Abstract
Repeat kidney transplantation (re-KT) is the preferred treatment for patients with graft failure. Changing allocation policies, widening risk profile of recipients, and improving dialysis care may have altered the survival benefit of re-KT. We characterized trends in re-KT survival benefit over three decades and tested whether it differed by age, race/ethnicity, sex, and PRA. Using SRTR data, we identified 25,419 patients who underwent re-KT from 1990–2019 and 25,419 waitlisted counterfactuals from the same year with the same waitlisted time following graft failure. In adjusted analysis re-KT was associated with a lower risk of death (aHR=0.63, 95%CI:0.61–0.65). Using the 1990–94 era as a reference (aHR=0.77, 95%CI:0.69–0.85), incremental improvements in the survival benefit were noted (1995–99:aHR=0.72, 95%CI:0.67–0.78; 2000–04:aHR=0.59, 95%CI:0.55–0.63; 2005–09:aHR=0.59, 95%CI:0.56–0.63; 2010–14:aHR=0.57, 95%CI:0.53–0.62; 2015–19:aHR=0.64, 95%CI: 0.57–0.73). The survival benefit of re-KT was noted in both younger (age=18–64 years:aHR=0.63, 95%CI:0.61–0.65) and older patients (age≥65 years:aHR=0.66, 95%CI:0.58–0.74, pinteraction=0.45). Patients of all races/ethnicities demonstrated similar benefits with re-KT. However, it varied by the sex of the recipient (female patients:aHR=0.60, 95%CI:0.56–0.63, male patients:aHR=0.66, 95%CI:0.63–0.68, pinteraction=0.004) and PRA (0–20:aHR=0.69, 95%CI:0.65–0.74; 21–80:aHR=0.61, 95%CI:0.57–0.66, pinteraction=0.02; >80:aHR=0.57, 95%CI:0.53–0.61, pinteraction<0.001). Our findings support the continued practice of re-KT and efforts to overcome the medical, immunologic, and surgical challenges of re-KT.
Introduction
When compared with dialysis, a repeat kidney transplantation (re-KT) is a superior treatment option for patients with a history of graft failure. A landmark paper in 1998 reported that in a cohort of 19,208 patients with graft failure, re-KT led to 23–45% reduction in 5-year mortality risk.(1) These results were replicated in a Canadian cohort,(2) and later by other scholars analyzing U.S. registry data.(3–5) These findings of a survival benefit have been extended to third and even fourth transplantations.(6) Thus, about 15% of the current waitlist in the U.S. includes kidney transplant recipients (KTRs) with a history of graft failure.(7)
While gradual improvements in KTR survival have been reported over time,(7, 8) the age, risk profiles and lifetime immunosuppression burden of patients undergoing re-KT have changed dramatically since these initial reports.(7, 9, 10) Simultaneously, the survival of patients on dialysis is improving.(7) Therefore, it remains to be seen if re-KT is associated with a survival benefit in recent eras, in particular following Kidney Allocation System (KAS) implementation.(11) KAS was introduced in 2014 by the United Network for Organ Sharing with the goals of longevity matching and priotizing sensitized patients and has.(11) However, this often entails allocating grafts with a higher Kidney Donor Profile Index to those with a history of graft failure. Re-KT with higher risk donors, such as expanded criteria donors, may not be associated with a survival advantage.(5) Thus, KAS may have diminished the survival benefit of re-KT. On the other hand, increasing waiting time before re-KT is associated with an increased risk of death,(12) and KAS may have shortened the wait time to re-KT, potentially improving outcomes. Overall, exploring the trends in the survival benefit of re-KT following 2014 is needed to assess the impact of this policy change.
In addition to era changes, a more granular evaluation of the survival benefit of re-KT by age, race/ethnicity, sex and panel reactive assay (PRA) of the KTR has yet to be conducted. Amongst first KTRs, many studies have shown inferior outcomes in women and in patients of non-White race/ethnicity;(13, 14) this has yet to be explored in recipients of re-KT. Also, each previous transplant leads to sensitization and higher PRA confers an incremental mortality risk in KTRs.(15) High PRA following graft failure and its association with the survival benefit of re-KT is not known. Most importantly, the survival advantage of re-KT in older patients has not been explored despite this being a rapidly rising cohort of patients.(10) Older patients are known to be more frail, have cognitive impairment and have multiple comorbidities; all are risk factors for mortality. Determining the importance of re-KT in these specific populations therefore will have clinical and policy implications.
Thus, we sought to estimate the survival benefit of re-KT over the past three decades and test whether it has changed over time, particularly during the KAS era. We also aimed to quantify the survival benefit of re-KT by age, race/ethnicity, sex, and PRA.
Methods
Data source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This dataset has previously been described elsewhere.(16) Death was ascertained from multiple sources including the Centers for Medicare and Medicaid Services and the National Technical Information Service Death Master File. This study was reviewed by the Johns Hopkins School of Medicine Institutional Review Board and was determined to be exempt. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Study population
We identified adult (≥18 years of age) patients who were waitlisted for re-KT between January 1, 1990, and December 31, 2019, after their first kidney-only graft failed. Grafts received from both deceased or living donors were included in the analysis. For each patient who had re-KT, we matched them with one randomly selected waitlisted patient with a history of first kidney-only graft failure from the same calendar year who spent the same amount of time on the waitlist; this comprised the waitlisted counterfactual cohort. We used the SRTR variable definition, i.e., the time origin for waitlisting as the date/time candidate was physically added to the waitlist to assess this.
