Abstract
Background and Objectives
In the United States, the risk of stroke is greater among Black compared with that among White individuals. However, the reasons for the difference in stroke incidence are not fully elucidated. We aimed to identify metabolites that account for higher prevalent hypertension and incident ischemic stroke among Black adults.
Methods
We used a stroke case cohort nested within the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Targeted metabolomic profiling of 162 plasma metabolites was performed by liquid chromatography–tandem mass spectrometry. We identified metabolites that were associated with prevalent hypertension and incident ischemic stroke and mediated the relationship between hypertension and ischemic stroke by weighted logistic regression, Cox proportional hazard model, and inverse odds ratio weighting mediation analysis.
Results
Incident ischemic stroke cases adjudicated through April 1, 2019 (n = 1,075) were included in the study. The random cohort sample was derived from the full cohort using stratified sampling (n = 968). Among 162 metabolites, gluconic acid was associated with prevalent hypertension in Black adults (odds ratio [OR] 1.86, 95% CI 1.39–2.47, p = 2.58 × 10−5) but not in White adults (OR 1.00, 95% CI 0.80–1.24, p = 0.97; p for interaction = 4.57 × 10−4). Gluconic acid also demonstrated an association with incident ischemic stroke among Black participants (hazard ratio [HR] 1.53, 95% CI 1.28–1.81, p = 1.76 × 10−6) but not White participants (HR 1.16, 95% CI 1.00–1.34, p = 0.057; p for interaction = 0.019). In mediation analysis, gluconic acid mediated 25.4% (95% CI 4.1%–46.8%, p = 0.02) of the association between prevalent hypertension and incident ischemic stroke among Black individuals. Specific socioeconomic factors were linked to elevated gluconic acid level among Black adults in multivariable analysis, including a Southern dietary pattern (β = 0.18, 95% CI 0.08–0.28, p < 0.001), lower educational attainment (β = 0.45, 95% CI 0.19–0.72, p = 0.001), and a lack of exercise (β = 0.26, 95% CI 0.01–0.51, p = 0.045).
Discussion
Gluconic acid is associated with prevalent hypertension and incident ischemic stroke and mediates the relationship between hypertension and ischemic stroke in Black but not White adults. Gluconic acid is a biomarker that is associated with social determinants of health including a Southern diet, low educational attainment, and low physical activity.
In the United States, the risk of stroke is consistently greater among Black compared with that among White individuals.1-3 However, the underlying mechanisms responsible for the racial disparities of stroke incidence remain to be fully elucidated. Previous research in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study have demonstrated that traditional stroke risk factors are more common among Black when compared with those among White individuals and account for approximately half of the difference in stroke incidence.4 Among these risk factors, hypertension is the most potent and accounts for 50% of the combined risk effect for incident stroke.4,5 Moreover, the impact of elevated blood pressure has shown to increase stroke risk three times greater in Black compared with that in White individuals.6
Metabolomic profiling can provide insight into the interaction between endogenous metabolic processes and environmental factors that influence disease development.7,8 This has led to novel insights into cardiovascular disease, stroke, and metabolic syndrome.9,10 Previous work by our group identified novel metabolites that associated with incident stroke independent of other traditional stroke risk factors.11 In this study, we hypothesized that circulating metabolites could provide insight into the racial disparities in the rates of hypertension and stroke. We further hypothesized that candidate metabolites may mediate the relationship between hypertension and stroke and account for a larger impact of hypertension on stroke among Black individuals.
The objectives of this study were first to identify metabolites that were associated with prevalent hypertension in the Black vs White population. Second, we sought to determine whether these metabolites also accounted for race-specific associations with incident stroke. Furthermore, we aimed to determine whether the candidate metabolites mediated the association between hypertension and the risk of incident of ischemic stroke among Black individuals. Finally, because racial categories may represent several identified socioeconomic factors and determinants of health, we assessed which factors were associated with the candidate metabolites.
