Abstract
Calcinosis, insoluble calcium compounds deposited in skin and other tissues, is a crippling sequela of dermatomyositis. Prolonged disease associated with ongoing inflammation, ischemia, repetitive trauma and certain autoantibodies are associated with calcinosis. Herein, we describe potential pathogenic mechanisms including the role of mitochondrial calcification. There are no widely effective treatments for calcinosis. We review available pharmacologic therapies for calcinosis including those targeting calcium and phosphorus metabolism; immunosuppressive/anti-inflammatory therapies; and vasodilators. Mounting evidence supports the use of various formulations of sodium thiosulfate in the treatment of calcinosis. Although early institution of aggressive immunosuppression may prevent calcinosis in juvenile dermatomyositis, only limited data support improvement once it has developed. Minocycline can be useful particularly for lesions associated with surrounding inflammation. Powerful vasodilators, such as prostacyclin analogues, may have promise in the treatment of calcinosis but further studies are necessary. Surgical removal of lesions when amenable is our treatment of choice.
Keywords: Calcinosis, juvenile dermatomyositis, adult dermatomyositis, composition, mechanism(s), mitochondrial calcification, treatment, surgical excision
A. Overview
Dermatomyositis (DM) is a rare autoimmune connective tissue disease characterized by distinct skin rashes and muscle inflammation. Calcinosis, the deposition of insoluble calcium salts in skin and other tissues, is a debilitating sequela of the disease. It occurs in 20-40% of juvenile dermatomyositis (JDM) [1, 2] and up to 20% of adult DM patients [3-5]. Delay in immunosuppression is strongly associated with calcinosis in JDM, while adult DM has been associated with long disease duration, fingertip ulcers, antibodies against Melanoma Differentiation Associated gene-5 (MDA-5) and Nuclear Matrix Protein-2 (NXP-2) [6, 7]. Calcinosis in DM can present as localized superficial calcareal (‘sponge-like’ in appearance) or superficial nodular, deeper tumoral deposits (deep calcareal or tumoral calcinosis), involving muscle or fascia (universalis), or rarely a generalized form covering an extensive area (exoskeleton) [8]. Extremities and pressure points are the most affected areas. Complications include ulceration, recurrent infection, and restriction of joint mobility, thereby resulting in significant morbidity. Imaging modalities, particularly plain x-rays, can assist in diagnosing and quantifying calcinosis. Other imaging modalities include ultrasound, computed tomography, positron emission tomography, and magnetic resonance imaging [9].
There are no widely effective medical therapies for calcinosis. In a survey performed by Orandi et al, 62-92% of experienced physicians chose non-immunosuppressive modalities to treat calcinosis [10]. However, since calcinosis is thought to be a sequela of inflammation, particularly in JDM, immunosuppression is often used to prevent the onset and progression of calcinosis. Our review focuses on recent advances in our knowledge underlying the origins of calcinosis and available treatment options.
B. Composition of calcified deposits in dermatomyositis
There have been several attempts to characterize the content of calcified deposits in DM, with some contrasting results. Early work from Landis and colleagues, using high-voltage electron-microscopic tomography (μCT), identified small individual spear-like hydroxyapatite crystals confined within individual collagen fibrils [11]. In his model, Landis proposed that the disordered fibrils, creating collagen holes, could act as nucleation sites for mineral deposits, while similarly limiting the growth of the crystals within the width of the hole, creating a tissue of hydroxyapatite crystals joined together by collagen [11]. Similar to the findings of Landis, later work by Eidelman confirmed the presence of small islands of mineralization, even less than 80 x 80 μm in size, in particular in early disease [12]. However, in longstanding disease, solid mineralization, with no islands, was seen throughout the deposit as assessed by μCT 3D projection. In contrast to the small islands described by Landis, Eidelman primarily observed collagen fibrils in the periphery of the deposit, presumably where the calcification was still ongoing, whereas the deposit itself was devoid of collagen. Thus, presence of collagen fibrils within the calcification deposit may indicate an early stage of calcification [12].
Work by Pachman and colleagues, using X-ray diffraction and synchrotron x-ray microdiffraction, confirmed that the mineral deposit contains hydroxyapatite [13]. However, in contrast to these findings, using the same technology, Eidelman found the only mineral present in all patients analyzed was carbonate apatite, and not hydroxyapatite [12], with no other calcium phosphate compounds present. They further observed small levels of sodium and magnesium, as well as trace elements of chlorine, aluminum, and sulfur to be present in the deposit [12]. Thus, the field has yet to resolve whether the mineral is carbonate apatite or hydroxyapatite.
Other than mineral, the calcified deposits also consist of several mineralization matrix proteins including pro-mineralization proteins such as bone sialoprotein, dentin matrix protein 1, and dentin phosphoprotein; as well as the mineralization inhibitors matrix G1a protein and osteopontin [13, 14]. Several of these components are also found in bone, however, reduced levels of osteopontin and increased levels of osteonectin are present in calcium deposits as compared to bone [13]. Given the important balance between pro-mineralization factors and mineralization inhibitors in development of calcification, levels of several inhibitors have been analyzed in circulation. Unexpectedly, levels of osteopontin were similar in healthy controls and JDM patients, including patients with calcinosis [15]. However, the authors noted that levels of another important mineralization inhibitor, fetuin-A, were markedly reduced in some of the JDM children with calcinosis, suggesting its involvement in calcinosis development. Further, levels of fetuin-A, a negative acute-phase reactant, were associated with age. Thus, the authors proposed that increasing levels of fetuin-A over time may, at least in part, explain why calcinosis is more prominent in juvenile as compared to adult DM [15].
Macrophages are known to infiltrate the calcified tissue and contribute to the local inflammatory milieu [16]. Similarly, many of the matrix proteins, including osteopontin, are likely to be released from local macrophages. Macrophages can also contribute to demineralization of ectopic calcification via the secretion of carbonic anhydrases that, in conjunction with H+-ATPases create a microacidic environment necessary for the dissolution of calcium phosphate crystals. Macrophages polarized towards M1 phenotype, commonly seen in inflammatory conditions, are characterized by decreased carbonic anhydrase 2 expression, suggesting that inflammation alters the decalcification capacity of macrophages [17]. Of note, under inflammatory conditions, macrophages may also differentiate into osteoclasts, several of which have been observed in close proximity to calcium deposits [14]. However, given the progressive nature of calcium deposits, osteoclasts are likely impaired in their capacity to resolve the mineral deposit or too few to efficiently counter the calcification process. Further studies are needed to address the role of osteoclasts in resolving calcified deposits in DM [14].
C. Mechanisms of calcification in dermatomyositis
Although the exact mechanism(s) contributing to calcinosis development are not fully understood, several studies have highlighted factors associated with calcinosis, which may provide insight into the pathogenesis (Figure 1).
Figure 1. Calcinosis pathogenesis.
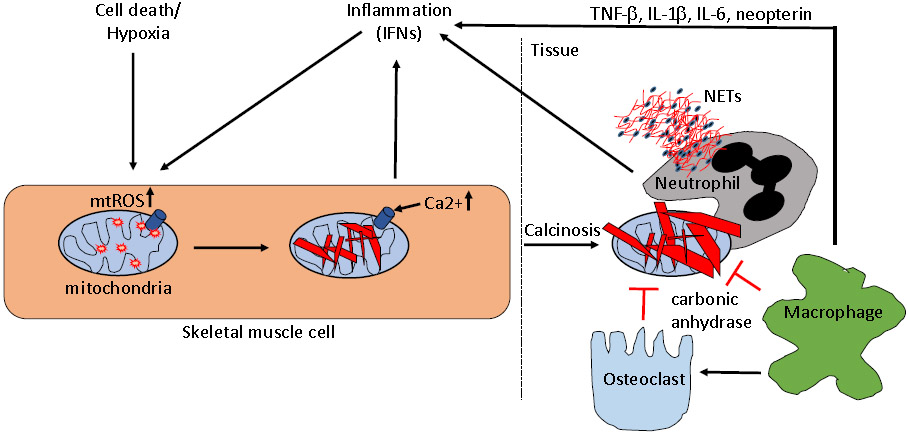
A depiction of main mechanisms suggested to be involved in the calcinosis pathogenesis in dermatomyositis. Various forms of cell death, trauma, and hypoxia, as well as inflammatory cytokines such as TNFα and IFNα, can promote mitochondrial generation of reactive oxygen species (mtROS) in skeletal muscle cells. Upon mtROS generation, calcium and phosphate accumulate in mitochondria precipitating as hydroxyapatite. This intramitochondrial calcification acts to promote inflammation, including type I IFNs and IL-1β. Calcified mitochondria are released into the extracellular space upon cell death, and accumulates in tissue to form large hydroxyapatite precipitates. Osteoclasts and macrophages attempt to degrade the calcium crystal through release of carbonic anhydrase, though this process seems to be impaired in dermatomyositis. Instead, infiltrating macrophages and neutrophils engage the calcium crystals resulting in induction of inflammatory cytokines and neutrophil cell death through formation of neutrophil extracellular traps (NETs), respectively, contributing to local tissue damage and inflammation. Targeting either of the depicted pathways, in particular mitochondrial ROS generation, is anticipated to result in reduced calcinosis development.
