Abstract
The gut microbiota (GM) plays a crucial role in maintaining the overall health and well-being of the host. Recent studies have demonstrated that the GM may significantly influence bone metabolism and degenerative skeletal diseases, such as osteoporosis (OP). Interventions targeting GM modification, including probiotics or antibiotics, have been found to affect bone remodeling. This review provides a comprehensive summary of recent research on the role of GM in regulating bone remodeling and seeks to elucidate the regulatory mechanism from various perspectives, such as the interaction with the immune system, interplay with estrogen or parathyroid hormone (PTH), the impact of GM metabolites, and the effect of extracellular vesicles (EVs). Moreover, this review explores the potential of probiotics as a therapeutic approach for OP. The insights presented may contribute to the development of innovative GM-targeted therapies for OP.
Subject terms: Bone, Metabolic bone disease, Metabolic disorders
Introduction
As a severe skeletal disease, osteoporosis (OP) has garnered considerable attention due to its characteristic symptoms of low bone mass and deterioration of bone microarchitecture, which increases fragility and the risk of fractures.1 Individuals over 50 years old, particularly women, are more susceptible to fractures in the hip, vertebral body, and wrist.2 Women face a greater risk of developing OP due to bone loss caused by the sharp decline in estrogen levels during menopause.3 Moreover, estrogen-independent mechanisms, such as secondary hyperparathyroidism, chronic inflammation, and senility, can also contribute to the development of OP.4–7
Bone tissue undergoes continuous renewal through bone remodeling, which is maintained by the equilibrium of osteoclast (OC)-mediated bone resorption and osteoblast (OB)-mediated bone formation.8 The development and application of clinical therapeutics for OP, including antiresorptive agents and anabolic agents, primarily depend on the structures and functions of OBs and OCs.2 In their review of the currently available drugs for OP, Khosla and Hofbauer noted that some drugs are not recommended for long-term use due to their severe side effects and complications. Consequently, there is an urgent need to develop more effective anti-OP drugs.9–11
The gut microbiota regulates bone remodeling
An imbalance in the gut microbiota (GM), known as dysbiosis, has been observed in individuals with OP.12–14 The use of ovariectomized (OVX) mice, a widely utilized animal model in OP studies, has revealed a clear association between GM and bone mass.14 Studies involving artificial interference of the GM in animals have demonstrated that the GM acts as a regulator of bone mineral density (BMD).15–22 These interventions generally include fecal microbiota transplantation (FMT) in germ-free (GF) mice15–19 and antibiotic treatment in conventionally raised mice.20–22 Additionally, under physiological conditions, the GM has been shown to influence bone development through “maternal vertical transmission” and “cohabitation transmission”.23 In this review, we summarize recent findings on the regulation of bone metabolism by the GM and examine its promising potential as a therapeutic target for OP.
Evidence from experiments with GF mice
The administration of FMT in GF mice has demonstrated that the GM plays a significant role in regulating bone mass.15–19 The initial study that investigated the effect of the GM on bone mass discovered that GF mice exhibited a higher BMD and fewer OCs than wild-type (WT) mice.15 Moreover, male GF mice had higher BMD, a higher ratio of bone volume to total volume (BV/TV), and an increased trabecular bone (Tb) number (Tb. N.) in the proximal tibia in comparison to specific pathogen-free (SPF) mice.16 Furthermore, the alveolar BMD of GF mice was found to be higher than that of SPF mice.17 However, Schwarzer et al. showed that GF mice had shorter femurs and lower cortical bone (Cb) thickness, Cb fraction, and Tb fraction than WT mice.18 The variability in these study results could potentially be attributed to the differences in the sex and genetic background of the mice used. The GM exhibited varying regulatory patterns depending on colonization time, as well as the sex and strain of mice. After one month of colonization, female GF mice experienced decreased Tb mass with increased levels of bone absorption marker C-terminal telopeptides of type I collagen (CTX-I) and bone formation marker procollagen type I N-terminal propeptide (P1NP). Furthermore, mice subjected to extended GM colonization had longer femurs than GF mice, despite comparable Tb mass, bone resorption markers, and bone formation markers.19
Evidence from experiments in antibiotic-treated mice
The administration of antibiotics is a model that is widely used to investigate the relationship between the GM and host metabolism.24 Weaned female mice exhibited a higher BMD after antibiotic therapy (penicillin, vancomycin, penicillin plus vancomycin, or chlortetracycline; 1 μg·g−1 body weight) for three weeks than untreated mice.21 Treating parental mice with antibiotics also altered the skeletal structure of their offspring in a gender-specific manner. Compared to the control group, male mice displayed decreased bone mineral content (BMC) and bone area (BA) but unchanged BMD, while female mice exhibited increased BMD with no significant changes in BMC or BA.20 One study showed that female mice exposed to therapeutic doses of amoxicillin, tylosin, or a mixture of the two (alternating courses of amoxicillin and tylosin) on days 10–15, 28–31, and 37–40 displayed an increase in BMC and BA across all administrations, with amoxicillin having the most significant effect.22 Female mice had increased BV/TV after one month of treatment with broad-spectrum antibiotics (ampicillin, vancomycin, metronidazole, and neomycin). Oral vancomycin alone also increased bone mass, suggesting that gram-positive bacteria may play a substantial role in bone remodeling.19 However, administering a four-week course of antibiotics has been shown to disrupt the Tb architecture of the femur.25
Limitations of using GF mice and antibiotic-treated mouse models
It has been shown that GF mice and antibiotic-treated mice serve as effective experimental models for demonstrating the regulation of bone metabolism by the GM. However, they are not considered suitable as screening models for identifying probiotics as potential treatments for OP. The use of GF animals as research models is limited by the absence of a normal GM, which may render the results of such studies potentially inapplicable to individuals or animals with a normal GM. The GM is critical for the postnatal development and maturation of the immune system, which is essential for bone physiology.26–29 The underdeveloped lymphoid organs of GF animals, due to a lack of antigenic stimulus, may not reflect real-life scenarios and result in inaccurate findings regarding immunity and related pathways.30,31
The use of broad-spectrum antibiotics can have a profound and long-lasting effect on GM composition. While the GM eventually returns to baseline within 8 to 31 months, the composition of bacterial communities often remains altered.32 In addition, antibiotics can significantly reduce the abundance of bacteria capable of producing butyrate,32 which has been shown to promote bone formation.33 Research has also revealed that antibiotic treatment can dramatically reduce the abundance of Bacteroidetes, whose reduction is associated with inflammatory bowel disease (IBD) and type 1 diabetes, both of which involve excessive bone loss.25,34,35
In summary, various factors, including the host’s sex and genetics,16–18 GM colonization time,19 and antibiotic treatment,21,25 may influence the composition of the GM. Variations in GM components could partially explain differences observed within the same animal model. Even the same strain of mice from different laboratories may have different GMs.23 As a result, it is crucial to focus on the function of individual bacterial strains when evaluating the potential of probiotics as a treatment for OP.
Regulatory mechanism of bone remodeling by the GM
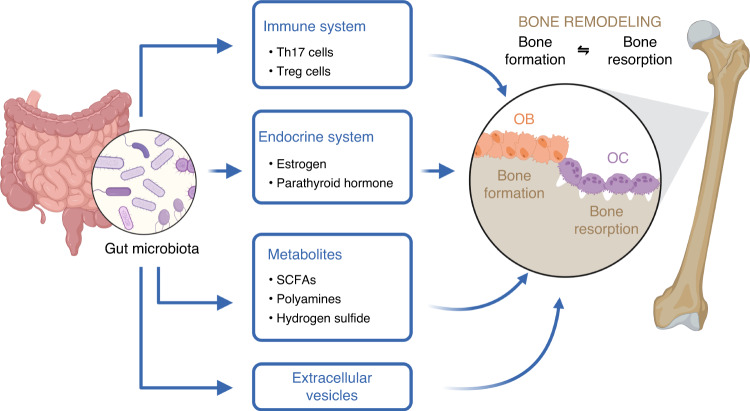
As illustrated in Fig. 1, the regulatory mechanism of bone metabolism by the GM includes direct and indirect effects. Directly, GM influences bone remodeling through the release of extracellular vesicles (EVs) or microbial metabolites such as short-chain fatty acids (SCFAs), polyamines, and hydrogen sulfide (H2S). Indirectly, the GM regulates bone remodeling by its interaction with immune cells, such as T helper cells 17 (Th17 cells) and T regulatory cells (Treg cells), or hormones such as estrogen and parathyroid hormone (PTH). This section delves into the various mechanisms through which the GM affects bone remodeling.
Fig. 1.
