Abstract
目的
探讨S100钙结合蛋白A10(S100A10)在肺腺癌(LUAD)的表达水平及对患者预后的影响,以及S100A10对肺癌细胞增殖和转移的调控作用和作用机制。
方法
采用免疫组化技术(IHC)检测S100A10在LUAD及癌旁组织中的表达水平。Western blot、CCK-8和EdU、Transwell实验分别测定S100A10蛋白的表达水平、细胞增殖和侵袭能力。通过基因集富集分析(GSEA)S100A10在肺腺癌中可能的调控通路。分别将A549-shS100A10和A549-shCtrl细胞,H1299-S100A10和H1299-GFP的细胞注射到裸鼠皮下,观察S100A10的表达水平对肿瘤增殖的影响。
结果
与癌旁组织相比,肿瘤组织中S100A10的表达水平显著上调,并且S100A10表达水平升高与淋巴结转移、肿瘤分期、远处器官转移有关(P < 0.05),而与分化程度、年龄、性别无关(P > 0.05)。生存分析显示,肿瘤组织中S100A10表达水平越高,患者的预后越差(P < 0.001)。体外实验显示,上调S100A10的表达,促进了肿瘤细胞的增殖和侵袭能力(P < 0.001)。GSEA分析显示S100A10高表达显著富集至糖代谢、糖酵解、mTOR信号通路等基因集。葡萄糖消耗及乳酸生成实验表明,上调S100A10促进葡萄糖消耗和乳酸的生成,相反,下调S100A10抑制葡萄糖消耗和乳酸的生成。Western blot实验显示,S100A10能够促进Akt-mTOR信号通路激活。裸鼠移植瘤实验结果表明,上调S100A10的表达促进肿瘤增殖,而下调S100A10的表达则抑制肿瘤增殖(P < 0.001)。
结论
S100A10通过激活Akt-mTOR-糖酵解信号通路,进而促进肿瘤细胞的增殖和侵袭能力。
Keywords: S100A10, 肺腺癌, 临床病理参数, Akt-mTOR, 糖酵解, 肿瘤细胞增殖和侵袭
Abstract
Objective
To investigate the effects of expression levels of S100 calcium-binding protein A10 (S100A10) in lung adenocarcinoma (LUAD) on patient prognosis and the regulatory role of S100A10 in lung cancer cell proliferation and metastasis.
Methods
Immunohistochemistry was used to detect the expression levels of S100A10 in LUAD and adjacent tissues, and the relationship between S100A10 expression and clinicopathological parameters and prognosis of the patients was statistically analyzed. The lung adenocarcinoma expression dataset in TCGA database was analyzed using gene enrichment analysis (GSEA) to predict the possible regulatory pathways of S100A10 in the development of lung adenocarcinoma. Lactate production and glucose consumption of lung cancer cells with S100A10 knockdown or overexpression were analyzed to assess the level of glycolysis. Western blotting, CCK-8 assay, EdU-594 assay, and Transwell assays were performed to determine the expression level of S100A10 protein, proliferation and invasion ability of lung cancer cells. A549 cells with S100A10 knockdown and H1299 cells with S100A10 overexpression were injected subcutaneously in nude mice, and tumor growth was observed.
Results
The expression level of S100A10 was significantly upregulated in LUAD tissues as compared with the adjacent tissues, and an elevated S100A10 expression level was associated with lymph node metastasis, advanced tumor stage and distant organ metastasis (P < 0.05), but not with tumor differentiation or the patients' age or gender (P > 0.05). Survival analysis showed that elevated S100A10 expressions in the tumor tissue was associated with a poor outcome of the patients (P < 0.001). In the lung cancer cells, S100A10 overexpression significantly promoted cell proliferation and invasion in vitro (P < 0.001). GSEA showed that the gene sets of glucose metabolism, glycolysis and mTOR signaling pathway were significantly enriched in high expressions of S100A10. In the tumor-bearing nude mice, S100A10 overexpression significantly promoted tumor growth, while S100A10 knockdown obviously suppressed tumor cell proliferation (P < 0.001).
