Abstract
Aims
Implantable cardioverter defibrillators (ICDs) prevent sudden cardiac death. Anxiety, depression, and post-traumatic stress disorder (PTSD) are underappreciated symptoms. We aimed to systematically synthesize prevalence estimates of mood disorders and symptom severities, pre- and post-ICD insertions. Comparisons were made with control groups, as well as within ICD patients by indication (primary vs. secondary), sex, shock status, and over time.
Methods
Databases (Medline, PsycINFO, PubMed, and Embase) were searched without limits from inception to 31 August 2022; 4661 articles were identified, 109 (39 954 patients) of which met criteria.
Results
Random-effects meta-analyses revealed clinically relevant anxiety in 22.58% (95%CI 18.26–26.91%) of ICD patients across all timepoints following insertion and depression in 15.42% (95%CI 11.90–18.94%). Post-traumatic stress disorder was seen in 12.43% (95%CI 6.90–17.96%). Rates did not vary relative to indication group. Clinically relevant anxiety and depression were more likely in ICD patients who experienced shocks [anxiety odds ratio (OR) = 3.92 (95%CI 1.67–9.19); depression OR = 1.87 (95%CI 1.34–2.59)]. Higher symptoms of anxiety were seen in females than males post-insertion [Hedges’ g = 0.39 (95%CI 0.15–0.62)]. Depression symptoms decreased in the first 5 months post-insertion [Hedges’ g = 0.13 (95%CI 0.03–0.23)] and anxiety symptoms after 6 months [Hedges’ g = 0.07 (95%CI 0–0.14)].
Conclusion
Depression and anxiety are highly prevalent in ICD patients, especially in those who experience shocks. Of particular concern is the prevalence of PTSD following ICD implantation. Psychological assessment, monitoring, and therapy should be offered to ICD patients and their partners as part of routine care.
Keywords: ICD, Anxiety, Depression, PTSD, Shocks, Cardiac
Structured Graphical Abstract
Structured graphical abstract.
What’s new?
We synthesized prevalence estimates of depression, anxiety, and post-traumatic stress disorder (PTSD) in 39 954 implantable cardioverter defibrillator (ICD) patients (across 109 papers).
Rates of mood disorders are high in ICD patients: 15% depression, 23% anxiety, and 12% PTSD.
Rates were higher in ICD patients who experienced shocks but did not vary relative to indication group (primary vs. secondary).
Introduction
Implantable cardioverter defibrillators (ICDs) prevent sudden cardiac death in vulnerable cardiac patient populations.1–3 Implantable cardioverter defibrillator procedures are increasing around the world, with over 4000 insertions carried out in the 2014/5 financial year in Australia4; 800 000 in the USA between 1993 and 20065; and in Italy, procedures increased from around 3000 to 24 000 between 2001 and 2017.6 Despite ICD procedures being common, patients display poor mental health outcomes, including high levels of anxiety and depression.7,8
Depression symptoms include depressed mood, apathy and anhedonia, agitation, and fatigue.9 General anxiety symptoms include worry, restlessness, and muscle tension.9 Generalized anxiety disorder and major depressive disorder are diagnosed when symptoms of anxiety and depression, respectively, significantly interrupt daily functioning.9 Post-traumatic stress disorder (PTSD) symptoms include intrusive thoughts, arousal abnormalities, and negative cognitive performance and mood, which follow an exposure to one or more traumatic events.9 Depression, anxiety, and PTSD cause significant distress to the person and their loved ones. The high rates of anxiety and depression pre-ICD reflect patient anxieties around their heart health and impending ICD procedure. The fear of experiencing a shock is a considerable psychological burden on ICD patients after insertion, especially as only around 30% of patients receive at least one shock in the first 2 years post-insertion.10
Poor mood outcomes in ICD patients are gaining increasing attention, as they are associated with lower quality of life and wellbeing, along with increased mortality.11,12 We aimed to systematically review and synthesize evidence in relation to levels of anxiety, depression, and PTSD in ICD patients, overall, across time (from pre-ICD to various timepoints following insertion) as well as relative to male vs. female, shock vs. no shock, and primary vs. secondary indication. We also considered the type of control group (e.g. partners and cardiac patients without intervention), where applicable. This knowledge will shape care planning and provision for future ICD patients.
Methods
This work was conducted according to the PRISMA 2020 statement13 and was registered prior to data extraction with OSF (https://osf.io/tz6wu). The PRISMA 2020 Checklist is available in Supplementary material online, Table S1.
Eligibility criteria
Original research papers (no reviews, opinion pieces, theses, and conference abstracts) published in English language were eligible for inclusion. Case studies were excluded. Studies must have included an adult sample (all patients > 18 years); reported appropriate data for a sample of participants who had only an ICD (without cardiac resynchronization therapy devices); included a measure of anxiety, PTSD, and/or depression symptomology or diagnoses; and included at least one of the following: (i) mood symptom or diagnosis data (continuous or categorical) for an ICD comparison group and a non-ICD comparison group without intervention (e.g. no pharmacological intervention or pacemaker and etc.), (ii) mood data for an ICD group at >1 timepoint (e.g. pre-ICD and post-ICD), or (iii) event rate (prevalence) data for a categorical mood measure(s) for an ICD group at ≥1 timepoint. Prospective and retrospective studies, cross-sectional or longitudinal studies, cohort, and randomized controlled trials (where data for non-intervention control ICD group could be extracted) were included.
Information sources and selection process
Databases (Medline, PsycINFO, PubMed, and Embase) were searched without database limits from inception to 31 August 2022. The complete search strategy is in the Supplementary Materials. All identified records were screened by title and abstract by two reviewers (R.S., J.M.A., E.S.G., or S.K.). Each retrieved report was then screened by two reviewers (R.S., J.M.A., E.S.G., or S.K.). Disagreements at both screening stages were resolved through discussion and consensus between reviewers.
Data collection and coding
Data were extracted for each study by two independent reviewers (E.S.G., J.M.A., R.S., or V.B.), with discrepancies resolved through discussion and consensus. Measures of association or descriptive statistics between ICDs and mood symptoms or disorders across time or in comparison to an appropriate non-ICD control group [partners, cardiac patients without intervention (e.g. ICD, pacemaker, etc.), and general population], or categorical data describing mood disorders or symptoms in ICD samples were extracted from included studies. We also extracted the following study characteristic variables: country; study design; sample size; sex (male/female); age; shocks (% who experienced); indication (% primary); timepoint of mood measure (in relation to ICD); and method used to measure or diagnose anxiety, depression, and PTSD.
Categorical mood data were categorized according to cut-off definition for presence into: (i) diagnosed mood disorder or clinically relevant cut-off on a measure of mood symptoms or (ii) at least mild mood symptoms. Symptom severity categorizations were based on commonly accepted or reported cut-offs for the tests used.
Risk of bias in individual studies
Risk of bias within included studies was assessed with the Risk of Bias for Non-randomized studies (RoBANS)14 tool for observational studies and the Risk of Bias 2.015 tool for randomized controlled trials. Two independent reviewers (E.S.G., J.M.A., and R.S.) assessed risk of bias with disagreements resolved through discussion and consensus.
