Abstract
Exposure of cells to ionizing radiation inhibits DNA replication in a dose-dependent manner. The dose response is biphasic and the initial steep component reflects inhibition of replicon initiation thought to be mediated by activation of the S-phase checkpoint. In mammalian cells, inhibition of replicon initiation requires the ataxia telagiectasia mutated (ATM) gene, a member of the phosphatidyl inositol kinase-like (PIKL) family of protein kinases. We studied the effect on replicon initiation of another member of the PI-3 family of protein kinases, the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) by measuring either total DNA synthesis, or size distribution of nascent DNA using alkaline sucrose gradient centrifugation. Exposure of human cells proficient in DNA-PKcs (HeLa or M059-K) to 10 Gy inhibited replicon initiation in a time-dependent manner. Inhibition was at a maximum 1 h after irradiation and recovered at later times. Similar treatment of human cells deficient in DNA-PKcs (M059-J) inhibited replicon initiation to a similar level and with similar kinetics; however, no evidence for recovery, or only limited recovery, was observed for up to 8 h after irradiation. In addition a defect was observed in the maturation of nascent DNA. Similarly, a Chinese hamster cell line deficient in DNA-PKcs (irs-20) showed little evidence for recovery of DNA replication inhibition up to 6 h after irradiation, whereas the parental CHO cells showed significant recovery and an irs-20 derivative expressing the human DNA-PKcs complete recovery within 4 h. Normal kinetics of recovery were observed in xrs-5 cells, deficient in Ku80; in 180BR cells, deficient in DNA ligase IV; as well as XR-1 cells, deficient in XRCC4, an accessory factor of DNA ligase IV. Since all these cell lines share the DNA double strand break rejoining defect of M059-J and irs20 cells, the lack of recovery of DNA replication in the latter cells may not be attributed entirely to the prolonged presence of unrepaired DNA dsb. We propose that DNA-PKcs, in addition to its functions in the rejoining of DNA dsb and in DNA replication, also operates in a pathway that in normal cells facilitates recovery of DNA replication after irradiation.
INTRODUCTION
Inhibition of DNA replication in eukaryotic cells is one of the earliest effects of radiation to be reported and quantitated (reviewed in 1–6). The response is biphasic with a steep (Do ~10 Gy) and a shallow (Do ~100 Gy) component that derive from an inhibition of replicon initiation and DNA chain elongation, respectively (7–9). Evidence accumulates that inhibition of replicon initiation is a regulated process that operates via the activation of a checkpoint in S-phase (5,6), and which can be viewed as part of the overall cellular response to DNA damage. In line with this hypothesis, evidence was presented for a radiation-induced trans-acting inhibitor of DNA replication (10–15), and for enhanced inhibition following upregulation of the ras signaling pathway (16–19).
The gene mutated in cells of patients suffering from Ataxia Telangiectasia (ATM) has been shown to be a central component of the pathway that regulates DNA replication after DNA damage (3,4,20,21). Recently, evidence has been presented that chk2, the human homolog of the Saccharomyces cerevisiae RAD53 and Schizosaccharomyces pombe Cds1, is downstream of ATM, probably acting by inhibiting Cdc25C through phosphorylation of serine-216 (22,23). It is thought that Cdc25C activates cyclin A/cdk2 which is required for sustained DNA replication and is down-regulated after irradiation (24–27). Recent data in yeast suggest that Rad53 may act directly to regulate DNA replication by phosphorylating Cdc7p/Dbf4p, a kinase required for initiation of eukaryotic DNA replication (28). Thus, one component of the regulation of DNA replication in irradiated cells may involve the activation of a signal transduction pathway that inhibits replicon initiation by preventing the activation of cyclin A/cdk2 and/or Cdc7/Dbf4. Downstream targets on the DNA replication machinery of these signaling pathways have not been identified, although it is long known that the 34 kDa subunit of RPA (RPA2) is phosphorylated by cyclin A/cdk2 (29–32). Yet, a role for this phosphorylation in the regulation of DNA replication after DNA damage remains to be established (reviewed in 33).
Biochemical experiments provide evidence for additional regulatory mechanisms for DNA replication following irradiation. Thus, extracts of cells exposed to ionizing radiation have a reduced ability in supporting DNA replication (11–13,15). Recent results indicate that this in vitro inhibition has two components. The first involves the activation of DNA-PK that targets and inactivates the SV40 TAg, an essential factor for the initiation (and elongation) steps of DNA replication in this in vitro assay (15). The other component involves RPA and is mediated mainly by a reduction in the levels rather than in the activity of the protein (15). Activation of DNA-PK has also been implicated in the regulation of DNA replication in cells exposed to ultraviolet light (34). DNA-PK is known to phosphorylate in vitro a number of proteins including RPA2, p53, TAg and several transcription factors (35–37). Several studies indicate that DNA-PK is the predominant RPA2 kinase both in vivo and in vitro (reviewed in 33). Taken together these results suggest a putative role for DNA-PK in the regulation of DNA replication in irradiated cells; however, the mechanism of this regulation remains to be elucidated.
Despite the biochemical evidence implicating DNA-PK in the regulation of DNA replication after DNA damage, genetic studies have failed so far to implicate this kinase in radiation-induced inhibition of DNA replication. Thus, cells from scid mice, known to be deficient in the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) (38) show an inhibition of DNA synthesis after irradiation that is comparable to that measured in cells from wild-type animals (39). Furthermore, inhibition of DNA synthesis after irradiation is similar in two cell lines established from a single tumor specimen obtained from a patient with glioblastoma (40), despite the fact that one of these cell lines (M059-J) has undetectable DNA-PK activity (41) due to a 20-fold reduction in DNA-PKcs message stability (42). These apparently contradictory observations from biochemical and genetic studies led us to initiate systematic experiments to investigate the effect of ionizing radiation on DNA synthesis in cells deficient in DNA-PKcs. Here, we report that while deficiency in DNA-PKcs has no directly measurable effect on DNA synthesis inhibition after irradiation, it causes a pronounced defect in the ability of cells to recover from this inhibition. These results are discussed vis-à-vis available biochemical data, and models for the regulation of DNA replication in irradiated cells are proposed.
