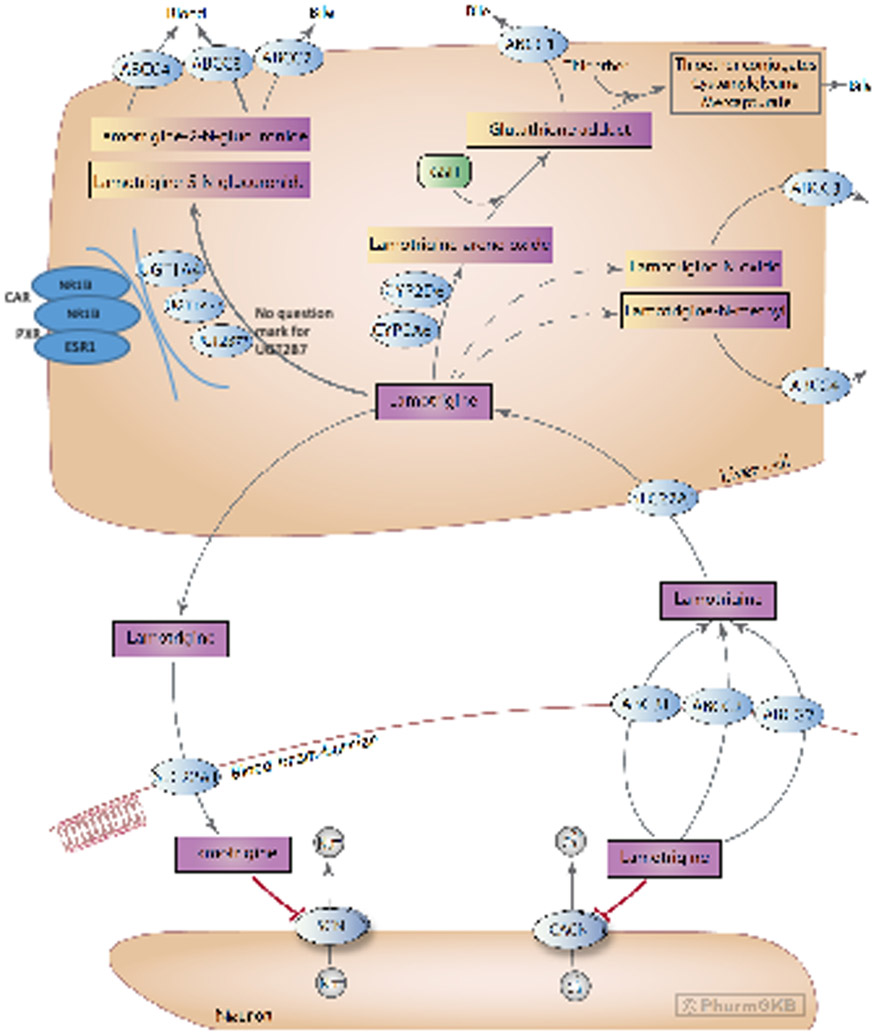
Background
Lamotrigine (LTG) is an antiseizure drug (ASD) which was approved by the US Food and Drug Administration (FDA) in 1994 to treat focal (partial) seizures, primary generalized tonic-clonic seizures, and generalized seizures of Lennox-Gastaut syndrome in both children and adults. It was evaluated for several mood disorders and subsequently also approved for maintenance of bipolar disorder in adults [1-5]. LTG was originally synthesized at Wellcome Laboratories as an antifolate analog, but screening in animal seizure models revealed potent activity and it was developed to improve upon existing antiepileptic drugs for refractory patients with a better safety and drug interaction profile [6]. LTG is a 2,5-diamino-triazine ASD which blocks voltage-gated sodium channels (VGSCs) [7-12] and voltage-gated calcium channels (VGCCs) [7, 13-15] both of which may contribute to the antiseizure activity of this drug. The common adverse effects for LTG are dizziness, diplopia, headache, ataxia, blurred vision, nausea, somnolence, vomiting, and hypersensitive skin rashes including severe reactions such as Stevens-Johnson syndrome (10% rash incidence) [16, 17]. Risk of severe cutaneous adverse reactions (Stevens-Johnson syndrome/Toxic Epidermal Necrolysis) due to LTG is related to HLA-B polymorphisms, including HLA-B*15:02 mostly in Asian (Han Chinese) population (p<0.05) [18-22]. Further, one study reported that HLA-B*15:02 allele was present in 33.3% of lamotrigine-induced SJS/TEN cases whereas 9.4% in lamotrigine-tolerant controls (P < 0.05,), however the sample size was small [23].
In this article we review the metabolic pathways and mechanism of action of LTG, along with a comprehensive summary of polymorphisms in pathway genes contributing to the inter-individual variability in LTG clearance and response.
Pharmacokinetics
A schematic representation of LTG disposition within the body is provided in Figure 1. The mechanism by which LTG crosses the blood-brain barrier (BBB) is not completely understood, but studies have implicated a role for organic cation transporters (OCT) [24, 25]. An in vitro study showed that LTG is a substrate for SLC22A1 (also known as OCT1) [24], and polymorphisms in the SLC22A1 gene have been associated with concentration differences in Chinese patients with epilepsy [25].
Stylized diagram showing lamotrigine metabolism in the liver and its mechanism of action at neurons. A fully clickable version of this figure can be found at https://www.pharmgkb.org/pathway/PA166183755.
Although controversial, a current hypothesis for pharmaco-resistant epilepsy implicates overexpression of efflux transporters at the BBB [26]. There is conflicting evidence for the role of the ATP-binding cassette (ABC) superfamily of transporters in LTG efflux, specifically P-glycoprotein (P-gp), encoded by the ABCB1 (MDR1) gene [26-28] and ABCG2 (BCRP), both located on the apical capillary endothelial membrane [25, 28-31]. Romermann and colleagues recently reported that LTG showed a ABCG2 mediated transport, where LTG exhibited a significant difference in the absence and presence of ABCG2 inhibition (P < 0.0001) [29]. Mechanistic study of Löscher and others reported that LTG was an efficient BCRP (ABCG2) substrate in transfected MDCK cells [29, 56]. A comprehensive literature review concluded that LTG was a P-gp substrate based on a combination of data from in vitro, in vivo, and predicted structure-activity relationship studies [26, 27, 32]. Therefore this drug is the first ASD which was identified as a dual substrate of the two major human efflux transporters at the BBB (synergistic or cooperative role of P-gp and ABCG2 in the efflux of dual substrates at the BBB) [29]. In contrast, Nakanishi et al. reported no difference in total brain-to-plasma concentration ratios of LTG in Mdr1a/1b/Bcrp triple-knockout mice [31]. Despite inconsistencies in model system results, correlations have been identified between polymorphisms in these genes and LTG concentrations in plasma; thus, transporters may play an important role for differential drug response [33-38]. Additionally, polymorphisms in ABCC2 (MRP2) are associated with drug resistance in different populations, which indicates this transporter may also play a role in drug efflux [39-41].
ABCC3 (MRP3) is located on the sinusoidal membrane of hepatocytes separating the cytosol from the bloodstream, and transports glucuronides from the liver cells into the bloodstream for elimination by the kidney [42, 43]. Since 80% of LTG glucuronides are eliminated in urine and the 2-N quaternary ammonium glucuronide is not able to cross membranes, ABCC3 or ABCC4 (MRP4) are likely responsible for transport from the liver to the blood [42-45]. In contrast, ABCC2 (MRP2) effluxes glutathione adducts from the liver cell out to the bile [46, 47]. Glucuronides may also be excreted by ABCC2 into bile, but the major route of excretion is via the urine. Given the ongoing disagreement in results, further research is needed for complete understanding of the actual role of efflux transporters in ASD treatment.
Previous studies mentioned that LTG is extensively metabolized, with over 80% of the total dose recovered in the urine [48-50]. Three main metabolites of LTG have been observed in humans: LTG-2-N-glucuronide, LTG-5-N-glucuronide, and a minor LTG-N-oxide [51-54]. Another minor metabolite, LTG-N-methyl, was also detected in human urine and in dogs but is a minor metabolite in human urine [51]. LTG-2-N-glucuronide is the major metabolite eliminated from the body with some 5-N-glucuronide, making up 80-90% of the drug recovered in urine; N-oxide was also detected in patients taking LTG along with the parent drug [50, 51, 55]. LTG-2-N-glucuronide is an inactive metabolite primarily formed by UDP-glucuronosyltransferase (UGT) 1A4 (UGT1A4) [52, 53, 56, 57]. Additional studies suggest that UGT1A3 (minor compared to 1A4) and UGT2B7 (based on in vitro inhibition with zidovudine [56]) may also play a role in the metabolism of LTG, though contradictory evidence exists because glucuronidation was not confirmed with cloned, expressed UGT2B7 [53]. However, there are studies reported that polymorphisms (such as UGT2B7_−161C>T, UGT2B7 372 GG) in UGT2B7 have been associated (p< 0.05) with LTG concentration-to-dose ratio and clearance which suggests a possible influence of UGT2B7 in LTG metabolism [58-61].