Data analysis
Data analyses were conducted in R using the metafor package.16 The data and code associated with this analysis are publicly available (https://github.com/ericaghezzi/ICD_mood_metaanalysis). Data for analyses were split relative to timepoint of mood measure: (i) pre-discharge from hospital for ICD; (ii) discharge to 6 months post-ICD; (iii) 6–12 months post-ICD; (iv) >12 months post-ICD; and (v) all post-ICD. Four mood measures were investigated: (i) anxiety, (ii) depression, (iii) PTSD, and (iv) any mood disorder (all combined). Finally, both mood symptomology (continuous) and two dichotomous measures of mood [presence or absence of (i) clinically significant symptoms/diagnosis and (2) at least mild symptoms] were investigated.
Separate meta-analyses were conducted for all analyses in which >2 studies reported appropriate data. Unless otherwise stated, effect sizes were calculated as Hedges’ g for continuous measures of mood symptomology and odds ratio (OR) for dichotomous measures of mood. Four types of analyses were conducted, as described below.
Pooled prevalence (as percentage with condition) was calculated for each dichotomous measure of mood for both ICD and non-ICD comparison groups.
Differences in ICD patients’ mood were investigated based on sex (female vs. male), shocks (vs. no shocks), and indication for ICD (primary vs. secondary). An OR > 1 represents greater likelihood of the presence of mood diagnoses or clinically relevant symptoms in the first subgroup (female, shocks, and primary indication) as compared to the second subgroup (male, no shock, and secondary indication). A positive Hedges’ g represents more mood symptomology in first subgroup compared to the second subgroup.
Differences in mood between ICD and non-ICD comparison groups (partners, non-ICD cardiac patients, and general population) were estimated. An OR > 1 represented greater likelihood of the presence of mood diagnoses in the ICD group compared to the non-ICD group. A positive Hedges’ g represented more mood symptomology in the ICD group compared to the non-ICD control group.
Differences in mood symptomology in ICD patients were investigated between the following timepoints: (i) pre-discharge vs. discharge—5 months post-ICD, (ii) discharge—6 months vs. 6–12 months post-ICD, (iii) 6–12 months vs. >12 months, and (iv) pre-ICD vs. post-ICD. Effect sizes were calculated as standardized mean change using raw score standardization (SMCR) with r2 = 0.6. A positive standardized mean change represented more mood symptoms at the first timepoint compared to the second timepoint.
Random-effects models were used, and statistical dependency was accounted for by averaging effect sizes and variances within studies to produce a single study-level estimate for each analysis. Between-study variance (quantified with tau2) was estimated using the Paule and Mandel method,17 and the Knapp and Hartung method18 was used to calculate the confidence intervals for all analyses. The proportion of between-study heterogeneity out of total variance was assessed using the I2 statistic; classified as low (25%), moderate (50%), or high (75%).19
Funnel plots of effect size vs. standard error were visually examined for symmetry to assess for bias across studies in primary analyses due to the small-study effect. In analyses with at least 10 studies, the small-study effect was formally tested using Egger’s intercept test. If evidence of asymmetry (one-tailed P < 0.1 on the Egger’s test) was found, Duval and Tweedie’s trim and fill method was used to quantify the magnitude of potential bias.
Certainty in the body of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.20 Overall certainty was categorized as high, moderate, low, or very low according to assessments of the eight GRADE criteria: risk of bias, inconsistency of results, indirectness of evidence, imprecision, publication bias, magnitude of effect, dose–response gradient, and influence of residual plausible confounding.
Results
Summary of studies
The database search identified 4661 articles. Following removal of duplicates, remaining articles were screened by title and abstract. A total of 609 were then reviewed at full-text stage, of which 500 were excluded (reasons outlined in Figure 1). Eight studies met inclusion criteria but were not captured by meta-analysis groupings.21–26 A total of 109 met criteria and were able to be meta-analysed (see Table 1 for study information). Due to reference limitations, included study references can be found in the Supplementary Materials. Most studies were from the USA,37 the Netherlands,23 Germany,10 and Canada.10 The tools and methods to measure mood across included studies are listed in Supplementary material online, Table S2. A total of 39 954 participants were included across all studies, with a mean age of 64 years and 91% being male across all included participants (notably, there was one study with 25 789 male participants). The average proportion of males within individual studies was 78%.
Figure 1.
PRISMA flow diagram.13 ICD, implantable cardioverter defibrillator.
Table 1.
Demographics of included studies
| Lead authora | Year | Country | Study design | n | Male, n | Age, mean | Age, SD | Shock (%) | Primary indication (%) | Mood measure included | Timepoints reportedb | Comparison group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amiaz | 2016 | Israel | Cross-sectional | 95 | 80 | 66.00 | 11.50 | 26.32 | 70.97 | Depression, anxiety, PTSD | 4, 7 | |
| Amiaz | 2017 | Israel | Prospective cohort | 158 | 134 | 64.00 | 12.40 | 6.38 | 91.18 | Depression | 1, 2, 4, 6, 7 | |
| Arteaga | 1995 | USA | Cross-sectional | 45 | 32 | 61.00 | 37.78 | Depression, anxiety | 3, 7 | Cardiac | ||
| Berg | 2019 | Denmark | Prospective cohort | 676 | 534 | 61.90 | 42.05 | 61.36 | Anxiety, depression | 1, 7 | ||
| Bilge | 2006 | Turkey | Cross-sectional | 91 | 79 | 53.00 | 14.00 | 61.54 | 21.98 | Anxiety, depression | 2, 3, 4, 5, 7 | |
