7, 9, 51].
As each response described above predicts increased risk of chronic disease incidence and/or progression [14, 77-88], it is reasonable to hypothesize that the combined pattern of responses may be associated with chronic disease risk. The metabolomic signature of chronic hypertonicity may be associated with the metabolomic signatures of aging [89], obesity, insulin resistance, high blood pressure, dyslipidemia [87, 90], Alzheimer’s disease [91], coronary artery disease [85] and colorectal cancer [92]. Chronic diseases are associated with a Warburg type of metabolic pattern [93], which, like the aestivation pattern, is characterized by increased protein breakdown and aerobic glycolysis as opposed to oxidative phosphorylation.
Gaps in knowledge about effects of whole-body hydration
Despite experiments demonstrating that incubation of cells in a hypotonic milieu alters cell metabolism, it is unknown if a corresponding chronic hypotonic exposure at the whole-body level changes metabolism. Clinical studies in healthy individuals have tested relatively short-term exposures, which do not generalize to free-living conditions of daily life, such as intravenous or desmopressin-induced hypoosmolality vs. hyperosmolality sustained over 17 hours [94, 95]. Given physiological homeostatic mechanisms that excrete excess body water and mitigate against plasma hypotonicity, it is difficult to conceive of a chronic hypotonic exposure at the whole-body level that exactly parallels long-term incubation of cells in hypotonic media. Regular consumption of hypotonic water might only intermittently dilute plasma and swell cells.
The Adapt Study tested effects of four weeks of higher intake (>+1 L/d above baseline) of plain drinking water in healthy young men [96, 97]. Weekly fasting blood tests showed that the sustained hypotonic exposure was associated with only a small, 2 mOsm/kg H2O, mean decrease in serum osmolality. The mean urine osmolality was, nevertheless, halved by the exposure, suggesting that the transient and/or minimal hemodilution was enough to swell osmoreceptor cells in the hypothalamus and suppress anti-diuretic hormone (ADH) release and urine concentrating activity. In association with the sustained higher water intake, the Adapt Study observed significant decreases in serum insulin, serum homeostasis model assessment index of insulin resistance (HOMA-IR), saliva cortisol, and urine arginine and glutamic acid [96, 97]. Though the results signal potential for an array of metabolic changes, prior analyses did not check for a broad array of metabolic effects [96, 97].
Beyond the problem of how to operationalize chronic hypotonicity as an exposure in clinical studies, it also remains to be determined if metabolic effects of chronic hypotonicity can be detected in urine or serum, and under what conditions. While osmotic stress effects are reported in cells from many body tissues, including the intestine [98], kidney [99], brain [100], liver [101], blood [102], vascular endothelium [103], fibroblasts [104], and muscle [105], the effects may be tissue-specific and depend on the presence of particular conditions or substrate [41, 104, 106]. Metabolomic profiles vary by specimen type [107].
To address gaps in knowledge about the effects of chronic whole-body hydration on metabolism, the present analysis revisited Adapt Study data to examine if chronic hypotonicity, induced by four weeks of sustained higher intake (>+1 L/d) of plain water, was associated with a major alteration in metabolism for the participants, akin to a switch in macronutrient and energy metabolism, redox balance, and inflammation, detectable in serum or urine. The analysis described changes in the serum and urine metabolomic profiles associated with chronic hypotonicity, and metabolic pathways potentially impacted by the changes. The analysis considered if and how results depended on specimen type (urine vs. serum) and condition (after overnight food and fluid restriction vs. 60 min after drinking a bolus of 750 mL water).
Materials and Methods
Study design & population
This secondary analysis used data from Week 1 and Week 6 of the Adapt study [96, 97]. Participation in the Adapt study involved a baseline period, followed by instruction to increase plain water intake by +1 L/d above baseline in Weeks 3 and 4 and increase plain water intake to +2 L/d above baseline in Weeks 5 and 6.
All Adapt study participants (n=5) were healthy men, aged 20–25 years, with sedentary physical activity level, normal weight status, and 3-day mean total water intake below 2 L/day before enrollment. They were non-smokers and infrequent consumers of caffeinated or alcoholic beverages (less than 2 servings/week). For the duration of the study, the participants were instructed to record the type and estimated portion size of all foods and drinks consumed, using 7-day diet records, and consume the same foods and beverages, as consumed at baseline, on a weekly cycle. They were instructed to maintain their baseline level of physical activity and wear an armband to monitor their physical activity (SenseWear Pro 3, BodyMedia, Pittsburg, PA, USA). Total energy and macronutrient intake and physical activity did not change significantly over time [96].
The present analysis focused on study participants who changed hydration classification. Between Week 1 and Week 6, urine osmolality decreased below 800 mOsm/kg H2O, saliva osmolality decreased below 100 mOsm/kg H2O, and serum osmolality remained below 295 mOsm/kg H2O. Urine osmolality of 800 mOsm/kg H2O or higher is frequently interpreted as suboptimal hydration, underhydration, or index of hyperosmotic stress on cells (cell dehydration) [108-112]. Saliva osmolality above 100 mOsm/kg H2O approximately corresponds with a total body water (TBW) deficit greater than 1% [113, 114]. Serum osmolality of 295 mOsm/kg H2O or higher is recognized as elevated [115, 116].
The present analysis excluded one of the five Adapt study participants who did not meet all three hydration criteria in Week 6. Each of the remaining four participants transitioned from not meeting one or more hydration criteria in Week 1 to meeting all three criteria in Week 6.
Specimen collection & processing
In Week 1 and Week 6, the study participants came to the research clinic in the morning after overnight food and water restriction for specimen collection and clinical measures. At home, the study participants collected first-morning urine in a pre-labeled container. They arrived at the clinic with the first-morning sample in a cooler with an ice pack at approximately 8 am. Fasting body weight was measured in duplicate using a calibrated clinical scale (Scale-Tronix, Carol Stream, Illinois, USA) after the participants voided and removed shoes and outer clothing. TBW was estimated from body weight using the Watson and Hume equations [117, 118].
The study participants collected unstimulated saliva by passively drooling through a straw after a few moments of not swallowing. Participants were next given 750 mL plain tap water to consume within approximately five to ten minutes. Urine was collected within 60 minutes after the water bolus. Blood was collected 90 minutes after the bolus into non-anticoagulated tubes.
Urine, saliva, and serum osmolality were determined in triplicate on fresh samples by freezing point depression osmometer (Advanced Instruments Model 3320, Norwood MA, USA). Freshly collected whole blood was used to determine the red blood cell (RBC) mean corpuscular volume (MCV) from manual hematocrit (HCT) and RBC concentration: MCV (fL) = HCT x 10/RBC concentration. The manual spun HCT (in %) was determined in triplicate and the RBC concentration (in millions/μL) was determined by the ADVIA 120 hematology analyzer (Bayer Healthcare, Tarrytown, NY, USA) at Children’s Hospital Oakland Research Institute (CHORI) (Oakland, CA, USA).
RBC deformability was also determined on fresh whole blood at CHORI (Oakland, CA, USA) by ektacytometry. Briefly, whole blood was suspended in a viscometer and exposed to shear stress in solutions ranging in osmolality from hypotonic to hypertonic by a NaCl gradient in polyvinylpyrrolidone (PVP) at a viscosity of 30 cP. On the hypotonic part of the range, the osmolality associated with minimal RBC deformability was determined. On the hypertonic part of the range, the osmolality where RBC deformability is half of maximal deformability was determined [119].
Aliquots of first-morning urine, post-bolus urine, serum, plasma, and white blood cells (WBC) were stored frozen at −80°C. Stored WBC were sent to TruDiagnostic (Lexington, KY, USA) for determination of the DunedinPACE index of aging [120].
Stored plasma was sent to the Victoria Hospital Kidney Clinical Research Unit, London ON, Canada, for determination of plasma copeptin by automated immunofluorescent assay. Serum creatinine (mg/dL) was determined by Quest Diagnostics (San Jose, CA, USA). The glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [121].
The RBC K:Na ratio, a proxy reflecting Na-K pump activity, was determined at CHORI as described previously. Briefly, heparinized whole blood was centrifuged, RBC pellets were washed in choline, lysed and digested in OmniTrace 70% HNO3 (EMD Chemicals) overnight (60°C, 150–200 rpm orbital shaking), and thereafter diluted to 5% HNO3 with OmniTrace water (EMD Chemicals, Gibbstown, NJ, USA). The acid lysates were centrifuged (10 min at 3000 x g) and introduced into a Vista Pro inductively coupled plasma – atomic emission spectrometer (ICP-AES; Varian Inc., Palo Alto, CA, USA) as described previously [122]. The ICP-AES was calibrated using National Institute of Standards and Technology (NIST)-traceable elemental standards and validated using NIST-traceable 1577b bovine liver reference material (BLRM). Na and K were determined at 568.821 nm and 766.491 nm, respectively. The detection range was 0.05–50 ppm. The coefficient of variations (CV) for intra-assay and inter-assay precision for elements measured with the NIST reference material were routinely <10%. Cesium (50 ppm) was used for ionization suppression and yttrium (5 ppm) was used as an internal standard for all samples. All reagents and plastic ware were certified or routinely tested for trace metal work. Elemental content data was summarized using native software (ICP Expert; Varian Inc.) and normalized to RBC volume determined by complete blood count (CBC).
Body water turnover was indexed using the 7-day deuterium elimination rate method. Once in each two-week study period, the 750 mL water bolus administered to the study participants contained 10 mL 2H2O (99.9 atom%, Donated from the Western Human Nutrition Research Center, UC Davis, CA, USA). The deuterium enrichment (D/H) was determined by mass spectrometry for one urine or plasma sample collected before each dose, one sample collected at least 90 min after each dose, and one sample collected 7 days after each dose. The D/H measurement was made directly in urine, but water from plasma was partially purified before analysis by centrifugation (30 min at 3, 000 x g, Amicon ultra 15, 10 kDa, NMWL, Millipore, Billerica, MA, USA). The D/H was analyzed on a Micromass Isoprime DI coupled with an Aquaprep system (Isoprime Ltd, Cheadle Hulme, UK) using the H2-water equilibration method in the presence of hydrophobic platinum as the catalyst as previously described [1]. The analyses were performed at the Laboratoire de Geochimie des isotopes stables (Centre GEOTOP-UQAM, Montréal, Canada). The D/H was expressed in ppm versus V-SMOW (155.76 ppm): D/H (ppm) = [(D/H in delta V-SMOW/1, 000) + 1] x 155.76. The half-life of D2O in the body (t ½in days) was estimated to index the turnover of the body water pool as [ln 2]/-kr, where kr is the deuterium elimination rate (Δ ln (D/H, ppm)/days), the rate constant of D2O disappearance from the body water pool [1]. As the exact weight of the 10 mL 2H2O dose was not recorded, it was not possible to reliably estimate the TBW.
Metabolomic analysis
Metabolomics analysis provides global assessment of low molecular weight metabolites, including sugars, lipids, steroids, vitamins, amino acids, fatty acids, organic acids, and small peptides, in biological samples, which reflect the downstream expression of genome, transcriptome, proteome, and phenotype of an organism, at a point in time, given exogenous factors, such as physical environment, time of day, and diet [107].
First-morning urine, post-bolus urine, and serum specimen were sent to the University of California Davis, West Coast Metabolomics Center (WCMC) for untargeted analysis of primary metabolism using validated automated liner exchange, cold injection system, gas chromatography with time-of-flight mass spectrometry (ALEX-CIS GCTOF MS). The WCMC uses 15-25 internal standards, a standardized protocol for removing noise from raw data, and a BinBase algorithm to match spectra with known compounds [123]. All specimen, first-morning urine, post-bolus urine, and serum from all time points, were analyzed in one batch. WCMC reported the peak height (mass-to-charge ratio, m/z) and peak retention times (rt; in seconds) for all features that were positively detected in at least 10% of the urine and serum samples. Known compounds were reported with their Kyoto Encyclopedia of Genes and Genomes (KEGG) ID.
