Abstract
Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous adverse reactions with high morbidity and mortality and not clearly established treatment protocol. This meta-analysis aimed to evaluate the efficacy and safety of three biologic TNF-α inhibitors (infliximab, etanercept, adalimumab) in the treatment of SJS, SJS-TEN overlap, and TEN.
Methods
Electronic databases were searched for original studies containing human participants diagnosed with SJS/TEN and treated with biologic TNF-α inhibitors. Individual patient data were collected and summarized to provide a comprehensive overview on therapeutic efficacy of different biologic TNF-α inhibitors for SJS, SJS-TEN overlap, and TEN, respectively. Meta-analyses on aggregated study data were conducted using random-effects model.
Results
Overall, 55 studies with 125 sets of individual patient data were included. Infliximab was used to treat 3 patients with SJS-TEN overlap and 28 patients with TEN, and the actual mortality rate was 33.3% and 17%, respectively. Etanercept was administered to 17 patients with SJS, 9 patients with SJS-TEN overlap, and 64 patients with TEN, and mortality rate was reported to be 0%, 0%, and 12.5%, respectively. For participants with TEN, no significant difference was found in time of reepithelialization, hospitalization time, and mortality rate comparing etanercept with infliximab. More sequelae were reported in patients receiving infliximab than in patients treated with etanercept (39.3% versus 6.4%). Adalimumab was administered to four patients with TEN, and mortality rate was 25%. Meta-analyses on aggregated study data revealed significantly shortened hospitalization time in etanercept compared with non-etanercept groups [weighted mean differences (WMD) −5.30; 95% confidence interval (CI) −8.65 to −1.96]. Etanercept was associated with a survival benefit for patients when compared with non-etanercept treatment, however, the analysis was not statistically significant (odds ratio 0.55; 95% CI 0.23–1.33).
Conclusions
On the basis of the current findings, etanercept is currently the most promising biologic therapy for SJS/TEN. Further evaluation in prospective studies is required to confirm its efficacy and safety.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00928-w.
Keywords: Stevens–Johnson syndrome, Toxic epidermal necrolysis, Biologic, TNF-α inhibitors, Etanercept
Key Summary Points
| Patient-level analysis indicated that infliximab had comparable efficacy to etanercept in treating patients with toxic epidermal necrolysis (TEN), as demonstrated by similar clinical outcomes, including time to reepithelialization, hospitalization time, and mortality rate. |
| Patient-level analysis showed that etanercept had a lower mortality rate compared with the average rate reported in epidemiological studies across patients with Stevens–Johnson syndrome (SJS), SJS-TEN overlap, and TEN. |
| Meta-analysis results showed that etanercept therapy provided comparable survival outcome and shorter hospital stays compared with non-etanercept treatments. |
| Patients with TEN who received infliximab had a higher rate of sequelae compared with those treated with etanercept. |
| Overall, etanercept appears to be the most promising biologic therapy for SJS/TEN on the basis of the current findings. |
Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but life-threatening cutaneous adverse reactions primarily caused by certain medications [1]. SJS and TEN are considered different severities of the same disease spectrum with distinct epidermal detachment areas: SJS is defined as < 10% skin detachment, SJS-TEN overlap is 10–30%, and TEN is > 30% [2]. The published overall mortality rate is approximately 5–10% for SJS, 10–25% for SJS-TEN overlap, and 25–50% for TEN [3–5].
As rare conditions, there is currently no well-established treatment protocol for SJS and TEN. The most commonly used therapeutic interventions are cyclosporine and glucocorticoid regimens [6]. The exact pathogenesis of SJS/TEN remains unclear. Skin lesions and blister fluid in SJS/TEN are known to contain high levels of TNF-α, which has led to the use of TNF-α inhibitors such as infliximab, etanercept, and adalimumab in the treatment of these conditions [7, 8].
Numerous studies have reported the beneficial effects of biologic TNF-α inhibitors in the treatment of SJS/TEN [9–13]. However, many of these studies were case reports and case series with small sample sizes, which lacked the statistical power to support the use of TNF-α inhibitors as standard of care. Several meta-analyses have demonstrated the significant effect of etanercept and infliximab in reducing mortality [14–16]. However, these previous meta-analyses frequently integrated the outcomes of SJS, SJS-TEN overlap, and TEN as a single entity, and seldom explored the impact of factors such as timing of intervention and combined therapy on treatment efficacy [14, 17]. These gaps motivated us to conduct this study, which aims to explore the efficacy and safety of biologic TNF-α inhibitors (infliximab, etanercept, adalimumab) for SJS, SJS-TEN overlap, and TEN, respectively.
The primary objective of this meta-analysis is to evaluate the effects of infliximab, etanercept, or adalimumab on mortality, length of hospitalization, and reepithelialization time in SJS, SJS-TEN overlap, and TEN, respectively. We also aim to explore the impact of age, sex, severity-of-illness score of toxic epidermal necrolysis (SCORTEN), days after onset to therapy given, and combined intervention on treatment efficacy. In this meta-analysis, aggregated study data (meta-analysis at the study level) and individual patient data (IPD) (meta-analysis at the patient level) were used to obtain effect estimates for different TNF-α inhibitors.
Methods
The present meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Ethical approval and patient consent were not necessary as this was a systematic review and meta-analysis of previously published studies.
Search Strategy
Two independent reviewers conducted a comprehensive search of PubMed, Embase, Web of Science, Scopus, and Cochrane Library for published articles reporting the effects of TNF-α inhibitors in the treatment of SJS/TEN. Search date was set from January 1990 to January 2023, as the internationally accepted consensus definition for diagnosing SJS/TEN was developed in 1990 [18]. The following keywords were used: (Stevens-Johnson syndrome OR Toxic epidermal necrolysis OR SJS OR TEN) AND (TNF-α inhibitors OR biologic OR etanercept OR adalimumab OR infliximab). Bibliographies of relevant articles and systematic reviews were manually searched for additional studies.
Definition
Diagnosis of SJS, SJS/TEN overlap and TEN was based on the internationally accepted consensus definition described by Roujeau et al. as follow: SJS, < 10% skin detachment of total body surface area; SJS-TEN overlap, 10–30% skin detachment of total body surface area; and TEN, > 30% skin detachment of total body surface area [18].
Eligible Criteria
Comparative studies [observational studies and randomized controlled trials (RCT)] and non-comparative studies (case reports and case series studies) of any age group assessing the effectiveness of TNF-α inhibitor (infliximab, etanercept, and adalimumab) interventions for SJS, SJS-TEN overlap, and TEN were included. Other inclusion criteria were as follows: (1) studies clearly differentiating severity of diseases (SJS, SJS-TEN overlap, and TEN) or providing raw data of body surface area involvement to categorize disease phenotypes; (2) studies having sufficient description of treatment inventions; (3) studies providing at least one of the outcomes regarding mortality, hospitalization time, and time of reepithelialization. Studies that did not meet the above listed criteria, duplicate studies, review articles, and non-research letters were excluded. In case of duplicate reports, the study with the most comprehensive, up-to-date and largest dataset was included. We contacted the corresponding author to obtain missing individual patient data and outcome data via email. If no response was received before drafting the manuscript, the study was excluded from analysis. The different steps to identify and assess the literature were independently performed by two reviewers. Any disagreement was resolved through consensus.
Data Extraction
Two reviewers independently extracted data from each study using a predefined form. Any discrepancies were resolved through discussion. Extracted data included general study characteristics (first author, publication year, study design, country, admission ward), basic patient demographics (age, sex, type of diagnose, body surface area, SCORTEN score involvement, comorbidities, and previous treatments), intervention characteristics (type of biologic therapy, dose, frequency, route of administration, days after onset to therapy given, and combination therapy), and data on outcome measures (mortality, hospitalization time, time of reepithelialization, and sequelae).
Quality Assessment
An instrument proposed by MacLehose et al. and modified by Zimmermann et al. was applied for quality assessment of case reports and case series studies [19, 20]. The instrument assesses studies on the basis of 13 aspects and assigns a total score ranging from 0 to 13 [20]. Studies with a score below 5 were considered high risk.