| Bourke | 1997 | United Kingdom | Retrospective cohort | 35 | 4 | 48.00 | 11.50 | Anxiety/depression | 4, 7 | |||
| Carroll | 2002 | USA | Prospective cohort | 70 | 51 | 64.10 | 27.00 | Anxiety, depression | 1, 3, 4, 6, 7 | |||
| Carroll | 2010 | Canada | Before–after | 30 | 15 | 66.80 | 9.73 | 51.61 | Anxiety | 1, 2, 6, 7 | ||
| Carroll | 2012 | Canada | Prospective Cohort | 70 | 61 | 64.76 | 9.37 | 100 | Depression | 1, 6 | ||
| Chevalier | 1996 | France | Cross-sectional | 32 | 28 | 54.47 | 14.35 | 46.88 | Depression, anxiety | 4, 7 | ||
| Cook | 2013 | USA, Canada | Cross-sectional | 70 | 45 | 39.35 | 15.93 | Depression | 5, 7 | Cardiac | ||
| Cross | 2010 | USA | Cross-sectional | 30 | 18 | 66.90 | 10.18 | 30.00 | Depression, anxiety, anxiety/depression | 4, 7 | Cardiac | |
| Crow | 1998 | USA | Prospective cohort | 35 | 52.00 | Depression, anxiety | 1, 7 | |||||
| D’Antono | 2013 | Canada | Before–after | 46 | 39 | 68.80 | 8.50 | 60.87 | Depression, anxiety | 5, 7 | ||
| de Groot | 2003 | The Netherlands | Cross-sectional | 150 | 122 | 59.63 | 12.22 | Anxiety, depression | 5, 7 | Partners | ||
| Dickerson | 2010 | USA | Prospective cohort | 80 | 61 | 62.40 | 11.50 | 68.42 | Anxiety | 1, 2, 6, 7 | ||
| Dougherty | 2004 | USA | RCT | 84 | 62 | 65.06 | 12.24 | 0 | Anxiety, depression | 2, 7 | ||
| Dougherty | 2016 | USA | Prospective cohort | 55 | 44 | 66.50 | 11.30 | 0 | Anxiety, depression | 1, 2, 3, 4, 7 | Partners | |
| Dougherty | 2022 | USA | RCT | 84 | 62 | 65.06 | 12.24 | 0 | Anxiety, depression | 3, 4, 2, 7 | ||
| Dunbar | 2009 | USA | RCT | 78 | 54 | 58.40 | 12.00 | 14.10 | Anxiety, depression | 1, 2, 3, 4, 7 | ||
| Duncker | 2015 | Germany | Before–after | 29 | 24 | 58.00 | 60.31 | 100 | Depression, anxiety/depression | 1, 2, 3, 6, 7 | ||
| Duru | 2001 | Switzerland | Cross-sectional | 76 | 64 | 58.27 | 13.03 | 59.21 | Depression, anxiety | 4, 7 | ||
| Edelman | 2007 | Australia | RCT | 22 | 19 | Depression, anxiety | 1, 6 | |||||
| Emons | 2019 | The Netherlands | Cross-sectional | 287 | 228 | 58.94 | 10.30 | 66.30 | Anxiety | 2, 7 | ||
| Fitchet | 2003 | UK | RCT | 16 | 14 | 58.00 | 10.00 | Depression, anxiety | 5, 7 | |||
| Flemme | 2012 | Sweden | Cross-sectional | 147 | 121 | 63.00 | 13.00 | 25.85 | 47.62 | Anxiety, depression | 4, 7 | |
| Ford | 2016 | Canada | RCT | 97 | 31.2 | 25.80 | PTSD | 2, 4, 7 | ||||
| Francis | 2009 | USA | Cross-sectional | 44 | 41 | 62.10 | 9.30 | Depression, anxiety | 4, 7 | |||
| Friedmann | 2006 | USA | Cross-sectional | 48 | 38 | 66.00 | 12.10 | 0 | Depression, anxiety | 5, 4, 7 | ||
| Garnero | 2014 | Italy | Cross-sectional | 43 | 68.70 | 8.40 | 100 | Depression | 4, 7 | Cardiac | ||
| Godemann | 2001 | Germany | Cross-sectional | 72 | 62 | 69.10 | 10.40 | 69.57 | Anxiety, depression | 4, 7 | ||
| Godemann | 2004 | Germany | Cross-sectional | 90 | 78 | 59.50 | 11.10 | 67.8 | Anxiety, depression | 4, 7 | ||
| Gostoli | 2016 | Italy | Prospective cohort | 117 | 87 | 63.10 | 13.70 | Depression, anxiety | 1, 6 | |||
| Habibović | 2012 | The Netherlands | Prospective cohort | 395 | 320 | 62.80 | 10.30 | Anxiety, PTSD | 2, 4, 7 | |||
| Habibović | 2013 | The Netherlands | Prospective cohort | 188 | 150 | 57.50 | 12.50 | 51.06 | Anxiety, depression | 2, 4, 7 | Cardiac | |
| Habibović | 2017 | The Netherlands | Prospective cohort | 249 | 204 | 58.93 | 9.84 | 9.24 | 69.08 | PTSD, anxiety | 2, 3, 4, 7 | |
| Habibović | 2020 | The Netherlands | Prospective cohort | 214 | 177 | 58.90 | 9.90 | 27.1 | 71.03 | Anxiety | 2, 7 | |
| Hallas | 2010 | UK | Prospective cohort | 52 | 45 | 60.63 | 11.97 | 29.55 | Depression, anxiety | 1, 2, 3, 4, 6, 7 | ||
| Hamilton | 2004 | USA | Prospective cohort | 70 | 51 | 63.81 | Anxiety, depression | 1, 3, 4, 6, 7 | ||||
| Hammash | 2019 | Australia, USA | Cross-sectional | 263 | 190 | 61.00 | 14.00 | 36.12 | Anxiety, depression | 4, 7 | ||
| Hegel | 1997 | USA | Prospective cohort | 38 | Depression, anxiety | 4, 7 | ||||||
| Herbst | 1999 | USA | Cross-sectional | 49 | 43 | 67.41 | 11.67 | 65.31 | Depression, anxiety | 4, 7 | Cardiac | |
| Herrmann | 1997 | Germany | Cross-sectional | 63 | 50 | 61.00 | 13.00 | Anxiety, depression | 4, 7 | Cardiac | ||
| Irvine | 2011 | Canada | RCT | 97 | 79 | 63.20 | 14.20 | Depression, anxiety, PTSD | 1, 3, 4, 7 | |||
| Israelsson | 2018 | Sweden | Cross-sectional | 990 | 772 | 65.60 | 12.30 | 39.6 | 0 | Anxiety, depression, anxiety/depression | 4, 5, 7 | General |
| Jacob | 2012 | USA | Case–control | 76 | 36 | 55.57 | 12.64 | 72.37 | Depression, anxiety | 5, 7 | ||
| James | 2012 | USA | Cross-sectional | 86 | 38 | 45.80 | 12.90 | 52.33 | 45.88 | Anxiety, depression | 4, 7 | |
| Köbe | 2017 | Germany | Case–control | 84 | 60 | 44.65 | 12.30 | 9.52 | 58.33 | PTSD, anxiety, depression | 4, 7 | |
| Kamphuis | 2002 | The Netherlands | Before–after | 133 | 98 | 55.24 | 13.70 | Anxiety, depression | 2, 3, 4, 7, 1, 6 | |||
| Kamphuis | 2003 | The Netherlands | Before–after | 132 | 97 | 55.24 | 13.70 | 26 | Anxiety, depression | 1, 2, 3, 4, 6, 7 | ||
| Kapa | 2010 | Canada, USA | Prospective cohort | 223 | 180 | 66.00 | 12.00 | 8.97 | 48.88 | Depression, anxiety, PTSD | 2, 3, 4, 7 | |
| Keren | 2011 | Cross-sectional | 143 | 119 | 67.68 | 11.80 | 37.59 | Anxiety, depression | 4, 7 | |||
| Kim | 2009 | Prospective cohort | 122 | 92 | 65.00 | 9.05 | 14.75 | Depression, anxiety | 5, 4, 7 | |||
| Kim | 2020a | South Korea | Prospective cohort | 74 | 58 | 56.53 | 12.43 | 45.90 | Anxiety, depression | 3, 7 | ||
| Kim | 2020b | South Korea | Prospective cohort | 34 | 25 | 56.20 | 12.00 | 55.88 | Anxiety, depression | 1, 2, 3, 4, 6, 7 | ||
| Knackstedt | 2014 | Germany | Before–after | 17 | 14 | 61.70 | 10.30 | 11.76 | Depression, anxiety | 2, 3, 7 | ||
| Lüderitz | 1993 | Germany | Prospective cohort | 57 | 50 | 59.00 | 13.00 | Anxiety | 1, 4, 6, 7 | |||
| Lache | 2007 | Germany | Cross-sectional | 55 | 43 | 61.00 | 12.00 | Anxiety, depression | 4, 7 | |||
| Lemon | 2007 | Sydney | Prospective cohort | 49 | 41 | 64.70 | 9.90 | Depression, anxiety | 1, 2, 3, 6, 7 | |||
