Abstract
Organoids have attracted increasing attention because they are simple tissue-engineered cell-based in vitro models that recapitulate many aspects of the complex structure and function of the corresponding in vivo tissue. They can be dissected and interrogated for fundamental mechanistic studies on development, regeneration, and repair in human tissues. Organoids can also be used in diagnostics, disease modeling, drug discovery, and personalized medicine. Organoids are derived from either pluripotent or tissue-resident stem (embryonic or adult) or progenitor or differentiated cells from healthy or diseased tissues, such as tumors. To date, numerous organoid engineering strategies that support organoid culture and growth, proliferation, differentiation and maturation have been reported. This Primer serves to highlight the rationale underlying the selection and development of these materials and methods to control the cellular/tissue niche; and therefore, structure and function of the engineered organoid. We also discuss key considerations for generating robust organoids, such as those related to cell isolation and seeding, matrix and soluble factor selection, physical cues and integration. The general standards for data quality, reproducibility and deposition within the organoid community is also outlined. Lastly, we conclude by elaborating on the limitations of organoids in different applications, and key priorities in organoid engineering for the coming years.
Introduction
Stem cells are critical in maintaining organ size, structure and function through cellular renewal, migration, differentiation and apoptosis1. Stem cells reside in a defined microenvironment commonly referred to as the stem cell niche, which provides the appropriate structural support, nutrients, and mechano-chemical cues to regulate stem cell fate2. Given the importance of these environmental cues, there have been numerous tissue engineering attempts to mimic the stem cell niche in vitro to achieve high spatiotemporal control over cell-cell and cell-matrix interactions and reproduce mechano-chemical cues using engineered hydrogels and micro-devices3,4. In 1977, Matrigel, a basement membrane extracellular matrix (ECM) containing a unique mix of ECM components and growth factors, was extracted from mouse sarcoma tumors and used to support in vitro cell culture5. Matrigel was later shown to allow breast epithelial cells to grow in three-dimensions (3D) and form lumens with milk protein secretion6, and adult intestinal stem cells embedded in Matrigel and in the presence of tissue-specific cocktail of growth factors were also shown to self-organize into 3D crypt-villus structures7. Organoid research interwined with 3D cell culture, stem cell and tissue engineering for over a century, with various debates on the definition, standard and scope.
In general, an organoid is a self-organized three-dimensional tissue that is typically derived from stem cells (pluripotent, fetal or adult), and which mimics the key functional, structural, and biological complexity of an organ8–12. Cells comprising organoids can be derived from induced pluripotent stem cells (iPSC), or tissue-derived cells (TDC), including normal stem/progenitor cells, differentiated cells and cancer cells13. Compared to conventional two-dimensional (2D) cultures and animal models, organoid cultures enable patient specificity in the model while recapitulating in vivo tissue-like structures and functions in vitro. Organoid cultures are more accessible for manipulation and in-depth biological studies14 than animal models. As such, organoid cultures have been leveraged for a wide variety of applications including drug discovery15,16, personalized companion diagnostics16 and cell therapy14.
However, organoid cultures exhibit significant heterogeneity, variable complexity in cellular composition, can undergo poorly-controlled morphogenesis in self-assembly process, and often lack stromal, vascular and immunological components4,13. Hence, there is a great need to improve organoid culture by leveraging our understanding of organogenesis as well as how cells interact with their cellular and physical microenvirontment, i.e., the stem cell niche. Based on these insights, bioengineering strategies could be developed to precisely control stem cell decisions during organoid development. For example, from early embryogenesis studies, it is known that morphogen gradients regulate tissue patterning and development17,18. Microfluidics systems can be used to create the required concentration gradients of these by diffusing morphogens, giving rise to the desired cell types with spatial patterning17. Beyond biochemical cues, it is now increasingly appreciated that stem cells also experience active and passive forces from their external microenvironment and convert these physical stimuli into biochemical responses19. These physical cues could arise from the matrix, external forces, and/or cell-cell interactions. Rather than relying on natural or biologically-derived ECM such as Matrigel with limited stiffness tunability, synthetic hydrogels or other ECM combinations can be leveraged to control the physical properties of the matrix. Liquid friction against the cell membrane can also exert shear stress on cells20. The dynamic biofluidic environment has diverse effects on different cell types depending on magnitude, direction, and frequency20. Hence, microfluidic systems and bioreactors can be applied to provide perfusion at both the micro-scale and macro-scales21–23. Lastly, it is now known that cells interact with their neighbors and respond to external stimuli in a collective manner24. Topographical cues, such as curvature and shape of neighboring cells, can affect stem cell decisions25. A recent neural tube model dissected the folding process and demonstrated that geometry constraints by micropatterning can control the final morphology of neural tube-like structures26.
