Abstract
Background
Disparities in pancreatic cancer incidence and outcomes exist in Native American populations. These disparities are multifactorial, difficult to quantify and are influenced by historical, socioeconomic and health-care structural factors. This article aims to assess these factors and offer a call to action to overcome them.
Methods
We reviewed published data on pancreatic cancer in Native American populations with a focus on disparities in incidence, outcomes, and research efforts. The historical context of the interactions between Native Americans and the United States healthcare system was also analyzed to form actionable items to build trust and collaboration.
Results
The incidence of pancreatic cancer in Native Americans is higher than the general United States population and has the worst survival of any major racial or ethnic group. These outcomes are influenced by a patient population with often poor access to high-quality cancer care, historical trauma potentially leading to reduced care utilization, and a lack of research focused on etiologies and comorbid conditions that contribute to these disparities.
Conclusion
A collaborative effort between non-tribal and tribal leaders and cancer centers is key to addressing disparities in pancreatic cancer outcomes and research. More population-level studies are needed to better understand the incidence, etiologies, and comorbid conditions of pancreatic cancer in Native Americans. Lastly, a concerted focused effort should be undertaken between non-tribal and tribal entities to increase the access of Native Americans to high-quality care for pancreatic cancer and other lethal malignancies.
Keywords: Pancreatic Cancer, Disparities, Call to Action, Native American, American Indian, Alaskan Native, Pancreatic Ductal Adenocarcinoma
Precis;
More population-level studies are needed to better understand the incidence, etiologies, and comorbid conditions of pancreatic cancer in Native Americans. A concerted focused effort should be undertaken between non-tribal and tribal entities to increase the access of Native Americans to high-quality care for pancreatic cancer and other lethal malignancies.
Introduction:
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy, accounting for 47,000 deaths yearly in the United States (US), and is currently the third leading cause of cancer-related death.1 The lethality of PDAC is underpinned by advanced stage at diagnosis, high morbidity from technically difficult surgical resection, and overall poor efficacy of systemic therapy relative to other cancers. Native American and Alaskan Native (NA/AN) populations have higher incidence of PDAC than US Whites, and the worst survival outcomes for PDAC among all major US racial/ethnic groups.2–4 Herein, we aimed to deconstruct the factors potentially leading to disparities in care and outcomes in NA/ANs with PDAC, using available evidence informed by the authors’ combined experiences as members of the Native American and/or healthcare communities.
Trust and Access to Care in Native American and Alaskan Native Patients
Historical Trauma
The NA/AN population carries historical traumas from their systematic genocide and the continued colonization of ancestral lands. Although the historical atrocities perpetuated against the Indigenous people of the Americas are largely hidden or ignored by the general population, they continue to have a dramatic effect on the lives of NA/AN people today. A complete list of these shared traumas is too numerous to name, but includes the possible use of biological warfare through small pox-laced blankets, starvation tactics by the US military through the destruction of food crops and the slaughter of buffalo to near extinction, and the legalization of the murder of NA/ANs through scalping bounties during the Dakota War of 1830–1862 as documented by Calloway in First Peoples.5 In addition, the Indian Removal Act forced Native Americans off their ancestral homelands, which they occupied for tens of thousands of years, intentionally severing ties to sacred sites, water sources, and plants which provided traditional medicines.5,6 These and other seized parcels of land have formed the endowments for multiple academic institutions and cancer centers throughout the United States through legislation such as the Morrill Land-Grant College Act of 1862 (Figure 1).
