Abstract
Lasia spinosa (L.) Thw. (L. spinosa) is widely used as a folk remedy for different physical ailments, and its neurological effects have yet to be assessed. Phytochemicals status of L. spinosa was evaluated by GC-MS analysis. Membrane stabilization test, elevated plus maze (EPM) tests and hole board tests (HBT), tail suspension tests (TST) and thiopental sodium-induced sleeping tests (TISTT) were used to assess anti-inflammatory, anxiolytic and anti-depressant activity. Fourteen compounds have been recorded from GC-MS analysis. The LSCTF showed 68.66 ± 2.46% hemolysis protections (p < 0.05) at 500 μg/mL, whereas LSCHF and LSNHF demonstrated efficiency rates of 68.6 ± 1.46% and 52.46 ± 5.28%, respectively. During EPM tests, LSNHF and LSCTF significantly (p < 0.001) increased the time spent in the open arm (59.88 ± 0.65 s and 50.77 ± 0.67 s, respectively) at the dosages of 400 mg/kg. In HBT, samples exhibited dose-dependent anxiolytic activity. LSNHF and LSCTF showed a significant (p < 0.001) hole poking tendency and a high number of head dips (78.66 ± 1.05 and 65.17 ± 0.96, respectively) at the higher dose. In TST, at 400 mg/kg dose demonstrated significantly (p < 0.001) smaller amounts of time immobile, at 81.33 ± 1.67 s and 83.50 ± 1.90 s, respectively, compared to the control group. A consistent finding was also observed in TISTT. The computer-assisted studies on the identified compounds strongly support the aforementioned biological activities, indicating that L. spinosa has potential as a source of medication for treating neuropsychiatric and inflammatory diseases.
Keywords: Lasia spinosa, Hemolysis inhibition, Anxiolytic activity, Antidepressant, Molecular docking, And PASS
Highlights
-
•
GC-MS analysis identified the major components of LSNHF.
-
•
LSNHF and LSCTF showed a significant hole poking tendency.
-
•
The in silico studies of identified compounds were strongly linked to anti-inflammatory, anxiolytic and antidepressant activities.
Abbreviations
- LSNHF
Lasia spinosa n-hexane fraction
- LSCTF
Lasia spinosa CCl4 fraction
- LSCHF
Lasia spinosaCHCl3 fraction
- GC-MS
Gas chromatography-mass spectrometry
- p.o.
per oral
- i.p.
intraperitoneal
- b.w.
body weight
- ANOVA
analysis of variance
- SEM
standard error mean
- SPSS
statistical package for social science
1. Introduction
450 million persons globally are estimated to be affected by neurodegenerative disorders, while approximately 0.12 billion people suffer from anxiety and depression. Depression and anxiety are thus regarded as the world's leading neurological disorders [1]. Anxiety, depression, and insomnia are psychological disorders that disrupt the normal activity of the people suffering from them. The precise etiology of these psychological problems is still unclear, but physiological problems, habitual, genetic variation, chronic diseases, and certain lifestyle habits may be responsible for these disorders [2]. Chronic pain, inflammation, and undiagnosed illnesses may also contribute [3]. In addition, scientific trials have demonstrated that genetic changes and neuronal stress and pain mechanisms also have a high degree of association [3,4]. Medications that target neurotransmitters such as serotonin and noradrenaline are often used to control activity and nociceptive pathways in the brain and central nervous system [5]. Currently, benzodiazepines, barbituric acid, and serotonin-norepinephrine reuptake inhibitors are among the most widely used medications for the management of anxious depressive disorders [6,7] despite their associated side effects, which include impotency, over-sleeping, euphoria, and increased risk of drug dependence and abuse [8]. Because such side effects may contraindicate the use of these medications in certain cases, it's important to continue exploring alternative sources of medication for neurological and psychiatric diseases.
In recent years, research on natural pharmaceutical compounds has demonstrated the wide range of medicinal values to be found in plants and herbs, from relaxation, pain relief [9,10], and inflammation management [[11], [12], [13]] to the treatment of rheumatoid arthritis [14], tumors [[15], [16], [17], [18]], and depression [5,19]. It is also evident that natural products derived from medicinal plants are the best source of alternatives conventional drugs for patients who are susceptible to them [[20], [21], [22]]. Several computational modeling techniques such as molecular docking, PASS, and ADME profiling offer numerous benefits in experimental research [23,24]. Molecular docking predicts the binding mode and affinity of a small molecule to a protein target, enabling drug discovery to identify potential lead compounds and optimize them for increased potency and selectivity. Similarly, PASS predicts the biological activity of a molecule based on its structure, prioritizing compounds for further testing or identifying potential off-target effects [25]. ADME profiling predicts the pharmacokinetic properties of a molecule, including absorption, distribution, metabolism, and excretion, optimizing the drug development process by identifying potential issues early on and guiding the design of compounds with improved properties [26]. In this study, a molecular docking analysis accurately predicts the interaction between Cyclooxygenase-1, Cyclooxygenase-2, potassium channel, and serotonin transporter L. spinosa compounds, providing insights into their ligand-receptor interaction and binding energy. L. spinosa, which belongs to the Lamiaceae family and is locally known as khattosh, is an evergreen, herbaceous perennial plant that grows to 1–2 m tall and spreads by means of a long, creeping, stoloniferous stem [27]. This plant's root extract exhibits notable analgesic, anti-inflammatory, and anti-diarrheal effects [28]. The plant is harvested for rhizomes, which are both edible and medicinal [29]. The stems and leaves of L. spinosa possessed significant antioxidant potentials and used as prospective functional foods [30]. It has been demonstrated that L. spinosa exhibits cardioprotective properties against doxorubicin-induced cardiotoxicity by normalizing specific biochemical markers, notably creatinine kinase, lactate dehydrogenase, and troponin I [31]. Although the herb has many therapeutic benefits for physical ailments, its therapeutic impact on neurological disorders has yet to be empirically assessed. The current study has therefore been designed to investigate and provide scientific evidence of Lasia spinosa (L.) Thw.‘s anxiolytic, anti-depressive, sedative, and hemolysis-inhibitive properties. To investigate the biological activities of the chemicals identified from LSNHF, computer-aided research such as molecular docking, ADME profile, toxicological characteristics and PASS prediction were performed.
2. Materials and methods
2.1. Chemicals
Carbon tetrachloride (CCl4), chloroform, n-hexane, methanol, disodium hydrogen phosphate, sodium dihydrogen phosphate, and Nitro blue tetrazolium (NBT) were procured from Sigma-Aldrich (USA via local supplier) Sodium citrate, sodium phosphate, hydrocortisone, albumin, and diclofenac sodium were purchased from Merck (Mumbai, India). Diazepam, Imipramine HCl, and Thiopental Sodium, manufactured by Incepta Pharmaceuticals Ltd., Bangladesh, were purchased from a local market.
2.2. Plant materials
Fresh rhizomes of the L. spinosa plant were collected in November 2018. They were identified by a taxonomist, and a specimen number of the sample was stored in the local herbarium.
2.3. Preparation of crude extract
The dried rhizomes of L. spinosa were powered (862 g) and macerated in 1500 mL 99.99% pure methanol. The macerated material was then put into an amber bottle for 7 days, during which time it was continuously shaken and stirred to isolate the crude extract. The resulting extract was filtered using Whatman grade 1 filter paper. The filtered supernatant (1000 mL) was evaporated using rotary evaporator (RE200 Biby Sterling, UK) and yielded 12.92 g dry crude extract, which was then preserved at 4 °C.
2.4. Extract preparation through solvent-solvent partitioning
The crude methanol extract was further fractioned using Kupchan and Tsou's method [32], following the modifications made by Wagenen et al. [33]. 5 g crude extract was used to prepare a mother solution and was dissolved and triturated in 10% methanol (aqueous). The ratio of methanol to water was 9:1 v/v. The mother solution was then partitioned by four different solvents – n-hexane, carbon tetrachloride (CCl4), chloroform, and aqueous fraction – in order of increasing polarity using a separation funnel (Mittal Overseas, Ambala-133,001, Haryana, India). Physical appearances of the fractions and their quantities after partitioning are shown in Fig. 1. Each fraction was dried, producing an n-hexane fraction (LSNHF), a CCl4 fraction (LSCTF), a chloroform fraction (LSCHF), and an aqueous fraction (LSAF). The obtained fraction extracts were preserved in a refrigerator at 4 °C.
Fig. 1.
Schematic representation of the modified Kupchan partitioning of methanol crude extract Lasia spinosa. The crude extract (5 g) was triturated and dissolved in 10% aqueous methanol (methanol:water; 9:1 v/v) to make the mother solution, which was then partitioned off successively by four solvents – n-hexane (LSNHF: 2.23 g), carbon tetra chloride (LSCTF: 1.80 g), chloroform (LSCHF: 120 mg)m and aqueous (LSAF: 223 mg) – in order of increasing polarity using a separating funnel.
2.5. Qualitative phytochemical screening
The qualitative phytochemical screening of L. spinosa extracts was performed according to the previously mentioned method [34] to determine the secondary metabolites, specifically alkaloids, glycosides, tannins, phenols, flavonoids, terpenoids, steroids, and quinones.
2.6. Experimental animals
Swiss albino mice aged 6–7 weeks were purchased from the animal research division of International Center for Diarrheal Disease and Research, Bangladesh (ICDDRB). Each mouse weighed 32–36 g, and the sex ratio within the sample was 50:50. Mice were kept in poly-carbonated cages at standard laboratory condition. A temperature of 25 °C and a humidity level of 55–56% were maintained in a 12-h/day light cycle. The mice had access to a pellet diet, libitum, and tap water.
2.7. Anti-inflammatory effect on human red blood cell (HRBC) membrane stabilization
In vitro anti-inflammatory assays examining membrane stabilization were performed following the procedure described earlier [9]. 5 mL blood was taken from healthy volunteers with prior consent and mixed with an equal volume of sterilized Alsever's solution (dextrose 2%, sodium citrate 0.8%, citric acid 0.5%, and NaCl 0.42%). This solution was then centrifuged at 3000 g. To prepare a 10% v/v suspension of red blood cells, the packed cells were washed with isosaline. Test solutions contained 1 mL 0.15 M phosphate buffer (pH 7), 2 mL hypotonic saline solution, and 0.5 mL relevant fraction of L. spinosa and reference drug diclofenac sodium at different concentration levels (62.5, 125, 250 and 500 μg/mL), and 0.5 mL 10% HRBC. The control solution contained 1 mL phosphate buffer, 2 mL distilled water, and 0.5 mL 10% HRBC in isotonic saline solution. Once prepared, the solutions were incubated for 30 min at 37 °C and then centrifuged at 3000 g. Discharge and hemoglobin content from the centrifuged supernatant was evaluated at 560 nm using a spectrophotometer. The percentage membrane stabilization projected through the inhibition of hemolysis was measured using the following equation: % inhibition of hemolysis = [(Ac–As)/Ac] × 100, where.