It is controversial whether engineered cell-based in vitro models such as organoids need to faithfully recapitulate bulk of the structures and functions of the in vivo organ-of-origin. One trend is to recapitulate as much in vivo tissue architecture and functions as possible in vitro in order to demonstrate the physiological relevance of the models of increasing complexity. For engineers, the artificially created in vitro models only need to recapitulate specific features of the in vivo tissue, relevant to the physiological or diseased functions of interest (FOI). There is an optimism to create highly complex models and expect them to accurately mimic the in vivo organ-of -origin. For majority users, simpler ones are more robust for mechanistic studies and applications27–29.
In this Primer, we focus on the rationale underlying the establishment of organoid cultures and provide guiding principles for the selection of suitable materials and methods for different applications. We first discuss the experimentation considerations for setting up organoid-based cultures, categorized into four major components that make up organoid cultures – cells, soluble factors, matrix, and physical cues and discuss approaches to integrate these components (FIG. 1). We also discuss key considerations for generating more complex yet robust organoids, such as those related to cell isolation and seeding, matrix and soluble factor selection, physical cues and integration. The general standards for data quality, reproducibility and deposition within the organoid community is also outlined. Lastly, we conclude by elaborating on the limitations of organoids in different applications, and key priorities in organoid engineering for the coming years.
Fig. 1. Components to engineer organoids.
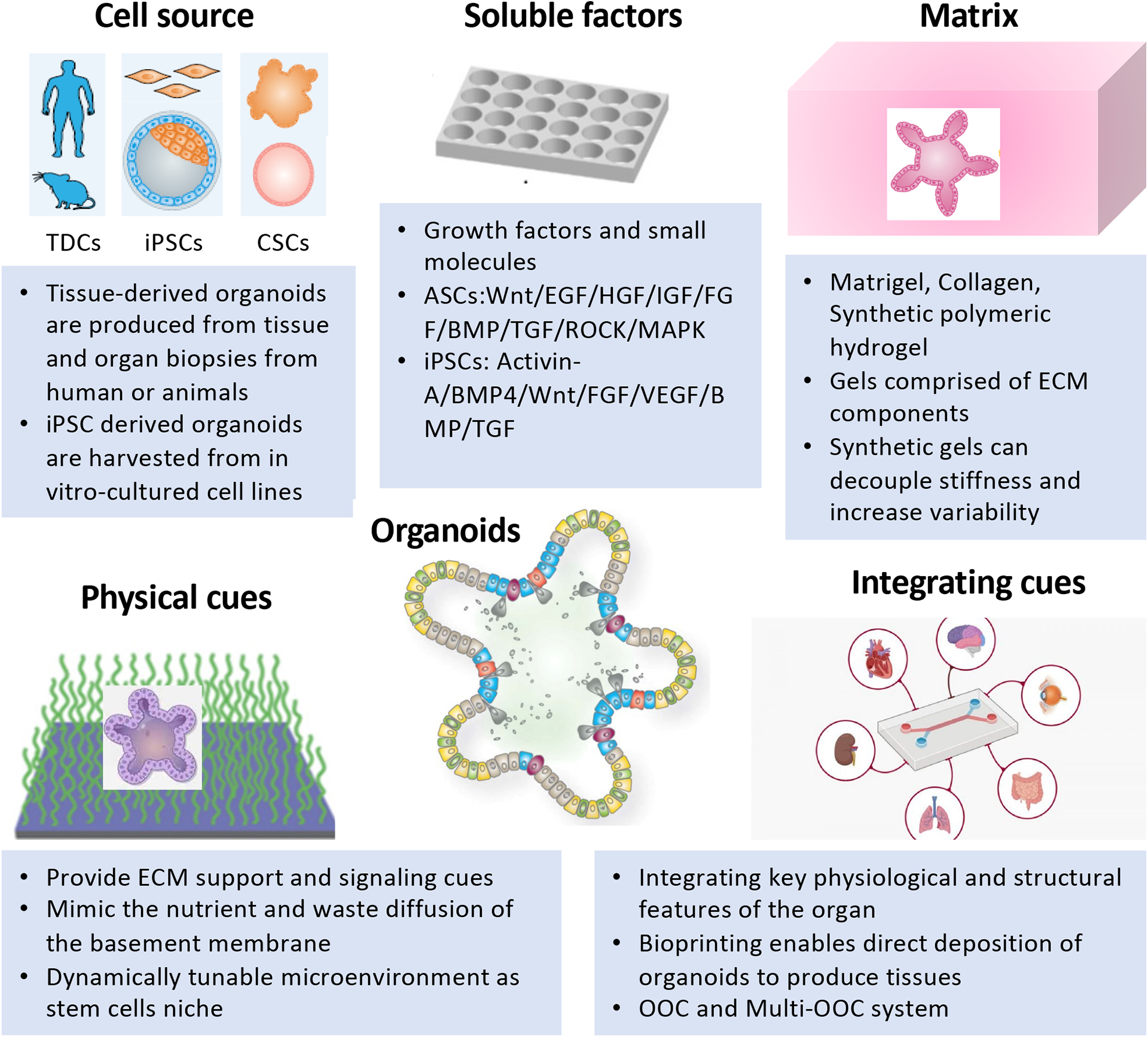
The set up of organoid-based culture requires considerations about four major components that make up organoid cultures – cells, soluble factors, matrix, and physical cues and how to integrate these components
Experimentation
Cell source
