ABSTRACT
The lack of effective first-line antibiotic treatments against Neisseria gonorrhoeae, and the worldwide dissemination of resistant strains, are the main drivers of a worsening global health crisis. β-lactam antibiotics have been the backbone of therapeutic armamentarium against gonococci. However, we are lacking critical insights to design rationally optimized therapies. In the present work, we generated the first PBP-binding data set on 18 currently available and clinically relevant β-lactams and 4 β-lactamase inhibitors in two N. gonorrhoeae ATCC type collection strains, 19424 and 49226 (PBP2 type XXII and A39T change in mtrR). PBP binding (IC50) was determined via the Bocillin FL binding assay in isolated membrane preparations. Three clusters of differential PBP IC50s were identified and were mostly consistent across both strains, but with quantitative differences. Carbapenems were coselective for PBP2 and PBP3 (0.01 to 0.03 mg/L). Third- and fourth-generation cephalosporins cefixime, cefotaxime, ceftazidime, cefepime, and ceftriaxone showed the lowest IC50 values for PBP2 (0.01 mg/L), whereas cefoxitin, ceftaroline, and ceftolozane required higher concentrations (0.04 to >2 mg/L). Aztreonam was selective for PBP2 in both strains (0.03 to 0.07 mg/L); amdinocillin bound this PBP at higher concentrations (1.33 to 2.94 mg/L). Penicillins specifically targeted PBP2 in strain ATCC 19424 (0.02 to 0.19 mg/L) and showed limited inhibition in strain ATCC 49226 (0.01 to >2 mg/L). Preferential PBP2 binding was observed by β-lactam-based β-lactamase inhibitors sulbactam and tazobactam (1.07 to 6.02 mg/L); meanwhile, diazabicyclooctane inhibitors relebactam and avibactam were selective for PBP3 (1.27 to 5.40 mg/L). This data set will set the bar for future studies that will help the rational use and translational development of antibiotics against multidrug-resistant (MDR) N. gonorrhoeae.
IMPORTANCE The manuscript represents the first N. gonorrhoeae PBP-binding data set for 22 chemically different drugs in two type strains with different genetic background. We have identified three clusters of drugs according to their PBP binding IC50s and highlighted the binding differences across the two strains studied. With the currently available genomic information and the PBP-binding data, we have been able to correlate the target attainment differences and the mutations that affect the drug uptake with the MIC changes. The results of the current work will allow us to develop molecular tools of great practical use for the study and the design of new rationally designed therapies capable of combating the growing MDR gonococci threat.
KEYWORDS: penicillin-binding proteins (PBP), N. gonorrhoeae, gonococcus, β-lactams, β-lactam resistance
INTRODUCTION
Sexually transmitted infections (STIs) are a major public health problem worldwide affecting quality of life and causing serious morbidity and mortality. Gonorrhea, one of the oldest recorded human diseases, is an STI caused by Neisseria gonorrhoeae (gonococcus), a facultative anaerobic, immobile, and nonencapsulated Gram-negative diplococcus. The obligate human pathogen N. gonorrhoeae is a significant global public health problem of growing magnitude with more than 106 million new cases being diagnosed every year worldwide (1). Without an effective vaccine, the lack of effective treatment for controlling gonorrhea has caused N. gonorrhoeae to be classified as an urgent public health threat globally. In fact, the Centers for Disease Control (CDC) classified N. gonorrhoeae as a “superbug,” and the World Health Organization (WHO) classified it as a “Priority 2” microorganism, announcing a near future in which gonorrhea would become untreatable (2 to 4).
Due to single dose therapy, successful horizontal gene transfer, genome plasticity, and rare resistance-derived fitness cost, N. gonorrhoeae has developed and retained resistance to every major antibiotic class, matching every historical milestone in antibiotic discovery. Worldwide emergence of gonococcal strains exhibiting multidrug resistance (MDR) and extensive drug resistance (XDR) is of great concern (5 to 8). In fact, as of 2012 cefixime is no longer recommended, and in many settings worldwide (with the current azithromycin resistance rates), ceftriaxone (single high dose) is the last remaining option for empirical first-line antimicrobial therapy (9 to 12).
Particularly, gonococci resistance determinants that affect current first-line treatments include (i) plasmid-mediated high-level resistance to penicillins determined by plasmids harboring TEM-1-type β-lactamases (13, 14); (ii) chromosomally mediated penicillin and extended spectrum cephalosporins (ESCs) resistance due to mutations affecting penicillin-binding proteins (PBPs) targets, frequently in the penA gene encoding PBP2, the main gonococcal lethal target for β-lactam antimicrobials (from single point mutations near the active site to mosaic penA genes that contain up to 60 to 70 amino acid changes) (5, 8, 11, 14 to 16); (iii) overexpression of the MtrCDE efflux pump conferring diminished sensitivity to hydrophobic antimicrobials, such as macrolides, β-lactams, ciprofloxacin, and tetracycline (17); (iv) mutations in outer membrane protein (OMP) PorB1b (penB determinant) associated with a decreased susceptibility to penicillin, cephalosporins, and tetracyclines (18, 19); and (v) reduced affinity for the 50S ribosomal macrolide target (23S rRNA SNPs) together with mtrR mutations, which constitute the macrolides’ main resistance determinants (5, 17, 20 to 22).
As a result of multiple resistance mechanisms coexisting in successful lineages, antimicrobial resistance rates and gonococcal treatment failures have increased worldwide (5, 7, 11, 23 to 27). Thus, nonoptimized dual-antimicrobial regimens might not be effective long-term solutions. Furthermore, the preclinical pipeline remains virtually empty of agents targeted to clinical development for N. gonorrhoeae treatment (10, 28 to 31).
During the last 80 years, β-lactams have recurrently been the cornerstone of our therapeutic arsenal against N. gonorrhoeae infections. However, the shortage of new antimicrobial compounds and the lack of mechanistic support of empirical combinations to treat resistant gonococcal isolates could be devastating for STIs’ antibiotic stewardship (9 to 11, 32 to 34).
The genome of N. gonorrhoeae encodes 5 PBPs. The two low molecular mass class C PBPs, PBP3 (dacB) and 4 (pbpG) (which catalyze carboxypeptidase and endopeptidase activity), are nonessential for cell viability but do play a role in cell morphology maintenance (35, 36). A third low molecular mass PBP with carboxypeptidase activity, dacC (not identified in labeled penicillin binding assays), has been also described. However, its binding ability and role in the intrinsic β-lactam resistance are still unknown (36, 37). The two high molecular mass transpeptidases, PBP1 (Class A) and PBP2 (Class B) are both essential. Gonococcal PBP1 (ponA) is the homolog of Escherichia coli PBP1a (mrcA), responsible for transglycosylation and transpeptidation, and participates in cell growth and elongation, while PBP2 (penA) is the homolog of E.coli PBP3 (ftsI), an essential peptidoglycan transpeptidase that catalyzes the cross-linking of peptidoglycan-adjacent strand functions during cell wall synthesis and division (38). PBP2, the primary clinical target, is inhibited at 10-fold lower concentrations than PBP1 in β-lactam-susceptible strains (5, 15, 16). PBP3 (dacB) is homologous to E. coli PBP4 (dacB), while PBP4 (pbpG) is most similar to E. coli PBP7 (pbpG) (35, 39).
Although the gonococcal PBP inhibitory concentrations of cephalosporins have been thoroughly characterized for the primary target PBP2 (33), we are not aware of any published PBP-binding IC50s data set in N. gonorrhoeae, and PBP binding profiles are known for roughly a few β-lactams (38, 40, 41). Therefore, the scientific basis to enhance β-lactam-based therapy to inactivate PBPs more efficiently is missing for this important pathogen (42).