Statistical Analysis
For descriptive statistics of individual patient data, continuous variables were reported as mean (SD) with standard deviations (SD) or median (range) with interquartile ranges (IQR) as appropriate according to distribution, while categorical variables were reported as absolute numbers (N) and percentages of the total. Demographic and clinical variables between patients receiving different biologic therapies were compared. Associations between biologic treatment efficacy and age, sex, days after onset to therapy given, and use of concomitant therapies were evaluated. Statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 28.0.1.0., Armonk, New York). A p-value < 0.05 was considered as statistically significant.
Study level meta-analysis was conducted using Stata software version 15.0 (Stata Corporation, College Station, TX, USA). Dichotomous variables were pooled as odds ratio (OR) with 95% confidence interval (CI), while continuous variables were generated using weighted mean differences (WMD) with a 95% CI. Statistical heterogeneity between the studies was measured by chi-squared Q-test and further quantified by I2 statistics. Heterogeneity was deemed significant when corresponding p-value was < 0.1 or when I2 was > 50%. When substantial heterogeneity was present, a random-effect model was used for analysis; otherwise, a fixed-effect model was applied. All reported p-values were two-sided and p < 0.05 was considered statistically significant.
Results
Search Results
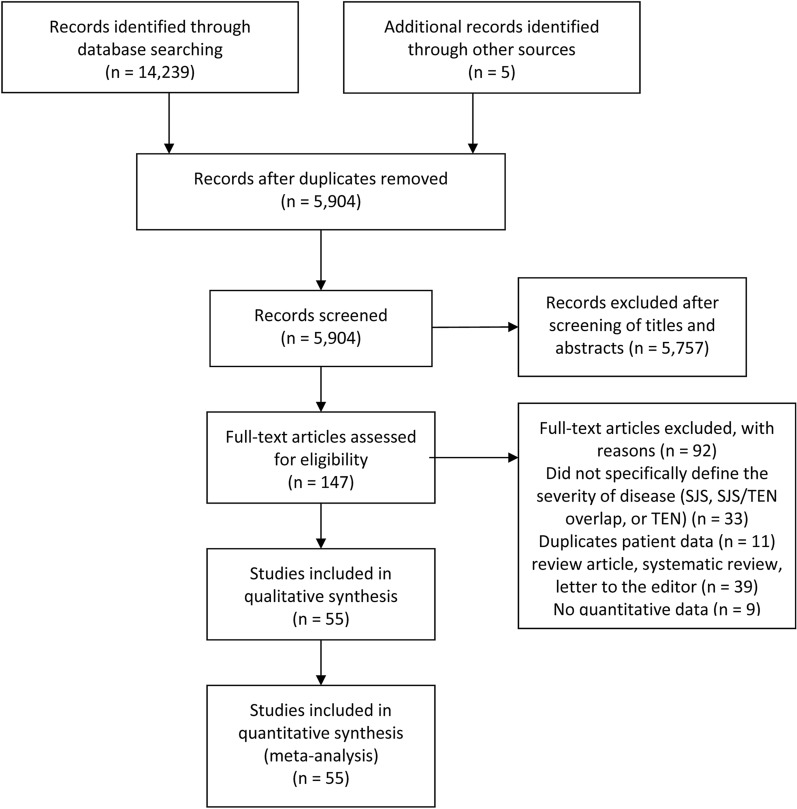
The initial database search yielded 5899 records (duplicates excluded), and an additional 5 articles were identified from reference check. Following title and abstract screening, 147 full-text articles were retrieved and assessed for eligibility and data extraction. Eventually, 55 studies were included in the meta-analysis. The selection process is summarized in Fig. 1.
Fig. 1.
The PRISMA flow diagram depicting the process of study selection
Study Characteristics
The 55 eligible studies were made up of 41 case reports, 6 case series, 6 prospective/retrospective cohort studies, and 2 RCTs [11–13, 21–72]. Seven of the studies were two-arm intervention studies. The majority of studies involved drug-induced conditions (50 out of 55 studies), while 3 studies reported vaccination-induced TEN, and 2 described infection-associated TEN. Infliximab was used in 21 studies, etanercept in 33 studies, and adalimumab in 1 case report. Treatment protocols of TNF-α inhibitors included monotherapy, following failure of other systemic treatments (28 studies), and in combination with systemic corticosteroid (9 studies), intravenous immunoglobulin (5 studies), corticosteroids + intravenous immunoglobulin (11 studies), or N-acetylcysteine (1 studies). The basic study characteristics were summarized in Table 1.
Table 1.
Basic characteristics of the included studies
| References | Region | Study design | Diagnosis | Sample size | Intervention | Combined therapies | Timing with combined therapies |
|---|---|---|---|---|---|---|---|
| Al-Shouli et al. [21] | Saudi Arabia | Case report | TEN | 1 | Infliximab | Supportive therapy | – |
| Ao et al. [22] | China | Cohort study | SJS, SJS-TEN overlap, TEN | 15 | Etanercept | Corticosteroids | Combined |
| Bakir et al. [23] | Saudi Arabia | Case report | TEN | 1 | Etanercept | – | – |
| Burduk et al. [24] | Poland | Case report | TEN | 1 | Infliximab | – | – |
| Cardenas et al. [25] | USA | Case report | TEN | 1 | Infliximab | IVIG + supportive therapy | After |
| Chafranska et al. [26] | Denmark | Case report | TEN | 1 | Infliximab | Supportive therapy | – |
| Chahal et al. [27] | USA | Case report | TEN | 1 | Etanercept | Supportive therapy | – |
| Choi et al. [28] | USA | Case report | TEN | 1 | Etanercept | Corticosteroids | Combined |
| Chong et al. [29] | USA | Case report | SJS-TEN overlap | 1 | Etanercept | Supportive therapy | – |
| Coulombe et al. [30] | Canada | Case report | EN | 1 | Etanercept | Corticosteroids | Combined |
| Didona et al. [31] | Italy | Case report | TEN | 1 | Etanercept | Corticosteroids | Combined |
| Dreyer et al. [32] | USA | Cohort study | SJS, SJS-TEN overlap, TEN | 13 | Etanercept | None or IVIG | Combined |
| Eliades et al. [33] | USA | Case series | SJS, SJS-TEN overlap, TEN | 4 | Etanercept | – | – |
| Estébanez et al. [34] | Spain | Case report | TEN | 1 | Etanercept | Corticosteroids + IVIG | After |
| Famularo et al. [35] | Italy | Case report | TEN | 1 | Etanercept | Corticosteroids | After |
| Faris et al. [36] | USA | Case report | TEN | 3 | Etanercept | Corticosteroids | After |
| Fischer et al. [37] | Germany | Case report | TEN | 1 | Infliximab | – | – |
| Frederiks et al. [38] | Australia | Case series | TEN | 4 | Etanercept | Corticosteroids + IVIG | Combined |
| Gaitanis et al. [39] | Greece | Case report | TEN | 3 | Infliximab | Corticosteroids + IVIG + supportive therapy | Combined |
| Gavigan et al. [40] | Canada | Case report | SJS-TEN overlap | 1 | Etanercept | Corticosteroids | Combined |
| Gubinelli et al. [41] | Italy | Case report | TEN | 1 | Etanercept | Corticosteroids + Supportive therapy | Combined |
| Hunger et al. [42] | Switzerland | Case report | TEN | 1 | Infliximab | – | – |
| Jiang et al. [43] | China | Case report | TEN | 1 | Infliximab | Corticosteroids + supportive therapy | After |
| Kherlopian et al. [12] | Australia | Case series | TEN | 4 | Adalimumab | – | – |
| Kreft et al. [45] | Germany | Case report | TEN | 1 | Infliximab | Corticosteroids | After |
| Kreft et al. [44] | Germany | Case report | TEN | 1 | Infliximab | Corticosteroids + IVIG | After |
| Kumar and Bhandari [80] | USA | Case report | TEN | 1 | Infliximab | Corticosteroids | After |
| Lee et al. [47] | Taiwan | Case report | TEN | 1 | Etanercept | Corticosteroids | After |
| López-Gómez et al. [48] | Spain | Case report | SJS-TEN overlap | 1 | Etanercept | Corticosteroids | Combined |
| Maximova et al. [49] | Italy | Case report | TEN | 1 | Etanercept | Corticosteroids + IVIG | Combined |
| Natsis et al. [50] | USA | Case report | TEN | 1 | Etanercept | – | – |
| Osawa et al. [51] | Japan | Case report | TEN | 1 | Etanercept | Corticosteroids + IVIG | Combined |
| Paquet et al. [52] | Belgium | RCT | TEN | 4 | Infliximab | N-acetylcysteine | Combined |
