Abstract
Cardiometabolic diseases and abnormalities have recently emerged as independent risk factors of coronavirus disease 2019 (COVID-19) severity, including hospitalizations, invasive mechanical ventilation, and mortality. Determining whether and how this observation translates to more effective long-term pandemic mitigation strategies remains a challenge due to key research gaps. Specific pathways by which cardiometabolic pathophysiology affects humoral immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and vice versa, remain unclear. This review summarizes current evidence of the bidirectional influences between cardiometabolic diseases (diabetes, adiposity, hypertension, CVDs) and SARS-CoV-2 antibodies induced from infection and vaccination based on human studies. Ninety-two studies among >408,000 participants in 37 countries on 5 continents (Europe, Asia, Africa, and North and South America) were included in this review. Obesity was associated with higher neutralizing antibody titers following SARS-CoV-2 infection. Most studies conducted prior to vaccinations found positive or null associations between binding antibodies (levels, seropositivity) and diabetes; after vaccinations, antibody responses did not differ by diabetes. Hypertension and CVDs were not associated with SARS-CoV-2 antibodies. Findings underscore the importance of elucidating the extent that tailored recommendations for COVID-19 prevention, vaccination effectiveness, screening, and diagnoses among people with obesity could reduce disease burden caused by SARS-CoV-2.
Keywords: SARS-CoV-2, antibodies, diabetes, obesity, cardiovascular diseases
Statement of Significance.
A growing body of evidence indicates that obesity and related cardiometabolic abnormalities are independent risk factors of COVID-19 severity; however, the specific mechanisms underlying these associations and robust subclinical correlates of protection remain unclear. To shed light on this major research gap, we systematically reviewed the current state of the evidence regarding the bidirectional influences between cardiometabolic diseases and SARS-CoV-2 antibodies induced from previous infection and vaccination.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is estimated to have caused >14.9 million excess deaths globally in 2020 and 2021 [1,2]. Designing more effective long-term pandemic mitigation strategies remains elusive given critical research gaps regarding which individuals are at elevated risk for COVID-19 severity, postacute sequelae of COVID-19, and vaccine breakthrough infections [3,4].
Cardiometabolic Diseases Are a Major Risk Factor for Greater COVID-19 Severity
There is growing evidence that cardiometabolic diseases (CMDs) and abnormalities (hyperglycemia, hyperinsulinemia, endothelial dysfunction) are independent risk factors of COVID-19 severity, including hospitalizations, intubations, invasive mechanical ventilation, complications, and deaths [[3], [4], [5], [6], [7], [8]]. A population study among >61 million UK participants showed that the odds of COVID-19 mortality during hospitalization were greater among those with type 1 diabetes (adjusted odds ratio [aOR]: 3.5; 95% CI: 3.2, 3.9) and type 2 diabetes (aOR: 2.0; 95% CI: 2.0, 2.1), relative to among those without diabetes [7]. One US study estimated that 63.5% of COVID-19 hospitalizations were attributable to obesity, diabetes mellitus (DM), hypertension, and heart failure [9]. Mendelian randomization studies have demonstrated that BMI is linked with COVID-19 severity [[10], [11], [12], [13]]. Moreover, the association between BMI and COVID-19 severity has been characterized as J-shaped in 3 large cohorts with a combined total of >28.5 million participants [[14], [15], [16]]. These prior studies highlight the potential for more effective and frequent screening for cardiometabolic risk as well as management of diabetes and hypertension through pharmacologic and nonpharmacologic approaches to reduce disease burden caused by SARS-CoV-2.
Large cohort studies have demonstrated a higher risk of incident diabetes and abnormal glycometabolism during the postacute phase of SARS-CoV-2 infection [[17], [18], [19]]. Putative mechanisms from experimental studies include the ability of SARS-CoV-2 to disrupt signaling pathways of insulin and insulin-like growth factor in metabolic tissues (adipose, hepatic) and pancreatic β cells [20,21]. A fundamental research gap is determining the underlying etiology that explains these observed bidirectional links between COVID-19 severity and CMD comorbidities. This unresolved question precludes the translation of initial evidence to specific recommendations that improve COVID-19 and CMD mitigation strategies. Key examples include tailored COVID-19 clinical management and vaccination guidelines among people with CMDs and monitoring for CMD indicators following acute SARS-CoV-2 infection.
Host Humoral Immune Dysregulation Caused by SARS-CoV-2: Exacerbations by Cardiometabolic Disease Status?
The interconnectedness of human immunologic and metabolic systems is evolutionarily conserved in invertebrate model organisms [22]. Diabetes, obesity, and cardiovascular comorbidities have bidirectional linkages with immune responses, which have been characterized by numerous previous studies [23, 24].
SARS-CoV-2 neutralization via host humoral immune response
SARS-CoV-2 infection and COVID-19 vaccines induce robust humoral immune responses among most immunocompetent individuals [25,26]. Severe and mild SARS-CoV-2 infections induce extrafollicular and germinal center B cell responses, resulting in increased antibody titers with viral neutralization capacity [[25], [26], [27]]. Spike glycoproteins, comprised of S1 and S2 subunits, are structural proteins that allow binding between ACE2 receptors on host cells and SARS-CoV-2, leading to subsequent membrane fusion and viral entry [26]. SARS-CoV-2 spike protein is the target antigen of most COVID-19 vaccines [26]. Nucleocapsid proteins have essential functions including packaging viral RNA, which is necessary for viral replication and proliferation [28]. The wide-ranging spectrum of protective immunity against SARS-CoV-2 by antibodies induced by natural infection and vaccination remain incompletely understood and are under active investigation [25,26,29,30].
Elevated proinflammatory response and metaflammation
Many individuals with excess adiposity or abnormal glucose–insulin homeostasis have chronic, low-grade systemic inflammation (Figures 1 and 2) [3]. Higher body mass index is correlated with adipose tissue dysregulation [31], increased adipocyte size parameters [32], the accumulation and proinflammatory (M1) polarization of macrophages in adipose tissues [33,34]. Macrophage–adipocyte cross-talk leads to further alterations of adipose tissue function and proinflammatory macrophage activation [35]. Metaflammation, defined as chronic metabolic inflammation [22], includes proinflammatory cytokines and signaling networks such as Type I IFN signaling.
FIGURE 1.
Underlying biological rationale for potential impacts of adiposity on humoral immune response against SARS-CoV-2. Elevated body mass index is associated with adipose tissue dysregulation [31], increased adipocyte size [32], as well as the increased infiltration and proinflammatory polarization of macrophages [33, 34]. Obesity induces toll-like-receptor signaling pathways, which subsequently upregulate Type I interferon (IFN) signaling and other proinflammatory responses. Obesity-associated chronic low-grade inflammation has been shown to reprogram adipocytes and immune cells, including by altered gene expression [33], resulting in higher proinflammatory cytokine production. Since Type I IFN is a key innate immune response against SARS-CoV-2 [36], it is hypothesized that underlying obesity triggers an excessive proinflammation response during SARS-CoV-2 infection, which results in greater local tissue damage, COVID-19 severity, and elevated neutralizing antibodies. BMI, body mass index; COVID-19, coronavirus disease 2019; IFN, interferon; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 2.
Putative biological rationale explaining bidirectional effects of glucose–insulin metabolism dysregulation and humoral immune response against SARS-CoV-2. Among individuals with uncontrolled diabetes, hyperglycemia and dysregulated glucose–insulin metabolism can lead to elevated proinflammatory mediators. Based on aging and geriatric studies, chronic proinflammation could accelerate physiologic aging of B cells, resulting in reduced functional capacity to produce high-affinity protective antibodies. Following SARS-CoV-2, a strong proinflammatory response could lead to hyperinflammation. ACE2, angiotensin converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Type I IFN responses are a major line of antiviral defense in the innate immune response against SARS-CoV-2 and other pathogenic human coronaviruses [36]. Dysregulated IFN signaling and elevated proinflammatory mediators are associated with impaired immune responses against SARS-CoV-2 and greater COVID-19 severity [[36], [37], [38]]. Therefore, it is hypothesized that obesity and diabetes result in excessive proinflammation responses during SARS-CoV-2 infection [39], explaining greater severity in COVID-19 and elevated neutralizing antibodies.
