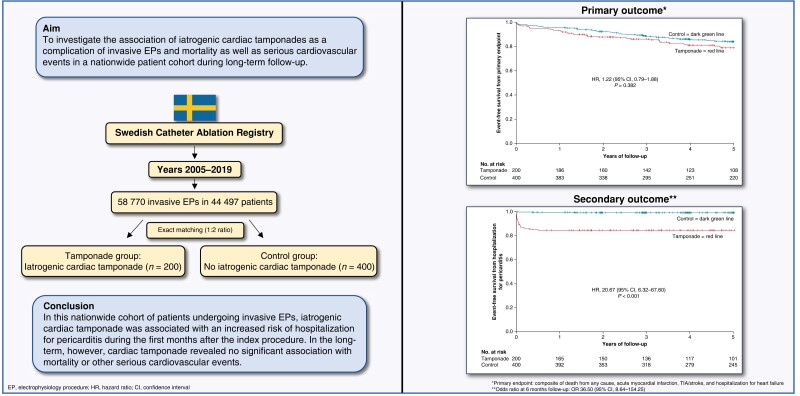
Abstract
Aims
To investigate the association of iatrogenic cardiac tamponades as a complication of invasive electrophysiology procedures (EPs) and mortality as well as serious cardiovascular events in a nationwide patient cohort during long-term follow-up.
Methods
From the Swedish Catheter Ablation Registry between 2005 and 2019, a total of 58 770 invasive EPs in 44 497 patients were analysed. From this, all patients with periprocedural cardiac tamponades related to invasive EPs were identified (n = 200; tamponade group) and matched (1:2 ratio) to a control group (n = 400). Over a follow-up of 5 years, the composite primary endpoint—death from any cause, acute myocardial infarction, transitory ischaemic attack (TIA)/stroke, and hospitalization for heart failure—revealed no statistically significant association with cardiac tamponade [hazard ratio (HR) 1.22 (95% CI, 0.79–1.88)]. All single components of the primary endpoint as well as cardiovascular death revealed no statistically significant association with cardiac tamponade. Cardiac tamponade was associated with a significantly higher risk with hospitalization for pericarditis [HR 20.67 (95% CI, 6.32–67.60)].
Conclusion
In this nationwide cohort of patients undergoing invasive EPs, iatrogenic cardiac tamponade was associated with an increased risk of hospitalization for pericarditis during the first months after the index procedure. In the long-term, however, cardiac tamponade revealed no significant association with mortality or other serious cardiovascular events.
Keywords: Cardiac arrhythmia, Catheter ablation, Cardiac tamponade, Swedish registries
Graphical Abstract
Graphical abstract.
What’s new?
This is the first nationwide study analysing the clinical long-term outcome of patients with cardiac tamponades related to interventional electrophysiology.
Iatrogenic cardiac tamponade was associated with an increased risk of hospitalization for pericarditis during the first months after index procedure but not with an increased risk of periprocedural cerebrovascular events.
In the long-term, cardiac tamponade revealed no significant association with mortality or other serious cardiovascular events.
Introduction
Catheter ablation has been established as an effective treatment for a variety of cardiac arrhythmias.1–5 However, periprocedural complications may occur. Cardiac tamponade is a dreaded complication with an overall rate around 1.0%, bearing a rare but potentially fatal outcome.6–11 It may occur during all types of invasive electrophysiology procedure (EP) but has a generally higher risk during catheter ablation of ventricular tachycardia (VT) as well as atrial fibrillation.6 For the acute management of cardiac tamponade including treatment via pericardiocentesis in unstable patients, there is comprehensive data available.12 However, the potential long-term impact of EP-related cardiac tamponade on relevant clinical outcomes other than arrhythmia recurrence has been scarcely investigated so far. In only one study, it has been shown that iatrogenic cardiac tamponade in patients undergoing invasive EPs was associated with a higher risk for cerebrovascular events as well as hospitalization for pericarditis during the first 2 weeks and first months, respectively, after the index procedure but with no increased risk for mortality or other serious cardiovascular events.7 Although this has been the largest patient cohort and longest follow-up regarding EP-related cardiac tamponades so far, this study was only performed at a single electrophysiology centre and results may not be applicable to other centres.13 Therefore, the purpose of this study was to investigate the association of iatrogenic cardiac tamponades and mortality as well as serious cardiovascular events during long-term follow-up in a nationwide multicentre cohort of patients undergoing invasive EPs.