Figure 1:
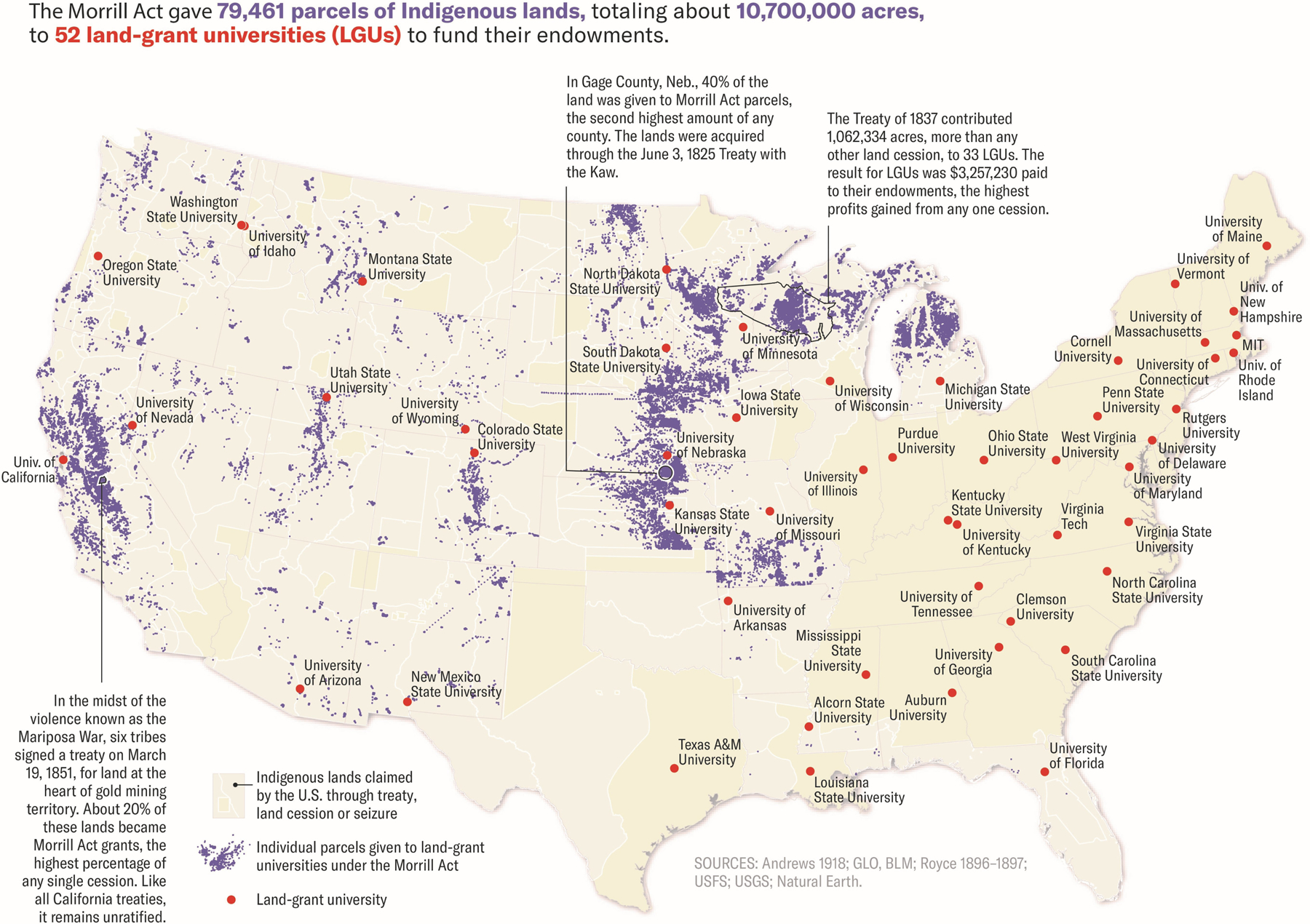
Map of NA/AN lands seized by the United States Government (tan), with further delineation of land seized and granted to academic universities to contribute to their endowments (purple). Universities receiving land-grants are identified (red dots). Map by Margaret Pearce for High Country News (https://www.hcn.org/issues/52.4/indigenous-affairs-education-land-grab-universities). Reprinted with permission from ref [6].
More contemporary examples of shared traumas include assimilation boarding schools where children experienced sexual and physical abuse, the Indian Relocation Act, forced sterilization of NA/AN women, the epidemic of missing and murdered Indigenous women, and the disproportionately high mortality rate from COVID-19 in NA/AN populations.5,7–9 The lasting cultural memory of historical traumas, combined with continued colonization of ancestral lands is likely responsible for the significant mistrust of the US healthcare system, and cancer care in particular, among NA/ANs.10,11 In a study by Guagagnolo and colleagues of Native American and White patients with cancer, Native American race was independently predictive of higher mistrust and lower satisfaction scores (P<0.001 for both). Additionally, Native American patients with cancer were significantly more likely to agree with the statements “In the past, clinics and hospitals have done harmful things to patients without their knowledge,” “I worry that doctors and nurses will do experimental studies on me without telling or asking me,” and “I have put off getting medical care in the past because I felt that I would be treated disrespectfully” (all P<0.0001). While based on a small cohort, this study is one of the few direct pieces of evidence showing that historical traumas have a real impact on trust and utilization of available healthcare resources. In order to address outcome disparities, it is clear that trust must be rebuilt, as must the lacking infrastructure allowing NA/ANs to access high quality cancer care.
