Abstract
Objective
Little is known about effective strategies to improve advance care planning (ACP) for persons with cognitive impairment in primary care, the most common setting of care. We describe a randomized controlled trial to test the efficacy of a multicomponent communication intervention, “Sharing Healthcare Wishes in Primary Care” (SHARE).
Participants
Planned enrollment of 248 dyads of adults 80 years and older with possible cognitive impairment and their care partner, from primary care clinics at 2 Mid-Atlantic health systems.
Methods
The treatment protocol encompasses an introductory letter from the clinic; access to a designated facilitator trained in ACP; person-family agenda-setting to align perspectives about the family’s role; and print education. The control protocol encompasses minimally enhanced usual care, which includes print education and a blank advance directive. Randomization occurs at the individual dyad-level. Patient and care partner surveys are fielded at baseline, 6-, 12-, and 24- months. Fidelity of interventionist delivery of the protocol is measured through audio-recordings of ACP conversations and post-meeting reports, and by ongoing monitoring and support of interventionists.
Outcomes
The primary outcome is quality of end-of-life care communication at 6 months; secondary outcomes include ACP process measures. An exploratory aim examines end-of-life care quality and bereaved care partner experiences for patients who die by 24 months.
Conclusions
Caregiver burden, clinician barriers, and impaired decisional capacity amplify the difficulty and importance of ACP discussions in the context of cognitive impairment: this intervention will comprehensively examine communication processes for this special subpopulation in a key setting of primary care.
Keywords: advance care planning, primary care, cognitive impairment, randomized controlled trial
1. Introduction
Advance care planning (ACP) is a communication process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care.[1] Early initiation of ACP is an imperative in the context of Alzheimer’s Disease and Related Dementias (ADRD) due to the long course of illness and its progressive and devastating effects on decision-making capacity. [2] Little attention has been directed at developing strategies to improve ACP for persons with ADRD in primary care, the most common setting of initial diagnosis and ongoing medical management.[3, 4]
Identifying and addressing goals and values are core elements of high-quality ADRD care,[5–7] but evidence-based models that meet the needs of this population in the primary care context do not exist. Preliminary studies establish the benefit of normalizing ACP in primary care[8] and engaging family (broadly, as defined by each person), in face-to-face primary care visits[9] and electronic interactions,[10, 11], but these strategies have thus far been deployed in isolation of one another. The Sharing Health Care Wishes in Primary Care (SHARE) trial (NCT04593472) tests the efficacy of a multicomponent communication intervention to proactively engage family and normalize ACP among older primary care patients with possible cognitive impairment.
2. Study Design
2.1. Overview of study design and procedures.
This is an intention to treat, single-blind, two-group randomized controlled trial testing an experimental condition of a multicomponent communication intervention, referred to as SHARE, versus a control condition of minimally enhanced usual care. We plan enrollment of 248 person-family dyads comprising primary care patients with cognitive impairment ages 80 years or older and the family “care partner” who helps them the most with medical decision making. We originally planned enrollment of 62 dyads from 4 primary care practices. Design changes due to the COVID-19 pandemic led to the expansion of study sites from 4 to 8 primary care practices, with commensurate shifts in planned enrollment. The study received approval by the Johns Hopkins Medicine Single Institutional Review Board and is overseen by a four-member Data Safety and Monitoring Board.
Upon obtaining informed consent and after completing baseline enrollment interviews, patient-care partner dyads are randomized to the experimental or control condition. Patients and care partners are fielded surveys at enrollment and 6-, 12-, and 24-months by phone, video conference, or online, based on preferred modality. Bereavement surveys are administered to enrolled care partners two to three months after the death of a patient.
2.2. Research Aims.
Primary Aim:
The primary objective is to test the efficacy of SHARE on the quality of communication about end-of-life care at 6 months. Our hypothesis is that, as compared with the control group, care partners in the experimental group will report better quality of communication about end-of-life care with primary care clinicians.
Secondary Aim:
Our secondary objective is to test the efficacy of SHARE on ACP processes at 6 months. We hypothesize that care partners in the experimental group will be more highly engaged in ACP than those in the control group as measured by having had one or more ACP conversation and readiness to engage in ACP, and that patients will be more likely to have a documented advance directive in their electronic health record.
Exploratory Aim:
For patients who die by 24 months, we assess quality of end-of-life care and bereaved care partner experiences with medical decision-making. Exploratory endpoints include end-of-life care quality, decisional conflict, decisional regret, symptoms of anxiety and depression, and potentially burdensome end-of-life care.
2.3. Conceptual Framework.
SHARE is motivated by the demonstrated significance of interpersonal communication within the context of serious illness, and the important role of family in medical decision-making within the context of serious illness, cognitive impairment and end-of-life care.[12–14] Our study seeks to improve communication by establishing a structured protocol to proactively engage family care partners in ongoing interactions with primary care clinicians and stimulate and support ACP (Exhibit 1). Our premise is that although patients expect clinician-initiated ACP,[15] patient, family, and system factors inhibit these conversations.[2, 16] SHARE seeks to better equip persons with possible cognitive impairment and care partners with the knowledge, skills, and support to engage in effective communication through structured processes that support ACP.
Exhibit 1.