MATERIALS AND METHODS
Cell culture and irradiation
S-HeLa cells were grown in modified Minimum Essential Medium (MEM) supplemented with 5% bovine calf serum and antibiotics. M059-J and M059-K cells were obtained from Dr Allalunis-Turner (Cross Cancer Institute, Edmonton, Canada) (40,41,43,44) and were cloned to obtain a homogeneous culture as determined by flow cytometry. Clone A from each cell line (M059-J/A, M059-K/A) was grown in a 1:1 F12/DMEM mixture supplemented with 10% bovine calf serum, antibiotics, non-essential amino acids and 2 mM glutamine. AT5BI cells were obtained from Dr Malcolm Paterson (Cross Cancer Institute, Edmonton, Canada), and were grown in MEM supplemented with 10% fetal bovine serum. 180BRM cells (45,46) were obtained by transfecting 180BR cells (kindly provided by Dr Colin Arlett, MRC Cell Mutation Unit, Brighton, UK and Dr Edmond Malaise, Institute Gustave-Roussy, Villejuif, France) with the pRNS-1 plasmid containing a replication deficient SV40 virus (47,48) and pooling clones resistant to 70 µg/ml G418. 180BRM cells have an extended life span, but are not immortalized. They were grown in MEM supplemented with 10% bovine calf serum. Untransformed 180BR cells were grown in MEM supplemented with 10% fetal bovine serum. Irs-20 is a Chinese hamster mutant cell-line with a defect in DNA-PKcs (49–51). K147 cells were obtained by transfecting irs-20 cells with a YAC containing the human DNA-PKcs (52). The above two cell lines as well as parental CHO cells were obtained from Dr Joel Bedford (Colorado State University, Fort Collins, CO) and were grown in McCoy’s 5A medium supplemented with 10% bovine calf serum. When required, 200 µg/ml G418 was added to the growth medium. Xrs-5 cells, a radiation sensitive Chinese hamster cell line with a defect in DNA dsb rejoining (53,54), were obtained from Dr Penny Jeggo (MRC Cell Mutation Unit, Brighton, UK) and are known to be deficient in Ku80 (55–57). They were grown in McCoy’s 5A medium supplemented with 10% fetal bovine serum and antibiotics. XR-1 cells, a radiation sensitive Chinese hamster cell line with a defect in DNA dsb rejoining (58–64), were obtained from Dr Thomas Stamato (Lankenau Hospital, Philadelphia, PA) and are known to be deficient in XRCC4, a cofactor of DNA ligase IV. They were grown in McCoy’s 5A medium supplemented with 10% bovine calf serum and antibiotics. All cell lines were grown at 37°C in an atmosphere of 5% CO2 and 95% air.
To evaluate DNA synthesis either by incorporation of radioactively labeled precursors into total DNA, or by alkaline sucrose gradient centrifugation (see below), 1.5 × 105 HeLa, or 1.0 × 105 CHO, irs-20, K147 cells, xrs-5, XR-1, M059-J/A or M059-K/A cells from a growing culture were seeded into 60-mm dishes and were allowed to grow for 2 days. In some experiments, 5.0 × 105 M059-J/A cells or 3.5 × 105 AT5BI cells were plated per 100 mm dish and were allowed to grow for 2 days. Initial numbers and dish size similar to those of AT5BI cells were also used for 180BRM, and 180BR cells. These conditions assured optimal growth characteristics and adequate material for our measurements. For reproducible evaluation of DNA synthesis after irradiation, it is particularly important to use actively growing cells. We have frequently observed a reduction in radiation-induced inhibition of DNA replication with cells used at a stage of reduced growth-rate.
Cells were exposed to X-rays at the indicated doses, at room temperature, and returned to 37°C. At various times thereafter, 3–5 µCi/ml of 3H-thymidine was added and cells were incubated at 37°C for 15 min to evaluate their DNA synthesis activity. After this period of incubation cells were processed either for measurement of total DNA synthesis, or for measurement of the size distribution of nascent DNA in alkaline sucrose density gradients as described below.
Irradiations were carried out with a Pantak X-ray machine operated at 320 kV, 10 mA with a 2 mm Al filter (effective photon energy ~90 kV), at a dose rate of 2.7 Gy/min. Dosimetry was performed with a Victoreen dosimeter that was used to calibrate an in-field ionization monitor.
Measurement of total DNA synthesis
The methods used to measure total DNA synthesis have been described (16,17). Briefly, cells were trypsinized, loaded on glass microfiber filters (Whatman GF/A), washed three times with a 10% solution of trichloroacetic acid (TCA), five times with deionized water, and incubated for 12 h in 0.5 ml of 0.5 N NaOH at 60°C. Samples were subsequently neutralized with 0.5 ml of 0.5 N HCl, supplied with scintillation fluid (Scintiverse, Fisher), and counted for 3H activity in a liquid scintillation counter (Packard TriCarb 2200CA). The rate of DNA synthesis for each sample was calculated from these measurements and is presented as the percentage of values obtained in sham-irradiated controls.
Alkaline sucrose gradient sedimentation
The size distribution of nascent DNA was measured in irradiated and non-irradiated cells with the help of 5–20% linear alkaline sucrose gradients (16,17). They were prepared in a buffer containing 0.1 M NaOH, 0.9 M NaCl, 0.01 M EDTA, pH 12.5, in 20 ml polyallomer tubes for a total volume of 17 ml. Cells were trypsinized and appropriately resuspended in PBS so that 50 µl of cell suspension contained 2.5 × 105 cells. They were gently layered on 0.3 ml of lytic solution (0.5 M NaOH, 0.02 M EDTA, pH 12.5 and 0.1% NP-40), in a cut 1 ml syringe, and incubated (after sealing to prevent evaporation) at room temperature for 3 h. Subsequently, lysate was gently layered on the top of the gradient and centrifugation was carried out for 90 min using an AH 627 rotor in a Sorval RC60 centrifuge, 26 000 r.p.m. at 20°C. After centrifugation, gradients were fractionated from the bottom in 1 ml fractions, DNA was precipitated with 10% TCA and loaded on glass fiber filters. Radioactivity into acid insoluble material was measured as described above. Results are presented as percentage of total 3H-thymidine activity per fraction. Where indicated, values obtained with irradiated cells were corrected by multiplying by the relative inhibition in total DNA synthesis to visualize, except for relative alterations in the size distribution of the DNA fragments, also the effect of radiation on the absolute abundance of these fragments.
RESULTS
Inhibition of DNA replication after irradiation
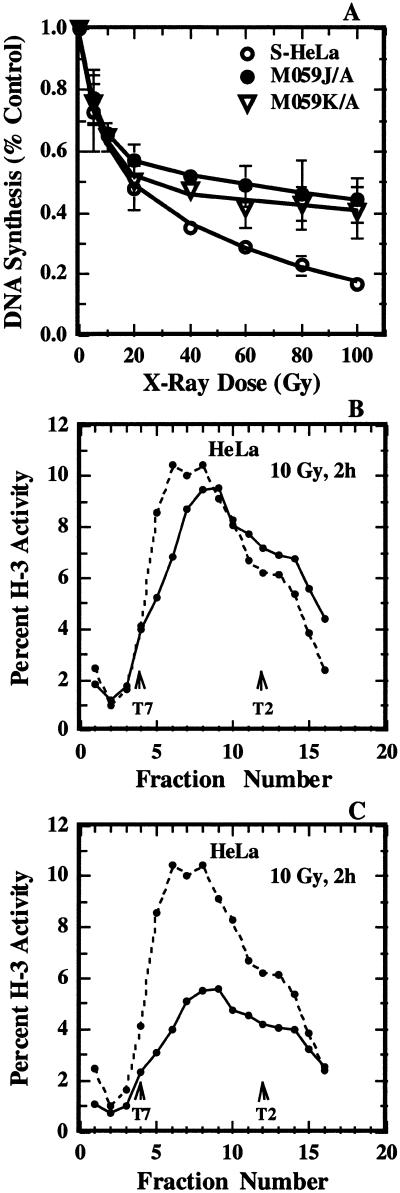
To evaluate inhibition of DNA replication, replicate cultures of S-HeLa, M059-K/A and M059-J/A cells were prepared and exposed to various doses of radiation. Cells were returned to 37°C for 1 h and subsequently 3H-thymidine was added for 15 min. The level of incorporation of radioactive precursor into acid insoluble material was measured as described in Materials and Methods and the results obtained are summarized in Figure 1A. As expected, radiation exposure inhibited ongoing DNA replication in HeLa cells in a dose dependent manner. The inhibition was biphasic with a steep and a shallow component, as reported previously for a great variety of human and rodent cell lines (1–6). A qualitatively similar response was obtained with M059-K/A and M059-J/A cells but the overall inhibition was slightly lower. There was no statistically significant difference in the levels of inhibition between M059-J/A and M059-K/A cells, despite the fact that the former cell line has no detectable levels of DNA-PK activity (41) due to a 20-fold reduction in DNA-PKcs message stability (42), whereas the latter cell line has normal levels of activity for this kinase. These results are in agreement with a previous report demonstrating that after irradiation, DNA replication is inhibited in M059-J cells with a sensitivity similar to that observed in DNA-PK proficient cells (M059-K) (40,41,65). It is interesting that the initial steep component of the inhibition, which reflects inhibition of replicon initiation, was similar in the three cell lines tested.
