Abstract
The term neuronutrition has been proposed as part of nutritional neuroscience, studying the effects of various dietary components on behavior and cognition. Other researchers underline that neuronutrition includes the use of various nutrients and diets to prevent and treat neurological disorders. The aim of this narrative review was to explore the current understanding of the term neuronutrition as the key concept for brain health, its potential molecular targets, and perspectives of its nutritional approach to the prevention and treatment of Alzheimer’s and Parkinson’s diseases, multiple sclerosis, anxiety, depressive disorders, migraine, and chronic pain. Neuronutrition can be defined as a part of neuroscience that studies the influence of various aspects of nutrition (nutrients, diet, eating behavior, food environment, etc.) on the development of nervous disorders and includes nutrition, clinical dietetics, and neurology. There is evidence that the neuronutritional approach can influence neuroepigenetic modifications, immunological regulation, metabolic control, and behavioral patterns. The main molecular targets in neuronutrition include neuroinflammation, oxidative/nitrosative stress and mitochondrial dysfunction, gut–brain axis disturbance, and neurotransmitter imbalance. To effectively apply neuronutrition for maintaining brain health, a personalized approach is needed, which includes the adaptation of the scientific findings to the genetic, biochemical, psycho-physiological, and environmental features of each individual.
Keywords: neuronutrition, neurological disorders, neuronutrients, brain health
1. Introduction
Chronic non-infectious diseases remain the leading causes of death and disability worldwide despite the extensive development of innovative pharmaceutical technologies that are generally increasing in frequency and, in many cases, decreasing in latency. Modifiable lifestyle factors play a significant role in the prevention and therapy of these disorders, among which diet and nutritional behavior occupy a special place [1]. Dietary recommendations for corrective eating behavior and nutrient status for gastroenterological and cardiovascular diseases have been developed already [2,3]. Numerous studies show that both neuronutrients and eating behavior, in general, could impact the pathogenesis of neurological disorders and also the cognitive and emotional states of the patients [4,5].
At the same time, researchers note that nutrition in neurology has always been considered narrowly in the context of managing neurological patients with malnutrition, dysphagia [6], and alcohol-related neurological disorders [7]. Additionally, micronutrient deficiencies, particularly B2 and B12 [8,9], iron [10], and copper deficiencies [11], may result in the onset of different neurological symptoms. On the other hand, an excess of micronutrients, such as copper, can lead to the development of other neurological disorders, such as Wilson’s disease [12]. One more notable domain of clinical nutrition in neurology pertains to the application of a ketogenic diet for the management of refractory epilepsy [13] and Glucose transporter type 1 (GLUT1) deficiency syndrome [14].
Another important and well-discussed nutritional aspect in neurology is ischemic stroke prevention, as it has a lot of common risk factors with other cardiovascular disorders, which is not the subject of this review and can be read elsewhere [15,16].
Although research in nutrition science has demonstrated the potential for beneficial effects of selected nutrients and diets on such conditions as depression, anxiety, cognitive decline, and neurodevelopmental disorders [17,18,19], these findings often remain theoretical and have little application in clinical practice. Moreover, there is now an identified need for research to develop practical recommendations on nutrition and the use of neuronutrients in the prevention and treatment of various neurological disorders.
The aim of the narrative review was to explore the state of the art of the term neuronutrition as the key concept for brain health, potential neuronutritional molecular targets, and interventions as an interdisciplinary approach to the prevention and treatment of Alzheimer’s diseases, multiple sclerosis, anxiety and depressive disorders, migraine, and chronic pain.
2. Neuronutrition
Nutrition has traditionally been viewed as a supplier of elements for building and maintaining the human body and as a source of energy for the body’s vital functions. Research in psychoneuroendocrinoimmunology (PNEI) has broadened the horizons of the role of nutrition. According to the PNEI concept, nutrition is a tool with which the environment methodically shapes the metabolome and epigenome, and various nutrients and eating behaviors have a multifaceted effect on self-regulation, metabolism, immune system, and brain function [20].
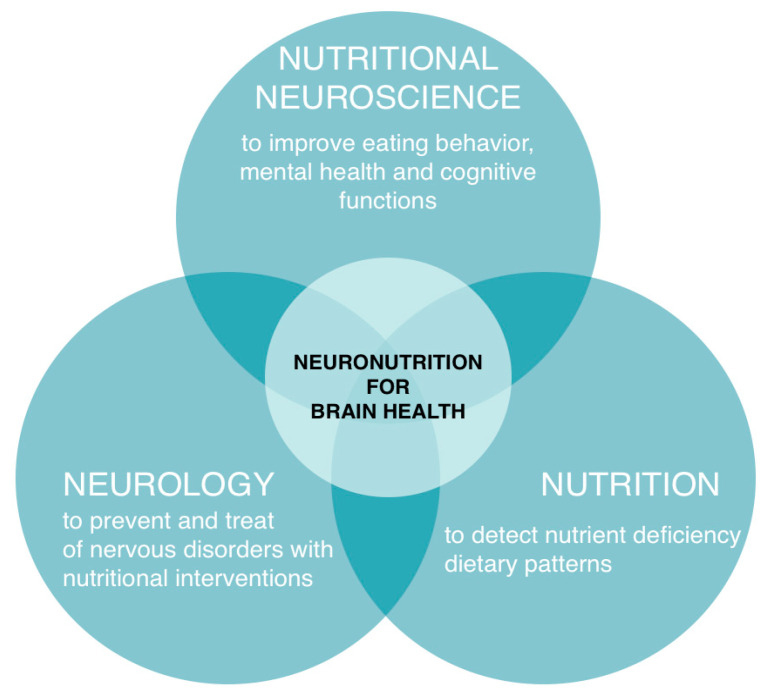
A new scientific field, nutritional neuroscience, which studies the effects of dietary components, such as proteins, carbohydrates, fats, and supplements, including phytonutrients, on the central and peripheral nervous system, neurochemistry, neurobiology, behavior, and cognition, has recently emerged [21]. Some researchers suggest using the term neuronutrition as part of the nutritional neuroscience of maintaining brain health and cognitive function through dietary influence [22]. Other researchers have defined neuronutrition as not only the use of diet but also the use of various nutrients to prevent and treat disorders of the central and peripheral nervous system [23]. The first references to neuronutrition were mentioned in the context of dietary patterns influence on Alzheimer’s disease development [24]. In a broader sense, neuronutrition is an interdisciplinary area that studies the influence of various aspects of nutrition (nutrients, diet, food behavior, food environment, etc.) on brain health [25], prevention, and treatment of neurological disorders across the lifespan (Figure 1).
Figure 1.
Neuronutrition definition. Neuronutrition includes a subset of nutritional neuroscience (approach to improving eating behavior [26], mental health [27], and cognitive functions [28] in healthy and sick individuals), nutrition (approach to detect nutritional status and dietary patterns in patients with neurological disorders [29]), and neurology (approach to prevent and treat neurological disorders with nutritional interventions) [30]).
The future of neuronutrition, as a part of personalized and preventive medicine, is to apply neuronutritional interventions to prevent and treat brain disorders (both neurological and psychiatric), including migraine, chronic pain syndrome, epilepsy, amyotrophic lateral sclerosis, anxiety, and depressive disorders, neurodegenerative diseases (Alzheimer’s, Parkinson’s), autoimmune conditions (multiple sclerosis), and others.
Since brain dysfunction (maladaptive response to stress) contributes to the formation/progression of other chronic diseases (metabolic syndrome, arterial hypertension, irritable bowel syndrome, etc.), the neuronutritional approach may also find applications in the prevention and ancillary treatment of various somatic pathological conditions [31].
3. Neuronutritional Interventions
Neuronutrition includes the use of diets, functional foods (food products with specific nutritional properties), food supplements/nutraceuticals, and medications (nutrients in supradietary doses), for the prevention and treatment of neurological and psychiatric disorders [23].
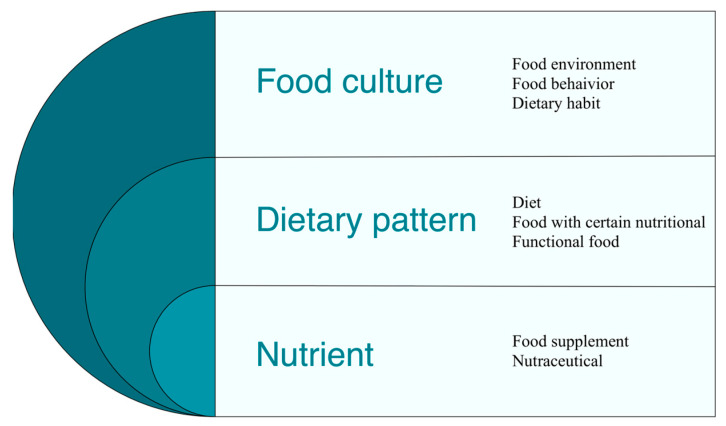
As practice shows, the nervous system state, as well as whole body function, depend on the effect of individual nutrients and diets, which is largely determined by food culture. Hence, studying and forming healthy dietary habits, optimal eating behavior, and a healthy food environment are also among the areas of neuronutrition as a science (Figure 2).
Figure 2.
Neuronutritional interventions can be implemented through the correction of dietary culture, dietary patterns, and nutrient intake to optimize neurological health and prevent/treat neurological disorders.
3.1. Nutrient Interactions
Brain health preservation and neurological disorder prevention are largely associated with the suppression of signaling pathways associated with aging. Phytonutrients, such as the polyphenols apigenin, quercetin, and proanthocyanidins, have been shown to modulate and suppress many of these signaling pathways [32]. Other neuronutrients affect neuroinflammation and oxidative/nitrosative stress and/or modify neurotransmitter chemistry [33,34]
Food is a complex combination of multiple nutrients and anti-nutrients, many of which have been shown to modulate inter alia gene expression and metabolic pathways [35].