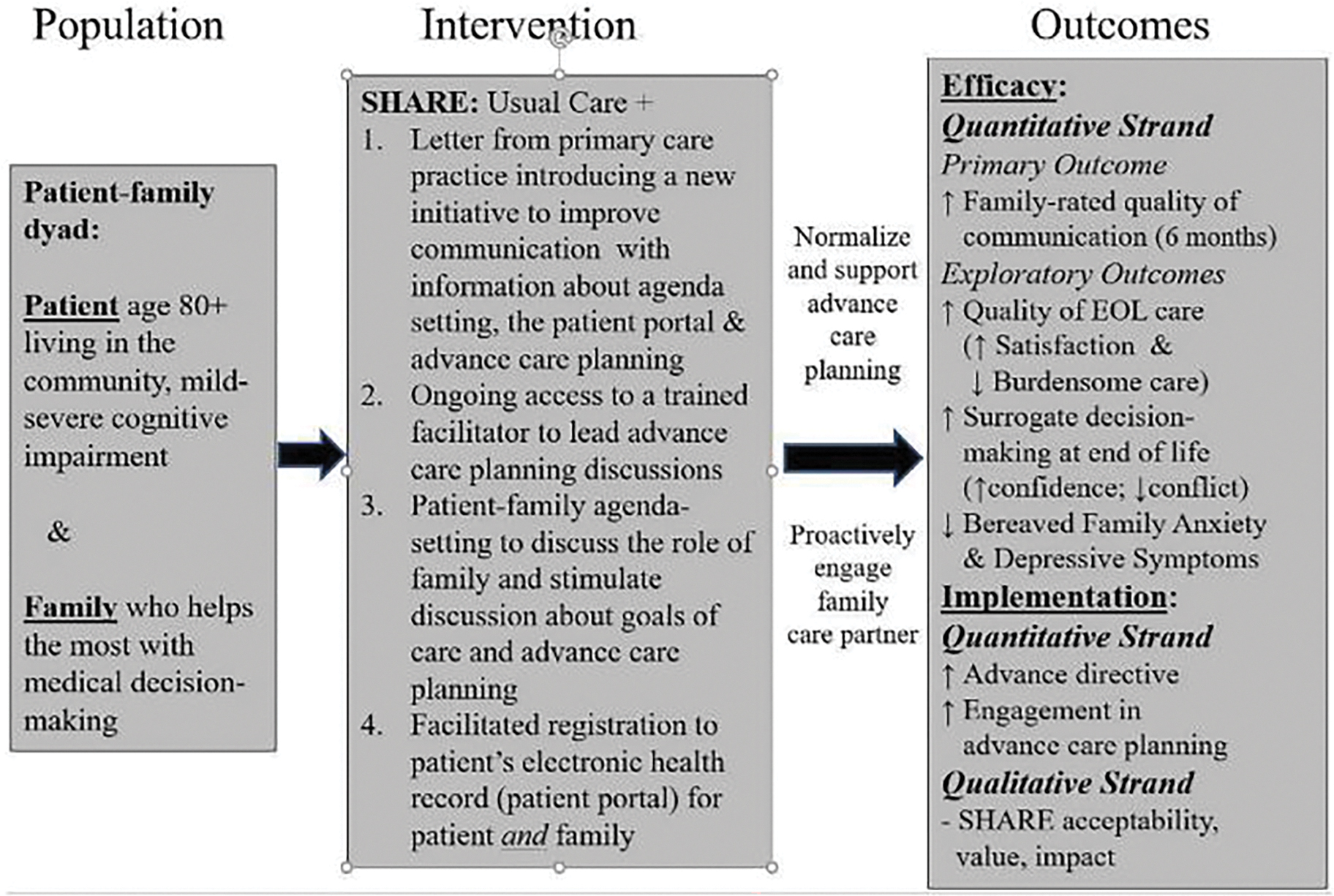
Conceptual Framework
Eligibility criteria
Eligibility criteria for clinicians, patients, and care partners are summarized in Exhibit 2. Clinician inclusion criteria are: 1) practicing primary care clinician, who is a physician, nurse practitioner, or physician assistant at a participating primary care clinic, and 2) care for patients ages 80 years or older.
Exhibit 2.
Inclusion and exclusion criteria for patients, family, and clinicians
| Participant | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Primary care clinician | • Practicing primary care physician, nurse practitioner, or physician assistant at participating practice • Care for patients ages 80+ |
• Does not care for patients ages 80+ • Plans to leave the practice |
| Patient | • Age 80+ • Under care of participating clinician • Involved care partner • Screens positive for possible cognitive impairment (mild-severe) • No care partner |
• Moving out of state within the year • Non-English speaking |
| Care partner | • Assists eligible patient and either family member or unpaid • 18 years or older • Able to hear well enough to communicate by telephone • Screens negative for possible cognitive impairment |
• Moving out of state within the year • Non-English speaking • Life-threatening illness |
Inclusion criteria for persons with possible cognitive impairment are: 1) 80 years or older, 2) English speaking, 3) able to provide informed consent themselves or through a legally authorized representative, 4) has a care partner who helps with activities such as doctor visits, managing medications, or participating in medical decision-making, 5) not planning to move out of state within the year, 6) possible cognitive impairment on the basis of one or more incorrect answers or not being able to respond to a validated 6-item telephone screening instrument, [17] and 7) under the care of a primary care clinician at a participating primary care clinic. The study initially planned hospitalization within the prior year as an inclusion criterion, but this requirement was dropped due to changes in care delivery throughout the COVID-19 pandemic.
Care partner inclusion criteria are: 1) assist an eligible person with possible cognitive impairment with health care activities, 2) 18 years and older, 3) English speaking, 4) able to hear well enough to communicate by telephone, 5) not planning to move out of the state within the year, 6) does not report having a life-threatening illness, 7) does not screen positive for possible cognitive impairment on the basis of the 6-item telephone screening instrument,[17] and provides informed consent.
The unit of analysis is the person-care partner dyad: both dyad members must meet eligibility criteria. We focus on persons with possible mild, moderate, and severe cognitive impairment regardless of clinical diagnosis because of the importance of addressing ACP early in the disease trajectory,[2] the under-diagnosis of ADRD [18–20] and the greater implementation potential of a protocol with broad applicability. Since the study focuses on a culturally complex topic, we exclude non-English-speaking individuals. SHARE has been designed to be broadly applicable to all primary care patients, but we focus on those 80 years and older to facilitate exploratory analyses of end-of-life endpoints.
3. Recruitment, enrollment, randomization, and follow-up
3.1. Recruitment
Recruitment follows a phased process, as in prior work. [21, 22] The office medical directors of primary care practices operated by two health care organizations in the Baltimore-Washington area are first approached by the leadership of their organizations to gauge interest in partnering on the study. Office medical directors who are receptive to participating are asked to allow the study team to present to clinicians in their practices about the objectives of the trial and invite them to contact the research team to learn more, determine whether they would like to participate, and provide informed consent.
Established patients of participating clinicians ages 80 and older are identified by the research team through the electronic health record. Research staff mail letters describing the study to potentially eligible patients one month in advance of a scheduled clinic visit. Patients who do not “opt out” by returning an opt-out card by mail are contacted by research staff to discuss study procedures and administer a telephone screening interview. For patients who are unable to interact by telephone due to cognitive or hearing impairment, eligibility is assessed through completing the screening survey with a knowledgeable informant. Finally, care partners of eligible patients are contacted by research staff to introduce the study, answer questions, and administer a telephone screening interview.
3.2. Enrollment
Research staff schedule a time for a telephone or video conference baseline enrollment meeting with patients and care partners who meet eligibility criteria and are interested in participating. For patients with two or more incorrect answers or who are not able to respond to a six-item telephone screening survey, [17] screening questions are asked of a knowledgeable informant and a legally authorized representative is identified according to Maryland law. Enrollment officially occurs at the time that both patient and care partner have provided informed consent, or at such a time that it is confirmed by research staff that both patient and caregiver have reviewed, completed, and signed either paper or e-consent forms or have completed an oral consent process. Upon enrollment, research staff field baseline assessments to both patients (or their knowledgeable informant) and care partners. All recruitment materials are IRB approved (00242431).
3.3. Randomization and Follow-Up
Randomization is at the level of the patient-care partner dyad, which allows examination of group differences by clinic. After obtaining informed consent and completing baseline interviews, each dyad is randomized in a 1:1 ratio using stratified, blocked randomization by primary care clinician with randomly varying block sizes of 4 or 6 dyads for each clinician. Randomization utilizes a statistical algorithm within REDCap developed by the project statistician (DLR) and unknown to research staff. Staff responsible for the randomization protocol are aware of participant assignment status only after it occurs. Allocation concealment is ensured by blinding the PI and staff conducting follow-up assessments and responsible for coding study outcomes. Experimental and control dyads are introduced to their respective protocol after randomization, upon completion of baseline enrollment procedures. Participants in both groups are told that the study is about communication in primary care. Patients and care partners individually receive $20 for each completed assessment, or up to $80 (baseline, 6, 12, and 24 months).