Conclusion
S100A10 overexpression promotes glycolysis by activating the Akt-mTOR signaling pathway to promote proliferation and invasion of lung adenocarcinoma cells.
Keywords: S100A10, lung adenocarcinoma, clinicopathological parameters, Akt-mTOR, glycolysis, tumor cell invasion and proliferation
肺癌是世界上最常见的肿瘤,每年导致约170万人死亡[1, 2]。肺癌包括非小细胞肺癌(NSCLC)和小细胞肺癌,大约80%~85%的肺癌属于非小细胞肺癌[3]。近年来,随着分子靶向治疗以及免疫检测点抑制剂的应用,肺癌患者的生存率有了提高,但是大多数肺癌患者诊断时已是晚期,因此五年生存率仍较差[4, 5]。
S100蛋白家族是一组相对分子质量较小的钙结合蛋白,通过与靶蛋白互作在胞内外参于调节细胞增殖与凋亡,细胞骨架与细胞基质重构及迁移等重要生物学过程[6, 7]。根据氨基酸序列和蛋白结构的相似性,已有20种该家族成员被发现,其中有17个家族成员编码在人体1q21表皮分化复合体(EDC)区域。EDC由数个基因或基因家族构成,这些基因编码的蛋白质决定了细胞表皮分化过程[7-10]。在肿瘤细胞中EDC的表达异常与上皮-间质转化(EMT)密切关联,因此,S100蛋白家族在肿瘤发生发展中发挥重要作用[8]。目前有研究表明,S100A10是EMT的关键调节因子,参与乳腺癌、肝癌、胰腺癌的发生,进展和转移[11-13]。然而,肺腺癌中S100A10的表达及其信号传递途径还未完全阐明。
蛋白激酶B(Akt)/哺乳动物雷帕霉素靶蛋白(mTOR)可以将细胞外和细胞内的信号进行整合聚集,从而在肿瘤细胞糖酵解、干性、转移和耐药性等病理过程中发挥关键性调控作用[14-18]。肿瘤细胞通过从微环境中摄取大量葡萄糖进行糖酵解,从而产生足够的能量满足其快速增殖的需求[19]。Akt-mTOR的过度激活与肿瘤的发生发展密切相关,以Akt-mTOR为靶点设计抗肿瘤药物是一大研究热点[20, 21]。因此,深入了解Akt-mTOR调节机制可能有助于肿瘤治疗策略的开发。本研究探讨S100A10的表达水平与肺腺癌(LUAD)临床病理参数的关系以及对患者预后的影响,通过体外和体内实验进一步研究S100A10调控肿瘤细胞增殖和侵袭的作用,并探讨S100A10促进肿瘤进展的相关机制。
1. 材料和方法
1.1. 细胞培养
人肺癌A549、H1299、H1972、SPC-A-1、HCC827细胞购于中国科学院细胞库。用含10%胎牛血清的RPMI 1640培养基培养,细胞在37℃、CO2体积分数为5%的条件下培养。取对数生长期的细胞用于试验。
1.2. 主要试剂
胎牛血清(Gibco),DMEM培养基,0.25%胰蛋白酶,S100A10多克隆抗体、脂质体Lipofect 2000 (Invitrogen),β‐actin抗体、Vimentin(D21H3)、ZEB1(E2G6Y)抗体、p-Akt(1∶1000,Ser-473)、mTOR(1∶ 1000,7C10)、p-mTOR (1∶ 1000,Ser2448)(Cell Signaling Technology)。S100A10过表达慢病毒颗粒(Sino Biological),S100A10shRNA(靶标序列: CTG AGGTTCAACCCGTTGAAA)(Sigma),RIPA裂解液、BCA试剂盒、胰酶、Transwell 24孔板(孔径为8 μm)、BeyoClick ™ EdU-594细胞增殖检测试剂盒、增强型CCK-8试剂盒(Beyotime Biotechnology),SP Rabbit & Mouse HRP Kit试剂盒(CoWin Biosciences),Matrigel(BD Biosciences),Transwell培养小室(Millipore),葡萄糖测定试剂盒(上海荣盛生物技术有限公司),D-Lactate Assay试剂盒(Abcam)。