Data analyses
MetaboAnalyst 5.0 software [124, 125] was used to normalize and analyze the metabolomic data. Feature peak heights from all three specimen types were normalized relative to creatinine (KEGG ID C00791, m/z/rt: 115/502599) to account for the known decreases in serum and urine osmolality between Week 1 and Week 6 [96]. Serum creatinine did not change significantly between Week 1 and Week 6. To include report of the physiological conditions experienced without control for hemodilution, serum metabolomic data were also analyzed, normalized relative to the pooled Week 1 sample. For all MetaboAnalyst analyses, in addition to normalization, the data were log transformed, mean-centered, and divided by the standard deviation of each variable to facilitate comparison of features.
Specific Aim 1: Describe change in metabolomic profiles associated with induced chronic hydration
To check for evidence of a major alteration in metabolism, the serum and urine metabolomic profiles were considered in terms of the number and percent of metabolic features altered, significant change in the overall profile, and number of metabolic pathways altered. Given that serum is considered a reasonably good metabolic proxy for the entire organism [107], analyses focused on change in the serum metabolomic profile relative to creatinine to check for altered whole body metabolism. The same analyses were repeated for each specimen type.
Change in feature abundance was expressed in terms of fold change from Week 1 to Week 6. The fold change of all metabolic features studied was summarized in a plot of log fold change. The number and percent of features that changed by 2-fold or more in abundance for three or more study participants were determined.
Change in the overall metabolomic profile was visualized using heatmaps, Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) 2-D score plots, and Principal Components Analysis (PCA) score plots. Each participant’s time point-specific metabolomic profile was represented as a column in a heatmap, point in OPLS-DA score plot, and point in PCA score plot. OPLS-DA is designed to distinguish within- and between-group variation, taking into account specified factors (time, in the present analysis) [126]. Statistically significant differences in metabolomic profile between Week 1 and Week 6 were defined in terms of non-overlapping 95% confidence regions on the OPLS-DA and PCA score plots [127]. PCA is an unsupervised method for distinguishing class differences in data, without specifying class factors in advance. Both OPLS-DA and PCA were used because OPLS-DA results can be statistically unreliable without PCA cross-validation, and PCA results can miss important factors [128, 129].
To check if multiple metabolic pathways were potentially impacted by the induced change in hydration between Week 1 and Week 6, KEGG IDs for known compounds that changed by 2-fold or more for at least three participants were uploaded to the Pathways module of MetaboAnalyst 5.0. Compounds that decreased were entered separately from compounds that increased to facilitate interpretation about the direction of potential change in the pathway. Using the Homo sapiens library as reference, the hypergeometric test was used to test if the specified group of compounds was overrepresented, i.e. occurred more frequently than would be expected by chance. In addition to p-value and p-value adjusted for multiple testing using False Discovery Rate (FDR), the software reports an index of pathway impact, which is calculated by adding up the centrality and enrichment measures of each of the matched metabolites and then dividing by the sum of the measures of all metabolites in the pathway. The analysis highlighted pathways where a statistically significant (raw p-value <0.05) number of intermediates were impacted or where the impacted intermediate(s) was central or important to the pathway (impact factor >0.20).
Specific Aim 2: Describe metabolic pathways potentially impacted by the induced change in hydration
Metabolic pathways identified as potentially impacted, based on the serum metabolomic results, were grouped by type of pathway and direction of change. Pathways were grouped by involvement in protein metabolism, carbohydrate metabolism, lipid metabolism, redox balance, or inflammation. The potential impacts were summarized in relation to the TCA cycle.
To check for a switch in energy metabolism, we described coordinated changes in multiple pathways resulting in concurrent changes in TCA cycle precursors. Given the hypotonic exposure, a metabolic switch away from protein breakdown was hypothesized. Given the association between chronic diseases and the Warburg-like metabolic pattern, oxidative glycolysis via the TCA cycle (also known as oxidative phosphorylation) was distinguished from glycolysis to lactate.
Specific Aim 3: Compare results derived from different specimen
To check if metabolomic effects of hydration depend on the type of specimen assayed, the agreement in results from different specimen was described: serum vs. urine, first-morning urine vs. post-bolus urine, and serum normalized relative to creatinine vs. serum relative to the pooled Week 1 sample. Agreement was described in terms of overall change in metabolomic profile, known compounds that changed by 2-fold or more, which metabolic pathways appeared potentially impacted, and direction of potential impact.
Results
Participant characteristics
Four healthy, normal weight men (ages 21-25 years) were included in this analysis. They maintained a total energy intake of approximately 2,000 kcal/d and did not significantly change their physical activity level over the study weeks. The four participants significantly increased plain water intake between Week 1 and Week 6. The increase in drinking water was associated with a decrease in other beverage intake of about 1 serving/d, but no significant change in total macronutrient or energy intake.
Between Week 1 and Week 6, the four study participants included in this analysis experienced a change in hydration classification (see Table 1). In Week 1, all four participants had a urine osmolality above 800 mOsm/kg H2O. Two of the four participants had saliva osmolality over 100 mOsm/kg H2O. In Week 6, all four participants had urine osmolality below 800 mOsm/kg H2O, saliva osmolality below 100 mOsm/kg H2O, and serum osmolality below 295 mOsm/kg H2O.
Table 1.
Characteristics of Adapt study participants who changed hydration classification between Week 1 and Week 6. The table describes four study participants. Mean (± SE) weekly nutrient intakes were estimated from 7-day diet records collected over six consecutive weeks. Post-bolus samples were collected 60-90 min after the participants consumed 750 mL drinking water. Table 1 presents indices of cell water or total body water that were available in the Adapt study database. For detail about the methods see [96, 97]. *Significantly different (p<0.05) from corresponding Week 1 value in fixed effect regression models.
| Week 1 | Weeks 3-6 | Week 6 | ||
|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | ||
| 7-Day water intake | ||||
| Plain drinking water | L/d | 0.4 (0.2) | 1.7 (0.1)* | 2.1 (0.1)* |
| mL/kg | 6.2 (3.2) | 25.1 (2.0)* | 30.8 (1.5)* | |
| Total water | L/d | 2.0 (0.5) | 2.9 (0.2)* | 3.3 (0.4)* |
| mL/kg | 29.8 (8.4) | 43.1 (3.3)* | 48.0 (5.9)* | |
| Water from other beverages | L/d | 0.7 (0.2) | 0.5 (0.1)* | 0.4 (0.2)* |
| mL/kg | 10.1 (3.2) | 7.0 (1.4)* | 5.8 (2.3)* | |
| Water from food | L/d | 0.9 (0.2) | 0.8 (0.6) | 0.8 (0.2) |
| mL/kg | 13.5 (2.6) | 11.0 (0.8) | 11.5 (2.4) | |
| 7-Day solute intake | ||||
| Total energy | kcal/d | 2106 (208) | 1879 (79) | 1991 (141) |
| Total protein | g/d | 95 (7) | 81 (5) | 85 (13) |
| Total carbohydrate | g/d | 247 (22) | 209 (10) | 225 (19) |
| Total fat | g/d | 87 (13) | 83 (5) | 87 (12) |
| Point in time osmolality | ||||
| Random fasting saliva | mOsm/kg H2O | 93.5 (12.7) | 86.3 (4.5) | 78.8 (7.0) |
| First-morning urine | mOsm/kg H2O | 979.1 (119.8) | 675.3 (36.3)* | 629.2 (59.0)* |
| Post-bolus urine | mOsm/kg H2O | 394.3 (199.6) | 309.6 (39.4) | 333.3 (91.2) |
| Post-bolus serum | mOsm/kg H2O | 290 (1.4) | 287.7 (0.6) | 286.9 (1.7) |
| Point in time anti-diuresis | ||||
| Plasma copeptin | pmol/L/crt | 0.43 (0.1) | 0.33 (0.03)* | 0.29 (0.02)* |
| Acute response to osmotic challenge | ||||
| Change in saliva after bolus | mOsm/kg H2O | −26.5 (12.6) | −14.7 (5.4) | −3.4 (5.9) |
| Change in urine after bolus | mOsm/kg H2O | −747.0 (73.8) | −365.7 (46.9)* | −295.9 (124.2)* |
| RBC minimal deformability | mOsm/kg H2O | 139.9 (3.7) | 135.6 (1.4) | 135.4 (3.5) |
| RBC half-maximal deformability | mOsm/kg H2O | 409.1 (6.9) | 393.9 (4.3) | 397.1 (5.9) |
| Water at the cell level | ||||
| RBC mean corpuscular volume | fL | 89.9 (1.7) | 91.8 (1.0)* | 91.8 (2.3) |
| RBC K:Na | ratio | 9.78 (0.69) | 8.82 (0.25) | 8.61 (0.34)* |
| Water at the whole-body level | ||||
| Half-life of water in the body | days | 7.3 (0.9) | 6.8 (0.3) | 6.5 (0.4)* |
| Deuterium elimination rate | Δ Ln ppm/days | 0.102 (0.013) | 0.105 (0.005) | 0.109 (0.007)* |
| Estimated GFR | mL/min/1.73m2 | 129.2 (2.8) | 124.6 (2.3) | 123.2 (7.6) |
| Weight change relative to Week 1 | % | + 1.6 (0.3)* | +2.1 (0.5)* | |
| TBW by Watson equation | L | 41.2 (0.3) | 41.5 (0.2)* | 41.6 (0.3)* |
| TBW by Hume equation | L | 38.3 (0.3) | 39.1 (0.2)* | 39.2 (0.3)* |
The sustained increase in water intake was associated with significantly decreased plasma copeptin and urine osmolality, over four weeks, and significantly greater red blood cell volume, deuterium elimination rate (shorter half-life of water in the body), estimated total body water, and body weight in Week 6 relative to Week 1.
In Week 1, the average decrease in urine osmolality following acute hypotonic challenge exceeded 700 mOsm/kg H2O. The response to acute challenge decreased significantly between Week 1 and Week 6, suggesting greater retention of the 750 mL water bolus, following overnight food and water restriction, in Week 6. Other responses to acute osmotic challenge suggested a trend towards greater tolerance of hypotonicity in Week 6 but did not reach statistical significance. In Week 6, the mean decrease in saliva osmolality following the water bolus was about one tenth the decrease observed in Week 1. The osmolality associated with half-maximal RBC deformability was about 10 mOsm/kg H2O lower in Week 6.
Significant changes in serum chemistry and indirect calorimetry signaled change in macronutrient and energy metabolism for the four study participants between Week 1 and Week 6. The fasting serum total protein and albumin decreased, while serum glucose increased. The fasting, recumbent respiratory quotient was significantly higher in Week 6 compared to Week 1 (see supplementary Table S1).
A downward trend in the HOMA index and significant mean (SE) decrease in the DunedinPACE index of 0.027 (0.008) further signaled reduced age-related chronic disease risk between Week 1 and Week 6.
Specific Aim 1: Check for a major alteration in metabolism
Metabolomic analysis identified a total of 562 features in serum, including 168 known compounds. From Week 1 to Week 6, the majority of features changed in abundance by 2-fold or more, relative to creatinine, for at least three of the four study participants. A total of 78 features decreased and 247 increased (supplementary Figure S1).
The Week 1 and Week 6 serum metabolomic profiles differed, based on qualitatively different heatmaps (supplementary Figure S2), and non-overlapping 95% confidence ellipses in OPLS-DA analysis accounting for time as a factor. The Week 1 and Week 6 profiles did not differ significantly in unsupervised PCA analysis, which does not account for clustering by time. The PCA score plots separated the serum metabolomic profiles of two participants in Week 1 from all other profiles (supplementary Figure S3).
Of the 168 known compounds detected in serum, 86 increased or decreased by 2-fold or more for three or more participants. Pathway analysis of the 86 known compounds identified over two dozen pathways potentially impacted by the 2-fold changes. Tables 2, 3, and 4 list the known compounds that changed by 2-fold or more by potentially impacted pathway. Supplementary Table S2 lists the log fold change of each known compound. Supplementary Table S3 lists impact factors and p-values for each metabolic pathway.