Exposure and Outcomes
We tested whether patient survival differed by re-KT by comparing patients who received re-KT with the waitlist controls. We also tested whether the association differed by the age at waitlisting (younger: 18–64 years and older: ≥65 years), sex (female and male), race/ethnicity (non-Hispanic White, non-Hispanic Black, and multiracial patients or patients of other races/ethnicities), peak PRA (0–20, 21–80 >80) and the era of re-KT (1990–94, 1995–99, 2000–04, 2005–09, 2010–14, 2015–19). The 2015–19 era defined the post-KAS era.
Statistical analysis
We summarized the characteristics at waitlisting of the two cohorts; for continuous variables, we report the medians and for categorical variables, we report the percentage of patients. We assessed the survival benefit of re-KT among KTRs who were waitlisted after graft failure using a stochastic extension of the sequential stratification method suggested by Schaubel.(17–22) At a specific time, t, when a patient received re-KT, a counterfactual was selected who spent the same amount of time on the waitlist but had not yet received re-KT up to and including time t.(21) The counterfactual waitlisted patient were selected without replacement and may receive re-KT at some later time and can be censored at that time.(21) Participants were then followed from the time of transplant (for re-KTRs) or from the time of matching (for waitlisted counterfactuals). We followed re-KTRs until death or administrative censoring date (October 31, 2020), whichever came first. We followed the waitlisted counterfactual patients until death, re-KT, or the administrative censoring date, whichever came first.
We applied the inverse probability weighting method to adjust for the following confounders: age, sex, race/ethnicity, education level, insurance, body mass index, hypertension, diabetes, malignancy, the lifetime of first KT, pre-emptive listing and peak PRA. A weighted Kaplan-Meier estimator was used to estimate the 1-, 3-, and 6-year patient survival. We then used Cox regression to compare the mortality risk between the re-KTR and their waitlisted counterfactual