Clinically, it has been recognized that patients with antibodies targeting NXP2 and PM/Scl have an increased risk of developing calcinosis [7, 16]. However, the functional property of these antibodies, and whether they directly contribute to calcinosis pathogenesis is not known. Given the antigenic targets of the antibodies, it is plausible that the antibodies, upon recognition of extracellular antigens, such as occurs upon cell death, may form nucleic acid-containing immune complexes that induce inflammation, similar to what is seen in systemic lupus erythematosus (SLE) (18). Indeed, cell death [19], as well as elevated levels of immune complexes have been observed in JDM [20].
Duration of untreated disease is associated with muscle weakness, elevated levels of creatine kinase and lactate dehydrogenase, as well as development of calcinosis [21]. It is possible that a longer duration of untreated disease could contribute to calcinosis due to the prolonged inflammatory environment in tissue. Indeed, chronic cutaneous inflammation has been linked to presence of calcification [13]. Moreover, muscle damage that rapidly accrues in untreated disease can trigger local calcification, as demonstrated in vivo in mouse models [22]. Consistent with this observation, calcifications often occur at areas of frequent trauma, such as the elbows in DM and thumbs in systemic sclerosis (SSc) [23-25]. Presence of digital ulcers is associated with development of calcinosis in SSc, indicating a role for vascular ischemia in calcinosis pathogenesis [26, 27]. Further support of this is the finding that ulnar artery occlusion on ultrasound evaluation is highly correlated with the presence of calcinosis of the hands in SSc patients [28]. Similarly, in adult DM, patients with calcinosis more frequently have fingertip ulcers [7]. Hypoxia, known to promote calcification in vascular smooth muscle cells (29), is commonly seen in JDM [30].
With regards to inflammation-mediated calcification, the TNFα-308A allele, enriched in children with JDM as compared to healthy individuals, has been reported to be associated with enhanced TNFα production by peripheral blood mononuclear cells, as well as with development of calcification [31]. Similar results were seen in a larger study with the TNFα-308A allele being a risk factor for JDM, and in particular for calcinosis [32].
To model the calcification pathogenesis in vivo, cardiotoxin can be injected in mice to simulate injury-driven damage to muscle cells. Calcification occurs with high penetrance even in wild-type (75%), and more so in mice lacking osteopontin (100%) [22]. However, in contrast to human disease, the calcifications are readily cleared by osteoclast-like cells within 28 days. Thus, it is likely that DM-related factors, such as inflammatory cytokines, or autoantibodies, may be essential to promote chronicity of the calcification.
Another important insight from the cardiotoxin mouse model is the calcification of mitochondria shortly after skeletal muscle injury, suggesting that this may be an early nucleation site [22, 33]. Consistent with these findings, we found mitochondrial calcification occurs in skeletal muscle cells of JDM [34]. Mechanistically, mitochondrial accumulation of calcium and phosphate was driven through inflammatory components, including type I interferons (IFN), inducing robust mitochondrial reactive oxygen species production, facilitating calcium influx and subsequent formation of hydroxyapatite [34] (Figure 1). The calcified mitochondria promoted inflammation, with release of several inflammatory cytokines, including IL-1β, as well as upregulation of several type I interferon-regulated genes, commonly seen in JDM [35]. Using electron microscopy, calcified mitochondria were observed in the extracellular space, likely derived from damaged and/or dying muscle cells, with mitochondrial components also present in circulation at elevated levels, in particular in patients with calcinosis [36]. Of note, given the immunogenicity of mitochondria, autoantibodies targeting mitochondria were commonly observed in JDM (40%), primarily in patients with calcinosis, with antibodies being present even prior to clinical diagnosis of calcinosis, suggesting a potential predictive value of this novel biomarker [36].
Inflammation is not only acting upstream of calcification, but also downstream, as the tissue-deposited hydroxyapatite crystals are highly inflammatory. We have recently shown, both in tissue of JDM patients and in vitro, that infiltrating neutrophils engulf calcium phosphate crystals, leading to a programmed form of cell death, namely neutrophil extracellular trap (NET) formation, with expulsion of neutrophil chromatin and cytosolic content, causing local tissue damage and inflammation [20]. Of note, levels of NETs are elevated in JDM and DM, and associated with muscle damage [37], and possibly calcinosis [20]. However, subsequent studies indicated that not only calcium crystals, but also other mechanisms, including immune complexes, may act to induce NET formation in DM, similar to what has been seen in SLE [38, 39]. Infiltrating macrophages have also been shown to engulf tissue-deposited hydroxyapatite in JDM [20]. Depending on the shape of the crystal, with small needle-shaped crystals being more efficient [40], macrophages will undergo NLRP3-dependent inflammasome activation, and secrete IL-1β in affected tissue [41]. IL-1β [42] and IL-18 production have been noted by infiltrating immune cells, such as macrophages, in both asymptomatic and affected tissue in DM patients, with a reduction of infiltrating IL-18+ cells upon effective immunosuppressive treatment [43]. Further, macrophages, as well as macrophage-derived cytokines, including TNF-α, IL-1β, IL-6, IL-18, and neopterin, have all been found in ‘milk of calcium’ from JDM patients, emphasizing macrophage activation occurring locally in tissue [16]. However, the contribution of macrophage (and neutrophil) activation to disease, and in particular to calcinosis, is yet to be determined.
Another important consideration, is the concept of crystal formation within the muscle cell, such as occurs during mitochondrial calcification. Data from our group demonstrate that such mitochondrial-derived crystals engage several inflammatory pathways to promote generation of inflammatory cytokines, including interferons and IL-1β [35]. Figure 1 summarizes the mechanisms thought to be involved in the pathogenesis of calcinosis in DM.
D. Therapeutics
In this section, we will discuss pharmacological and non-pharmacological treatment of calcinosis. We have subdivided the pharmacological therapies, broadly into 4 categories based on their mechanism of action: those affecting calcium and phosphorus metabolism, anti-inflammatory/immunosuppressive therapies, vasodilatory therapies, and other mechanism of action. Table 1 summarizes the existing pharmacological therapies for the treatment of calcinosis in DM.
Table 1.