Regulation mechanisms of bone metabolism by gut microbiota. GM indirectly impacts bone metabolism by interacting with the immune system, maintaining a balance between Th17 cells and Treg cells. Additionally, GM or its metabolites have been demonstrated to be pivotal in regulating bone remodeling through their effect on estrogen and PTH. Moreover, GM regulates bone remodeling directly through its microbial metabolites, such as SCFAs, polyamines, and H2S, as well as EVs. EVs Extracellular vesicles, H2S Hydrogen sulfide, SCFAs Short-chain fatty acids, Th17 cells T helper cells 17, Treg cells T Regulatory cells. Created with BioRender.com
The GM regulates bone remodeling through the immune system
The intestine is the largest lymphatic organ and is host to a diverse array of microorganisms. The instrumental role of the GM in immune maturation, homeostasis, and inflammatory diseases has been widely recognized.36 A dysbiotic GM is associated with increased intestinal permeability, characterized by decreased expression of intestinal tight junction proteins and leading to bacterial translocation, chronic inflammation, and the migration of inflammatory cells.25,30 This persistence of intestinal inflammation often results in chronic inflammatory diseases that are associated with bone destruction, even if the inflammatory site is not located in the bone, as seen in IBD or Crohn’s disease (CD).37 Significant bone loss has been observed in various models of intestinal inflammation, including in dextran sulfate sodium (DSS)-induced chemical injury, adoptive T-cell transfer of colitis, and Salmonella enterica infection.38 Several chemokines and inflammatory cytokines in the femur, including granulocyte colony-stimulating factor (G-CSF), tumor necrosis factor-α (TNF-α), interleukin (IL)-12p40, MCP-1/CCL-2, RANTES/CCL-5, and keratinocyte-derived chemokine/CXCL1, increased dramatically across multiple colitis models, resulting in the expansion of osteoclast precursor cells (pre-OC).38 The receptor activator of nuclear factor kappa-B ligand (RANKL), due to its expression in both activated T cells and mesenchymal lineage cells, is considered an irreplaceable molecule that bridges the immune system and skeletal system.39–43 The term “osteoimmunology” highlights the reciprocal interactions between the skeletal and immune systems.44,45
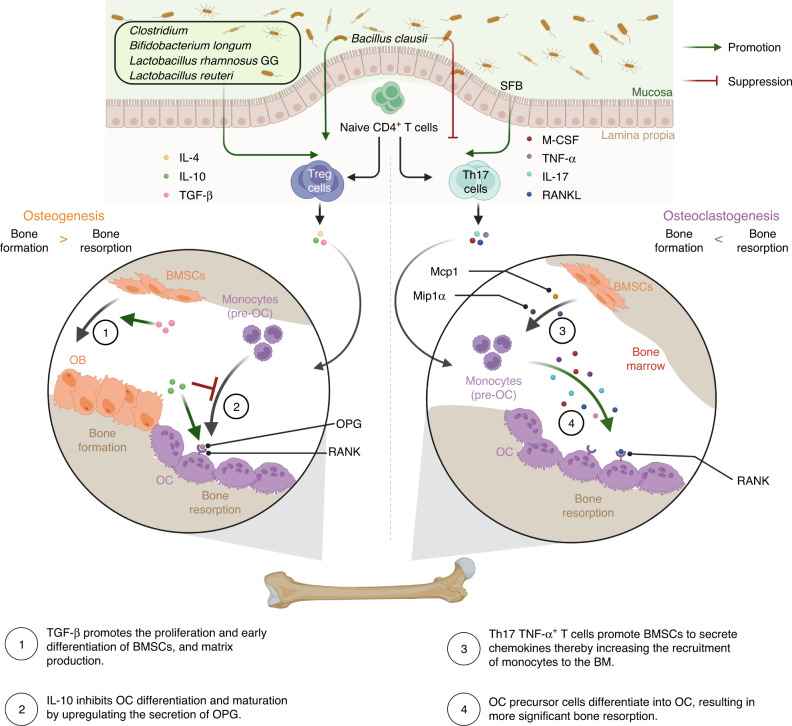
T helper 17 cells
Early studies indicated that activated T cells were an essential source of RANKL.46 Subsequent research, however, revealed that not all activated T cells have the capability to stimulate OC differentiation. Only Th17 TNF-α+ cells selectively express macrophage colony-stimulating factor (M-CSF) and RANKL, but not interferon (IFN)-γ, which acts as an inhibitor of OC differentiation.35,46 The mechanism by which Th17 cells regulate bone metabolism is depicted in Fig. 2. Bone marrow (BM) Th17 TNF-α+ cells not only promote OC differentiation in the absence of exogenous osteoclastogenic factors but also stimulate bone marrow mesenchymal stem cells (BMSCs) to secrete chemokines (Mcp1, Mip1α, and RANKL) and recruit inflammatory monocytes (pre-OC) to the BM, resulting in increased bone resorption.35 Certain bacteria, such as segmented filamentous bacteria (SFB), Bifidobacterium adolescentis, and Eggerthella lenta, have been reported to expand Th17 cells.47–49 As a gram-positive commensal bacterium, SFB stimulates an increase in Th17 cells and the secretion of IL-17 in the gut.47,50–52 SFB has been demonstrated to disrupt BMD through maternal vertical transmission and cohabitation transmission.23,53,54
Fig. 2.
Gut microbiota regulates bone metabolism through the immune system. Th17 and Treg cells exhibit opposite effects on bone remodeling. GM influences bone remodeling by modulating the balance between Th17 and Treg cells. Th17 cells promote OC differentiation, leading to heightened bone resorption. Conversely, Treg cells inhibit osteoclastogenesis and increase bone formation by secreting anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β. BM Bone marrow, BMSCs Bone marrow mesenchymal stem cells, IL Interleukin, M-CSF Macrophage colony-stimulating factor, OB Osteoblasts, OC Osteoclasts, OPG Osteoprotegerin, RANK Receptor activator of nuclear factor kappa-B, RANKL Receptor activator of nuclear factor kappa-B ligand, SFB Segmented filamentous bacteria, TGF-β Transforming growth factor-β, Th17 cells T helper cells 17, TNF-α Tumor necrosis factor-α, Treg cells T Regulatory cells. Created with BioRender.com
Th17 cells play a vital role in OVX-induced bone loss.30,55 These cells stimulate osteoclastogenesis and bone resorption through the elevated production of IL-17, RANKL, and TNF-α.56 Premenopausal women who underwent OVX have higher numbers of activated circulating CD3+ CD69+ T cells and CD3+ TNF+ cells in their peripheral blood than healthy individuals.57 An increase in Th17 TNF-α+ T-cell-mediated TNF-α is considered the predominant factor involved in PTH-induced bone loss.5 Th17 cells, which contain the vβ14+ chain on their T-cell receptor, are typically only found in the lamina propria under noninflammatory conditions.58 However, the administration of continuous parathyroid hormone (cPTH) has been shown to increase the number of vβ14+ Th17 cells in the BM, which depends on the presence of SFB.5 TNF increases Th17 cells (CD4+ IL-17A+ T cells) in the gut in concert with SFB and guides the migration of intestinal Th17 cells to BM through the upregulation of CCL20.5 A recent study found that aging leads to an accumulation of immune cells (including neutrophils, monocytes-macrophages, and M1-like macrophages) in the BM of 18-month-old rats.59 The abundance of grancalcin (GCA) secreted by neutrophils and monocytes-macrophages in the BM disrupts skeletal microarchitecture by promoting adipogenic differentiation and suppressing bone turnover at the expense of ossification, which are well-known characteristics of senescent BMSCs.59,60
The following treatment options can be considered based on the above research: 1. Inhibition of the transportation of immune cells to the BM. FTY720, an inhibitor of sphingosine 1 phosphate (S1P) receptor-1, can arrest lymphocyte transportation from Peyer’s patches (PPs) and mesenteric lymph nodes without affecting lymphocyte function. FTY720 has been shown to reduce the number of Th17 cells and Vβ14+ Th17 cells in the BM.5 2. Transport BM immune cells back to the intestinal tract. Oral administration of synthetic retinoid AM80 upregulates the expression of gut-homing molecule α4β7 on T cells, transferring T follicular helper cells (CD19− CD4+ CXCR+ PD-1+ T cells) from the inflammation site to PPs and thereby reducing the inflammatory response.61 Increasing the migration of T cells from bone to intestine-related lymphoid tissues theoretically minimizes T-cell-derived RANKL. However, some drugs used to treat IBD target anti-α4β7 integrin (vedolizumab), which acts therapeutically by blocking the gut homing of T lymphocytes.62–64 Consequently, methods that address both conditions (OP or IBD) are urgently needed. 3. Neutralize inflammatory factors with antibodies. A neutralizing IL-17A antibody can inhibit RANKL expression and bone loss in OVX mice. Deficiency of IL-17RA or Act1, an IL-17RA-interacting protein, protects mice from OVX-induced bone loss.65
T Regulatory cells
Contrary to the impact of Th17 cells, Treg cells, which are CD4+ T cells with immunosuppressive functions, have a beneficial impact on bone remodeling.48 Certain microbes regulate bone remodeling by altering the balance between Th17 cells and Treg cells. For example, oral administration of Bacillus clausii has been shown to significantly increase the population of CD4+ Foxp3+ Treg cells and decrease the proportion of CD4+ Rorγt+ Th17 cells in the BM and spleen, thereby preventing OVX-induced bone loss.66 Clostridia have demonstrated considerable benefits in Treg cell populations.67–70 Furthermore, specific bacterial species such as Lactobacillus rhamnosus GG (LGG), Lactobacillus reuteri (L. reuteri), Bifidobacterium breve (B. breve) AH1205, Bifidobacterium longum (B. longum) AH1206 and probiotic mixture VSL#3 (a mixture of B. breve, B. longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum (L. plantarum), Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophilus) have the potential to impact both the abundance and functionality of Treg cells.33,71–74
The mechanism by which Treg cells regulate bone metabolism is illustrated in Fig. 2. Treg cells inhibit osteoclastogenesis and promote bone formation by secreting anti-inflammatory cytokines such as IL-4, IL-10, and transforming growth factor-β (TGF-β).44,75,76 IL-10, an inhibitory cytokine, helps to downregulate the expression of RANKL and M-CSF and enhances the secretion of osteoprotegerin (OPG), thereby inhibiting OC differentiation and maturation.77 TGF-β plays a critical role in regulating various stages of OB differentiation, promoting the proliferation and early differentiation of OB progenitor cells and stimulating matrix production but inhibiting later differentiation and matrix mineralization.78 A mixture of Clostridia strains belonging to clusters IV, XIVa, and XVIII can activate intestinal epithelial cells to produce TGF-β in the colon.69 Furthermore, Treg cells have the capacity to stimulate CD8+ T cells to secrete the Wnt ligand Wnt10b, which stimulates bone formation by activating Wnt signaling in OBs.33 The administration of LGG did not result in any increase in BV and bone formation in mice depleted of Treg cells through anti-CD25 antibody treatment, highlighting the crucial role of Treg cells in the bone anabolic activity of LGG.33
The role of the GM in estrogen regulation of bone metabolism
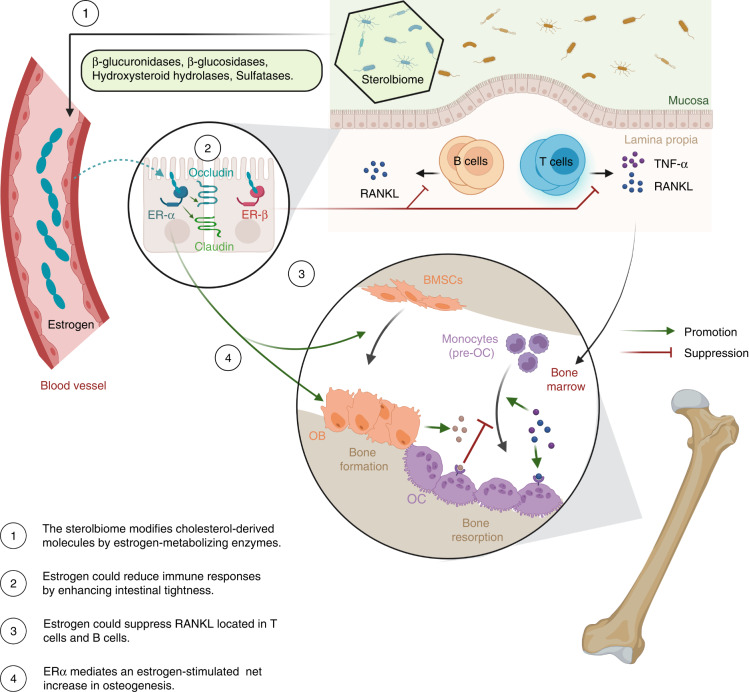
Accumulated research has revealed the irreplaceable role of estrogen in osteogenesis and osteoclastogenesis.46,79–83 As illustrated in Fig. 3, estrogen receptor α (ERα) mediates an estrogen-stimulated net increase in bone formation.84,85 A lack of ERα results in reduced femur length in female adult mice.86 The regulation of bone metabolism by estrogen also has close ties to the immune system, as it protects bone mass through the downregulation of immune responses and modulation of the balance between OB and OC.87 Estrogen suppresses RANKL located in CD3+ T cells and CD20+ B cells88 and elevates the production of OPG in osteoblastic cells.89,90 Bone loss resulting from decreased estrogen is due to the T-cell-mediated increase in TNF-α, which indirectly enhances OC differentiation.91–94 The activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in response to sex steroid deficiency promotes bone resorption and impedes bone formation.90
Fig. 3.
