In this study, Son et al. describe the functional and evolutionary significance of the N-terminal proline-rich disordered domain (PRD) of DROSHA, a key factor for miRNA processing. They show that the PRD preferentially facilitates the maturation of rapidly evolving, primary miRNA hairpins located within introns, thus providing new insights into regulation of miRNA biogenesis.
Keywords: DROSHA, disordered domain, intron, microRNA, proline-rich domain
Abstract
DROSHA serves as a gatekeeper of the microRNA (miRNA) pathway by processing primary transcripts (pri-miRNAs). While the functions of structured domains of DROSHA have been well documented, the contribution of N-terminal proline-rich disordered domain (PRD) remains elusive. Here we show that the PRD promotes the processing of miRNA hairpins located within introns. We identified a DROSHA isoform (p140) lacking the PRD, which is produced by proteolytic cleavage. Small RNA sequencing revealed that p140 is significantly impaired in the maturation of intronic miRNAs. Consistently, our minigene constructs demonstrated that PRD enhances the processing of intronic hairpins, but not those in exons. Splice site mutations did not affect the PRD's enhancing effect on intronic constructs, suggesting that the PRD acts independently of splicing reaction by interacting with sequences residing within introns. The N-terminal regions from zebrafish and Xenopus DROSHA can replace the human counterpart, indicating functional conservation despite poor sequence alignment. Moreover, we found that rapidly evolving intronic miRNAs are generally more dependent on PRD than conserved ones, suggesting a role of PRD in miRNA evolution. Our study reveals a new layer of miRNA regulation mediated by a low-complexity disordered domain that senses the genomic contexts of miRNA loci.
MicroRNAs (miRNAs) are ∼22-nt RNAs that guide post-transcriptional gene silencing (Bartel 2018). The miRNA sequence is embedded in an RNA hairpin and transcribed by RNA polymerase II (pol II) as part of a long primary transcript (pri-miRNA) (Lee et al. 2002, 2004; Cai et al. 2004). Pri-miRNAs are recognized and cleaved by Microprocessor, a heterotrimeric complex composed of one molecule of DROSHA and two copies of DGCR8 (Lee et al. 2003; Denli et al. 2004; Gregory et al. 2004; Han et al. 2004; Landthaler et al. 2004; Nguyen et al. 2015; Herbert et al. 2016). DROSHA, an RNase III-type endoribonuclease, crops the hairpin by introducing a staggered cut in the lower part of the stem, releasing an ∼60- to 80-nt precursor miRNA (pre-miRNA). The pre-miRNA is subsequently cut by cytoplasmic RNase III DICER, yielding a mature miRNA duplex of ∼22 nt (Bernstein et al. 2001; Grishok et al. 2001; Hutvágner et al. 2001; Ketting et al. 2001). The duplex is loaded into Argonaute protein (AGO), in which one strand remains as a mature miRNA (Mourelatos et al. 2002; Kobayashi and Tomari 2016).
Pri-miRNAs have unique features that distinguish pri-miRNAs from numerous other hairpins encoded in the genome. A canonical and optimal pri-miRNA contains a hairpin with a stem of ∼35 bp, an unstructured apical loop, and single-stranded RNA (ssRNA) segments flanking the hairpin (Zeng and Cullen 2005; Zeng et al. 2005; Han et al. 2006; Ma et al. 2013). In addition to these structural features, pri-miRNAs often carry position-specific sequence motifs: the UGU/GUG (UGUG) motif at the apical junction, the UG motif at the basal junction, the mismatched GHG (mGHG) motif in the lower stem, and the CNNC motif in the 3′ flanking segment (Auyeung et al. 2013; Fang and Bartel 2015; Nguyen et al. 2018; Kwon et al. 2019).
While these local features of miRNA hairpins have been rigorously investigated, the surrounding genomic contexts of miRNA hairpins are less well understood (Olena and Patton 2010). Some miRNA hairpins are clustered in the genome and transcribed in a single “polycistronic” nascent transcript (Lagos-Quintana et al. 2001; Lau et al. 2001; Lee et al. 2002; Mourelatos et al. 2002). Processing of suboptimal hairpins can be assisted by their optimal neighbors in a process known as “cluster assistance,” suggesting that genomic organization relative to the other miRNA hairpins has an impact on DROSHA processing (Truscott et al. 2016; Lataniotis et al. 2017; Fang and Bartel 2020; Hutter et al. 2020; Shang et al. 2020).
Another genomic context that may potentially impact miRNA biogenesis is their positions relative to introns. More than half of human miRNA hairpins are found in intronic loci of coding or noncoding transcripts, and introns are thought to be hotspots for de novo miRNA evolution (Guerra-Assunção and Enright 2012; Meunier et al. 2013). Multiple lines of evidence support that processing of intronic miRNAs occurs cotranscriptionally and is temporally coordinated with splicing (Kim and Kim 2007; Morlando et al. 2008; Pawlicki and Steitz 2008; Ballarino et al. 2009; Kataoka et al. 2009; Nojima et al. 2015; Yin et al. 2015; Liu et al. 2016). Both DROSHA and DGCR8 were reported to associate with pol II, supporting the cotranscriptional processing model (Gromak et al. 2013; Church et al. 2017). Efficiently processed pri-miRNAs tend to be enriched in the insoluble fraction of the nuclear extract, presumably associated with transcription machinery (Morlando et al. 2008; Pawlicki and Steitz 2008). Both in vitro and in vivo experiments indicated that DROSHA crops the intronic hairpin prior to the splicing reaction, but cleaved introns remain tethered via spliceosomes and get trans-spliced. The splicing reaction is not a prerequisite for intronic miRNA processing, judging from the observations that a purified Microprocessor complex can process minimal pri-miRNAs without intronic context and that intronic miRNAs are processed in the absence of ATP, which is essential for splicing (Lee et al. 2003; Kataoka et al. 2009). However, splice site mutations appear to reduce pri-miRNA processing both in cells and in vitro, albeit modestly (Kim and Kim 2007; Kataoka et al. 2009). Moreover, in the case of intronic pri-mir-211, located within melastatin intron 6, the mutation of 5′ splice sites and the depletion of U1 snRNP components resulted in a decrease of pri-mir-211 processing, suggesting a positive influence of splicing machinery on at least some intronic pri-miRNAs (Janas et al. 2011). Exonic pri-miRNAs show variable nucleocytoplasmic distribution, are cropped from either unspliced or spliced transcripts, and are thought to be processed less efficiently than intronic miRNAs (Pawlicki and Steitz 2008; Slezak-Prochazka et al. 2013; Dai et al. 2016). Overall, the associations of Microprocessor with transcription and splicing machinery are intriguing. However, it remains unknown how these machineries are mechanistically coordinated and, specifically, what role DROSHA plays in this process.
Structural and biochemical studies have elucidated how DROSHA and DGCR8 contribute to the recognition of a pri-miRNA. DROSHA recognizes the basal ss–dsRNA junction, UG motif, and mGHG motif (Nguyen et al. 2015; Kwon et al. 2016; Jin et al. 2020; Partin et al. 2020), while DGCR8 interacts with the apical regions, including the terminal loop and the UGUG motif (Nguyen et al. 2015, 2018; Partin et al. 2017, 2020). These studies clarified that the central and C-terminal regions of DROSHA and DGCR8 (390–1365 of DROSHA and 223–751 of DGCR8) are sufficient to cover most parts of the hairpin (Kwon et al. 2016; Jin et al. 2020; Partin et al. 2020), supporting earlier biochemical data that the N termini of DROSHA and DGCR8 are dispensable for pri-miRNA processing in vitro (Han et al. 2004; Nguyen et al. 2015). More recently, it was found that the N-terminal part of DGCR8 contains a partially conserved segment that binds to ERH, which is required for cluster assistance (Kwon et al. 2020).
