Abstract
Introduction:
Previous studies of mercury (Hg) in pregnant women in the area of La Paz, Baja California Sur (BCS), Mexico found a proportion of individuals had concentrations of total Hg ([THg]) above some thresholds of concern set by health agencies. The [THg] were associated with fish and seafood consumption as well as other factors; although it was unclear which marine diet items could potentially be contributing to the concentrations observed.
Method:
We examined [THg] and monomethylmercury concentration ([MeHg+]) in the archived hair of 70 pregnant women from BCS as well as in diet items including fish, shellfish, and staple items (rice, beans, corn, and flour). We measured stable isotopes of carbon and nitrogen and employed a Bayesian stable isotope mixing model to investigate the proportion of fish and seafood in the isotopic profiles of archived hair samples.
Results:
Concentrations of Hg species were low in staple foods and ranged from below detection limit to 5.71 parts per billion (ppb) wet weight. In hair, geometric mean [THg] was 658 ppb and [MeHg+] was 395 ppb, which were lower than previous reports. Percent MeHg+ was positively correlated with higher δ15N values.
Conclusions:
The largest carbon contributors to the diet of the study participants were corn and rice, and our analysis of fish contribution to diet varyingly agreed with the self-reported fish consumption. This report highlights the ability to discriminate potential sources of Hg from a diverse diet and the limitations of dietary recall studies.
Keywords: Fish consumption, Mercury, Methylmercury, Mixing models, Stable isotopes
1. Introduction
Mercury (Hg) is a global pollutant. Human exposure to monomethylmercury (MeHg+) is generally through the consumption of freshwater and marine fish (vertebrates) and seafood1 (invertebrates) (Clarkson and Magos, 2006). Inorganic mercury (Hg2+) is poorly absorbed by mammals compared to MeHg+ which tends to effectively bioaccumulate and biomagnify through trophic levels (Clarkson and Magos, 2006; Eagles-Smith et al., 2016). Aquatic environments typically have higher concentrations of MeHg+ than terrestrial environments due to the methylation of inorganic Hg species which occurs via sulfate and iron-reducing bacteria in hypoxic or anaerobic environments such as those found in marine or freshwater sediment (Hu et al., 2013).
Inorganic Hg species are known to cause nephrotoxicity (Clarkson and Magos, 2006) while MeHg+ is especially neurotoxic to the developing fetus and neonate as it crosses the placental barrier (Ceccatelli et al., 2010). Although the neurotoxicity of MeHg+ in animal models and humans is well documented (Clarkson and Magos, 2006; Bernhoft, 2012), there is considerable disagreement and conflicting data regarding long term effects of fish consumption during pregnancy (Sheehan et al., 2014; Myers et al., 2015). In some populations with high proportions of marine diet items (i.e. Seychelles) there have been only a few reports of negative associations between developmental outcomes and maternal [MeHg+] (Myers et al., 2003; van Wijngaarden et al., 2013), and the most recent examination of the original study cohort (at 22–24 years) found no convincing evidence for adverse effects of fish consumption during pregnancy (van Wijngaarden et al., 2017). In contrast, a similar longitudinal study found evidence of neurological deficits in children of mothers who had concentrations of 10–20 ppm [THg] in hair (Grandjean et al., 1998) with numerous caveats to consider but are not reviewed here.
Although there is some evidence that low-dose MeHg+ exposure via fish consumption is correlated with developmental deficits in children, the lack of observed toxicity in longitudinal studies may be due, at least in part, to nutritional components of fish which may counteract MeHg+ induced toxicity and may support proper fetal development (Egeland and Middaugh, 1997; Gribble et al., 2016; Oken et al., 2016). Fish contain high concentrations of antioxidants including selenium (Se) and long chain omega-3 polyunsaturated fatty acids (PUFAs), which are essential for normal brain development and function. In an animal model, pups from pregnant mice concurrently exposed to both MeHg+ and Se did not show the same developmental deficits as mice exposed solely to MeHg+. Several studies have found a positive correlation between fish consumption and developmental endpoints (Sakamoto et al., 2004; Oken et al., 2008; Mahaffey et al., 2011; Strain et al., 2012), although some have noted that selecting fish with high concentrations of omega-3 PUFAs and low [MeHg+] and other toxicants (i.e. organohalogens, biotoxins) would be optimal (Egeland and Middaugh, 1997; Gribble et al., 2016). In humans, benefits in birth and developmental outcomes with the consumption of fish oil supplements during pregnancy have been reported (Sakamoto et al., 2004; Olsen et al., 1992; Dunstan et al., 2008).
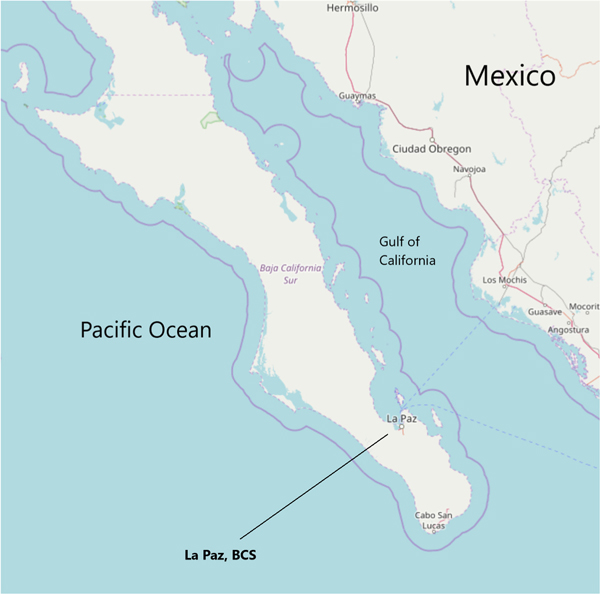
The two states of Baja California peninsula in Mexico (Baja California and Baja California Sur (BCS), Fig. 1) are bordered by the Pacific Ocean and the Gulf of California (also known as Sea of Cortez), which together constitute the majority of fisheries production for the country (Erisman et al., 2010). Fish and seafood are important dietary components for many residents of BCS, although the frequency and identity of species consumed are not well known (Gaxiola-Robles et al., 2013, 2014). Initial findings examining total mercury concentrations ([THg]) in the hair of a cohort of pregnant women found that a sizeable proportion (54 out of 75 women) had hair [THg] above the EPA advisory guideline of 1000 parts per billion (ppb) and some (6 of 75 women) had concentrations above 5000 ppb (Gaxiola-Robles et al., 2014). In a companion study, nitrogen isotope composition (δ15N) was used as an indicator of fish consumption that was associated with higher [THg], although interestingly lower mean [THg] was seen in the women who reported consuming fish and shellfish most frequently (Bentzen et al., 2014). Other factors significantly associated with [THg] playing a potential role were passive tobacco exposure and body mass index.
Fig. 1.
A map of the southern part of the Baja California peninsula, Mexico. La Paz, Baja California Sur (BCS) is situated on the Gulf of California, although it lies close to the Pacific Ocean near the fishing villages of Todos Santos and El Pescadero. Commercial fish are harvested from both coasts of the peninsula. The continuous line approximately denotes the 12-mile territorial zone. Map is from OpenStreetMaps.
Reports of Hg concentrations in marine fish landed and consumed from the Pacific Ocean waters in Mexico have been mainly focused on top predatory fish, some of which had concentrations higher than the U.S. Food and Drug Administration (FDA) action level of 1000 ppb wet weight (García-Hernández et al., 2007; Ruelas-Inzunza et al., 2008; Barrera-García et al., 2012). Mexico ranks as the 6th largest producer and 6th largest importer of shark products, with a large portion of the meat destined for domestic consumption (Dent and Clarke, 2015). Per capita consumption estimates of shark are relatively low compared to other categories of seafood; however, MeHg+ concentrations in large predatory taxonomic groups, such as sharks and tunas, can be several orders of magnitude higher than more commonly consumed items, such as shrimp and tilapia (Murillo-Cisneros et al., 2018). It is important to note that not all fish with high Hg concentrations are large predatory species some fish appear to store MeHg+ in muscle over liver (based on concentration) and in long-lived species this can lead to relatively high [MeHg+] (Harley et al., 2015). At the time of writing, we could find no comprehensive surveys of frequency of species consumption for fish consumers in this region, although landing and per capita consumption data for the country are compiled by the Comisión Nacional de Acuacultura y Pesca (CONAPESCA).
Stable isotopes of carbon (δ13C) and nitrogen are used to reconstruct diet through multidimensional mixing models (Moore and Semmens, 2008). These models are based on the principles that lighter isotopes of C and N are more easily incorporated into biochemical processes (heavier isotopes preferentially excreted), resulting in tissular enrichment of heavier isotopes. Across trophic levels this results in significant enrichment of N15 (usually 3—4‰ per trophic level in most mammalian tissues, known as the trophic enrichment factor, TEF) and smaller but measurable enrichment of C13 (1—2‰ TEF) with increasing trophic levels (Kelly, 2000). Food web movement of stable isotopes in marine systems is somewhat complicated by the fact that different environments (i.e. benthic versus pelagic) display different δ13C values in primary producers (France, 1995).