Figure 1.
Effects of radiation on DNA synthesis in human cell lines. (A) Total DNA synthesis after exposure of S-HeLa (open circles), M059-J/A (closed circles) or M059-K/A (triangles) cells to different doses of radiation. DNA replication activity was measured by a 15 min pulse with 3H-thymidine, given 1 h after irradiation. The mean and standard error from three experiments is shown. (B) Alkaline sucrose density gradient profile of HeLa cells exposed to either 0 (broken line) or 10 Gy (solid line) of X-rays and analyzed 2 h later. Plotted is the percentage of total activity in each fraction as a function of the fraction number. Sedimentation is from left to right. Early fractions contain small nascent DNA produced at the very early stages of DNA replication, and their reduction after irradiation reflects an inhibition of replicon initiation. The locations of sedimentation maxima for T7 (37 kb) and T2 (167 kb) phage DNA are also shown for calibration purposes. (C) The results shown in (B) have been replotted after consideration of the effect of radiation on total DNA synthesis.
Since in vitro data strongly implicate DNA-PK in the regulation of DNA replication in irradiated cells (15), we inquired whether DNA-PK deficiency generates subtle defects in the regulatory pathway that cannot be discerned in experiments measuring the effect of radiation on total DNA synthesis. For this purpose we carried out experiments using alkaline sucrose density centrifugation to evaluate specifically inhibition of replicon initiation and the consequences of DNA-PK deficiency on this endpoint. Figure 1 (B and C) shows representative results obtained with HeLa cells exposed to 10 Gy X-rays and analyzed 2 h later. Plotted in the figure is the percentage of total 3H-thymidine activity measured in each fraction as a function of fraction number. The upper panel in the figure shows the results obtained when gradients from non-irradiated and irradiated cells are analyzed independently, whereas the lower panel shows the same results normalized to consider the inhibition of total DNA synthesis.
As expected, radiation exposure inhibited replicon initiation manifested by a reduction in the activity of early fractions, which represent the top of the gradient and therefore low molecular weight material. Inhibition of chain elongation is negligible after exposure to this dose of radiation (see Introduction). Similar observations were also made in the results shown in the lower panel, but the inhibition of replicon initiation is more difficult to discern due to the overall reduction in DNA replication (50% in this experiment). Because the non-normalized way of presentation allows direct visualization of the effect of radiation on replicon initiation, it was adopted in all subsequent experiments.
Effect of DNA-PKcs on the regulation of DNA replication after DNA damage
First, we studied the effect of DNA-PKcs on inhibition of replicon initiation in cells exposed to different doses of X-rays. Results obtained with HeLa cells were compared to those obtained with M059-J cells and showed comparable levels of inhibition of replicon initiation for the same radiation dose (results not shown), confirming the results of Figure 1A.
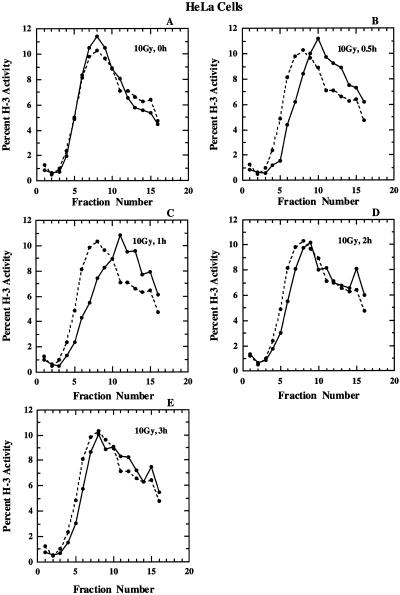
As a next step we compared kinetics of inhibition of replicon initiation, as well as of the ensuing recovery between M059-J/A and HeLa cells. Results obtained with HeLa cells exposed to 10 Gy and analyzed at different times thereafter are shown in Figure 2. In all panels results obtained with non-irradiated cells are included for comparison. There is no effect on the size distribution of nascent DNA immediately after exposure to 10 Gy. A pronounced inhibition becomes apparent 30 min after irradiation and is maintained for up to 1 h thereafter. A clear recovery in the size distribution of nascent DNA is observed 2 h after irradiation and leads at 3 h to a nascent DNA size distribution similar to that observed in non-irradiated control cells. We note here for later reference that the size distribution of nascent DNA after 3 h is similar between irradiated and non-irradiated cells both in the low molecular weight and as well as in the high molecular weight region of the gradient. This suggests that in HeLa cells the DNA metabolism returns to normal both in terms of replicon initiation, as well as in terms of maturation of nascent DNA into chromatin.
Figure 2.
Alkaline sucrose density gradient profiles for S-HeLa cells exposed to 10 Gy X-rays and analyzed at various times thereafter as indicated (solid line). Shown in all panels for comparison is the profile obtained with non-irradiated cells (broken line).
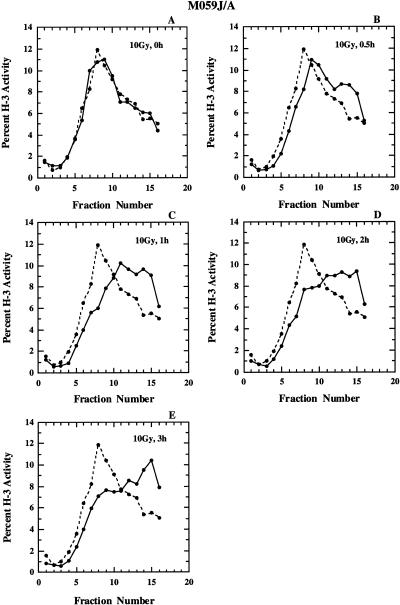
Figure 3 shows results similar to those of Figure 2 for M059-J/A cells. Similar to HeLa cells, irradiation has no effect on the nascent DNA size distribution when measured immediately after irradiation. A measurable effect is observed 30 min after irradiation that reaches a maximum at 1 h after irradiation. However, contrary to observations with HeLa cells, incubation of M059-J/A cells for longer times at 37°C does not lead to recovery as indicated by the persistence of lower levels of activity in early fractions. Furthermore, inspection of the gradients in the high molecular weight region (Fig. 3C–E) indicates the accumulation of nascent DNA at sizes larger than those observed in the non-irradiated controls suggesting defects in the maturation of nascent DNA.
Figure 3.