The N-terminal region of DROSHA possesses a proline-rich (P-rich) domain (amino acids 1–212) and an arginine/serine-rich (RS-rich) domain (amino acids 219–316) (Fig. 1A). The RS-rich domain is believed to function as a regulatory platform associated with subcellular localization and stability control of DROSHA. Phosphorylation of serines in the RS-rich domain (S300 and S302) by glycogen synthase kinase 3 β (GSK3β) is required for the nuclear localization of DROSHA (Tang et al. 2010, 2011). Consistent with this, a smaller isoform of DROSHA (∼130 kDa) lacking the nuclear localization signal in the RS-rich domain (due to alternative splicing) was found in the cytoplasm of quiescent human cells (Martinez et al. 2017). The RS-rich domain is also subject to phosphorylation by p38 MAPK in stress conditions, which induces DROSHA protein degradation (Yang et al. 2015), while lysine acetylation in the RS-rich domain stabilizes DROSHA by preventing ubiquitination (Tang et al. 2013).
Figure 1.
Proteolytic cleavage yields a DROSHA isoform (p140) lacking the proline-rich domain. (A) Domain structure of human DROSHA. The lower horizontal lines indicate the epitopes of three antibodies against DROSHA used in this study. SPOT-disorder-single and Valdar01 methods were used for disorderliness prediction and amino acid conservation scoring, respectively (see the Supplemental Material for details). (P-rich) Proline-rich, (RS-rich) arginine/serine-rich, (RIIID) RNase III domain, (dsRBD) double-stranded RNA-binding domain. (B) Identification of an N-terminal-truncated form of DROSHA (p140). HEK293T cells were transfected with control (siCtrl) or DROSHA (siDro) siRNAs for 2 d and then subjected to subcellular fractionation followed by Western blotting. (C) Localization of DROSHA (red) visualized using antibodies against the N terminus (left) or C terminus (right) in HEK293T cells. Costaining was done with an antibody against GM130 (green), a Golgi apparatus marker. The insets are enlarged views from the white rectangles. Scale bars, 10 µm. (D) Detection of the N-terminal fragment of ∼25 kDa (p25) in the membrane fraction. These blots are from the lower parts of the same blots in B. The asterisks indicate cross-reacting bands. (E) Fragments generated from ectopically expressed DROSHA. A plasmid with DROSHA coding sequence linked to the HA and FLAG tags was transfected to HEK293T cells. After 48 h, subcellular fractionation was performed, and the membrane fractions were used for Western blotting. (F) Schematics of the observed DROSHA fragments.
In contrast, the P-rich disordered domain (PRD) of DROSHA, located at the very N terminus, is the least conserved and most disordered region in DROSHA. Although several groups have observed DROSHA isoforms that may lack an intact PRD (Gregory et al. 2004; Han et al. 2004; Yang et al. 2015; Dai et al. 2016), the function of the short isoforms and the functional relevance of PRD remain unknown.
Here, we identified a major DROSHA isoform of ∼140 kDa lacking the PRD (DROSHA-p140) and found that DROSHA-p140 is produced by proteolytic cleavage even in unperturbed cells. By comparing this smaller isoform and full-length protein, we discovered that the PRD of DROSHA is required for efficient processing of pri-miRNAs in intronic regions.
Results
Proteolytic cleavage yields a DROSHA isoform (p140) lacking the proline-rich domain
To understand the functional significance of the PRD of DROSHA, we first interrogated natural isoforms of DROSHA that may lack the N terminus. We performed Western blotting using three antibodies targeting different parts of DROSHA (Fig. 1A). Apart from the full-length DROSHA protein (∼170 kDa), we detected a prominent band of ∼140 kDa with two antibodies against the C-terminal region (Ab-Ct#1 and Ab-Ct#2) (Fig. 1B). This ∼140-kDa band was not observed when DROSHA was knocked down or knocked out or when the N terminus targeting antibody (Ab-Nt) was used (Fig. 1B; Supplemental Fig. S1A,B). Thus, this band represents a smaller isoform of DROSHA lacking the N-terminal region. This 140-kDa isoform was observed in all human cell lines that we examined, including MCF7 (human breast cancer cell line) and HCT116 (human colon cancer cell line) (Supplemental Fig. S1A,B). We refer to this isoform as “DROSHA-p140” (Fig. 1F).
Intriguingly, DROSHA-p140 (p140) was found mainly in the membrane fraction as well as in the nucleus, unlike the full-length protein, which is predominantly nuclear (Fig. 1B; Supplemental Fig. S1A,B). We also performed immunofluorescence (IF) staining using Ab-Ct#1 that can recognize both the full length and p140 (Fig. 1C, right; Supplemental Fig. S1C,F). The DROSHA signal was detected in the nucleus, as expected from the well-established nuclear function of DROSHA (Lee et al. 2002; Kuehbacher et al. 2007; Tang et al. 2010; Bellemer et al. 2012). However, intriguingly, we also detected clear DROSHA signals in specific perinuclear areas in close proximity to GM130, a marker for the Golgi apparatus (Fig. 1C, right; Supplemental Fig. S1C,F). This peri-Golgi signal as well as the nucleoplasmic signal were lost in DROSHA knockout (KO) cells (Supplemental Fig. S1D), indicating that DROSHA indeed localizes both to the nucleus and near the Golgi. Given that p140 is the major isoform in the membrane according to the subcellular fractionation experiment (Fig. 1B; Supplemental Fig. S1A,B), this peri-Golgi signal may originate mainly from p140 rather than full-length DROSHA. Notably, the antibody Ab-Nt, targeting the N terminus of DROSHA, also gave clear signals in the Golgi (Fig. 1C, left; Supplemental Fig. S1E,F). We further found that although this antibody cannot capture p140, it detects an ∼25-kDa protein that is mainly in the membrane fraction (Fig. 1D; Supplemental Fig. S1G). This band was not detected by the C-terminal antibody or when DROSHA was ablated, indicating that this ∼25-kDa protein (“DROSHA-p25”) detected by Ab-Nt was derived from the N terminus of DROSHA (Fig. 1D; Supplemental Fig. S1G).
The presence of the separate DROSHA-p25 (p25) protein implied that p140 may be produced by proteolytic cleavage rather than alternative splicing. To test this possibility, we ectopically expressed full-length DROSHA tagged with HA and FLAG at the N and C termini, respectively (Fig. 1E). p25 was detected specifically with anti-HA antibody, while p140 was observed exclusively with anti-FLAG antibody, which indicates that p25 and p140 arise from proteolytic cleavage of the full-length protein, rather than from alternative splicing (Fig. 1F).
Identification of residues critical for proteolytic cleavage
Given the size of p25, it is likely that the cleavage occurs at the boundary between the PRD and the RS-rich domain (amino acids 180–280). To identify the residues necessary for protein cleavage, we generated deletion mutants and analyzed their products by Western blotting. Since both domains are predicted to be unstructured (Fig. 1A; Hanson et al. 2018), and our attempts to solve the structure experimentally have been unsuccessful, we had no structural guidance. Therefore, we made a series of 20 amino acid deletions within this region (Fig. 2A). The mutant lacking amino acids 180–199 failed to produce p140 and p25 (Fig. 2B, Δ180–199). To further narrow down the region, we created another set of mutants with 10 amino acid deletions (Fig. 2A) and identified two mutants that were significantly affected (Fig. 2C, Δ190–199 and Δ195–204). A substitution mutant (Asn197–Asn198 replaced with alanines) also showed a defect, unlike similar substitutions in neighboring positions, highlighting the importance of two asparagines located at the end of PRD (Fig. 2D, 197–198 NN → AA). Although we attempted to map the exact cleavage site using mass spectrometry-based methods, our efforts were unsuccessful, likely because the N-terminal sequences are unsuitable for tryptic digestion necessary for mass spectrometry analysis (data not shown).
Figure 2.
Identification of the residues critical for proteolytic cleavage of DROSHA. (A) Schematics of the DROSHA constructs harboring deleted or substituted regions. (B–D) Western blot analyses of DROSHA mutants with 20 amino acid deletions (B), 10 amino acid deletions (C), or three amino acid substitutions (D) collectively indicate that the region around the 200th residue is crucial for the proteolytic cleavage. Subcellular fractionation was performed 48 h post-transfection, and the membrane fractions were used for Western blotting. The asterisk indicates a nonspecific band.
Together, our results demonstrate that proteolytic cleavage removes ∼200 amino acids from the N terminus, including the PRD. The membrane association of DROSHA fragments and their colocalization with the Golgi marker GM130 (Fig. 1B,C) imply that a fraction of DROSHA molecules associates with the Golgi and is cleaved by a Golgi-associated protease, although the responsible protease has not been identified yet. Our discovery of p140 lacking the PRD provided an opportunity to investigate the role of the PRD.