Nutraceuticals are food and/or herbal extracts utilized to ameliorate health, delay senescence, prevent diseases, and support the proper functioning of the human body [36]. This definition leads to a partial overlap with the definition of a food supplement; however, while nutraceuticals are made from food or part of a food, food supplements are single substances used alone or in mixtures with the scope of adding micronutrients when the body needs them [37].
Many food supplements and nutraceuticals have been studied in relation to nervous disease treatment and prevention. Magnesium, coenzyme Q10, feverfew, riboflavin, and phycocyanins have shown modest efficacy but a very good safety and tolerability profile in migraine treatment [38]. Diets with a low nutrient density are linked to a higher risk of cognitive decline [39]. Conversely, diets with a higher nutrient density are associated with a nutraceutical component in the Mediterranean diet and are associated with a degree of neuroprotection [40].
Food supplements were initially used to prevent and/or treat deficiencies in some essential micronutrients, thus reducing their adverse health consequences. Nowadays, this practice is more widespread, meaning that adding supplements not only covers the deficit but also helps gain a positive effect on health [41]. Many studies on food supplements’ role in the prevention and treatment of various nervous diseases are being conducted as they are safe but, at the same time, can be efficient in some areas where pharmaceutical pharmacology has been unproductive. Nicotinamide riboside supplementation, for example, was shown to augment the NAD metabolome and induced transcriptional upregulation of processes related to mitochondrial, lysosomal, and proteasomal function in blood cells and/or skeletal muscle and improve some clinical symptoms in patients with Parkinson’s disease [42]. Pro/prebiotics can be useful in Alzheimer’s disease prevention [43].
3.2. Dietary Pattern
A dietary pattern is defined as the amount, proportion, variety, or combination of different foods, drinks, and nutrients in the diet and the frequency of their consumption [44].
Neuronutrition’s aim is to replace maladaptive, unhealthy dietary patterns that increase chronic disease risk development with healthy dietary patterns that promote brain health [45].
According to the neuronutrition concept, the dietary pattern includes functional foods, foods with certain nutritional properties, and specialized diets that have shown effectiveness in maintaining brain health and in the prevention and treatment of neurological disorders.
The antidepressant food rating was developed to identify individual foods with the highest nutrient density for depressive disorder prevention and treatment. The highest-ranking foods were oysters, mussels, leafy greens, peppers, and cruciferous vegetables [46].
Functional foods are novel foods that have been formulated so that they contain substances or live microorganisms that have possible health-enhancing or disease-preventing values and at a concentration that is both safe and sufficiently high to achieve the intended benefit. The added ingredients may include nutrients, dietary fiber, phytochemicals, fatty acids, or probiotics [47]. In Japan, a functional product in the form of yogurt based on beta lactoline that improves memory has been developed. Taking beta-lactoline for 6 weeks improved brain blood circulation, increased concentration, and memory [48].
A growing body of evidence has been accumulated on the protective effects of the Mediterranean diet in neurodegenerative disease prevention [49,50]. Adherence to a calorie-restricted diet was found to improve the quality of life and emotional state of patients with multiple sclerosis [51].
3.3. Food Culture
Food culture is what we do, think, and feel around food as an individual or group within contemporary social and environmental constructs [52]. This part of neuronutrition includes aspects of dietary habits, food behavior, and food environment that affect neurological disorders prevention and treatment.
Dietary habits are habitual decisions of a person or a group of people ranging from the selection of individual foods to methods of cooking and eating [53]. Dietary habit formation involves the reward system of the brain and the nucleus accumbens and other hypothalamic nuclei, which are involved in food consumption motivation, pleasure from food intake, appetite, and satiety [54]. Unhealthy dietary habits, such as regular excessive consumption of refined carbohydrates and inadequate fiber intake, that contribute to hypothalamic dysregulation and damage [55] are risk factors for Alzheimer’s disease [56], Parkinson’s disease [57], and depression [58].
Food behavior is a complex interplay of physiological, psychological, social, and genetic factors that influence meal timing, amount of food consumed, food preferences, and food choices [59]. Regulation of hunger and satiety is controlled by hypothalamic neurons. Their signals are converted into motivated behavior to meet the homeostatic needs of a person [60]. Eating disorders contribute not only to metabolic dysregulation and obesity [61] but also to chronic pain [62] and dementia patients’ condition worsening [63]. Eating disorders, for example, have also been found in patients with migraines, and skipping meals may be an early symptom of an attack rather than a migraine trigger [64].
The food environment includes both urban and domestic environments, in which a person makes the decision about nutrition, as well as healthy and unhealthy foods available in it [65]. The environment has a great influence on food choices, which are largely determined by the context in which they are made. There is evidence that higher access to fast food restaurants near a person’s home has been associated with a higher body mass index [66]. Higher grocery shopping and lower fast food restaurant availability, as well as higher income and college education, have also been found to be independently associated with higher consumption of fresh fruits and vegetables, lower consumption of fast food and soda, and lower risk of being overweight and obese. [67].
Another part of food culture is chrononutrition, a branch of nutritional science focused on studying how nutrients or mealtimes themselves can influence the circadian rhythm system in health and disease [68]. A growing body of evidence suggests that nutrient and food consumption timing can affect circadian rhythms functioning, and circadian rhythms desynchronization can negatively affect the timing and choice of food [69]. Eating at inappropriate times can disrupt circadian rhythm organization and contribute to metabolic dysregulation and chronic disease development [70]; there is a close relationship between human personality, chrononutrition, and cardiometabolic health [71]. Data have also been published on the possibilities of chrononutrition use in medicine, with intermittent fasting improving chronic pain as an example [72].
4. The Molecular Targets of Neuronutrition
The mechanisms underlying the effects of nutrition on the nervous system and neurological diseases are still poorly understood. There is evidence for the effects of such aspects of nutrition as vitamin and mineral intake on the synthesis of neurotrophic factors and neurotransmitters, neuroplasticity, myelination, and microglia activity [73,74].
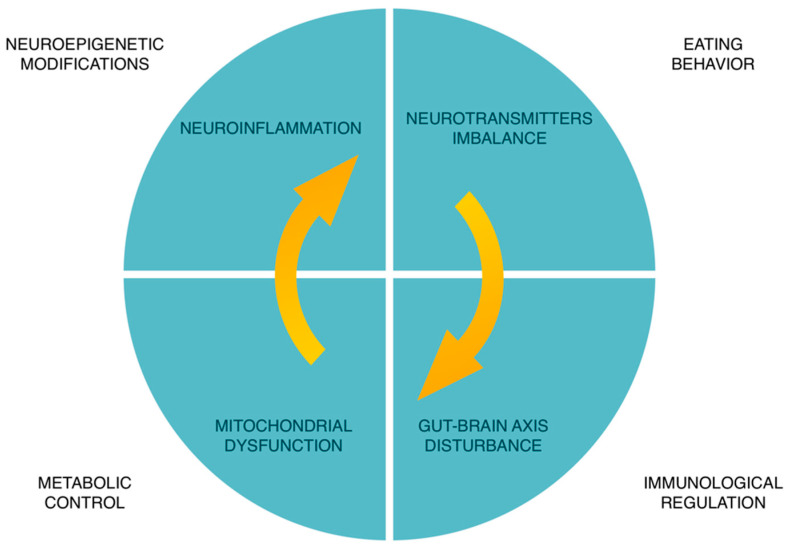
At the moment, it is assumed that neuronutritional interventions can also influence neuroepigenetics modifications, immune regulation, metabolic control, and eating behavior of patients with neurological disorders and brain health [30] (Figure 3).
Figure 3.
Molecular targets of neuronutrition. The main molecular targets in neuronutrition are neuroinflammation, mitochondrial dysfunction, oxidative/nitrosative stress, gut–brain axis disturbance, and neurotransmitters imbalance [75,76].
There are also disease-specific neuronal targets; for example, in migraine, it is the calcitonin gene-related peptide (CGRP) and its receptors [77]; in chronic neuropathic pain—central sensitization, and in nociplastic pain—fatty acid amines [78].
4.1. Neuroepigenetics Modifications
Interactions between nutrition and genes are involved in brain development and function, affecting cell membranes, neurotransmitters, neurogenesis, synaptic plasticity, and metabolism in neurons [79]. Results from studies in the field of neuroepigenetics of nutrition show that diets high in sugar, trans-fats, and methionine cause changes in DNA methylation and histone modifications in brain regions, such as the hypothalamus, hippocampus, striatum, and cortex [80]. Overeating or malnutrition contributes to a chronic stressful environment and leads to neuroepigenetic reprogramming that contributes to cognitive disorders and other degenerative condition development [81]. Nutrition, being a powerful epigenetic regulator, plays an important role in preserving brain health and preventing neurological disorders through gene modification.
4.2. Neuroinflammation
Neuroinflammation is involved in most neurodegenerative processes [82] and pain mechanisms [83] and represents one of the common mechanisms involved in brain aging. Neuroinflammation is characterized by hyperactivation of peripheral glia, including Schwann cells, satellite glial cells in the posterior horn of the spinal cord, and trigeminal nerve ganglia, and central glia, including microglia, astrocytes, and oligodendrocytes in the spinal cord and brain [82]. A diet high in processed foods and saturated and trans fats may contribute to the promotion of low-grade inflammation and increase the risk of the development of non-communicable diseases, including neurological disorders [84].
A prospective cohort study of more than 70,000 participants shows that high consumption of ultra-processed foods was associated with a higher risk of dementia [85].