Poor Access to Care
In addition to distrust of the US healthcare system, NA/ANs battle reduced access to cancer screening, high quality cancer care, and palliative care.12 According to the First Nations Development Institute, 68% of NA/ANs live on or near Indian reservations or tribal lands.12,13 These reservations and tribal lands are largely rural; healthcare facilities near these populations are operated by the Indian Health Service (IHS), and are underfunded, understaffed, and lack the highly specialized care necessary to diagnose, treat, or follow patients with PDAC compared to the capabilities of NCI Comprehensive Cancer Centers. Per the National Congress of American Indians, the IHS was allocated $2,849 per capita for patient expenditures in 2013, compared to $7,717 per patient for health care spending at the national level. The average IHS federal dollar allocation only covers 59% of the calculated cost per patient.14 Because of the difficulty in recruiting providers to rural locations and underfunding of the IHS care sites, they are understaffed by 25% on average.15
NA/ANs clearly face reduced access to and utilization of high-quality cancer care. While this may not have a dramatic effect for all malignancies, it has the potential to significantly impact disease trajectory in PDAC due to its inherently poor prognosis and the level of expertise required to deliver quality care. NA/ANs will be among the last to benefit from new technologies/therapeutics that are often first adopted or developed by NCI Cancer Centers, many of which have directly or indirectly benefited from the seizure of NA/AN lands (Figure 1).6 Furthermore, no NCI Cancer Centers exist in many states with significant NA/AN populations (Alaska, North Dakota, South Dakota, Montana, Nevada, and Wyoming), undoubtedly creating a barrier to accessing high-quality cancer care.
The Ideal and Reality: Referral, Treatment, and Outcomes in Native American and Alaskan Native Patients with Pancreatic Cancer
To understand the real differences in treatment and outcomes in NA/AN patients with PDAC, it is necessary to examine the impact of access to care and care utilization on outcomes across the spectrum of PDAC phases of care, including diagnosis, curative-intent resection, perioperative systemic chemotherapy and surveillance, and palliative chemotherapy.
Early Detection
Overall, approximately 75% of patients with PDAC succumb to their disease within 1 year of diagnosis, and less than 8% survive beyond 5 years.1 Surgery remains the only hope of cure, however surgical resection is only possible in the 15–20% of medically fit patients with nonmetastatic tumors.16 Therefore, increasing the proportion of patients diagnosed at an early stage is crucial to improving outcomes. Early detection is hampered by many factors, including the rarity of PDAC raising the threshold for a screening test to be cost-effective, as well as by asymptomatic disease until progression to a locally advanced or metastatic stage. Diagnosis at an early stage is therefore essential, however is ultimately predicated on sufficient access to and utilization of healthcare. It is known that NA/AN patients are more likely to present with higher stage disease at diagnosis for the four most common cancers (lung, breast, prostate, colon).17,18 Unfortunately, no studies investigating this phenomenon for PDAC within the NA/AN population exist; as such, the extent to which delayed detection contributes to observed poor outcomes for PDAC in NA/ANs is unclear, however mandates further investigation.
Resection at a High-Volume Center
Centers offering resection for PDAC are not created equal, owing to the extremely technical nature of pancreatectomies and the high likelihood of postoperative complications. Ample evidence has shown the benefit of surgical treatment at high-volume centers, including lower margin positivity, improved lymphadenectomy, shorter hospitalizations, and reduced improved complication rates such as 30-day mortality and readmission.19,20 Overall, these benefits translate to improved overall survival despite more advanced disease in patients treated at high-volume centers.19,20 Once again, there is a paucity of data on the receipt of surgery for PDAC specifically in NA/AN patients relative to Whites. What is known, however, is that non-White Americans with PDAC are less likely to receive care at high-volume centers, contributing to poorer outcomes.21 Even after accounting for hospital-specific practice differences, this finding also holds true for non-White patients with borderline resectable PDAC, which is best managed at high-volume centers due to the technical difficulty of vascular reconstruction and postoperative care.22 Finally, it is known that NA/AN patients are significantly less likely to receive curative-intent resections for breast, colon, lung, and prostate cancer than White counterparts, even after matching.17 Together, these data strongly suggest that NA/AN patients with PDAC face significant systemic barriers to curative-intent resection for nonmetastatic PDAC, however the paucity of PDAC-specific data mandates further investigation.