Methods
Study protocol and setting
The Swedish Catheter Ablation Registry has been previously described.14 Since 2004, data on catheter ablations performed in Sweden are prospectively included in the registry.15 Since 2006, all centres performing catheter ablation of cardiac arrhythmias in Sweden [11 ablation centres (7 university institutions, 3 community hospitals, and 1 private institution)] report to this registry. Baseline characteristics together with procedural characteristics, as well as data on adverse events including any cardiac tamponade or pericardial effusion requiring pericardiocentesis, are provided. Patient consent was obtained by information of entry and allowance to opt out. The completeness of key variables [personal identification number, age, gender, date of ablation, type of arrhythmia, procedural time, and energy delivery (radiofrequency or cryo energy)] is high. Coverage and register and data completeness are exceeding 98% throughout the study period (see Supplementary material online, Table S1 for details).
The National Patient Registry, the Cause of Death Registry, and Dispensed Drug Registry, administered by the Swedish Board of Health and Welfare, provided information on date and cause of death, further baseline comorbidities, prescribed drugs dispensed at pharmacies, and the cause-specific hospitalization as outcome defined according to the ICD-10 codes. The personal identification number that all permanent Swedish citizens own served as unique identifier for each patient allowing merging of different registries.
Establishment of the Swedish Catheter Ablation Registry and this analysis with linking of the above registries was approved by the Swedish Ethical Review Authority and was conducted in accordance with the Declaration of Helsinki. For this study, individual patient consent was not required.
Study population and post-ablation follow-up
For the current study, consecutive patients (≥18 years old at the time of index procedure) undergoing invasive EP at one of the catheter ablation centres in Sweden between 1 January 2005 and 31 December 2019 were enrolled. Electrophysiology procedures were performed according to conventional and local standards as described previously.14
For the tamponade group, all patients suffering from a cardiac tamponade or pericardial effusion related to invasive EP requiring pericardiocentesis were included. Cardiac tamponades had to be detected up to 30 days from invasive EP.
A control group not experiencing any kind of cardiac effusion was generated from the same catheter ablation cohort and matched with cardiac tamponade patients in a ratio of 1:2 based on age, gender, treated arrhythmia type at index procedure, and timepoint of index procedure (range of 5 years for the first and last criteria).
All patients were followed up from the time of their index procedure until the date of death, emigration, or the end of the study (31 December 2020).
Study outcomes
The primary endpoint was defined as composite of death from any cause, acute myocardial infarction, transitory ischaemic attack (TIA), or stroke and heart failure that led to an unplanned overnight hospitalization. Transitory ischaemic attack or stroke was diagnosed during ambulatory or inpatient visit.
Secondary endpoints were the single components of the primary endpoint as well as cardiovascular death as previously defined16 and unplanned overnight hospitalization for pericarditis. Hospitalization for pericarditis was defined as new admission to hospital due to pericarditis.
Statistical analysis
The matching was performed in MATLAB (‘MATrix LABoratory’) version 2020b (MathWorks, Natick, Massachusetts, USA). All continuous variables are presented as mean +/− standard deviation or median with interquartile range (IQR), were appropriate, and were compared by using Student’s t-test or Mann–Whitney U test, respectively, according to their distributions. Categorical variables are expressed as frequencies/percentages and were compared by χ2 tests. Clinical outcomes were examined as a time-to-first-event analysis within 5 years which was deemed a meaningful period to reflect long-term follow-up and during which time more than half of patients were still at risk except for hospitalization for pericarditis. Endpoints which do not include mortality patients were censored at death. Hazard ratios with 95% confidence intervals and P values from Cox regression analyses, stratifying for matched pairs, are provided. In case of zero events in one group, P values from log-rank analysis are provided. For hospitalization for pericarditis, a binary logistic regression analysis at 6 months was performed in addition to Cox regression analysis, and odds ratios (ORs) with 95% confidence intervals as well as P values are provided. As all available tamponade patients were included, no sample size calculation was performed. All statistical tests and confidence intervals were two-sided, with a significance level of 0.05. Supplementary material online, Table S2 displays the definition of the variables used in the current study. Statistical analyses were performed using SPSS software, version 27 (IBM Corp., Armonk, New York).