The role of gut microbiota in estrogen regulation of bone metabolism. Estrogen has an irreplaceable role in osteogenesis and osteoclastogenesis. Some “sterolbiome”, which could modify these cholesterol-derived molecules, regulates bone remodeling by interaction with estrogen. BMSCs Bone marrow mesenchymal stem cells, ER Estrogen receptor, OB Osteoblasts, OC Osteoclasts, OPG Osteoprotegerin, pre-OC Osteoclast precursor cells, RANK Receptor activator of nuclear factor kappa-B, RANKL Receptor activator of nuclear factor kappa-B ligand, TNF-α Tumor necrosis factor-α. Created with BioRender.com
The role of the GM in regulating estrogen metabolism is attracting growing interest. The GM directly contributes to the control of host sex steroid levels.95 The term “sterolbiome” refers to the collection of gut microbes that modify these cholesterol-derived molecules.96 The most critical estrogen-metabolizing enzymes encoded by the sterolbiome are β-glucuronidases, β-glucosidases, hydroxysteroid hydrolases, and sulfatases, which deconjugate estrogens to enhance intestinal reuptake.97,98 These enzymes and their activities are well represented in the human GM and are modulated by diet and bacterial density, leading to changes in local and systemic estrogen levels.99,100 Li et al. reported that sex steroid deficiency-associated bone loss was dependent on the GM. Estrogen deficiency induced by leuprolide, a gonadotrophin-releasing hormone agonist, was insufficient to increase bone resorption and Tb loss in GF mice. Mechanistically, estrogen deficiency led to decreased expression of intestinal tight junction proteins, increased intestinal permeability, and elevated serum endotoxin levels; however, these phenotypes were unaffected in GF mice. Only conventionally raised mice showed increased expression of osteoclastogenic cytokines in both the BM and small intestine after estrogen deprivation,30 thus indicating that the bone loss associated with estrogen deficiency is linked to GM-driven inflammatory signaling.
The role of the GM in parathyroid hormone regulation of bone metabolism
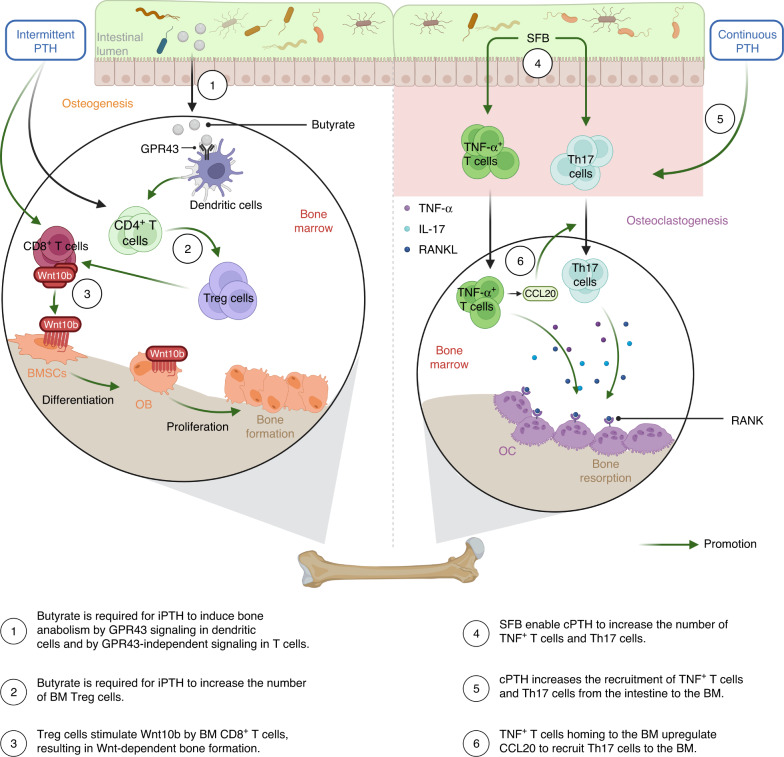
As a pivotal hormone regulating calcium balance, PTH plays a critical role in postnatal skeletal development.101 The effect of PTH on bone remodeling, as depicted in Fig. 4, is contingent upon the pattern of exposure of target cells to PTH - whether it is continuous or intermittent.
Fig. 4.
The role of gut microbiota in PTH regulation of bone metabolism. PTH plays a vital role in postnatal skeletal development. SFB enhances the inflammatory responses by interacting with cPTH in the gut and BM, leading to bone loss. However, the GM metabolite butyrate is essential for iPTH to stimulate bone formation. BM: Bone marrow, BMSCs Bone marrow mesenchymal stem cells, cPTH Continuous parathyroid hormone, iPTH Intermittent parathyroid hormone, GPR G-protein-coupled receptor, IL Interleukin, OB Osteoblasts, OC Osteoclasts, PTH Parathyroid hormone, RANK Receptor activator of nuclear factor kappa-B, RANKL Receptor activator of nuclear factor kappa-B ligand, SFB Segmented filamentous bacteria, Th17 cells T helper cells 17, TNF-α Tumor necrosis factor-α, Treg cells T Regulatory cells. Created with BioRender.com
Continuous parathyroid hormone
Primary hyperparathyroidism, a condition characterized by chronic continuous overproduction of PTH by the parathyroid glands,102 can be modeled in animals through cPTH infusion.103 This condition, which is a common cause of OP and fractures,104,105 is characterized by the critical role of osteocyte-derived RANKL and T-cell-derived IL-17A in promoting bone catabolism.106 However, cPTH was unable to induce bone loss in either antibiotic-treated or GF mice. The presence of SFB in the GM enabled cPTH to increase the number of intestinal TNF+ T cells and Th17 cells, as well as the recruitment of these cells from the intestine to the BM. The TNF+ T cells in the BM upregulated CCL20, a chemoattractant that facilitated the recruitment of Th17 cells from the intestine to the BM.5
Intermittent parathyroid hormone
Maximizing the bone anabolic effects of PTH can be achieved by administering daily injections of intermittent PTH (iPTH) in young mice.107,108 This intervention results in a marked increase in both BV and strength by stimulating bone formation.109,110 The activation of Wnt signaling in osteoblastic cells drives these effects, which are characterized by enhanced OB formation, extended lifespan, and reactivation of bone lining cells.110,111
A recent study revealed that the permissive levels of butyrate produced by the GM are essential for iPTH to induce bone anabolism in mice.54 In the absence of the GM, the anabolic effects of iPTH and antibiotic treatment resulted in no increase in Treg cells in the gut and BM. However, the administration of butyrate restored the bone anabolic activity of iPTH and increased the number of Treg cells. By binding to G-protein-coupled receptor 43 (GPR43) located in dendritic cells, butyrate stimulates Treg differentiation, which then triggers the expression of the Wnt ligand Wnt10b in BM CD8+ T cells and activates Wnt-dependent bone formation.54
The GM regulates bone physiology via metabolites
The GM exerts positive effects on distal organs through the production of secondary metabolites, which serve as essential regulators of anatomically distant organs.112 These metabolites are now commonly referred to as “postbiotics”.113
Short-chain fatty acids (SCFAs)
Vegetable fiber is a dietary element that plays an important role in human health and microbial composition.112 SCFAs, including acetate, propionate, and butyrate, are produced through microbial fermentation of nondigestible dietary fiber.114 While acetate can be produced by various bacterial species, the production pathways for propionate and butyrate are more limited and involve specific bacterial strains.115 Akkermansia muciniphila (A. muciniphila) generates propionate by digesting intestinal mucin.116 Additionally, several bacterial species, including Eubacterium dolichum, Ruminococcus bromii (R. bromii), Bacteroides eggerthii, Bacteroides fragilis, and Veillonella parvula, have been linked to propionate production in the intestine.117 The majority of butyrate-producing bacteria in the human gut belong to the families Clostridiaceae, Eubacteriaceae, Lachnospiraceae, and Ruminococcaceae.118 Representative species of these families, such as Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii, R. bromii, and Clostridium butyricum, have been identified as the key butyrate producers.119–121
Acetate, propionate, and butyrate significantly increased bone mass through distinct mechanisms. Acetate inhibits OC numbers in a T-cell- and B-cell-dependent manner,120 while propionate and butyrate effectively prevent OVX-induced bone loss by decreasing OC number and serum CTX-I levels. However, acetate did not show any protection against OVX-induced bone loss.120 Additionally, acetate, propionate, and butyrate also suppressed OC differentiation in vitro,120 which may be related to their inhibition of histone deacetylase activity.112,122–124 Butyrate inhibits osteoclastogenesis by suppressing c-Fos, tumor necrosis factor receptor associated Factor 6 (TRAF6), and nuclear factor of activated T cells cytoplasmic 1 (NFATc1) expression.120,125,126
Studies of SCFAs in bone formation have mainly focused on butyrate. After treatment with butyrate, mice exhibited a substantial increase in BV/TV, serum osteocalcin (OCN), and mineral apposition rate (MAR) after two and four weeks compared to those treated with a control vehicle.33 The increase in femoral BV/TV by butyrate disappeared following inhibition of Treg cells with a CD25 antibody, demonstrating that the capacity of butyrate to stimulate bone formation was Treg-dependent.33 While Lucas et al. reported that butyrate improves bone mass by inhibiting osteoclastogenesis instead of promoting bone formation,120 early studies in the 2000s showed that butyrate promotes osteogenic differentiation of human BMSCs.127,128 These studies demonstrate butyrate’s ability to promote bone formation, although the mechanisms appear contradictory. While studies have shown that the administration of butyrate increased BMSC proliferation in WT mice but not in mice treated with CD25 antibodies,33 it remains unclear whether CD25 antibodies affect OB mineralization by butyrate in vitro. Although initial studies have explored the mechanism of butyrate’s promotion of bone formation in BMSCs, further research is necessary using various cell lines or animal models to provide a complete understanding. Studies of acetate and propionate on OB are limited. Acetate was recently reported to increase the differentiation of aging BMSCs by restoring cytosolic acetyl-CoA levels and remodeling the chromatin landscape.60
The effective regulation of SCFAs in bone remodeling highlights their potential role in novel therapeutic strategies in OP treatment. Researchers have reported that a vegetarian diet or a Mediterranean diet is beneficial to bone health.129,130 Maximizing SCFA levels by supplementing a diet rich in vegetable fiber or SCFA-producing bacteria may provide an approach to prevent against OP.