Ac = optical density of hypotonic-buffered saline solution alone and.
As = optical density of test sample in hypotonic solution.
2.8. Study design
Experimental animals were categorized into six groups (control, standard, and test groups). Each group consisted of six mice. The treatment groups were administered both LSNHF and LSCTF at the doses of 200 and 400 (mg/kg b. w, p. o.), whereas the control group received 10 mL/kg b. w. 1% Tween 80 in water, p. o. Diazepam (1 mg/kg, b. w, i. p) was used as the standard drug for the elevated plus maze tests, hole board tests, and thiopental sodium-induced sleeping time tests, and tail suspension tests. The standard drugs were given 15 min prior to the tests, and LSNHF200, LSNHF400, LSCTF200, LSCTF400, and 1% Tween 80 were administered 30 min prior.
2.9. Anxiolytic activity
2.9.1. Elevated plus maze test (EPM)
The elevated plus maze apparatus was made of two open arms (35 cm × 5 cm) and two closed arms (30 cm × 5 cm × 15 cm) that join together in a common central ground (5 cm × 5 cm). The entire apparatus is elevated about 25 cm above the floor. The walls of the closed arms were wood, and the floors were painted black. Mice (35–40 g) were rested for 10 days before the experiment in the apparatus, and the researcher handled the mice on alternate days to reduce their tension. At the time of the experiment, each mouse was positioned individually in the center of the maze facing one of the enclosed arms 30 min after being administered the control and treatment solutions or 15 min after receiving the reference drug diazepam. The mice were then filmed for 6 min using a video camera; the first min was set aside for behavioral adjustment, and in the following 5 min, time spent in the open arm and the total numbers of entries into the open arm were recorded. Throughout the experimental process, a pleasant and congenial atmosphere was maintained to ensure consistent results [35]. Experimental animals of all groups (control, standard, and test groups) were dosed with their respective solutions as described in the study design.
2.9.2. Hole board test (HBT)
The hole board tests (HBT) were performed almost identically to the methodology mentioned earlier, but with minor modification [1,36]. In our experiment, the hole board was 20 cm × 40 cm and contained 16 evenly spaced holes with a diameter of 3 cm. The board was 1.8 cm thick and suspended 15 cm above the base. Experimental mice were divided into different groups; mice in the control and treatment groups were treated 30 min prior to the experiment, and mice in the standard groups were treated 15 min prior. Each mouse was placed individually in the center of the board, and its exploratory activities were recorded with a video camera for 6 min, of which 5 min were assessed for data collection. To avoiding disturbing the mice, the surrounding area was kept silent. A head dip was noted only when both eyes went down into a hole, and time spent looking through the hole was also recorded. Experimental animals in all groups (control, standard, and test groups) were dosed with their respective solutions as described in the study design.
2.10. Antidepressant activity
2.10.1. Tail suspension test (TST)
Tail suspension tests were carried out following the described method with minor modification [37,38]. Mice were transferred to the laboratory from their habitat colony in their own cages and rested for 1–2 h after transfer to adapt to the laboratory environment. The test compartment was an open cylinder made of transparent glass. The compartment was 10 cm (diameter) x 25 cm (height) and was kept at a temperature of 25 ± 1 °C. Throughout the experiment, each mouse was isolated both visually and auditorily from the others. The behavior of the mouse was recorded for a total of 6 min, of which the final 5 min were assessed for immobility. Mice were considered immobile when passively motionless, except for those motions required to hold its head above the water. The test was executed in a calm environment and low light condition. Experimental animals in all groups (control, standard, and test groups) were dosed with their respective solutions as described in the study design.
2.11. Sedative activity
2.11.1. Thiopental sodium-induced sleeping time test
The thiopental sodium-induced sleeping time test was performed according to the method described [39,40]. Thiopental sodium (40 mg/kg b. w, i. p.) was injected into individual mice 30 min after administering the treatment drug for induced sleeping. Time from the thiopental sodium injection to loss of righting reflex and duration of sleep were both recorded through careful observation. Percentage of effect for each treatment drug was calculated using the following equation: Effect (%) = (Average duration of loss of righting reflex in the test group/Average duration of loss of righting reflex in the control) × 100.
2.12. GS-MS analysis of LSNHF
The bioactive chemicals in LSNHF were evaluated using GC-MS according to the method described previously [10,41].
2.13. In silico molecular docking
2.13.1. Protein preparation
The 3D structure of cyclooxygenase-1 (PDB: 2OYE) [42], cyclooxygenase-2 (PDB: 1CX2) [43], potassium channel (PDB: 4UUJ) [44], serotonin transporter (PDB: 5I6X) [45] were downloaded in PDB format form Protein data Bank [46]. The protein structures were prepared in accordance with the approach outlined by Uddin et al. previously in their study [19].
2.13.2. Ligand preparation
The structures of the identified compounds from LSNHF and the standard drugs were downloaded from PubChem online databases. Using the LigPrep wizard of Schrödinger-maestro (v11.1), these identified compounds were converted into minimized 3D structure. Using Epik, we were able to establish a possible ionization state at the desired pH of 7.0 ± 2.0 to accurately count tautomers and determine the protonation state in biological status. Up to 32 stereoisomers were preserved per ligand. The forcefield were set to OPLS3 [47].
2.13.3. Receptor grid generation
For the prepared proteins, receptor grids were made so that different ligand would bind in the predicted active site during docking [5,48]. In Glide, grids were made with the van der Waals scaling factor set to 1.00 and the charge cutoff set to 0.25, and the OPLS3 force field was used. For docking experiments, the bounding box was set to 14 Å × 14 Å × 14 Å.
2.13.4. Ligand docking
After completing the prerequisite stages, the SP glide of Schrödinger-maestro (v11.1) was used to run the docking simulation. For ligand atoms, the van der Waals scaling factor and partial charge cutoff were set to 0.80 and 0.15 respectively.
2.14. Pharmacokinetic parameters determination
The SwissADME (http://www.swissadme.ch/) was used to evaluate at the pharmacokinetic properties of the isolated compound. In this study, an orally active drug should meet the drug-likeness parameters [49] to show their pharmaceutical fidelity. These include the molecular weight of the compounds, their lipophilicity (LogP), the number of hydrogen-bond acceptors, the number of hydrogen-bond donors, their topological polar surface area (TPSA), and the number of rotatable bond (nRB) based on Lipinski's rule and Veber's rule.
2.15. Toxicity prediction by AdmetSAR
Toxicological characteristics of the identified compounds were assessed utilizing the AdmetSAR online tool (http://lmmd.ecust.edu.cn/admetsar1/predict/), since toxicity is a major issue during the development of novel pharmaceuticals.
2.16. Prediction of activity spectra for substances (PASS)
The identified compounds from LSNHF fraction were tested for evaluation the anti-inflammatory, anxiolytic, and antidepressant activities by using PASS online (http://www.pharmaexpert.ru/passonline/).
2.17. Statistical analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's test to estimate significant differences between the tests and control groups for this experiment using GraphPad Prism Data Editor for Windows (Version 6.01, GraphPad Software Inc., San Diego, CA, USA). Values are represented as Mean ± SEM. P values < 0.05, 0.01, and 0.001 were considered statistically significant.
3. Results
3.1. Qualitative phytochemical assay
Phytochemical assay of the different fractions revealed the presence of secondary bioactive isolates, namely alkaloids, carbohydrates, proteins, phenols, and flavonoids (Table 1).
Table 1.
Phytochemical screening of LSNHF, LSCHF, and LSCTF fractions of Lasia spinosa.
| Name of the test | Procedure name | Observation |
||
|---|---|---|---|---|
| LSNHF | LSCHF | LSCTF | ||
| Alkaloids | Wagner's test | +++ | ++ | +++ |
| Carbohydrates (Monosaccharide) | Molisch's test | + | + | ++ |
| Carbohydrates (Polysaccharide) | Iodine test | _ | _ | _ |
| Protein | Xanthoproteic test | + | + | ++ |
| Flavonoids | Alkaline Reagent test | + | + | + |
| Phenols | FeCL3 test | ++ | ++ | ++ |
| Saponins | Froth test | _ | _ | _ |
Where.
Bioavailability Key.
(−) = not present; (+) = present in low concentration; (++) = present in moderately high concentration; (+++) = present in very high concentration.
3.2. Anti-inflammatory effect on human red blood cell (HRBC) membrane stabilization
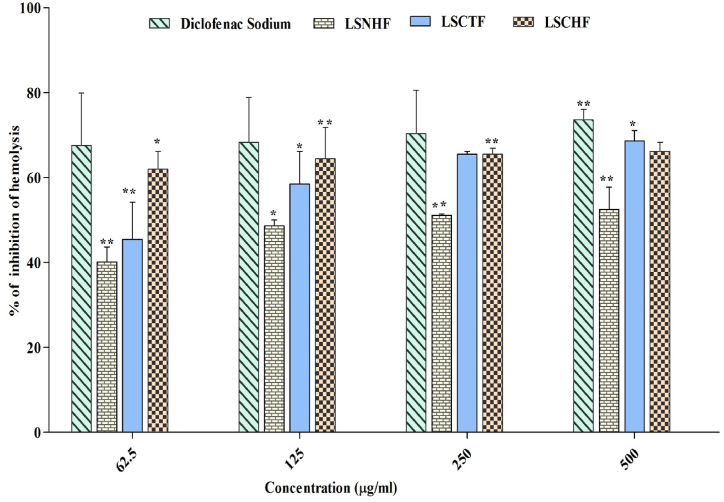
The results of the membrane stabilization test with LSCTF, LSCHF, and LSNHF are shown in Fig. 2. Different fractions revealed concentration-dependent increases in the percentage of protection for all concentrations (62.5–500 μg/mL), but all effects were less than that of the reference drug diclofenac sodium. The outcome percentage associated with LSCTF was 68.662 ± 2.46% (p*< 0.05) at the highest concentration (500 μg/mL), but the same concentration of LSCHF showed about 66.19 ± 2.11% membrane stabilization (p**< 0.01), and LSNHF only stabilized 52.46 ± 5.28% at 500 μg/mL (p**< 0.01). By comparison, the standard drug diclofenac sodium showed maximum stabilization of 73.60 ± 2.46% at 500 μg/mL.