Table 2.
Metabolic pathways that appeared decreased in serum and increased in post-bolus urine. Red and blue font highlight known compounds that changed by 2-fold or more between Week 1 and Week 6. Red font represents compounds that increased. Blue font represents compounds that decreased. Urine results are based on analyses that normalized urine feature abundance relative to creatinine. c Serum results based on analyses that normalized serum feature abundance relative to creatinine. p Serum results based on analyses that normalized serum feature abundance relative to the pooled Week 1 sample.
| First-morning urine |
Post-bolus urine |
Post-bolus serumc |
Post-bolus serump |
|
|---|---|---|---|---|
| Alanine, aspartate & glutamate metabolism |
L-Glutamine
N-acetyl-L-aspartate Fumarate Succinate |
L-Glutamine
L-Alanine L-Glutamate |
L-Glutamine
L-Alanine L-Glutamate |
L-Glutamine
L-Alanine L-Glutamate |
| Aminoacyl-tRNA biosynthesis |
L-Glutamine
L-Tryptophan L-Lysine L-Leucine L-Cysteine L-Serine L-Tyrosine |
L-Glutamine
L-Tryptophan L-Valine L-Alanine L-Threonine L-Proline L-Glutamate |
L-Glutamine
L-Tryptophan L-Valine L-Alanine L-Threonine L-Proline L-Glutamate L-Phenylalanine L-Isoleucine L-Lysine, L-Leucine |
L-Glutamine
L-Tryptophan L-Valine L-Alanine L-Threonine L-Proline L-Glutamate L-henylalanine L-Isoleucine L-Lysine, L-Leucine L-Tyrosine L-Methionine |
| Arachidonic acid metabolism |
Arachidonate
Prostaglandin F2alpha |
Arachidonate | Arachidonate | |
| Arginine & proline metabolism |
L-Proline
L-Glutamate L-Ornithine |
Urea L-Proline
L-Glutamate L-Ornithine |
Urea L-Proline
L-Glutamate L-Ornithine |
|
| Arginine metabolism |
L-Glutamine
L-Citrulline Fumarate |
L-Glutamate
L-Ornithine L-Glutamine |
L-Glutamate
L-Ornithine L-Glutamine |
L-Glutamate
L-Ornithine L-Glutamine |
| Retinol metabolism |
Retinal
L-Glutamine |
Retinal | Retinal | Retinal |
| Glyoxylate & dicarboxylate metabolism |
cis-Aconitate
Glycolate L-Serine D-Glycerate |
L-Glutamine
L-Glutamate |
||
| Linoleic acid metabolism | Linoleic acid | Linoleic acid | Linoleic acid | |
| Nitrogen metabolism |
L-Glutamine
L-Glutamate |
L-Glutamine
L-Glutamate |
L-Glutamine
L-Glutamate |
|
| Butanoate metabolism |
(R)-3-Hydroxy-butanoate
L-Glutamate |
(R)-3-Hydroxy-butanoate
L-Glutamate |
||
| D-Glutamine & D-Glutamate metabolism |
L-Glutamine
L-Glutamate |
L-Glutamine
L-Glutamate |
L-Glutamine
L-Glutamate |
|
| Glutathione metabolism |
L-Glutamate
L-Ornithine |
L-Glutamate
L-Ornithine 5-Oxoproline L-Threonine |
||
| Valine, leucine & isoleucine biosynthesis |
L-Threonine
L-Valine |
L-Threonine
L-Valine L-Isoleucine L-Leucine |
L-Threonine
L-Valine L-Isoleucine L-Leucine |
Table 3.
Metabolic pathways that appeared increased in serum and decreased in urine. Red and blue font highlight known compounds that changed by 2-fold or more between Week 1 and Week 6. Red font represents compounds that increased. Blue font represents compounds that decreased. Urine results are based on analyses that normalized urine feature abundance relative to creatinine. c Serum results based on analyses that normalized serum feature abundance relative to creatinine. p Serum results based on analyses that normalized serum feature abundance relative to the pooled Week 1 sample.
| First-morning urine |
Post-bolus urine |
Post-bolus serumc |
Post-bolus serump |
|
|---|---|---|---|---|
| Ascorbate & aldarate |
UDP-glucuronate
L-Gulonate D-Glucarate myo-inositol |
UDP-glucuronate
L-Gulonate D-Glucarate |
UDP-glucuronate
L-Gulonate D-Glucarate |
UDP-glucuronate
L-Gulonate D-Glucarate |
| Pentose & glucuronate interconversions |
UDP-glucuronate
L-Arabitol L-Gulonate Xylitol D-Xylose |
UDP-Glucuronate L-Arabitol
L-Gulonate Xylitol D-Xylose |
UDP-glucuronate
L-Arabitol L-Gulonate D-Xylose |
UDP-glucuronate
L-Arabitol L-Gulonate |
| Galactose metabolism |
D-Sorbitol
1D-myo-inositol 3-beta-D-Galactosyl-sn-glycerol alpha-D-Galactosyl-(1->3)- Glycerol Lactose myo-inositol |
D-Sorbitol 1D-myo-inositol
3-beta-D-Galactosyl-sn-glycerol alpha-D-Galactosyl-(1->3)- Sucrose Lactose |
D-Sorbitol
1D-myo-inositol 3-beta-D-Galactosyl-sn-glycerol alpha-D-Galactosyl-(1->3)-Sucrose |
Table 4.
Metabolic pathways that appeared potentially impacted in urine or serum but not both specimen types. Red and blue font highlight known compounds that changed by 2-fold or more between Week 1 and Week 6. Red font represents compounds that increased. Blue font represents compounds that decreased. Urine results are based on analyses that normalized urine feature abundance relative to creatinine. c Serum results based on analyses that normalized serum feature abundance relative to creatinine. p Serum results based on analyses that normalized serum feature abundance relative to the pooled Week 1 sample.
| First-morning urine |
Post-bolus urine |
Post-bolus serumc |
Post-bolus serump |
|
|---|---|---|---|---|
| Pantothenate & CoA biosynthesis | L-Cysteine Uracil | |||
| Glycerolipid metabolism |
1-Acylglycerol Glycerol
D-Glycerate |
1-Acylglycerol | ||
| Glycine serine & threonine metabolism |
L-Serine
L-Cysteine D-Glycerate |
L-Serine
L-Cysteine |
||
| Beta-Alanine metabolism | beta-Alanine Uracil | beta-Alanine | ||
| Unsaturated fatty acids biosynthesis |
(9Z)-Octadecenoic acid
Linoleate Arachidonate |
|||
| Phenylalanine tyrosine & Tryptophan biosynthesis | L-Tyrosine | L-Phenylalanine | L-Phenylalanine L-Tyrosine | |
| Purine metabolism | L-Glutamine Adenine Inosine |
Specific Aim 2: Check for a switch in macronutrient and energy metabolism, redox balance, and inflammation
Figure 1 summarizes all of the pathways identified as potentially impacted. Pathways where the intermediate(s) decreased (blue font) were distinguished from those where the intermediate(s) increased (red font).
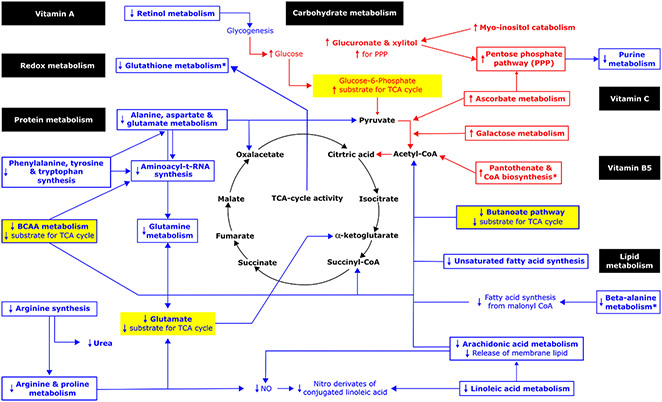
Figure 1.
Metabolic pathways potentially impacted by the induced change in chronic hydration based on changes in serum metabolomic profile between Week 1 and Week 6. Pathway impact was analyzed for known compounds that changed by 2-fold or more in serum. Metabolomic pathways identified only in serum when normalized relative to the pooled Week 1 samples are marked with an asterisk (*). Bold font denotes 2-fold or greater change observed in this analysis. Red and blue font represent 2-fold or greater increases and decreases between Week 1 and Week 6, respectively. Metabolic pathways potentially impacted are shown enclosed in a box. Metabolic intermediates that changed are shown without a box. Hypothesized correlated changes, which might be inferred but were not observed in this analysis, are shown without bold font. Example sources of substrate for the TCA cycle are highlighted in yellow. See Table 3 for detail about metabolic pathways potentially impacted.
Pathways identified as potentially impacted include pathways involved in macronutrient and energy metabolism, redox balance, and inflammation. Collectively, the pathway analyses suggest concurrent changes in metabolism away from protein degradation, favoring oxidation of carbohydrate in the TCA cycle instead of glucose metabolism to lactate.
Protein metabolism
Observed changes in the serum abundance of amino acids implied decreased amino acid-derived carbons for the TCA cycle, including pyruvate, oxaloacetate, succinyl-CoA, and α-ketoglutarate:
Decreased transamination of L-alanine reduces pyruvate.
Decreased transamination of beta-alanine reduces malonate semialdehyde which is converted to acetyl CoA.
Decreased catabolism of alanine, aspartate and glutamate reduces oxaloacetate.
Decreased catabolism of BCAA reduces glutamate-derived succinyl-CoA.
Decreased metabolism of phenylalanine, tyrosine and tryptophan decreases alanine metabolism.
Decreased metabolism of arginine and glutamate reduces α-ketoglutarate.
Decreased aminoacyl-tRNA synthesis decreases substrate for acylation-mediated glucose sensing, signaling and transcription.
Carbohydrate metabolism
Changes in the serum abundance of carbohydrates, such as UDP-glucuronate, favored oxidation of glucose in the TCA cycle:
Increased glucuronate and xylitol, produced from catabolism of myo-inositol, increases substrate for the pentose phosphate pathway.
Increased metabolism of ascorbate.
Decreased retinol metabolism increases glucose availability by decreasing glycogen synthesis from acetate, lactate, and glycerol.
Increased galactose metabolism.
Purine metabolism
Observed decreases in serum L-glutamine, adenine and inosine implied reduced purine synthesis and/or degradation.
Glutamine availability limits de novo purine biosynthesis.
Nucleic acid breakdown results in the release of free purine nucleobases in the form of adenine, guanine, and the hypoxanthine base of inosine monophosphate.
Lipid metabolism
Changes in the serum lipid abundance indicated decreased reliance on ketone body and fatty acid precursors for the TCA cycle, consistent with increased glucose utilization:
Decreased linoleic and arachidonic acid metabolism imply decreased release of membrane lipid.
Decreased unsaturated fatty acid synthesis limits lipid-derived acetyl CoA.
Decreased butanoate (butyrate) metabolism reduces R-3-hydroxybutyrate.
Redox balance & inflammation
Increased serum abundance of vitamin C suggested potential impact on redox balance.
Decreased beta-alanine suggested increased antioxidant capacity from carnosine and anserine.
Decreased serum arachidonate suggested decreased inflammation.
Beta-alanine is produced from the breakdown of carnosine and/or anserine.
Specific Aim 3: Check for agreement between specimen types
This study analyzed two kinds of specimen (serum and urine), which were collected under two kinds of conditions (first-morning and following hypotonic challenge) and normalized with two kinds of methods (relative to creatinine or relative to the pooled Week 1 sample). The following results were similar across all specimen types, conditions, and normalization methods:
The majority of features changed in abundance between Week 1 and Week 6. In the first-morning urine and post-bolus urine, 67% and 73% of the 562 features changed by 2-fold or more, respectively.