In the present work, we aimed to characterize the PBP binding profiles for 18 currently available and clinically relevant β-lactams and four β-lactamase inhibitors in two N. gonorrhoeae type collection strains, ATCC 19424 and ATCC 49226. Our PBP binding data set for a comprehensive set of chemically diverse β-lactam antibiotics and β-lactamase inhibitors will help us rank order the drugs according to their PBP selectivity and rationally design new compounds and combinations. Our results warrant future research to rationally optimize β-lactam-based combination antibiotic therapy against resistant isolates.
RESULTS
Membrane fractions from N. gonorrhoeae ATCC 19424 and ATCC 49226 were labeled with Bocillin FL for 30 min. We were able to identify PBP1, 2, and 3, which had the same apparent molecular mass in both strains (Fig. 1A). In accordance with previous works using radiolabeled penicillin, we were not able to detect dacC with our experimental approach (40). Stefanova et al. showed (not as evident as PBP1 to 3) iodopenicillin (125I) binding to PBP4 (pbpG), though it was not visible in our assay (35). Two autofluorescence bands (no binding to β-lactams) and one potential proteolytic artifact without apparent antibiotic binding properties were observed with no interference with our analysis (Fig. 1B) (48). None of the ATCC strains expresses a β-lactamase; however, they possess different allelic variants for the genes intimately related with β-lactam resistance: PBPs, porin PorB, and efflux pump regulator mrtR (Table 1). Both strains possess the porB1a porin allele, which has been correlated with lower MICs.
FIG 1.
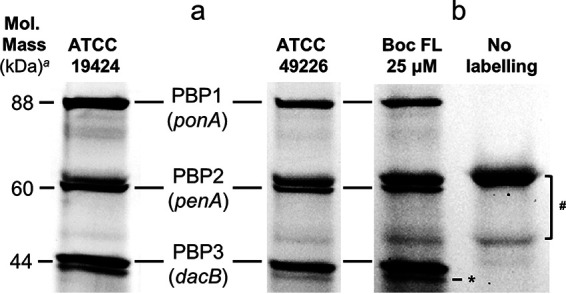
Penicillin-binding protein profiles of N. gonorrhoeae strains ATCC 19424 and ATCC 49226. PBPs were labeled with Bocillin FL, separated by SDS-PAGE, and quantified via the ImageQuantTL program. aApparent molecular mass relative to Precision Plus Protein Dual Color Standards (range 10 to 250 kDa) (Bio-Rad Laboratories, Hercules, CA). (a) Comparison of the PBP profiles of the two studied N. gonorrhoeae strains. (b) Membrane preparation loaded with and without Bocillin FL labelling. #Two autofluorescence bands were present in both strains with and without Bocillin FL labelling (not bound by any of the drugs tested); they were excluded from any further analysis. *The band below PBP3 is a potential proteolytic band and was excluded from further analysis.
TABLE 1.
Amino acid changes and allelic variants of N. gonorrhoeae ATCC 19424 and ATCC 49226
| Straina | Mutationsb |
||||||
|---|---|---|---|---|---|---|---|
| ST (NG-MAST) |
PBP1 (ponA) |
PBP2 (penA) |
PBP3 (dacB) |
PorB1a (penB) |
mtrR | ||
| Allelic changes | Type (mosaic) | ||||||
| ATCC 19424 | 266 | WT | H541N | XV (no) | T252S, H278Q, S285A, I364M | A121G | WT |
| ATCC 49226 | 1572 | A375T, F666S | R345_D346insD, F504L, A505V, A516G, H541N, P552V, K555Q, I556V, I566V, T573_A574insN, A574V | XXII (no) | T252S, H278Q, S285A, I364M | G120D, A121G | A39T |
The sequences and complete genomes for the N. gonorrhoeae ATCC strains 19424 and 49226 were obtained from the ATCC (American Type Culture Collection) genome portal.
The wild-type PBP profiles from N. gonorrhoeae were obtained from the following: PBP1 from strain FA19 (GenBank accession number U72876), PBP2 from strain LM306 (GenBank accession number M32091), PBP3 from strain FA1090 (GenBank accession number AF071224), and mtrR from strain FA19 (GenBank accession number CP012026.1). Alignments were performed using the blastp suite (protein-protein BLAST).
We generated the binding profiles and inhibition concentrations (IC50s) of 18 different β-lactams and four β-lactamase inhibitors in triplicate for two wild-type reference N. gonorrhoeae strains, ATCC 19424 (Fig. 2; Fig. S1 in the supplemental material) and ATCC 49226 (Fig. 3; Fig. S2).
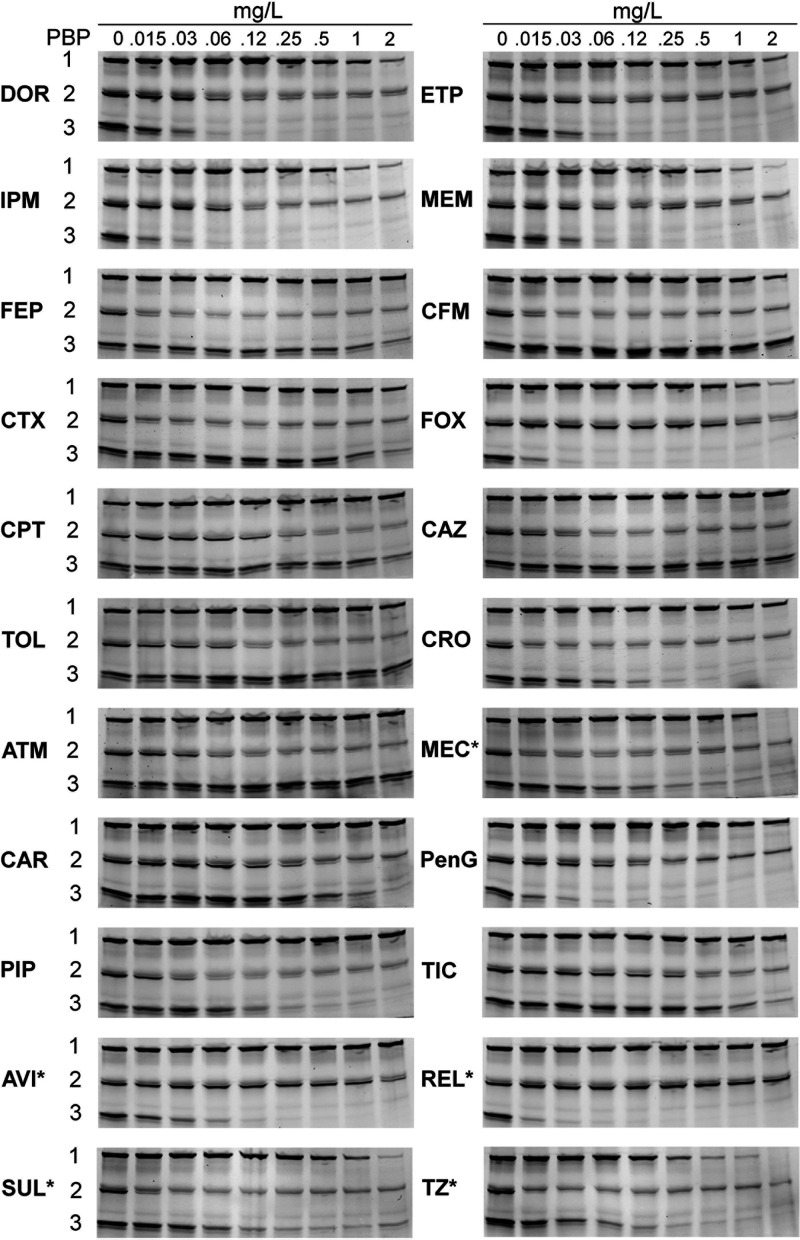
FIG 2.