Under defined physicochemical conditions, tissues such as small intestine7, colon30,31, stomach32,33, esophagus30, tongue34, liver35–38, lung15, pancreas39–41, heart42, ear43, and skin44 have been obtained from iPSCs, adult or fetal cells, either stem/progenitor cells or differentiated cells. The starting cellular population for any given organoid is of prime importance and not only can affect the variability and heterogeneity in the structures obtained but the function of the tissue they aim to model. Hence, to establish tissue-derived organoids or cancer organoids, we obtain tissue resident stem/progenitor/differentiated cells or tumour cells, respectively, through an optimized tissue dissociation method. In contrast, for iPSC-derived organoids, we establish and fully characterize iPSC lines as the starting cells. Patient/tissue-derived stem cells will be obtained through an optimized tissue dissociation method and then embedded into a 3D matrix mimicking stem cell niches. iPSC can be maintained and expanded as undifferentiated clonal populations on feeder cells. To exemplify the generation of tissue-derived organoids we will use intestinal organoids as an example (FIG. 2A), as this was the first tissue-derived-organoid type established7. The small intestine and colon are opened longitudinally, washed, then cut into 2–4 mm fragments to increase surface area for enzymatic digestion or further mechanical dissociation. EDTA treatment is used to chelate calcium, disrupting cell-cell adhesion and tissue integrity45. Larger tissue fragments and whole cells are remoed from collected crypt fractions, and the harvested primary intestinal crypts are used for seeding and generation of intestinal organoid cultures.
Fig. 2. Flowchart of the procedures.
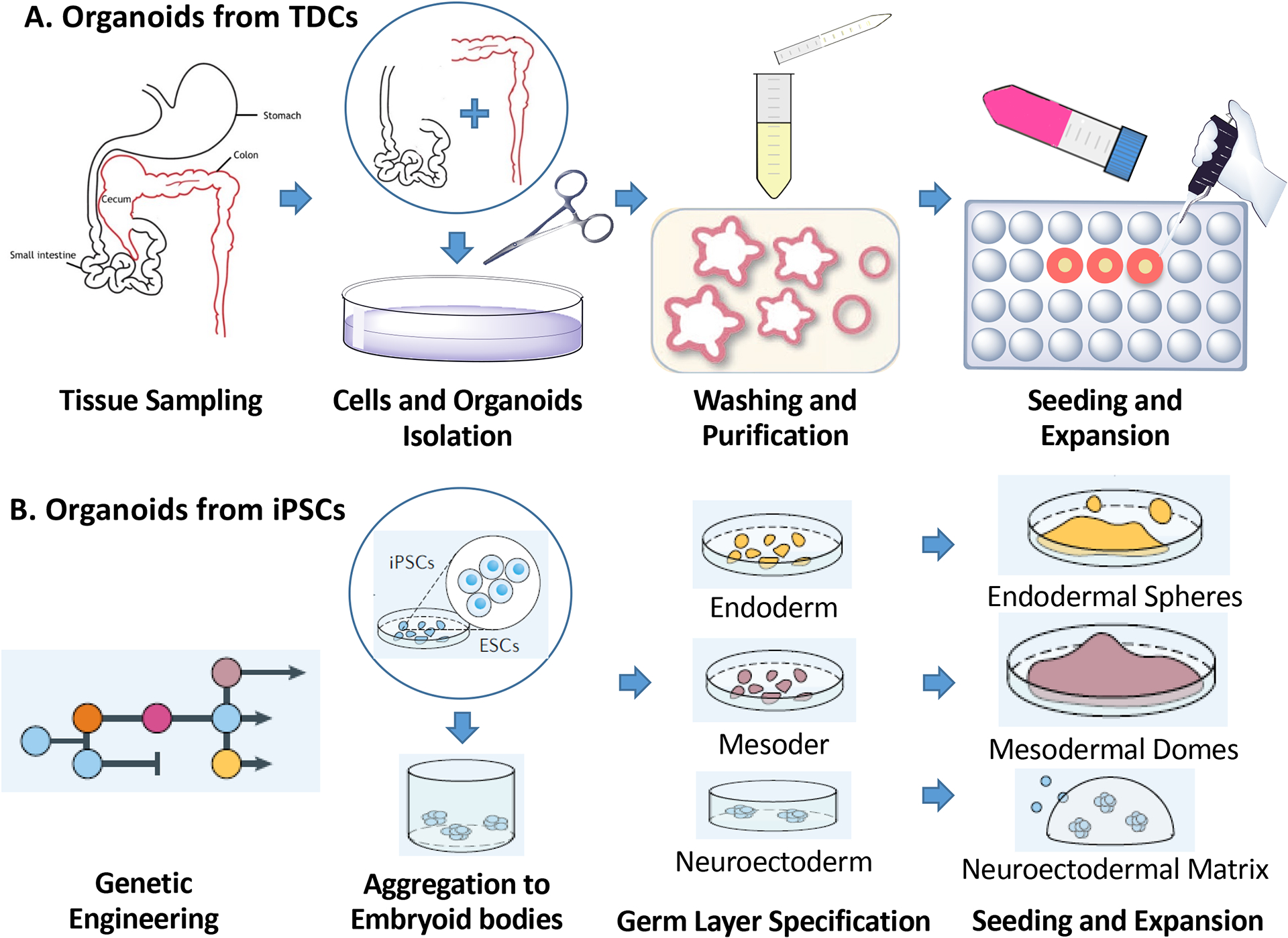
Organoids can be generated from TDC or iPSC.
The starting cellular populations for organoid cultures can generally be obtained from adult or fetal tissue biopsies. The most commonly used tissue dissociation method is enzymatic digestion, which disolves the ECM46. The composition of the enzymatic cocktail and efficacy of the enzymatic dissociation process varies with tissue type47, and in certain cases, DNase can be added to remove excessive DNA released from necrotic cells48. Depending on the tissue type, the tissue fragments can be further incubated with enzymes such as collagenase, elastase, or dispase to generate single-cell suspensions and then seeded in Matrigel.The enzymatic dissociation method may affect the cell state of retrieved cells as it may require extended durations in the enzymatic mix to dissociate the majority of the tissue-resident stem cells. Tissue dissociation can also be achieved