1.3. 蛋白印迹法检测(WB)
使用RIPA裂解液裂解提取总蛋白,BCA试剂盒检测蛋白浓度。经SDS-PAGE分离蛋白后,用半干转膜仪转移蛋白质至PVDF膜,然后用脱脂牛奶室温封闭蛋白2 h,加入一抗在4 ℃封闭过夜,再加入对应二抗室温封闭1h,滴反应液曝光。
1.4. 慢病毒感染
H1299细胞接种于24孔培养板培养至密度约80%时,根据预试验确定的感染条件,按感染复数(MOI)为150加入S100A10 Lentiviral cDNA慢病毒液感染细胞。S100A10沉默慢病毒(sh-S100A10)、S100A10过表达慢病毒(OE-S100A10)和阴性对照慢病毒(sh-NC)。由于病毒质粒中含有编码绿色荧光蛋白(GFP)的基因,利用荧光显微镜可根据荧光强度估算细胞感染效率。感染病毒4 d后每组细胞内加入氨苄青霉素进行筛选,6 d细胞长满后,进行细胞传代,2周后获得稳定转染的Lentiviral S100A10 overexpression的H1299细胞系。
1.5. 免疫组织化学检测
肺癌和癌旁组织芯片脱蜡水化:60 ℃烤片1h,二甲苯脱蜡;再依次浸入梯度乙醇和蒸馏水进行水化。抗原修复用柠檬酸缓冲液。在室温下用正常山羊血清覆盖芯片约10 min进行抗原封闭,然后在4 ℃下与anti-S100A1孵育过夜。随后用生物素标记羊抗兔二抗工作液孵育,最后应用DAB显色工作液进行显色,随后将芯片用苏木精复染。S100A10阳性细胞的百分比进行评分:0分(0%~25%),1分(25%~50%),2分(50%~ 75%)和3分(> 75%)。将0和1分定义为低表达,而将2和3分定义为高表达。
1.6. 细胞增殖测定
转染后,使用BeyoClick™ EdU-594细胞增殖检测试剂盒、增强型CCK-8检测试剂盒检测A549敲低组和过表达的H1299及控制对照组的增殖情况,具体实验方法严格按照说明书进行操作。
1.7. Transwell侵袭检测
将基质胶置于Transwell小室以明确定细胞的侵袭能力。收集生长良好的过表达S100A10的H1299和A549敲低组及控制对照组,消化离心后,用无血清的RPMI 1640培养基重悬细胞密度为4×105/mL,取200 μL细胞悬液接种于24孔板Transwell上室(孔径为8 μm),而下腔室则加入500 μL含有10%FBS的RPMI 1640培养基。在37 ℃下孵育24 h后,取出24孔板中的Transwell小室,用棉签轻轻擦去上层的细胞。用4%多聚甲醛固定20 min后,用0.1%结晶紫染色20 min,PBS液冲洗干净,倒扣小室自然风干。每组实验重复3次。在倒置显微镜下随机取5个视野,并计算细胞的个数及平均值。
1.8. 葡萄糖消耗的测量
转染后,将过表达及敲除S100A10、控制对照组的A549和H1299细胞接种到6孔板中。6 h后,将培养基更换为完全培养基并培养48 h。葡萄糖测定试剂盒收集培养基以测量葡萄糖浓度。葡萄糖消耗计算为新鲜培养基中的原始葡萄糖浓度与收集的培养基中测量的葡萄糖浓度之间的差异。所有结果均标准化为相应的蛋白质浓度值。实验至少重复3次。
1.9. 乳酸产量的测量
根据制造商的说明书,收集培养物上清液,评估培养上清液中的乳酸产生量。所有结果均标准化为相应的蛋白质浓度值。实验至少重复了3次。
1.10. 基因集富集分析(GSEA)