The same association was found between having depressive symptoms and a high intake of ultra-processed foods among young individuals [86]
Positive effects of nutrition on neuroinflammatory signaling pathways regulation have been found with the consumption of whole plant foods, such as berries, mushrooms, turmeric, and garlic [87]. Interactions between different components of whole foods and plant foods contribute to a synergistic effect for neuroinflammation regulation and possible prevention of neurodegeneration.
4.3. Immunological Regulation: Vitamin D
Vitamin D plays a crucial role in immune system regulation and can impact various neurological conditions via this mechanism. Research indicates that low levels of vitamin D are linked to cognitive decline [88], Parkinson’s disease [89], depression, Alzheimer’s disease [90], and other neurological disorders.
The connection between microbiome and vitamin D is also significant. Studies have shown that vitamin D deficiency and the microbiome can contribute to systemic and chronic inflammation, which, in turn, can increase the risk of neurological conditions development [91].
Given these findings, there is potential for vitamin D supplementation to slow down cognitive decline in Alzheimer’s disease, particularly in its early stages [92]. However, more research is needed to determine the optimal dosage of vitamin D for preventing and treating neurological disorders, as well as its mechanisms of action. Additionally, individual patient characteristics, such as age, gender, presence of some medical conditions, and other factors that may affect vitamin D levels and its impact on the body, must be taken into account.
4.4. Gut–Brain Axis Disturbance
Many neurological diseases, namely, Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and chronic stress, can cause changes in the bidirectional gut–brain axis, leading to abnormalities in both gut function, such as irritable bowel syndrome, and brain function [76]. In addition, dietary regimens, antibiotic intake, and bacterial and viral infections are often associated with altered gut bacterial composition and disruption of the gut–brain axis, which may contribute to the development of neurological diseases [93]. There is evidence that pro-inflammatory gut bacteria, especially Salmonella, Bacillus, Mycobacterium, E. coli, and Staphylococcus, mediated by dysbiosis, may contribute to neuroinflammation in patients with Alzheimer’s disease [94].
Gut microbiota mediators can directly regulate the excitability of primary sensory neurons of the dorsal ganglion of the spinal cord through activation or sensitization of pain-related receptors or ion channels [95]. Consumption of fruits and vegetables stimulates the production of butyrate produced by bacterial fermentation of dietary fiber in the colon, which reduces mucosal inflammation [96]. Increased permeability of the intestinal barrier is observed in the early stages of Parkinson’s disease [97]. A large database has been accumulated on the effectiveness of probiotics in patients with Parkinson’s disease for the treatment of constipation, and new studies on their positive effects on motor and cognitive disorders in such patients are appearing [98].
4.5. Oxidative/Nitrosative Stress and Mitochondrial Dysfunction
In addition to the negative effects of ultra-processed foods on neuroinflammation, a pro-inflammatory diet, including added sugar and saturated fats, may also contribute to oxidative stress and mitochondrial dysfunction [99].
Metabolic changes in the brain are increasingly recognized as key risk factors for the development of cognitive impairment as well as for the chronification of migraine [100]. The main aspects of these changes are energy metabolism, reactive oxygen species metabolism, and lipid metabolism [30]. Oxidative/nitrosative stress is implicated in trauma-induced brain injury, which appears to be increasingly common in contact sports [101]. The prevention and reduction in oxidative/nitrosative stress via inter alia omega-3 PUFA/amphiphilic polyphenol combinations present an intriguing and potentially valuable way forward [102].
Decreased brain energy metabolism includes mitochondrial dysfunction and systemic metabolic dysregulation, such as insulin resistance [103]. Polyphenol resveratrol can stimulate mitochondrial biogenesis and enhance autophagy, contributing to ATP production and restoration of neuronal function [104].
The development of metabolic flexibility for the prevention and therapy of neurological disorders is also promising. Several preclinical studies have already explored the potential of metabolic reprogramming of microglia in diseases, such as Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, and brain aging, by affecting glucose, amino acids, or fatty acids [105]. Clinical studies on the use of nutrients, such as L-carnitine, alpha-lipoic acid, CoQ10, B vitamins, and riboflavin to correct mitochondrial dysfunction have shown their effectiveness in reducing the number and duration of attacks in migraine patients [100].
4.6. Neurotransmitter Imbalance
Neurotransmitter imbalance is observed in patients with Alzheimer’s disease, in which the presence of intracellular neurofibrillary tangles and senile plaques are found, including in neurons that synthesize and use acetylcholine [106]. A decrease in GABA activity has been found in anxiety disorders [107]. In depressive disorders, often associated with many neurological diseases, complex disorders of cholinergic, dopaminergic, and serotonergic transmission have been shown [108]. It is also necessary to consider that high levels of stress contribute to abnormalities in the neurotransmitter system, and as a consequence, to cognitive disorders [109]. It has been shown that nutrition can influence emotional state and cognitive functions depending on the presence of neurotransmitter precursors contained in plant and animal foods [110]. Consumption of GABA-containing tea was found to decrease stress levels in young people while increasing heart rate variability [111].
5. Neuronutrition and Migraine
Recently, there has been growing evidence of neuronutritional interventions to address pathogenetic mechanisms and comorbidity of migraine, a multifactorial disease that is one of the main causes of disability in the adult population worldwide [112]. On the one hand, the well-known tools of neuronutrition for migraine are the correction of eating behavior, including compliance with regular meals, weight management, adequate hydration, and elimination of common food triggers, such as alcohol, coffee, and chocolate. On the other hand, personalization in migraine nutritional management consists of the identification and correction of nutrient deficiencies and the impact of neuroinflammation and mitochondrial dysfunction [113,114].
Correction of metabolic dysregulation in patients with migraine is possible via the modern diagnostic methods of metabolomics and the targeted effect of nutraceuticals. A forward-looking interdisciplinary approach is using continuous glucose monitoring for patients with migraine to apply personal dietary patterns as the key step to developing metabolic flexibility [115].
Current evidence also suggests that the gut–brain axis influences migraine through changes in inflammatory mediators, gut microbiota profile and its metabolites, neuropeptides and serotonin pathway, stress hormones, and nutrients [116]. In addition, neuronutritional interventions have the potential to influence other links in migraine pathogenesis, including serotonergic dysfunction, CGRP levels, nitric oxide, adiponectin and leptin, hypothalamic function, and platelet aggregation [117]. In Table 1, we summarized all relevant information on the neuronutritional approach to preventive migraine management.
Table 1.
Neuronutritional interventions for preventive migraine management.
| The Molecular Target of Neuronutrition |
Neuronutritional Interventions | |
|---|---|---|
| Dietary Patterns | Nutrients | |
| Mitochondrial dysfunction and metabolic control |
Low Glycemic Index Diet [118] Low-fat diet [119] Ketogenic diet [120] EPA 1 + DHA 2 (1.5 g/day) and reduction in omega-6 in the diet [121] |
CoQ10 3 (400 mg/day) [122] - CoQ10 (30 mg/day) + L-carnitine (500 mg/day) [123] Riboflavin (400 mg/day) [124] CoQ10 (150 mg/day), riboflavin (400 mg/day), magnesium (600 mg/day) [125] Omega-3 (EPA (400 mg/day) + DHA (350 mg/day)) [126] |
| Gut–brain axis disturbance |
Elimination diet based on immunological testing (IgG+ products) [127] Gluten-free diet [128] Plant-based diet [129] |
Multispecies probiotics (Bifidobacterium and Lactobacterium) [130] |
| Neuroepigenetics modifications | Epigenetic diet (a diet rich in methyl-donor nutrients) [131] |
B6 (25 mg/day) + B9 (2 mg/day) + B12 (400 mcg/day) [132] Curcumin (1 g/day) [133] |
| CGRP 4 levels and CGRP receptor activity | MIND 5-diet [134] | Ginger extract (600 mg/day) [135] Magnesium citrate (600 mg/day) [136] Vitamin D (2000 IU/day) [137] Melatonin (3 mg/day) [138] |
1 EPA—Eicosapentaenoic acid. 2 DHA—Docosahexaenoic acid. 3 CoQ10—Coenzyme Q10. 4 CGRP—calcitonin gene-related peptide. 5 MIND—Mediterranean–DASH Intervention for Neurodegenerative Delay.
6. Neuronutrition and Alzheimer’s Disease
The pathogenesis of Alzheimer’s disease was found to be related to dietary factors; in particular, excessive saturated fat intake and vitamin E deficiency may contribute to neurodegeneration [139]. A diet low in omega-3 polyunsaturated fatty acids and antioxidants supports neuroinflammation in patients with Alzheimer’s disease and contributes to its progression [140]. In view of the lack of effective drug treatment for Alzheimer’s disease, new therapeutic targets are being actively sought, and mitochondrial dysfunction is one of the promising ones [141]. A ketogenic diet, previously used in the therapy of epilepsy, and antioxidant nutrients can affect mitochondrial dysfunction and improve the cognitive status of patients with Alzheimer’s disease [142]. Such neuronutriton interventions on the cholinergic system as vitamin B12 and folic acid supplementation, have shown effectiveness in improving the cognitive performance of patients with Alzheimer’s disease [143,144]. In Table 2, we have shown the relevant data on the neuronutritional approach to Alzheimer’s disease.
Table 2.
