Abstract
The transplantation of organs across species offers the potential to solve the shortage of human organs. While activation of human platelets by human von Willebrand factor (vWF) requires vWF activation by shear stress, contact between human platelets and porcine vWF (pvWF) leads to spontaneous platelet adhesion and activation. This non-physiologic interaction may contribute to the thrombocytopenia and coagulation pathway dysregulation often associated with xenotransplantation of pig organs in nonhuman primates. Pigs genetically modified to decrease antibody and complement-dependent rejection (GTKO.hCD46) were engineered to express humanized pvWF (h*pvWF) by replacing a pvWF gene region that encodes the glycoprotein Ib-binding site with human cDNA orthologs. This modification corrected for non-physiologic human platelet aggregation on exposure to pig plasma, while preserving in vitro platelet activation by collagen. Organs from pigs with h*pvWF demonstrated reduced platelet sequestration during lung (p≤ .01) and liver (p≤ .038 within 4 h) perfusion ex vivo with human blood and after pig-to-baboon lung transplantation (p≤ .007). Residual platelet sequestration and activation were not prevented by the blockade of canonical platelet adhesion pathways. The h*pvWF modification prevents physiologically inappropriate activation of human or baboon platelets by porcine vWF, addressing one cause of the thrombocytopenia and platelet activation observed with xenotransplantation.
Keywords: genetic engineering, lung transplantation, pig, platelets, von Willebrand factor
1 |. INTRODUCTION
If scientific barriers can be surmounted, xenotransplantation utilizing pigs would provide the unique opportunity to develop a safe and readily available supply of organs, allowing treatment of many of the greater than 111 000 people currently on United Network for Organ Sharing (UNOS) waitlists in the United States alone.1 Genetic modifications have effectively reduced antibody-, complement-, and coagulation-mediated mechanisms of pig organ xenograft injury.2–6 However, these modifications fail to attenuate thrombocytopenia during the first days following transplant in pig-to-primate xenotransplantation models,7–13 and platelet deposition is prominent within failing cell and organ xenografts.3,14–17 Activation and aggregation of human platelets are particularly prominent within minutes after lung or liver perfusion with human blood or engraftment in non-human primates and are mediated in part by non-physiologic interaction between porcine von Willebrand factor (pvWF) and human or baboon platelet glycoprotein Ib (GPIb).16,18–21
vWF is stored in the Weibel–Palade body of endothelial cells (ECs) and the α-granules of megakaryocytes and platelets. The mature functional vWF comprises a series of multimers with molecular weight from 500 kDa (dimers) to over 10 × 106 kDa.22 Large multimers of vWF, present in ECs, subendothelium, and platelets, efficiently promote platelet adhesion and aggregation through binding to GPIb and GPIIbIIIa.22 When the endothelium is activated, vWF is expressed on ECs and released into the circulating blood, initiating platelet adhesion to ECs and to other platelets thereby leading to thrombus formation.23 Human vWF (hvWF) must undergo a shear stress-dependent activation step to bind to the human platelet GPIb receptor. In contrast, native pvWF interacts with human GPIb independently of a prerequisite shear stress-dependent conformational change.24,25
We have previously shown that blocking the human GPIb-binding site for hvWF attenuates platelet activation and sequestration during perfusion of GTKO.hCD46 pig (also referred to as “pvWF”) lungs and livers with human blood, implicating the pvWF-GPIb interaction.19–21 However, this approach blocks both physiologic and non-physiologic pig–human interactions and may not inhibit pvWF-mediated interactions topologically remote from the binding site of the anti-GPIb Fab used in those studies. We developed a CRISPR-Cas9-based strategy for genetic editing of the pig vWF gene to provide “humanization” of pvWF (h*pvWF) in α1,3 galactosyltransferase knockout cells expressing human CD46 (GTKO.hCD46). Here, we evaluate the effects associated with h*pvWF with respect to physiologically inappropriate human platelet aggregation by pig plasma and various pathophysiologic features associated with lung and liver xenograft injury in organ transplant models.
2 |. MATERIAL AND METHODS
2.1 |. In vitro
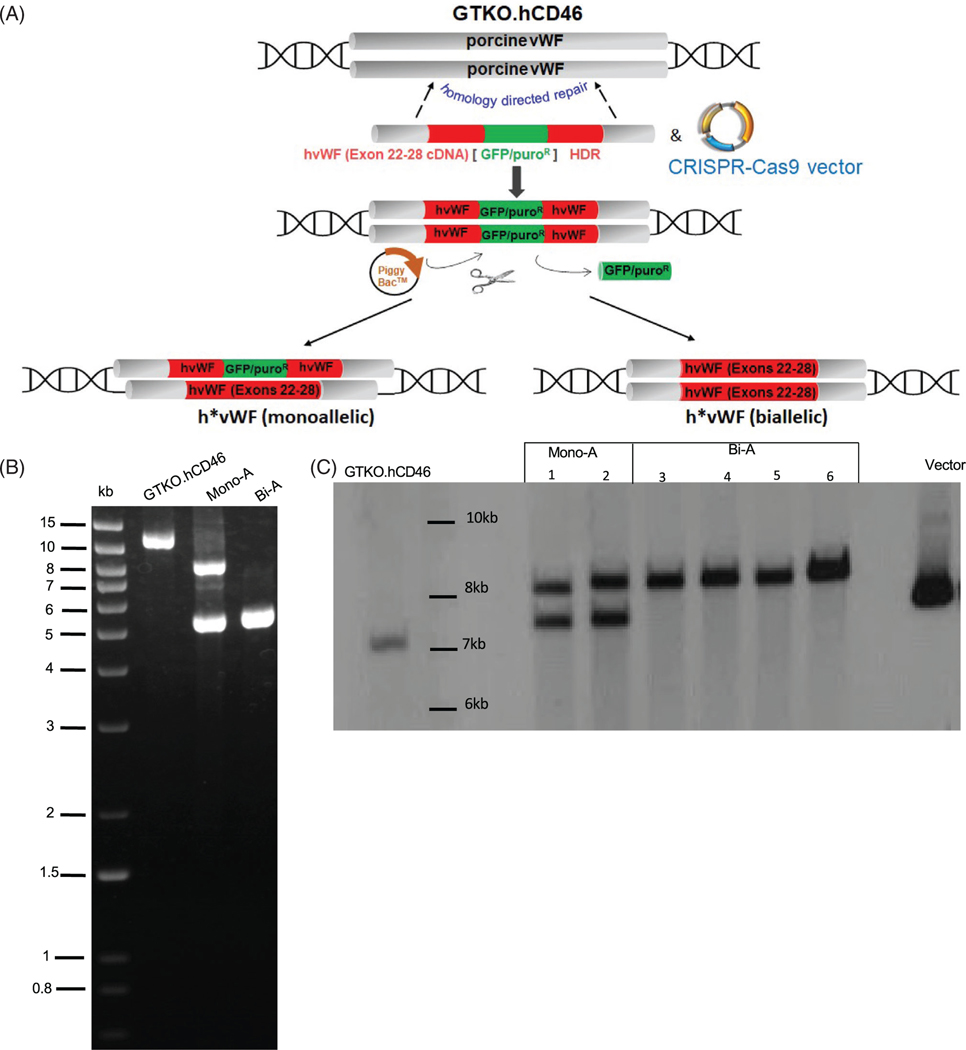
Pigs with knockout of α1,3 galactosyltransferasegene (GGTA1; GTKO) and expressing human CD46 (hCD46), or GTKO.hCD46, were used in all experiments.14,26 To create h*pvWF pigs, a targeting vector with a cDNA fragment including hvWF exons 22–28 replaced pvWF exons 22–28 via homology-directed repair in porcine fetal fibroblasts. Screening for proper targeting at the colony level was performed by polymerase chain reaction (PCR). Cells used for somatic cell nuclear transfer (SCNT) to generate fetuses, and fibroblasts from these were transfected with a piggyBac transposase vector to excise the green fluorescent protein (GFP)/puromycin cassette and restore in-frame expression of the hvWF cDNA.27 A second round of SCNT was done using single-cell clones with the correct vWF edit (without random integrations) to yield pigs carrying the “humanized” vWF knock-in sequence as identified by long-range PCR and confirmed by Southern blot analysis.