Results
Baseline characteristics of patients with and without periprocedural cardiac tamponade
Between 1 January 2005 and 31 December 2019, a total of 58 770 invasive EPs in 44 497 patients were performed. In this cohort, a total of 200 patients with periprocedural cardiac tamponades/pericardial effusions requiring pericardiocentesis were identified and included in the study. The overall procedural risk of cardiac tamponade/pericardial effusion was 0.34%. Among patients undergoing catheter ablation for atrial fibrillation and VT, the procedural risk of tamponade was 0.61% (133 tamponades among 21 629 atrial fibrillation catheter ablations) and 1.1% (20 tamponades among 1787 VT catheter ablations), respectively. The control group comprised 400 patients without cardiac tamponade/pericardial effusion requiring pericardiocentesis during the index procedure. In addition to the matched variables, the remaining clinical baseline characteristics of both groups were similar without any statistically significant difference (Table 1).
Table 1.
Baseline characteristics of patients with and without periprocedural cardiac tamponade
| Baseline characteristics | Tamponade group (n = 200) | Control group (n = 400) | P value |
|---|---|---|---|
| Age (years) a | 63.7 +/− 10.7 | 63.6 +/− 10.6 | 0.989 |
| Male, n (%) a | 106 (53.0) | 212 (53.0) | 1.000 |
| Ischaemic cardiomyopathy | 34 (17.0) | 51 (12.8) | 0.173 |
| Heart failure, n (%) | 28 (14.0) | 46 (11.5) | 0.430 |
| Arterial hypertension, n (%) | 104 (52.0) | 202 (50.5) | 0.795 |
| Diabetes mellitus, n (%) | 20 (10.0) | 25 (6.3) | 0.103 |
| Hyperlipidaemia, n (%) | 40 (20.0) | 77 (19.3) | 0.828 |
| Previous TIA/stroke, n (%) | 21 (10.5) | 25 (6.3) | 0.074 |
| CHA₂DS₂−VASc score ≥2, n (%) | 126 (63.0) | 227 (56.8) | 0.159 |
| Oral anticoagulation/antiplatelet therapy, n (%) | 0.561 | ||
| None | 26 (13.0) | 61 (15.3) | |
| Warfarin | 105 (52.5) | 225 (56.3) | |
| NOAC | 43 (21.5) | 70 (17.5) | |
| ASA or ADP inhibitor | 17 (8.5) | 33 (8.3) | |
| ASA + ADP inhibitor | 2 (1.0) | 1 (0.3) | |
| ASA + warfarin or ASA + NOAC | 7 (3.5) | 10 (2.5) |
Matched variable.
NOAC, novel oral anticoagulant; ASA, acetylsalicylic acid; ADP, adenosine diphosphate receptor.
The mean duration of follow-up was 6.7 +/− 3.9 years in the tamponade and 7.2 +/− 3.9 years in the control group (P = 0.145).
Index procedure–related characteristics in patients with and without periprocedural cardiac tamponade
All timepoints (year) of index electrophysiology procedures in tamponade and control patients are provided in Supplementary material online, Table S3. Procedure time was significantly longer in the tamponade as compared with that in the control group (P = 0.015). The remainder of index procedure–related characteristics was not different between the groups as presented in Table 2.
Table 2.
