Abstract
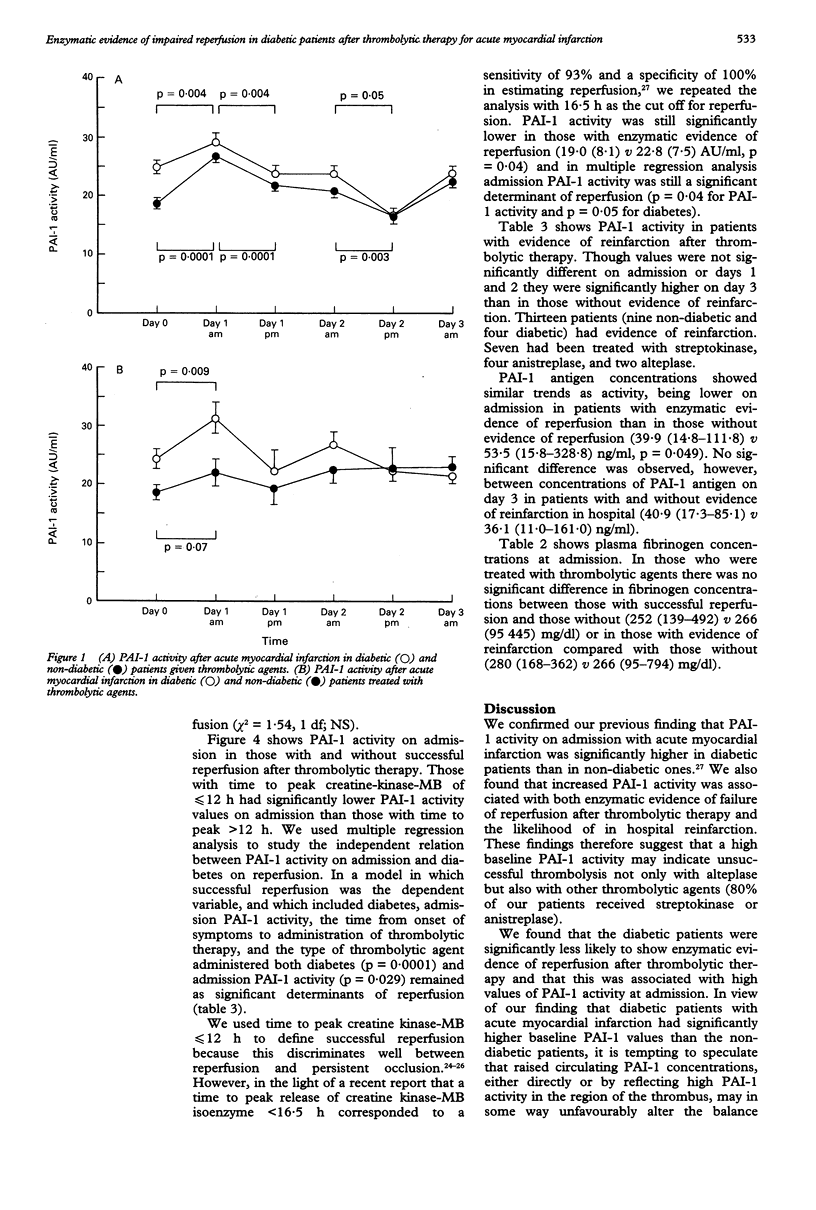
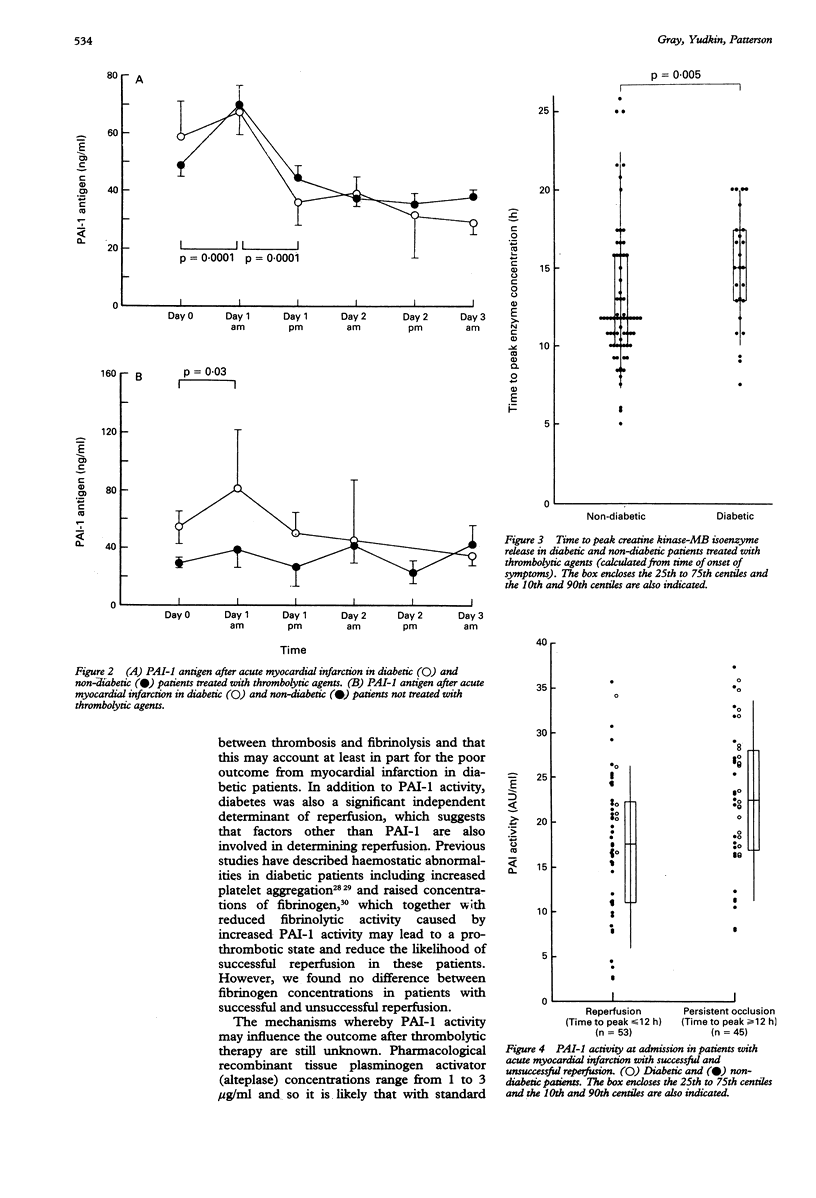
OBJECTIVE--To compare the activity of plasminogen activator inhibitor (PAI-1) in diabetic and non-diabetic patients admitted with acute myocardial infarction and to determine whether PAI-1 activity influences reperfusion after thrombolytic therapy. DESIGN--Prospective study of patients admitted with acute myocardial infarction. SETTING--District general hospital. MAIN OUTCOME MEASURES--Reperfusion assessed by time to peak release of creatine kinase-MB isoenzyme. RESULTS--Baseline PAI-1 activity and antigen concentrations were significantly higher in diabetic patients (n = 45) than in non-diabetic patients (n = 110) (24.6 (6.9) v 18.6 (7.9) AU/ml (AU = arbitrary units) (p = 0.0001) and 58.8 (13.1-328.8) v 41.0 (10.9-125.4) ng/ml (p = 0.004). Time to peak release of creatine kinase-MB was calculated in 123 (80%) patients. In 98 who received thrombolytic therapy the median time to peak enzyme release was 15.5 h (7.5-24 h) in diabetic patients (n = 26) and 12 h (5-26 h) in non-diabetic patients (n = 72) (p = 0.005). In those with a time to peak release of < or = 12 h, indicating likely successful reperfusion, PAI-1 activity was 17.5 (7.8) AU/ml compared with 22.8 (7.7) AU/ml in those with a time to peak release of > 12 h (p = 0.001). In multiple regression analysis both diabetes (p = 0.0001) and PAI-1 activity at admission (p = 0.029) were independently related to successful reperfusion. In 13 patients with evidence of reinfarction in hospital PAI-1 activity on day 3 was 26.7 (6.4) AU/ml compared with 21.7 (6.3) AU/ml in those without evidence of reinfarction (p = 0.032). CONCLUSION--Both raised PAI-1 activity on admission and diabetes were associated with a reduced likelihood of enzymatic evidence of reperfusion after thrombolytic therapy. Increased PAI-1 activity on day 3 was associated with an increased risk of reinfarction. Diabetic patients had higher PAI-1 activity on admission. This may partly explain their reduced likelihood of reperfusion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auwerx J., Bouillon R., Collen D., Geboers J. Tissue-type plasminogen activator antigen and plasminogen activator inhibitor in diabetes mellitus. Arteriosclerosis. 1988 Jan-Feb;8(1):68–72. doi: 10.1161/01.atv.8.1.68. [DOI] [PubMed] [Google Scholar]
- Barbash G. I., Hod H., Roth A., Miller H. I., Rath S., Zahav Y. H., Modan M., Zivelin A., Laniado S., Seligsohn U. Correlation of baseline plasminogen activator inhibitor activity with patency of the infarct artery after thrombolytic therapy in acute myocardial infarction. Am J Cardiol. 1989 Dec 1;64(19):1231–1235. doi: 10.1016/0002-9149(89)90559-6. [DOI] [PubMed] [Google Scholar]