In wildlife, stable isotope mixing models (SIMMs) are increasingly utilized as tools for assessing the diet and ecology of difficult to observe species and habitats (Parnell et al., 2013; Phillips et al., 2014a). In human populations, C and N stable isotopes have been validated for use as an assessment of dietary intake of sugars (Nash et al., 2014) and marine items (O’Brien et al., 2017). In omnivorous species SIMMs are often complicated by the fact that dietary items can have highly variable concentrations of C and N, however several models can account for difference in nutrient content across diet items (Phillips et al., 2014b). It might seem imprudent to conduct chemical diet assessments in situations where dietary recall and communication with participants is possible, self-reported consumption and diet recalls may be of limited use and are occasionally conflicting with chemical analyses (O’Brien, 2015). In addition, Hg in hair represents several weeks or months of accumulation based on the length analyzed (growth rate ~1 cm month−1 (LeBeau et al., 2011)), and dietary assessments aimed at assessing consumption over long time periods (i.e. food frequency questionnaires, FFQs) have multiple shortcomings and inaccuracies and their merit is highly debated (Shim et al., 2014). Thus, a more detailed chemical feeding ecology based on assessment of Hg exposure in conjunction with dietary recall data is warranted (Bentzen et al., 2014).
Fish consumption is almost always considered the primary route of Hg exposure for non-occupational sources and pathways (Clarkson and Magos, 2006), although some reports have indicated that other dietary sources of Hg should be considered. While fish may be commonly consumed for some individuals, it is likely that consumption of staple foods derived from corn, rice, and beans are higher in both frequency of consumption and portion size. MeHg+ has been found in appreciable concentrations in some sources of rice (Qiu et al., 2008; Zhang et al., 2010) as well as high-fructose corn syrup (HFCS), which the authors suggest considering in dietary assessments (Dufault et al., 2009).
In the present study, our objective was to evaluate potential sources of dietary Hg exposure by measuring [THg] and [MeHg+] in hair and diet items and stable isotope compositions of C and N. We report [THg] and [MeHg+] in pregnant women from La Paz and in several species of fish, some of which have not been previously assessed for Hg in this region. Using stable isotopes we examined the relative contributions of diet items using Bayesian SIMMs. We also evaluate whether major dietary sources of Hg can be evaluated using chemical analysis by comparing the results of our SIMMs to data from self-reported dietary surveys.
2. Methods
2.1. Sample collection
Fish and seafood samples were purchased (generally as whole eviscerated fish) from fish markets in La Paz between March 2013 and May 2015. The full list of species examined is presented in Table 1. Samples of corn flour, wheat flour, and tortillas were purchased from supermarkets in La Paz.
Table 1.
Mercury concentrations for fish and seafood. Data presented as geometric mean ± standard error. Percent MeHg+ for each species is calculated using geometric mean of individually calculated [MeHg+]/[THg]*100.
| Common name |
Species |
Tissue |
Number |
[THg] in ppb ww |
[MeHg+] in ppb ww |
%MeHg |
|
|---|---|---|---|---|---|---|---|
| Spanish | English | ||||||
|
| |||||||
| Almeja chocolata | chocolate clam | Megapitaria squalida | mantle | 5 | 2.37 ± 0.05 | 1.40 ± 0.04 | 59% |
| abductor | 5 | 2.91 ± 0.21 | 2.37 ± 0.22 | 82% | |||
| foot | 5 | 2.96 ± 0.17 | 2.24 ± 0.15 | 75% | |||
| Cangrejo verde | green crab | Callinectes bellicosus | carapace | 6 | 27.50 ± 21.00 | 25.63 ± 20.30 | 93% |
| claw | 8 | 20.62 ± 12.11 | 19.30 ± 10.75 | 94% | |||
| Cabrilla | leopard grouper | Mycteroperca rosacea | muscle | 4 | 86.80 ± 18.80 | 78.90 ± 19.22 | 91% |
| Cochito | finescale triggerfish | Balister polylepis | muscle | 4 | 23.00 ± 20.56 | 21.90 ± 20.87 | 95% |
| Huachinanago | red snapper | Lutjanus peru | muscle | 4 | 30.09 ± 2.89 | 29.44 ± 2.98 | 98% |
| Mojarra | Peruvian mojarra | Diapterus peruvianus | muscle | 8 | 196.68 ± 36.04 | 178.54 ± 30.74 | 91% |
| Pargo | yellow snapper | Lutjanus argentiventris | muscle | 5 | 77.64 ± 13.91 | 71.48 ± 14.16 | 92% |
| Pierna | ocean whitefish | Caulolatilus princeps | muscle | 6 | 51.57 ± 26.76 | 46.76 ± 30.57 | 91% |
| Sierra | Pacific sierra | Scomberomorus sierra | muscle | 6 | 73.42 ± 2.57 | 70.21 ± 4.01 | 96% |
[THg] – concentration of total mercury.
[MeHg+] – concentration of methylmercury.
ppb – parts per billion (μg/kg).
ww – wet weight.
%MeHg – [MeHg+]/[THg]*100.
Deidentified hair samples were provided from archived samples by Dr. Gaxiola-Robles (CIBNOR, IMSS) collected under the approval of the Comisión Nacional de Investigación Científica (permit number 2016–785-013). Occipital hair samples were obtained according to the protocol established in McDowell et al. (2004). Rice and bean samples donated by each study participant were collected to reflect the type of staple items likely to be served in their own home.
A questionnaire was provided to each participant containing questions about the frequency of consumption and portion sizes of various foods including finfish, seafood (invertebrates), pork, beef, beans, rice and other staples (a questionnaire is provided as supplemental material). The form also contained questions regarding the participant’s occupation, smoking habits, dental amalgams, potential pesticide exposure, and other questions potentially relevant to Hg exposure. Archived questionnaire data was matched to the hair samples using deidentified numerical designations.
2.2. Sample processing
Hair samples were washed in order to remove external surface contamination using Triton X-100 detergent and Milli-Q water (Millipore, Bedford, Maryland, USA). Purchased whole fish remained frozen in a cooler until arrival at the University of Alaska Fairbanks (UAF), at which point they were stored at −20 °C until processing. Once thawed, morphometric measurements were obtained for each fish/invertebrate including length and mass. For invertebrates, total mass was recorded (including exoskeleton) and length was measured as carapace width at the widest point. Species identification was done via morphologic examination and consultation with local fish biologists. For the green crab (Callinectes bellicosus) samples, the frozen specimens were disarticulated upon their arrival at UAF and claws could not be matched to the main body. However, since muscle from both parts are consumed, these were analyzed independently. Sex was not determined since most of the fish purchased from the markets were without internal organs, making visual sex identification impossible. A small portion of the cranial fillet was obtained from fish samples (1—6 g) with the skin removed. From the chocolate clam (Megapitaria squalida) samples of the mantle, foot, and abductor muscle were obtained, and from the crabs muscle samples were obtained from the carapace (peropodal) and claw (cheliped). All samples were placed into Whirl-Paks (Nasco, Fort Atkinson, Wisconsin, USA) prior to analysis.
2.3. Freeze drying and homogenization
Samples including finfish, seafood, tortillas, and hair were freeze dried in order to facilitate Hg and stable isotopes of C and N measurements. Samples in Whirl-Paks were placed into glass vacuum jars and were freeze-dried using a Labconco FreeZone 6 L lyophilizer (Labconco, Kansas City, Missouri, USA). Samples were freeze-dried for 36–78 h depending on the water content. In the case of finfish, seafood, and tortilla samples percent moisture was calculated as the proportion of sample mass lost during freeze drying. Rice, beans, and flour were analyzed in their dried form (without cooking). In order to increase reproducibility and precision, all samples were homogenized using a steel-ball Cryomill (Retsch Inc, Newton, Pennsylvania, USA) for 1–2 min at 25 Hz.
2.4. Total mercury analysis
[THg] was determined using a Direct Mercury Analyzer (DMA-80, Milestone Inc, Milestone, Shelton, Connecticut, USA) which combines sample decomposition, catalysis, and atomic absorption spectrophotometry (Harley et al., 2015). The detection level was set to the lowest point on the calibration curve as 0.5 ng. Standard reference materials (SRMs) were selected in order to approximate both expected [THg] as well as simulate the wide variety of matrices analyzed in this study. SRMs used were IAEA 085 and IAEA 086 (hair matrix, International Atomic Energy Agency, Vienna, Austria), DORM-4 (fish protein, National Research Council (NRC) Canada, Ottawa, Canada), NIST-1570a (spinach, National Institute of Standards and Technology), Gaithersburg, Maryland, USA) and TORT-3 (lobster hepatopancreas, NRC Canada). An internal laboratory standard (Hg in 3.7% HCl) was also used. Recovery of SRMs for THg were between 87 and 103%. Samples were run in triplicate, except in the event of greater than 15% relative standard deviation (RSD) in which case they were run in quintuplicate. Samples for which two replicates were below the detection level of the machine (0.5 ng) were only run in duplicate. Replicates were averaged using arithmetic mean prior to use in reporting and statistics. Samples that were below detection level (BDL) were factored in to mean calculations (unless otherwise noted) by dividing the minimum detection limit (MDL) by the square root of 2. MDLs were calculated for each sample as the detection level of the machine divided by the mass of the sample ran (approximately 0.03 g–0.15 g). Different matrices have different recommended masses (i.e. corn versus fish tissue) in laboratory and manufacturer protocols which resulted in slightly different MDLs for various tissues (~2ppb–16 ppb).
2.5. Methylmercury analysis
Approximately 0.001–0.05 g of sample was digested using two different methods. Nitric acid (30% v/v) was used in hair digestion as recommended by Brooks Rand Inc. Briefly, subsamples were weighed out into 40 mL TraceClean vials (VWR, Radnor PA) and 10 mL of 30% HNO3 was added. Samples were digested in a hot water bath at 65 °C for 36−48 h. Samples were removed from the water bath and 20 mL of milliQ water was added to bring the total digestion volume to 30 mL.