Index procedure–related characteristics in patients with and without periprocedural cardiac tamponade
| Baseline characteristics | Tamponade group (n = 200) | Control group (n = 400) | P value |
|---|---|---|---|
| Type of arrhythmia to be treated, n (%) a | 1.000 | ||
| Atrial fibrillation | 133 (66.5) | 266 (66.5) | |
| VES/VT | 29 (14.5) | 58 (14.5) | |
| AVRT | 12 (6.0) | 24 (6.0) | |
| AVNRT | 11 (5.5) | 22 (5.5) | |
| Atrial tachycardia excluding CTI-dependent flutter | 7 (3.5) | 14 (3.5) | |
| His ablation | 4 (2.0) | 8 (2.0) | |
| CTI-dependent flutter | 4 (2.0) | 8 (2.0) | |
| Energy delivery, n (%) b | 0.065 | ||
| RF energy | 165 (82.5) | 343 (85.8) | |
| Cryo energy | 12 (6.0) | 34 (8.5) | |
| RF and cryo energy | 2 (1.0) | 3 (0.8) | |
| Unclassified | 21 (10.5) | 20 (5.0) | |
| Procedure time (min) c | 188.8 +/− 78.5 | 173.0 +/− 69.5 | 0.015 |
Matched variable.
Data available in 93% of all patients.
Data available in 96% of all patients.
VES, ventricular extrasystole; VT, ventricular tachycardia; AVNRT, atrioventricular node re-entry tachycardia; AVRT, atrioventricular re-entrant tachycardia; CTI, cavotricuspid isthmus; RF, radiofrequency.
Primary endpoint
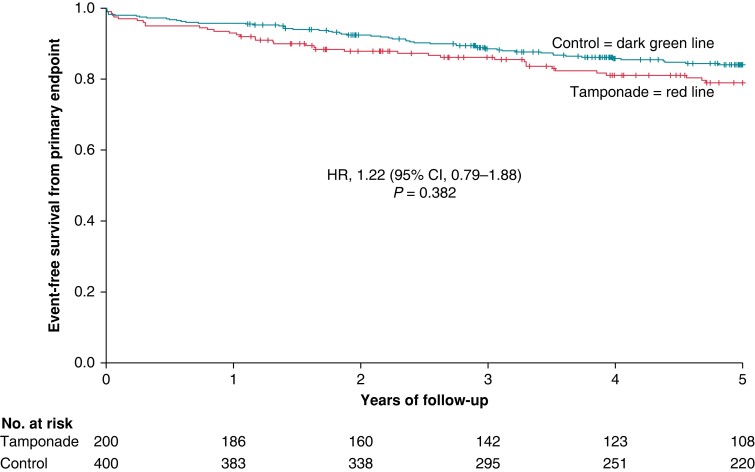
After a follow-up of 5 years, the composite primary endpoint—death from any cause, acute myocardial infarction, TIA/stroke, and hospitalization for heart failure—occurred in 38 tamponade patients (19.0%) vs. 58 control patients (14.5%) resulting in no statistically significant association with cardiac tamponade (hazard ratio [HR] 1.22 [95% CI, 0.79–1.88]) (Table 3). The Kaplan–Meier curve of the primary endpoint is provided in Figure 1.
Table 3.
Primary and secondary clinical endpoints after a follow-up of 5 years
| Endpoint | Tamponade group (n = 200) | Control group (n = 400) | Hazard ratio (95% CI) | P value Cox regression |
|---|---|---|---|---|
| Number (percent) | Number (percent) | |||
| Primary a | 38 (19.0) | 58 (14.5) | 1.22 (0.79–1.88) | 0.382 |
| Secondary | ||||
| Death from any cause | 21 (10.5) | 22 (5.5) | 1.67 (0.89–3.10) | 0.111 |
| Acute myocardial infarction | 4 (2.0) | 6 (1.5) | 1.24 (0.35–4.43) | 0.739 |
| TIA/stroke | 11 (5.5) | 18 (4.5) | 1.26 (0.59–2.70) | 0.549 |
| Hospitalization for heart failure | 15 (7.5) | 23 (5.8) | 1.14 (0.56–2.31) | 0.715 |
| Cardiovascular death | 11 (5.5) | 7 (1.8) | 2.27 (0.84–6.16) | 0.106 |
| Hospitalization for pericarditis | 31 (15.5) | 3 (0.8) | 20.67 (6.32–67.60) | <0.001 |
Composite of death from any cause, acute myocardial infarction, TIA/stroke, and hospitalization for heart failure.
