Abstract
Background
Antimicrobial resistance is an increasing challenge in low and middle-income countries as it is widespread in these countries and is linked to an increased mortality. Apart from human and environmental factors, animal-related drivers of antimicrobial resistance in low- and middle-income countries have special features that differ from high-income countries. The aim of this narrative review is to address the zoonotic sources and the spread of antimicrobial resistance from the perspective of low- and middle-income countries.
Main body
Contamination with extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli is highest in poultry (Africa: 8.9–60%, Asia: 53–93%) and there is a risk to import ESBL-producing E. coli through poultry meat in Africa. In aquacultures, the proportion of ESBL-producers among E. coli can be high (27%) but the overall low quality of published studies limit the general conclusion on the impact of aquacultures on human health. ESBL-producing E. coli colonization of wildlife is 1–9% in bats or 2.5–63% birds. Since most of them are migratory animals, they can disperse antimicrobial resistant bacteria over large distances. So-called ‘filth flies’ are a relevant vector not only of enteric pathogens but also of antimicrobial resistant bacteria in settings where sanitary systems are poor. In Africa, up to 72.5% of ‘filth flies’ are colonized with ESBL-producing E. coli, mostly conferred by CTX-M (24.4–100%). While methicillin-resistant Staphylococcus aureus plays a minor role in livestock in Africa, it is frequently found in South America in poultry (27%) or pork (37.5–56.5%) but less common in Asia (poultry: 3%, pork: 1–16%).
Conclusions
Interventions to contain the spread of AMR should be tailored to the needs of low- and middle-income countries. These comprise capacity building of diagnostic facilities, surveillance, infection prevention and control in small-scale farming.
Graphical abstract
Keywords: Antimicrobial resistance, Extended-spectrum Beta-lactamase, Methicillin-resistant Staphylococcusaureus
Background
The increase in antimicrobial resistance (AMR) poses a considerable threat to health and livelihoods. An estimated 4.95 million deaths associated with AMR occurred in 2019 [1]. Most AMR associated deaths occur in low- and middle-income countries (LMIC) and are therefore linked to poverty, income inequalities, difficulties in healthcare access, and inadequate or lacking policies for preventing the development and transmission of AMR [1–3]. AMR is, however, a global problem affecting both high-income and LMIC alike. Beyond the direct effect on human health, AMR in livestock can affect livelihoods particularly among the most vulnerable people [4].
The aim of this narrative review is (i) to shed light on the various zoonotic sources from which resistant bacteria can be transmitted in LMIC, and (ii) to address multilateral health policies to contain this spread effectively. This work addresses AMR in the context of food production from animal rearing (livestock and aquaculture) as well as at the interface between humans and wildlife. Specifically we are discussing the prevalence of resistant bacteria that may lead to transmission and dissemination either directly from animal contact or consumption of animal products (e.g. AMR in wildlife and in wild animals used for food), or indirectly (e.g. through ‘filth flies’). We mainly focus exemplarily on extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and methicillin-resistant Staphylococcus aureus (MRSA) in LMIC (excluding China) and the most important pathogens associated with aquaculture as these are of critical importance for understanding AMR particularly in the community setting in LMIC. China was largely excluded as this topic has recently been reviewed elsewhere [5]. For country income classification, the definitions from the World Bank were used [6].
Antimicrobial resistance: mechanisms and transmission
Antimicrobial agents either inhibit (bacteriostatic agents, e.g., macrolides, lincosamides) or kill bacteria (bactericidal agents, such as beta-lactams). Beta-lactam antimicrobials (e.g. penicillins, aminopenicillins, cephalosporins) inhibit the synthesis of the peptidoglycan layer of the cell wall. Resistance to beta-lactams is mediated either by the expression of bacterial enzymes that lyse the beta-lactam ring in Gram-negative rods (beta-lactamases) or by alterations of the cellular target, e.g. penicillin-binding proteins in Gram-positive cocci. In Enterobacterales (e.g., Escherichia coli), beta-lactamases, such as ESBL and/or AmpC beta-lactamases can hydrolyse not only narrow-spectrum penicillins, but also cephalosporins and beta-lactam/beta-lactamase inhibitor combinations. AMR can result through mutations of chromosomal or extrachromosomal genes or the acquisition of resistance genes from other organisms. Selective pressure such as that exerted by antimicrobial treatment, leads to the generation, survival, and proliferation of resistant clones and can promote the exchange of resistance determinants within and between bacterial species [7]. Plasmids are of particular importance as they can carry resistance genes that confer resistance to multiple antimicrobial classes and are responsible for the global dissemination of resistance to key antimicrobials such as carbapenems and third-generation cephalosporins [8–12]. Genetic material can also be exchanged by transformation or transduction via bacteriophages [7].
Drivers of antimicrobial resistance
The development, selection, and transmission of AMR can be attributed to multiple factors. Inappropriate and excessive antimicrobial use (AMU) are well-recognised drivers of AMR (Fig. 1) [7]. In humans, antimicrobial consumption has increased by 65% between 2000 and 2015 [13]. This increase has been driven in particular by LMIC and is closely related to increases in gross domestic product [13]. Beyond their use for treating infections in animals, antimicrobials are used for prophylaxis and growth promotion sometimes as measures for counteracting lacking hygiene [4]. Antimicrobials used in animals often belong to the same classes as those used in humans and transmission of resistant bacteria between animals and from animals to humans has been extensively reported.
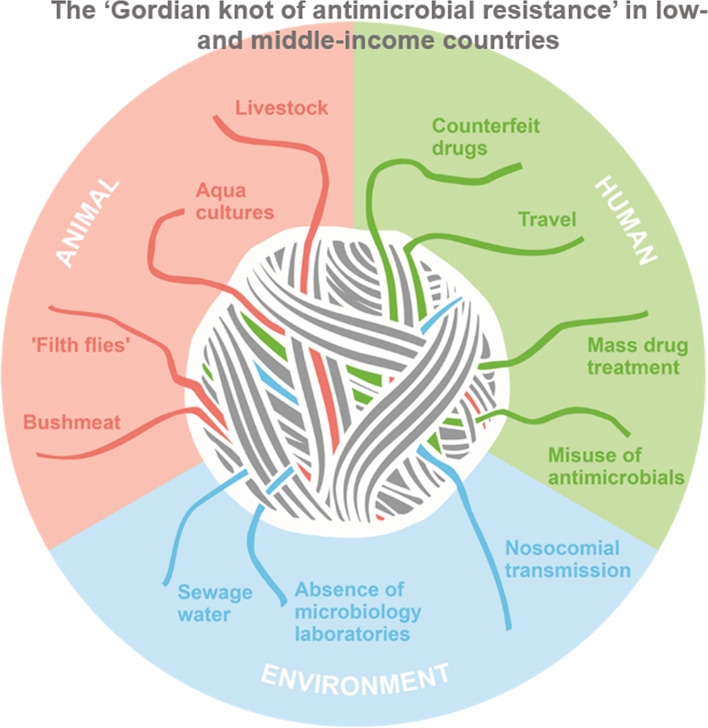
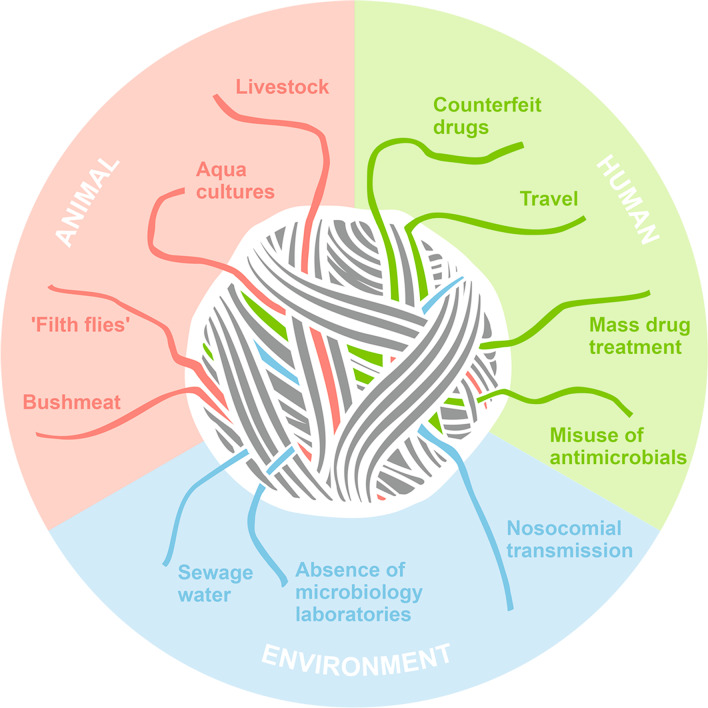
Fig. 1.
The ‘Gordian Knot of antimicrobial resistance’ in low- and middle-income countries (LMIC) describing factors that contribute to the transmission of antimicrobial resistance at the human-animal interface. Reservoirs and sources of antimicrobial resistance (AMR) are in the environment, in animals and humans, which is considered in the One Health concept. This review focuses on zoonotic reservoirs and sources in LMIC. Additional drivers of AMR in LMIC are related to human health (travel [157], mass drug treatment, e.g. for trachoma [158], misuse of antimicrobials [7], counterfeit drugs [159], hospital transmission, absence of microbiological laboratories) and the environment (sewage water [160])
According to the World Organization for Animal Health (WOAH) report, 69,455 tonnes of antimicrobial agents were used in animals in 2018 [14]. The highest antimicrobial quantities, adjusted for animal biomass, were used in countries from Asia and the lowest in Africa. Encouragingly, a decrease by 27% was observed in antimicrobials used in animals between 2016 and 2018 although most countries did not report AMU data for estimating time trends [14]. Similarly, fewer countries than in previous years reported on using antimicrobials for growth promotion [14]. This is in line with the WHO recommendations to completely restrict the use of antimicrobial drug classes important to human health for growth promotion in animals [15]. It is hoped that AMU in food production will decline in the next years due to the implementation and enforcement of regulations restricting use, a decrease in meat consumption, and user fees for AMU in food-production animals [4]. However, the reliability of AMU estimates can be uncertain. Other authors estimate that AMU in animals will in fact increase by 8% between 2020 and 2030, when it will reach 107,472 tonnes with Asia accounting for 67% of the global use and Africa for less than 1% [16]. A recent scoping review highlighted the lack of standardization of data from research studies on AMU in animals [17]. Furthermore, participants were occasionally unable to differentiate antimicrobials from other substances suggesting a lack of reliability of reported data [17]. Interventions that restrict the use of antimicrobials in animals have been shown to reduce the burden of AMR in animals and are likely to also reduce AMR in humans [18].
Antimicrobials and their residues can accumulate in the environment through contamination with human or animal waste, inadequate disposal of waste products resulting from the manufacture of antimicrobials, and the use of antimicrobials as pesticides (Fig. 1) [19]. Contact of animals with contaminated water and soil leads to transmission of resistant bacteria to and from food animals and within food chains. In addition, other factors such as lacking access to diagnostics, healthcare transmission, mobility of humans (e.g. travel), goods, freight, foods and plants further contribute to the transmission of resistant organisms [7]. A well-known example of global dissemination resulting from travel is that of the plasmid-mediated colistin resistance conferred by the gene mcr-1 [20]. Plasmid-transmitted mcr-1 was first described in 2016 in Southern China, and likely resulted from the use of colistin for growth promotion in animals [10]. In the following years, the presence of mcr-1 has been reported in multiple bacterial species across the world [12]. Furthermore, imported meat [21, 22] and wildlife [23] have also been reported to carry AMR across borders.
Main text
Livestock for food production
A source for transmission to humans can be antimicrobial resistant bacteria in livestock such as livestock-associated MRSA (LA-MRSA), or ESBL-producing Enterobacterales [24, 25]. The extent and direction of transmission is, however, often unclear as longitudinal genotyping studies are largely missing to track transmission events, particularly in LMIC [26]. Therefore, transmission is often assumed if resistant isolates from animals and humans share indistinguishable genomic profiles without knowing the direction of transmission. While livestock in LMIC can be colonized with antimicrobial resistant pathogens, transmission most likely occurs while handling livestock products such as meat. In the following section, we therefore focus on the contamination of meat and other animal products for consumption and largely do not consider faecal colonisation studies of livestock.