Population-level relevance
Evaluation of SARS-CoV-2 specific antibodies has several advantages for population-level monitoring of previous infection and vaccination. First, antibodies remain detectable for months [40]. Antibody screening allows differentiation between vaccine-induced and infection-induced responses based on antibody specificity for the spike protein only (vaccination) compared with spike and nucleocapsid proteins (natural infection). SARS-CoV-2 nucleic acid amplification tests are considered gold standard diagnostics; however, they are less feasible for large-scale surveillance due to key constraints (eg, cost, shorter detection windows) [41]. Second, a substantial proportion of SARS-CoV-2 infections are asymptomatic and mildly symptomatic [42, 43]; because asymptomatic cases are less likely to seek testing, it is presumed that these individuals are underreported.
Therefore, our study objective was to concisely review recent evidence regarding bidirectional influences between CMDs (DM, adiposity, hypertension, CVDs) and SARS-CoV-2 antibodies induced from previous infection and vaccination (Supplemental Figures 1, 2; Supplemental Table 1).
Serologic Evidence of Previous SARS-CoV-2 Infection and Cardiometabolic Diseases
Since interpretations of SARS-CoV-2 antibodies differ based on COVID-19 vaccination history, we summarized studies below stratified by pre- or postvaccination time periods. Given that the US FDA, CDC, UK, and WHO first issued emergency use authorizations or approvals for COVID-19 vaccines throughout December 2020, we categorized studies as “prevaccination” if data collection occurred prior to December 2020 or if the authors explicitly stated that participants had not yet received COVID-19 vaccinations. We considered studies as “postvaccination” if participant vaccination status was a primary research question (eg, humoral immune response following BNT162b2 mRNA vaccine doses) or part of the study design (eg, inclusion criterion) and if data collection occurred after December 2020.
Prevaccination era
Among studies with data collection before the rollout of COVID-19 vaccinations, we considered neutralizing and binding antibodies against any SARS-CoV-2 proteins (nucleocapsid [anti-N], spike [anti-S], mixtures) as evidence of previous infection.
Among 7 cohort studies evaluating neutralizing SARS-CoV-2 antibodies and elevated BMI [[44], [45], [46], [47], [48], [49], [50]], 5 studies found that higher neutralizing antibody titers were associated with elevated BMI [[44], [45], [46], [47], [48]] (Table 1). This positive association remained robust across studies with heterogeneous assays (viral neutralization tests [VNTs] based on plaque reduction [46,47] or cytopathic effect [44] and pseudovirus VNTs [pVNTs] with lentiviral particles [45,48]) and adjustment for time between symptom onset and antibody measurement [47]. Because previous studies have found that neutralizing antibodies are positively correlated with COVID-19 symptom severity [51,52], we hypothesize people with obesity have greater severity of COVID-19 and relatedly elevated viral load as well as neutralizing antibodies. Interestingly, a longitudinal study among symptomatic, hospitalized COVID-19 patients found that neutralizing antibody titers did not significantly change between 2 timepoints among those with obesity (P = 0.65), compared with the increase among those with normal weight (P < 0.01); at the first timepoint, neutralizing antibody titers were higher among participants with obesity, relative to normal weight [50]. This finding emphasizes the need for future studies to corroborate whether obesity affects neutralizing antibody kinetics throughout acute infection and postrecovery phases. We note that most studies had modest sample sizes (N ranging from 78 to 329), emphasizing the need for larger studies representative of the general population. As a summary, elevated BMI was positively associated with neutralizing antibodies against SARS-CoV-2, which correlates with protection against symptomatic infection from SARS-CoV-2 variants [53].
Table 1.
Summary of longitudinal studies evaluating SARS-CoV-2 neutralizing antibodies and BMI
| Author | Year | N | Participant characteristics | Collection dates | Pre- vs. post- vaccination | Neutralization assay type1 | SARS-CoV-2 strain/protein | Association with BMI and/or obesity |
Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| Directionality | Notes | |||||||||
| De Giorgi | 2021 | 202 | COVID-19 convalescent plasma donors | April–November 2020 | Pre | VNT (plaque and fluorescence reduction) | SARS-CoV-2 (2019-nCoV/USA-WA1-A12/2020) | + | nAb titers positively correlated with BMI (P = 0.0018) | [46] |
| Grzelak | 2021 | 308 | HCWs with previous SARS-CoV-2 infections and mild symptoms (6 mo post-symptom onset) | December 20202 | Pre | pVNT (lentiviral particles) | SARS-CoV-2 S protein | + | BMI > 25 kg/m2 associated with nAbs (+14.09 [95% CI: 8.34, 19.84]; P < 0.0001 | [45] |
| Karuna | 2021 | 329 | HIV seronegative adults convalescing from SARS-CoV-2 infection | May–October 2020 | Pre | pVNT (lentivirus backbone vector) | SARS-CoV-2 S protein | + (ID80) | BMI ≥ 30 kg/m2 associated with nAb titer: geometric mean ratio 1.44 (95% CI: 1.07, 1.94); q = 0.04 | [48] |
| 0 (ID50) | BMI ≥ 30 kg/m2 associated with nAb titer: geometric mean ratio 1.37 (95% CI: 0.92, 2.04); q = 0.23 | |||||||||
| Nilles | 2021 | 4469 | Industry employees | April–July 2020 | Pre | pVNT (lentiviral particles) | SARS-CoV-2 S protein | 0 | Viral neutralizing activity in 12% of people with obesity and 11.5% among those without obesity (P = 0.95) | [49] |
| Racine-Brzostek | 2021 | 1055 | Convalescent adult outpatients with previous positive SARS-CoV-2 serology | April–June 2020 | Pre | pVNT (lentiviral particles) | SARS-CoV-2 S protein | —4 | —4 | [47] |
| VNT (plaque reduction) | SARS-CoV-2 strain (USA-WA1/2020 [BEI Resources, NR181 52281]) | + | nAb titers higher among individuals with BMI ≥ 30 kg/m2 (P = 0.0055) | |||||||
| Teresa Valenzuela | 2021 | 117 | COVID-19 confirmed patients | May–August 2020 | Pre | VNT (cytopathic effect) | SARS-CoV-2 strain (33782CL) | − | Comparing changes in nAbs at 3–4 versus 1–2 wk after COVID-19 symptom onset, individuals without obesity had increases in nAbs (P < 0.001), whereas those with obesity had no increases (P = 0.647) | [50] |
| Wendel | 2021 | 78 | Male COVID-19 convalescent plasma donors | Nov 20202 | Pre | VNT (cytopathic effect) | SARS-CoV-2 (GenBank: MT350282) | + | Positive correlation between weight and nAb titers. Weight (>90 kg) and BMI (overweight, obese) associated with maintaining higher nAb titers (≥ 160; P < 0.02) |
[44] |
| Amjadi | 2021 | 113 | COVID-19 convalescent individuals | May 20212 | Post3 | VNT (plaque forming units; crystal violet stain for living cells) | SARS-CoV-2 strain (SARS-CoV-2/UW-001/Human/2020/Wisconsin) | + | nAb titers differed across BMI categories (P = 0.027) | [126] |
| Frasca | 2022 | 30 | Patients with SARS-CoV-2 infection (confirmed with RT-PCR) | July 20212 | Post3 | VNT (surrogate plaque reduction) | SARS-CoV-2 S (RBD; DYNEX Agility automated ELISA system) | − | After SARS-CoV-2 infection, nAbs present in samples of all lean individuals but only a few individuals with obesity (P < 0.0001) | [127] |
| Levin | 2021 | 4868 | HCWs with vaccinations | December 2020–July 2021 | Post | pVNT (GFP-pseudotyped virus with a vesicular stomatits virus backbone coated with SARS-CoV-2 S protein) | SARS-CoV-2 S protein | + | nAb titer higher among individuals with obesity, compared to without: ratio of mean titer 1.31 (95% CI: 1.14, 1.51) | [83] |
| Malavazos | 2021 | 1060 | Individuals after 1st and 2nd BNT162b2 mRNA vaccinations, stratified by abdominal obesity and previous infection status | January 2021–March 2022 | Post | IgG neutralizing antibodies against trimeric complex (CLIA) | SARS-CoV-2 S protein trimer, including RBD and NTD sites from 3 S1 subunits (LIAISON assay) | − | After 2nd vaccine doses, abdominal obesity was associated with decreased IgG-TrimericS levels in infection-naïve individuals. The interaction between abdominal adiposity and SARS-CoV-2 infection was significant (P = 0.002) | [84] |
| + | At baseline (prior to vaccines) among participants with previous infection, those with abdominal obesity had higher IgG-TrimericS levels, compared to those without abdominal obesity (P < 0.001) | |||||||||
GFP, green fluorescent protein; HCW, healthcare worker; nAb, neutralizing antibody; NTD, N-terminal domain; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Neutralizing antibody detection tests categorized as: virus neutralization test (VNT), pseudovirus neutralization test (pVNT), and competitive neutralization test (cVNT), per the Interim Guidelines for COVID-19 Antibody Testing from the CDC (https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html)
None stated. Categorization based on the date (see italicized text) manuscript submitted to journal.