OPLS-DA plots with non-overlapping 95% confidence regions suggested significantly different metabolomic profiles in Week 6 vs. Week 1.
PCA analysis separated the profiles of two participants in Week 1 from the rest of the data from Week 1 and Week 6.
Of the 20 pathways that appeared impacted based on serum analyses, 17 also appeared impacted in urine analyses (see Tables 2, 3 and S3). Results of the heatmap, OPLS-DA and PCA analyses of each specimen type are detailed in the Supplementary Materials (see Figures S1-S3].
Differences between urine and serum
Unlike the OPLS-DA results for the serum data, the OPLS-DA results for the urine data suggested reduced between-person variation in the urine metabolomic profile in Week 6 (supplementary Figure S3). Potential impacts on glycine, serine and threonine metabolism, glyoxylate and dicarboxylate metabolism, and glycerolipid metabolism were only detected in urine. The pantothenate & CoA biosynthesis, glutathione metabolism, and purine pathways were not identified as potentially impacted based on urine (Tables 2-4).
The urine and serum metabolomic results differed in terms of directions of effect. The majority of low molecular weight features increased in abundance relative to creatinine, in serum, but decreased relative to creatinine, in urine (Table 3). The ascorbate & aldarate, pentose & glucuronate, and galactose pathways, which appeared upregulated based on the serum metabolomic profile, appeared downregulated based on the urine metabolomic profiles. Metabolic pathways which appeared increased in post-bolus urine (Table 2), appeared to decrease in the other specimen types: Alanine, aspartate & glutamate metabolism, aminoacyl-tRNA biosynthesis, arachidonic acid metabolism, arginine & proline metabolism, arginine biosynthesis, retinol metabolism, and glyoxylate & dicarboxylate metabolism.
Differences by timing and normalization method
Many features which decreased in abundance in the first-morning urine from Week 1 to Week 6 increased in abundance in the post-bolus urine (see Table 2). The acute change in condition, from overnight food and water restriction to 60 min post-ingestion of 750 mL drinking water, was associated with a reversal in the direction of potential impact of the chronic hydration exposure on alanine, aspartate, glutamate and glyoxylate metabolism and arginine and aminoacyl-tRNA synthesis.
Most of the metabolic pathways (12 out of 14) that were identified as potentially impacted based on the serum results, when normalized by creatinine, also appeared potentially impacted when normalized by the pooled Week 1 sample. The two exceptions, butanoate and galactose pathways, did not appear impacted when normalization did not account for hemodilution. Normalization by the pooled sample, which might better reflect the in vivo or physiological condition, identified three pathways, which were not identified by normalization by creatinine. Potential impacts on glutathione and beta-alanine metabolism and pantothenate & CoA biosynthesis were observed without correction for hemodilution.
Discussion
Inspired by experimental literature indicating that chronic hypotonicity results in a “major alteration” in metabolism at the cell level [11, 15], this clinical study sought to check if chronic hypotonicity is associated with a broad array of metabolic changes at the whole person level. The study took advantage of frozen specimen collected from healthy young men for the Adapt Study, before and after a chronic hypotonic exposure, induced by four weeks of sustained higher intake of drinking water. This paper reports results of ancillary urine and serum metabolomic analyses.
The present study population experienced four weeks of physiologically relevant hypotonicity, at the cell level, based on significantly decreased plasma copeptin and urine osmolality in Weeks 3-6 vs. Week 1. Plasma copeptin is a proxy for ADH because it is released in equimolar amounts with ADH [130]. Hypotonic swelling of osmoreceptor cells in the hypothalamus inhibits ADH release and urine concentration [131].
Significantly altered acute response to hypotonic challenge in Week 6 vs. Week 1 suggests adaptation to chronic hypotonicity in this study population. Significant decreases in RBC K:Na (altered intracellular osmolytes) co-occurred with significant increases in cell water (as indexed by MCV) and increased body water (as indexed by percent change in body weight), despite significantly faster water turnover and decreased effort to retain body water via urine concentration.
Check for a major alteration in metabolism
Consistent with results from controlled experiments at the cell level [11, 15], chronic hypotonicity did appear to be associated with a major alteration in metabolism in the present study. In absolute terms, hundreds of metabolic features increased or decreased in abundance by 2-fold or more. In relative terms, over half of the known and unknown features detected in serum changed by 2-fold or more. The overall metabolic profile changed significantly based on OPLS-DA. Over two dozen metabolic pathways were potentially impacted.
Check for a switch in macronutrient and energy metabolism, redox balance, and inflammation
Results of the present study indicated a switch in metabolism away from an aestivation-like pattern. Aestivation is characterized by protein breakdown and accumulation of organic osmolytes, including urea [4, 5, 7, 9]. At the cell level, adaptation to chronic hypotonicity switches metabolism by downregulating protein breakdown and metabolic pathways that accumulate intracellular products of low molecular weight [41]. In the present study, protein-breakdown and urea metabolism decreased between Week 1 and Week 6, consistent with experimental literature at the cell level.
The present results are also consistent with a switch away from a Warburg-like pattern, to favor oxidation of carbohydrate via the TCA cycle instead of aerobic oxidation of carbohydrate to produce lactate. Between Week 1 and Week 6, protein-derived precursors for the TCA cycle decreased, while carbohydrate-derived precursors increased. Serum lactic acid decreased by 87% (supplementary Table S2 gives values as log-fold changes). Fasting serum glucose and the fasting whole body respiratory quotient increased significantly (see supplementary Table S1), despite no change in the mean weekly total energy and carbohydrate intake and no apparent decrease in insulin sensitivity. Fasting plasma insulin and the HOMA index of insulin resistance trended down from Week 1 to Week 6.
Consistent with experimental data [67], the chronic hypotonic exposure was associated with potential impacts on the pentose phosphate pathway. The pentose phosphate pathway produces glucose-6-phosphate, which in turn can either be used for glycolysis or recycled through the pentose phosphate pathway [132, 133]. Flux of glucuronate and xylitol through the pentose phosphate pathways favors oxidation of glucose via glycolysis. The pentose phosphate pathway increases ascorbic acid recycling [134].
Concurrent changes in vitamin C, retinoic acid, proline abundance, and galactose metabolism suggested coordinated impact on oxidative glycolysis. Vitamin C boosts the conversion of pyruvate into acetyl CoA within the mitochondria by inhibiting pyruvate dehydrogenase kinase-1 (PDK-1), a key mitochondrial enzyme that redirects glucose metabolism from oxidative phosphorylation toward aerobic glycolysis [135]. Under fasting conditions, lower retinoic acid may contribute to metabolic flexibility [136-138]. Decreased retinol metabolism increases glucose availability by decreasing glycogen synthesis from acetate, lactate, and glycerol. Reduced proline abundance decreases net glycogen deposition because proline simultaneously increases glycogen synthase flux and inhibits phosphorylase flux [139]. Increased galactose metabolism favors mitochondrial oxidative glycolysis [140].
The Warburg effect is characterized by increased production of lactate because of changes that include inhibition of the pyruvate dehydrogenase complex by pyruvate dehydrogenase kinases and high expression of NF-κB [93]. The Warburg effect is also characterized by increased purine metabolism, as glycolytic intermediates (3-phosphoglycerate and fructose-6-phosphate) are routed to the non-oxidative branch of the pentose phosphate pathway, which generates ribose-5-phosphate, substrate for purine metabolism. The Warburg effect is associated with low ATP concentrations [141], which in turn can reflect low phosphate levels [142]. In the present study, purine metabolism was decreased in Week 6 vs. Week 1. Serum phosphate increased by 20% relative to creatinine between Week 1 and Week 6.
The switch favoring oxidative glycolysis, in this study, appeared to co-occur with a switch in dominant antioxidants with no adverse effect on inflammation. As vitamin C and TCA cycle metabolism increased, glutathione, arginine, linoleic acid and beta-alanine metabolism decreased. Vitamin C is considered the most effective aqueous-phase antioxidant in human blood plasma, which unlike other plasma antioxidants, completely protects plasma lipids against detectable peroxidative damage induced by aqueous peroxyl radicals [143]. Higher TCA cycle activity causes an increase in TCA cycle acids, which are antioxidants [144]. Glutathione, nitric oxide produced from arginine, and conjugated linoleic acid isomers, though important antioxidants [145-147], may be less compatible with sustained hypotonic conditions where protein and lipid breakdown are reduced, nitric oxide-mediated vasodilation is less needed, and NF-κB-dependent cytokine expression [148, 149] is downregulated by hypotonic conditions [150, 151]. Reduced breakdown of the dipeptides anserine and carnosine to beta-alanine favors anserine and carnosine antioxidant capacity. The observed decreases in arachidonic acid metabolism, which imply decreased inflammation [152], suggest no loss of antioxidant protection from the decrease in glutathione, arginine, and linoleic acid metabolism.
The coordinated metabolomic changes may have been at least partially mediated by chronic effects on gene transcription, given that synthesis of aminoacyl-tRNA decreased. Decreases in aminoacyl-tRNA synthesis decrease substrate for acylation-mediated glucose sensing, signaling and transcription which determine glucose uptake, energy utilization and de novo lipogenesis [153].
Check for agreement between specimen types
General agreement between specimen types, with respect to potential impacts on macronutrient and energy metabolism, suggests that any of the four specimen types might reasonably be used to check for hydration effects on metabolomic profile (impact vs. no impact). Inference regarding specific pathways or directions of effect appeared to depend on specimen type and condition, however.
Many low molecular weight features increased in serum, relative to creatinine, possibly reflecting counterregulatory accumulation of osmolytes to gradually retain water and increase the total body water pool. In the Adapt Study participants, the induced change in hydration was associated with significant decreases in RBC K:Na and increases in plasma aldosterone, serum sodium, RBC volume, and body weight [96, 97].
In contrast to observed increased abundance in serum, the majority of low molecular weight features decreased in abundance in post-bolus urine. Between-person differences in metabolomic profile also decreased. The decreases in urine feature abundance, relative to creatinine, may be consistent with chronic relative hypotonicity limiting the intracellular accumulation of low molecular weight osmolytes and reducing the efflux of osmolytes following an acute hypoosmotic challenge. Homeostatic mechanisms that prevent serum concentrations from varying outside a narrow range might account for the different response in urine compared to serum.
Opposite directions of potential pathway impact in serum and urine metabolomic studies are attributed to homeostatic regulation and local tissue effects [154]. The serum metabolomic profile or metabolic ‘fingerprint’ is known to be distinct from the metabolic ‘footprint’ observable in urine [107]. While serum may reflect whole body metabolism, urine is thought to mainly reflect the renal cellular and functional processes and state of the kidney [107]. Opposite effects on serum and urine metabolomic profiles may not be inconsistent effects if increased renal excretion contributes to or explains decreased serum abundance.
In general, the abundance of a given free metabolite in serum or urine is determined by the net balance of its appearance in (by production, influx and/or release from sequestration) and disappearance from (by conversion, efflux, and/or sequestration) the fluid. In serum the abundance of glutamine, for example, is determined by its release from cells (e.g. from skeletal muscle, liver, brain, lung, gut, adipose tissue), by its uptake into cells (e.g. kidney, small intestine, liver, endothelia, blood cells, immune cells, cancer cells), by paracellular exchange between compartments (e.g. blood/gut, blood/peritoneal cavity, across blood brain barrier) and by its direct loss via urine which is determined by its glomerular filtration and tubular reabsorption [155-157].
Contributions from the various inputs to the net glutamine balance may change considerably during adaptation to altered conditions [158]. Here, glutamine abundance decreased in first-morning urine and post-bolus serum but increased in post-bolus urine when compared Week 6 to Week 1. The decrease in first-morning urine could reflect decreased glutamine in plasma and hence less glutamine filtered by the kidney and/or increased tubular reabsorption of glutamine. A decrease in serum glutamine concentration would be consistent with a net shift of glutamine to protein synthesizing tissues like liver or skeletal muscle, increased synthesis of glutathione, or decreased urea production. As plasma protein and albumin decreased from Week 1 to Week 6, the data suggest increased protein synthesis in skeletal muscle. Also BUN and BUN:creatinine trended to decrease. The decreased serum glutamine might also be explained by enhanced gluconeogenesis from glutamine.