Alkaline sucrose density profiles of M059-J/A cells. Other details as in Figure 2.
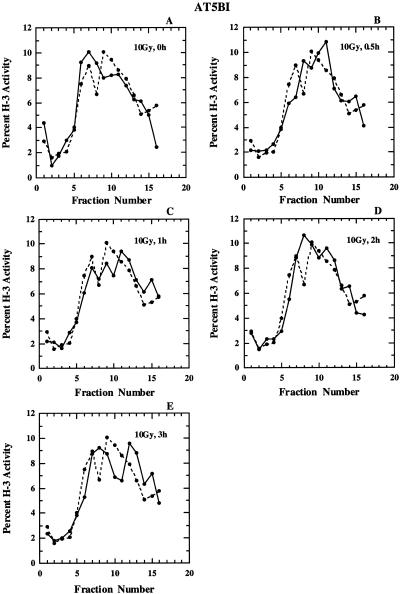
Cells from ataxia telagiectasia patients are sensitive to killing by ionizing radiation and show reduced inhibition of replicon initiation (3,4,20,21). In an attempt to compare the response of M059-J cells to that of AT cells under our experimental conditions, we exposed AT5BI cells to 10 Gy X-rays and measured the size distribution of nascent DNA at various times thereafter. The results obtained are shown in Figure 4. As expected, AT cells show no measurable variations in the nascent DNA size distribution at all times examined, in line with a lack of inhibition of replicon initiation. Also, the maturation of nascent DNA into high molecular weight material appears normal in AT cells. These results confirm earlier observations with these cells (see Introduction) and validate the methods used in the present study.
Figure 4.
Alkaline sucrose density profiles of AT5BI cells. Other details as in Figure 2.
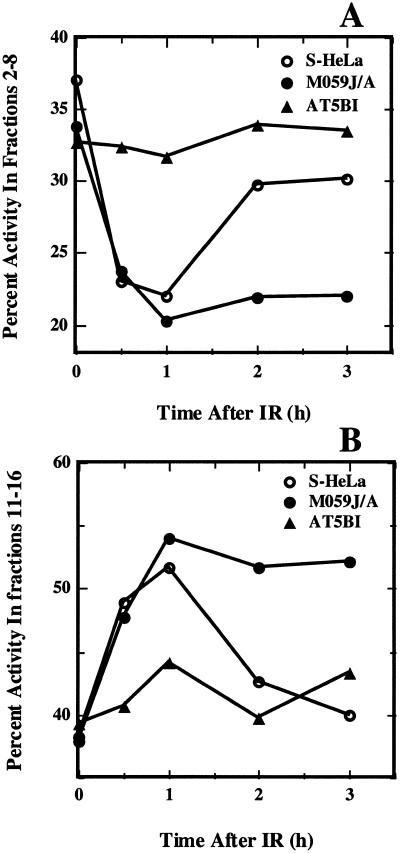
For a direct comparison of the effect of radiation on replicon initiation in the experiments shown in Figures 2–4, we calculated the percentage of total activity in fractions 2–8 as a function of time after irradiation and the results obtained are shown in Figure 5A. Activity variations in these fractions reflect mainly changes in replicon initiation. In HeLa cells, irradiation leads to a progressive reduction in percentage activity in this portion of the gradient that reaches a minimum at ~1 h. At later times, an increase in percentage activity is observed indicating recovery from this inhibition. The lack of complete recovery probably reflects some residual inhibition, or, more likely, redistribution of irradiated cells in the S-phase–late S-phase cells are known to initiate less frequently. In line with results in Figure 4, no significant inhibition is observed in AT cells (AT5BI) at all times examined. In M059-J/A cells, on the other hand, inhibition reaches a maximum at 1 h but does not show signs of recovery for the duration of the experiment.
Figure 5.
Analysis of alkaline sucrose gradient profiles shown in Figures 2–4. (A) Percentage of total activity in fractions 2–8 as a function of the time after exposure to 10 Gy of HeLa, M059-J/A and AT5BI cells. (B) Percentage of total activity in fractions 11–16 as a function of the time after exposure to 10 Gy in HeLa, M059-J/A and AT5BI cells.
In Figure 5B results similar to those of Figure 5A are shown for percentage activity at the bottom of the gradient (fractions 11–16). This part of the gradient harbors large molecular-weight pieces of nascent DNA and the reduction observed reflects the maturation of nascent DNA into full size DNA that sediments to the bottom of the gradient. There is a small, transient increase in percentage activity as a function of time in this region of the gradient in AT cells. A larger, yet still transient, increase is also observed in HeLa cells, and percentage activity returns to near control levels 3 h after irradiation. A significant increase in percentage activity is also observed in M059-J/A cells reaching a maximum at 1 h after irradiation. However, in contrast to the results with HeLa or AT5BI cells, no return to normal size DNA is observed at later times, and percentage activity in this portion of the gradient remains at a plateau for the 3 h of follow-up. These results indicate that cells deficient in DNA-PKcs experience a persistent inhibition of replicon initiation and a deficiency in the maturation of nascent DNA into chromosomal DNA.
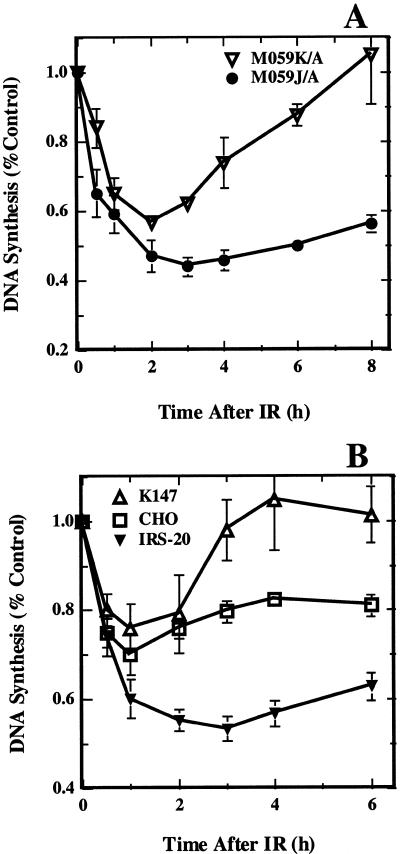
Since in the above described experiments the response of M059-J/A cells was compared to that of HeLa cells, we carried out experiments with M059-K/A cells that have been derived from the same tumor as M059-J/A and are therefore a better control (43). Alkaline sucrose gradient centrifugation experiments were impractical in M059-K/A cells due to the low levels of total (3H)-thymidine incorporation that lead to low activity per fraction. Therefore, we measured total DNA synthesis instead but extended the period of observation to 8 h to better cover the recovery phase. Figure 6A shows the results obtained. In agreement with results presented in Figures 3 and 5A total DNA synthesis is rapidly inhibited in M059-J/A cells reaching a minimum 2–3 h after irradiation. At later times a slight recovery is observed, but replication remains largely inhibited up to 8 h. In M059-K/A cells, on the other hand, DNA replication is inhibited with similar kinetics, reaches a minimum 2 h after irradiation and recovers to control levels 8 h post-irradiation.
Figure 6.