Existing Pharmacological Therapies for Calcinosis in Dermatomyositis
| Name of drug | Dose | Mechanism of action | Side effects | Level of evidence/source |
|---|---|---|---|---|
| Diltiazem (oral) | 330-360mg/day in adult DM 5-6mg/kg/day in juvenile DM |
Reduces calcium efflux from dystrophic muscle | Malaise, Nausea, headache, lower extremity edema, and lowering the blood pressure. | Bertorini TE et al(47) Vinen CS et al(48) Abdallah Lotf et all (49) S.J. Balin et al(50) |
| Amlodipine (oral) | 10mg a day | Peripheral vasodilation | Same as above | S.J. Balin et al(50) |
| Sodium thiosulfate (topical) | 25% sodium thiosulfate compounded in zinc oxide ointment-applied 2 to 3 times daily to affected areas (base can be substituted with cold cream or petrolatum) | Chelates calcium, enhances solubility and clearance of calcinosis | Transient burning sensation after application | Topham C et al(57) Eleryan MG et al (58) Del Barrio-Díaz P et al (59) Ma JE et al(60) Nowaczyk J et al(61) |
| Sodium thiosulfate (intralesional) | Volume-0.1ml/cm^2 of affected area Concentration-40 mg/ml | Infection, Local pain or transient burning sensation following injection | Goossens J et al(62) Baumgartner-Nielsen J et al(63) López-Sundh AE et al(64) Winter AR et al(69) |
|
| Sodium thiosulfate (intravenous) | 12.5g-25g sodium thiosulfate three times a week | Fatigue, nausea, vomiting, metabolic acidosis not requiring treatment | Badawi AH et al(68) Mageau A et al(69) Song P et al(70) |
|
| Bisphosphonates Pamidronate (intravenous) | 1mg/kg/day for 3 days and given every 3 months | Inhibit macrophage activity, decrease bone turnover, prevent formation of calcium phosphate crystals | flu-like symptoms in first 24-72 hrs after infusion | Tayfur AC et al(75) Marco Puche A et al(76) Slimani S et al(77) I. Saini et al(78) |
| Alendronate (oral) | 10mg/day | Esophagitis | M.L. Bianchi et al(80) | |
| Probenecid (oral) | 250mg/day 1.5g/day 1g/day |
Increase phosphate excretion- decrease serum calcium phosphate product | flushing, headache, dizziness, anorexia | Skuterud E et al(89) Eddy MC et al(90) Nakamura H et al(91) |
| TNF-alpha inhibitors-Infliximab (intravenous) | 3mg/kg at 0,2,6 week and then every 8 weeks | Suppression of cytokine release | Infection of calcinotic lesion, allergic reaction | Riley P et al(92) Campanilho-Marques R et al(93) Tosounidou S et al(94) |
| JAK-inhibitor Tofacitinib (oral) Baricitinib(oral) |
5mg twice a day or 11mg extended release od 6mg bid |
Decreases ROS-mediated mitochondrial calcium accumulation | Hypercalcemia, weight gain, vascular thrombosis, malignancy and serious infections | Wendel S et al(100) Betül Sözeri et al(101) Papadopoulou C et al(102) Sabbagh S et al(103) |
| Rituximab (intravenous) | 375-500 mg/m^2 body surface area, 2-4 infusions | Suppress immunity via anti-CD20 action | Infections | Bader-Meunier B et al(96) |
| IVIG | 2g/kg/month given over 3-4 days | Competes with C5-C9 complex deposition in tissues | Headache, nausea, thrombosis | Aggarwal R et al(107) Touimy M et al(108) Peñate Y et al(109) Shahani L et al(110) Galimberti F et al(111) |
| Warfarin (oral) | 1mg/day | Reduces gamma carboxylation | Monitor INR if bleeding happens | Cukierman T et al(123) |
| Minocycline (oral) | 50-100mg/day | Anti-inflammatory and anti-microbial | Dizziness, fatigue | Robertson LP et al(103) S.J. Balin et al(50) Carmen Fonseca et al(104) |
| Treprostinil (oral) | 0.125mg three times a day and can be increased gradually as tolerated | Prostaglandin analogue/vasodilator |
Headache and gastrointestinal disturbance like GERD | Chung, Melody et al(118) |
Calcium and phosphorus metabolism
Calcium channel blockers
It is hypothesized that efflux of calcium from fibroblasts or muscle cells heralds the formation of calcinosis in inflammatory myopathy. Calcium channel blockers, particularly diltiazem, reduce the calcium content in dystrophic muscle and inhibit the mitochondrial sodium-calcium exchange (calcium efflux). Calcium channel blockers are also vasodilators so may be beneficial to calcinosis via multiple mechanisms [44-46]. Bertorini et al described a case series of 6 patients with JDM and calcinosis who received 6 mg/kg/day of diltiazem. Four patients completed the treatment for two years and two of them (50%) showed regression of calcinosis [47]. Several independent case reports in adult DM have described successful use of 330-360 mg/day of diltiazem to treat calcinosis [48, 49]. A retrospective study of 78 patients (1996-2009) with calcinosis associated with connective tissue diseases (CTD), performed at Mayo Clinic, found that diltiazem showed a favorable response in 9 out of 17 treated patients (53%) [50]. Overall, calcium channel blockers are well-tolerated, with the most common side effects being headache, lower extremity edema, and lowering of blood pressure.
Sodium Thiosulfate
Sodium thiosulfate acts as a calcium chelating agent by producing calcium thiosulfate, increasing the solubility, and hastening the clearance of the precipitated deposit. However, its mechanism in reversing calcinosis is presumed to be related to cation-chelating properties [51].
Topical:
Topical sodium thiosulfate has shown promise in treating calcinosis secondary to different pathologies [52-54]. There are several case reports of topical sodium thiosulfate in the treatment of calcinosis lesions in patients with CTDs, including systemic sclerosis (SSc) [55, 56] and amyopathic DM [57]. A combination approach using fractionated CO2 laser to deliver a lower dose of sodium thiosulfate demonstrated improvement in calcinosis lesions in two DM patients [58]. Different formulas of sodium thiosulfate have also shown positive impact in treating calcinosis lesions in DM patients [59]. In a retrospective study at Mayo clinic, 28 patients with calcinosis due to CTD received sodium thiosulfate 25% compounded in zinc oxide ointment applied two or three times daily [60]. Most patients (69%) had clinical improvement and two patients had complete resolution of calcinosis lesions without recurrence. A systematic review identified 136 patients (71 SSc, 16 DM, and 7 overlap syndrome patients) treated with topical or intralesional sodium thiosulfate [61]. Most patients experienced improvement, 81% with topical sodium thiosulfate and 74% with intralesional sodium thiosulfate.
Intralesional:
Most reports about intralesional sodium thiosulfate use in treating calcinosis are from case reports and case series, with the majority demonstrating positive results [62-64]. A prospective cohort evaluated the efficacy of intralesional sodium thiosulfate in treating dystrophic calcinosis in a double-blind, placebo-controlled fashion [65]. Five patients (two DM, two SSc, and one morphea) were enrolled. Two lesions were selected for each patient, and normal saline or sodium thiosulfate 40 mg/ml was injected into lesions at baseline and 1- and 2-month follow-up. One patient was lost to follow-up after one month of therapy and excluded from the analysis. The study terminated earlier than anticipated due to funding issues. Only one patient had improvement in the calcinosis lesion size and physician global assessment after 12 weeks. The lower dose of sodium thiosulfate (40 mg/ml) used in this study and the shorter period of treatment might have affected the results.
Intravenous:
Intravenous (IV) sodium thiosulfate therapy has shown beneficial effects in treatment of calciphylaxis in patients with end stage renal disease on hemodialysis [66, 67]. There are multiple case reports describing improvement in calcinosis size, pain and functional ability after IV sodium thiosulfate therapy for DM-related calcinosis [68, 69]. One study reported no significant benefit with IV sodium thiosulfate for management of long-standing and extensive CTD-associated calcinosis in three patients, two with DM and one with mixed CTD [70]. There is an ongoing open-label clinical trial assessing the efficacy of IV sodium thiosulfate for treatment of calcinosis associated with juvenile and adult DM.
Side effects:
The most common adverse reactions during sodium thiosulfate treatment for calcinosis and calciphylaxis were fatigue, nausea and vomiting. Rare side effects (<2%) including bad taste with periorbital tingling, fatigue, hypotension, and decreased hearing have been reported with IV sodium thiosulfate use.
Bisphosphonates
Bisphosphonates may counteract calcinosis by two mechanisms: they can hinder the activity of macrophages and also reduce bone turnover [71]. Tayfur et al reported a case series of six JDM patients with calcinosis who were treated with bisphosphonates. They were all on background immunosuppression with agents including methotrexate, IVIG and corticosteroids. Four out of the six patients (66%) showed improvement within three months of treatment and complete resolution in one year [72]. The patients were on weight-based dosing of IV pamidronate or oral alendronate 70mg per week. Puche A et al described a similar response in a case series of three patients who received 1 mg/kg/day of IV pamidronate for three consecutive days every three months [73]. Calcinosis universalis, which is considered the most extensive form of calcinosis, also responded to IV pamidronate given at a dose of 2 mg/kg/year in a young patient with JDM [74]. Intravenous bisphosphonates can cause flu-like symptoms for the first 24-72 hours after infusion but can be remediated easily with acetaminophen or ibuprofen. Oral bisphosphonates can cause esophagitis and gastrointestinal ulcers [75].
Probenecid
Biochemical studies have shown that probenecid increases renal phosphate clearance, thereby reducing the plasma calcium phosphate product, resulting in dissolution of calcinosis [76]. Probenecid was initially used for treatment of calcinosis in SSc [77-79]. Skuterud et al described a case of JDM and calcinosis, who showed clinical response within a few weeks of adding probenecid 250 mg daily to existing immunosuppression with D-penicillamine [80]. Eddy et al described a JDM case of refractory calcinosis universalis that responded to probenecid 1.5 g daily in addition to dietary restriction of calcium and phosphorus [81]. Most reports used doses ranging from 1-2 g daily and treatment response was evident after 6 months of treatment [82]. Side effects include flushing, headache, dizziness, and anorexia.