Previous studies have also reported a minor pathway of LTG bioactivation to a reactive arene oxide on the dichlorophenyl ring [55, 62]. The P450 isoenzymes mainly responsible for the formation of the arene oxide are CYP2A6 and CYP2D6 [54, 55, 62]. Chen et al. identified a glutathione conjugate in human and rat liver microsomal incubations in the presence of NADPH and glutathione (GSH) [62]. Four radiolabeled metabolites were identified and quantified radiometrically in rat bile (biliary metabolites) by HPLC; of these four, the most polar metabolite was the protonated molecule of a glutathione adduct of LTG (i.e. the primary thioether addition product of an arene-oxide). Glutathione-derived adducts produced thioether conjugates, such as a cysteinyl-glycine adduct of LTG and a cysteine adduct of LTG. [55, 62]. However, glutathione-derived N-acetylcysteine conjugates (mercapturic acids) have not been measured in human urine [55, 62]. The enzyme likely responsible for formation of mercapturic acids from cysteine S-conjugates has been identified as NAT8 [63]. Formation of reactive metabolites (formed from the arene oxide) has also been observed in keratinocytes and may be responsible for mild skin rashes and more severe cutaneous reactions observed with LTG administration [46, 62].
One study reported that concomitant administration with UGT-inducing antiseizure drugs such as phenytoin, phenobarbital, or carbamazepine, the elimination half-life of LTG decreases by ~40-50% [64]. In contrast, another study reported that valproic acid (VPA) inhibits LTG metabolism, increasing half-life linearly (from 24 h to ~72 h) with increasing dose [65]. Addition of VPA may counteract the change in kinetics caused by co-administered UGT-inducing antiseizure drugs [64]. In vitro studies with VPA have shown the increased area under the LTG plasma-concentration time curve to be due to competitive inhibition of UGT1A4 or UGT2B7 by VPA. [56]. VPA has higher affinity for UGT2B7, but it is also a substrate of UGT1A4, albeit with a higher Km of 3.1 mM, compared to 1.2 mM for UGT2B7 [53, 66]. Thus, the LTG-VPA interaction may be mediated by competitive binding to these two UGT isoforms.
Decreased LTG plasma concentrations caused by an increase in drug clearance have been reported during pregnancy [67-71]. A population pharmacokinetic study identified two subpopulations of women who experience different changes in LTG clearance during pregnancy, which may indicate that a genetic mutation is responsible for these differences [61, 72]. It is hypothesized that the increase in 17β-estradiol during pregnancy results in activation of estrogen-receptor-α (encoded by the ESR1 gene) in the liver, resulting in upregulation of UGT1A4 expression and increased LTG clearance [73]. However, evidence for direct binding of estradiol to estrogen response elements in the UGT1A4 promoter is lacking, but an indirect effect of ERα on the SP1 transcription factor (specificity protein-1) is more likely responsible for the induction observed during pregnancy [73]. Furthermore, many studies showed similar increases in LTG clearance occurs with concomitant use of oral contraceptives containing estrogen [74-76], but not in progesterone-only based contraceptives [77]. An additional piece of evidence includes the observation that UGT1A4 & UGT2B7 expression correlate with ESR1 expression [78, 79].
Expression of genes in the UGT1A and UGT2B families are also induced by activators of two nuclear receptors pregnane-X-receptor (NR1I2, also known as PXR), and constitutive androstane receptor (NR1I3, also known as CAR) [79-81]. Many studies showed that, UGT1A4 specifically has been shown to be co-regulated by CAR, PXR, and aryl hydrocarbon receptor (AhR) [78, 81-84], while UGT2B7 is co-regulated by CAR and PXR [78, 79, 81, 85]. Carbamazepine, phenytoin, and phenobarbital are prototypical CAR activators e.g. leading to induction of CYP2B6, and these drugs increase LTG when co-administered for epileptic seizures [57].
Pharmacodynamics
A stylized depiction of the potential mechanism of action of LTG is provided in Figure 1. The exact mechanism through which LTG elicits its therapeutic effect is unknown; however, a likely mechanism is through antagonizing type 2 voltage-gated sodium channels (VGSCs; encoded by the SCN gene family) [7-9], similar to the mechanism of the older antiseizure drugs phenytoin and carbamazepine [8, 86]. VGSCs are large membrane-spanning proteins consisting of a large alpha subunit, which sometimes interacts with a smaller regulatory beta subunit. At resting potential, the ion pore exists in the closed state, but once neuronal membranes containing VGSCs are sufficiently depolarized (typically by an action potential), there is a structural change in the protein causing the ion pore to open. Within milliseconds the inactivation loop moves to block the flow of ions through the pore, during which point the channel is said to be inactivated. LTG preferentially binds to the inactivated state of VGSCs, acting as an antagonist [10, 87, 88]. The binding site for LTG is located on the extracellular side of the alpha subunit and is shared by carbamazepine and phenytoin [11].
LTG also acts as an antagonist to high-voltage-activated N-, P-, and Q-type calcium channels (VGCCs; encoded by the CACN gene family) [7, 13-15, 89], which may be an additional mechanism through which LTG elicits its antiseizure properties. VGCCs are structurally similar to VGSCs, however they do not have the intracellular inactivation loop present in VGSCs. As such, VGCCs can only exist in an open and closed state, and they are normally closed at the resting membrane potential [90, 91]. The blockade of voltage-gated sodium and calcium channels modulate the release of neurotransmitters. Studies consistently show a reduction in release of the excitatory neurotransmitter, glutamate, upon LTG administration [92-97]. However, LTG has been reported to elicit both increased [92, 98] and decreased [7, 93-95] release of the inhibitory neurotransmitter GABA. The role of calcium as a second messenger within the cell may also lead to multifactorial changes as a result of altered ion flux. For example, an in vitro study using primary mouse neuronal cultures found CaM kinase II activity to be affected as a result of LTG administration, leading to altered intracellular calcium concentrations [99]. Further studies may fully elucidate the downstream effects of LTG administration and their role in the observed antiseizure activity.
Pharmacogenomics
Metabolizing enzyme variants
Inter-individual variability in clinical efficacy or adverse effects following treatment with LTG has been shown to be associated with genetic variants within drug metabolizing enzymes, drug transporters, and drug targets [59, 100, 101]. As LTG is metabolized by UGTs, single-nucleotide polymorphisms (SNPs) in UGT1A4 and UGT2B7 may play a role in the inter-individual variability in LTG metabolism [100, 102]. Two promoter polymorphisms in UGT1A4, −219C>T (rs3732219) and −163G>A (rs3732218), have been associated with altered LTG pharmacokinetics [103]. Several studies reported that these two promoter SNPs in the 5’-untranslated region of UGT1A4 (rs3732219, rs3732218) are in high linkage disequilibrium with rs2011425 (Leu48Val) with a minor allele frequency (MAF) of 0.082 in Europeans, 0.226 in East Asians and 0.102 in Africans (gnomAD, dbSNP) [103-105].Further, studies showed that these polymorphisms are associated with lower enzymatic activity (p<0.05) as well as significantly higher LTG concentration (p<0.01) as compared to wild-type [103, 106]. Mechanistic study showed that, the two UGT1A4 promoter polymorphisms (rs3732218 −163G>A allele and rs373221 −219C>T allele) have been associated with a reduction in basal UGT1A4 luciferase reporter activity by 40-50% in MCF7 breast cancer cells and 30-40% in HepG2 hepatoma cells. [106, 107]. Additionally, UGT1A4 142T>G (rs2011425) in the coding region was significantly associated with increased LTG concentrations, lower LTG clearance, and better efficacy in treating epilepsy for patients with the TT genotype compared to GT and GG genotypes [101, 108-111]. Further, in vitro studies with cloned, expressed UGT1A4 variant enzymes with either the 142 A>G (Leu48Val, UGT1A4*3) or the Pro24Thr (UGT1A4*2) mutation had approximately 50% reduced intrinsic clearance (Vmax/Km) compared to the wild-type enzyme [110]. Several studies reported that pregnancy increases LTG clearance by >50% and UGT1A4 rs2011425 (UGT1A4*3) was associated with reductions in the LTG concentration-to-dose ratio (C/D ratio) during pregnancy [61, 68, 73]. The rs2011425 polymorphism also showed significant effects on efficacy in pediatric epilepsy patients treated with LTG [112]. Mechanistic study described that, individuals who are homozygous for the rs2011425 GG genotype show higher glucuronidation activity compared to individuals with the TT genotype using human liver microsomes isolated from 80 genotyped livers [83, 113].The minor allele frequency of rs2011425 is 0.082 in Europeans, 0.101 in Africans, and 0.213 in East Asians (gnomAD, dbSNP). Saeki et al. defined the UGT1A4*3a haplotype (containing rs3732219, rs3732218, rs2011425 together with the synonymous SNPs 448T>C (L150L), 804G>A (P268P) and IVS1+43C>T) with a frequency of 12.5%, whereas the rare UGT1A4*7a haplotype (MAF = 0.002) is comprised of rs3732219, rs3732218, rs2011425 plus 448T>C, 804G>A and 1VS1+43C>T together with the non-synonymous 271C>T (R91C) [105, 114]. Although information on SNPs and haplotypes of UGT1A4 is available {https://www.pharmacogenomics.pha.ulaval.ca/ugt-alleles-nomenclature/}, unfortunately, the impact of the different haplotypes on LTG pharmacokinetics has not been comprehensively investigated. UGT1A4 70C>A (Pro24Thr, rs6755571), located at the end of the signal peptide with an MAF of 0.052 in Europeans, 0.000 in East Asians, and 0.015 in Africans (gnomAD, dbSNP), was associated with higher LTG serum concentrations and lower clearance in vitro even during pregnancy [61, 101, 109, 110]. The rare UGT1A4 1091C>T SNP (rs34946978, Pro365Leu) located in the UDPGA binding region of all UGT1A isoforms was associated with a general reduction in glucuronidation activity of the entire UGT1A family (MAF = 0.011 in East Asian, <0.0002 in Africans and Europeans) [115, 116].