Binding patterns of β-lactams in N. gonorrhoeae PBPs from strain ATCC 19424. DOR, doripenem; ETP, ertapenem; IPM, imipenem; MEM, meropenem; FEP, cefepime; CFM, cefixime; CTX, cefotaxime; FOX, cefoxitin; CPT, ceftaroline; CAZ, ceftazidime; TOL, ceftolozane; CRO, ceftriaxone; ATM, aztreonam; MEC, amdinocillin (amdinocillin); CAR, carbenicillin; PenG, penicillin G; PIP, piperacillin; TIC, ticarcillin; AVI, avibactam; REL, relebactam; SUL, sulbactam; TZ, tazobactam. The membrane preparations were incubated with the indicated β-lactams for 30 min before Bocillin FL labeling. Labeled PBPs were separated by SDS-PAGE and detected using a fluorimager. The range of concentrations tested was 0.015 to 2 mg/L. *MEC and BLIs studied ranged from 2 to 256 mg/L.
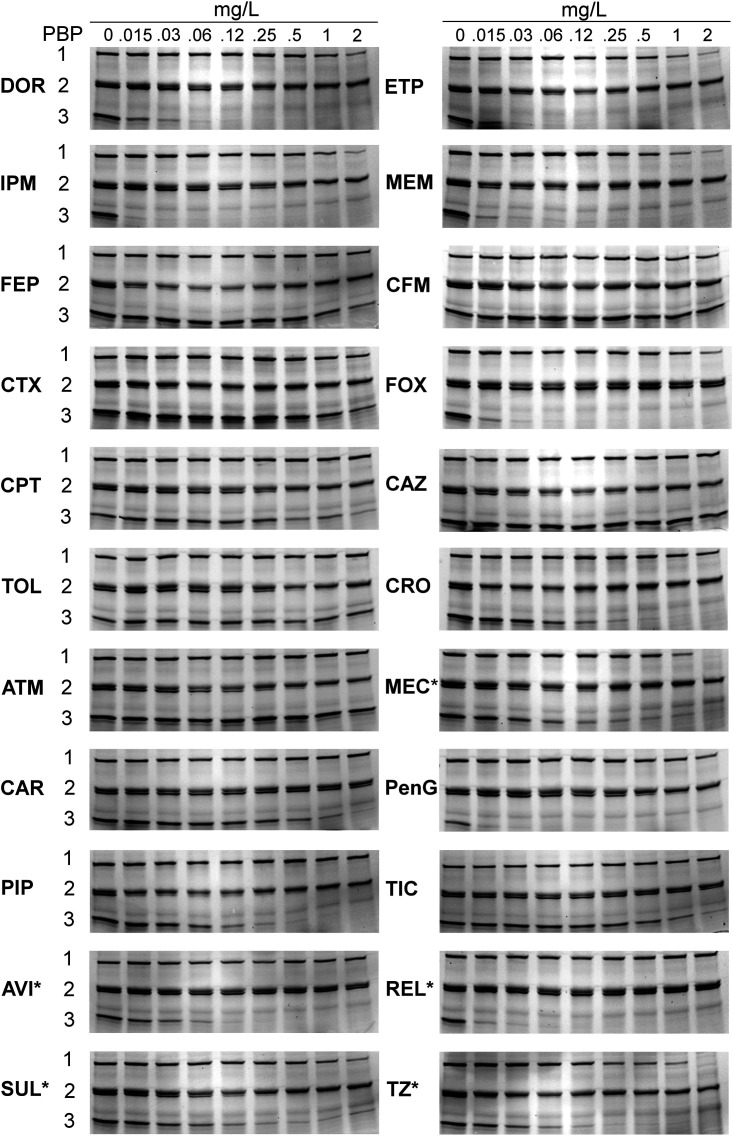
FIG 3.
Binding patterns of β-lactams for N. gonorrhoeae PBPs from strain ATCC 49226. The membrane preparations were incubated with the indicated β-lactams for 30 min before Bocillin FL labeling. The range of concentrations tested was 0.015 to 2 mg/L. Please see Fig. 2 for abbreviations. *MEC and BLIs studied ranged from 2 to 256 mg/L.
A drug was considered selective for a PBP if the IC50 was at least 4-fold lower than that of the next most inhibited PBP (Table S1) (49). If any additional PBP binding was below the 4-fold threshold, the compound was then considered coselective (50, 51). The MIC/min IC50 parameter (Table S1) represents the ratio of the MIC to the minimum IC50 value. Higher ratios indicate lower antibacterial potency and may correlate with a lower therapeutic efficacy. Drugs with minimum IC50 values for PBP3 displayed the higher MIC values.
In both strains, all carbapenems but imipenem were coselective for PBP2 and PBP3 (i.e., Bocillin FL signal reduction occurred at very low carbapenem concentrations) with overall very low IC50s (Table 2; Table S2) (52). The carbapenems IC50 for PBP3 were the lowest among all the drugs tested (0.01 to 0.03 mg/L). Ertapenem was the carbapenem with the lowest IC50. The MICs for the carbapenems ranged from 0.004 mg/L to 0.032 mg/L in the two ATCC strains, with the exception of imipenem. Imipenem showed the highest MIC (0.032 to 0.064 mg/L) and IC50 (PBP2 = 0.07 to 0.17 mg/L) values for all the targets.
TABLE 2.