For all other sample digestions we followed the established method outlined in Bloom (1989) using potassium hydroxide (KOH) dissolved in methanol (25%). Samples were weighed in TraceClean vials and 10 mL of KOH in methanol was added. Samples were digested in the dark at room temperature for approximately 48 h, at which point 20 mL of methanol was added to bring the total digestion volume to 30 mL. All digestions were analyzed within two weeks.
Aliquots of samples and SRMs (liquid standard, IAEA-085 and IAEA-086) were run on the Brooks Rand methylmercury cold vapor atomic fluorescence spectroscopy (CVAFS) detection system (including the MERX autosampler) using a 7 point calibration curve (Taylor et al., 2011). Methylmercury was analyzed using the Brooks Rand Model III detector (Brooks Rand, Seattle, Washington, USA) using a modified method of EPA Method 1630 in order to incorporate the MERX autosampler. Briefly, 40 mL glass vials were filled with milliQ water, 300 μL of acetate buffer, and a 30–100 μL aliquot of digestion. An ethylating reagent consisting of 2% sodium tetraethylborate (NaBEt4) in 2% KOH/water was added and the septa caps were screwed on tightly to the vials. Each vial was then sequentially pulled into a bubbler, after which point we followed EPA Method 1630 as outlined in Harley et al. (2015). Recovery of SRMs for MeHg+ was 95.8 ± 7.6%. Percent MeHg+ was calculated as the slope of a robust linear regression between [THg] and [MeHg+] as described in Wagemann et al. where [MeHg+] = a • [THg] + b (Wagemann et al., 1997). This method is a robust estimator to calculate average ratios of highly correlated variables.
Similar to THg, samples were run in triplicate, except in the event of greater than 15% RSD. Samples where two replicates were below the detection level of the machine (0.5 pg) were only run in duplicate. Replicates were averaged using arithmetic mean prior to use in reporting and statistics. Concentrations are reported in wet (fresh) weight unless otherwise noted. For fish tissues and tortilla samples this represents the concentration in dry weight (dw) which was back-calculated to reflect the concentration of the tissue as to represent the fish purchased by the consumer (without freeze drying). However, for staple foods the wet weight will refer to the uncooked food (e.g. dried beans, rice). This concentration of cooked staple foods at the “end of the fork” (i.e. as it reaches the consumer) is likely to be lower than what is reported here since the foods can take on water during cooking and are often mixed with other ingredients.
2.6. C and N stable isotopes analysis
Stable isotopes of carbon and nitrogen were analyzed following the methods reported by Bentzen et al. (2014). Approximately 0.2–0.5 mg of dry sample was folded into pressed tin capsules (Elemental Microanalysis, Cambridge, UK) and analyzed using a DeltaVPlus continuous-flow isotopic ratio mass spectrometer (CFIRMS, Thermo Scientific, Bremen, Germany) and elemental analyzer (Costech Scientific, Valencia, CA, USA) at the Alaska Stable Isotope Facility (ASIF) at UAF. Standard reference materials (meat based protein peptone, Sigma Chemical, St. Louis, Missouri, USA) were analyzed approximately every 10 samples to ensure ongoing precision, and blank samples were analyzed approximately every 20 samples to detect drifting values or contamination. Recovery of the peptone stable isotope SRM was 101 ± 2.1%.
Stable isotope values are presented in notation; that is, expressed as a part per thousand () deviation from internationally recognized standards (carbon, Vienna PeeDee belemnite; nitrogen, atmospheric air). Thus.
Equations (1) and (2)
| (1) |
and
| (2) |
Samples were run in two or more replicates. In cases where the RSD for values exceeded 20%, samples were reanalyzed in triplicate or higher. The arithmetic mean of replicates was obtained for each sample and used in further analyses.
2.7. Stable isotope mixing model (SIMM)
In order to conduct a non-biased quantitative assessment of the potential contributions of different items in the participants’ diet, we employed a mixing model based on a Bayesian framework (simmr in R) (Parnell et al., 2010). We followed the best practices for SIMM as outlined in Phillips et al. (2014a). In addition to the traditional Bayesian two-dimensional mixing model approach, this method allows corrections for the relative concentrations of C and N in our source data. Although not always recommended, this correction has been suggested in instances where there are large differences in elemental concentrations, as is sometimes found in omnivorous diets (Phillips et al., 2014a; Koch and Phillips, 2002). Since we sampled both staple foods (relatively low N concentrations) as well as seafood (relatively high N concentrations), we had large variation in elemental concentrations of our sources; we therefore employed the concentration dependent correction to our model. We also employed a k-neighbor joining algorithm to group sources according to similarity in 15N and 13C values (Rosing et al., 1998). Mixing models are dependent on a trophic enrichment factor (TEF) defined as the difference between the stable isotope ratios between the diet items and the tissue of interest in the consumer. These are defined using notation as.
Equations (3) and (4)
| (3) |
| (4) |
The stable isotope values for the diet items need to be adjusted based on the TEF in order to accurately assess the contribution of each item to the consumer’s diet. It has been suggested that the TEF is the most important factor in SIMMs, and small variations in TEF values can lead to large differences in assessments (Martínez del Rio et al., 2009; Caut et al., 2009). Values based on experimentally controlled feeding studies have indicated that TEFs are species, tissue, sex, and diet specific (Roth and Hobson, 2000). Unfortunately, there are few experimental studies examining TEFs for human hair, and even fewer that have examined females specifically. One study examined 15N and 13C in women (n = 20) from Fiji with a similar diet to the women in our study (fish, staples, meat, shellfish) (Hedges et al., 2009). TEFs for nitrogen in the literature are somewhat variable, but the convention has been a 3–4‰ increase per trophic level in 15N, although most studies examining hair or keratin have found this value to be slightly higher (Hedges et al., 2009; Hola et al., 2015 ). In this study we use TEFs as provided in Caut et al. for mammalian hair, where and (Caut et al., 2009).
We utilized the simmr package developed for the R programming language (R Core Team. R, 2017) using 100,000 iterations and 1000 burn-ins (Parnell et al., 2010). In order to determine if our chemical analysis correlated with self-reported consumption habits, we repeated these mixing models separating groups according to their frequency of seafood consumption. To simplify this analysis, we refined seafood consumption frequency groups into three categories (1) once per month or less, (2) once every other week, and (3) once per week or more. Using these values, we were able to achieve good convergence based on the Gelmen diagnostics. All further statistical analysis was done in R (R Core Team. R, 2017). All graphical representations were generated using the ggplot2 package (Wickham, 2009).
3. Results
Of the bean and rice samples that were donated from our study participants, no bean samples (0/70, 0%) had concentrations above minimum detection level for MeHg+, while nearly all rice samples (65/70, 93%) had detectable [MeHg+]. The geometric mean (±standard error (SE)) of [MeHg+] in rice was 2.16 ± 0.09 ppb ww, with the minimum concentration BDL and the highest concentration of 5.17 ppb. No bean or rice samples had [THg] above the detection limit for this assay, approximately 16 ppb. We also tested store-bought items including 10 flour samples (5 corn and 5 wheat) and 27 tortillas (15 flour and 12 flour). None of the tortilla or flour samples had consistently detectable [MeHg+]. Percent MeHg+ was not calculated for staple foods due to many samples being below the detection limit for [THg] and the disparate detection levels of the respective assays. Our MDL for [THg] was approximately 16 ppb for most matrices, while the MDL for [MeHg+] was at least an order of magnitude less (approximately 0.5 ppb). Thus, some samples had detectable concentrations of [MeHg+] but concentrations of [THg] that were BDL.
THg and MeHg+ concentrations in green crabs and chocolate clams were typically low, as presented in Table 1. Mean [THg] was lowest in invertebrate species (<3 ppb in chocolate clam, < 30 ppb in green crab) and highest in mojarra (Diapterus peruvianus, 202 ppb). Methylmercury concentrations were highly correlated to [THg] and the average %MeHg+ of THg was 94% (95% confidence interval 93.1%–94.3%), indicating average %MeHg+ of [THg] was approximately 94% for all marine samples. Standard errors for aggregated crab samples were elevated by one carapace and one claw sample that had concentrations >100 ppb for both THg and MeHg+. The %MeHg+ for these samples were 94% and 89%, respectively.
