Abstract
Background
Rapid adaptation to new environments can facilitate species invasions and range expansions. Understanding the mechanisms of adaptation used by invasive disease vectors in new regions has key implications for mitigating the prevalence and spread of vector-borne disease, although they remain relatively unexplored.
Results
Here, we integrate whole-genome sequencing data from 96 Aedes aegypti mosquitoes collected from various sites in southern and central California with 25 annual topo-climate variables to investigate genome-wide signals of local adaptation among populations. Patterns of population structure, as inferred using principal components and admixture analysis, were consistent with three genetic clusters. Using various landscape genomics approaches, which all remove the confounding effects of shared ancestry on correlations between genetic and environmental variation, we identified 112 genes showing strong signals of local environmental adaptation associated with one or more topo-climate factors. Some of them have known effects in climate adaptation, such as heat-shock proteins, which shows selective sweep and recent positive selection acting on these genomic regions.
Conclusions
Our results provide a genome wide perspective on the distribution of adaptive loci and lay the foundation for future work to understand how environmental adaptation in Ae. aegypti impacts the arboviral disease landscape and how such adaptation could help or hinder efforts at population control.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-023-09402-5.
Keywords: Aedes mosquitoes, Genome scan, Landscape genomics, Selection, Adaptive loci
Background
Biological invasions, involving the introduction, establishment, and spread of species outside their native zone, present one of the main threats to biodiversity, ecosystem integrity, agriculture, fisheries, and public health; with economic costs amounting to hundreds of billions of dollars per year worldwide [1, 2]. During biological invasions, species often spread over a wide and climatically diverse range of environments. Although plasticity and broad ecological tolerance have been shown to facilitate the spread of invaders across such heterogeneous conditions [3, 4], increasing evidence suggests that rapid adaptation to local conditions is commonplace in invasive populations and can enable the establishment and spread of these species in the face of novel selection pressures [5–10]. As such, invasive species represent an ideal model to investigate contemporary adaptive processes, which is key in an era of rapid, human-induced, environmental change.
The establishment and persistence of vectors within new ecological niches poses a serious threat from emerging and endemic arboviral diseases [11]. Dengue fever is among the most widespread vector-borne infectious diseases in the world and is re-emerging in the United States of America after many years of absence [12, 13]; the same trend is also reported elsewhere around the world such as Brazil, Cuba and China [14]. The risk of dengue infection coincides with the distribution of mosquitoes capable of transmitting dengue virus (DENV). Aedes aegypti, the yellow fever mosquito, is the primary urban vector of dengue viruses worldwide is prevalent throughout the tropics and sub-tropics and is closely associated with human habitats outside its native range in Africa. The state of California has maintained an active and extensive mosquito surveillance program initiated in the early 1900s [15] and has previously only detected sporadic specimens of Ae. aegypti near airports [16]. Confirmed breeding populations of Ae. aegypti in California were never reported prior to the summer of 2013, when they were detected in three cities in the central valley counties of Fresno and Madera and the coastal county of San Mateo [16, 17]. Subsequent reports indicate that Ae. aegypti has now become established and is spreading throughout large regions of California [18]. Recent studies demonstrated that Northern and Southern California populations of Ae. aegypti were presumably introduced from two independent introductions which came from the South-Central US and Southwest US/northern Mexico region specifically [19]. Introduced populations of Ae.aegypti to USA have also undergone behavioural and genetic changes in comparison to their ancestral African form, including the evolution of house-entering behaviour and a preference for human odour and blood-feeding [20, 21].
Although it is known that the environment is a key element in driving and altering the life-history traits of Aedes mosquitoes [22–24], there remains a limited understanding of how their genomic background changes across a heterogeneous landscape. A landscape genomics approach is an important first step to associate population structure with the environment and to narrow down candidate genomic targets for further investigation of local environmental adaptation [25]. In the present study, we applied landscape genomics approaches to test the possibility of rapid adaptation to heterogeneous environments by identifying loci with unusual allelic associations to different environmental conditions. We produced evidence relevant to the question of whether adaptation is predominantly mono- or polygenic by conducting genotype-environment association (EAA) analysis using whole genome re-sequencing (WGS) data and by characterizing population structure to account for potentially confounding effects in EAA tests. Our new insights into the evolution of rapid adaptation observed in Ae. aegypti in California will improve our knowledge of evolutionary forces and processes during the invasion of disease vectors, which is crucial for advancing dynamic mitigation strategies aimed at reducing disease risk worldwide [26, 27].
Materials and methods
Mosquito collections
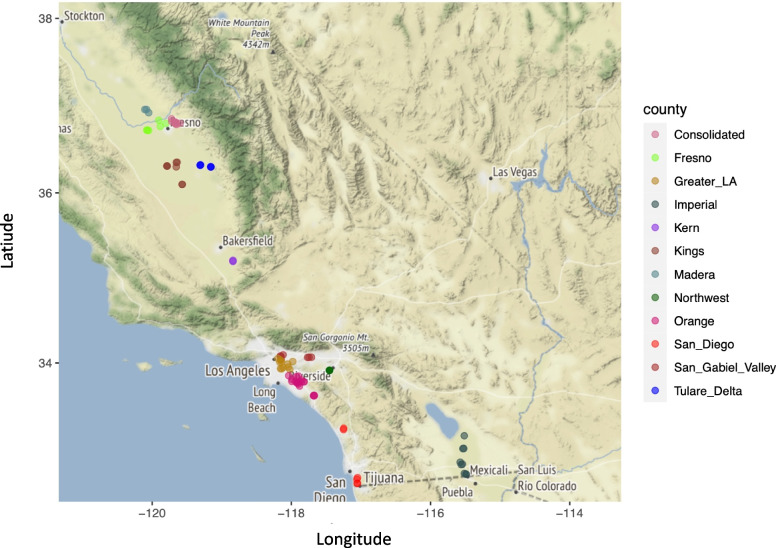
A total of 96 individual adult female Ae. aegypti from 12 geographic districts were collected across southern and central California between 2013–2017 (Fig. 1, Supplementary Table 1). These mosquitoes were collected using BG Sentinel traps baited with CO2. All collections on private properties were conducted after obtaining permission from residents and/or owners. Mosquito samples were individually preserved in 80% ethanol and held at either -20 or -80 °C prior to DNA extraction.
Fig. 1.
Sampling locations of 96 Ae. aegypti mosquitoes collected across central and southern California between 2013 and 2017. Map was created using R Project for Statistical Computing v. 3.3.1 [28] and package maps v. 3.2.0 [29]. Colors indicate the origin mosquito abatement or vector control district of populations. Consolidated refers to the name of a mosquito abatement district in central California
Whole-genome resequencing
Genomic DNA was extracted and sequenced using established protocols as described by Nieman et al. 2015 [30]. Genomic DNA concentrations for each sample were quantified using the Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA) on a Qubit instrument (Life Technologies, Carlsbad, CA). A genomic DNA library was constructed for each individual mosquito using 20 ng DNA, the Qiaseq FX 96 kit (Qiagen, Valencia, CA), and Ampure SPRI beads (Beckman Coulter, Brea, CA) following an established protocol by Nieman et al. 2015 [30]. Library concentrations were measured using Qubit (Life Technologies, Carlsbad, CA) as described above. Libraries were sequenced as 150-bp pair-end reads, each in one lane of an Illumina HiSeq 4000 platform at the UC Davis DNA Technologies Core and according to the manufacturer’s standard protocols (summary statistics of Illumina re-sequencing data per sample is available in Supplementary Table 1).