PBP IC50 and MICs of β-lactam antibiotics and BLIs in N. gonorrhoeae ATCC 19424 and ATCC 49226
| Straina | IC50 and MIC of the indicated drug (mg/L)b |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DOR | ETP | IPM | MEM | FEP | CFM | CTX | FOX | CPT | CAZ | TOL | |
| ATCC 19424 | |||||||||||
| PBP1 | 1.13 ± 0.21 | 0.48 ± 0.22 | 0.4 ± 0.24 | 0.24 ± 0.07 | >2 | 0.92 ± 0.28 | 1.85 ± 0.24 | 0.58 ± 0.1 | >2 | >2 | >2 |
| PBP2 | 0.06 ± 0.03 | 0.04 ± 0.02 | 0.07 ± 0.02 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0 | 0.01 ± 0.00 | 0.39 ± 0.05 | 0.15 ± 0.09 | 0.01 ± 0.00 | 0.04 ± 0.01 |
| PBP3 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 1.57 ± 0.42 | >2 | 1.43 ± 0.63 | 0.01 ± 0.00 | 1.9 ± 1.0 | >2 | >2 |
| MIC | 0.004 | 0.004 | 0.032 | 0.008 | 0.008 | 0.004 | 0.004 | 0.25 | 0.032 | 0.016 | 0.032 |
| ATCC 49226 | |||||||||||
| PBP1 | 1.08 ± 0.22 | 0.57 ± 0.06 | 0.8 ± 0.56 | 0.45 ± 0.21 | >2 | >2 | >2 | 0.91 ± 0.02 | >2 | >2 | >2 |
| PBP2 | 0.08 ± 0.03 | 0.01 ± 0.00 | 0.17 ± 0.08 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | >2 | 0.21 ± 0.01 | 0.01 ± 0.00 | 0.27 ± 0.09 |
| PBP3 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 1.18 ± 0.38 | >2 | 1.02 ± 0.34 | 0.01 ± 0.00 | 1.29 ± 0.03 | >2 | >2 |
| MIC | 0.032 | 0.008 | 0.064 | 0.016 | 0.032 | 0.016 | 0.008 | 0.5 | 0.5 | 0.032 | 0.250 |
| CRO | ATM | MECc | CAR | PenG | PIP | TIC | AVIc | RELc | SULc | TZc | |
| ATCC 19424 | |||||||||||
| PBP1 | 1.87 ± 0.53 | >2 | 102 ± 2.26 | >2 | 1.89 ± 0.39 | >2 | >2 | >512 | >512 | 55 ± 17 | 19 ± 11 |
| PBP2 | 0.01 ± 0.00 | 0.03 ± 0.01 | 1.33 ± 0.15 | 0.13 ± 0.04 | 0.05 ± 0.02 | 0.02 ± 0.00 | 0.19 ± 0.14 | 117 ± 7.22 | >512 | 1.18 ± 0.04 | 1.07 ± 0.02 |
| PBP3 | 0.07 ± 0.02 | >2 | 11.92 ± 1.47 | 0.57 ± 0.13 | 0.02 ± 0.01 | 0.11 ± 0.04 | 1.05 ± 0.22 | 2.33 ± 1.12 | 1.27 ± 0.12 | 9.31 ± 4.17 | 4.72 ± 1.81 |
| MIC | 0.004 | 0.032 | 1 | 0.032 | 0.064 | 0.001 | 0.016 | 128 | >256 | 0.25 | 0.125 |
| ATCC 49226 | |||||||||||
| PBP1 | 1.9 ± 0.19 | >2 | 155 | 155 ± 24 | >2 | >2 | >2 | >256 | >256 | 203 ± 118 | 25 ± 6.94 |
| PBP2 | 0.01 ± 0.00 | 0.07 ± 0.03 | 5.88 | 5.88 ± 1.94 | 0.26 ± 0.08 | 0.01 ± 0.00 | >2 | >256 | >256 | 6.02 ± 0.34 | 1.44 ± 0.05 |
| PBP3 | 0.07 ± 0.02 | >2 | 9.23 | 9.23 ± 3.52 | 0.01 ± 0.00 | 0.09 ± 0.02 | 0.63 ± 0.10 | 5.40 ± 1.87 | 1.31 ± 0.26 | 6.36 ± 0.21 | 5.69 ± 0.51 |
| MIC | 0.002 | 0.125 | 8 | 0.5 | 0.125 | 0.125 | 0.5 | >256 | >256 | 4 | 1 |
| PIP/TZd | CAZ/AVId | TOL/TZd | |||||||||
| ATCC 19424 | |||||||||||
| MIC | <0.016 | <0.016 | <0.016 | ||||||||
| ATCC 49226 | |||||||||||
| MIC | 0.032 | 0.032 | <0.016 | ||||||||
N. gonorrhoeae strains ATCC 19424 and ATCC 49226. PBP, penicillin-binding proteins.
Concentration of β-lactam that inhibits 50% of Bocillin FL compared to that of a control containing no drug. Please see Fig. 2 for abbreviations.
When none of the PBPs were inhibited using the regular concentrations, an extended range of 2 to 256 mg/L was used (MEC, AVI, SUL, REL, and TZ).
MIC strips contain stable concentration gradient for piperacillin, ceftazidime, or ceftolozane (0.016 to 256 mg/L) in the presence of a fixed concentration of the BLI tazobactam or avibactam (4 mg/L).
Excluding imipenem, carbapenem MICs were 1.25- to 7.5-fold lower than the IC50s for PBP2 (Table S1), suggesting that inactivation of PBP2 at very low carbapenem concentrations causes considerable bacterial inhibition. Compared with ceftriaxone (same PBP2 IC50 = 0.01), a higher extent of PBP1 and PBP3 inactivation did not greatly enhance the effectivity of these compounds.
Third- and fourth-generation cephalosporins cefixime, cefotaxime, ceftazidime, cefepime, and ceftriaxone had the lowest IC50 values for PBP2 (0.01 to 0.02 mg/L) (Table 2), which were consistent between the two strains; however, a 2- to 4-fold MIC increase was observed for ATCC 49226. Second- and new-generation cephalosporins (cefoxitin, ceftaroline, and ceftolozane) were able to bind PBP2 at higher concentrations (IC50 = 0.21 to >2 mg/L). Cefoxitin inactivated the nonlethal target PBP3 with very low values in both strains (0.01 mg/L). Accordingly, the observed MIC values were 25- to 50-fold higher (0.25 and 0.5 mg/L) than the IC50s for this PBP (Table S1).
Aztreonam was selective for PBP2 in both strains (0.03 to 0.07 mg/L), but MIC values were 0.032 (ATCC 19424) and 0.125 mg/L (ATCC 49226). Amdinocillin, on the other hand, showed limited PBP2 inhibition (IC50 = 1.33 and 5.88 mg/L) and ranked the higher MIC values (1 and 8 mg/L) among β-lactam compounds.
Penicillins were selective for PBP2 in strain ATCC 19424 (0.01 to 0.19 mg/L), and with the sole exception of piperacillin, they caused little to no inactivation in strain ATCC 49226 (0.26 to >2 mg/L). While the PBP IC50s were consistent for both strains, the piperacillin MIC was substantially higher for strain ATCC 49226 (0.125 versus 0.001 mg/L, respectively).
Diazabicyclooctane (DBO) non-β-lactam β-lactamase inhibitors (avibactam and relebactam) were the only drugs showing PBP3 selective binding, while β-lactam-derived β-lactamase inhibitors (sulbactam and tazobactam) were selective for PBP2 (Table 2). The MICs of the DBO β-lactamase inhibitors (BLIs) were 55- to 400-fold higher than their IC50s for PBP3. Conversely, the MIC values for the β-lactam BLIs were 1.5- to 8-fold lower than their PBP2 IC50 values. Such remarkable difference was likely caused by the difference in target selectivity (PBP2 versus PBP3) between the β-lactamase inhibitors (Table S1).
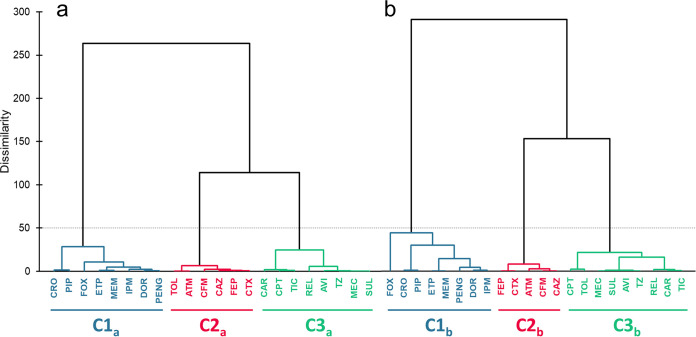
The principal-component analysis of the log-transformed PBP IC50s showed that the first two eigenvectors explained 89.9% of the total variance of the two strains for the 22 compounds (not shown). Agglomerative hierarchical clustering (AHC) identified the same three clusters with distinct PBP occupancy patterns in both strains (Fig. 4). The first cluster contained β-lactams that predominantly targeted PBP3 (0.01 to 0.11 mg/L), while also binding PBP2 at low concentrations in both strains (0.01 to 0.26 mg/L) (carbapenems, cefoxitin, ceftriaxone, penicillin G, and piperacillin). The second cluster comprised compounds that exclusively inactivated PBP2 (third-generation cephalosporins [but ceftriaxone] and aztreonam). Ceftolozane differed in the two analyses, belonging to cluster 3 in the ATCC 49226 strain. The third cluster included compounds that had PBP2, PBP3, or both as their primary targets but only at substantially higher concentrations (new-generation cephalosporins, imipenem, BLIs, carbenicillin, ticarcillin, and amdinocillin).
FIG 4.