Anti-inflammatory/Immunosuppressive Therapies
Tumor Necrosis Factor-α inhibitors (TNFi)
Patients with dystrophic calcification have high levels of TNF-α. Activated macrophages and T cells increase the production of TNF- α in active JDM, which subsequently triggers an inflammatory cascade with release of cytokines and endothelial adhesion molecules [31]. Therefore, TNF- α inhibitors may play a role in reducing calcinosis.
In 2008, Riley et al described softening and regression of calcinosis in 3 out of 5 children (60%) with refractory JDM after infliximab infusions given for 18 months. Infliximab 3 mg/kg was infused at 0, 2 and 6 weeks, and then every 8 weeks thereafter [83]. A retrospective analysis of 60 children with JDM who received either infliximab or adalimumab for at least 3 months was performed. Twenty-eight children had calcinosis and 54% showed improvement in size of calcinotic lesions after a median treatment time of 2.7 years [84]. Two case reports have confirmed a favorable response in adult patients, one with SSc/myositis overlap, and the other with adult DM [85,86]. The most common side effects include infections and allergic reactions.
Rituximab
Rituximab is a monoclonal antibody against the CD20 antigen on the surface of B-lymphocytes. It causes B-cell depletion by activating complement-dependent B-cell cytotoxicity and through antibody-mediated cellular toxicity. A multicenter prospective registry– Rituximab for Autoimmune diseases (AIR)– identified 9 patients with severe JDM from 2005-2010 who received 2-4 infusions of rituximab, each dose ranging from 375-500 mg/m2. Six of the nine patients had calcinosis and none of them showed improvement after 3 years of follow-up [87]. Post-hoc analysis of the Rituximab in Myositis (RIM) trial, included 7 adult and 22 JDM patients with calcinosis and refractory myositis. Although there was significant improvement in visual analog scale for cutaneous disease activity in adults (3.22 to 1.72) and children (3.26 to 1.56), there was no improvement in calcinosis after 3.6 years of follow-up [88]. Common side effects of rituximab included infection of calcinotic lesions, and occasionally infusion related adverse events [87].
Janus Kinase inhibitors (JAKi)
JAK-STAT is a signal transduction pathway that regulates inflammation-induced ROS-mediated mitochondrial accumulation, an important process mediating calcinosis. Shneyderman et al reported a case series of three adults with refractory DM who showed partial regression of calcinosis with 5 mg tofacitinib given twice a day for three months [89]. Wendel et al reported successful use of a similar treatment regimen given over 28 months, in two adult DM patients with extensive calcinosis and lung involvement [90]. Sozeri et al reported a case series of two JDM patients with recalcitrant calcinosis who showed complete resolution in one of them and more than 50% reduction in calcinosis in the other [91]. There are two other case reports in JDM, where JAKi halted the progression of calcinosis despite interrupted treatment [92] with significant regression after 6 months of treatment [93]. JAKi have several potential side effects including vascular thrombosis, malignancy and serious infections.
Intravenous Immunoglobulin (IVIG)
In DM, auto-antibodies against microvascular components activate the classic complement pathway and lead to deposition of membrane attack complex (C5b-C9) in the affected tissues. [94]. IVIG hampers this process by competing for the same receptors in the tissue and also decreases T-cell activation [95,96]. A randomized controlled trial of IVIG in adult DM showed a favorable response in composite response criteria in the treatment arm (78.7%) compared to placebo (43.8%) starting at the 3rd or 4th infusion [97]. There is some evidence supporting the utility of IVIG in the treatment of calcinosis specifically, but the mechanism is unclear, and could be related to better control of the underlying inflammatory disease. Few case reports have shown that IVIG improves recalcitrant calcinosis in juvenile and adult DM after 5-6 courses (each dosed at 2 g/kg/month) [98-100]. In 2015, a retrospective analysis found that five out of eight DM patients treated with IVIG, experienced improvement in their calcinosis [101]. Fluid overload symptoms like headache and nausea are commonly reported by patients and there is an increased risk for thrombotic complications.
Minocycline
Minocycline inhibits collagenolytic enzymes, particularly matrix metalloproteinase, resulting in anti-inflammatory effects. In addition, minocycline chelates calcium, which could be useful for the treatment of calcinosis [102]. Robertson et al described clinical and radiological improvement in 8 of 9 patients in an open-label study of calcinosis associated with SSc when minocycline was given at a dose of 50-100 mg daily [103]. However, a retrospective analysis by Balin et al showed that only 1 of 6 patients treated with minocycline had partial clinical improvement in calcinosis [50]. The largest study reported thus far is an observational study of a single center in the UK which reported that 34 of 78 SSc patients (43.6%) with refractory calcinosis improved on repeated courses of 50-200 mg daily minocycline for 6-12 weeks duration [104]. Common side effects include dizziness and fatigue.
Vasodilators
Persistent ischemia, as evidenced by associations with long duration of Raynaud’s phenomenon, digital ulcer burden and acro-osteolysis, is likely involved in the pathogenesis of calcinosis in SSc and DM [7, 25,105]. Therefore, drugs targeting vascular instability like phosphodiesterase 5 inhibitors, prostacyclin analogues, and endothelin receptor antagonists should be explored as potential options for calcinosis treatment.
Calcium channel blockers
Diltiazem has been discussed above. In the retrospective analysis done by Balin et al, there was only one patient who used amlodipine (at 10 mg/day) for calcinosis treatment and had a favorable response [50].
Treprostinil
Treprostinil is a prostacyclin analogue and a potent vasodilator. A recent pilot study of oral treprostinil in 12 SSc patients with calcinosis of the hands showed non-progression using a validated radiographic scoring system in 80% of patients who completed the treatment [106]. Headaches and dose-dependent gastrointestinal side effects were commonly reported.
Miscellaneous
Warfarin
Patients with calcinosis have high levels of calcium-binding amino acid gamma-carboxyglutamic acid in involved tissues and urine [107,108]. Low dose warfarin (1 mg/day) inhibits γ-carboxylation which might improve calcinosis, particularly smaller lesions that may depend relatively more on γ-carboxyglutamic acid for crystal growth. There have been two clinical trials in patients with calcinosis universalis associated with SSc or DM. Patients were treated with warfarin at 1 mg daily for 18 months. Extra-skeletal uptake of technetium 99m-diphosphonate (compared to bone uptake) was reduced in two of four patients in a non-blinded trial, and two of three patients in the treatment arm of a double-blinded trial. However, none of the patients showed clinical improvement in calcinosis [109,110]. Small calcinotic lesions (less than two cm) responded to long term treatment (1 year) of low dose warfarin in a case report and two of three patients in a case series of patients with SSc [110,111]. Although low dose warfarin is safe with no bleeding risks, evidence supporting its use in treatment of calcinosis is limited. Incidentally, matrix Gla proteins (MGP), the endogenous inhibitors of tissue mineralization, depend on the γ-carboxylation of glutamine residues for their ability to bind to calcium and hydroxyapatite, thus preventing calcium crystal growth [112]. Furthermore, MGP proteins are also known for binding and inhibiting the osteo-inductive function of bone morphogenetic proteins [113,114]. Hence, the potential limitation and risk involved with warfarin treatment is enhancement in tissue mineralization, particularly vascular calcification, by inhibiting the activity of MGP [115,116].
Non-Pharmacological treatment
Surgical Excision
Surgical excision can be an effective approach for recalcitrant calcinosis that causes disability due to severe pain, recurrent ulceration, nerve compression, or due to its proximity to a joint. Surgical treatment of symptomatic lesions in 28 CTD patients with calcinosis, had favorable results: 5 patients experienced partial improvement and 22 patients experienced complete resolution after surgical removal [50]. Although an attractive option with immediate results, potential risks associated with surgical removal of calcinotic lesions include infection, delayed wound healing, local neuromuscular injury, and recurrence. In addition, not all lesions are accessible and amenable to surgery. Despite these limitations, surgical removal is our treatment of choice for accessible lesions in agreeable patients.
Extracorporeal shockwave treatment
Extracorporeal shockwave therapy is a minimally invasive and safe treatment for nephrolithiasis, which has been explored to break down subcutaneous calcinosis. In a study done by Sultan-Bichat et al, pain relief was achieved in two out of three SSc patients, and also in the only included DM patient, possibly due to the release of locally compressed nerves [117]. Blumhardt et al performed a 12-week study involving four SSc patients, of whom two achieved significant pain relief. This may be considered as an alternative option for analgesia in recalcitrant calcinosis lesions associated with SSc and DM [118,119].