4. Measurements
Exhibit 3 summarizes primary and secondary outcome measures, covariates, and descriptive measures. Sociodemographic characteristics are assessed at baseline only, while health status, quality of life, and caregiving circumstances (e.g., responsibilities, appraisal) are assessed at each time point. Outcomes are operationalized as described by source instrument. As our sample encompasses persons with possible cognitive impairment, some of whom may be unable to respond to study assessments, survey-based outcomes are primarily assessed from the perspective of the care partner.
Exhibit 3.
Detailed Measurement Battery: Detailed Schedule of Instruments Fielded and Schedule of Assessments
| Construct | Measure (Source: Author, Year) | Items | Range | Validity | Source | Outcome | BL | 6 M | 12 M | 24 M | EOL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes @ 6 Months, Patient and Family Experience | |||||||||||
| QOC (Primary) | Quality of Communication About End of Life Care (Engelberg 2006; Reiff 2022) General QOC Subscale (Secondary) | 7 7 |
0–70 | α=0.79 α=0.91 |
P, F | F@6M | X | X | X | X | |
| ACP (Secondary) | 4-item ACP engagement μ (Sudore 2017; Van Scoy 2019) | 4 | 0–20 | α=0.84 * | P, F | F@6M | X | X | X | X | |
| Documented Advance Directive | -- | Y/N | -- | EMR | E@6m | X | X | X | X | ||
| Outcomes @ EOL, Quality of Care and Surrogate Decision-Making Experience (Exploratory) | |||||||||||
| Quality of care | Satisfaction w/EOL Care in Dementia (Volicer 2001) | 10 | 10–40 | α=0.90 | F | F@EOL | X | ||||
| Surrogate Decision Making | Decisional conflict scale (O’Connor 1995) | 16 | 0–100 | α=0.78 | F | F@EOL | X | ||||
| Decisional regret – (Degner 1992; Mack 2016) | 5 | 0–100 | α=0.81–0.92 | F | F@EOL | X | |||||
| Anxiety: GAD-7 (Spitzer 2006) | 7 | 0–21 | α=>0.92 | F | F@EOL | X | X | X | X | X | |
| EOL care | Potentially burdensome care | EMR, F | EMR | X | |||||||
| Primary Care Interactions and Communication | |||||||||||
| Therapeutic alliance | The Human Connection Scale (Mack 2009; Huff 2015) | 16 | 16–64 | α=0.90 original |
F | - | X | X | X | X | |
| Shared Decision Making | CollaboRATE μ (Barr, 2014) | 3 | 0–100 | α=0.89 | P, F | - | X | X | X | X | |
| Primary Care Interactions | Frequency/Mode of Contacts (Wolff 2016; 2019) | 5 | -- | --- | F, E | - | X | X | X | X | X |
| Registration & use of patient portal (Wolff 2020) | -- | -- | --- | EMR | - | X | X | X | X | X | |
| Implementation, Fidelity/Implementation Processes, Clinic Context (Note: these items will be limited to intervention participants) | |||||||||||
| Acceptability | Acceptability: Recruitment & retention | Consort diagram | - | X | |||||||
| Perceptions of SHARE purpose, value, impact | Interviews w/ patients, family, clinicians | X | |||||||||
| Fidelity | Number of ACP conversations | 5 | Y/N | P, F, I | - | X | X | X | X | ||
| Fidelity to Respecting Choices and SHARE protocol (Paladino 2019; Vaccaro 2019) | P,F,I, A | - | X | X | X | X | |||||
| Sustainability | Clinic continues intervention | Interviews w/ health system leadership | X | ||||||||
| Patient and Family Characteristics, Caregiving Circumstances | |||||||||||
| Demographics | Age, Gender, Education, Race/Ethnicity, Family employment status | 13 | P/F, F | - | X | ||||||
| Health literacy (Wallace 2006) | 1 | 0–4 | P, F | - | X | ||||||
| Living arrangement | 1 | P/F, F | - | X | X | X | X | X | |||
| Relationship between patient and family | 1 | P, F | X | ||||||||
| Interpersonal relationships | Caregiver/receiver Mutuality (Archbold 1990) | 15 | 0–4 | α=0.93 | P, F | - | X | ||||
| Family Apgar (Smilkstein 1978) |
5 | 0–20 | α=0.94 | P, F | - | X | |||||
| Health | Modified Telephone Interview for Cognitive Status (TICS-m; Brandt 1988) | 12 | 0–50 | P | - | X | |||||
| Self-rated health | 1 | Likert | P/F, F | - | X | X | X | X | X | ||
| PHQ-2 (Arroll 2010) | 2 | 0–6 | P, F | - | X | X | X | X | X | ||
| GAD-2 (Sapra 2020) | 2 | 0–6 | P | X | X | X | X | ||||
| Quality of Life | Quality of Life-AD (Logsdon 2002) | 13 | 13–52 | α=0.84; ICC>0.75 |
P/F | - | X | X | X | X | X |
| EuroQOL-5D (EuroQOL 1990) | 6 | F | - | X | X | X | X | X | |||
| Caregiving Circumstances | Intensity: Frequency, Type of Help | 10 | Homegrown | F | - | X | X | X | X | X | |
| Caregiver self efficacy (Fortinsky 2002) | 4 | 5–20 | N/A | F | - | X | X | X | X | ||
| 12-Item Zarit Burden Int + 1 global (Bedard 2001) | 13 | 0–60 | 0.87 | F | - | X | X | X | X | X | |
Source: P=patient; F=family caregiver; E=EMR; I=interventionist facilitator; A=audiotaped advance care planning conversations; C=clinic Note: μ=Modified to reflect care partner perspective.
Note that psychometric properties for this information are listed for the original, long-form version of the instrument, rather than the short-form being fielded in this study for patients, or the adapted version which reflects the care partner perspective.
4.1. Primary outcome
Our primary outcome is the end-of-life subscale (n=7 items) of the quality of communication instrument at 6 months follow-up.[23, 24] We focus on the end-of-life subscale as it is most pertinent to ACP, and we examine the care partner perspective due to incapacity of some patients to self-report. Data procedures for analysis follow established procedures and question wording follow that stipulated by Engelberg (2006), with the incorporation of skip patterns to reduce cognitive demand and improve measurement precision, as described by Reiff (See Appendix 1). [25]
4.2. Secondary outcomes
Readiness to engage in ACP is assessed from the patient perspective using the 4-item ACP engagement survey.[26, 27] Readiness to engage in ACP is assessed from the care partner survey using items from work by Van Scoy [26, 28, 29][22, 30] who identified parallel items for surrogate decision-makers. We rely on 6 items corresponding to the 4-item patient survey and the role of care partners in SHARE. Appendix 2 provides the question wording for both patient and care partner surveys.