Methods
Study Design and Population
The REGARDS study is a national population-based, prospective cohort study of 30,239 non-Hispanic Black and White community-dwelling participants aged 45 years or older. Details of the study design have been described elsewhere.12 In brief, participants were recruited and enrolled between January 2003 and October 2007. Black individuals and those living in southeastern United States, “The Stroke Belt,” including Alabama, Arkansas, Georgia, Indiana, Kentucky, Louisiana, Mississippi, North and South Carolina, Tennessee, and Virginia, were oversampled.13 Demographic, clinical, and lifestyle information was collected by computer-assisted telephone interview and a baseline in-person visit. During the in-person visit, blood samples were collected, sent to a central laboratory, and stored at −80°C until metabolomic profiling.11 Participants or their proxies were followed up by telephone interview at 6-month intervals to ascertain hospitalizations and identify suspected stroke events.
In this analysis, we used the stroke case cohort nested within the REGARDS study. The case cohort consisted of all stroke cases and a random cohort sampled from the overall cohort. Stroke cases included all participants who had an incident stroke from the time of enrollment through April 1, 2019. The random cohort samples (controls) included participants who were sampled from the full REGARDS cohort. The controls were selected by a stratified sampling approach based on age, race, and sex to ensure sufficient representation of high-risk groups. This sampling design has been used and described in prior REGARDS studies.11,14-18 In this analysis, participants who were lost to follow-up, had a history of stroke at the time of enrollment or had no plasma available for metabolite measurements were excluded. Participants with hemorrhagic stroke or no record of stroke type were also excluded from the final analysis.
Prevalent Hypertension and Incident Ischemic Stroke
Prevalent hypertension was defined by either (1) blood pressure measurement at baseline during the in-person visit, (2) self-reported usage of antihypertensive medications, or (3) a self-reported history of hypertension. The blood pressure was recorded as the average of 2 measurements after the participant had been seated for 5 minutes. A systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥80 mm Hg was used to define hypertension based on the 2017 American College of Cardiology/American Heart Association guideline definition.19
Methods for incident stroke ascertainment in the REGARDS study have been previously described in detail.2 In brief, hospitalization records were obtained for any participants with symptoms potentially consistent with stroke or who died. After an initial review by a study nurse, adjudicated stroke events were determined by a team of stroke experts after reviewing medical records. Ischemic stroke was defined as focal neurologic deficit lasting >24 hours or nonfocal neurologic symptoms consistent with stroke on neuroimaging according to the definition from the World Health Organization.20
Covariates
Covariates evaluated in this study were based on risk factors described in the Framingham Stroke Risk Score.21 In the REGARDS study, race was self-identified by the participants during the baseline interview and were given the choice of White, Black or African American, Asian, Native Hawaiian or Other Pacific Islander, American Indian, Alaska Native, or other race. Potential participants were also asked whether they were Hispanic or Latino. Only non-Hispanic Black or White participants were eligible for and enrolled in the REGARDS study. Information on sex and smoking status was also obtained by questioning the participants in the baseline interview. Diabetes mellitus was defined by self-reported current usage of diabetic medication, a fasting blood glucose measurement ≥126 mg/dL, or a nonfasting blood glucose ≥200 mg/dL. Coronary heart disease was determined by a self-reported history of myocardial infarction, coronary revascularization procedure, or electrocardiographic evidence of prior myocardial infarction at baseline. Atrial fibrillation was based on a self-reported diagnosis by physician or electrocardiographic evidence of atrial fibrillation on the baseline electrocardiogram. Left ventricular hypertrophy was defined by electrocardiographic criteria on the baseline electrocardiogram.
Metabolomic Profiling
A total of targeted 162 circulating plasma metabolites were measured by liquid chromatography–tandem mass spectrometry. Details of the metabolite measurement are provided elsewhere.11,22-24 In brief, polar metabolite extraction was performed using protein precipitation from 30 µL of EDTA plasma.11 Metabolites were separated using dual infinity II 1290 high-performance liquid chromatography pumps (Agilent, Santa Clara, CA) on an Xbridge Amide column (2.1 × 100 mm 3.5 µm; Waters, Milford, MA). For detection, a 6495 triple-quadrupole mass spectrometer was used (Agilent). Peak integration and analysis were performed using MassHunter QQQ Quantitative Analysis software (Agilent). Peaks were quality controlled and normalized to the nearest human pooled plasma samples that were injected at regular intervals after every 10 injections. Because the metabolite levels did not conform to typical distributional assumptions, all values were rank based inverse normal transformed before statistical analysis.