- Boyle D. M., Barber J. M., McIlmoyle E. L., Salathia K. S., Evans A. E., Cran G., Elwood J. H., Shanks R. G. Effect of very early intervention with metoprolol on myocardial infarct size. Br Heart J. 1983 Mar;49(3):229–233. doi: 10.1136/hrt.49.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Declerck P. J., Alessi M. C., Verstreken M., Kruithof E. K., Juhan-Vague I., Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988 Jan;71(1):220–225. [PubMed] [Google Scholar]
- Eisenberg P. R., Sherman L. A., Jaffe A. S. Paradoxic elevation of fibrinopeptide A after streptokinase: evidence for continued thrombosis despite intense fibrinolysis. J Am Coll Cardiol. 1987 Sep;10(3):527–529. doi: 10.1016/s0735-1097(87)80194-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Catella F., Roy L., FitzGerald G. A. Marked platelet activation in vivo after intravenous streptokinase in patients with acute myocardial infarction. Circulation. 1988 Jan;77(1):142–150. doi: 10.1161/01.cir.77.1.142. [DOI] [PubMed] [Google Scholar]
- Fuller J. H., Keen H., Jarrett R. J., Omer T., Meade T. W., Chakrabarti R., North W. R., Stirling Y. Haemostatic variables associated with diabetes and its complications. Br Med J. 1979 Oct 20;2(6196):964–966. doi: 10.1136/bmj.2.6196.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore J. M., Roberts R., Ball S. P., Montero A., Goldberg R. J., Dalen J. E. Peak creatine kinase as a measure of effectiveness of thrombolytic therapy in acute myocardial infarction. Am J Cardiol. 1987 Jun 1;59(15):1234–1238. doi: 10.1016/0002-9149(87)90896-4. [DOI] [PubMed] [Google Scholar]
- Grande P., Granborg J., Clemmensen P., Sevilla D. C., Wagner N. B., Wagner G. S. Indices of reperfusion in patients with acute myocardial infarction using characteristics of the CK-MB time-activity curve. Am Heart J. 1991 Aug;122(2):400–408. doi: 10.1016/0002-8703(91)90992-q. [DOI] [PubMed] [Google Scholar]
- Grande P., Naestoft J., Christiansen C. An easy and reliable estimation of acute myocardial infarct size from serum CK-MB measurements. Eur J Cardiol. 1980 Jan;11(1):71–77. [PubMed] [Google Scholar]
- Grant P. J., Stickland M. H., Booth N. A., Prentice C. R. Metformin causes a reduction in basal and post-venous occlusion plasminogen activator inhibitor-1 in type 2 diabetic patients. Diabet Med. 1991 May;8(4):361–365. doi: 10.1111/j.1464-5491.1991.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Gray R. P., Yudkin J. S., Patterson D. L. Plasminogen activator inhibitor: a risk factor for myocardial infarction in diabetic patients. Br Heart J. 1993 Mar;69(3):228–232. doi: 10.1136/hrt.69.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris J. C., Schved J. F., Feugeas O., Aguilar-Martinez P., Arnaud A., Sanchez N., Sarlat C. Impact of smoking, physical training and weight reduction on FVII, PAI-1 and hemostatic markers in sedentary men. Thromb Haemost. 1990 Dec 28;64(4):516–520. [PubMed] [Google Scholar]