Advance directive completion is defined as having a documented durable power of attorney or a living will in the primary care electronic health record at the end of the study. We exclude the Medical Order for Life Sustaining Treatment (MOLST) in our outcome as the completion of a Maryland MOLST is mandatory in certain situations (e.g., on transfer between settings) and is not indicative of having had an ACP discussion or naming a durable power of attorney; the Maryland MOLST does not conform to the National POLST Paradigm.[31, 32]
4.3. Exploratory outcomes
Exploratory analyses assess bereaved care partner experiences for patients who die by 24 months. Measures include decisional conflict,[33] decisional regret,[34], symptoms of anxiety[35], and satisfaction with care.[36] Potentially burdensome care is measured by any intensive care unit use or life prolonging care (cardiopulmonary resuscitation, mechanical ventilation, tracheostomy, dialysis, artificial nutrition, chemotherapy) within 30 days of death[37, 38] using dates and services abstracted from medical records and the Chesapeake Regional Information System (CRISP), Maryland’s Health Information Exchange. This measure assesses services received rather than whether the care was perceived as disproportionately burdensome.
5. Experimental arm
SHARE encompasses four components (see Exhibit 4).
A mailed letter from the primary care clinic introducing the initiative (see Exhibit 5).
Access to a facilitator trained in the protocol and leading ACP conversations. Facilitators were certified in the Respecting Choices advance care planning curriculum (http://respecting-choices.org) and structured conversation guide exploring personal values, identifying an appropriate health care decision-maker, and communicating preferences for end-of-life care.[39] This curriculum was supplemented by training to deliver ACP in the context of cognitive impairment and to support care partner involvement, including: facilitating family meetings, communicating difficult news, assessing cognitive capacity, registering patients and families for the patient portal, and procedures for documenting the occurrence and outcomes of advance care planning discussions in the electronic health record.
Person-family agenda-setting to align perspectives about the role of family and stimulate discussion about health care issues and ACP.
Educational materials about communication and ACP, including: a 44-page brochure developed by the National Institute on Aging entitled “A Guide for Older People: Talking with your Doctor”, a blank advance directive, and information about and facilitated registration for the patient portal to enable and extend electronic interactions and information access to patients and family.
Exhibit 4.
SHARE Therapeutic Components: Content, Rationale, Evidence of Effectiveness
| Content | Rationale | Evidence of Effectiveness |
|---|---|---|
| 1. Primary care initiated voluntary ACP | Most older adults and families appreciate when primary care practices engage them in ACP.[15] Proactively introducing ACP normalizes these discussions. | Primary care initiatives to increase advance directive completion are effective and well-received.[8] |
| 2. ACP education and availability of nonclinician led ACP discussions | ACP education and resources increase patient & family awareness, knowledge and skill. Respecting Choices is a structured educational program to train non-clinicians to facilitate ACP discussions. [65, 66] | ACP and Respecting Choices are associated with increased advance directive documentation and patient satisfaction in primary care and those with serious illness. [67–70] |
| 3. Person-Family Agenda Setting | Individuals & families often have different concerns. Agenda-setting stimulates discussions about ACP & the role of family. | Agenda-setting helps clarify concerns, goals, and expectations, and increase engagement in care. [9, 71] |
| 4. Resources about communication with a primary care clinician, including the patient portal | The patient portal facilitates timely and accurate information about patient health, diagnoses, test results, & prescribed treatments. Families can have their own identity credentials to access information and communicate with clinicians. | The patient portal operates through mechanisms of convenience, continuity, activation, and understanding.[72] Prior studies find clinical benefit of supporting family through technology.[73] |
ACP=advance care planning; EOL=end-of-life
Exhibit 5.
Introductory Letter to Patients
| Dear [Patient Name]: At [Clinic Name/ Health System], it is important for us to understand your wishes about your care. Here are some ways you can help. |
| 1. Tell us your concerns We want to know what is important to you. Attached is a checklist and resources about communication that may be helpful. If you want, you can complete the attached checklist and bring it to your next appointment at [Clinic Name/ Health System]. |
| 2. Share your health information with family or a friend Giving a family member or friend access to your health information can help them better understand your health and treatments. If you would like to share your health information with family or a friend, you can complete the attached PROXY ACCESS form and give it to the front desk at [Clinic Name/ Health System]. |
| 3. Talk with your health team about your wishes We want to make sure your wishes are respected if you have a serious illness or injury. If you want, you can complete the attached advance directive and bring it to your next appointment to talk to your doctor about it. You may also bring a copy of your own advance directive. |
| We look forward to talking with you on future visits and addressing your questions. |
| Sincerely, |
| [Clinic Name/ Health System/ Primary Care Provider] |
Within 2 business days of enrollment, research staff provide SHARE facilitators the contact information of participants randomized to the experimental arm. SHARE facilitators then contact patients by telephone to inquire about their interest in scheduling an introductory conversation via telephone or video conference, with a goal of completing at least one ACP conversation within 4 weeks of enrollment. During the initial meeting the facilitator reviews: (1) recent changes in the individual’s health status, (2) whether the individual has identified a health care agent (3) individual goals, values, and preferences for future medical care; and (4) offers to assist with completing or updating advance directives as needed.
At the conclusion of the initial meeting the facilitator seeks patient and care partner input regarding the frequency (e.g., monthly, quarterly) and mode (by phone, via secure electronic messaging through the patient portal) of future “check-in” contacts to assess interest in scheduling future meetings. Facilitators document the meeting in REDCap and share impressions (e.g., significant symptom burden, major changes in health, potential eligibility for hospice care) with the primary care clinician or related point of contact. SHARE facilitators document the occurrence and content of subsequent contacts and ACP conversations in REDCap as well as the patient’s medical record.
6. Control arm
Dyads assigned to the control group receive a protocol of minimally enhanced usual care, encompassing an introductory letter, print educational brochure, and a blank easy to complete advance directive. Control dyads are told that they are participating in a study about communication in primary care. We chose an active control arm because providing an advance directive can be considered as the standard of care even if it is often not ‘usual care’, to ensure that positive results can be attributed to the intervention, and to mitigate perceptions among dyads randomized to the control group that they are not being offered anything beyond usual care. All patients may register for the patient portal, provide their primary care clinician with a completed advance directive, or avail themselves of ACP with their primary care clinician or other clinic staff. However, these processes generally occur on an ad-hoc basis and do not involve a systematized approach to engaging with family caregivers or ACP.
7. Fidelity plan
Guided by the NIH Behavior Change Consortium[40] we address fidelity through design (by selecting distinct therapeutic elements based on theory), training (by relying on a protocolized curriculum to train ACP facilitators), and by assessment of interventionist delivery of the protocol (by review of audio-recordings of ACP conversations and post-meeting reports, and ongoing monitoring and support of interventionists).
The original plan was that facilitators be nurse case managers or social workers employed by one of 4 participating primary clinics: each facilitator would have a caseload of up to 31 patients. Shifts toward remote modalities necessitated by the COVID-19 outbreak led us to instead rely on nurse case managers, social workers, or lay facilitators employed by the health systems operating participating primary care practices. As facilitators were embedded in the health system rather than primary care practice, their theoretical caseload was as many as 62 patients, though the staggered pace of accrual left their actual caseload much lower, an average of 24 (fluctuating from 14 to 41) at any given in time.