Agglomerative hierarchical clustering for logarithmic IC50 data of the tested 22 drugs with penicillin-binding proteins in isolated membranes of N. gonorrhoeae strains (a) ATCC 19424 and (b) ATCC 49226 using the XLSTAT program. C1 to C3 represent clusters 1 to 3. Please see Fig. 2 for abbreviations.
DISCUSSION
With few preclinical candidates targeting MDR gonococci in the drug development pipeline and vaccine development being an unlikely solution in the short term due to high antigenic variability in clinical isolates, public health control of gonorrhea relies totally on appropriate antimicrobial treatment (2, 4, 10, 31).
The current emergence of resistance to first-line empirical gonococcal therapy (including ESCs) calls for the design and optimization of effective combination therapies (17, 27, 32, 33). The optimal first-line therapy should be widely available, highly effective (if possible as a single dose), lack toxicity, and with a microbiologic cure rate of >95% of patients (3). To gain insights into the molecular basis for optimizing drug combinations that maximize treatment efficacy and suppress resistance emergence, we characterized the occupancy patterns for 18 currently available and clinically relevant β-lactams and four β-lactamase inhibitors in N. gonorrhoeae.
Although no major differences were observed for the signal intensities of the three studied PBPs and PBP selectivity across both strains, remarkable differences were found for the binding IC50s and MIC values. In general, PBP profiles and occupancy patterns in the two studied strains were comparable to previously published data (40).
Carbapenems (except imipenem) were the only compounds that were coselective for PBP2 and PBP3 in both strains. Expectedly, compared with the first-line drug ceftriaxone, the increased PBP3 inhibition didn’t enhance bacterial growth inhibition (36, 41). In contrast to what has been observed in Enterobacterales and P. aeruginosa, imipenem showed the lowest PBP inhibition and the highest MIC values amid carbapenems (53, 54). Moreover, like doripenem, it is not likely to retain activity against cephalosporin-resistant N. gonorrhoeae isolates (55). In contrast, ertapenem and meropenem were 8 times more active and showed the lowest IC50s for PBP2 and 3 in both strains, mostly unaltered by the PBP2 type XXII and the mtrR mutation from strain ATCC 49226. Ertapenem and meropenem are the most effective drugs of this class, and potential candidates for possible N. gonorrhoeae alternative treatment for the ESC-resistant isolates. Furthermore, ertapenem could possibly be part of dual antimicrobial therapy (once a day) if the genetic resistance determinants are properly characterized (10, 14, 29).
As previously reported, third-generation cephalosporins cefixime, cefotaxime, and ceftazidime (acylation rates comparable to ceftriaxone) showed the greatest PBP2 inhibition in both strains. All of the drugs from this subclass showed a marginal 2-fold MIC increase except cefixime, which displayed a 4-fold higher MIC for strain ATCC 49226. Besides its epistatic nature, it seems that cefixime resistance may be more affected by penA and mtrR mutations compared to ceftriaxone, a possible explanation for the earlier therapeutic failures (16, 33, 42).
Cefoxitin inactivated PBP3 and PBP1 (slow PBP2 acylation rates). Although it could be a treatment option (plus probenecid) for penicillinase-producing isolates, there is not enough clinical evidence to support its efficacy against isolates with mutations in PBP2 or mtrR (33).
New-generation cephalosporins required the highest concentrations to inactivate PBP2 and demonstrated the lowest antibacterial activity in this subclass. Ceftaroline, with low IC50 values, exhibited reduced antimicrobial efficacy for strain ATCC 49226. The A39T missense mutation in mtrR and the PBP2 variant (type XXII) present in this strain may contribute to the 16-fold MIC increase (together with epistatic loci affecting antibiotic resistance) (16). Ceftolozane has better outer membrane permeability and stability toward class C beta-lactamases, leading to improved efficacy against P. aeruginosa. However, this feature limits PBP2 acylation capability and thus growth inhibition in N. gonorrhoeae (MIC = 0.25 mg/L) (33). A time of free drug above MIC (fT>MIC) of 20 to 24 h (longer for pharyngeal gonorrhea) needed for therapeutic efficacy, would rarely be achieved with ESCs with MICs ≥0.25 mg/L (42).
Aztreonam selectivity for PBP2 was markedly affected by the presence of the PBP2 type XXII and mtrR mutations. In a recent small clinical trial, a single 2-g aztreonam dose was an effective ESC-sparing alternative to treat gonococcal urethritis. However, in isolates with an MIC above 0.5 mg/L, it only achieved a 50% cure rate and was not a reliable treatment for pharyngeal infections (56). Amdinocillin, with limited PBP2 inhibition, has demonstrated poor therapeutic activity for isolates that are nonsusceptible to common first-line antigonococcal antimicrobials (30).
Like in previous reports for penicillins, modifications of the penA gene (type XXII) elicited a significant reduction of PBP2 binding, with the sole exception of piperacillin, with comparable IC50 values among the two strains. The observed 125-fold higher piperacillin MIC for strain ATCC 49226 suggested that reduced uptake (porin or altered efflux) could be the underlying mechanism for the MIC shift (17, 29). The porB1a allele has been associated with lower resistance levels compared to porB1b; however, the impact of the double mutation G120D/A121G on β-lactams resistance needs to be further explored (57 to 59). Furthermore, the A375T substitution in the ponA gene present in the strain ATCC 49226 has been identified in N. gonorrhoeae clinical isolates with high-level resistance to penicillins (37).
Since their introduction, continuous β-lactam development has allowed combinations of two different compounds that are not affected by the same resistance determinants (12, 60, 61). Several β-lactam-derived BLIs have been developed to treat infections by β-lactamase-producing organisms (clavulanate, sulbactam, and tazobactam) combined with β-lactams. Recently, two novel classes of non-β-lactam BLIs have been introduced to the clinic: the boronate-based (vaborbactam) and the diazabicyclooctanes (DBOs) (avibactam, relebactam, zidebactam, nacubactam, etc.). These compounds have shown improved spectrum or β-lactam enhancing activity (PBP2-binding), acting synergistically with the partner β-lactam (62). With the current low penicillinase-producing N. gonorrhoeae prevalence, combination therapies could focus on enhancing the partner activity (additive [PBP2] or complementary [PBP1] PBP-binding) (13).
As previously described in other Gram-negatives, novel BLIs (DBOs) showed neither an extensive PBP occupancy nor a significant increase or decrease of their primary target attainment in any of the studied strains (63). The potential therapeutic efficacy of sulbactam or tazobactam alone was better than that of the DBO-derived BLIs (avibactam and relebactam), against the two strains. When combined with their clinical partner β-lactam (piperacillin-tazobactam and ceftolozane-tazobactam), β-lactam BLIs displayed a notable increase of the antibacterial activity against both strains (4- to >16-fold MIC reduction). DBO-based β-lactamase inhibitor avibactam showed a modest ceftazidime MIC reduction. Neither of the studied strains expresses a β-lactamase, so the improved efficacy could rely on additive (PBP2; tazobactam) target binding (34, 62).
Clinically available combinations, piperacillin-tazobactam, ceftolozane-tazobactam, and to a lesser extent ceftazidime-avibactam, though unsuitable for empirical use (short half-lives and parenteral route of administration), could be considered for individual patients with ceftriaxone-resistant gonococcal infection once MICs (or preferably genetic point-of-care antimicrobial resistance tests) are available (34).
Dissimilar antimicrobial stewardship programs worldwide and the lack of clinical data and breakpoints to support the use of rationally improved antimicrobial therapies, challenge the treatment against N. gonorrhoeae (9, 42). Our study presents the first data set on PBP occupancy in N. gonorrhoeae for 22 clinically relevant β-lactams and BLIs. A limitation of our study is the use of two N. gonorrhoeae strains without a mosaic PBP2 allele and lack of studies on isogenic strains with knockout or overexpression of efflux, and porins. However, our comprehensive data set comprised 22 drugs studied at least in triplicate in two N. gonorrhoeae strains used for clinical control assessment procedures.