Contradictory evidence exists for a role for UGT2B7 in the formation of the major metabolite LTG-2-N-glucuronide [56]. No activity with commercial cloned, expressed UGT2B7 in Supersomes® was found (RP Remmel, personal communication). Polymorphisms in this gene may play a role in inter-individual variability in LTG concentrations and dose in patients with epilepsy [100, 102]. In a study (n = 53), the SNP −161C>T (rs7668258) SNP in UGT2B7 was found to be significantly associated with lower LTG concentration-to-dose ratios in epilepsy patients with the TT genotype compared to patients with the CC genotype [58]. These findings were corroborated by another small study from Thailand (n = 75) that found that the TT and CT genotypes had on average 18% lower clearance than patients carrying the CC genotype [59]. Patients with epilepsy on stable dosing with LTG with the UGT2B7 −161G>T (rs7668258) TT genotype had a reduced clearance compared with the GT and GG genotypes. Clearance was 247% higher in Slovenian epilepsy patients with the UGT2B7 −372A>G GG genotype as compared to the AA genotype [60]. Furthermore, a case study of a 38-year-old woman treated with LTG suggests that the UGT2B7 −372A>G polymorphism may have a role in body rash and multi-organ failure [117]. A recent study reported that UGT2B7 802C>T (rs7439366) was associated with reductions of LTG concentration-to-dose ratio (C/D ratio) during pregnancy [61].
Transporter variants
ATP-binding cassette efflux transporters are overexpressed at the BBB, where they reduce the penetration of ASDs into the brain. ABCB1 and ABCG2 have been implicated to play a key role in LTG transport. This would suggest that genetic variants of drug transporter genes associated with functional variations in efflux activity may contribute to the inter-individual variation in ASD drug resistance [33, 35, 118-120].
Polymorphisms in transporter proteins have indeed been shown to significantly influence pharmacokinetics and bioavailability of many drugs. There is several evidence that ABCB1 1236 C>T (rs1128503), 2677 G>T/A (rs2032582), and the synonymous, high frequency 3435 C>T SNP (rs1045642) influence LTG serum concentration (1236C-2677G-3435C carriers had higher LTG concentrations than 1236T-2677G-3435T carriers followed by 1236T-2677T-3435C carriers) and drug response in patients [33-35, 38, 121]. Polymorphisms in ABCG2 (rs2231142 and rs3114020) were found to be significantly associated with LTG concentration dose-normalized by body weight [29, 38]. A patient’s cohort study with 131 LTG monotherapy patients reported that presence of ABCG2 421C>A (rs2231142) resulted in modestly lower LTG trough concentration (CI-95%) compared to wild type [122]. Further, ABCG2 rs2231142 was also shown to be responsible for approximately 4.8% of the variability in LTG trough concentration variation in Chinese epilepsy patients [25, 38]. Additionally, ABCC2 −24C>T (rs717620) polymorphism in exon 1 was reported to be associated with resistance to LTG (P<0.001) and other ASDs (VPA, phenobarbital, carbamazepine, and oxcarbazepine) in a German-Caucasian as well as an Asian patient cohort, hypothesized to be a result of compensatory upregulation of ABCB1 [39, 41]. Further, linkage disequilibrium (LD) test showed that the ABCC2 rs717620 were in strong LD with rs2273697 (D'= 0.694) and rs3740066 (D'= 0.699) and frequencies of haplotypes (ABCC2 −24C>T/ABCC2 1249G>A/ABCC2 3972C>T) in resistant patients was significantly higher (P < 0.05) in Chinese epilepsy patients (n=537) [41] .However, ABCC2 −24C>T was not associated with drug resistance in Han Chinese, Croatian, or Austrian-Caucasian epilepsy patients [123-125].
Opposing the force of efflux transporters are influx transporters that act to carry LTG across the BBB into the brain. One such transporter is SLC22A1. A study of Chinese epilepsy patient’s cohort (n= 112) found an association between plasma concentrations of LTG and SLC22A1 1A>G (rs628031) genotype, GG genotype having significantly lower LTG dose-normalized concentrations in plasma (P<0.05) which indicated that polymorphisms in the SLC22A1 gene may have association with LTG concentration differences [25].
Pharmacodynamic variants
The primary hypothesis for the antiseizure effect of LTG is through binding to voltage-gated sodium channels [7-9]. Voltage-gated sodium channels are heteromeric complexes that regulate sodium exchange between intracellular and extracellular spaces. The alpha subunits are encoded by the SCN gene, of which there are four predominant isoforms in the human brain: SCN1A, SCN2A, SCN3A, and SCN8A. A polymorphism in SCN1A, 1G>A (rs3812718), was significantly associated with effective dose of LTG. Peak plasma concentrations corresponded to effective doses were almost half for wild type compared to variant [126]. At the time of writing, polymorphisms in the SCN gene and their influence on ASDs including LTG had not been not well investigated.
Although not thought to be a direct target of LTG, GABA receptors are the principal inhibitory receptor in the central nervous system (CNS), and heterogeneity in the ionotropic GABAA receptor is associated with epilepsy [127]. Several antiseizure drugs like barbiturates and benzodiazepine-like agents bind to GABAA and cause alteration in receptor subunits to regulate drug response [128]. Polymorphisms of the genes encoding different subunits of GABAA receptors may be associated with ASD response and resistance. GABRA1 rs6883877, GABRA1 rs1157122, GABRA1 rs6892782, GABRA1 rs10068980, GABRA2 rs511310, GABRA3 rs4828696, and GABRA3 rs1112122 were all found to be significantly associated with resistance to LTG, along with other ASDs such as carbamazepine, phenytoin, and VPA [129]. However, due to a lack of studies, the influence of variations in GABA receptors is not well understood, therefore they are not represented in Figure 1.