The PRD is required for efficient processing of a subset of pri-miRNAs in cells
To examine the significance of the PRD, we assessed the activity of p140 in comparison with the full-length protein. We expressed the DROSHA mutants in the DROSHA KO cells using a rescue strategy (Fig. 3A). The Δ195–204 mutant that is most strongly defective in protein cleavage was used as a full-length mimic (referred to here as “FLm”) (Fig. 2C). We selected the ΔN200 mutant lacking the first 200 amino acids as a p140 mimic (referred to here as “p140m”). We carefully assessed the transfection conditions to match protein expression levels from two plasmids (Supplemental Fig. S2B). Both FLm and p140m proteins were localized to the nucleus, as determined by immunofluorescence (Supplemental Fig. S2C). Subcellular fractionation and Western blotting also showed that the nuclear localization of p140m is comparable with that of FLm (Supplemental Fig. S2D). These results confirm the previous observations that the N-terminal 1–270 amino acids (including the PRD) are dispensable for nuclear localization (Tang et al. 2010; Bellemer et al. 2012). Furthermore, FLm and p140m interact with DGCR8 to similar degrees (Supplemental Fig. S2E), consistent with the earlier finding that DGCR8 binds to the RIIIDs of DROSHA rather than the N terminus (Kwon et al. 2016).
Figure 3.
The PRD is necessary for the processing of a subset of pri-miRNAs in cells. (A) Experimental design to compare miRNA expression levels in cells expressing full-length DROSHA or p140. The Δ195–204 or ΔN200 DROSHA construct was transiently transfected to HCT116 or HEK293E DROSHA KO cells. Total RNAs and cell lysates were extracted for small RNA sequencing and Western blotting, respectively. (B) Differentially expressed miRNAs from HCT116 (left) or HEK293E (right). Vertical dotted lines were set based on the maximum fold changes of spike-in RNAs and indicate 2.9-fold and 2.1-fold enrichment in HCT116 and HEK293E, respectively. Horizontal dotted lines denote an adjusted P-value of 0.05 (n = 3). Only miRNAs that passed the cutoffs are colored. Numbers at the top corners indicate the numbers of significantly down-regulated or up-regulated miRNAs in each group. Fold changes and P-values for each miRNA are listed in Supplemental Table S2. (C) Comparison between log2 fold changes measured in two cell lines. In the case of canonical miRNAs, two values are highly correlated. (r) Pearson correlation coefficient. (D) Relative expression levels of mature and pri-miRNAs for selected miRNAs. Validating the sequencing results (gray bars), mature miRNAs decreased when measured by qRT-PCR (purple bars). Pri-miRNA levels determined by RT-qPCR increased (dark blue), suggesting a defect in pri-miRNA processing. U6 snRNA and GAPDH were used as internal controls for mature miRNAs and pri-miRNAs, respectively. The abundance of pri-mir-92a-1 was measured because of the low expression of pri-mir-92a-2. The error bars indicate mean ± standard deviations (n = 3). Two-tailed unpaired Student's t-test: (*) P < 0.05, (**) P < 0.005.
To functionally assess FLm and p140m, we used two different KO cell lines generated from HCT116 and HEK293E cells (Supplemental Figs. S1B, S2A; Kim et al. 2016). After transient transfection of FLm or p140m, small RNA sequencing was carried out using the AQ-seq protocol, which allows accurate miRNA quantification (Kim et al. 2019). Three independent sequencing libraries showed high reproducibility between biological replicates (Supplemental Fig. S2F).
Interestingly, when rescued with p140m, some miRNAs were produced at significantly lower levels compared with the FLm-expressing condition (Fig. 3B; Supplemental Fig. S2G). Noncanonical DROSHA-independent miRNAs were not affected or even modestly increased in p140m-expressing cells, as expected. Similar results were obtained in HCT116 and HEK293E cells, especially for canonical DROSHA-dependent miRNAs (Fig. 3C). For validation, we quantified several mature miRNAs (miR-19a, miR-15a, miR-25, miR-221, and miR-92a) and their pri-miRNAs by RT-qPCR (Fig. 3D). While mature miRNA levels were lower with p140m than with FLm, pri-miRNA levels showed the opposite patterns, indicating that the differences are attributable to the pri-miRNA-processing step. These results demonstrate that the PRD of DROSHA is required for the processing of a subset of miRNAs in cells.
The above observation appears to contradict our previous finding that the DROSHA ΔN390, which lacks the PRD as well as the RS-rich domain, is as active as the full-length protein in vitro, at least for pri-let-7a-1, pri-mir-16-1, and pri-mir-30a used in the studies (Han et al. 2004; Nguyen et al. 2015). To investigate further, we examined whether the PRD dependency can be demonstrated in vitro using pri-mir-186 and pri-mir-125a, which are PRD-dependent and PRD-independent, respectively, based on the rescue and sequencing data (Fig. 4A,B). We prepared these two representative pri-miRNAs as a minimal form of 125 nt, covering only the hairpin and its immediate surrounding sequences, by in vitro transcription and incubated them with varying concentrations of the Microprocessor complex composed of either FLm or p140m (Fig. 4A,B; Supplemental Fig. S3A; Kim and Kim 2022). In this assay, p140m processed both pri-miRNAs as efficiently as FLm (Fig. 4A,B). Thus, the purified Microprocessor and the 125-nt pri-miRNA transcripts cannot recapitulate the PRD dependency. This suggests that the PRD may require additional trans-acting factor(s) and/or cis-acting element(s) to exert its effect.
Figure 4.
The PRD dependency cannot be recapitulated in vitro with the purified Microprocessor. (A,B, top) Representative gel images from in vitro processing assays with Microprocessor composed of either recombinant p140 or full-length DROSHA mimic proteins (8–120 fmol). The log2(p140m/FLm) value of miR-186 is −2.541, and that of miR-125a is 0.165 according to the rescue and sequencing data shown in Figure 3. F2 corresponds to pre-miRNA, while F1 and F3 are the flanking fragments. (Bottom) Quantification of pre-miRNA (F2) band intensities from three independent replicates. The bars indicate mean ± standard deviations (n = 3) after minimum–maximum normalization.
Investigating the factors contributing to PRD dependency
To identify the elements that determine the PRD dependency of certain pri-miRNAs, we analyzed various features of pri-miRNAs and their association with the PRD dependency. Initially, we examined four sequence motifs (basal UG, apical UGUG, flanking CNNC, and mGHG) known to facilitate pri-miRNA processing but could not find any significant association between these motifs and PRD dependency (Fig. 5A,B; Supplemental Fig. S4A; Auyeung et al. 2013; Fang and Bartel 2015; Kwon et al. 2019). We also analyzed the secondary structures of pri-miRNAs but could not find substantial differences between the PRD-dependent and PRD-independent miRNAs (Supplemental Fig. S4B; Reuter and Mathews 2010). Moreover, there was no significant difference in processing efficiency between the two groups, as measured by high-throughput in vitro assays with mini-pri-miRNAs of 125 nt, which contain all the essential local elements (Supplemental Fig. S4C; Kim et al. 2021). These observations suggest that the intrinsic properties of pri-miRNA hairpins that reside locally within 125 nt are unlikely to be the major determinants of the specificity of the PRD.
Figure 5.
Searching for pri-miRNA features related to PRD dependency. (A) Schematic diagram of local features of a pri-miRNA hairpin. (B–D) PRD dependency measured in HCT116 as quantified by log2 miRNA expression fold changes (p140m/FLm). miRNAs were grouped based on each sequence motif (B), clustered genomic arrangement (C), or intronic/exonic locations (D). The box indicates the upper and lower quartiles. The horizontal line within the box plot denotes the median. Whiskers represent 1.5× interquartile range. The values from the two groups were compared using the two-tailed Mann–Whitney U-test: (n.s.) not significant (P = 0.05). (E) The PRD dependency of intronic and exonic miRNAs in HCT116 and HEK293E cells. (F) Analysis of DROSHA and miRNA expressions on HCT116 wild-type (WT) cells exposed to serum-starved conditions for 72 h. (G) Serum deprivation induces accumulation of p140 and p25. (H,I) Log2 fold changes of miRNA expression levels under normal or serum-starved conditions. miRNAs were grouped based on the intronic/exonic locations (H) or PRD dependency (I). Two-tailed Mann–Whitney U-test (n = 3).