- Hamsten A., de Faire U., Walldius G., Dahlén G., Szamosi A., Landou C., Blombäck M., Wiman B. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet. 1987 Jul 4;2(8549):3–9. doi: 10.1016/s0140-6736(87)93050-9. [DOI] [PubMed] [Google Scholar]
- Jang I. K., Gold H. K., Ziskind A. A., Fallon J. T., Holt R. E., Leinbach R. C., May J. W., Collen D. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation. 1989 Apr;79(4):920–928. doi: 10.1161/01.cir.79.4.920. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I., Roul C., Alessi M. C., Ardissone J. P., Heim M., Vague P. Increased plasminogen activator inhibitor activity in non insulin dependent diabetic patients--relationship with plasma insulin. Thromb Haemost. 1989 Jun 30;61(3):370–373. [PubMed] [Google Scholar]
- Katus H. A., Diederich K. W., Scheffold T., Uellner M., Schwarz F., Kübler W. Non-invasive assessment of infarct reperfusion: the predictive power of the time to peak value of myoglobin, CKMB, and CK in serum. Eur Heart J. 1988 Jun;9(6):619–624. doi: 10.1093/oxfordjournals.eurheartj.a062551. [DOI] [PubMed] [Google Scholar]
- Kerins D. M., Roy L., FitzGerald G. A., Fitzgerald D. J. Platelet and vascular function during coronary thrombolysis with tissue-type plasminogen activator. Circulation. 1989 Dec;80(6):1718–1725. doi: 10.1161/01.cir.80.6.1718. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Nicolosa G., Bachmann F. Plasminogen activator inhibitor 1: development of a radioimmunoassay and observations on its plasma concentration during venous occlusion and after platelet aggregation. Blood. 1987 Nov;70(5):1645–1653. [PubMed] [Google Scholar]
- Kruithof E. K., Tran-Thang C., Bachmann F. Studies on the release of a plasminogen activator inhibitor by human platelets. Thromb Haemost. 1986 Apr 30;55(2):201–205. [PubMed] [Google Scholar]
- Kwaan H. C., Colwell J. A., Cruz S., Suwanwela N., Dobbie J. G. Increased platelet aggregation in diabetes mellitus. J Lab Clin Med. 1972 Aug;80(2):236–246. [PubMed] [Google Scholar]
- Lucore C. L., Fujii S., Sobel B. E. Dependence of fibrinolytic activity on the concentration of free rather than total tissue-type plasminogen activator in plasma after pharmacologic administration. Circulation. 1989 Jun;79(6):1204–1213. doi: 10.1161/01.cir.79.6.1204. [DOI] [PubMed] [Google Scholar]
- Ong L., Reiser P., Coromilas J., Scherr L., Morrison J. Left ventricular function and rapid release of creatine kinase MB in acute myocardial infarction. Evidence for spontaneous reperfusion. N Engl J Med. 1983 Jul 7;309(1):1–6. doi: 10.1056/NEJM198307073090101. [DOI] [PubMed] [Google Scholar]