vWF was detected in platelet-poor plasma (PPP) from GTKO.hCD46.h*pvWF pigs, GTKO.hCD46 pigs, and humans via simple Western (WES), with capillaries probed with either human-specific anti-vWF antibody or antibody detecting both human and pig vWF. vWF multimers were visualized on sodium dodecyl sulfate (SDS) agarose gels.15 Plasma levels of hvWF, h*pvWF, and pvWF were measured by enzyme-linked immunosorbent assay (ELISA). The REAADS vWF Antigen Test kit detected both hvWF and pvWF, whereas the REAADS vWF Activity Test utilized a monoclonal antibody specific for the hvWF A1 domain that did not cross-react with pvWF.
vWF-dependent platelet function was assessed by exposing whole blood from human donors, h*pvWF pigs, and GTKO.hCD46 pigs to collagen and measuring aggregation by whole blood aggregometry.15 Human platelet aggregation was assessed following platelet-rich plasma (PRP) mixture with PPP from humans, h*pvWF pigs, or GTKO.hCD46 pigs.
Factor VIII activity of whole blood from h*pvWF and GTKO.hCD46 pigs was measured using human plasma standards and controls (Antech Laboratories).
Tissue samples collected from GTKO.hCD46 and h*pvWF pigs were incubated with either human-specific or human/pig cross-reactive anti-vWF antibody for immunohistochemistry (IHC) using commercially available sections of the human lung as a reference.
2.2 |. Animal care and use
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland, Baltimore, and were conducted in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.3 |. Ex vivo lung and liver perfusions
Organ recovery was preceded by the routine administration of antihistamines, thromboxane synthase inhibitor, and 1-deamino-8-Dargenine vasopressin (DDAVP, to pre-deplete the porcine endothelium of vWF)28 to the pig, followed by organ perfusion with clinical cold preservation solutions and topical cooling with saline slush.18,21,29–32
Each lung was separately ventilated and perfused with freshly donated pooled human blood for up to 8 h on side-by-side ex vivo circuits as previously described.19,30 In each pair of lungs, one lung received a standard “base regimen” (n = 5 h*pvWF, n = 5 reference), including 1-BIA (thromboxane synthase inhibitor), anti-GPIb Fab (6B4, blocks human GPIb-binding site for vWF), and histamine receptor blockade; while the contralateral lung was “treated” with the base regimen and additional integrin (H52/IB4) and selectin (GMI-1271, PSGL-1) blocking cocktail (n = 5 h*pvWF, n = 5 reference).19,33 Pulmonary artery (PA) flow and PA and airway pressures were used to calculate pulmonary vascular resistance (PVR).14 Blood samples were collected from each lung circuit before perfusion and from pulmonary vein effluent at regular intervals throughout perfusion. Platelets were quantified by flow cytometry as previously described.14
The liver was perfused with human blood on an ex vivo circuit as previously described.32 All experiments received a “base regimen” of remodulin (treprostinil), heparin, and insulin. Additional treatment with famotidine, BIA, and anti-GPIb were given to the “treated” groups (n = 7 h*pvWF, n = 4 reference). Experiments were electively terminated after 12 h if livers had not failed earlier due to rapidly escalating hepatic arterial and/or portal venous vascular resistance.
2.4 |. In vivo lung transplantation
GTKO.hCD46.h*pvWF left lungs were transplanted into baboon recipients (n = 3 h*pvWF) as previously described.10,30 Genetic reference group (GTKO.hCD46.pvWF: n = 5) were performed earlier in the lab’s experience, and recipients, therefore, received a different drug regimen than the GTKO.hCD46.h*pvWF transplants. Recipients of lungs from pigs with additional genetic modifications presumed not to directly modulate platelet adhesion (GTKO.hCD46.A20, GTKO.hCD46.HLA-E, and GTKO.hCD46.HLA-E.β4GalKO: n = 5) comprised the regimen reference group, which received anti-histamines, a thromboxane synthase inhibitor, and some or all of the anti-inflammatory (PSGL/GMI1271, +/− Interleukin 8 (IL8) receptor antagonist, IL6 receptor antagonist, tumor necrosis factor inhibitor, C1 esterase inhibitor, cobra venom factor) and immunosuppressive (prednisolone, anti-thymocyte globulin, αCD40, αCD20, mycophenolate mofetil) treatments given to the GTKO.hCD46.h*pvWF lung recipients and to the group listed in Table S1).30,33–38
After transplantation, the baboon remained intubated and anesthetized overnight and was evaluated with chest x-ray and bronchoscopy the following morning. If clinically stable, the baboon was extubated and vital signs and behavior were monitored closely. X-ray and bronchoscopy surveillance were performed every 2 to 3 days. When the baboon developed concerning behavioral or clinical changes for graft failure, the transplanted and native lungs were evaluated in the operating room prior to euthanasia, followed by macroscopic assessment and histological analyses.
2.5 |. Statistical analyses
Statistical analyses were performed using Prism 8.0. Survival was represented using the Kaplan–Meier curve, and statistical significance was calculated using log-rank analysis. Unless otherwise stated, all other data were expressed as mean and standard error of the mean within study groups. Variables that were normally distributed were assessed with Student’s t-tests to compare two groups. Variables that were not normally distributed were assessed with Mann–Whitney tests. Variables compared across multiple conditions (such as change in platelets for treated and untreated conditions) were analyzed with two-way analysis of variance with multiple comparisons, with Geisser– Greenhouse correction as indicated. ps < .05 were considered statistically significant. Extended methods are described in the Supplemental data.
3 |. RESULTS
3.1 |. Generation of humanized vWF pigs
“Humanization” of GPIb platelet-binding domain in pvWF was done by replacing porcine exons 22–28 with their human counterparts and was confirmed in porcine fibroblasts (Figure 1). Selected cells were used for SCNT, and ET produced a total of 27 pigs, 20 (74%) harboring bi-allelically (Bi-A) targeted, marker-excised h*vWF; in seven others (26%), the targeting fragment was bi-allelic, but the marker excised from only one allele (Mono-A) as demonstrated by long-range PCR and illustrated by representative Southern blots (Figure 1B,C). Further analyses were done on pigs with mono-allelic or bi-allelic modification of h*vWF. Genotypes of pigs used in this study are described in the supplemental material (Table S1).
FIGURE 1.