采用GSEA 4.0进行GSEA,寻找S100A10影响肿瘤发生发展可能的信号通路。采用GSEA网站的MsigDB数据库获取c2.cp.kegg.v7.0.symbols.gmt数据集,设置分析置换次数为1000次,显著富集基因集须满足一下条件:错误发生率(FDR) < 0.25,P < 0.05。
1.11. 裸鼠移植瘤实验
首先,我们使用S100A10敲低组及控制对照组的A549细胞,S100A10过表达组及控制对照组的H1299细胞接种在6孔板中,培养过夜,并用慢病毒感染。感染后72 h用荧光显微镜测定慢病毒感染率。分别将上述细胞注射到裸鼠皮下(南京青龙山6~8周龄),每只老鼠两个部位。使用游标卡尺每周测量肿瘤两次,根据体积公式计算肿瘤体积:V=(S2×L)/2,其中V是体积,S是最短直径,L是最长直径。在第30天把小鼠处死,获得肿瘤,并测量肿瘤大小和质量。本研究动物实验通过皖南医学院医学伦理委员会审查批准(2021-71)。
1.12. 统计方法
采用SPSS 16.0软件(Chicago,IL,USA)对数据进行统计分析处理,S100A10的表达水平与临床病理参数的关系采用chi-square检验,生存分析采用Kaplan-Meier生存曲线。用均数±标准差表示计量资料,两组间的比较采用student t检验,利用GraphPad Prism 7(San Diego,CA,USA)软件对数据进行作图。P < 0.05为差异具有统计学意义。
2. 结果
2.1. S100A10表达水平与肺腺癌的临床病理参数密切相关
免疫组化评分结果显示,S100A10的表达水平与肿瘤大小(P=0.009)、淋巴结转移(P=0.025)、肿瘤分期(P=0.002)、远处转移(P=0.004)密切有关,而与患者年龄(P=0.223)、肿瘤分化程度(P=0.431)无关,提示S100A10的表达水平与肿瘤负荷密切关联(图 1A,表 1)。Western blot法检测3例肺腺癌患者癌组织和癌旁组织S100A10的蛋白表达水平,结果提示肿瘤组织S100A10的表达水平显著高于癌旁组织(图 1B)。Kaplan-Meier生存分析显示,与S100A10低表达的患者相比,高表达S100A10的患者的生存期显著降低,差异具有统计学意义(P < 0.001,图 1C)。
图 1.
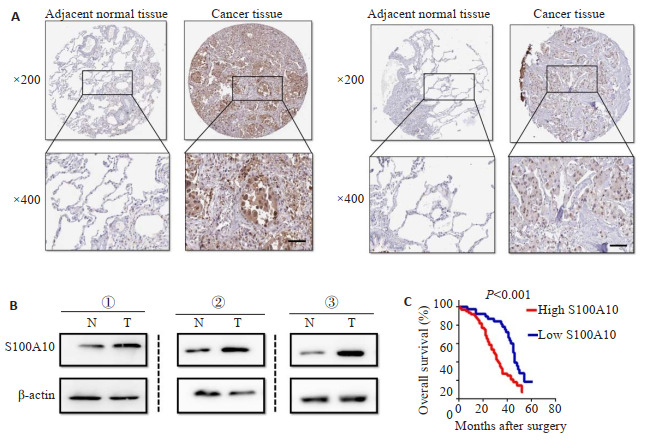
肺腺癌组织高表达S100A10并且可以作为患者预后的标记
High expression of S100A10 in lung adenocarcinoma tissues can be used as a prognostic marker. A: Immunohistochemistry for detecting the expression of S100A10 in adjacent tissues and tumor tissues in a tissue microarray. Representative images of two cases of lung adenocarcinoma show significantly higher expression level of S100A10 in tumor tissues than in adjacent tissues. B: Western blotting showing higher S100A10 expression in cancer tissues than in adjacent tissues from 3 patients with lung cancer. C: Kaplan-Meier survival analysis curves showing poor prognosis in patients with high expression of S100A10.
表 1.