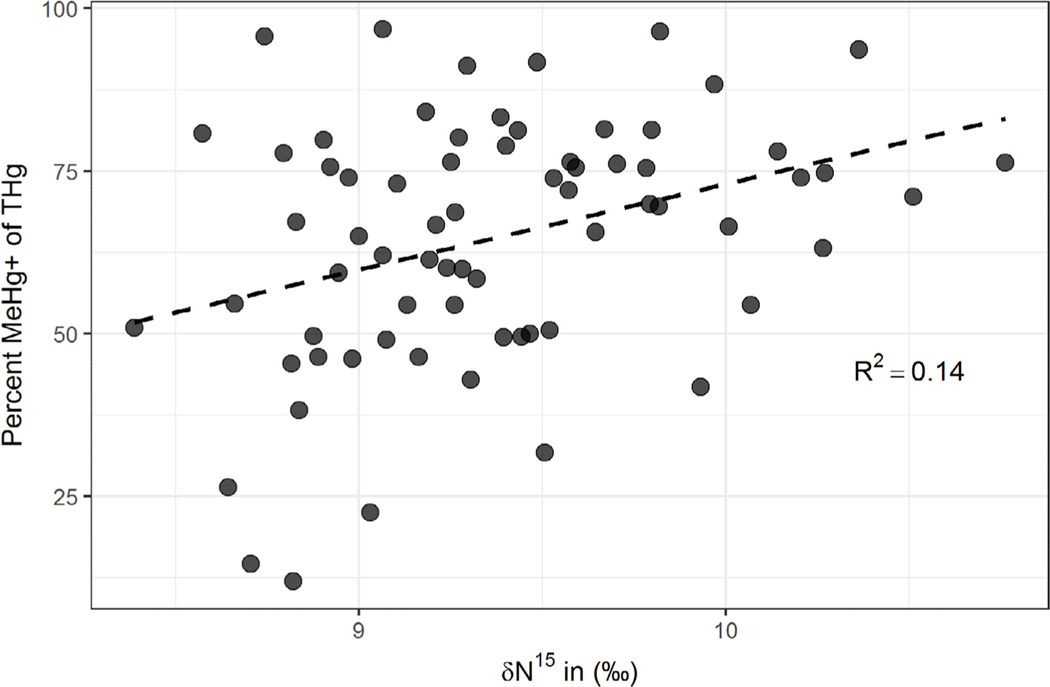
Ages of participants ranged from 18 to 56 years (median = 27) and body mass index (BMI) ranged from 18 to 63 (median = 28). Concentrations of Hg in hair samples are presented in Table 2. Geometric mean (±SE) of [THg] was 658 ± 74 and [MeHg+] was 395 ± 56 ppb ww. There were 17 individuals with [THg] above the EPA reference concentration of 1.0 ppm (Rice et al., 2003; Trasande et al., 2016). Average percent MeHg+, derived using robust linear regression as described above, indicated that on average 76% of the [THg] in hair was MeHg+ (R2= 0.74), although 17 individuals had % MeHg+ less than 50% and 3 individuals had %MeHg+ less than 25%. The [THg] was significantly lower in this study than in Gaxiola-Robles et al. when compared using a student’s t-test (p = 0.001) (Gaxiola-Robles et al., 2014). Carbon isotope compositions (δ13C) ranged from −15.2‰ to −18.3‰ (median = −17.0‰, standard error = 0.1) and nitrogen (δ15N) ranged from 8.4‰ to 10.8‰ (median 9.2‰, SE = 0.1). The 13C and 15N values were not well correlated (r = 0.18). There was a significant positive association between [THg] and as well as [MeHg+] and, although considerably more variability in [MeHg+] was explained by 15N. Percent MeHg+ was also positively associated with 15N values (, Fig. 2) although not with 13C values.
Table 2.
Mercury concentrations in human hair. Values expressed as geometric mean in parts per billion (μg/kg) wet weight. Data are compared to thresholds (1 ppm and 5 ppm) similar to Gaxiola-Robles et al. (2014).
| Study | Hg Species | Median | Geometric mean | Standard error | n > 1 ppm | n > 5 ppm |
|---|---|---|---|---|---|---|
|
| ||||||
| This study | [THg] | 688 | 658 ± | 74 | 17/70 (24%) | 0 (0%) |
| [MeHg+] | 432 | 395 ± | 56 | 10/70 (14%) | 0 (0%) | |
| %MeHg+a | 76.3% | 60.4% | 2.3% | |||
| Gaxiola-Robles et al. (Harley et al., 2015) | [THg] | 1520 | 1389 | 463 | 54/75 (72%) | 6/75(8%) |
[THg] – concentration of total mercury.
[MeHg+] – concentration of methylmercury.
Percent MeHg+ calculated as the slope of the regression (a) where [MeHg] = a • [THg] + b. For further explanation see text and Wagemann et al. (1997).
Fig. 2.
Linear relationship between %MeHg+ of [THg] and δ15N, an estimator of fish consumption, in human hair samples (n = 70). Seventeen individuals had %MeHg+ less than 50%. MeHg+ - monomethylmercury, THg – total mercury, δ15N - stable isotope ratio of N.
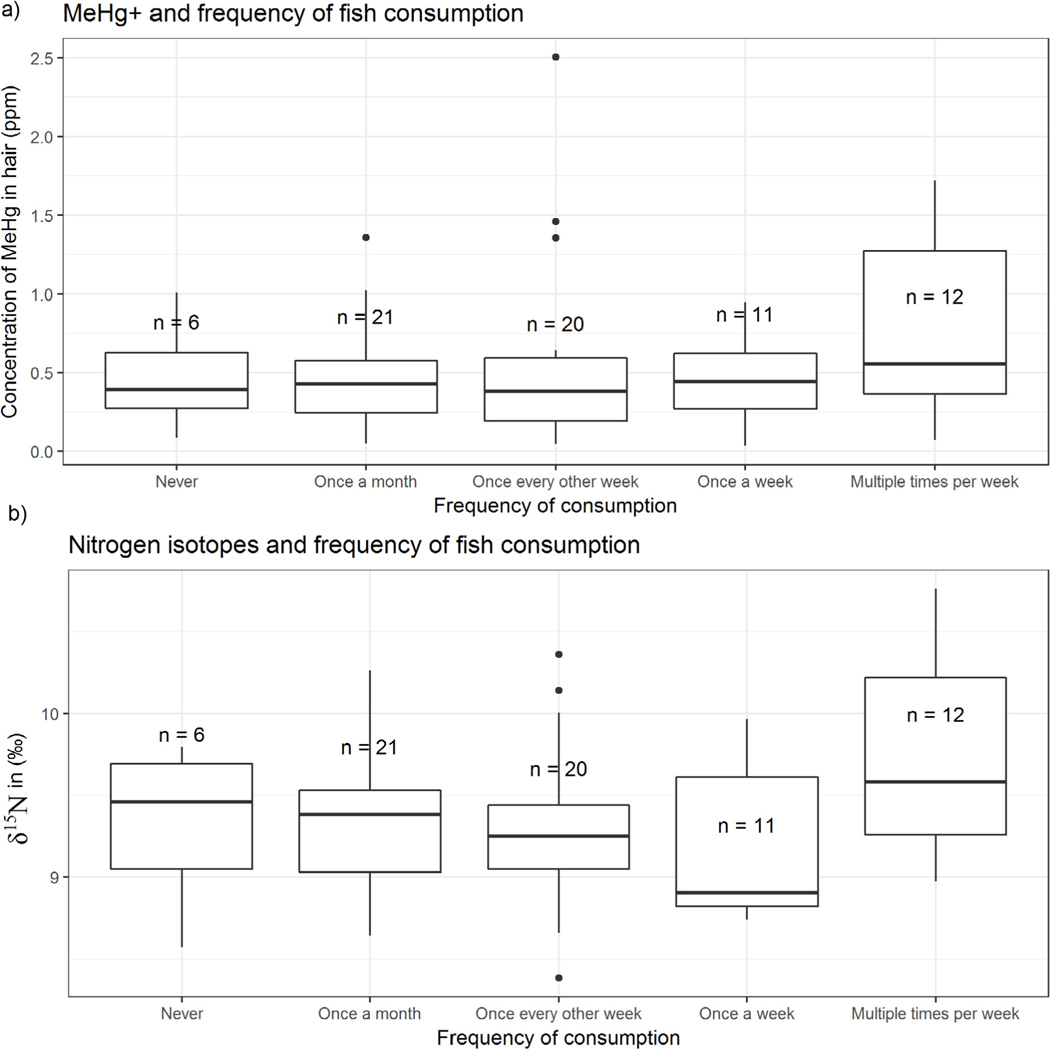
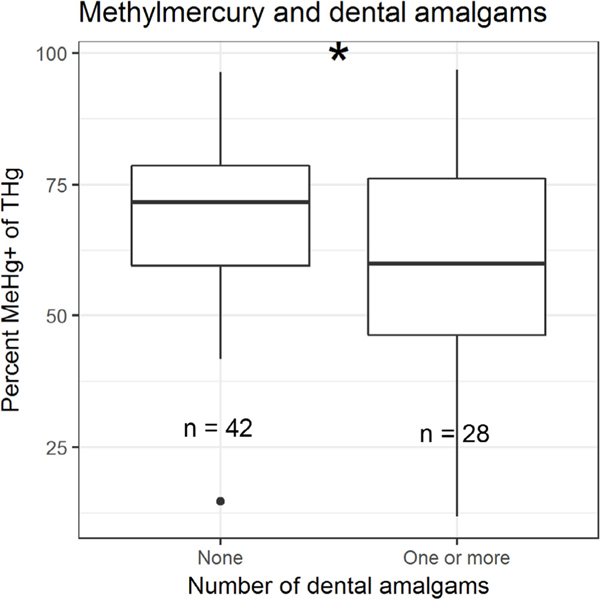
Neither [MeHg+] nor [THg] varied significantly between groups of self-reported fish consumption (Fig. 3a). We also noted no significant differences between fish consumption groups and δ15N values (Fig. 3b). This was the case for groups based on the frequency of consumption of seafood (not shown). There was a significant difference in %MeHg+ between participants who had zero dental amalgams and participants who had one or more (students t-test, p = 0.02, Fig. 4). Due to homogeneity of responses, we did not make comparisons of Hg species related to smoking.
Fig. 3.
(a) Concentrations of MeHg+ in hair samples grouped by self-reported fish consumption, and (b) δ15N in hair based on self-reported consumption of fish. Boxplots represent first quartile, median, and third quartile while whiskers represent the highest and lowest datum within 1.5 interquartile range (IQR). Individual points are data outside 1.5*IQR. MeHg+ - monomethylmercury, δ15N - stable isotope ratio of N.
Fig. 4.