ACP facilitators are certified in the Respecting Choices ACP curriculum which includes 6 online modules and synchronous instruction to gain competency with scripted interview tools, communication techniques, and demonstrated proficiency through role-plays. The Respecting Choices curriculum is supplemented by 0.5 days of training in the study protocol, which was delivered and reinforced by co-investigator JGC using traditional didactics, case scenarios, and modeling and mentored role play.
ACP facilitators document all patient and care partner contacts. After each ACP meeting, facilitators document their impressions of the meeting content and progress using the post-ACP report form that includes a checklist of key fidelity components. ACP conversations are audio-recorded and reviewed to monitor adherence to the SHARE protocol, as previously described.[41] Following Vaccaro and Seaman, [42, 43] at least two trained unblinded research staff listen to and rate each audio-recorded ACP conversation. To achieve consistency between auditors, an ACP audit tool was developed to guide assessment of specific content covered in the ACP Facilitator training such as use of motivational interviewing techniques, empathetic language, and inclusion of both patient and caregiver in the conversation, aspects of advance directive documentation and ACP processes, and contextual information such as the duration of the visit. The audit tool mirrors items asked of facilitators in post-ACP meeting reports to ensure that auditors and facilitators are attending to the same core ACP conversation elements.
Facilitators are convened weekly by phone to review progress updates, discuss challenges encountered, engage in collaborative problem-solving, review adherence issues and strategies for resolutions, and revisit specific topics as needed. The structure and content of the supervision meetings is tailored to the specific fidelity-related needs and issues of the facilitators. Additional elements of fidelity maintenance include monitoring completion of post-ACP meeting reports after each facilitated meeting.
8. Data collection
Data from screening calls, ACP contacts, and surveys are entered into REDCap forms that the data manager checks for completion and accuracy. The number, duration, and mode of contacts with the ACP facilitator are monitored weekly. This level of detail permits the research team to identify areas where full implementation is not being achieved and enable corrective actions. Measures of advance directive completion, use of the patient portal, and potentially burdensome care will be extracted from the electronic health record and the Maryland CRISP by research staff masked to treatment group at the end of the trial. Audio-recordings of ACP conversations are transcribed and audited by unblinded research staff to evaluate fidelity to the SHARE protocol.
9. Sample size and analysis
The sample size is based on our ability to detect a distribution-based clinically meaningful effect for our primary outcome at 6 months.[44] From prior trials we assume an unadjusted intervention effect of 0.30 standard deviation units (SDUs).[13, 45] Incorporating baseline QOC scores as a covariate and assuming correlation of 0.65 between baseline and 6-month QOC scores yields a covariate-adjusted effect size of 0.39 SDUs. Based on an enrolled sample of 248 dyads and attrition of 10%, a retained sample of 222 will provide more than 80% power to detect a covariate-adjusted effect of 0.39 using a two-sided test and a type 1 error rate of 0.05.
9.1. Analyses of primary outcome.
The primary outcome is care partner responses to the 7-item end-of-life subscale of the quality of communication about end-of-life care (QOC) questionnaire at 6 months.[23, 24]. We will use intention-to-treat analyses as the primary method of analysis and use analyses of covariance on the 6-month QOC score with treatment group as the primary independent variable and baseline QOC score as the primary covariate. Although randomization was implemented at the individual patient level, there may still be clustering of outcomes within practice sites and practice-level variables may also be related to outcomes. Therefore, hierarchical, multilevel models with patients embedded with practices may be employed if such patterns and associations are observed. Baseline individual- and practice-level variables will be covariates if there is imbalance on these measures by treatment group or if they are significantly correlated with 6-month QOC after adjusting for baseline QOC score. If a multilevel design is used, this will be implemented using SAS Proc MIXED with dyads (level 1) nested within clinics (level 2) that will allow us to enter and control for clinic-level covariates (size and characteristics of patient panel, staff, location).
Descriptive analyses will carefully examine the presence of missing outcome data, the reasons for missingness (e.g., patient death vs. lost to follow-up), and the predictors of missingness. Sensitivity analyses using multiple imputation of outcome data may be conducted if missingness is more than trivial and the pattern of missingness supports such imputation methods
9.2. Secondary outcomes and supplemental analyses.
Analyses of secondary and exploratory outcomes, implementation measures, and prespecified subgroups will be conducted. Maintenance of intervention effects extend the multilevel model to include a longitudinal or within-person level. Trajectories of treatment impact over time will be estimated with linear longitudinal models. For dichotomous outcomes (e.g., newly documented advance directive) we will construct multilevel logistic regression models using SAS Proc GLIMMIX. We examine the consistency of intervention effects for subgroups identified a priori including by primary clinic, by caregiving relationship (spouse vs. non-spouse) and possible cognitive impairment severity (mild vs. moderate/severe using the Modified Telephone Interview for Cognitive Status (TICS-m). [46–48] We will examine rates of recruitment and retention, the timeliness and completeness of collected data,[49] and relative attrition rates (SHARE versus control protocol).[50]
9.3. Qualitative Strand.
Upon the completion of the final follow-up survey, we purposively sample 12 dyads who received SHARE for in-depth interviews and all interventionists and fidelity raters. We assess perspectives about SHARE delivery characteristics and its purpose, value, and impact. Post-intervention in-depth interviews will be audio-recorded, transcribed verbatim, and entered in NVivo textual data analysis software. Qualitative analyses will be conducted by the research team concurrent to data collection using thematic analysis [51] to identify, analyze, describe, and report emergent themes in our data. We will use a mixed methods approach with quantitative analyses of implementation and efficacy to elucidate the mechanism by which effects or lack of effects of SHARE are observed and to identify how SHARE might be further refined and improved.
10. Discussion
Alzheimer’s Disease and Related Dementias (ADRD) are among the most disabling and costly of all health conditions[52] and a leading cause of death.[53] Family care partners are at the forefront of managing ADRD, and clinicians rely on the substituted judgement of family for persons who lack decisional capacity toward the end of life.[54] However, family are not routinely included in discussions about prognosis[55] and are too often poorly prepared to engage in surrogate decision-making.[54, 56] Compared to persons without ADRD, those with ADRD are less likely to complete an advance directive or formally designate a surrogate decision-maker,[57] placing them at heightened risk for potentially burdensome and costly end-of-life care.[58]
The investigation has several novel design elements. The study incorporates elements of efficacy and implementation research. Implementation science posits that hybrid mixed-methods studies may improve the speed of knowledge creation and translation by increasing the usefulness, relevance, and public health impact of complex behavioral interventions.[59–61] Our study quantitatively examines clinically meaningful outcomes and qualitatively assesses diverse individual, family, and primary care stakeholder perspectives and contextual factors. By audio-recording ACP conversations, we are able to comprehensively assess the structure and content of conversations and the ability of facilitators to adhere to the protocol as intended. Comprehensive tracking of ACP contacts and outcomes of conversations will afford comprehensive examination of process measures in relation to person- and family-reports of the quality of communication about end-of-life care. Caregiver burden, stigma, and impaired decisional capacity amplify the difficulty and importance of ACP discussions in the context of ADRD[2] yet few interventions have been equipped to comprehensively examine conversation processes and outcomes in persons with ADRD outside of the nursing home context, as in this trial.