S100A10的表达水平与临床病理参数的关系(%)
Relationship between the expression level of S100A10 and clinicopathological parameters (%)
| Clinicopathologic parameters | S100A10 | |||
| n | Low | High | P | |
| Age (year) | 0.223 | |||
| ≤60 | 36 | 18 | 18 | |
| > 60 | 54 | 20 | 34 | |
| Gender | 0.500 | |||
| Male | 32 | 12 | 20 | |
| Female | 58 | 26 | 32 | |
| Histologic differentiation | 0.431 | |||
| Well | 12 | 7 | 5 | |
| Moderate | 53 | 22 | 31 | |
| Poor | 25 | 9 | 16 | |
| T classification | 0.009 | |||
| T1-T2 | 29 | 18 | 11 | |
| T3-T4 | 61 | 20 | 41 | |
| Lymph node metastasis | 0.025 | |||
| N0-N1 | 33 | 19 | 14 | |
| N2-N3 | 57 | 19 | 38 | |
| Distant metastasis (M) | 0.004 | |||
| No (M0) | 61 | 32 | 29 | |
| Yes (M1) | 29 | 6 | 23 | |
| TNM stage | 0.002 | |||
| Ⅰ-Ⅱ | 40 | 24 | 16 | |
| Ⅲ-Ⅳ | 50 | 14 | 36 | |
2.2. S100A10过表达促进肺癌细胞的增殖和转移
Western blot检测S100A10在肺腺癌细胞系的基线表达水平,结果显示A549细胞高表达S100A10,而H1299细胞低表达S100A10(图 2A)。随后我们应用S100A10shRNA敲除A549细胞中的S100A10的表达,敲除效率见图 2B。CCK8和EdU实验结果均显示,敲除A549细胞中的S100A10可显著抑制A549细胞的增殖能力(P < 0.001,图 2C、D)。与此一致,Transwell侵袭实验结果表明,敲除S100A10的表达同样遏制了肿瘤细胞的侵袭能力(P < 0.001,图 2E)。为了进一步证实上述结论,我们应用S100A10的过表达慢病毒颗粒感染H1299细胞以上调S100A10的表达,Western blot检测过表达水平(图 2B),结果表明,与控制对照组相比,过表达S100A10可明显促进H1299细胞的增殖和侵袭能力(P < 0.001,图 2C~E)。
图 2.

S100A10调控肺癌细胞的增殖和转移能力
S100A10 regulates the proliferation and metastasis of lung cancer cells. A: Baseline expression of S100A10 in lung adenocarcinoma cell lines detected by Western blotting. B: Expression of S100A10 detected by Western blotting in A549 cells with S100A10 knockdown (shS100A10#1 and shS100A10#2) and in H1299 cells infected with S100A10- overexpressing lentiviral vector. C: Proliferation of A549 cells with S100A10 knockdown and H1299 cells with S100A10 overexpression detected by CCK8 assay. D: EdU assay showing that S100A10 knockdown inhibits proliferation of A549 cells and overexpression of S100A10 promotes proliferation of H1299 cells. E: Transwell assay showing S100A10 knockdown and overexpression inhibits and promotes the invasion of the tumor cells, respectively. Data are presented as Mean±SE.**P < 0.001.
2.3. S100A10通过激活Akt-mTOR信号通路影响肿瘤细胞糖酵解过程
基因集富集分析(GESA)表明,S100A10显著富集于糖酵解、糖异生、磷酸戊糖途径、mTOR信号通路(图 3A、B)。分别上调或下调肿瘤细胞S100A10结果显示,下调S100A10的表达抑制了肿瘤细胞的乳酸产量、葡萄糖消耗量,上调S100A10的表达则导致的相反的效应(P < 0.001,图 3C、D)。Western blot检测结果显示,上调S100A10促进了Akt-mTOR信号通路的激活,而下调S100A10则抑制了Akt-mTOR信号通路的激活(图 3E、F)。
图 3.