CI, confidence interval; TIA, transitory ischaemic attack.
Figure 1.
Kaplan–Meier curve showing event-free survival from the primary endpoint (death from any cause, acute myocardial infarction, TIA/stroke, and hospitalization for heart failure) in the tamponade compared with the control group after a follow-up of 5 years. HR, hazard ratio; CI, confidence interval.
Secondary endpoints
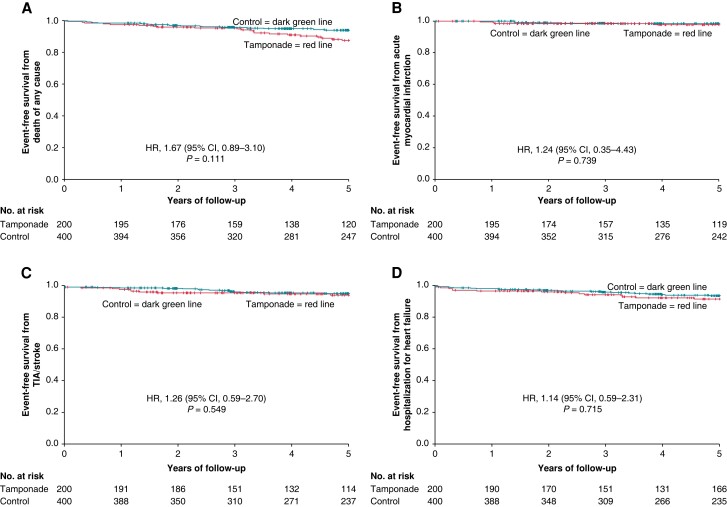
All single components of the primary endpoint as wells as cardiovascular death revealed no statistically significant association after a follow-up of 5 years (Table 3). The Kaplan–Meier curves of the single components of the primary endpoint are presented in Figure 2A–D.
Figure 2.
Kaplan–Meier curve showing event-free survival from (A) death from any cause, (B) acute myocardial infarction, (C) TIA/stroke, and (D) hospitalization for heart failure in the tamponade compared with the control group after a follow-up of 5 years. Day 0 is the timepoint of index procedure. HR, hazard ratio; CI, confidence interval.
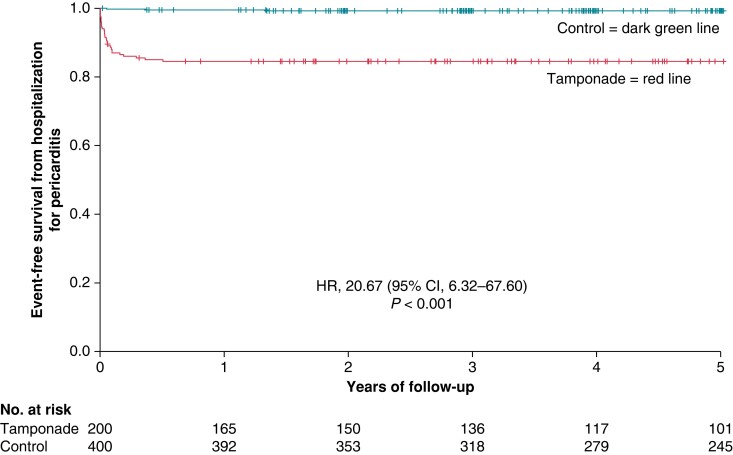
Hospitalization for pericarditis occurred in more patients in the tamponade than in the control group resulting in a significantly higher risk with periprocedural cardiac tamponade [15.5% vs. 0.8%; HR 20.67 (95% CI, 6.32–67.60)] (Table 3). The Kaplan–Meier curve of this endpoint is provided in Figure 3. Hospitalizations for pericarditis occurred during the first 6 months of follow-up in both groups (median 14 days, range 1–184 days) except for one control patient in which hospitalization for pericarditis occurred after 487 days post-index EP. Mean duration of hospital stay due to pericarditis was 5.2 +/− 4.3 days (range 1–17 days).