Perioperative Systemic Chemotherapy
In the modern era, all patients with PDAC are presumed to have micrometastatic disease at diagnosis, due to 5-year recurrence rates approaching or exceeding 80%, even in patients undergoing a margin-negative resection.23 As such, recommendations are that all resected patients receive perioperative chemotherapy, regardless of final tumor characteristics or nodal status.24 Currently, most patients receive systemic chemotherapy in the adjuvant setting; as previously mentioned, however, complications from pancreatic surgery are often severe, and frequently lead to delays in receipt of adjuvant chemotherapy by months or disqualify patients altogether, shortening recurrence-free survival.25 While, there is no direct evidence that a lack of access to high-volume pancreatic centers results in worse utilization of adjuvant chemotherapy for NA/AN patients; ample evidence of this phenomenon exists when comparing White and African-American patients.26–28 Additionally, receipt of adjuvant therapy has been shown to be significantly worse for NA/AN patients with breast and colon cancer compared with matched White counterparts.17 There are no data on disparities for any ethnic group in the delivery of neoadjuvant therapy for PDAC, despite emerging evidence of its potential survival advantages.29,30 In the absence of direct evidence we conclude that NA/AN patients likely face barriers to adequate perioperative systemic therapy, however once again dedicated investigation is warranted.
Postoperative Surveillance
During and following adjuvant therapy, adequate postoperative surveillance is essential, as even with best therapy 50% of patients with radiographically localized disease who undergo complete resection still recur within 1 year, most commonly as distant metastases.31 Postoperative surveillance for PDAC is associated with improved survival and is cost effective.32 Asymptomatic recurrence is associated with improved survival compared to patients with recurrences detected via symptom-guided workup.33 While no direct evidence of disparities in postoperative surveillance for PDAC exist, this is true for both prostate and breast cancer, where NA/AN patients are less likely to receive appropriate surveillance than matched White counterparts.17 Additional study of disparities in this critical aspect of PDAC survivorship is needed.
Palliative Chemotherapy
Despite intensive surveillance, palliative chemotherapy is often the only therapeutic option for patients with distant recurrence, as well as for patients with metastatic disease at diagnosis. Similarly to patients with resectable disease, both diagnosis and treatment of metastatic PDAC at a high-volume hospital are associated with improved survival.34 However, even in states with an NCI Comprehensive Cancer Center, such as New Mexico, NA/AN patients had less than half the likelihood of receiving or being offered chemotherapy and were more likely to die at one month (25.8% versus 7.5%) and at one year following diagnosis (26.2% versus 48.3%).35 To the authors’ knowledge, no data exist on palliative chemotherapy disparities in other states with substantial NA/AN populations but without Comprehensive Cancer Centers.
It is clear that little direct evidence exists for disparities in NA/AN treatment and outcomes during specific phases of PDAC care. Evidence of these disparities in African-American and Hispanic populations with PDAC is ample, as is evidence for disparities in resection rates, adjuvant therapy, and surveillance in NA/AN patients with breast, colon, prostate, and lung cancer.17,36 It is paramount to address the disparities in PDAC care for NA/AN patients that likely exist, however this can only be accomplished by first addressing the profound disparity in research that exists, which also extends to population-level research.
The State of Population-Level Research in Native Americans and Alaskan Natives with Pancreatic Cancer
Much is unknown about the etiologies and comorbid conditions that underpin PDAC in NA/AN populations. What is known is that PDAC incidence is higher in NA/AN populations than in Whites in the United States.3,37,38 The reasons for this are unclear and, most importantly, uninvestigated. The multiethnic cohort study (MECS) was a large population-level investigation started in the mid-1990s designed to investigate the contribution of lifestyle factors and comorbid conditions to the development of cancer, as well as multiple other diseases.39 Results from the MECS have shed light into contributing factors to multiple disease, including PDAC, among many ethnic groups, including White, Black, Latinx, American Japanese, and Native Hawaiians.40 Unfortunately, despite the fact that NA/ANs constitute 1.3% of the United States population, relative to 0.2% for Native Hawaiians/Pacific Islanders, NA/ANs were not included in the MECS. A similar cohort study in the Southern United States, termed the Southern Community Cohort Study (SCCS), predominantly focused on studying individuals of African-American descent compared to a non-African-American cohort.41 While some individuals of NA/AN descent were included, they ultimately comprised 1.3% of the non-African-American cohort, or 300 individuals. Even fewer inclusion of NA/ANs is seen in other historical large cohort studies, such as the CLUE II cohort which was 98.8% White42, or the National Institute of Health-American Association of Retired Persons Diet and Health Study, which was 99% White, Hispanic, and African American, with the remaining 1% labeled as Asian/Other.43 A full list of population-level studies in the United States that have not included NA/AN populations is outside the scope of this article. Suffice it to say, however, no large population-level cohort studies have been published or are currently enrolling with a focus or even representative proportion of NA/AN participants.