S100A10通过调控Akt-mTOR信号通路激活影响糖酵解过程
S100A10 affects glycolysis by regulating the activation of AKT-mTOR signaling pathway. A, B: GSEA analysis showing that a high expression of S100A10 in lung adenocarcinoma cells was significantly enriched in glucose metabolism, glycolysis, mTOR signaling pathway and other gene sets. C, D: Inhibition of S100A10 inhibits glycolysis, and overexpression of S100A10 promotes glycolysis. E, F: Western blot analysis showing that S100A10 could regulate the activation of AKTmTOR signaling pathway
2.4. S100A10促进肺腺癌的发生发展
A549-shS100A10和A549-shCtrl细胞移植到裸鼠皮下,统计分析上述两组小鼠的肿瘤体积和肿瘤体质量,与对照组相比,敲除S100A10表达可明显抑制肿瘤的增殖(P < 0.001,图 4A、B)。Western blot结果显示,敲除S100A10的表达显著抑制了肿瘤细胞的上皮细胞-间质转化(EMT)转化(图 4C),上调S100A10的表达则促进了肿瘤的增殖(P < 0.001)和EMT转化(图 4D~F)。
图 4.

体内S100A10促进肿瘤增殖
S100A10 promotes tumor proliferation in vivo. A: Tumor tissues derived from S100A10 knockout A549 cells and control A549 cells collected 30 days after injection in nude mice and the tumor growth curves. B: Tumor weight in S100A10 knockout group and control group. C: EMT transformation markers detected by Western blotting. D: Tumor tissues derived from H1299 cells overexpressing S100A10 and control H1299 cells in nude mice and the tumor growth curves. E: Tumor weight in S100A10 overexpression and GFP control groups. F: EMT transformation markers detected by Western blotting. All data are presented as Mean±SE. **P < 0.001.
3. 讨论
目前研究表明,S100蛋白家族在调控肿瘤发生发展过程中发挥关键作用,S100蛋白家族通过调控肿瘤细胞内Ca2+稳态、细胞增殖和凋亡信号通路、细胞代谢通路、肿瘤微血管生成等发挥其病理生理功能[22]。新近研究显示[23],肿瘤组织S100蛋白表达异常与患者肿瘤负荷以及不良预后密切关联,S100蛋白家族可能参与肿瘤免疫抑制性微环境的形成。在NSCLC患者中,肿瘤组织S100A8、S100A9的表达水平显著高于其癌旁组织,COX多因素生存分析显示S100A8、S100A9的表达水平显著影响患者的PFS、OS,患者S100A8、S100A9表达水平上调其PFS、OS相应减少[24]。进一步的研究显示,肿瘤相关中性粒细胞胞质中的存在大量S100A8和S100A9蛋白,中性粒细胞通过分泌S100A8/9并结合到肿瘤细胞表面Toll样受体(Toll-like receptors, TLR)从而介导细胞的促增殖和抗凋亡信号通路,导致肿瘤恶性进展[25]。另有研究显示,NSCLC患者血清中S100A10的表达水平与患者的肿瘤负荷、远处转移、不良预后存在关联[26]。本研究中,我们的数据显示,肺腺癌患者肿瘤组织S100A10的表达水平显著高于其癌旁组织,并且S100A10的表达水平与患者的肿瘤体积、淋巴结转移以及远处转移有关,生存分析显示,与S100A10低表达患者相比,S100A10高表达患者其OS显著缩短,这些数据表明,S100A10作为关键的癌蛋白可能促进了肺腺癌的发生发展。
新近的研究发现,S100A10在多种肿瘤组织中表达水平上调,如乳腺癌、胰腺癌、肝癌等,S100A10表达水平升高与肿瘤患者的临床病理参数密切关联[11-13]。在乳腺癌中,低氧诱导因子HIF-1可以促进S100A10的表达,S100A10通过结合到干细胞转录因子Oct-4的转录结合位点,进而促进多能性癌基因SOX2、KLF4的表达,诱导乳腺癌细胞干性[11]。在肝癌患者中,S100A10可以促进肿瘤细胞外泌体的合成和分泌,S100A10可以作为肝癌的治疗靶点[12]。在胰腺癌患者中,肿瘤组织S100A10表达水平显著高于其癌旁组织,S100A10的表达水平可以作为预测患者预后的独立危险因素,通过下调胰腺癌细胞的S100A10的表达水平则抑制了肿瘤细胞的增殖和转移,与此相反,上调S100A10的表达则促进了肿瘤细胞的增殖和转移能力,机制研究表明,S100A10通过促进JNK信号通路活化进而导致肿瘤进展[13]。与此一致,本研究中,通过上调肺腺癌细胞S100A10的表达水平促进肿瘤细胞的增殖和转移能力,然而,下调S100A10的表达则抑制了肿瘤细胞的增殖和转移,进一步的体内实验同样证明了这一结论,这些数据表明S100A10能够促进肺腺癌细胞的增殖和转移能力。