Future studies evaluating the target site penetration of β-lactams in reduced outer membrane permeability isolates and to systematically link PBP occupancy patterns to bacterial killing (especially in isolates producing mosaic PBP2 alleles) are warranted. We think that the present work provides relevant data to allow selecting β-lactams and combinations for future synergy studies with β-lactamase inhibitors and other antibiotic classes against resistant N. gonorrhoeae isolates.
Without a doubt, a coordinated effort among basic, translational, and clinical research is needed to develop in vitro and in silico pharmacokinetic/pharmacodynamics (PK/PD) models that evaluate and predict antibacterial activity and resistance emergence. Future mechanistically informed clinical trials evaluating rationally designed therapies could help advance the standard of care toward the much-needed individually tailored treatment.
MATERIALS AND METHODS
In vitro susceptibility testing.
The MIC of β-lactams and β-lactamase inhibitors (BLIs) was determined by the standard Clinical and Laboratory Standards Institute (CLSI) broth microdilution method (43). Imipenem was purchased from Fresenius Kabi (Barcelona, Spain); meropenem from Aurovitas (Madrid, Spain); ertapenem from Merck Sharp & Dohme (Haarlem, Netherlands); penicillin G from Laboratorio Reig Jofré SA (Barcelona, Spain); ceftaroline from Pfizer Pharmaceuticals (Ringsend, Ireland); cefotaxime, ceftriaxone, cefoxitin, and ceftazidime from Laboratorios Normon (Madrid, Spain); cefepime from Accord Healthcare (Barcelona, Spain); aztreonam from Bristol-Myers Squibb (Madrid, Spain); and doripenem, cefixime, ceftolozane, amdinocillin, carbenicillin, piperacillin, ticarcillin, avibactam, relebactam, sulbactam, and tazobactam from MedChem Express (Sollentuna, Sweden). Ceftazidime-avibactam and ceftolozane-tazobactam MICs were determined by MIC test strip from Liofilchem (Rosetto degli Abruzzi, Italy). MIC values were determined from at least three independent experiments.
PBP-binding assays.
To enhance the strength of our PBP-binding (reported as the 50% inhibitory concentration; IC50) data sets and evaluate strain-to-strain variability, we studied two wild-type reference N. gonorrhoeae (NG) strains, ATCC 19424 and ATCC 49226, type strain and CLSI-recommended strain for quality control assessment procedures, respectively. The PBP-binding IC50s were determined in membrane preparations from the reference strains following previously described protocols (40).
N. gonorrhoeae cultures were grown in phosphate-buffered gonococcal medium (GCP) broth supplemented with 1/100 volume 4.2% sodium bicarbonate and 1/100 volume Kellogg’s supplement on a 37°C shaking incubator (200 rpm). Approximately 400 mL late-log phase (OD600nm = 1) was collected by centrifugation, washed, and resuspended in 20 mM KH2PO4 with 140 mM NaCl pH 7.5. Cells were sonicated and centrifuged at 4,000 × g for 20 min. Bacterial membranes were collected by ultracentrifugation. Binding reactions were conducted for 22 chemically diverse β-lactams and β-lactamase inhibitors using 20 micrograms of membrane preparation proteins for 30 min at 37°C (range of concentrations tested = 0.0156 to 2 mg/L). When no measurable binding was observed for any of the PBPs, an upper or lower extended concentration range was used (0.001 to 0.125 or 2 to 256 mg/L when indicated). The initial incubation allows that each of the drugs binds to each of the PBP target receptors depending on its second-order acylation rate constant (k2/Ks; inhibitory potency of a β-lactam) (44 to 46). After the initial incubation, membrane preparations were labeled with 25 μM Bocillin FL for 30 min (Bocillin FL is a fluorophore-conjugated penicillin V analog that binds to all PBPs). Unbound PBP molecules are then available to bind to Bocillin FL. For a given antibiotic concentration, the weaker the Bocillin FL signal, the greater the binding capacity for each of the PBPs (47). PBPs were separated on SDS-PAGE (Bio-Rad Laboratories, Hercules, CA), visualized using a Typhoon FLA 9500 biomolecular imager, and IC50s quantified using ImageQuantTL 8.1 (GE Healthcare Bio-Sciences AB, Björkgatan, 30 751 84 Uppsala). The amount of a PBP bound by a particular beta-lactam is determined by the remaining PBP molecules available to react with Bocillin FL, in comparison to those bound by Bocillin FL in the absence of the beta-lactam. Binding affinities were reported as the β-lactam or BLI concentrations that half-maximally inhibited Bocillin FL binding (IC50s) and were determined from at least three independent experiments.
Data analysis.
Agglomerative hierarchical clustering (AHC) was performed in the XLSTAT software (v2021.5; Addinsoft). We analyzed the logarithmic PBP-binding data of twenty-two drugs in both N. gonorrhoeae strains to identify different clusters of the studied drugs based on their binding data. Agglomerative hierarchical clustering was used based on the Euclidean distance for calculating the degree of dissimilarity. The Ward's method was used for agglomeration to generate the dendrogram, and three differentiated clusters were observed in each strain.
ACKNOWLEDGMENTS
B.M. received funds from a RADIX17/3-1 fellowship and RADIX17/3-2 grant, programs within the FUTURMed project IdISBa Research Institute of Health Sciences of the Balearic Islands, Hospital Universitario Son Espases, Palma, Spain. Call funded by the 2017 annual plan of the sustainable tourism tax. Govern de les Illes Balears, by the Miguel Servet Research Contract Program CP20/00138 from the National Institutes of Health Carlos III (ISCIII) and by the Agencia Estatal de Investigación (AEI - State Research Agency), Spain, through the Plan Estatal de Investigación Científica PROYECTOS DE I+D+i PID2020-112654RB-I00 / AEI / 10.13039/501100011033. The assay development part of this work was supported by the award R01AI136803 to B.M. from the National Institute of Allergy and Infectious Diseases (NIAID). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health (NIH).
A.O. received funds from the Ministerio de Economía y Competitividad of Spain, Instituto de Salud Carlos III, cofinanced by European Regional Development Fund “A Way to Achieve Europe” ERDF, through the Spanish Network for the Research in Infectious Diseases (RD16/0016).
A.O. has received fees as speaker and/or research grants from MSD, Pfizer, and Wockhardt.
Footnotes
Supplemental material is available online only.
Contributor Information
Bartolome Moya, Email: bartolome.moya@ssib.es.
Pablo Power, Universidad de Buenos Aires.