Fig. 2.
Effect of L.spinosa on inhibition of hemolysis. All values are expressed as mean ± SEM (n = 3). Data were analyzed with the software statistical package for social science (SPSS), Version 16.0. Chicago, SPSS Inc. Using two-way analysis of variance (ANOVA) followed by the Bonferroni posthoc test. P**< 0.001 vs diclofenac sodium.
3.3. In vivo anxiolytic activity
3.3.1. Elevated plus-maze test (EPM)
As depicted in Fig. 3, all doses of LSNHF and LSCTF increased both the time spent in open arm and percent of entries into the open arms. In particular, LSNHF (p***< 0.001) increased the time spent in the open arms 36.66 ± 0.82 s and 59.88 ± 0.65 s at the dosages of 200 and 400 mg/kg, p. o., respectively, whereas the same doses of LSCTF resulted in times of 43.83 ± 0.72 s and 50.77 ± 0.67 s. The LSNHF significantly (p***< 0.001) increased the percent of entries (61.85 ± 1.36) into the open arms at 400 mg/kg, and the LSCTF corresponded to 68.76 ± 0.89 entries at the same dose. In contrast, mice treated with diazepam (1 mg/kg, i. p.) spent a significantly (p*** < 0.001) longer period in the open arms (42.33 ± 0.87 s) and higher number of entries (79.8 ± 1.05).
Fig. 3.
Effect of different treatments on time spent in open arm in elevated plus maze test (a). Effect of different treatment on % of time entry into the open arms in elevated plus maze test (b). Values are expressed as mean ± SEM. Values are considered statistically significant at × p < 0.05, **p < 0.01, and ***p < 0.001. Values were analyzed by the software statistical package for social sciences (SPSS), Version 16.0. Chicago, SPSS Inc., using one way analysis of variance (ANOVA) followed Dunnett's test (n = 6, per group). Control: 1% Tween 80 in water (10 mL/kg p. o.) diazepam (1 mg/kg, i. p.): reference standard drug; LSNHF200: Lasia spinosa n-hexane fraction (200 mg/kg, p. o.); LSNHF400: Lasia spinosa n-hexane fraction (400 mg/kg, p. o.); LSCTF200: Lasia spinosa CCl4 fraction (200 mg/kg, p. o.) and LSCTF400: Lasia spinosa CCl4 fraction (400 mg/kg, p. o.).
3.3.2. Hole board test (HBT)
LSNHF and LSCTF demonstrated significant dose-dependent anxiolytic effects in this test, where a greater number of hole pokes indicates reduced anxiety (results displayed in Fig. 4). Mice treated with 400 mg/kg, p. o. LSNHF performed significantly more hole pokes (78.66 ± 1.05, p***< 0.001), and the LSCTF-treated mice showed increased head dips (39.50 ± 1.33 and 65.17 ± 0.96 at the dosages of 200 and 400 mg/kg, p. o. respectively). Mice in the standard group, treated with diazepam 1 mg/kg, i. p., also demonstrated significantly more (p***< 0.001) head dips (64.33 ± 2.32) than the mice in the control group (26.33 ± 1.44).
Fig. 4.
Effect of different treatments on number of head dips in hole board test. Values are expressed as mean ± SEM. Values are considered statistically significant at × p < 0.05, **p < 0.01, and ***p < 0.001. Values were analyzed by the software statistical package for social sciences (SPSS), Version 16.0. Chicago, SPSS Inc., using one way analysis of variance (ANOVA) followed by Dunnett's test (n = 6, per group). Control: 1% Tween 80 in water (10 mL/kg p. o.), diazepam (1 mg/kg, i. p.): reference standard drug; LSNHF200: Lasia spinosa n-hexane fraction (200 mg/kg, p. o.); LSNHF400: Lasia spinosa n-hexane fraction (400 mg/kg, p. o.); LSCTF200: Lasia spinosa CCl4 fraction (200 mg/kg, p. o.) and LSCTF400: Lasia spinosa CCl4 fraction (400 mg/kg, p. o.).
3.4. Anti-depressant activity
3.4.1. Tail suspension test (TST)
The anti-depressant activity of LSNHF and LSCTF as demonstrated by the immobility time during TST is displayed in Fig. 5. During the observational period, depressive behavior (measured by time spent immobile) decreased significantly in a dose-dependent manner. Mice treated with 400 mg/kg, p. o. Doses of LSNHF and LSCTF showed significant (p***< 0.001) decreases in immobility time (81.33 ± 1.67 s and 83.50 ± 1.90 s, respectively), and mice treated with the reference drug imipramine hydrochloride significantly showed similar immobility times (88.3 ± 2.07 s, p***< 0.001). In contrast, mice in the control group demonstrated longer immobility time (205 ± 1.06 s).
Fig. 5.
Effect of different treatments on behavior during tail suspension test. Values are expressed as mean ± SEM. Values are considered statistically significant at × p < 0.05, **p < 0.01, and ***p < 0.001. Values were analyzed by the software statistical package for social sciences (SPSS), Version 16.0. Chicago, SPSS Inc., using one way analysis of variance (ANOVA) followed by Dunnett's test (n = 6, per group). Control: 1% Tween 80 in water (10 mL/kg p. o.) diazepam (1 mg/kg, i. p.): reference standard drug; LSNHF200: Lasia spinosa n-hexane fraction (200 mg/kg, p. o.); LSNHF400: Lasia spinosa n-hexane fraction (400 mg/kg, p. o.); LSCTF200: Lasia spinosa CCl4 fraction (200 mg/kg, p. o.) and LSCTF400: Lasia spinosa CCl4 fraction (400 mg/kg, p. o.).
3.5. Sedative activity
3.5.1. Thiopental sodium induced sleeping time test
It was found that both LSNHF and LSCTF significantly (**p < 0.001) enhanced the onset of sleep and dose-dependently increased the duration of sleep in thiopental-induced sleeping time tests. The duration of sleeping time associated with LSNHF at the doses of 200 and 400 mg/kg, p. o. Was 73 ± 1.71 and 119.83 ± 0.85, respectively, while LSCTF corresponded to sleeping times of 84.67 ± 1.58 and 124.83 ± 1.32 at the same respective doses. Mice treated with diazepam (1 mg/kg, i. p.) showed a sleeping time of 109.83 ± 2.34, and mice in the control group slept for 42.50 ± 1.17. These results are displayed in Table 2.
Table 2.
Effect of different treatments on onset and duration of sleep in thiopental sodium-induced sleeping time test.
| Treatment | Onset of Sleep (Min) (mean ± SEM) | Duration of Sleep (Min) (mean ± SEM) |
|---|---|---|
| Control | 48.16 ± 0.79 | 42.50 ± 1.17 |
| RSD | 12.66 ± 0.71*** | 109.83 ± 2.34*** |
| LSNHF200 | 1.83 ± 0.33** | 73 ± 1.71** |
| LSNHF400 | 2.08 ± 0.49** | 119.83 ± 0.85** |
| LSCTF200 | 1.83 ± 0.27*** | 84.67 ± 1.58*** |
| LSCTF400 | 1.91 ± 0.30** | 124.83 ± 1.32** |
Values are expressed as mean ± SEM. *p < 0.05, **p < 0.01 and ***p < 0.001, significantly different from control; one way ANOVA followed Dunnett's test (n = 6, per group). Control: 1% tween 80 in water (10 mL/kg p.o.) RSD: reference standard drug (diazepam 1 mg/kg, i.p.); LSNHF200: Lasia spinosa n-hexane fraction (200 mg/kg, p.o.); LSNHF400: Lasia spinosa n-hexane fraction (400 mg/kg, p.o.); LSCTF200: Lasia spinosa CCl4 fraction (200 mg/kg, p.o.) and LSCTF400: Lasia spinosa CCl4 fraction (400 mg/kg, p.o.).
3.6. GC-MS analysis
The analysis of phytochemical substances in LSNHF found a number of medicinally active chemicals, as reported in Table 3. The total ionic chromatogram (TIC) is displayed in Fig. 6.
Table 3.
Compounds identified from LSNHF fraction by GC-MS.
| Name of the compounds | R. Time | m/z | Area | Conc. (%) |
|---|---|---|---|---|
| Butanoic acid, 2-methyl- | 5.411 | 74.00 | 581,879 | 38.364 |
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | 13.580 | 164.00 | 60,890 | 4.015 |
| 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | 15.654 | 57.00 | 5688 | 0.357 |
| Undec-10-ynoic acid, tetradecyl ester | 16.175 | 55.00 | 1304 | 0.086 |
| 7-Hexadecenal, (Z)- | 17.900 | 57.00 | 3678 | 0.242 |
| 5-Caranol, trans,trans-(+)- | 19.127 | 57.00 | 28,952 | 1.909 |
| 7-Hexadecenal, (Z)- | 20.471 | 69.00 | 27,797 | 1.833 |
| 2-Tridecenoic acid, (E)- | 22.087 | 55.00 | 195,830 | 12.911 |
| Oleic Acid | 23.783 | 55.00 | 142,209 | 9.376 |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | 24.011 | 55.00 | 112,831 | 7.439 |
| Eicosanoic acid | 24.219 | 55.00 | 112,614 | 7.425 |
| Undec-10-ynoic acid, tetradecyl ester | 25.787 | 55.00 | 48,793 | 3.217 |
| Docosane, 1,22-dibromo- | 26.752 | 57.00 | 69,125 | 4.557 |
| Cholesterol margarate | 29.903 | 57.00 | 113,164 | 7.461 |
Fig. 6.
Total ionic chromatogram (TIC) of LSNHF using GC-MS.