While no direct epidemiologic evidence exists on specific population-level challenges facing the NA/AN community, inferences can be made on interplay between diabetes, tobacco use, and obesity with pancreatic cancer. NA/AN populations suffer from higher proportions of type II diabetes than their counterparts44–46, higher rates of obesity in adults and children47–49, and higher rates of tobacco use than non-White counterparts.50,51 These are the three most prevalent modifiable risk factors for the development of pancreatic cancer.52–56 Unlike cohort studies in predominantly White populations, however, data directly linking these risk factors to pancreatic cancer in NA/AN populations is lacking, and stymies implementation of population-level interventions to alter these risk factors.
A lack of NA/AN participants in large cohort studies has real impact on the ability to understand not only the impact of environment, lifestyle, and comorbid conditions on the incidence and prognosis of PDAC, but of genetic factors as well. Genome-wide association studies investigating genetic risk factors diseases similarly pool data from population-level studies, and have historically focused on populations of Asian or European ancestry.57–66 A study by Bougumil et al. pooled data from the MECS and SCCS to validate 31 genetic risk variants for PDAC reported by prior studies, and found different subsets were predictive in each race/ethnicity; only 11 were relevant across all racial groups studied.67 While this study highlighted the existence of inter-racial differences in genetic risks, as well as the importance of diverse patient populations when formulating polygenic risk scores, NA/AN individuals were not analyzable due to the population limitations of the MECS/SCCS.
It is clear from the available data that race/ethnicity-specific differences exist in both genetic and behavioral/comorbidity-related risk factors for PDAC; unfortunately, data for NA/AN populations is virtually nonexistent in both areas. Until effort is made to address the chronic under-investigation of health and disease, including cancer, in NA/AN populations, the medical profession cannot begin to redress past wrongs and rebuild trust.
The Complicity of Observation Without Intervention: A Call to Action
Although the present article has focused on PDAC, one of the most lethal cancers in US populations, similar disparities in diagnosis and treatment of NA/AN patients exist for other hepato-pancreato-biliary malignancies, such as gallbladder and primary liver cancer3,68,69, as well as for colorectal70, gastric69, renal71, lung69, and gynecologic cancers.72 It is clear that patients with relatively rare yet lethal malignancies—such as PDAC—stand to benefit most from specialized high-volume centers, novel therapeutics, and surgical techniques that are often developed at such centers, as well as from population-level research to discover lifestyle associations with incidence and outcomes from such malignancies. Unfortunately, the US healthcare system has much work to do for NA/AN populations to be treated equally. Furthermore, the limited research into NA/AN-specific disparities is often just that: research without follow-through, observation without intervention. This begs the question: where do we begin to intervene?
The authors feel that rebuilding trust and collaborating with NA/AN people are essential to achieving parity in research, treatment, and outcomes for NA/AN individuals with PDAC and other poor-outcome malignancies. To reconcile historical atrocities, it is incumbent upon the US healthcare system to lead the effort to eliminate these disparities. Specific actions include: collaborating with NA/AN communities and organizations, raising provider awareness of disparities in PDAC care in NA/AN populations, expanding access to comprehensive cancer care, increasing the number of providers in NA/AN communities, enhancing the knowledge of PDAC among providers who serve NA/AN populations, addressing institutional and unconscious racial biases in healthcare, and engaging in cross-cultural education, and engage in efforts to recruit NA/ANs into the healthcare and oncology fields.
Rebuilding trust and collaborating with NA/AN communities will enhance engagement of NA/AN populations with healthcare providers, lead to improved screening, early diagnosis and referral, and increase the likelihood that NA/AN individuals will receive referrals to high-volume centers. Improved trust is also necessary to enroll NA/AN patients in much-needed population-level studies to improve understanding of cancer and other diseases. Unfortunately, there is significant heterogeneity among the more than 575 tribes in their relationship with the United States healthcare system, and no silver bullet exists. Utilizing the broad reach of national NA/AN organizations (Table 1) and local tribal communities is essential in closing this gap in care. It is incumbent upon the institutions located nearest to Indian reservations to engage with tribal communities, organizations, and clinics. In many cases, this will require inter-state outreach by cancer centers for tribes in states without robust cancer care infrastructure.