Variants associated with adverse effects
Drug-induced skin injuries, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug-induced hypersensitivity syndrome (DIHS) are all reported LTG-related adverse events [130-138]. SJS/TEN in LTG-treated pediatric patients (n=486) was reported in many studies and 97% of cases occurred within 8 weeks of initiation of LTG therapy, with a median time to onset of 15 days [139]. Human leukocyte antigen (HLA) genes produce proteins which identify foreign particles in the immune system [140]. The complex of drug antigen/metabolite-human leukocyte antigen (HLA)-T cell receptor (TCR) initiates immune reactions for SJS/TEN. Specific HLA alleles or variant predisposition and interaction with a drug allow the presentation of drug-bound HLA to the TCR, which further triggers the activation of CD8+ cytotoxic lymphocytes and a series of specific immune reactions which ultimately cause keratinocyte apoptosis and severe drug-induced skin injuries [141-145]. HLA-B genotypes and LTG-induced cutaneous adverse drug reaction (LTG-cADR) associations have been described in several reports. HLA-B*15:02 is associated with SJS in response to LTG administration in multiple populations [18-23]. Another study reported that HLA-B*38:01 was significantly associated with LTG-related SJS/TEN in a Spanish Caucasian population (p<0.001) [146]. HLA-DRB1*04:05 and HLA-DQB1*04:01 alleles occurred in a higher frequency in Japanese patients with LTG-cADRs. These two alleles are in linkage disequilibrium in this population, along with HLA-DQA1*03:03, which is also associated with LTG-cADRs [147]. Studies in Korean populations assume a relationship between HLA-B*44:03 and LTG-induced SJS/TEN (odds ratio: 12.75; CI 1.03-157.14; p=0.053), however the number of patients for these study was very low (n=9, n=5 respectively) [148, 149] and further detailed study is need to explore the role of HLA-B*44:03 in LTG induce SJS/TEN. HLA-A*24:02 allele and LTG-induced maculopapular eruptions (MPE) (OR 3.949, p=0.005) [150, 151]. Additionally, HLA-A*24:02 showed significant association with DRESS (drug reactions with eosinophilia and systemic symptoms), in a Spanish Caucasian population (p<0.001, n=12) [146]. Furthermore, a recent study reported HLA-A*24:02 was also associated significantly with SJS induced by LTG in a southern Han Chinese population (p = 0.005, n=91) [151]. Two other novel SNPs, rs12668095 near CRAMP1L/TMEM204 and rs79007183 near TNS3, were associated (P=4.89×10(−7), P=3.15×10(−10) respectively) with LTG-induced MPE in a Korean population (n=34 discovery cohort and n=59 validation cohort) [152]. HLA-A*02:01:01/-B*35:01:01/-C*04:01:01 haplotypes were also associated with LTG-induced MPE in a Mexican Mestizo population (p<0.0001, though n=21) [153]. A recent study in Thai patients reported that HLA-A*02:07 and HLA-B*15:02 allele carriers were significantly higher in the LTG-induced skin injuries group than in tolerant controls. Additionally, HLA-A*33:03, HLA-B*15:02, and HLA-B*44:03 were significantly higher in the LTG-induced MPE group (though the study only included a small group of patients), and the authors note that these alleles could be useful screening markers for preventing drug-induced skin injuries before LTG treatment in Thai patients [154]. Another recent study found that frequency of the HLA-A*31:01 allele was significantly higher (p<0.001, n>50) in the LTG-induced SCAR (severe cutaneous adverse reactions) group compared to the LTG-tolerant group in Korean population. Therefore, HLA-A*31:01 might be a risk allele for LTG-induced SCAR in Korean population [155].
Conclusions
There are several pharmacogenomic studies that reveal important aspects of pharmacokinetics, pharmacodynamics, and mode of action of LTG; however, complete understanding of PK/PD and mechanism of action of LTG through further research is still necessary to improve its therapeutic efficacy. The primary motive for utilizing pharmacogenetics in administration of LTG lies in the ability to understand the influence of genetic polymorphisms in the pharmacokinetics and pharmacodynamics of the drug being metabolized. This will allow physicians to optimize therapeutic doses for patients to provide maximum efficacy and optimal seizure control. We have outlined likely gene candidates in this article, but further studies need to be done to identify a specific set of clinically relevant gene signatures.
Acknowledgements
This work was supported by NIH/NIGMS grant R24 GM61374, NIH/NINDS grant P50 NS16308, the Epilepsy Foundation, and the Patricia L Nangle Fund.
Footnotes
Conflicts of Interest: RBA is a stockholder in Personalis Inc. and 23andMe, and a paid advisor for Youscript. The remaining authors have no conflicts to declare.
References
- 1.Eriksson AS, Nergardh A, and Hoppu K, The efficacy of lamotrigine in children and adolescents with refractory generalized epilepsy: a randomized, double-blind, crossover study. Epilepsia, 1998. 39(5): p. 495–501. [DOI] [PubMed] [Google Scholar]
- 2.Jawad S, Richens A, Goodwin G, and Yuen WC, Controlled trial of lamotrigine (Lamictal) for refractory partial seizures. Epilepsia, 1989. 30(3): p. 356–63. [DOI] [PubMed] [Google Scholar]
- 3.Messenheimer J, Ramsay RE, Willmore LJ, Leroy RF, Zielinski JJ, Mattson R, et al. , Lamotrigine therapy for partial seizures: a multicenter, placebo-controlled, double-blind, cross-over trial. Epilepsia, 1994. 35(1): p. 113–21. [DOI] [PubMed] [Google Scholar]
- 4.Veggiotti P, Cieuta C, Rex E, and Dulac O, Lamotrigine in infantile spasms. Lancet, 1994. 344(8933): p. 1375–6. [DOI] [PubMed] [Google Scholar]
- 5.Besag FM, Dulac O, Alving J, and Mullens EL, Long-term safety and efficacy of lamotrigine (Lamictal) in paediatric patients with epilepsy. Seizure, 1997. 6(1): p. 51–6. [DOI] [PubMed] [Google Scholar]
- 6.Weisler RH, Calabrese JR, Bowden CL, Ascher JA, DeVeaugh-Geiss J, and Evoniuk G, Discovery and development of lamotrigine for bipolar disorder: a story of serendipity, clinical observations, risk taking, and persistence. J Affect Disord, 2008. 108(1-2): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 7.Lees G and Leach MJ, Studies on the mechanism of action of the novel anticonvulsant lamotrigine (Lamictal) using primary neurological cultures from rat cortex. Brain Res, 1993. 612(1-2): p. 190–9. [DOI] [PubMed] [Google Scholar]
- 8.Qiao X, Sun G, Clare JJ, Werkman TR, and Wadman WJ, Properties of human brain sodium channel alpha-subunits expressed in HEK293 cells and their modulation by carbamazepine, phenytoin and lamotrigine. Br J Pharmacol, 2014. 171(4): p. 1054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakatani Y, Masuko H, and Amano T, Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci, 2013. 123(2): p. 203–6. [DOI] [PubMed] [Google Scholar]
- 10.Kuo CC and Lu L, Characterization of lamotrigine inhibition of Na+ channels in rat hippocampal neurones. Br J Pharmacol, 1997. 121(6): p. 1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo CC, A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Mol Pharmacol, 1998. 54(4): p. 712–21. [PubMed] [Google Scholar]
- 12.Yang YC, Hsieh JY, and Kuo CC, The external pore loop interacts with S6 and S3-S4 linker in domain 4 to assume an essential role in gating control and anticonvulsant action in the Na(+) channel. J Gen Physiol, 2009. 134(2): p. 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefani A, Spadoni F, Siniscalchi A, and Bernardi G, Lamotrigine inhibits Ca2+ currents in cortical neurons: functional implications. Eur J Pharmacol, 1996. 307(1): p. 113–6. [DOI] [PubMed] [Google Scholar]
- 14.von Wegerer J, Hesslinger B, Berger M, and Walden J, A calcium antagonistic effect of the new antiepileptic drug lamotrigine. Eur Neuropsychopharmacol, 1997. 7(2): p. 77–81. [DOI] [PubMed] [Google Scholar]
- 15.Grunze H, von Wegerer J, Greene RW, and Walden J, Modulation of calcium and potassium currents by lamotrigine. Neuropsychobiology, 1998. 38(3): p. 131–8. [DOI] [PubMed] [Google Scholar]