Figure 3.
Kaplan–Meier curve showing event-free survival from hospitalization for pericarditis in the tamponade compared with the control group after a follow-up of 5 years. Day 0 is the timepoint of index procedure. HR, hazard ratio; CI, confidence interval.
Consistency analysis
To investigate whether the observed events are index procedure related, we performed a consistency analysis considering only 30 days and first year of follow-up. The results of the primary endpoint and its subcomponents from this analysis were consistent with the results of the primary analysis showing no statistically significant association with cardiac tamponade [HR 1.18 (95% CI, 0.39–3.63) and HR 1.67 (95% CI, 0.81–3.43), respectively]. All results for the primary and secondary endpoints after 30 days and 1 year follow-up are provided in Supplementary material online, Tables S4 and S5.
To investigate whether the observed events are also found in the largest subgroup of arrhythmia, we performed a consistency analysis only considering patients treated for atrial fibrillation. From this analysis, the results of the primary endpoint and its subcomponents after a 5 year follow-up were similar to the results of the primary analysis showing no statistically significant association with cardiac tamponade [HR 1.20 (95% CI, 0.68–2.11)]. All results for the primary and secondary endpoints in patients treated for atrial fibrillation at index EP after a 5 year follow-up are provided in Supplementary material online, Table S6.
As almost all hospitalizations for pericarditis occurred during early follow-up, a binary logistic regression analysis at 6 months in addition to Cox regression analysis was performed. The results of this analysis [OR 36.50 (95% CI, 8.64–154.25)] were consistent with the results of the primary analysis.
Discussion
In this nationwide study analysing patients undergoing invasive EPs, iatrogenic cardiac tamponade was associated with an increased risk of hospitalization for pericarditis during the first months after index procedure. In the long-term, however, it revealed no significant association with mortality or other serious cardiovascular events. Results were consistent during shorter follow-up as well as in the largest arrhythmia subgroup, i.e. in patients with atrial fibrillation.
Unlike recent single-centre analysis investigating invasive EPs only from Karolinska University Hospital’s database,7 this study applied the Swedish Catheter Ablation Registry which includes all centres performing catheter ablation of cardiac arrhythmias in Sweden. To the best of our knowledge, this is the largest cohort and first multicentre study analysing the clinical long-term outcome of patients with EP-related cardiac tamponades.
Patient characteristics were well-balanced between the study groups except for procedure time which was significantly longer in the tamponade group probably due to the more complex course. Consistent with previous data, the largest arrhythmia subgroup associated with cardiac tamponade was atrial fibrillation with 66.5%.6 The procedural risk of cardiac tamponade/pericardial effusion requiring pericardiocentesis was overall 0.34%, among atrial fibrillation patients 0.61%, and VT patients 1.1%. These rates were comparable or even lower with previous data with 0.6%, 0.8%, and 1.1%, respectively.6,8,9
Different from recent single-centre analysis,7 the present multicentre study revealed no statistically significant association for the composite primary endpoint with cardiac tamponade. In the previous study, this was mainly driven by a statistically significant increased risk of TIA/stroke especially during the first 2 weeks after index procedure. In the current larger multicentre study, the rate of TIA/stroke in the tamponade group was generally lower compared with the previous study [11 patients (5.5%) vs. 5 patients (8.3%)], and this secondary endpoint did not reveal a statistically significant association with cardiac tamponade. Several factors might have contributed to the previously increased TIA/stroke risk as discussed in the former study. One factor was insufficient anticoagulation in patients ablated for VT and atrioventricular node re-entry tachycardia (AVNRT) with concomitant atrial fibrillation during ‘the early years’ but only protected by acetylsalicylic acid (ASA)7 which was in line with clinical routine at that time. Tamponade patients of the former study were also included in the present study but not those from ‘the early years’ since the present study started from January 2005 onwards which might be an explanation for the lower TIA/stroke risk in the current study. Further presumably contributing factors to the overall lower TIA/stroke risk especially during the first 30 days in the current study [two patients (1.0%) vs. four patients (6.7%)] might be the more frequently used uninterrupted anticoagulation strategy pre-ablation as well as the larger proportion of novel oral anticoagulants (NOACs). While in the present study we do not have detailed data on periprocedural or reversal of anticoagulation, blood reinfusion, or cardiac surgery as in the former study which was derived from the electronic medical record7 and not from national registries as in the current study, a thorough adjustment of anticoagulation especially in the initial phase after cardiac tamponade requiring pericardiocentesis is generally advisable.