Table 1:
National NA/AN Healthcare Organizations
Conclusion:
Despite the significant disparities in cancer care and research that exist for NA/AN populations, there is a light at the end of the tunnel as witnessed during the recent outbreak of the SARS-CoV-2 virus in the United States. The Navajo Nation, with a population of more than 300,000 enrolled members, was hard-hit by the initial COVID wave, outpacing the infection rate in New York by May 2020.73,74 As we are recommending, a collaborative effort was made between tribal and non-tribal leaders. Together, through successful outreach, they built trust, educated communities on public health measures to slow the virus’ spread, and set up and encouraged testing.73,75 Once vaccines became available, the Navajo Nation conducted a rapid vaccination campaign, far outpacing the vaccination rate of neighboring states and the United States as a whole.76 Altogether, this resulted in a sharp decline in cases, and on March 22nd, 2021 the Navajo Nation reported zero new cases or deaths.77 It is clear that with sufficient collaborative effort and community outreach progress can be made in addressing healthcare disparities in NA/AN populations. It is the authors’ hope that the same strategies and infrastructure used to address the SARS-CoV-2 pandemic can be replicated for future efforts to address the significant disparities that exist in NA/AN populations for PDAC care and research, as well as with other lethal malignancies.
Funding:
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. T.L. Sutton received salary support from the Brenden-Colson Center for Pancreatic Care at Oregon Health & Science University. J. R. Brody is also supported by funding from the Pancreatic Cancer Cure Foundation, Pancreatic Cancer Action Network-AACR Research Acceleration Network Grant, grant ID 15–90-25-BROD, R01CA212600–01 (to J.R. Brody), and in part by NIH U01CA224012. Additional support was provided by the Knight Cancer Institute P30CA069533.
Footnotes
Declarations of Interest: There are no conflicts of interest or financial ties to disclose for any of the authors.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Anama-Green C, Quinn M. Survival Analysis of Demographic Factors Associated With 5+ Year Survival of Pancreatic Carcinoma. Cureus 2021;13:e13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts SR, Kelly JJ, Ashokkumar R, Lanier AP. Occurrence of pancreatic, biliary tract, and gallbladder cancers in Alaska Native people, 1973–2007. Int J Circumpolar Health 2012;71:17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health 2014;104 Suppl 3:S377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calloway CG. First peoples : a documentary survey of American Indian history 3rd ed. ed. Boston: Boston: : Bedford/St. Martin’s; 2008. [Google Scholar]
- 6.Ahtone T, Lee R. Looking Forward from Land-Grab Universities. Native American and Indigenous Studies 2021;8:176–82. [Google Scholar]
- 7.Investigation of Allegations Concerning Indian Health Service In: Office GA, ed.1974. [Google Scholar]
- 8.Missing and Murdered Indigenous Women & Girls Urban Indian Health Institute; 2018. [Google Scholar]
- 9.Arrazola J MM, Joshi S. COVID-19 Mortality Among American Indian and Alaska Native Persons — 14 States, January–June 2020. MMWR Morb Mortal Wkly Rep 2020:1853–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guadagnolo BA, Petereit DG, Helbig P, et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clin Trials 2009;6:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guadagnolo BA, Cina K, Helbig P, et al. Medical mistrust and less satisfaction with health care among Native Americans presenting for cancer treatment. J Health Care Poor Underserved 2009;20:210–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guadagnolo BA, Petereit DG, Coleman CN. Cancer Care Access and Outcomes for American Indian Populations in the United States: Challenges and Models for Progress. Semin Radiat Oncol 2017;27:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bureau. UC. American Indian and Alaska Native Heritage Month: November 2012 US Department of Commerce; Washington, DC; 2011. [Google Scholar]
- 14.National Congress of American Indians. (January 2016). Fiscal Year 2017 Indian Country Budget Requests: Upholding the Promises RTGFtGotPW [Google Scholar]
- 15.Indian Health Service: Agency Faces Ongoing Challenges Filling Provider Vacancies In: Office GA, ed.2018. [Google Scholar]
- 16.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004;363:1049–57. [DOI] [PubMed] [Google Scholar]
- 17.Javid SH, Varghese TK, Morris AM, et al. Guideline-concordant cancer care and survival among American Indian/Alaskan Native patients. Cancer 2014;120:2183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 2004;101:3–27. [DOI] [PubMed] [Google Scholar]
- 19.Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG 3rd. Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann Surg 2017;266:333–8. [DOI] [PubMed] [Google Scholar]