Generation of humanized pig von Willebrand factor (vWF) by homology directed repair. (A) GTKO.CD46 porcine fetal fibroblasts were transfected with a targeting vector containing cDNA of human vWF (hvWF) exons 22–28 and a transposase-excisable GFP/PuroR selectable marker and plasmids expressing target-specific CRISPR-Cas9. Single cell clones of targeted fibroblasts were subjected to somatic cell nuclear transfer (SCNT), followed by embryo transfer to recipient pigs from which fetuses were collected on gestation Day 32. Fibroblasts from these fetuses were transfected with transposase-expressing plasmids to excise the selectable marker and subjected to a second round of SCNT to generate pigs. (B) Long-range polymerase chain reaction (PCR) on genomic DNA of GTKO.hCD46 and vWF-edited pigs. Primers are located just outside of the 5′ and 3′ homology arm sequences. The wild type (WT) allele produces a band of 11.7 kb, while the monoallelic (Mono-A) modification produces two bands, one of 8.2 kb, which contains the “humanization of pvWF (h*pvWF) insert plus the unexcised GFP/PuroR selectable marker, and another of 5.3 kb produced by the allele in which the marker was excised. The bi-allelic modification (Bi-A) produces a single band of 5.3 kb. This was sequenced to confirm precise integration of hvWF exons 22–28 in pvWF and precise excision of the selectable marker. (C) Southern blot was performed by digesting genomic DNA with HindIII/Psil restriction enzymes and using a DIG-labeled hvWF probe (exon 24–28, with partial sequence homology to pig vWF). pvWF generates a single band of 7 kb; Mono-A generates two bands of 7.5 kb and 8.4 kb, representing alleles in which the GFP/PuroR marker was unexcised or excised, respectively; and Bi-A generates a single band of 8.4 kb. Targeting vector serves as a positive control
Ten of 18 h*pvWF piglets that reached transplant age (2–4 months) exhibited a phenotype prone to abnormal bleeding after routine procedures such as ear tattoo and tail sampling. Two of these animals exhibited chronic low volume epistaxis or melena consistent with gastrointestinal blood loss. Stress-induced bleeding in over half of h*pvWF pigs was empirically treated with oral desmopressin (100–200 μg daily) and vitamin K2 (0.5–1.0 mg daily) in an effort to prevent hemorrhagic complications.
3.2 |. Molecular characterization of h*pvWF protein expression
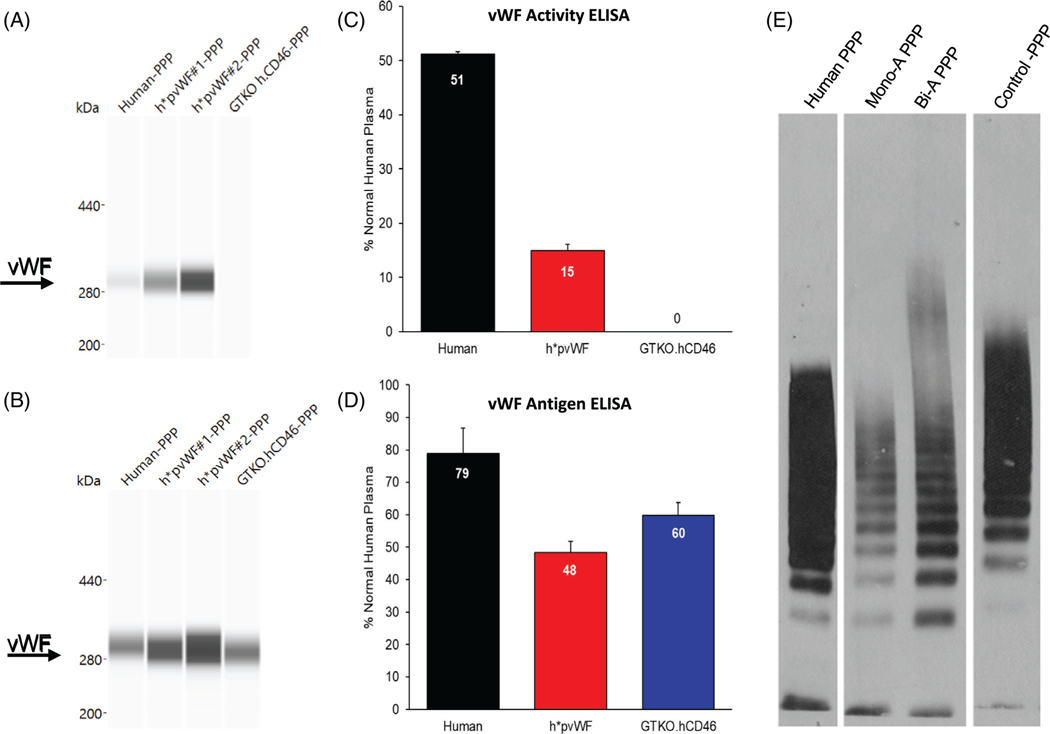
A human-specific monoclonal antibody detected hvWF and h*pvWF but not pvWF, in PPP by Western blot (Figure 2A), whereas a polyclonal antibody recognized all three forms of vWF protein (Figure 2B). The molecular mass of h*pvWF was similar to hvWF and pvWF at just over 280 kDa (Figure 2A,B). In addition, each species of vWF formed multimers with similar “laddering” patterns (Figure 2E). Together, these data indicated that the human exon 22–28 cDNA fragment was expressed in-frame with pvWF to encode a full-length human-porcine chimeric protein capable of forming large multimers, a functionally important characteristic of vWF in both pig and human.39,40
FIGURE 2.
vWF protein expression by Western blot and ELISA in plasma. Capillary Western blots were run on the WES system using platelet-poor plasma (PPP). (A) Monoclonal antibody specific to hvWF glycoprotein Ib (GPIb)-binding domain detected hvWF and h*pvWF but not pvWF from GTKO.hCD46 pigs. (B) Polyclonal antibody to full-length hvWF detected hvWF, h*pvWF, and GTKO.hCD46 porcine vWF (pvWF). vWF activity and antigen were assayed on PPP using two hvWF ELISA kits. Each assay included plasma from three h*pvWF pigs. Human (n = 1) and GTKO.hCD46 pig (n = 1) samples were included as controls. Samples were run twice in duplicate. Mean and SD assay results are shown. (C) The vWF activity ELISA measures vWF using a monoclonal antibody specific to the hvWF GPIb-binding domain. Measurable vWF activity was found in plasma from human and h*pvWF pigs but not from control GTKO.hCD46 pigs. vWF activity in both human and h*pvWF PPP was in the normal human range for the assay (1.7%–57.98%; mean 26.5%). (D) The vWF antigen ELISA measures hvWF using an antibody that cross-reacts with pvWF. Measurable vWF antigen was found in plasma from human, h*pvWF pigs, and GTKO.hCD46 pigs. vWF antigen levels in human, h*pvWF, and GTKO.hCD46 pigs was within the normal human range for the assay (47%–197%; mean 106%). (E) vWF multimer patterns in PPP. Membranes were probed with hvWF antibody. vWF from human, h*pvWF, and control GTKO.hCD46 pigs were capable of forming multimers in this assay
3.3 |. h*pvWF is secreted and functional
The human-specific vWF activity test detected vWF in human and h*pvWF plasma but not in plasma from GTKO.hCD46 pigs (Figure 2C), while the non-species-specific vWF antigen test produced a positive result for all samples tested (Figure 2D). Although vWF activity and antigen quantity in h*pvWF plasma was lower than in normal human plasma by both assays, h*pvWF results were within the kits’ normal ranges for human blood. Similarly, plasma FVIII activity was detected in h*pvWF plasma, at levels generally in the range of that seen with GTKO.hCD46, demonstrating absence of hemophilia A phenotype (Figure S1).