Vaccination status of participants not stated
Comparison not reported
Among cohort and cross-sectional studies assessing associations between binding SARS-CoV-2 antibodies and BMI, the majority found positive associations between BMI and antibody levels [45,46,[54], [55], [56], [57], [58]] or seropositivity [[59], [60], [61], [62], [63]]. The remaining showed null associations between BMI and antibody levels [[64], [65], [66]], seropositivity [67,68], or both [49]. The association between binding antibodies and diabetes is less clear. Six studies reported positive associations, including greater antibody levels [47,66] and percentage of seropositivity [62,[69], [70], [71]], among people with metabolic abnormalities (DM and related indicators [glucose, HbA1c]), relative to those without. Five studies showed no differences in seropositivity by DM status [49,[72], [73], [74], [75]], whereas one study found an inverse association between seropositivity and DM [76]. In summary, SARS-CoV-2 binding antibody levels and seropositivity were either positively or not associated with BMI and DM. Explaining the discrepant directionality of these findings is a priority research area moving forward, including accounting for the diversity of SARS-CoV-2 antigen-specific antibodies (subclass, isotype, FcR binding capacity) [77], DM and adiposity subtypes, and clinical manifestations [78,79] and nonlinearity of associations.
Eight studies showed that SARS-CoV-2 binding antibody seropositivity [69,73], specifically anti-N [67,[74], [75], [76]] and anti-S receptor binding domain (RBD) [49], and anti-S titers [66] did not differ by hypertension or elevated blood pressure. Two studies reported positive associations between SARS-CoV-2 Ig levels and hypertension [47,80]; 2 studies found inverse associations between neutralizing antibody titers or binding antibody seropositivity and hypertension [48,76]. A study found that seroprevalence of SARS-CoV-2 binding antibodies (IgM) did not differ by coronary heart disease and congestive heart failure, respectively, among patients with COVID-19 [73]. Conversely, one study (N=341) showed that CVDs were associated with an elevated odds of seropositivity for SARS-CoV-2 binding antibodies [72]. Overall, most studies found that previous infection, based on seropositivity for binding antibodies against SARS-CoV-2, did not differ by hypertension. Few studies evaluated SARS-CoV-2 antibodies and CVDs.
Postvaccination era
Following COVID-19 vaccination approvals, we considered anti-N as evidence of previous SARS-CoV-2 infection. COVID-19 mRNA vaccines induce anti-S antibodies among immunocompetent individuals; therefore, interpretation of anti-S seropositivity requires further information to distinguish between whether seropositivity was induced by vaccination or previous infection. Two cross-sectional studies evaluated associations between anti-N and CMDs. Seropositivity of anti-N IgG and BMI were not associated in one study [81]. In the second study, diabetes was associated with a greater odds of anti-N seropositivity during 3 study phases (all P < 0.05) but not the fourth phase [82]. SARS-CoV-2 variants, vaccination status, and timing (eg, since infection or vaccination) of participants throughout the data collection period (May 2020–June 2021) were not accounted for in the analysis, which could potentially explain the discrepant results.
Vaccination-Induced Humoral Immunity and Cardiometabolic Diseases
Since most COVID-19 vaccinations target SARS-CoV-2 spike proteins [26], we summarized antibody responses specific to spike proteins following COVID-19 vaccinations.
Neutralizing antibodies
In 2 studies that studied humoral responses after BNT162b2 (Pfizer-BioNtech) mRNA vaccination doses, both found that neutralizing antibodies differed by adiposity [83, 84]. In a prospective cohort study among 4868 health care workers (HCWs), obesity (BMI ≥ 30 kg/m2) was associated with elevated neutralizing antibody titers, which were assessed by pVNTs, at 6 mo after receiving 2 vaccine doses [83]. Comparing 3 mo with 1 mo postvaccination, there was a greater fold decrease of IgG neutralizing antibodies against trimeric S complex levels among individuals with abdominal obesity compared with those without abdominal obesity [84]. These results potentially suggest that body composition and types of adipose tissue may differentially impact neutralizing antibodies induced by COVID-19 vaccinations.
Two studies showed that after BNT162b2 mRNA vaccine doses, neutralizing antibody titers and seropositivity did not differ by DM [85], CVDs [84, 85], or hypertension [84]. Both studies were among HCWs, including 2607 in Israel [85] and 1060 in Italy [84].
Anti-S binding antibodies
Fourteen cohort and cross-sectional studies assessed associations between anti-S and adiposity (BMI, waist circumference) following COVID-19 vaccinations. The directionality of associations was inconsistent [86]; specifically, 7 studies showed null associations [[87], [88], [89], [90], [91], [92], [93]], 3 found positive associations [81,94,95], and 3 showed inverse associations [[96], [97], [98]]. Anti-S levels were evaluated in all studies except for one that assessed seroprevalence [88]. Based on the previous observation of the J-shaped association between BMI and mortality, future studies should consider the nonlinearity of this association. Studies included mRNA (mRNA-1273 [Moderna], BNT162b2) and inactivated (CoronaVac [Sinovac]) vaccines with varying doses (eg, 1 compared with 2 full doses) and lengths of time since receipt of vaccinations; these factors potentially explain these incongruous associations.
Among 5 cohort studies comparing postvaccination anti-S by DM [85,[99], [100], [101], [102]], 4 found null associations between anti-S seropositivity [85,100,102] and levels [101]. Two studies showed inverse associations between DM and seronegativity [99] and IgA [85]. We note that null associations remained robust across heterogeneous vaccination doses. Among 5 vaccination studies (BNT162b2, ChAdOx1-S, CoronaVac), anti-S responses (levels, ratios) did not differ by hypertension in 3 studies [90,91,103] or cardiac comorbidities in one study [89]. In a study among 86 HCWs, hypertension was associated with lower anti-S titers adjusting for BMI, age, and sex [97].
In summary, neutralizing antibody and anti-S responses after vaccination differed by adiposity in the most studies, however inconsistent directionality underscores the need to further elucidate mechanisms and effect modification. Future priority questions include determining specific influences of body composition (adipose tissue type) and time since previous infection(s) and vaccination(s), particularly antibody waning. Postvaccination humoral responses, based on neutralizing and anti-S binding antibody levels and seropositivity, were generally similar by DM, CVD, and hypertension.
Postacute Sequelae of COVID-19: New-Onset Type 1 Diabetes Mellitus?
Prevaccination era
We categorized studies among adolescents and children with data collection prior to May 2021 as “prevaccination” studies, based on the US FDA approval date of COVID-19 vaccine use among 12- to 15-year-old adolescents. Bidirectional etiology has been hypothesized albeit based on ecologic observations of increased new-onset type 1 DM cases and severity of diabetic ketoacidosis following the COVID-19 pandemic, including among children and adolescents in the United Kingdom [104] and Germany [105]. In Finland, the incidence of type 1 DM was higher among children during the COVID-19 pandemic, relative to a reference cohort among children prior to the pandemic [106]. One cohort study among new-onset (N=129) or established (N=94) type 1 DM cases, relative to healthy controls (N=664), found no intergroup differences in seroprevalence of SARS-CoV-2 antibodies [107]. Among individuals with SARS-CoV-2 seropositivity, none progressed to incident type 1 DM [108]; vice versa, among those with new-onset type 1 DM, none had anti-SARS-CoV-2 seropositivity [108,109].