Opposite directions of potential impact in the urine metabolome, before and after hypotonic challenge, may be useful for distinguishing chronic from acute effects of hypotonicity and generating hypotheses about mechanisms of adaptation. The agreement in direction of potential impact, i.e. the null effect of the acute challenge, on the ascorbate, pentose and glucuronate interconversions, galactose, glycerolipid, and glycine pathways suggests chronic or regulated change in these pathways.
Potential implications for chronic disease and aging
The results of this study imply potential for hydration metabolomic profiles to be inversely associated with aging and risk for chronic diseases, including obesity, diabetes, atherosclerosis, and cancer [152]. The DunedinPACE index, a DNA methylation marker of the pace of aging, decreased significantly. The metabolomic patterns shifted away from aestivation-like and Warburg-like profiles. Aestivation-related metabolism, including elevated BCAA and urea cycle metabolites, is associated with increased risk of insulin resistance, obesity, hypertension, dyslipidemia and coronary artery disease [84, 85, 87, 90, 159]. The Warburg metabolic profile is implicated in inflammation and the pathogenesis of cancer and atherosclerosis [152].
Alignment of the present results in free-living young men with experimental data at the cell level suggests causal mechanisms to explain observational effects of sustained higher intake of drinking water on chronic disease and mortality. The present results imply that sustained hypotonic exposure may reduce chronic disease risk via multiple mechanisms, simultaneously, including NF-κB signaling and gene transcription, redox balance, and Renin–Angiotensin–Aldosterone System (RAAS) activation.
Between Week 1 and Week 6, concurrent with decreases in extracellular osmolality, leukocyte SGK1 mRNA decreased (see [96, 160]). Hyperosmotic stress, SGK1 and retinoic acid trigger the NF-κB signaling cascade and gene transcription [150, 151]. SGK1 accounts for a large part of the pathophysiological consequences of dehydration [161, 162]. Retinoic acid, via NF-κB signaling, affects transcription of over 500 genes involved in cell proliferation, differentiation, and apoptosis [163].
The observed increase in serum abundance of vitamin C and decrease in arachidonic metabolism are consistent with reduced oxidative stress and inflammation. Vitamin C is hypothesized to prevent or delay the development of certain cancers, cardiovascular disease, and neurodegenerative diseases [164] by limiting the damaging effects of free radicals through its antioxidant activity. The arachidonic acid pathways are implicated in obesity, diabetes, CVD, cancer and inflammatory diseases including asthma and arthritis [165, 166].
The present results carry implications for clinical and epidemiological research. If hydration causes major alteration in the whole metabolic profile, focus on one hydration effect at a time may miss meaningful correlated variation. Single hydration effects may be confounded or modified by other aspects of the profile. The ‘optimal’ hydration reference group may be misclassified if individuals respond to or compensate for sub-optimal hydration differently. Acute and chronic effects of hydration may interact.
Limitations & Future directions
Inconsistent findings across studies
Results of the present analysis conflict with earlier Adapt Study analyses to some extent. In the present analysis, BCAA abundance appeared to decrease between Week 1 and Week 6 (based on pathway impact p-value <0.001 and FDR <0.001), whereas in earlier analyses [96], mean plasma amino acid concentrations did not change significantly between these time points. The difference in findings may be attributable to the fact that earlier analyses evaluate the effect of time (e.g. Week 6 vs. Week 1) as opposed to the effect of different hydration conditions. The earlier analysis included all Adapt Study participants, unlike the present one, which included only participants who experienced a measured change in hydration classification. Significant between-participant differences in saliva osmolality, deuterium elimination rate, and change in serum sodium signal heterogeneity at baseline, which may have modified effects of the hydration exposure [97].
Apparent decreases in protein catabolism associated with four weeks of sustained hypotonic water intake in the present analysis are consistent with previously reported significant decreases in whole body protein breakdown associated with experimentally induced acute hypoosmolality vs. isoosmolality [94]. The observed prioritization of glucose as substrate for the TCA cycle in this long-term study needs to be reconciled, however, with a reported decrease in glucose clearance and increase in lipid oxidation associated with acute hypotonic exposure [94]. Also, unlike previous short-term experiments, but like other metabolomic analyses, this metabolic pathway analysis did not detect change in glycogen synthesis or breakdown [167]. LC-MS methods may not accurately index glycogen because they cannot distinguish hexoses with the same molecular formula [167].
Features observed in other Adapt Study analyses of serum and urine, including sodium, potassium, vitamin D, and taurine, were not detected by the WCMC and not available in the metabolomic analysis dataset. Absence of their mention in the present analysis may reflect WCMC methods, which are tailored to describe primary metabolism, such as protocol that removes features considered to contribute noise, like sodium, or limitations related to ionization voltage so high that molecules like taurine fragment into multiple products [168, 169].
Methods limitations
Several aspects of study design limit interpretation of the present results. Aspects of primary metabolism were described only for the four study participants included in this analysis, given the conditions specified in the Adapt protocol. Participants were non-acutely ill, free-living, sedentary, young men, who consumed the same foods on a weekly basis, and who initially reported less than 2 L/d total water intake [96, 97]. Due to the small sample size and non-random recruitment strategy, the results may not generalize to non-acutely ill, sedentary young men, in general. The results may not generalize to women, children, older adults, athletes, patients, or the general population. It remains to be determined if instruction to consume an additional 2 L/d or 30 mL/kg/d plain drinking water results in a similar switch in metabolism in other population groups, under other conditions.
Despite intention to enroll participants with similar sub-optimal hydration status, the ‘true’ long-term hydration status of each participant at baseline was unknown. Urine osmolality is sensitive to acute change in hydration but vulnerable to misclassification error with respect to chronic status. Results of the unsupervised PCA, which separated the data for two participants from all other data points, suggest that two participants had different hydration status at baseline.
Despite intention to test a homogeneous exposure, each individual’s ‘true’ water intake requirement was unknown. The Adapt Study exposure was expressed in absolute terms (L/d above baseline) as opposed to mL/kg or a tailored volume to replace individual-specific deficit. The fact that at least one Adapt study participant did not change hydration classification suggests heterogeneity in the dose, timing and/or conditions required to achieve the same hydration outcome for all.
The effects of the present study may be confounded by unobserved variability in protocol adherence, diet, physical activity and/or other determinants of water intake requirements. Although each participant served as his own control, the study design was not randomized. Although participants were instructed to maintain the same diet over time, without researcher-provided meals, standardized diet cannot be guaranteed. Controlled diet experiments would be needed to confirm and characterize independent effects of water on changes in metabolism.
Due to the small sample size, analyses had limited statistical power to test for significant change over time or pathway impact, accounting for multiple comparisons. The pathway analyses considered metabolite abundance measured at two points in time, as opposed to metabolic ‘flux’, and were observational, not causal, analyses. It is not possible to distinguish reduced pathway inputs from reduced pathway activity. Decreased purine metabolism, for example, may reflect reduced purine synthesis and/or reduced purine catabolism. Analyses did not consider change in cell membrane composition and function, water/nutrient transporters, tissue-specific effects, or features not detectable by ALEX-CIS GCTOF mass spectrometry.
Potential measurement error limits interpretation of the results. MCV and percent weight change were the only available indices of cell volume and total body water volume in the Adapt Study database. Future studies should include measures of intracellular fluid volume and total body water along with measures of glycogen utilization and gluconeogenesis. As noted above, the present metabolomic results reflect protocol which cannot detect all metabolites and algorithms which may misclassify metabolites as noise.
Feature abundance was normalized by creatinine in this study to reduce intraindividual variation [170, 171] as well as account for known decreases in serum and urine osmolality [96, 97]. Normalization by creatinine can introduce error, however, if the rate of creatinine production is not constant. Creatinine production can differ between individuals if people vary in age, sex, and body size, and can differ within-individuals if there are changes in renal function, metabolism or muscle mass [172, 173]. Study participants ranged in weight from 62.1-69.7 kg. If protein metabolism changed significantly between Week 1 and Week 6, in relation to the induced change in hydration, then assumptions required to normalize the outcome of interest by creatinine may not hold. The similar pattern of results observed when normalizing relative to the pooled Week 1 samples suggest that this source of error was negligible.
Conclusion
This analysis described healthy young men before and after four weeks of sustained higher intake of drinking water. It juxtaposed acute and chronic effects of hypoosmotic challenge. Despite the small sample size and non-randomized design, the detailed descriptive data support hypothesis generation and warrant further research.
The results suggest the hypothesis that, for sedentary healthy young men, >1 L/d additional drinking water can lead to measurable changes in hydration at the cell and whole-body levels, if sustained for four weeks. The results suggest the hypothesis that sustained lower extracellular osmolality downregulates metabolic pathways that alter macronutrient and energy metabolism to accumulate end-products of low molecular weight, including sorbitol, lactose, glycerol, and free amino acids, in healthy, young adult men. The results suggest that chronic cell hydration acts as a metabolic switch, which alters many metabolic pathways in concert, at the whole-person level. The types of metabolic pathways identified as potentially impacted by this analysis as well as the observed direction of potential pathway impact are consistent with experimental literature at the cell level. Finally, the results warrant further work to determine if sustained higher intake of plain drinking water shifts metabolism away from aestivation-like and Warburg-like patterns, and limits multiple chronic disease risk factors, simultaneously.
Supplementary Material
Acknowledgements
Thank you to Janice Hamer, Christine Chi, Annie Higa and Vivian Ng for sample collection and processing, Sandra Larkin for Advia hematology analyses and ektacyometry, and Kathryn Gallagher and J.J. Fung for sample preparation for the WCMC. Thank you to the Reviewers for helpful comments.
Funding
Data collection for the original Adapt study was provided by an unrestricted grant from Danone Research in 2007, NIH CTSA grant UL1 RR024131, and NIH RAS Award ID No. A106017. The sample preparation and metabolomic assays at WCMC were paid for by JDS. The data analysis and manuscript preparation were unfunded.
Abbreviations
- ALEX-CIS GCTOF MS
automated liner exchange, cold injection system, gas chromatography with time-of-flight mass spectrometry
- BCAA
branched chain amino acid
- BLRM
bovine liver reference material
- BUN
blood urea nitrogen
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CHORI
Children’s Hospital Oakland Research Institute
- CKD
chronic kidney disease
- CoA
coenzyme A
- CV
coefficient of variations
- CBC
complete blood count
- DunedinPACE
Dunedin Pace of Aging Calculated from the Epigenome
- FDR
False Discovery Rate
- HCT
hematocrit
- HOMA-IR
Homeostasis Model Assessment Index of Insulin Resistance
- IL
interleukin
- ICP-AES
inductively coupled plasma atomic emission spectroscopy
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LC-MS
liquid chromatography–mass spectrometry
- MCV
mean corpuscular volume
- NIST
National Institute of Standards and Technology
- NFAT5
Nuclear factor of activated T cells 5
- NF-κB
nuclear factor-kappa B
- Nrf2
nuclear factor-E2-p45-related factor2
- OPLS-DA
Orthogonal Partial Least Squares Discriminant Analysis
- PCA
Principal Component Analysis
- RBC
red blood cell
- SGK1
serum & glucocorticoid inducible kinase
- TCA
tricarboxylic acid
- TNF
tumor necrosis factor
- TonEBP
tonicity-responsive-enhancer-binding-protein
- WCMC
West Coast Metabolomics Center
Footnotes
Statement of Ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/). The protocol was reviewed and approved by the Institutional Review Board of Children’s Hospital & Research Center Oakland, CA. All participants gave written informed consent.
Disclosure Statement
The authors have no conflicts to disclose.