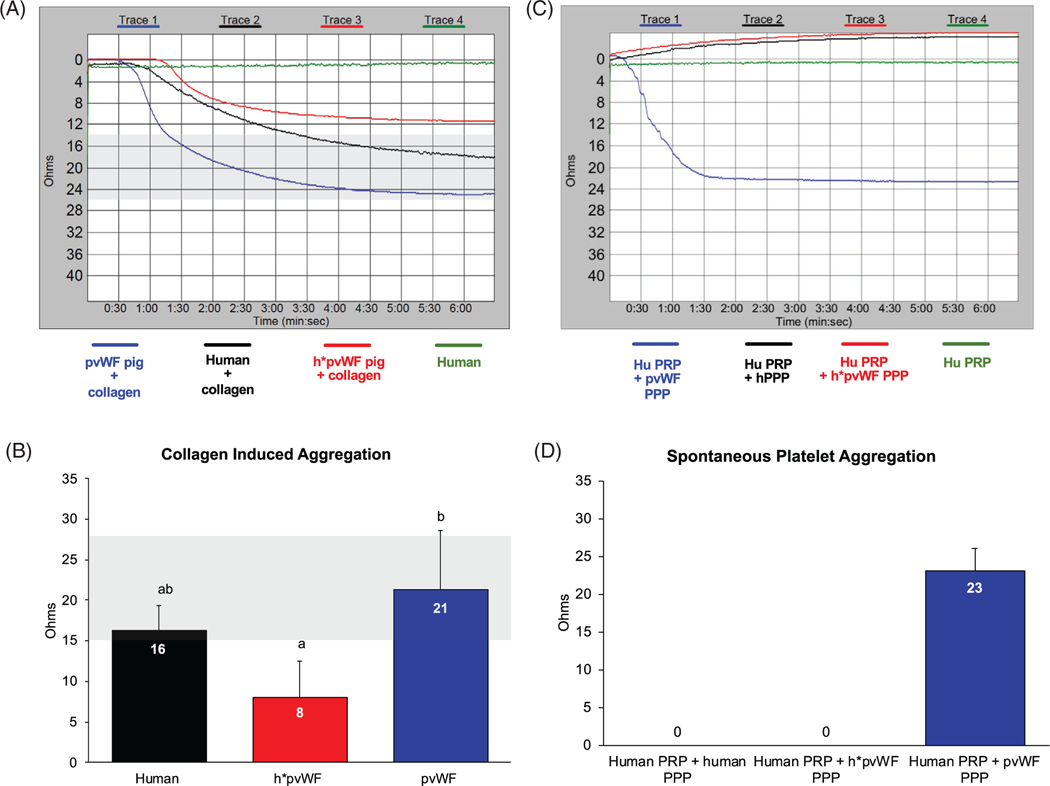
By whole blood aggregometry, collagen-induced platelet aggregation in blood from human donors, h*pvWF pigs, and pvWF pigs each aggregated in response to collagen, indicating that h*pvWF is functional (Figure 3A,B). h*pvWF aggregation did not differ from human blood but was lower than in porcine blood from pvWF pigs (p < .001).
FIGURE 3.
Collagen-induced platelet aggregation. Fresh-drawn, citrated whole blood from human (n = 2), h*pvWF pigs (n = 13) and GTKO.hCD46 pigs (n = 6) was tested using a Chrono-log Whole Blood Aggregometer. (A) Collagen (2 mg/ml) was utilized as an agonist to induce platelet aggregation. Gray-shaded area represents normal range for collagen-induced platelet aggregation in humans (15–27 Ohms). (B) Blood from h*pvWF pigs was similar to human blood in its functional ability to stimulate platelet aggregation (a;ab p = .14). Platelet aggregation was lower in blood from h*pvWF versus control pvWF (a;b p < .001). Mean results were compared using one-way ANOVA and Tukey’s multiple comparison test. Spontaneous platelet aggregation: Human platelet-rich plasma (hPRP) was prepared from blood of a healthy donor. PPP was prepared from h*pvWF and GTKO.hCD46 pigs. (C) hPRP was mixed with human/porcine PPP (1:1) and platelet aggregation was monitored without adding an agonist. (D) PPP from h*pvWF pigs and human did not trigger platelet aggregation whereas PPP from GTKO.hCD46 pigs triggered platelet aggregation within 1–2 s. Representative tracing from one experiment shown using a bi-allelic h*pvWF pig (A & C), while histogram bars represent mean and SD of both mono- and bi-allelic pigs
3.4 |. h*pvWF prevents spontaneous platelet aggregation by pig plasma
In an in vitro platelet aggregation assay, neither human PRP (hPRP) nor human PPP induced spontaneous aggregation of human platelets, whereas porcine PPP (pPPP) caused robust “non-physiologic” spontaneous platelet aggregation (Figure 3C,D) as shown previously.19 In contrast, platelet aggregation was not observed in hPRP upon addition of pPPP from an h*pvWF pig. Together, these data demonstrate that h*pvWF curtails dysregulated, physiologically inappropriate (in the absence of shear stress) platelet aggregation of human platelets by pvWF in pig plasma.
3.5 |. Tissue expression and localization of h*pvWF by IHC and Western blot
Representative h*pvWF pig lung and liver tissue samples obtained before organ perfusion or transplant confirm the h*pvWF expression by IHC and Western blot using the human-specific monoclonal anti-vWF antibody (Figure S2B). The pattern of h*pvWF distribution primarily in endothelium was indistinguishable from that observed with the cross-reactive polyclonal antibody in h*pvWF or pvWF pig organs (Figure S2A) or in human lung (Figure S2D), indicating that expression of the h*pvWF protein was not significantly altered by the h*pvWF modification. Western blot with the human-specific antibody detected vWF at the expected molecular weight in both lung and liver tissue lysates from h*pvWF pigs but not in tissues from pvWF pigs (Figure S2E,F).