Our team has encountered challenges executing the SHARE study. The protocol was initially designed, funded, and developed in a period preceding the COVID-19 outbreak. The study proposed in-person enrollment and baseline assessments in home or community settings preferred by older adults and their care partners, to accommodate the needs of the target population. The study planned to train existing primary care staff to assume the ACP facilitator role, so as to physically embed the model in primary care practices and facilitate subsequent scaling. Although the study was successfully reconfigured to accommodate the remote deployment, delays in the postal service, logistical complexities associated with moving research staff to a remote work environment, and the necessity of relying on telephone and video conferencing modalities for contacts with study participants have led to slower than expected accrual. The study initially planned having a hospitalization within the prior year as inclusion criteria, but this eligibility criteria was dropped due to changes in care delivery throughout the pandemic. Facilitating ACP remotely has been a challenge given the high degree of sensory impairment and more limited technology experience of our study population.
11. Conclusions.
This clinical trial examines effects of a multicomponent communication intervention to proactively engage family and normalize ACP in primary care among persons with possible cognitive impairment ranging from mild to severe. Our protocol is aligned with contemporary initiatives to improve care quality for persons with ADRD and recommendations for advancing interventions with high implementation and sustainability potential.[62] Our study is especially timely given the addition of Medicare billing codes for ACP with non-physicians, alignment with recommendations of American Medical Association and National Quality Forum consensus committees emphasizing ACP in ADRD quality measurement,[5, 63] and a National Academies of Sciences, Engineering, and Medicine report calling for strategies to proactively engage and support families in care delivery.[64] Our protocol is consistent with principles for approaching ACP in older adults with cognitive impairment in primary care[2] and if successful, our trial will provide supporting evidence regarding the feasibility and benefit in support of specific recommendations and dissemination in practice.
Supplementary Material
Acknowledgements
We thank our diligent and thoughtful research data collection team, Kezia Alexander, Margo Chapin, Kora Coker, Nicole Dodson-Loucks, Amy Guo, Amrutha Kadali, Abigail Russman, Ambrym Smith, and Kate Zou for assistance with the transition of study processes into the remote environment during the COVID-19 pandemic, and for assistance with recruitment, enrollment and data collection throughout the COVID-19 variant waves and unique challenges the pandemic presented to our target population.
Funders
This study was supported by National Institute of Aging grants R01AG058671 and T32AG066576 which partially supported the effort of Jenni Reiff. The sponsor of this research was not involved in its study concept or design, recruitment of subjects or acquisition of data, data analysis or interpretation, or in the preparation of this manuscript.
Funding
National Institute on Aging R01AG058671 and T32AG066576
Footnotes
Competing Interests
The authors declare that they do not have any competing interests to report.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests: None.
Registration: ClinicalTrials.gov: NCT04593472.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sudore RL, Lum HD, You JJ, Hanson LC, Meier DE, Pantilat SZ, Matlock DD, Rietjens JA, Korfage IJ, Ritchie CS, Kutner JS, Teno JM, Thomas J, McMahan RD, Heyland DK, Defining Advance Care Planning for Adults: A Consensus Definition from a Multidisciplinary Delphi Panel, Journal of pain and symptom management (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].deLima Thomas J, Sanchez-Reilly S, Bernacki R, O’Neill L, Morrison LJ, Kapo J, Periyakoil VS, Carey EC, Advance Care Planning in Cognitively Impaired Older Adults, Journal of the American Geriatrics Society 66(8) (2018) 1469–1474. [DOI] [PubMed] [Google Scholar]
- [3].Yang M, Chang CH, Carmichael D, Oh ES, Bynum JP, Who Is Providing the Predominant Care for Older Adults With Dementia?, Journal of the American Medical Directors Association 17(9) (2016) 802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geldmacher DS, Kerwin DR, Practical Diagnosis and Management of Dementia Due to Alzheimer’s Disease in the Primary Care Setting: An Evidence-Based Approach, The primary care companion to CNS disorders 15(4) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].NQF, Priority setting for health care performance measurement: Addressing performance measure gaps for dementia, including Alzheimer’s Disease, National Quality Forum, Washington, DC, 2014. [Google Scholar]
- [6].NASEM, Reducing the Impact of Dementia in America, National Academy Press, Washington DC, 2021, p. 286. [Google Scholar]
- [7].NASEM, Meeting the challenge of caring for persons living with dementia and their care partners and caregivers: a way forward, National Academies Press, Washington DC, 2021. [PubMed] [Google Scholar]
- [8].Luu NP, Nigrin C, Peairs K, Dy SM, Sawyer M, Pitts S, Petty B, Increasing advance care planning completion at an academic internal medicine outpatient clinic, Journal of pain and symptom management 54(3) (2017) 383–386. [DOI] [PubMed] [Google Scholar]
- [9].Wolff JL, Roter DL, Barron J, Boyd CM, Leff B, Finucane T, Gallo JJ, Rabins PV, Roth DL, Gitlin LN, A tool to strengthen the older patient-companion partnership in primary care: Results from a pilot study, Journal of the American Geriatrics Society 62(2) (2014) 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wolff JL, Darer JD, Berger A, Clarke D, Green JA, Stametz RA, Delbanco T, Walker J, Inviting patients and care partners to read doctors’ notes: OpenNotes and shared access to electronic medical records, Journal of the American Medical Informatics Association : JAMIA (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wolff JL, Berger A, Clarke D, Green JA, Stametz RA, Yule C, Darer JD, Patients, care partners, and shared access to the patient portal: online practices at an integrated health system, Journal of the American Medical Informatics Association : JAMIA 23(6) (2016) 1150–1158. [DOI] [PubMed] [Google Scholar]
- [12].Wolff JL, Roter DL, Family presence in routine medical visits: A meta-analytical review, Social science & medicine 72(6) (2011) 823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hanson LC, Zimmerman S, Song MK, Lin FC, Rosemond C, Carey TS, Mitchell SL, Effect of the Goals of Care Intervention for Advanced Dementia: A Randomized Clinical Trial, JAMA internal medicine 177(1) (2016) 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brundage MD, Feldman-Stewart D, Tishelman C, How do interventions designed to improve provider-patient communication work? Illustrative applications of a framework for communication, Acta Oncol 49(2) (2010) 136–43. [DOI] [PubMed] [Google Scholar]