| Paradisi et al. [11] | Italy | Case series | TEN | 10 | Etanercept | Supportive therapy | – |
| Paradisi et al. [53] | Italy | Case series | TEN | 17 | Etanercept | Supportive therapy | – |
| Patmanidis et al. [54] | Greece | Case report | TEN | 1 | Infliximab | Corticosteroids + supportive therapy | Combined |
| Pham et al. [55] | USA | Case series | TEN | 13 | Etanercept | – | – |
| Plant et al. [56] | UK | Case report | TEN | 1 | Infliximab | IVIG + supportive therapy | After |
| Scott-Lang et al. [57] | Scotland | Case report | TEN | 1 | Infliximab | IVIG + supportive therapy | Combined |
| Scott-Lang et al. [58] | Scotland | Case report | TEN | 1 | Infliximab | IVIG + supportive therapy | Combined |
| Shen et al. [59] | China | Case report | TEN | 1 | Etanercept | Corticosteroids | After |
| Sibbald et al. [60] | USA | Cohort study | TEN | 1 | Etanercept | Corticosteroids + IVIG | Combined |
| Tian et al. [61] | China | Cohort study | SJS, SJS-TEN overlap, TEN | 11 | Etanercept | Corticosteroids + IVIG | Combined |
| Torres‐Navarro et al. [62] | Spain | Cohort study | SJS, SJS-TEN overlap, TEN | 4 | Etanercept | Corticosteroids + IVIG | Combined |
| Vivar et al. [63] | USA | Case report | TEN | 1 | Infliximab | Corticosteroids + IVIG | Combined |
| Wallenborn et al. [64] | Germany | Case report | TEN | 1 | Infliximab | Corticosteroids | After |
| Wang et al. [65] | China | RCT | SJS, SJS-TEN overlap, TEN | 48 | Etanercept | – | – |
| Wang et al. [66] | China | Case report | SJS | 1 | Etanercept | IVIG | Combined |
| Wang et al. [67] | China | Case report | SJS-TEN overlap | 1 | Etanercept | Corticosteroids + IVIG | Combined |
| Wojtkiewicz et al. [68] | Poland | Case report | TEN | 1 | Infliximab | Corticosteroids + supportive therapy | Combined |
| Worsnopet al. [69] | UK | Case report | TEN | 1 | Infliximab | IVIG | After |
| Zander et al. [70] | USA | Case report | TEN | 1 | Etanercept | Corticosteroids + supportive therapy | Combined |
| Zárate-Correa et al. [13] | Colombia | Case report | TEN | 4 | Infliximab | IVIG + supportive therapy | Combined |
| Zhang et al. [71] | China | Case report | TEN | 1 | Etanercept | Corticosteroids + IVIG | Combined |
| Zhang et al. [72] | China | Cohort study | SJS, SJS-TEN overlap, TEN | 242 | Etanercept | Corticosteroids | Combined |
IVIG intravenous immunoglobulin, RCT randomized controlled trials, SJS Stevens–Johnson syndrome, TEN toxic epidermal necrolysis
The average quality score of the included studies was 6.86 (range 5.33–11.67). Supplementary Table 1 provides detailed results of the quality assessment for the included 55 publications.
Patient-Level Analysis
Individual patient data were available for 125 patients from 51 studies. Of these patients, 31 (24.8%) received infliximab, 90 (72%) were treated with etanercept, and 4 (3.2%) received adalimumab. Detailed information on individual patient characteristics, such as age, sex, body surface area, SCORTEN score, disease severity, biologic therapy, dose, frequency, route of administration, and combination therapy, were summarized in Supplementary Table 2.
Among the 125 patients with individual data, 17 were diagnosed with SJS (< 10% BSA). All of them were treated with etanercept. The mean age was 39.42 years. The mean time of reepithelialization was 5.67 days, the mean hospitalization time was 11.36 days, the actual mortality reported was 0%, and the number of patient-reported sequelae was 0% (Table 2).
Table 2.
Results of individual patient data analysis
| SJS | Infliximab | Etanercept | Adalimumab | p value |
|---|---|---|---|---|
| Number of cases (n) | 17 | |||
| Age (years) | 39.42 ± 18.76 | |||
| Sex (male/female/missing) (n) | 6/6/5 | |||
| BSA (%) | 8.40 ± 2.07 (7/10) | |||
| SCORTEN (n) | ||||
| 0–1 | 8 | |||
| 2 | 3 | |||
| 3 | 1 | |||
| 4 | 3 | |||
| ≥ 5 | 2 | |||
| Undefined | 0 | |||
| Days after symptom onset therapy given (days) | 11.50 ± 3.54 (2/17) | |||
| Time of reepithelialization (days) | 5.67 ± 2.31 (3/17) | |||
| Hospitalization time (days) | 11.36 ± 2.87 (11/17) | |||
| Mortality rate (%) | 0% (0/17) | |||
| Number of patients reported sequelae (%) | 0% (0/17) |
| SJS/TEN overlap | Infliximab | Etanercept | Adalimumab | p value |
|---|---|---|---|---|
| Number of cases (n) | 3 | 9 | ||
| Age (years) | 70.33 ± 4.73 | 38.57 ± 27.47 | ||
| Sex (male/female/missing) (n) | 2/1/0 | 1/6/2 | ||
| BSA (%) | 20.0 ± 5.0 | 19.75 ± 2.76 | ||
| SCORTEN (n) | ||||
| 0–1 | 2 | |||
| 2 | 2 | |||
| 3 | 1 | 1 | ||
| 4 | 1 | 1 | ||
| ≥ 5 | ||||
| Undefined | 1 | 3 | ||
| Days after symptom onset therapy given (days) | 3.67 ± 2.52 (3/3) | 4.60 ± 2.88 (5/9) | ||
| Time of reepithelialization (days) | NA | 11.83 ± 9.58 (6/9) | ||
| Hospitalization time (days) | 20.50 ± 2.12 (2/3) | 14.4 ± 7.06 (5/9) | ||
| Mortality rate (%) | 33.3% (1/3) | 0% (0/9) | ||
| Number of patient-reported sequelae (%) | 0% (0/3) | 0% (0/9) |
| TEN | Infliximab | Etanercept | Adalimumab | p value |
|---|---|---|---|---|
| Number of cases (n) | 28 | 64 | 4 | |
| Age (years) | 40.71 ± 25.68 | 48.61 ± 25.70 | 38.75 ± 19.79 | 0.348 |
| Sex (male/female/missing) (n) | 12/16/0 | 26/33/5 | 0/0/4 | 0.915 |
| BSA (%) | 58.33 ± 25.95 | 58.25 ± 21.04 | 66.25 ± 24.28 (4/4) | 0.805 |
| SCORTEN (n) | 0.405 | |||
| 0–1 | 0 | 5 | 1 | |
| 2 | 4 | 8 | 1 | |
| 3 | 2 | 16 | 1 | |
| 4 | 3 | 11 | 1 | |
| ≥ 5 | 1 | 11 | 0 | |
| Undefined | 18 | 13 | 0 | |
| Days after symptom onset therapy given (days) | 5.67 ± 3.45 (24/28) | 4.60 ± 7.01 (53/64) | 1.50 ± 3.00 (4/4) | 0.424 |
| Time of reepithelialization (days) | 12.5 ± 13.98 (20/28) | 10.47 ± 4.98 (45/64) | 17.24 ± 12.69 (4/4) | 0.104 |
| Hospitalization time (days) | 22.58 ± 13.87 (12/28) | 21.24 ± 11.96 (21/64) | 22.75 ± 8.81 (4/4) | 0.944 |
| Mortality rate (%) | 14.3% (4/28) | 12.5% (8/64) | 0% (0/4) | 0.673 |
| Number of patient-reported sequelae (%) | 39.3% (11/28) | 6.4% (10/64) | 0% (0/4) | 0.022 |
NA not applicable, SJS Stevens–Johnson syndrome, TEN toxic epidermal necrolysis
p value: etanercept versus infliximab
Among the 125 patients with individual data, 96 were diagnosed with TEN (> 30% BSA). The mean age of participants with TEN who underwent infliximab (28 of 96 patients) therapy was 40.71 years. The mean time of reepithelialization was 12.5 days, the mean hospitalization time was 22.58 days, the actual mortality reported was 14.3%, and the number of patients reported sequelae was 39.3% (Table 2).