3.7. Molecular docking study
3.7.1. Molecular docking study for anti-inflammatory activity
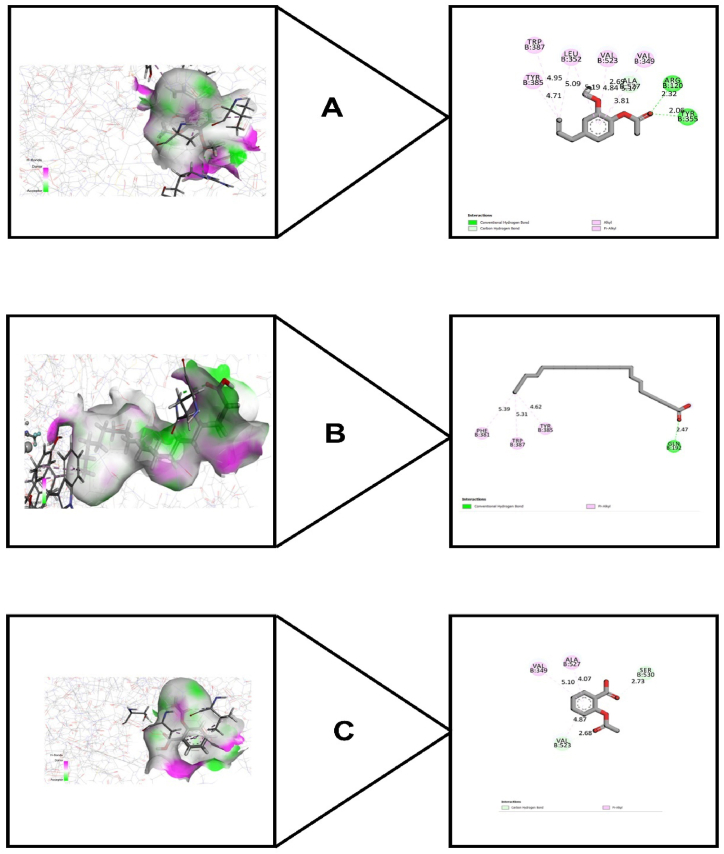
To find out the anti-inflammatory activity of the identified chemical compounds, Cyclooxygenase-1 (PDB: 2OYE) and Cyclooxygenase-2 (PDB: 1CX2) were utilized. The results are summarized in Table 4. For cyclooxygenase 1, phenol, 2-methoxy-4-(2-propenyl)-, acetate possessed best docking score (−7.072) where aspirin showed (−6.057 kcal/mol). Docosane, 1,22-dibromo- also showed a strong binding affinity having a docking score (−6.553 kcal/mol). For cyclooxygenase 2, phenol, 2-methoxy-4-(2-propenyl)-, acetate also showed the strongest binding affinity (−6.907 kcal/mol), while aspirin showed (−5.631 kcal/mol). Eicosanoic acid showed a docking score of (−5.014 kcal/mol). The structures of interaction of best docking scores along with aspirin are shown in Fig. 7 (A-C) and Fig. 8 (A-C) for cyclooxygenase-1 and cyclooxygenase-2 respectively. In addition to that, the hydrogen bond and hydrophobic interaction for cyclooxygenase-1 and cyclooxygenase-2 are depicted in Table 5 and Table 6 respectively.
Table 4.
Molecular-docking scores for the identified compounds from LSNHF.
| Compounds | Docking score (kcal/mol) |
|||
|---|---|---|---|---|
| PDB:2OYE | PDB:1CX2 | PDB: 4UUJ | PDB: 5I6X | |
| Butanoic acid, 2-methyl- | −4.314 | −4.413 | −2.847 | −3.478 |
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | −7.072 | −6.907 | −2.999 | −5.541 |
| 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | – | – | – | – |
| Undec-10-ynoic acid, tetradecyl ester | – | – | −2.205 | −4.405 |
| 7-Hexadecenal, (Z)- | −1.498 | −1.677 | +1.271 | −1.341 |
| 2-Tridecenoic acid, (E)- | −2.003 | −1.746 | +0.966 | −0.136 |
| Oleic Acid | −2.189 | −2.241 | +0.522 | −0.945 |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | – | – | +0.163 | – |
| Eicosanoic acid | −5.127 | −5.014 | −3.398 | −4.629 |
| Docosane, 1,22-dibromo- | −6.553 | – | −2.577 | −5.318 |
| Cholesterol margarate | – | – | −2.827 | – |
| Standard drug | Aspirin (−6.057) | Aspirin (−5.631) | Diazepam (−3.036) | Fluoxetine hydrochloride (−9.556) |
Fig. 7.
3D and 2D images of best docking scores between (A) Cyclooxygenase-1 (PDB: 2OYE) and Phenol, 2-methoxy-4-(2-propenyl)-, acetate; (B) Cyclooxygenase-1 (PDB 2OYE) and Docosane, 1,22-dibromo-; (C) Cyclooxygenase-1 (PDB: 2OYE) and Aspirin.
Fig. 8.
3D and 2D images of best docking scores between (A) Cyclooxygenase-2 (PDB: 1CX2) and Phenol, 2-methoxy-4-(2-propenyl)-, acetate; (B) Cyclooxygenase-2 (PDB 1CX2) and Eicosanoic acid; (C) Cyclooxygenase-2 (PDB: 1CX2) and Aspirin.
Table 5.
Interaction and bond distances of identified compounds from LSNHF with cyclooxygenase-1 (PDB: 2OYE)-binding sites for anti-inflammatory activity.
| Protein | Ligand | Hydrogen-bond interactions |
Hydrophobic interactions |
||
|---|---|---|---|---|---|
| Amino acid residue | Distance (Å) | Amino acid residue | Distance (Å) | ||
| 2OYE | Butanoic acid, 2-methyl- | MET522 | 2.035 | LEU352 | 5.064 |
| LEU384 | 4.542 | ||||
| PHE381 | 5.096 | ||||
| TYR385 | 3.634 | ||||
| TRP387 | 5.300 | ||||
| TRP387 | 4.915 | ||||
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | LEU352 | 4.591 | |||
| ILE523 | 4.308 | ||||
| PHE518 | 5.009 | ||||
| LEU352 | 5.193 | ||||
| ALA527 | 4.518 | ||||
| 7-Hexadecenal, (Z)- | BOG751 | 2.564 | LEU384 | 4.154 | |
| TYR385 | 3.423 | ||||
| TRP387 | 4.203 | ||||
| TRP387 | 4.256 | ||||
| 2-Tridecenoic acid, (E)- | ILE517 | 3.032 | LEU384 | 4.169 | |
| PHE518 | 2.408 | PHE381 | 5.492 | ||
| HIS90 | 2.438 | TYR385 | 3.222 | ||
| SER516 | 2.380 | TRP387 | 4.320 | ||
| TRP387 | 4.367 | ||||
| Oleic Acid | MET522 | 1.846 | PRO86 | 5.339 | |
| ILE523 | 4.401 | ||||
| HIS90 | 5.143 | ||||
| Eicosanoic acid | LEU384 | 2.0662 | ILE89 | 4.615 | |
| GLY526 | 2.713 | ||||
| Docosane, 1,22-dibromo- | LEU99 | 4.608 | |||
| LEU384 | 3.884 | ||||
| MET522 | 4.390 | ||||
| TRP100 | 4.406 | ||||
| TRP387 | 5.101 | ||||
| TRP387 | 5.093 | ||||
Table 6.
Interaction and bond distances of identified compounds from LSNHF with cyclooxygenase-2 (PDB: 1CX2)-binding sites for anti-inflammatory activity.
| Protein | Ligand | Hydrogen-bond interactions |
Hydrophobic interactions |
||
|---|---|---|---|---|---|
| Amino acid residue | Distance (Å) | Amino acid residue | Distance (Å) | ||
| 1CX2 | Butanoic acid, 2-methyl- | SER530 | 2.0574 | ALA527 | 4.140 |
| ALA527 | 2.607 | LEU352 | 5.174 | ||
| SER530 | 2.713 | VAL523 | 3.948 | ||
| TYR385 | 4.383 | ||||
| TRP387 | 7.909 | ||||
| PHE518 | 5.458 | ||||
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | ARG120 | 2.317 | LEU352 | 5.092 | |
| TYR355 | 2.057 | TYR385 | 4.708 | ||
| ALA527 | 2.687 | TRP387 | 4.946 | ||
| VAL349 | 5.169 | ||||
| LEU352 | 5.190 | ||||
| VAL523 | 4.842 | ||||
| ALA527 | 3.808 | ||||
| 7-Hexadecenal, (Z)- | ARG120 | 2.470 | LEU352 | 5.215 | |
| MET522 | 4.835 | ||||
| VAL523 | 4.703 | ||||
| TRP387 | 5.205 | ||||
| PHE518 | 3.928 | ||||
| 2-Tridecenoic acid, (E)- | ARG513 | 2.794 | LEU384 | 5.034 | |
| LEU352 | 2.091 | MET522 | 4.473 | ||
| ARG513 | 3.084 | TRO387 | 4.827 | ||
| ARG513 | 2.914 | PHE518 | 4.883 | ||
| Oleic Acid | PHE518 | 2.131 | LEU352 | 5.039 | |
| MET522 | 4.941 | ||||
| TRP387 | 5.036 | ||||
| PHE518 | 3.922 | ||||
| Eicosanoic acid | GLN192 | 2.469 | PHE381 | 5.392 | |
| TYR385 | 4.620 | ||||
| TRP387 | 5.311 | ||||
3.7.2. Molecular docking study for anxiolytic activity
The molecular docking results related to anxiolytic activity were summarized in Table 4. For anxiolytic activity, potassium channel (PDB: 4UUJ) were used and eicosanoic acid showed the strongest binding affinity (−3.398 kcal/mol), while Diazepam showed a docking score of (−3.036 kcal/mol). Phenol, 2-methoxy-4-(2-propenyl)-, acetate also showed a binding affinity having docking score (−2.999 kcal/mol). The structures of interaction of best docking scores are depicted in Fig. 9 (A-C). In addition, the hydrogen bond and hydrophobic interaction for potassium channel are depicted in Table 7.
Fig. 9.
3D and 2D images of best docking scores between (A) Potassium channel (PDB: 4UUJ) and Eicosanoic acid; (B) potassium channel (PDB 4UUJ) and Phenol, 2-methoxy-4-(2-propenyl)-, acetate; (C) Potassium channel (PDB: 4UUJ) and Diazepam.
Table 7.