Postvaccination era
In a multicenter prospective cohort study (N=247), anti-S (RBD) antibody levels (binding antibody units/mL) were similar between participants with type 1 DM, type 2 DM, and healthy controls after the second COVID-19 vaccine doses [101]. A case-control study found that anti-S (RBD; IgA, IgG, IgM) responses after 1 and 2 vaccine doses were similar between patients with and without type 1 DM [110].
Overall, cross-sectional and cohort studies have not shown associations between anti-SARS-CoV-2 antibodies and type 1 DM. Although immunofluorescence, immunostaining, and some single cell RNAseq studies have shown that the ACE2 receptor is expressed on almost all human pancreatic cell types [111,112], a recent in vivo single cell RNAseq study indicated that infection impacts were limited and generally noncytopathic [112], which could explain the null findings in epidemiologic studies. Future studies, including in vivo studies with larger study populations, are needed to shed light on discrepant results and resolve this ongoing controversy of whether SARS-CoV-2 infection can result in new-onset type 1 DM [113].
Research Gaps and Future Perspectives
There are major challenges in interpreting previous findings, which stem from interstudy methodologic differences and remaining research gaps.
Accounting for temporally dependent, extrinsic, and intrinsic factors at the population and individual level
One challenge is accounting for the dynamic escalation in the host–pathogen arms race via emergent SARS-CoV-2 variants and subvariants of concern with improved immune evasion and transmissibility [114] and vaccine development, availability, uptake, and effectiveness (Figure 3). Humoral immune responses following SARS-CoV-2 infection and COVID-19 vaccination differ by multiple temporally dependent factors. At the population level, transmission and COVID-19 severity risk has substantially fluctuated during different surges of SARS-CoV-2 variants of concern and vaccination rollout strategies. Host immune mechanisms are differentially sensitive and effective against SARS-CoV-2 variants [114,115], which differ by phylogenetic lineage and mutations, often in spike protein [114]. Since epitopes on SARS-CoV-2 spike protein are primary targets of neutralizing antibodies, particular variants (eg, omicron) have improved immune escape responses relative to other variants (eg, alpha) [114,115]. At the individual level, the potential impacts from the length of time since acute infection and vaccination (including full doses and boosters), temporality of hyperglycemia and DM diagnosis (eg, transience, delayed detection) need to be considered in future studies and comparisons of studies with variable durations and assessment timepoints.
FIGURE 3.
Key temporally dependent factors affecting links between cardiometabolic health and COVID-19 outcomes. Comparing prior findings regarding humoral immunity and cardiometabolic health is challenging due to the temporal dependence of related extrinsic and intrinsic factors at the population and individual level. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2 outcomes and baseline immune competency comparators: moving targets
The relevance of comparator groups has dynamically shifted throughout the pandemic, which poses a substantial challenge in determining the influences of CMDs on humoral immune responses against SARS-CoV-2. During the early phase of the pandemic, naïve individuals without evidence of previous SARS-CoV-2-specific immunity were predominantly considered the comparator group of interest, and SARS-CoV-2 infection was a major outcome of interest. Since then, a growing number of people have previous exposure to SARS-CoV-2 infection(s) or received COVID-19 vaccination (full dosages, booster[s]). Studies have shown that >80% of people in the United States had SARS-CoV-2 spike or nucleocapsid antibodies, indicating previous infection, vaccination, or both [116]; key outcomes have shifted to COVID-19 severity, reinfections, and vaccine breakthrough infections in many studies, particularly against emergent variants of concern. Given the highly variable impacts of SARS-CoV-2 in communities across the United States and globally, different reference groups (eg, previous vaccination, infection, hybrid immunity) are arguably more relevant to certain target populations.
An important future research priority is identifying a correlate of protection, or panel of biomarkers, against risk of infection or severe disease that reflects baseline status of immunity specific to SARS-CoV-2. The ideal correlate of protection will be quantifiable, feasible for scaling up to population-level surveillance, and sensitive to cardiometabolic health, which would provide valuable insights for prevention approaches.
Role of confounders: COVID-19 severity and socioeconomic factors
One central unresolved question is determining the role of COVID-19 severity in the association between CMDs and SARS-CoV-2 antibodies. COVID-19 severity could be: 1) a confounder that distorts the true association, which could potentially explain inconsistent findings in previous studies that have not adjusted for disease severity; 2) an effect modifier, which suggests that findings not reported as stratum-specific estimates could introduce bias; or 3) an intermediate variable or mediator, which suggests that adjusting for severity could bias results. Lastly, COVID-19 severity could result in elevated humoral immune responses independent of CMDs, or alternatively, CMDs could lead to more severe COVID-19 independent of antibodies. A key initial step is improving the evaluation of disease severity.
Socioeconomic status is another major potential confounder. A growing body of literature has characterized differences in SARS-CoV-2 infection risk [117], COVID-19 cases [118], and CMDs [119] by socioeconomic and neighborhood factors.
Interpreting SARS-CoV-2 antibody assays
Comparing findings across studies with variegated SARS-CoV-2 antibody assays is challenging. Few studies used quantitative antibody assays or neutralizing antibodies, particularly with calibration against the WHO standard [120]. Many studies reported relative quantitation values of signal intensity and utilized differing cutoff values to define seropositivity. A number of studies used commercially available immunoassays and rapid diagnostic tests (lateral flow assays) that report SARS-CoV-2-specific antibodies without distinguishing between neutralizing versus nonneutralizing antibodies, antigen epitopes (binding sites of nucleocapsid, spike [S1, S2, RBD] proteins of SARS-CoV-2), and Ig isotypes (IgG, IgM, IgA). SARS-CoV-2 binding antibody assays correlate with neutralization titers [121]; however, other antibody effector functions beyond neutralization are still under active investigation [122]. CMD status and indicators are also variable, rendering meta-analyses of associations with SARS-CoV-2 antibodies difficult or not possible in some cases.
Generalizability to other populations
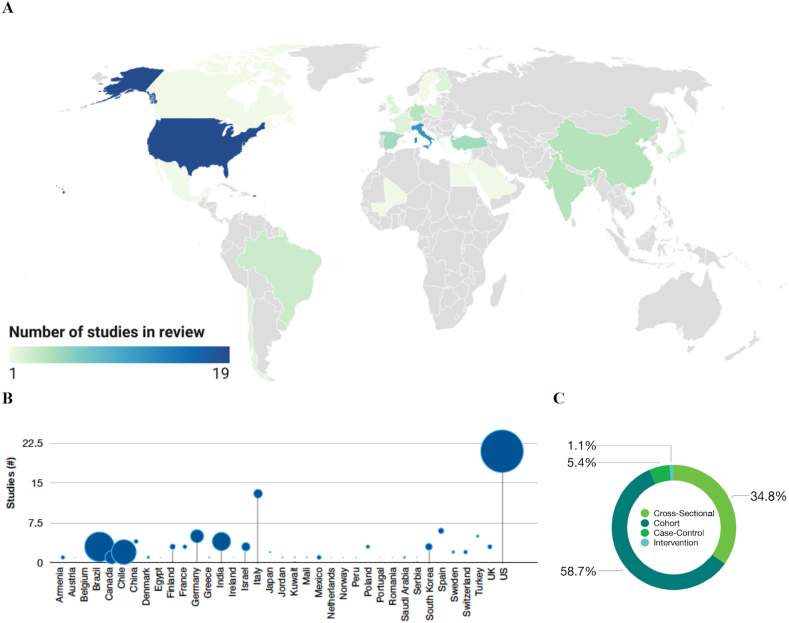
This review included 92 studies with over 408,000 participants from 37 different countries on 5 continents (Europe, Asia, Africa, and North and South America; Figure 4A, 4B). Approximately one-third of studies were from Europe (eg, Belgium, Denmark, Finland, France, Germany, Greece, Italy, Poland, Spain, Sweden, Switzerland, United Kingdom), and nearly one-quarter of studies conducted in North America (United States, Mexico). Future studies among low- and middle-income countries are needed. Most studies were cohorts (58.7%), followed by cross-sectional (34.8%), case-control studies (5.4%), and an intervention study (1.1%; Figure 4C).