REFERENCES
- 1.World Health. Organization (WHO). 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Hook EW, Kirkcaldy RD. 2018. A brief history of evolving diagnostics and therapy for gonorrhea: lessons learned. Clin Infect Dis 67:1294–1299. doi: 10.1093/cid/ciy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). 2012. Cephalosporin-resistant Neisseria gonorrhoeae public health response plan. CDC, Atlanta, GA. [Google Scholar]
- 4.Zielke RA, Wierzbicki IH, Weber JV, Gafken PR, Sikora AE. 2014. Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Mol Cell Proteomics 13:1299–1317. doi: 10.1074/mcp.M113.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67:1858–1860. doi: 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsson S, Mason C, Khan N, Meo P, Unemo M. 2019. In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae: future treatment option for gonorrhoea? J Antimicrob Chemother 74:1591–1594. doi: 10.1093/jac/dkz060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin I, Sawatzky P, Allen V, Lefebvre B, Hoang L, Naidu P, Minion J, Van Caeseele P, Haldane D, Gad RR, Zahariadis G, Corriveau A, German G, Tomas K, Mulvey MR. 2019. Multidrug-resistant and extensively drug-resistant. Can Commun Dis Rep 45:45–53. doi: 10.14745/ccdr.v45i23a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaskolskiy B, Dementieva E, Kandinov I, Filippova M, Petrova N, Plakhova X, Chestkov A, Kubanov A, Deryabin D, Gryadunov D. 2019. Resistance of Neisseria gonorrhoeae isolates to beta-lactam antibiotics (benzylpenicillin and ceftriaxone) in Russia, 2015–2017. PLoS One 14:e0220339. doi: 10.1371/journal.pone.0220339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenyon C, Laumen J, Van Den Bossche D, Van Dijck C. 2019. Where have all the susceptible gonococci gone? A historical review of changes in MIC distribution over the past 75 years. BMC Infect Dis 19:1085. doi: 10.1186/s12879-019-4712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis DA. 2019. New treatment options for Neisseria gonorrhoeae in the era of emerging antimicrobial resistance. Sex Health 16:449. doi: 10.1071/SH19034. [DOI] [PubMed] [Google Scholar]
- 11.Lahra MM, Martin I, Demczuk W, Jennison AV, Lee K-I, Nakayama S-I, Lefebvre B, Longtin J, Ward A, Mulvey MR, Wi T, Ohnishi M, Whiley D. 2018. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 24:735–743. doi: 10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unemo M, Workowski K. 2018. Dual antimicrobial therapy for gonorrhoea: what is the role of azithromycin? Lancet Infect Dis 18:486–488. doi: 10.1016/S1473-3099(18)30162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micaëlo M, Goubard A, La Ruche G, Denamur E, Tenaillon O, Cambau E, Jacquier H, Bercot B. 2017. Molecular epidemiology of penicillinase-producing Neisseria gonorrhoeae isolates in France. Clin Microbiol Infect 23:968–973. doi: 10.1016/j.cmi.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi M, Ono E, Shimuta K, Watanabe H, Okamura N. 2010. Identification of TEM-135 β-lactamase in penicillinase-producing Neisseria gonorrhoeae strains in Japan. Antimicrob Agents Chemother 54:3021–3023. doi: 10.1128/AAC.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berenger BM, Demczuk W, Gratrix J, Pabbaraju K, Smyczek P, Martin I. 2019. Genetic characterization and enhanced surveillance of ceftriaxone-resistant Neisseria gonorrhoeae strain, Alberta, Canada, 2018. Emerg Infect Dis 25:1660–1667. doi: 10.3201/eid2509.190407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomberg J, Unemo M, Davies C, Nicholas RA. 2010. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry 49:8062–8070. doi: 10.1021/bi101167x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohneck EA, Zalucki YM, Johnson PJT, Dhulipala V, Golparian D, Unemo M, Jerse AE, Shafer WM. 2011. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio 2:e00187-11. doi: 10.1128/mBio.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill MJ, Simjee S, Al-Hattawi K, Robertson BD, Easmon CS, Ison CA. 1998. Gonococcal resistance to β-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob Agents Chemother 42:2799–2803. doi: 10.1128/AAC.42.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olesky M, Hobbs M, Nicholas RA. 2002. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:2811–2820. doi: 10.1128/AAC.46.9.2811-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan C, Li Y, Le W-J, Liu Y-R, Li S, Wang B-X, Rice PA, Su X-H. 2018. Increasing resistance to azithromycin in Neisseria gonorrhoeae in Eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from Nanjing. Antimicrob Agents Chemother 62:e02499-17. doi: 10.1128/AAC.02499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beggs GA, Zalucki YM, Brown NG, Rastegari S, Phillips RK, Palzkill T, Shafer WM, Kumaraswami M, Brennan RG. 2019. Structural, biochemical, and in vivo characterization of MtrR-mediated resistance to innate antimicrobials by the human pathogen Neisseria gonorrhoeae. J Bacteriol 201:e00401-19. doi: 10.1128/JB.00401-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Wang F, Zhu C, Xiu L, Li Y, Li L, Liu B, Li Y, Zeng Y, Guo B, Peng J. 2020. Determining antimicrobial resistance profiles and identifying novel mutations of Neisseria gonorrhoeae genomes obtained by multiplexed MinION sequencing. Sci China Life Sci 63:1063–1070. doi: 10.1007/s11427-019-1558-8. [DOI] [PubMed] [Google Scholar]
- 23.Xiu L, Yuan Q, Li Y, Zhang C, Tang L, Peng J. 2020. Emergence of ceftriaxone-resistant Neisseria gonorrhoeae strains harbouring a novel mosaic penA gene in China. J Antimicrob Chemother 75:907–910. doi: 10.1093/jac/dkz530. [DOI] [PubMed] [Google Scholar]
- 24.Public Health England. 2018. UK case of Neisseria gonorrhoeae with high-level resistance to azithromycin and resistance to ceftriaxone acquired abroad. In Health protection report—volume 12. Public Health England, London, UK. [Google Scholar]
- 25.Unemo M, Del Rio C, Shafer WM. 2016. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr 4:4.3.35. doi: 10.1128/microbiolspec.EI10-0009-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tapsall JW, Ndowa F, Lewis DA, Unemo M. 2009. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther 7:821–834. doi: 10.1586/eri.09.63. [DOI] [PubMed] [Google Scholar]
- 27.Ibargoyen García U, Nieto MC, Azpeitia EM, Imaz M, Hernandez L, Álava JA, Cámara MM, López de Munain J, Muñoz J, Díaz de Tuesta JL, Cisterna R. 2019. Epidemiological surveillance study of gonococcal infection in Northern Spain. Enferm Infecc Microbiol Clin 38:59–64. doi: 10.1016/j.eimc.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, Tapsall JW. 2012. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob Agents Chemother 56:3603–3609. doi: 10.1128/AAC.00326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs F, Wille J, Hamprecht A, Parcina M, Lehmann C, Schwarze-Zander C, Seifert H, Higgins PG. 2019. In vitro activity of mecillinam and nitroxoline against Neisseria gonorrhoeae—re-purposing old antibiotics in the multi-drug resistance era. J Med Microbiol 68:991–995. doi: 10.1099/jmm.0.001014. [DOI] [PubMed] [Google Scholar]
- 31.Hook EW, Newman L, Drusano G, Evans S, Handsfield HH, Jerse AE, Kong FYS, Lee JY, Taylor SN, Deal C. 2019. Development of new antimicrobials for urogenital gonorrhea therapy: clinical trial design considerations. Clin Infect Dis 70:1495–1500. doi: 10.1093/cid/ciz899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Dhital S, Naderer T. 2019. Efficacy and safety of injectable and oral antibiotics in treating gonorrhea: a systematic review and network meta-analysis. J Clin Med 8:2182. doi: 10.3390/jcm8122182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner JM, Connolly KL, Aberman KE, Fonseca JC, Singh A, Jerse AE, Nicholas RA, Davies C. 2021. Molecular features of cephalosporins important for activity against antimicrobial-resistant Neisseria gonorrhoeae. ACS Infect Dis 7:293–308. doi: 10.1021/acsinfecdis.0c00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jean S-S, Lu M-C, Shi Z-Y, Tseng S-H, Wu T-S, Lu P-L, Shao P-L, Ko W-C, Wang F-D, Hsueh P-R. 2018. In vitro activity of ceftazidime-avibactam, ceftolozane-tazobactam, and other comparable agents against clinically important Gram-negative bacilli: results from the 2017 Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). Infect Drug Resist 11:1983–1992. doi: 10.2147/IDR.S175679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanova ME, Tomberg J, Olesky M, Höltje J-V, Gutheil WG, Nicholas RA. 2003. Neisseria gonorrhoeae penicillin-binding protein 3 exhibits exceptionally high carboxypeptidase and β-lactam binding activities. Biochemistry 42:14614–14625. doi: 10.1021/bi0350607. [DOI] [PubMed] [Google Scholar]