Differences in the percent MeHg+ of [THg] between study participants with and without dental amalgams. Asterisk indicates significance at α = 0.05 level (student’s t-test). %.? MeHg+ - monomethylmercury, THg – total mercury.
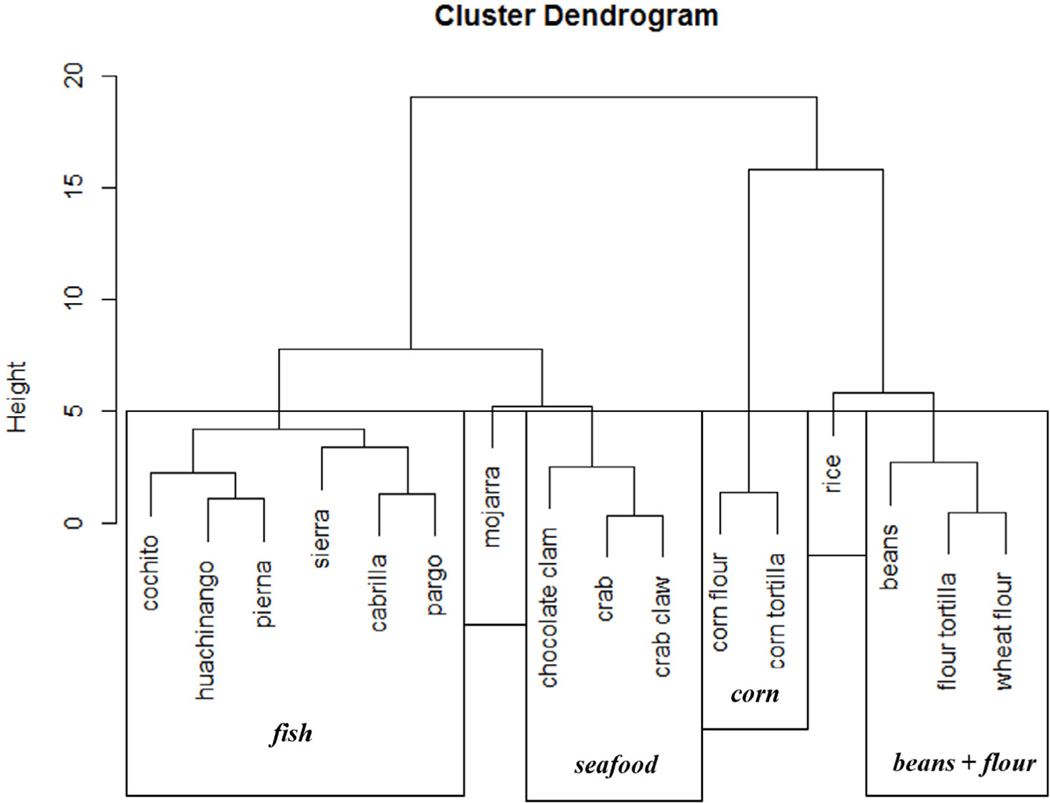
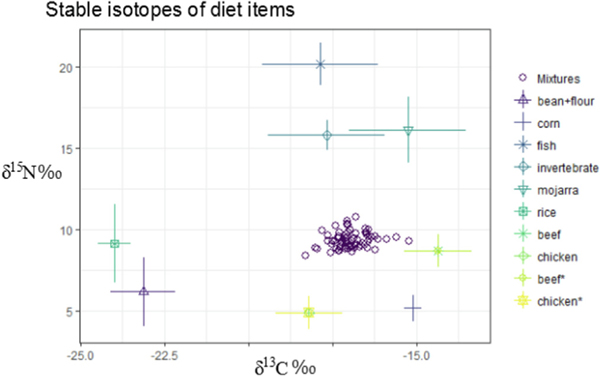
We determined δ15N and δ13C values from each finfish, seafood, and staple food sample analyzed and executed a mixing model to attempt to account for the relative contribution of each diet item in the source. Our initial model contained each fish species (i.e. Table 1) and staple food (rice, corn, beans, etc.) as a separate source. However, this model was inhibited by too many sources due to overlapping stable isotope ranges of many food items. We employed a k-nearest neighbor algorithm (Rosing et al., 1998) to group sources according to similarity in δ15N and δ13C values (Fig. 5) and we identified six discrete groups which were fed back into the SIMM. We restricted our model to six sources rather than further grouping because smaller groups, while potentially mathematically sound, would differ largely from groupings based on ecological and dietary considerations (for instance, mojarra would be inappropriately grouped with crabs and clams). The δ15N and δ13C values (±standard deviation (SD)) for each diet group are presented in Fig. 6. Literature stable isotope values for beef and chicken (from (Jahren and Kraft, 2008)) are included in this figure to display the potential for them to exhibit a corn-based composition, however these values were not generated from our analysis.
Fig. 5.
Results of clustering k-neighbor joining clustering algorithm based on δ15N and δ13C values. Groupings (n = 6) used in further analysis are shown in boxes. δ15N - stable isotope ratio of N, δ13C - stable isotope ratio of C.
Fig. 6.
Average stable isotope values of grouped dietary items (grouped according to k-neighbor clusters presented in Fig. 5). Values for beef and chicken are provided from Jarhen and Kraft (Jahren and Kraft, 2008) to show the corn signature present in these protein sources, however they were not included in the mixing model. Mixtures provided are individual hair samples (n = 70) from pregnant women in La Paz, Baja California, Mexico.
The results of the SIMM output are presented in Table 3. The percent of diet attributed to mojarra (mean proportion = 0.01, SD = 0.01) and fish (mean proportion = 0.02, SD = 0.01) were quite low, while beans + flour and rice make up significant, although somewhat variable, proportions of diet. Corn made up a significant proportion of all participants diet (mean proportion = 0.71, SD = 0.01).
Table 3.
Mean proportions and standard deviations (SD) of items in the diet of all study individuals generated by the mixing model (simmr).
| Food Item | Proportion | SD |
|---|---|---|
|
| ||
| corn | 0.71 | 0.01 |
| rice | 0.15 | 0.04 |
| bean + flour | 0.08 | 0.04 |
| invertebrates | 0.03 | 0.01 |
| fish | 0.02 | 0.01 |
| mojarra | 0.01 | 0.01 |
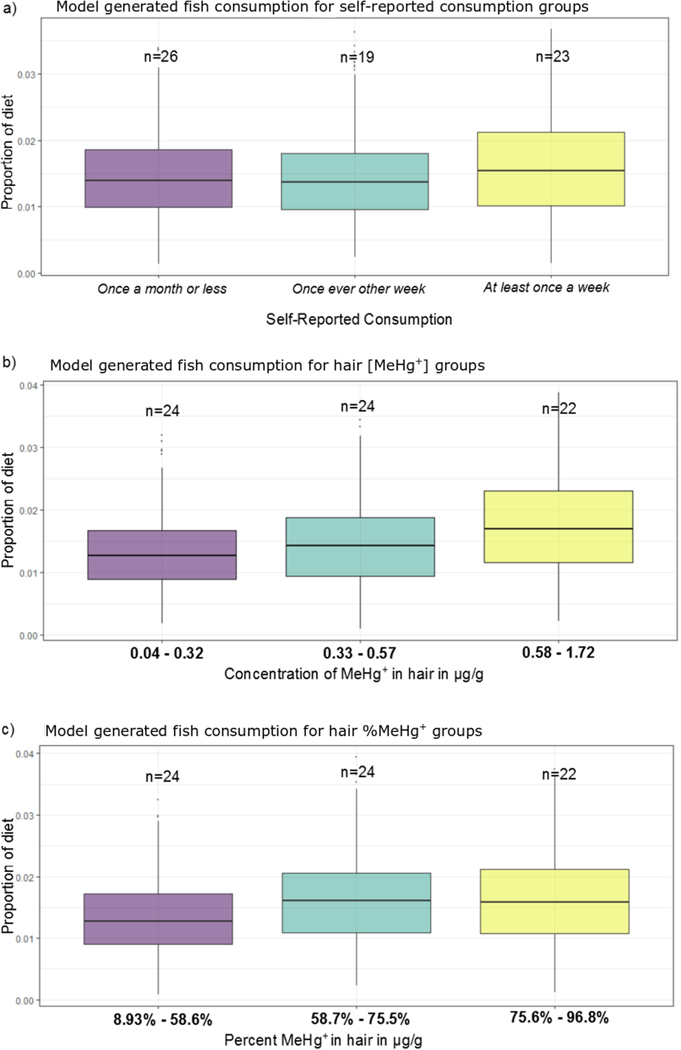
In order to compare our chemical analysis with self-reported consumption habits, we repeated these mixing models separating groups according to their frequency of seafood consumption. We found only small differences in the estimated portions of fish in the diet of each of the three groups (Fig. 7a). Using the results of each model iteration (n = 100,000) each self-reported consumption group was ranked in terms of proportion of fish consumption according to the SIMM (i.e. at least once a week > once every other week > once a month or less) and found that the most frequent consumption group (at least once per week) was selected as the highest fish consumers in 39% of iterations, as compared to 31% and 30% for the other two groups.
Figure 7.
a) simmr model output proportions of fish in the diet of each self-reported fish consumption group, b) model output proportions of fish consumption based on groups according to [MeHg+] in hair, and c) model output proportions for groups based on %MeHg+ in hair. MeHg+ - monomethylmercury.
We also ran our SIMM to compare fish consumption across groups generated according to their [MeHg+] and %MeHg+ in hair (Fig. 7b). Using the three approximately equal sized groupings of participants, we found that the model selected the high [MeHg+] group as the highest fish consumers in approximately 50% of iterations. The model selected the high %MeHg+ group in 43% of iterations (Fig. 7c).