after adjusting for confounding. We then tested whether the association differed by patient’s age at waitlisting for re-KT, race/ethnicity, sex, peak PRA, and eras of interest using a Wald test. To analyze the impact of donor type (living and deceased donor), we conducted a stratified analysis of the survival benefit of re-KT by donor types when compared with waitlisted counterfactual in each cohort. Confidence intervals are reported as per the method of Louis and Zeger.(23) All analyses were performed using Stata 17.0/MP for Unix (College Station, Texas).
Results
Cohort characteristics
From 1990–2019, 50,838 patients who were waitlisted for re-KT met our inclusion criteria. Of these, 25,419 underwent re-KT and we identified 25,419 waitlisted counterfactuals (Figure 1). The covariate balance plot is provided in Supplemental Figure 1. The absolute number of patients with graft failure being waitlisted for re-KT increased steadily from 1990 but stagnated after 2007 and started to decrease in the KAS era. However, the percentage of patients receiving re-KT doubled in the KAS era (Figure 2). The median age at listing was 43 years (IQR: 33, 53) in both cohorts and only a small fraction were older adults (4.3% vs. 4.4%). The characteristics of patients in the two cohorts were similar except for the following key differences. There were more White (59.3% vs. 46.4%) and fewer Black (23.2% vs. 34.8%) patients in the re-KT cohort. Also, fewer patients in the re-KT cohort had B or O blood type (53.5% vs. 62.0%) and a PRA>80 (28.7% vs. 46.7%) while a higher proportion was waitlisted pre-emptively (38.3% vs. 33.0%). Lastly, the median graft survival of the first transplant was higher in the re-KT cohort (6.4 vs. 4.9 years) (Table 1).
Figure 1:
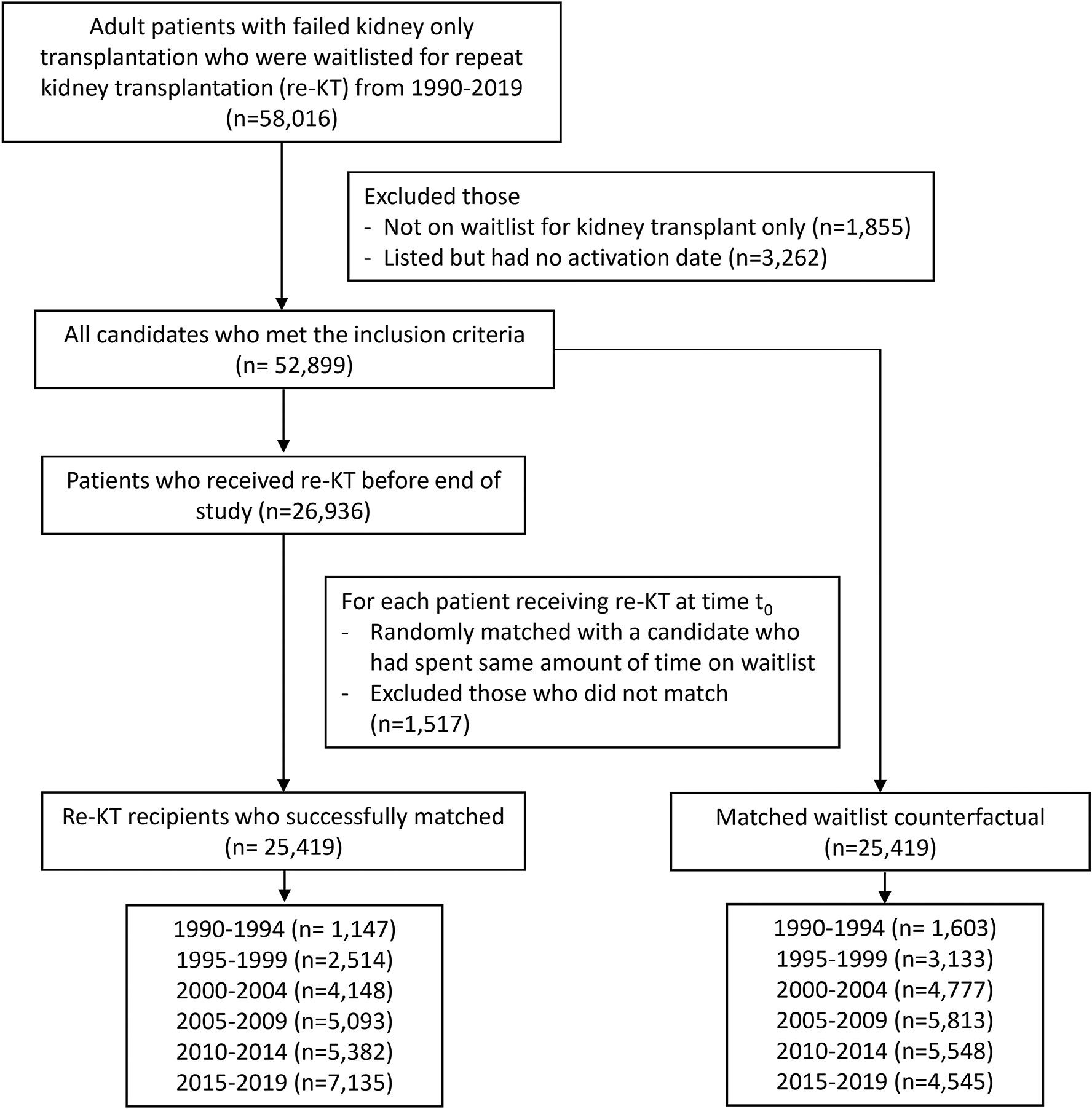
Study flow diagram and cohort derivation for the study of repeat kidney transplantation survival benefit
Figure 2:
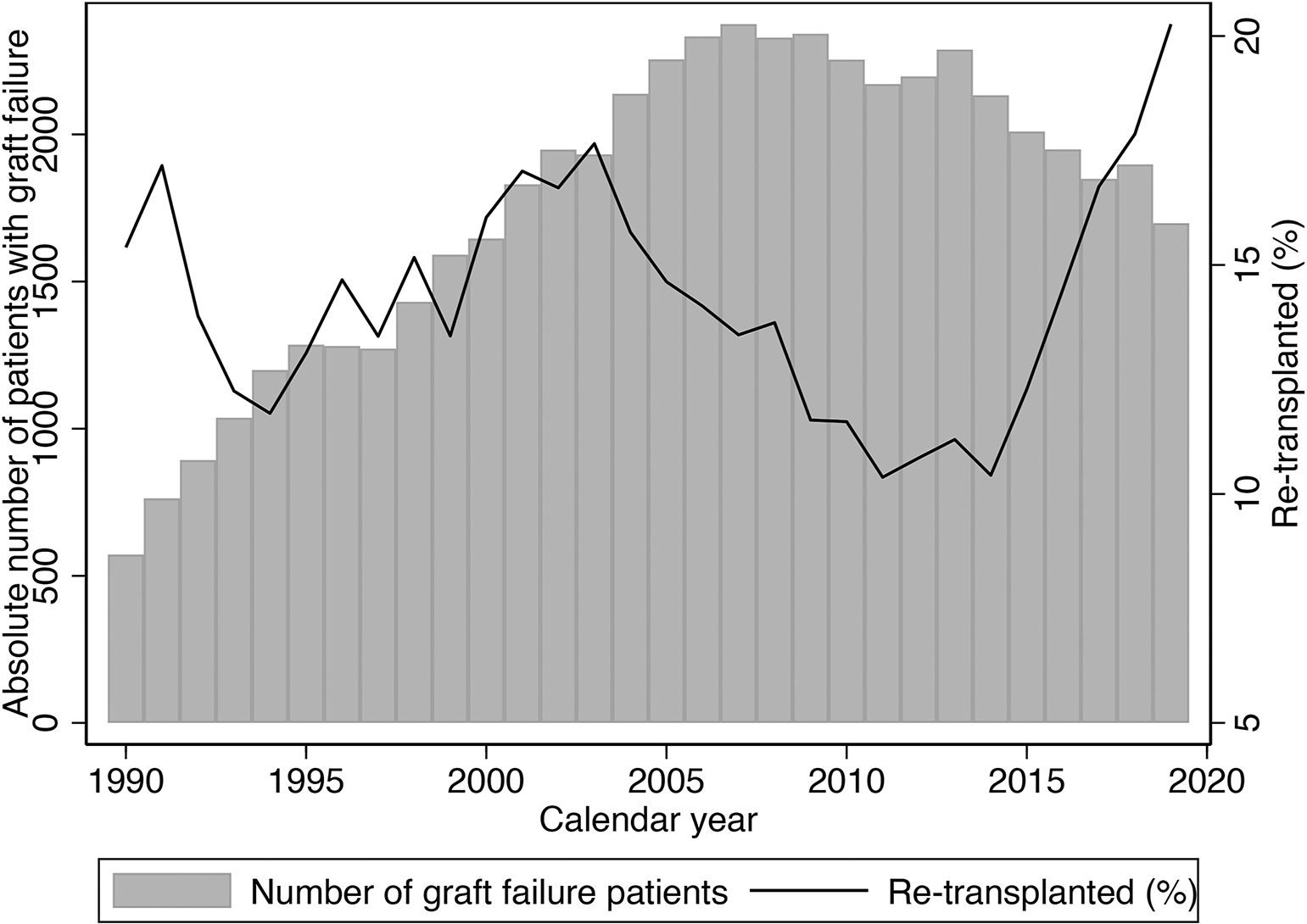
Absolute number of patients with graft failure who were waitlisted for a repeat kidney transplantation and the percentage that received a repeat transplant by calendar year
Table 1.