Statistical Analyses
Baseline characteristics were presented as mean ± SD and percentage for continuous and categorical variables, respectively. Sample weighting was used in all analyses to account for the stratified sampling of the random cohort sample as previously described.11,16,25
The goal of this study was to identify race-specific metabolites for prevalent hypertension that accounted for higher incident ischemic stroke among Black adults. To approach this, we divided our analysis into sequential steps. First, we examined a race-by-metabolite interaction in a weighted logistic regression adjusted for age, sex, and race to identify metabolites that differed in association with prevalent hypertension by race. At this stage, race was assumed to be an effect modifier in the association between metabolites and prevalent hypertension. To minimize the risk for false discovery, the Benjamini-Hochberg procedure was used to set a 10% false discovery rate (FDR) for 162 tests.26
Next, to identify whether the candidate metabolites that met the statistical significance criteria in the first step also differed in association with incident stroke by race, we assessed a race-by-metabolite interaction in a weighted Cox proportional hazard model. At this stage, race was also considered an effect modifier. A base model included adjustment for age, sex, race, and age-by-race interaction similar to previous REGARDS studies.11,14,15 A fully adjusted model included the following stroke risk factors: systolic blood pressure, diabetes mellitus, current smoking status, atrial fibrillation, left ventricular hypertrophy, and coronary heart disease. Participants in the random cohort who developed ischemic stroke during the observation period during analyses were censored during stroke onset as detailed in prior studies.11,27 Common metabolites that showed association with prevalent hypertension and incident stroke in Black but not White participants were carried forward in subsequent mediation analysis.
Third, to determine whether candidate metabolites were a mediator in the relationship between hypertension and risk of incident ischemic stroke, mediation analysis was performed using the inverse odds ratio (OR) weighting method.28 Because the goal was to identify metabolites that could account for the higher stroke risk in Black adults, mediation was conducted in the Black race stratum. In brief, a logistic regression model between hypertension and the metabolite mediator was fit, adjusting for age and sex. Then the inverse odds weight was calculated by taking the inverse of the predicted OR from the logistic regression model. The direct and total effects of hypertension on incident stroke were calculated by fitting the Cox proportional hazard model of incident ischemic stroke on hypertension adjusted for age and sex, with and without applying inverse odds weight, respectively. The indirect effect (i.e., the percent mediation of metabolite on the relationship between prevalent hypertension and incident stroke) was calculated from the total and direct effect difference. The 95% CI of the indirect effect was calculated from bootstrapping (n = 500).
Because self-identification of race is a social construct encompassing socioeconomic status, geographic origin, and lifestyle factors,29-31 we sought to explore how these factors associated with the level of candidate metabolites using weighted linear regression adjusted for age and sex among Black adults. Factors included in the analysis were income (below vs above $35,000), level of education (high school level vs greater than high school level), having health insurance, living region (belt, buckle, and nonbelt), living area (rural, mixed, and urban), exercise (any vs none), and adherence to a Southern diet.32,33 Income, education, and exercise were dichotomized, similar to that of a previous REGARDS study.33 All statistical analyses were performed using STATA version 14.2 (StataCorp, LLC, College Station, TX)
Standard Protocol Approvals, Registrations, and Patient Consents
All participants provided written informed consent. The study was approved by all participating institutional review boards. The metabolomics analysis was approved by the Mass General Brigham institutional review board. This study is in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement.
Data Availability
Data for this study are available to qualified investigators on reasonable request to the corresponding author.