The mean age of participants with TEN who underwent etanercept (64 of 96 patients) therapy was 48.61 years. The mean time of reepithelialization was 10.47 days, the mean hospitalization time was 21.24 days, the actual mortality reported was 12.5%, and the number of patient-reported sequelae was 6.4% (Table 2).
The mean age of participants with TEN who underwent adalimumab (4 of 96 patients) therapy was 38.75 years. The mean time of reepithelialization was 17.24 days, the mean hospitalization time was 22.75 days, the actual mortality reported was 0%, and the number of patient-reported sequelae was 0% (Table 2).
Demographic and clinical variables between patients with TEN receiving infliximab and etanercept were compared. No significant difference was found between the assessed variables, except that the number of patient-reported sequelae was significantly higher in patients treated with infliximab compared with patients who received etanercept (p = 0.022) (Table 2).
Among the 125 patients with individual data, 12 were diagnosed with SJS-TEN overlap (10%–30% BSA). The mean age of SJS-TEN overlap participants who underwent infliximab (3 of 12 patients) therapy was 70.33 years. The mean hospitalization time was 20.50 days, the actual mortality reported was 33.3%, and the number of patient-reported sequelae was 0% (Table 2).
The mean age of participants with SJS-TEN overlap who underwent etanercept (9 of 12 patients) therapy was 38.57 years. The mean time of reepithelialization was 11.83 days, the mean hospitalization time was 14.4 days, the actual mortality reported was 0%, and the number of patient-reported sequelae was 0% (Table 2).
Subgroup Analysis
Associations between biologic treatment efficacies (time of reepithelialization, hospitalization time, actual mortality, and sequelae) and demographic as well as intervention characteristics [age (< 40 vs ≥ 40), sex (male versus female), days after onset to therapy given (< 7 days versus ≥ 7 days), and use of concomitant therapies (none or supportive therapy versus corticosteroids versus immunoglobulin versus corticosteroids + immunoglobulin)] were evaluated using the data on patients with TEN.
For patients treated with infliximab, none of the assessed demographic and intervention variables showed a significant effect on time of reepithelialization, hospitalization time, actual mortality, and sequelae (all p > 0.05) (Table 3).
Table 3.
Subgroup analysis using individual patient data
| Group | Infliximab | Etanercept | p value | ||||
|---|---|---|---|---|---|---|---|
| Number of reported cases | p value | Number of reported cases | p value | ||||
| Time of reepithelialization (days) | |||||||
| Age (years) | 0.788 | 0.553 | 0.566 | ||||
| < 40 | 12 | 10.67 ± 8.92 | 11 | 11.91 ± 5.38 | |||
| ≥ 40 | 8 | 7.75 ± 8.61 | 30 | 10.17 ± 4.60 | |||
| Sex | 0.106 | 0.224 | 0.751 | ||||
| Male | 9 | 12.67 ± 11.09 | 20 | 9.50 ± 3.58 | |||
| Female | 11 | 12.36 ± 16.52 | 21 | 11.71 ± 5.63 | |||
| SCORTEN | – | 0.901 | – | ||||
| 0–1 | – | – | 2 | 13.00 ± 1.41 | |||
| 2 | 4 | 13.25 ± 9.88 | 7 | 9.29 ± 5.99 | |||
| 3 | – | – | 10 | 9.60 ± 4.60 | |||
| 4 | – | – | 7 | 9.86 ± 4.91 | |||
| ≥ 5 | – | – | 10 | 9.50 ± 4.06 | |||
| Days after symptom onset therapy given (days) | – | 0.984 | – | 0.751 | |||
| < 7 | 13 | 11.85 ± 9.35 | 40 | 10.63 ± 4.88 | |||
| ≥ 7 | 7 | 13.71 ± 21.01 | 1 | – | |||
| Concomitant therapies | 0.716 | 0.092 | 0.426 | ||||
| None or supportive therapy | 5 | 6.80 ± 1.48 | 34 | 9.47 ± 2.24 | |||
| Corticosteroids | 9 | 11.00 ± 12.30 | 7 | 13.29 ± 6.21 | |||
| IVIG | 4 | 9.00 ± 5.74 | 1 | – | |||
| Corticosteroids + IVIG | 1 | – | 2 | 13.50 ± 3.54 | |||
| Hospitalization time (days) | |||||||
| Age (years) | 0.329 | 0.561 | 0.802 | ||||
| < 40 | 7 | 18.13 ± 9.52 | 16 | 20.69 ± 12.47 | |||
| ≥ 40 | 4 | 16.50 ± 9.75 | 5 | 23.00 ± 11.29 | |||
| Sex | 0.177 | 0.320 | 0.837 | ||||
| Male | 8 | 17.88 ± 8.49 | 9 | 21.89 ± 13.76 | |||
| Female | 4 | 17.00 ± 11.83 | 12 | 20.75 ± 11.04 | |||
| SCORTEN | – | 0.668 | – | ||||
| 0–1 | 0 | – | 5 | 22.60 ± 13.30 | |||
| 2 | 2 | 27.00 ± 1.14 | 1 | – | |||
| 3 | 1 | – | 5 | 20.80 ± 12.38 | |||
| 4 | 1 | – | 3 | 26.33 ± 22.23 | |||
| ≥ 5 | 0 | – | 0 | – | |||
| Days after symptom onset therapy given (days) | 1.894 | – | – | ||||
| < 7 | 9 | 19.67 ± 7.75 | 16 | 22.13 ± 12.81 | |||
| ≥ 7 | 3 | 31.33 ± 25.75 | 0 | NA | |||
| Concomitant therapies | 0.677 | 0.039 | 0.128 | ||||
| None or supportive therapy | 2 | 10.50 ± 0.71 | 7 | 17.29 ± 9.01 | |||
| Corticosteroids | 4 | 19.75 ± 9.54 | 10 | 18.60 ± 8.63 | |||
| IVIG | 4 | 19.00 ± 11.96 | 1 | – | |||
| Corticosteroids + IVIG | 1 | – | 4 | 34.75 ± 16.28 | |||
| Mortality rate (%) | |||||||
| Age (years) | 0.066 | 0.117 | 0.846 | ||||
| < 40 | 14 | 0% | 19 | 0% | |||
| ≥ 40 | 14 | 35.7% | 40 | 20% | |||
| Sex | 0.691 | 0.684 | 0.335 | ||||
| Male | 12 | 25% | 26 | 15.4% | |||
| Female | 16 | 16% | 34 | 11.8% | |||
| SCORTEN | |||||||
| 0–1 | – | – | – | 5 | 0% | 0.589 | NA |
| 2 | 4 | 0% | 8 | 0% | |||
| 3 | – | – | 16 | 6.25% | |||
| 4 | – | – | 11 | 9.1% | |||
| ≥ 5 | – | – | 11 | 18.2% | |||
| Days after symptom onset therapy given (days) | 0.786 | 0.014 | 0.631 | ||||
| < 7 | 18 | 16.7% | 47 | 10.6% | |||
| ≥ 7 | 8 | 12.5% | 5 | 60% | |||
| Concomitant therapies | 0.100 | 0.084 | 0.442 | ||||
| None or supportive therapy | 7 | 0% | 39 | 5.1% | |||
| Corticosteroids | 11 | 27.3% | 15 | 20% | |||
| IVIG | 6 | 0% | 1 | 0% | |||
| Corticosteroids + IVIG | 4 | 50% | 8 | 25% | |||
| Number of patient-reported sequelae (%) | |||||||
| Age (years) | 0.663 | 0.010 | 0.657 | ||||
| < 40 | 14 | 27.1% | 19 | 36.8% | |||
| ≥ 40 | 14 | 21.4% | 40 | 7.5% | |||
| Sex | 0.381 | 0.642 | 0.012 | ||||
| Male | 12 | 25% | 26 | 19.2% | |||
| Female | 16 | 50% | 34 | 14.7% | |||
| SCORTEN | – | 0.451 | – | ||||
| 0–1 | – | – | 5 | 40% | |||
| 2 | – | – | 8 | 0% | |||
| 3 | – | – | 16 | 6.3% | |||
| 4 | 4 | 75% | 11 | 18.2% | |||
| ≥ 5 | – | – | 11 | 0% | |||
| Days after symptom onset therapy given (days) | 0.946 | 0.459 | 0.080 | ||||
| < 7 | 18 | 38.9% | 47 | 21.3% | |||
| ≥ 7 | 8 | 37.8% | 5 | 0% | |||
| Concomitant therapies | 0.234 | 0.040 | 0.265 | ||||
| None or supportive therapy | 7 | 57.1% | 39 | 7.7% | |||
| Corticosteroids | 11 | 36.4% | 15 | 13.3% | |||
| IVIG | 6 | 50% | 1 | 0% | |||
| Corticosteroids + IVIG | 4 | 25% | 8 | 62.5% | |||
IVIG intravenous immunoglobulin
For patients treated with etanercept, the use of concomitant therapies was found to have a significant influence on hospitalization time and sequelae, with patients receiving etanercept with corticosteroids + immunoglobulin having the longest hospitalization time (p = 0.039) and highest sequelae rate (p = 0.040) (Table 3).