- 20.Ahola R, Sand J, Laukkarinen J. Centralization of Pancreatic Surgery Improves Results: Review. Scand J Surg 2020;109:4–10. [DOI] [PubMed] [Google Scholar]
- 21.Chang DC, Zhang Y, Mukherjee D, et al. Variations in referral patterns to high-volume centers for pancreatic cancer. J Am Coll Surg 2009;209:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molina G, Clancy TE, Tsai TC, Lam M, Wang J. Racial Disparity in Pancreatoduodenectomy for Borderline Resectable Pancreatic Adenocarcinoma. Ann Surg Oncol 2021;28:1088–96. [DOI] [PubMed] [Google Scholar]
- 23.Fatima J, Schnelldorfer T, Barton J, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg 2010;145:167–72. [DOI] [PubMed] [Google Scholar]
- 24.Traverso LW. Pancreatic cancer: surgery alone is not sufficient. Surg Endosc 2006;20 Suppl 2:S446–9. [DOI] [PubMed] [Google Scholar]
- 25.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372–7. [DOI] [PubMed] [Google Scholar]
- 26.Shavers VL, Harlan LC, Jackson M, Robinson J. Racial/ethnic patterns of care for pancreatic cancer. J Palliat Med 2009;12:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham A, Al-Refaie WB, Parsons HM, Dudeja V, Vickers SM, Habermann EB. Disparities in pancreas cancer care. Ann Surg Oncol 2013;20:2078–87. [DOI] [PubMed] [Google Scholar]
- 28.Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection: a key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer 2009;115:3979–90. [DOI] [PubMed] [Google Scholar]
- 29.Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020;38:1763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eijck CHJV, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy to improve overall survival in pancreatic cancer: Long-term results of the multicenter randomized phase III PREOPANC trial. Journal of Clinical Oncology 2021;39:4016-. [Google Scholar]
- 31.Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg 2018;267:936–45. [DOI] [PubMed] [Google Scholar]
- 32.Tzeng CW, Abbott DE, Cantor SB, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol 2013;20:2197–203. [DOI] [PubMed] [Google Scholar]
- 33.Nordby T, Hugenschmidt H, Fagerland MW, Ikdahl T, Buanes T, Labori KJ. Follow-up after curative surgery for pancreatic ductal adenocarcinoma: asymptomatic recurrence is associated with improved survival. Eur J Surg Oncol 2013;39:559–66. [DOI] [PubMed] [Google Scholar]
- 34.Haj Mohammad N, Bernards N, Besselink MG, et al. Volume matters in the systemic treatment of metastatic pancreatic cancer: a population-based study in the Netherlands. J Cancer Res Clin Oncol 2016;142:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenbaum A, Alkhalili E, Rodriguez R, et al. Pancreatic Adenocarcinoma in New Mexico Native Americans: Disparities in Treatment and Survival. J Health Care Poor Underserved 2019;30:609–17. [DOI] [PubMed] [Google Scholar]
- 36.Noel M, Fiscella K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity 2019;3:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paltoo DN, Chu KC. Patterns in cancer incidence among American Indians/Alaska Natives, United States, 1992–1999. Public Health Rep 2004;119:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervantes A, Waymouth EK, Petrov MS. African-Americans and Indigenous Peoples Have Increased Burden of Diseases of the Exocrine Pancreas: A Systematic Review and Meta-Analysis. Dig Dis Sci 2019;64:249–61. [DOI] [PubMed] [Google Scholar]
- 39.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang BZ, Stram DO, Le Marchand L, et al. Interethnic differences in pancreatic cancer incidence and risk factors: The Multiethnic Cohort. Cancer Med 2019;8:3592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved 2010;21:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez DS, Peskoe SB, Tsilidis KK, et al. Association of variants in genes related to the immune response and obesity with BPH in CLUE II. Prostate Cancer Prostatic Dis 2014;17:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, et al. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev 2007;16:2664–75. [DOI] [PubMed] [Google Scholar]
- 44.Cavanaugh CL, Taylor CA, Keim KS, Clutter JE, Geraghty ME. Cultural perceptions of health and diabetes among Native American men. J Health Care Poor Underserved 2008;19:1029–43. [DOI] [PubMed] [Google Scholar]
- 45.Acton KJ, Burrows NR, Moore K, Querec L, Geiss LS, Engelgau MM. Trends in diabetes prevalence among American Indian and Alaska native children, adolescents, and young adults. Am J Public Health 2002;92:1485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gohdes D, Kaufman S, Valway S. Diabetes in American Indians. An overview. Diabetes Care 1993;16:239–43. [DOI] [PubMed] [Google Scholar]
- 47.Story M, Evans M, Fabsitz RR, Clay TE, Holy Rock B, Broussard B. The epidemic of obesity in American Indian communities and the need for childhood obesity-prevention programs. Am J Clin Nutr 1999;69:747s–54s. [DOI] [PubMed] [Google Scholar]