Neuronutritional interventions for treatment of Alzheimer’s disease.
| The Molecular Target of Neuronutrition |
Neuronutritional Interventions | |
|---|---|---|
| Dietary Patterns | Nutrients | |
| Neuroinflammation | Mediterranean diet [145] | Omega-3 fatty acids (2.3 g/day) [146] Correction of vitamin D status [147] Selenium (200 mcg/day) + probiotics (Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum) [148] |
| Mitochondrial dysfunction |
Ketogenic diet [149] Olive oil [150] |
Thiamine (400 mg/day) [151] Alpha-lipoic acid (600 mg/day) + Omega-3 fatty acids (3 g/day) [152] |
| Neurotransmitter imbalance |
MIND 1 diet [153] MCT 2 oil (42 g/day) [154] |
Ginko biloba (240 mg/day) [155] Saffron (30 mg/day) [156] Correction of magnesium deficiency [157] |
1 MIND—Mediterranean–DASH Intervention for Neurodegenerative Delay. 2 MCT—Medium Chain Triglycerides.
7. Neuronutrition and Anxiety and Depressive Disorders
Anxiety and depression are very common disorders, which not only often coexist in one patient but can be confounding factors in many other somatic disorders that can lead to a poor prognosis for the patient [158]. Nutrition plays an important role in prevention and treatment of both anxiety and depression. Diet is a modifiable risk factor for depression; thus, improving diet can reduce the burden of depressive disorders [159]. It has been found that the increase in depressive disorders in recent decades has been paralleled by a decrease in healthy lifestyles, including a deterioration in the quality of diet [160]. Nutrients, including tryptophan, vitamin B6, vitamin B12, folic acid, phenyl-alanine, tyrosine, histidine, choline, and glutamic acid, are essential for the production of neurotransmitters, such as serotonin, dopamine, and noradrenaline, which are involved in regulating neurotransmitters that determine mood, appetite, and cognitive function [161]. Marine omega-3 fatty acids regulate dopaminergic and serotonergic neurotransmission, which can reduce both depression [162] and anxiety [163]. In Table 3, there is a summary of perspective dietary patterns and nutrients that could affect the neuronutrition molecular targets involved in anxiety and depression disorders.
Table 3.
Neuronutritional interventions for management of anxiety and depressive disorders.
| The Molecular Target of Neuronutrition |
Neuronutritional Interventions | |
|---|---|---|
| Dietary Patterns | Nutrients | |
| Neurotransmitter i mbalance |
Modified Mediterranean diet [164] Diet rich in tryptophan (10 mg/kg/day) [165] |
Correction of zinc deficiency [166] Vitamin B6 (80 mg/day) [167] L-theanine (200 mg/day) [168] Magnesium (300 mg/day) + vitamin B6 (30 mg/day) [169] |
| Neuroinflammation | Calorie restriction [170] Mediterranean diet [171] |
Omega-3 fatty acids (DHA 2 (720 mg/day) + EPA 1 (480 mg/day) [172] Correction of vitamin D deficiency [173] |
| Gut–brain axis disturbance |
High intake of dietary fiber [174] |
Probiotics (Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98) [175] Galactooligosaccharides (7.5 g/day) [176] |
1 EPA—Eicosapentaenoic acid. 2 DHA—Docosahexaenoic acid.
8. Conclusions
Neuronutrition is at the intersection of neuronutrtional neuroscience, nutrition, and neurology. In a broader context, it covers the impact of aspects of nutrition (food culture, dietary patterns, nutrients) on brain health at different stages of the life span. Leading molecular targets in neuronutrition are neuroinflammation, mitochondrial dysfunction, neurotransmitter imbalances, and gut–brain axis disturbance. Changing food culture, improving dietary patterns, and the use of selected neuronutrients depending on the specific neuronutritional target is a promising multidisciplinary approach to brain health, prevention, and treatment of neurological disorders. Integrating a neuronutritional approach to the management of migraine, Alzheimer’s disease, anxiety, and depressive disorders can increase the patients’ quality of life and the burden of disease, as confirmed by randomized studies.
To effectively apply neuronutrition in clinical practice, a personalized approach is needed that will cover the genetic, biochemical, psychophysiological, and environmental factors of each patient. Additionally, more studies and clinical evidence are needed to identify individual patient phenotypes, taking into account the neuronutritional targets and such neuronutritional interventions as functional foods, diets, food supplements, and nutraceuticals.
Author Contributions
Conceptualization, A.B.D. and A.V.B.; methodology, A.B.D. and P.C.; writing—original draft preparation, A.V.B., Y.D.V. and V.N.N.; writing—review and editing, A.A.M., A.V.K. and A.F.T.; supervision, P.C.; visualization, V.N.N.; funding acquisition, A.B.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vodovotz Y., Barnard N., Hu F.B., Jakicic J., Lianov L., Loveland D., Buysse D., Szigethy E., Finkel T., Sowa G., et al. Prioritized Research for the Prevention, Treatment, and Reversal of Chronic Disease: Recommendations From the Lifestyle Medicine Research Summit. Front. Med. 2020;7:585744. doi: 10.3389/fmed.2020.585744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein A.H., Appel L.J., Vadiveloo M., Hu F.B., Kris-Etherton P.M., Rebholz C.M., Sacks F.M., Thorndike A.N., Van Horn L., Wylie-Rosett J., et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation. 2021;144:e472–e487. doi: 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 3.Corsello A., Pugliese D., Gasbarrini A., Armuzzi A. Diet and Nutrients in Gastrointestinal Chronic Diseases. Nutrients. 2020;12:2693. doi: 10.3390/nu12092693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira-De-Almeida C.A., Zotarelli-Filho I.J., Nogueira-De-Almeida M.E., Souza C.G., Kemp V.L., Ramos W.S. Neuronutrients And Central Nervous System: A Systematic Review. Central Nerv. Syst. Agents Med. Chem. 2022;23:1–12. doi: 10.2174/1871524923666221121123937. [DOI] [PubMed] [Google Scholar]
- 5.Spencer S.J., Korosi A., Layé S., Shukitt-Hale B., Barrientos R.M. Food for thought: How nutrition impacts cognition and emotion. NPJ Sci. Food. 2017;1:7. doi: 10.1038/s41538-017-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgos R., Bretón I., Cereda E., Desport J.C., Dziewas R., Genton L., Gomes F., Jésus P., Leischker A., Muscaritoli M., et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018;37:354–396. doi: 10.1016/j.clnu.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 7.De La Monte S.M., Kril J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrakumar A., Bhardwaj A., ‘t Jong G.W. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J. Basic Clin. Physiol. Pharmacol. 2018;30:153–162. doi: 10.1515/jbcpp-2018-0075. [DOI] [PubMed] [Google Scholar]
- 9.Calderón-Ospina C.A., Nava-Mesa M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020;26:5–13. doi: 10.1111/cns.13207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benkirane A., Warlop T., Ivanoiu A., Baret P., Wiame E., Haufroid V., Duprez T., Hantson P. Case report: Motor neuron disease phenotype associated with symptomatic copper deficiency: Challenging diagnosis and treatment. Front. Neurol. 2023;13:1063803. doi: 10.3389/fneur.2022.1063803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinke J.S.J., Ziengs A.L., Buunk A.M., van Sonderen L., Gomes-Neto A.W., Berger S.P., Bakker S.J.L., Eisenga M.F., Spikman J.M., De Borst M.H., et al. Nephrology, Dialysis, Transplantation: Official Publication of The European Dialysis and Transplant Association. European Renal Association; Oxford, UK: 2023. Iron Deficiency and Cognitive Functioning in Kidney Transplant Recipients: Findings of the TransplantLines Biobank and Cohort Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penning L.C., Berenguer M., Czlonkowska A., Double K.L., Dusek P., Espinós C., Lutsenko S., Medici V., Papenthin W., Stremmel W., et al. A Century of Progress on Wilson Disease and the Enduring Challenges of Genetics, Diagnosis, and Treatment. Biomedicines. 2023;11:420. doi: 10.3390/biomedicines11020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spence J.D. Nutrition and Risk of Stroke. Nutrients. 2019;11:647. doi: 10.3390/nu11030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarnowska I.M. Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients. 2020;12:2616. doi: 10.3390/nu12092616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwantje M., Verhagen L.M., van Hasselt P.M., Fuchs S.A. Glucose transporter type 1 deficiency syndrome and the ketogenic diet. J. Inherit. Metab. Dis. 2020;43:216–222. doi: 10.1002/jimd.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foroughi M., Akhavanzanjani M., Maghsoudi Z., Ghiasvand R., Khorvash F., Askari G. Stroke and nutrition: A review of studies. Int. J. Prev. Med. 2013;4((Suppl. 2)):S165–S179. [PMC free article] [PubMed] [Google Scholar]
- 17.Su K.-P., Tseng P.-T., Lin P.-Y., Okubo R., Chen T.-Y., Chen Y.-W., Matsuoka Y.J. Association of Use of Omega-3 Polyunsaturated Fatty Acids With Changes in Severity of Anxiety Symptoms: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2018;1:e182327. doi: 10.1001/jamanetworkopen.2018.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markun S., Gravestock I., Jäger L., Rosemann T., Pichierri G., Burgstaller J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients. 2021;13:923. doi: 10.3390/nu13030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph M., Graham A.M., Feczko E., Miranda-Dominguez O., Rasmussen J.M., Nardos R., Entringer S., Wadhwa P.D., Buss C., Fair D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018;21:765–772. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottaccioli F., Bottaccioli A.G., Marzola E., Longo P., Minelli A., Abbate-Daga G. Nutrition, Exercise, and Stress Management for Treatment and Prevention of Psychiatric Disorders. A Narrative Review Psychoneuroendocrineimmunology-Based. Endocrines. 2021;2:226–240. doi: 10.3390/endocrines2030022. [DOI] [Google Scholar]