Interaction and bond distances of identified compounds from LSNHF with potassium channel (PDB: 4UUJ)-binding sites for anxiolytic activity.
| Protein | Ligand | Hydrogen-bond interactions |
Hydrophobic interactions |
||
|---|---|---|---|---|---|
| Amino acid residue | Distance (Å) | Amino acid residue | Distance (Å) | ||
| 4UUJ | Butanoic acid, 2-methyl- | ARG89 | 2.505 | LEU86 | 5.238 |
| PO411333 | 1.587 | LEU86 | 3.912 | ||
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | TRP87 | 2.192 | LEU86 | 4.467 | |
| THR85 | 2.302 | LEU86 | 5.352 | ||
| PO411333 | 2.344 | ||||
| PO411333 | 2.601 | ||||
| Undec-10-ynoic acid, tetradecyl ester | CYS90 | 2.819 | MET96 | 4.249 | |
| CYS90 | 3.934 | ||||
| VAL93 | 3.878 | ||||
| 7-Hexadecenal, (Z)- | ARG89 | 2.146 | |||
| LEU86 | 2.986 | ||||
| ARG89 | 2.847 | ||||
| 2-Tridecenoic acid, (E)- | PO411333 | 1.533 | ARG89 | 4.483 | |
| CYS90 | 4.702 | ||||
| VAL93 | 4.417 | ||||
| Oleic acid | ARG89 | 2.278 | TRP68 | 5.100 | |
| PO41133 | 1.843 | ||||
| PO41133 | 1.711 | ||||
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | THR75 | 2.455 | |||
| Eicosanoic acid | ARG89 | 2.300 | TRP68 | 5.303 | |
| PO41133 | 1.442 | ||||
| Docosane, 1,22-dibromo- | THR75 | 2.775 | LEU86 | 4.949 | |
| ARG89 | 2.964 | ARG89 | 3.956 | ||
| Cholesterol margarate | ARG89 | 2.825 | ARG89 | 4.703 | |
| ARG89 | 4.877 | ||||
| VAL93 | 5.248 | ||||
| MET96 | 4.052 | ||||
3.7.3. Molecular docking study for antidepressant activity
To determine the antidepressant activity, serotonin transporter (PDB: 5I6X) were used and the results are represented in Table 4. Phenol, 2-methoxy-4-(2-propenyl)-, acetate showed the strongest binding affinity having a docking score (−5.541 kcal/mol) and fluoxetine hydrochloride showed (−9.556 kcal/mol) docking score. Docosane, 1,22-dibromo- also interacted and showed (−5.318 kcal/mol) docking score. The hydrogen bond and hydrophobic interaction for serotonin transporter are shown in Table 8 and the structures of compounds having best docking score are displayed in Fig. 10 (A-C).
Table 8.
Interaction and bond distances of identified compounds from LSNHF with serotonin transporter (PDB: 5I6X)-binding sites for antidepressant activity.
| Protein | Ligand | Hydrogen-bond interactions |
Hydrophobic interactions |
||
|---|---|---|---|---|---|
| Amino acid residue | Distance (Å) | Amino acid residue | Distance (Å) | ||
| 5I6X | Butanoic acid, 2-methyl- | ASP98 | 1.588 | TYR95 | 4.358 |
| SER336 | 2.746 | TYR95 | 5.235 | ||
| GLY338 | 3.047 | ||||
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | SER336 | 2.572 | TYR95 | 5.311 | |
| TYR95 | 2.590 | TYR176 | 5.444 | ||
| ALA172 | 4.469 | ||||
| ILE172 | 4.739 | ||||
| TRY176 | 4.891 | ||||
| ILE172 | 5.227 | ||||
| Undec-10-ynoic acid, tetradecyl ester | ALA173 | 4.039 | |||
| ILE172 | 4.252 | ||||
| VAL501 | 4.181 | ||||
| PHE341 | 4.365 | ||||
| 7-Hexadecenal, (Z)- | ASN177 | 2.076 | PHE341 | 2.879 | |
| ALA173 | 2.964 | ILE172 | 3.835 | ||
| SER439 | 2.872 | ||||
| 2-Tridecenoic acid, (E)- | TYR95 | 1.855 | PHE335 | 4.186 | |
| PHE556 | 4.970 | ||||
| Oleic acid | ASP98 | 1.574 | PHE335 | 4.327 | |
| Eicosanoic acid | TYR95 | 1.575 | LEU99 | 4.359 | |
| ALA96 | 2.589 | ILE179 | 5.177 | ||
| LEU406 | 4.430 | ||||
| LEU431 | 4.109 | ||||
| PHE407 | 3.970 | ||||
| Docosane, 1,22-dibromo- | ALA173 | 3.541 | |||
| LEU443 | 4.273 | ||||
| LEU99 | 3.915 | ||||
| ILE179 | 5.039 | ||||
| LEU406 | 4.836 | ||||
| LEU431 | 3.847 | ||||
| PHE407 | 4.264 | ||||
Fig. 10.
3D and 2D images of best docking scores between (A) serotonin transporter (PDB: 5I6X) and Phenol, 2-methoxy-4-(2-propenyl)-, acetate; (B) serotonin transporter (PDB: 5I6X) and Docosane, 1,22-dibromo-; (C) serotonin transporter (PDB: 5I6X) and Fluoxetine hydrochloride.
3.7.4. Pharmacokinetic parameters determination and toxicity prediction analysis
The ADME properties of the identified compounds were analyzed using Lipinski's rule and Veber's rule. Table 9 displays the data for each parameter has been taken from the SwissADME web server. Butanoic acid, 2-methyl-; Phenol, 2-methoxy-4-(2-propenyl)-, acetate; 9,9-Dimethoxybicyclo [3.3.1] nona-2,4-dione; Undec-10-ynoic acid, tetradecyl ester; 7-Hexadecenal, (Z)- and 2-Tridecenoic acid, (E)- did not violate the Lipinski's rule of five. Oleic Acid; Eicosanoic acid and Docosane, 1,22-dibromo- violated single parameter of Lipinski's rule of five. l-(+)-Ascorbic acid 2,6-dihexadecanoate and Cholesterol margarate violated two parameters of Lipinski's rule of five. Through the online AdmetSAR web server, each identified compound's toxicity profile was assessed and is shown in Table 10.
Table 9.
Physicochemical properties of identified compounds from LSNHF for good oral bioavailability.
| Compounds | Lipinski's rule |
Lipinski's violation (≤1) | Veber rules |
||||
|---|---|---|---|---|---|---|---|
| MW (<500 g/mol) | HBA (<10) | HBD (<5) | Log P (≤5) | nRB (≤10) | TPSA (≤140 Å2) | ||
| Butanoic acid, 2-methyl- | 102.13 | 2 | 1 | 0.97 | 0 | 2 | 37.30 |
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | 206.24 | 3 | 0 | 2.55 | 0 | 5 | 35.53 |
| 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | 212.24 | 4 | 0 | 1.04 | 0 | 2 | 52.60 |
| Undec-10-ynoic acid, tetradecyl ester | 378.63 | 2 | 0 | 7.97 | 0 | 22 | 26.30 |
| 7-Hexadecenal, (Z)- | 238.41 | 1 | 0 | 5.11 | 0 | 13 | 17.07 |
| 2-Tridecenoic acid, (E)- | 212.33 | 2 | 1 | 3.93 | 0 | 10 | 37.3 |
| Oleic Acid | 282.46 | 2 | 1 | 5.71 | 1 | 15 | 37.30 |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | 652.94 | 8 | 2 | 9.57 | 2 | 34 | 119.36 |
| Eicosanoic acid | 312.53 | 2 | 1 | 6.62 | 1 | 18 | 37.30 |
| Docosane, 1,22-dibromo- | 468.39 | 0 | 0 | 9.10 | 1 | 21 | 0.00 |
| Cholesterol margarate | 639.09 | 2 | 1 | 12.44 | 2 | 22 | 26.30 |
Abbreviation: MW: Molecular weight; HBA:hydrogen-bond acceptors; HBD: hydrogen-bond donors; Log P: lipophilicity; nRB: number of rotatable bonds; TPSA: topological polar surface area.
Table 10.
Toxicological properties of identified compounds from LSNHF.
| Compounds | Parameters |
|||
|---|---|---|---|---|
| AMES toxicity | Carcinogens | Acute oral toxicity | Rat acute toxicity | |
| Butanoic acid, 2-methyl- | Non AMES toxic | Carcinogens | III | 1.7348 |
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | Non AMES toxic | Non-carcinogens | III | 2.0606 |
| 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | Non AMES toxic | Non-carcinogens | III | 2.1281 |
| Undec-10-ynoic acid, tetradecyl ester | Non AMES toxic | Carcinogens | III | 2.0360 |
| 7-Hexadecenal, (Z)- | Non AMES toxic | Carcinogens | III | 1.6468 |
| 2-Tridecenoic acid, (E)- | Non AMES toxic | Non-carcinogens | III | 1.9685 |
| Oleic Acid | Non AMES toxic | Non-carcinogens | IV | 1.3991 |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | Non AMES toxic | Non-carcinogens | III | 2.2891 |
| Eicosanoic acid | Non AMES toxic | Non-carcinogens | IV | 1.3275 |
| Docosane, 1,22-dibromo- | AMES toxic | carcinogens | II | 3.2432 |
| Cholesterol margarate | Non AMES toxic | Non-carcinogens | III | 2.0248 |
3.7.5. PASS prediction by PASS online
Using the structure-based Pass Online biological activity prediction program, we examined the PASS of the identified compounds. The collected PASS prediction data from PASS online for the identified compounds has been shown in Table 11. A chemical compound is regarded as having pharmacological potential if the probable activity (Pa) value exceeds the probable inactivity (Pi) value.
Table 11.
Biological activities predicted for identified compounds from LSNHF by PASS online.