Africa
In Africa, the contamination of meat with ESBL-producing E. coli depends on the animal species and is highest in poultry (8.9–60%) [27–29] followed by pork and beef (2.3–22%, Table 1) [30–32]. In sub-Saharan Africa, poultry meat is often imported from high-income countries (e.g. USA, Europe) and particularly poultry from Europe and Brazil (Table 1) is contaminated with ESBL-producing E. coli (up to 54%) [29, 33–35]. Whether imported poultry meat can be a source for colonisation with ESBL-producing bacteria in humans is controversial as cefoxitinase CTX-M subtypes in poultry meat (blaCTX-M-1, blaCTX-M-14) differed from those in humans (blaCTX-M-15) in one study from Gabon [29]. In contrast, a study on faecal samples in Ghana revealed that locally raised poultry and hospitalized patients share the same lineages of ESBL-producing E. coli (mainly blaCTX-M-15, sequence type [ST]38 and ST58) suggesting transmission or the spread of global clones both in animals and humans [36]. Another study from Ghana showed that imported poultry is less frequently contaminated with ESBL-producers than locally produced poultry (31 vs. 44%) [33]. Meat from LMIC can also be exported to high income countries: Up to 95% of broiler meat from Brazil was contaminated with ESBL/AmpC-producing E. coli on the Swedish market (AmpC is a beta-lactamases that hydrolyse penicillins, and second- and third-generation cephalosporines) [21]. Similarly, 29.5% of chicken meat batches imported to the UK from South America were contaminated with ESBL-producers mostly deriving from Brazil (blaCTX-M-2, blaCTX-M-8) [37]. Thus, international poultry trade can pose a risk to import or export ESBL producing Enterobacterales. There is evidence that the “pathogen reduction treatment” with chlorinated water in the US can reduce the risk of contamination with ESBL-producers in poultry [29, 38]. Here, eviscerated carcasses are washed with chlorinated water to remove harmful bacteria such as Salmonella sp.
Table 1.
Contamination of livestock meat with ESBL-producing Escherichia coli
| Region | Major resistance genes | Year | Country | Samples (n) | Prevalence [% (n/N)] | References |
|---|---|---|---|---|---|---|
| Africa | blaCTX-M-14, blaCTX-M-15 | 2013 | Egypt | Poultry (112) | 8.9% (10/112) | [27] |
| Not done | 2016 | Ethiopia | Beef (88) | 6% (5/88) | [126] | |
| Not done | 2020 | Ethiopia | Beef (556) | 2.3% (13/556) | [30] | |
| blaCTX-M-1, blaCTX-M-14 | 2011–2012 | Gabon | Poultry (60) | 23% (14/60) | [29] | |
| blaCTX-M-15 | 2013 | Ghana | Poultry (188) | 10.6% (20/188) | [34] | |
| blaCTX-M-1, blaCTX-M-14, blaCTX-M-15 | 2015 | Ghana | Poultry (200) | 23% (46/200) | [33] | |
| blaTEM | 2019 | Ghana | Goat (108), beef (81), sheep (16) | 2% (4/205) | [127] | |
| blaCTX-M-164 | 2015 | Mozambique | Poultry (99) | 17% (17/99) | [35] | |
| Not applicable | 2009–2014 | Nigeria | Poultry (unknown) | 0% (0/unknown) | [128] | |
| blaCTX-M-1, blaCTX-M-8, blaCTX-M-14, blaTEM-1b, blaSHV-5 | 2006 | Tunisia | Beef (23), poultry (10), sheep (1), fish (4) | 29% (11/38) | [32] | |
| Not applicable | 2004–2005 | Tunisia | Sheep (8), poultry (7), beef (4), fish (3), pork (1) | 0% (0/23)a | [129] | |
| blaCTX-M-1, blaTEM-1b, blaTEM-20 | 2007 | Tunisia | Sheep (28), poultry (26), beef (14), fish (10), horse (1) | 13% (10/79) | [31] | |
| Not applicable | Before 2009 | Tunisia | Poultry (55) | 0% (0/55)a | [130] | |
| blaCTX-M | Before 2016 | Zambia | Poultry (384) | 20.1% (77/384) | [28] | |
| South-East Asia | blaCTX-M | 2016 | Cambodia | Pork (60) | 75% (45/60) | [43] |
| blaCTX-M | 2016 | Cambodia | Poultry (30) | 53% (16/30) | [43] | |
| blaCTX-M-15 | 2014–2015 | Cambodia | Pork (110) | 0% (0/110) | [45] | |
| blaCTX-M-15 | 2014–2015 | Cambodia | Poultry (87) | 0% (0/87) | [45] | |
| blaCTX-M-15 | 2014–2015 | Thailand | Pork (175) | 4% (7/175) | [45] | |
| blaCTX-M-15 | 2014–2015 | Thailand | Poultry (189) | 0% (0/189) | [45] | |
| blaTEM, blaSHV, blaCTX-M | 2012–2013 | Malaysia | Poultry (160) | 54% (86/160) | [131] | |
| blaCTX-M, blaTEM, blaSHV | Before 2022 | Pakistan | Poultry and livestock (250) | 30% (75/250) | [132] | |
| blaTEM | Before 2022 | Thailand | Minced meat (150) | 52% (78/150) | [133] | |
| blaCTX-M, blaTEM, blaSHV | 2012–2014 | Vietnam | Poultry (82) | 93% (76/82) | [44] | |
| blaCTX-M, blaTEM, blaSHV | 2012–2014 | Vietnam | Pork (92) | 35% (32/92) | [44] | |
| blaCTX-M, blaTEM, blaSHV | 2012–2014 | Vietnam | Beef (74) | 34% (18/74) | [44] | |
| blaCTX-M-1, blaCTX-M-9, blaTEM, | 2015–2017 | Vietnam | Poultry (116) | 66% 77/116 | [47] | |
| blaCTX-M-1, blaCTX-M-9, blaTEM, | 2015–2017 | Vietnam | Pork (112) | 55% (62/112) | [47] | |
| blaCTX-M, blaTEM, blaSHV | Before 2022 | Vietnam | Poultry (60) | 90% (54/60) | [46] | |
| Latin America | blaCTX-M-2, blaCTX-M-15 | 2014 | Brazil | Poultry (100) | 6% (6/100) | [56] |
| blaCTX-M-2, blaCTX-M-55 | 2019 | Brazil | Poultry (50) | 42% (21/50)a | [58] | |
| blaCTX-M-2, blaCTX-M-55 | 2019 | Brazil | Pork (50) | 12% (6/50) | [58] | |
| blaCTX-M-2, blaCTX-M-8, blaCTX-M-14, blaCTX-M-55 | 2018–2019 | Brazil | Lamb (25) | 56% (14/25) | [134] | |
| blaCTX-M-3, blaCTX-M-55 | 2017–2018 | Ecuador | Poultry (335) | 72.8% (244/335)a | [57] |
This table focuses on pathogens isolated from meat. It is anticipated that faecal samples from livestock would yield similar levels of colonization with ESBL-E. coli
aDeduced from third generation cephalosporin resistance
MRSA is less commonly detected in meat products in Africa and contamination rates are estimated to be 7.8% in a recent review with highest contamination rates in pork (12%) followed by poultry (6.8%) and beef (6.1%) [39]. While LA-MRSA clonal complex (CC) 398 is common in Europe, particularly in pork (up to 25%) [40, 41], it is rarely detected in Africa. So far, LA-MRSA CC398 in meat was only reported in Tunisia (poultry, veal) [42].
Asia
In Asia, the prevalence of ESBL-producing E. coli in meat samples and livestock is considerably higher than in Africa (Table 1). ESBL-producing E. coli was isolated from 53–93% chicken meat samples [43, 44] and 35–75% pork meat [43, 44]. In contrast, one study conducted in Thailand and Cambodia reported very low prevalence of contamination of less than 4% for both poultry and pork [45]. In addition to ESBL, meat products from Asia are relatively often contaminated with organisms harbouring mcr-1 genes [46, 47].
Conversely, the prevalence of MRSA is relatively low in livestock and animal products in Asia. MRSA was found in 3% of chicken meat samples, but not in pork or goat meat in India [48]. In contrast, in a study from Pakistan, MRSA was isolated from 11% of eggs from retail shops and all isolates were positive for the Panton-Valentine leukocidin (PVL) [49]. PVL is produced by some S. aureus strains and is a virulence factor leading to cell lysis and tissue damage. PVL is widespread in the tropics, particularly in Africa and associated with severe skin and soft tissue infections [50].
While CC398 is a common MRSA clonal complex in livestock in Europe, CC9 is the predominant MRSA clone in pig farming in Asia [51, 52]. In Sri Lanka, MRSA prevalence in pigs was considerably higher than in poultry or cattle (16% vs. 9.3% and 6.2%, respectively) [53]. A higher prevalence of MRSA among pig farmers than among poultry and cattle farmers was also observed suggesting possible transmission of MRSA between humans and pigs [53]. Several studies from Thailand also report on the prevalence of MRSA colonization among pigs and pig farmers. A study investigated 104 pig farms from two provinces in Thailand and found that in almost 10%, MRSA could be isolated from pigs, farmers, or the farm environment [54]. Among individual pigs, 2.5% were MRSA-colonised, all with ST9 clones, harbouring staphylococcal cassette chromosome (SCC)-mec IV [54]. In another study from the same country, the prevalence of LA-MRSA was less than 1% [52].
Latin America
Latin America, particularly Brazil and Argentina, is a major meat exporting region providing beef and poultry for the African and Asian markets. While numerous studies analysed the burden of AMR in humans, comparably little is known on AMR in food items such as meat in South America [55]. Contamination rates of poultry meat with ESBL-producing E. coli (6–72.8%) are comparable with other LMIC (Table 1) [56–58]. Not only ESBL but also plasmid-mediated AmpC beta-lactamases can be widespread in E. coli from Brazilian poultry meat [59]. It is therefore not surprising, that ESBL/AmpC-producing E. coli are disseminated through meat from South America to Europe and to LMIC as outlined above.
Poultry meat from South America has the highest MRSA prevalence in the world (27% vs. 1% in North America) [60]. This is in line with contamination rates reported for pork meat, which are also much higher in Brazil (37.5%) [61] and Chile (43.1–56.5%) [62] compared to Africa and South-East Asia. In conclusion, the global meat trade can promote the spread of AMR (e.g. ESBL-E coli, MRSA) from, in and between LMIC.
Aquaculture for food production
According to the Food and Agriculture Organization (FAO), aquaculture is defined as the farming of aquatic organisms including fish, molluscs, crustaceans and aquatic plants in inland and coastal areas [63]. In 2020, the global aquaculture production was estimated at 87,501 thousand tonnes of live animal weight with the Asian region accounting for 88% of the total production (77,377 thousand tonnes) and including a considerably high production in China contributing 56.7% [63]. Production from the Latin American and African regions amounted to 3781 (4%) and 2354 (3%) thousand tonnes, respectively [63]. It has been discussed whether aquaculture has the ability to improve livelihoods and health in LMIC by contributing to the progress of a number of inter-related sustainable development goals (SDG) such as SDG2 “zero hunger” and SDG3 “good health and well-being” [64, 65]. In 2020, aquaculture production reached an all-time record of 214 million tonnes including 178 million tonnes of aquatic animals [63]. The rapid growth in aquaculture production in recent decades has been facilitated by a transition from extensive to intensive farming [63, 66, 67]. Hence, multiple changes have arisen from increased aquaculture production that include, among others, major adverse effects associated with improper site selection, use of chemicals and anti-infective agents, increased land use, and a global increase of inland water use from 12% (in the late 1980s) to 37% in 2020 [63, 64, 66–68]. Since intensified aquaculture is inevitably associated with disease occurrence in large-scale animal production settings, antimicrobials are used for therapeutic and prophylactic purposes [69–71]. In many countries, aquaculture production systems are not separated from the environment leading to the accumulation of antimicrobial residues in the waters used for animal farming and adjacent waters affecting wild fish, plankton and sediments [71, 72]. This leads to the selection of antimicrobial resistant bacteria and changes the composition of environmental bacteria [67]. The number of antimicrobial substances approved for the use in aquaculture varies markedly between different countries (e.g. two in Brazil, 30 in Vietnam) [73]. The technical guidelines on the prudent and responsible use of veterinary medicines in aquaculture (provided by the FAO) address the need for appropriate environmental assessment and monitoring of drug and chemical use and its impact [74]. However, standardized AMU indicators for aquaculture are not available, counteracting any efforts to compare antimicrobial use on a local or global scale [75].
