Abstract
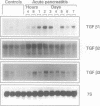
BACKGROUND: Transforming growth factor beta isoforms (TGF beta s) belong to a family of multifunctional regulators of cellular growth and differentiation. They are mitogenic and chemotactic for fibroblasts and are potent stimulators of extracellular matrix production (collagen) and deposition. Upregulation of TGF beta transcription has been reported for several in vivo systems during repair after injury. AIMS: To study the expression of the three mammalian isoforms of TGF beta (TGF beta 1-3) and their relation to collagen expression as a marker for fibroblast response in acute oedematous pancreatitis in rats. METHODS: Using northern blot analysis and immunohistochemistry, the expression and localisation of TGF beta isoforms, collagen, and amylase were analysed during the course of acute oedematous pancreatitis in rats, experimentally induced by intravenous caerulein infusion. RESULTS: Induction of acute pancreatitis resulted in a biphasic peak pattern of expression of TGF beta 1, beta 2, and beta 3 mRNA, with a pronounced increase from day 1 to day 3 (sixfold, 2.5-fold, fivefold, respectively) and again from day 5 to day 7 (three-fold, 2.3-fold, 3.5-fold, respectively). The temporal changes in TGF beta mRNA identically paralleled the expression in collagen mRNA. In contrast, amylase mRNA expression, used as a general indicator of acinar cell integrity, was slightly decreased after induction of acute pancreatitis. Immunohistochemical analysis of pancreatitis tissue showed that increased expression of TGF beta s was mainly present in the pancreatic acinar and ductal cells; this was evident within one day after pancreatitis induction. CONCLUSION: Overexpression of TGF beta s after induction of acute pancreatitis suggests a role for these proteins in pancreatic repair and remodelling. The increased levels of TGF beta s may help suppress immune activation, and may contribute to the increase in the extracellular matrix including collagen and to the repair of the pancreatic parenchyma.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Hupp T., Kern H. F. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch A Pathol Anat Histol. 1979 May 14;382(1):31–47. doi: 10.1007/BF01102739. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J., Fee F., Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988 Jan 28;331(6154):363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- Barnard J. A., Warwick G. J., Gold L. I. Localization of transforming growth factor beta isoforms in the normal murine small intestine and colon. Gastroenterology. 1993 Jul;105(1):67–73. doi: 10.1016/0016-5085(93)90011-z. [DOI] [PubMed] [Google Scholar]
- Beauchamp R. D., Lyons R. M., Yang E. Y., Coffey R. J., Jr, Moses H. L. Expression of and response to growth regulatory peptides by two human pancreatic carcinoma cell lines. Pancreas. 1990 Jul;5(4):369–380. doi: 10.1097/00006676-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas. 1989;4(2):169–178. doi: 10.1097/00006676-198904000-00005. [DOI] [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas. 1986;1(5):421–429. doi: 10.1097/00006676-198609000-00006. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Friess H., Weber A., Büchler M. Standards in monitoring acute experimental pancreatitis. Eur Surg Res. 1992;24 (Suppl 1):1–13. doi: 10.1159/000129234. [DOI] [PubMed] [Google Scholar]
- Friess H., Yamanaka Y., Büchler M., Ebert M., Beger H. G., Gold L. I., Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993 Dec;105(6):1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- Gress T., Müller-Pillasch F., Elsässer H. P., Bachem M., Ferrara C., Weidenbach H., Lerch M., Adler G. Enhancement of transforming growth factor beta 1 expression in the rat pancreas during regeneration from caerulein-induced pancreatitis. Eur J Clin Invest. 1994 Oct;24(10):679–685. doi: 10.1111/j.1365-2362.1994.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer B., Friess H., Weber A., Baczako K., Kisling P., Schilling M., Uhl W., Dervenis C., Büchler M. W. Hyperlipaemia intensifies the course of acute oedematous and acute necrotising pancreatitis in the rat. Gut. 1996 May;38(5):753–758. doi: 10.1136/gut.38.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenwallner W., Stein W., Hafkenscheid J. C., Kruse-Jarres J. D., Kaiser C., Hubbuch A., Klein G. Reference ranges for alpha-amylase in serum and urine with 4,6-ethylidene-(G7)-1-4-nitrophenyl-(G1)-alpha,D-maltoheptaoside as substrate. J Clin Chem Clin Biochem. 1989 Feb;27(2):97–101. doi: 10.1515/cclm.1989.27.2.97. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Joyce M. E., Terek R. M., Jingushi S., Bolander M. E. Role of transforming growth factor-beta in fracture repair. Ann N Y Acad Sci. 1990;593:107–123. doi: 10.1111/j.1749-6632.1990.tb16104.x. [DOI] [PubMed] [Google Scholar]