Demographic and other characteristics of patients at waitlisting. The cohorts are those received repeat kidney transplantation between 1990–2019 and their waitlisted counterfactual from the same calendar year who spent the same amount of time on the waitlist
| Characteristics | Re-transplant recipients (n=25,419) | Waitlisted counterfactual (n=25,419) |
|---|---|---|
|
| ||
| Age at listing (years) | 43.0 (33.0, 53.0) | 43.0 (33.0, 53.0) |
| Age at listing ≥65 | 4.3% | 4.4% |
| Female | 41.2% | 41.6% |
| Race/ethnicity | ||
| Non-Hispanic White | 59.3% | 46.4% |
| Non-Hispanic Black | 23.2% | 34.8% |
| Hispanic | 12.4% | 12.5% |
| Other/multi-racial | 5.0% | 6.3% |
| Attended college | 44.1% | 40.0% |
| Insurance | ||
| Public | 55.9% | 64.9% |
| Private | 43.8% | 34.6% |
| Other | 0.3% | 0.4% |
| BMI, kg/m2 | ||
| 0–24.9 | 48.8% | 48.4% |
| 25–29.9 | 29.3% | 29.6% |
| ≥30 | 21.9% | 22.0% |
| B or O blood type | 53.5% | 62.0% |
| Medical co-morbidities | ||
| Hypertension | 84.1% | 84.3% |
| Diabetes | 18.3% | 20.7% |
| Angina | 8.1% | 8.8% |
| Symptomatic CVD | 1.9% | 2.2% |
| Any previous malignancy | 7.5% | 6.1% |
| First graft lifetime (year) | 6.4 (2.5, 11.5) | 4.9 (1.7, 9.6) |
| Pre-emptive listing | 38.3% | 33.0% |
| Peak PRA (%) | ||
| 0–20 | 43.9% | 27.1% |
| 21–80 | 27.4% | 26.2% |
| >80 | 28.7% | 46.7% |
| Era (n) | ||
| 1990–1994 | 1,147 | 1,603 |
| 1995–1999 | 2,514 | 3,133 |
| 2000–2004 | 4,148 | 4,777 |
| 2005–2009 | 5,093 | 5,813 |
| 2010–2014 | 5,382 | 5,548 |
| 2015–2019 | 7,135 | 4,454 |
Continuous variables are reported as median (interquartile range).
CVD: cerebrovascular disease
Survival benefit of re-KT
Patients who received re-KT had a better survival (1 year: 96.5% vs. 95.4%, 3-year: 92.0% vs. 85.4%, 6-year: 83.8% vs. 71.4%) (Figure 3A). In adjusted analysis, re-KT was associated with 37% lower mortality risk when compared with waitlisted counterfactuals (adjusted hazard ratio [aHR]: 0.63, 95% confidence interval [CI]:0.61–0.65) (Table 2).
Figure 3:
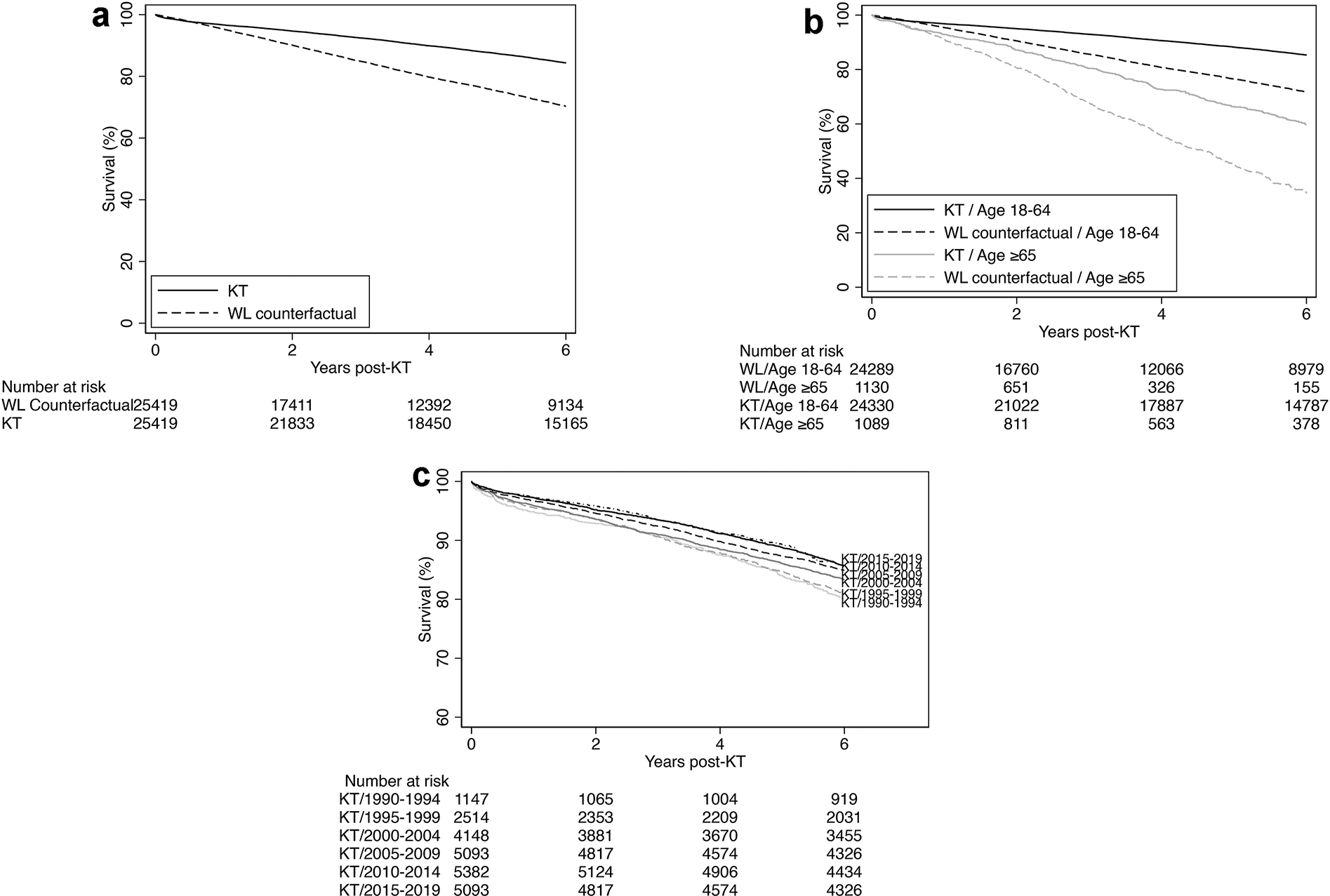
Survival of repeat kidney transplant (KT) recipients compared with waitlisted (WL) counterfactuals (patients from the same calendar year who spent the same amount of time on the waitlist) for the a) total cohort; b) stratified by age; and c) survival of repeat kidney transplant recipients over time. We defined time origin as the transplant date for both the re-transplant recipient and their waitlisted counterfactual and used the sequential stratification method for survival analysis
Table 2:
Survival benefit of repeat kidney transplantation (KT) (n=25,419) when compared with waitlisted counterfactual (n=25,419) in the overall cohort and stratified by era and recipient age at waitlisting, sex, race/ethnicity, and peak PRA. Waitlisted counterfactuals were patients from the same calendar year who spent the same amount of time on the waitlist.
| aHR (95% CI) | Interaction P | |
|---|---|---|
|
| ||
| Overall | 0.610.630.65 | - |