FIGURE 4.
Comparison of geography and study design across studies in review. Among 92 studies included in this review, geographic distribution is visually illustrated by a heatmap (A) and the total number of participants in studies, stratified by country (B). Blue dots are proportionally scaled to represent the number of participants (B). The percentage of studies with differing study designs (cohort, cross-sectional, intervention) are illustrated (C). a 88 studies are included in this map. Four studies were excluded from this visualization as they included data from participants in multiple countries, including Austria, Peru, Ireland, Netherlands, Portugal, Romania, Serbia, and Norway. Created with Datawrapper. b 90 studies were included. Two multi-site studies were excluded because the number of participants was reported in aggregate and not stratified by geographic location [77,101].
Concluding Remarks
Obesity was associated with higher neutralizing antibody titers following SARS-CoV-2 infection. Further studies need to confirm the extent that neutralizing antibody titers explain immunomodulatory influences of adiposity on COVID-19 severity risk as well as account for extensive heterogeneity among people with elevated BMI. Prior to vaccine rollout, most studies showed positive or null associations between binding antibodies against SARS-CoV-2 and DM; after vaccination, antibody responses did not differ by DM. Most studies found that SARS-CoV-2 antibody seropositivity did not differ by hypertension, suggesting that hypertension does not affect SARS-CoV-2 infection risk.
Key strengths of this review include the diverse participants in 5 continents of available studies, evaluation of SARS-CoV-2 antibodies as relatively noninvasive indicators appropriate for population-level assessment, and systematic search strategy. However, major limitations and challenges include the inability to draw causal inferences from observational human studies, highly heterogeneous study designs (particularly SARS-CoV-2 antibody assays, cardiometabolic indicators), and need to further evaluate etiology, effect modification, and confounders (eg, SARS-CoV-2 variants, vaccination history details, and baseline immunocompetence).
Future priority research questions include: How do we accurately evaluate population-scale impacts of underlying cardiometabolic health on humoral immunity against SARS-CoV-2 variants and vaccinations (Figure 5)? Is the efficacy and effectiveness of COVID-19 vaccination affected by CMDs? To what extent should COVID-19 vaccination recommendations, such as booster dosages and timing, be tailored among people with CMDs? How does acute SARS-CoV-2 infection (symptomatic, asymptomatic) affect the risk of short- and long-term metabolic postacute sequelae of COVID-19? Are there metabolically sensitive correlates of protection that provide earlier detection of individuals at greater risk for COVID-19 severity and postacute sequelae of COVID-19 [123]? What is the role of COVID-19 severity in the association between CMDs and SARS-CoV-2 neutralizing antibodies? How do COVID-19 treatments (eg, nirmatrelvir/ritonavir, remdesivir, dexamethasone) modify associations between cardiometabolic diseases and COVID-19 severity? How do socioeconomic and neighborhood factors influence the association between CMDs and COVID-19 severity? Can lifestyle and pharmacologic interventions that improve modifiable cardiometabolic risk factors effectively reduce population burden due to COVID-19 severity [124]?
FIGURE 5.
Overview of key knowledge gaps, challenges, and opportunities. Despite growing evidence of cardiometabolic diseases as independent risk factors of COVID-19 severity, including mortality, intubations, and hospitalizations (A), the etiology (B) as well as the impact on transmissibility (C) of SARS-CoV-2 vaccines remain unclear (D). Several large cohort studies have shown that postacute sequelae of COVID-19 include metabolic abnormalities in some individuals, although implications have yet to be determined given key remaining research gaps. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In summary, the current state of the evidence implicates the role of elevated BMI in modulating SARS-CoV-2 neutralizing antibodies. This finding underscores the importance of more effective obesity prevention efforts [125] and determining the potential benefits of tailored recommendations according to preinfection BMI on improved prevention, protection from vaccination, and treatment of COVID-19.
Acknowledgments
FIGURE 1, FIGURE 2, FIGURE 3, FIGURE 5 and 5 were created with BioRender.com. The authors’ responsibilities were as follows—EAY: conceptualized the review objectives, served as the primary reviewer of literature, drafted the initial manuscript and figures; RPJ, KMVN, MJG: provided interpretations of the findings and critical revisions that substantially improved the manuscript and figures; RPJ: conceptualized and designed the final version of the first figure; and all authors: read and approved the final manuscript.
Author disclosures
MJG received research funding from Gilead Sciences and Regeneron, serves as a consultant to ReAlta Life Sciences and receives royalties from Springer and UpToDate. MJG served as a Data Safety and Monitoring Board chair for Swedish Orphan Biovitrum (Sobi) and Enzychem, and as a consultant for Regeneron. EAY, RPJ, and KMVN have no conflicts of interest.
Funding
KMVN was partly supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers P30DK111024 and P30111024-05S1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2023.06.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Knutson V., Aleshin-Guendel S., Karlinsky A., Msemburi W., Wakefield J. World Health Organization; 2022. Estimating global and country-specific excess mortality during the COVID-19 pandemic. [Google Scholar]
- 2.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022;399(10334):1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steenblock C., Schwarz P.E.H., Ludwig B., Linkermann A., Zimmet P., Kulebyakin K., et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefan N. Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat. Rev. Endocrinol. 2022;18(2):75–76. doi: 10.1038/s41574-021-00608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefan N., Birkenfeld A.L., Schulze M.B. Global pandemics interconnected — obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021;17(3):135–149. doi: 10.1038/s41574-020-00462-1. [DOI] [PubMed] [Google Scholar]
- 6.Drucker D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33(3):479–498. doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021;17(1):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hearn M., Liu J., Cudhea F., Micha R., Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J. Am. Heart Assoc. 2021;10(5) doi: 10.1161/JAHA.120.019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 Host Genetics Initiative Mapping the human genetic architecture of COVID-19. Nature. 2021;600(7889):472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu H.Q., Qu J., Glessner J., Hakonarson H. Mendelian randomization study of obesity and type 2 diabetes in hospitalized COVID-19 patients. Metabolism. 2022;129 doi: 10.1016/j.metabol.2022.155156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freuer D., Linseisen J., Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: A two-sample multivariable Mendelian randomization study. Metabolism. 2021;118 doi: 10.1016/j.metabol.2021.154732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrera-Mendoza B., Wendt F.R., Pathak G.A., De Angelis F., De Lillo A., Koller D., et al. The association of obesity-related traits on COVID-19 severity and hospitalization is affected by socio-economic status: a multivariable Mendelian randomization study. Int. J. Epidemiol. 2022;51(5):1371–1383. doi: 10.1093/ije/dyac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates T., Summerfield A., Razieh C., Banerjee A., Chudasama Y., Davies M.J., et al. A population-based cohort study of obesity, ethnicity and COVID-19 mortality in 12.6 million adults in England. Nat. Commun. 2022;13(1):624. doi: 10.1038/s41467-022-28248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao M., Piernas C., Astbury N.M., Hippisley-Cox J., O’Rahilly S., Aveyard P., et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piernas C., Patone M., Astbury N.M., Gao M., Sheikh A., Khunti K., et al. Associations of BMI with COVID-19 vaccine uptake, vaccine effectiveness, and risk of severe COVID-19 outcomes after vaccination in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2022;10(8):571–580. doi: 10.1016/S2213-8587(22)00158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montefusco L., Ben Nasr M., D’Addio F., Loretelli C., Rossi A., Pastore I., et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021;3(6):774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathmann W., Kuss O., Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022;65(6):949–954. doi: 10.1007/s00125-022-05670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y., Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelesidis T., Mantzoros C.S. Cross-talk between SARS-CoV-2 infection and the insulin/IGF signaling pathway: implications for metabolic diseases in COVID-19 and for post-acute sequelae of SARS-CoV-2 infection. Metabolism. 