We then investigated the genomic arrangements of pri-miRNAs and found no difference in PRD dependency between “clustered” and “stand-alone” miRNAs (Fig. 5C; Supplemental Fig. S4D). Moreover, the PRD dependency did not correlate with the depletion effects of ERH or SAFB2, which are known to facilitate the processing of clustered suboptimal miRNAs (Supplemental Fig. S4E; Fang and Bartel 2020; Hutter et al. 2020; Kwon et al. 2020). These results are consistent with the previous report that the N-terminal region of DROSHA is dispensable for cluster assistance (Fang and Bartel 2020).
Interestingly, when we classified miRNAs based on their positions relative to the intron/exon structure, intronic miRNAs tended to be more strongly influenced by the PRD compared with exonic miRNAs (Fig. 5D; Supplemental Fig. S4F). This trend was observed in both HCT116 and HEK293E cells (Fig. 5E), suggesting that the PRD may contribute to the maturation of intronic miRNAs.
To investigate the impact of changes in DROSHA isoform levels on miRNA biogenesis, we cultured HCT116 under serum-starved conditions, which are known to induce the production of a smaller isoform of DROSHA of ∼130 kDa (Fig. 5F; Martinez et al. 2017). Our results showed an increase in both the p140 and p25 isoforms, indicating that serum starvation triggers proteolytic cleavage of DROSHA (Fig. 5G; Supplemental Fig. S4G). The full-length protein level was largely unaffected, possibly due to enhanced expression of DROSHA and/or differential stability of the isoforms under these conditions, resulting in a modest net increase in total DROSHA abundance. Small RNA sequencing data showed that exonic miRNAs and “PRD-independent miRNAs” were up-regulated when p140 level increased, unlike intronic miRNAs and “PRD-dependent miRNAs,” which were largely insensitive to the up-regulation of p140 (Fig. 5H,I; Supplemental Fig. S4H). This is consistent with the other data that PRD-dependent miRNAs cannot be processed efficiently by p140.
Intronic pri-miRNAs depend on the P-rich domain for processing
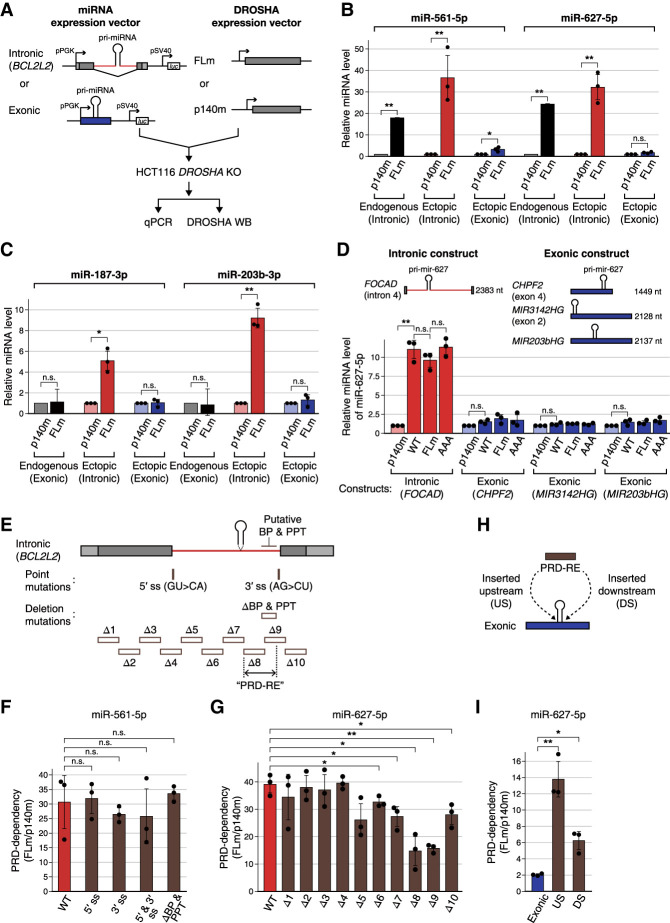
Because the endogenous miRNA abundance can be influenced by multiple factors and secondary effects, we constructed minigene plasmids that contain miRNA hairpins (of 125 nt) in either an intronic or exonic context (Fig. 6A). The plasmids were cotransfected with either FLm or p140m expression plasmids into HCT116 DROSHA KO cells (Fig. 6A; Supplemental Fig. S5A). We selected miRNAs with low endogenous levels in HCT116 to reliably quantify miRNAs produced from the plasmids. When pri-mir-561 and pri-mir-627, which are originally intronic, were ectopically expressed from intronic constructs (Fig. 6B, red bars), their mature miRNA levels were substantially higher with FLm than with p140m. However, in exonic contexts, FLm only modestly induced miR-561 and miR-627 expression compared with p140 (Fig. 6B, blue bars). These findings suggest that the PRD promotes intronic pri-miRNA processing preferentially.
Figure 6.
The PRD is required for intronic miRNA maturation. (A) Schematics of intronic and exonic constructs. The intronic constructs include exon 3, intron 3, and exon 4 of the BCL2L2 gene. A 125-nt pri-miRNA was inserted into the middle of the intron. In the exonic constructs, pri-miRNAs were inserted into multiple cloning sites downstream from the PGK promoter. Each construct was designed to express the Renilla luciferase (luc) from an independent promoter downstream from the miRNA cassette to normalize transfection efficiency. (B,C) Relative expression levels of miRNAs transcribed from endogenous genomic loci (as determined by small RNA sequencing), intronic constructs, or exonic constructs (as determined by miRNA TaqMan qPCR). Endogenous genomic locations of miRNA genes in B and C are intronic and exonic, respectively. All miRNAs become more dependent on the PRD in the intronic environment, regardless of the original genomic location. miRNA levels from exonic or intronic constructs were normalized to U6 snRNA and Renilla luciferase transcript. The miRNA levels in the p140m-expressing condition were set to 1. The error bars indicate mean ± standard deviations (n = 3). Two-tailed unpaired Student's t-test: (*) P < 0.05, (**) P < 0.005, (n.s.) not significant. (D) The relative expression levels of miR-627 transcribed from different intronic or exonic backbones. The number at the right of the schematic indicates the length of the corresponding intron or exon. The dependency is not affected by the length of the transcript. Normalization and statistical tests were performed in the same way as in B and C. (E) Point and deletion mutants of the BCL2L2 intronic construct. Mutations were introduced into the 5′/3′splice site (ss), polypyrimidine tract (PPT), or branch point (BP). Δ1–Δ10 have a 120-nt length deletion. Δ4 includes the 5′ splice site, while Δ9 includes the 3′ splice site. (F) PRD dependency of intronic constructs with mutations in the core splicing elements. miRNA levels from exonic or intronic constructs were normalized to U6 snRNA and Renilla luciferase transcript. The error bars indicate mean ± standard deviations (n = 3). Two-tailed unpaired Student's t-test: (n.s.) not significant (P = 0.05). (G) PRD dependency of deletion mutants Δ1–Δ10. Normalization and statistical tests were applied in the same way as in F. Two-tailed unpaired Student's t-test: (*) P < 0.05, (**) P < 0.005. The bars without an asterisk do not show statistically significant differences. (H) “PRD-RE” (spanning Δ8 and a part of Δ9 regions) was inserted upstream (US) of or downstream (DS) from the exonic construct. (I) Measured PRD dependency of insertion mutants. Normalization and statistical tests were done in the same way as in G.
To further validate this notion, we generated minigene constructs with different miRNAs (miR-187-3p, miR-203b-3p, miR-146b-5p, and miR-193a-3p) that are originally exonic and independent of PRD (Fig. 6C; Supplemental Fig. S5A,B). Remarkably, when engineered into an intron, these miRNAs were produced more by FLm than by p140m (Fig. 6C, red bars; Supplemental Fig. S5B). In contrast, in exonic contexts, the same hairpins were not influenced significantly by the PRD (Fig. 6C, blue bars; Supplemental Fig. S5B). Thus, the PRD dependency was mainly determined by the surrounding context of the hairpin rather than by the hairpin sequences.