Polyamines
Ubiquitous in all organisms, polyamines are naturally occurring organic polycations derived from amino acids and are involved in various biological processes, such as proliferation, differentiation, and apoptosis.131 With a fast plasma turnover and the ability to quickly reach target tissues,132 the majority of the host polyamine pool is comprised of polyamines originating from the GM.133 Gut microbes primarily produce polyamines through the transamination of ingested amino acids, particularly arginine, by catalytic enzymes.134,135 The decrease in total bacteria by antibiotic administration resulted in the depletion of spermine levels in the intestine.133 Additionally, an increased abundance of A. muciniphila may be associated with polyamine biosynthesis.8
Polyamines have been shown to promote osteogenic differentiation of goat adipose tissue‐derived mesenchymal stem cells (ADSCs) and mouse BMSCs.8,136,137 Lee et al. demonstrated that exogenous polyamines regulated osteogenic and adipogenic differentiation in a reciprocal manner,131 upregulating osteogenic gene expression (including runt-related transcription Factor 2 (RUNX2), alkaline phosphatase (ALP), osteopontin, and OCN) and downregulating adipogenic gene expression (such as peroxisome proliferator-activated receptor (PPAR-γ)), thereby reducing fat accumulation and promoting extracellular matrix mineralization and osteogenesis in human BMSCs.131 Additionally, polyamines are known inhibitors of osteoclastogenesis, with oral administration of spermidine or spermine directly preventing an increase in the OC surface/bone surface ratio and a decrease in the BV in OVX mice.138 Either spermine or spermidine could reduce the number of multinucleated tartrate-resistant acid phosphatase (TRAP)+ cells in a concentration-dependent manner in vitro.138 More recently, daily oral supplementation of a diet containing polyamine-rich yeast was found to inhibit osteoclastic activation in OVX mice.139 However, inhibition of polyamine biosynthesis in vivo has limited the beneficial effects of spermine and spermidine on bone strength.8
Excessive spermidine concentrations were associated with an increased risk of osteoporotic fracture.140 Spermine synthase is responsible for synthesizing spermine from spermidine. Deficiency of spermine synthase causes excessive spermidine accumulation and a lack of spermine.141 Patients with Snyder-Robinson syndrome, a syndrome caused by loss-of-function mutations of the spermine synthase gene, exhibit severe OP and kyphoscoliosis and have BMSCs with impaired capacities for osteogenic differentiation and mineralization.142
Hydrogen sulfide
As a vital endogenous gasotransmitter, H2S is produced through endogenous cysteine catabolism and sulfate-reducing bacteria (SRB).112,143–145 Germ-free mice have been found to have significantly lower levels of free H2S in the cecum and colon than conventional mice.143 Cysteine catabolic bacteria, including Fusobacterium, Clostridium, Escherichia, Salmonella, Klebsiella, Streptococcus, Desulfovibrio, and Enterobacter, convert cysteine to H2S, pyruvate, and ammonia through the action of cysteine desulfhydrase. Sulfate-reducing bacteria, including Desulfovibrio, Desulfobacter, Desulfobulbus, and Desulfotomaculum, are responsible for producing H2S, with Desulfovibrio being the dominant genus, and Desulfovibrio piger and Desulfovibrio desulfuricans being the dominant species.145 H2S serves as a critical metabolite for GM-mediated bone remodeling.
H2S has been implicated in bone formation and postnatal skeletal development, with the ability to maintain the self-renewal and osteogenic differentiation of BMSCs through the Wnt/β-catenin signaling pathway.146 Sodium hydrosulfide (NaHS), a common H2S donor, has been shown to decrease the RANKL/OPG mRNA ratio in human BMSCs.147 In pre-OCs, NaHS inhibited OC differentiation by reducing intracellular reactive oxygen species (ROS) levels and triggering nuclear factor erythroid 2-related Factor 2 (NRF2)-dependent antioxidant responses.147 In pathologic bone loss, OVX decreased the concentration of serum H2S and two key H2S-generating enzymes (cystathione β-synthase and cystathione γ-lyase) in the BM.148 H2S deficiency in cystathione β-synthase knockout mice leads to a consistently osteoporotic phenotype149 with impaired BMSCs and impaired bone formation.146 Restoration of H2S levels by the H2S donor GYY4317 reversed the osteopenia phenotype and osteogenic deficiency of BMSCs.146 The administration of GYY4137 increases serum H2S levels and bone formation by activating Wnt signaling via increased Wnt10b production and prevents the loss of Tb.148
Gut microbe-derived extracellular vesicles
Extracellular vesicles (EVs), which bacteria release as a means of interspecies communication, are spherical lipid bilayer nanostructures with diameters ranging from 10 to 400 nm. These nanostructures are comprised of various components, including bioactive proteins, lipids, nucleic acids, and virulence factors.150 Their unique nanoscale structure ensures the long-distance transport of EVs and their interior molecules throughout the intracellular compartments in a concentrated, protected, and targeted manner.151 Moreover, EVs released by different microbes exhibit different characteristics, such as different morphology, composition, and biogenesis. Additionally, environmental conditions can impact the protein profiles of EVs.152
Tong et al. isolated EVs from LGG that had a diameter of 161.9 ± 54.8 nm and expressed the exosomal protein TSG101. Oral administration of LGG-released EVs effectively ameliorated DSS-induced colitis by inhibiting Toll-like receptor (TLR) 4/NF-κB/nod-like receptor family 3 (NLRP3) axis activation. Treatment with LGG-EVs also reshaped the GM in colitis mice, with characteristic beneficial bacteria such as A. muciniphila and Bifidobacterium animalis clustering in the LGG-EV group.153 Administration of L. reuteri-EVs attenuated LPS-induced inflammation in broilers, thereby improving growth performance, reducing mortality, and reducing intestinal injury.154 Administration of EVs released from Lactobacillus sakei NBRC15893 was reported to enhance immunoglobulin A production by activating host TLR2 signaling.155 In addition, EVs derived from A. muciniphila were effective in protecting against DSS-induced colitis.156 Administration of A. muciniphila-released EVs was also found to regulate lipid metabolism and inhibit inflammation in the colon, adipose tissue, and liver, thereby ameliorating high-fat diet-induced obesity.157,158 The “gut-bone axis” has received limited attention regarding research on EVs, with only one study demonstrating that the protective effect of A. muciniphila on bone is mediated by the secretion of EVs. These nanovesicles enter and accumulate in bone tissues, inhibiting osteoclastogenesis and promoting osteogenesis.159
Due to the potential dangers of genetically modified organisms in clinical settings, EVs are considered an alternative to probiotics in some immunocompromised individuals.152 Chronic diseases such as inflammation or obesity are recognized as being closely related to bone metabolism. Further investigation is required to clarify the mechanism of the GM in the “gut-bone axis” from the perspective of EVs. However, producing sufficient quantities of EVs with high purity and reproducibility remains a critical challenge for subsequent studies.160
The prospect of probiotics in osteoporosis therapy
Fecal microbiota transplantation may not be an effective option for the treatment of OP due to the presence of harmful bacteria within the transplant material. Studies have shown that the therapeutic effects of FMT for IBD are also inconsistent.36 Therefore, it is essential to thoroughly study the function of bacterial species and evaluate their safety. Probiotics are defined as viable microorganisms that provide a health benefit when administered in adequate quantities.161 As a potential new therapy for OP, probiotics are gaining attention. The effects and involved mechanisms of probiotics on bone remodeling are summarized in Table 1.
Table 1.
The effects and underlying mechanisms of probiotics on bone remodeling in animal models and population-based studies
| Genus | Species | Research models or population | Effects | Mechanisms | References |
|---|---|---|---|---|---|
| Lactobacillus | L. reuteri ATCC PTA 6475 | OVX Balb/c mice |
Femur and vertebrae BV/TV, BMD, BMC, Tb. N. ↑ , Tb spacing ↓ |
TRAP5 and RANKL mRNA expression ↓ , BM CD4+ T cells, OC differentiation in vitro ↓ |
173 |
| L. reuteri ATCC PTA 6475 | Type 1 diabetic C57BL/6 mice |
Tb. BV/TV, Tb. N., Tb. Th., BMD, BMC ↑ , Tb spacing ↓ |
MAR, OB surface, serum OCN, Wnt10b expression and Wnt10b+ cells in gut and bone ↑ , BM adipocyte area and size ↓ | 34 | |
| L. reuteri ATCC PTA 6475 | BALB/c mice with OP |
Femur and vertebrae BV/TV, BMD, BMC, Tb. N., MAR, serum OCN ↑ , Tb spacing, serum TRAP 5b ↓ |
Colon permeability, colon TNF-α/IL-10 ratio ↓ | 25 | |
| L. reuteri ATCC PTA 6475 | Rag knockout C57BL/6 mice (deficient in mature T- and B- cells) | Femur BV/TV, BMC, Tb. Th. in WT mice ↑ |
IL-10, IFN-γ, TGF-β, osterix gene expression ↑ , L. reuteri requires lymphocytes to exert beneficial effects on bone. |
181 | |
| L. rhamnosus GG | OP C57BL/6 J mice induced by leuprolide administration | Spine and femur BV/TV, serum OCN ↑ , serum CTX ↓ |
Intestinal barrier integrity ↑ , TNF-α, RANKL, IL-17 mRNA expression ↓ |
30 | |
| L. rhamnosus GG | Conventionally raised C57BL/6 mice | Femur BV/TV, Spine BV/TV ↑ , serum CTX ↓ |
MAR, P1NP, Wnt10b expression in BM and CD8+ T cells, Foxp3+ T cells in BM, spleen, and PP ↑ |
33 | |
| L. acidophilus ATCC 4356 | OVX Balb/c mice |
Bone mass ↑ , Femur resorption pits/lacunae ↓ |
CD4+ Foxp3+ Treg cells, CD8+ Foxp3+ Treg cells, serum IL-10, IFN-γ ↑ , CD4+Rorγt+ Th17 cells, serum IL-17, TNF-α, RANKL ↓ |
174 | |
| L. sakei | RA DBA/1 J mice | Arthritis score, TRAP+-, RANK+-, RANKL+ -cells ↓ | TNF-α, IL-1β, IL-6, IL-17 ↓ | 180 | |
| L. fermentum ZS40 | OP Wistar rats | BV/TV, Tb. N., Tb. Th., BMD ↑, the number of OC, Tb spacing ↓ |
β-catenin, Wnt10b, Lrp5, Lrp6, Runx2, ALP, RANKL, OPG mRNA expression ↑ , DKK1, RANK, TRAP, CTSK mRNA expression ↓ |
175 | |
| L. Plantarum HFY15 | OP Wistar rats | BV/TV, Tb. N., Tb. Th., BMD ↑, the number of OC, Tb spacing ↓ |
β-Catenin, Wnt10b, Lrp5, Lrp6, Runx2, ALP mRNA expression ↑ , DKK1, RANK, TRAP, CTSK mRNA expression ↓ |
176 | |
| L. helveticus ATCC 27558 | OVX Sprague–Dawley rats | Femur BMD, breaking forces, serum OCN ↑ , serum CTX ↓ |
Runx2 and BMP2 mRNA expression ↑ , Lactobacillus enumeration in the feces ↑ |
177 | |
| L. curvatus Wikim 38 | OVX C57BL/6 mice | BV/TV, BMD, Tb. Th. ↑ |