A recent systematic review and meta-analysis of 749 point-prevalence surveys published between 2019 and 2000 and reporting on AMR bacteria from aquatic food animals intended for human consumption in Asia revealed concerning levels of resistance to medically important antimicrobials in foodborne pathogens such as Vibrio sp., Aeromonas sp., Streptococcus sp., Edwardsiella sp. and E. coli [66]. The overall prevalence of resistance to the highest priority critically important antimicrobials for human medicine defined by WHO [76] were 34% for macrolides, 18% for third- and fourth-generation cephalosporins and 16% for quinolones [66]. For E. coli, the prevalence of resistance to third-generation cephalosporins (indicative ESBL/AmpC production) was 27%, 10% to fosfomycin and 5.2% to colistin across the investigated Asian sub-regions [66]. Other studies from Southeast Asia reported that ESBL-producing E. coli was isolated from 20–53% of fish and shrimp samples [43, 44, 47].
However, available studies on AMR among the most important pathogens associated with aquaculture or its products have considerable differences regarding study design, strategy for analysis, sampling procedures, methods for pathogen identification (species level), and characterisation that often hinder a systematic comparative analysis [77]. The frequent occurrence of significant errors regarding testing methodologies, quality controls, and the use of appropriate interpretive criteria in the performance and reporting of susceptibility testing results of bacteria isolated from aquatic animals were recently addressed [78]. Chromosomal genes conferring resistance towards beta-lactams in Aeromonas spp. [79] led, for instance, to the recommendation to consider respective isolates from human clinical samples (i.e. members of Aeromonas caviae complex, Aeromonas hydrophila complex, and Aeromonas veronii complex) as uniformly resistant to ampicillin, amoxicillin-clavulanic acid, and cefazolin [80]. It is important to consider expected resistant phenotypes, formerly ‘intrinsic resistances’, when reporting prevalence, especially when combining results for multiple species, because results can be misleading and, may therefore interfere with our general understanding regarding the origin and spread of AMR [81]. The methods used for antimicrobial susceptibility testing of Vibrio sp. associated with aquaculture were recently analysed and revealed, that although 203 studies reported on the prevalence of resistance, 185 of them did not provided the criteria they used to determine resistance, used criteria that had not been validated, or were inappropriate [77].
A recent review highlighted that, although most WHO member states have developed a national action plan on AMR compliant with the “One Health” perspective, almost 40% do not acknowledge aquaculture as a critical component where AMR should be further investigated and contained along the whole production chain [82]. Hence, aquaculture will continue to pose challenges in terms of the rapid dissemination of antimicrobial resistant pathogens and AMR determinants since the aquatic environment provides a conducive environment for drug residues, microbial pathogens, and antimicrobial resistance gene dispersions [71].
AMR in wildlife and bushmeat
Bushmeat or wild meat from non-domesticated animals (e.g., bats, monkeys, reptiles, squirrels) is, apart from livestock meat and aquaculture, an important protein source in the Global South. Approximately 5 million tonnes of bushmeat [83] are consumed annually which is the same amount of meat produced in Canada in 2020 (5.2 million tonnes) [84]. The handling and consumption of bushmeat is a risk factor for the transmission of (emerging) zoonotic diseases (e.g. Ebola, Rickettsia, Brucella, mpox) and can expose humans to antimicrobial resistant bacteria.
AMR in wildlife is mostly an indicator of environmental contamination (e.g., surface water, food) with antimicrobial resistant bacteria [85]. For instance, colonization with antimicrobial resistant bacteria in rats and shrews was significantly higher in the vicinity of farms than in more remote areas in Vietnam (forest or rice paddies) [86].
Latest models suggest that the risk for exposure to emerging infectious diseases caused by drug resistant pathogens in LMIC is highest in West Africa and East Asia [87]. Similar to livestock meat, E. coli and S. aureus are the most relevant antimicrobial resistant Gram-negative and Gram-positive species in bushmeat [88]. Apart from one study in Peru [89], transmission of ESBL-producing E. coli between wild animals/bushmeat and humans or livestock have not yet been traced, but numerous observations suggest a certain role of bushmeat/wildlife in the spread of AMR. For instance, bats (1–9%) or birds (2.5–63%) are among the major species to be colonized with ESBL-producing organisms (Table 2), they live in proximity to humans (e.g. Eidolon helvum, Fregata magnificens) [90–92] and are very mobile and could therefore spread pathogens over large distances. For instance, the fruit bat E. helvum can migrate up to 3000 km [93], while some individuals of frigatebird (F. magnificens) can move over 4400 km from their breeding sites [94]. Thus, they can be considered as “flying bridges” [91] to disperse antimicrobial resistant bacteria even between continents, as shown for Franklin’s gull [95].
Table 2.
Contamination of bushmeat or wildlife with antimicrobial resistant bacteria
| Bacterial species | Wildlife | Resistance genes | Year | Country | Samples (n) | Prevalence [% (n/N)] | References |
|---|---|---|---|---|---|---|---|
| ESBL-Escherichia coli | Bats | blaCTX-M-15, blaSHV-11 | 2017 | Gabon | Faeces (68) | 9% (6/68) | [135] |
| Bats | blaCTX-M-15, blaCTX-M-14, blaCTX-M-3, blaCTX-M-55 | 2015–2018 | Peru | Rectal swabs (388) | 5.2% (20/388) | [89] | |
| Bats | blaCTX-M-15 | Before 2021 | Nigeria | Viscera (180) | 1% (2/180) | [136] | |
| Bears | blaCTX-M | 2015–2016 | India | Faeces (21) | 76% (16/21) | [137] | |
| Chimpanzees | blaCTX-M-15 | 2018 | Uganda | Faeces (86) | 11% (9/86) | [138] | |
| Chimpanzees | Not applicable | 2012 | Côte d’Ivoire | Faeces (43) | 0% (0/43) | [139] | |
| Condor | blaCTX-M-14, blaCTX-M-55 | 2019 | Chile | Faecal swabs (27) | 63% (17/27) | [140] | |
| Gorillas | Not applicable | 2011 | Central African Republic | Faeces (65) | 0% (0/65) | [141] | |
| Gull | blaCTX-M-14, blaCTX-M-15 | 2010 | Bangladesh | Faeces (150) | 19.3% (29/150) | [142] | |
| Macaques | Not applicable | 2014–2015 | Algeria | Faeces (126) | 0% (0/126) | [143] | |
| Owl | blaCTX-M-8 | 2018 | Chile | Cloacal swabs (5) | 60% (3/5) | [144] | |
| Rats | Not applicable | 2018–2019 | Iran | Faeces (100) | 0%a (0/100) | [145] | |
| Rats, shrews | Not done | 2013 | Vietnam | Faeces (234) | 0.4% (1/234) | [86] | |
| Seabirds | blaCTX-M-8, blaCTX-M-55 | Before 2022 | Brazil | Cloacal swabs (204) | 2.5% (5/204) | [91] | |
| Seabirds | blaCTX-M-15, blaCTX-M-2, blaCTX-M-22 | 2011 | Chile | Faecal swabs (124) | 54% (67/124) | [95] | |
| Wildlife | Not done | 2018–2019 | Sri Lanka | Faeces (47) | 4% (2/47) | [146] | |
| Wild birds | blaCTX-M | 2010–2013 | Brazil | Faeces (112) | 12.5% (14/112) | [92] | |
| Wild birds | blaCTX-M-15, blaCTX-M-32 | 2012 | Nicaragua | Faeces (100) | 10% (10/100) | [97] | |
| Wild birds | blaCTX-M-9 | 2010 | Mongolia | Cloacal swabs (91) | 6% (5/91) | [147] | |
| Wild birds | blaCTX-M-14, blaCTX-M-15, blaCTX-M-24 | 2015 | Mongolia | Cloacal swabs (63) | 14% (9/63) | [96] | |
| Wild boars | blaCTX-M-15 | 2014–2015 | Algeria | Faeces (90) | 33% (30/90) | [143] | |
| Methicillin-resistant S. aureus | Chimpanzees | Not applicable | 2008 | Côte d’Ivoire | Fruit wadges (21) | 0% (0/21) | [148] |
| Lemurs | Not applicable | 2012 | Madagascar | mucous membrane (25) | 0% (0/25) | [148] | |
| Macaques | mecA | 2017 | Nepal | Saliva (59) | 7% (4/59) | [99] | |
| Marsupials | Not applicable | 2018–2019 | Brazil | Faeces (23) | 0% (0/23) | [149] | |
| Monkeys | Not applicable | Before 2011 | Gabon | Nose (16) | 0% (0/16) | [150] | |
| Rodents | Not applicable | 2018–2019 | Brazil | Faeces (136) | 0% (0/136) | [149] | |
| Seabirds | mecA | Before 2021 | Brazil | Cloacal and tracheal swabs (18) | 6% (1/18) | [105] |
aDeduced from third generation cephalosporin resistance
Some migratory birds in the Mongolian desert were colonized with ESBL-producing E. coli that did not carry the ESBL determinants (blaCTX-M-14, blaCTX-M-15, blaCTX-M-24) on plasmids but on chromosomes, suggesting a more stable integration in the bacterial genome [96].
In addition, there is evidence that bats and pigs are colonized with near-identical ESBL-producing E. coli [89]. Similarly, ESBL-producing E. coli from poultry, humans and wild birds in Nicaragua shared the same resistance genes (blaCTX-M-15), and were detected in same clusters possibly indicating transmission [97].
Despite numerous studies ascertaining colonization, none (in Africa) or very few MRSA (in Latin America and Asia) were detected in free-living wildlife or bushmeat so far (Table 2) [98, 99]. Few studies suggest that S. aureus can be transmitted from humans to gorillas in captivity, including one fatal case, macaques in temple areas, human-habituated monkeys [100] or chimpanzees living in sanctuaries [99, 101, 102]. This highlights, that wildlife is not only a risk for humans but humans can also be a threat for wildlife particularly if animals are reintroduced into the wild [103]. Noteworthy, MRSA in wildlife can also emerge independently of close contact to humans or anthropogenic antimicrobial selective pressure [104]. For instance, coagulase-negative Staphylococcus sciuri were identified as the probable reservoir and source of mecA in a MRSA isolate from non-migratory seabirds on a remote Brazilian island [105].
‘Filth flies’ as reservoirs and vectors for AMR
So called ‘filth flies’ (e.g., Muscidae, Calliphoridae) belong to the order Diptera (true flies) and are coprophagous insects. By consuming faeces from larger animals and humans, and in this way also bacteria, ‘filth flies’ can be both a reservoir and a vector of antimicrobial resistant bacteria. Thus, ‘filth flies’ could be a link between humans, livestock, wildlife, and bushmeat, particularly in LMIC where adequate sanitation systems are often lacking. The capacity to be a reservoir is, however, limited as the alimentary tract of the flies is a hostile environment to the majority of bacterial species (e.g. E. coli, Pseudomonas aeruginosa) [106]. Although the concentration of bacteria decline exponentially during the intestinal passage, defaecation can still be a way of bacterial transmission as bacteria proliferate in faecal droplets [107]. The same is true for regurgitation. The third way of transmission is translocation from the exoskeleton (e.g., insect legs, antennae, labium): approximately 103 of viable bacterial cells (colony forming units) can be transmitted from the exoskeleton per landing [106]. Among antimicrobial resistant species, ESBL-producing E. coli is the most relevant in ‘filth flies’ in LMIC (e.g. Ethiopia, Nigeria, Thailand, India, Zambia,) with colonization rates between 0.8 and 72.5% (Table 3). Similar to data from humans, CTX-M is the most common ESBL in E. coli from flies (24.4–100%) [107–110]. It appears that colonization rates with ESBL-producing E. coli are much higher in the hospital environment than in rural areas as shown in Nigeria and Ethiopia [107, 111]. This suggests that flies could serve as a vector to spread antimicrobial resistant bacteria from the hospital into the community setting. Noteworthy, flies are not only mechanical vectors but could be considered as “reactors of AMR” as resistance genes (e.g. blaCTX-M, blaCMY-2) can be horizontally transferred between different bacterial strains within flies [112]. Not only single genes but also combinations of resistance genes can be co-transferred between bacterial cells via plasmids containing mcr-1 and blaTEM-1 [109].