- Oswald G. A., Yudkin J. S. Hyperglycaemia following acute myocardial infarction: the contribution of undiagnosed diabetes. Diabet Med. 1987 Jan-Feb;4(1):68–70. doi: 10.1111/j.1464-5491.1987.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Rapold H. J., Grimaudo V., Declerck P. J., Kruithof E. K., Bachmann F. Plasma levels of plasminogen activator inhibitor type 1, beta-thromboglobulin, and fibrinopeptide A before, during, and after treatment of acute myocardial infarction with alteplase. Blood. 1991 Sep 15;78(6):1490–1495. [PubMed] [Google Scholar]
- Reilly C. F., Fujita T., Mayer E. J., Siegfried M. E. Both circulating and clot-bound plasminogen activator inhibitor-1 inhibit endogenous fibrinolysis in the rat. Arterioscler Thromb. 1991 Sep-Oct;11(5):1276–1286. doi: 10.1161/01.atv.11.5.1276. [DOI] [PubMed] [Google Scholar]
- Sane D. C., Stump D. C., Topol E. J., Sigmon K. N., Kereiakes D. J., George B. S., Mantell S. J., Macy E., Collen D., Califf R. M. Correlation between baseline plasminogen activator inhibitor levels and clinical outcome during therapy with tissue plasminogen activator for acute myocardial infarction. Thromb Haemost. 1991 Mar 4;65(3):275–279. [PubMed] [Google Scholar]
- Schröder R., Biamino G., von Leitner E. R., Linderer T., Brüggemann T., Heitz J., Vöhringer H. F., Wegscheider K. Intravenous short-term infusion of streptokinase in acute myocardial infarction. Circulation. 1983 Mar;67(3):536–548. doi: 10.1161/01.cir.67.3.536. [DOI] [PubMed] [Google Scholar]
- Schwartz H., Leiboff R. L., Katz R. J., Wasserman A. G., Bren G. B., Varghese P. J., Ross A. M. Arteriographic predictors of spontaneous improvement in left ventricular function after myocardial infarction. Circulation. 1985 Mar;71(3):466–472. doi: 10.1161/01.cir.71.3.466. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Bresnahan G. F., Shell W. E., Yoder R. D. Estimation of infarct size in man and its relation to prognosis. Circulation. 1972 Oct;46(4):640–648. doi: 10.1161/01.cir.46.4.640. [DOI] [PubMed] [Google Scholar]
- Vague P., Juhan-Vague I., Alessi M. C., Badier C., Valadier J. Metformin decreases the high plasminogen activator inhibition capacity, plasma insulin and triglyceride levels in non-diabetic obese subjects. Thromb Haemost. 1987 Jun 3;57(3):326–328. [PubMed] [Google Scholar]
- White H. D., Norris R. M., Brown M. A., Takayama M., Maslowski A., Bass N. M., Ormiston J. A., Whitlock T. Effect of intravenous streptokinase on left ventricular function and early survival after acute myocardial infarction. N Engl J Med. 1987 Oct 1;317(14):850–855. doi: 10.1056/NEJM198710013171402. [DOI] [PubMed] [Google Scholar]
- Yudkin J. S., Oswald G. A. Determinants of hospital admission and case fatality in diabetic patients with myocardial infarction. Diabetes Care. 1988 Apr;11(4):351–358. doi: 10.2337/diacare.11.4.351. [DOI] [PubMed] [Google Scholar]