F-actin ring formation, bone resorption, osteoclastogenesis, RANKL/TRAF6/ NF-κB/MAPK gene expression ↓ |
178 | |
| L. paracasei DSM13434, or a mixture of L. paracasei DSM13434, L. plantarum DSM 15312 and DSM 15313 | OVX C57BL/6 N mice | Cb. BMC., Cb. Area ↑ | BM TNF-α, IL-1β, OPG ↑ | 179 | |
| L. reuteri ATCC PTA 6475 | Postmenopausal women with osteopenia | The loss of total BMD ↓ | 189 | ||
| L. casei Shirota | Elderly patients with an acute distal radius fracture | DASH (disabilities of the arm, shoulder, and hand) score, pain, complex regional pain syndrome score, wrist flexion, and grip strength of patients receiving probiotics exhibited a significantly faster pace of improvement. | 190 | ||
| L. paracasei DSM 13434, DSM 15312, and DSM 15313 | Postmenopausal women with osteopenia | Lumbar spine bone loss ↓ | 188 | ||
| Bifidobacterium | B. longum | OVX Sprague-Dawley rats |
Serum OCN, femur BMD, Tb. Th., femur strength ↑ , Serum CTX ↓ |
Sparc and BMP2 mRNA expression ↑ , Total Bifidobacteria in the feces ↑ |
184 |
| B. longum 35624 | OC precursors from C57BL/6 N mice | OC differentiation ↓ | Surface exopolysaccharide of B. longum 35624 mediated inhibition of osteoclast formation is TLR2-dependent. | 186 | |
| B. adolescentis | Fractured C57BL/6 mice | Serum P1NP, callus cartilage remodeling ↑ , post-traumatic bone loss ↓ |
Tight junction protein ↑ , Systemic inflammation ↓ |
187 | |
| B. pseudocatenulatum CECT 7765 | Obesity C57BL/6 mice |
Tb. N., Serum OCN ↑ , Tb spacing, Serum CTX ↓ ; |
Canonical Wnt/β-catenin pathway ↑ | 185 | |
|
VSL#3 (B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. bulgaricus, and Streptococcus thermophilus) |
OP C57BL6/J mice induced by leuprolide administration | Femur BV/TV, Cb. V., serum OCN ↑ | Intestinal tight junction ↑ | 30 | |
| Akkermansia | A. muciniphila | OVX C57BL/6 mice |
BMD, Tb. BV/TV., Tb. N., serum OCN, MAR ↑ , Tb spacing, the number and size of OC ↓ |
EVs are required for the A. muciniphila‐induced bone protective effects. | 159 |
| A. muciniphila | C57/BL6 mice with experimental periodontitis | Alveolar bone loss ↓ | M2 macrophages, IL-10 gene expression ↑ | 201 | |
| A. muciniphila | Fractured C57BL/6 mice | BV/TV, tissue mineral density of callus, femoral ultimate load, serum OCN, bone fracture healing ↑ |
Type H vessel formation in callus ↑, inflammatory responses in fracture healing, intestinal permeability, and inflammation ↓ |
203 |
↑: up-regulated or increased compared to those mice without treatment
↓: down-regulated or decreased compared to those mice without treatment
ALP Alkaline phosphatase, BM Bone marrow, BMC Bone mineral content, BMD Bone mineral density, BMP2 Bone morphogenetic protein 2, BV/TV The ratio of bone volume to total volume, Cb Cortical bone, CTSK Cathepsin K, CTX-I C-terminal telopeptides of type I collagen, DKK1 Dickkopf-related protein 1, EVs Extracellular vesicles, IFN-γ Interferon-γ, IL Interleukin, LRP Low-density lipoprotein receptor-related protein, MAPK Mitogen-activated protein kinase, MAR Mineral apposition rate, OB Osteoblasts, OC Osteoclasts, OCN Osteocalcin, OP Osteoporosis, OPG Osteoprotegerin, OVX Ovariectomized, P1NP Procollagen type I N-terminal propeptide, PPs Peyer’s patches, RA Rheumatoid arthritis, RANKL Receptor activator of nuclear factor kappa-B ligand, Tb. N. Trabecular bone number, Tb. Th. Trabecular bone thickness, Tb Trabecular bone, TGF-β Transforming growth factor-β, TLR Toll-like receptor, TNF-α Tumor necrosis factor-α, TRAF6 Tumor necrosis factor receptor associated factor 6, TRAP Tartrate-resistant acid phosphatase, WT Wild-type
Conventional probiotics: Lactobacillus and Bifidobacterium
As a conventional probiotic, Lactobacillus has a long history of use.162 It is associated with various health benefits, including relief from diarrhea, irritable bowel syndrome (IBS), IBD, lactose intolerance, and obesity.163–167 Lactobacillus-fermented products, such as milk, soy skim milk, and kefir (fermented milk similar to yogurt with a history of over one hundred years), have been reported to have a beneficial effect on bone health.168–172 Lactobacillus supplementation has been shown to prevent bone loss induced by OVX,25,30,34,173–179 reduce the number of TRAP+ cells, receptor activator of nuclear factor kappa-B (RANK)+ cells, and RANKL+ cells in rheumatoid arthritis (RA) mice,180 and improve skeletal health in intact animals.33,181 Bifidobacterium, on the other hand, was markedly reduced in the senescence-induced OP model.182 Mice fed a low-calcium diet also had lower BMD and a decreased abundance of Bifidobacterium.183 Bifidobacterium has been proven to prevent bone loss induced by OVX or obesity,184,185 inhibit pre-OC differentiation in vitro,186 and accelerate callus cartilage remodeling in fractured mice.187 Administration of a probiotic cocktail (VSL#3 or a mixture of Lactobacillus paracasei (L. paracasei) DSM 13434, L. plantarum DSM 15312, and DSM 15313) could increase femur BV/TV and Cb. BMC in OVX mice.30,179 The mixture of L. paracasei DSM 13434, DSM 15312, and DSM 15313 has been used in clinical trials and proven to prevent lumbar spine bone loss in postmenopausal women with OP.188 Treatment with L. reuteri ATCC PTA 6475 or Lactobacillus casei Shirota (L. casei Shirota) alone could reduce BMD or accelerate distal radius fracture healing in the elderly.189,190
In addition, Lactobacillus and Bifidobacterium have been engineered as delivery vectors for specific molecules.191,192 The premise is that such bacterial vectors do not produce any virulence factors and are tolerated by the host.162 For example, Lactococcus lactis (L. lactis) was engineered to deliver IL-10 to control allergen sensitivity.193 Oral administration of L. lactis, engineered to express and deliver elafin (a natural protease inhibitor with pleiotropic anti-inflammatory properties), could decrease inflammation and restore intestinal homeostasis in mouse models of acute and chronic colitis.191 Another human commensal bacterium, Bacteroides ovatus, was also engineered for in situ delivery of TGF-β and treatment of colitis.192 Further studies are needed to explore the role of such engineered bacteria in bone metabolism.
Next-generation probiotics
The development of new probiotics, also known as “next-generation probiotics,” has gradually gained traction recently.162 One such probiotic is A. muciniphila, a newly identified genus in the phylum Verrucomicrobia that is a symbiotic bacterium in the mucus layer and utilizes mucus as a single nutrient source.116,194 Given its safety and pivotal roles in alleviating obesity and IBD, A. muciniphila is widely considered a next-generation probiotic.194–200
Research has shown that A. muciniphila is abundant in children’s GM, which may explain why FMT from children provides better protection against OVX-induced bone loss compared to GM transplantation from older people.159 A. muciniphila is also correlated with bone physiology, and there is a direct correlation to bone formation. For example, Chevalier et al. reported that warm temperature exposure (34 °C), rather than room temperature (RT) conditions, improved the tibial BV/TV and the abundance of Akkermansia in 24-week-old female mice.8 In contrast, OVX mice had a lower abundance of A. muciniphila than mice with sham operations (Sham).159 Warm-exposed-FMT increased tibial breaking strength and BV in OVX mice compared with RT-exposed-FMT.8 A. muciniphila has also been reported to promote the healing of bone fractures and mitigate Porphyromonas gingivalis-induced alveolar bone destruction.201–203 Accumulated research has revealed that both live and pasteurized A. muciniphila could prevent or treat obesity-related metabolic disorders and systemic inflammation.194–199,204–208 However, pasteurized A. muciniphila provides no protection against OVX-induced bone loss.209
Despite the growing interest in the use of next-generation probiotics as a potential therapy for OP, research in this field remains in its infancy. Many novel probiotics, which were discussed in the review by O’Toole et al., have not been used in bone research.162 For instance, Clostridium butyricum (C. butyricum) is a strain of the Clostridium genus found in various environments, including soil, cultured milk products, vegetables, and the human colon.210 C. butyricum can utilize undigested dietary fibers and generate SCFAs, specifically butyrate and acetate.121 C. butyricum MIYAIRI 588 (CBM 588) is widely used as a novel food ingredient or a treatment for diarrhea due to its safe, nonpathogenic, and nontoxic profile.210–212 Given the benefits of butyrate on bone formation, we suggest that CBM 588 or other SCFA-producing GM can be applied to research on the gut-bone axis. Faecalibacterium prausnitzii (F. prausnitzii) is a major member of Firmicutes phylum. The reduction in F. prausnitzii is associated with a higher risk of postoperative recurrence of ileal CD. The metabolites of F. prausnitzii suppress NF-κB activation and IL-8 production.213,214 F. prausnitzii increased plasma anti-Th17 cytokines (IL-10 and IL-12) and decreased plasma IL-17 levels in colorectal colitis rats. The culture supernatant of F. prausnitzii also suppressed Th17 cell differentiation in vitro.215 Given that immune cytokines regulate bone metabolism, it is necessary to explore the effect of these anti-inflammatory probiotics in protecting against bone loss.
Problems with probiotic screening
Khosla and Hofbauer conducted a comprehensive review of the advancements and challenges in OP treatment and indicated that the progression of recent OP drugs and new pharmacological approaches will probably be based on mechanisms of rare diseases and fundamental bone biology.9 The development of these new medications provides a basis for the exploration of probiotic treatments. The prolonged use of anti-resorption drugs, however, is associated with certain risks. For example, combined hormone therapy increases the risk of cardiovascular disease in patients over 70 years old, denosumab is associated with the risk of osteonecrosis of the jaw and atypical femur fractures, and bisphosphonates have been shown to suppress bone turnover markers for at least five years after discontinuation.2,9 Apart from these limitations, most drugs, except bisphosphonates and strontium ranelate, have failed to sustain their bone-anabolic effects following discontinuation.9 Probiotic treatments are expected to address such drawbacks and provide sustained benefits in terms of bone mass, provided they are effectively colonized in the host body. Nonetheless, further research is necessary to fully comprehend the mechanisms of host colonization and the long-term effects of such colonization.