E. Future Therapeutic Targets
Prior work from Meyer and colleagues demonstrated that patients with DM have increased reactive oxygen species (ROS) production originating from mitochondria, likely driven by inflammatory mediators such as IFN-β [120]. Further, the authors demonstrated, using transcriptomic analysis, that mitochondrial genes were down-regulated in early untreated DM muscles. Using in vitro systems, we recently demonstrated that type I IFN-driven mitochondrial ROS generation facilitates intramitochondrial calcification, with mitochondrial ROS scavengers reducing both calcification and type I IFN signature in muscle cells [20, 35, 36]. Importantly, the ROS scavenger N-acetyl cysteine reduced mitochondrial dysfunction, type I IFN signature, and muscle weakness in an experimental autoimmune myositis model [120]. Work from our group, as well as others, have similarly implicated ROS, in particular mitochondrial ROS, in promoting inflammation, autoimmunity, and organ damage in vivo in lupus-prone mice [39, 121], clearly suggesting that mitochondrial ROS may have implications as a therapeutic target in several inflammatory conditions. Studies investigating the role of mitochondrial ROS in development of calcinosis in vivo are warranted. Considering the important role of inflammatory cytokines, including type I IFNs, in mitochondrial dysfunction, muscle damage and subsequent calcinosis, targeting inflammatory cytokines may alleviate disease progression, including calcinosis. Consistent with this interpretation, targeting the JAK/STAT signaling pathway, essential for type I IFN signaling, has shown potential in treating calcinosis [90].
Similar to bisphosphonates [72,122], other drugs able to prevent osteoclast-mediated bone resorption, including denosumab [123], have been associated with reduction in calcinosis. In contrast, teriparatide, acting through parathyroid hormone to promote new bone formation, has been associated with worsening of calcinosis, including in DM [124, 125]. Although bisphosphonates are known to prevent osteoclast-mediated bone resorption, which is helpful in the treatment of osteoporosis, the beneficial effect on calcification is likely derived from the capacity of bisphosphonates to bind to hydroxyapatite crystals, preventing calcification [126,127]. Further, bisphosphonates, similar to pyrophosphate, can prevent formation and aggregation of calcium phosphate crystals, as well as inhibit amorphous calcium phosphate to transform into insoluble hydroxyapatite [128]. Whether a combination of bisphosphonate and an immunosuppressive drug would have additive value in resolving calcifications is yet to be determined.
F. Approach to Calcinosis in our Center
Herein, we will discuss our treatment approach for calcinosis in adult DM patients who we manage in our multi-disciplinary Rheumatologic Dermatology Clinic. We aggressively treat our DM patients with active skin, muscle, lung, and/or joint disease with immunomodulatory agents including corticosteroids, mycophenolate mofetil, IVIG, hydroxychloroquine, methotrexate, azathioprine, tofacitinib, tacrolimus, and/or rituximab. For calcinosis specifically, we refer patients to our Hand and Plastic surgeons for surgical removal of calcinosis whenever feasible and acceptable to patients. This approach has resulted in immediate favorable results for most patients. While most surgical debridements are outpatient procedures and do not require medication adjustment, occasionally extensive debridements are done where we follow the perioperative guidelines on immunosuppression by ACR [129] in order to minimize delayed healing and infections. For small lesions at the surface of the skin, we give a trial of compounded topical sodium thiosulfate for at least 2-3 months. If there is erythema or inflammation associated with the calcinotic lesion(s) we try minocycline at 50-100 mg daily for at least 3-6 months. In the presence of infection, antimicrobial treatment with cephalexin or appropriate antibiotics based on culture is given for 10-14 days. Wound care is beneficial for non-healing ulcers. For those patients who have large areas of calcinosis (deep calcareal or calcinosis universalis) we refer patients for IV sodium thiosulfate infusions given 3 times a week, overseen by a nephrologist. Despite such multidisciplinary care, including rheumatology, dermatology, wound care, dermatologic or plastic surgery, and nephrology, some patients continue to experience recalcitrant or recurrent lesions. New and effective therapeutic avenues need to be explored for this debilitating condition.
Summary
Although calcinosis contributes to considerable disability and morbidity in affected patients with dermatomyositis, it is not well-studied. Imbalance between pro-mineralization factors and inhibitors, protracted local inflammation mediated through macrophages, and calcified mitochondria in inflamed muscle associated with inflammatory cytokines are some theories proposed to explain its pathogenesis. The available pharmacologic treatment options reviewed in this chapter are based on observational studies and anecdotal case reports. Randomized controlled trials using validated outcome measures are needed to guide physicians appropriately. Surgical excision should be considered for accessible calcinotic lesions.
Practice Points.
Pharmacologic treatment options for calcinosis in DM are supported by low level of evidence only.
Early institution of immunosuppressive therapy can prevent development and progression of calcinosis particularly in JDM.
Surgical removal of accessible calcinotic lesions can provide immediate relief and improved functionality.
Research Agenda.
Large scale randomized controlled trials with validated outcome measures are needed to develop evidence-based treatment guidelines for calcinosis associated with DM.
Drugs targeting type 1 interferon pathway and vasodilators, such as prostacyclin analogues, need to be explored further.
Financial disclosure
The authors received no funding or sponsorship in preparing this manuscript except those mentioned below:
Srijana Davuluri received T32 training grant from NIH- 2T32AR050942 which supports her postdoctoral research fellowship.
Christian Lood received funding from CureJM, Seattle Children’s Juvenile Myositis Center of Excellence, NIH R21AR077565 and NIH R21AR079542.
Lorinda Chung receives funding from the Scleroderma Research Foundation.
Footnotes
Declaration of competing interest
The authors declare no conflict of interest regarding this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Srijana Davuluri, Stanford School of Medicine, Division of Immunology &Rheumatology, 1000 Welch Road, Suite 204, Palo Alto, California-94304.
Bhargavi Duvvuri, University of Washington, Department of Medicine, Division of Rheumatology, 750 Republican Street, Seattle, WA-98109.
Christian Lood, University of Washington, Division of Rheumatology, 750 Republican Street, Room E-545, Seattle WA 98109.
Sara Faghihi-Kashani, Stanford School of Medicine, Division of Immunology &Rheumatology, 1000 Welch Road, Suite 204, Palo Alto, California-94304.
Lorinda Chung, Stanford School of Medicine & Palo Alto VA Health Care System, Division of Immunology &Rheumatology, 1000 Welch Road, Suite 203, Palo Alto, California-94304.