Comparative Analysis
Seven two-arm intervention studies were included, of which six compared etanercept with non-etanercept treatments (supportive therapy, corticosteroid, and immunoglobulin) [22, 32, 55, 61, 65, 72], and one study compared infliximab + N-acetylcysteine with N-acetylcysteine [52].
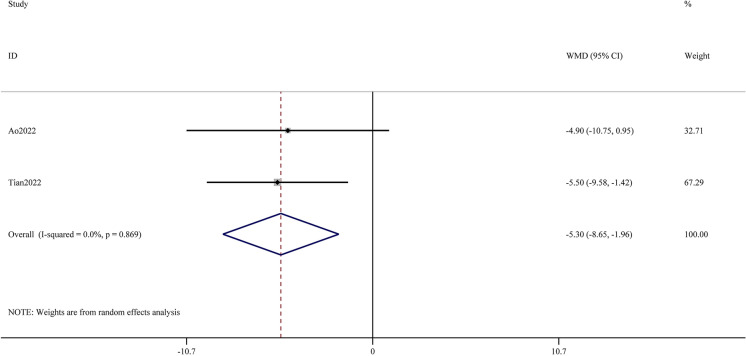
Hospitalization time was reported in two studies, and results of meta-analysis showed that etanercept significantly reduced hospitalization time compared with non-etanercept treatments (WMD −5.30; 95% CI −8.65 to −1.96; p = 0.002; I2 = 0%) (Fig. 2).
Fig. 2.
Meta-analysis of hospitalization time comparing etanercept therapy with non-etanercept treatments
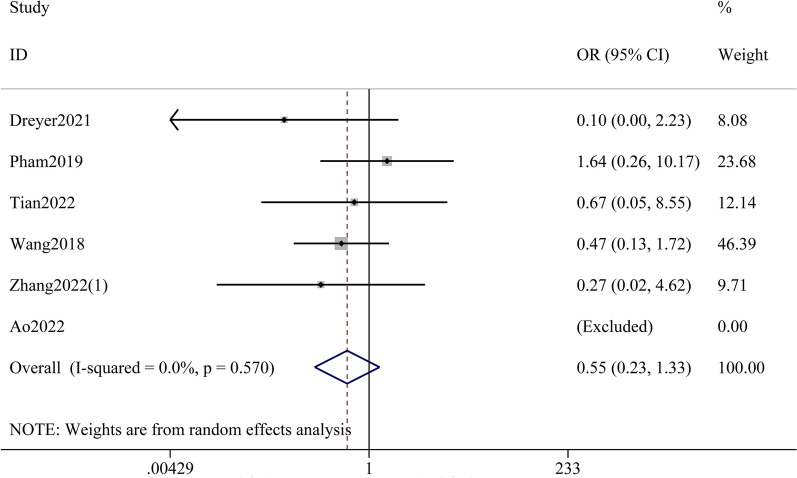
Mortality rate was reported in six studies, and although the meta-analysis showed a trend toward lower mortality with etanercept, the result was not statistically significant (OR 0.55; 95% CI 0.23–1.33; p = 0.185; I2 = 0%) (Fig. 3).
Fig. 3.
Meta-analysis of mortality rate comparing etanercept therapy with non-etanercept treatments
A RCT conducted by Paquet et al. compared the effect of infliximab + N-acetylcysteine with N-acetylcysteine alone in the treatment of TEN. Among the ten treated patients (five in each group), no unexpected drug-induced adverse event was noticed. However, the combination treatment of infliximab with N-acetylcysteine did not appear to reverse the evolving TEN process, with an actual mortality rate of 40% in the combination group versus 20% in the N-acetylcysteine group.
Discussion
Numerous therapeutic approaches for SJS and TEN have been proposed, however, the optimal regimen has yet to be determined. TNF-α has been implicated in the pathogenesis of SJS and TEN. It has generally been suggested that the mechanism underlying SJS and TEN involves an immunological disorder mediated by cytotoxic T cells activated by culprit drugs presented by human leukocyte antigen class I molecules on keratinocytes [72]. The granulysin molecule, which is released by cytotoxic T cells, is an important cytotoxic molecule [73]. TNF-α acts as an upstream regulator of the granulysin gene and promotes its production. Evidence has shown that the concentration of TNF-α in serum and blister fluid of patients with SJS/TEN is elevated and can be markedly decreased after TNF-α blockade treatment, along with rapid resolution of the condition, which further supports the use of TNF-α inhibitors for treating SJS/TEN [21, 42, 65].
The present meta-analysis focused on assessing the therapeutic efficacy of different biologic TNF-α inhibitors in treating SJS/TEN by conducting a patient-level as well as a study-level analysis. Previous studies have evaluated the role of TNF-α inhibitors in treating SJS/TEN using individual patient level data, but they combined the data of SJS, SJS-TEN overlap, and TEN as an entirety, and only conducted a subgroup analysis on the basis of whether TNF-α inhibitors were used as monotherapy [14, 17]. In contrast, our study pooled patient data separately on the basis of the severity of diseases: SJS, SJS-TEN overlap, and TEN. We have also assessed the effect of five confounding factors, including different combination therapies. Furthermore, our study is the largest and most up-to-date meta-analysis targeting the topic of biologic therapies for SJS/TEN, including 55 studies with 20 published between 2020 and 2022. We believed that our study provides a more precise and comprehensive overview of the efficacy of each TNF-α inhibitors than the previous reviews.
This meta-analysis has identified 22 publications (made up of 31 patients) that use infliximab for the treatment of SJS-TEN overlap and TEN. Patient-level analysis revealed that infliximab had comparable efficacy to etanercept in treating patients with TEN, as indicated by similar clinical outcomes, including time to reepithelialization, hospitalization time, and mortality rate (Table 1) Infliximab was the first TNF-α inhibitor proven to be effective in treating TEN [37], however, recent publications regarding its use are lacking, with only seven cases reported between 2015 and 2022 [15, 25, 26, 43, 46, 63, 64]. In 2004, Paquet et al. conducted a pilot study comparing the effect of infliximab combined with N-acetylcysteine versus N-acetylcysteine along for patients with TEN, and their findings countered the success of infliximab in the treatment of TEN [52]. Furthermore, the present analysis revealed a higher sequelae rate in patients with TEN receiving infliximab than in those treated with etanercept (39.3% versus 6.4%, p = 0.022), further discouraging the use of infliximab as a standardized treatment for SJS/TEN.