Table 3.
Colonization of ‘filth flies’ with antimicrobial resistant Escherichia coli and Staphylococcus aureus in LMIC. LMIC Low- and middle-income countries
| Bacterial species | Country | Year | Colonization rate in flies, % (n of isolates/n of flies) | Setting | Fly species (n) | References |
|---|---|---|---|---|---|---|
| ESBL-E. coli | China | 2011 | 3% (37/1228)a | Airport | Chrysomya megacephala (276), Aldrichina graham (247) and others (705) | [151] |
| Ethiopia | 2019 | 6% (5/85) | Hospital, butchery | Not specified (85) | [111] | |
| Nigeria | 2017 | 0.8% (16/2000) | Urban, semi-urban and rural | Not specified (2000) | [107] | |
| Thailand | Before 2021 | 55.7% (334/600) | Urban and rural | Chrysomya megacephala (600) | [110] | |
| Thailand | 2013–2015 | 22.6% (53/235) | Urban and rural | houseflies (177), blowflies (32), flesh flies (8), not identified (18) | [109] | |
| India | Before 2022 | 11% (17/150) | Milk and meat shops | Musca domestica (150) | [108] | |
| Thailand | 2018 | 100% (25/25) | Markets | Not specified (25) | [152] | |
| Zambia | 2015 | 13.4% (56/418) | Food market | House flies (418) | [153] | |
| Methicillin-resistant Staphylococcus aureus | Bangladesh | 2017–2018 | 25.3% (101/400) | Hospital | House flies (400) | [154] |
| Botwana | 2018–2019 | 1% (10/970) | Hospital | House flies (970) | [155] | |
| Libya | Before 2015 | 1.3% (2/150) | Urban | Musca domestica (150) | [156] | |
| Nigeria | 2017 | 0.2% (4/2000) | Urban, semi-urban and rural | Not specified (2000) | [106] |
MRSA colonization in flies is poorly investigated and varies markedly between geographic regions (0.2–25.3%) in LMIC (Table 3).
AMR surveillance and integration across sectors
To address the issue of AMR, in 2015, the World Health Organization (WHO) put forward the Global Action Plan for AMR (GAP-AMR) which sets five strategic objectives that aim to reduce the burden of AMR and preserve our ability to treat infections effectively [113]. In these efforts, WHO was joined by the WOAH and the FAO to support countries in strengthening their efforts against AMR. The Tripartite organisations (FAO, WOAH, and WHO) are in the process of developing several tools for monitoring and surveillance. The joint Tripartite AMR country self-assessment survey (TrACSS) tracks country progress towards development and implementation of National Action Plans for AMR [114]. To date, almost 150 countries have joined this initiative and developed National Action Plans for AMR [115]. In 2022, FAO has started developing a global system to aid countries in collecting, analysing and comparing AMR data from animals and food. The International FAO AMR Monitoring (InFARM) platform aims to enable comparisons between settings and facilitate public sharing and harmonisation of AMR data [116]. The FAO has developed an Assessment Tool for Laboratories and AMR Surveillance Systems (FAO-ATLASS) to support countries in strengthening laboratories and improving national AMR surveillance systems for the food and agriculture sectors [117].
The Tripartite Integrated Surveillance System on AMR/AMU (TISSA) is a platform that integrates surveillance data across different organisations and areas. Specifically TISSA is meant to integrate four monitoring and surveillance systems across the three organizations (Global Antimicrobial Resistance and Use Surveillance System (GLASS), WOAH surveillance data on antimicrobial use in animals, ATLASS, and TrACSS) thus encompassing human and animal health, plants, and the environment [118].
To further emphasize the interconnectivity across sectors in the efforts to combat AMR, the WHO is planning the Tricycle project which will integrate surveillance in humans, animals and the environment (Fig. 1). The project is aimed at low-resource countries and focuses on a single pathogen namely ESBL-producing E. coli. By employing standard methodologies for surveillance and the project will enable participating countries to develop further surveillance systems involving other pathogens and resistance mechanisms [119].
In addition, the Tripartite organizations have developed a One Health priority research agenda to promote scientific interest, increase investment and inform policies related to AMR [120]. One Health provides an integrated and unified approach across sectors aiming to sustainably improve the health of humans, animals and ecosystems [121]. In November 2022, the Antimicrobial Resistance Multi-Stakeholder Partnership Programme was launched in November 2022 by FAO, WHO, WOAH, and the UN Environment Programme (Quadripartite) to strengthen the global efforts regarding One Health integrated surveillance on the human, animal, food and environmental sectors [122].
Mitigating AMR emergence and transmission
Several measures can be taken to mitigate the emergence and transmission of AMR. Strengthening AMR surveillance and research within but also across sectors can lead to a better understanding of the burden of resistance and enable the implementation of setting-specific measures to prevent further spread. This can only be achieved by increasing diagnostic capacity, by developing reliable integrated systems for reporting AMR data across countries, laboratories and sectors (e.g., including data from human, animal, food, and environmental samples), and by ensuring access to funding for undertaking these activities particularly in LMIC. Of critical importance is also ensuring that data are representative and thus correctly reflect the local/regional AMR landscape. Similarly, reliable data on AMU in animals are needed to inform policy and plan educational interventions for effecting behaviour change and reducing AMU.
Developing new antimicrobial classes and systems (e.g. plasmids, phages), that are used only in food production, would make the emergence of resistance to these antimicrobials less problematic to human health [123]. Further, improving hygiene in animal-rearing facilities, education and veterinary care, as well as adequate waste disposal and treatment of sewage would prevent transmission of AMR determinants between animals, environmental contamination, and entry into the food chain [11]. In addition, research to identify how and where contamination with resistant organisms occurs during food production and commercialization and how it is transmitted to and from humans would aid in developing mitigating interventions.
Vaccines are a promising tool for combating AMR. These act directly by reducing the incidence of infections overall, and thus of resistant infections, and indirectly by reducing AMU. For instance, vaccination of pigs against Lawsonia intracellularis led to an important reduction in AMU and improved productivity [124] Vaccines have been developed and are being used in aquaculture for preventing infections in high-value Atlantic salmon, however, vaccines for low-value fish which are farmed primarily in LMIC are still needed or underused [125].
Policies and regulations controlling and restricting the use of antimicrobials critical for human health, the uncontrolled purchase of antimicrobials and their use for growth-promotion in animals would lead to safeguarding the future of antimicrobials [11]. Promoting the labelling of animal products according to the use of antimicrobials in food production may also encourage farmers to reduce AMU.
Conclusions
This review set out to provide an overview on the zoonotic sources of AMR, associated challenges and relevance to LMIC. Livestock farming accounts for substantial AMU in both high- and low-income settings. While regulations to control and restrict excessive use of antimicrobials have been implemented in high-resource settings, efforts should be made to support LMIC in developing strategies to better monitor and optimise AMU. Furthermore, bushmeat and aquaculture are relevant sources of animal meat in LMIC and should be targeted for AMR surveillance and research. Therefore, measures tailored to the specific features of LMIC need to implemented to contain the spread of AMR. Addressing AMR across sectors and settings, will prevent the development and transmission of resistance as well as preserve our ability to effectively treat infections in humans and animals.
Acknowledgements
We thank Eske Lübbers for designing Fig. 1.
Abbreviations
- AMR
Antimicrobial resistance
- AMU
Antimicrobial use
- ATLASS
Assessment Tool for Laboratories and AMR Surveillance Systems
- CC
Clonal complex
- ESBL
Extended-spectrum beta-lactamases
- FAO
Food and Agriculture Organization
- GLASS
Global Antimicrobial Resistance and Use Surveillance System
- InFARM
International FAO AMR Monitoring
- LMIC
Low- and middle-income countries
- MRSA
Methicillin-resistant Staphylococcus aureus
- PVL
Panton-Valentine leukocidin
- SDG
Sustainable development goals
- ST
Sequence type
- TISSA
Tripartite Integrated Surveillance System
- WHO
World Health Organization
- WOAH
World Organization for Animal Health
Author contributions
IDO: Conceptialization, Investigation, Writing—Original Draft, Writing—Review and Editing; BW: Conceptialization, Investigation, Writing—Original Draft, Writing—Review and Editing; FS: Conceptialization, Investigation, Writing—Original Draft, Writing—Review and Editing, Supervision, Visualization.
Funding
The study has no funding. We acknowledge the open access fund of the University of Münster.
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors do not have any competing interests.
References
- 1.Antimicrobial Resistance C. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Review on Antimicrobial Resistance, Chaired by Jim O'Neill. Antimicrobial resistance: Tackling a crisis for the health andwealth of nations”. 2014. Available from: http://amr-review.org/Publications.html. Accessed 02 Jan 2023.
- 3.Alvarez-Uria G, Gandra S, Laxminarayan R. Poverty and prevalence of antimicrobial resistance in invasive isolates. Int J Infect Dis. 2016;52:59–61. doi: 10.1016/j.ijid.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, Levin SA, Bonhoeffer S, Laxminarayan R. Reducing antimicrobial use in food animals. Science. 2017;357(6358):1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao Y, Wang Y, Yuan Y, Xie Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci Total Environ. 2021;798:149205. doi: 10.1016/j.scitotenv.2021.149205. [DOI] [PubMed] [Google Scholar]
- 6.The World Bank. World Bank Country and Lending Groups. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 16 Jan 2023.
- 7.Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, Guerin PJ, Piddock LJ. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 8.Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD. Global extraintestinal pathogenic Escherichiacoli (ExPEC) lineages. Clin Microbiol Rev. 2019; 32(3). [DOI] [PMC free article] [PubMed]
- 9.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011;11(5):355–362. doi: 10.1016/S1473-3099(11)70059-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, et al. Emergence of plasmid-mediated colistin resistance mechanism mcr-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 11.Nadimpalli M, Delarocque-Astagneau E, Love DC, Price LB, Huynh BT, Collard JM, Lay KS, Borand L, Ndir A, Walsh TR, et al. Combating global antibiotic resistance: emerging One Health concerns in lower- and middle-income countries. Clin Infect Dis. 2018;66(6):963–969. doi: 10.1093/cid/cix879. [DOI] [PubMed] [Google Scholar]
- 12.Nang SC, Li J, Velkov T. The rise and spread of mcr plasmid-mediated polymyxin resistance. Crit Rev Microbiol. 2019;45(2):131–161. doi: 10.1080/1040841X.2018.1492902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Organization for Animal Health. OIE Annual report on antimicrobial agents intended for use in animals. Sixth Edition, 2022. Available from https://www.woah.org/en/document/annual-report-on-antimicrobial-agents-intended-for-use-in-animals/. Accessed 02 Jan 2023.
- 15.World Health Organization. WHO guidelines on use of medically important antimicrobials in food-producing animals. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. Available from https://www.who.int/publications/i/item/9789241550130. Accessed 02 Jan 2023.
- 16.Mulchandani R, Wang Y, Gilbert M, Van Boeckel TP. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Global Public Health. 2023;3(2):e0001305. doi: 10.1371/journal.pgph.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malijan GM, Howteerakul N, Ali N, Siri S, Kengganpanich M, Group O-DS. Nascimento R, Booton RD, Turner KME, Cooper BS, et al. A scoping review of antibiotic use practices and drivers of inappropriate antibiotic use in animal farms in WHO Southeast Asia region. One Health. 2022;15:100412. doi: 10.1016/j.onehlt.2022.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang KL, Caffrey NP, Nobrega DB, Cork SC, Ronksley PE, Barkema HW, Polachek AJ, Ganshorn H, Sharma N, Kellner JD, et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017;1(8):E316–E327. doi: 10.1016/S2542-5196(17)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Initiatives for Addressing Antimicrobial Resistance in the Environment: Current Situation and Challenges. 2018. https://wellcome.ac.uk/sites/default/files/antimicrobial-resistance-environment-report.pdf. Accessed 02 Jan 2023.