Alignment, variant calling and annotation
Raw reads were trimmed using Trimmomatic [31] version 0.36 and high-quality trimmed reads were mapped to the AaegL5 reference genome [32] using BWA-MEM version 0.7.15 with default parameters. Mapping statistics were calculated using Qualimap [33] version 2.2 (Supplementary Table 1). The marked duplicate reads were removed using Picard tools version 2.1.1 (http://broadinstitute.github.io/picard/).
We called variants using Freebayes [34] version 1.0.1 with standard filters and population priors disabled. We required a minimum read depth of 8 to call variants for each individual following the recommendation of Crawford and Lazzaro to minimize bias in population inference [35]. To improve the reliability of calls, we required variants to be supported by both forward and reverse reads overlapping the loci (Erik Garrison, Welcome Trust Sanger Institute and Cambridge University, personal communication, Dec. 2014). The repeat regions were “soft-masked” (repeated and low complexity regions in the genome replaced with lowercased versions of their nucleic base) in the AaegL5 reference genome and single nucleotide polymorphisms (SNPs) in these regions were excluded from analysis. SNPs with minor allele frequency (MAF) of < 3% and individuals with > 20% missing genotypes after filtering for genotype quality were excluded from the analysis to minimize bias from sequencing error [22].
Analysis of population structure
We started by generating linkage disequilibrium (LD) pruned SNP sets as follows. We set sliding widows of size 50 (that is the number of markers used for linkage disequilibrium testing at a time) and window increments of 5 markers. For any pair of SNPs in a window we defined, the first marker of the pair was discarded when the correlation coefficient (r2) between markers exceeded 0.2 using an R package, SNPRelate [36]. This yielded 100,089 independent SNPs that were retained for downstream population structure analysis.
Analysis of population structure was performed using the quality-control-positive linkage-disequilibrium-pruned set of 100,089 autosomal SNPs. Principle component analysis (PCA) [37] was conducted across all populations using EIGENSTART (v. 6.1.4) and results were visualized in RStudio [38]. We applied unsupervised hierarchal clustering of individuals using the maximum likelihood method implemented in ADMIXTURE (v. 1.3.0) [39] using default input parameters. ADMIXTURE estimates ancestry coefficients from K modelled ancestral populations by assigning individuals to subpopulations after maximizing Hardy–Weinberg equilibrium of allele frequencies. The ‘—cv’ flag was added to perform the cross-validation procedure and to calculate the optimal number of K. A good value of K exhibits a low cross-validation error compared to other K values.
Environmental data
A total of 25 biologically relevant topo-climate variables (Supplementary Table 2) were used in the analyses. Climate data for each geographic district were collected from geographic coordinates of the locations where the samples were collected using the software package ClimateNA [40]. All variables were annual and collected from 2010 to 2017 available by ClimateNA software. We calculated the average of the annual climate variables over 2010–2017 for the subsequent EAA analysis.
Screening for SNPs associated with local adaptation
To identify putative loci with a signal of selection, we used three approaches with different underlying algorithms and assumptions. To identify loci associated with a signal of selection, we used two EAA approaches, BayPass [41] and latent factor mixed model (LFMM) [42].
BayPass package version 2.1 [43] provides a re-implementation of the Bayesian hierarchical model and explicitly accounts for the covariance structure among population allele frequencies that arises from the shared populations history. This was achieved by estimating a population covariance matrix, which renders the identification of SNPs subjected to selection less sensitive to the confounding impact of neutral genetic structure [41]. Population structure was estimated by choosing a random and unlinked set of 10 K SNPs across all populations selected for this study using the BayPass core model when no covariate data (i.e., no climate data) is provided. The main parameter of the interest is the scaled covariance matrix of population allele frequencies estimated for individuals collected from each geographic district used for this study. We then used the auxiliary (AUX) covariate model to assess the association of SNPs with topo-climate variables. For each SNP, the Bayes factor (denoted BFis as in Gautier, 2015 [43]) relies on the importance sampling algorithm proposed by Coop et al. 2010 [44] and uses Markov Chain Monte Carlo (MCMC) samples obtained under the core model. Aux model involves the introduction of a Bayesian auxiliary variable for each regression coefficient and the auxiliary variable indicates whether a specific SNP can be regarded as associated climate variable or not. It is then straightforward to derive a Bayes Factor to compare both core and AUX models. BFis was expressed in deciban units (db, tenths of a power of 10) via the transformation 10 log10 (BF). As a decision rule and to calculate a significance threshold for outlier identification, pseudo-observed data (POD) were employed with the same random 10 kb SNPs used for the core model, and a 1% empirical threshold was calculated for the observed Bayes factor. To produce a narrower set of outlier loci, we then followed the popular Jeffreys’ rule [45] that identified outlier loci with BF ≥ 10. The Latent Factor Mixed Model (LFMM) is a variant of the Bayesian principal component analysis in which residual background population structure and confounding variables are introduced via latent factors. We used a model with three latent factors (representing three major genetic clusters) to account for neutral population structure in the data based on the result we obtained from PCA and ADMIXTURE. We ran 105 MCMC integrations with 5 burn-in steps with 10 replicate runs. Z-scores from replicate runs were combined and adjusted using the genomic inflation factor which estimates the excess of the false discovery rate due to multiple testing, and it is defined as the ratio of the observed and the expected median of the distribution of the test statistic [46]. Lambda was calculated according to Delvin and Roeder (1999) [46]:
We corrected for multiple testing by fixing the false discovery rate to 5%. Only SNPs with FDR < 5% were retained as those significantly associated with topo-climate variables.
In addition to two EAAs methods, PCAdapt was used to find loci putatively under selection pressure as they deviate from the typical distribution of the test statistic Z [47]. Similar to LFMM, three K populations were chosen to account for neutral population structure. PCAdapt examines the correlations (measured as the squared loadings p2jk, which is the squared correlation between the jth SNP and Kth principal component) between genetic variants and specific PCs without any prior definition of populations. Assuming a chi-square distribution (degree of freedom = 1) for the squared loadings p2j1, as suggested by Luu et al. 2017 [47], we used PCAdapt to calculate P values for all SNPs and then estimated the FDR to generate a list of candidate SNPs showing significant associations to population structure. Only SNPs with FDR < 5% were retained as those significantly involved in local adaptation.