- 21.Zamroziewicz M.K., Barbey A.K. Nutritional Cognitive Neuroscience: Innovations for Healthy Brain Aging. Front. Neurosci. 2016;10:240. doi: 10.3389/fnins.2016.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devi A., Narayanan R. A Review on Neuronutrition. Asian J. Dairy Food Res. 2019;38:128–133. doi: 10.18805/ajdfr.DR-1454. [DOI] [Google Scholar]
- 23.Topcuoglu M.A., Arsava E.M. Nutrition in Neurologic Disorders. Springer; Berlin/Heidelberg, Germany: 2017. Neuronutrition: An Emerging Concept; pp. 155–206. [DOI] [Google Scholar]
- 24.Ramesh B.N., Rao T.S., Prakasam A., Sambamurti K., Rao K.S. Neuronutrition and Alzheimer’s Disease. NIH Public Access. 2010;19:1123–1139. doi: 10.3233/JAD-2010-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y., Demnitz N., Yamamoto S., Yaffe K., Lawlor B., Leroi I. Defining brain health: A concept analysis. Int. J. Geriatr. Psychiatry. 2021;37:1–13. doi: 10.1002/gps.5564. [DOI] [PubMed] [Google Scholar]
- 26.Giuliani N.R., Merchant J.S., Cosme D., Berkman E.T. Neural predictors of eating behavior and dietary change. Ann. N. Y. Acad. Sci. 2018;1428:208–220. doi: 10.1111/nyas.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grajek M., Krupa-Kotara K., Białek-Dratwa A., Sobczyk K., Grot M., Kowalski O., Staśkiewicz W. Nutrition and mental health: A review of current knowledge about the impact of diet on mental health. Front. Nutr. 2022;9:943998. doi: 10.3389/fnut.2022.943998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalile B., Kim C., Challinor A., Geurts L., Gibney E.R., Galdos M.V., La Fata G., Layé S., Mathers J.C., Vauzour D., et al. The EAT–Lancet reference diet and cognitive function across the life course. Lancet Planet. Health. 2022;6:e749–e759. doi: 10.1016/S2542-5196(22)00123-1. [DOI] [PubMed] [Google Scholar]
- 29.Arumugam A., Thiyagarajan D. Role of Nutrients in Neurological Disorders. Springer; Singapore: 2022. Role of Nutrition in Pathogenesis of Neurological Disorders; pp. 143–158. [DOI] [Google Scholar]
- 30.Mao X.-Y., Yin X.-X., Guan Q.-W., Xia Q.-X., Yang N., Zhou H.-H., Liu Z.-Q., Jin W.-L. Dietary nutrition for neurological disease therapy: Current status and future directions. Pharmacol. Ther. 2021;226:107861. doi: 10.1016/j.pharmthera.2021.107861. [DOI] [PubMed] [Google Scholar]
- 31.Wallace R.L. Integrative and Functional Medical Nutrition Therapy. Humana Press; Totowa, NJ, USA: 2020. Nutrition and Behavioral Health/Mental Health/Neurological Health; pp. 473–492. [DOI] [Google Scholar]
- 32.Satyam S.M., Bairy L.K. Neuronutraceuticals Combating Neuroinflammaging: Molecular Insights and Translational Challenges—A Systematic Review. Nutrients. 2022;14:3029. doi: 10.3390/nu14153029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasmi A., Nasreen A., Menzel A., Benahmed A.G., Pivina L., Noor S., Peana M., Chirumbolo S., Bjørklund G. Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules. 2022;28:210. doi: 10.3390/molecules28010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Michalak M., Agellon L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018;91:95–103. [PMC free article] [PubMed] [Google Scholar]
- 35.Moskalev A. Healthy Ageing and Longevity. Springer Nature; Cham, Switzerland: 2021. Nutritional Regulation of Aging and Longevity; pp. 439–464. [DOI] [Google Scholar]
- 36.Sachdeva V., Roy A., Bharadvaja N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020;21:884–896. doi: 10.2174/1389201021666200130113441. [DOI] [PubMed] [Google Scholar]
- 37.Santini A., Novellino E. To Nutraceuticals and Back: Rethinking a Concept. Foods. 2017;6:74. doi: 10.3390/foods6090074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana S., Russo M., Torelli P. Nutraceuticals and migraine: Further strategy for the treatment of specific conditions. Neurol. Sci. 2022;43:6565–6567. doi: 10.1007/s10072-022-06250-1. [DOI] [PubMed] [Google Scholar]
- 39.Gonçalves N.G., Ferreira N.V., Khandpur N., Steele E.M., Levy R.B., Lotufo P.A., Bensenor I.M., Caramelli P., de Matos S.M.A., Marchioni D.M., et al. Association Between Consumption of Ultraprocessed Foods and Cognitive Decline. JAMA Neurol. 2023;80:142. doi: 10.1001/jamaneurol.2022.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon O.M., Ranson J.M., Gregory S., Macpherson H., Milte C., Lentjes M., Mulligan A., McEvoy C., Griffiths A., Matu J., et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: Findings from the UK Biobank prospective cohort study. BMC Med. 2023;21:81. doi: 10.1186/s12916-023-02772-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Féart C. Dietary Supplements: Which Place between Food and Drugs? Nutrients. 2020;12:204. doi: 10.3390/nu12010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brakedal B., Dölle C., Riemer F., Ma Y., Nido G.S., Skeie G.O., Craven A.R., Schwarzlmüller T., Brekke N., Diab J., et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson’s disease. Cell Metab. 2022;34:396–407.e6. doi: 10.1016/j.cmet.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Pluta R., Ułamek-Kozioł M., Januszewski S., Czuczwar S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging. 2020;12:5539–5550. doi: 10.18632/aging.102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cespedes E.M., Hu F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015;101:899–900. doi: 10.3945/ajcn.115.110213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayedi A., Soltani S., Abdolshahi A., Shab-Bidar S. Healthy and unhealthy dietary patterns and the risk of chronic disease: An umbrella review of meta-analyses of prospective cohort studies. Br. J. Nutr. 2020;124:1133–1144. doi: 10.1017/S0007114520002330. [DOI] [PubMed] [Google Scholar]
- 46.LaChance L.R., Ramsey D. Antidepressant foods: An evidence-based nutrient profiling system for depression. World J. Psychiatry. 2018;8:97–104. doi: 10.5498/wjp.v8.i3.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Temple N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022;9:957516. doi: 10.3389/fnut.2022.957516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ano Y., Kita M., Kobayashi K., Koikeda T., Kawashima R. Effects of β-Lactolin on Regional Cerebral Blood Flow within the Dorsolateral Prefrontal Cortex during Working Memory Task in Healthy Adults: A Randomized Controlled Trial. J. Clin. Med. 2021;10:480. doi: 10.3390/jcm10030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Armeli F., Bonucci A., Maggi E., Pinto A., Businaro R. Mediterranean Diet and Neurodegenerative Diseases: The Neglected Role of Nutrition in the Modulation of the Endocannabinoid System. Biomolecules. 2021;11:790. doi: 10.3390/biom11060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu J., Tan L.-J., Lee J.E., Shin S. Association between the mediterranean diet and cognitive health among healthy adults: A systematic review and meta-analysis. Front. Nutr. 2022;9:946361. doi: 10.3389/fnut.2022.946361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitzgerald K.C., Vizthum D., Henry-Barron B., Schweitzer A., Cassard S.D., Kossoff E., Hartman A.L., Kapogiannis D., Sullivan P., Baer D.J., et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2018;23:33–39. doi: 10.1016/j.msard.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mingay E., Hart M., Yoong S., Hure A. Why We Eat the Way We Do: A Call to Consider Food Culture in Public Health Initiatives. Int. J. Environ. Res. Public Health. 2021;18:11967. doi: 10.3390/ijerph182211967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preedy V.R., Watson R.R. Handbook of Disease Burdens and Quality of Life Measures. Springer; New York, NY, USA: 2010. [DOI] [Google Scholar]
- 54.Meunier N., Briand L., Jacquin-Piques A., Brondel L., Pénicaud L. COVID 19-Induced Smell and Taste Impairments: Putative Impact on Physiology. Front. Physiol. 2021;11:625110. doi: 10.3389/fphys.2020.625110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samodien E., Johnson R., Pheiffer C., Mabasa L., Erasmus M., Louw J., Chellan N. Diet-induced hypothalamic dysfunction and metabolic disease, and the therapeutic potential of polyphenols. Mol. Metab. 2019;27:1–10. doi: 10.1016/j.molmet.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentreau M., Chuy V., Féart C., Samieri C., Ritchie K., Raymond M., Berticat C., Artero S. Refined carbohydrate-rich diet is associated with long-term risk of dementia and Alzheimer’s disease in apolipoprotein E ε4 allele carriers. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2020;16:1043–1053. doi: 10.1002/alz.12114. [DOI] [PubMed] [Google Scholar]
- 57.Boulos C., Yaghi N., El Hayeck R., Heraoui G.N., Fakhoury-Sayegh N. Nutritional Risk Factors, Microbiota and Parkinson’s Disease: What Is the Current Evidence? Nutrients. 2019;11:1896. doi: 10.3390/nu11081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fatahi S., Matin S.S., Sohouli M.H., Găman M.-A., Raee P., Olang B., Kathirgamathamby V., Santos H.O., Guimarães N.S., Shidfar F. Association of dietary fiber and depression symptom: A systematic review and meta-analysis of observational studies. Complement. Ther. Med. 2021;56:102621. doi: 10.1016/j.ctim.2020.102621. [DOI] [PubMed] [Google Scholar]
- 59.Hummel E., Hoffmann I. Complexity of nutritional behavior: Capturing and depicting its interrelated factors in a cause-effect model. Ecol. Food Nutr. 2016;55:241–257. doi: 10.1080/03670244.2015.1129325. [DOI] [PubMed] [Google Scholar]