4. Discussion
Hair mercury concentrations in this study were lower than previously reported in Gaxiola-Robles et al. (2014), from the same region and an order of magnitude lower than concentrations in pregnant women from known high fish consumption communities (i.e. the Seychelles (van Wijngaarden et al., 2017)). Concentrations of THg reported here are less than the 95 percentile of frequent fish consumers reported in the National Health and Nutrition Examination Survey (NHANES) study (McDowell et al., 2004), although nearly all (65 out of 70, 96%) of the individuals in this study had hair [THg] higher than the median concentration of all participants in that study.
There are several potential reasons for the different average [THg] hair concentrations between the two BCS-bases studies. Although these were not the same women examined in the previous study (Gaxiola-Robles et al., 2014), they were from a similar area, age, and socio-economic class. One explanation could be a general decrease in fish consumption, although our 15N values did not differ significantly from those of Bentzen et al. (2014). However, a switch in preferred species from one with higher [THg] (i.e. mojarra, elasmobranchs) to one with lower [THg] (i.e. cochito) could explain differences in [THg] that were not associated with changes in 15N values. Although species-specific consumption was not assessed in the previous studies, in our study a large portion of respondents reported consuming pierna (Caulolatilus princeps) (41%) and cochito (Balister polyepis) (30%), two species that we found to have relatively low [THg].
Hair [MeHg+] is not commonly measured, and is generally assumed that the percent MeHg+ of total Hg in hair is at least 80% (Cernichiari et al., 1995). However, to our knowledge there is no standardized method of calculating average %MeHg+, with some reports using simple arithmetic means (Harada et al., 1998), while others have advocated for a robust linear regression (Wagemann et al., 1997) making it difficult to compare results across studies. The median %MeHg+ in our study was 67%, although 17 individuals had %MeHg+ less than 50%. While we support the calculation of the average ratio of MeHg+:THg using methods other than arithmetic mean, we suggest here that there were individuals whose %MeHg+ values differ greatly from the estimate of average %MeHg+ using the slope parameter. Different tissues can have different %MeHg+ related to the protein (cysteine) content, lipid type and content, or in situ demethylation; thus, it is conceivable that hair %MeHg+ could vary from blood or urine (Squadrone et al., 2015). An alternative explanation is that these individuals have exposure to sources of inorganic Hg in addition to MeHg+ through diet or other routes of exposure (e.g., amalgams, dentistry occupation, hair and skin products).
Shallow hydrothermal activity in the Gulf of California discharges water enriched with a number of minerals including Hg, which may contribute to marine inorganic or organic (following methylation) Hg concentrations (Prol-Ledesma et al., 2004). Although other sources of inorganic mercury exposure have been implicated for Mexican citizens including cosmetic and beauty supplies, some of which contain mercury concentrations between 20,000 and 36,000 ppm (Dickenson et al., 2013). Imported cosmetic products were identified as a potential source of inorganic mercury exposure in adults from New York City (McKelvey et al., 2011). It has been suggested that inorganic mercury is absorbed through skin across the epidermis via sweat glands and sebaceous glands (Chan, 2011; Park and Zheng, 2012), which would explain high concentrations of Hg found in urine of patients displaying symptoms of Hg toxicity after self-reported use of a Mexican skin lightning cream (Weldon et al., 2000). Indeed, a study in 2011 found that 6 of 15 creams produced in Mexico had detectable concentrations of Hg, some as high as 36,000 ppm (Peregrino et al., 2011), well above the FDA’s allowable limit of 1 ppm. Although the dermal absorption rate of the Hg (unknown form) from lightning creams is not well described, there is strong evidence that there is at least partial absorption (Clarkson and Magos, 2006; Peregrino et al., 2011) and even poor absorption efficiencies could lead to potentially toxic exposures.
Concentrations of THg and MeHg+ in the chocolate clam were quite low (2−5 ppb), which is consistent with the current concentration reported by the FDA and Atwell et al. (1998). To our knowledge, this is the first report of Hg concentrations in this species from this region. Concentrations of THg and MeHg+ were slightly higher in the crab samples, and although Hg concentrations are not commonly measured in crab species, our values were more or less consistent with previous reports (Burger et al., 2007). One crab sample of both carapace and claw muscle tissue had [THg] and [MeHg+] 5–10 times higher than the other samples (135 [MeHg+] and 112 [THg] ppb). Interestingly, this individual had the lowest 15N and 13C values of the crabs, nearly a full trophic level (3‰) lower than the mean 15N. Crabs and other detritivores are likely exposed to variable concentrations and forms of Hg due to opportunistic feeding on detritus, so variation in [Hg] and C and N stable isotopes are perhaps not unexpected. While it is surprising to see significantly higher concentrations in an individual feeding on a lower trophic level, we will not draw any conclusions from one sample on a poorly studied species. Further analysis of [Hg] in detritivores from the region are warranted, especially given reports of hydrothermal activity in the region (Prol-Ledesma et al., 2004).
Concentrations of Hg in finfish were generally low and based on their C and N stable isotope profiles most appeared to be low trophic level feeders. However, even the higher trophic level species (cabrilla (Mycteroperca rosacea) and sierra (Scomberomorus sierra), mean 15N > 18) had geometric mean concentrations less than 100 ppb. We caution that despite the low concentrations reported here, there could be seasonal or spatial variation in Hg concentrations within a species. More robust sample sizes over larger temporal and spatial settings should be assessed prior to considering consumption recommendations. Of the species mentioned as most frequently consumed in self-reporting, we analyzed 5 of 6 (83%) of finfish species and 2 of 4 (50%) shellfish species. There were a few species that were reported to be consumed by the study participants that were not measured for Hg including shrimp and scallops; however, these items are generally not known to have high [THg]. Some studies from this region have shown high [THg] in sharks and elasmobranchs (Barrera-García et al., 2012; Murillo-Cisneros et al., 2018). These species were not asked about specifically but were not mentioned in self-reporting.
The highest concentrations of THg and MeHg+ in this study were in mojarra (Table 1). This species has been noted to have mean concentrations of 580 ppb ww from the Sinaloa coast of Mexico, a value which is higher than the general guidance level for fish mercury from both the World Health Organization (WHO) and the European Commission (EC) (Ruelas-Inzunza et al., 2008). Another study found mean [THg] in D. peruvianus to be the highest (2556 ppb dry weight) of 19 species analyzed from the Gulf of California (García-Hernández et al., 2007). The [THg] found in D. peruvianus are slightly unusual considering the size and ecology of the species. The mean length of D. peruvianus in this study (16.7 cm, n = 12) was in agreement with previous reports on the species (16.8 cm) (Spanopoulos-Zarco et al., 2015), yet it was notably smaller than other fish species assessed in this study. There are only a few resources regarding dietary assessments for the species; however, most indicate that they are benthic carnivorous or omnivorous fish whose prey include mainly bivalves and marine worms (Phyla Mollusca and Echinodermata), although Lopez-Peralta and Arcila (2002) also note detritus as a dietary component in their stomach content analysis. Further investigations into the feeding ecology, life history, and metabolism of this species is warranted to assess their role in human Hg exposure.
Stable isotopes of C and N from hair samples were analyzed in order to assess potential sources of Hg through diet (Bentzen et al., 2014). Overall, our isotope values were very similar to Bentzen et al. although in this study we did not observe nearly as broad a range in [THg] in human hair (Bentzen et al., 2014). Interestingly, %MeHg+ appeared to be positively associated with 15N values. Bentzen et al. found that 15N values were associated with [THg] that was likely driven by finfish and seafood consumption (Bentzen et al., 2014). We also found a positive association between 15N values and [THg], and a stronger association between 15N and [MeHg+] and % MeHg+. This supports the notion that the seafood and fish are sources of MeHg+ exposure; however, the low %MeHg+ among infrequent fish consumers (15N < 9) could also potentially be explained by these individuals having exposure to Hg (potentially inorganic) via routes other than seafood. The 47% of infrequent fish consumers had one or more dental amalgams, while 26% of moderate consumers and 43% of frequent consumers had amalgams. Dental amalgams have also been known to increase tissular inorganic Hg concentrations, and other studies have found that some of the variation in %MeHg+ can be explained by the presence of dental amalgams (Vieira et al., 2013). In our study, %MeHg+ was indeed significantly lower in individuals with one or more dental amalgams, although there were no significant differences in [THg] or [MeHg+] between those with amalgams and those without. There are numerous factors to consider regarding Hg exposure via dental amalgams including the timing of the procedure as well as the type of amalgam used. It would be worthwhile to design a specific study examining Hg exposure via dental amalgams and those working in the dentistry profession in this population that would need to strategically account for Hg exposure from fish.
Corn has a fairly unique C isotope composition among human staple foods due to its C4 carbon fixation which results in lower δ13C compared to other terrestrial plants (O’Brien, 2015). We note that, while all respondents had significant contribution of corn in their diets, this does not imply that only corn products (i.e. flour) are contributing. Many livestock species including cattle and chicken are fed diets with large proportion of corn, thus their δ13C values are quite similar to corn (Jahren and Kraft, 2008). We did not measure stable isotopes in these sources, although many of the study participants reported frequent consumption of beef, chicken, and pork, which would contribute to corn-like δ13C values we observe here (see Fig. 6).