| Name | Characteristics | Biological properties | Pa | Pi |
|---|---|---|---|---|
| Butanoic acid, 2-methyl- | Anti-inflammatory | Anti-inflammatory | 0.374 | 0.109 |
| Non-steroidal anti-inflammatory agent | 0.290 | 0.046 | ||
| Anti-inflammatory, intestinal | 0.467 | 0.010 | ||
| Anti-inflammatory, ophthalmic | 0.429 | 0.005 | ||
| Anxiolytic | GABA receptor agonist | 0.159 | 0.199 | |
| Antidepressant | – | – | – | |
| Phenol, 2-methoxy-4-(2-propenyl)-, acetate | Anti-inflammatory | Anti-inflammatory | 0.621 | 0.027 |
| Non-steroidal anti-inflammatory agent | 0.502 | 0.012 | ||
| Anti-inflammatory, intestinal | 0.351 | 0.040 | ||
| Anti-inflammatory, ophthalmic | 0.324 | 0.049 | ||
| Cyclooxygenase 1 inhibitor | 0.246 | 0.021 | ||
| Cyclooxygenase 2 inhibitor | 0.155 | 0.026 | ||
| Anxiolytic | – | – | – | |
| Antidepressant | – | – | – | |
| 9,9-Dimethoxybicyclo [3.3.1]nona-2,4-dione | Anti-inflammatory | Anti-inflammatory, intestinal | 0.392 | 0.025 |
| Anti-inflammatory, ophthalmic | 0.356 | 0.025 | ||
| Anxiolytic | – | – | – | |
| Antidepressant | Antidepressant, Imipramin-like | 0.136 | 0.099 | |
| Undec-10-ynoic acid, tetradecyl ester | Anti-inflammatory | Anti-inflammatory | 0.612 | 0.029 |
| Anti-inflammatory, intestinal | 0.480 | 0.009 | ||
| Non-steroidal anti-inflammatory agent | 0.475 | 0.014 | ||
| Anti-inflammatory, ophthalmic | 0.274 | 0.123 | ||
| Anxiolytic | GABA receptor agonist | 0.214 | 0.062 | |
| Antidepressant | Antidepressant, Imipramin-like | 0.239 | 0.041 | |
| 7-Hexadecenal, (Z)- | Anti-inflammatory | Anti-inflammatory | 0.250 | 0.212 |
| Non-steroidal anti-inflammatory agent | 0.235 | 0.074 | ||
| Anti-inflammatory, intestinal | 0.427 | 0.017 | ||
| Anti-inflammatory, ophthalmic | 0.283 | 0.107 | ||
| Anxiolytic | – | – | – | |
| Antidepressant | – | – | – | |
| 2-Tridecenoic acid, (E)- | Anti-inflammatory | Anti-inflammatory | 0.627 | 0.025 |
| Anti-inflammatory, intestinal | 0.569 | 0.004 | ||
| Anti-inflammatory, ophthalmic | 0.370 | 0.018 | ||
| Non-steroidal anti-inflammatory agent | 0.272 | 0.053 | ||
| Anxiolytic | GABA receptor agonist | 0.223 | 0.057 | |
| Antidepressant | – | – | – | |
| Oleic acid | Anti-inflammatory | Anti-inflammatory | 0.614 | 0.029 |
| Anti-inflammatory, ophthalmic | 0.394 | 0.011 | ||
| Non-steroidal anti-inflammatory agent | 0.352 | 0.029 | ||
| Anti-inflammatory, intestinal | 0.685 | 0.003 | ||
| Anxiolytic | GABA receptor agonist | 0.193 | 0.079 | |
| Antidepressant | – | – | – | |
| l-(+)-Ascorbic acid 2,6-dihexadecanoate | Anti-inflammatory | Anti-inflammatory | 0.802 | 0.007 |
| Non-steroidal anti-inflammatory agent | 0.500 | 0.012 | ||
| Anti-inflammatory, intestinal | 0.429 | 0.016 | ||
| Anti-inflammatory, ophthalmic | 0.240 | 0.203 | ||
| Anxiolytic | – | – | – | |
| Antidepressant | – | – | – | |
| Eicosanoic acid | Anti-inflammatory | Anti-inflammatory | 0.515 | 0.052 |
| Anti-inflammatory, intestinal | 0.727 | 0.002 | ||
| Anti-inflammatory, ophthalmic | 0.403 | 0.010 | ||
| Non-steroidal anti-inflammatory agent | 0.310 | 0.040 | ||
| Anxiolytic | GABA receptor agonist | 0.204 | 0.070 | |
| Antidepressant | – | – | – | |
| Docosane, 1,22-dibromo- | Anti-inflammatory | Anti-inflammatory, ophthalmic | 0.413 | 0.008 |
| Anti-inflammatory, intestinal | 0.385 | 0.027 | ||
| Cyclooxygenase 2 inhibitor | 0.080 | 0.068 | ||
| Anxiolytic | – | – | – | |
| Antidepressant | Antidepressant, Imipramin-like | 0.178 | 0.072 | |
| Cholesterol margarate | Anti-inflammatory | Anti-inflammatory | 0.573 | 0.038 |
| Anti-inflammatory, ophthalmic | 0.387 | 0.013 | ||
| Anxiolytic | – | – | – | |
| Antidepressant | – | – | – |
4. Discussion
Although L. spinosa has a long history of folk use, its pharmacological value in the treating of central nervous system disorders remains unknown. To this end, the present experimental study was designed to comprehensively investigate the neurological impacts of this plant. The anti-inflammatory potential of this plant was investigated through membrane stabilization. Membrane-stabilizing properties of L. spinosa fractions might be related to its interference in the release of neutrophils' lysosomal contents. A preventive impact on erythrocyte breakdown may be recognized as a significant anti-inflammatory property of L. spinosa fractions, but this study found no significant improvement in hemolysis inhibition between the reference drug diclofenac sodium and any L. spinosa fraction. The presence of various bioactive compounds such as flavonoids, terpenoids, alkaloids, and phenolic are responsible for anti-inflammatory activity. For example, flavonoids such as quercetin, kaempferol, and luteolin have been shown to possess anti-inflammatory properties by inhibiting the activity of pro-inflammatory enzymes such as cyclooxygenase (COX) and lipoxygenase (LOX), which are involved in the synthesis of inflammatory mediators like prostaglandins and leukotrienes [50]. Moreover, phenolic compounds, such as resveratrol found in grapes and curcumin found in turmeric, have also been shown to possess anti-inflammatory properties by inhibiting the activity of various pro-inflammatory enzymes and cytokines. The presence of alkaloids, flavonoids, steroids, tannins, carbohydrates, and protein in L. spinosa suggests that the anti-inflammatory activity of its extracts is attributed to the combined effects of these bioactive compounds, which are capable of targeting multiple pathways involved in inflammation [30,51]. Anxiety and depression are major health challenges worldwide [7,52,53], and so this study sought to investigate the pharmaceutical applications of L. spinosa in the treatment of such neurobiological disorders through mouse model behavioral assessments [19] such as the tail suspension test (TST), which evaluates depressive tendencies [54], and the elevated plus maze test (EPM) and hole board test (HBT), which measure anxiety [36]. In addition, the thiopental sodium-induced sleeping test was used to measure the effect of L. spinosa on sleep induction and duration. The EPM test is used to assess the anxiolytic effect of medicines on mice [55] and is a popular screening tool to investigate novel benzodiazepine-like therapeutics [56]. In this study, we found that mice treated with various fractions of L. spinosa were significantly more likely to enter into and spend time in the open arms of the maze, indicating increased calmness and reduced anxiety [57]. Both dosages (200 mg/kg b. w., p. o. And 400 mg/kg b. w., p. o.) of LSNHF and LSCTF correlated to these behaviors. Although similar activity was displayed by the mice treated with the reference drug, the specific effect of LSNHF and LSCTF may occur because of the exertion caused by the GABA-A/benzodiazepine receptor complex. The LSNHF and LSCTF demonstrated anxiolytic activity, due to their ability to modulate neurotransmitter systems such as the serotonergic, GABAergic, and dopaminergic systems. These systems are involved in regulating anxiety and other emotional behaviors [51]. The open arms of the EPM apparatus trigger anxiety in rodents, resulting in minimal exploration of these arms. Anxiolytics reduce open arm exploration, whereas anxiogenics reduce open arm exploration [58]. The hole board test is used to assess the attitude of animals to an unfamiliar situation, which can elicit anxious behavior. Several studies have shown that HBT is an accurate indicator of animals’ emotional states in such situations [59]. Our experimental data demonstrated that both LSNHF and LSCTF were associated with a greater propensity for head dipping at the dose of 400 mg/kg b. w., p. o. Anxiety arises due to either an abnormal activity of neurotransmitters such as serotonin, dopamine, or GABA receptors, or due to an irregularity caused by glutamatergic, serotonergic, GABA-ergic, or noradrenergic transmission [60]. In this context, we believe that LSNHF and LSCTF may enact anxiolytic changes by signaling chemical transmission.
LSNHF and LSCTF were tested in tail suspension tests (TST) to evaluate these fractions' antidepressant potential. The tests showed an association between LSNHF and LSCTF (at both 200 and 400 mg/kg b. w., p. o. doses) and increased propellant behaviors, such as minimized immobility and expanded battling propensity, during the test. These effects may be explained by the fractions’ ability to mitigate monoamine reuptake in the brain.
Previous studies have reported that Benzodiazepin enacts its anxiolytic effect by binding with GABA receptor discrete from the binding site of GABA receptor [61]. When thiopental sodium binds with the GABA receptor complex, it provides synergistic action through hyperpolarization of post-synaptic neuronal cell [62]. To assess the role of GABA-ergic systems in LSNHF- and LSCTF-induced sedation, thiopental sodium was injected into mice along with (but prior to) LSNHF, LSCTF, or diazepam. Overall, the findings of this study thus confirm that L. spinosa may be a potential source of medication for the treatment of mental disorders such as depression and anxiety. When it comes to discovering new lead compounds, computer-aided studies are regarded as the most essential approaches since they not only reduce the time and money that would have been spent doing a clinical trial but also most significantly save money [63]. Molecular docking is an important way to figure out how ligands interact with their targets because it shows how small molecules behave at the active sites of target proteins and helps researchers to better understand the mechanisms behind many pharmacological reactions [64,65]. Thus, molecular docking has been used to better understand these pharmacological responses. Four target proteins were used to test the anti-inflammatory, anti-anxiety, and antidepressant effects of the chosen compounds which includes cyclooxygenase-1 (PDB: 2OYE), cyclooxygenase-2 (PDB: 1CX2), potassium channel (PDB: 4UUJ) and human serotonin transporter (PDB: 5I6X). To determine the most potential molecule from LSNHF for anti-inflammatory, anxiolytic, and antidepressant activity. The docking analysis in Schrodinger Suite v11.1 was used to examine the compound's interaction with the active sites. For anti-inflammatory activity, among all the identified compounds, phenol, 2-methoxy-4-(2-propenyl)-, acetate exhibits the best docking score against cyclooxygenase-1 and cyclooxygenase-2 receptor which are (−7.072 kcal/mol) and (−6.907 kcal/mol) respectively. Again, for anxiolytic activity, eicosanoic acid possessed the best docking score (−3.398 kcal/mol) against potassium channel receptor. In addition to that, for determining the antidepressant, phenol, 2-methoxy-4-(2-propenyl)-, acetate exhibit the best docking score (−5.541 kcal/mol). The ADME study reveals that all the identified compounds did not violated any of the parameter of Lipinski's rule except Oleic Acid; l-(+)-Ascorbic acid 2,6-dihexadecanoate; Eicosanoic acid; Docosane, 1,22-dibromo- and Cholesterol margarate. In addition, among the selected compounds, Butanoic acid, 2-methyl-; Phenol, 2-methoxy-4-(2-propenyl)-, acetate and 9,9-Dimethoxybicyclo [3.3.1] nona-2,4-dione did not violate any of the parameters of Veber's rule. The observance of these two guidelines is a strong predictor of both good oral bioavailability and safety [66,67]. In addition, to establish our pharmacological investigations, we carried out an analysis of the compounds with the help of computer-aided web server known as PASS. The values of Pa and Pi may range anywhere from 0.000 to 1.000. If Pa is more than Pi, then the lead molecule is regarded to be experimentally active. If Pa is more than 0.6, there is a good chance that the compound has pharmacological potential, while values between 0.5 and 0.6 imply that the pharmacological potential is considerable. Pa values less than 0.5 suggest reduced pharmacological activity, which may point to the possibility of the discovery of a novel chemical [68,69]. Our study findings suggest that L. spinosa has potential therapeutic benefits for treating neurological disorders such as anxiety and depression. The in silico analysis identified molecular targets that may contribute to the observed biological activities. These findings provide scientific evidence supporting the traditional use of L. spinosa in ethnomedicine and emphasize the need for further research on the active constituents of this plant for the development of new drugs.