Compared with infliximab and adalimumab, there is mounting evidence supporting the use of etanercept in the treatment of SJS/TEN. According to our collected data, etanercept has been used to treat SJS, SJS-TEN overlap, and TEN, and has yielded a lower mortality rate than the average rate reported in epidemiology studies [74–77]. Since etanercept has been widely accepted in the treatment of SJS/TEN, the focus should now be on determining the most suitable etanercept regimen to achieve the greatest efficacy. According to our subgroup analysis using individual patient data, patients receiving etanercept monotherapy had a similar mortality rate compared with those treated with etanercept in combination with corticosteroids or corticosteroids + immunoglobulin. However, patients receiving etanercept combined with corticosteroids and immunoglobulin appeared to have a longer hospitalization time and higher sequelae rate compared with etanercept monotherapy. However, since only four sets of patient data were pooled for hospitalization time, and eight for sequelae rate, the results of our analysis is preliminary. The RCT performed by Wang et al. found that etanercept monotherapy yielded fewer side effects compared with corticosteroids monotherapy [65]. The latest and largest observational study conducted by Zhang et al. showed that combination therapy of etanercept and corticosteroids demonstrated higher effectiveness in shortening the reepithelialization time and reducing mortality compared with corticosteroid monotherapy [72]. They suggested that the combined treatment might avoid common adverse events related to the higher dosage of corticosteroids in SJS/TEN [72]. The findings of Dreyer et al. suggested that in some patients, etanercept monotherapy is not an adequate intervention, but the addition of immunoglobulin may be helpful [32]. Nevertheless, studies directly comparing the efficacy of etanercept monotherapy with combination treatment are lacking. The findings of this study may inspire future studies to assess this aspect.
It is suggested that the time gap between the development of symptoms and initiation of therapy could affect the mortality across the treatment groups [78]. On the basis of the results of our subgroup analysis, patients with TEN who received etanercept within 7 days after symptom onset demonstrated significantly lower mortality (10.6% versus 60%) than patients treated after more than 7 days. However, this result was preliminary, with only five patients in the ≥ 7 day subgroup. Although the optimum time for starting etanercept after onset of SJS/TEN has not been sufficiently discussed, most experts advocate early initiation [79]. In our study, we collected data on 60 individual patients regarding the time elapsed between symptom onset and therapy initiation, with 51 of them (85%) receiving etanercept within 7 days of symptom onset. This finding indicates that starting etanercept within 7 days of SJS/TEN symptom onset is currently the most common choice.
In contrast to case reports and case series, comparative studies can provide more compelling evidence regarding the efficacy of etanercept for the treatment of SJS/TEN. Our analysis has identified six studies that compared the treatment effect of etanercept with non-etanercept therapies for SJS/TEN. Meta-analysis results showed that etanercept therapy provided comparable survival outcome and quicker hospital discharge compared with non-etanercept treatments, further confirming the use of etanercept for the treatment of SJS/TEN. However, there is only one RCT targeting this topic thus far, while the remaining five studies were retrospectively designed, which has weakened the credibility of the meta-analysis. Overall, the present study provides evidence that supports the use of etanercept as an effective alternative for the treatment of SJS/TEN. Whether etanercept should be recommended as the first-line treatment for SJS/TEN still requires further confirmation by large sample size and prospective RCT.
The interpretation of the present findings should be approached with caution due to several limitations. The included studies were predominantly case reports, case series, and observational studies with no randomization, which might introduce significant heterogeneity and bias. However, since SJS and TEN are rare diseases, meta-analysis of these types of studies is one of the few options available to assess treatment efficacy and feasibility of TNF-α inhibitors. Despite patient data analysis being conducted, some subgroups, such as time between disease onset and treatment initiation, SCORTEN, and concomitant therapies, had insufficient reported information, and the results of subgroup analyses were preliminary and not conclusive. Nonetheless, this study offers a broad summary of biologic treatments and outcomes for SJS/TEN, which might pave the way for future investigations. Further studies with a strong level of evidence, such as RCTs, are needed to validate and confirm the present findings.
Conclusions
The current findings suggest that infliximab, etanercept, and adalimumab are effective treatments for SJS/TEN. However, the use of infliximab may be limited by high reported side effects and adalimumab by lack of validate evidence. Etanercept appears to be the most promising biologic TNF-α inhibitors for SJS/TEN among the three options. Patient-level analysis demonstrates that etanercept is safe and effective for treating different severities of SJS/TEN. In study-level analysis, etanercept results in shorter hospitalization time and lower risk of mortality than non-etanercept treatments. Further studies, such as RCT, are required to provide high-level evidence to support the clinical application of biologic TNF-α inhibitors in SJS/TEN and to explore the optimal regimen, timing, and contraindications for biologic therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Conceptualization: JiaLi.C.; Methodology: Xuan.Z., XinZhu.X. and Jie.F.; Formal analysis and investigation: Xuan.Z., XinZhu.X. and Jie.F.; Writing–original draft preparation: JiaLi.C.; Manuscript editing: JiaLi.C.; Manuscript review: All authors.
Disclosures
The authors declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
References
- 1.Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. J Plast Reconstr Aesthet Surg. 2016;69(6):e119–e153. doi: 10.1016/j.bjps.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. 2015;16(6):475–493. doi: 10.1007/s40257-015-0158-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang MS, Lee JY, Kim J, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS One. 2016;11(11):e0165933. doi: 10.1371/journal.pone.0165933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016;136(7):1387–1397. doi: 10.1016/j.jid.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Chaby G, Maldini C, Haddad C, et al. Incidence of and mortality from epidermal necrolysis (Stevens-Johnson syndrome/toxic epidermal necrolysis) in France during 2003–16: a four-source capture-recapture estimate. Br J Dermatol. 2020;182(3):618–624. doi: 10.1111/bjd.18424. [DOI] [PubMed] [Google Scholar]
- 6.McPherson T, Exton LS, Biswas S, et al. British Association of Dermatologists' guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in children and young people, 2018. Br J Dermatol. 2019;181(1):37–54. doi: 10.1111/bjd.17841. [DOI] [PubMed] [Google Scholar]
- 7.Caproni M, Torchia D, Schincaglia E, et al. Expression of cytokines and chemokine receptors in the cutaneous lesions of erythema multiforme and Stevens-Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol. 2006;155(4):722–728. doi: 10.1111/j.1365-2133.2006.07398.x. [DOI] [PubMed] [Google Scholar]