This phenomenon was also observed with additional constructs containing sequences from different genes (WWP2, IGF2, CTDSP2, and FOCAD) (Supplemental Fig. S5C,D). All of these heterologous intronic constructs exhibited PRD dependency. Additionally, to rule out the possibility that the length of the hosting transcript influences the PRD dependency, we made longer exonic constructs (CHPF2, MIR3142HG, and MIR203bHG) and a different intronic construct (FOCAD) of comparable lengths (Fig. 6D; Supplemental Fig. S5F). This time, in addition to FLm, we also used WT DROSHA and the cleavage-deficient substitution mutant (197–198 NN → AA) (Fig. 2D). None of the long exonic constructs showed PRD dependency, while the intronic construct exhibited strong PRD dependency (Fig. 6D). We made similar observations using another set of exon/intron constructs that contain pri-mir-203b (Supplemental Fig. S5E,F), confirming that the length of the hosting transcript is not a significant factor and that exonic regions do not contain a determinant for PRD dependency.
Next, to investigate whether splicing is required for PRD's action, we introduced mutations to the 5′ splice site (changing GU to CA), 3′ splice site (changing AG to CU), branch point, and polypyrimidine tract (deletion) (Fig. 6E). RT-PCR analysis confirmed that these mutations indeed abrogated splicing (Supplemental Fig. S6A). However, these splicing-defective mutants still showed PRD dependency, which is comparable with the wild-type construct (Fig. 6F; Supplemental Fig. S6B), indicating that splicing events are not required for this regulation.
To identify the sequence determinant for PRD-mediated regulation, we generated a series of mutants with 120-nt deletions within the BCL2L2 backbone (Fig. 6E). Some of the mutants exhibited impaired PRD responsiveness (Fig. 6G), indicating that the deleted regions may contribute to PRD-mediated regulation. In particular, the segment in the 3′ part of the intron spanning the Δ8 and Δ9 regions showed the strongest effect. To investigate whether this intronic segment could confer PRD dependency independently of splicing, we then transplanted the intronic segment (spanning the Δ8 and Δ9 regions except the splice site and downstream exon, referred to here as “PRD-RE”) to an exonic construct upstream of or downstream from the miR-627 hairpin (Fig. 6H). Interestingly, the exonic constructs with PRD-RE insertion became dependent on the PRD, indicating that this intronic segment is at least partly responsible for the PRD dependency of the BCL2L2 constructs (Fig. 6I; Supplemental Fig. S6C). PRD-RE does not contain the 3′ splice site, and, accordingly, transcripts containing PRD-RE were not spliced (Supplemental Fig. S6D). These results imply that the PRD may interact with intron-enriched sequences independently of splicing.
Evolutionary conservation of the DROSHA PRD
The N-terminal sequences of human DROSHA (1–390 amino acids) are poorly conserved and do not align well with its homologs (Fig. 1A; Supplemental Fig. S7C; Valdar 2002; Waterhouse et al. 2009; Sievers et al. 2011). Nevertheless, the Drosha homologs (at least those in chordates) seem to preserve some common features in their N termini; they are intrinsically disordered with an unusually high proportion of proline residues (Fig. 7A; Supplemental Fig. S7A; Hanson et al. 2018). Human DROSHA contains 63 proline residues out of the first 200 amino acids (32%).
Figure 7.
Evolutionary implications of the DROSHA PRD. (A) Comparison of the N-terminal part of Drosha homologs across species. The N-terminal part was defined by taking the upstream 391st amino acid of human DROSHA and the corresponding N-terminal parts from the homologs in other species. The disorderliness was predicted by SPOT-disorder-single (Hanson et al. 2018). UniProt protein IDs are listed in Supplemental Table S4. (NTD) N-terminal domain. (B, top) Hybrid DROSHA proteins with the N-terminal part from other species and the C-terminal part from human DROSHA. (Bottom) The exonic or intronic construct containing pri-mir-627 was transfected into HCT116 DROSHA KO cells together with one of the hybrid proteins. FLm was used for the H. sapiens NTD. miRNA levels were normalized to U6 snRNA and the Renilla luciferase transcript. The miRNA levels in the p140m-expressing condition were set to 1. The error bars indicate mean ± standard deviations (n = 3). Two-tailed unpaired Student's t-test: (**) P < 0.005, (n.s.) not significant (P = 0.05). (C, top) The P > A mutant in which all prolines within the first 1–200 amino acids were replaced with alanines was cloned. miRNA expression tests were done as in B. (D) A model for the function of the DROSHA PRD in pri-miRNA processing. The PRD may interact with the intronic sequences directly or indirectly via an intron-binding protein(s), thereby assisting the Microprocessor to access the intronic miRNA hairpins. (E) Conservation analysis of DROSHA-dependent canonical miRNAs. Conservation was quantified as the average of phyloP scores using the pre-miRNA sequence. Different miRNA groups were compared using the two-tailed Mann–Whitney U test: (***) P < 0.0005, (n.s.) not significant.
To examine the functionality of the N termini of DROSHA homologs, we generated hybrid DROSHA constructs by replacing the N terminus of human DROSHA with its counterpart from fish (D. rerio) or frogs (X. tropicalis) (Fig. 7B; Supplemental Fig. S7B). After ectopic expression of these hybrid proteins in DROSHA KO HCT116 cells, we measured the level of miR-627-5p from minigene constructs (Fig. 7B; Supplemental Fig. S7B). Despite the sequence divergence, the PRD from fish and frog homologs (dreN and xtrN) can replace the human counterpart (Fig. 7B). Thus, the proline-rich N terminus of DROSHA retains its function, at least in vertebrates, even without strict conservation in their primary sequences (Supplemental Fig. S7C).
As proline richness is one of the most noticeable common features of this domain (Fig. 7A), we generated a mutant human DROSHA by substituting all prolines with alanines in the PRD (Fig. 7C). This mutant is strongly defective in processing the intronic construct (Fig. 7C; Supplemental Fig. S7D), indicating that the proline residues in this disordered domain are indeed important for DROSHA's function, particularly in intronic pri-miRNA processing.
Discussion
In this study, we have discovered the critical and conserved roles of the DROSHA PRD in the maturation of intronic miRNAs (Figs. 5, 6). The PRD has been overlooked because it is dispensable for cleavage activity in vitro and nuclear localization in cells (Fig. 4; Han et al. 2004; Tang et al. 2010; Bellemer et al. 2012; Nguyen et al. 2015; Kwon et al. 2016). Our current findings suggest that the PRD enables the Microprocessor to readily access the intron of nascent pri-miRNAs, facilitating the processing of intronic hairpins (see Fig. 7D for a model). This finding is in line with earlier observations that intronic pri-miRNA processing can occur cotranscriptionally and prior to splicing (Kim and Kim 2007; Morlando et al. 2008; Ballarino et al. 2009; Kataoka et al. 2009). Our mutagenesis experiments on the BCL2L2 minigene constructs suggest that the main cis-acting determinant(s) reside in the 3′ part of the intron, although we do not exclude a possibility that sequences outside this region also contribute to PRD dependency. DROSHA immunoprecipitation followed by liquid chromatography with tandem mass spectrometry (LC-MS/MS) provided a list of potential interactors of full-length DROSHA or p140 (Supplemental Fig. S8A; Supplemental Table S5). However, we do not yet understand how the intronic motif(s) are recognized by the PRD and/or its protein interactors. Future studies could reveal which specific sequence(s) interact with the PRD, whether other PRD-responsive pri-miRNAs contain similar motif(s), and whether this interaction is direct or indirect through an RNA-binding protein(s).