A decreased diversity in the GM is regarded as an indicator of various pathological conditions, such as inflammatory and metabolic disorders.216,217 The causal relationship between GM composition and OP remains controversial. Huang et al. conducted an analysis of 12 prior studies to examine the differences in GM abundance between OP patients and healthy individuals, which included fecal GM data from 2 033 people (604 with OP and 1 429 healthy controls).218 The findings revealed that the relative abundance of Lactobacillus and Ruminococcus increased in the OP group, while the relative abundance of Bacteroides in the Bacteroidetes phylum increased (with the exception of Ireland). Conversely, the relative abundance of the genera Blautia, Alistipes, Megamonas, and Anaerostipes decreased in Chinese OP patients.218 However, as mentioned by the author, most of the included studies exhibited significant heterogeneity, and individual differences, such as sample size, race, residence, diet, medication, age, gender, physical exercise, and stress levels, often impacted GM composition. As a result, it remains uncertain whether changes in GM composition can serve as a biomarker for OP. Nonetheless, these findings provide valuable insights for the selection of probiotics. For instance, Blautia is a genus of anaerobic bacteria that can generate SCFAs from dietary fiber and modulate the immune response of Treg cells.219,220 Alistipes, which belongs to the Bacteroidetes phylum, was reported to generate acetate and propionate.221,222 Alistipes finegoldii is suggested to be protective against colitis.223 Anaerostipes was suggested to have potential benefits due to its ability to produce SCFAs.224,225 It is important to determine the changes in GM composition, particularly with regards to bacteria that have potential benefits, following probiotic administration. For instance, the relative abundance of phylum Verrucomicrobia and genus Akkermansia was decreased in OP mice,25,159 but transplantation of GM rich in A. muciniphila or administration of LGG could restore their abundance.25,159 Administration of LGG has also been reported to alter microbial diversity and increase the proportion of SCFA-producing Clostridia in conventionally raised mice.33 Furthermore, feeding rats Lactobacillus helveticus was associated with a significant increase in the number of Lactobacillus colonies in their feces compared to the sham and OVX groups.177
Studies that assess the impact of probiotics on bone health have primarily used OVX animals as experimental models. It is important to consider not only preventing adverse health outcomes but also promoting positive ones, especially increasing bone mass during skeletal maturity (peak BMD). Maintaining peak BMD is crucial for good bone health, as a 10% increase in peak BMD has been estimated to delay the onset of OP by 13 years.226
Conclusion
Research on the GM in the gut–bone axis has yielded novel insights into the pathogenesis of OP and the potential for using gut microbes as a treatment strategy. However, the inconsistency of test environments, the host’s genetic backgrounds, and the sources of gut microbes pose significant challenges in controlling variables in research. Furthermore, the response of the host GM to FMT or probiotic stimulation could potentially interfere with the colonization of exogenous gut microbes. Therefore, the transition from basic research to clinical research and the practical application of probiotics remains a challenge. It is imperative to continue the search for effective probiotics for OP treatment and to meticulously evaluate their quality, safety, dosage, stability, and interactions with other medications.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFD1300400) and the 2115 Talent Development Program of China Agricultural University.
Author contributions
All authors wrote, discussed, and reviewed the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.Sang C, et al. TNF-α promotes osteoclastogenesis through JNK signaling-dependent induction of Semaphorin3D expression in estrogen-deficiency induced osteoporosis. J. Cell. Physiol. 2017;232:3396–3408. doi: 10.1002/jcp.25784. [DOI] [PubMed] [Google Scholar]
- 4.Tan J, et al. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res. 2015;3:15003. doi: 10.1038/boneres.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu M, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF(+) T cells and Th17 cells. Nat. Commun. 2020;11:468. doi: 10.1038/s41467-019-14148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton ES, Hochberg MC. Osteoporosis and osteoarthritis, rheumatoid arthritis and spondylarthropathies. Curr. Osteoporos. Rep. 2013;11:257–262. doi: 10.1007/s11914-013-0172-1. [DOI] [PubMed] [Google Scholar]
- 7.Gulati AM, et al. Osteoporosis in psoriatic arthritis: a cross-sectional study of an outpatient clinic population. Rmd. Open. 2018;4:e000631. doi: 10.1136/rmdopen-2017-000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevalier C, et al. Warmth prevents bone loss through the gut microbiota. Cell. Metab. 2020;32:575–90.e7. doi: 10.1016/j.cmet.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. doi: 10.1016/S2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei H, et al. Identification of fibroblast activation protein as an osteogenic suppressor and anti-osteoporosis drug target. Cell. Rep. 2020;33:108252. doi: 10.1016/j.celrep.2020.108252. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Dang K, Huai Y, Qian A. Osteoimmunology: the regulatory roles of T lymphocytes in osteoporosis. Front. Endocrinol. 2020;11:465. doi: 10.3389/fendo.2020.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos. Int. 2019;30:1003–1013. doi: 10.1007/s00198-019-04855-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ. 2017;5:e3450. doi: 10.7717/peerj.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen K, et al. Fecal and serum metabolomic signatures and microbial community profiling of postmenopausal osteoporosis mice model. Front. Cell. Infect. Microbiol. 2020;10:535310. doi: 10.3389/fcimb.2020.535310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjögren K, et al. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novince CM, et al. Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci. Rep. 2017;7:5747. doi: 10.1038/s41598-017-06126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida Y, et al. Commensal microbiota enhance both osteoclast and osteoblast activities. Molecules. 2018;23:1517. doi: 10.3390/molecules23071517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzer M, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351:854–857. doi: 10.1126/science.aad8588. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA. 2016;113:E7554–e63. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox LM, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nobel YR, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat. Commun. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagi AM, et al. The gut microbiota is a transmissible determinant of skeletal maturation. Elife. 2021;10:e64237. doi: 10.7554/eLife.64237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yatsunenko T, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepper JD, et al. Probiotic Lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. J. Bone Miner. Res. 2019;34:681–698. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willers M, et al. S100A8 and S100A9 are important for postnatal development of gut microbiota and immune system in mice and infants. Gastroenterology. 2020;159:2130–45.e5. doi: 10.1053/j.gastro.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Nash MJ, Frank DN, Friedman JE. Early microbes modify immune system development and metabolic homeostasis-The “Restaurant” Hypothesis Revisited. Front. Endocrinol. 2017;8:349. doi: 10.3389/fendo.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin R, et al. Early life: gut microbiota and immune development in infancy. Benef. Microbes. 2010;1:367–382. doi: 10.3920/BM2010.0027. [DOI] [PubMed] [Google Scholar]
- 29.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li JY, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016;126:2049–2063. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 32.Haak BW, et al. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 2019;74:782–786. doi: 10.1093/jac/dky471. [DOI] [PubMed] [Google Scholar]
- 33.Tyagi AM, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49:1116–31.e7. doi: 10.1016/j.immuni.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, et al. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015;156:3169–3182. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciucci T, et al. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut. 2015;64:1072–1081. doi: 10.1136/gutjnl-2014-306947. [DOI] [PubMed] [Google Scholar]
- 36.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 37.Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am. J. Med. 2009;122:599–604. doi: 10.1016/j.amjmed.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peek CT, et al. Intestinal inflammation promotes MDL-1(+) osteoclast precursor expansion to trigger osteoclastogenesis and bone loss. Cell. Mol. Gastroenterol. Hepatol. 2022;14:731–750. doi: 10.1016/j.jcmgh.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong BR, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell. 1999;4:1041–1049. doi: 10.1016/S1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 40.Wong BR, Josien R, Choi Y. TRANCE is a TNF family member that regulates dendritic cell and osteoclast function. J. Leukoc. Biol. 1999;65:715–724. doi: 10.1002/jlb.65.6.715. [DOI] [PubMed] [Google Scholar]
- 41.Josien R, Wong BR, Li HL, Steinman RM, Choi Y. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J. Immunol. 1999;162:2562–2568. doi: 10.4049/jimmunol.162.5.2562. [DOI] [PubMed] [Google Scholar]
- 42.Wong BR, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 43.Wong BR, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J. Exp. Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto K, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 2017;97:1295–1349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- 45.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 46.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 47.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanov, I. I., Tuganbaev, T., Skelly, A. N. & Honda, K. T cell responses to the microbiota. Annu. Rev. Immunol., (2022). [DOI] [PMC free article] [PubMed]
- 49.Alexander M, et al. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe. 2022;30:17–30.e9. doi: 10.1016/j.chom.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atarashi K, et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamopoulos IE, et al. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res. Ther. 2010;12:R29. doi: 10.1186/ar2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duque G, et al. Interferon-γ plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J. Bone Miner. Res. 2011;26:1472–1483. doi: 10.1002/jbmr.350. [DOI] [PubMed] [Google Scholar]
- 53.Hathaway-Schrader JD, et al. Specific commensal bacterium critically regulates gut microbiota osteoimmunomodulatory actions during normal postpubertal skeletal growth and maturation. JBMR Plus. 2020;4:e10338. doi: 10.1002/jbm4.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li JY, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J. Clin. Investig. 2020;130:1767–1781. doi: 10.1172/JCI133473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JY, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc. Natl. Acad. Sci. USA. 2011;108:768–773. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adeel S, et al. Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. J. Investig. Med. 2013;61:1178–1183. doi: 10.2310/JIM.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goto Y, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li, C.-J. et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell. Metab., (2021). [DOI] [PubMed]
- 60.Pouikli A, et al. Chromatin remodeling due to degradation of citrate carrier impairs osteogenesis of aged mesenchymal stem cells. Nat. Aging. 2021;1:810–825. doi: 10.1038/s43587-021-00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naskar D, Teng F, Felix KM, Bradley CP, Wu HJ. Synthetic retinoid AM80 ameliorates lung and arthritic autoimmune responses by inhibiting T follicular helper and Th17 cell responses. J. Immunol. 2017;198:1855–1864. doi: 10.4049/jimmunol.1601776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheridan C. First integrin inhibitor since Tysabri nears approval for IBD. Nat. Biotechnol. 2014;32:205–207. doi: 10.1038/nbt0314-205. [DOI] [PubMed] [Google Scholar]
- 63.Aden K, et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology. 2019;157:1279–92.e11. doi: 10.1053/j.gastro.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 64.Schleier L, et al. Non-classical monocyte homing to the gut via α4β7 integrin mediates macrophage-dependent intestinal wound healing. Gut. 2020;69:252–263. doi: 10.1136/gutjnl-2018-316772. [DOI] [PubMed] [Google Scholar]