- 8.Nassif A, Moslehi H, Le Gouvello S, et al. Evaluation of the potential role of cytokines in toxic epidermal necrolysis. J Invest Dermatol. 2004;123(5):850–855. doi: 10.1111/j.0022-202X.2004.23439.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang HC, Wang TJ, Lin MH, Chen TJ. A review of the systemic treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Biomedicines. 2022 doi: 10.3390/biomedicines10092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen A, Olabi B, Langley A, et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev. 2022;3(3):Cd013130. doi: 10.1002/14651858.CD013130.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71(2):278–283. doi: 10.1016/j.jaad.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Kherlopian A, Mewton E, Fong G, Fischer G. Highlighting adalimumab as a treatment option for systemic treatment of toxic epidermal necrolysis: a case series from a tertiary specialised burns centre. Australas J Dermatol. 2022;63(4):497–504. doi: 10.1111/ajd.13911. [DOI] [PubMed] [Google Scholar]
- 13.Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, Serrano-Reyes C. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23(1):61–63. [PubMed] [Google Scholar]
- 14.Zhang S, Tang S, Li S, Pan Y, Ding Y. Biologic TNF-alpha inhibitors in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a systemic review. J Dermatolog Treat. 2020;31(1):66–73. doi: 10.1080/09546634.2019.1577548. [DOI] [PubMed] [Google Scholar]
- 15.Patel TK, Patel PB, Thakkar S. Comparison of effectiveness of interventions in reducing mortality in patients of toxic epidermal necrolysis: a network meta-analysis. Indian J Dermatol Venereol Leprol. 2021;87(5):628–644. doi: 10.25259/IJDVL_605_19. [DOI] [PubMed] [Google Scholar]
- 16.Krajewski A, Maciejewska-Markiewicz D, Jakubczyk K, et al. Impact of multiple medical interventions on mortality, length of hospital stay and reepithelialization time in toxic epidermal necrolysis, Steven-Johnsons syndrome, and TEN/SJS overlap—metanalysis and metaregression of observational studies. Burns. 2022;48(2):263–280. doi: 10.1016/j.burns.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Sachdeva M, Maliyar K, Ponzo MG. A systematic review of efficacy and safety of monotherapy and combination therapy with biologic for Stevens-Johnson syndrome and toxic epidermal necrolysis. J Cutan Med Surg. 2021;25(6):598–615. doi: 10.1177/1203475421993779. [DOI] [PubMed] [Google Scholar]
- 18.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92–96. doi: 10.1001/archderm.1993.01680220104023. [DOI] [PubMed] [Google Scholar]
- 19.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann S, Sekula P, Venhoff M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(6):514–522. doi: 10.1001/jamadermatol.2016.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Shouli S, Abouchala N, Bogusz MJ, Al Tufail M, Thestrup-Pedersen K. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85(6):534–535. doi: 10.1080/00015550510037062. [DOI] [PubMed] [Google Scholar]
- 22.Ao S, Gao X, Zhan J, et al. Inhibition of tumor necrosis factor improves conventional steroid therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis in a cohort of patients. J Am Acad Dermatol. 2022;86(6):1236–1245. doi: 10.1016/j.jaad.2022.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Bakir M, Almeshal H, Alturki R, Obaid S, Almazroo A. Toxic epidermal necrolysis post COVID-19 vaccination—first reported case. Cureus. 2021;13(8):e17215. doi: 10.7759/cureus.17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burduk PK, Seredyka-Burduk M, Kaźmierczak W, Malukiewicz G, Kołtan A. Nasolacrimal duct obstruction after toxic epidermal necrolysis. Otolaryngol Polska. 2012;66(2):148–51. doi: 10.1016/S0030-6657(12)70763-1. [DOI] [PubMed] [Google Scholar]
- 25.Cardenas J, Mahadevaiah S, Bender N. A challenging case of pediatric toxic epidermal necrolysis. Crit Care Med. 2021;49(1 SUPPL 1):344. doi: 10.1097/01.ccm.0000728676.21202.7f. [DOI] [Google Scholar]
- 26.Chafranska L, Saunte DM, Behrendt N, et al. Pediatric toxic epidermal necrolysis treated successfully with infliximab. Pediatr Dermatol. 2019;36(3):342–345. doi: 10.1111/pde.13778. [DOI] [PubMed] [Google Scholar]
- 27.Chahal D, Aleshin M, Turegano M, Chiu M, Worswick S. Vaccine-induced toxic epidermal necrolysis: a case and systematic review. Dermatol Online J. 2018 doi: 10.5070/D3241037941. [DOI] [PubMed] [Google Scholar]
- 28.Choi R, Garritano J, Laird M, et al. Treatment of toxic epidermal necrolysis and concurrent COVID-19-associated hyperinflammatory syndrome with systemic corticosteroids and etanercept. JAAD Case Rep. 2022;29:139–141. doi: 10.1016/j.jdcr.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong I, Chao A. Stevens-Johnson syndrome/toxic epidermal necrolysis and treatment with a biologic: a case report. Perm J. 2017;21:16–060. doi: 10.7812/TPP/16-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coulombe J, Belzile E, Duhamel A, et al. Pediatric SJS/TEN subdued by a combination of dexamethasone, cyclosporine, and etanercept. J Cutan Med Surg. 2019;23(5):547–550. doi: 10.1177/1203475419861078. [DOI] [PubMed] [Google Scholar]
- 31.Didona D, Paolino G, Garcovich S, Caposiena Caro RD, Didona B. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30(10):e83–e84. doi: 10.1111/jdv.13330. [DOI] [PubMed] [Google Scholar]
- 32.Dreyer SD, Torres J, Stoddard M, et al. Efficacy of etanercept in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Cutis. 2021;107(6):E22–E28. doi: 10.12788/cutis.0288. [DOI] [PubMed] [Google Scholar]
- 33.Eliades P, Fonseca M, Harp J. Use of etanercept in a series of pediatric patients with Stevens-Johnson syndrome-toxic epidermal necrolysis spectrum disease. JAMA Dermatol. 2020;156(8):921–922. doi: 10.1001/jamadermatol.2019.3731. [DOI] [PubMed] [Google Scholar]
- 34.Estebanez A, Saez-Martin LC, Munoz JI, et al. Levetiracetam-induced pediatric toxic epidermal necrolysis successfully treated with etanercept. Pediatr Dermatol. 2020;37(4):701–705. doi: 10.1111/pde.14179. [DOI] [PubMed] [Google Scholar]
- 35.Famularo G, Di Dona B, Canzona F, Girardelli CR, Cruciani G. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41(6):1083–1084. doi: 10.1345/aph.1K001. [DOI] [PubMed] [Google Scholar]
- 36.Faris J, Wilson J, Dolman HS, et al. A cautionary tale of etanercept use in patients with toxic epidermal necrolysis. J Burn Care Res. 2021;42(3):586–589. doi: 10.1093/jbcr/iraa194. [DOI] [PubMed] [Google Scholar]
- 37.Fischer M, Fiedler E, Marsch WC, Wohlrab J. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146(4):707–709. doi: 10.1046/j.1365-2133.2002.46833.x. [DOI] [PubMed] [Google Scholar]
- 38.Frederiks AJ, Kumarasinghe SP, Wood F, et al. Toxic epidermal necrolysis in adult patients: experience from the West Australian Collaboration. Australas J Dermatol. 2022;63(4):437–451. doi: 10.1111/ajd.13903. [DOI] [PubMed] [Google Scholar]
- 39.Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatology (Basel, Switzerland) 2012;224(2):134–139. doi: 10.1159/000338202. [DOI] [PubMed] [Google Scholar]
- 40.Gavigan GM, Kanigsberg ND, Ramien ML. Pediatric Stevens-Johnson syndrome/toxic epidermal necrolysis halted by etanercept. J Cutan Med Surg. 2018;22(5):514–515. doi: 10.1177/1203475418758989. [DOI] [PubMed] [Google Scholar]
- 41.Gubinelli E, Canzona F, Tonanzi T, Raskovic D, Didona B. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36(3):150–153. doi: 10.1111/j.1346-8138.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 42.Hunger RE, Hunziker T, Buettiker U, Braathen LR, Yawalkar N. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116(4):923–924. doi: 10.1016/j.jaci.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 43.Jiang YY, Nguyen GH, Jin HZ, Zeng YP. Methazolamide-induced toxic epidermal necrolysis in a man carrying HLA-B*59:01: successful treatment with infliximab and glucocorticoid. Int J Dermatol. 2018;57(4):494–496. doi: 10.1111/ijd.13924. [DOI] [PubMed] [Google Scholar]
- 44.Kreft B, Lieser U, Haase R, Marsch WC, Wohlrab J. Extensive hypertrophic scarring after toxic epidermal necrolysis in a child. Pediatr Dermatol. 2014;31(4):527–528. doi: 10.1111/j.1525-1470.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- 45.Kreft B, Wohlrab J, Bramsiepe I, Eismann R, Winkler M, Marsch C. Etoricoxib-induced toxic epidermal necrolysis: Successful treatment with infliximab. J Dermatol. 2010;37(10):904–906. doi: 10.1111/j.1346-8138.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 46.Kumar Das K, Khondokar S, Rahman A, Chakraborty A. Unidentified drugs in traditional medications causing toxic epidermal necrolysis: a developing country experience. Int J Dermatol. 2014;53(4):510–515. doi: 10.1111/ijd.12253. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y-Y, Ko J-H, Wei C-H, Chung W-H. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31(2):78–81. doi: 10.1016/j.dsi.2012.06.005. [DOI] [Google Scholar]