Our research has identified p140 as a major natural isoform of DROSHA, produced from proteolytic cleavage. It remains to be determined whether p140 is equivalent to the small isoforms of DROSHA described earlier (Gregory et al. 2004; Han et al. 2004; Grund et al. 2012; Yang et al. 2015; Dai et al. 2016, 2020; Link et al. 2016; Martinez et al. 2017). We found that p140 is impaired in intronic miRNA processing but can cleave exonic pri-miRNAs at a level comparable with that of the full-length protein (Fig. 6). p140 may act post-transcriptionally on exonic pri-miRNAs in the nucleoplasm or in the cytoplasm, possibly near the Golgi apparatus (Fig. 7D). The production of p140 may control miRNA biogenesis, favoring PRD-independent miRNAs (Fig. 5F–I). It is possible that p140 may provide a layer of miRNA regulation by escaping degradation via the E3 ubiquitin ligase NEDD4, which interacts with the PPxY motif of DROSHA (Jiang et al. 2021). This selective degradation of the full-length protein would alter the protein isoform ratio, resulting in changes to the miRNA profile. Additionally, p140 may have yet unknown function(s), possibly near the Golgi apparatus. It was previously proposed that DROSHA has an antiviral activity by impeding the RNA-dependent RNA polymerases (RdRps) of some positive stranded RNA viruses in the cytoplasmic membrane fraction (Aguado et al. 2017). Given that p140 is abundant in the membrane fraction and retains the region conferring steric hindrance on RdRps (Figs. 1B,F, 2; Supplemental Fig. S1A,B; Aguado et al. 2017), p140 may act effectively on viral RdRps. Alternatively but not mutually exclusively, p140 may bind and act on unknown cellular target RNAs localized near the membrane compartments. Further investigation will be needed to explore these intriguing hypotheses.
Our finding that the PRD is functionally conserved has evolutionary implications. Chordate Drosha homologs share several characteristics, such as length, disorderliness, and proline proportion (Fig. 7A). It was recently proposed that intrinsically disordered regions (IDRs) with shared molecular characteristics such as length, complexity, amino acid composition, and net charge can perform similar functions even if they do not exhibit detectable similarity in amino acid sequence alignments (Zarin et al. 2017, 2019). Such molecular features can be under evolutionary constraint (Zarin et al. 2017, 2019). It is noteworthy that the number of miRNA genes has increased during animal evolution (Fromm et al. 2015), and the recently emerged ones are overrepresented in intronic regions (Meunier et al. 2013). In our rescue sequencing experiments, we observed that rapidly evolving miRNAs tend to be processed more efficiently by the full-length protein than p140, while conserved miRNAs do not show such differences, which suggests that “young” miRNA genes are more dependent on PRD than the “established” ones (Fig. 7E; Pollard et al. 2010). The two groups—PRD-dependent and PRD-independent intronic miRNAs—do not significantly differ in their processing efficiency as measured in vitro with the 125-nt fragments (Supplemental Fig. S7E; Kim et al. 2021), excluding the possibility that their PRD dependency is determined simply by their suboptimal local features. The mechanism underlying the differential PRD dependency should be investigated in detail in the future. However, it is tempting to speculate that the acquisition of the DROSHA PRD during evolution may have assisted the processing of emerging hairpins within introns so that the young miRNAs can establish a biological function and coevolve with their genomic contexts (Figs. 5, 6).
Materials and methods
Cell lines and cultures
All cells were cultured under standard conditions: HCT116 in McCoy's 5A (Welgene), HEK293T and HEK293E in DMEM (Welgene), and A549 and MCF7 in RPMI (Welgene), all of which were supplemented with 9.1% fetal bovine serum (Welgene). HCT116 DROSHA KO cells were from a previous study (Kim et al. 2016), and HEK293E DROSHA KO cells were generated by CRISPR/Cas9 technology. All cell lines were authenticated using short tandem repeat (STR) profiling by ATCC.
RNA interference
To knock down DROSHA, cells seeded 1 d before were transfected with final 30 nM siRNAs using the Lipofectamine 3000 reagent (Thermo Fisher Scientific) and harvested after 2 d. For HEK293T cells, single siRNAs (siDro-1 or siDro-2) were treated individually, whereas for MCF7 cells, a mixed siRNA pool (siDro-1, siDro-2, siDro-3, and siDro-4) was used. The control siRNA used was “AccuTarget negative control siRNA.” All siRNAs were purchased from Bioneer. The sequences of synthetic siRNAs are listed in Supplemental Table S1.
Subcellular fractionation
The fractionation procedure for cytoplasm was performed following the previously described method (Holden and Horton 2009). Briefly, the cultured cells were harvested in ice-cold PBS and placed in a 1.5-mL microtube. After centrifugation at 100g for 10 min, the PBS was removed, and the cell pellet volume was measured. The pellet was resuspended in its 10-fold volume of digitonin buffer (150 mM NaCl, 50 mM HEPES at pH 7.5, 150∼200 µg/mL digitonin) supplemented with protease inhibitor cocktail (Calbiochem) by gentle pipetting. Different concentrations of digitonin (Sigma-Aldrich) were used in digitonin buffer, depending on the cell lines: 150 µg/mL for HEK293T, MCF7, and HEK293E, and 200 µg/mL for HCT116 cells. After end-over-end incubation for 10 min at 4°C, the lysate was centrifuged at 2000g for 10 min at 4°C. The supernatant was collected as the cytoplasmic fraction. The remaining pellet was washed once with 1 mL of PBS, collected by centrifugation, and used to prepare the membrane and nucleus fractions with the subcellular protein fractionation kit for cultured cells (Thermo Fisher Scientific). We followed the manufacturer's instructions with an additional PBS wash step after extracting the membrane fraction.
Small RNA sequencing
Sequencing libraries were generated as previously described (Kim et al. 2019). In brief, we mixed 20 µg of total RNAs with 10 nmol of 30 equimolar spike-in RNAs described in our previous study (Kim et al. 2019). Small RNAs were enriched by size fractionation by 15% urea–polyacrylamide gel electrophoresis and ligated to the randomized adaptor at the 3′ and 5′ ends. The ligated RNAs were reverse-transcribed using SuperScript IV reverse transcriptase (Thermo Fisher Scientific), amplified using Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific), and subjected to high-throughput sequencing on the MiSeq platform (Illumina).
TaqMan miRNA qPCR
Total RNAs were isolated using TRIzol (Thermo Fisher Scientific) and treated with DNase I (Takara Bio), and 10 ng of total RNAs was used for one reaction. Complementary DNAs (cDNAs) were then synthesized using the TaqMan microRNA reverse transcription kit (Thermo Fisher Scientific) and subjected to quantitative real-time PCR with the TaqMan microRNA assay on a StepOnePlus real-time PCR system (Thermo Fisher Scientific) or QuantStudio 3 real-time PCR system (Thermo Fisher Scientific). The catalog numbers are listed in Supplemental Table S1.
Data availability
All high-throughput sequencing data generated in this study (small RNA sequencing) have been deposited in GEO (accession no. GSE230544). Custom codes are publicly available online (https://www.github.com/wwc420/drosha_nterm).
Supplementary Material
Acknowledgments
We appreciate the mass spectrometry service team's effort, especially Jeesoo Kim and Jong-Seo Kim. We are also grateful to Eunji Kim, Da-Eun Choi, and Sunyoung Bang for their technical help. We thank Kijun Kim, Kyungmin Baeg, Buyeon Um, Harim Jang, Haedong Kim, Young-Yoon Lee, Myeonghwan Kim, Youngran Park, Yeon Choi, Jong Woo Bae, Yeonwoo Park, Seungchan Baek, and Hyejun Kim for helpful discussions and advice. This research was supported by the Institute for Basic Science from the Ministry of Science and Information and Communication Technologies of Korea (IBS-R008-D1 to S.S., B.K., J.Y., and V.N.K.) and BK21 research fellowships from the Ministry of Education of Korea (to S.S.).
Author contributions: S.S., B.K., and V.N.K. conceived the study. S.S. and B.K. performed experiments. J.Y. generated the HEK293E DROSHA knockout cell line. S.S. conducted the bioinformatic analyses and visualized the data, except for the mass analysis. B.K. analyzed the mass data. S.S. and V.N.K. wrote the manuscript. V.N.K. supervised the research and collected financial support.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.350275.122.
Competing interest statement
The authors declare no competing interests.