- 20.Schaumburg F, Sertic SM, Correa-Martinez C, Mellmann A, Köck R, Becker K. Acquisition and colonization dynamics of antimicrobial-resistant bacteria during international travel: a prospective cohort study. Clin Microbiol Infect. 2019;25(10):1287 e1281–1287 e1287. doi: 10.1016/j.cmi.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Egervarn M, Borjesson S, Byfors S, Finn M, Kaipe C, Englund S, Lindblad M. Escherichia coli with extended-spectrum beta-lactamases or transferable AmpC beta-lactamases and Salmonella on meat imported into Sweden. Int J Food Microbiol. 2014;171:8–14. doi: 10.1016/j.ijfoodmicro.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Jansen W, Muller A, Grabowski NT, Kehrenberg C, Muylkens B, Al Dahouk S. Foodborne diseases do not respect borders: zoonotic pathogens and antimicrobial resistant bacteria in food products of animal origin illegally imported into the European Union. Vet J. 2019;244:75–82. doi: 10.1016/j.tvjl.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Ma ZB, Zeng ZL, Yang XW, Huang Y, Liu JH. The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool Res. 2017;38(2):55–80. doi: 10.24272/j.issn.2095-8137.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuny C, Köck R, Witte W. Livestock associated MRSA (LA-MRSA) and its relevance for humans in Germany. Int J Med Microbiol. 2013;303(6–7):331–337. doi: 10.1016/j.ijmm.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Köck R, Herr C, Kreienbrock L, Schwarz S, Tenhagen BA, Walther B. Multiresistant Gram-negative pathogens—a zoonotic problem. Dtsch Arztebl Int. 2021;118(35–36):579–589. doi: 10.3238/arztebl.m2021.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarus B, Paterson DL, Mollinger JL, Rogers BA. Do human extraintestinal Escherichia coli infections resistant to expanded-spectrum cephalosporins originate from food-producing animals? A systematic review. Clin Infect Dis. 2015;60(3):439–452. doi: 10.1093/cid/ciu785. [DOI] [PubMed] [Google Scholar]
- 27.Abdallah HM, Reuland EA, Wintermans BB, Al Naiemi N, Koek A, Abdelwahab AM, Ammar AM, Mohamed AA, Vandenbroucke-Grauls CM. Extended-spectrum beta-lactamases and/or carbapenemases-producing Enterobacteriaceae isolated from retail chicken meat in Zagazig, Egypt. PLoS ONE. 2015;10(8):e0136052. doi: 10.1371/journal.pone.0136052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chishimba K, Hang'ombe BM, Muzandu K, Mshana SE, Matee MI, Nakajima C, Suzuki Y. Detection of extended-spectrum beta-lactamase-producing Escherichia coli in market-ready chickens in Zambia. Int J Microbiol. 2016;2016:5275724. doi: 10.1155/2016/5275724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaumburg F, Alabi AS, Frielinghaus L, Grobusch MP, Köck R, Becker K, Issifou S, Kremsner PG, Peters G, Mellmann A. The risk to import ESBL-producing Enterobacteriaceae and Staphylococcus aureus through chicken meat trade in Gabon. BMC Microbiol. 2014;14:286. doi: 10.1186/s12866-014-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worku W, Desta M, Menjetta T. High prevalence and antimicrobial susceptibility pattern of salmonella species and extended-spectrum beta-lactamase producing Escherichia coli from raw cattle meat at butcher houses in Hawassa city, Sidama regional state, Ethiopia. PLoS ONE. 2022;17(1):e0262308. doi: 10.1371/journal.pone.0262308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben Slama K, Jouini A, Ben Sallem R, Somalo S, Saenz Y, Estepa V, Boudabous A, Torres C. Prevalence of broad-spectrum cephalosporin-resistant Escherichia coli isolates in food samples in Tunisia, and characterization of integrons and antimicrobial resistance mechanisms implicated. Int J Food Microbiol. 2010;137(2–3):281–286. doi: 10.1016/j.ijfoodmicro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Jouini A, Vinue L, Slama KB, Saenz Y, Klibi N, Hammami S, Boudabous A, Torres C. Characterization of CTX-M and SHV extended-spectrum beta-lactamases and associated resistance genes in Escherichia coli strains of food samples in Tunisia. J Antimicrob Chemother. 2007;60(5):1137–1141. doi: 10.1093/jac/dkm316. [DOI] [PubMed] [Google Scholar]
- 33.Eibach D, Dekker D, Gyau Boahen K, Wiafe Akenten C, Sarpong N, Belmar Campos C, Berneking L, Aepfelbacher M, Krumkamp R, Owusu-Dabo E, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in local and imported poultry meat in Ghana. Vet Microbiol. 2018;217:7–12. doi: 10.1016/j.vetmic.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 34.Rasmussen MM, Opintan JA, Frimodt-Møller N, Styrishave B. Beta-lactamase producing Escherichia coli isolates in imported and locally produced chicken meat from Ghana. PLoS ONE. 2015;10(10):e0139706. doi: 10.1371/journal.pone.0139706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faife SL, Zimba T, Sekyere JO, Govinden U, Chenia HY, Simonsen GS, Sundsfjord A, Essack SY. Beta-lactam and fluoroquinolone resistance in Enterobacteriaceae from imported and locally-produced chicken in Mozambique. J Infect Dev Ctries. 2020;14(5):471–478. doi: 10.3855/jidc.10924. [DOI] [PubMed] [Google Scholar]
- 36.Falgenhauer L, Imirzalioglu C, Oppong K, Akenten CW, Hogan B, Krumkamp R, Poppert S, Levermann V, Schwengers O, Sarpong N, et al. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front Microbiol. 2018;9:3358. doi: 10.3389/fmicb.2018.03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhanji H, Murphy NM, Doumith M, Durmus S, Lee SS, Hope R, Woodford N, Livermore DM. Cephalosporin resistance mechanisms in Escherichia coli isolated from raw chicken imported into the UK. J Antimicrob Chemother. 2010;65(12):2534–2537. doi: 10.1093/jac/dkq376. [DOI] [PubMed] [Google Scholar]
- 38.Loretz M, Stephan R, Zweifel C. Antimicrobial activity of decontamination treatments for poultry carcasses: a literature survey. Food Control. 2010;21(6):791–804. doi: 10.1016/j.foodcont.2009.11.007. [DOI] [Google Scholar]
- 39.Thwala T, Madoroba E, Basson A, Butaye P. Prevalence and characteristics of Staphylococcus aureus associated with meat and meat products in African countries: a review. Antibiotics (Basel) 2021;10(9):1108. doi: 10.3390/antibiotics10091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhegghe M, Crombe F, Luyckx K, Haesebrouck F, Butaye P, Herman L, Heyndrickx M, Rasschaert G. Prevalence and genetic diversity of livestock-associated methicillin-resistant Staphylococcus aureus on Belgian pork. J Food Prot. 2016;79(1):82–89. doi: 10.4315/0362-028X.JFP-15-266. [DOI] [PubMed] [Google Scholar]
- 41.Mama OM, Morales L, Ruiz-Ripa L, Zarazaga M, Torres C. High prevalence of multidrug resistant S. aureus-CC398 and frequent detection of enterotoxin genes among non-CC398 S. aureus from pig-derived food in Spain. Int J Food Microbiol. 2020;320:108510. doi: 10.1016/j.ijfoodmicro.2020.108510. [DOI] [PubMed] [Google Scholar]
- 42.Chairat S, Gharsa H, Lozano C, Gomez-Sanz E, Gomez P, Zarazaga M, Boudabous A, Torres C, Ben Slama K. Characterization of Staphylococcus aureus from raw meat samples in Tunisia: detection of clonal lineage ST398 from the African continen. Foodborne Pathog Dis. 2015;12(8):686–692. doi: 10.1089/fpd.2015.1958. [DOI] [PubMed] [Google Scholar]
- 43.Nadimpalli M, Vuthy Y, de Lauzanne A, Fabre L, Criscuolo A, Gouali M, Huynh BT, Naas T, Phe T, Borand L, et al. Meat and fish as sources of extended-spectrum beta-lactamase-producing Escherichia coli, Cambodia. Emerg Infect Dis. 2019;25(1):126–131. doi: 10.3201/eid2501.180534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen do P, Nguyen TA, Le TH, Tran NM, Ngo TP, Dang VC, Kawai T, Kanki M, Kawahara R, Jinnai M, et al. Dissemination of extended-spectrum beta-lactamase- and AmpC beta-lactamase-producing Escherichia coli within the food distribution system of Ho Chi Minh City, Vietnam. Biomed Res Int. 2016;2016:8182096. doi: 10.1155/2016/8182096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trongjit S, Angkittitrakul S, Chuanchuen R. Occurrence and molecular characteristics of antimicrobial resistance of Escherichia coli from broilers, pigs and meat products in Thailand and Cambodia provinces. Microbiol Immunol. 2016;60(9):575–585. doi: 10.1111/1348-0421.12407. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama T, Le Thi H, Thanh PN, Minh DTN, Hoang ON, Hoai PH, Yamaguchi T, Jinnai M, Do PN, Van CD, et al. Abundance of colistin-resistant Escherichia coli harbouring mcr-1 and extended-spectrum beta-lactamase-producing E. coli co-harbouring bla(CTX-M-55) or (-65) with bla(TEM) isolates from chicken meat in Vietnam. Arch Microbiol. 2022;204(2):137. doi: 10.1007/s00203-021-02746-0. [DOI] [PubMed] [Google Scholar]
- 47.Le PQ, Awasthi SP, Hatanaka N, Hinenoya A, Hassan J, Ombarak RA, Iguchi A, Tran NTT, Dao KVT, Vien MQ, et al. Prevalence of mobile colistin resistance (mcr) genes in extended-spectrum beta-lactamase-producing Escherichia coli isolated from retail raw foods in Nha Trang, Vietnam. Int J Food Microbiol. 2021;346:109164. doi: 10.1016/j.ijfoodmicro.2021.109164. [DOI] [PubMed] [Google Scholar]
- 48.Zehra A, Gulzar M, Singh R, Kaur S, Gill JPS. Prevalence, multidrug resistance and molecular typing of methicillin-resistant Staphylococcus aureus (MRSA) in retail meat from Punjab, India. J Glob Antimicrob Resist. 2019;16:152–158. doi: 10.1016/j.jgar.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Syed MA, Shah SHH, Sherafzal Y, Shafi-Ur-Rehman S, Khan MA, Barrett JB, Woodley TA, Jamil B, Abbasi SA, Jackson CR. Detection and molecular characterization of methicillin-resistant Staphylococcus aureus from table eggs in Haripur, Pakistan. Foodborne Pathog Dis. 2018;15(2):86–93. doi: 10.1089/fpd.2017.2336. [DOI] [PubMed] [Google Scholar]