2022;134 doi: 10.1016/j.metabol.2022.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin J., Toyoda S., Nishitani S., Onodera T., Fukuda S., Kita S., et al. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism. 2022;133 doi: 10.1016/j.metabol.2022.155236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 23.Donath M.Y., Dinarello C.A., Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat. Rev. Immunol. 2019;19(12):734–746. doi: 10.1038/s41577-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 24.Lackey D.E., Olefsky J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 25.Laidlaw B.J., Ellebedy A.H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 2022;22(1):7–18. doi: 10.1038/s41577-021-00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotaki R., Adachi Y., Moriyama S., Onodera T., Fukushi S., Nagakura T., et al. SARS-CoV-2 omicron-neutralizing memory B cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci. Immunol. 2022;7(70) doi: 10.1126/sciimmunol.abn8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutomski C.A., El-Baba T.J., Bolla J.R., Robinson C.V. Multiple roles of SARS-CoV-2 N protein facilitated by proteoform-specific interactions with RNA, host proteins, and convalescent antibodies. JACS Au. 2021;1(8):1147–1157. doi: 10.1021/jacsau.1c00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23(2):210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 30.Pape K.A., Dileepan T., Kabage A.J., Kozysa D., Batres R., Evert C., et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021;37(2) doi: 10.1016/j.celrep.2021.109823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheja L., Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019;15(9):507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 32.Honecker J., Weidlich D., Heisz S., Lindgren C.M., Karampinos D.C., Claussnitzer M., et al. A distribution-centered approach for analyzing human adipocyte size estimates and their association with obesity-related traits and mitochondrial function. Int. J. Obes. (Lond). 2021;45(9):2108–2117. doi: 10.1038/s41366-021-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas D., Apovian C. Macrophage functions in lean and obese adipose tissue. Metabolism. 2017;72:120–143. doi: 10.1016/j.metabol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong L.R., Perlman S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses — are we our own worst enemy? Nat. Rev. Immunol. 2022;22(1):47–56. doi: 10.1038/s41577-021-00656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoggins J.W. Interferon-stimulated genes: what do they all do? Annu. Rev. Virol. 2019;6(1):567–584. doi: 10.1146/annurev-virology-092818-015756. [DOI] [PubMed] [Google Scholar]
- 40.Cromer D., Juno J.A., Khoury D., Reynaldi A., Wheatley A.K., Kent S.J., et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat. Rev. Immunol. 2021;21(6):395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kevadiya B.D., Machhi J., Herskovitz J., Oleynikov M.D., Blomberg W.R., Bajwa N., et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021;20(5):593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sah P., Fitzpatrick M.C., Zimmer C.F., Abdollahi E., Juden-Kelly L., Moghadas S.M., et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc. Natl. Acad. Sci. U. S. A. 2021;118(34) doi: 10.1073/pnas.2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC COVID-19 Vaccine Breakthrough Case Investigations Team COVID-19 vaccine breakthrough infections reported to CDC — United States, January 1–April 30, 2021. MMWR Mortal Wkly. Rep. 2021;70(21):792–793. doi: 10.15585/mmwr.mm7021e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wendel S., Fontão-Wendel R., Fachini R., Candelaria G., Scuracchio P., Achkar R., et al. A longitudinal study of convalescent plasma (CCP) donors and correlation of ABO group, initial neutralizing antibodies (nAb), and body mass index (BMI) with nAb and anti-nucleocapsid (NP) SARS-CoV-2 antibody kinetics: proposals for better quality of CCP collections. Transfusion. 2021;61(5):1447–1460. doi: 10.1111/trf.16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grzelak L., Velay A., Madec Y., Gallais F., Staropoli I., Schmidt-Mutter C., et al. Sex differences in the evolution of neutralizing antibodies to severe acute respiratory syndrome coronavirus 2. J. Infect. Dis. 2021;224(6):983–988. doi: 10.1093/infdis/jiab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Giorgi V., West K.A., Henning A.N., Chen L., Holbrook M.R., Gross R., et al. Anti-SARS-CoV-2 serology persistence over time in COVID-19 convalescent plasma donors. medRxiv. 2021 doi: 10.1101/2021.03.08.21253093. [DOI] [Google Scholar]
- 47.Racine-Brzostek S.E., Yang H.S., Jack G.A., Chen Z., Chadburn A., Ketas T.J., et al. Postconvalescent SARS-CoV-2 IgG and neutralizing antibodies are elevated in individuals with poor metabolic health. J. Clin. Endocrinol. Metab. 2021;106(5):e2025–e2034. doi: 10.1210/clinem/dgab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karuna S., Li S.S., Grant S., Walsh S.R., Frank I., Casapia M., et al. Neutralizing antibody responses over time in demographically and clinically diverse individuals recovered from SARS-CoV-2 infection in the United States and Peru: a cohort study. PLOS Med. 2021;18(12) doi: 10.1371/journal.pmed.1003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilles E.J., Siddiqui S.M., Fischinger S., Bartsch Y.C., de St Aubin M., Zhou G., et al. Epidemiological and immunological features of obesity and SARS-CoV-2. Viruses. 2021;13(11):2235. doi: 10.3390/v13112235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teresa Valenzuela M., Urquidi C., Rodriguez N., Castillo L., Fernández J., Ramírez E. Development of neutralizing antibody responses against SARS-CoV-2 in COVID-19 patients. J. Med. Virol. 2021;93(7):4334–4341. doi: 10.1002/jmv.26939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko J.H., Joo E.J., Park S.J., Baek J.Y., Kim W.D., Jee J., et al. Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients. J. Clin. Med. 2020;9(7):2268. doi: 10.3390/jcm9072268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sancilio A., Schrock J.M., Demonbreun A.R., D’Aquila R.T., Mustanski B., Vaught L.A., et al. COVID-19 symptom severity predicts neutralizing antibody activity in a community-based serological study. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-15791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shields A.M., Faustini S.E., Perez-Toledo M., Jossi S., Allen J.D., Al-Taei S., et al. Serological responses to SARS-CoV-2 following non-hospitalised infection: clinical and ethnodemographic features associated with the magnitude of the antibody response. BMJ Open Respir. Res. 2021;8(1) doi: 10.1136/bmjresp-2020-000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerhards C., Thiaucourt M., Kittel M., Becker C., Ast V., Hetjens M., et al. Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescence from a COVID-19 infection. Int. J. Infect. Dis. 2021;107:221–227. doi: 10.1016/j.ijid.2021.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Greef J., Scohy A., Zech F., Aboubakar F., Pilette C., Gerard L., et al. Determinants of IgG antibodies kinetics after severe and critical COVID-19. J. Med. Virol. 2021;93(9):5416–5424. doi: 10.1002/jmv.27059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt J., Berghaus S., Blessing F., Wenzel F., Herbeck H., Blessing J., et al. Serological and viral genetic features of patients with COVID-19 in a selected German patient cohort-correlation with disease characteristics. Geroscience. 2021;43(5):2249–2264. doi: 10.1007/s11357-021-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epsi N.J., Richard S.A., Laing E.D., Fries A.C., Millar E., Simons M.P., et al. Clinical, immunological, and virological SARS-CoV-2 phenotypes in obese and nonobese military health system beneficiaries. J. Infect. Dis. 2021;224(9):1462–1472. doi: 10.1093/infdis/jiab396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Tong C.H., Bare L.A., Devlin J.J. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw. Open. 2021;4(5) doi: 10.1001/jamanetworkopen.2021.