References
- Aguado LC, Schmid S, May J, Sabin LR, Panis M, Blanco-Melo D, Shim JV, Sachs D, Cherry S, Simon AE, et al. 2017. RNase III nucleases from diverse kingdoms serve as antiviral effectors. Nature 547: 114–117. 10.1038/nature22990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung VC, Ulitsky I, McGeary SE, Bartel DP. 2013. Beyond secondary structure: primary-sequence determinants license pri-miRNA hairpins for processing. Cell 152: 844–858. 10.1016/j.cell.2013.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarino M, Pagano F, Girardi E, Morlando M, Cacchiarelli D, Marchioni M, Proudfoot NJ, Bozzoni I. 2009. Coupled RNA processing and transcription of intergenic primary microRNAs. Mol Cell Biol 29: 5632–5638. 10.1128/MCB.00664-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. 2018. Metazoan microRNAs. Cell 173: 20–51. 10.1016/j.cell.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemer C, Bortolin-Cavaillé M-L, Schmidt U, Jensen SMR, Kjems J, Bertrand E, Cavaillé J. 2012. Microprocessor dynamics and interactions at endogenous imprinted C19MC microRNA genes. J Cell Sci 125: 2709–2720. 10.1242/jcs.100354 [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. 2001. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409: 363–366. 10.1038/35053110 [DOI] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR. 2004. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 10: 1957–1966. 10.1261/rna.7135204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church VA, Pressman S, Isaji M, Truscott M, Cizmecioglu NT, Buratowski S, Frolov MV, Carthew RW. 2017. Microprocessor recruitment to elongating RNA polymerase II is required for differential expression of MicroRNAs. Cell Rep 20: 3123–3134. 10.1016/j.celrep.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Chen K, Youngren B, Kulina J, Yang A, Guo Z, Li J, Yu P, Gu S. 2016. Cytoplasmic Drosha activity generated by alternative splicing. Nucleic Acids Res 44: 10454–10466. 10.1093/nar/gkw668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Hallmark L, Bofill De Ros X, Crouch H, Chen S, Shi T, Yang A, Lian C, Zhao Y, Tran B, et al. 2020. Novel, abundant Drosha isoforms are deficient in miRNA processing in cancer cells. RNA Biol 17: 1603–1612. 10.1080/15476286.2020.1813439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, Hannon GJ. 2004. Processing of primary microRNAs by the Microprocessor complex. Nature 432: 231–235. 10.1038/nature03049 [DOI] [PubMed] [Google Scholar]
- Fang W, Bartel DP. 2015. The menu of features that define primary microRNAs and enable de novo design of microRNA genes. Mol Cell 60: 131–145. 10.1016/j.molcel.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Bartel DP. 2020. MicroRNA clustering assists processing of suboptimal microRNA hairpins through the action of the ERH protein. Mol Cell 78: 289–302.e6. 10.1016/j.molcel.2020.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm B, Billipp T, Peck LE, Johansen M, Tarver JE, King BL, Newcomb JM, Sempere LF, Flatmark K, Hovig E, et al. 2015. A uniform system for the annotation of vertebrate microRNA genes and the evolution of the human microRNAome. Annu Rev Genet 49: 213–242. 10.1146/annurev-genet-120213-092023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RI, Yan K-P, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. 2004. The Microprocessor complex mediates the genesis of microRNAs. Nature 432: 235–240. 10.1038/nature03120 [DOI] [PubMed] [Google Scholar]
- Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC. 2001. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106: 23–34. 10.1016/S0092-8674(01)00431-7 [DOI] [PubMed] [Google Scholar]
- Gromak N, Dienstbier M, Macias S, Plass M, Eyras E, Cáceres JF, Proudfoot NJ. 2013. Drosha regulates gene expression independently of RNA cleavage function. Cell Rep 5: 1499–1510. 10.1016/j.celrep.2013.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund SE, Polycarpou-Schwarz M, Luo C, Eichmüller SB, Diederichs S. 2012. Rare Drosha splice variants are deficient in microRNA processing but do not affect general microRNA expression in cancer cells. Neoplasia 14: 238–248. 10.1593/neo.111586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Assunção JA, Enright AJ. 2012. Large-scale analysis of microRNA evolution. BMC Genomics 13: 218. 10.1186/1471-2164-13-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom K-H, Kim Y-K, Jin H, Kim VN. 2004. The Drosha–DGCR8 complex in primary microRNA processing. Genes Dev 18: 3016–3027. 10.1101/gad.1262504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom K-H, Nam J-W, Heo I, Rhee J-K, Sohn SY, Cho Y, Zhang B-T, Kim VN. 2006. Molecular basis for the recognition of primary microRNAs by the Drosha–DGCR8 complex. Cell 125: 887–901. 10.1016/j.cell.2006.03.043 [DOI] [PubMed] [Google Scholar]
- Hanson J, Paliwal K, Zhou Y. 2018. Accurate single-sequence prediction of protein intrinsic disorder by an ensemble of deep recurrent and convolutional architectures. J Chem Inf Model 58: 2369–2376. 10.1021/acs.jcim.8b00636 [DOI] [PubMed] [Google Scholar]
- Herbert KM, Sarkar SK, Mills M, Delgado De la Herran HC, Neuman KC, Steitz JA. 2016. A heterotrimer model of the complete Microprocessor complex revealed by single-molecule subunit counting. RNA 22: 175–183. 10.1261/rna.054684.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden P, Horton WA. 2009. Crude subcellular fractionation of cultured mammalian cell lines. BMC Res Notes 2: 243. 10.1186/1756-0500-2-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter K, Lohmüller M, Jukic A, Eichin F, Avci S, Labi V, Szabo TG, Hoser SM, Hüttenhofer A, Villunger A, et al. 2020. SAFB2 enables the processing of suboptimal stem-loop structures in clustered primary miRNA transcripts. Mol Cell 78: 876–889.e6. 10.1016/j.molcel.2020.05.011 [DOI] [PubMed] [Google Scholar]
- Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293: 834–838. 10.1126/science.1062961 [DOI] [PubMed] [Google Scholar]
- Janas MM, Khaled M, Schubert S, Bernstein JG, Golan D, Veguilla RA, Fisher DE, Shomron N, Levy C, Novina CD. 2011. Feed-forward microprocessing and splicing activities at a microRNA-containing intron. PLoS Genet 7: e1002330. 10.1371/journal.pgen.1002330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Prabhakar A, Van der Voorn SM, Ghatpande P, Celona B, Venkataramanan S, Calviello L, Lin C, Wang W, Black BL, et al. 2021. Control of ribosomal protein synthesis by the microprocessor complex. Sci Signal 14: eabd2639. 10.1126/scisignal.abd2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Wang J, Liu C-P, Wang H-W, Xu R-M. 2020. Structural basis for pri-miRNA recognition by Drosha. Mol Cell 78: 423–433.e5. 10.1016/j.molcel.2020.02.024 [DOI] [PubMed] [Google Scholar]
- Kataoka N, Fujita M, Ohno M. 2009. Functional association of the Microprocessor complex with the spliceosome. Mol Cell Biol 29: 3243–3254. 10.1128/MCB.00360-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. 2001. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev 15: 2654–2659. 10.1101/gad.927801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-K, Kim VN. 2007. Processing of intronic microRNAs. EMBO J 26: 775–783. 10.1038/sj.emboj.7601512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim VN. 2022. High-throughput in vitro processing of human primary microRNA by the recombinant microprocessor. STAR Protoc 3: 101042. 10.1016/j.xpro.2021.101042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-K, Kim B, Kim VN. 2016. Re-evaluation of the roles of DROSHA, Exportin 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci 113: E1881–E1889. 10.1073/pnas.16025321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim J, Kim K, Chang H, You K, Kim VN. 2019. Bias-minimized quantification of microRNA reveals widespread alternative processing and 3′ end modification. Nucleic Acids Res 47: 2630–2640. 10.1093/nar/gky1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Baek SC, Lee Y-Y, Bastiaanssen C, Kim J, Kim H, Kim VN. 2021. A quantitative map of human primary microRNA processing sites. Mol Cell 81: 3422–3439.e11. 10.1016/j.molcel.2021.07.002 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Tomari Y. 2016. RISC assembly: coordination between small RNAs and Argonaute proteins. Biochim Biophys Acta 1859: 71–81. 10.1016/j.bbagrm.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. 2007. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 101: 59–68. 10.1161/CIRCRESAHA.107.153916 [DOI] [PubMed] [Google Scholar]
- Kwon SC, Nguyen TA, Choi Y-G, Jo MH, Hohng S, Kim VN, Woo J-S. 2016. Structure of human DROSHA. Cell 164: 81–90. 10.1016/j.cell.2015.12.019 [DOI] [PubMed] [Google Scholar]