3.6 |. Ex vivo perfusion of h*pvWF pig lungs with human blood
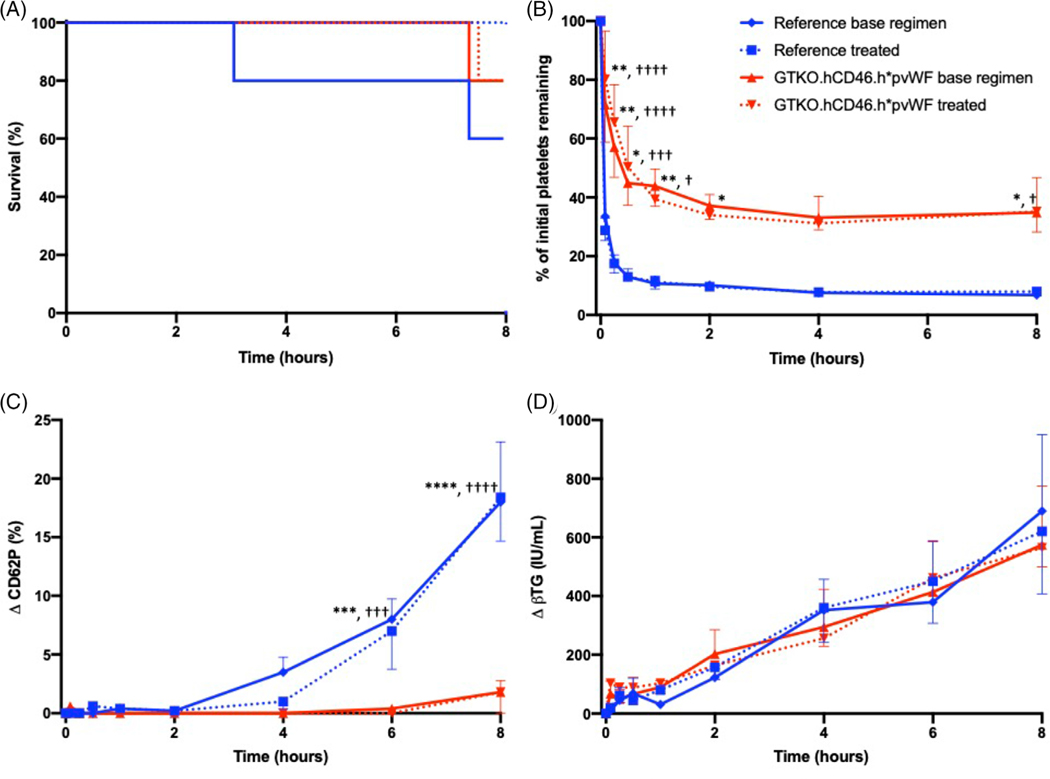
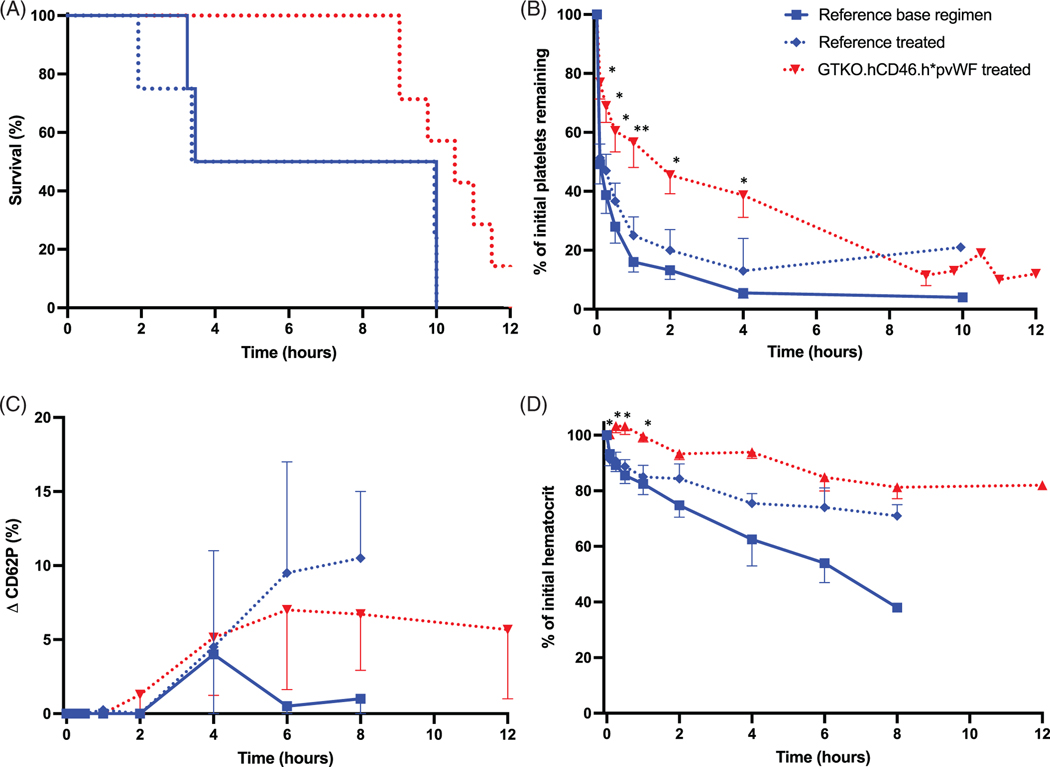
Lungs from GTKO.hCD46.h*pvWF pigs (n = 10) exhibited similar duration of organ function compatible with supporting the life of a lung recipient (survival), compared to GTKO.hCD46 lungs (n = 10) during ex vivo perfusion with human blood (median > 8 h) (p = .44), with most experiments reaching the time of elective termination (Figure 4A). PVR remained very low and constant through the duration of perfusion in both cohorts (Figure S3), suggesting h*pvWF has no adverse effect on lung xenograft physiology.
FIGURE 4.
Ex vivo perfusion of pig lungs. Humanized vWF (h*pvWF, red) were compared to reference lungs with pvWF (blue), with base regimen (solid; n = 5 h*pvWF, n = 5 pvWF), or additional treatment (dashed; n = 5 h*pvWF, n = 5 pvWF). (A) Survival was similar for both groups regardless of treatment, with most experiments reaching elective termination at 480 min (p = .44). (B) There was significant improvement in platelet sequestration in the humanized vWF lungs, compared to reference lungs with pvWF, regardless of treatment (p ≤ .01). The reduction in platelet sequestration in the h*pvWF lungs was both rapidly significant (5 min) and sustained (8 h), regardless of treatment. (C) Platelet activation as demonstrated by platelet expression of P-selectin (CD62P) was significantly attenuated in human blood perfusing h*pvWF lungs, compared to pvWF lungs at 6 h, regardless of treatment (p < .001). (D) There was no difference in rise in plasma beta thromboglobulin levels (βTG), regardless of genetics or treatment. Error bars represent standard error of the mean.
* base regimen, † treated *p < .05, **p < .01, ***p < .001, ****p < .0001
Platelet sequestration was significantly reduced and delayed in lungs expressing h*pvWF, compared to those with pvWF (p ≤ .01) (Figure 4B), but was not further modulated by additional selectin (GMI 1260; PSGL-1) and integrin (H52; IB4) receptor blockade (treated) relative to a reference anti-inflammatory drug treatment regimen derived from our prior work (BIA, famotidine, GPIb: “base regimen”).19 The reduction in platelet sequestration was most significant within the first 5 min of xenoperfusion (5 min: base regimen p = .0017, drug-treated p < .0001) and persisted throughout the duration of the experiment (8 h: base regimen p = .0348, drug-treated p = .0279; Figure 4B). Expression of CD62P by platelets was also significantly attenuated in the h*pvWF lungs beginning at 6 h of perfusion for both treated and base regimen groups (p < .001; Figure 4C). Platelet sequestration and activation (as CD62P, Figure 4C, and βTG, Figure 4D) were not significantly modulated by additional administration of selectin- and integrin–blocking molecules (“treated” groups), implicating alternative adhesive mechanisms. No significant difference was observed in association with lung phenotype or drug treatment groups for neutrophil counts (Figure S4A), or F1 + 2 levels (Figure S4B).
3.7 |. Ex vivo perfusion of h*pvWF pig livers with human blood
During ex vivo perfusion, “survival” (vascular resistance compatible with liver perfusion) of h*pvWF livers (n = 7) treated with famotidine, BIA, and anti-GPIb was similar to GTKO.hCD46 livers both with (reference “treated” group, n = 4) and without (reference “base regimen” group, n = 4; Figure 5A).41 Platelet sequestration was significantly attenuated with treated h*pvWF livers, compared to the treated reference group through the first 4 h of perfusion (p ≤ .0378; Figure 5B), although activation of circulating platelets (CD62P, Figure 5C) was not modulated. The decline in circuit hematocrit, reflecting RBC damage and sialoadhesin-mediated endocytosis by Kupffer cells, was significantly delayed for the first 2 h in h*pvWF livers (p = .0166) (Figure 5D), indirectly implicating pvWF-GPIb interactions in this phenomenon.42
FIGURE 5.