- 65.DeSelm CJ, et al. IL-17 mediates estrogen-deficient osteoporosis in an Act1-dependent manner. J. Cell. Biochem. 2012;113:2895–2902. doi: 10.1002/jcb.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dar HY, et al. Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition. 2018;54:118–128. doi: 10.1016/j.nut.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Stefka AT, et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA. 2014;111:13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 69.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiba T, Seno H. Indigenous clostridium species regulate systemic immune responses by induction of colonic regulatory T cells. Gastroenterology. 2011;141:1114–1116. doi: 10.1053/j.gastro.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 71.Lyons A, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin. Exp. Allergy. 2010;40:811–819. doi: 10.1111/j.1365-2222.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 72.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J. Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 73.Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- 74.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Hontecillas R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One. 2012;7:e34676. doi: 10.1371/journal.pone.0034676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fujisaki J, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo CY, Wang L, Sun C, Li DJ. Estrogen enhances the functions of CD4(+)CD25(+)Foxp3(+) regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell. Mol. Immunol. 2011;8:50–58. doi: 10.1038/cmi.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grafe I, et al. TGF-β family signaling in mesenchymal differentiation. Cold Spring Harb. Perspect. Biol. 2018;10:a022202. doi: 10.1101/cshperspect.a022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang J, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat. Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Migliaccio A, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. Embo. J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kousteni S, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 82.Kousteni S, et al. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J. Clin. Investig. 2003;111:1651–1664. doi: 10.1172/JCI200317261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robinson LJ, et al. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Exp. Cell Res. 2009;315:1287–1301. doi: 10.1016/j.yexcr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samuels A, Perry MJ, Goodship AE, Fraser WD, Tobias JH. Is high-dose estrogen-induced osteogenesis in the mouse mediated by an estrogen receptor? Bone. 2000;27:41–46. doi: 10.1016/S8756-3282(00)00289-1. [DOI] [PubMed] [Google Scholar]
- 85.McDougall KE, et al. Estrogen receptor-alpha dependency of estrogen’s stimulatory action on cancellous bone formation in male mice. Endocrinology. 2003;144:1994–1999. doi: 10.1210/en.2002-0074. [DOI] [PubMed] [Google Scholar]
- 86.Lindberg MK, et al. Estrogen receptor specificity in the regulation of the skeleton in female mice. J. Endocrinol. 2001;171:229–236. doi: 10.1677/joe.0.1710229. [DOI] [PubMed] [Google Scholar]
- 87.Carlsten H. Immune responses and bone loss: the estrogen connection. Immunol. Rev. 2005;208:194–206. doi: 10.1111/j.0105-2896.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 88.Eghbali-Fatourechi G, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Investig. 2003;111:1221–1230. doi: 10.1172/JCI200317215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofbauer LC, et al. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 90.Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012;23:576–581. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J. Bone Miner. Res. 2007;22:724–729. doi: 10.1359/jbmr.070207. [DOI] [PubMed] [Google Scholar]
- 92.Roggia C, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc. Natl. Acad. Sci. USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cenci S, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee SK, et al. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J. Bone Miner. Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- 95.Pace F, Watnick PI. The interplay of sex steroids, the immune response, and the intestinal microbiota. Trends Microbiol. 2020;29:849–859. doi: 10.1016/j.tim.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ridlon JM, Bajaj JS. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharm. Sin. B. 2015;5:99–105. doi: 10.1016/j.apsb.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J. Natl. Cancer Inst. 2016;108:djw029. doi: 10.1093/jnci/djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parida S, Sharma D. The microbiome-estrogen connection and breast cancer risk. Cells. 2019;8:1642. doi: 10.3390/cells8121642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008;66:487–495. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 100.Flores R, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl. Med. 2012;10:253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wein MN, Kronenberg HM. Regulation of bone remodeling by parathyroid hormone. Cold. Spring. Harb. Perspect. Med. 2018;8:a031237. doi: 10.1101/cshperspect.a031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018;14:115–125. doi: 10.1038/nrendo.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iida-Klein A, et al. Short-term continuous infusion of human parathyroid hormone 1-34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J. Endocrinol. 2005;186:549–557. doi: 10.1677/joe.1.06270. [DOI] [PubMed] [Google Scholar]
- 104.Silverberg SJ, et al. Skeletal disease in primary hyperparathyroidism. J. Bone Miner. Res. 1989;4:283–291. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 105.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 106.Li JY, et al. IL-17 receptor signaling in osteoblasts/osteocytes mediates PTH-induced bone loss and enhances osteocytic RANKL production. J. Bone Miner. Res. 2019;34:349–360. doi: 10.1002/jbmr.3600. [DOI] [PubMed] [Google Scholar]
- 107.Silva BC, Costa AG, Cusano NE, Kousteni S, Bilezikian JP. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J. Endocrinol. Investig. 2011;34:801–810. doi: 10.3275/7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1-34) on rat bone. Bone. 1995;16:477–484. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 110.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol. Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Zaiss MM, Jones RM, Schett G, Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J. Clin. Investig. 2019;129:3018–3028. doi: 10.1172/JCI128521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kishimoto T, et al. Peptidoglycan and lipopolysaccharide synergistically enhance bone resorption and osteoclastogenesis. J. Periodontal. Res. 2012;47:446–454. doi: 10.1111/j.1600-0765.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- 114.Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74:13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- 115.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut. microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 117.Shimizu J, et al. Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS One. 2018;13:e0203657. doi: 10.1371/journal.pone.0203657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019;59:S130–s52. doi: 10.1080/10408398.2018.1542587. [DOI] [PubMed] [Google Scholar]
- 119.Mariño E, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017;18:552–562. doi: 10.1038/ni.3713. [DOI] [PubMed] [Google Scholar]
- 120.Lucas S, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018;9:55. doi: 10.1038/s41467-017-02490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J. Anim. Sci. Biotechnol. 2020;11:24. doi: 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- 123.Riggs MG, Whittaker RG, Neumann JR, Ingram VM. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 124.Kim DS, et al. Attenuation of rheumatoid inflammation by sodium butyrate through reciprocal targeting of HDAC2 in osteoclasts and HDAC8 in T cells. Front. Immunol. 2018;9:1525. doi: 10.3389/fimmu.2018.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rahman MM, et al. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood. 2003;101:3451–3459. doi: 10.1182/blood-2002-08-2622. [DOI] [PubMed] [Google Scholar]
- 126.Kim HN, et al. Trichostatin A inhibits osteoclastogenesis and bone resorption by suppressing the induction of c-Fos by RANKL. Eur. J. Pharmacol. 2009;623:22–29. doi: 10.1016/j.ejphar.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 127.Chen TH, Chen WM, Hsu KH, Kuo CD, Hung SC. Sodium butyrate activates ERK to regulate differentiation of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007;355:913–918. doi: 10.1016/j.bbrc.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 128.Katono T, et al. Sodium butyrate stimulates mineralized nodule formation and osteoprotegerin expression by human osteoblasts. Arch. Oral. Biol. 2008;53:903–909. doi: 10.1016/j.archoralbio.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 129.Marsh AG, Sanchez TV, Midkelsen O, Keiser J, Mayor G. Cortical bone density of adult lacto-ovo-vegetarian and omnivorous women. J. Am. Diet. Assoc. 1980;76:148–151. doi: 10.1016/S0002-8223(21)05128-2. [DOI] [PubMed] [Google Scholar]
- 130.Rivas A, et al. Mediterranean diet and bone mineral density in two age groups of women. Int. J. Food Sci. Nutr. 2013;64:155–161. doi: 10.3109/09637486.2012.718743. [DOI] [PubMed] [Google Scholar]
- 131.Lee MJ, et al. Exogenous polyamines promote osteogenic differentiation by reciprocally regulating osteogenic and adipogenic gene expression. J. Cell. Biochem. 2013;114:2718–2728. doi: 10.1002/jcb.24620. [DOI] [PubMed] [Google Scholar]
- 132.Pegg AE. Mammalian polyamine metabolism and function. Iubmb. Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Q, et al. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome. 2021;9:224. doi: 10.1186/s40168-021-01157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tofalo R, Cocchi S, Suzzi G. Polyamines and gut microbiota. Front. Nutr. 2019;6:16. doi: 10.3389/fnut.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ramos-Molina B, Queipo-Ortuño MI, Lambertos A, Tinahones FJ, Peñafiel R. Dietary and gut microbiota polyamines in obesity- and age-related diseases. Front. Nutr. 2019;6:24. doi: 10.3389/fnut.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tjabringa GS, et al. Polyamines modulate nitric oxide production and COX-2 gene expression in response to mechanical loading in human adipose tissue-derived mesenchymal stem cells. Stem. Cells. 2006;24:2262–2269. doi: 10.1634/stemcells.2005-0625. [DOI] [PubMed] [Google Scholar]
- 137.Tjabringa GS, et al. The polymine spermine regulates osteogenic differentiation in adipose stem cells. J. Cell. Mol. Med. 2008;12:1710–1717. doi: 10.1111/j.1582-4934.2008.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamamoto T, et al. The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br. J. Pharm. 2012;166:1084–1096. doi: 10.1111/j.1476-5381.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamada T, et al. Daily intake of polyamine-rich Saccharomyces cerevisiae S631 prevents osteoclastic activation and bone loss in ovariectomized mice. Food Sci. Biotechnol. 2019;28:1241–1245. doi: 10.1007/s10068-019-00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kong SH, Kim JH, Shin CS. Serum spermidine as a novel potential predictor for fragility fractures. J. Clin. Endocrinol. Metab. 2021;106:e582–e591. doi: 10.1210/clinem/dgaa745. [DOI] [PubMed] [Google Scholar]
- 141.Albert JS, et al. Impaired osteoblast and osteoclast function characterize the osteoporosis of Snyder - Robinson syndrome. Orphanet J. Rare Dis. 2015;10:27. doi: 10.1186/s13023-015-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murray-Stewart, T., Dunworth, M., Foley, J. R., Schwartz, C. E. & Casero, R. A., Jr. Polyamine homeostasis in Snyder-Robinson syndrome. Med. Sci. 6, 112 (2018). [DOI] [PMC free article] [PubMed]