Identification of top candidate genes
Loci that selected as outliers by all three implemented methods, BayPass, LFMM and PCAdapt, were identified. For each gene, we counted the number of outlier SNPs (a) and the total number of SNPs (n). To identify top-candidate genes for each variable, we compared the number of outlier SNPs per gene to the 0.9999 quantile of the binomial expectation where the expected frequency of SNPs per gene is (summation over i genes), calculating p separately for each environmental variable and excluding genes with no outliers from the calculation of p . Any genes with p values falling above this cutoff threshold were then identified as “top candidate genes” [48]. The position and function of the candidate genes identified by this approach were mined using the mosquito genomics resource of VectorBase [49].
Signature of positive selection around candidate genes
Two standard methods were further applied to search for signs of selective sweep in different groups of populations. Pairwise nucleotide diversity (π) [50], which is expected to have local reduction following a selective sweep, was calculated using a sliding window approach with window size of 10kbp and moving step of 5kbp using the software R package PopGenome [51] separately for each of the three groups detected by the PCA and admixture analyses. Weir and Cockerham’s Fst, which measures genetic divergence between pairs of three groups of populations, was calculated using a sliding-window size of 10 kb and moving step of 5 kb by VCFtools [52].
Gene annotation and enrichment analysis
To explore which biological processes (BP) top candidate genes are involved in, we performed a Gene Ontology (GO) and enrichment analysis for the top candidate genes we identified using topGO package in R [53]. Significance for each individual GO-identifier was computed with Fisher’s exact test and significance threshold of 1%. We also performed BLAST [54] searches of the predicted genes against the homologous genes in the annotated Drosophila melanogaster genome in order to potentially obtain more precise information on their functional annotation.
Results
Characterization of sequence variation in Ae. aegypti
We performed whole genome re-sequencing of all 101 Ae. aegypti samples and obtained, on average, over 110 million Illumina raw reads with an average sequencing depth of ~ 10X per individual covering > 85% of the reference genome. After variant calling and applying appropriate filtering, we identified a total of 1,968,198 single nucleotide polymorphisms (SNPs) with a minor allele frequency (MAF) > 3% which were subjected to downstream analysis. Supplementary Table 1 summarizes the per-individual read counts and coverage depths.
Analysis of local population structure
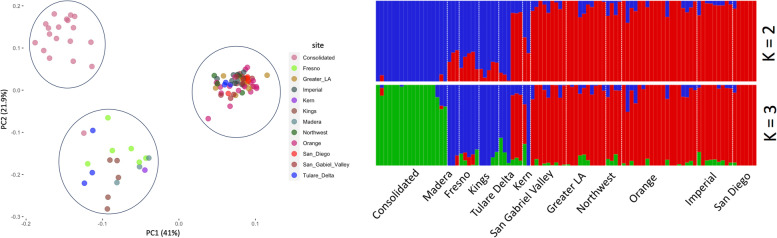
We examined population structure and identified ancestral components with an autosomal marker dataset (100,089) using PCA and ADMIXTURE. We found a strong local population structure across the entire range of collections by PCA. The two-first axes (principal components 1 and 2) explained a large proportion of the variation, cumulatively accounting for 63.9% of the variance in SNP genotypes and three main genetic clusters were determined from this analysis (Fig. 2a). The first cluster (Ae.a1) included samples collected from various sites in Consolidated Mosquito Abatement District in central California. The second cluster (Ae.a2) primarily included samples from other mosquito districts in central California (Madera, Fresno, Kings, Tulare_Delta, and Kern) and the third cluster (Ae.a3) consisted of samples collected from southern California mosquito districts (San_Diego, Imperial, Orange, Northwest, Greater_LA, and San_Gabriel_Valley).
Fig. 2.
a. The first two principal components of a principal component analysis (PCA) of individual genotypes based on the LD pruned dataset describing the relationship among populations. Color code refers to origin mosquito abatement or vector control district of populations. b. Clustering assignments of each genotype inferred using the software Admixture for K = 2 and K = 3 populations. Each color represents one genetic cluster and each vertical bar represents one genotype
Admixture analysis highlighted a significant population structure. According to cross validation error (Supplementary Fig. 1), k = 3 was the most well-presented population structure for our dataset which distinguished individuals from southern California, central California, and Consolidated as genetically distinct groups (Fig. 2b). There were some individuals positioned between the three main clusters suggesting a potential admixture between different populations (Fig. 2a/b). Our results generally recapitulate the broad inferences of a previous study by Lee et al. 2019 [18].
Genomic evidence for local adaptation in response to environmental heterogeneity in California
If the CA populations of Ae.aegypti were locally adapted, we would expect to see that these populations of Ae. aegypti harbor genomic loci with signals of selection correlated to heterogeneous environmental conditions after taking the underlying population structure into account. In order to find genomic regions that are associated with local adaptation and to assess how candidate variation is portioned among different environmental variables, we carried out three complementary approaches which take into account the neutral genetic structure.
We performed PCA analysis for the 25 topo-climate variables extracted from ClimateNA (Supplementary Table 2). The two-first axes (PC1 and PC2) explained a large proportion of the variation, 56% and 35% respectively. Twelve Ae. aegypti populations, mainly distributed along the second PC axis, were linked to both temperature and precipitation variables (Supplementary Fig. 2). We then started by identifying SNPs that showed strong associations with the topo-climate variables using LFMM and BayPass [42, 43]. The number of latent factors was set to three based on the results of Admixture and PCA, as explained above. Under K = 3 genetic clusters, LFMM identified 17,519 outlier SNPs with a genomic signal of local adaptation at the FDR of 5% across all variables. Among all variables, we found the highest number of outliers associated with both temperature and humidity (climatic moisture deficit, degree-days above 18 °C, and annual heat-moisture index with 4,406; 4,078; and 3,685 outlier SNPs respectively).
LFMM is robust in identifying adaptive processes that result from weak, multi-locus effects across various demographic scenarios and sampling schemes. However, it is important to recognize that a subset of the 17,519 candidate loci identified through this single analysis are likely to be false positives. We therefore explored associations with the Bayesian method available in BayPass under the AUX covariate model. We selected this model over others because it is more precise and efficient when estimating the covariance matrix (Ω) and more sensitive for identifying SNPs displaying weak association signals resulting from soft selective sweeps often involved in polygenic characters [43]. Analysis of the data set under the BayPass core model allowed us to estimate the scaled covariance matrix of population allele frequencies Ω that quantifies the genetic relationship among each pair of populations. The resulting estimates of Ω accurately reflected the known structure between samples, that is, a clustering at the higher level by population geographic origin (Supplementary Fig. 3a and b). BayPass analysis identified 16,976 SNPs with a signature of selection widespread across the genome and associated with various topo-climatic factors we tested. Among the analyzed variables, latitude, annual heat-moisture index, mean annual temperature, and climatic moisture deficit were the variables with the highest number of outlier SNPs detected by the BayPass AUX model.