References
- 1.Peronnet F, Mignault D, du Souich P, Vergne S, Le Bellego L, Jimenez L, Rabasa-Lhoret R: Pharmacokinetic analysis of absorption, distribution and disappearance of ingested water labeled with D2O in humans. Eur J Appl Physiol 2012;112:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritz P, Salle A, Simard G, Dumas JF, Foussard F, Malthiery Y: Effects of changes in water compartments on physiology and metabolism. Eur J Clin Nutr 2003;57 Suppl 2:S2–S5. [DOI] [PubMed] [Google Scholar]
- 3.Delpire E, Gagnon KB: Water homeostasis and cell volume maintenance and regulation. Curr Top Membr 2018;81:3–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovarik JJ, Morisawa N, Wild J, Marton A, Takase-Minegishi K, Minegishi S, Daub S, Sands JM, Klein JD, Bailey JL, Kovalik JP, Rauh M, Karbach S, Hilgers KF, Luft F, Nishiyama A, Nakano D, Kitada K, Titze J: Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol 2021;232:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minegishi S, Luft FC, Titze J, Kitada K: Sodium handling and interaction in numerous organs. Am J Hypertens 2020;33:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L, Johannes B, Marton A, Muller DN, Rauh M, Luft FC, Titze J: Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest 2017;127:1932–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, Lantier L, LaRocque LM, Marton A, Neubert P, Schroder A, Rakova N, Jantsch J, Dikalova AE, Dikalov SI, Harrison DG, Muller DN, Nishiyama A, Rauh M, Harris RC, Luft FC, Wassermann DH, Sands JM, Titze J: High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest 2017;127:1944–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schroder A, Luft FC: Spooky sodium balance. Kidney Int 2014;85:759–767. [DOI] [PubMed] [Google Scholar]
- 9.Marton A, Kaneko T, Kovalik JP, Yasui A, Nishiyama A, Kitada K, Titze J: Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nature Rev Nephr 2021;17:65–77. [DOI] [PubMed] [Google Scholar]
- 10.Wild J, Jung R, Knopp T, Efentakis P, Benaki D, Grill A, Wegner J, Molitor M, Garlapati V, Rakova N, Marko L, Marton A, Mikros E, Munzel T, Kossmann S, Rauh M, Nakano D, Kitada K, Luft F, Waisman A, Wenzel P, Titze J, Karbach S: Aestivation motifs explain hypertension and muscle mass loss in mice with psoriatic skin barrier defect. Acta Physiol 2021;232:1–18. [DOI] [PubMed] [Google Scholar]
- 11.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D: Functional significance of cell volume regulatory mechanisms. Physiol Rev 1998;78:247–306. [DOI] [PubMed] [Google Scholar]
- 12.Hosseiniyan Khatibi SM, Zununi Vahed F, Sharifi S, Ardalan M, Mohajel Shoja M, Zununi Vahed S: Osmolytes resist against harsh osmolarity: Something old something new. Biochimie 2019;158:156–164. [DOI] [PubMed] [Google Scholar]
- 13.Stookey JD, Kavouras S, Suh H, Lang F: Underhydration is associated with obesity, chronic diseases, and death within 3 to 6 years in the U.S. population aged 51-70 years. Nutrients 2020;12:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang F, Guelinckx I, Lemetais G, Melander O: Two liters a day keep the doctor away? Considerations on the pathophysiology of suboptimal fluid intake in the common population. Kidney Blood Press Res 2017;42:483–494. [DOI] [PubMed] [Google Scholar]
- 15.Burg MB, Ferraris JD, Dmitrieva NI: Cellular response to hyperosmotic stresses. Physiol Rev 2007;87:1441–1474. [DOI] [PubMed] [Google Scholar]
- 16.Brocker C, Thompson David C, Vasiliou V: The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 2012;3:345–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casali CI, Erjavec LC, Fernandez-Tome MDC: Sequential and synchronized hypertonicity-induced activation of Rel-family transcription factors is required for osmoprotection in renal cells. Heliyon 2018;4:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilborg A, Sabath N, Wiesel Y, Nathans J, Levy-Adam F, Yario TA, Steitz JA, Shalgi R: Comparative analysis reveals genomic features of stress-induced transcriptional readthrough. Proc Natl Acad Sci USA 2017;114:E8362–E8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cvetkovic L, Perisic S, Titze J, Jack HM, Schuh W: The impact of hyperosmolality on activation and differentiation of B lymphoid cells. Frontiers Immunol 2019;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunes P, Ernandez T, Roth I, Qiao X, Strebel D, Bouley R, Charollais A, Ramadori P, Foti M, Meda P, Feraille E, Brown D, Hasler U: Hypertonic stress promotes autophagy and microtubule-dependent autophagosomal clusters. Autophagy 2013;9:550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritter M, Bresgen N, Kerschbaum HH: From pinocytosis to methuosis-fluid consumption as a risk factor for cell death. Front Cell Dev Biol 2021;9:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakab M, Ritter M: Cell volume regulatory ion transport in the regulation of cell migration. Contrib Nephrol 2006;152:161–180. [DOI] [PubMed] [Google Scholar]
- 23.Schwab A, Fabian A, Hanley PJ, Stock C: Role of ion channels and transporters in cell migration. Physiol Rev 2012;92:1865–1913. [DOI] [PubMed] [Google Scholar]
- 24.Zierler S, Frei E, Grissmer S, Kerschbaum HH: Chloride influx provokes lamellipodium formation in microglial cells. Cell Physiol Biochem 2008;21:55–62. [DOI] [PubMed] [Google Scholar]
- 25.Furtner T, Zierler S, Kerschbaum HH: Blockade of chloride channels suppresses engulfment of microspheres in the microglial cell line, BV-2. Brain Res 2007;1184:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Dmitrieva NI, Burg MB: Hypertonic stress response. Mutat Res 2005;569:65–74. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara S, Ichijo H, Watanabe K: A novel lens for cell volume regulation: Liquid-liquid phase separation. Cell Physiol Biochem 2021;55:135–160. [DOI] [PubMed] [Google Scholar]
- 28.Jakab M, Furst J, Gschwentner M, Botta G, Garavaglia ML, Bazzini C, Rodighiero S, Meyer G, Eichmueller S, Woll E, Chwatal S, Ritter M, Paulmichl M: Mechanisms sensing and modulating signals arising from cell swelling. Cell Physiol Biochem 2002;12:235–258. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann EK, Lambert IH, Pedersen SF: Physiology of cell volume regulation in vertebrates. Physiol Rev 2009;89:193–277. [DOI] [PubMed] [Google Scholar]
- 30.Beck FX, Schmolke M, Guder WG: Osmolytes. Curr Opin Nephrol Hypertens 1992;1:43–52. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Perez A, Burg MB: Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membrane Biol 1991;119:1–13. [DOI] [PubMed] [Google Scholar]
- 32.Chadwick SR, Wu JZ, Freeman SA: Solute transport controls membrane tension and organellar volume. Cell Physiol Biochem 2021;55:1–24. [DOI] [PubMed] [Google Scholar]
- 33.Warskulat U, Newsome W, Noe B, Stoll B, Haussinger D: Anisoosmotic regulation of hepatic gene expression. Biol Chem Hoppe Seyler 1996;377:57–65. [DOI] [PubMed] [Google Scholar]
- 34.Warskulat U, Zhang F, Haussinger D: Modulation of phagocytosis by anisoosmolarity and betaine in rat liver macrophages (Kupffer cells) and RAW 264.7 mouse macrophages. FEBS Letters 1996;391:287–292. [DOI] [PubMed] [Google Scholar]
- 35.Häussinger D, Lang F: Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta 1991;1071:331–350. [DOI] [PubMed] [Google Scholar]
- 36.Schliess F, Haussinger D: The cellular hydration state: a critical determinant for cell death and survival. Biol Chem 2002;383:577–583. [DOI] [PubMed] [Google Scholar]
- 37.Haussinger D: Neural control of hepatic osmolytes and parenchymal cell hydration. Anat Rec A Discov Mol Cell Evol Biol 2004;280:893–900. [DOI] [PubMed] [Google Scholar]
- 38.Jentsch TJ: VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 2016;17:293–307. [DOI] [PubMed] [Google Scholar]
- 39.Bonus M, Haussinger D, Gohlke H: Liver cell hydration and integrin signaling. Biol Chem 2021;402(9):1033–1045. [DOI] [PubMed] [Google Scholar]
- 40.Häussinger D: Regulation of metabolism by changes in cellular hydration. Clin Nutr 1995;14:4–12. [Google Scholar]
- 41.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN: Living with water stress: evolution of osmolyte systems. Science 1982;217:1214–1222. [DOI] [PubMed] [Google Scholar]
- 42.Model MA, Hollembeak JE, Kurokawa M: Macromolecular crowding, a hidden link between cell volume and everything else. Cell Physiol Biochem 2021;55:25–40. [DOI] [PubMed] [Google Scholar]
- 43.Mudrak NJ, Rana PS, Model MA: Calibrated brightfield-based imaging for measuring intracellular protein concentration. Cytometry Part A, J Int Soc Analytical Cytol 2018;93:297–304. [DOI] [PubMed] [Google Scholar]
- 44.Kuper C, Bartels H, Fraek ML, Beck FX, Neuhofer W: Ectodomain shedding of pro-TGF-alpha is required for COX-2 induction and cell survival in renal medullary cells exposed to osmotic stress. Am J Physiol Cell Physiol 2007;293:C1971–C1982. [DOI] [PubMed] [Google Scholar]
- 45.Cheung CY, Ko BC: NFAT5 in cellular adaptation to hypertonic stress - regulations and functional significance. J Mol Signal 2013;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SD, Choi SY, Lim SW, Lamitina ST, Ho SN, Go WY, Kwon HM: TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress, organic osmolyte-dependent and -independent pathways. Am J Physiol Renal Physiol 2011;300:F707–F715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi SY, Lee-Kwon W, Kwon HM: The evolving role of TonEBP as an immunometabolic stress protein. Nature Rev Nephrol 2020;16:352–364. [DOI] [PubMed] [Google Scholar]
- 48.Jalihal AP, Schmidt A, Gao G, Little SR, Pitchiaya S, Walter NG: Hyperosmotic phase separation, Condensates beyond inclusions, granules and organelles. J Biol Chem 2021;296:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majumder S, Jain A: Osmotic Stress Triggers Phase Separation. Mol Cell 2020;79:876–877. [DOI] [PubMed] [Google Scholar]
- 50.Haussinger D, Lang F, Bauers K, Gerok W: Interactions between glutamine metabolism and cell-volume regulation in perfused rat liver. Eur J Biochem 1990;188:689–695. [DOI] [PubMed] [Google Scholar]
- 51.Storey KB, Storey JM: Aestivation: signaling and hypometabolism. J Exp Biol 2012;215:1425–1433. [DOI] [PubMed] [Google Scholar]
- 52.Fumarola C, La Monica S, Guidotti GG: Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: Role of glutamine and of cell shrinkage. J Cell Physiol 2005;204:155–165. [DOI] [PubMed] [Google Scholar]
- 53.Gual P, Le Marchand-Brustel Y, Tanti J: Positive and negative regulation of glucose uptake by hyperosmotic stress. Diab Metab 2003;29:566–575. [DOI] [PubMed] [Google Scholar]
- 54.Bratusch-Marrain PR, DeFronzo RA: Impairment of insulin-mediated glucose metabolism by hyperosmolality in man. Diab 1983;32:1028–1034. [DOI] [PubMed] [Google Scholar]
- 55.Gual P, Gonzalez T, Gremeaux T, Barres R, Le Marchand-Brustel Y, Tanti JF: Hyperosmotic stress inhibits insulin receptor substrate-1 function by distinct mechanisms in 3T3-L1 adipocytes. J Biol Chem 2003;278:26550–26557. [DOI] [PubMed] [Google Scholar]
- 56.Haussinger D, Roth E, Lang F, Gerok W: Cellular hydration state, an important determinant of protein catabolism in health and disease. Lancet 1993;341:1330–1332. [DOI] [PubMed] [Google Scholar]
- 57.Lornejad-Schafer MR, Schafer C, Graf D, Haussinger D, Schliess F: Osmotic regulation of insulin-induced mitogen-activated protein kinase phosphatase (MKP-1) expression in H4IIE rat hepatoma cells. Biochem J 2003;371:609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schliess F, von Dahl S, Haussinger D: Insulin resistance induced by loop diuretics and hyperosmolarity in perfused rat liver. Biol Chem 2001;382:1063–1069. [DOI] [PubMed] [Google Scholar]
- 59.Winges A, Garcia TB, Prager P, Wiedemann P, Kohen L, Bringmann A, Hollborn M: Osmotic expression of aldose reductase in retinal pigment epithelial cells, involvement of NFAT5. Graefe Arch Clin Exp Ophthal 2016;254:2387–2400. [DOI] [PubMed] [Google Scholar]
- 60.Dall’Asta V, Rossi PA, Bussolati O, Gazzola GC: Regulatory volume decrease of cultured human fibroblasts involves changes in intracellular amino-acid pool. Biochim Biophys Acta 1994;1220:139–145. [DOI] [PubMed] [Google Scholar]
- 61.Dall’Asta V, Bussolati O, Sala R, Parolari A, Alamanni F, Biglioli P, Gazzola GC: Amino acids are compatible osmolytes for volume recovery after hypertonic shrinkage in vascular endothelial cells. Am J Physiol 1999;276:C865–C872. [DOI] [PubMed] [Google Scholar]