- 60.Parker J.A., Bloom S.R. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18–30. doi: 10.1016/j.neuropharm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Donofry S.D., Stillman C.M., Erickson K.I. A review of the relationship between eating behavior, obesity and functional brain network organization. Soc. Cogn. Affect. Neurosci. 2020;15:1157–1181. doi: 10.1093/scan/nsz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Y., De Araujo I., Stanley G., Small D., Geha P. Chronic pain precedes disrupted eating behavior in low-back pain patients. PLoS ONE. 2022;17:e0263527. doi: 10.1371/journal.pone.0263527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fostinelli S., De Amicis R., Leone A., Giustizieri V., Binetti G., Bertoli S., Battezzati A., Cappa S.F. Eating Behavior in Aging and Dementia: The Need for a Comprehensive Assessment. Front. Nutr. 2020;7:604488. doi: 10.3389/fnut.2020.604488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martins-Oliveira M., Tavares I., Goadsby P.J. Was it something I ate? Understanding the bidirectional interaction of migraine and appetite neural circuits. Brain Res. 2021;1770:147629. doi: 10.1016/j.brainres.2021.147629. [DOI] [PubMed] [Google Scholar]
- 65.Downs S.M., Ahmed S., Fanzo J., Herforth A. Food Environment Typology: Advancing an Expanded Definition, Framework, and Methodological Approach for Improved Characterization of Wild, Cultivated, and Built Food Environments toward Sustainable Diets. Foods. 2020;9:532. doi: 10.3390/foods9040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atanasova P., Kusuma D., Pineda E., Anjana R.M., De Silva L., Hanif A.A., Hasan M., Hossain M., Indrawansa S., Jayamanne D., et al. Food environments and obesity: A geospatial analysis of the South Asia Biobank, income and sex inequalities. SSM Popul. Health. 2022;17:101055. doi: 10.1016/j.ssmph.2022.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Althoff T., Nilforoshan H., Hua J., Leskovec J. Large-scale diet tracking data reveal disparate associations between food environment and diet. Nat. Commun. 2022;13:267. doi: 10.1038/s41467-021-27522-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flanagan A., Bechtold D.A., Pot G.K., Johnston J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2021;157:53–72. doi: 10.1111/jnc.15246. [DOI] [PubMed] [Google Scholar]
- 69.Crispim C.A., Mota M.C. New perspectives on chrononutrition. Biol. Rhythm. Res. 2019;50:63–77. doi: 10.1080/09291016.2018.1491202. [DOI] [Google Scholar]
- 70.Franzago M., Alessandrelli E., Notarangelo S., Stuppia L., Vitacolonna E. Chrono-Nutrition: Circadian Rhythm and Personalized Nutrition. Int. J. Mol. Sci. 2023;24:2571. doi: 10.3390/ijms24032571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al Abdi T., Andreou E., Papageorgiou A., Heraclides A., Philippou E. Personality, Chrono-nutrition and Cardiometabolic Health: A Narrative Review of the Evidence. Adv. Nutr. Int. Rev. J. 2020;11:1201–1210. doi: 10.1093/advances/nmaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caron J.P., Kreher M.A., Mickle A.M., Wu S., Przkora R., Estores I.M., Sibille K.T. Intermittent Fasting: Potential Utility in the Treatment of Chronic Pain across the Clinical Spectrum. Nutrients. 2022;14:2536. doi: 10.3390/nu14122536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poulose S.M., Miller M.G., Scott T., Shukitt-Hale B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. Int. Rev. J. 2017;8:804–811. doi: 10.3945/an.117.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gómez-Pinilla F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holton K.F. Micronutrients May Be a Unique Weapon Against the Neurotoxic Triad of Excitotoxicity, Oxidative Stress and Neuroinflammation: A Perspective. Front. Neurosci. 2021;15:726457. doi: 10.3389/fnins.2021.726457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suganya K., Koo B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020;21:7551. doi: 10.3390/ijms21207551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Slavin M., Bourguignon J., Jackson K., Orciga M.-A. Impact of Food Components on in vitro Calcitonin Gene-Related Peptide Secretion—A Potential Mechanism for Dietary Influence on Migraine. Nutrients. 2016;8:406. doi: 10.3390/nu8070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nijs J., Elma Ö., Yilmaz S.T., Mullie P., Vanderweeën L., Clarys P., Deliens T., Coppieters I., Weltens N., Van Oudenhove L., et al. Nutritional neurobiology and central nervous system sensitisation: Missing link in a comprehensive treatment for chronic pain? Br. J. Anaesth. 2019;123:539–543. doi: 10.1016/j.bja.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Samonte F.G. The Role of Nutritional Status in Neuroepigenetic Modification. Am. J. Med. Biol. Res. 2017;5:1–8. [Google Scholar]
- 80.Vaziri A., Dus M. Brain on food: The neuroepigenetics of nutrition. Neurochem. Int. 2021;149:105099. doi: 10.1016/j.neuint.2021.105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dauncey M.J. Nutrition, the brain and cognitive decline: Insights from epigenetics. Eur. J. Clin. Nutr. 2014;68:1179–1185. doi: 10.1038/ejcn.2014.173. [DOI] [PubMed] [Google Scholar]
- 82.Kwon H.S., Koh S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mishra A., Bandopadhyay R., Singh P.K., Mishra P.S., Sharma N., Khurana N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021;36:1591–1626. doi: 10.1007/s11011-021-00806-4. [DOI] [PubMed] [Google Scholar]
- 84.Asensi M.T., Napoletano A., Sofi F., Dinu M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients. 2023;15:1546. doi: 10.3390/nu15061546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H., Li S., Yang H., Zhang Y., Zhang S., Ma Y., Hou Y., Zhang X., Niu K., Borné Y., et al. Association of Ultraprocessed Food Consumption With Risk of Dementia: A Prospective Cohort Study. Neurology. 2022;99:e1056–e1066. doi: 10.1212/WNL.0000000000200871. [DOI] [PubMed] [Google Scholar]
- 86.Godos J., Bonaccio M., Al-Qahtani W.H., Marx W., Lane M.M., Leggio G.M., Grosso G. Ultra-Processed Food Consumption and Depressive Symptoms in a Mediterranean Cohort. Nutrients. 2023;15:504. doi: 10.3390/nu15030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang R., Zhu Z., Wu Q., Bekhit A.E.-D.A., Wu S., Chen M., Wang J., Ding Y. Whole-plant foods and their macromolecules: Untapped approaches to modulate neuroinflammation in Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2021;63:2388–2406. doi: 10.1080/10408398.2021.1975093. [DOI] [PubMed] [Google Scholar]
- 88.Balion C., Griffith L.E., Strifler L., Henderson M., Patterson C., Heckman G., Llewellyn D.J., Raina P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pignolo A., Mastrilli S., Davì C., Arnao V., Aridon P., Mendes F.A.D.S., Gagliardo C., D’amelio M. Vitamin D and Parkinson’s Disease. Nutrients. 2022;14:1220. doi: 10.3390/nu14061220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Annweiler C., Rolland Y., Schott A.M., Blain H., Vellas B., Herrmann F.R., Beauchet O. Higher Vitamin D Dietary Intake Is Associated With Lower Risk of Alzheimer’s Disease: A 7-Year Follow-up. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012;67:1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 91.Murdaca G., Gerosa A., Paladin F., Petrocchi L., Banchero S., Gangemi S. Vitamin D and Microbiota: Is There a Link with Allergies? Int. J. Mol. Sci. 2021;22:4288. doi: 10.3390/ijms22084288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murdaca G., Banchero S., Tonacci A., Nencioni A., Monacelli F., Gangemi S. Vitamin D and Folate as Predictors of MMSE in Alzheimer’s Disease: A Machine Learning Analysis. Diagnostics. 2021;11:940. doi: 10.3390/diagnostics11060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rogers G.B., Keating D., Young R., Wong M.-L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 95.Lin B., Wang Y., Zhang P., Yuan Y., Zhang Y., Chen G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain. 2020;21:103. doi: 10.1186/s10194-020-01170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elma Ö., Yilmaz S.T., Deliens T., Coppieters I., Clarys P., Nijs J., Malfliet A. Nutritional factors in chronic musculoskeletal pain: Unravelling the underlying mechanisms. Br. J. Anaesth. 2020;125:e231–e233. doi: 10.1016/j.bja.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 97.Forsyth C.B., Shannon K.M., Kordower J.H., Voigt R.M., Shaikh M., Jaglin J.A., Estes J.D., Dodiya H.B., Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tan A.H., Hor J.W., Chong C.W., Lim S. Probiotics for Parkinson’s disease: Current evidence and future directions. JGH Open Open Access J. Gastroenterol. Hepatol. 2020;5:414–419. doi: 10.1002/jgh3.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang S., Liu H., Li C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods. 2021;10:1854. doi: 10.3390/foods10081854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gross E.C., Lisicki M., Fischer D., Sándor P.S., Schoenen J. The metabolic face of migraine—from pathophysiology to treatment. Nat. Rev. Neurol. 2019;15:627–643. doi: 10.1038/s41582-019-0255-4. [DOI] [PubMed] [Google Scholar]
- 101.Owens T.S., Calverley T.A., Stacey B.S., Iannatelli A., Venables L., Rose G., Fall L., Tsukamoto H., Berg R.M.G., Jones G.L., et al. Contact events in rugby union and the link to reduced cognition: Evidence for impaired redox-regulation of cere-brovascular function. Exp. Physiol. 2021;106:1971–1980. doi: 10.1113/EP089330. [DOI] [PubMed] [Google Scholar]