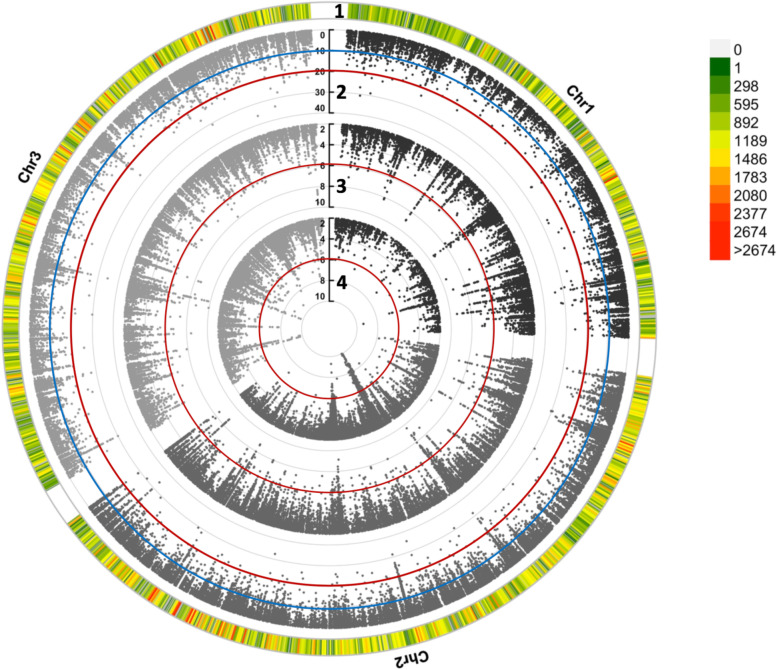
The PCAdapt [47] method is considered less sensitive to confounding demography due to its ability to account for population structure or unobserved spatial autocorrelation in the data [55]. Compared to 17,519 and 16,976 outlier SNPs detected by LFMM and BayPass respectively, PCAdapt identified a of total of 8,637 SNPs with a signature of selection widespread across the genome. Figure 3 shows an example of a circular Manhattan plot for a single environmental variable: mean warmest month temperature (MWMT). Across all three implemented methods, there were 1,991 SNPs consistently identified as outliers with a signal of selection and correlated with topo-climate variables, providing higher confidence that these loci are located within, or close to, regions involved in adaptation to heterogeneous environments.
Fig. 3.
Circular Manhattan plot of genome-wide association analysis performed using three different methods. Ring 1 shows distribution of high-quality SNPs over three different Ae. aegypti chromosomes. It indicates the number of SNPs within 1 Mb windows and reflects the SNP density on each chromosome for genome-wide association with climate variables. Ring 2 shows SNPs association with MWMT (mean temperature of the warmest month) detected by BayPass. The significance level of association based on Bayes Factors (BF) is presented by blue (BF = 10) and red (BF = 20) circles. Rings 3 and 4 show SNPs detected by LFMM and PCAdapt respectively. The significance level (–logP for LFMM and PcAdapt) is represented by a red circle
Candidate gene functions and molecular pathways
We identified top candidate genes as those where an exceptional proportion of their total SNPs were outliers across all the three methods used, as explained above and in the methods section (Fig. 4). In total, we found 112 top candidate genes and many of these genes were supported by multiple environmental variables (Supplementary Table 3). AHM (annual heat-moisture index),CMD (Hargreaves climate moisture deficit) and DD18 (degree days below 18 °C) were the three top variables with the largest number of top candidate genes respectively. The vast majority of the genes detected as top candidates are annotated as being involved in a variety of biological processes, including AAEL001245 (EBgn0262737) which is known to encode a protein involved in thermo-sensory behavior and regulation of alternative splicing in Drosophila and AAEL019772 (FBgn0015245) and AAEL008641 (FBgn0001122) which encode heat shock proteins in Drosophila [56] (complete list of candidate genes with Drosophila homologs are described in Supplementary Table 3).
Fig. 4.
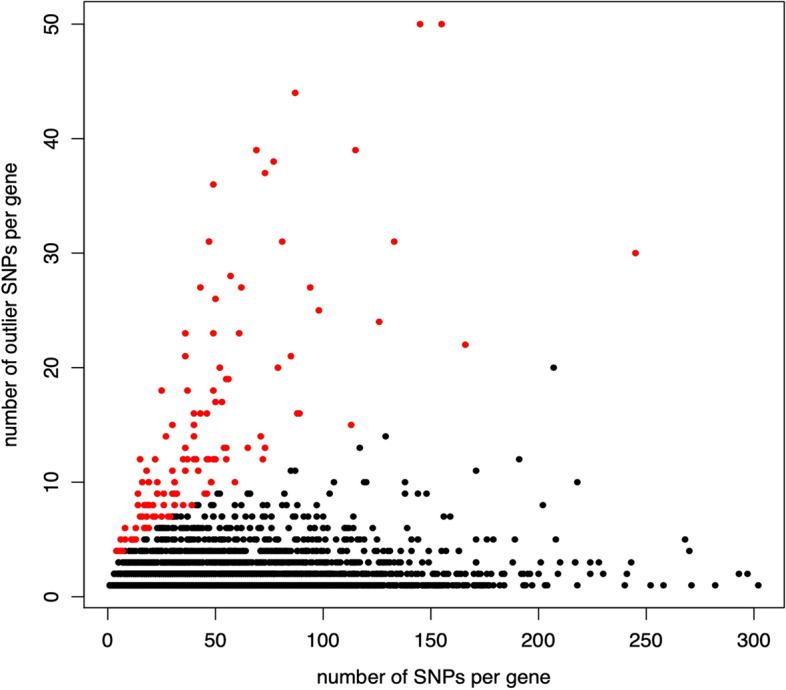
Top candidate genes for mean warmest month temperature (MWMT) identified as those with an extreme number of outlier SNPs relative to binomial expectation, shown in red. The same method was used to identify top candidate genes for each of the 25 topo-climate variable tests
To understand the biological function of the top candidate genes, we performed GO enrichment analysis. From the 112 genes identified as top candidates, we identified 10 significantly overrepresented biological processes including metabolism, cell growth, response to stress, DNA repair, membrane assembly, transport through the endomembrane system, and mRNA transcription which all play important roles in adaption (Table 1).
Table 1.