- 50.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(1):43–54. doi: 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vestergaard M, Cavaco LM, Sirichote P, Unahalekhaka A, Dangsakul W, Svendsen CA, Aarestrup FM, Hendriksen RS. SCCmec type IX element in Methicillin resistant Staphylococcus aureus spa type t337 (CC9) isolated from pigs and pork in Thailand. Front Microbiol. 2012;3:103. doi: 10.3389/fmicb.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinlapasorn S, Lulitanond A, Angkititrakul S, Chanawong A, Wilailuckana C, Tavichakorntrakool R, Chindawong K, Seelaget C, Krasaesom M, Chartchai S, et al. SCCmec IX in meticillin-resistant Staphylococcus aureus and meticillin-resistant coagulase-negative staphylococci from pigs and workers at pig farms in Khon Kaen, Thailand. J Med Microbiol. 2015;64(9):1087–1093. doi: 10.1099/jmm.0.000119. [DOI] [PubMed] [Google Scholar]
- 53.Jayaweera J, Kumbukgolla WW. Antibiotic resistance patterns of methicillin-resistant Staphylococcus aureus (MRSA) isolated from livestock and associated farmers in Anuradhapura, Sri Lanka. Germs. 2017;7(3):132–139. doi: 10.18683/germs.2017.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patchanee P, Tadee P, Arjkumpa O, Love D, Chanachai K, Alter T, Hinjoy S, Tharavichitkul P. Occurrence and characterization of livestock-associated methicillin-resistant Staphylococcus aureus in pig industries of northern Thailand. J Vet Sci. 2014;15(4):529–536. doi: 10.4142/jvs.2014.15.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bastidas-Caldes C, Romero-Alvarez D, Valdez-Velez V, Morales RD, Montalvo-Hernandez A, Gomes-Dias C, Calvopina M. Extended-spectrum beta-lactamases producing Escherichia coli in South America: a systematic review with a One Health perspective. Infect Drug Resist. 2022;15:5759–5779. doi: 10.2147/IDR.S371845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardozo MV, Liakopoulos A, Brouwer M, Kant A, Pizauro LJL, Borzi MM, Mevius D, de Avila FA. Occurrence and molecular characteristics of extended-spectrum beta-lactamase-producing Enterobacterales recovered from chicken, chicken meat, and human infections in Sao Paulo State, Brazi. Front Microbiol. 2021;12:628738. doi: 10.3389/fmicb.2021.628738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ortega-Paredes D, de Janon S, Villavicencio F, Ruales KJ, De La Torre K, Villacis JE, Wagenaar JA, Matheu J, Bravo-Vallejo C, Fernandez-Moreira E, et al. Broiler farms and carcasses are an important reservoir of multi-drug resistant Escherichia coli in Ecuador. Front Vet Sci. 2020;7:547843. doi: 10.3389/fvets.2020.547843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soncini JGM, Cerdeira L, Sano E, Koga VL, Tizura AT, Tano ZN, Nakazato G, Kobayashi RKT, Aires CAM, Lincopan N, et al. Genomic insights of high-risk clones of ESBL-producing Escherichia coli isolated from community infections and commercial meat in southern Brazil. Sci Rep. 2022;12(1):9354. doi: 10.1038/s41598-022-13197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koga VL, Maluta RP, da Silveira WD, Ribeiro RA, Hungria M, Vespero EC, Nakazato G, Kobayashi RKT. Characterization of CMY-2-type beta-lactamase-producing Escherichia coli isolated from chicken carcasses and human infection in a city of South Brazil. BMC Microbiol. 2019;19(1):174. doi: 10.1186/s12866-019-1550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ribeiro CM, Stefani LM, Lucheis SB, Okano W, Cruz JCM, Souza GV, Casagrande TAC, Bastos PAS, Pinheiro RR, Arruda MM, et al. Methicillin-resistant Staphylococcus aureus in poultry and poultry meat: a meta-analysis. J Food Prot. 2018;81(7):1055–1062. doi: 10.4315/0362-028X.JFP-17-445. [DOI] [PubMed] [Google Scholar]
- 61.Costa WL, Ferreira Jdos S, Carvalho JS, Cerqueira ES, Oliveira LC, Almeida RC. Methicillin-resistant Staphylococcus aureus in raw meats and prepared foods in public hospitals in Salvador, Bahia, Brazil. J Food Sci. 2015;80(1):M147–150. doi: 10.1111/1750-3841.12723. [DOI] [PubMed] [Google Scholar]
- 62.Velasco V, Vergara JL, Bonilla AM, Munoz J, Mallea A, Vallejos D, Quezada-Aguiluz M, Campos J, Rojas-Garcia P. Prevalence and characterization of Staphylococcus aureus strains in the pork chain supply in Chile. Foodborne Pathog Dis. 2018;15(5):262–268. doi: 10.1089/fpd.2017.2381. [DOI] [PubMed] [Google Scholar]
- 63.Food and Agriculture Organization of the United Nations, (FAO). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome, FAO. 10.4060/cc0461en. Accessed 1 Feb 2023.
- 64.Gonzalez Parrao C, Moratti M, Shisler S, Snilstveit B, Eyers J. PROTOCOL: aquaculture for improving productivity, income, nutrition and women's empowerment in low‐ and middle‐income countries: a systematic review and meta‐analysis. Campbell Syst Rev. 2021; 17(3). [DOI] [PMC free article] [PubMed]
- 65.Troell M, Naylor RL, Metian M, Beveridge M, Tyedmers PH, Folke C, Arrow KJ, Barrett S, Crepin AS, Ehrlich PR, et al. Does aquaculture add resilience to the global food system? Proc Natl Acad Sci USA. 2014;111(37):13257–13263. doi: 10.1073/pnas.1404067111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schar D, Zhao C, Wang Y, Larsson DGJ, Gilbert M, Van Boeckel TP. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat Commun. 2021;12(1):5384. doi: 10.1038/s41467-021-25655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schar D, Klein EY, Laxminarayan R, Gilbert M, Van Boeckel TP. Global trends in antimicrobial use in aquaculture. Sci Rep. 2020;10(1):21878. doi: 10.1038/s41598-020-78849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giri S, Daw TM, Hazra S, Troell M, Samanta S, Basu O, Marcinko CLJ, Chanda A. Economic incentives drive the conversion of agriculture to aquaculture in the Indian Sundarbans: Livelihood and environmental implications of different aquaculture types. Ambio. 2022;51(9):1963–1977. doi: 10.1007/s13280-022-01720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaiyapuri M, Pailla S, Rao Badireddy M, Pillai D, Chandragiri Nagarajarao R, Prasad Mothadaka M. Antimicrobial resistance in Vibrios of shrimp aquaculture: incidence, identification schemes, drivers and mitigation measures. Aquac Res. 2021;52(7):2923–2941. doi: 10.1111/are.15142. [DOI] [Google Scholar]
- 70.Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. 2015;112(18):5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santos L, Ramos F. Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents. 2018;52(2):135–143. doi: 10.1016/j.ijantimicag.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Lulijwa R, Rupia EJ, Alfaro AC. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquac. 2019;12(2):640–663. doi: 10.1111/raq.12344. [DOI] [Google Scholar]
- 73.Okeke ES, Chukwudozie KI, Nyaruaba R, Ita RE, Oladipo A, Ejeromedoghene O, Atakpa EO, Agu CV, Okoye CO. Antibiotic resistance in aquaculture and aquatic organisms: a review of current nanotechnology applications for sustainable management. Environ Sci Pollut Res Int. 2022;29(46):69241–69274. doi: 10.1007/s11356-022-22319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Food and Agriculture Organization. Aquaculture development. 8. Recommendations for prudent and responsible use of veterinary medicines in aquaculture. FAO Technical Guidelines for Responsible Fisheries; 2019. No. 5. Suppl. 8. Rome. Available from https://www.fao.org/3/ca7029en/CA7029EN.pdf. Accessed 13 Feb 2023.
- 75.Narbonne JA, Radke BR, Price D, Hanington PC, Babujee A, Otto SJG. Antimicrobial use surveillance indicators for finfish aquaculture production: a review. Front Vet Sci. 2021;8:595152. doi: 10.3389/fvets.2021.595152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR). Critically important antimicrobials for human medicine. WHO, Geneva, Switzerland, 2019. Available from https://www.who.int/publications/i/item/9789241515528. Accessed 1 Feb 2023.
- 77.Smith P. Critical review of methods used in published studies of susceptibility of Vibrio spp.; lessons to be learnt. Asian Fisheries Sci. 2020; 33S.
- 78.Smith P. Eight rules for improving the quality of papers on the antimicrobial susceptibility of bacteria isolated from aquatic animals. Dis Aquat Organ. 2020;139:87–92. doi: 10.3354/dao03476. [DOI] [PubMed] [Google Scholar]
- 79.Chen PL, Ko WC, Wu CJ. Complexity of beta-lactamases among clinical Aeromonas isolates and its clinical implications. J Microbiol Immunol Infect. 2012;45(6):398–403. doi: 10.1016/j.jmii.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Clinical Laboratory Standards Institute. M45 Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. CLSI USA, 2016. Available from www.clsi.org. Accessed 1 Feb 2023.
- 81.Peterson E, Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front Microbiol. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caputo A, Bondad-Reantaso MG, Karunasagar I, Hao B, Gaunt P, Verner-Jeffreys D, Fridman S, Dorado-Garcia A. Antimicrobial resistance in aquaculture: a global analysis of literature and national action plans. Rev Aquac. 2022;15:568–578. doi: 10.1111/raq.12741. [DOI] [Google Scholar]
- 83.Peros CS, Dasgupta R, Kumar P, Johnson BA. Bushmeat, wet markets, and the risks of pandemics: exploring the nexus through systematic review of scientific disclosures. Environ Sci Policy. 2021;124:1–11. doi: 10.1016/j.envsci.2021.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Food and Agriculture Organization of the United Nations. FAOSTAT Data. Available from https://www.fao.org/faostat/en/#data. Accessed 31 Jan 2023.