Results
Study Population and Baseline Characteristics
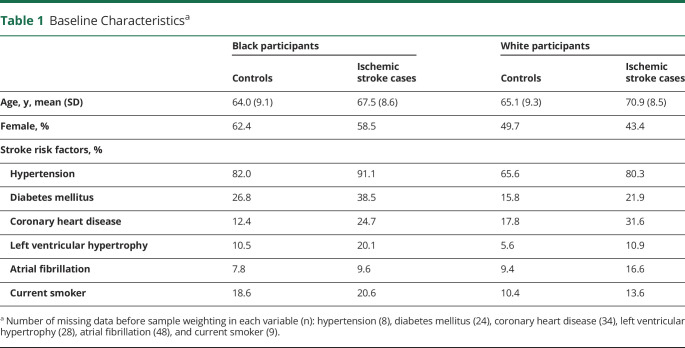
In this case cohort study, there were 1,404 stroke cases adjudicated through April 1, 2019 that were compared with 1,127 random cohort participants during an average follow-up time of 7.1 ± 4.5 years. Participants who were lost to follow-up (n = 16), had a medical history of stroke (n = 308), had no plasma available (n = 110), or had hemorrhagic stroke (n = 122) were excluded. Final analyses included a total of 2,043 participants, which included 1,075 ischemic stroke cases (41% Black individuals) and 968 participants in the random cohort sample (48% Black individuals; Figure 1). In comparison with White adults, Black adults had a higher prevalence of all stroke risk factors with an exception for coronary heart disease and atrial fibrillation (Table 1). Participants who developed ischemic stroke were older and had a higher rate of stroke risk factors compared with the random cohort sample (Table 1).
Figure 1. Flow Diagram of Study Population.
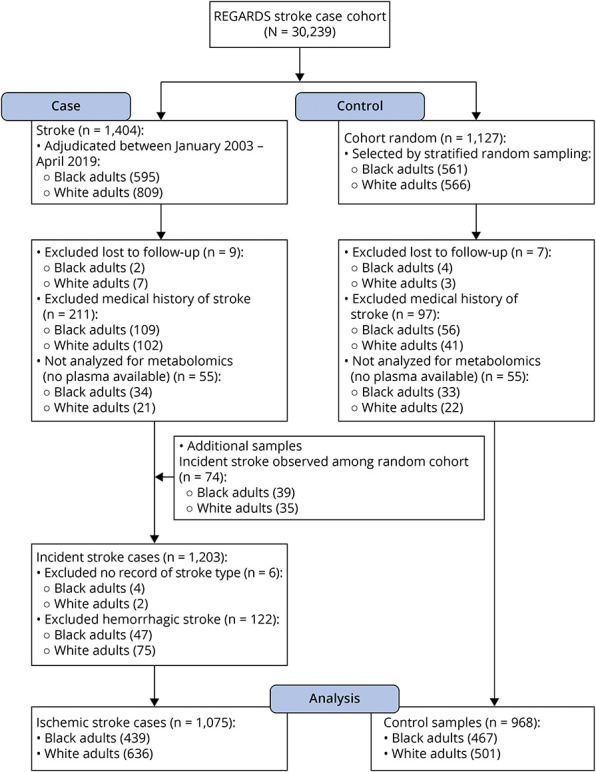
REGARDS = REasons for Geographic and Racial Differences in Stroke.
Table 1.