In the current study, iatrogenic cardiac tamponade revealed no significant association with all-cause or cardiovascular (CV) mortality, hospitalization for heart failure, or acute myocardial infarction which is in line with the former single-centre study.7 Also, none of the tamponade patients died during hospital stay.
Iatrogenic cardiac tamponade was associated with a strongly increased risk of hospitalization for pericarditis during the first 6 months after index procedure as described previously.7 Hence, being aware of the high occurrence of hospitalization for post-traumatic pericarditis in the tamponade group, a routine application of non-steroidal anti-inflammatory drug (NSAIDs), colchicine, or oral/intrapericardial administration of steroids after pericardiocentesis might be beneficial to prevent or at least attenuate the intrapericardial inflammatory reactions to trauma and bleeding trigger.17,18 Inflammatory processes in the pericardium may lead to fibrinous scarring and the development of pericardial constriction with symptoms of heart failure.12 However, in this study iatrogenic cardiac tamponade was not significantly associated with hospitalization for heart failure after long-term follow-up.
Limitations
This is a registry-based cohort study. From the catheter ablation registry database, it cannot be distinguished between in- or out-of-hospital cardiac tamponades/pericardial effusions. Most of the reported complications in the database occur during the in-hospital period, but also out-of-hospital cardiac tamponades might have been included in the study.
We performed an exact matching over a propensity score matching as only a subset of variables was available in the initial ablation database. Despite this limitation, our matching resulted in a well-matched cohort.
In some of the secondary analyses, e.g. acute myocardial infarction, there were low event rates potentially impeding proper statistical analysis and hence they should be considered with caution.
Underreporting may occur in registry studies; however, for serious complications, such as cardiac tamponade, it is assumed to be rare.
Conclusion
In this nationwide cohort of patients undergoing invasive EPs, iatrogenic cardiac tamponade was associated with an increased risk of hospitalization for pericarditis during the first months after index procedure. In the long-term, however, cardiac tamponade revealed no significant association with mortality or other serious cardiovascular events.
Supplementary Material
Contributor Information
Gesa von Olshausen, Department of Cardiology, Karolinska University Hospital, Solna, S1:02, SE-17176 Stockholm, Sweden; Medical Department I (Cardiology, Angiology, Pneumology), Klinikum rechts der Isar, Technical University of Munich, Ismaninger Str. 22, DE-81675 Munich, Germany.
Fariborz Tabrizi, Department of Clinical Sciences, Karolinska Institute, Arrhythmia Center Stockholm, South Hospital, Sjukhusbacken 10, SE-11883, Stockholm, Sweden.
Rúna Sigurjónsdóttir, Department of Cardiology, Sahlgrenska University Hospital, Gothenburg, Blåa Stråket 3, 141345 Göteborg, Sweden.
Michael Ringborn, Thoracic Center, Blekinge County Hospital, Lasarettsvägen, SE-371 85, Karlskrona, Sweden.
Niklas Höglund, Department of Public Health and Clinical Medicine, Umeå University, SE-90187 Umeå, Sweden.
Anders Hassel Jönsson, Department of Cardiology, Linköping University Hospital, SE-581 85 Linköping, Sweden.
Fredrik Holmqvist, Department of Cardiology, Skåne University Hospital Lund, SE-221 85 Lund, Sweden.