- Kwon SC, Baek SC, Choi Y-G, Yang J, Lee Y-S, Woo J-S, Kim VN. 2019. Molecular basis for the single-nucleotide precision of primary microRNA processing. Mol Cell 73: 505–518.e5. 10.1016/j.molcel.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Kwon SC, Jang H, Shen S, Baek SC, Kim K, Yang J, Kim J, Kim J-S, Wang S, Shi Y, et al. 2020. ERH facilitates microRNA maturation through the interaction with the N-terminus of DGCR8. Nucleic Acids Res 48: 11097–11112. 10.1093/nar/gkaa827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. 2001. Identification of novel genes coding for small expressed RNAs. Science 294: 853–858. 10.1126/science.1064921 [DOI] [PubMed] [Google Scholar]
- Landthaler M, Yalcin A, Tuschl T. 2004. The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol 14: 2162–2167. 10.1016/j.cub.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Lataniotis L, Albrecht A, Kok FO, Monfries CAL, Benedetti L, Lawson ND, Hughes SM, Steinhofel K, Mayr M, Zampetaki A. 2017. CRISPR/cas9 editing reveals novel mechanisms of clustered microRNA regulation and function. Sci Rep 7: 8585. 10.1038/s41598-017-09268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau NC, Lim LP, Weinstein EG, Bartel DP. 2001. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294: 858–862. 10.1126/science.1065062 [DOI] [PubMed] [Google Scholar]
- Lee Y, Jeon K, Lee J-T, Kim S, Kim VN. 2002. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 21: 4663–4670. 10.1093/emboj/cdf476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, et al. 2003. The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419. 10.1038/nature01957 [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom K-H, Lee S, Baek SH, Kim VN. 2004. MicroRNA genes are transcribed by RNA polymerase II. EMBO J 23: 4051–4060. 10.1038/sj.emboj.7600385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link S, Grund SE, Diederichs S. 2016. Alternative splicing affects the subcellular localization of Drosha. Nucleic Acids Res 44: 5330–5343. 10.1093/nar/gkw400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Liang C, Kollipara RK, Matsui M, Ke X, Jeong B-C, Wang Z, Yoo KS, Yadav GP, Kinch LN, et al. 2016. HP1BP3, a chromatin retention factor for co-transcriptional microRNA processing. Mol Cell 63: 420–432. 10.1016/j.molcel.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Wu Y, Choi J-G, Wu H. 2013. Lower and upper stem–single-stranded RNA junctions together determine the Drosha cleavage site. Proc Natl Acad Sci 110: 20687–20692. 10.1073/pnas.1311639110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I, Hayes KE, Barr JA, Harold AD, Xie M, Bukhari SIA, Vasudevan S, Steitz JA, DiMaio D. 2017. An Exportin-1-dependent microRNA biogenesis pathway during human cell quiescence. Proc Natl Acad Sci 114: E4961–E4970. 10.1073/pnas.1618732114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Lemoine F, Soumillon M, Liechti A, Weier M, Guschanski K, Hu H, Khaitovich P, Kaessmann H. 2013. Birth and expression evolution of mammalian microRNA genes. Genome Res 23: 34–45. 10.1101/gr.140269.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando M, Ballarino M, Gromak N, Pagano F, Bozzoni I, Proudfoot NJ. 2008. Primary microRNA transcripts are processed co-transcriptionally. Nat Struct Mol Biol 15: 902–909. 10.1038/nsmb.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. 2002. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev 16: 720–728. 10.1101/gad.974702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TA, Jo MH, Choi Y-G, Park J, Kwon SC, Hohng S, Kim VN, Woo J-S. 2015. Functional anatomy of the human microprocessor. Cell 161: 1374–1387. 10.1016/j.cell.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Nguyen TA, Park J, Dang TL, Choi Y-G, Kim VN. 2018. Microprocessor depends on hemin to recognize the apical loop of primary microRNA. Nucleic Acids Res 46: 5726–5736. 10.1093/nar/gky248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima T, Gomes T, Grosso ARF, Kimura H, Dye MJ, Dhir S, Carmo-Fonseca M, Proudfoot NJ. 2015. Mammalian NET-seq reveals genome-wide nascent transcription coupled to RNA processing. Cell 161: 526–540. 10.1016/j.cell.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olena AF, Patton JG. 2010. Genomic organization of microRNAs. J Cell Physiol 222: 540–545. 10.1002/jcp.21993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin AC, Ngo TD, Herrell E, Jeong B-C, Hon G, Nam Y. 2017. Heme enables proper positioning of Drosha and DGCR8 on primary microRNAs. Nat Commun 8: 1737. 10.1038/s41467-017-01713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin AC, Zhang K, Jeong B-C, Herrell E, Li S, Chiu W, Nam Y. 2020. Cryo-EM structures of human Drosha and DGCR8 in complex with primary microRNA. Mol Cell 78: 411–422.e4. 10.1016/j.molcel.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlicki JM, Steitz JA. 2008. Primary microRNA transcript retention at sites of transcription leads to enhanced microRNA production. J Cell Biol 182: 61–76. 10.1083/jcb.200803111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Hubisz MJ, Rosenbloom KR, Siepel A. 2010. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res 20: 110–121. 10.1101/gr.097857.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JS, Mathews DH. 2010. RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics 11: 129. 10.1186/1471-2105-11-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang R, Baek SC, Kim K, Kim B, Kim VN, Lai EC. 2020. Genomic clustering facilitates nuclear processing of suboptimal pri-miRNA loci. Mol Cell 78: 303–316.e4. 10.1016/j.molcel.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539. 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak-Prochazka I, Kluiver J, de Jong D, Kortman G, Halsema N, Poppema S, Kroesen B-J, van den Berg A. 2013. Cellular localization and processing of primary transcripts of exonic microRNAs. PLoS One 8: e76647. 10.1371/journal.pone.0076647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhang Y, Tucker L, Ramratnam B. 2010. Phosphorylation of the RNase III enzyme Drosha at serine300 or serine302 is required for its nuclear localization. Nucleic Acids Res 38: 6610–6619. 10.1093/nar/gkq547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Li M, Tucker L, Ramratnam B. 2011. Glycogen synthase kinase 3 β (GSK3β) phosphorylates the RNAase III enzyme Drosha at S300 and S302. PLoS One 6: e20391. 10.1371/journal.pone.0020391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Wen S, Zheng D, Tucker L, Cao L, Pantazatos D, Moss SF, Ramratnam B. 2013. Acetylation of drosha on the N-terminus inhibits its degradation by ubiquitination. PLoS One 8: e72503. 10.1371/journal.pone.0072503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott M, Islam ABMMK, Frolov MV. 2016. Novel regulation and functional interaction of polycistronic miRNAs. RNA 22: 129–138. 10.1261/rna.053264.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdar WSJ. 2002. Scoring residue conservation. Proteins 48: 227–241. 10.1002/prot.10146 [DOI] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Li W, She H, Dou J, Duong DM, Du Y, Yang S-H, Seyfried NT, Fu H, Gao G, et al. 2015. Stress induces p38 MAPK-mediated phosphorylation and inhibition of Drosha-dependent cell survival. Mol Cell 57: 721–734. 10.1016/j.molcel.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Yu Y, Reed R. 2015. Primary microRNA processing is functionally coupled to RNAP II transcription in vitro. Sci Rep 5: 11992. 10.1038/srep11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarin T, Tsai CN, Nguyen Ba AN, Moses AM. 2017. Selection maintains signaling function of a highly diverged intrinsically disordered region. Proc Natl Acad Sci 114: E1450–E1459. 10.1073/pnas.1614787114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarin T, Strome B, Nguyen Ba AN, Alberti S, Forman-Kay JD, Moses AM. 2019. Proteome-wide signatures of function in highly diverged intrinsically disordered regions. Elife 8: e46883. 10.7554/eLife.46883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cullen BR. 2005. Efficient processing of primary microRNA hairpins by Drosha requires flanking nonstructured RNA sequences. J Biol Chem 280: 27595–27603. 10.1074/jbc.M504714200 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Yi R, Cullen BR. 2005. Recognition and cleavage of primary microRNA precursors by the nuclear processing enzyme Drosha. EMBO J 24: 138–148. 10.1038/sj.emboj.7600491 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All high-throughput sequencing data generated in this study (small RNA sequencing) have been deposited in GEO (accession no. GSE230544). Custom codes are publicly available online (https://www.github.com/wwc420/drosha_nterm).