Top-ranked biological processes that were significantly overrepresented in the top candidate genes in Ae. aegypti
| GO.ID | Term | Annotated | Significant | Expected | elimFisher | p.adj |
|---|---|---|---|---|---|---|
| GO:0006468 | protein phosphorylation | 241 | 73 | 31.87 | 1.10E-12 | 0 |
| GO:0006355 | regulation of transcription | 403 | 100 | 53.29 | 4.70E-11 | 0 |
| GO:0007186 | G protein-coupled receptor signaling pathway | 151 | 46 | 19.97 | 1.70E-08 | 0 |
| GO:0035556 | Intracellular signal transduction | 184 | 55 | 24.33 | 5.70E-05 | 0 |
| GO:0035023 | Regulation of rho protein signal | 28 | 12 | 3.1 | 1.00E-04 | 0 |
| GO:0006813 | Potassium ion transport | 39 | 18 | 5.16 | 2.20E-04 | 0 |
| GO:0007165 | Signal transduction | 600 | 154 | 79.34 | 0.00028 | 0 |
| GO:0007169 | Transmembrane receptor protein tyrosine | 9 | 6 | 1.19 | 0.00031 | 0 |
| GO:0071805 | Potassium ion transmembrane transport | 13 | 7 | 1.72 | 0.00057 | 0 |
| GO:0007264 | Small GPTase mediated signal transduction | 17 | 25 | 9.52 | 0.00312 | 0 |
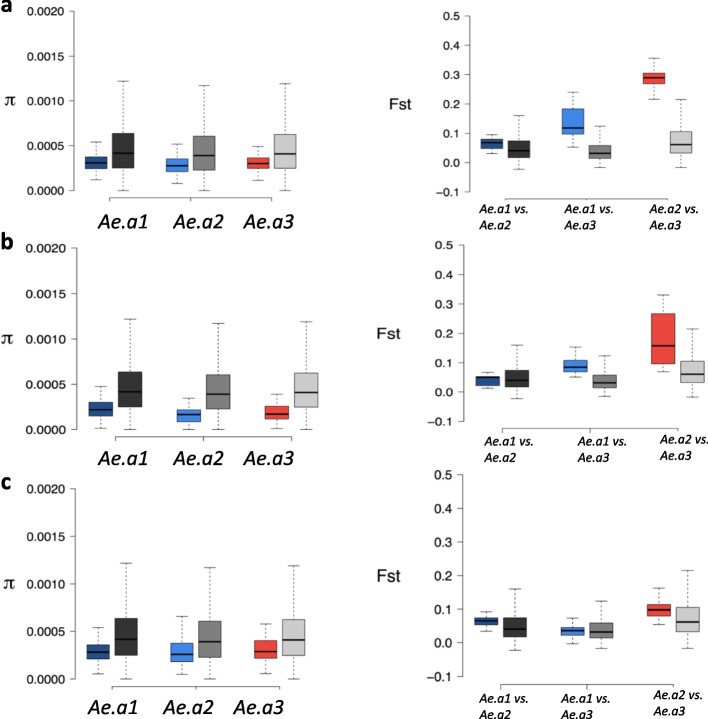
In order to gain further insight into the evolutionary history of adaptation, we performed nucleotide and differentiation-based tests to examine the presence of recent positive selection for the three genes with known activity in thermal adaptation (AAEL001245, AAEL019772 and AAEL008641). The nucleotide diversities (π) at the selected genes were significantly below the genome-wide averages in coding regions in all three groups (Fig. 5), which was consistent with the expectation of a strong selective event and rapid adaptive evolution [57]. Additionally, the level of genetic differentiation (Fst) among populations was higher at the selected genes compared with genetic background, especially between Ae. aegypti 2 and Ae. aegypti 3 groups (Fig. 5) implying that spatially varying selection has likely driven differentiation in these genes between the groups.
Fig. 5.
Nucleotide diversity (π) and genetic differentiation (Fst) for three genes, a. AAEL001245, b. AAEL019772 and c. AAEL008641, with significant signature of adaptation and well-known roles in thermal stress adaptation in Drosophila melanogaster
Discussion
Invasive species cause considerable ecological and economic harms worldwide [58, 59]. Despite the broad impacts they have on diversity and agriculture, the genetic basis of adaptations and near-term evolution of invading populations are poorly understood. Ae. aegypti is the major vector of multiple diseases, such as dengue, Zika, and chikungunya and its geographical range is continuously expanding; presumably due to anthropogenic conveyance, ongoing climate change, and increasing global transportation. The goal of the present study was to describe the fine-scale genomic architecture of this invasive mosquito within habitats characterized by different abiotic environmental conditions and to probe the underlying genetic basis for rapid adaptation of this species to new environments.
Our investigation of putative signals of selection and local adaptation of Ae. aegypti (in total 96 mosquitoes from 12 geographic sites) that were recently introduced and established in various locations in central and southern California (Fig. 1) found signals of selection, distributed along the genome. In a stepwise approach that included applying landscape genomics, identification of top candidate genes and GO enrichment analysis, we identified a set of candidate genes with various biological functions associated with adaptation to local abiotic environmental conditions in central and southern California.
The study by Pless et al. [19] showed northern and southern populations of Ae.aegypti were likely founded by two independent introductions which came from the south central US and southwest US/northern Mexico regions respectively. We found three major genetic clusters among 96 individuals collected from 12 geographic sites across central and southern California. Our results were consistent with a previous study by Lee et al. [18] where they also found three major genetic clusters. In their analysis, samples from southeast USA (Florida) clustered with populations from the town of Exeter in central California and southern California. Our finding along with the previous findings by Lee et al. and previous report support the hypothesis that populations of Ae.aegypti distributed in California originated from multiple independent introductions from genetically distinct source populations; although the exact origin of the introductions remains uncertain and open for the future investigations.
To find genomic regions that have been targets of natural selection, we identified SNPs that are putatively selected for and strongly associated with topo-climatic variables using various landscape genomics methods. The methods we employed substantially controlled for neutral population genetic structure such as genetic drift or gene flow. We chose outlier SNPs as those which were consistently identified by all applied methods, allowing us to eliminate stochastic variation that could affect the results. We assumed that outlier loci detected along the genome are likely to be under selection, either directly or through hitchhiking [60]; although we acknowledge that other processes, including regions with reduced recombination, inversions, and chromosomal rearrangements, may also be responsible for the results we obtained [61]. Therefore, further studies of linkage disequilibrium and the regions with reduced recombination and genome structure could illuminate the possible role of these factors in shaping adaptation as has been shown in other mosquitoes such as Anopheles [62].
Natural selection plays a key role in shaping the available genetic variation of populations and thereby produces adaptation [63]. By applying EAA methodology we scanned the genome to uncover genomic selection footprints. We detected loci which were associated with both temperature and precipitation related variables (Supplementary Table 3), which implies the significance of both of these elements in shaping selection pressure and forming local adaptation in Ae. aegypti. Our results are in accordance with previous reports identifying these abiotic variables as major predictors in Aedes distribution patterns [14, 25]. Temperature has been known to govern reproduction, maturation, and mortality rates and to be important for egg laying, development and survival of Ae. aegypti in larval habitats [64]. These variables are also likely to elevate selection for thermal tolerance at the adult stage to increase resistance to diurnal and inter-seasonal variation [14, 64, 65]. Precipitation affects the distribution of Ae. aegypti since rainfall generates breeding grounds for adults. Unlike other mosquito species, Ae. aegypti eggs are laid above the water surface and hatch only when the water level rises and wets them [66].