REFERENCES
- 1.Mathiesen P, Hegaard H, Herlin T, et al. Long-term outcome in patients with juvenile dermatomyositis: a cross-sectional follow-up study. Scand J Rheumatol 2012;41(1):50–8. [DOI] [PubMed] [Google Scholar]
- 2.Clemente G, Piotto DG, Barbosa C, et al. High frequency of calcinosis in juvenile dermatomyositis: a risk factor study. Rev Bras Reumatol 2012;52(4):549–53. [PubMed] [Google Scholar]
- 3.Gutierrez A Jr., Wetter DA. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol Ther 2012;25(2):195–206. [DOI] [PubMed] [Google Scholar]
- 4.Walsh JS, Fairley JA. Calcifying disorders of the skin. J Am Acad Dermatol 1995;33(5 Pt 1):693–706. quiz 7-10. [DOI] [PubMed] [Google Scholar]
- 5.Cook CD, Rosen FS, Banker BQ. Dermatomyositis and focal scleroderma. Pediatr Clin 1963;10:979–1016. [DOI] [PubMed] [Google Scholar]
- 6.Fisler RE, Liang MG, Fuhlbrigge RC, et al. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol 2002;47(4):505–11. [DOI] [PubMed] [Google Scholar]
- 7.Valenzuela A, Chung L, Casciola-Rosen L, Fiorentino D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol 2014;150(7):724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blane CE, White SJ, Braunstein EM, et al. Patterns of calcification in childhood dermatomyositis. AJR Am J Roentgenol 1984;142(2):397–400. [DOI] [PubMed] [Google Scholar]
- 9.Mar D, Valenzuela A, Stevens KJ, et al. A narrative review of imaging in calcinosis associated with systemic sclerosis. Clin Rheumatol 2021;40(10):3867–74. [DOI] [PubMed] [Google Scholar]
- 10.Orandi A, Baszis K, Dharnidharka V, et al. Assessment, classification and treatment of calcinosis as a complication of juvenile dermatomyositis: a survey of pediatric rheumatologists by the childhood arthritis and rheumatology research alliance (CARRA). Pediatr Rheumatol 2017;15(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landis WJ. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 1995;16(5):533–44. [DOI] [PubMed] [Google Scholar]
- 12.Eidelman N, Boyde A, Bushby AJ, et al. Microstructure and mineral composition of dystrophic calcification associated with the idiopathic inflammatory myopathies. Arthritis Res Ther 2009;11(5):R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pachman LM, Veis A, Stock S, et al. Composition of calcifications in children with juvenile dermatomyositis: association with chronic cutaneous inflammation. Arthritis Rheum 2006;54(10):3345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urganus AL, Zhao YD, Pachman LM. Juvenile dermatomyositis calcifications selectively displayed markers of bone formation. Arthritis Rheum 2009;61(4):501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marhaug G, Shah V, Shroff R, et al. Age-dependent inhibition of ectopic calcification: a possible role for fetuin-A and osteopontin in patients with juvenile dermatomyositis with calcinosis. Rheumatology (Oxford) 2008;47(7):1031–7. [DOI] [PubMed] [Google Scholar]
- 16.Chung MP, Richardson C, Kirakossian D, et al. Calcinosis biomarkers in adult and juvenile dermatomyositis. Autoimmun Rev 2020;19(6):102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barinda AJ, Ikeda K, Hirata KI, Emoto N. Macrophages highly express carbonic anhydrase 2 and play a significant role in demineralization of the ectopic calcification. Kobe J Med Sci 2017;63(2):E45–e50. [PMC free article] [PubMed] [Google Scholar]
- 18.Lövgren T, Eloranta ML, Båve U, et al. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 2004;50(6):1861–72. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Fedczyna TO, McVicker V, et al. Apoptosis in the skeletal muscle of untreated children with juvenile dermatomyositis: impact of duration of untreated disease. Clin Immunol 2007;125(2):165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duvvuri B, Pachman LM, Morgan G, et al. Neutrophil extracellular traps in tissue and periphery in juvenile dermatomyositis. Arthritis Rheumatol 2020;72(2):348–58. [DOI] [PubMed] [Google Scholar]
- 21.Pachman LM, Abbott K, Sinacore JM, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J Pediatr 2006;148(2):247–53. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Urganus AL, Spevak L, et al. Characterization of dystrophic calcification induced in mice by cardiotoxin. Calcif Tissue Int 2009;85(3):267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeltzel MF, Oberle EJ, Robinson AB, et al. The presentation, assessment, pathogenesis, and treatment of calcinosis in juvenile dermatomyositis. Curr Rheumatol Rep 2014;16(12):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pachman LM, Boskey AL. Clinical manifestations and pathogenesis of hydroxyapatite crystal deposition in juvenile dermatomyositis. Curr Rheumatol Rep 2006;8(3):236–43. [DOI] [PubMed] [Google Scholar]
- 25.Valenzuela A, Baron M, Rodriguez-Reyna TS, et al. Calcinosis is associated with ischemic manifestations and increased disability in patients with systemic sclerosis. Semin Arthritis Rheum 2020;50(5):891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valenzuela A, Baron M, Herrick AL, et al. Calcinosis is associated with digital ulcers and osteoporosis in patients with systemic sclerosis: A Scleroderma Clinical Trials Consortium study. Semin Arthritis Rheum 2016;46(3):344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avouac J, Mogavero G, Guerini H, et al. Predictive factors of hand radiographic lesions in systemic sclerosis: a prospective study. Ann Rheum Dis 2011;70(4):630–3. [DOI] [PubMed] [Google Scholar]
- 28.Fairchild R, Chung M, Sharpless L, et al. Ultrasound detection of calcinosis and association with ulnar artery occlusion in patients with systemic sclerosis. Arthritis Care Res (Hoboken) 2021;73(9):1332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mokas S, Larivière R, Lamalice L, et al. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int 2016;90(3):598–609. [DOI] [PubMed] [Google Scholar]
- 30.Preuße C, Allenbach Y, Hoffmann O, et al. Differential roles of hypoxia and innate immunity in juvenile and adult dermatomyositis. Acta Neuropathol Commun 2016;4(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pachman LM, Liotta-Davis MR, Hong DK, et al. TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum 2000;43(10):2368–77. [DOI] [PubMed] [Google Scholar]
- 32.Mamyrova G, O'Hanlon TP, Sillers L, et al. Cytokine gene polymorphisms as risk and severity factors for juvenile dermatomyositis. Arthritis Rheum 2008;58(12):3941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duvvuri B, Lood C. Mitochondrial calcification. Immunometabolism 2021;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvvuri B, Pachman L, Moore R, et al. Mitochondrial ROS as a regulator of calcinosis in juvenile dermatomyositis. American College of Rheumatology Convergence; 2020. [Google Scholar]
- 35.Duvvuri B, Pachman L, Wang T, et al. Mitochondrial calcification-induced inflammation in human skeletal muscle and immune Cells. American college of rheumatology convergence. Arthritis Rheumatol; 2021. [Google Scholar]
- 36.Duvvuri B, Pachman L, Moore R, et al. Mitochondrial contribution to juvenile dermatomyositis pathogenesis. ACR/ARP Annual Meeting: Arthritis Rheumatol; 2019. [Google Scholar]
- 37.Seto N, Torres-Ruiz JJ, Carmona-Rivera C, et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight 2020;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lood C, Arve S, Ledbetter J, Elkon KB. TLR7/8 activation in neutrophils impairs immune complex phagocytosis through shedding of FcgRIIA. J Exp Med 2017;214(7):2103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lood C, Blanco LP, Purmalek MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22(2):146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebre F, Sridharan R, Sawkins MJ, et al. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci Rep 2017;7(1):2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin C, Frayssinet P, Pelker R, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci U S A 2011;108(36):14867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Authier FJ, Mhiri C, Chazaud B, et al. Interleukin-1 expression in inflammatory myopathies: evidence of marked immunoreactivity in sarcoid granulomas and muscle fibres showing ischaemic and regenerative changes. Neuropathol Appl Neurobiol 1997;23(2):132–40. [PubMed] [Google Scholar]
- 43.Helmers SB, Bruton M, Loell I, et al. Expression of interleukin-18 in muscle tissue of patients with polymyositis or dermatomyositis and effects of conventional immunosuppressive treatment. Rheumatology (Oxford) 2018;57(12):2149–57. [DOI] [PubMed] [Google Scholar]