为了进一步研究S100A10促进肺腺癌进展的可能机制,我们通过基因富集分析发现,S100A10显著富集于糖酵解、糖异生、磷酸戊糖途径、mTOR信号通路。糖代谢的异常改变是肿瘤细胞的主要特征之一,Akt-mTOR信号通路通过促进肿瘤细胞糖酵解过程中关键酶的表达,从而导致肿瘤细胞葡萄糖消耗增加和糖酵解途径异常活跃,促使肿瘤进展[27]。Akt-mTOR处于肿瘤细胞信号通路的关键位置,Akt-mTOR通路异常激活与肿瘤的增殖、凋亡以及耐药性密切相关,因而Akt、mTOR抑制剂成为肿瘤靶向治疗的热点[28-30]。基于这些研究数据,我们推测S100A10通过调控Akt-mTOR信号通路的激活从而促进糖酵解途径。我们的实验数据表明,抑制肺癌细胞S100A10的表达则降低了乳酸的产量、葡萄糖的消耗量,与此相反,上调S100A10的表达则促进了乳酸的产量、葡萄糖的消耗量,与此一致,Western blot蛋白定量分析同样表明,下调S100A10的表达则抑制了Akt-mTOR信号通路的激活,而上调S100A10的表达则促进了Akt-mTOR信号通路的激活。因此,S100A10通过促进肺腺癌的糖酵解途径,进而诱导肿瘤的增殖和转移。
综上所述,肺腺癌S100A10的表达上调,其表达水平与患者的临床病理参数、不良预后密切相关,S100A10通过调控Akt-mTOR信号通路的激活进而增强肿瘤细胞的糖酵解途径,从而促进肿瘤细胞的增殖和转移能力。因此,S100A10或许可以作为预测肺腺癌肿瘤负荷的重要标记或者靶向治疗的靶点。
Biography
王会杰,在读硕士研究生,E-mail: whj@stu.wnmc.edu.cn
Funding Statement
安徽省自然科学基金(2008085QH351);安徽省教育厅重点项目(KJ2021A0838)
Contributor Information
王 会杰 (Huijie WANG), Email: whj@stu.wnmc.edu.cn.
耿 彪 (Biao GENG), Email: wnyxy1@163.com.
References
- 1.Yang CY, Yang JCH, Yang PC. Precision management of advanced non-small cell lung cancer. Annu Rev Med. 2020;71:117–36. doi: 10.1146/annurev-med-051718-013524. [DOI] [PubMed] [Google Scholar]
- 2.Tan DL, Wang S, Zhang P, et al. LncRNA SNHG12 decreases non-small cell lung cancer cell sensitivity to cisplatin by repressing miR-525-5p and promoting XIAP. Ann Clin Lab Sci. 2023;53(1):64–75. [PubMed] [Google Scholar]
- 3.-Q Li W, -W Cui J. Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol. 2020;146(9):2329–38. doi: 10.1007/s00432-020-03296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbst RS, Morgensztern D, Boshoff C. The biology and manage-ment of non-small cell lung cancer. Nature. 2018;553(7689):446–54. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 5.Dlamini SB, Sartorius B, Ginindza TG. Pre- and post-intervention survey on lung cancer awareness among adults in selected communities in KwaZulu-Natal, South Africa: a quasi-experimental study. J Public Health Afr. 2023;14(1):2131. doi: 10.4081/jphia.2023.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren YJ, Chen B, Zhang M, et al. Comprehensive analysis of the prognosis of S100 family members and their relationship with tumor-infiltrating immune cells in human pancreatic adenocarcinoma. Medicine. 2023;102(8):e32976. doi: 10.1097/MD.0000000000032976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15(2):96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, Du G, Wang D. The S100 protein family in lung cancer. Clin Chim Acta. 2021;520:67–70. doi: 10.1016/j.cca.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Low RRJ, Fung KY, Gao H, et al. S100 family proteins are linked to organoid morphology and EMT in pancreatic cancer[J]. Cell Death Differ, 2023 Feb 24. doi: 10.1038/s41418-023-01126-z.