- [15].Hancock K, Clayton JM, Parker SM, Wal der S, Butow PN, Carrick S, Currow D, Ghersi D, Glare P, Hagerty R, Tattersall MH, Truth-telling in discussing prognosis in advanced life-limiting illnesses: a systematic review, Palliative medicine 21(6) (2007) 507–17. [DOI] [PubMed] [Google Scholar]
- [16].Lakin JR, Block SD, Billings JA, Koritsanszky LA, Cunningham R, Wichmann L, Harvey D, Lamey J, Bernacki RE, Improving Communication About Serious Illness in Primary Care: A Review, JAMA internal medicine 176(9) (2016) 1380–1387. [DOI] [PubMed] [Google Scholar]
- [17].Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC, Six-item screener to identify cognitive impairment among potential subjects for clinical research, Medical care 40(9) (2002) 771–81. [DOI] [PubMed] [Google Scholar]
- [18].Bradford A, Kunik ME, Schulz P, Williams SP, Singh H, Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors, Alzheimer disease and associated disorders 23(4) (2009) 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chodosh J, Petitti DB, Elliott M, Hays RD, Crooks VC, Reuben DB, Galen Buckwalter J, Wenger N, Physician recognition of cognitive impairment: evaluating the need for improvement, Journal of the American Geriatrics Society 52(7) (2004) 1051–9. [DOI] [PubMed] [Google Scholar]
- [20].Connolly A, Gaehl E, Martin H, Morris J, Purandare N, Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence, Aging & mental health 15(8) (2011) 978–84. [DOI] [PubMed] [Google Scholar]
- [21].Wolff JL, Roter DL, Boyd CM, Roth DL, Echavarria D, Aufill J, Vick J, Gitlin LN, Patient-Family Agenda Setting for Primary Care Patients With Cognitive Impairment: The SAME Page Trial, Journal of general internal medicine 33(9) (2018) 1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wolff JL, Scerpella D, Cockey K, Hussain N, Funkhouser T, Echavarria D, Aufill J, Guo A, Sloan DH, Dy SM, Smith KM, Investigators SC, SHARING Choices: A Pilot Study to Engage Family in Advance Care Planning of Older Adults With and Without Cognitive Impairment in the Primary Care Context, The American journal of hospice & palliative care (2020) 1049909120978771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Engelberg R, Downey L, Curtis JR, Psychometric characteristics of a quality of communication questionnaire assessing communication about end-of-life care, Journal of palliative medicine 9(5) (2006) 1086–98. [DOI] [PubMed] [Google Scholar]
- [24].Curtis JR, Patrick DL, Caldwell E, Greenlee H, Collier AC, The quality of patient-doctor communication about end-of-life care: a study of patients with advanced AIDS and their primary care clinicians, AIDS 13(9) (1999) 1123–31. [DOI] [PubMed] [Google Scholar]
- [25].Reiff JS, Cagle J, Zhang T, Roth DL, Wolff JL, Fielding the quality of communication questionnaire to persons with cognitive impairment and their family in primary care: a pilot study, Journal of the American Geriatrics Society (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sudore RL, Stewart AL, Knight SJ, McMahan RD, Feuz M, Miao Y, Barnes DE, Development and validation of a questionnaire to detect behavior change in multiple advance care planning behaviors, PloS one 8(9) (2013) e72465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sudore RL, Heyland DK, Barnes DE, Howard M, Fassbender K, Robinson CA, Boscardin J, You JJ, Measuring Advance Care Planning: Optimizing the Advance Care Planning Engagement Survey, Journal of pain and symptom management 53(4) (2017) 669–681 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sudore RL, Boscardin J, Feuz MA, Mcmahan RD, Katen MT, Barnes DE, Effect of the PREPARE Website vs. an Easy-to-Read Advance Directive on Advance Care Planning Documentation and Engagement Among Veterans: A Randomized Clinical Trial, JAMA internal medicine in press (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sudore RL, Knight SJ, McMahan RD, Feuz M, Farrell D, Miao Y, Barnes DE, A novel website to prepare diverse older adults for decision making and advance care planning: a pilot study, Journal of pain and symptom management 47(4) (2014) 674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Scoy LJ, Day AG, Howard M, Sudore R, Heyland DK, Adaptation and Preliminary Validation of the Advance Care Planning Engagement Survey for Surrogate Decision Makers, Journal of pain and symptom management 57(5) (2019) 980–988 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tarzian AJ, Cheevers NB, Maryland’s Medical Orders for Life-Sustaining Treatment Form Use: Reports of a Statewide Survey, Journal of palliative medicine (2017). [DOI] [PubMed] [Google Scholar]
- [32].NPPTF, National POLST Paradigm: Appropriate POLST Paradigm Form Use Policy, polst.org, National Polst Paradigm Task Force, 2017. [Google Scholar]
- [33].O’Connor A, Validation of a decisional conflict scale, Medical decision making : an international journal of the Society for Medical Decision Making 15(1) (1995) 25–30. [DOI] [PubMed] [Google Scholar]
- [34].Degner L, Sloan J, Decision making during serious illness: what role do patients really want to play?, Journal of clinical epidemiology 45(9) (1992) 941–50. [DOI] [PubMed] [Google Scholar]
- [35].Spitzer RL, Kroenke K, Williams JB, Lowe B, A brief measure for assessing generalized anxiety disorder: the GAD-7, Archives of internal medicine 166(10) (2006) 1092–7. [DOI] [PubMed] [Google Scholar]
- [36].Volicer L, Hurley AC, Blasi ZV, Scales for evaluation of End-of-Life Care in Dementia, Alzheimer disease and associated disorders 15(4) (2001) 194–200. [DOI] [PubMed] [Google Scholar]
- [37].Hoerger M, Epstein RM, Winters PC, Fiscella K, Duberstein PR, Gramling R, Butow PN, Mohile SG, Kaesberg PR, Tang W, Plumb S, Walczak A, Back AL, Tancredi D, Venuti A, Cipri C, Escalera G, Ferro C, Gaudion D, Hoh B, Leatherwood B, Lewis L, Robinson M, Sullivan P, Kravitz RL, Values and options in cancer care (VOICE): study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers, BMC cancer 13 (2013) 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Epstein RM, Duberstein PR, Fenton JJ, Fiscella K, Hoerger M, Tancredi DJ, Xing G, Gramling R, Mohile S, Franks P, Kaesberg P, Plumb S, Cipri CS, Street RL Jr., Shields CG, Back AL, Butow P, Walczak A, Tattersall M, Venuti A, Sullivan P, Robinson M, Hoh B, Lewis L, Kravitz RL, Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial, JAMA oncology (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Choices R, Return on Investment: Implementation of Respecting Choices Model of Advance Care Planning, in: System GH (Ed.) Gundersen Health, LaCrosse, WI. [Google Scholar]
- [40].Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S, N.I.H.B.C.C. Treatment Fidelity Workgroup of the, Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium, Health Psychol 23(5) (2004) 443–51. [DOI] [PubMed] [Google Scholar]
- [41].Cagle JG, Reiff JS, Smith A, Echavarria D, Scerpella D, Zhang T, Roth DL, Hanna V, Wolff JL, A Fidelity Tool to Assess Advance Care Planning in the Context of Cognitive Impairment: The SHARE Trial, under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vaccaro L, Butow PN, Lee D, Johnson SB, Bell M, Clayton J, Detering KM, Tattersall M, Fidelity is fundamental: intervention predictors in advance care plans in terminal cancer, BMJ supportive & palliative care (2019). [DOI] [PubMed] [Google Scholar]