Relative DNA synthesis as a function of time after exposure of DNA-PKcs deficient cells to 10 Gy X-rays. (A) Results obtained with M059-J/A and M059-K/A cells. Shown is the mean and standard error from three independent experiments. (B) Results obtained with irs-20 cells, the parental CHO cells, and a cell line derived from irs-20 cells by transfection with a YAC 147 expressing the human DNA-PKcs. Shown is the mean and standard error from four independent experiments.
The above results in aggregate demonstrate that M059-J/A cells show a normal initial inhibition of DNA replication in response to radiation but a deficiency in recovering from this inhibition. Because in M059-J/A cells the DNA-PKcs cannot be detected, a correlation is suggested between DNA-PKcs and recovery from DNA replication inhibition. To examine the generality of this observation we tested the response of irs-20 cells in experiments similar to those described in Figure 6A. Irs-20 cells are of Chinese hamster origin with a mutation in DNA-PKcs that renders them defective in DNA-PK activity (49–52). As controls we used CHO cells, and a cell line derived from irs-20 cells by transfection with a YAC containing the human DNA-PKcs (K147) (52). The results obtained are shown in Figure 6B. Similar to observations with M059-J/A cells, irs-20 cells show a strong inhibition of total DNA synthesis that is at maximum 2–3 h after irradiation and which recovers only slightly at later times. Less inhibition and more recovery is seen in the parental CHO cells, whereas K147 cells show an initial inhibition to levels similar to those of CHO cells but full recovery 4 h after irradiation. Because the level of DNA-PKcs is practically zero for irs-20 cells, low in CHO cells (a typical observation in rodent cells) and high in K147 cells (52), a direct correlation is again indicated between DNA-PKcs and the recovery phase from DNA replication inhibition. The slight increase in the maximum levels of inhibition in DNA-PK deficient, as compared to DNA-PK proficient, cell lines 1–2 h cell after irradiation probably reflects, at least partly, this impaired recovery. It should be noted here that changes in total DNA synthesis at different times after irradiation also reflect alterations in the fractions of cells in S-phase, which is affected by the activation of checkpoints in other phases of the cell cycle, e.g. G1. This effect may be limited in our experiments, as all cell lines, except 180BR and AT5BI, have either mutant or inactivated p53.
Recovery of DNA replication after DNA damage in cells deficient in non-homologous end-joining (NHEJ)
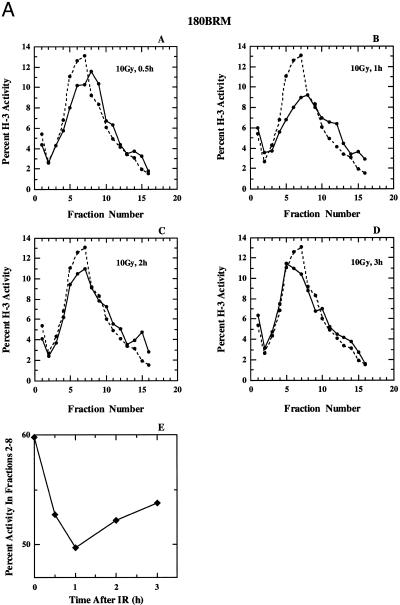
Because M059-J/A and irs-20 cells are deficient in the rejoining of DNA dsb (40,41,51), mainly NHEJ, one could reason that the lack of recovery from DNA replication inhibition is secondary to this deficiency. It is, for example, possible that the prolonged presence of DNA dsb generates a continuous signal that activates the checkpoint response and prevents resumption of DNA replication. To examine this possibility we tested three other cell lines known to be deficient in DNA dsb rejoining but proficient in DNA-PKcs and the results obtained are summarized in Figure 7A–C. Figure 7A shows results obtained with 180BRM cells, a derivative of 180BR that is known to be severely deficient in the rejoining DNA dsb (45,46), probably as a result of a mutation in DNA ligase IV (66). These cells respond to radiation in a manner similar to that of HeLa cells. Replicon initiation is inhibited 1 h after irradiation but recovers at later times. There is no evidence for persistent inhibition of replicon initiation, nor of increased incorporation in the high molecular weight region. Similar results were obtained with the parental 180BR cells (not shown) suggesting that the expression of TAg does not measurably affect the regulation of DNA replication after irradiation.
Figure 7.
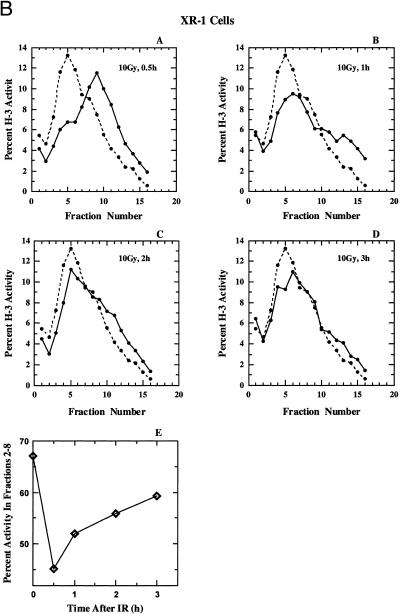
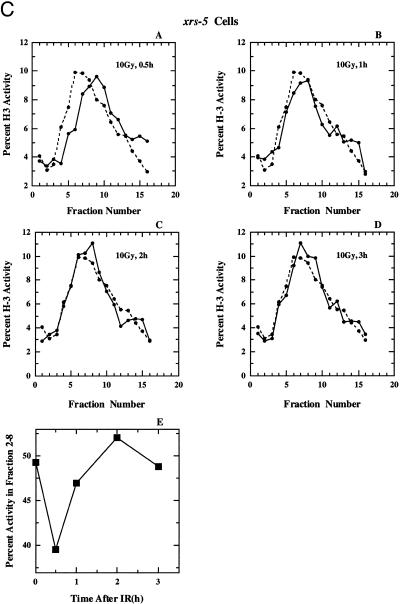
(A) (Panels A–D) Alkaline sucrose density profiles of 180BRM cells. Other details as in Figure 2. (Panel E) Percentage of total activity in fractions 2–8 as a function of the time after exposure to 10 Gy calculated from the results shown in panels A–D. (B) Alkaline sucrose density profiles of XR-1 cells. Other details as in Figure 2. (C) Alkaline sucrose density profiles of xrs-5 cells. Other details as in Figure 2.
The results of a similar experiment performed with XR-1 cells is shown in Figure 7B. These cells are radiosensitive to killing and deficient in DNA dsb rejoining as a result of a mutation in XRCC4, an accessory factor of DNA ligase IV (58–64). In these cells a strong shift to high molecular weight DNA is observed 0.5 h after irradiation that returns to near normal levels after 2–3 h. Finally, Figure 7C shows results obtained with xrs-5 cells, a radiosensitive mutant of CHO cells with a defect in DNA dsb rejoining as a result of a mutation in Ku80 (53–57). Here, a modest inhibition in replicon initiation is observed 0.5 h after irradiation that returns to normal at 1 h and remains at this level for up to 3 h. Our results are compatible to those previously reported for Ku deficient cells (67) that showed proficiency in the recovery from DNA replication inhibition in several Ku80 mutants (xrs-1, xrs-6 and xrs-7).