| By era | ||
| 1990–94 | 0.690.770.85 | Reference |
| 1995–99 | 0.670.720.78 | 0.31 |
| 2000–04 | 0.550.590.63 | <0.001 |
| 2005–09 | 0.560.590.63 | <0.001 |
| 2010–14 | 0.530.570.62 | <0.001 |
| 2015–19 | 0.570.640.73 | 0.03 |
| By recipient age, years | ||
| 18–64 | 0.610.630.65 | Reference |
| ≥65 | 0.580.660.74 | 0.45 |
| By recipient sex | ||
| Male | 0.630.660.68 | Reference |
| Female | 0.560.600.63 | 0.004 |
| By recipient | ||
| race/ethnicity | ||
| Non-Hispanic White | 0.620.640.67 | Reference |
| Non-Hispanic Black | 0.590.630.66 | 0.40 |
| Hispanic | 0.530.590.65 | 0.12 |
| Other/multi-racial | 0.500.580.68 | 0.21 |
| By Peak PRA, % | ||
| 0–20 | 0.650.690.74 | Reference |
| 21–80 | 0.570.610.66 | 0.02 |
| >80 | 0.530.570.61 | <0.001 |
Adjusted for age, sex, race/ethnicity, education level, insurance, body mass index, hypertension, diabetes, malignancy, peak PRA, lifetime of first KT, and pre-emptive listing using the inverse probability weighting method.
Survival benefit by age at waitlisting
In patients aged 18–64 years old at waitlisting for re-KT, re-KTRs had better survival than those who were waitlisted: 1-, 3-, and 6-year survival were 96.6% vs. 95.5%, 92.6% vs. 86.1% and 84.8% vs. 72.9% among re-KTRs and waitlisted counterfactuals, respectively. In patients aged ≥65 years at waitlisting for re-KT, re-KTRs also had better survival than those who were waitlisted: 1-, 3-, and 6-year survival were 92.4% vs. 91.5%, 79.5% vs. 68.8% and 58.8% vs. 36.2% among re-KTRs and waitlisted counterfactuals, respectively (Figure 3B). The survival benefit of re-KT did not vary by age of the recipient at listing. Both younger (aHR=0.63, 95%CI:0.61–0.65) and older (aHR=0.66, 95%CI:0.58–0.74) patients demonstrated a similar reduction in the risk of death with re-KT when compared with waitlisted counterfactuals (pinteraction=0.45) (Table 2).
Era analysis
When stratified by era, the overall survival of re-KT recipients seemed to improve over time (Figure 3C). Furthermore, the survival benefit of re-KT existed across all eras including the KAS era. In the 1990–94 era, re-KT was associated with a 23% lower risk of death than dialysis (aHR=0.77, 95%CI:0.69–0.85) and in the KAS era (2015–19), re-KT was associated with a 36% lower risk of death (aHR=0.64, 95%CI: 0.57–0.73). Similar results were noted from 1995–2014; 1995–99: aHR=0.72 (95%CI:0.67–0.78), 2000–04: aHR=0.59 (95%CI:0.55–0.63), 2005–09: aHR=0.59 (95%CI:0.56–0.63), and 2010–14: aHR=0.57, 95%CI:0.53–0.62). When using the 1990–94 era as a reference, except the 1995–99 era (Pinteraction=0.31), the survival benefit of re-KT was superior in the latter eras including the KAS era (2000–04:pinteraction<0.001; 2005–09:pinteraction<0.001; 2010–14:pinteraction<0.001; 2015–19: pinteraction=0.03). However, the survival benefit of re-KT during the KAS era, i.e., 2015–2019, was not different than the 2010–2014 (pinteraction=0.12) and the 2005–2009 (pinteraction=0.27) era (Table 2).