- [43].Seaman JB, Arnold RM, Buddadhumaruk P, Shields AM, Gustafson RM, Felman K, Newdick W, SanPedro R, Mackenzie S, Morse JQ, Chang CH, Happ MB, Song MK, Kahn JM, Reynolds CF 3rd, Angus DC, Landefeld S, White DB, Protocol and Fidelity Monitoring Plan for Four Supports. A Multicenter Trial of an Intervention to Support Surrogate Decision Makers in Intensive Care Units, Annals of the American Thoracic Society 15(9) (2018) 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kallogjeri D, Spitznagel EL Jr., Piccirillo JF, Importance of Defining and Interpreting a Clinically Meaningful Difference in Clinical Research, JAMA Otolaryngol Head Neck Surg 146(2) (2020) 101–102. [DOI] [PubMed] [Google Scholar]
- [45].Au DH, Udris EM, Engelberg RA, Diehr PH, Bryson CL, Reinke LF, Curtis JR, A randomized trial to improve communication about end-of-life care among patients with COPD, Chest 141(3) (2012) 726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Seo EH, Lee DY, Kim SG, Kim KW, Kim DH, Kim BJ, Kim MD, Kim SY, Kim YH, Kim JL, Kim JW, Moon SW, Park JH, Ryu SH, Yoon JC, Lee NJ, Lee CU, Jhoo JH, Choo LH, Woo JI, Validity of the telephone interview for cognitive status (TICS) and modified TICS (TICSm) for mild cognitive imparment (MCI) and dementia screening, Archives of gerontology and geriatrics 52(1) (2011) e26–30. [DOI] [PubMed] [Google Scholar]
- [47].Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJ, Petersen RC, Rocca WA, Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia, Neuroepidemiology 34(1) (2010) 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Jager CA, Budge MM, Clarke R, Utility of TICS-M for the assessment of cognitive function in older adults, International journal of geriatric psychiatry 18(4) (2003) 318–24. [DOI] [PubMed] [Google Scholar]
- [49].Leon A, Davis L, Kraemer H, The role and interpretation of pilot studies in clinical research, J Psychiatr Res (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lancaster GA, Campbell MJ, Eldridge S, Farrin A, Marchant M, Muller S, Perera R, Peters TJ, Prevost AT, Rait G, Trials in primary care: statistical issues in the design, conduct and evaluation of complex interventions, Statistical methods in medical research 19(4) (2010) 349–77. [DOI] [PubMed] [Google Scholar]
- [51].Braun V, Clarke V, Thematic analysis: a practical guide, SAGE, Thousand Oaks, CA, 2022. [Google Scholar]
- [52].Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM, Monetary costs of dementia in the United States, The New England journal of medicine 368(14) (2013) 1326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Satariano WA, Kealey M, Hubbard A, Kurtovich E, Ivey SL, Bayles CM, Hunter RH, Prohaska TR, Mobility Disability in Older Adults: At the Intersection of People and Places, The Gerontologist 56(3) (2016) 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wendler D, Rid A, Systematic review: the effect on surrogates of making treatment decisions for others, Annals of internal medicine 154(5) (2011) 336–46. [DOI] [PubMed] [Google Scholar]
- [55].Cagle JG, McClymont KM, Thai JN, Smith AK, “If You Don’t Know, All of a Sudden, They’re Gone”: Caregiver Perspectives About Prognostic Communication for Disabled Elderly Adults, Journal of the American Geriatrics Society 64(6) (2016) 1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].White DB, Ernecoff N, Buddadhumaruk P, Hong S, Weissfeld L, Curtis JR, Luce JM, Lo B, Prevalence of and Factors Related to Discordance About Prognosis Between Physicians and Surrogate Decision Makers of Critically Ill Patients, JAMA : the journal of the American Medical Association 315(19) (2016) 2086–94. [DOI] [PubMed] [Google Scholar]
- [57].Harrison KL, Adrion ER, Ritchie CS, Sudore RL, Smith AK, Low Completion and Disparities in Advance Care Planning Activities Among Older Medicare Beneficiaries, JAMA internal medicine 176(12) (2016) 1872–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nicholas LH, Bynum JP, Iwashyna TJ, Weir DR, Langa KM, Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia, Health affairs 33(4) (2014) 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Glasgow RE, Lichtenstein E, Marcus AC, Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition, American journal of public health 93(8) (2003) 1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C, Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact, Medical care 50(3) (2012) 217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gitlin LN, Czaja SJ, Behavioral intervention research: Designing, evaluating, and implementing, Springer Publishing; 2016. [Google Scholar]
- [62].Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M, Reenvisioning Clinical Science: Unifying the Discipline to Improve the Public Health, Clinical psychological science : a journal of the Association for Psychological Science 2(1) (2014) 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Johnson J, Odenheimer G, Dementia Performance Measurement Set: From the Physician Consortium for Performance Improvement, American Medical Association, 2011, pp. 1–79. [Google Scholar]
- [64].NASEM, Families Caring for an Aging America, National Academies of Sciences, Engineering, and Medicine, Washington, DC, 2016. [Google Scholar]
- [65].Hammes BJ, Rooney BL, Death and end-of-life planning in one midwestern community, Archives of internal medicine 158(4) (1998) 383–90. [DOI] [PubMed] [Google Scholar]
- [66].Detering KM, Hancock AD, Reade MC, Silvester W, The impact of advance care planning on end of life care in elderly patients: randomised controlled trial, Bmj 340 (2010) c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, Mitchell SL, Jackson VA, Block SD, Maciejewski PK, Prigerson HG, Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment, JAMA : the journal of the American Medical Association 300(14) (2008) 1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mack JW, Cronin A, Keating NL, Taback N, Huskamp HA, Malin JL, Earle CC, Weeks JC, Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30(35) (2012) 4387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Silveira MJ, Kim SY, Langa KM, Advance directives and outcomes of surrogate decision making before death, The New England journal of medicine 362(13) (2010) 1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Nicholas LH, Langa KM, Iwashyna TJ, Weir DR, Regional variation in the association between advance directives and end-of-life Medicare expenditures, JAMA : the journal of the American Medical Association 306(13) (2011) 1447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gobat N, Kinnersley P, Gregory JW, Robling M, What is agenda setting in the clinical encounter? Consensus from literature review and expert consultation, Patient education and counseling 98(7) (2015) 822–9. [DOI] [PubMed] [Google Scholar]
- [72].Otte-Trojel T, de Bont A, Rundall TG, van de Klundert J, How outcomes are achieved through patient portals: a realist review, Journal of the American Medical Informatics Association : JAMIA 21(4) (2014) 751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wolff JL, Aufill J, Echavarria D, Blackford AL, Connolly RM, Fetting JH, Jelovac D, Papathakis K, Riley C, Stearns V, Zafman N, Thorner E, Levy HP, Guo A, Dy SM, Wolff AC, A randomized intervention involving family to improve communication in breast cancer care, NPJ Breast Cancer 7(1) (2021) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