- 16.Pellock JM, Overview of lamotrigine and the new antiepileptic drugs: the challenge. J Child Neurol, 1997. 12 Suppl 1: p. S48–52. [DOI] [PubMed] [Google Scholar]
- 17.Bourgeois BF, New antiepileptic drugs. Arch Neurol, 1998. 55(9): p. 1181–3. [DOI] [PubMed] [Google Scholar]
- 18.Man CB, Kwan P, Baum L, Yu E, Lau KM, Cheng AS, et al. , Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia, 2007. 48(5): p. 1015–8. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Ye YM, Palikhe NS, Kim JE, and Park HS, Genetic and ethnic risk factors associated with drug hypersensitivity. Curr Opin Allergy Clin Immunol, 2010. 10(4): p. 280–90. [DOI] [PubMed] [Google Scholar]
- 20.Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MH, and Kwan P, HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia, 2013. 54(7): p. 1307–14. [DOI] [PubMed] [Google Scholar]
- 21.Hassan I, HLA-B*1502 screening in carbamazepine and lamotrigine candidates of Asian background. Aust N Z J Psychiatry, 2012. 46(11): p. 1106–7. [DOI] [PubMed] [Google Scholar]
- 22.Neuman MG, Cohen L, Nanau RM, and Hwang PA, Genetic and immune predictors for hypersensitivity syndrome to antiepileptic drugs. Transl Res, 2012. 159(5): p. 397–406. [DOI] [PubMed] [Google Scholar]
- 23.Zeng T, Long YS, Min FL, Liao WP, and Shi YW, Association of HLA-B*1502 allele with lamotrigine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese subjects: a meta-analysis. Int J Dermatol, 2015. 54(4): p. 488–93. [DOI] [PubMed] [Google Scholar]
- 24.Dickens D, Owen A, Alfirevic A, Giannoudis A, Davies A, Weksler B, et al. , Lamotrigine is a substrate for OCT1 in brain endothelial cells. Biochem Pharmacol, 2012. 83(6): p. 805–14. [DOI] [PubMed] [Google Scholar]
- 25.Shen CH, Zhang YX, Lu RY, Jin B, Wang S, Liu ZR, et al. , Specific OCT1 and ABCG2 polymorphisms are associated with Lamotrigine concentrations in Chinese patients with epilepsy. Epilepsy Res, 2016. 127: p. 186–190. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Kwan P, Zuo Z, and Baum L, The transport of antiepileptic drugs by P-glycoprotein. Adv Drug Deliv Rev, 2012. 64(10): p. 930–42. [DOI] [PubMed] [Google Scholar]
- 27.Luna-Tortos C, Fedrowitz M, and Loscher W, Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology, 2008. 55(8): p. 1364–75. [DOI] [PubMed] [Google Scholar]
- 28.Rivers F, O'Brien TJ, and Callaghan R, Exploring the possible interaction between anti-epilepsy drugs and multidrug efflux pumps; in vitro observations. Eur J Pharmacol, 2008. 598(1-3): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 29.Romermann K, Helmer R, and Loscher W, The antiepileptic drug lamotrigine is a substrate of mouse and human breast cancer resistance protein (ABCG2). Neuropharmacology, 2015. 93: p. 7–14. [DOI] [PubMed] [Google Scholar]
- 30.Cerveny L, Pavek P, Malakova J, Staud F, and Fendrich Z, Lack of interactions between breast cancer resistance protein (bcrp/abcg2) and selected antiepileptic agents. Epilepsia, 2006. 47(3): p. 461–8. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi H, Yonezawa A, Matsubara K, and Yano I, Impact of P-glycoprotein and breast cancer resistance protein on the brain distribution of antiepileptic drugs in knockout mouse models. Eur J Pharmacol, 2013. 710(1-3): p. 20–8. [DOI] [PubMed] [Google Scholar]
- 32.Loscher W, Luna-Tortos C, Romermann K, and Fedrowitz M, Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? Problems and approaches in determining which antiepileptic drugs are affected. Curr Pharm Des, 2011. 17(26): p. 2808–28. [DOI] [PubMed] [Google Scholar]
- 33.Lovric M, Bozina N, Hajnsek S, Kuzman MR, Sporis D, Lalic Z, et al. , Association between lamotrigine concentrations and ABCB1 polymorphisms in patients with epilepsy. Ther Drug Monit, 2012. 34(5): p. 518–25. [DOI] [PubMed] [Google Scholar]
- 34.Hung CC, Chen CC, Lin CJ, and Liou HH, Functional evaluation of polymorphisms in the human ABCB1 gene and the impact on clinical responses of antiepileptic drugs. Pharmacogenet Genomics, 2008. 18(5): p. 390–402. [DOI] [PubMed] [Google Scholar]
- 35.Bialecka M, Hnatyszyn G, Bielicka-Cymerman J, and Drozdzik M, [The effect of MDR1 gene polymorphism in the pathogenesis and the treatment of drug-resistant epilepsy]. Neurol Neurochir Pol, 2005. 39(6): p. 476–81. [PubMed] [Google Scholar]
- 36.Leschziner G, Jorgensen AL, Andrew T, Pirmohamed M, Williamson PR, Marson AG, et al. , Clinical factors and ABCB1 polymorphisms in prediction of antiepileptic drug response: a prospective cohort study. Lancet Neurol, 2006. 5(8): p. 668–76. [DOI] [PubMed] [Google Scholar]
- 37.Szoeke C, Sills GJ, Kwan P, Petrovski S, Newton M, Hitiris N, et al. , Multidrug-resistant genotype (ABCB1) and seizure recurrence in newly treated epilepsy: data from international pharmacogenetic cohorts. Epilepsia, 2009. 50(7): p. 1689–96. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Wang X, Li H, Zhang J, Chen Z, Xie W, et al. , Polymorphisms of ABCG2, ABCB1 and HNF4alpha are associated with Lamotrigine trough concentrations in epilepsy patients. Drug Metab Pharmacokinet, 2015. 30(4): p. 282–7. [DOI] [PubMed] [Google Scholar]
- 39.Ufer M, Mosyagin I, Muhle H, Jacobsen T, Haenisch S, Hasler R, et al. , Non-response to antiepileptic pharmacotherapy is associated with the ABCC2 −24C>T polymorphism in young and adult patients with epilepsy. Pharmacogenet Genomics, 2009. 19(5): p. 353–62. [DOI] [PubMed] [Google Scholar]
- 40.Qian L, Fang S, Yan YL, Zeng SS, Xu ZJ, and Gong ZC, The ABCC2 c.−24C>T polymorphism increases the risk of resistance to antiepileptic drugs: A meta-analysis. J Clin Neurosci, 2017. 37: p. 6–14. [DOI] [PubMed] [Google Scholar]
- 41.Qu J, Zhou BT, Yin JY, Xu XJ, Zhao YC, Lei GH, et al. , ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci Ther, 2012. 18(8): p. 647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, and Brouwer KL, Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur J Pharm Sci, 2006. 27(5): p. 447–86. [DOI] [PubMed] [Google Scholar]
- 43.Keppler D, Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol, 2011(201): p. 299–323. [DOI] [PubMed] [Google Scholar]
- 44.Zamek-Gliszczynski MJ, Chu X, Polli JW, Paine MF, and Galetin A, Understanding the transport properties of metabolites: case studies and considerations for drug development. Drug Metab Dispos, 2014. 42(4): p. 650–64. [DOI] [PubMed] [Google Scholar]
- 45.Keppler D and Konig J, Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis, 2000. 20(3): p. 265–72. [DOI] [PubMed] [Google Scholar]
- 46.Ballatori N, Krance SM, Marchan R, and Hammond CL, Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol Aspects Med, 2009. 30(1-2): p. 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole SP and Deeley RG, Transport of glutathione and glutathione conjugates by MRP1. Trends Pharmacol Sci, 2006. 27(8): p. 438–46. [DOI] [PubMed] [Google Scholar]
- 48.Remmel RP and Sinz MW, A quaternary ammonium glucuronide is the major metabolite of lamotrigine in guinea pigs. In vitro and in vivo studies. Drug Metab Dispos, 1991. 19(3): p. 630–6. [PubMed] [Google Scholar]
- 49.Mikati MA, Schachter SC, Schomer DL, Keally M, Osborne-Shafer P, Seaman CA, et al. , Long-term tolerability, pharmacokinetic and preliminary efficacy study of lamotrigine in patients with resistant partial seizures. Clin Neuropharmacol, 1989. 12(4): p. 312–21. [DOI] [PubMed] [Google Scholar]
- 50.Cohen AF, Land GS, Breimer DD, Yuen WC, Winton C, and Peck AW, Lamotrigine, a new anticonvulsant: pharmacokinetics in normal humans. Clin Pharmacol Ther, 1987. 42(5): p. 535–41. [DOI] [PubMed] [Google Scholar]
- 51.Doig MV and Clare RA, Use of thermospray liquid chromatography-mass spectrometry to aid in the identification of urinary metabolites of a novel antiepileptic drug, Lamotrigine. J Chromatogr, 1991. 554(1-2): p. 181–9. [DOI] [PubMed] [Google Scholar]
- 52.Sinz MW and Remmel RP, Isolation and characterization of a novel quaternary ammonium-linked glucuronide of lamotrigine. Drug Metab Dispos, 1991. 19(1): p. 149–53. [PubMed] [Google Scholar]