- Kagami S., Border W. A., Ruoslahti E., Noble N. A. Coordinated expression of beta 1 integrins and transforming growth factor-beta-induced matrix proteins in glomerulonephritis. Lab Invest. 1993 Jul;69(1):68–76. [PubMed] [Google Scholar]
- Keski-Oja J., Leof E. B., Lyons R. M., Coffey R. J., Jr, Moses H. L. Transforming growth factors and control of neoplastic cell growth. J Cell Biochem. 1987 Feb;33(2):95–107. doi: 10.1002/jcb.240330204. [DOI] [PubMed] [Google Scholar]
- Korc M., Chandrasekar B., Yamanaka Y., Friess H., Buchier M., Beger H. G. Overexpression of the epidermal growth factor receptor in human pancreatic cancer is associated with concomitant increases in the levels of epidermal growth factor and transforming growth factor alpha. J Clin Invest. 1992 Oct;90(4):1352–1360. doi: 10.1172/JCI116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Saksela O., Keski-Oja J. Transforming growth factor-beta induction of type-1 plasminogen activator inhibitor. Pericellular deposition and sensitivity to exogenous urokinase. J Biol Chem. 1987 Dec 25;262(36):17467–17474. [PubMed] [Google Scholar]
- Levine J. H., Moses H. L., Gold L. I., Nanney L. B. Spatial and temporal patterns of immunoreactive transforming growth factor beta 1, beta 2, and beta 3 during excisional wound repair. Am J Pathol. 1993 Aug;143(2):368–380. [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pelton R. W., Saxena B., Jones M., Moses H. L., Gold L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991 Nov;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchumoni C. S., Agarwal N., Jain N. K. Systemic complications of acute pancreatitis. Am J Gastroenterol. 1988 Jun;83(6):597–606. [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghow R., Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987 Apr;79(4):1285–1288. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson J. H. Acute pancreatitis: pathogenesis, outcome and treatment. Clin Gastroenterol. 1984 Sep;13(3):843–863. [PubMed] [Google Scholar]
- Spormann H., Sokolowski A., Letko G. Effect of temporary ischemia upon development and histological patterns of acute pancreatitis in the rat. Pathol Res Pract. 1989 May;184(5):507–513. doi: 10.1016/S0344-0338(89)80143-8. [DOI] [PubMed] [Google Scholar]
- Steer M. L. Classification and pathogenesis of pancreatitis. Surg Clin North Am. 1989 Jun;69(3):467–480. doi: 10.1016/s0039-6109(16)44831-0. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Allen J. B., Dougherty E. B., Dougherty S. F. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Mergenhagen S. E. Inflammatory and immunomodulatory roles of TGF-beta. Immunol Today. 1989 Aug;10(8):258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Wong H. L., Wahl S. M. Selective induction of Fc gamma RIII on human monocytes by transforming growth factor-beta. J Immunol. 1990 May 1;144(9):3444–3448. [PubMed] [Google Scholar]
- Willemer S., Elsässer H. P., Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992;24 (Suppl 1):29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- Willemer S., Elsässer H. P., Kern H. F., Adler G. Tubular complexes in cerulein- and oleic acid-induced pancreatitis in rats: glycoconjugate pattern, immunocytochemical, and ultrastructural findings. Pancreas. 1987;2(6):669–675. doi: 10.1097/00006676-198711000-00008. [DOI] [PubMed] [Google Scholar]
- Willemer S., Klöppel G., Kern H. F., Adler G. Immunocytochemical and morphometric analysis of acinar zymogen granules in human acute pancreatitis. Virchows Arch A Pathol Anat Histopathol. 1989;415(2):115–123. doi: 10.1007/BF00784348. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Noble N. A., Miller D. E., Border W. A. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994 Mar;45(3):916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- Zhang K., Garner W., Cohen L., Rodriguez J., Phan S. Increased types I and III collagen and transforming growth factor-beta 1 mRNA and protein in hypertrophic burn scar. J Invest Dermatol. 1995 May;104(5):750–754. doi: 10.1111/1523-1747.ep12606979. [DOI] [PubMed] [Google Scholar]