Baseline Characteristicsa
Metabolite Association With Prevalent Hypertension
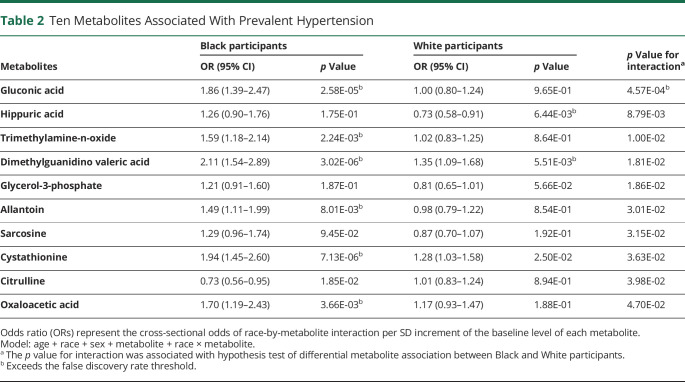
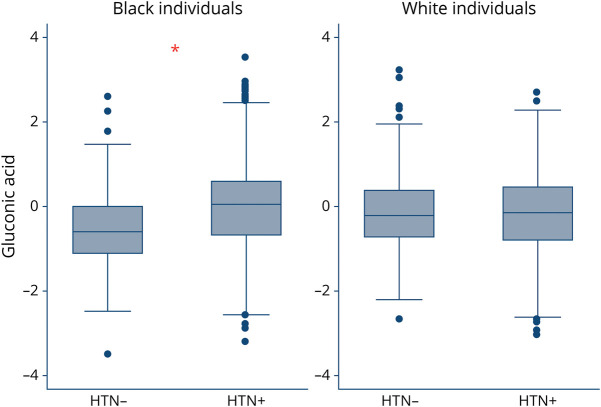
Among 162 metabolites, there were 10 metabolites that demonstrated a race-by-metabolite interaction in association with prevalent hypertension with a nominal p value <0.05. These metabolites were gluconic acid, hippuric acid, trimethylamine-n-oxide, dimethylguanidino valeric acid, glycerol-3-phosphate, allantoin, sarcosine, cystathionine, citrulline, and oxaloacetic acid. However, only gluconic acid was associated with prevalent hypertension after FDR adjustment. Accordingly, gluconic acid was associated with hypertension among Black participants (OR 1.86, 95% CI 1.39–2.47, p = 2.58 × 10−5) but not White participants (OR 1.00, 95% CI 0.80–1.24, p = 0.97); p for interaction = 4.57 × 10−4 (Table 2). Figure 2 demonstrates the box plots for gluconic acid levels among Black adults with and without hypertension (p < 0.001) and White adults with and without hypertension (p = 0.62). We also performed a sensitivity analysis to rule out the possibility that residual confounding among the stroke case group was driving the gluconic acid associations. Similar to our main findings, gluconic acid was the only metabolite that demonstrated a race-by-metabolite interaction in association with prevalent hypertension that surpassed the FDR threshold in random cohort sample (eTable 1, links.lww.com/WNL/C746).
Table 2.
Ten Metabolites Associated With Prevalent Hypertension
Figure 2. Gluconic Acid Level in Vary by Race and Hypertension Status.
Box and whisker plots of plasma gluconic acid level in Black and White individuals without hypertension (HTN−) and with hypertension (HTN+). *p < 0.001.
Metabolite Association With Incident Ischemic Stroke
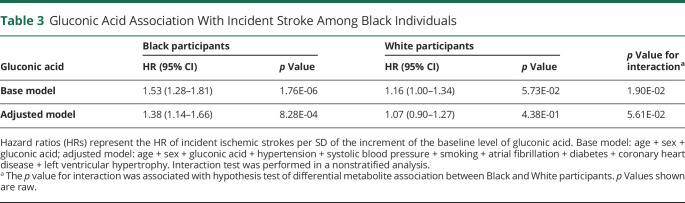
We next determined whether gluconic acid had a difference by race in its association with incident stroke. In Black individuals, gluconic acid was associated with incident ischemic stroke in both a base model (hazard ratio [HR] 1.53, 95% CI 1.28–1.81, p = 1.76 × 10−6) and a model adjusted for traditional stroke risk factors including systolic blood pressure, diabetes mellitus, current smoking status, atrial fibrillation, left ventricular hypertrophy, and coronary heart disease (HR 1.38, 95% CI 1.14–1.66, p = 8.28 × 10−4) (Table 3). However, gluconic acid was not associated with incident ischemic stroke among White individuals, and accordingly, gluconic acid demonstrated a race-by-metabolite interaction (base model, p for interaction = 0.02), although not in the fully adjusted model (p for interaction = 0.056).
Table 3.
Gluconic Acid Association With Incident Stroke Among Black Individuals
Gluconic Acid as a Mediator Between Hypertension and Ischemic Stroke
Mediation analysis was performed among Black adults to determine whether gluconic acid was a mediator of the relationship between hypertension and incident ischemic stroke. Using inverse OR weighting mediation, gluconic acid mediated 25.4% (95% CI 4.1%–46.8%; p = 0.02) of the association between prevalent hypertension and incident ischemic stroke among Black individuals (eTable 2, links.lww.com/WNL/C746).