- 53.Argikar UA and Remmel RP, Variation in glucuronidation of lamotrigine in human liver microsomes. Xenobiotica, 2009. 39(5): p. 355–63. [DOI] [PubMed] [Google Scholar]
- 54.Lu W and Uetrecht JP, Possible bioactivation pathways of lamotrigine. Drug Metab Dispos, 2007. 35(7): p. 1050–6. [DOI] [PubMed] [Google Scholar]
- 55.Maggs JL, Naisbitt DJ, Tettey JN, Pirmohamed M, and Park BK, Metabolism of lamotrigine to a reactive arene oxide intermediate. Chem Res Toxicol, 2000. 13(11): p. 1075–81. [DOI] [PubMed] [Google Scholar]
- 56.Rowland A, Elliot DJ, Williams JA, Mackenzie PI, Dickinson RG, and Miners JO, In vitro characterization of lamotrigine N2-glucuronidation and the lamotrigine-valproic acid interaction. Drug Metab Dispos, 2006. 34(6): p. 1055–62. [DOI] [PubMed] [Google Scholar]
- 57.Argikar UA, Senekeo-Effenberger K, Larson EE, Tukey RH, and Remmel RP, Studies on induction of lamotrigine metabolism in transgenic UGT1 mice. Xenobiotica, 2009. 39(11): p. 826–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanca Sanchez M, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizan EM, et al. , UGT2B7_−161C>T polymorphism is associated with lamotrigine concentration-to-dose ratio in a multivariate study. Ther Drug Monit, 2010. 32(2): p. 177–84. [DOI] [PubMed] [Google Scholar]
- 59.Singkham N, Towanabut S, Lertkachatarn S, and Punyawudho B, Influence of the UGT2B7 −161C>T polymorphism on the population pharmacokinetics of lamotrigine in Thai patients. Eur J Clin Pharmacol, 2013. 69(6): p. 1285–91. [DOI] [PubMed] [Google Scholar]
- 60.Milosheska D, Lorber B, Vovk T, Kastelic M, Dolzan V, and Grabnar I, Pharmacokinetics of lamotrigine and its metabolite N-2-glucuronide: Influence of polymorphism of UDP-glucuronosyltransferases and drug transporters. Br J Clin Pharmacol, 2016. 82(2): p. 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrenaite V, Ohman I, Ekstrom L, Saebye D, Hansen TF, Tomson T, et al. , UGT polymorphisms and lamotrigine clearance during pregnancy. Epilepsy Res, 2018. 140: p. 199–208. [DOI] [PubMed] [Google Scholar]
- 62.Chen H, Grover S, Yu L, Walker G, and Mutlib A, Bioactivation of lamotrigine in vivo in rat and in vitro in human liver microsomes, hepatocytes, and epidermal keratinocytes: characterization of thioether conjugates by liquid chromatography/mass spectrometry and high field nuclear magnetic resonance spectroscopy. Chem Res Toxicol, 2010. 23(1): p. 159–70. [DOI] [PubMed] [Google Scholar]
- 63.Veiga-da-Cunha M, Tyteca D, Stroobant V, Courtoy PJ, Opperdoes FR, and Van Schaftingen E, Molecular identification of NAT8 as the enzyme that acetylates cysteine S-conjugates to mercapturic acids. J Biol Chem, 2010. 285(24): p. 18888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jawad S, Yuen WC, Peck AW, Hamilton MJ, Oxley JR, and Richens A, Lamotrigine: single-dose pharmacokinetics and initial 1 week experience in refractory epilepsy. Epilepsy Res, 1987. 1(3): p. 194–201. [DOI] [PubMed] [Google Scholar]
- 65.Morris RG, Black AB, Lam E, and Westley IS, Clinical study of lamotrigine and valproic acid in patients with epilepsy: using a drug interaction to advantage? Ther Drug Monit, 2000. 22(6): p. 656–60. [DOI] [PubMed] [Google Scholar]
- 66.Argikar UA and Remmel RP, Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos, 2009. 37(1): p. 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomson T, Ohman I, and Vitols S, Lamotrigine in pregnancy and lactation: a case report. Epilepsia, 1997. 38(9): p. 1039–41. [DOI] [PubMed] [Google Scholar]
- 68.Tran TA, Leppik IE, Blesi K, Sathanandan ST, and Remmel R, Lamotrigine clearance during pregnancy. Neurology, 2002. 59(2): p. 251–5. [DOI] [PubMed] [Google Scholar]
- 69.Pennell PB, Newport DJ, Stowe ZN, Helmers SL, Montgomery JQ, and Henry TR, The impact of pregnancy and childbirth on the metabolism of lamotrigine. Neurology, 2004. 62(2): p. 292–5. [DOI] [PubMed] [Google Scholar]
- 70.de Haan GJ, Edelbroek P, Segers J, Engelsman M, Lindhout D, Devile-Notschaele M, et al. , Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology, 2004. 63(3): p. 571–3. [DOI] [PubMed] [Google Scholar]
- 71.Richards N, Reith D, Stitely M, and Smith A, Are doses of lamotrigine or levetiracetam adjusted during pregnancy? Epilepsia Open, 2018. 3(1): p. 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polepally AR, Pennell PB, Brundage RC, Stowe ZN, Newport DJ, Viguera AC, et al. , MODEL-BASED LAMOTRIGINE CLEARANCE CHANGES DURING PREGNANCY: CLINICAL IMPLICATION. Ann Clin Transl Neurol, 2014. 1(2): p. 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H, Yang K, Choi S, Fischer JH, and Jeong H, Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos, 2009. 37(9): p. 1841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christensen J, Petrenaite V, Atterman J, Sidenius P, Ohman I, Tomson T, et al. , Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia, 2007. 48(3): p. 484–9. [DOI] [PubMed] [Google Scholar]
- 75.Ohman I, Luef G, and Tomson T, Effects of pregnancy and contraception on lamotrigine disposition: new insights through analysis of lamotrigine metabolites. Seizure, 2008. 17(2): p. 199–202. [DOI] [PubMed] [Google Scholar]
- 76.Sabers A, Ohman I, Christensen J, and Tomson T, Oral contraceptives reduce lamotrigine plasma levels. Neurology, 2003. 61(4): p. 570–1. [DOI] [PubMed] [Google Scholar]
- 77.Reimers A, Helde G, and Brodtkorb E, Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia, 2005. 46(9): p. 1414–7. [DOI] [PubMed] [Google Scholar]
- 78.Neumann E, Mehboob H, Ramirez J, Mirkov S, Zhang M, and Liu W, Age-Dependent Hepatic UDP-Glucuronosyltransferase Gene Expression and Activity in Children. Front Pharmacol, 2016. 7: p. 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu W, Ramirez J, Gamazon ER, Mirkov S, Chen P, Wu K, et al. , Genetic factors affecting gene transcription and catalytic activity of UDP-glucuronosyltransferases in human liver. Hum Mol Genet, 2014. 23(20): p. 5558–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen S, Yueh MF, Evans RM, and Tukey RH, Pregnane-x-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 mice. Hepatology, 2012. 56(2): p. 658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fritz A, Busch D, Lapczuk J, Ostrowski M, Drozdzik M, and Oswald S, Expression of clinically relevant drug-metabolizing enzymes along the human intestine and their correlation to drug transporters and nuclear receptors: An intra-subject analysis. Basic Clin Pharmacol Toxicol, 2019. 124(3): p. 245–255. [DOI] [PubMed] [Google Scholar]
- 82.Moscovitz JE, Kalgutkar AS, Nulick K, Johnson N, Lin Z, Goosen TC, et al. , Establishing Transcriptional Signatures to Differentiate PXR-, CAR-, and AhR-Mediated Regulation of Drug Metabolism and Transport Genes in Cryopreserved Human Hepatocytes. J Pharmacol Exp Ther, 2018. 365(2): p. 262–271. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, Wong T, Hashizume T, Dickmann LZ, Scian M, Koszewski NJ, et al. , Human UGT1A4 and UGT1A3 conjugate 25-hydroxyvitamin D3: metabolite structure, kinetics, inducibility, and interindividual variability. Endocrinology, 2014. 155(6): p. 2052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gufford BT, Robarge JD, Eadon MT, Gao H, Lin H, Liu Y, et al. , Rifampin modulation of xeno- and endobiotic conjugating enzyme mRNA expression and associated microRNAs in human hepatocytes. Pharmacol Res Perspect, 2018. 6(2): p. e00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yueh MF, Mellon PL, and Tukey RH, Inhibition of human UGT2B7 gene expression in transgenic mice by the constitutive androstane receptor. Mol Pharmacol, 2011. 79(6): p. 1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czapinski P, Blaszczyk B, and Czuczwar SJ, Mechanisms of action of antiepileptic drugs. Curr Top Med Chem, 2005. 5(1): p. 3–14. [DOI] [PubMed] [Google Scholar]
- 87.Lang DG, Wang CM, and Cooper BR, Lamotrigine, phenytoin and carbamazepine interactions on the sodium current present in N4TG1 mouse neuroblastoma cells. J Pharmacol Exp Ther, 1993. 266(2): p. 829–35. [PubMed] [Google Scholar]