- 102.Serini S., Calviello G. Reduction of Oxidative/Nitrosative Stress in Brain and its Involvement in the Neuroprotective Effect of n-3 PUFA in Alzheimer’s Disease. Curr. Alzheimer Res. 2016;13:123–134. doi: 10.2174/1567205012666150921101147. [DOI] [PubMed] [Google Scholar]
- 103.Watts M.E., Pocock R., Claudianos C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018;11:216. doi: 10.3389/fnmol.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chuang Y.-C., Chen S.-D., Hsu C.-Y., Chen S.-F., Chen N.-C., Jou S.-B. Resveratrol Promotes Mitochondrial Biogenesis and Protects against Seizure-Induced Neuronal Cell Damage in the Hippocampus Following Status Epilepticus by Activation of the PGC-1α Signaling Pathway. Int. J. Mol. Sci. 2019;20:998. doi: 10.3390/ijms20040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang S., Qin C., Hu Z.-W., Zhou L.-Q., Yu H.-H., Chen M., Bosco D.B., Wang W., Wu L.-J., Tian D.-S. Microglia reprogram metabolic profiles for phenotype and function changes in central nervous system. Neurobiol. Dis. 2021;152:105290. doi: 10.1016/j.nbd.2021.105290. [DOI] [PubMed] [Google Scholar]
- 106.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nuss P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015;11:165–175. doi: 10.2147/NDT.S58841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y., Zhao J., Guo W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018;9:2201. doi: 10.3389/fpsyg.2018.02201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kumar A., Rinwa P., Kaur G., Machawal L. Stress: Neurobiology, consequences and management. J. Pharm. Bioallied Sci. 2013;5:91–97. doi: 10.4103/0975-7406.111818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Briguglio M., Dell’osso B., Panzica G., Malgaroli A., Banfi G., Dina C.Z., Galentino R., Porta M. Dietary Neurotransmitters: A Narrative Review on Current Knowledge. Nutrients. 2018;10:591. doi: 10.3390/nu10050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hinton T., Jelinek H.F., Viengkhou V., Johnston G.A., Matthews S. Effect of GABA-Fortified Oolong Tea on Reducing Stress in a University Student Cohort. Front. Nutr. 2019;6:27. doi: 10.3389/fnut.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khan J., Al Asoom L.I., Al Sunni A., Rafique N., Latif R., Al Saif S., Almandil N.B., Almohazey D., AbdulAzeez S., Borgio J.F. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed. Pharmacother. 2021;139:111557. doi: 10.1016/j.biopha.2021.111557. [DOI] [PubMed] [Google Scholar]
- 113.Del Moro L., Rota E., Pirovano E., Rainero I. Migraine, Brain Glucose Metabolism and the “Neuroenergetic” Hypothesis: A Scoping Review. J. Pain. 2022;23:1294–1317. doi: 10.1016/j.jpain.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 114.Slavin M., Bs H. (.L.; Frankenfeld, C.; Cheskin, L.J. What is Needed for Evidence-Based Dietary Recommendations for Migraine: A Call to Action for Nutrition and Microbiome Research. Headache. 2019;59:1566–1581. doi: 10.1111/head.13658. [DOI] [PubMed] [Google Scholar]
- 115.Schröder T., Kühn G., Kordowski A., Jahromi S.R., Gendolla A., Evers S., Gaul C., Thaçi D., König I.R., Sina C. A Digital Health Application Allowing a Personalized Low-Glycemic Nutrition for the Prophylaxis of Migraine: Proof-of-Concept Data from a Retrospective Cohort Study. J. Clin. Med. 2022;11:1117. doi: 10.3390/jcm11041117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arzani M., On behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS) Jahromi S.R., Ghorbani Z., Vahabizad F., Martelletti P., Ghaemi A., Sacco S., Togha M. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain. 2020;21:1–12. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jahromi S.R., On behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS) Ghorbani Z., Martelletti P., Lampl C., Togha M. Association of diet and headache. J. Headache Pain. 2019;20:106. doi: 10.1186/s10194-019-1057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Evcili G., Utku U., Öğün M.N., Özdemir G. Early and Long Period Follow-up Results of Low-Glycemic Index Diet for Migraine Prophylaxis. J. Turk. Soc. Algol. 2018;30:8–11. doi: 10.5505/agri.2017.62443. [DOI] [PubMed] [Google Scholar]
- 119.Ferrara L.A., Pacioni D., Di Fronzo V., Russo B., Speranza E., Carlino V., Gargiulo F., Ferrara F. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr. Metab. Cardiovasc. Dis. 2015;25:370–375. doi: 10.1016/j.numecd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 120.Valente M., Garbo R., Filippi F., Antonutti A., Ceccarini V., Tereshko Y., Di Lorenzo C., Gigli G.L. Migraine Prevention through Ketogenic Diet: More than Body Mass Composition Changes. J. Clin. Med. 2022;11:4946. doi: 10.3390/jcm11174946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramsden C.E., Zamora D., Faurot K.R., MacIntosh B., Horowitz M., Keyes G.S., Yuan Z.-X., Miller V., Lynch C., Honvoh G., et al. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: Randomized controlled trial. BMJ. 2021;37:n1448. doi: 10.1136/bmj.n1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dahri M., Tarighat-Esfanjani A., Asghari-Jafarabadi M., Hashemilar M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2019;22:607–615. doi: 10.1080/1028415X.2017.1421039. [DOI] [PubMed] [Google Scholar]
- 123.Hajihashemi P., Askari G., Khorvash F., Reza Maracy M., Nourian M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia. 2019;39:648–654. doi: 10.1177/0333102418821661. [DOI] [PubMed] [Google Scholar]
- 124.Rahimdel A., Zeinali A., Yazdian-Anari P. Effectiveness of Vitamin B2 versus Sodium Valproate in Migraine Prophylaxis: A randomized clinical trial. Electron. Physician. 2015;7:1344–1348. doi: 10.14661/1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gaul C., Diener H.-C., Danesch U., on behalf of the Migravent® Study Group Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain. 2015;16:32. doi: 10.1186/s10194-015-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soares A.D.A., Louçana P.M.C., Nasi E.P., Sousa K.M.D.H., Sa O., Silva-Néto R.P. A double- blind, randomized, and placebo-controlled clinical trial with omega-3 polyunsaturated fatty acids (OPFA ɷ-3) for the prevention of migraine in chronic migraine patients using amitriptyline. Nutr. Neurosci. 2018;21:219–223. doi: 10.1080/1028415X.2016.1266133. [DOI] [PubMed] [Google Scholar]
- 127.Mitchell N., Hewitt C.E., Jayakody S., Islam M., Adamson J., Watt I., Torgerson D.J. Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr. J. 2011;10:85. doi: 10.1186/1475-2891-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beuthin J., Veronesi M., Grosberg B., Evans R.W. Gluten-Free Diet and Migraine. Headache. 2020;60:2526–2529. doi: 10.1111/head.13993. [DOI] [PubMed] [Google Scholar]
- 129.Perzia B.M., Dunaief J.L., Dunaief D.M. Chronic migraine reversal and prevention with the LIFE diet: A nutrient dense whole food plant-based diet (WFPBD) BMJ Case Rep. 2021;14:e243987. doi: 10.1136/bcr-2021-243987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martami F., Togha M., Seifishahpar M., Ghorbani Z., Ansari H., Karimi T., Jahromi S.R. The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia Int. J. Headache. 2019;39:841–853. doi: 10.1177/0333102418820102. [DOI] [PubMed] [Google Scholar]
- 131.Fila M., Chojnacki C., Chojnacki J., Blasiak J. Is an “Epigenetic Diet” for Migraines Justified? The Case of Folate and DNA Methylation. Nutrients. 2019;11:2763. doi: 10.3390/nu11112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Menon S., Nasir B., Avgan N., Ghassabian S., Oliver C., Lea R., Smith M., Griffiths L. The effect of 1 mg folic acid supplementation on clinical outcomes in female migraine with aura patients. J. Headache Pain. 2016;17:60. doi: 10.1186/s10194-016-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rezaie S., Askari G., Khorvash F., Tarrahi M.J., Amani R. Effects of curcumin supplementation on clinical fea-tures and inflammation, in migraine patients: A double-blind controlled, placebo randomized clinical trial. Int. J. Prev. Med. 2021;12:161. doi: 10.4103/ijpvm.IJPVM_405_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Askarpour M., Yarizadeh H., Sheikhi A., Khorsha F., Mirzaei K. Associations between adherence to MIND diet and severity, duration and frequency of migraine headaches among migraine patients. BMC Res. Notes. 2020;13:1–6. doi: 10.1186/s13104-020-05181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen L., Cai Z. The efficacy of ginger for the treatment of migraine: A meta-analysis of randomized controlled studies. Am. J. Emerg. Med. 2021;46:567–571. doi: 10.1016/j.ajem.2020.11.030. [DOI] [PubMed] [Google Scholar]
- 136.Köseoglu E., Talaslioglu A., Gönül A.S., Kula M. The effects of magnesium prophylaxis in migraine without aura. Magnes. Res. 2008;21:101–108. [PubMed] [Google Scholar]
- 137.Ghorbani Z., Rafiee P., Fotouhi A., Haghighi S., Magham R.R., Ahmadi Z.S., Djalali M., Zareei M., Jahromi S.R., Shahemi S., et al. The effects of vitamin D supplementation on interictal serum levels of calcitonin gene-related peptide (CGRP) in episodic migraine patients: Post hoc analysis of a randomized double-blind placebo-controlled trial. J. Headache Pain. 2020;21:22. doi: 10.1186/s10194-020-01090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ebrahimi-Monfared M., Sharafkhah M., Abdolrazaghnejad A., Mohammadbeigi A., Faraji F. Use of melatonin versus valproic acid in prophylaxis of migraine patients: A double-blind randomized clinical trial. Restor. Neurol. Neurosci. 2017;35:385–393. doi: 10.3233/RNN-160704. [DOI] [PubMed] [Google Scholar]