A summary of the results of Figure 7 (panels A–D), for the portion of the gradients that reflects effects on replicon initiation (fractions 2–8) is shown in Figure 7, panel E, and confirms recovery, albeit to a different extent and with different kinetics, for all cell lines. The mechanistic analysis of the quantitative differences observed between the cell lines will require further investigations. These results in aggregate suggest that cells deficient in DNA dsb rejoining as a result of defects either in Ku, XRCC4 or DNA ligase IV, recover DNA replication 1–2 h after radiation exposure. Thus, the lack of recovery observed in cells deficient in DNA-PKcs cannot be a simple reflection of a deficiency in DNA dsb rejoining.
DISCUSSION
The results presented above provide evidence for a contribution of DNA-PKcs in the regulation of DNA replication in cells exposed to ionizing radiation. Whereas no effect on the initial inhibition of DNA replication can be directly measured, the effect on the recovery from this inhibition is large and reproducible and observable in DNA-PKcs deficient cell lines from human or Chinese hamster origin. Alkaline sucrose gradient centrifugation studies further indicate that DNA-PKcs deficiency causes a prolonged inhibition in replicon initiation, and an inhibition in the maturation of nascent DNA. The observation that the latter responses are normal in cells that are DNA-PKcs proficient but deficient in DNA dsb rejoining, argues against a simple correlation between unrepaired DNA dsb and the level of DNA replication inhibition and suggests a requirement for this kinase either in the maintenance of the inhibition, or in the recovery from this inhibition. In the following paragraphs, we discuss the salient features of the results presented above and offer interpretations that also accommodate recent findings at the biochemical level on the role of DNA-PKcs in the regulation of DNA replication in irradiated cells (15).
Inhibition of DNA replication in irradiated DNA-PKcs deficient cells
Recent biochemical data obtained with the SV40-based in vitro DNA replication assay directly implicate DNA-PK in the regulation of DNA replication in the presence of DNA dsb (15). In this in vitro system, DNA-PK probably acts, by inhibiting through phosphorylation, the initiating functions of TAg. We speculated that in intact irradiated cells, DNA-PK may also regulate DNA replication by regulating the activities of the functional homologs of TAg. On the basis of this interpretation, one would therefore expect that cells lacking DNA-PKcs will show reduced levels of inhibition of DNA replication after irradiation (a quasi AT phenotype) which, as mentioned above, is not confirmed by the results presented here, or by previously published reports (39,40). However, considering the limitations of the methods used to evaluate inhibition of DNA replication in vivo and the properties of DNA-PK one can argue that the discrepancy is more apparent than real.
In vitro inhibition of SV40 DNA replication requires the activation of DNA-PK by double-stranded DNA ends. In vivo, therefore, a similar activation of DNA-PK will require the proximity of a DNA dsb. As a consequence, activation of DNA-PK will be a rather local event, as DNA dsb are relatively rare in cells exposed to doses of radiation in the range 0–20 Gy. Thus, the inhibition will only prevent the firing of the limited number of replicons (or clusters of replicons) that are located in the immediate vicinity of the break and the DNA-PK complex. It may not be possible to quantitate this local, and therefore presumably also limited, inhibition by measuring total DNA synthesis or by sizing nascent DNA. However, such a pathway of regulation of DNA replication will complement in an essential way the checkpoint functions of ATM since it will be specific for DNA dsb, local in action and immediate in response. The checkpoint functions of the ATM pathway (20,21,68), on the other hand, require up to 1 h for maximum response, are activated by other forms of DNA damage in addition to DNA dsb, and cause a global inhibition of DNA replication.
Thus, it is possible to develop plausible rationales that reconcile observations in vivo with observations in vitro, but more experiments will be required to test the validity of the proposed models. In any event, the known requirement for DNA-PK in the efficient rejoining of DNA dsb (35,69,70) and the speculated involvement of DNA-PK in the local inhibition of DNA replication after irradiation suggest a role for DNA-PK in the coordination of two vital nuclear processes: DNA replication and DNA dsb end-joining (71). The advantages of such steps in genomic stability are obvious, as DNA replication will not initiate on fragmented DNA templates, thus avoiding the potentially serious consequences of replication fork disruption.
Recovery of DNA replication in irradiated DNA-PK deficient cells
The results presented here implicate DNA-PKcs in the recovery from radiation-induced inhibition of DNA replication. This effect was consistently observed in all experiments performed, could be quantitated either by measurements of total DNA synthesis (Fig. 6), or of replicon initiation (Figs 2–5), and was confirmed in DNA-PKcs deficient cell lines of human or Chinese hamster origin (Fig. 6). The simple interpretation that the reduced recovery in DNA-PKcs deficient cell lines is a consequence of their deficiency in rejoining DNA dsb (35,69,70) seems unlikely since three mutants with deficiencies in other known components of the NHEJ pathway show clear recovery (Fig. 7). However, an additional argument can be developed against this interpretation. Radiation-induced lesions other than DNA dsb (e.g. DNA single strand breaks, base damage and DNA- or DNA-protein cross-links) are thought to contribute to the signal that leads to the inhibition of DNA replication, and repair of these lesions is normal in DNA-PKcs deficient cells (69). Persistent inhibition of DNA replication as a sole result of persistence of DNA dsb in the genome would practically eliminate all these lesions from the signal generating pathway.
The above observations are compatible with a model according to which DNA-PKcs functions to mediate the recovery from the inhibition of DNA replication in irradiated cells. The observation that cells deficient in Ku are able to recover, dissociates this component of DNA-PK from a function in the recovery of DNA replication and suggests that DNA-PKcs operates in this pathway independently of Ku. DNA-PKcs has been directly implicated in NHEJ, as well as in the in vitro regulation of SV40 DNA replication (15). The results presented here add yet another potential function: as an active component of a regulated recovery process.
It appears paradoxical that DNA-PKcs can function to inhibit DNA replication (15), but also to mediate recovery from such inhibition only ~1 h later (this report). However, available results hint to such opposing roles for this kinase. Thus, DNA-PKcs (and possibly also DNA-PK) is active under normal in vitro DNA replication conditions and strongly phosphorylates RPA2 (72 and unpublished data) without inhibiting replication. Nevertheless, under the same conditions, DNA-PK strongly inhibits DNA replication if DNA dsb are present (15). Thus, the kinase must change substrate specificity and roles in the regulation of DNA replication depending upon the structure of the activating DNA. Evidence for such differential effects of DNA-PKcs has been obtained in experiments investigating the mechanism of inhibition of DNA replication in cells exposed to camptothecin (73). These authors present evidence that DNA-PKcs normally binds RPA during DNA replication, but RPA is replaced by Ku in the complex when DNA damage is present. More work will be needed to elucidate the basis of these substrate specificities of DNA-PKcs and to understand the role of the activating DNA structures. It will also be important to examine the mechanism by which DNA replication remains inhibited in DNA-PKcs deficient cells, to investigate the role of ATM in the effect, as well as possible functional interactions between DNA-PKcs and ATM.
Delayed maturation of nascent DNA in cells deficient in DNA-PKcs
In addition to the deficiency in recovering from DNA replication inhibition, cells deficient in DNA-PKcs also show a deficiency in the maturation of nascent DNA. As a result of this deficiency, an accumulation of nascent DNA in the high molecular weight region is observed in alkaline sucrose gradients. The effect is also specific for DNA-PKcs deficient cells, and is not observed in other cell lines deficient in NHEJ. Thus, it may not be a simple consequence of the DNA dsb rejoining defect. This observation suggests that DNA-PKcs may somehow assist in the maturation of nascent DNA, particularly in cells exposed to ionizing radiation. It is not clear at present how this function is mediated; it is however a further indication of the multiplicity of functions of DNA-PKcs in DNA metabolism. This novel observation deserves further investigation.