- 48.Schell LM, Gallo MV. Overweight and obesity among North American Indian infants, children, and youth. Am J Hum Biol 2012;24:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bullock A, Sheff K, Moore K, Manson S. Obesity and Overweight in American Indian and Alaska Native Children, 2006–2015. Am J Public Health 2017;107:1502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odani S, Armour BS, Graffunder CM, Garrett BE, Agaku IT. Prevalence and Disparities in Tobacco Product Use Among American Indians/Alaska Natives - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2017;66:1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stearns AE, Spivak AL, Givel MS. Behind the smokescreen: Native American tobacco use in Oklahoma. Int Q Community Health Educ 2012;33:305–18. [DOI] [PubMed] [Google Scholar]
- 52.Andersen DK, Korc M, Petersen GM, et al. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017;66:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li D. Diabetes and pancreatic cancer. Mol Carcinog 2012;51:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 1996;156:2255–60. [PubMed] [Google Scholar]
- 55.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 2012;51:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and Pancreatic Cancer: Overview of Epidemiology and Potential Prevention by Weight Loss. Pancreas 2018;47:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet 2009;41:986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Childs EJ, Mocci E, Campa D, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet 2015;47:911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein AP, Wolpin BM, Risch HA, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun 2018;9:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin Y, Nakatochi M, Hosono Y, et al. Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat Commun 2020;11:3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 2010;42:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolpin BM, Rizzato C, Kraft P, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet 2014;46:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet 2011;44:62–6. [DOI] [PubMed] [Google Scholar]
- 64.Zhang M, Wang Z, Obazee O, et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget 2016;7:66328–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X, Lin X, Na R, et al. An evaluation study of reported pancreatic adenocarcinoma risk-associated SNPs from genome-wide association studies in Chinese population. Pancreatology 2017;17:931–5. [DOI] [PubMed] [Google Scholar]
- 66.Ueno M, Ohkawa S, Morimoto M, et al. Genome-wide association study-identified SNPs (rs3790844, rs3790843) in the NR5A2 gene and risk of pancreatic cancer in Japanese. Sci Rep 2015;5:17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bogumil D, Conti DV, Sheng X, et al. Replication and Genetic Risk Score Analysis for Pancreatic Cancer in a Diverse Multiethnic Population. Cancer Epidemiol Biomarkers Prev 2020;29:2686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melkonian SC, Jim MA, Reilley B, et al. Incidence of primary liver cancer in American Indians and Alaska Natives, US, 1999–2009. Cancer Causes Control 2018;29:833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melkonian SC, Jim MA, Haverkamp D, et al. Disparities in Cancer Incidence and Trends among American Indians and Alaska Natives in the United States, 2010–2015. Cancer Epidemiol Biomarkers Prev 2019;28:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly JJ, Lanier AP, Schade T, Brantley J, Starkey BM. Cancer disparities among Alaska native people, 1970–2011. Prev Chronic Dis 2014;11:E221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Weir HK, Jim MA, King SM, Wilson R, Master VA. Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990–2009. Am J Public Health 2014;104 Suppl 3:S396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruegl AS, Joshi S, Batman S, Weisenberger M, Munro E, Becker T. Gynecologic cancer incidence and mortality among American Indian/Alaska Native women in the Pacific Northwest, 1996–2016. Gynecol Oncol 2020;157:686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kakol M, Upson D, Sood A. Susceptibility of Southwestern American Indian Tribes to Coronavirus Disease 2019 (COVID-19). J Rural Health 2021;37:197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ufberg M. The Navajo Nation Is Being Decimated by This Virus. GEN 2020. [Google Scholar]
- 75.Kovich H. Rural Matters - Coronavirus and the Navajo Nation. N Engl J Med 2020;383:105–7. [DOI] [PubMed] [Google Scholar]
- 76.Treisman R. Outpacing The U.S., Hard-Hit Navajo Nation Has Vaccinated More Than Half Of Adults. NPR 2021. [Google Scholar]
- 77.Mendez R. Navajo Nation reports no new daily Covid cases, deaths for the first time in six months. CNBC 2021. [Google Scholar]