Race/ethnicity, sex, and PRA
When compared with non-Hispanic White patients (aHR=0.64, 95%CI:0.62–0.67), non-Hispanic Black patients (aHR=0.63, 95%CI:0.59–0.66, pinteraction=0.40), Hispanic patients (aHR=0.59, 95%CI:0.53–0.65, pinteraction=0.12) and multiracial or patients of other ethnicities (aHR=0.58, 95%CI:0.50–0.68, pinteraction=0.21) all experienced a similar survival benefit. However, the survival benefit varied by the sex of the recipient. Female patients (aHR=0.60, 95%CI:0.56–0.63, pinteraction=0.004) experienced a higher survival advantage with re-KT than male patients (aHR=0.66, 95%CI:0.63–0.68). Also, the survival benefit of re-KT varied by the peak PRA; 0–20: aHR=0.69 (95%CI: 0.65–0.74), 21–80: aHR=0.61 (95%CI:0.57–0.66 pinteraction=0.02), and >80: aHR=0.57 (95%CI: 0.53–0.61, pinteraction<0.001) (Table 2).
Stratified analysis
After stratifying by donor type, there were 7,670 recipients of living donor re-KT. The survival benefit of re-KT was noted in both the living and deceased donor cohorts; aHR=0.48, 95%CI:0.45–0.52 and aHR=0.67, 95%CI:0.65–0.70, respectively.
Discussion
In this national study of 25,419 re-KTRs from 1990–2019 and 25,419 waitlisted counterfactual patients, we report that re-KT was associated with a 37% lower risk of death. There has been an incremental improvement in the observed survival benefit of re-KT since 1990 except it plateaued during the KAS era. In the KAS era, re-KT had a 36% lower risk of death, which was similar to the survival benefit observed in the earlier eras of 2005–09 and 2010–14, where patients experienced a 41% and 43% lower risk of death with re-KT, respectively. We also report that the survival benefit of re-KT did not vary by age and race/ethnicity of the recipient but did vary by PRA and sex; higher PRA and female sex were associated with a significantly better survival benefit. Overall, our findings demonstrate that re-KT is associated with a significant survival benefit for all patients and that KAS has not significantly changed the outcomes of re-KTRs with a history of graft failure.
In the 1990s, the reduction in mortality with re-KT was reported to be 23–45% in a US cohort and 50% in a Canadian cohort.(1, 2) More recently, Clark and colleagues analyzed 27,459 KTRs who initiated dialysis after graft failure from 1995–14 and reported that re-KT was associated with a 68% lower adjusted risk of death.(3) We report a lower survival benefit likely due to the sequential stratification method that has created a more balanced comparison group referred to as the waitlisted counterfactuals.(24) We also report significantly improved survival benefit of re-KT over time. In the U.S., adjusted annual mortality rates of all KTRs declined from 48.8 to 28.6/1,000 patient-years at risk from 2001 to 2017.(7) Simultaneously, these rates for patients on dialysis also decreased from 230.6 to 165.1/1,000 patientyears at risk.(7) The observed improvements were attributed to better diagnostic and therapeutic tools and advances in the medical management of co-morbidities,(8) and significant improvements in the care of patients on dialysis.(9) Despite this, mortality remains unacceptably high for patients on dialysis,(9) and re-KT is the preferred treatment in patients with graft failure.
Although an earlier analysis included some re-KTs conducted during the KAS era, the specific impact of this policy change on the survival benefit of re-KT was not analyzed.(3) In our analysis, an incremental increase in the survival benefit was noted until the KAS era. KAS helped improve access to re-KT.(11) On the other hand, grafts with higher kidney donor profile index are likely allocated to patients with lower expected post-transplant survival that includes KTRs with graft failure. Others have shown higher mortality among KTR in the post-KAS era when compared with the pre-KAS era disproportionately borne by middle-aged recipients.(25) The number of patients on the kidney transplant waiting list has declined during KAS, as it ties the start of waiting time to the date of dialysis initiation.(7, 26) This is speculated to have reduced the incentive to list dialysis patients until they are actively ready for KT, and we report this to be the case with KTRs with graft failure as well.(26) Thus, a multitude of factors can explain these findings. Regardless, even during the KAS era re-KT was associated with significantly lower mortality risk.
Some other findings merit discussion. Despite patients of all races/ethnicities demonstrating an equal advantage with re-KT, the proportion of Black patients in the re-KT cohort was much lower than White patients, further adding to the growing body of literature on disparities in access to transplantation. Second, female sex derived a better survival advantage with re-KT than male sex. There are sex differences in survival on hemodialysis,(27) and gender-level factors, such as, lack of support, stereotyping by clinicians, and entrenched social and economic disadvantages can impact their outcomes on dialysis(28). Discussions pertaining to sex and gender issues are beyond the scope of this article; however, our observation needs to be further explored. Third, while others have shown adverse graft and patient outcomes with higher PRA,(15) we report that patients with higher PRA derive a better survival advantage with re-KT and should be prioritized in allocation schemes. Also, as is well known in the field of KT, living donor re-KT is be a better therapeutic choice than deceased donor re-KT as mortality risk was 52% lower when the cohort of living donor recipients were compared with waitlisted counterfactuals. In the deceased donor cohort mortality risk was 33% lower. Last, medical comorbidities, cognitive impairment and frailty, lack of patient interest, or regressive attitudes towards re-KT may negatively affect access to re-KT among older patients; however, our data support improving access to re-KT among older adults as they derive an equal survival advantage as younger patients.