- 48.López-Gómez V, Yarza R, Muñoz-González H, et al. Ribociclib-related Stevens-Johnson syndrome: oncologic awareness, case report, and literature review. J Breast Cancer. 2019;22(4):661–666. doi: 10.4048/jbc.2019.22.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maximova N, Granzotto M, Kiren V, Zanon D, Comar M. First description of Merkel Cell polyomavirus DNA detection in a patient with Stevens-Johnson syndrome. J Med Virol. 2013;85(5):918–923. doi: 10.1002/jmv.23550. [DOI] [PubMed] [Google Scholar]
- 50.Natsis N, Ikediobi O, Dorschner R. 31783 etanercept in toxic epidermal necrolysis: in support of standard of care. J Am Acad Dermatol. 2022;87(3):AB56. doi: 10.1016/j.jaad.2022.06.256. [DOI] [Google Scholar]
- 51.Osawa K, Kiniwa Y, Shimosato Y, et al. Toxic epidermal necrolysis caused by apalutamide: a case report of treatment using etanercept with conventional steroid therapy. Acta Derm Venereol. 2022;102:adv00723. doi: 10.2340/actadv.v102.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paquet P, Jennes S, Rousseau AF, Libon F, Delvenne P, Pierard GE. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. A proof-of-concept study. Burns. 2014;40(8):1707–1712. doi: 10.1016/j.burns.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 53.Paradisi A, Abeni D, Didona D, Ricci F, Canzona F, Didona B. A new case series on etanercept treatment for toxic epidermal necrolysis. Eur J Dermatol. 2020;30(5):561–568. doi: 10.1684/ejd.2020.3883. [DOI] [PubMed] [Google Scholar]
- 54.Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314. doi: 10.1155/2012/915314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pham CH, Gillenwater TJ, Nagengast E, McCullough MC, Peng DH, Garner WL. Combination therapy: etanercept and intravenous immunoglobulin for the acute treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis. Burns. 2019;45(7):1634–1638. doi: 10.1016/j.burns.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 56.Plant A, Livesey A, Cooper H. Long-term complications of toxic epidermal necrolysis successfully treated with infliximab. Br J Dermatol. 2020;182(4):e120. [Google Scholar]
- 57.Scott-Lang V, McKay D, Tidman M. Toxic epidermal necrolysis successfully treated with infliximab. J Am Acad Dermatol. 2012;66(4):AB168. doi: 10.1111/pde.12029. [DOI] [PubMed] [Google Scholar]
- 58.Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31(4):532–534. doi: 10.1111/pde.12029. [DOI] [PubMed] [Google Scholar]
- 59.Shen MH, Liu MT, Chung WH, Lu CW. Toxic epidermal necrolysis induced by human herpesvirus 7 treated with a tumor necrosis factor-α inhibitor. J Dermatol. 2020;47(10):1179–1181. doi: 10.1111/1346-8138.15493. [DOI] [PubMed] [Google Scholar]
- 60.Sibbald C, Putterman E, Micheletti R, Treat J, Castelo-Soccio L. Retrospective review of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis cases at a pediatric tertiary care institution. Pediatr Dermatol. 2020;37(3):461–466. doi: 10.1111/pde.14118. [DOI] [PubMed] [Google Scholar]
- 61.Tian CC, Ai XC, Ma JC, et al. Etanercept treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis. Ann Allergy Asthma Immunol. 2022;129(3):360–5.e1. doi: 10.1016/j.anai.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Torres-Navarro I, Briz-Redon A, Botella-Casas G, et al. Accuracy of SCORTEN and ABCD-10 to predict mortality and the influence of renal function in Stevens-Johnson syndrome/toxic epidermal necrolysis. J Dermatol. 2020;47(10):1182–1186. doi: 10.1111/1346-8138.15490. [DOI] [PubMed] [Google Scholar]
- 63.Vivar KL, Deschaine M, Messina J, et al. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44(4):381–384. doi: 10.1111/cup.12876. [DOI] [PubMed] [Google Scholar]
- 64.Wallenborn J, Fischer M. Intensive care in a patient with toxic epidermal necrolysis. Case Rep Crit Care. 2017;2017:3246196. doi: 10.1155/2017/3246196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3):985–996. doi: 10.1172/JCI93349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Gao X, Chen X, Tang X, Chen H, Han J. Successful treatment of interstitial lung disease related to Stevens-Johnson syndrome/toxic epidermal necrolysis overlap with etanercept: a case report and published work review. J Dermatol. 2019;46(11):1035–1038. doi: 10.1111/1346-8138.15058. [DOI] [PubMed] [Google Scholar]
- 67.Wang R, Zhong S, Tu P, Li R, Wang M. Rapid remission of Stevens-Johnson syndrome by combination therapy using etanercept and intravenous immunoglobulin and a review of the literature. Dermatol Ther. 2019;32(4):e12832. doi: 10.1111/dth.12832. [DOI] [PubMed] [Google Scholar]
- 68.Wojtkiewicz A, Wysocki M, Fortuna J, Chrupek M, Matczuk M, Koltan A. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88(4):420–421. doi: 10.2340/00015555-0462. [DOI] [PubMed] [Google Scholar]
- 69.Worsnop F, Wee J, Natkunarajah J, Moosa Y, Marsden R. Reaction to biological drugs: infliximab for the treatment of toxic epidermal necrolysis subsequently triggering erosive lichen planus. Clin Exp Dermatol. 2012;37(8):879–881. doi: 10.1111/j.1365-2230.2012.04357.x. [DOI] [PubMed] [Google Scholar]
- 70.Zander E, Hintze TD, Sallee B, Allen P, Miller JL, Sagdeo M. Treatment of toxic epidermal necrolysis with etanercept in a pediatric patient. J Pediatr Pharmacol Ther. 2021;26(7):758–761. doi: 10.5863/1551-6776-26.7.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S, Liu XY, Zhang JZ, Cai L, Zhou C. Drug-induced toxic epidermal necrolysis with secondary aspergillus fumigatus infection: a case report. J Peking Univ Health Sci. 2019;51(5):977–80. doi: 10.19723/j.issn.1671-167X.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J, Lu CW, Chen CB, et al. Evaluation of combination therapy with etanercept and systemic corticosteroids for Stevens-Johnson syndrome and toxic epidermal necrolysis: a multicenter observational study. J Allergy Clin Immunol Pract. 2022;10(5):1295–304.e6. doi: 10.1016/j.jaip.2022.01.038. [DOI] [PubMed] [Google Scholar]
- 73.Wang CW, Chung WH, Cheng YF, et al. A new nucleic acid-based agent inhibits cytotoxic T lymphocyte-mediated immune disorders. J Allergy Clin Immunol. 2013;132(3):713–22.e11. doi: 10.1016/j.jaci.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 74.Frey N, Jossi J, Bodmer M, et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. J Invest Dermatol. 2017;137(6):1240–1247. doi: 10.1016/j.jid.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 75.Woolum JA, Bailey AM, Baum RA, Metts EL. A review of the management of Stevens-Johnson syndrome and toxic epidermal necrolysis. Adv Emerg Nurs J. 2019;41(1):56–64. doi: 10.1097/TME.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 76.Charlton OA, Harris V, Phan K, Mewton E, Jackson C, Cooper A. Toxic epidermal necrolysis and Steven-Johnson syndrome: a comprehensive review. Adv Wound Care (New Rochelle) 2020;9(7):426–439. doi: 10.1089/wound.2019.0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torres-Navarro I, Briz-Redón Á, Botella-Estrada R. Systemic therapies for Stevens-Johnson Syndrome and toxic epidermal necrolysis: a SCORTEN-based systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(1):159–171. doi: 10.1111/jdv.16685. [DOI] [PubMed] [Google Scholar]
- 78.de Filippis R, Soldevila-Matías P, Guinart D, et al. Unravelling cases of clozapine-related drug reaction with eosinophilia and systemic symptoms (DRESS) in patients reported otherwise: a systematic review. J Psychopharmacol (Oxford, England) 2021;35(9):1062–1073. doi: 10.1177/02698811211021587. [DOI] [PubMed] [Google Scholar]
- 79.Gupta LK, Martin AM, Agarwal N, et al. Guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis: an Indian perspective. Indian J Dermatol Venereol Leprol. 2016;82(6):603–625. doi: 10.4103/0378-6323.191134. [DOI] [PubMed] [Google Scholar]
- 80.Kumar R, Bhandari S. Pembrolizumab induced toxic epidermal necrolysis. Curr Probl Cancer. 2020;44(2):100478. doi: 10.1016/j.currproblcancer.2019.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.