Ex vivo perfusion of pig livers. Humanized vWF (red), compared to reference livers with pvWF (blue), with base regimen (solid; n = 4 pvWF), or additional treatment (dashed; n = 7 h*pvWF, n = 4 pvWF). (A) Survival was similar for all groups (p = .12). (B) There was significant improvement in platelet sequestration in the treated humanized vWF livers, compared to reference livers with pvWF for the first 4 h (p < .04). The reduction in platelet sequestration in the h*pvWF livers was rapidly significant (5 min). (C) There was no significant difference in the CD62P rise on human platelets. (D) h*pvWF livers demonstrated initial protection from drop in hematocrit (p < .02 in first 2 h), but this effect did not persist for the duration of the experiment. Error bars represent standard error of the mean. *p < .05, **p < .01 versus treated
3.8 |. In vivo orthotopic h*pvWF pig lung xenotransplants
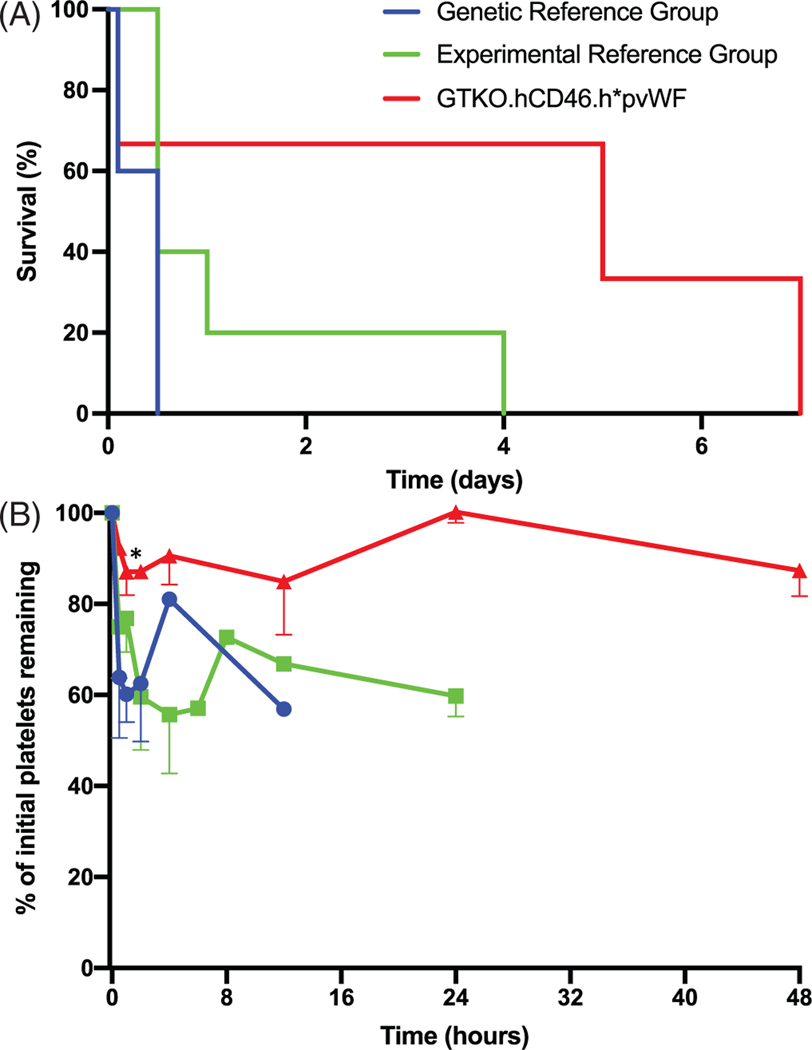
GTKO.hCD46.h*pvWF pig lung transplants into baboons with additional mechanism-directed drug treatments (Table S1) did not exhibit life-supporting lung function at the end of implantation (n = 3). By comparison, two of five recipients of GTKO.hCD46 pig lungs without these drug treatments (genetic reference group) and three of five GTKO.hCD46 with other genetic modifications and with these drug treatments (experimental regimen reference group) were transiently fully or partially life-supporting. Reference lung recipients typically exhibited loss of xenograft vascular barrier function with tracheobronchial flooding and/or recipient hemodynamic instability within 24 h (nine of 10; Figure 6A); one, a GTKO.hCD46.HLA-E.β4GalKO regimen reference lung recipient, survived for 4 days before xenograft barrier function failure. Two of the three GTKO.hCD46.h*pvWF lung recipients survived for 5 and 7 days before the xenograft lost barrier function and became consolidated and hemorrhagic; survival duration was not significantly different between genetic and experimental regimen reference groups (p = .074). Recipients of both genetic and regimen reference pvWF lungs exhibited 30%–40% decline in platelets within 2 h after xenograft revascularization, and platelet counts typically remained in this range until graft failure. In association with h*pvWF lungs, platelet counts declined 10%–20% within 2 h and remained stable over the first 48 h in the surviving recipients (p ≤ .007; Figure 6B). The platelet counts did not decline further over the remaining day(s) of survival of these recipients.
FIGURE 6.
In vivo pig-to-baboon lung xenotransplantation. (A) There was a nonsignificant trend toward improved survival of baboon recipients of pig lungs with h*pvWF (GTKO.hCD46.h*pvWF, red), compared to lungs with pvWF (GTKO.hCD46, blue; GTKO.hCD46 with additional genetics, green); as well as (B) improvement in platelet sequestration in the first 48 h after transplantation (p < .01). Error bars represent standard error of the mean. *p < .05 versus genetic reference
4 |. DISCUSSION
Using CRISPR-Cas9-assisted homology-directed repair and SCNT, we successfully replaced the region on pvWF that included the site that participates in GPIb-binding with orthologous hvWF cDNA sequences. This modification was associated with amelioration of the dysregulated platelet activation otherwise observed when human platelets are exposed to pig vWF in vitro, ex vivo, and in vivo. Prior pvWF knockout pigs have been shown to have prolonged bleeding (> 500 min) and require chronic plasma supplementation to prevent lethal bleeding, despite coagulation and other hematological parameters within the normal range.43 In contrast, h*pvWF pigs were viable and developed normally for over 20 weeks when stressful interventions are minimized, allowing maturation to an age and size appropriate for organ evaluation. While stress-induced bleeding was empirically treated with oral desmopressin and vitamin K2, it is unclear if these treatments were helpful. At present, we cannot rule out that the bleeding phenotype is (a) a vWF-independent artifact of pig cloning (since bleeding is also observed in multiple different strains of cloned pigs), (b) due to incomplete epigenetic reprogramming at the vWF locus or its regulatory regions, or (c) a combination of ineffective vWF molecular hybrids associated with some particular transgene insertion sites, potentially aggravated by vWF-independent cloning artifacts. However, the fact that half of the pigs had no clinically apparent bleeding issues indicates that the h*pvWF is indeed functional and hemostatically active in multiple different genotypic constructs. We infer that the human GPIb region substitution used here does not fully complement the physiological function of pvWF in h*pvWF pigs.
During both ex vivo lung perfusion with human blood and in vivo lung xenotransplants into baboon recipients, h*pvWF lungs were associated with a significant reduction in platelet sequestration when compared to lungs with pvWF and either GTKO.hCD46 “reference” genetics or with similar experimental drug treatment regimens and additional genetic modifications. In vitro analyses confirmed the functionality of the human GPIb-binding domain in these genetically modified pigs, as well as physiologically appropriate interactions with human platelets. Specifically, plasma from pigs with h*pvWF did not cause spontaneous “physiologically inappropriate” aggregation of human platelets as did porcine plasma-containing pvWF, while whole blood from h*pvWF pigs had a physiologically appropriate aggregation response to collagen.