- 88.Xie X, Lancaster B, Peakman T, and Garthwaite J, Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch, 1995. 430(3): p. 437–46. [DOI] [PubMed] [Google Scholar]
- 89.Stefani A, Spadoni F, and Bernardi G, Voltage-activated calcium channels: targets of antiepileptic drug therapy? Epilepsia, 1997. 38(9): p. 959–65. [DOI] [PubMed] [Google Scholar]
- 90.Morris CE, Voltage-gated channel mechanosensitivity: fact or friction? Front Physiol, 2011. 2: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guda P, Bourne PE, and Guda C, Conserved motifs in voltage-sensing and pore-forming modules of voltage-gated ion channel proteins. Biochem Biophys Res Commun, 2007. 352(2): p. 292–8. [DOI] [PubMed] [Google Scholar]
- 92.Cunningham MO and Jones RS, The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology, 2000. 39(11): p. 2139–46. [DOI] [PubMed] [Google Scholar]
- 93.Leach MJ, Marden CM, and Miller AA, Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia, 1986. 27(5): p. 490–7. [DOI] [PubMed] [Google Scholar]
- 94.Waldmeier PC, Baumann PA, Wicki P, Feldtrauer JJ, Stierlin C, and Schmutz M, Similar potency of carbamazepine, oxcarbazepine, and lamotrigine in inhibiting the release of glutamate and other neurotransmitters. Neurology, 1995. 45(10): p. 1907–13. [DOI] [PubMed] [Google Scholar]
- 95.Teoh H, Fowler LJ, and Bowery NG, Effect of lamotrigine on the electrically-evoked release of endogenous amino acids from slices of dorsal horn of the rat spinal cord. Neuropharmacology, 1995. 34(10): p. 1273–8. [DOI] [PubMed] [Google Scholar]
- 96.Calabresi P, Centonze D, Marfia GA, Pisani A, and Bernardi G, An in vitro electrophysiological study on the effects of phenytoin, lamotrigine and gabapentin on striatal neurons. Br J Pharmacol, 1999. 126(3): p. 689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang SJ, Huang CC, Hsu KS, Tsai JJ, and Gean PW, Presynaptic inhibition of excitatory neurotransmission by lamotrigine in the rat amygdalar neurons. Synapse, 1996. 24(3): p. 248–55. [DOI] [PubMed] [Google Scholar]
- 98.Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, et al. , Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology, 2002. 58(3): p. 368–72. [DOI] [PubMed] [Google Scholar]
- 99.Lee ES, Ryu JH, Kim EJ, Kim GT, Cho YW, Park HJ, et al. , Lamotrigine increases intracellular Ca(2+) levels and Ca(2+)/calmodulin-dependent kinase II activation in mouse dorsal root ganglion neurones. Acta Physiol (Oxf), 2013. 207(2): p. 397–404. [DOI] [PubMed] [Google Scholar]
- 100.Lopez M, Dorado P, Monroy N, Alonso ME, Jung-Cook H, Machin E, et al. , Pharmacogenetics of the antiepileptic drugs phenytoin and lamotrigine. Drug Metabol Drug Interact, 2011. 26(1): p. 5–12. [DOI] [PubMed] [Google Scholar]
- 101.Chang Y, Yang LY, Zhang MC, and Liu SY, Correlation of the UGT1A4 gene polymorphism with serum concentration and therapeutic efficacy of lamotrigine in Han Chinese of Northern China. Eur J Clin Pharmacol, 2014. 70(8): p. 941–6. [DOI] [PubMed] [Google Scholar]
- 102.Kacirova I, Grundmann M, and Brozmanova H, Serum levels of lamotrigine during delivery in mothers and their infants. Epilepsy Res, 2010. 91(2-3): p. 161–5. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q, Liang M, Dong Y, Yun W, Qiu F, Zhao L, et al. , Effects of UGT1A4 genetic polymorphisms on serum lamotrigine concentrations in Chinese children with epilepsy. Drug Metab Pharmacokinet, 2015. 30(3): p. 209–13. [DOI] [PubMed] [Google Scholar]
- 104.Benoit-Biancamano MO, Adam JP, Bernard O, Court MH, Leblanc MH, Caron P, et al. , A pharmacogenetics study of the human glucuronosyltransferase UGT1A4. Pharmacogenet Genomics, 2009. 19(12): p. 945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saeki M, Saito Y, Jinno H, Sai K, Hachisuka A, Kaniwa N, et al. , Genetic variations and haplotypes of UGT1A4 in a Japanese population. Drug Metab Pharmacokinet, 2005. 20(2): p. 144–51. [DOI] [PubMed] [Google Scholar]
- 106.Edavana VK, Dhakal IB, Williams S, Penney R, Boysen G, Yao-Borengasser A, et al. , Potential role of UGT1A4 promoter SNPs in anastrozole pharmacogenomics. Drug Metab Dispos, 2013. 41(4): p. 870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Edavana VK, Penney RB, Yao-Borengasser A, Williams S, Rogers L, Dhakal IB, et al. , Fulvestrant up regulates UGT1A4 and MRPs through ERalpha and c-Myb pathways: a possible primary drug disposition mechanism. Springerplus, 2013. 2: p. 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gulcebi MI, Ozkaynakci A, Goren MZ, Aker RG, Ozkara C, and Onat FY, The relationship between UGT1A4 polymorphism and serum concentration of lamotrigine in patients with epilepsy. Epilepsy Res, 2011. 95(1-2): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 109.Reimers A, Sjursen W, Helde G, and Brodtkorb E, Frequencies of UGT1A4*2 (P24T) and *3 (L48V) and their effects on serum concentrations of lamotrigine. Eur J Drug Metab Pharmacokinet, 2016. 41(2): p. 149–55. [DOI] [PubMed] [Google Scholar]
- 110.Zhou J, Argikar UA, and Remmel RP, Functional analysis of UGT1A4(P24T) and UGT1A4(L48V) variant enzymes. Pharmacogenomics, 2011. 12(12): p. 1671–9. [DOI] [PubMed] [Google Scholar]
- 111.Liu L, Zhao L, Wang Q, Qiu F, Wu X, and Ma Y, Influence of valproic acid concentration and polymorphism of UGT1A4*3, UGT2B7 −161C > T and UGT2B7*2 on serum concentration of lamotrigine in Chinese epileptic children. Eur J Clin Pharmacol, 2015. 71(11): p. 1341–7. [DOI] [PubMed] [Google Scholar]
- 112.Du Z, Jiao Y, and Shi L, Association of UGT2B7 and UGT1A4 Polymorphisms with Serum Concentration of Antiepileptic Drugs in Children. Med Sci Monit, 2016. 22: p. 4107–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ehmer U, Vogel A, Schutte JK, Krone B, Manns MP, and Strassburg CP, Variation of hepatic glucuronidation: Novel functional polymorphisms of the UDP-glucuronosyltransferase UGT1A4. Hepatology, 2004. 39(4): p. 970–7. [DOI] [PubMed] [Google Scholar]
- 114.Mori A, Maruo Y, Iwai M, Sato H, and Takeuchi Y, UDP-glucuronosyltransferase 1A4 polymorphisms in a Japanese population and kinetics of clozapine glucuronidation. Drug Metab Dispos, 2005. 33(5): p. 672–5. [DOI] [PubMed] [Google Scholar]
- 115.Takeuchi K, Kobayashi Y, Tamaki S, Ishihara T, Maruo Y, Araki J, et al. , Genetic polymorphisms of bilirubin uridine diphosphate-glucuronosyltransferase gene in Japanese patients with Crigler-Najjar syndrome or Gilbert's syndrome as well as in healthy Japanese subjects. J Gastroenterol Hepatol, 2004. 19(9): p. 1023–8. [DOI] [PubMed] [Google Scholar]
- 116.Mimura Y, Maruo Y, Ohta Y, Sato H, and Takeuchi Y, Effect of common exon variant (p.P364L) on drug glucuronidation by the human UDP-glucuronosyltransferase 1 family. Basic Clin Pharmacol Toxicol, 2011. 109(6): p. 486–93. [DOI] [PubMed] [Google Scholar]
- 117.Provenzani A, Labbozzetta M, Notarbartolo M, Poma P, Polidori P, Vizzini G, et al. , Rash and multiorgan dysfunction following lamotrigine: could genetic be involved? Int J Clin Pharm, 2015. 37(5): p. 682–6. [DOI] [PubMed] [Google Scholar]
- 118.Cascorbi I, ABC transporters in drug-refractory epilepsy: limited clinical significance of pharmacogenetics? Clin Pharmacol Ther, 2010. 87(1): p. 15–8. [DOI] [PubMed] [Google Scholar]
- 119.Loscher W, Klotz U, Zimprich F, and Schmidt D, The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia, 2009. 50(1): p. 1–23. [DOI] [PubMed] [Google Scholar]
- 120.Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. , Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med, 2003. 348(15): p. 1442–8. [DOI] [PubMed] [Google Scholar]
- 121.Glauser TA, Holland K, O'Brien VP, Keddache M, Martin LJ, Clark PO, et al. , Pharmacogenetics of antiepileptic drug efficacy in childhood absence epilepsy. Ann Neurol, 2017. 81(3): p. 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Klarica Domjanovic I, Lovric M, Trkulja V, Petelin-Gadze Z, Ganoci L, Cajic I, et al. , Interaction between ABCG2 421C>A polymorphism and valproate in their effects on steady-state disposition of lamotrigine in adults with epilepsy. Br J Clin Pharmacol, 2018. 84(9): p. 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwan P, Wong V, Ng PW, Lui CH, Sin NC, Wong KS, et al. , Gene-wide tagging study of the association between ABCC2, ABCC5 and ABCG2 genetic polymorphisms and multidrug resistance in epilepsy. Pharmacogenomics, 2011. 12(3): p. 319–25. [DOI] [PubMed] [Google Scholar]