Our study has several strengths. We analyzed the survival benefit of re-KT in a large national cohort of patients over three decades. Examining secular trends in the survival benefit accounted for positive changes in transplant and dialysis care over time. Most prior studies have used time-dependent Cox models in survival analysis, with transplantation being treated as a time-varying exposure. This method involves making parametric assumptions about the relationship between time since transplant and the relative hazard of transplant versus waitlisting which might not hold,(29) and it often relies on timevarying hazard ratios, which introduce an inherent selection bias.(30) Moreover, such methods do not allow for estimates of absolute survival for the counterfactual population. However, as with most observational study designs, we can only account for the confounding of variables that were measured. Specifically, in the context of this study, more granular ascertainment of comorbidities is not available from national registry data in the United States. We acknowledge that KTRs with graft failure who are waitlisted for re-KT represent a highly selected group, predisposed to have good outcomes, and may not include transplant candidates who were never referred/listed. Thus, findings may not be generalizable to all patients with graft failure.
Despite this, our findings are extremely important. We report that despite significant improvements in dialysis care over the past three decades, re-KT continues to be associated with a significant survival benefit. Also, as the number of patients receiving first KT increases every year, so does the number that experience graft failure. Even in older adults, there has been a significant rise in annual rates of KT since 1990 and many were re-KTRs.(10) We report that despite the widening risk profiles of patients being considered for re-KT,(7, 9, 10), it is the preferred therapeutic option for KTRs with graft kidney failure.
In conclusion, re-KT is associated with a significant survival benefit over dialysis among all patients including older patients. Patients of different race/ethnicity derive the same survival benefit with re-KT but a higher survival benefit was observed among the female sex and those with higher PRA and this has practice and policy implications. Overtime, incremental improvements in the survival benefit of re-KT were noted except that it plateaued during the KAS era. Given that graft failure is now the fourth leading cause of kidney failure in patients on dialysis our findings support the continued practice of re-KT despite the widening risk profile of recipients and improving dialysis care. We recommend that ongoing efforts focus on overcoming the medical, immunologic and surgical challenges of re-KT.
Supplementary Material
Funding:
This study was supported by NIH R01DK120518 (McAdams-DeMarco) and K24AI144954 (Segev). Mara McAdams-DeMarco was also supported by K02AG076883 and R01AG055781 from the National Institute on Aging and R01DK114074 from NIDDK. Dr. Sandal is supported by the Chercheur boursier clinicien - Junior 1 award from the Fonds de recherche du Québec – Santé. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, writing, review, or approval of the manuscript.
Abbreviations
- aHR
adjusted hazard ratio
- CI
confidence interval
- KT
kidney transplantation
- KTR
kidney transplant recipients
- PRA
panel reactive assay
- Re-KT
repeat kidney transplantation
Footnotes
Disclosure: Dr. Segev receives speaking honoraria from Sanofi and Novartis. Dr. Sandal has received an education grant from Amgen Canada. Dr. McAdams-DeMarco received speaking honoraria from Chiesi. The rest of the authors have no disclosures. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing:
This an analysis of a publicly available dataset.
References
- 1.Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB: Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation, 66: 1651–1659, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Rao PS, Schaubel DE, Wei G, Fenton SS: Evaluating the survival benefit of kidney retransplantation. Transplantation, 82: 669–674, 2006. 10.1097/01.tp.0000235434.13327.11 [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Kadatz M, Gill J, Gill JS: Access to Kidney Transplantation after a Failed First Kidney Transplant and Associations with Patient and Allograft Survival: An Analysis of National Data to Inform Allocation Policy. Clin J Am Soc Nephrol, 14: 1228–1237, 2019. 10.2215/cjn.01530219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panchal H, Muskovich J, Patterson J, Schroder PM, Ortiz J: Expanded criteria donor kidneys for retransplantation United Network for Organ Sharing update: proceed with caution. Transpl Int, 28: 990–999, 2015. 10.1111/tri.12584 [DOI] [PubMed] [Google Scholar]
- 5.Miles CD, Schaubel DE, Jia X, Ojo AO, Port FK, Rao PS: Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant, 7: 1140–1147, 2007. 10.1111/j.1600-6143.2007.01742.x [DOI] [PubMed] [Google Scholar]
- 6.Sandal S, Ahn JB, Segev DL, Cantarovich M, McAdams-DeMarco MA: Comparing outcomes of third and fourth kidney transplantation in older and younger patients. Am J Transplant, 21: 4023–4031, 2021. 10.1111/ajt.16786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of kidney disease in the United States., Available at: https://www.usrds.org/annual-data-report/. Accessed Febreuary 23
- 8.Hariharan S, Israni AK, Danovitch G: Long-Term Survival after Kidney Transplantation. N Engl J Med, 385: 729–743, 2021. 10.1056/NEJMra2014530 [DOI] [PubMed] [Google Scholar]