Although significantly reduced both ex vivo and in vivo, platelet sequestration was not completely prevented in association with the h*pvWF modification. βTG, a plasma biomarker that quantifies cumulative platelet activation in the lung and elsewhere in the perfusion circuit, was not reduced in association with h*pvWF or the additional interventions tested here. Blockade of the platelet agonist thromboxane (using BIA) and canonical P-selectin binding (with PSGL1)—pathways that mediate platelet activation and adhesion in other models—in addition to inhibiting the vWF/GPIb integrin axis (using GPIb-blocking Fab 6B4)19,36 failed to inhibit residual platelet aggregation, implicating alternative platelet activation (adenosine diphosphate (ADP), or PAR1) and non-canonical platelet ligation mechanisms in the residual sequestration; current work is exploring whether FcγR, sialoadhesin, ASGR1 and/or galectins play a role.44 Several physiologic incompatibilities between pig and human coagulation pathway proteins have been described, which have been addressed by the expression of human coagulation cascade regulatory proteins in GTKO.hCD46 transgenic pigs.45 However, platelet activation was not fully prevented by these approaches in pigs expressing pvWF.21,32,41,46 We presume that the observed delay in activation of platelets residual in the circuit in the reference group(s) probably reflect differential susceptibility of platelets in the blood to activation stimuli since platelets are of different ages and experience different interactions during their course through the lung and perfusion circuit. While we cannot specifically explain why some platelets exhibit delayed activation in the reference lungs (or livers), the fact that activation is further delayed when the lung or liver expresses h*pvWF demonstrates that vWF interactions contribute to this phenomenon, directly or indirectly. Determining whether adding hTBM, hEPCR, hTFPI, or hCD39 will address residual platelet sequestration and aggregation seen here with humanized pvWF will depend on generation of GTKO.hCD46 cells and organs that combine h*pvWF with one or more of these additional thromboregulatory gene modifications.
Thrombocytopenia is a prominent feature of every in vivo organ xenograft model, although the severity varies with different organs and cells, and recovery is generally observed if anti-pig antibody elaboration is constrained by effective immunosuppression. This has been demonstrated by multiple groups, although a causal contribution of thrombocytopenia to the coagulation dysregulation often seen in xenograft recipients has not been proven.7–13 Of interest, delayed xenograft rejection (thrombotic microangiopathy, consumptive coagulopathy, including thrombocytopenia) is diminished by expression of human thrombomodulin in GTKO.hCD46 pig organs.47–49 We posit that platelet adhesion is pathogenic and pro-inflammatory for every cell and organ xenograft, albeit with variable consequences for each graft and in different models. Importantly, we show here that humanizing pvWF partially attenuates initial platelet sequestration in two organ systems. Inflammation and coagulation are both linked to xenograft injury and likely trigger increased immune injury and resistance to tolerance induction.15,45,50 Further work will be needed to determine whether humanizing vWF is desirable to enable cell or organ xenotransplantation, to increase the prevalence or ease of achieving long-term xenograft survival with normal function, or necessary to successful clinical translation, particularly for liver and lung xenografts. In either case, the h*pvWF genetic modification is effective to partially modulate platelet sequestration and is consistent with viable animals.
Prior studies have used von Willebrand diseased (vWD) pig organs in kidney or lung xenotransplant models.46,51 These pigs are severely deficient in vWF due to genetic mutations. This Type III vWD is a very severe, autosomal recessive hemophilia phenotype manifesting as hemarthrosis and purpura fulminans.52,53 While this line of animals could in theory provide a source of organs devoid of pvWF for xenotransplantation, due to their fragility, these animals are not suitable for the breeding and shipment processes that are required to provide organs for transplantation. We are optimistic that half of our h*pvWF pigs had no clinically apparent bleeding issues, and the rest had only mild signs of bleeding noted, suggesting that h*pvWF is indeed functional and hemostatically active. Humanizing the pvWF gene prevents physiologically abnormal triggering of human platelets by pig vWF while preserving other critical homeostatic thromboregulatory functions of the vWF molecule. h*pvWF pigs exhibit a normal range of circulating Factor VIII levels and activity and aggregation since aggregation of pig platelets by collagen is preserved in h*pvWF pigs. Reduced vWF levels in ELISA assays reflect either decreased expression or altered conformational folding of h*pvWF relative to hvWF. In either case, h*pvWF exhibited preserved functional capabilities including multimerization and endothelial-specific expression similar to pvWF and hvWF. Meanwhile, the spontaneous human platelet aggregation seen in assays and perfusions with pvWF organs was mitigated with the h*pvWF pigs.
Limitations of this study include the relatively small numbers of h*pvWF animals studied in vivo and potentially confounding variables associated with evolving genetics and recipient drug treatment regimens studied, which were modified over 7 years in response to our interim ex vivo and in vivo observations, and reports by others working in the field. Additionally, the use of both mono- and bi-allelic pigs is a potential limitation since the mono-allelic pigs may express a fusion protein with unknown properties. While we cannot exclude that lung microvascular expression of hCD46, for example, may have differed between groups, our extensive unpublished data characterizing endothelial protein expression in the pig lines reported here, and multiple other genetically modified pig strains, do not support that alternative explanation for our findings.
This is the first report showing that humanizing pig vWF reduces the thrombodysregulation caused by non-physiologic interaction between pvWF and primate GPIb and partially attenuates the platelet sequestration and activation that is associated with injury to lung and liver xenografts from GTKO.hCD46 pigs. Although h*pvWF does not provide a singular solution to thrombocytopenia in two pig organ xenograft models, studies incorporating this modification into donor pigs with additional mechanism-directed genetic modifications may reveal a synergistic role for h*pvWF, particularly in the setting of thromboregulatory molecule expression, or in combination with drug regimens that block as-yet-unidentified pathways driving thrombocytopenia. Improved understanding of these pathways could prove pivotal not only for lung and liver xenografts but may also facilitate successful transplantation of other organs and cells where thrombocytopenia is typically seen but the consequences of adhesive and pro-inflammatory platelet interactions appear to be less obvious, such as the heart, kidney, and islets.
Supplementary Material
ACKNOWLEDGMENTS
These studies were supported by U19 AI090959, P01 HL107152 and by unrestricted educational gifts from and sponsored research agreements with United Therapeutics’ subsidiary, Lung Biotechnology LLC. This work was supported by the University of Maryland Clinical Translational Science Institute and the University of Maryland General Clinical Research Center.
Fundinginformation
NIH, Grant/Award Numbers: U19 AI090959, P01 HL107152
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
CONFLICT OF INTEREST
Richard N Pierson III has served without compensation on Revivicor’s Scientific Advisory Board. David Ayares is Executive VP and CSO and a full-time employee of Revivicor, Inc. Kasinath Kuravi, Lori Sorrells, Benson Morrill, Amy Dandro, Todd Vaught, Jeffery Monahan, Carol Phelps, and Willard Eyestone are employees at Revivicor, Inc. Revivicor, Inc. is a wholly owned subsidiary of United Therapeutics, Inc.
REFERENCES
- 1.Unos Data. Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/# Accessed June 5, 2020.