- 139.Giles C., Takechi R., Mellett N.A., Meikle P.J., Dhaliwal S., Mamo J.C. The Effects of Long-Term Saturated Fat Enriched Diets on the Brain Lipidome. PLoS ONE. 2016;11:e0166964. doi: 10.1371/journal.pone.0166964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Devassy J.G., Leng S., Gabbs M., Monirujjaman M., Aukema H.M. Omega-3 Polyunsaturated Fatty Acids and Oxylipins in Neuroinflammation and Management of Alzheimer Disease. Adv. Nutr. Int. Rev. J. 2016;7:905–916. doi: 10.3945/an.116.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Misrani A., Tabassum S., Yang L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021;13:617588. doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tabaie E.A., Reddy A.J., Brahmbhatt H., Psychiatry M.G.H. A narrative review on the effects of a ketogenic diet on patients with Alzheimer’s disease. AIMS Public Health. 2021;9:185–193. doi: 10.3934/publichealth.2022014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.El-Mezayen N.S., el Moneim R.A., El-Rewini S.H. Vitamin B12 as a cholinergic system modulator and blood brain barrier integrity restorer in Alzheimer’s disease. Eur. J. Pharm. Sci. 2022;174 doi: 10.1016/j.ejps.2022.106201. [DOI] [PubMed] [Google Scholar]
- 144.Chen H., Liu S., Ge B., Zhou D., Li M., Li W., Ma F., Liu Z., Ji Y., Huang G. Effects of Folic Acid and Vitamin B12 Supplementation on Cognitive Impairment and Inflammation in Patients with Alzheimer’s Disease: A Randomized, Single-Blinded, Placebo-Controlled Trial. J. Prev. Alzheimer’s Dis. 2021;8:249–256. doi: 10.14283/jpad.2021.22. [DOI] [PubMed] [Google Scholar]
- 145.Ballarini T., van Lent D.M., Brunner J., Schröder A., Wolfsgruber S., Altenstein S., Brosseron F., Buerger K., Dechent P., Dobisch L., et al. Mediterranean Diet, Alzheimer Disease Biomarkers, and Brain Atrophy in Old Age. Neurology. 2021;96:e2920–e2932. doi: 10.1212/WNL.0000000000012067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tofiq A., Zetterberg H., Blennow K., Basun H., Cederholm T., Eriksdotter M., Faxén-Irving G., Hjorth E., Jernerén F., Schultzberg M., et al. Effects of Peroral Omega-3 Fatty Acid Supplementation on Cerebrospinal Fluid Biomarkers in Patients with Alzheimer’s Disease: A Randomized Controlled Trial—The OmegAD Study. J. Alzheimer’s Dis. 2021;83:1291–1301. doi: 10.3233/JAD-210007. [DOI] [PubMed] [Google Scholar]
- 147.Yang K., Chen J., Li X., Zhou Y. Vitamin D concentration and risk of Alzheimer disease: A meta-analysis of prospective cohort studies. Medicine. 2019;98:e16804. doi: 10.1097/MD.0000000000016804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tamtaji O.R., Heidari-Soureshjani R., Mirhosseini N., Kouchaki E., Bahmani F., Aghadavod E., Tajabadi-Ebrahimi M., Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019;38:2569–2575. doi: 10.1016/j.clnu.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 149.Phillips M.C.L., Deprez L.M., Mortimer G.M.N., Murtagh D.K.J., McCoy S., Mylchreest R., Gilbertson L.J., Clark K.M., Simpson P.V., McManus E.J., et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021;13:51. doi: 10.1186/s13195-021-00783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rigacci S. Olive Oil Phenols as Promising Multi-targeting Agents Against Alzheimer’s Disease. Adv. Exp. Med. Biol. 2015;863:1–20. doi: 10.1007/978-3-319-18365-7_1. [DOI] [PubMed] [Google Scholar]
- 151.Gibson G.E., Luchsinger J.A., Cirio R., Chen H., Franchino-Elder J., Hirsch J.A., Bettendorff L., Chen Z., Flowers S.A., Gerber L.M., et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimer’s Dis. 2020;78:989–1010. doi: 10.3233/JAD-200896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shinto L., Quinn J., Montine T., Dodge H.H., Woodward W., Baldauf-Wagner S., Waichunas D., Bumgarner L., Bourdette D., Silbert L., et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014;38:111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dhana K., James B.D., Agarwal P., Aggarwal N.T., Cherian L.J., Leurgans S.E., Barnes L.L., Bennett D.A., Schneider J.A. MIND Diet, Common Brain Pathologies, and Cognition in Community-Dwelling Older Adults. J. Alzheimer’s Dis. 2021;83:683–692. doi: 10.3233/JAD-210107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Juby A.G., Blackburn T.E., Mager D.R. Use of medium chain triglyceride (MCT) oil in subjects with Alzheimer’s disease: A randomized, double-blind, placebo-controlled, crossover study, with an open-label extension. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2022;8:e12259. doi: 10.1002/trc2.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xie L., Zhu Q., Lu J. Can We Use Ginkgo biloba Extract to Treat Alzheimer’s Disease? Lessons from Preclinical and Clinical Studies. Cells. 2022;11:479. doi: 10.3390/cells11030479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Akhondzadeh S., Sabet M.S., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 157.Du K., Zheng X., Ma Z.-T., Lv J.-Y., Jiang W.-J., Liu M.-Y. Association of Circulating Magnesium Levels in Patients With Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022;13:799824. doi: 10.3389/fnagi.2021.799824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Salcedo B. National Alliance on Mental Illness. NAMI; Arlington, VA, USA: 2018. The Comorbidity of Anxiety And Depression; pp. 19325–19333. [Google Scholar]
- 159.Kris-Etherton P.M., Petersen K.S., Hibbeln J.R., Hurley D., Kolick V., Peoples S., Rodriguez N., Woodward-Lopez G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021;79:247–260. doi: 10.1093/nutrit/nuaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 161.Sarris J., Logan A.C., Akbaraly T.N., Amminger G.P., Balanzá-Martínez V., Freeman M.P., Hibbeln J., Matsuoka Y., Mischoulon D., Mizoue T., et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]
- 162.Lin P.-Y., Huang S.-Y., Su K.-P. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol. Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 163.Grosso G., Pajak A., Marventano S., Castellano S., Galvano F., Bucolo C., Drago F., Caraci F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jacka F.N., O’neil A., Opie R., Itsiopoulos C., Cotton S., Mohebbi M., Castle D., Dash S., Mihalopoulos C., Chatterton M.L., et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial) BMC Med. 2017;15:23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoepner C.T., McIntyre R.S., Papakostas G.I. Impact of Supplementation and Nutritional Interventions on Pathogenic Processes of Mood Disorders: A Review of the Evidence. Nutrients. 2021;13:767. doi: 10.3390/nu13030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Anbari-Nogyni Z., Bidaki R., Madadizadeh F., Sangsefidi Z.S., Fallahzadeh H., Karimi-Nazari E., Nadjarzadeh A. Relationship of zinc status with depression and anxiety among elderly population. Clin. Nutr. ESPEN. 2020;37:233–239. doi: 10.1016/j.clnesp.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 167.Khodadad M., Bahadoran P., Kheirabadi G.R., Sabzghabaee A.M. Can Vitamin B6 Help to Prevent Postpartum Depression? A Randomized Controlled Trial. Int. J. Prev. Med. 2021;12:136. doi: 10.4103/ijpvm.IJPVM_240_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hidese S., Ogawa S., Ota M., Ishida I., Yasukawa Z., Ozeki M., Kunugi H. Effects of L-Theanine Administration on Stress-Related Symptoms and Cognitive Functions in Healthy Adults: A Randomized Controlled Trial. Nutrients. 2019;11:2362. doi: 10.3390/nu11102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Noah L., Dye L., De Fer B.B., Mazur A., Pickering G., Pouteau E. Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post-hoc analysis of a randomised controlled trial. Stress Health J. Int. Soc. Investig. Stress. 2021;37:1000–1009. doi: 10.1002/smi.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Manchishi S.M., Cui R.J., Zou X.H., Cheng Z.Q., Li B.J. Effect of caloric restriction on depression. J. Cell. Mol. Med. 2018;22:2528–2535. doi: 10.1111/jcmm.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bizzozero-Peroni B., Godoy-Cumillaf A., Fernández-Rodríguez R., Rodríguez-Gutiérrez E., Jiménez-López E., Giakoni-Ramírez F., Duclos-Bastías D., Mesas A.E. Mediterranean Diet Interventions for Depressive Symptoms in Adults with Depressive Disorders: A Protocol for a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2022;19:14437. doi: 10.3390/ijerph192114437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ravi S., Khalili H., Abbasian L., Arbabi M., Ghaeli P. Effect of Omega-3 Fatty Acids on Depressive Symptoms in HIV-Positive Individuals. Ann. Pharmacother. 2016;50:797–807. doi: 10.1177/1060028016656017. [DOI] [PubMed] [Google Scholar]
- 173.Eid A., Khoja S., AlGhamdi S., Alsufiani H., Alzeben F., Alhejaili N., Tayeb H.O., Tarazi F.I. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD) Metab. Brain Dis. 2019;34:1781–1786. doi: 10.1007/s11011-019-00486-1. [DOI] [PubMed] [Google Scholar]
- 174.Saghafian F., Hajishafiee M., Rouhani P., Saneei P. Dietary fiber intake, depression, and anxiety: A systematic review and meta-analysis of epidemiologic studies. Nutr. Neurosci. 2022;26:108–126. doi: 10.1080/1028415X.2021.2020403. [DOI] [PubMed] [Google Scholar]
- 175.Lee H.J., Hong J.K., Kim J.-K., Kim D.-H., Jang S.W., Han S.-W., Yoon I.-Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2021;13:2660. doi: 10.3390/nu13082660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Johnstone N., Milesi C., Burn O., van den Bogert B., Nauta A., Hart K., Sowden P., Burnet P.W.J., Kadosh K.C. Anxiolytic effects of a galacto-oligosaccharides prebiotic in healthy females (18–25 years) with corresponding changes in gut bacterial composition. Sci. Rep. 2021;11:8302. doi: 10.1038/s41598-021-87865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]