5. Conclusion
Based on the pharmacological findings, L. spinosa, particularly its LSNHF and LSCTF components, could provide novel therapeutic options for treating neuropsychiatric disorders like anxiety, depression, and insomnia. Additionally, L. spinosa has demonstrated potential in inhibiting hemolysis in humans, which may be attributed to its ability to alter inflammatory mediators, making it a potential treatment for inflammation-initiated neurodegenerative disorders. In silico studies on the identified compounds from LSNHF indicate their drug likeness, safe toxicological properties, and probable pharmacological activity, with docking analysis revealing high binding affinity for receptors associated with anti-inflammatory, anxiolytic, and antidepressant activities. These findings suggest that L. spinosa's bioactive compounds could be used to develop more potent and cost-effective drugs for managing various life-threatening diseases, including inflammation, anxiety, and depression. However, further research is necessary to identify and refine the novel bioactive components and understand the molecular mechanisms underlying the observed pharmacological effects.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical standards of animal handling were maintained according to the guidelines of Institutional Animal Ethics Committee of the Department of Pharmacy, International Islamic University Chittagong (Pharm-P&D/95:2018).
Author contribution statement
Mahfuz Ahmed Sakib: Performed the experiments; Wrote the paper.
Mst. Samima Nasrin, Mohammad Forhad Khan, Md. Amjad Hossen: Performed the experiments; Analyzed and interpreted the data.
Jishan Khan: Performed the experiments.
Md. Hazrat Ali, PhD: Contributed reagents, materials, analysis tools or data.
A. S. M. Ali Reza: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Md. Anwarul Haque, PhD: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
The authors are grateful to the Department of Pharmacy at the International Islamic University of Chittagong for their assistance in performing this research, and to Dr. Sheikh Bokhtear Uddin, a professor in the Department of Botany at the University of Chittagong, for identifying the plant.
References
- 1.Goni O., Khan M.F., Rahman M.M., Hasan M.Z., Kader F.B., Sazzad N., Sakib M.A., Romano B., Haque M.A., Capasso R. Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113664. [DOI] [PubMed] [Google Scholar]
- 2.Berton O., Nestler E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7(2):137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 3.Adnan M., Chy M.N.U., Kamal A.M., Chowdhury K.A.A., Rahman M.A., Reza A.A., Moniruzzaman M., Rony S.R., Nasrin M.S., Azad M.O.K. Intervention in neuropsychiatric disorders by suppressing inflammatory and oxidative stress signal and exploration of in silico studies for potential lead compounds from Holigarna caustica (Dennst.) Oken leaves. Biomolecules. 2020;10(4):561. doi: 10.3390/biom10040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam M.R., Reza A.A., Hossain M.S., Farhana M.K. In vitro evaluation of cytotoxic and thrombolytic activities of oroxylum indicum (linn.) Bangladesh Pharm. J. 2015;17(1):70–74. [Google Scholar]
- 5.Uddin M.J., Ali Reza A., Abdullah-Al-Mamun M., Kabir M.S., Nasrin M.S., Akhter S., Arman M.S.I., Rahman M.A. Antinociceptive and anxiolytic and sedative effects of methanol extract of Anisomeles indica: an experimental assessment in mice and computer aided models. Front. Pharmacol. 2018;9:246. doi: 10.3389/fphar.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moniruzzaman M., Mannan M.A., Hossen Khan M.F., Abir A.B., Afroze M. The leaves of Crataeva nurvala Buch-Ham. modulate locomotor and anxiety behaviors possibly through GABAergic system. BMC Compl. Alternative Med. 2018;18(1):1–12. doi: 10.1186/s12906-018-2338-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tareq A.M., Farhad S., Uddin A.N., Hoque M., Nasrin M.S., Uddin M.M.R., Hasan M., Sultana A., Munira M.S., Lyzu C. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon. 2020;6(6) doi: 10.1016/j.heliyon.2020.e04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S.-M., Han C., Bahk W.-M., Lee S.-J., Patkar A.A., Masand P.S., Pae C.-U. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam J. Med. 2018;54(2):101–112. doi: 10.4068/cmj.2018.54.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansari P., Uddin M.J., Rahman M.M., Abdullah-Al-Mamun M., Islam M.R., Ali M.H., Reza A.A. Anti-inflammatory, anti-diarrheal, thrombolytic and cytotoxic activities of an ornamental medicinal plant: persicaria orientalis. J. Basic Clin. Physiol. Pharmacol. 2017;28(1):51–58. doi: 10.1515/jbcpp-2016-0023. [DOI] [PubMed] [Google Scholar]
- 10.Hossain K.H., Rahman M.A., Taher M., Tangpong J., Hajjar D., Alelwani W., Makki A.A., Reza A.A. Hot methanol extract of Leea macrophylla (Roxb.) manages chemical-induced inflammation in rodent model. J. King Saud Univ. Sci. 2020;32(6):2892–2899. [Google Scholar]
- 11.Babar Z., Jaswir I., Tareq A., Ali Reza A.M., Azizi W., Hafidz M., Ahfter F., Hasan M., Farhad S., Uddin M.R. In vivo anxiolytic and in vitro anti-inflammatory activities of water-soluble extract (WSE) of Nigella sativa (L.) seeds. Nat. Prod. Res. 2021;35(16):2793–2798. doi: 10.1080/14786419.2019.1667348. [DOI] [PubMed] [Google Scholar]
- 12.Hossain M.S., Reza A.A., Rahaman M.M., Nasrin M.S., Rahat M.R.U., Islam M.R., Uddin M.J., Rahman M.A. Evaluation of morning glory (Jacquemontia tamnifolia (L.) Griseb) leaves for antioxidant, antinociceptive, anticoagulant and cytotoxic activities. J. Basic Clin. Physiol. Pharmacol. 2018;29(3):291–299. doi: 10.1515/jbcpp-2017-0042. [DOI] [PubMed] [Google Scholar]
- 13.Ali Reza A., Nasrin M.S., Hossen M.A., Rahman M.A., Jantan I., Haque M.A., Sobarzo-Sánchez E. Mechanistic insight into immunomodulatory effects of food-functioned plant secondary metabolites. Crit. Rev. Food Sci. Nutr. 2021:1–31. doi: 10.1080/10408398.2021.2021138. [DOI] [PubMed] [Google Scholar]
- 14.Mobarak H., Meah M.S., Sikder N., Tareq M., Azad A., Khatun R., Nasrin M.S., Raihan M.O., Reza A.A. Investigation of preliminary phytochemicals, analgesic, anti-arthritic, thrombolytic and Cytotoxic Activities of Begonia Roxburghii (Miq.) DC. Leaves. Med One. 2018;3(1) [Google Scholar]
- 15.Haque M.A., Reza A.A., Nasrin M.S., Rahman M.A. Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling. Integr. Cancer Ther. 2020;19 doi: 10.1177/1534735420969809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque M.A., Islam M.A.U. Pleurotus highking mushroom induces apoptosis by altering the balance of proapoptotic and antiapoptotic genes in breast cancer cells and inhibits tumor sphere formation. Medicina. 2019;55(11):716. doi: 10.3390/medicina55110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman M.M., Reza A.M.A., Khan M.A., Sujon K.M., Sharmin R., Rashid M., Sadik M.G., Reza M.A., Tsukahara T., Capasso R. Unfolding the apoptotic mechanism of antioxidant enriched-leaves of tabebuia pallida (lindl.) miers in EAC cells and mouse model. J. Ethnopharmacol. 2021 doi: 10.1016/j.jep.2021.114297. [DOI] [PubMed] [Google Scholar]
- 18.Reza A.A., Haque M.A., Sarker J., Nasrin M.S., Rahman M.M., Tareq A.M., Khan Z., Rashid M., Sadik M.G., Tsukahara T. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Sci. Nutr. 2021;9(7):3777–3805. doi: 10.1002/fsn3.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali Reza A., Hossain M.S., Akhter S., Rahman M., Nasrin M., Uddin M., Sadik G., Khurshid Alam A. In vitro antioxidant and cholinesterase inhibitory activities of Elatostema papillosum leaves and correlation with their phytochemical profiles: a study relevant to the treatment of Alzheimer's disease. BMC Compl. Alternative Med. 2018;18(1):1–8. doi: 10.1186/s12906-018-2182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z.-J. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004;75(14):1659–1699. doi: 10.1016/j.lfs.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Hossen M.A., Ali Reza A., Amin M.B., Nasrin M.S., Khan T.A., Rajib M.H.R., Tareq A.M., Haque M.A., Rahman M.A., Haque M.A. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer‐aided model. Food Sci. Nutr. 2021;9(7):3836–3851. doi: 10.1002/fsn3.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman M., Uddin M., Reza A., Tareq A.M., Emran T.B., Simal-Gandara J. Ethnomedicinal value of antidiabetic plants in Bangladesh: a comprehensive review. Plants. 2021;10(4):729. doi: 10.3390/plants10040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahan I., Sakib S.A., Alam N., Majumder M., Sharmin S., Reza A.A. Pharmacological insights into Chukrasia velutina bark: experimental and computer‐aided approaches. Anim. Model. Exp. Med. 2022;5(4):377–388. doi: 10.1002/ame2.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossen M.A., Reza A.A., Ahmed A.A., Islam M.K., Jahan I., Hossain R., Khan M.F., Maruf M.R.A., Haque M.A., Rahman M.A. Pretreatment of Blumea lacera leaves ameliorate acute ulcer and oxidative stress in ethanol-induced Long-Evan rat: a combined experimental and chemico-biological interaction. Biomed. Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111211. [DOI] [PubMed] [Google Scholar]
- 25.Nasrin S., Islam M.N., Tayab M.A., Nasrin M.S., Siddique M.A.B., Emran T.B., Reza A.A. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: an experimental chemico-biological interaction. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112842. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A.A., Rahman M.A., Hossen M.A., Reza A.A., Islam M.S., Rashid M.M., Rafi M.K.J., Siddiqui M.T.A., Al-Noman A., Uddin M.N. Epiphytic Acampe ochracea orchid relieves paracetamol-induced hepatotoxicity by inhibiting oxidative stress and upregulating antioxidant genes in in vivo and virtual screening. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112215. [DOI] [PubMed] [Google Scholar]