- 2.Ye Y, Cooper DKC. Experimental xenotransplantation in nonhuman primates using distantly related donor species. Cooper DKC, Kemp E, Reemtsma K, White DJG, eds. Xenotransplantation. Springer Berlin; Heidelberg; 1991:389–393. [Google Scholar]
- 3.Bach FH, Robson SC, Ferran C, et al. Endothelial cell activation and thromboregulation during xenograft rejection. Immunol Rev. 1994;141(1):5–30. [DOI] [PubMed] [Google Scholar]
- 4.Ekser B, Rigotti P, Gridelli B, Cooper DKC. Xenotransplantation of solid organs in the pig-to-primate model. Transpl Immunol. 2009;21(2):87–92. [DOI] [PubMed] [Google Scholar]
- 5.Platt JL. Genetic engineering for xenotransplantation. Transplant Proc. 1999;31(3):1488–1490. [DOI] [PubMed] [Google Scholar]
- 6.Zeyland J, Lipiński D, Słomski R. The current state of xenotransplantation. J Appl Genet. 2015;56(2):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10(2):273–285. [DOI] [PubMed] [Google Scholar]
- 8.Yeh H, Machaidze Z, Wamala I, et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21(5):454–464. [DOI] [PubMed] [Google Scholar]
- 9.Ekser B, Klein E, He J, et al. Genetically-engineered pig-to-baboon liver xenotransplantation: histopathology of xenografts and native organs. PLoS One. 2012;7(1):e29720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen B-NH, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. JThoracCardiovasc Surg. 2007;133:1354–1363. [DOI] [PubMed] [Google Scholar]
- 11.Iwase H, Jagdale A, Yamamoto T, et al. Indicators of impending pig kidney and heart xenograft failure: relevance to clinical organ xenotransplantation–Review article. Int J Surg. 2019;70:84–91. [DOI] [PubMed] [Google Scholar]
- 12.Iwase H, Ekser B, Hara H, et al. Regulation of human platelet aggregation by genetically modified pig endothelial cells and thrombin inhibition. Xenotransplantation. 2014;21(1):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan J, Singh A, Thomas M, Ayares D, Horvath K, Mohiuddin M. Abstract 14062: Cardiac xenotransplantation-associated consumptive thrombocytopenia is attenuated with use of anti-cd40 antibody co-stimulation blockade. Circulation. 2016;134:A14062. [Google Scholar]
- 14.Burdorf L, Stoddard T, Zhang T, et al. Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am J Transplant. 2014;14(5):1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwase H, Ekser B, Zhou H, Dons EM, Cooper DKC, Ezzelarab MB. Platelet aggregation in humans and nonhuman primates: relevance to xenotransplantation. Xenotransplantation. 2012;19(4):233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Q, Yeh H, Wei L, et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS One. 2012;7(10):e47273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172(6):1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird C, Burdorf L. Lung xenotransplantation: a review. CurrOpinOrgan Transplant. 2016;21(3):272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCD46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplantation. 2016;23(3):222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer S, Zorn GL 3rd, Zhang J-PP, et al. Hyperacute lung rejection in the pig-to-human model. III. Platelet receptor inhibitors synergistically modulate complement activation and lung injury. Transplantation. 2003;75(7):953–959. [DOI] [PubMed] [Google Scholar]
- 21.LaMattina JC, Burdorf L, Zhang T, et al. Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein Ib blockade. Xenotransplantation. 2014;21(3):274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer D, Girma JP. von Willebrand factor: structure and function. Thromb Haemost. 1993;70(1):99–104. [PubMed] [Google Scholar]
- 23.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–297. [DOI] [PubMed] [Google Scholar]
- 24.Mazzucato M, De Marco L, Pradella P, Masotti A, Pareti FI. Porcine von Willebrand factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75(4): 655–660. [PubMed] [Google Scholar]
- 25.Schulte am Esch J 2nd, Cruz MA, Siegel JB, Anrather J, Robson SC. Activation of human platelets by the membrane-expressed A1 domain of von Willebrand factor. Blood. 1997;90(11):4425–4437. [PubMed] [Google Scholar]
- 26.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giraldo AM, Ball S, Bondioli KR. Production of transgenic and knockout pigs by somatic cell nuclear transfer. Methods Mol Biol. 2012;885:105–123. [DOI] [PubMed] [Google Scholar]
- 28.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15(1):27–35. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen B-NH, Azimzadeh AM, Schroeder C, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18(2):94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burdorf L, Azimzadeh AM. Xenogeneic lung transplantation models. Methods Mol Biol. 2020;2110:173–196. [DOI] [PubMed] [Google Scholar]
- 31.Collins BJ, Blum MG, Parker RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol. 2001;90(6):2257–2268. [DOI] [PubMed] [Google Scholar]
- 32.Cimeno A, Hassanein W, French BM, et al. N-glycolylneuraminic acid knockout reduces erythrocyte sequestration and thromboxane elaboration in an ex vivo pig-to-human xenoperfusion model. Xenotransplantation. 2017;24(6):e12339. 10.1111/xen.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burdorf L, Harris D, Dahi S, et al. Thromboxane and histamine mediate PVR elevation during xenogeneic pig lung perfusion with human blood. Xenotransplantation. 2019;26(2):e12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schröder C, Pfeiffer S, Wu G, et al. Effect of complement fragment 1 esterase inhibition on survival of human decay-accelerating factor pig lungs perfused with human blood. J Hear Lung Transplant. 2003;22(12):1365–1375. [DOI] [PubMed] [Google Scholar]
- 36.Fontayne A, Meiring M, Lamprecht S, et al. The humanized anti-glycoprotein Ib monoclonal antibody h6B4-Fab is a potent and safe antithrombotic in a high shear arterial thrombosis model in baboons. Thromb Haemost. 2008;100(4):670–677. [PubMed] [Google Scholar]
- 37.Laird CT, Hassanein W, O’Neill NA, et al. P- and E-selectin receptor antagonism prevents human leukocyte adhesion to activated porcine endothelial monolayers and attenuates porcine endothelial damage. Xenotransplantation. 2018;25(2):e12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.French BM, Sendil S, Sepuru KM, et al. Interleukin-8 mediates neutrophil-endothelial interactions in pig-to-human xenogeneic models. Xenotransplantation. 2018;25(2):e12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols TC, Bellinger DA, Merricks EP, et al. Porcine and canine von willebrand factor and von willebrand disease: hemostasis, thrombosis, and atherosclerosis studies. Thrombosis. 2010;2010:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 2014;25(3):206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cimeno A, Barth RN, LaMattina JC. Advances in liver xenotransplantation. Curr Opin Organ Transplant. 2018;23(6):615–620. [DOI] [PubMed] [Google Scholar]
- 42.Brock LG, Delputte PL, Waldman JP, Nauwynck HJ, Rees MA. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. Am J Transplant. 2012;12(12):3272–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hai T, Teng F, Guo R, Li W, Zhou Q. One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res. 2014;24(3):372–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.French BM, Sendil S, Pierson RN, Azimzadeh AM. The role of sialic acids in the immune recognition of xenografts. Xenotransplantation. 2017;24(6):e12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg. 2015;23(Pt B):296–300. [DOI] [PubMed] [Google Scholar]
- 46.Cantu E, Balsara KR, Li B, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7(1):66–75. [DOI] [PubMed] [Google Scholar]
- 47.Singh AK, Chan JL, DiChiacchio L, et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation. 2019;26(2):e12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564(7736):430–433. [DOI] [PubMed] [Google Scholar]
- 49.Kopp CW, Grey ST, Siegel JB, et al. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation. 1998;66(2):244–251. [DOI] [PubMed] [Google Scholar]
- 50.Ezzelarab MMB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22(1):32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyer C, Wolf P, Romain N, et al. Use of von Willebrand diseased kidney as donor in a pig-to-primate model of xenotransplantation. Transplantation. 1999;67(1):38–45. [DOI] [PubMed] [Google Scholar]
- 52.Sadler JE. A revised classification of von Willebrand disease. Thromb Haemost. 1994;71(4):520–525. [PubMed] [Google Scholar]
- 53.Bowie EJ, Owen CAJ, Zollman PE, Thompson JHJ, Fass DN. Tests of hemostasis in swine: normal values and values in pigs affected with von Willebrand’s disease. Am J Vet Res. 1973;34(11):1405–1407. [PubMed] [Google Scholar]
- 54.Wang B, Xu H, Li J, et al. Complement depletion with cobra venom factor alleviates acute hepatic injury induced by ischemia‑reperfusion. Mol Med Rep. 2018;18(5):4523–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.