- 85.Khan SA, Imtiaz MA, Sayeed MA, Shaikat AH, Hassan MM. Antimicrobial resistance pattern in domestic animal—wildlife—environmental niche via the food chain to humans with a Bangladesh perspective; a systematic review. BMC Vet Res. 2020;16(1):302. doi: 10.1186/s12917-020-02519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nhung NT, Cuong NV, Campbell J, Hoa NT, Bryant JE, Truc VN, Kiet BT, Jombart T, Trung NV, Hien VB, et al. High levels of antimicrobial resistance among escherichia coli isolates from livestock farms and synanthropic rats and shrews in the Mekong Delta of Vietnam. Appl Environ Microbiol. 2015;81(3):812–820. doi: 10.1128/AEM.03366-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Devnath P, Karah N, Graham JP, Rose ES, Asaduzzaman M. Evidence of antimicrobial resistance in bats and its planetary health impact for surveillance of zoonotic spillover events: a scoping review. Int J Environ Res Public Health. 2022;20(1):243. doi: 10.3390/ijerph20010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benavides JA, Godreuil S, Opazo-Capurro A, Mahamat OO, Falcon N, Oravcova K, Streicker DG, Shiva C. Long-term maintenance of multidrug-resistant Escherichia coli carried by vampire bats and shared with livestock in Peru. Sci Total Environ. 2022;810:152045. doi: 10.1016/j.scitotenv.2021.152045. [DOI] [PubMed] [Google Scholar]
- 90.Olatimehin A, Shittu AO, Onwugamba FC, Mellmann A, Becker K, Schaumburg F. Staphylococcus aureus complex in the straw-colored fruit bat (Eidolon helvum) in Nigeria. Front Microbiol. 2018;9:162. doi: 10.3389/fmicb.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ewbank AC, Fuentes-Castillo D, Sacristan C, Cardoso B, Esposito F, Fuga B, de Macedo EC, Lincopan N, Catao-Dias JL. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli survey in wild seabirds at a pristine atoll in the southern Atlantic Ocean, Brazil: first report of the O25b-ST131 clone harboring bla(CTX-M-8) Sci Total Environ. 2022;806(Pt 2):150539. doi: 10.1016/j.scitotenv.2021.150539. [DOI] [PubMed] [Google Scholar]
- 92.Batalha de Jesus AA, Freitas AAR, de Souza JC, Martins N, Botelho LAB, Girao VBC, Teixeira LM, Riley LW, Moreira BM. High-level multidrug-resistant Escherichia coli isolates from wild birds in a large urban environment. Microb Drug Resist. 2019;25(2):167–172. doi: 10.1089/mdr.2018.0180. [DOI] [PubMed] [Google Scholar]
- 93.Ossa G, Kramer-Schadt S, Peel AJ, Scharf AK, Voigt CC. The movement ecology of the straw-colored fruit bat, Eidolon helvum, in sub-Saharan Africa assessed by stable isotope ratios. PLoS ONE. 2012;7(9):e45729. doi: 10.1371/journal.pone.0045729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weimerskirch H, Le Corre M, Marsac F, Barbraud C, Tostain O, Chastel O. Postbreeding movements of frigatebirds tracked with satellite telemetry. The Condor. 2006;108(1):220–225. doi: 10.1093/condor/108.1.220. [DOI] [Google Scholar]
- 95.Baez J, Hernandez-Garcia M, Guamparito C, Diaz S, Olave A, Guerrero K, Canton R, Baquero F, Gahona J, Valenzuela N, et al. Molecular characterization and genetic diversity of ESBL-producing Escherichia coli colonizing the migratory Franklin's gulls (Leucophaeus pipixcan) in Antofagasta, North of Chile. Microb Drug Resist. 2015;21(1):111–116. doi: 10.1089/mdr.2014.0158. [DOI] [PubMed] [Google Scholar]
- 96.Guenther S, Semmler T, Stubbe A, Stubbe M, Wieler LH, Schaufler K. Chromosomally encoded ESBL genes in Escherichia coli of ST38 from Mongolian wild birds. J Antimicrob Chemother. 2017;72(5):1310–1313. doi: 10.1093/jac/dkx006. [DOI] [PubMed] [Google Scholar]
- 97.Hasan B, Laurell K, Rakib MM, Ahlstedt E, Hernandez J, Caceres M, Jarhult JD. Fecal carriage of extended-spectrum beta-lactamases in healthy humans, poultry, and wild birds in Leon, Nicaragua—a shared pool of bla(CTX-M) genes and possible interspecies clonal spread of extended-spectrum beta-lactamases-producing Escherichia coli. Microb Drug Resist. 2016;22(8):682–687. doi: 10.1089/mdr.2015.0323. [DOI] [PubMed] [Google Scholar]
- 98.Lozano C, Gharsa H, Ben Slama K, Zarazaga M, Torres C. Staphylococcus aureus in animals and food: methicillin resistance, prevalence and population structure. A review in the African continent. Microorganisms. 2016;4(1):12. doi: 10.3390/microorganisms4010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts MC, Joshi PR, Greninger AL, Melendez D, Paudel S, Acharya M, Bimali NK, Koju NP, No D, Chalise M et al. The human clone ST22 SCCmec IV methicillin-resistant Staphylococcusaureus isolated from swine herds and wild primates in Nepal: is man the common source? FEMS Microbiol Ecol. 2018; 94(5). [DOI] [PMC free article] [PubMed]
- 100.Senghore M, Bayliss SC, Kwambana-Adams BA, Foster-Nyarko E, Manneh J, Dione M, Badji H, Ebruke C, Doughty EL, Thorpe HA, et al. Transmission of Staphylococcus aureus from humans to green monkeys in the Gambia as revealed by whole-genome sequencing. Appl Environ Microbiol. 2016;82(19):5910–5917. doi: 10.1128/AEM.01496-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagel M, Dischinger J, Turck M, Verrier D, Oedenkoven M, Ngoubangoye B, Le Flohic G, Drexler JF, Bierbaum G, Gonzalez JP. Human-associated Staphylococcus aureus strains within great ape populations in Central Africa (Gabon) Clin Microbiol Infect. 2013;19(11):1072–1077. doi: 10.1111/1469-0691.12119. [DOI] [PubMed] [Google Scholar]
- 102.Schaumburg F, Mugisha L, Peck B, Becker K, Gillespie TR, Peters G, Leendertz FH. Drug-resistant human Staphylococcus aureus in sanctuary apes pose a threat to endangered wild ape populations. Am J Primatol. 2012;74(12):1071–1075. doi: 10.1002/ajp.22067. [DOI] [PubMed] [Google Scholar]
- 103.Messenger AM, Barnes AN, Gray GC. Reverse zoonotic disease transmission (zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PLoS ONE. 2014;9(2):e89055. doi: 10.1371/journal.pone.0089055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Larsen J, Raisen CL, Ba X, Sadgrove NJ, Padilla-Gonzalez GF, Simmonds MSJ, Loncaric I, Kerschner H, Apfalter P, Hartl R, et al. Emergence of methicillin resistance predates the clinical use of antibiotics. Nature. 2022;602(7895):135–141. doi: 10.1038/s41586-021-04265-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saraiva MMS, de Leon C, Silva N, Raso TF, Serafini PP, Givisiez PEN, Gebreyes WA, Oliveira CJB. Staphylococcus sciuri as a reservoir of mecA to Staphylococcus aureus in non-migratory seabirds from a remote Oceanic island. Microb Drug Resist. 2021;27(4):553–561. doi: 10.1089/mdr.2020.0189. [DOI] [PubMed] [Google Scholar]
- 106.Onwugamba FC, Fitzgerald JR, Rochon K, Guardabassi L, Alabi A, Kühne S, Grobusch MP, Schaumburg F. The role of 'filth flies' in the spread of antimicrobial resistance. Travel Med Infect Dis. 2018;22:8–17. doi: 10.1016/j.tmaid.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Onwugamba FC, Mellmann A, Nwaugo VO, Süselbeck B, Schaumburg F. Antimicrobial resistant and enteropathogenic bacteria in 'filth flies': a cross-sectional study from Nigeria. Sci Rep. 2020;10(1):16990. doi: 10.1038/s41598-020-74112-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chandrakar C, Shakya S, Patyal A, Jain A, Ali SL, Mishra OP. ERIC-PCR-based molecular typing of multidrug-resistant Escherichia coli isolated from houseflies (Musca domestica) in the environment of milk and meat shops. Lett Appl Microbiol. 2022;75(6):1549–1558. doi: 10.1111/lam.13821. [DOI] [PubMed] [Google Scholar]
- 109.Fukuda A, Usui M, Okubo T, Tagaki C, Sukpanyatham N, Tamura Y. Co-harboring of cephalosporin (bla)/colistin (mcr) resistance genes among Enterobacteriaceae from flies in Thailand. FEMS Microbiol Lett. 2018; 365(16). [DOI] [PubMed]
- 110.Punyadi P, Thongngen P, Kiddee A, Assawatheptawee K, Tansawai U, Bunchu N, Niumsup PR. Prevalence of bla(CTX-M) and emergence of bla(CTX-M-5)-carrying Escherichia coli in Chrysomya megacephala (Diptera: Calliphoridae), Northern Thailand. Microb Drug Resist. 2021;27(5):698–705. doi: 10.1089/mdr.2020.0249. [DOI] [PubMed] [Google Scholar]
- 111.Tufa TB, Fuchs A, Wienemann T, Eggers Y, Abdissa S, Schneider M, Jensen BO, Bode JG, Pfeffer K, Haussinger D, et al. Carriage of ESBL-producing Gram-negative bacteria by flies captured in a hospital and its suburban surroundings in Ethiopia. Antimicrob Resist Infect Control. 2020;9(1):175. doi: 10.1186/s13756-020-00836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukuda A, Usui M, Okubo T, Tamura Y. Horizontal transfer of plasmid-mediated cephalosporin resistance genes in the intestine of houseflies (Musca domestica) Microb Drug Resist. 2016;22(4):336–341. doi: 10.1089/mdr.2015.0125. [DOI] [PubMed] [Google Scholar]
- 113.World Health Organization. Global Action Plan on Antimicrobial Resistance. WHO, Geneva, Switzerland, 2015. Available from: https://www.who.int/publications/i/item/9789241509763. Accessed 02 Jan 2023.
- 114.Tripartite AMR Country Self-Assessment Survey (TrACSS) 2020–2021. Global monitoring of country progress on addressing antimicrobial resistance. FAO, OIE and WHO, 16 March 2021. Available from https://www.who.int/publications/m/item/tripartite-amr-country-self-assessment-survey-(tracss)-2020-2021. Accessed 02 Jan 2023.
- 115.World Health Organization. Library of AMR national action plans. Available from https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans. Accessed 02 Jan 2023.
- 116.Food and Agriculture Organization. The International FAO Antimicrobial Resistance Monitoring (InFARM) System. Available from https://www.fao.org/antimicrobial-resistance/resources/database/infarm/en/. Accessed 02 Jan 2023.
- 117.Food and Agriculture Organization. FAO Assessment Tool for Laboratories and AMR Surveillance Systems (FAO-ATLASS). Available from https://www.fao.org/antimicrobial-resistance/resources/tools/fao-atlass/en/. Accessed 02 Jan 2023.
- 118.World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE). Monitoring and evaluation of the Global Action Plan on Antimicrobial Resistance. Framework and recommended indicators, 2019. Available from https://www.who.int/publications/i/item/monitoring-and-evaluation-of-the-global-action-plan-on-antimicrobial-resistance. Accessed 02 Jan 2023.
- 119.World Health Organization. WHO integrated global surveillance on ESBL-producing E. coli using a “One Health” approach: implementation and opportunities. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. Available from https://www.who.int/publications/i/item/who-integrated-global-surveillance-on-esbl-producing-e.-coli-using-a-one-health-approach. Accessed 02 Jan 2023.
- 120.World Health Organization. Invitation to participate in a survey on research questions for the development of a One Health Priority Research Agenda on Antimicrobial Resistance. Available from https://www.who.int/news-room/articles-detail/invitation-to-participate-in-a-survey-on-research-questions-for-the-development-of-a-one-health-priority-research-agenda-on-antimicrobial-resistance. Accessed 06 Jan 2023.
- 121.Joint Tripartite (FAO, OIE, WHO) and UNEP Statement. Tripartite and UNEP support OHHLEP's definition of "One Health". Joint news release, December 2021. Available from https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health. Accessed 13 Feb 2023.
- 122.World Health Organization News Release. Quadripartite launches a new platform to tackle antimicrobial resistance threat to human and animal health and ecosystems. November 2022. Available from https://www.who.int/news/item/18-11-2022-quadripartite-launches-a-new-platform-to-tackle-antimicrobial-resistance-threat-to-human-and-animal-health-and-ecosystems. Accessed 7 Feb 2023.