The introduction of Aedes aegypti into California would most certainly have been through some anthropogenic means which is a well-known mode of dispersal in this species. [14] A rapid evolutionary response, as we observed in this population, would therefore have been largely based on preexisting standing genetic variation. We have identified signals of local environmental adaptation across a relatively small number of loci distributed along the genome. Our lack of ability to detect more putative regions under selection can be explained by analytical limitations in distinguishing weak multi-locus signatures from the genomic differentiation introduced by genetic drift and demography [67, 68]. It has been shown that there is an extensive genetic differentiation and a limited amount of gene flow among CA populations of Ae.aegypti and also a relatively limited number of generations after introduction to California. Therefore, small number of regions with signature of adaptation can be stemmed from biological limitations and not just analytical limitations [69]. These limited regions are expected to have a strong impact on the fitness such as viability, reproductive success, cold tolerance and phenology traits such as diapause in one environment over the other because the allele with the highest fitness is expected to spread to all populations if this condition is not met. This can be tested in a common garden with a reciprocal transplant experiment in the future.
By applying top candidate gene methods, we discovered 112 genes that contain SNPs highly associated with at least one topo-climatic variable (Supplementary Table 3). To better understand the role of each selected top candidate gene, we found their homolog genes in Drosophila. Several genes of heat shock protein (HSP) families are known to be selected in mosquitoes, which aid in overcoming stress induced by elevated temperature [70]. Nucleotide diversity at these genes was below the genome-wide averages and the level of genetic differentiation was high among populations which further confirms that these genes are likely targets of the positive selection. In general, these results present some promising avenues for future works; especially if the markers detected here are linked to the actual targets of selection. The congruence between the observed genome scans and the genes assigned biological functions makes them ideal targets for further experimental validation. From an evolutionary perspective, coding regions are key genomic spots to look for the signatures of selection, as these directly influence functional elements in contrast to the non-coding genome regions. However, it is important to note that selection can also act on noncoding regions since they may be located, for example, in promoters, enhancers, or small RNAs where they affect gene expression. In this context, SNPs residing in non-coding regions of the genome may be of interest for future studies.
Conclusions
The study of invasive adaptation and genome evolution is an emerging field that is developing rapidly and offers countless opportunities to investigate adaptive processes. Understanding the genomic basis of adaptive evolution in invasive species is important for predicting future invasion scenarios, identifying candidate genes involved in invasions, and, more generally, for understanding how populations can evolve rapidly in response to novel and changing environments.
Here we used a landscape genomics approaches to identify genomic regions and candidate genes potentially involved in adaptation. The identified genes showed footprints of selection and were correlated with environmental factors that differed between sites, as expected under a scenario of environment-mediated selection in natural populations of Ae. aegypti in California. Our findings help to elucidate the role of rapid evolution in the establishment and spread of invasive species. We detected evidence indicating local adaptation to various environmental conditions in populations of Ae. aegypti just a few years after its introduction into California, adaptations which may translate into a fitness advantage for specific populations.
Supplementary Information
Additional file 2: Supplementary table 2. Environmental data collected from ClimateNA database for each sampling site.
Additional file 3: Supplementary table 3. List of genes selected as top candidates by top candidate gene approach, their homologs and biological function and topo-climate variables associated with each candidate gene.
Additional file 4: Supplementary Figure 1. Cross validation error plot showing the selection of K = 3 is the most well-presented population structure for this dataset.
Additional file 5: Supplementary Figure 2. a. Heatmap indicating correlation among 25 topo-climate variables collected from ClimateNA. b. Principle component analysis of the topo-climate variables for 12 populations used for the association analysis, with the projection of the correlations on the first PCA plan. The full names of the topo-climate variables are available in supplementary table 2.
Additional file 6: Supplementary Figure 3. Inferred relationship among 12 populations of the Ae.aegypti represented by a. a hierarchical clustering tree derived from the matrix Ω estimated under the core model and b. a correlation plot.
Acknowledgements
We thank personnel from Consolidated Mosquito Abatement District, Delta, Greater Los Angeles County, San Bernadino County, and San Mateo County Vector Control Districts and Coachella, Fresno, Madera County and Orange County Mosquito and Vector Control Districts, Community Health Division of the Department of Environmental Health, San Diego County Environmental Health, and Dr. Christopher Barker (UC Davis) for providing specimens used in this study. We thank Youki Yamasaki, Allison Chang, Parker Houston, Allison Weakley, Kendra Person, and Hans Gripkey for assisting DNA extraction and library preparations for this study. Thanks to Melika Hajkazemian, Melina Campos and Christine Coleman for their comments to the manuscript.
Authors’ contributions
ShS and GCL conceived the idea, designed the study, interpreted the data and wrote the manuscript. ShS performed the analysis and made figures. YL and AJC helped with gathering mosquitos. TCC performed alignment and variant calling. MC performed DNA extraction and library preparation. All authors read and approved the final version of manuscript.
Funding
We acknowledge funding support from the Pacific Southwest Regional Center of Excellence for Vector-Borne Diseases funded by the U.S. Centers for Disease Control and Prevention (Cooperative Agreement 1U01CK000516).
Availability of data and materials
All scripts used for the analysis described are available on GitHub (https://github.com/shaghayeghsoudi/genomics_of_adaptation_A.aegypti_scripts) The datasets supporting the conclusions of this article are included within the article and its additional files.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bradshaw CJA, Leroy B, Bellard C, Roiz D, Albert C, Fournier A, et al. Massive yet grossly underestimated global costs of invasive insects. Nat Commun. 2016;7(1):12986. doi: 10.1038/ncomms12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paini DR, Sheppard AW, Cook DC, De Barro PJ, Worner SP, Thomas MB. Global threat to agriculture from invasive species. Proc Natl Acad Sci. 2016;113(27):7575–7579. doi: 10.1073/pnas.1602205113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geng Y-P, Pan X-Y, Xu C-Y, Zhang W-J, Li B, Chen J-K, et al. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biol Invasions. 2007;9(3):245–256. doi: 10.1007/s10530-006-9029-1. [DOI] [Google Scholar]
- 4.Zhang Y-Y, Zhang D-Y, Barrett SCH. Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol Ecol. 2010;19(9):1774–86. 10.1111/j.1365-294X.2010.04609.x. [DOI] [PubMed]
- 5.Chown SL, Hodgins KA, Griffin PC, Oakeshott JG, Byrne M, Hoffmann AA. Biological invasions, climate change and genomics. Evol Appl. 2015;8(1):23–46. doi: 10.1111/eva.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colautti RI, Barrett SCH. Rapid Adaptation to Climate Facilitates Range Expansion of an Invasive Plant. Science. 2013;342(6156):364–366. doi: 10.1126/science.1242121. [DOI] [PubMed] [Google Scholar]
- 7.Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra Ls. Rapid Evolution of a Geographic Cline in Size in an Introduced Fly. Science. 2000;287(5451):308–9. 10.1126/science.287.5451.308. [DOI] [PubMed]
- 8.Lachmuth S, Durka W, Schurr FM. Differentiation of reproductive and competitive ability in the invaded range of Senecio inaequidens: the role of genetic Allee effects, adaptive and nonadaptive evolution. New Phytol. 2011;192(2):529–541. doi: 10.1111/j.1469-8137.2011.03808.x. [DOI] [PubMed] [Google Scholar]
- 9.Turner KG, Hufbauer RA, Rieseberg LH. Rapid evolution of an invasive weed. New Phytol. 2014;202(1):309–321. doi: 10.1111/nph.12634. [DOI] [PubMed] [Google Scholar]
- 10.van Boheemen LA, Atwater DZ, Hodgins KA. Rapid and repeated local adaptation to climate in an invasive plant. New Phytol. 2019;222(1):614–627. doi: 10.1111/nph.15564. [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. The Lancet. 2012;380(9857):1946–1955. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musso D, Rodriguez-Morales AJ, Levi JE, Cao-Lormeau V-M, Gubler DJ. Unexpected outbreaks of arbovirus infections: lessons learned from the Pacific and tropical America. Lancet Infect Dis. 2018;18(11):e355–e361. doi: 10.1016/S1473-3099(18)30269-X. [DOI] [PubMed] [Google Scholar]
- 13.Weaver SC. Arrival of Chikungunya Virus in the New World: Prospects for Spread and Impact on Public Health. PLOS Neglect Trop Dis. 2014;8(6):e2921. 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed]
- 14.Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife. 2015;4:e08347. 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed]
- 15.Wekesa, JW. A Century of Mosquito Control in California: 1915 - 2015. Wing Beats. 2015;26:33–46.