- 36.Obergfell KP, Schaub RE, Priniski LL, Dillard JP, Seifert HS. 2018. The low-molecular-mass, penicillin-binding proteins DacB and DacC combine to modify peptidoglycan cross-linking and allow stable Type IV pilus expression in Neisseria gonorrhoeae. Mol Microbiol 109:135–149. doi: 10.1111/mmi.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nokchan N, Wongsurawat T, Jenjaroenpun P, Nitayanon P, Tribuddharat C. 2022. Whole-genome sequence analysis of high-level penicillin-resistant strains and antimicrobial susceptibility of Neisseria gonorrhoeae clinical isolates from Thailand. PLoS One 17:e0271657. doi: 10.1371/journal.pone.0271657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spratt BG. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173–176. doi: 10.1038/332173a0. [DOI] [PubMed] [Google Scholar]
- 39.Stefanova ME, Tomberg J, Davies C, Nicholas RA, Gutheil WG. 2004. Overexpression and enzymatic characterization of Neisseria gonorrhoeae penicillin-binding protein 4. Eur J Biochem 271:23–32. doi: 10.1046/j.1432-1033.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- 40.Barbour AG. 1981. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob Agents Chemother 19:316–322. doi: 10.1128/AAC.19.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dougherty TJ, Koller AE, Tomasz A. 1981. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Neisseria gonorrhoeae. Antimicrob Agents Chemother 20:109–114. and doi: 10.1128/AAC.20.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chisholm SA, Mouton JW, Lewis DA, Nichols T, Ison CA, Livermore DM. 2010. Cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J Antimicrob Chemother 65:2141–2148. doi: 10.1093/jac/dkq289. [DOI] [PubMed] [Google Scholar]
- 43.CLSI. 2019. Performance standards for antimicrobial susceptibility testing; twenty-seventh informational supplement. CLSI document M100-S29. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 44.Tipper DJ, Strominger JL. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci USA 54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frère JM, Joris B. 1985. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol 11:299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- 46.Frère JM, Ghuysen JM, Reynolds PE, Moreno R. 1974. Binding of β-lactam antibiotics to the exocellular dd-carboxypeptidase-transpeptidase of Streptomyces R39. Biochem J 143:241–249. doi: 10.1042/bj1430241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. 1999. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother 43:1124–1128. doi: 10.1128/AAC.43.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henderson TA, Dombrosky PM, Young KD. 1994. Artifactual processing of penicillin-binding proteins 7 and 1b by the OmpT protease of Escherichia coli. J Bacteriol 176:256–259. doi: 10.1128/jb.176.1.256-259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kocaoglu O, Carlson EE. 2015. Profiling of β-lactam selectivity for penicillin-binding proteins in Escherichia coli strain DC2. Antimicrob Agents Chemother 59:2785–2790. doi: 10.1128/AAC.04552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spratt BG. 1975. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA 72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dougherty TJ, Kennedy K, Kessler RE, Pucci MJ. 1996. Direct quantitation of the number of individual penicillin-binding proteins per cell in Escherichia coli. J Bacteriol 178:6110–6115. doi: 10.1128/jb.178.21.6110-6115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gómez J, García-Vázquez E, Hernández-Torres A. 2015. Betalactams in clinical practice. Rev Esp Quimioter 28:1–9. (In Spanish). [PubMed] [Google Scholar]
- 53.Davies TA, Shang W, Bush K, Flamm RK. 2008. Affinity of doripenem and comparators to penicillin-binding proteins in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:1510–1512. doi: 10.1128/AAC.01529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moya B, Bhagwat S, Cabot G, Bou G, Patel M, Oliver A. 2020. Effective inhibition of PBPs by cefepime and zidebactam in the presence of VIM-1 drives potent bactericidal activity against MBL-expressing Pseudomonas aeruginosa. J Antimicrob Chemother 75:1474–1478. doi: 10.1093/jac/dkaa036. [DOI] [PubMed] [Google Scholar]
- 55.Hiyama Y, Takahashi S, Sato T, Shinagawa M, Fukushima Y, Nakajima C, Suzuki Y, Masumori N, Yokota S-I. 2019. Evaluation of susceptibilities to carbapenems and faropenem against cephalosporin-resistant Neisseria gonorrhoeae clinical isolates with penA mosaic alleles. Microb Drug Resist 25:427–433. doi: 10.1089/mdr.2018.0263. [DOI] [PubMed] [Google Scholar]
- 56.Barbee LA, Soge OO, Ocbamichael N, LeClair A, Golden MR. 2020. Single-arm open-label clinical trial of two grams of aztreonam for the treatment of Neisseria gonorrhoeae. Antimicrob Agents Chemother 65:e01739-20. doi: 10.1128/AAC.01739-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demczuk W, Sidhu S, Unemo M, Whiley DM, Allen VG, Dillon JR, Cole M, Seah C, Trembizki E, Trees DL, Kersh EN, Abrams AJ, de Vries HJC, van Dam AP, Medina I, Bharat A, Mulvey MR, Van Domselaar G, Martin I. 2017. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol 55:1454–1468. doi: 10.1128/JCM.00100-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balashov S, Mordechai E, Adelson ME, Gygax SE. 2013. Multiplex bead suspension array for screening Neisseria gonorrhoeae antibiotic resistance genetic determinants in noncultured clinical samples. J Mol Diagn 15:116–129. doi: 10.1016/j.jmoldx.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Serra-Pladevall J, Barberá MJ, Rodriguez S, Bartolomé-Comas R, Roig G, Juvé R, Andreu A. 2016. Neisseria gonorrhoeae antimicrobial susceptibility in Barcelona: penA, ponA, mtrR, and porB mutations and NG-MAST sequence types associated with decreased susceptibility to cephalosporins. Eur J Clin Microbiol Infect Dis 35:1549–1556. doi: 10.1007/s10096-016-2696-7. [DOI] [PubMed] [Google Scholar]
- 60.Singh V, Bala M, Bhargava A, Kakran M, Bhatnagar R. 2018. In vitro efficacy of 21 dual antimicrobial combinations comprising novel and currently recommended combinations for treatment of drug resistant gonorrhoea in future era. PLoS One 13:e0193678. doi: 10.1371/journal.pone.0193678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiao Y, Moya B, Chen M-J, Zavascki AP, Tsai H, Tao X, Sutaria DS, Louie A, Boyce JD, Deveson Lucas D, Kim TH, Tsuji BT, Bonomo RA, Drusano GL, Bulitta JB. 2019. Comparable efficacy and better safety of double β-lactam combination therapy versus β-lactam plus aminoglycoside in gram-negative bacteria in randomized, controlled trials. Antimicrob Agents Chemother 63:e00425-19. doi: 10.1128/AAC.00425-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, Oliver A. 2017. WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61:e02529-16. doi: 10.1128/AAC.02529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Argüello S, Montaner M, Oliver A, Moya B. 2021. Molecular basis of ampC β-lactamase induction by avibactam in Pseudomonas aeruginosa: PBP occupancy, live cell binding dynamics and impact on resistant clinical isolates harboring PDC-X variants. Int J Mol Sci 22:3051. doi: 10.3390/ijms22063051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2 and Table S1. Download spectrum.00692-23-s0001.pdf, PDF file, 0.4 MB (394.5KB, pdf)