| Levesque | 2020 | Argentina, Australia, Belgium, Canada, France, India, Italy, Japan, Malta, Norway, Taiwan, the Netherlands, Sweden, Switzerland, USA | Cross-sectional | 107 | 53 | 40.10 | 12.40 | 38.32 | Anxiety, depression | 4, 7 | Cardiac | |
| Lewin | 2009 | UK | RCT | 121 | 100 | 63.40 | 12.10 | 13.1 | Anxiety, depression | 1, 3, 6, 7 | ||
| Luyster | 2006 | USA | Cross-sectional | 100 | 81 | 67.90 | 11.70 | 26 | 51.00 | Depression, anxiety | 4, 7 | |
| Luyster | 2009 | USA | Cross-sectional | 88 | 68 | 70.00 | 10.70 | 11.36 | Depression, anxiety | 4, 7 | ||
| Marchlinski | 2016 | USA | Prospective cohort | 228 | 234 | 67.40 | 25.36 | Depression, anxiety | 5, 7 | |||
| Mohammadi | 2019 | Iran | Cross-sectional | 95 | 65 | 55.79 | 13.99 | Anxiety | 1, 2, 6, 7 | |||
| Morken | 2014 | Norway | Cross-sectional | 167 | 133 | 64.40 | 13.30 | 34.13 | 36.53 | PTSD | 4, 7 | |
| Morris | 1991 | USA | Cross-sectional | 20 | 15 | 60.90 | 8.78 | Depression, anxiety | 3, 7 | |||
| Newall | 2007 | New Zealand | Cross-sectional | 46 | 32 | 56.20 | 30.43 | Depression, anxiety | 4, 7 | |||
| Ofman | 2018 | USA | Retrospective cohort | 25 678 | 25 365 | 65.50 | 10.30 | Anxiety, depression | 5, 7 | |||
| Opić | 2012 | The Netherlands, Belgium | Cross-sectional | 61 | 43 | 60.07 | 16.98 | 45.9 | 31.15 | Anxiety, depression | 4, 7 | Cardiac |
| Pannag | 2020 | Canada | RCT | 10 | 10 | 69.90 | 11.30 | 100 | Anxiety | 1, 2, 6, 7 | ||
| Pannag | 2021 | Canada | RCT | 10 | 10 | 69.90 | 11.30 | 100 | Anxiety | 1, 2, 6, 7 | ||
| Pasyar | 2022 | Iran | Cross-sectional | 96 | 59 | 51.10 | 26.57 | Depression, anxiety | 4, 7 | |||
| Pauli | 1999 | Germany | Cross-sectional | 61 | 49 | 55.70 | 9.00 | 45.76 | Anxiety, depression | 4, 7 | General | |
| Pedersen | 2018 | The Netherlands | Prospective cohort | 134 | 111 | 60.00 | 10.00 | 6.72 | 51.00 | Depression | 2, 4, 7 | |
| Pedersen | 2019 | Europe, New Zealand | Case–control | 334 | 242 | 54.50 | 14.59 | 12.28 | 71.26 | Depression, anxiety | 1, 2, 3, 4, 7 | |
| Pushkarev | 2018 | Russia | Prospective cohort | 260 | 216 | 57.00 | 10.05 | Depression | 4, 7 | |||
| Pushkarev | 2022 | Russia | Cross-sectional | 95 | 215 | 57.10 | 10.00 | Anxiety | 4, 7 | |||
| Pycha | 1990 | USA | Cross-sectional | 42 | 38 | Depression, anxiety | 4, 7 | Partners | ||||
| Rafsanjani | 2020 | Iran | Cross-sectional | 100 | 60 | 59.30 | 11.70 | Depression, anxiety | 4, 7 | General | ||
| Rahmawati | 2016 | Japan | Cross-sectional | 179 | 145 | 60.51 | 15.87 | 59.78 | 29.05 | Anxiety, depression, PTSD | 4, 7 | |
| Redhead | 2010 | UK | Case–control | 100 | 143 | 69.00 | 49 | 100 | Anxiety, depression | 4, 7 | Partners | |
| Roberts | 2016 | USA | Cross-sectional | 50 | 33 | 62.28 | 15.68 | PTSD | 5, 7 | |||
| Rottmann | 2018 | The Netherlands | Before–after | 286 | 227 | 59.30 | 11.00 | 12.24 | 66.40 | Anxiety, depression | 1, 4, 6, 7 | Partners |
| Salmoirago-Blotcher | 2012 | USA | Cross-sectional | 46 | 32 | 65.00 | 10.50 | 13.04 | Anxiety/depression, depression, anxiety | 4, 7 | ||
| Salmoirago-Blotcher | 2013 | USA | RCT | 22 | 18 | 62.90 | 10.20 | 86.36 | Depression, anxiety | 5, 7 | ||
| Sandhu | 2022 | USA | Retrospective cohort | 66 | 51 | 58.52 | 17.18 | 69.70 | 31.82 | Anxiety, depression | 5, 7 | |
| Schulz | 2013 | Germany | Prospective cohort | 54 | 42 | 57.18 | 13.90 | 40.74 | Anxiety, depression | 1, 4, 6, 7 | ||
| Schuster | 1998 | USA | Cross-sectional | 39 | 31 | 65.00 | 11.00 | 56.41 | Anxiety | 4, 7 | ||
| Sowell | 2007 | USA | Cross-sectional | 40 | 31 | 66.00 | 11.28 | 27.00 | Anxiety | 4, 7 | Partners | |
| Spindler | 2009 | Denmark | Cross-sectional | 535 | 438 | 61.50 | 14.40 | 42.06 | 5.20 | Anxiety, depression | 4, 7 | |
| Starrenburg | 2014a | The Netherlands | Prospective cohort | 300 | 250 | 62.30 | 11.00 | 19.67 | 70.67 | Anxiety, depression | 1, 6 | |
| Starrenburg | 2014b | The Netherlands | Prospective cohort | 300 | 247 | 62.00 | 11.10 | 8.67 | 70.67 | Anxiety, depression | 1, 2, 3, 4, 6, 7 | |
| Thomas | 2009 | USA, Canada, New Zealand | Prospective cohort | 57 | 47 | 59.80 | 11.80 | 21.05 | 100 | Depression, anxiety | 5, 4, 7 | |
| Timal | 2021 | The Netherlands | RCT | 80 | 61 | 68.35 | 8.29 | 100 | Depression | 5, 4, 7 | Cardiac | |
| Undavia | 2008 | USA | Cross-sectional | 43 | 28 | 64.67 | 14.90 | 41.86 | 39.53 | Anxiety, depression | 4, 7 | |
| van den Broek | 2006 | The Netherlands | Prospective cohort | 33 | 27 | 60.00 | 11.00 | 24.24 | Anxiety | 5, 7 | ||
| van den Broek | 2008 | The Netherlands | Prospective cohort | 308 | 254 | 62.60 | 10.10 | 5.19 | 54.22 | Anxiety | 2, 7 | |
| van den Broek | 2009a | The Netherlands | Prospective cohort | 205 | 179 | 62.10 | 10.60 | 4.24 | 49.76 | Anxiety | 2, 7 | |
| van den Broek | 2009b | The Netherlands | Prospective cohort | 391 | 315 | 62.30 | 10.40 | 57.29 | Depression, anxiety | 2, 7 | ||
| van den Heuvel | 2022 | Australia | Prospective cohort | 40 | 26 | 46.30 | 14.20 | 12.50 | 92.50 | Anxiety, depression | 1, 2, 3, 4, 6, 7 | |
| Verkerk | 2015 | The Netherlands | Prospective cohort | 35 | 18 | 36.70 | 8.60 | 8.57 | 100 | Depression, anxiety | 1, 2, 3, 4, 6, 7 | |
| Versteeg | 2012 | The Netherlands | Cross-sectional | 272 | 225 | 59.20 | 11.90 | 9.19 | 72.06 | Anxiety, depression | 2, 4, 7 | |
| Versteeg | 2017 | France, Germany, Spain, Switzerland, The Netherlands | Cross-sectional | 351 | Anxiety/depression | 2, 7 | ||||||
| Visser | 2017 | The Netherlands | Case–control | 120 | 56 | 56.55 | 29.17 | 0 | Depression | 4, 7 | ||
| Wallace | 2002 | USA | Cross-sectional | 58 | 44 | 67.00 | 65.00 | Anxiety, depression | 4, 7 | |||
| Whang | 2005 | USA | Prospective cohort | 645 | 527 | 64.11 | 12.59 | 11.94 | Depression | 4, 7 | ||
| Wheeler | 2009 | USA | Prospective cohort | 33 | 26 | 63.48 | 10.64 | 18.18 | Anxiety, depression | 1, 7 | ||
| Zangger | 2018 | Denmark | Cross-sectional | 358 | 293 | 65.50 | 11.00 | 52.51 | Anxiety, depression | 3, 7 |
ICD, implantable cardioverter defibrillator; PTSD, post-traumatic stress disorder; RCT, randomized controlled trial.