- 44.Wrogemann K, Nylen EG. Mitochondrial calcium overloading in cardiomyopathic hamsters. J Mol Cell Cardiol 1978;10(2):185–95. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz A. Molecular studies of the calcium antagonist binding site on calcium channels. Am J Cardiol 1994;73(6):B12–B4. [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharya SK, Palmieri GM, Bertorini TE, Nutting DF. The effects of diltiazem in dystrophic hamsters. Muscle Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 1982;5(1):73–8. [DOI] [PubMed] [Google Scholar]
- 47.Bertorini TE, Sebes JI, Palmieri G, et al. Diltiazem in the treatment of calcinosis in juvenile dermatomyositis. J Clin Neuromuscul Dis 2001;2(4):191–3. [DOI] [PubMed] [Google Scholar]
- 48.Vinen C, Patel S, Bruckner F. Regression of calcinosis associated with adult dermatomyositis following diltiazem therapy. Rheumatology 2000;39(3):333–4. [DOI] [PubMed] [Google Scholar]
- 49.Abdallah-Lotf M, Grasland A, Vinceneux P, Sigal-Grinberg M. Regression of cutis calcinosis with diltiazem in adult dermatomyositis. Eur J Dermatol: EJD. 2005;15(2):102–4. [PubMed] [Google Scholar]
- 50.Balin SJ, Wetter DA, Andersen LK, Davis MD. Calcinosis cutis occurring in association with autoimmune connective tissue disease: the Mayo Clinic experience with 78 patients, 1996-2009. Arch Dermatol 2012;148(4):455–62. [DOI] [PubMed] [Google Scholar]
- 51.Generali JA, Cada DJ. Sodium thiosulfate: calciphylaxis. Hosp Pharm 2015;50(11):975–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbott J, Kowalski EH, Klein S, et al. Iatrogenic calcinosis cutis secondary to calcium chloride successfully treated with topical sodium thiosulfate. JAAD Case Reports 2020;6(3):181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhari N, Bharti P. Dystrophic calcinosis cutis in autosomal recessive dystrophic epidermolysis bullosa. BMJ Case Reports CP 2019;12(9):e231287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doneray H, Ozden A, Gurbuz K. The successful treatment of deep soft-tissue calcifications with topical sodium thiosulphate and acetazolamide in a boy with hyperphosphatemic familial tumoral calcinosis due to a novel mutation in FGF23. Journal of Clinical Research in Pediatric Endocrinology 2021;14(2):239–43. 10.4274/jcrpe.galenos.2021.2020.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Hodenberg C, Neufeld M, Wohlrab J, et al. Topical sodium thiosulfate: a reliable treatment for digital calcinosis cutis–a case series with six patients. JDDG J der Deutschen Dermatol Gesellschaft 2020;18(10):1181–3. [DOI] [PubMed] [Google Scholar]
- 56.Tajalli M, Qureshi AA. Successful treatment of calcinosis cutis of fingertip in the setting of CREST syndrome with topical 20% sodium thiosulfate. JAAD Case Reports 2019;5(11):988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Topham C, Haynes D, Frigerio A, Hull C. Linear amyopathic dermatomyositis with calcinosis cutis responsive to topical sodium thiosulfate. Pediatr Dermatol 2019;36(4):e102–e3. [DOI] [PubMed] [Google Scholar]
- 58.Eleryan MG, Awosika O, Akhiyat S, et al. Treatment of calcinosis associated with adult and juvenile dermatomyositis using topical sodium thiosulfate via fractionated CO2 laser treatment. Clin Exp Rheumatol 2019;37(6):1092–3. [PMC free article] [PubMed] [Google Scholar]
- 59.Del Barrio-Díaz P, Moll-Manzur C, Álvarez-Veliz S, Vera-Kellet C. Topical sodium metabisulfite for the treatment of calcinosis cutis: a promising new therapy. Br J Dermatol 2016;175(3):608–11. [DOI] [PubMed] [Google Scholar]
- 60.Ma J, Ernste F, Davis M, Wetter D. Topical sodium thiosulfate for calcinosis cutis associated with autoimmune connective tissue diseases: the Mayo Clinic experience, 2012–2017. Clin Exp Dermatol 2019;44(5):e189–e92. [DOI] [PubMed] [Google Scholar]
- 61.Nowaczyk J, Zawistowski M, Fiedor P. Local, non-systemic, and minimally invasive therapies for calcinosis cutis: A systematic review. Arch Dermatol Res 2021:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goossens J, Courbebaisse M, Caudron E, et al. Efficacy of intralesional sodium thiosulfate injections for disabling tumoral calcinosis: two cases. Seminars in Arthritis and Rheumatism. Elsevier; 2017. [DOI] [PubMed] [Google Scholar]
- 63.Baumgartner-Nielsen J, Olesen AB. Treatment of skin calcifications with intra-lesional injection of sodium thiosulphate: a case series. Acta Derm Venereol 2016;96(2):257–8. [DOI] [PubMed] [Google Scholar]
- 64.López-Sundh AE, Quintana-Sancho A, Durín-Vian C, et al. Clinical and ultrasound response to intralesional sodium thiosulfate for the treatment of calcinosis cutis in the setting of systemic sclerosis. A case-based review. Clin Rheumatol 2021;40(7):2985–9. [DOI] [PubMed] [Google Scholar]
- 65.Winter AR, Klager S, Truong R, et al. Efficacy of intralesional sodium thiosulfate for the treatment of dystrophic calcinosis cutis: A double-blind, placebo-controlled pilot study. JAAD international 2020; 1(2):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephro 2013;8(7):1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malbos S, Ureña-Torres P, Bardin T, Ea H-K. Sodium thiosulfate is effective in calcific uremic arteriolopathy complicating chronic hemodialysis. Joint Bone Spine 2016;83(1):89–92. [DOI] [PubMed] [Google Scholar]
- 68.Badawi AH, Patel V, Warner AE, Hall JC. Dystrophic calcinosis cutis: treatment with intravenous sodium thiosulfate. Cutis 2020;106(2):E15–E7. [DOI] [PubMed] [Google Scholar]
- 69.Mageau A, Guigonis V, Ratzimbasafy V, et al. Intravenous sodium thiosulfate for treating tumoral calcinosis associated with systemic disorders: report of four cases. Joint Bone Spine 2017;84(3):341–4. [DOI] [PubMed] [Google Scholar]
- 70.Song P, Fett N, Lin J, et al. Lack of response to intravenous sodium thiosulfate in three cases of extensive connective tissue disease-associated calcinosis cutis. Br J Dermatol 2018;178(6):1412–5. [DOI] [PubMed] [Google Scholar]
- 71.Mönkkönen J, Taskinen M, Auriola SO, Urtti A. Growth inhibition of macrophage-like and other cell types by liposome-encapsulated, calcium-bound, and free bisphosphonates in vitro. J Drug Target 1994;2(4):299–308. [DOI] [PubMed] [Google Scholar]
- 72.Tayfur AC, Topaloglu R, Gulhan B, Bilginer Y. Bisphosphonates in juvenile dermatomyositis with dystrophic calcinosis. Mod Rheumatol 2015;25(4):615–20. [DOI] [PubMed] [Google Scholar]
- 73.Puche M, Penades C. Effectiveness of the treatment with intravenous pamidronate in calcinosis in juvenile dermatomyositis. Clin Exp Rheumatol 2010;28(1):135–40. [PubMed] [Google Scholar]
- 74.Slimani S, Abdessemed A, Haddouche A, Ladjouze-Rezig A. Complete resolution of universal calcinosis in a patient with juvenile dermatomyositis using pamidronate. Joint Bone Spine 2010;77(1):70–2. [DOI] [PubMed] [Google Scholar]
- 75.Bianchi ML, Cimaz R, Bardare M, et al. Efficacy and safety of alendronate for the treatment of osteoporosis in diffuse connective tissue diseases in children: a prospective multicenter study. Arthritis Rheum 2000;43(9):1960–6. [DOI] [PubMed] [Google Scholar]
- 76.Harel L, Harel G, Korenreich L, et al. Treatment of calcinosis in juvenile dermatomyositis with probenecid: the role of phosphorus metabolism in the development of calcifications. J Rheumatol 2001;28(5):1129–32. [PubMed] [Google Scholar]
- 77.Dent CE, Stamp TC. Treatment of calcinosis circumscripta with probenecid. Br Med J 1972;1(5794):216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.MacKie R. Probenecid in calcification of the hands. Br Med J 1972;2(5816):768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meyers D. Treatment of calcinosis circumscripta and Raynaud's phenomenon. Med J Aust 1976;2(12):457. [DOI] [PubMed] [Google Scholar]
- 80.Skuterud E, Sydnes OA, Haavik TK. Calcinosis in dermatomyositis treated with probenecid. Scand J Rheumatol 1981;10(2):92–4. [DOI] [PubMed] [Google Scholar]
- 81.Eddy MC, Leelawattana R, McAlister WH, Whyte MP. Calcinosis universalis complicating juvenile dermatomyositis: resolution during probenecid therapy. J Clin Endocrinol Metab 1997;82(11):3536–42. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura H, Kawakami A, Ida H, et al. Efficacy of probenecid for a patient with juvenile dermatomyositis complicated with calcinosis. J Rheumatol 2006;33(8):1691–3. [PubMed] [Google Scholar]
- 83.Riley P, McCann LJ, Maillard SM, et al. Effectiveness of infliximab in the treatment of refractory juvenile dermatomyositis with calcinosis. Rheumatology (Oxford) 2008;47(6):877–80. [DOI] [PubMed] [Google Scholar]
- 84.Campanilho-Marques R, Deakin CT, Simou S, et al. Retrospective analysis of infliximab and adalimumab treatment in a large cohort of juvenile dermatomyositis patients. Arthritis Res Ther 2020;22(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tosounidou S, MacDonald H, Situnayake D. Successful treatment of calcinosis with infliximab in a patient with systemic sclerosis/myositis overlap syndrome. Rheumatology (Oxford) 2014;53(5):960–1. [DOI] [PubMed] [Google Scholar]