- 16.Gloria-Soria A, Brown JE, Kramer V, Hardstone Yoshimizu M, Powell JR. Origin of the Dengue Fever Mosquito, Aedes aegypti, in California. PLOS Neglect Trop Dis. 2014;8(7):e3029. 10.1371/journal.pntd.0003029. [DOI] [PMC free article] [PubMed]
- 17.Cornel AJ, Holeman J, Nieman CC, Lee Y, Smith C, Amorino M, et al. Surveillance, insecticide resistance and control of an invasive Aedes aegypti (Diptera: Culicidae) population in California. F1000Res. 2016;5:194. 10.12688/f1000research.8107.3. [DOI] [PMC free article] [PubMed]
- 18.Lee Y, Schmidt H, Collier TC, Conner WR, Hanemaaijer MJ, Slatkin M, et al. Genome-wide divergence among invasive populations of Aedes aegypti in California. BMC Genomics. 2019;20(1):204. doi: 10.1186/s12864-019-5586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pless E, Gloria-Soria A, Evans BR, Kramer V, Bolling BG, Tabachnick WJ, et al. Multiple introductions of the dengue vector, Aedes aegypti, into California. PLOS Neglect Trop Dis. 2017;11(8):e0005718. 10.1371/journal.pntd.0005718. [DOI] [PMC free article] [PubMed]
- 20.Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, et al. Human impacts have shaped historical and recent evolution in Aedes Aegypti, the dengue and yellow fever mosquito. Evolution. 2014;68(2):514–525. doi: 10.1111/evo.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515(7526):222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- 23.Leisnham PT, Juliano SA. Interpopulation differences in competitive effect and response of the mosquito Aedes aegypti and resistance to invasion by a superior competitor. Oecologia. 2010;164(1):221–230. doi: 10.1007/s00442-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tun-Lin W, Burkot TR, Kay BH. Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland. Aust Med Vet Entomol. 2000;14(1):31–37. doi: 10.1046/j.1365-2915.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennett KL, McMillan WO, Loaiza JR. The genomic signal of local environmental adaptation in Aedes aegypti mosquitoes. Evol Appl. 2021;14(5):1301–1313. doi: 10.1111/eva.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colautti RI, Lau JA. Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol. 2015;24(9):1999–2017. doi: 10.1111/mec.13162. [DOI] [PubMed] [Google Scholar]
- 27.Moran EV, Alexander JM. Evolutionary responses to global change: lessons from invasive species. Ecol Lett. 2014;17(5):637–649. doi: 10.1111/ele.12262. [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2022. https://www.R-project.org/.
- 29.Becker RA, Wilks, AR, Original S code. R version by Ray Brownrigg. Enhancements by Thomas P Minka and Alex Deckmyn. maps. 2017. p. R package v. 3.2.0. https://cran.r-project.org/web/packages/maps/.
- 30.Nieman CC, Yamasaki Y, Collier TC, Lee Y. A DNA extraction protocol for improved DNA yield from individual mosquitoes. F1000Research. 2015;4:1314. 10.12688/f1000research.7413.1. [DOI] [PMC free article] [PubMed]
- 31.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563(7732):501–507. doi: 10.1038/s41586-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okonechnikov K, Conesa A, García-Alcalde F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 2016;32(2):292–294. doi: 10.1093/bioinformatics/btv566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv:1207.3907v2 [q-bio.GN]2012. https://arxiv.org/abs/1207.3907.
- 35.Crawford J, Lazzaro B. Assessing the Accuracy and Power of Population Genetic Inference from Low-Pass Next-Generation Sequencing Data. Front Genet. 2012;3. 10.3389/fgene.2012.00066. [DOI] [PMC free article] [PubMed]
- 36.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28(24):3326–3328. doi: 10.1093/bioinformatics/bts606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 38.RStudio Team. RStudio: Integrated Development for R. RStudio. Boston: PBC; 2020. http://www.rstudio.com/.