Study references provided in Supplementary Materials.
Timepoint 1 = pre-discharge; timepoint 2 = discharge—5 months post-ICD; timepoint 3 = 6–12 months post-ICD; timepoint 4 = >12 months post-ICD; timepoint 5 = unspecified post-ICD timepoint; timepoint 6 = all pre-ICD; timepoint 7 = all post-ICD.
Prevalence of mood disorders
A total of 90 studies were included in prevalence analyses, with individual prevalence analyses containing 5 to 61 studies. Prevalence estimates for anxiety, depression, PTSD, and any mood disorder (all pooled) for all cut-off definitions (clinically significant, at least mild symptoms) at each timepoint are displayed in Figure 2, with all data in Supplementary material online, Table S3.
Figure 2.
Prevalence of clinically relevant symptoms or diagnosis as well as at least mild symptoms of anxiety, depression, and PTSD in ICD patients across timepoints (before and after ICD implantation). ICD, implantable cardioverter defibrillator; PTSD, post-traumatic stress disorder.
Anxiety in implantable cardioverter defibrillator patients
Prevalence estimates for the presence of a diagnosis or clinically relevant symptomology of anxiety were 30.43% (95%CI 18.42–42.43%, k = 15) prior to discharge from hospital for ICD implantation; 32.29% (95%CI 23.96–40.61%, k = 15) from discharge to 5 months after implantation; 28.98% (95%CI 14.32–43.63%, k = 8) from 6 months to 12 months post-implantation; and 22.39% (95%CI 17.04–27.74%, k = 28) beyond 12 months post-implantation. The overall prevalence of a diagnosis or clinically relevant anxiety post-implantation was 22.58% (95%CI 18.26–26.91%, k = 50). Similar, or higher, rates of at least mild anxiety symptomology were seen across analyses (see Figure 2 and Supplementary material online, Table S3).
Depression in implantable cardioverter defibrillator patients
Prevalence estimates for the presence of a diagnosis or clinically relevant symptomology of depression were 16.81% (95%CI 10.61–23.02%, k = 15) prior to discharge from hospital for ICD implantation; 22.56% (95%CI 11.41–33.71%, k = 7) from discharge to 5 months after implantation; 20.52% (95%CI 6.85–34.2%, k = 6) from 6 months to 12 months post-implantation; and 13.60% (95%CI 9.64–17.57%, k = 24) beyond 12 months post-implantation. The overall prevalence of a diagnosis or clinically relevant depression post-implantation was 15.42% (95%CI 11.90–18.94%, k = 38). Similar, or higher, rates of at least mild depressive symptomology were seen across analyses (see Figure 2 and Supplementary material online, Table S3).
Post-traumatic stress disorder in implantable cardioverter defibrillator patients
Prevalence estimates for the presence of a diagnosis or clinically relevant symptomology of PTSD were 11.68% (95%CI 5.54–17.83%, k = 8) beyond 12 months post-implantation. The overall prevalence of a diagnosis or clinically relevant PTSD post-implantation was 12.43% (95%CI 6.90–17.96%, k = 9).
Mood symptoms and disorders in partner control groups
Pooled prevalence of clinically significant mood symptoms or diagnosed mood disorders in the partners of ICD patients post-ICD was able to be calculated (3 studies, 225 participants). An estimated 22.88% (95%CI—29.96–75.72%), 14.11% (95%CI—17.78–46.00%), and 18.52% (95%CI—23.52–60.56%) of partners had clinically relevant anxiety, depression, or any mood disorder respectively following their partner’s ICD implantation (see Supplementary material online, Table S4). It is important to note that these estimates have wide confidence intervals, and large heterogeneity was seen in these analyses.
Subgroup analyses
Female vs. male implantable cardioverter defibrillator patients
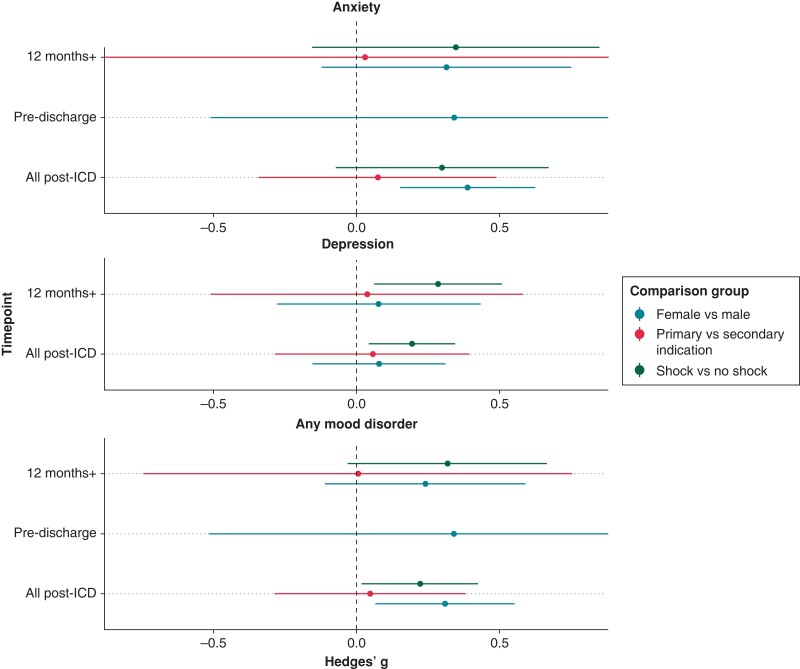
The difference in mood between female and male ICD patients was reported in 10 studies. Sufficient data were available to investigate sex differences both pre- and post-ICDs for anxiety symptoms, but only post-ICD for depression symptoms and clinically significant mood disorders. Significantly higher symptoms of anxiety were found in female ICD patients compared to male ICD patients post-ICD insertion (Hedges’ g = 0.39, 95%CI 0.15–0.62, k = 7, see Figure 3 and Supplementary material online, Table S5). There were no significant differences pre-ICD for anxiety symptoms, post-ICD for depression symptoms, or by presence of clinically significant mood disorder post-ICD (see Figures 3 and 4 and Supplementary material online, Tables S5 and S6).
Figure 3.
Hedges’ g for difference in mood symptoms (anxiety, depression, and all combined) between ICD groups based on sex, shocks, and indication across timepoints. Note: Hedges’ g > 0 indicates more mood symptoms in the first subgroup (female, primary, and shock) compared to the second subgroup (male, secondary, and no shock). ICD, implantable cardioverter defibrillator.
Figure 4.