Frieder Braunschweig, Department of Cardiology, Karolinska University Hospital, Solna, S1:02, SE-17176 Stockholm, Sweden.
Supplementary material
Supplementary material is available at Europace online.
Funding
This project was supported by the Deutsche Forschungsgemeinschaft to G.v.O. (OL 605/1–1).
Data availability
The data underlying this article cannot be shared publicly due to privacy of individuals that were investigated in the study. The data will be shared on reasonable request to the corresponding author provided that this in accordance with the institutional ethical guidelines as well as regulation and legislation.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom-Lundqvist Cet al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia: the task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 3. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 4. Aktaa S, Tzeis S, Gale CP, Ackerman MJ, Arbelo E, Behr ERet al. European Society of Cardiology quality indicators for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2023;25:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konig S, Schroter T, Borger MA, Bertagnolli L, Nedios S, Darma Aet al. Outcomes following cardiac sympathetic denervation in patients with structural heart disease and refractory ventricular arrhythmia. Europace 2022;24:1800–8. [DOI] [PubMed] [Google Scholar]
- 6. Fink T, Sciacca V, Feickert S, Metzner A, Lin T, Schluter Met al. Outcome of cardiac tamponades in interventional electrophysiology. Europace 2020;22:1240–51. [DOI] [PubMed] [Google Scholar]
- 7. von Olshausen G, Bourke T, Schwieler J, Drca N, Bastani H, Tapanainen Jet al. Long-term outcome of patients with invasive electrophysiology procedure-related cardiac tamponade. Europace 2020;22:1547–57. [DOI] [PubMed] [Google Scholar]
- 8. Bollmann A, Ueberham L, Schuler E, Wiedemann M, Reithmann C, Sause Aet al. Cardiac tamponade in catheter ablation of atrial fibrillation: German-wide analysis of 21 141 procedures in the Helios atrial fibrillation ablation registry (SAFER). Europace 2018;20:1944–51. [DOI] [PubMed] [Google Scholar]
- 9. Hamaya R, Miyazaki S, Taniguchi H, Kusa S, Nakamura H, Hachiya Het al. Management of cardiac tamponade in catheter ablation of atrial fibrillation: single-centre 15 year experience on 5222 procedures. Europace 2018;20:1776–82. [DOI] [PubMed] [Google Scholar]
- 10. Doldi F, Gessler N, Anwar O, Kahle AK, Scherschel K, Rath Bet al. In-hospital mortality and major complications related to radiofrequency catheter ablations of over 10 000 supraventricular arrhythmias from 2005 to 2020: individualized case analysis of multicentric administrative data. Europace 2023;25:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner Aet al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert Jet al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tonchev IR, Nam MCY, Gorelik A, Kumar S, Haqqani H, Sanders Pet al. Relationship between procedural volume and complication rates for catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2021;23:1024–32. [DOI] [PubMed] [Google Scholar]
- 14. Holmqvist F, Kesek M, Englund A, Blomstrom-Lundqvist C, Karlsson LO, Kenneback Get al. A decade of catheter ablation of cardiac arrhythmias in Sweden: ablation practices and outcomes. Eur Heart J 2019;40:820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kesek M. Ablation procedures in Sweden during 2007: results from the Swedish Catheter Ablation Registry. Europace 2009;11:152–4. [DOI] [PubMed] [Google Scholar]
- 16. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn Bet al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018;137:961–72. [DOI] [PubMed] [Google Scholar]
- 17. Maisch B, Ristic AD, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone; the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J 2002;23:1503–8. [DOI] [PubMed] [Google Scholar]
- 18. Imazio M, Brucato A, Rovere ME, Gandino A, Cemin R, Ferrua Set al. Colchicine prevents early postoperative pericardial and pleural effusions. Am Heart J 2011;162:527–532.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy of individuals that were investigated in the study. The data will be shared on reasonable request to the corresponding author provided that this in accordance with the institutional ethical guidelines as well as regulation and legislation.