There are a number of assumptions and sources of error built into SIMMs, and while we attempted to control for most of these, there are a few confounding factors that should be addressed. First, there is evidence that individual variation in uptake and metabolism can lead to variation in C and N stable isotope values in consumers. For instance, Sponheimer et al. found large variation (up to 3‰) in δ15N values in hair of mammals that were fed identical diets (Sponheimer et al., 2003). It is also possible that individuals in this study could have exogenously altered the chemical composition of their hair, i.e. via bleaching or washing. Indeed, a number of samples had evidence of artificial coloration. However, it appears that stable isotope values of human hair are unaffected by shampoo and only slightly affected by aggressive bleaching (O’Connell and Hedges, 1999), thus, we do not see this as a large source of error.
Overall, our SIMM agreed with self-reported fish consumption. We did not see significantly different δ15N values across fish consumption groups; however, using a stable isotope mixing model we found that frequent fish consumers indeed had slightly higher proportions of fish in their diet. Our model also indicated a relatively high proportion of rice in the diet (mean proportion = 0.15) which supports self-reporting in that 80% of respondents reported eating rice more than once a week. The ubiquity of these staple foods, similarity of stable isotope values, and the lack of data concerning portion sizes inhibited a more thorough examination of these staple foods in terms of daily Hg intake. Our model comparing groups based on MeHg+ concentrations and %MeHg+ in hair was more successful at distinguishing the chemical signature of fish consumption, which provides further evidence that chemical analysis of diet is warranted even in circumstances where dietary recall is possible.
5. Conclusion
We have presented [THg] and [MeHg+] in commonly consumed fish species from La Paz, Baja California, Mexico. To our knowledge, this is the first report of Hg in some of these species from this region. We found lower Hg concentrations in hair samples from pregnant women than previous reports (Gaxiola-Robles et al., 2014), which could indicate a change in dietary preference. We show here that chemical analyses and stable isotope modelling are a valuable tool in evaluating human diet and can be used to determine potential sources of contaminants. Further monitoring of human Hg exposure and Hg dynamics in the local environment and biota could provide a more complete picture of Hg exposure for humans in this area.
HIGHLIGHTS.
[THg] in hair (pregnant women) was lower than previous reports, and [THg] in fish were low compared to thresholds.
Stable isotope mixing models reveal limitations in dietary recall studies and the value of chemical analyses.
Percent methylmercury was positively associated with δ15 N values.
Acknowledgements
The authors would like to thank J. Margaret Castellini, Forrest Campnell, and Pablo Hernández-Almaraz for their assistance with this project.
Funding source
This study was carried out with the support of Consejo Nacional de Ciencia y Tecnología (SALUD, 2010-C01-140272 and SALUD, 2015-CO1-261224), SEMARNAT-CONACyT 249423 Centro de Investigaciones Biológicas del Noroeste (PC2.0, PC0.10, PC0.5) and Comisión Nacional de Investigación en Salud IMSS 2016-785-013. J. Harley (graduate student) was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number RL5GM118990. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support for J.Harley was also provided by the UAF Graduate School.
Footnotes
Ethics approval and consent to participate
Hair samples and survey information were collected under the approval of the Comisión Nacional de Investigación Cienti fica del Instituto Mexicano del Seguro Social (permit number 2016–785-013) and Consejo Nacional de Bioetica (CONBIOETICA-09-CEI-009–2016060).
Availability of data and material
The datasets generated and analyzed during the current study are not publicly available due to concurrent parallel studies but are available from the corresponding author on reasonable request.
In Baja California the term marisco, which translates to seafood, describes various invertebrate species such as shrimp, crabs, clams, and scallops.
Conflicts of interest
The authors declare that they have no competing interests.
References
- Atwell L, Hobson KA, Welch HE, 1998. Biomagnification and bioaccumulation of mercury in an arctic marine food web: insights from stable nitrogen isotope analysis. Can. J. Fish. Aquat. Sci 55, 1114–1121. [Google Scholar]
- Barrera-García A, O’Hara T, Galván-Magaña F, Mendéz-Rodríguez LC, Castellini JM, Zenteno-Savín T, 2012. Oxidative stress indicators and trace elements in the blue shark (Prionace glauca) off the east coast of the Mexican Pacific Ocean. Comp. Biochem. Physiol. C Toxicol. Pharmacol 156, 59–66. [DOI] [PubMed] [Google Scholar]
- Bentzen R, Castellini JM, Gaxiola-Robles R, Zenteno-Savín T, Mendéz-Rodríguez LC, O’Hara T, 2014. Relationship between self-reported fish and shellfish consumption, carbon and nitrogen stable isotope values and total mercury concentrations in pregnant women (II) from Baja California Sur, Mexico. Toxicol Rep 1, 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhoft RA, 2012. Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012. 10.1155/2012/460508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom N, 1989. Determination of picogram levels of methylmercury by aqueous phase ethylation, followed by cryogenic gas chromatography with cold vapour atomic fluorescence detection. Can. J. Fish. Aquat. Sci 46, 1131–1140. [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, et al. , 2007. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci. Total Environ 384, 93–105. [DOI] [PubMed] [Google Scholar]
- Caut S, Angulo E, Courchamp F, 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol 46, 443–453. [Google Scholar]
- Ceccatelli S, Daré E, Moors M, 2010. Methylmercury-induced neurotoxicity and apoptosis. Chem. Biol. Interact 188, 301–308. [DOI] [PubMed] [Google Scholar]
- Cernichiari E, Toribara TY, Liang L, Marsh DO, Berlin MW, Myers GJ, et al. , 1995. The biological monitoring of mercury in the Seychelles study. Neurotoxicology 16, 613–628. [PubMed] [Google Scholar]
- Chan TYK, 2011. Inorganic mercury poisoning associated with skin-lightning cosmetic products. Clin. Toxicol 49, 886–891. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, 2006. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol 36, 609–662. [DOI] [PubMed] [Google Scholar]
- Dent F, Clarke S, 2015. State of the Global Market for Shark Products. Fisheries and Aquaculture Technical Paper. Food and Agriculture Organization of the United Nations. [Google Scholar]
- Dickenson CA, Woodruff TJ, Stotland NE, Dobraca D, Das R, 2013. Elevated mercury levels in pregnant woman linked to skin cream from Mexico. Am. J. Obstet. Gynecol 209, e4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufault R, LeBlanc B, Schnoll R, Cornett C, Schweitzer L, Wallinga D, et al. , 2009. Mercury from chlor-alkali plants: measured concentrations in food product sugar. Environ. Health 8, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan JA, Simmer K, Dixon G, Prescott SL, 2008. Cognitive assessment of children at age 2½ years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed 93, F45–F50. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Wiener JG, Eckley CS, Willacker JJ, Evers DC, Marvin-DiPasquale M, et al. , 2016. Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci. Total Environ 568, 1213–1226. [DOI] [PubMed] [Google Scholar]
- Egeland GM, Middaugh JP, 1997. Balancing fish consumption benefits with mercury exposure. Science 278, 1904–5. [DOI] [PubMed] [Google Scholar]
- Erisman B, Mascarenas I, Paredes G, Sadovy de Mitcheson Y, Aburto-Oropeza O, Hastings P, 2010. Seasonal, annual, and long-term trends in commercial fisheries for aggregating reef fishes in the Gulf of California, Mexico. Fish. Res 106, 279–288. [Google Scholar]
- France R, 1995. Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar. Ecol. Prog. Ser 124, 307–312. [Google Scholar]
- García-Hernández J, Cadena-Cárdenas Lázaro, Betancourt-Lozano M, García-De-La-Parra LM, García-Rico L, Márquez-Farías F, 2007. Total mercury content found in edible tissues of top predator fish from the Gulf of California, Mexico. Toxicol. Environ. Chem 89, 507–522. [Google Scholar]
- Gaxiola-Robles R, Zenteno-Savín T, Labrada-Martagón V, Celis de la Rosa A. de J., Acosta Vargas B, Méndez-Rodríguez LC, 2013. Mercury concentration in breast milk of women from northwest Mexico; possible association with diet, tobacco and other maternal factors. Nutr. Hosp 28, 934–942. [DOI] [PubMed] [Google Scholar]
- Gaxiola-Robles R, Bentzen R, Zenteno-Savín T, Labrada-Martagón V, Castellini JM, Celis A, et al. , 2014. Marine diet and tobacco exposure affects mercury concentrations in pregnant women (I) from Baja California Sur, Mexico. Toxicol Rep 1, 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, 1998. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ. Res 77, 165–172. [DOI] [PubMed] [Google Scholar]
- Gribble MO, Karimi R, Feingold BJ, Nyland JF, O’Hara TM, Gladyshev MI, et al. , 2016. Mercury, selenium and fish oils in marine food webs and implications for human health. J. Mar. Biol. Assoc. U. K 96 (01), 43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Nakanishi J, Konuma S, Ohno K, Kimura T, Yamaguchi H, et al. , 1998. The present mercury contents of scalp hair and clinical symptoms in inhabitants of the minamata area. Environ. Res 77, 160–164. [DOI] [PubMed] [Google Scholar]
- Harley J, Lieske C, Bhojwani S, Castellini JM, Lopez JA, O’Hara TM, 2015. Mercury and methylmercury distribution in tissues of sculpins from the Bering Sea. Polar Biol. 38, 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R, Rush E, Aalbersberg W, 2009. Correspondence between human diet, body composition and stable isotopic composition of hair and breath in Fijian villagers. Isot. Environ. Health Stud 45, 1–17. [DOI] [PubMed] [Google Scholar]