Odds ratio for likelihood of clinically relevant mood disorders (anxiety, depression, and all combined) across timepoints between ICD groups based on sex and shocks. Note: odds ratio > 1 indicates higher likelihood of clinically relevant mood disorders in the first subgroup (female, primary, and shock) compared to the second subgroup (male, secondary, and no shock). ICD, implantable cardioverter defibrillator; OR , odds ratio.
Shock vs. no shock in implantable cardioverter defibrillator patients
The difference in mood between ICD patients who did and did not experience shocks was reported in 10 studies. Sufficient data were available to investigate differences in mood symptomology and the presence of clinically significant mood disorders for both anxiety and depression post-ICD, but not pre-ICD. Significantly higher symptoms of depression were found post-ICD in the ICD patients who experienced shocks compared to those who did not (Hedges’ g = 0.19, 95%CI 0.04–0.35, k = 6, see Figure 3 and Supplementary material online, Table S5). Implantable cardioverter defibrillator patients who experienced shocks also had significantly higher odds of having clinically significant, or diagnosed, anxiety (OR = 3.92, 95%CI 1.67–9.19, k = 5) and depression (OR = 1.86, 95%CI 1.34–2.59, k = 4) post-ICD (see Figure 4 and Supplementary material online, Table S6). No significant differences were found between groups in anxiety symptoms post-ICD (see Figure 3 and Supplementary material online, Table S5).
Primary vs. secondary implantable cardioverter defibrillator indication
The difference in mood symptoms between primary and secondary indication ICD patients was reported in five studies. Meta-analysis of differences in anxiety and depression symptoms post-ICD was conducted, with no significant differences (see Figure 3 and Supplementary material online, Table S5).
Comparisons with non-implantable cardioverter defibrillator groups
A total of 19 included studies reported data for an appropriate non-ICD group (partners, patients with cardiac conditions, and general population) and were entered into analyses. Partners of the ICD patients were reported in 6 studies, patients with cardiac conditions (without ICD or other cardiac intervention) were reported in 10, and a general or unspecified population was reported in 3. Sufficient data were only available to compare partners and non-ICD cardiac patients to ICD patients post-ICD. There were insufficient data to compare the ICD group to the general or unspecified non-ICD comparison group at any timepoint, although these data are included in the comparisons with all non-ICD controls.
Pooled estimates for these differences are reported in Supplementary material online, Tables S7 and S8 and displayed in Supplementary material online, Figures S1 and S2. No significant differences between groups for any mood measures were found. Heterogeneity across analyses varied from low to high (I2 range: 0–82.72%).
Comparisons within implantable cardioverter defibrillator patients over time
A total of 27 included studies reported continuous data for ICD patients across at least one timepoint comparison. Symptoms of depression decreased significantly from pre- to post-ICD implantation (SMCR = 0.20, 95%CI 0.10–0.30) and from pre-discharge for ICD implantation to up to 6 months post-ICD (SMCR = 0.13, 95%CI 0.03–0.23). Symptoms of anxiety also significantly decreased from up to 6 months post-ICD insertion compared to 6–12 months post-ICD insertion (SMCR = 0.07, 95%CI 0.00–0.14) (see Supplementary material online, Table S9 and Supplementary material online, Figure S3). No significant differences were seen in anxiety or depression symptoms between any other timepoints, and there were insufficient data to assess change in PTSD over time. Notably, there was one study identified as an outlier (which showed large increases in symptoms over time) across these analyses by visual inspection of the funnel plots (see Supplementary material online, Table S12). Sensitivity analyses in which this outlier was removed showed a different pattern of results, whereby there were also significant reductions in anxiety from pre- to post-ICDs. The other significant results reported remained stable.
Risk of bias
Risk of bias across domains for included non-randomized studies is shown in Figure 5, with individual study assessments in Supplementary material online, Table S10. Almost half of the included studies had a high risk of detection bias, often due to a lack of blinding of assessors for interview or diagnostic-based measures, or the repeated use of self-report measures over time (judged as potential bias). Approximately one quarter of the studies also had a high risk of selection bias relating to confounding variables, due to inadequate consideration of major confounders. Almost one half of studies had unclear risk of bias relating to the selection of participants due to some not confirming the absence of outcomes at the start of the study.
Figure 5.
Summary of risk of bias assessments for observational studies using Risk of Bias for Non-randomized studies (RoBANS) tool.14
Risk of bias across domains for included randomized studies is shown in Figure 6 with individual study assessments in Supplementary material online, Table S11. High risk of bias in the measurement of the outcome was identified across all included randomized controlled trials, as all employed self-report measures. There was a high risk of bias in the randomization process of just over half of the studies, generally because it was not clear whether the allocation sequence was concealed until participant enrolment. In just over half of studies, a high risk of bias was identified in missing outcome data, due to higher levels of attrition (>10%) and the potential for mood to have affected attrition (people with higher levels of mood symptoms or disorders may be more likely to withdraw). There was unclear risk of bias for all in the selection of the reported result due to the absence of pre-specified analysis plans.
Figure 6.
Summary of risk of bias assessments for randomized controlled trials using the Risk of Bias 2.0 tool.15
Reporting biases
Potential small study effect was found in several analyses. Overall, trim and fill estimation led to small changes in effect size. Particularly relevant to the significant results presented in previous sections, evidence of small study effect was found in the analyses of post-ICD prevalence for both clinically relevant anxiety and depression. In both cases, trim and fill estimation led to no change in effect estimate. All funnel plots and trim and fill estimations, where necessary, are displayed in Supplementary material online, Table S12.
Certainty of evidence
Using the GRADE approach, the overall certainty in the body of evidence presented here was deemed to be low. This means that there can be low confidence in reported effect estimates. The true effects might be markedly different from the estimated effect. With most included evidence being drawn from non-randomized studies, there is potential bias from lack of randomization (i.e. confounding and selection bias). Some evidence of this was shown in risk of bias assessments too, so we did adjust the level of certainty further based on risk of bias.
Discussion
The rates of anxiety and depression in ICD patients are high, regardless of whether for primary prevention or secondary prevention. Notably, these rates were not statistically higher than cardiac patients who did not undergo an ICD (or other) cardiac intervention, highlighting that depression and anxiety are high in cardiac patients generally. Clinically relevant anxiety was seen in around 30% of ICD patients within the first year after insertion, and depression in around 20%. This is higher than general populations, where the point prevalences of depression and anxiety have been estimated to be 13%27 and 7%28, respectively. Anxiety symptom burdens were higher in females, and those who received a shock were more likely to have clinically relevant anxiety and depression after insertion. The most striking finding relates to PTSD, apparent in 12% of ICD patients more than 12 months after insertion. For context, the point-prevalence of PTSD is around 1–2% in the general population29 and 12% in US military veterans30; although we did not compare severity or duration, which may be higher in veteran samples. Comparison groups employed across included studies were primarily partners or other cardiac patients and showed similar rates of anxiety and depression, demonstrating that mood disorders are major issues for these groups too.