- 27.Li J. Flora of China. Harv. Pap. Bot. 2007;13(2):301–302. [Google Scholar]
- 28.Deb D., Dev S., Das A.K., Khanam D., Banu H., Shahriar M., Ashraf A., Choudhuri M., Basher S. Antinociceptive, anti-inflammatory and anti-diarrheal activities of the hydroalcoholic extract of Lasia spinosa Linn.(Araceae) roots. Lat. Am. J. Pharm. 2010;29 [Google Scholar]
- 29.Brach A.R., Song H. eFloras: new directions for online floras exemplified by the Flora of China Project. Taxon. 2006;55(1):188–192. [Google Scholar]
- 30.Islam M.S., Rashid M.M., Ahmed A.A., Reza A.A., Rahman M.A., Choudhury T.R. The food ingredients of different extracts of Lasia spinosa (L.) Thwaites can turn it into a potential medicinal food. NFS J. 2021;25:56–69. [Google Scholar]
- 31.Akter R., Rahman M.A., Rafi M.K.J., Siddique T.A., Bithy F.Y., Akter S., Nisa F.Y., Khan M.A.N., Sultana F. The protective effect of Lasia spinosa (Linn.) dissipates chemical-induced cardiotoxicity in an animal model. Cardiovasc. Toxicol. 2023:1–14. doi: 10.1007/s12012-022-09775-1. [DOI] [PubMed] [Google Scholar]
- 32.Kupchan S., Tsou G. Tumor inhibitors. A new antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973;38:178–179. doi: 10.1021/jo00941a049. [DOI] [PubMed] [Google Scholar]
- 33.VanWagenen B.C., Larsen R., Cardellina J.H., Randazzo D., Lidert Z.C., Swithenbank C. Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J. Org. Chem. 1993;58(2):335–337. [Google Scholar]
- 34.Islam N., Khan M.F., Khatun M.R., Nur S., Hanif N.B., Kulsum U., Arshad L., Lyzu C., Cacciola N.A., Capasso R. Neuropharmacological insights of African oil palm leaf through experimental assessment in rodent behavioral model and computer-aided mechanism. Food Biosci. 2021;40 [Google Scholar]
- 35.Uddin M., Ali Reza A., Abdullah-Al-Mamun M., Kabir M.S., Nasrin M., Akhter S., Arman M., Islam S., Rahman M. Antinociceptive and anxiolytic and sedative effects of methanol extract of anisomeles indica: an experimental assessment in mice and computer aided models. Front. Pharmacol. 2018;9:246. doi: 10.3389/fphar.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abreu T.M., Monteiro V.S., Martins A.B.S., Teles F.B., da Conceição Rivanor R.L., Mota É.F., Macedo D.S., de Vasconcelos S.M.M., Júnior J.E.R.H., Benevides N.M.B. Involvement of the dopaminergic system in the antidepressant-like effect of the lectin isolated from the red marine alga Solieria filiformis in mice. Int. J. Biol. Macromol. 2018;111:534–541. doi: 10.1016/j.ijbiomac.2017.12.132. [DOI] [PubMed] [Google Scholar]
- 37.Khan M.F., Kader F.B., Arman M., Ahmed S., Lyzu C., Sakib S.A., Tanzil S.M., Zim A.I.U., Imran M.A.S., Venneri T. Pharmacological insights and prediction of lead bioactive isolates of Dita bark through experimental and computer-aided mechanism. Biomed. Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110774. [DOI] [PubMed] [Google Scholar]
- 38.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 39.Ali M.S., Dash P.R., Nasrin M. Study of sedative activity of different extracts of Kaempferia galanga in Swiss albino mice. BMC Compl. Alternative Med. 2015;15(1):1–5. doi: 10.1186/s12906-015-0670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellow S., File S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986;24(3):525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 41.Bulbul M., Hassan R., Rahman M., Emran T.B., Afroze M., Khan M., Chowdhury M.A.H., Ibrahim M.A., Chowdhury M.S. Leea macrophylla (Roxb.) root extract reverses CCl4 induced liver injury through upregulation of antioxidative gene expression: a molecular interaction for therapeutic inception. Adv. Tradit. Med. 2020;20(1):35–52. [Google Scholar]
- 42.Harman C.A., Turman M.V., Kozak K.R., Marnett L.J., Smith W.L., Garavito R.M. Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-α-substituted indomethacin ethanolamides. J. Biol. Chem. 2007;282(38):28096–28105. doi: 10.1074/jbc.M701335200. [DOI] [PubMed] [Google Scholar]
- 43.Kurumbail R.G., Stevens A.M., Gierse J.K., McDonald J.J., Stegeman R.A., Pak J.Y., Gildehaus D., Penning T.D., Seibert K., Isakson P.C. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 44.Lenaeus M.J., Burdette D., Wagner T., Focia P.J., Gross A. Structures of KcsA in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry. 2014;53(32):5365–5373. doi: 10.1021/bi500525s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coleman J.A., Green E.M., Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532(7599):334–339. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berman H.M., Battistuz T., Bhat T.N., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002;58(6):899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- 47.Harder E., Damm W., Maple J., Wu C., Reboul M., Xiang J.Y., Wang L., Lupyan D., Dahlgren M.K., Knight J.L. OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theor. Comput. 2016;12(1):281–296. doi: 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- 48.Bristy T.A., Barua N., Montakim Tareq A., Sakib S.A., Etu S.T., Chowdhury K.H., Jyoti M.A., Aziz M.A.I., Reza A.A., Caiazzo E. Deciphering the pharmacological properties of methanol extract of Psychotria calocarpa leaves by in vivo, in vitro and in silico approaches. Pharmaceuticals. 2020;13(8):183. doi: 10.3390/ph13080183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 50.Behl T., Rana T., Alotaibi G.H., Shamsuzzaman M., Naqvi M., Sehgal A., Singh S., Sharma N., Almoshari Y., Abdellatif A.A. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112545. [DOI] [PubMed] [Google Scholar]
- 51.Hoque M.A., Ahmad S., Chakrabarty N., Khan M.F., Kabir M.S.H., Brishti A., Raihan M.O., Alam A.K., Haque M.A., Nasrin M.S. Antioxidative role of palm grass rhizome ameliorates anxiety and depression in experimental rodents and computer-aided model. Heliyon. 2021;7(10) doi: 10.1016/j.heliyon.2021.e08199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rani T., Behl T., Sharma N., Makeen H.A., Albratty M., Alhazmi H.A., Meraya A.M., Bhatia S., Bungau S.G. Exploring the role of biologics in depression. Cell. Signal. 2022;98 doi: 10.1016/j.cellsig.2022.110409. [DOI] [PubMed] [Google Scholar]
- 53.Rana T., Behl T., Sehgal A., Mehta V., Singh S., Sharma N., Bungau S. Elucidating the possible role of FoxO in depression. Neurochem. Res. 2021;46:2761–2775. doi: 10.1007/s11064-021-03364-4. [DOI] [PubMed] [Google Scholar]
- 54.Pollak D.D., Rey C.E., Monje F.J. Rodent models in depression research: classical strategies and new directions. Ann. Med. 2010;42(4):252–264. doi: 10.3109/07853891003769957. [DOI] [PubMed] [Google Scholar]
- 55.Bourin M., B Petit-Demoulière, Nic Dhonnchadha B., Hascöet M. Animal models of anxiety in mice. Fund. Clin. Pharmacol. 2007;21(6):567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 56.Aj A., Adeyemi O. Anxiolytic and sedative effects of byrsocarpus coccineus schum. And thonn.(connaraceae) extract. Int. J. Appl. Res. Nat. Products. 2010;3:28–36. [Google Scholar]
- 57.Saivasanthi V., Swathi K., Sowmya Rani G.A. Evaluation of Caralluma fimbrita for analgesic, anti-Inflammatory, and anxiolytic activities. Int. J. Pharm. 2011;1(1):40–45.. [Google Scholar]
- 58.Walf A.A., Frye C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebert B., Wafford K.A., Deacon S. Treating insomnia: current and investigational pharmacological approaches. Pharmacol. Ther. 2006;112(3):612–629. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Mennini T., Caccia S., Garattini S. Mechanism of action of anxiolytic drugs. Prog. Drug Res./Fortschr. Arzneim./Prog. Rech. Pharm. 1987:315–347. doi: 10.1007/978-3-0348-9289-6_10. [DOI] [PubMed] [Google Scholar]
- 61.Whiting P.J. GABA-A receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov. Today. 2003;8(10):445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- 62.Fernández S., Wasowski C., Paladini A.C., Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004;77(2):399–404. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Singh J., Chuaqui C.E., Boriack-Sjodin P.A., Lee W.-C., Pontz T., Corbley M.J., Cheung H.-K., Arduini R.M., Mead J.N., Newman M.N. Successful shape-based virtual screening: the discovery of a potent inhibitor of the type I TGFβ receptor kinase (TβRI) Bioorg. Med. Chem. Lett. 2003;13(24):4355–4359. doi: 10.1016/j.bmcl.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 64.Adnan M., Nazim Uddin Chy M., Mostafa Kamal A., Azad M.O.K., Paul A., Uddin S.B., Barlow J.W., Faruque M.O., Park C.H., Cho D.H. Investigation of the biological activities and characterization of bioactive constituents of Ophiorrhiza rugosa var. prostrata (D. Don) & Mondal leaves through in vivo, in vitro, and in silico approaches. Molecules. 2019;24(7):1367. doi: 10.3390/molecules24071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng X.-Y., Zhang H.-X., Mezei M., Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011;7(2):146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012;64:4–17. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 67.Veber D.F., Johnson S.R., Cheng H.-Y., Smith B.R., Ward K.W., Kopple K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45(12):2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 68.Goel R.K., Singh D., Lagunin A., Poroikov V. PASS-assisted exploration of new therapeutic potential of natural products. Med. Chem. Res. 2011;20(9):1509–1514. [Google Scholar]
- 69.Khurana N., Ishar M.P.S., Gajbhiye A., Goel R.K. PASS assisted prediction and pharmacological evaluation of novel nicotinic analogs for nootropic activity in mice. Eur. J. Pharmacol. 2011;662(1–3):22–30. doi: 10.1016/j.ejphar.2011.04.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.