- 123.Hernando-Amado S, Coque TM, Baquero F, Martinez JL. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat Microbiol. 2019;4(9):1432–1442. doi: 10.1038/s41564-019-0503-9. [DOI] [PubMed] [Google Scholar]
- 124.Bak H, Rathkjen PH. Reduced use of antimicrobials after vaccination of pigs against porcine proliferative enteropathy in a Danish SPF herd. Acta Vet Scand. 2009;51(1):1. doi: 10.1186/1751-0147-51-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sommerset I, Krossoy B, Biering E, Frost P. Vaccines for fish in aquaculture. Expert Rev Vaccines. 2005;4(1):89–101. doi: 10.1586/14760584.4.1.89. [DOI] [PubMed] [Google Scholar]
- 126.Abayneh M, Tesfaw G, Woldemichael K, Yohannis M, Abdissa A. Assessment of extended-spectrum beta-lactamase (ESBLs) - producing Escherichia coli from minced meat of cattle and swab samples and hygienic status of meat retailer shops in Jimma town, Southwest Ethiopia. BMC Infect Dis. 2019;19(1):897. doi: 10.1186/s12879-019-4554-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dsani E, Afari EA, Danso-Appiah A, Kenu E, Kaburi BB, Egyir B. Antimicrobial resistance and molecular detection of extended spectrum beta-lactamase producing Escherichia coli isolates from raw meat in Greater Accra region, Ghana. BMC Microbiol. 2020;20(1):253. doi: 10.1186/s12866-020-01935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ojo OE, Schwarz S, Michael GB. Detection and characterization of extended-spectrum beta-lactamase-producing Escherichia coli from chicken production chains in Nigeria. Vet Microbiol. 2016;194:62–68. doi: 10.1016/j.vetmic.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 129.Jouini A, Ben Slama K, Saenz Y, Klibi N, Costa D, Vinue L, Zarazaga M, Boudabous A, Torres C. Detection of multiple-antimicrobial resistance and characterization of the implicated genes in Escherichia coli isolates from foods of animal origin in Tunis. J Food Prot. 2009;72(5):1082–1088. doi: 10.4315/0362-028X-72.5.1082. [DOI] [PubMed] [Google Scholar]
- 130.Soufi L, Abbassi MS, Saenz Y, Vinue L, Somalo S, Zarazaga M, Abbas A, Dbaya R, Khanfir L, Ben Hassen A, et al. Prevalence and diversity of integrons and associated resistance genes in Escherichia coli isolates from poultry meat in Tunisia. Foodborne Pathog Dis. 2009;6(9):1067–1073. doi: 10.1089/fpd.2009.0284. [DOI] [PubMed] [Google Scholar]
- 131.Aliyu AB, Saleha AA, Jalila A, Zunita Z. Risk factors and spatial distribution of extended spectrum beta-lactamase-producing- Escherichia coli at retail poultry meat markets in Malaysia: a cross-sectional study. BMC Public Health. 2016;16:699. doi: 10.1186/s12889-016-3377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shafiq M, Rahman SU, Bilal H, Ullah A, Noman SM, Zeng M, Yuan Y, Xie Q, Li X, Jiao X. Incidence and molecular characterization of ESBL-producing and colistin-resistant Escherichia coli isolates recovered from healthy food-producing animals in Pakistan. J Appl Microbiol. 2022;133(3):1169–1182. doi: 10.1111/jam.15469. [DOI] [PubMed] [Google Scholar]
- 133.Sornsenee P, Chimplee S, Arbubaker A, Kongchai S, Madimong H, Romyasamit C. Occurrence, antimicrobial resistance profile, and characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates from minced meat at local markets in Thailand. Foodborne Pathog Dis. 2022;19(3):232–240. doi: 10.1089/fpd.2021.0059. [DOI] [PubMed] [Google Scholar]
- 134.Gozi KS, Deus Ajude LPT, Barroso MDV, Silva CRD, Peiro JR, Mendes LCN, Nogueira MCL, Casella T. Potentially pathogenic multidrug-resistant Escherichia coli in lamb meat. Microb Drug Resist. 2021;27(8):1071–1078. doi: 10.1089/mdr.2020.0488. [DOI] [PubMed] [Google Scholar]
- 135.Mbehang Nguema PP, Onanga R, Ndong Atome GR, Obague Mbeang JC, Mabika Mabika A, Yaro M, Lounnas M, Dumont Y, Zohra ZF, Godreuil S, et al. Characterization of ESBL-producing Enterobacteria from fruit bats in an unprotected area of Makokou, Gabon. Microorganisms. 2020;8(1):138. doi: 10.3390/microorganisms8010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Obodoechi LO, Carvalho I, Chenouf NS, Martinez-Alvarez S, Sadi M, Nwanta JA, Chah KF, Torres C. Antimicrobial resistance in Escherichia coli isolates from frugivorous (Eidolon helvum) and insectivorous (Nycteris hispida) bats in Southeast Nigeria, with detection of CTX-M-15 producing isolates. Comp Immunol Microbiol Infect Dis. 2021;75:101613. doi: 10.1016/j.cimid.2021.101613. [DOI] [PubMed] [Google Scholar]
- 137.VinodhKumar OR, Karikalan M, Ilayaraja S, Sha AA, Singh BR, Sinha DK, Chandra Mohan S, Pruthvishree BS, Pawde AM, Sharma AK. Multi-drug resistant (MDR), extended spectrum beta-lactamase (ESBL) producing and carbapenem resistant Escherichia coli in rescued Sloth bears (Melursus ursinus) India Vet Res Commun. 2021;45(2–3):163–170. doi: 10.1007/s11259-021-09794-3. [DOI] [PubMed] [Google Scholar]
- 138.Bager SL, Kakaala I, Kudirkiene E, Byarugaba DK, Olsen JE. Genomic characterization of multidrug-resistant extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae from chimpanzees (pan troglodytes) from wild and sanctuary locations in Uganda. J Wildl Dis. 2022;58(2):269–278. doi: 10.7589/JWD-D-21-00068. [DOI] [PubMed] [Google Scholar]
- 139.Albrechtova K, Papousek I, De Nys H, Pauly M, Anoh E, Mossoun A, Dolejska M, Masarikova M, Metzger S, Couacy-Hymann E, et al. Low rates of antimicrobial-resistant Enterobacteriaceae in wildlife in Tai National Park, Cote d'Ivoire, surrounded by villages with high prevalence of multiresistant ESBL-producing Escherichia coli in people and domestic animals. PLoS ONE. 2014;9(12):e113548. doi: 10.1371/journal.pone.0113548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fuentes-Castillo D, Esposito F, Cardoso B, Dalazen G, Moura Q, Fuga B, Fontana H, Cerdeira L, Dropa M, Rottmann J, et al. Genomic data reveal international lineages of critical priority Escherichia coli harbouring wide resistome in Andean condors (Vultur gryphus Linnaeus, 1758) Mol Ecol. 2020;29(10):1919–1935. doi: 10.1111/mec.15455. [DOI] [PubMed] [Google Scholar]
- 141.Janatova M, Albrechtova K, Petrzelkova KJ, Dolejska M, Papousek I, Masarikova M, Cizek A, Todd A, Shutt K, Kalousova B, et al. Antimicrobial-resistant Enterobacteriaceae from humans and wildlife in Dzanga-Sangha Protected Area, Central African Republic. Vet Microbiol. 2014;171(3–4):422–431. doi: 10.1016/j.vetmic.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 142.Hasan B, Melhus A, Sandegren L, Alam M, Olsen B. The gull (Chroicocephalus brunnicephalus) as an environmental bioindicator and reservoir for antibiotic resistance on the coastlines of the Bay of Bengal. Microb Drug Resist. 2014;20(5):466–471. doi: 10.1089/mdr.2013.0233. [DOI] [PubMed] [Google Scholar]
- 143.Bachiri T, Bakour S, Ladjouzi R, Thongpan L, Rolain JM, Touati A. High rates of CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae in wild boars and Barbary macaques in Algeria. J Glob Antimicrob Resist. 2017;8:35–40. doi: 10.1016/j.jgar.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 144.Fuentes-Castillo D, Farfan-Lopez M, Esposito F, Moura Q, Fernandes MR, Lopes R, Cardoso B, Munoz ME, Cerdeira L, Najle I, et al. Wild owls colonized by international clones of extended-spectrum beta-lactamase (CTX-M)-producing Escherichia coli and Salmonella Infantis in the Southern Cone of America. Sci Total Environ. 2019;674:554–562. doi: 10.1016/j.scitotenv.2019.04.149. [DOI] [PubMed] [Google Scholar]
- 145.Azimi T, Azimi L, Fallah F, Pourmand MR, Ostadtaghizadeh A, Abai MR, Rahimi Foroushani A. Detection and characterization of Enterobacteriaceae family members carried by commensal Rattus norvegicus from Tehran, Iran. Arch Microbiol. 2021;203(4):1321–1334. doi: 10.1007/s00203-020-02126-0. [DOI] [PubMed] [Google Scholar]
- 146.Bamunusinghage NPD, Neelawala RG, Magedara HP, Ekanayaka NW, Kalupahana RS, Silva-Fletcher A, Kottawatta SA. Antimicrobial resistance patterns of fecal Escherichia coli in wildlife, urban wildlife, and livestock in the Eastern Region of Sri Lanka, and differences between carnivores, omnivores, and herbivores. J Wildl Dis. 2022;58(2):380–383. doi: 10.7589/JWD-D-21-00048. [DOI] [PubMed] [Google Scholar]
- 147.Guenther S, Aschenbrenner K, Stamm I, Bethe A, Semmler T, Stubbe A, Stubbe M, Batsajkhan N, Glupczynski Y, Wieler LH, et al. Comparable high rates of extended-spectrum-beta-lactamase-producing Escherichia coli in birds of prey from Germany and Mongolia. PLoS ONE. 2012;7(12):e53039. doi: 10.1371/journal.pone.0053039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schaumburg F, Mugisha L, Kappeller P, Fichtel C, Köck R, Kondgen S, Becker K, Boesch C, Peters G, Leendertz F. Evaluation of non-invasive biological samples to monitor Staphylococcus aureus colonization in great apes and lemurs. PLoS ONE. 2013;8(10):e78046. doi: 10.1371/journal.pone.0078046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Santana JA, Colombo SA, Silva BA, Diniz AN, de Almeida LR, Oliveira Junior CA, Lobato FCF, de Souza TG, Paglia AP, Silva ROS. Clostridioides difficile and multi-drug-resistant staphylococci in free-living rodents and marsupials in parks of Belo Horizonte, Brazil. Braz J Microbiol. 2022;53(1):401–410. doi: 10.1007/s42770-021-00640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schaumburg F, Alabi AS, Köck R, Mellmann A, Kremsner PG, Boesch C, Becker K, Leendertz FH, Peters G. Highly divergent Staphylococcus aureus isolates from African non-human primates. Environ Microbiol Rep. 2012;4(1):141–146. doi: 10.1111/j.1758-2229.2011.00316.x. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y, Yang Y, Zhao F, Fan X, Zhong W, Qiao D, Cao Y. Multi-drug resistant Gram-negative enteric bacteria isolated from flies at Chengdu Airport, China. Southeast Asian J Trop Med Public Health. 2013;44(6):988–996. [PubMed] [Google Scholar]
- 152.Thamlikitkul V, Tiengrim S, Thamthaweechok N, Buranapakdee P, Chiemchaisri W. Contamination by antibiotic-resistant bacteria in selected environments in Thailand. Int J Environ Res Public Health. 2019;16(19):3753. doi: 10.3390/ijerph16193753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Songe MM, Hangombe BM, Knight-Jones TJ, Grace D. Antimicrobial Resistant Enteropathogenic Escherichia coli and Salmonella spp. in houseflies Infesting fish in food markets in Zambia. Int J Environ Res Public Health. 2016;14(1):21. doi: 10.3390/ijerph14010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sobur MA, Islam MS, Haque ZF, Orubu ESF, Toniolo A, Choudhury MA, Rahman MT. Higher seasonal temperature enhances the occurrence of methicillin resistance of Staphylococcus aureus in house flies (Musca domestica) under hospital and environmental settings. Folia Microbiol (Praha) 2022;67(1):109–119. doi: 10.1007/s12223-021-00922-9. [DOI] [PubMed] [Google Scholar]
- 155.Seetswane E, Loeto D, Muzila M, Tshekiso K, Gomba A, Baruti K, Jongman M. Phenotypic and genotypic profiling reveals a high prevalence of methicillin-resistant Staphylococcusaureus isolated from hospitals, houseflies and adjacent informal food retailers in Botswana. Microbiology (Reading). 2022; 168 (10). [DOI] [PubMed]
- 156.Rahuma N, Ghenghesh KS, Ben Aissa R, Elamaari A. Carriage by the housefly (Musca domestica) of multiple-antibiotic-resistant bacteria that are potentially pathogenic to humans, in hospital and other urban environments in Misurata, Libya. Ann Trop Med Parasitol. 2005;99(8):795–802. doi: 10.1179/136485905X65134. [DOI] [PubMed] [Google Scholar]
- 157.Furuya-Kanamori L, Stone J, Yakob L, Kirk M, Collignon P, Mills DJ, Lau CL. Risk factors for acquisition of multidrug-resistant Enterobacterales among international travellers: a synthesis of cumulative evidence. J Travel Med. 2020; 27(1). [DOI] [PubMed]
- 158.Rambliere L, Guillemot D, Delarocque-Astagneau E, Huynh BT. Impact of mass and systematic antibiotic administration on antibiotic resistance in low- and middle-income countries? A systematic review. Int J Antimicrob Agents. 2021;58(1):106364. doi: 10.1016/j.ijantimicag.2021.106364. [DOI] [PubMed] [Google Scholar]
- 159.Ozawa S, Evans DR, Bessias S, Haynie DG, Yemeke TT, Laing SK, Herrington JE. Prevalence and estimated economic burden of substandard and falsified medicines in low- and middle-income countries: a systematic review and meta-analysi. JAMA Netw Open. 2018;1(4):e181662. doi: 10.1001/jamanetworkopen.2018.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zaatout N, Bouras S, Slimani N. Prevalence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae in wastewater: a systematic review and meta-analysis. J Water Health. 2021;19(5):705–723. doi: 10.2166/wh.2021.112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.