There appears to be a bidirectional relationship between PTSD symptoms and cardiac events. Life-threatening illness and events such as cardiac arrhythmias, ICD insertions, and critical care stays can trigger PTSD.31 Acute coronary syndromes, which often feature comorbid arrhythmias, can also trigger PTSD, which may in turn increase patient risk for subsequent cardiac events and mortality.32 Edmondson et al.32 reported a 12% prevalence of acute coronary-syndrome-induced-PTSD, equivalent to our rate of 12% after 12 months in ICD patients. A systematic review33 (no meta-analysis) also reported an average cardiac-disease-induced PTSD prevalence rate of 12% following a cardiac event (e.g. myocardial infarction, coronary artery bypass grafting, ICD, or heart transplantation). Importantly, with included studies not reporting PTSD prevalence prior to ICD insertion, we were unable to investigate the specific effect of ICD on PTSD rates above that of a cardiac disease population without intervention. There is limited investigation of the impact of the diagnosis itself as a potentially traumatic event. In a mitral regurgitation patient sample, higher rates of PTSD have been found among patients who received a diagnosis compared to a control group.34 Relatively high rates of PTSD (7%) have also been found in the lead up to coronary artery bypass grafting surgery,35 before the surgery has even occurred.
We found that those who did experience a shock demonstrated higher depression symptom severity, along with higher rates of clinically relevant depression and anxiety, as compared to those who did not experience a shock. Experiencing an ICD shock should trigger additional psychological assessment and support. Further, anxiety appears to be consistently more prevalent within ICD patients, as compared to depression, and is therefore likely the primary psychological issue revolving around a fear of recurrence (of arrhythmia and shock). Anxiety symptom burden was significantly higher in women as compared to men post-ICD insertion but not pre-ICD insertion.
There were high rates of depression and anxiety in partners of ICD patients. This finding is similar to a 2010 systematic review (no meta-analysis), reporting similar levels of distress between ICD patients and their partners.36 Qualitative data indicate that mood symptoms in partners primarily relate to increased caring responsibilities along with role changes, helplessness, and uncertainties related to shocks, sexual activities, and driving.36 Partners are a group of concern that need to be considered,37 and they need to be offered psychological therapies alongside the ICD patient. Including partners in post-ICD psychological interventions improves outcomes for patients.38 It is of note that most ICD patients are male, and most of their partners are female, which needs to be considered when comparing patient and control groups, as females typically have higher rates of depression and anxiety. Additionally, we did not investigate rates of mood disorders in partners of patients with cardiac disease, but without ICD (or other intervention), and included studies did not report rates of depression and anxiety in partners prior to implantation. It may be that a loved one having a serious cardiac disease (not specifically an ICD) is responsible for the increased rates of depression and anxiety in partners.
In general, rates of mood disorders in ICD patients and cardiac comparison groups did not statistically differ. The presence of cardiac disease is associated with mental health problems. This relationship appears bidirectional, with evidence for increased risk of cardiovascular disease in those with depression and anxiety39 as well as increased prevalence of anxiety disorders in those with cardiac disease.40
Interestingly, results presented here indicate a trend for anxiety and depression symptom severity reduction over time in ICD patients following insertion. However, the mechanisms of change are unclear, and biased attrition, whereby healthier people remain in longitudinal research, is likely a contributing factor.
This meta-analysis is not without limitation. We only included articles published in English. While 109 studies were included across analyses, fewer studies reported data for individual analyses. In some cases, insufficient data were available for comparisons. For instance, prevalence or change in PTSD over time, especially prior to implantation, was unable to be assessed. There were also limited data available across included studies for suitable control groups, particularly those from the general population (without cardiac disease). Additionally, males were overrepresented across our pooled sample, which is in keeping with previously published literature. Future studies need to consider gender along with cultural diversity more so, as they are important factors when assessing type and severity of mood symptoms but are understudied in ICD patients. Implantable cardioverter defibrillator implantation has known physical risks including infection, unnecessary shocks, device malfunction, and procedural complications.41 This meta-analysis provides strong empirical data demonstrating that depression, anxiety, and PTSD are psychological risks for ICD patients and their partners. Despite these high rates of mood symptoms and disorders, 70% of ICD patients with poor mental health outcomes receive no treatment.42
This large meta-analysis, which summarized the data of 39 954 ICD patients, is pivotal in demonstrating the high prevalence rates of depression, anxiety, and PTSD experienced by ICD patients. Increased awareness and monitoring for depression, anxiety, and PTSD are needed in ICD patients (particularly those who experience shocks), their partners, and cardiac patients in general. Psychological assessment and therapy must be integrated into ICD patient care pathways.
Supplementary Material
Contributor Information
Erica S Ghezzi, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, GPO Box 2741, Adelaide SA 5000, Australia.
Rhianna L S Sharman, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, GPO Box 2741, Adelaide SA 5000, Australia.
Joseph B Selvanayagam, Department of Cardiovascular Medicine, Flinders Medical Centre, GPO Box 2100, Adelaide SA 5001, Australia; Department of Medicine, School of Medicine and Public Health, Flinders University, GPO Box 2100, Adelaide SA 5001, Australia; Lifelong Health Theme, South Australian Health and Medical Research Institute, North Terrace, Adelaide SA 5000, Australia.
Peter J Psaltis, Lifelong Health Theme, South Australian Health and Medical Research Institute, North Terrace, Adelaide SA 5000, Australia; Adelaide Medical School, University of Adelaide, North Terrace, Adelaide SA 5005, Australia; Department of Cardiology, Royal Adelaide Hospital, Port Road, Adelaide SA 5000, Australia.
Prashanthan Sanders, Lifelong Health Theme, South Australian Health and Medical Research Institute, North Terrace, Adelaide SA 5000, Australia; Adelaide Medical School, University of Adelaide, North Terrace, Adelaide SA 5005, Australia; Department of Cardiology, Royal Adelaide Hospital, Port Road, Adelaide SA 5000, Australia.
Jack M Astley, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, GPO Box 2741, Adelaide SA 5000, Australia.
Sara Knayfati, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, GPO Box 2741, Adelaide SA 5000, Australia.
Vrinda Batra, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, GPO Box 2741, Adelaide SA 5000, Australia.
Hannah A D Keage, Cognitive Ageing and Impairment Neurosciences Laboratory, Justice and Society, University of South Australia, GPO Box 2741, Adelaide SA 5000, Australia.
Supplementary material
Supplementary material is available at Europace online.
Funding
L2 Future Leadership Fellowship from the National Heart Foundation of Australia (grant number FLF102056) to P.J.P. L2 Career Development Fellowship from the National Health and Medical Research Council (grant number CDF1161506) to P.J.P. Funding from the National Health and Medical Research Council and the National Heart Foundation of Australia to P.S.
Conflict of interest: P.J.P. has received research support from Abbott Vascular, consulting fees from Amgen and Esperion, and speaker honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck Schering-Plough, Novartis, Pfizer, and Sanofi.
J.B.S. has received research support from Biotronik, Bayer, Sanofi, Actelion, and Pfizer and speaker honoraria from Bureau-AstraZeneca, Boehringer-Ingelheim, Novartis, Abbot, Bayer, Sanofi, Biotronik, CircleCVI, and Takeda. J.B.S. is also on the advisory board for BMS, Sanofi, Faraday, and Recardio Pharmaceuticals.
P.S. reports having served on the advisory board of Medtronic, Abbott Medical, Boston Scientific, CathRx, and PaceMate. P.S. reports that the University of Adelaide has received on his behalf research funding, lecture, and/or consulting fees from Medtronic, Abbott Medical, Boston Scientific, and Becton-Dickenson.
Data availability
The data underlying this article were accessed from pre-existing literature and are publicly available in GitHub at https://github.com/ericaghezzi/ICD_mood_metaanalysis, along with the code used for data analysis.