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sami S., Akinbami L.J., Petersen L.R., Crawley A., Lukacs S.L., Weiss D., et al. Prevalence of SARS-CoV-2 antibodies in first responders and public safety personnel, New York City, New York, USA, May-July 2020. Emerg. Infect. Dis. 2021;27(3):796–804. doi: 10.3201/eid2703.204340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pathela P., Crawley A., Weiss D., Maldin B., Cornell J., Purdin J., et al. Seroprevalence of severe acute respiratory syndrome coronavirus 2 following the largest initial epidemic wave in the United States: findings from New York City, 13 May to 21 July 2020. J. Infect. Dis. 2021;224(2):196–206. doi: 10.1093/infdis/jiab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kabagambe E.K., Velasco-Gonzalez C., Henry M.B., Fort D., Wu Q., Sossaman G., et al. Prevalence, distribution and IgG antibody levels associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among health-system and community-based employees and patients. Am. J. Med. Sci. 2022;363(1):18–24. doi: 10.1016/j.amjms.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lombardi A., Mangioni D., Consonni D., Cariani L., Bono P., Cantù A.P., et al. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: a cross-sectional study. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-047216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jain S., Garg K., Tran S.M., Rask I.L., Tarczon M., Nandi V., et al. Characteristics of coronavirus disease 19 convalescent plasma donors and donations in the New York metropolitan area. Transfusion. 2021;61(8):2374–2383. doi: 10.1111/trf.16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krzywański J., Mikulski T., Krysztofiak H., Pokrywka A., Młyńczak M., Małek Ł.A., et al. Elite athletes with COVID-19 - predictors of the course of disease. J. Sci. Med. Sport. 2022;25(1):9–14. doi: 10.1016/j.jsams.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kutsuna S., Asai Y., Matsunaga A., Kinoshita N., Terada M., Miyazato Y., et al. Factors associated with anti-SARS-CoV-2 IgG antibody production in patients convalescing from COVID-19. J. Infect. Chemother. 2021;27(6):808–813. doi: 10.1016/j.jiac.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nah E.H., Cho S., Park H., Hwang I., Cho H.I. Nationwide seroprevalence of antibodies to SARS-CoV-2 in asymptomatic population in South Korea: a cross-sectional study. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2021-049837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahallawi W.H., Ibrahim N.A., Aljohani A.S., Shaikh E.A., Nafe R.H., Khan A.M., et al. Assessment of SARS-CoV-2 anti-spike IgG antibody in women and children in Madinah, Saudi Arabia: a single-center study. Int. J. Environ. Res. Public Health. 2021;18(19):9971. doi: 10.3390/ijerph18199971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macedo-Ojeda G., Muñoz-Valle J.F., Yokogawa-Teraoka P., Machado-Sulbarán A.C., Loza-Rojas M.G., García-Arredondo A.C., et al. COVID-19 screening by anti-SARS-CoV-2 antibody seropositivity: clinical and epidemiological characteristics, comorbidities, and food intake quality. Int. J. Environ. Res. Public Health. 2021;18(17):8895. doi: 10.3390/ijerph18178995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mesenburg M.A., Hallal P.C., Menezes A.M.B., Barros A.J.D., Horta B.L., Barros F.C., et al. Chronic non-communicable diseases and COVID-19: EPICOVID-19 Brazil results. Rev. Saude Publica. 2021;55:38. doi: 10.11606/s1518-8787.2021055003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma N., Sharma P., Basu S., Bakshi R., Gupta E., Agarwal R., et al. Second wave of the COVID-19 pandemic in Delhi, India: high seroprevalence not a deterrent? Cureus. 2021;13(10) doi: 10.7759/cureus.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Santi M., Diotallevi A., Brandi G. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in an Italian cohort in Marche region. Italy, Acta Biomed. 2021;92(1) doi: 10.23750/abm.v92i1.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding T., Zhang N. Clinical value analysis of IgM and IgG antibodies detected by nucleic acid in patients with COVID-19. Am. J. Transl. Res. 2021;13(6):7089–7103. [PMC free article] [PubMed] [Google Scholar]
- 74.Inbaraj L.R., George C.E., Chandrasingh S. Seroprevalence of COVID-19 infection in a rural district of South India: a population-based seroepidemiological study. PLOS ONE. 2021;16(3) doi: 10.1371/journal.pone.0249247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berenguer J., Díez C., Martín-Vicente M., Micán R., Pérez-Elías M.J., García-Fraile L.J., et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin. Microbiol. Infect. 2021;27(11):1678–1684. doi: 10.1016/j.cmi.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zejda J.E., Brożek G.M., Kowalska M., Barański K., Kaleta-Pilarska A., Nowakowski A., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in a random sample of inhabitants of the Katowice region, Poland. Int. J. Environ. Res. Public Health. 2021;18(6):3188. doi: 10.3390/ijerph18063188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schnittman S.R., Jung W., Fitch K.V., Zanni M.V., McCallum S., Lee J.S., et al. Effect of host factors and COVID-19 infection on the humoral immune repertoire in treated HIV. JCI Insight. 2023;8(5) doi: 10.1172/jci.insight.166848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahlqvist E., Prasad R.B., Groop L. Subtypes of type 2 diabetes determined from clinical parameters. Diabetes. 2020;69(10):2086–2093. doi: 10.2337/dbi20-0001. [DOI] [PubMed] [Google Scholar]
- 79.Sun W., Modica S., Dong H., Wolfrum C. Plasticity and heterogeneity of thermogenic adipose tissue. Nat. Metab. 2021;3(6):751–761. doi: 10.1038/s42255-021-00417-4. [DOI] [PubMed] [Google Scholar]
- 80.Dobaño C., Ramírez-Morros A., Alonso S., Rubio R., Ruiz-Olalla G., Vidal-Alaball J., et al. Sustained seropositivity up to 20.5 months after COVID-19. BMC Med. 2022;20(1):379. doi: 10.1186/s12916-022-02570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabater Vidal S., Bellido-Cambrón M.C., Arnedo-Pena A., Palomares-Gallego M.I., Larrea-González R.M., Carballido-Fernández M., et al. [Vaccine response to SARS-CoV-2 in hospital workers] Arch. Prev. Riesgos. Labor. 2021;24(4):383–403. doi: 10.12961/aprl.2021.24.04.05. [DOI] [PubMed] [Google Scholar]
- 82.Beretta O., Casati Pagani S., Lazzaro M., Merlani G., Bouvier Gallacchi M. Seroprevalence of the SARS-CoV-2 virus in the population of the southern Switzerland (Canton Ticino) - cohort study, results at 12 months. Swiss Med. Wkly. 2021;151 doi: 10.4414/smw.2021.w30116. [DOI] [PubMed] [Google Scholar]
- 83.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malavazos A.E., Basilico S., Iacobellis G., Milani V., Cardani R., Boniardi F., et al. Antibody responses to BNT162b2 mRNA vaccine: infection-naïve individuals with abdominal obesity warrant attention. Obesity (Silver Spring) 2022;30(3):606–613. doi: 10.1002/oby.23353. [DOI] [PubMed] [Google Scholar]
- 85.Lustig Y., Sapir E., Regev-Yochay G., Cohen C., Fluss R., Olmer L., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bayart J.L., Morimont L., Closset M., Wieërs G., Roy T., Gerin V., et al. Confounding factors influencing the kinetics and magnitude of serological response following administration of BNT162b2. Microorganisms. 2021;9(6):1340. doi: 10.3390/microorganisms9061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greco M., Cucci F., Portulano P., Lazzari R.A., Caldararo C., Sicuro F., et al. Effects of influenza vaccination on the response to BNT162b2 messenger RNA COVID-19 vaccine in healthcare workers. J. Clin. Med. Res. 2021;13(12):549–555. doi: 10.14740/jocmr4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gümüş H.H., Ödemiş İ., Alışka H.E., Karslı A., Kara S., Özkale M., et al. Side effects and antibody response of an inactive severe acute respiratory syndrome coronavirus 2 vaccine among health care workers. Rev. Assoc. Med. Bras. 2021;67(12):1825–1831. doi: 10.1590/1806-9282.20210755. 1992. [DOI] [PubMed] [Google Scholar]