- 39.Peter BM. Admixture, Population Structure, and F-Statistics. Genetics. 2016;202(4):1485–1501. doi: 10.1534/genetics.115.183913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Hamann A, Spittlehouse D, Carroll C. Locally Downscaled and Spatially Customizable Climate Data for Historical and Future Periods for North America. PLOS ONE. 2016;11(6):e0156720. 10.1371/journal.pone.0156720. [DOI] [PMC free article] [PubMed]
- 41.Günther T, Coop G. Robust Identification of Local Adaptation from Allele Frequencies. Genetics. 2013;195(1):205–220. doi: 10.1534/genetics.113.152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frichot E, Schoville SD, Bouchard G, François O. Testing for Associations between Loci and Environmental Gradients Using Latent Factor Mixed Models. Mol Biol Evol. 2013;30(7):1687–1699. doi: 10.1093/molbev/mst063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gautier M. Genome-Wide Scan for Adaptive Divergence and Association with Population-Specific Covariates. Genetics. 2015;201(4):1555–1579. doi: 10.1534/genetics.115.181453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coop G, Witonsky D, Di Rienzo A, Pritchard JK. Using Environmental Correlations to Identify Loci Underlying Local Adaptation. Genetics. 2010;185(4):1411–1423. doi: 10.1534/genetics.110.114819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffreys H. Theory of probability. 3. Oxford, UK: Oxford University Press; 1961. [Google Scholar]
- 46.Devlin B, Roeder K. Genomic Control for Association Studies. Biometrics. 1999;55(4):997–1004. doi: 10.1111/j.0006-341X.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 47.Luu K, Bazin E, Blum MGB. pcadapt: an R package to perform genome scans for selection based on principal component analysis. Mol Ecol Resour. 2017;17(1):67–77. doi: 10.1111/1755-0998.12592. [DOI] [PubMed] [Google Scholar]
- 48.Yeaman S, Hodgins KA, Lotterhos KE, Suren H, Nadeau S, Degner JC, et al. Convergent local adaptation to climate in distantly related conifers. Science. 2016;353(6306):1431–1433. doi: 10.1126/science.aaf7812. [DOI] [PubMed] [Google Scholar]
- 49.Amos B, Aurrecoechea C, Barba M, Barreto A, Basenko Evelina Y, Bażant W, et al. VEuPathDB: the eukaryotic pathogen, vector and host bioinformatics resource center. Nucleic Acids Res. 2021;50(D1):D898–D911. doi: 10.1093/nar/gkab929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123(3):585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer B, Wittelsbürger U, Ramos-Onsins SE, Lercher MJ. PopGenome: An Efficient Swiss Army Knife for Population Genomic Analyses in R. Mol Biol Evol. 2014;31(7):1929–1936. doi: 10.1093/molbev/msu136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korneliussen TS, Albrechtsen A, Nielsen R. ANGSD: Analysis of Next Generation Sequencing Data. BMC Bioinformatics. 2014;15(1):356. doi: 10.1186/s12859-014-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexa A, Rahnenführer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22(13):1600–1607. doi: 10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
- 54.Camacho CG, Coulouris G, Avagyan V, Ma N, Papadopoulos J. BLAST+: architecture and applications. BMC Bioinform. 2009;10:1–9. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Villemereuil P, Frichot É, Bazin É, François O, Gaggiotti OE. Genome scan methods against more complex models: when and how much should we trust them? Mol Ecol. 2014;23(8):2006–2019. doi: 10.1111/mec.12705. [DOI] [PubMed] [Google Scholar]
- 56.Lakhotia SC, Srivastava P, Prasanth KV. Regulation of heat shock proteins, Hsp70 and Hsp64, in heat-shocked Malpighian tubules of Drosophila melanogaster larvae. Cell Stress Chaperones. 2002;7(4):347–356. doi: 10.1379/1466-1268(2002)007<0347:rohsph>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen R. Molecular Signatures of Natural Selection. Annu Rev Genet. 2005;39(1):197–218. doi: 10.1146/annurev.genet.39.073003.112420. [DOI] [PubMed] [Google Scholar]
- 58.Pyšek P, Richardson DM. Invasive Species, Environmental Change and Management, and Health. Annu Rev Environ Resour. 2010;35(1):25–55. doi: 10.1146/annurev-environ-033009-095548. [DOI] [Google Scholar]
- 59.Marbuah G, Gren I-M, McKie B. Economics of Harmful Invasive Species: A Review. Diversity. 2014;6(3):500–523. doi: 10.3390/d6030500. [DOI] [Google Scholar]
- 60.Strasburg JL, Sherman NA, Wright KM, Moyle LC, Willis JH, Rieseberg LH. What can patterns of differentiation across plant genomes tell us about adaptation and speciation? Philos Trans R Soc B Biol Sci. 2012;367(1587):364–373. doi: 10.1098/rstb.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeaman S. Genomic rearrangements and the evolution of clusters of locally adaptive loci. Proc Natl Acad Sci. 2013;110(19):E1743–E1751. doi: 10.1073/pnas.1219381110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayala D, Ullastres A, González J. Adaptation through chromosomal inversions in Anopheles. Front Genetics. 2014;5. 10.3389/fgene.2014.00129. [DOI] [PMC free article] [PubMed]
- 63.Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecol Lett. 2004;7(12):1225–1241. doi: 10.1111/j.1461-0248.2004.00684.x. [DOI] [Google Scholar]
- 64.Brady OJ, Golding N, Pigott DM, Kraemer MUG, Messina JP, Reiner Jr RC, et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors. 2014;7(1):338. 10.1186/1756-3305-7-338. [DOI] [PMC free article] [PubMed]
- 65.Brady OJ, Johansson MA, Guerra CA, Bhatt S, Golding N, Pigott DM, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6(1):351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdez LD, Sibona GJ, Condat CA. Impact of rainfall on Aedes aegypti populations. Ecol Model. 2018;385:96–105. doi: 10.1016/j.ecolmodel.2018.07.003. [DOI] [Google Scholar]
- 67.Forester BR, Lasky JR, Wagner HH, Urban DL. Comparing methods for detecting multilocus adaptation with multivariate genotype–environment associations. Mol Ecol. 2018;27(9):2215–2233. doi: 10.1111/mec.14584. [DOI] [PubMed] [Google Scholar]
- 68.Hoban S, Kelley JL, Lotterhos KE, Antolin MF, Bradburd G, Lowry DB, et al. Finding the Genomic Basis of Local Adaptation: Pitfalls, Practical Solutions, and Future Directions. Am Nat. 2016;188(4):379–397. doi: 10.1086/688018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savolainen O, Lascoux M, Merilä J. Ecological genomics of local adaptation. Nat Rev Genet. 2013;14(11):807–820. doi: 10.1038/nrg3522. [DOI] [PubMed] [Google Scholar]
- 70.Sivan A, Shriram AN, Muruganandam N, Thamizhmani R. Expression of heat shock proteins (HSPs) in Aedes aegypti (L) and Aedes albopictus (Skuse) (Diptera: Culicidae) larvae in response to thermal stress. Acta Trop. 2017;167:121–127. doi: 10.1016/j.actatropica.2016.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Supplementary table 2. Environmental data collected from ClimateNA database for each sampling site.
Additional file 3: Supplementary table 3. List of genes selected as top candidates by top candidate gene approach, their homologs and biological function and topo-climate variables associated with each candidate gene.
Additional file 4: Supplementary Figure 1. Cross validation error plot showing the selection of K = 3 is the most well-presented population structure for this dataset.
Additional file 5: Supplementary Figure 2. a. Heatmap indicating correlation among 25 topo-climate variables collected from ClimateNA. b. Principle component analysis of the topo-climate variables for 12 populations used for the association analysis, with the projection of the correlations on the first PCA plan. The full names of the topo-climate variables are available in supplementary table 2.
Additional file 6: Supplementary Figure 3. Inferred relationship among 12 populations of the Ae.aegypti represented by a. a hierarchical clustering tree derived from the matrix Ω estimated under the core model and b. a correlation plot.
Data Availability Statement
All scripts used for the analysis described are available on GitHub (https://github.com/shaghayeghsoudi/genomics_of_adaptation_A.aegypti_scripts) The datasets supporting the conclusions of this article are included within the article and its additional files.