- 62.Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, Ehling P, Meuth SG, Roth J, Kuhlmann T, Wiendl H, Klotz L: Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun 2016;67:90–101. [DOI] [PubMed] [Google Scholar]
- 63.Neuhofer W: Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr Genom 2010;11:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shapiro L, Dinarello CA: Hyperosmotic stress as a stimulant for proinflammatory cytokine production. Exp Cell Res 1997;231:354–362. [DOI] [PubMed] [Google Scholar]
- 65.Offensperger WB, Offensperger S, Stoll B, Gerok W, Haussinger D: Effects of anisotonic exposure on duck hepatitis B virus replication. Hepatol 1994;20:1–7. [DOI] [PubMed] [Google Scholar]
- 66.Zhu X, Ma K, Zhou K, Liu J, Nurnberg B, Lang F: Vasopressin-stimulated ORAI1 expression and store-operated Ca(2+) entry in aortic smooth muscle cells. J Mol Med 2021;99:373–382. [DOI] [PubMed] [Google Scholar]
- 67.Saha N, Stoll B, Lang F, Haussinger D: Effect of anisotonic cell-volume modulation on glutathione-S-conjugate release, t-butylhydroperoxide metabolism and the pentose-phosphate shunt in perfused rat liver. Eur J Biochem 1992;209:437–444. [DOI] [PubMed] [Google Scholar]
- 68.Haussinger D, Stoll B, Morimoto Y, Lang F, Gerok W: Anisoosmotic liver perfusion: redox shifts and modulation of α-ketoisocaproate and glycine metabolism. Biol Chem Hoppe Seyler 1992;373:723–734. [DOI] [PubMed] [Google Scholar]
- 69.Devin A, Guerin B, Rigoulet M: Dependence of flux size and efficiency of oxidative phosphorylation on external osmolarity in isolated rat liver mitochondria, role of adenine nucleotide carrier. Biochim Biophys Acta 1996;1273:13–20. [DOI] [PubMed] [Google Scholar]
- 70.Banerjee B, Bhuyan G, Saha N: Influence of environmental hypertonicity on the induction of ureogenesis and amino acid metabolism in air-breathing walking catfish. Indian J Exp Biol 2014;52:728–738. [PubMed] [Google Scholar]
- 71.Reinehr R, Haussinger D: Hyperosmotic activation of the CD95 death receptor system. Acta Physiol 2006;187:199–203. [DOI] [PubMed] [Google Scholar]
- 72.Okada Y, Sabirov RZ, Sato-Numata K, Numata T: Cell death induction and protection by activation of ubiquitously expressed anion/cation channels, Part 1: Roles of VSOR/VRAC in cell volume regulation, release of double-edged signals and apoptotic/necrotic cell death. Front Cell Dev Biol 2020;8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Foller M, Lang F: Ion transport in eryptosis, the suicidal death of erythrocytes. Front Cell Dev Biol 2020;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lang F, Lang E, Foller M: Physiology and pathophysiology of eryptosis. Trans Med Hemotherapy 2012;39:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lang F, Lang KS, Lang PA, Huber SM, Wieder T: Osmotic shock-induced suicidal death of erythrocytes. Acta Physiol 2006;187:191–198. [DOI] [PubMed] [Google Scholar]
- 76.Choi SY, Lee-Kwon W, Lee HH, Lee JH, Sanada S, Kwon HM: Multiple cell death pathways are independently activated by lethal hypertonicity in renal epithelial cells. Am J Physiol Cell Physiol 2013;305:C1011–C1020. [DOI] [PubMed] [Google Scholar]
- 77.Droge W: Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47–95. [DOI] [PubMed] [Google Scholar]
- 78.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM: Chronic inflammation in the etiology of disease across the life span. Nature Med 2019;25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nistala R, Whaley-Connell A, Sowers JR: Redox control of renal function and hypertension. Antioxid Redox Signal 2008;10:2047–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pasini E, Corsetti G, Aquilani R, Romano C, Picca A, Calvani R, Dioguardi FS: Protein-amino acid metabolism disarrangements, The hidden enemy of chronic age-related conditions. Nutrients 2018;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brewer GJ: Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol 2010;45:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williamson JR, Yasuo I: Linking diabetic complications to sorbitol oxidation, oxidative stress and metabolic suppression. J Diab Metab 2012;3:1–15. [Google Scholar]
- 83.Sookoian S, Pirola CJ: Alanine and aspartate aminotransferase and glutamine-cycling pathway, their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012;18:3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White PJ, McGarrah RW, Herman MA, Bain JR, Shah SH, Newgard CB: Insulin action, type 2 diabetes, and branched-chain amino acids, A two-way street. Mol Metab 2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE: Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010;3:207–214. [DOI] [PubMed] [Google Scholar]
- 86.Du T, Han J: Arginine metabolism and its potential in treatment of colorectal cancer. Front Cell Dev Biol 2021;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, Magnusson M, Fox CS, O’Donnell CJ, Vasan RS, Melander O, Clish CB, Gerszten RE, Wang TJ: Metabolite profiling identifies pathways associated with metabolic risk in humans. Circ 2012;125:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G: Serine and glycine metabolism in cancer. Trends Biochem Sci 2014;39:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adav SS, Wang Y: Metabolomics Signatures of Aging: Recent Advances. Aging Dis 2021;12:646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, Muehlbauer M, Patel MJ, Stevens RD, Appel LJ, Newby LK, Svetkey LP: Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabol Clin Exp 2013;62:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe Y, Kasuga K, Tokutake T, Kitamura K, Ikeuchi T, Nakamura K: Alterations in glycerolipid and fatty acid metabolic pathways in Alzheimer’s disease identified by urinary metabolic profiling, A pilot study. Front Neurol 2021;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ning W, Qiao N, Zhang X, Pei D, Wang W: Metabolic profiling analysis for clinical urine of colorectal cancer. Asia Pac J Clin Oncol 2021;17:403–413. [DOI] [PubMed] [Google Scholar]
- 93.Burns JS, Manda G: Metabolic pathways of the Warburg Effect in health and disease: perspectives of choice, chain or chance. Int J Mol Sci 2017;18:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berneis K, Ninnis R, Haussinger D, Keller U: Effects of hyper- and hypoosmolality on whole body protein and glucose kinetics in humans. Am J Physiol 1999;276:E188–E195. [DOI] [PubMed] [Google Scholar]
- 95.Bilz S, Ninnis R, Keller U: Effects of hypoosmolality on whole-body lipolysis in man. Metabolism 1999;48:472–476. [DOI] [PubMed] [Google Scholar]
- 96.Stookey JD, Klein A, Hamer J, Chi C, Higa A, Ng V, Arieff A, Kuypers FA, Larkin S, Perrier E, Lang F: RBC deformability and amino acid concentrations after hypo-osmotic challenge may reflect chronic cell hydration status in healthy young men. Physiol Rep 2013;1:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stookey JD, Hamer J, Killilea DW: Change in hydration indices associated with an increase in total water intake of more than 0.5 L/day, sustained over 4 weeks, in healthy young men with initial total water intake below 2 L/day. Physiol Rep 2017;5:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satsu H, Miyamoto Y, Shimizu M: Hypertonicity stimulates taurine uptake and transporter gene expression in Caco-2 cells. Biochim Biophys Acta 1999;1419:89–96. [DOI] [PubMed] [Google Scholar]
- 99.Burg MB, Ferraris JD: Intracellular organic osmolytes: function and regulation. J Biol Chem 2008;283:7309–7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Law RO: Regulation of mammalian brain cell volume. J Exp Zool 1994;268:90–96. [DOI] [PubMed] [Google Scholar]
- 101.Haussinger D: Osmoregulation of liver cell function: signalling, osmolytes and cell heterogeneity. Contrib Nephrol 1998;123:185–204. [DOI] [PubMed] [Google Scholar]
- 102.Aramburu J, Lopez-Rodriguez C: Brx shines a light on the route from hyperosmolarity to NFAT5. Sci Signal 2009;2:pe20. [DOI] [PubMed] [Google Scholar]
- 103.Kempson SA, Hoshaw MJ, Hinesley RS, McAteer JA: Hyperosmotic stress up-regulates amino acid transport in vascular endothelial cells. Kidney Int 1997;52:1332–1339. [DOI] [PubMed] [Google Scholar]
- 104.Dall’Asta V, Rossi PA, Bussolati O, Gazzola GC: Response of human fibroblasts to hypertonic stress. Cell shrinkage is counteracted by an enhanced active transport of neutral amino acids. J Biol Chem 1994;269:10485–10491. [PubMed] [Google Scholar]
- 105.Alfieri RR, Bonelli MA, Cavazzoni A, Brigotti M, Fumarola C, Sestili P, Mozzoni P, De Palma G, Mutti A, Carnicelli D, Vacondio F, Silva C, Borghetti AF, Wheeler KP, Petronini PG: Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol 2006;576:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brocker C, Thompson DC, Vasiliou V: The role of hyperosmotic stress in inflammation and disease. Biomol Concepts 2012;3:345–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wishart DS: Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev 2019;99:1819–1875. [DOI] [PubMed] [Google Scholar]
- 108.Bar-David Y, Urkin J, Kozminsky E: The effect of voluntary dehydration on cognitive functions of elementary school children. Acta Paed 2005;94:1667–1673. [DOI] [PubMed] [Google Scholar]
- 109.Stookey JD, Brass B, Holliday A, Arieff A: What is the cell hydration status of healthy children in the USA? Preliminary data on urine osmolality and water intake. Public Health Nutr 2012;15:2148–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kenney EL, Long MW, Cradock AL, Gortmaker SL: Prevalence of inadequate hydration among US children and disparities by gender and race/ethnicity: National Health and Nutrition Examination Survey, 2009-2012. Am J Pub Health 2015;105:e113–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Armstrong LE, Kavouras SA, Walsh NP, Roberts WO: Diagnosing dehydration? Blend evidence with clinical observations. Curr Opin Clin Nutr Metab Care 2016;19:434–438. [DOI] [PubMed] [Google Scholar]
- 112.Dias FC, Boilesen SN, Tahan S, Melli LC, Morais MB: Prevalence of voluntary dehydration according to urine osmolarity in elementary school students in the metropolitan region of Sao Paulo, Brazil. Clinics 2019;74:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taylor NA, van den Heuvel AM, Kerry P, McGhee S, Peoples GE, Brown MA, Patterson MJ: Observations on saliva osmolality during progressive dehydration and partial rehydration. Eur J Appl Physiol 2012;112:3227–3237. [DOI] [PubMed] [Google Scholar]
- 114.Walsh NP, Montague JC, Callow N, Rowlands AV: Saliva flow rate, total protein concentration and osmolality as potential markers of whole body hydration status during progressive acute dehydration in humans. Arch Oral Biol 2004;49:149–154. [DOI] [PubMed] [Google Scholar]
- 115.Matz R: Dehydration in older adults. JAMA 1996;275:911–912. [DOI] [PubMed] [Google Scholar]
- 116.Tuazon SA, Cardona R, Tuazon JA, Staros EB: Serum osmolality. Medscape 2019;Nov 20(https://emedicine.medscape.com/article/2099042-overview). [Google Scholar]
- 117.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 1980;33:27–39. [DOI] [PubMed] [Google Scholar]
- 118.Hume R, Weyers E: Relationship between total body water and surface area in normal and obese subjects. J Clin Pathol 1971;24:234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clark MR, Mohandas N, Shohet SB: Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood 1983;61:899–910. [PubMed] [Google Scholar]
- 120.Belsky DW, Caspi A, Corcoran DL, Sugden K, Poulton R, Arseneault L, Baccarelli A, Chamarti K, Gao X, Hannon E, Harrington HL, Houts R, Kothari M, Kwon D, Mill J, Schwartz J, Vokonas P, Wang C, Williams BS, Moffitt TE: DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife 2022:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI: A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zyba SJ, Shenvi SV, Killilea DW, Holland TC, Kim E, Moy A, Sutherland B, Gildengorin V, Shigenaga MK, King JC: A moderate increase in dietary zinc reduces DNA strand breaks in leukocytes and alters plasma proteins without changing plasma zinc concentrations. Am J Clin Nutr 2017;105:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lai Z, Tsugawa H, Wohlgemuth G, Mehta S, Mueller M, Zheng Y, Ogiwara A, Meissen J, Showalter M, Takeuchi K, Kind T, Beal P, Arita M, Fiehn O: Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat Methods 2018;15:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MetaboAnalyst: www.metaboanalyst.ca.