In summary, cells deficient in DNA-PKcs show initial levels of DNA replication inhibition after irradiation that are quite similar to those of wild-type cells. The discordance between this observation and in vitro data implicating DNA-PK in this endpoint can be resolved by considering the expected local, and therefore limited, nature of the inhibition via this pathway in vivo. The novel observation here is that cells deficient in DNA-PKcs show a significant defect in the recovery from DNA replication inhibition, as well as a defect in the maturation of nascent DNA. These results suggest that important aspects of the DNA metabolism after DNA damage somehow depend on DNA-PKcs and expand further its potential functions in the cell.
Acknowledgments
ACKNOWLEDGEMENTS
The authors wish to thank Drs Colin Arlett and Edmond Malaise for 180BR cells and Dr Allalunis-Turner for M059J and M059-K cells, Dr Joel Bedford for irs20, K147, and CHO cells, and Dr Thomas Stamato for XR-1 cells. Special thanks go to Ms Nancy Mott for secretarial help. This work was supported by Grants CA 56706, CA 42026 and P30 CA56036-03 awarded from NIH, DHHS.
REFERENCES
- 1.Okada S. (1970) In Altman,K.I., Gerber,G.B. and Okada,S. (eds), RadiationBiochemistry. Academic Press, New York, Vol. 1, pp. 190–239. [Google Scholar]
- 2.Tolmach L.J., Hawkins,R.B. and Busse,P.M. (1980) In Meyn,R.E. and Withers,H.R. (eds), Radiation Biology inCancer Research. Raven Press, New York, pp. 125–142. [Google Scholar]
- 3.Painter R.B. (1986) Int. J. Radiat. Biol., 49, 771–781. [DOI] [PubMed] [Google Scholar]
- 4.Lavin M.F. and Schroeder,A.L. (1988) Mutat. Res., 193, 193–206. [DOI] [PubMed] [Google Scholar]
- 5.Iliakis G. (1997) Semin. Oncol., 24, 602–615. [PubMed] [Google Scholar]
- 6.Larner J.M., Lee,H. and Hamlin,J.L. (1997) Cancer Surveys, 29, 25–45. [PubMed] [Google Scholar]
- 7.Watanabe I. (1974) Radiat. Res., 58, 541–556. [PubMed] [Google Scholar]
- 8.Makino F. and Okada,S. (1975) Radiat. Res., 62, 37–51. [PubMed] [Google Scholar]
- 9.Larner J.M., Lee,H. and Hamlin,J.L. (1994) Mol. Cell. Biol., 14, 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb J.R., Petit-Frere,C., Broughton,B.C., Lehmann,A.R. and Green,M.H.L. (1989) Int. J. Radiat. Biol., 56, 125–130. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Huq,M.S., Cheng,X. and Iliakis,G. (1995) Radiat. Res., 142, 169–175. [PubMed] [Google Scholar]
- 12.Wang Y., Huq,M.S. and Iliakis,G. (1996) Radiat. Res., 145, 408–418. [PubMed] [Google Scholar]
- 13.Wang Y., Perrault,A.R. and Iliakis,G. (1997) Cancer Res., 57, 1654–1659. [PubMed] [Google Scholar]
- 14.Mirzayans R., Enns,L. and Paterson,M.C. (1997) Radiat. Res., 147, 13–21. [PubMed] [Google Scholar]
- 15.Wang Y., Zhou,X.Y., Wang,H.-Y. and Iliakis,G. (1999) J. Biol. Chem., 274, 22060–22064. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y. and Iliakis,G. (1992) Cancer Res., 52, 508–514. [PubMed] [Google Scholar]
- 17.Wang Y., Cheong,N. and Iliakis,G. (1993) Cancer Res., 53, 1213–1217. [PubMed] [Google Scholar]
- 18.Wang X. and Iliakis,G. (1993) Int. J. Radiat. Biol., 64, 165–168. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Cheong,N., Miura,M. and Iliakis,G. (1996) Radiat. Environ. Biophys., 36, 117–123. [DOI] [PubMed] [Google Scholar]
- 20.Rotman G. and Shiloh,Y. (1998) Hum. Mol. Genet., 1998, 1555–1563. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen T.J. and Shiloh,Y. (1996) Int. J. Radiat. Biol., 69, 527–537. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka S., Huang,M. and Elledge,S.J. (1998) Science, 282, 1893–1897. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi P., Eng,W.K., Zhu,Y., Mattern,M.R., Mishra,R., Hurle,M.R., Zhang,X., Lu,Q., Faucette,L.F., Scott,G.F., Li,X., Johnson,R.K., Winkler,J.D. and Zhou,B.-B.S. (1999) Oncogene, 18, 4047–4054. [DOI] [PubMed] [Google Scholar]
- 24.Muschel R.J., Zhang,H.B. and McKenna,W.G. (1993) Cancer Res., 53, 1128–1135. [PubMed] [Google Scholar]
- 25.Beamish H., Williams,R., Chen,P. and Lavin,M.F. (1996) J. Biol. Chem., 271, 20486–20493. [DOI] [PubMed] [Google Scholar]
- 26.D’Anna J.A., Valdez,J.G., Habbersett,R.C. and Crissman,H.A. (1997) Radiat. Res., 148, 260–271. [PubMed] [Google Scholar]
- 27.Xie G., Habbersett,R.C., Jia,Y., Peterson,S.R., Lehnert,B.E., Bradbury,E.M. and D’Anna,J.A. (1998) Oncogene, 16, 721–736. [DOI] [PubMed] [Google Scholar]
- 28.Weinreich M. and Stillman,B. (1999) EMBO J., 18, 5334–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Z.-Q., Amin,A.A., Gibbs,E., Niu,H. and Hurwitz,J. (1994) Proc. Natl Acad. Sci. USA, 91, 8343–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Z.-Q., Park,C.-H., Amin,A.A., Hurwitz,J. and Sancar,A. (1995) Proc. Natl Acad. Sci. USA, 92, 4636–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutta A., Din,S., Brill,S.J. and Stillman,B. (1991) Cold Spring Harbor Symp. Quant. Biol., LVI, 315–324. [DOI] [PubMed] [Google Scholar]
- 32.Dutta A. and Stillman,B. (1992) EMBO J., 11, 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wold M.S. (1997) Annu. Rev. Biochem., 66, 61–92. [DOI] [PubMed] [Google Scholar]
- 34.Park J.-S., Park,S.-J., Peng,X., Wang,M., Yu,M.-A. and Lee,S.-H. (1999) J. Biol. Chem., 274, 32520–32527. [DOI] [PubMed] [Google Scholar]