References
- 1. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau Ret al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein Het al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med 1996;335:1933–40. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DSet al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 4. Blanch B, Lago LP, Sy R, Harris PJ, Semsarian C, Ingles J. Implantable cardioverter-defibrillator therapy in Australia, 2002–2015. Med J Aust 2018;209:123–9. [DOI] [PubMed] [Google Scholar]
- 5. Kurtz SM, Ochoa JA, Lau E, Shkolnikov Y, Pavri BB, Frisch Det al. Implantation trends and patient profiles for pacemakers and implantable cardioverter defibrillators in the United States: 1993–2006. Pacing Clin Electrophysiol 2010;33:705–11. [DOI] [PubMed] [Google Scholar]
- 6. Zecchin M, Torre M, Carrani E, Sampaolo L, Ciminello E, Ortis Bet al. Seventeen-year trend (2001–2017) in pacemaker and implantable cardioverter-defibrillator utilization based on hospital discharge database data: an analysis by age groups. Euro J Intern Med 2021;84:38–45. [DOI] [PubMed] [Google Scholar]
- 7. Andersen CM, Theuns DAMJ, Johansen JB, Pedersen SS. Anxiety, depression, ventricular arrhythmias and mortality in patients with an implantable cardioverter defibrillator: 7 years’ follow-up of the MIDAS cohort. Gen Hosp Psychiatry 2020;66:154–60. [DOI] [PubMed] [Google Scholar]
- 8. Perini AP, Kutyifa V, Veazie P, Daubert JP, Schuger C, Zareba Wet al. Effects of implantable cardioverter/defibrillator shock and antitachycardia pacing on anxiety and quality of life: a MADIT-RIT substudy. Am Heart J 2017;189:75–84. [DOI] [PubMed] [Google Scholar]
- 9. Diagnostic and statistical manual of mental disorders 5TR. USA: American Psychiatric Association; 2022. [Google Scholar]
- 10. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MHet al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kikkenborg Berg S, Caspar Thygesen L, Hastrup Svendsen J, Vinggaard Christensen A, Zwisler AD. Anxiety predicts mortality in ICD patients: results from the cross-sectional national CopenHeartICD survey with register follow-up. Pacing Clin Electrophysiol 2014;37:1641–50. [DOI] [PubMed] [Google Scholar]
- 12. HabiboviC M, Pedersen SS, van den Broek KC, Theuns DAMJ, Jordaens L, van der Voort PHet al. Anxiety and risk of ventricular arrhythmias or mortality in patients with an implantable cardioverter defibrillator. Psychosom Med 2013;75:36–41. [DOI] [PubMed] [Google Scholar]
- 13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim SY, Park JE, Lee YJ, Seo H-J, Sheen S-S, Hahn Set al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408–14. [DOI] [PubMed] [Google Scholar]
- 15. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron Iet al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 16. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 17. Paule RC, Mandel J. Consensus values and weighting factors. J Res Natl Bur Stand (1977) 1982;87:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003;22:2693–710. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello Pet al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cinar FI, Tosun N, Kose S. Evaluation of an education and follow-up programme for implantable cardioverter defibrillator-implanted patients. J Clin Nurs 2013;22:2474–86. [DOI] [PubMed] [Google Scholar]
- 22. Crössmann A, Pauli P, Dengler W, Kühlkamp V, Wiedemann G. Stability and cause of anxiety in patients with an implantable cardioverter-defibrillator: a longitudinal two-year follow-up. Heart Lung 2007;36:87–95. [DOI] [PubMed] [Google Scholar]
- 23. Dougherty CM, Burr RL, Kudenchuk PJ, Glenny RW. Aerobic exercise effects on quality of life and psychological distress after an implantable cardioverter defibrillator. J Cardiopulm Rehabil Prev 2020;40:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Habibović M, Denollet J, Cuijpers P, Spek VR, van den Broek KC, Warmerdam Let al. E-health to manage distress in patients with an implantable cardioverter-defibrillator: primary results of the WEBCARE trial. Psychosom Med 2014;76:593–602. [DOI] [PubMed] [Google Scholar]
- 25. Kuhl EA, Sears SF, Vazquez LD, Conti JB. Patient-assisted computerized education for recipients of implantable cardioverter defibrillators: a randomized controlled trial of the PACER program. J Cardiovasc Nurs 2009;24:225–31. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal B, Pender A, Mosca L, Mochari-Greenberger H. Factors associated with medication adherence among heart failure patients and their caregivers. J Nurs Educ Pract 2015;5:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018;8:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med 2013;43:897–910. [DOI] [PubMed] [Google Scholar]
- 29. Perkonigg A, Kessler RC, Storz S, Wittchen HU. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand 2000;101:46–59. [DOI] [PubMed] [Google Scholar]
- 30. Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry 2014;1:269–77. [DOI] [PubMed] [Google Scholar]
- 31. Righy C, Rosa RG, da Silva RTA, Kochhann R, Migliavaca CB, Robinson CCet al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Critical Care 2019;23:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One 2012;7:e38915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vilchinsky N, Ginzburg K, Fait K, Foa EB. Cardiac-disease-induced PTSD (CDI-PTSD): a systematic review. Clin Psychol Rev 2017;55:92–106. [DOI] [PubMed] [Google Scholar]
- 34. Bayer-Topilsky T, Trenerry MR, Suri R, Topilsky Y, Antiel RM, Marmor Yet al. Psycho-emotional manifestations of valvular heart diseases: prospective assessment in mitral regurgitation. Am J Med 2013;126:916–24. [DOI] [PubMed] [Google Scholar]
- 35. Oxlad M, Stubberfield J, Stuklis R, Edwards J, Wade TD. Psychological risk factors for cardiac-related hospital readmission within 6 months of coronary artery bypass graft surgery. J Psychosom Res 2006;61:775–81. [DOI] [PubMed] [Google Scholar]
- 36. Van Den Broek KC, Habibović M, Pedersen SS. Emotional distress in partners of patients with an implantable cardioverter defibrillator: a systematic review and recommendations for future research. Pacing Clin Electrophysiol 2010;33:1442–50. [DOI] [PubMed] [Google Scholar]
- 37. Dunbar SB, Dougherty CM, Sears SF, Carroll DL, Goldstein NE, Mark DBet al. Educational and psychological interventions to improve outcomes for recipients of implantable cardioverter defibrillators and their families. Circulation 2012;126:2146–72. [DOI] [PubMed] [Google Scholar]
- 38. Liberato ACS, Thompson EA, Dougherty CM. Intervention mediating effects of self-efficacy on patient physical and psychological health following ICD implantation. J Behav Med 2021;44:842–52. [DOI] [PubMed] [Google Scholar]
- 39. Seldenrijk A, Vogelzangs N, Batelaan NM, Wieman I, van Schaik DJF, Penninx BJWH. Depression, anxiety and 6-year risk of cardiovascular disease. J Psychosom Res 2015;78:123–9. [DOI] [PubMed] [Google Scholar]
- 40. Vogelzangs N, Seldenrijk A, Beekman ATF, van Hout HPJ, de Jonge P, Penninx BWJH. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord 2010;125:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol 2008;52:1111–21. [DOI] [PubMed] [Google Scholar]
- 42. Hoogwegt MT, Kupper N, Theuns DA, Zijlstra WP, Jordaens L, Pedersen SS. Undertreatment of anxiety and depression in patients with an implantable cardioverter-defibrillator: impact on health status. Health Psychol 2012;31:745–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were accessed from pre-existing literature and are publicly available in GitHub at https://github.com/ericaghezzi/ICD_mood_metaanalysis, along with the code used for data analysis.