Association of Gluconic Acid Level and Socioeconomic Behavioral Factors
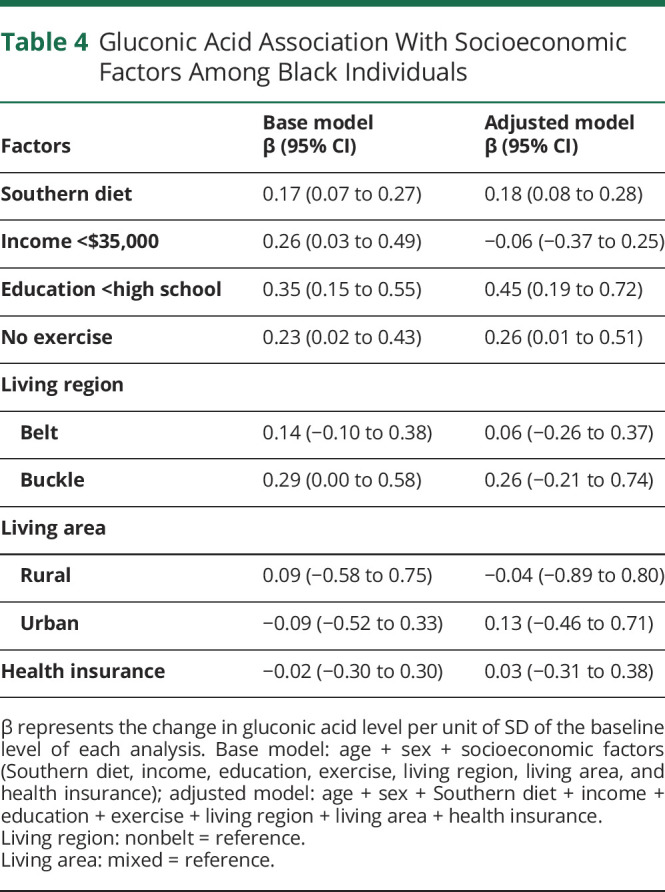
We examined whether race categories were associated with the level of gluconic acid in a linear regression model adjusted for age and sex in the overall participants. Gluconic acid level was not associated with race (β = 0.05, 95% CI −0.09 to 0.18, p = 0.497). To gain additional insight into the socioeconomic, geographic, and lifestyle factors underlying racial disparities of stroke,28-30 we assessed the association between these factors and gluconic acid level in Black participants. Adherence to a Southern diet (β = 0.17, 95% CI 0.07–0.27, p = 0.001), a lower income category (β = 0.26, 95% CI 0.03–0.49, p = 0.029), lower education (β = 0.35, 95% CI 0.15–0.55, p = 0.001), and lack of exercise (β = 0.23, 95% CI 0.02–0.43, p = 0.032) were each associated with a higher gluconic acid level. Other socioeconomic factors including living region, living area, and health insurance status were not associated with gluconic acid level (Table 4). In multivariable regression, a Southern dietary pattern (β = 0.18, 95% CI 0.08–0.28, p < 0.001), lower educational attainment (β = 0.45, 95% CI 0.19–0.72, p = 0.001), and lack of exercise (β = 0.26, 95% CI 0.01–0.51, p = 0.045) remained associated with gluconic acid level (Table 4).
Table 4.
Gluconic Acid Association With Socioeconomic Factors Among Black Individuals
Discussion
In this study, we identified gluconic acid as a circulating metabolite that was associated with hypertension and ischemic stroke among Black but not White adults. Gluconic acid also partially mediated the relationship between hypertension and ischemic stroke among Black adults. Finally, we found that gluconic acid level was associated with a Southern diet, low educational attainment, and less physical activity. These findings highlight gluconic acid as a disease risk biomarker for racial differences in stroke that are associated with several social determinants of health.