- 9.Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M: The current and future landscape of dialysis. Nature Reviews Nephrology, 16: 573–585, 2020. 10.1038/s41581-020-0315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandal S, Ahn JB, Cantarovich M, Chu NM, Segev DL, McAdams-DeMarco MA: Evolving trends in risk profiles and outcomes in older adults undergoing kidney re-transplantation. Transplantation, 2021. 10.1097/tp.0000000000003842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CJ, Wetmore JB, Israni AK: Old versus new: Progress in reaching the goals of the new kidney allocation system. Hum Immunol, 78: 9–15, 2017. 10.1016/j.humimm.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 12.Wong G, Chua S, Chadban SJ, Clayton P, Pilmore H, Hughes PD, Ferrari P, Lim WH: Waiting Time Between Failure of First Graft and Second Kidney Transplant and Graft and Patient Survival. Transplantation, 100: 1767–1775, 2016. 10.1097/tp.0000000000000953 [DOI] [PubMed] [Google Scholar]
- 13.Melk A, Babitsch B, Borchert-Mörlins B, Claas F, Dipchand AI, Eifert S, Eiz-Vesper B, Epping J, Falk CS, Foster B, Geyer S, Gjertson D, Greer M, Haubitz M, Lau A, Maecker-Kolhoff B, Memaran N, Messner HA, Ostendorf K, Samuel U, Schmidt BMW, Tullius SG, West L, Wong G, Zimmermann T, Berenguer M: Equally Interchangeable? How Sex and Gender Affect Transplantation. Transplantation, 103: 1094–1110, 2019. 10.1097/tp.0000000000002655 [DOI] [PubMed] [Google Scholar]
- 14.Purnell TS, Luo X, Kucirka LM, Cooper LA, Crews DC, Massie AB, Boulware LE, Segev DL: Reduced Racial Disparity in Kidney Transplant Outcomes in the United States from 1990 to 2012. J Am Soc Nephrol, 27: 2511–2518, 2016. 10.1681/asn.2015030293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapir-Pichhadze R, Tinckam KJ, Laupacis A, Logan AG, Beyene J, Kim SJ: Immune Sensitization and Mortality in Wait-Listed Kidney Transplant Candidates. J Am Soc Nephrol, 27: 570–578, 2016. 10.1681/asn.2014090894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massie AB, Kucirka LM, Segev DL: Big data in organ transplantation: registries and administrative claims. Am J Transplant, 14: 1723–1730, 2014. 10.1111/ajt.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaubel DE, Wolfe RA, Port FK: A Sequential Stratification Method for Estimating the Effect of a Time-Dependent Experimental Treatment in Observational Studies. Biometrics, 62: 910–917, 2006. 10.1111/j.1541-0420.2006.00527.x [DOI] [PubMed] [Google Scholar]
- 18.Komaba H, Wang M, Taniguchi M, Yamamoto S, Nomura T, Schaubel DE, Smith AR, Zee J, Karaboyas A, Bieber B, Fukagawa M, Tentori F: Initiation of Sevelamer and Mortality among Hemodialysis Patients Treated with Calcium-Based Phosphate Binders. Clin J Am Soc Nephrol, 12: 1489–1497, 2017. 10.2215/cjn.13091216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JM, Shen J, Kennedy EH, Wang L, Schaubel DE: Comparison of methods for estimating the effect of salvage therapy in prostate cancer when treatment is given by indication. Stat Med, 33: 257–274, 2014. 10.1002/sim.5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter A, Féray C, Antoine C, Azoulay D, Daurès JP, Landais P: Matching Graft Quality to Recipient’s Disease Severity Based on the Survival Benefit in Liver Transplantation. Sci Rep, 10: 4111, 2020. 10.1038/s41598-020-60973-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aalen OO: Armitage lecture 2010: Understanding treatment effects: the value of integrating longitudinal data and survival analysis. Stat Med, 31: 1903–1917, 2012. 10.1002/sim.5324 [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Schaubel DE, He K: Matching methods for obtaining survival functions to estimate the effect of a time-dependent treatment. Stat Biosci, 6: 105–126, 2014. 10.1007/s12561-013-9085-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis TA, Zeger SL: Effective communication of standard errors and confidence intervals. Biostatistics: 1-2, 2009. kxn014 [pii] 10.1093/biostatistics/kxn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado G, Greenland S: Estimating causal effects. International Journal of Epidemiology, 31: 422–429, 2002. 10.1093/ije/31.2.422 [DOI] [PubMed] [Google Scholar]
- 25.Butler CR, Perkins JD, Johnson CK, Blosser CD, Bakthavatsalam R, Leca N, Sibulesky L: Burden of excess mortality after implementation of the new kidney allocation system may be borne disproportionately by middle-aged recipients. PLoS One, 14: e0210589, 2019. 10.1371/journal.pone.0210589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Executive Summary of the US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States, Available at: https://www.usrds.org/media/2371/2019-executive-summary.pdf. Accessed February 23
- 27.Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, Ramirez SP, Gillespie BW, Pisoni RL, Robinson BM, Port FK: Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med, 11: e1001750, 2014. 10.1371/journal.pmed.1001750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong A, Evangelidis N, Kurnikowski A, Lewandowski M, Bretschneider P, Oberbauer R, Baumgart A, Scholes-Robertson N, Stamm T, Carrero JJ, Pecoits-Filho R, Hecking M: Nephrologists’ Perspectives on Gender Disparities in CKD and Dialysis. Kidney International Reports, 7: 424–435, 2022. 10.1016/j.ekir.2021.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.and LDF, Lin DY: TIME-DEPENDENT COVARIATES IN THE COX PROPORTIONAL-HAZARDS REGRESSION MODEL. Annual Review of Public Health, 20: 145–157, 1999. 10.1146/annurev.publhealth.20.1.145 [DOI] [PubMed] [Google Scholar]
- 30.Hernán MA: The hazards of hazard ratios. Epidemiology, 21: 13–15, 2010. 10.1097/EDE.0b013e3181c1ea43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This an analysis of a publicly available dataset.