- Holá M, Ježek M, Kušta T, Košatová M, 2015. Trophic discrimination factors of stable carbon and nitrogen isotopes in hair of corn fed wild boar. PLoS One 10. 10.1371/journal.pone.0125042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Lin H, Zheng W, Tomanicek SJ, Johs A, Feng X, et al. , 2013. Oxidation and methylation of dissolved elemental mercury by anaerobic bacteria. Nat. Geosci 6, 751–754. [Google Scholar]
- Jahren AH, Kraft RA, 2008. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc. Natl. Acad. Sci. Unit. States Am 105, 17855–17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, 2000. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool 78, 1–27. [Google Scholar]
- Koch PL, Phillips DL, 2002. Incorporating concentration dependence in stable isotope mixing models: a reply to robbins, hilderbrand and farley (2002). Oecologia 133, 14–18. [DOI] [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, Brewer JD, 2011. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci. Int 210, 110–116. [DOI] [PubMed] [Google Scholar]
- Lopez-Peralta RH, Arcila CAT, 2002. Diet composition of fish species from the southern continental shelf of Colombia. Naga Worldfish Cent Q 25, 23–29. [Google Scholar]
- Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Mariën K, et al. , 2011. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr. Rev 69, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez del Rio C, Wolf N, Carleton SA, Gannes LZ, 2009. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev 84, 91–111. [DOI] [PubMed] [Google Scholar]
- McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, et al. , 2004. Hair mercury levels in U.S. Children and women of childbearing age: reference range data from NHANES 1999e2000. Environ. Health Perspect 112, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey W, Jeffery N, Clark N, Kass D, Parsons PJ, 2011. Population-based inorganic mercury biomonitoring and the identification of skin care products as a source of exposure in New York city. Environ. Health Perspect 119, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JW, Semmens BX, 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett 11, 470–480. [DOI] [PubMed] [Google Scholar]
- Murillo-Cisneros DA, O’Hara TM, Castellini JM, Sanchez-Gonzalez A, Elorriaga-Verplancken FR, Marmolejo-Rodríguez AJ, et al. , 2018. Mercury concentrations in three ray species from the Pacific coast of Baja California Sur, Mexico: variations by tissue type, sex and length. Mar. Pollut. Bull 126, 77–85. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. , 2003. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. The Lancet 361, 1686–1692. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Watson GE, van Wijngaarden E, Thurston SW, Strain J, et al. , 2015. Methylmercury exposure and developmental neurotoxicity. Bull. World Health Organ 93, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash SH, Kristal AR, Hopkins SE, Boyer BB, O’Brien DM, 2014. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup’Ik study population. J. Nutr 144, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. , 2008. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 Years in a US cohort. Am. J. Epidemiol 167, 1171e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Rifas-Shiman SL, Amarasiriwardena C, Jayawardene I, Bellinger DC, Hibbeln JR, et al. , 2016. Maternal prenatal fish consumption and cognition in mid childhood: mercury, fatty acids, and selenium. Neurotoxicol. Teratol 57, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SF, Dalby Srensen J, Secher NJ, Hedegaard M, Brink Henriksen T, Hansen HS, et al. , 1992. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. The Lancet 339, 1003–1007. [DOI] [PubMed] [Google Scholar]
- O’Brien DM, 2015. Stable isotope ratios as biomarkers of diet for health Research. Annu. Rev. Nutr 35, 565e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DM, Thummel KE, Bulkow LR, Wang Z, Corbin B, Klejka J, et al. , 2017. Declines in traditional marine food intake and vitamin D levels from the 1960s to present in young Alaska Native women. Publ. Health Nutr 20, 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell TC, Hedges RE, 1999. Investigations into the effect of diet on modern human hair isotopic values. Am. J. Phys. Anthropol 108, 409–425. [DOI] [PubMed] [Google Scholar]
- Park J-D, Zheng W, 2012. Human exposure and health effects of inorganic and elemental mercury, human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health J Prev Med Public Health 45, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell AC, Inger R, Bearhop S, Jackson AL, 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS One 5, e9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell AC, Phillips DL, Bearhop S, Semmens BX, Ward EJ, Moore JW, et al. , 2013. Bayesian stable isotope mixing models. Environmetrics 24, 387–399. [Google Scholar]
- Peregrino CP, Moreno MV, Miranda SV, Rubio AD, Leal LO, 2011. Mercury levels in locally manufactured Mexican skin-lightning creams. Int. J. Environ. Res. Public Health 8, 2516–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, et al. , 2014. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool 92, 823–835. [Google Scholar]
- Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, et al. , 2014. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool 92, 823–835. [Google Scholar]
- Prol-Ledesma RM, Canet C, Torres-Vera MA, Forrest MJ, Armienta MA, 2004. Vent fluid chemistry in Bahía Concepcion coastal submarine hydrothermal system, Baja California Sur, Mexico. J. Volcanol. Geotherm. Res 137, 311–328. [Google Scholar]
- Qiu G, Feng X, Li P, Wang S, Li G, Shang L, et al. , 2008. Methylmercury accumulation in rice (oryza sativa L.) grown at abandoned mercury mines in guizhou, China. J. Agric. Food Chem 56, 2465–2468. [DOI] [PubMed] [Google Scholar]
- R Core Team R, 2017. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. [Google Scholar]
- Rice DC, Schoeny R, Mahaffey K, 2003. Methods and rationale for derivation of a reference dose for methylmercury by the U.S. EPA. Risk Anal 23, 107–115. [DOI] [PubMed] [Google Scholar]
- Rosing MN, Ben-David M, Barry RP, 1998. Analysis of stable isotope data: a K nearest-neighbors randomization test. J. Wildl. Manag 62, 380–388. [Google Scholar]
- Roth JD, Hobson KA, 2000. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: implications for dietary reconstruction. Can. J. Zool 78, 848–852. [Google Scholar]
- Ruelas-Inzunza J, Meza-López G, Péez-Osuna F, 2008. Mercury in fish that are of dietary importance from the coasts of Sinaloa (SE Gulf of California). J. Food Compos. Anal 21, 211–218. [Google Scholar]
- Sakamoto M, Kubota M, Liu XJ, Murata K, Nakai K, Satoh H, 2004. Maternal and fetal mercury and n-3 polyunsaturated fatty acids as a risk and benefit of fish consumption to fetus. Environ. Sci. Technol 38, 3860–3863. [DOI] [PubMed] [Google Scholar]
- Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA, 2014. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull. World Health Organ 92, 254–269F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J-S, Oh K, Kim HC, 2014. Dietary assessment methods in epidemiologic studies, 36. Epidemiol Health. 10.4178/epih/e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanopoulos-Zarco P, Ruelas-Inzunza J, Jara-Marini ME, Meza-Montenegro M, 2015. Bioaccumulation of arsenic and selenium in bycatch fishes Diapterus peruvianus, Pseudupeneus grandisquamis, and Trachinotus kennedyi from shrimp trawling in the continental shelf of Guerrero, Mexico. Environ. Monit. Assess 187, 700. [DOI] [PubMed] [Google Scholar]
- Sponheimer M, Robinson T, Ayliffe L, Roeder B, Hammer J, Passey B, et al. , 2003. Nitrogen isotopes in mammalian herbivores: hair d15N values from a controlled feeding study. Int. J. Osteoarchaeol 13, 80–87. [Google Scholar]
- Squadrone S, Chiaravalle E, Gavinelli S, Monaco G, Rizzi M, Abete MC, 2015. Analysis of mercury and methylmercury concentrations, and selenium:mercury molar ratios for a toxicological assessment of sperm whales (Physeter macrocephalus) in the most recent stranding event along the Adriatic coast (Southern Italy, Mediterranean Sea). Chemosphere 138, 633–641. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Thurston SW, Harrington D, Mulhern MS, McAfee AJ, et al. , 2012. Maternal PUFA status but not prenatal methylmercury exposure is associated with children’s language functions at age five years in the Seychelles. J. Nutr 142, 1943–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VF, Carter A, Davies C, Jackson BP, 2011. c. Anal Methods 3, 1143–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, DiGangi J, Evers DC, Petrlik J, Buck DG, Samanek J, et al. , 2016. Economic implications of mercury exposure in the context of the global mercury treaty: hair mercury levels and estimated lost economic productivity in selected developing countries. J. Environ. Manag 183, 229–235. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden E, Thurston SW, Myers GJ, Strain JJ, Weiss B, Zarcone T, et al. , 2013. Prenatal methyl mercury exposure in relation to neurodevelopment and behavior at 19 years of age in the Seychelles Child Development Study. Neurotoxicol. Teratol 39, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Thurston SW, Myers GJ, Harrington D, Cory-Slechta DA, Strain J, et al. , 2017. Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24 years. Neurotoxicol. Teratol 59, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira SM, de Almeida R, Holanda IBB, Mussy MH, Galvão RCF, Crispim PTB, et al. , 2013. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int. J. Hyg Environ. Health 216, 682–689. [DOI] [PubMed] [Google Scholar]
- Wagemann R, Trebacz E, Hunt R, Boila G, 1997. Percent methylmercury and organic mercury in tissues of marine mammals and fish using different experimental and calculation methods. Environ. Toxicol. Chem 16, 1859–1866. [Google Scholar]
- Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, et al. , 2000. Mercury poisoning associated with a Mexican beauty cream. West. J. Med 173, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2009. ggplot2: Elegant Graphics for Data Analysis. Springer New York, New York. http://had.co.nz/ggplot2/book. [Google Scholar]
- Zhang H, Feng X, Larssen T, Shang L, Li P, 2010. Bioaccumulation of methyl-mercury versus inorganic mercury in rice (oryza sativa L.) grain. Environ. Sci. Technol 44, 4499–4504. [DOI] [PubMed] [Google Scholar]