Gluconic acid is a polyhydroxycarboxylic acid that is formed by glucose fermentation through glucose oxidase activity and is potentially produced by the gut microbiome.34 The production of hydrogen peroxide during gluconic acid formation reflects the potential role of gluconic acid as a biomarker of oxidative stress.23 Due to its availability in food, production by the gut microbiome, and relation to oxidative stress, gluconic acid can be considered as a dietary-related oxidative stress marker. Gluconic acid has been demonstrated to be involved in the pathophysiology of diseases related to oxidative stress. For example, gluconic acid was associated with cytotoxic brain injury after acute ischemic stroke.23 This finding supports the potential role of gluconic acid in the pathogenesis of ischemic stroke. This interpretation is further supported by a previous study where we found that stroke-related metabolites were likely related to environmental exposures rather than genetic variation.11 Moreover, we extended these findings to show that many of these metabolites provided a link between dietary patterns and the risk of stroke.18
In this study, we found that the association with gluconic acid was specific to Black participants. There was no difference in gluconic acid between all Black and White participants, suggesting that there is not an intrinsic biological difference. Because race is a social construct rather than a biological variable and encompasses several potential social determinants of health that affect stroke risk,29,35 metabolomics is well-suited to gain an insight into these disparities. Variations in socioeconomic patterns,29,31 lifestyle, and diet can alter metabolism and have an impact on disease manifestation.36 In this study, we demonstrated that racial disparities in hypertension and stroke could be reflected in plasma metabolites. Among the potential social determinants of health, gluconic acid level was strongly associated with a Southern diet, low education, and lack of exercise. As such, gluconic acid is a metabolite biomarker that is associated with social and lifestyle factors including dietary pattern, education, and physical activity. Collectively, this analysis suggests that some of the racial disparities in hypertension and stroke incidence are reflected in the quantitative measurement of a blood biomarker, which is associated with differences in lifestyle. Because these factors are potentially modifiable, we envision that our findings could provide a potential roadmap for future personalized dietary and behavioral interventions that use plasma metabolites such as gluconic acid as a marker. For example, it is currently unknown how substantial a dietary change or the dose or intensity of exercise is needed to reduce the risk of stroke. A surrogate marker such as gluconic acid could provide a targetable trait for stroke prevention and future interventional studies.
The strengths in this study include the case cohort design, which is nested within the biracial REGARDS study, a cohort that is specifically designed to address the underpinnings of racial disparities of stroke. There were a large number of ischemic stroke cases in our study. In addition, the targeted metabolomic profiling approach using liquid chromatography and tandem quadrupole mass spectrometry allows the detection of a substantial list of metabolites with high sensitivity and specificity, although there are tradeoffs regarding the number of metabolites covered. However, there are also some limitations in this study. The association between metabolite biomarker and prevalent hypertension in this study is cross-sectional. Therefore, the temporality of the metabolite and prevalent hypertension cannot be definitively established. It is also likely that social determinants of health precede hypertension and metabolites are mediators of the association. A future cohort study design that provides information regarding incident hypertension would bridge the gap in this study. There may also be other factors associated with gluconic acid level. Because the main purpose of this study is to examine potential metabolites that could account for racial disparities, we focus our analysis on factors associated with gluconic acid, primarily on socioeconomic and lifestyle-related factors.
In conclusion, this study demonstrated that gluconic acid is a circulating metabolite that was associated with hypertension and ischemic stroke among Black US adults. Gluconic acid is a candidate biomarker that associated with diet, low educational attainment, and lack of exercise and predicts the risk of future stroke.
Acknowledgment
The authors thank the other investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: uab.edu/soph/regardsstudy/.
Glossary
- FDR
false discovery rate
- HR
hazard ratio
- OR
odds ratio
- REGARDS
REasons for Geographic and Racial Differences in Stroke
Appendix. Authors
Study Funding
This work was supported by the NIH R01 NS099209 (W.T. Kimberly), American Heart Association 17CSA33550004 (W.T. Kimberly). The REGARDS is supported by cooperative agreement U01 NS041588 cofunded by the National Institute of Neurological Disorders and Stroke and the National Institute on Aging, NIH, Department of Health and Human Service.