- 86.Xie F, Williams P, Batchelor R, et al. Successful treatment of dermatomyositis and associated calcinosis with adalimumab. Clin Exp Dermatol 2020;45(7):945–9. [DOI] [PubMed] [Google Scholar]
- 87.Bader-Meunier B, Decaluwe H, Barnerias C, et al. Safety and efficacy of rituximab in severe juvenile dermatomyositis: results from 9 patients from the French Autoimmunity and Rituximab registry. J Rheumatol 2011;38(7):1436–40. [DOI] [PubMed] [Google Scholar]
- 88.Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology (Oxford) 2017;56(2):247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shneyderman M, Ahlawat S, Christopher-Stine L, Paik JJ. Calcinosis in refractory dermatomyositis improves with tofacitinib monotherapy: a case series. Rheumatology (Oxford) 2021;60(11):e387–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wendel S, Venhoff N, Frye BC, et al. Successful treatment of extensive calcifications and acute pulmonary involvement in dermatomyositis with the Janus-Kinase inhibitor tofacitinib - A report of two cases. J Autoimmun 2019;100:131–6. [DOI] [PubMed] [Google Scholar]
- 91.Sozeri B, Demir F. A striking treatment option for recalcitrant calcinosis in juvenile dermatomyositis: tofacitinib citrate. Rheumatology (Oxford) 2020;59(12):e140–e1. [DOI] [PubMed] [Google Scholar]
- 92.Papadopoulou C, Hong Y, Omoyinmi E, et al. Janus kinase 1/2 inhibition with baricitinib in the treatment of juvenile dermatomyositis. Brain 2019;142(3):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabbagh S, Almeida de Jesus A, Hwang S, et al. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain 2019;142(11):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med 1986;314(6):329–34. [DOI] [PubMed] [Google Scholar]
- 95.Dwyer JM. Manipulating the immune system with immune globulin. N Engl J Med 1992;326(2):107–16. [DOI] [PubMed] [Google Scholar]
- 96.Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 1993;329(27):1993–2000. [DOI] [PubMed] [Google Scholar]
- 97.Aggarwal R, Schessl J, Bata-Csorgo Z, et al. Efficacy and safety of IVIg (octagam 10%) in patients with active dermatomyositis. Results of a randomized, double-blind, placebo-controlled phase III trial (ProDERM study). ACR Convergence Virtual 2020. [Google Scholar]
- 98.Touimy M, Janani S, Rachidi W, et al. Calcinosis universalis complicating juvenile dermatomyositis: improvement after intravenous immunoglobulin therapy. Joint Bone Spine 2013;80(1):108–9. [DOI] [PubMed] [Google Scholar]
- 99.Peñate Y, Guillermo N, Melwani P, et al. Calcinosis cutis associated with amyopathic dermatomyositis: response to intravenous immunoglobulin. J Am Acad Dermatol 2009;60(6):1076–7. [DOI] [PubMed] [Google Scholar]
- 100.Shahani L. Refractory calcinosis in a patient with dermatomyositis: response to intravenous immune globulin. BMJ Case Rep 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Galimberti F, Li Y, Fernandez AP. Intravenous immunoglobulin for treatment of dermatomyositis-associated dystrophic calcinosis. J Am Acad Dermatol 2015;73(1):174–6. [DOI] [PubMed] [Google Scholar]
- 102.Cohen H, Solomon V, Alferiev IS, et al. Bisphosphonates and tetracycline: experimental models for their evaluation in calcium-related disorders. Pharm Res (N Y) 1998;15(4):606–13. [DOI] [PubMed] [Google Scholar]
- 103.Robertson LP, Marshall RW, Hickling P. Treatment of cutaneous calcinosis in limited systemic sclerosis with minocycline. Ann Rheum Dis 2003;62(3):267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carmen Fonseca VO, Denton Christopher. Minocycline for refractory calcinosis in systemic sclerosis: a single-centre observational cohort study. American College of Rheumatology 2019; November 12:2019. New Orleans, Lousiana, USA: 2019. [Google Scholar]
- 105.Davies CA, Herrick AL, Cordingley L, et al. Expression of advanced glycation end products and their receptor in skin from patients with systemic sclerosis with and without calcinosis. Rheumatology (Oxford) 2009;48(8):876–82. [DOI] [PubMed] [Google Scholar]
- 106.Chung MP, Valenzuela A, Li S, et al. A pilot study to evaluate the safety and efficacy of treprostinil in the treatment of calcinosis in systemic sclerosis. Oxford): Rheumatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lian JB, Skinner M, Glimcher MJ, Gallop P. The presence of gamma-carboxyglutamic acid in the proteins associated with ectopic calcification. Biochem Biophys Res Commun 1976;73(2):349–55. [DOI] [PubMed] [Google Scholar]
- 108.Lian JB, Pachman LM, Gundberg CM, et al. GAMMA-carboxyglutamate excretion and calcinosis in juvenile dermatomyositis. Arthritis Rheum 1982;25(9):1094–100. [DOI] [PubMed] [Google Scholar]
- 109.Berger RG, Featherstone GL, Raasch RH, et al. Treatment of calcinosis universalis with low-dose warfarin. Am J Med 1987;83(1):72–6. [DOI] [PubMed] [Google Scholar]
- 110.Lassoued K, Saiag P, Anglade MC, et al. Failure of warfarin in treatment of calcinosis universalis. Am J Med 1988;84(4):795–6. [DOI] [PubMed] [Google Scholar]; (b). Cukierman T, Elinav E, Korem M, Chajek-Shaul T. Low dose warfarin treatment for calcinosis in patients with systemic sclerosis. Ann Rheum Dis 2004;63(10):1341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshida S, Torikai K. The effects of warfarin on calcinosis in a patient with systemic sclerosis. J Rheumatol 1993;20(7):1233–5. [PubMed] [Google Scholar]
- 112.Schurgers LJ, Teunissen KJ, Knapen MH, et al. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol 2005;25(8):1629–33. [DOI] [PubMed] [Google Scholar]
- 113.Urist MR, Huo YK, Brownell AG, et al. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci U S A 1984;81(2):371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boström K, Tsao D, Shen S, et al. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem 2001;276(17):14044–52. [DOI] [PubMed] [Google Scholar]
- 115.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol 2000;20(2):317–27. [DOI] [PubMed] [Google Scholar]
- 116.Han KH, O'Neill WC. Increased peripheral arterial calcification in patients receiving warfarin. J Am Heart Assoc 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sultan-Bichat N, Menard J, Perceau G, et al. Treatment of calcinosis cutis by extracorporeal shock-wave lithotripsy. J Am Acad Dermatol 2012;66(3):424–9. [DOI] [PubMed] [Google Scholar]
- 118.Blumhardt S, Frey DP, Toniolo M, et al. Safety and efficacy of extracorporeal shock wave therapy (ESWT) in calcinosis cutis associated with systemic sclerosis. Clin Exp Rheumatol 2016;34(5):177–80. [PubMed] [Google Scholar]
- 119.Chan AY, Li E. Electric shock wave lithotripsy (ESWL) as a pain control measure in dermatomyositis with calcinosis cutis-old method, new discovery. Clin Rheumatol 2005;24(2):172–3. [DOI] [PubMed] [Google Scholar]
- 120.Meyer A, Laverny G, Allenbach Y, et al. IFN-β-induced reactive oxygen species and mitochondrial damage contribute to muscle impairment and inflammation maintenance in dermatomyositis. Acta Neuropathol 2017;134(4):655–66. [DOI] [PubMed] [Google Scholar]
- 121.Fortner KA, Blanco LP, Buskiewicz I, et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med 2020;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Platter M, Pugmire B, Patel R. Resolution of calcinosis using bisphosphonates in overlap syndrome - a case report. BMC Rheumatol 2021;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen CL, Chen NC, Wu FZ, Wu MT. Impact of denosumab on cardiovascular calcification in patients with secondary hyperparathyroidism undergoing dialysis: a pilot study. Osteoporos Int 2020;31(8):1507–16. [DOI] [PubMed] [Google Scholar]
- 124.Echeverri AF, Ospina FE, Cañas CA, et al. Worsening of calcinosis cutis with teriparatide treatment in two osteoporotic patients. Br J Dermatol 2016;175(5):1049–51. [DOI] [PubMed] [Google Scholar]
- 125.Htet TD, Eisman JA, Elder GJ, Center JR. Worsening of soft tissue dystrophic calcification in an osteoporotic patient treated with teriparatide. Osteoporos Int 2018;29(2):517–8. [DOI] [PubMed] [Google Scholar]
- 126.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008;83(9):1032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jung A, Bisaz S, Fleisch H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif Tissue Res 1973;11(4):269–80. [DOI] [PubMed] [Google Scholar]
- 128.Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev 1998;19(1):80–100. [DOI] [PubMed] [Google Scholar]
- 129.Goodman SM, Springer B, Guyatt G, et al. American college of rheumatology/American association of hip and knee surgeons guideline for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty. Arthritis Rheumatology 2017;69(8):1538–51. [DOI] [PubMed] [Google Scholar]