- 35.Smith G.C.M. and Jackson,S.P. (1999) Genes Dev., 13, 916–934. [DOI] [PubMed] [Google Scholar]
- 36.Anderson C.W. and Carter,T.H. (1996) Curr. Topics Microbiol. Immunol., 217, 91–111. [DOI] [PubMed] [Google Scholar]
- 37.Lees-Miller S.P. (1996) Biochem. Cell Biol., 74, 503–512. [DOI] [PubMed] [Google Scholar]
- 38.Kirchgessner C.U., Patil,C.K., Evans,J.W., Cuomo,C.A., Fried,L.M., Carter,T., Oettinger,M.A. and Brown,J.M. (1995) Science, 267, 1178–1183. [DOI] [PubMed] [Google Scholar]
- 39.Komatsu K., Yoshida,M. and Okumura,Y. (1993) Int. J. Radiat. Biol., 63, 725–730. [DOI] [PubMed] [Google Scholar]
- 40.Allalunis-Turner M.J., Zia,P.K.Y., Barron,G.M., Mirzayans,R. and Day,R.S.,III (1995) Radiat. Res., 144, 288–293. [PubMed] [Google Scholar]
- 41.Lees-Miller S.P., Godbout,R., Chan,D.W., Weinfeld,M., Day,R.S.,III, Barron,G.M. and Allalunis-Turner,J. (1995) Science, 267, 1183–1185. [DOI] [PubMed] [Google Scholar]
- 42.Galloway A.M., Spencer,C.A., Anderson,C.W. and Allalunis-Turner,M.J. (1999) Oncogene, 18, 1361–1368. [DOI] [PubMed] [Google Scholar]
- 43.Allalunis-Turner M.J., Barron,G.M., Day,R.S., Dobler,K.D. and Mirzayans,R. (1993) Radiat. Res., 134, 349–354. [PubMed] [Google Scholar]
- 44.Allalunis-Turner J., Barron,G.M. and Day,R.S.,III (1997) Radiat. Res., 147, 284–287. [PubMed] [Google Scholar]
- 45.Badie C., Iliakis,G., Foray,N., Alsbeih,G., Pantelias,G.E., Okayasu,R., Cheong,N., Russell,N.S., Begg,A.C., Arlett,C.F. and Malaise,E.P. (1995) Cancer Res., 55, 1232–1234. [PubMed] [Google Scholar]
- 46.Badie C., Goodhardt,M., Waugh,A., Doyen,N., Foray,N., Calsou,P., Singleton,B., Gell,D., Salles,B., Jeggo,P., Arlett,C.F. and Malaise,E.-P. (1997) Cancer Res., 57, 4600–4607. [PubMed] [Google Scholar]
- 47.Small M.B., Gluzman,Y. and Ozer,H.L. (1982) Nature, 296, 671–672. [DOI] [PubMed] [Google Scholar]
- 48.Neufeld D.S., Ripley,S., Henderson,A. and Ozer,H.L. (1987) Mol. Cell. Biol., 7, 2794–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stackhouse M.A. and Bedford,J.S. (1993) Radiat. Res., 136, 241–249. [PubMed] [Google Scholar]
- 50.Stackhouse M.A. and Bedford,J.S. (1993) Radiat. Res., 136, 250–254. [PubMed] [Google Scholar]
- 51.Stackhouse M.A. and Bedford,J.S. (1994) Int. J. Radiat. Biol., 65, 571–582. [DOI] [PubMed] [Google Scholar]
- 52.Priestley A., Beamish,H.J., Gell,D., Amatucci,A.G., Muhlmann-Diaz,M.C., Singleton,S.P., Smith,G.C.M., Blunt,T., Schalkwyk,L.C., Bedford,J.S., Jeggo,P.A. and Taccioli,G.E. (1998) Nucleic Acids Res., 26, 1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeggo P.A. and Kemp,L.M. (1983) Mutat. Res., 112, 313–327. [DOI] [PubMed] [Google Scholar]
- 54.Kemp L.M., Sedgwick,S.G. and Jeggo,P.A. (1984) Mutat. Res., 132, 189–196. [DOI] [PubMed] [Google Scholar]
- 55.Getts R.C. and Stamato,T.D. (1994) J. Biol. Chem., 269, 15981–15984. [PubMed] [Google Scholar]
- 56.Rathmell W.K. and Chu,G. (1994) Proc. Natl Acad. Sci. USA, 91, 7623–7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singleton B.K., Priestley,A., Steingrimsdottir,H., Gell,D., Blunt,T., Jackson,S.P., Lehmann,A.R. and Jeggo,P.A. (1997) Mol. Cell. Biol., 17, 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giaccia A., Weinstein,R., Hu,J. and Stamato,T.D. (1985) Somatic Cell Mol. Genet., 11, 485–491. [DOI] [PubMed] [Google Scholar]
- 59.Taccioli G.E., Rathbun,G., Oltz,E., Stamato,T., Jeggo,P.A. and Alt,F.W. (1993) Science, 260, 207–210. [DOI] [PubMed] [Google Scholar]
- 60.Li Z., Otevrel,T., Gao,Y., Cheng,W.-L., Seed,B., Stamato,T.D., Taccioli,G.E. and Alt,F.W. (1995) Cell, 83, 1079–1089. [DOI] [PubMed] [Google Scholar]
- 61.Grawunder U., Zimmer,D., Kulesza,P. and Lieber,M.R. (1998) J. Biol. Chem., 273, 24708–24714. [DOI] [PubMed] [Google Scholar]
- 62.Grawunder U., Wilm,M., Wu,X., Kulesza,P., Wilson,T.E., Mann,M. and Lieber,M.R. (1997) Nature, 388, 492–495. [DOI] [PubMed] [Google Scholar]
- 63.Bryans M., Valenzano,M.C. and Stamato,T.D. (1999) Mutat. Res., 433, 53–58. [DOI] [PubMed] [Google Scholar]
- 64.Nocentini S. (1999) Radiat. Res., 151, 423–432. [PubMed] [Google Scholar]
- 65.Fried L.M., Koumenis,C., Peterson,S.R., Green,S.L., van Zijl,P., Allalunis-Turner,J., Chen,D.J., Fishel,R., Giaccia,A.J., Brown,J.M. and Kirchgessner,C.U. (1996) Proc. Natl Acad. Sci. USA, 93, 13825–13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riballo E., Critchlow,S.E., Teo,S.-H., Doherty,A.J., Priestley,A., Broughton,B., Kysela,B., Beamish,H., Plowman,N., Arlett,C.F., Lehmann,A.R., Jackson,S.P. and Jeggo,P.A. (1999) Curr. Biol., 9, 699–702. [DOI] [PubMed] [Google Scholar]
- 67.Jeggo P.A. (1985) Mutat. Res., 145, 171–176. [DOI] [PubMed] [Google Scholar]
- 68.Lavin M.F. and Shiloh,Y. (1997) Annu. Rev. Immunol., 15, 177–202. [DOI] [PubMed] [Google Scholar]
- 69.Jeggo P.A. (1998) Adv. Genet., 38, 186–218. [DOI] [PubMed] [Google Scholar]
- 70.Dynan W.S. and Yoo,S. (1998) Nucleic Acids Res., 26, 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lees-Miller S.P. and Anderson,C.W. (1991) Cancer Cells, 3, 341–345. [PubMed] [Google Scholar]
- 72.Brush G.S., Anderson,C.W. and Kelly,T.J. (1994) Proc. Natl Acad. Sci. USA, 91, 12520–12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shao R.-G., Cao,C.-X., Zhang,H., Kohn,K.W., Wold,M.S. and Pommier,Y. (1999) EMBO J., 18, 1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]