Disclosure
The authors declare that they have no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111(10):1327-1331. [DOI] [PubMed] [Google Scholar]
- 2.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. [DOI] [PubMed] [Google Scholar]
- 4.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42(12):3369-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44(6 suppl 1):S126-S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard G, Lackland DT, Kleindorfer DO, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173(1):46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson JK, Wilson ID. Understanding ‘global’ systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2(8):668-676. [DOI] [PubMed] [Google Scholar]
- 8.Sidorov E, Sanghera DK, Vanamala JKP. Biomarker for ischemic stroke using metabolome: a clinician perspective. J Stroke. 2019;21(1):31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126(9):1110-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vojinovic D, Kalaoja M, Trompet S, et al. Association of circulating metabolites in plasma or serum and risk of stroke. Neurology. 2021;96(8):e1110-e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ament Z, Patki A, Chaudhary N, et al. Nucleosides associated with incident ischemic stroke in the REGARDS and JHS cohorts. Neurology. 2022;98(21):e2097-e2107. doi: 10.1212/WNL.0000000000200262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. [DOI] [PubMed] [Google Scholar]
- 13.Howard VJ, Madsen TE, Kleindorfer DO, et al. Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol. 2019;76(2):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushman M, Judd SE, Howard VJ, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke. 2014;45(6):1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaudhary NS, Bridges SL Jr, Saag KG, et al. Severity of hypertension mediates the association of hyperuricemia with stroke in the REGARDS case cohort study. Hypertension. 2020;75(1):246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson NC, Cushman M, Judd SE, et al. Associations of coagulation factors IX and XI levels with incident coronary heart disease and ischemic stroke: the REGARDS study. J Thromb Haemost. 2017;15(6):1086-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panwar B, Jenny NS, Howard VJ, et al. Fibroblast growth factor 23 and risk of incident stroke in community-living adults. Stroke. 2015;46(2):322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhave VM, Ament Z, Patki A, et al. Plasma metabolites link dietary patterns to stroke risk. Ann Neurol. 2023;93(3):500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. [DOI] [PubMed] [Google Scholar]
- 20.Stroke—1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20(10):1407-1431. [DOI] [PubMed] [Google Scholar]
- 21.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312-318. [DOI] [PubMed] [Google Scholar]
- 22.Nelson SE, Ament Z, Wolcott Z, Gerszten RE, Kimberly WT. Succinate links atrial dysfunction and cardioembolic stroke. Neurology. 2019;92(8):e802-e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ament Z, Bevers MB, Wolcott Z, Kimberly WT, Acharjee A. Uric acid and gluconic acid as predictors of hyperglycemia and cytotoxic injury after stroke. Transl Stroke Res. 2021;12(2):293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimberly WT, O'Sullivan JF, Nath AK, et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight. 2017;2(9):e92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakai NA, Judd SE, Alexander K, et al. ABO blood type and stroke risk: the REasons for Geographic and Racial Differences in Stroke study. J Thromb Haemost. 2014;12(4):564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811-818. [DOI] [PubMed] [Google Scholar]
- 27.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165-1172. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol. 2015;181(5):349-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keeys M, Baca J, Maybank A. Race, racism, and the policy of 21st century medicine. Yale J Biol Med. 2021;94(1):153-157. [PMC free article] [PubMed] [Google Scholar]
- 30.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35(4):407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann NY Acad Sci. 2010;1186(1):69-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judd SE, Gutiérrez OM, Newby PK, et al. Dietary patterns are associated with incident stroke and contribute to excess risk of stroke in black Americans. Stroke. 2013;44(12):3305-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard G, Cushman M, Moy CS, et al. Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA. 2018;320(13):1338-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harder JM, Guymer C, Wood JPM, et al. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or rapamycin. Proc Natl Acad Sci USA. 2020;117(52):33619-33627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant BE, Jordan A, Clark US. Race as a social construct in psychiatry research and practice. JAMA Psychiatry. 2022;79(2):93-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guasch-Ferré M, Bhupathiraju SN, Hu FB. Use of metabolomics in improving assessment of dietary intake. Clin Chem. 2018;64(1):82-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study are available to qualified investigators on reasonable request to the corresponding author.