- 124.Hilger E, Reinthaler EM, Stogmann E, Hotzy C, Pataraia E, Baumgartner C, et al. , Lack of association between ABCC2 gene variants and treatment response in epilepsy. Pharmacogenomics, 2012. 13(2): p. 185–90. [DOI] [PubMed] [Google Scholar]
- 125.Sporis D, Bozina N, Basic S, Lovric M, Babic T, Susak I, et al. , Lack of association between polymorphism in ABCC2 gene and response to antiepileptic drug treatment in Croatian patients with epilepsy. Coll Antropol, 2013. 37(1): p. 41–5. [PubMed] [Google Scholar]
- 126.Krikova EV, Val'dman EA, Avakian GN, Andreev Ia A, Denisov EV, Rider FK, et al. , [Association study of the SCN1 gene polymorphism and effective dose of lamotrigine]. Zh Nevrol Psikhiatr Im S S Korsakova, 2009. 109(10): p. 57–62. [PubMed] [Google Scholar]
- 127.Fritschy JM, Epilepsy, E/I Balance and GABA(A) Receptor Plasticity. Front Mol Neurosci, 2008. 1: p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rogawski MA and Loscher W, The neurobiology of antiepileptic drugs. Nat Rev Neurosci, 2004. 5(7): p. 553–64. [DOI] [PubMed] [Google Scholar]
- 129.Hung CC, Chen PL, Huang WM, Tai JJ, Hsieh TJ, Ding ST, et al. , Gene-wide tagging study of the effects of common genetic polymorphisms in the alpha subunits of the GABA(A) receptor on epilepsy treatment response. Pharmacogenomics, 2013. 14(15): p. 1849–56. [DOI] [PubMed] [Google Scholar]
- 130.Blaszczyk B, Lason W, and Czuczwar SJ, Antiepileptic drugs and adverse skin reactions: An update. Pharmacol Rep, 2015. 67(3): p. 426–34. [DOI] [PubMed] [Google Scholar]
- 131.Kavitha S, Anbuchelvan T, Mahalakshmi V, Sathya R, Sabarinath TR, Gururaj N, et al. , Stevens-Johnson syndrome induced by a combination of lamotrigine and valproic acid. J Pharm Bioallied Sci, 2015. 7(Suppl 2): p. S756–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ordonez L, Salgueiro E, Jimeno FJ, and Manso G, Spontaneous reporting of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antiepileptic drugs. Eur Rev Med Pharmacol Sci, 2015. 19(14): p. 2732–7. [PubMed] [Google Scholar]
- 133.Wang XQ, Xiong J, Xu WH, Yu SY, Huang XS, Zhang JT, et al. , Risk of a lamotrigine-related skin rash: current meta-analysis and postmarketing cohort analysis. Seizure, 2015. 25: p. 52–61. [DOI] [PubMed] [Google Scholar]
- 134.Chou YC, Chao FH, and Chou YH, Rapid development of severe skin rash after adding valproic acid in a case of bipolar depression treated with low-dose lamotrigine. J Formos Med Assoc, 2014. 113(3): p. 195–6. [DOI] [PubMed] [Google Scholar]
- 135.Serrani Azcurra DJ, Lamotrigine rechallenge after a skin rash. A combined study of open cases and a meta-analysis. Rev Psiquiatr Salud Ment, 2013. 6(4): p. 144–9. [DOI] [PubMed] [Google Scholar]
- 136.Kiyohara T, Sawai T, Ido H, and Kumakiri M, Toxic epidermal necrolysis with some features of acute generalized exanthematous pustulosis. Acta Derm Venereol, 2013. 93(2): p. 212–4. [DOI] [PubMed] [Google Scholar]
- 137.Comfere NI, Sartori-Valinotti JC, Bruce AJ, and Drage LA, Successful treatment of lamotrigine-associated drug hypersensitivity syndrome with intravenous IgG. J Am Acad Dermatol, 2012. 66(6): p. e249–50. [DOI] [PubMed] [Google Scholar]
- 138.Frey N, Bodmer M, Bircher A, Ruegg S, Jick SS, Meier CR, et al. , The risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptic drugs. Epilepsia, 2017. 58(12): p. 2178–2185. [DOI] [PubMed] [Google Scholar]
- 139.Egunsola O, Star K, Juhlin K, Kardaun SH, Choonara I, and Sammons HM, Retrospective review of paediatric case reports of Stevens-Johnson syndrome and toxic epidermal necrolysis with lamotrigine from an international pharmacovigilance database. BMJ Paediatr Open, 2017. 1(1): p. e000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Orr HT, Lopez de Castro JA, Lancet D, and Strominger JL, Complete amino acid sequence of a papain-solubilized human histocompatibility antigen, HLA-B7. 2. Sequence determination and search for homologies. Biochemistry, 1979. 18(25): p. 5711–20. [DOI] [PubMed] [Google Scholar]
- 141.Dao RL, Su SC, and Chung WH, Recent advances of pharmacogenomics in severe cutaneous adverse reactions: immune and nonimmune mechanisms. Asia Pac Allergy, 2015. 5(2): p. 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pirmohamed M, Ostrov DA, and Park BK, New genetic findings lead the way to a better understanding of fundamental mechanisms of drug hypersensitivity. J Allergy Clin Immunol, 2015. 136(2): p. 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Usui T and Naisbitt DJ, Human leukocyte antigen and idiosyncratic adverse drug reactions. Drug Metab Pharmacokinet, 2017. 32(1): p. 21–30. [DOI] [PubMed] [Google Scholar]
- 144.Michels AW and Ostrov DA, New approaches for predicting T cell-mediated drug reactions: A role for inducible and potentially preventable autoimmunity. J Allergy Clin Immunol, 2015. 136(2): p. 252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bloch KM, Sills GJ, Pirmohamed M, and Alfirevic A, Pharmacogenetics of antiepileptic drug-induced hypersensitivity. Pharmacogenomics, 2014. 15(6): p. 857–68. [DOI] [PubMed] [Google Scholar]
- 146.Ramirez E, Bellon T, Tong HY, Borobia AM, de Abajo FJ, Lerma V, et al. , Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res, 2017. 115: p. 168–178. [DOI] [PubMed] [Google Scholar]
- 147.Ito A, Shimada H, Ishikawa K, Takeo N, Hatano Y, Katagiri K, et al. , Association between HLA-DRB1*0405, -DQB1*0401 and -DQA1*0303 alleles and lamotrigine-induced cutaneous adverse drug reactions. A pilot case-control study from Japan. J Affect Disord, 2015. 179: p. 47–50. [DOI] [PubMed] [Google Scholar]
- 148.Park HJ, Kim YJ, Kim DH, Kim J, Park KH, Park JW, et al. , HLA Allele Frequencies in 5802 Koreans: Varied Allele Types Associated with SJS/TEN According to Culprit Drugs. Yonsei Med J, 2016. 57(1): p. 118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park HJ, Kim SR, Leem DW, Moon IJ, Koh BS, Park KH, et al. , Clinical features of and genetic predisposition to drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a single Korean tertiary institution patients-investigating the relation between the HLA -B*4403 allele and lamotrigine. Eur J Clin Pharmacol, 2015. 71(1): p. 35–41. [DOI] [PubMed] [Google Scholar]
- 150.Moon J, Park HK, Chu K, Sunwoo JS, Byun JI, Lim JA, et al. , The HLA-A*2402/Cw*0102 haplotype is associated with lamotrigine-induced maculopapular eruption in the Korean population. Epilepsia, 2015. 56(10): p. e161–7. [DOI] [PubMed] [Google Scholar]
- 151.Shi YW, Min FL, Zhou D, Qin B, Wang J, Hu FY, et al. , HLA-A*24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology, 2017. 88(23): p. 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jang HW, Kim SW, Cho YJ, Heo K, Lee BI, Lee SK, et al. , GWAS identifies two susceptibility loci for lamotrigine-induced skin rash in patients with epilepsy. Epilepsy Res, 2015. 115: p. 88–94. [DOI] [PubMed] [Google Scholar]
- 153.Fricke-Galindo I, Martinez-Juarez IE, Monroy-Jaramillo N, Jung-Cook H, Falfan-Valencia R, Ortega-Vazquez A, et al. , HLA-A*02:01:01/-B*35:01:01/-C*04:01:01 haplotype associated with lamotrigine-induced maculopapular exanthema in Mexican Mestizo patients. Pharmacogenomics, 2014. 15(15): p. 1881–91. [DOI] [PubMed] [Google Scholar]
- 154.Koomdee N, Pratoomwun J, Jantararoungtong T, Theeramoke V, Tassaneeyakul W, Klaewsongkram J, et al. , Association of HLA-A and HLA-B Alleles with Lamotrigine-Induced Cutaneous Adverse Drug Reactions in the Thai Population. Front Pharmacol, 2017. 8: p. 879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim BK, Jung JW, Kim TB, Chang YS, Park HS, Moon J, et al. , HLA-A*31:01 and lamotrigine-induced severe cutaneous adverse drug reactions in a Korean population. Ann Allergy Asthma Immunol, 2017. 118(5): p. 629–630. [DOI] [PubMed] [Google Scholar]