- 10.Singh P, Ali SA. Multifunctional role of S100 protein family in the immune system: an update. Cells. 2022;11(15):2274–301. doi: 10.3390/cells11152274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu HQ, Xie Y, Tran L, et al. Chemotherapy-induced S100A10 recruits KDM6A to facilitate OCT4-mediated breast cancer stemness. J Clin Invest. 2020;130(9):4607–23. doi: 10.1172/JCI138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Huang HY, Sze KMF, et al. S100A10 promotes HCC development and progression via transfer in extracellular vesicles and regulating their protein cargos. Gut. 2023:gutjnl-2022-327998. doi: 10.1136/gutjnl-2022-327998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H, Yang PF, Li BX, et al. S100A10 promotes pancreatic ductal adenocarcinoma cells proliferation, migration and adhesion through JNK/LAMB3-LAMC2 axis. Cancers. 2022;15(1):202. doi: 10.3390/cancers15010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tewari D, Patni P, Bishayee A, et al. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2022;80:1–17. doi: 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Ghareghomi S, Atabaki V, Abdollahzadeh N, et al. Bioactive PI3-kinase/Akt/mTOR inhibitors in targeted lung cancer therapy. Adv Pharm Bull. 2023;13(1):24–35. doi: 10.34172/apb.2023.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: At the bench and bedside. Semin Cancer Biol. 2019;59:125–32. doi: 10.1016/j.semcancer.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 18.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu WZ, Wang X, Liao LS, et al. The TRPM7 channel reprograms cellular glycolysis to drive tumorigenesis and angiogenesis. Cell Death Dis. 2023;14(3):183–93. doi: 10.1038/s41419-023-05701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sirico M, D'Angelo A, Gianni C, et al. Current state and future challenges for PI3K inhibitors in cancer therapy. Cancers. 2023;15(3):703–34. doi: 10.3390/cancers15030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan ZH, Yang Y, Zheng HM, et al. Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer. J Cancer. 2022;13(13):3434–43. doi: 10.7150/jca.77619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donato R, Cannon BR, Sorci G, et al. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57. doi: 10.2174/156652413804486214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez LL, Garrie K, Turner MD. Role of S100 proteins in health and disease. Biochim Biophys Acta Mol Cell Res. 2020;1867(6):118677. doi: 10.1016/j.bbamcr.2020.118677. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Huang QD, Tang TY, et al. Clinical significance of calcium-binding protein S100A8 and S100A9 expression in non- small cell lung cancer. Thorac Cancer. 2018;9(7):800–4. doi: 10.1111/1759-7714.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprenkeler EGG, Zandstra J, van Kleef ND, et al. S100A8/A9 is a marker for the release of neutrophil extracellular traps and induces neutrophil activation. Cells. 2022;11(2):236. doi: 10.3390/cells11020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou YL, Zhang JH, Guo JB, et al. Clinical significance of serum S100A10 in lung cancer. J Int Med Res. 2021;49(10):3000605211049653. doi: 10.1177/03000605211049653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiaoting, H u. PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab Investig. 2020;100(6):801–11. doi: 10.1038/s41374-020-0404-9. [DOI] [PubMed] [Google Scholar]
- 28.Li QF, Li ZH, Luo T, et al. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol Biomed. 2022;3(1):47. doi: 10.1186/s43556-022-00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebrahimi S, Hosseini M, Shahidsales S, et al. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treat-ment of pancreatic cancer. Curr Med Chem. 2017;24(13):1321–31. doi: 10.2174/0929867324666170206142658. [DOI] [PubMed] [Google Scholar]
- 30.Xu WD, Berning P, Erdmann T, et al. mTOR inhibition amplifies the anti-lymphoma effect of PI3Kβ/δ blockage in diffuse large B-cell lymphoma. Leukemia. 2023;37(1):178–89. doi: 10.1038/s41375-022-01749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