- 125.Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques PE, Li S, Xia J: MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 2021;49:W388–W396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trygg J, Wold S: Orthogonal projections to latent structures (O-PLS). J Chemomet 2002;16:119–128. [Google Scholar]
- 127.Worley B, Halouska S, Powers R: Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal Biochem 2013;433:102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Worley B, Powers R: PCA as a practical indicator of OPLS-DA model reliability. Curr Metabolomics 2016;4:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JPM, van Dorsten FA: Assessment of PLSDA cross validation. Metabolomics 2008;4:81–89. [Google Scholar]
- 130.Refardt J, Winzeler B, Christ-Crain M: Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin Endocrinol 2019;91:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Danziger J, Zeidel ML: Osmotic homeostasis. Clin J Am Soc Nephrol 2015;10:852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kohlmeier M. Xylitol. In: Kohlmeier M, editor. Nutrient Metabolism. London: Academic Press; 2003. p. 223–227. [Google Scholar]
- 133.Hankes LV, Politzer WM, Touster O, Anderson L: Myo-inositol catabolism in human pentosurics: the predominant role of the glucuronate-xylulose-pentose phosphate pathway. Ann NY Acad Sci 1970;165:564–576. [PubMed] [Google Scholar]
- 134.Linster CL, Van Schaftingen E: Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J 2007;274:1–22. [DOI] [PubMed] [Google Scholar]
- 135.Cenigaonandia-Campillo A, Serna-Blasco R, Gomez-Ocabo L, Solanes-Casado S, Banos-Herraiz N, Puerto-Nevado LD, Canas JA, Acenero MJ, Garcia-Foncillas J, Aguilera O: Vitamin C activates pyruvate dehydrogenase (PDH) targeting the mitochondrial tricarboxylic acid (TCA) cycle in hypoxic KRAS mutant colon cancer. Theranostics 2021;11:3595–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klyuyeva AV, Belyaeva OV, Goggans KR, Krezel W, Popov KM, Kedishvili NY: Changes in retinoid metabolism and signaling associated with metabolic remodeling during fasting and in type I diabetes. J Biol Chem 2021;296:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen W, Chen G: The roles of vitamin A in the regulation of carbohydrate, lipid, and protein metabolism. J Clin Med 2014;3:453–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kiefer FW, Orasanu G, Nallamshetty S, Brown JD, Wang H, Luger P, Qi NR, Burant CF, Duester G, Plutzky J: Retinaldehyde dehydrogenase 1 coordinates hepatic gluconeogenesis and lipid metabolism. Endocrinology 2012;153:3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gustafson LA, Jumelle-Laclau MN, van Woerkom GM, van Kuilenburg ABP, Meijer AJ: Cell swelling and glycogen metabolism in hepatocytes from fasted rats. Biochim Biophys Acta 1997;1318:184–190. [DOI] [PubMed] [Google Scholar]
- 140.Kenakin TP. Safety Pharmacology. In: Kenakin TP, editor. Pharmacology in Drug Discovery and Development. Academic Press; 2017. p. 251–273. [Google Scholar]
- 141.Moreira JD, Hamraz M, Abolhassani M, Bigan E, Peres S, Pauleve L, Nogueira ML, Steyaert JM, Schwartz L: The redox status of cancer cells supports mechanisms behind the Warburg Effect. Metabolites 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pesta DH, Tsirigotis DN, Befroy DE, Caballero D, Jurczak MJ, Rahimi Y, Cline GW, Dufour S, Birkenfeld AL, Rothman DL, Carpenter TO, Insogna K, Petersen KF, Bergwitz C, Shulman GI: Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J 2016;30:3378–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frei B, England L, Ames BN: Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 1989;86:6377–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.El Sayed SM, Mahmoud AA, El Sawy SA, Abdelaal EA, Fouad AM, Yousif RS, Hashim MS, Hemdan SB, Kadry ZM, Abdelmoaty MA, Gabr AG, Omran FM, Nabo MM, Ahmed NS: Warburg effect increases steady-state ROS condition in cancer cells through decreasing their antioxidant capacities (anticancer effects of 3-bromopyruvate through antagonizing Warburg effect). Med Hypotheses 2013;81:866–870. [DOI] [PubMed] [Google Scholar]
- 145.Hummel SG, Fischer AJ, Martin SM, Schafer FQ, Buettner GR: Nitric oxide as a cellular antioxidant: a little goes a long way. Free Radic Biol Med 2006;40:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB: Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 2001;3:203–213. [DOI] [PubMed] [Google Scholar]
- 147.Fagali N, Catala A: Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys Chem 2008;137:56–62. [DOI] [PubMed] [Google Scholar]
- 148.Adkins Y, Kelley DS: Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 2010;21:781–792. [DOI] [PubMed] [Google Scholar]
- 149.Evans NP, Misyak SA, Schmelz EM, Guri AJ, Hontecillas R, Bassaganya-Riera J: Conjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgamma. J Nutr 2010;140:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Segars JH, Nagata T, Bours V, Medin JA, Franzoso G, Blanco JC, Drew PD, Becker KG, An J, Tang T: Retinoic acid induction of major histocompatibility complex class I genes in NTera-2 embryonal carcinoma cells involves induction of NF-kappa B (p50-p65) and retinoic acid receptor beta-retinoid X receptor beta heterodimers. Mol Cell Biol 1993;13:6157–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roth I, Leroy V, Kwon HM, Martin P-Y, Féraille E, Hasler U: Osmoprotective transcription factor NFAT5/TonEBP modulates nuclear factor-κB activity. Mol Biol Cell 2010;21:3459–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang CY, Mauro C: Similarities in the metabolic reprogramming of immune system and endothelium. Front Immunol 2017;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim K, Yoo HC, Kim BG, Kim S, Sung Y, Yoon I, Yu YC, Park SJ, Kim JH, Myung K, Hwang KY, Kim S, Han JM: O-GlcNAc modification of leucyl-tRNA synthetase 1 integrates leucine and glucose availability to regulate mTORC1 and the metabolic fate of leucine. Nat Commun 2022;13:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wojtowicz W, Zabek A, Deja S, Dawiskiba T, Pawelka D, Glod M, Balcerzak W, Mlynarz P: Serum and urine (1)H NMR-based metabolomics in the diagnosis of selected thyroid diseases. Sci Rep 2017;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P: Glutamine, metabolism and immune function, supplementation and clinical translation. Nutrients 2018;10:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cynober LA: Plasma amino acid levels with a note on membrane transport, characteristics, regulation, and metabolic significance. Nutrition 2002;18:761–766. [DOI] [PubMed] [Google Scholar]
- 157.Liao SF, Regmi N, Wu G: Homeostatic regulation of plasma amino acid concentrations. Front Biosci 2018;23:640–655. [DOI] [PubMed] [Google Scholar]
- 158.Cersosimo E, Williams PE, Radosevich PM, Hoxworth BT, Lacy WW, Abumrad NN: Role of glutamine in adaptations in nitrogen metabolism during fasting. Am J Physiol 1986;250:E622–E628. [DOI] [PubMed] [Google Scholar]
- 159.Wurtz P, Soininen P, Kangas AJ, Ronnemaa T, Lehtimaki T, Kahonen M, Viikari JS, Raitakari OT, Ala-Korpela M: Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diab Care 2013;36:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stookey JD, Allu PKR, Chabas D, Pearce D, Lang F: Hypotheses about sub-optimal hydration in the weeks before coronavirus disease (COVID-19) as a risk factor for dying from COVID-19. Med Hypotheses 2020;144:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V: (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 2006;86:1151–1178. [DOI] [PubMed] [Google Scholar]
- 162.Waldegger S, Barth P, Raber G, Lang F: Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci USA 1997;94:4440–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Balmer JE, Blomhoff R: Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773–1808. [DOI] [PubMed] [Google Scholar]
- 164.Covarrubias-Pinto A, Acuna AI, Beltran FA, Torres-Diaz L, Castro MA: Old things new view: ascorbic acid protects the brain in neurodegenerative disorders. Int J Mol Sci 2015;16:28194–28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang B, Wu L, Chen J, Dong L, Chen C, Wen Z, Hu J, Fleming I, Wang DW: Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther 2021;6:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I: Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci 2018;19:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang S-F, Fischer S, Koshkin A, Laczko E, Fischer D, Ogunshola OO: Cell-specific metabolomic responses to injury: novel insights into blood-brain barrier modulation. Sci Rep 2020;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Uekusa S, Onozato M, Sakamoto T, Umino M, Ichiba H, Okoshi K, Fukushima T: Development of a derivatization reagent with a 2-Nitrophenylsulfonyl moiety for UHPLC-HRMS/MS and its application to detect amino acids including taurine. Molecules 2021;26:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chaimbault P, Alberic P, Elfakir C, Lafosse M: Development of an LC-MS-MS method for the quantification of taurine derivatives in marine invertebrates. Anal Biochem 2004;332:215–225. [DOI] [PubMed] [Google Scholar]
- 170.Delanaye P, Rozet E, Krzesinski JM, Cavalier E: Urinary NGAL measurement: biological variation and ratio to creatinine. Clin Chim Acta 2011;412:390. [DOI] [PubMed] [Google Scholar]
- 171.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV: Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int 2010; 77:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002;62:237–244. [DOI] [PubMed] [Google Scholar]
- 173.Greenblatt DJ, Ransil BJ, Harmatz JS, Smith TW, Duhme DW, Koch-Weser J: Variability of 24-hour urinary creatinine excretion by normal subjects. J Clin Pharmacol 1976;16:321–328. [DOI] [PubMed] [Google Scholar]
- 174.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetol 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 175.van Haeften TW, Matsumoto K, Yano M, Ueki Y, Miyake S: Glucose tolerance, insulin sensitivity, and the homeostasis model assessment method/Response. Diab Care 1998;21:673–674. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.