- 143.Shen X, et al. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med. 2013;60:195–200. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Linden DR. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 2014;20:818–830. doi: 10.1089/ars.2013.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo FF, Yu TC, Hong J, Fang JY. Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front. Physiol. 2016;7:156. doi: 10.3389/fphys.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y, et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell. Stem. Cell. 2014;15:66–78. doi: 10.1016/j.stem.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gambari L, et al. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism. Pharmacol. Res. 2014;87:99–112. doi: 10.1016/j.phrs.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 148.Grassi F, et al. Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency. J. Bone Miner. Res. 2016;31:949–963. doi: 10.1002/jbmr.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Behera J, et al. Hydrogen sulfide promotes bone homeostasis by balancing inflammatory cytokine signaling in CBS-deficient mice through an epigenetic mechanism. Sci. Rep. 2018;8:15226. doi: 10.1038/s41598-018-33149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015;40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 151.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Molina-Tijeras JA, Gálvez J, Rodríguez-Cabezas ME. The immunomodulatory properties of extracellular vesicles derived from probiotics: a novel approach for the management of gastrointestinal diseases. Nutrients. 2019;11:1038. doi: 10.3390/nu11051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tong L, et al. Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in DSS-induced colitis mice. Nutrients. 2021;13:3319. doi: 10.3390/nu13103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu R, et al. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021;12:25. doi: 10.1186/s40104-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamasaki-Yashiki S, Miyoshi Y, Nakayama T, Kunisawa J, Katakura Y. IgA-enhancing effects of membrane vesicles derived from Lactobacillus sakei subsp. sakei NBRC15893. Biosci. Microbiota. Food Health. 2019;38:23–29. doi: 10.12938/bmfh.18-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kang CS, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ashrafian F, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front. Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ashrafian F, et al. Extracellular vesicles and pasteurized cells derived from Akkermansia muciniphila protect against high-fat induced obesity in mice. Microb. Cell. Fact. 2021;20:219. doi: 10.1186/s12934-021-01709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu JH, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv. Sci. 2021;8:2004831. doi: 10.1002/advs.202004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Klimentová J, Stulík J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015;170:1–9. doi: 10.1016/j.micres.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 161.Jones RM, Mulle JG, Pacifici R. Osteomicrobiology: the influence of gut microbiota on bone in health and disease. Bone. 2018;115:59–67. doi: 10.1016/j.bone.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 162.O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 163.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 165.Hsu CL, et al. Antiobesity and uric acid-lowering effect of Lactobacillus plantarum GKM3 in high-fat-diet-induced obese rats. J. Am. Coll. Nutr. 2019;38:623–632. doi: 10.1080/07315724.2019.1571454. [DOI] [PubMed] [Google Scholar]
- 166.Hatakka K, et al. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. shermanii JS on potentially carcinogenic bacterial activity in human colon. Int. J. Food Microbiol. 2008;128:406–410. doi: 10.1016/j.ijfoodmicro.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 167.Hao H, et al. Effect of extracellular vesicles derived from Lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front. Immunol. 2021;12:777147. doi: 10.3389/fimmu.2021.777147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chiang SS, Pan TM. Antiosteoporotic effects of Lactobacillus -fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J. Agric. Food Chem. 2011;59:7734–7742. doi: 10.1021/jf2013716. [DOI] [PubMed] [Google Scholar]
- 169.Ong AM, Kang K, Weiler HA, Morin SN. Fermented milk products and bone health in postmenopausal women: a systematic review of randomized controlled trials, prospective cohorts, and case-control studies. Adv. Nutr. 2020;11:251–265. doi: 10.1093/advances/nmz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tu MY, et al. Kefir peptides prevent estrogen deficiency-induced bone loss and modulate the structure of the gut microbiota in ovariectomized mice. Nutrients. 2020;12:3432. doi: 10.3390/nu12113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee CS, et al. Lactobacillus-fermented milk products attenuate bone loss in an experimental rat model of ovariectomy-induced post-menopausal primary osteoporosis. J. Appl. Microbiol. 2021;130:2041–2062. doi: 10.1111/jam.14852. [DOI] [PubMed] [Google Scholar]
- 172.Tu MY, et al. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS One. 2015;10:e0144231. doi: 10.1371/journal.pone.0144231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Britton RA, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014;229:1822–1830. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dar HY, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep. 2018;8:46–56. doi: 10.1016/j.bonr.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu X, et al. Lactobacillus Fermentum ZS40 prevents secondary osteoporosis in Wistar Rat. Food Sci. Nutr. 2020;8:5182–5191. doi: 10.1002/fsn3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu X, et al. Lactobacillus Plantarum HFY15 Helps prevent retinoic acid-induced secondary osteoporosis in Wistar rats. Evid. Based Complement. Altern. Med. 2020;2020:2054389. doi: 10.1155/2020/2054389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parvaneh M, et al. Lactobacillus helveticus (ATCC 27558) upregulates Runx2 and Bmp2 and modulates bone mineral density in ovariectomy-induced bone loss rats. Clin. Interv. Aging. 2018;13:1555–1564. doi: 10.2147/CIA.S169223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jang AR, et al. Cell-free culture supernatant of Lactobacillus curvatus Wikim38 inhibits RANKL-induced osteoclast differentiation and ameliorates bone loss in ovariectomized mice. Lett. Appl. Microbiol. 2021;73:383–391. doi: 10.1111/lam.13525. [DOI] [PubMed] [Google Scholar]
- 179.Ohlsson C, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9:e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jhun J, et al. Lactobacillus sakei suppresses collagen-induced arthritis and modulates the differentiation of T helper 17 cells and regulatory B cells. J. Transl. Med. 2020;18:317. doi: 10.1186/s12967-020-02477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Collins FL, et al. Beneficial effects of Lactobacillus reuteri 6475 on bone density in male mice is dependent on lymphocytes. Sci. Rep. 2019;9:14708. doi: 10.1038/s41598-019-51293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li L, et al. Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging. 2019;11:9348–9368. doi: 10.18632/aging.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ai T, et al. Konjac oligosaccharides modulate the gut environment and promote bone health in calcium-deficient mice. J. Agric. Food Chem. 2021;69:4412–4422. doi: 10.1021/acs.jafc.0c07839. [DOI] [PubMed] [Google Scholar]
- 184.Parvaneh K, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Biomed. Res. Int. 2015;2015:897639. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fernández-Murga ML, Olivares M, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reverses the adverse effects of diet-induced obesity through the gut-bone axis. Bone. 2020;141:115580. doi: 10.1016/j.bone.2020.115580. [DOI] [PubMed] [Google Scholar]
- 186.Wallimann A, et al. An exopolysaccharide produced by Bifidobacterium longum 35624® inhibits osteoclast formation via a TLR2-dependent mechanism. Calcif. Tissue Int. 2021;108:654–666. doi: 10.1007/s00223-020-00790-4. [DOI] [PubMed] [Google Scholar]
- 187.Roberts JL, et al. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed. Pharmacother. 2020;132:110831. doi: 10.1016/j.biopha.2020.110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jansson P-A, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:e154–e162. doi: 10.1016/S2665-9913(19)30068-2. [DOI] [PubMed] [Google Scholar]
- 189.Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018;284:307–317. doi: 10.1111/joim.12805. [DOI] [PubMed] [Google Scholar]
- 190.Lei M, Hua LM, Wang DW. The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial. Benef. Microbes. 2016;7:631–637. doi: 10.3920/BM2016.0067. [DOI] [PubMed] [Google Scholar]
- 191.Motta JP, et al. Food-grade bacteria expressing elafin protect against inflammation and restore colon homeostasis. Sci. Transl. Med. 2012;4:158ra44. doi: 10.1126/scitranslmed.3004212. [DOI] [PubMed] [Google Scholar]
- 192.Hamady ZZ, et al. Treatment of colitis with a commensal gut bacterium engineered to secrete human TGF-β1 under the control of dietary xylan 1. Inflamm. Bowel Dis. 2011;17:1925–1935. doi: 10.1002/ibd.21565. [DOI] [PubMed] [Google Scholar]
- 193.Frossard CP, Steidler L, Eigenmann PA. Oral administration of an IL-10-secreting Lactococcus lactis strain prevents food-induced IgE sensitization. J. Allergy Clin. Immunol. 2007;119:952–959. doi: 10.1016/j.jaci.2006.12.615. [DOI] [PubMed] [Google Scholar]
- 194.Naito Y, Uchiyama K, Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018;63:33–35. doi: 10.3164/jcbn.18-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017;106:171–181. doi: 10.1016/j.micpath.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 196.Schneeberger M, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front. Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 200.Depommier C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mulhall H, et al. Akkermansia muciniphila and its Pili-like protein Amuc_1100 modulate macrophage polarization in experimental periodontitis. Infect. Immun. 2020;89:e00500–20. doi: 10.1128/IAI.00500-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huck O, et al. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J. Clin. Periodontol. 2020;47:202–212. doi: 10.1111/jcpe.13214. [DOI] [PubMed] [Google Scholar]
- 203.Liu JH, et al. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis. Model. Mech. 2020;13:dmm043620. doi: 10.1242/dmm.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Depommier C, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut. microbes. 2020;11:1231–1245. doi: 10.1080/19490976.2020.1737307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut. 2020;69:1988–1997. doi: 10.1136/gutjnl-2019-320105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Druart C, et al. Toxicological safety evaluation of pasteurized Akkermansia muciniphila. J. Appl. Toxicol. 2021;41:276–290. doi: 10.1002/jat.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mulhall H, DiChiara JM, Huck O, Amar S. Pasteurized Akkermansia muciniphila reduces periodontal and systemic inflammation induced by Porphyromonas gingivalis in lean and obese mice. J. Clin. Periodontol. 2022;49:717–729. doi: 10.1111/jcpe.13629. [DOI] [PubMed] [Google Scholar]
- 208.Wu Z, et al. Pasteurized Akkermansia muciniphila reduces fat accumulation via nhr-49-mediated nuclear hormone signaling pathway in Caenorhabditis elegans. Molecules. 2022;27:6159. doi: 10.3390/molecules27196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lawenius L, et al. Pasteurized Akkermansia muciniphila protects from fat mass gain but not from bone loss. Am. J. Physiol. Endocrinol. Metab. 2020;318:E480–e91. doi: 10.1152/ajpendo.00425.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stoeva MK, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut. microbes. 2021;13:1–28. doi: 10.1080/19490976.2021.1907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Isa K, et al. Safety assessment of the Clostridium butyricum MIYAIRI 588® probiotic strain including evaluation of antimicrobial sensitivity and presence of Clostridium toxin genes in vitro and teratogenicity in vivo. Hum. Exp. Toxicol. 2016;35:818–832. doi: 10.1177/0960327115607372. [DOI] [PubMed] [Google Scholar]
- 212.Seki H, et al. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr. Int. 2003;45:86–90. doi: 10.1046/j.1442-200X.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 213.Sokol H, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rossi O, et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 2016;6:18507. doi: 10.1038/srep18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang M, et al. Faecalibacterium prausnitzii inhibits interleukin-17 to ameliorate colorectal colitis in rats. PLoS One. 2014;9:e109146. doi: 10.1371/journal.pone.0109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Scher JU, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016;12:154–167. doi: 10.1038/nrendo.2015.218. [DOI] [PubMed] [Google Scholar]
- 218.Huang R, et al. Changes in the gut microbiota of osteoporosis patients based on 16S rRNA gene sequencing: a systematic review and meta-analysis. J. Zhejiang Univ. Sci. B. 2022;23:1002–1013. doi: 10.1631/jzus.B2200344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu X, et al. Blautia-a new functional genus with potential probiotic properties? Gut. microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rey FE, et al. Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem. 2010;285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Polansky O, et al. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2015;82:1569–1576. doi: 10.1128/AEM.03473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS One. 2016;11:e0146162. doi: 10.1371/journal.pone.0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bui TPN, et al. Conversion of dietary inositol into propionate and acetate by commensal Anaerostipes associates with host health. Nat. Commun. 2021;12:4798. doi: 10.1038/s41467-021-25081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schwiertz A, et al. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 2002;25:46–51. doi: 10.1078/0723-2020-00096. [DOI] [PubMed] [Google Scholar]
- 226.Hernandez CJ, Beaupré GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos. Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]