- 89.Herzberg J., Vollmer T., Fischer B., Becher H., Becker A.K., Honarpisheh H., et al. SARS-CoV-2-antibody response in health care workers after vaccination or natural infection in a longitudinal observational study. Vaccine. 2022;40(2):206–212. doi: 10.1016/j.vaccine.2021.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campo F., Venuti A., Pimpinelli F., Abril E., Blandino G., Conti L., et al. Antibody persistence 6 months post-vaccination with BNT162b2 among health care workers. Vaccines (Basel). 2021;9(10):1125. doi: 10.3390/vaccines9101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee S.W., Moon J.Y., Lee S.K., Lee H., Moon S., Chung S.J., et al. Anti-SARS-CoV-2 spike protein RBD antibody levels after receiving a second dose of ChAdOx1 nCov-19 (AZD1222) vaccine in healthcare workers: lack of association with age, sex, obesity, and adverse reactions. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.779212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gaborit B., Fernandes S., Loubet P., Ninove L., Dutour A., Cariou B., et al. Early humoral response to COVID-19 vaccination in patients living with obesity and diabetes in France. The COVPOP OBEDIAB study with results from the ANRS0001S COV-POPART cohort. Metabolism. 2023;142 doi: 10.1016/j.metabol.2023.155412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra S.K., Pradhan S.K., Pati S., Sahu S., Nanda R.K. Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: a prospective longitudinal study. Cureus. 2021;13(11) doi: 10.7759/cureus.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michos A., Tatsi E.B., Filippatos F., Dellis C., Koukou D., Efthymiou V., et al. Association of total and neutralizing SARS-CoV-2 spike -receptor binding domain antibodies with epidemiological and clinical characteristics after immunization with the 1st and 2nd doses of the BNT162b2 vaccine. Vaccine. 2021;39(40):5963–5967. doi: 10.1016/j.vaccine.2021.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Early onset of SARS-COV-2 antibodies after first dose of BNT162b2: correlation with age, gender and BMI. Vaccines (Basel). 2021;9(7):685. doi: 10.3390/vaccines9070685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watanabe M., Balena A., Tuccinardi D., Tozzi R., Risi R., Masi D., et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022;38(1) doi: 10.1002/dmrr.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Özgür D., Tütüncü E.E. [Antibody response after two doses of inactivated SARS-CoV-2 vaccine in healthcare workers with and without previous COVID-19 infection: a prospective observational study] Mikrobiyol. Bul. 2022;56(1):36–48. doi: 10.5578/mb.20229904. [DOI] [PubMed] [Google Scholar]
- 99.Ben-Dov I.Z., Oster Y., Tzukert K., Alster T., Bader R., Israeli R., et al. Impact of tozinameran (BNT162b2) mRNA vaccine on kidney transplant and chronic dialysis patients: 3-5 months follow-up. J. Nephrol. 2022;35(1):153–164. doi: 10.1007/s40620-021-01210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alqassieh R., Suleiman A., Abu-Halaweh S., Santarisi A., Shatnawi O., Shdaifat L., et al. Pfizer-BioNTech and Sinopharm: a comparative study on post-vaccination antibody titers. Vaccines (Basel). 2021;9(11):1223. doi: 10.3390/vaccines9111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sourij C., Tripolt N.J., Aziz F., Aberer F., Forstner P., Obermayer A.M., et al. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: the prospective COVAC-DM cohort study. Diabetes Obes. Metab. 2022;24(5):849–858. doi: 10.1111/dom.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cucchiari D., Egri N., Bodro M., Herrera S., Del Risco-Zevallos J., Casals-Urquiza J., et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karamese M., Tutuncu E.E. The effectiveness of inactivated SARS-CoV-2 vaccine (CoronaVac) on antibody response in participants aged 65 years and older. J. Med. Virol. 2022;94(1):173–177. doi: 10.1002/jmv.27289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Unsworth R., Wallace S., Oliver N.S., Yeung S., Kshirsagar A., Naidu H., et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43(11):e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 105.Kamrath C., Mönkemöller K., Biester T., Rohrer T.R., Warncke K., Hammersen J., et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324(8):801–804. doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knip M., Parviainen A., Turtinen M., But A., Härkönen T., Hepojoki J., et al. SARS-CoV-2 and type 1 diabetes in children in Finland: an observational study. Lancet Diabetes Endocrinol. 2023;11(4):251–260. doi: 10.1016/S2213-8587(23)00041-4. [DOI] [PubMed] [Google Scholar]
- 107.Jia X., Gesualdo P., Geno Rasmussen C., Alkanani A.A., He L., Dong F., et al. Prevalence of SARS-CoV-2 antibodies in children and adults with type 1 diabetes. Diabetes Technol. Ther. 2021;23(7):517–521. doi: 10.1089/dia.2020.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hippich M., Holthaus L., Assfalg R., Zapardiel-Gonzalo J., Kapfelsperger H., Heigermoser M., et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med. 2021;2(2):149–163.e4. doi: 10.1016/j.medj.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salmi H., Heinonen S., Hästbacka J., Lääperi M., Rautiainen P., Miettinen P.J., et al. New-onset type 1 diabetes in Finnish children during the COVID-19 pandemic. Arch. Dis. Child. 2022;107(4):180–185. doi: 10.1136/archdischild-2020-321220. [DOI] [PubMed] [Google Scholar]
- 110.D’Addio F., Sabiu G., Usuelli V., Assi E., Abdelsalam A., Maestroni A., et al. Immunogenicity and safety of SARS-CoV-2 mRNA vaccines in a cohort of patients with type 1 diabetes. Diabetes. 2022;71(8):1800–1806. doi: 10.2337/db22-0053. [DOI] [PubMed] [Google Scholar]
- 111.Atkinson M.A., Powers A.C. Distinguishing the real from the hyperglycaemia: does COVID-19 induce diabetes? Lancet Diabetes Endocrinol. 2021;9(6):328–329. doi: 10.1016/S2213-8587(21)00087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Heide V., Jangra S., Cohen P., Rathnasinghe R., Aslam S., Aydillo T., et al. Limited extent and consequences of pancreatic SARS-CoV-2 infection. Cell Rep. 2022;38(11) doi: 10.1016/j.celrep.2022.110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kendall E.K., Olaker V.R., Kaelber D.C., Xu R., Davis P.B. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw. Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tao K., Tzou P.L., Nouhin J., Gupta R.K., de Oliveira T., Kosakovsky Pond S.L., et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021;22(12):757–773. doi: 10.1038/s41576-021-00408-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M., et al. SARS-CoV-2 variants, vaccines, and host immunity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jones J.M., Stone M., Sulaeman H., Fink R.V., Dave H., Levy M.E., et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA. 2021;326(14):1400–1409. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hong B., Bonczak B.J., Gupta A., Thorpe L.E., Kontokosta C.E. Exposure density and neighborhood disparities in COVID-19 infection risk. Proc. Natl. Acad. Sci. U. S. A. 2021;118(13) doi: 10.1073/pnas.2021258118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levy B.L., Vachuska K., Subramanian S.V., Sampson R.J. Neighborhood socioeconomic inequality based on everyday mobility predicts COVID-19 infection in San Francisco, Seattle, and Wisconsin. Sci. Adv. 2022;8(7) doi: 10.1126/sciadv.abl3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schultz W.M., Kelli H.M., Lisko J.C., Varghese T., Shen J., Sandesara P., et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu F., Althaus T., Tan C.W., Costantini A., Chia W.N., Van Vinh Chau N., et al. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe. 2022;3(2):e81–e82. doi: 10.1016/S2666-5247(21)00307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dogan M., Kozhaya L., Placek L., Gunter C., Yigit M., Hardy R., et al. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun. Biol. 2021;4(1):129. doi: 10.1038/s42003-021-01649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beaudoin-Bussières G., Chen Y., Ullah I., Prévost J., Tolbert W.D., Symmes K., et al. A Fc-enhanced NTD-binding non-neutralizing antibody delays virus spread and synergizes with a nAb to protect mice from lethal SARS-CoV-2 infection. Cell Rep. 2022;38(7) doi: 10.1016/j.celrep.2022.110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Castañé H., Iftimie S., Baiges-Gaya G., Rodríguez-Tomàs E., Jiménez-Franco A., López-Azcona A.F., et al. Machine learning and semi-targeted lipidomics identify distinct serum lipid signatures in hospitalized COVID-19-positive and COVID-19-negative patients. Metabolism. 2022;131 doi: 10.1016/j.metabol.2022.155197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nguyen N.N., Ho D.S., Nguyen H.S., Ho D.K.N., Li H.Y., Lin C.Y., et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: a meta-analysis. Metabolism. 2022;131 doi: 10.1016/j.metabol.2022.155196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boutari C., Mantzoros C.S. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133 doi: 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Amjadi M.F., O’Connell S.E., Armbrust T., Mergaert A.M., Narpala S.R., Halfmann P.J., et al. Specific COVID-19 symptoms correlate with high antibody levels against SARS-CoV-2. Immunohorizons. 2021;5(6):466–476. doi: 10.4049/immunohorizons.2100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frasca D., Reidy L., Romero M., Diaz A., Cray C., Kahl K., et al. The majority of SARS-CoV-2-specific antibodies in COVID-19 patients with obesity are autoimmune and not neutralizing. Int. J. Obes. (Lond). 2022;46(2):427–432. doi: 10.1038/s41366-021-01016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.