mechanically; although mechanical dissociation is much faster and less expensive, cell yield and viability can be inconsistent47. Mechanical and enzymatic dissociation can be combnined to generate better cell yield. After tissue dissociation, TDCs for organoid development are identified and collected using known biomarkers or physical characteristics46. Tissue-specific stem cell markers are typically used to identify and isolate the desired stem cells to generate organoids1,2. Fluorescence-activated or magnetic-activated cell sorting (FACS or MACS) isolates cells based on multiple parameters, including size, shape, and cell-surface marker expression46,47. Other isolation techniques include laser capture microdissection and manual cell picking46.
iPSC can be maintained and expanded as undifferentiated clonal populations over many generations. Undifferentiated human iPSCs are typically maintained on feeder cells or defined ECM substrates. Since single iPSC does not survive well in vitro, iPSC are typically harvested as cell aggregates which preserve cell-cell contact, yielding cell populations with higher viability. Physical scraping can also compensate for the lack of uniformity of cell aggregates. The dissociation enzymatic mixture should be chosen based on level of cell sensitivity45 and whether the cultured cells secrete excessive ECM, making it difficult to detach the cells from the cell culture plate (FIG. 2B).
Tumor tissue, derived from either biopsies or surgical resections, is also typically processed akin to normal tissue to isolate tumour cells to grow as organoids16,49–51. Tumor cells isolated from liquid samples such as peripheral blood52, ascites50,53, and pleural effusions54 can also be used as starting material to generate organoids. Patient-derived tumor organoids can be generated from samples obtained from minimally invasive Pap brush material55,56 . Due to their low numbers, tumor cells from biopsies or liquid samples can be firstly expanded in animal models as xenografts in order to obtain sufficient cells for organoid generation57. In the case of tumor tissue, it is preferable to limit tissue dissociation so that cell clusters rather than single cells are isolated. A critical factor that can influence the generation of tumour-derived organoids is the fact that isolated cells from tissue typically contain both cancer and normal cells. While for some tumors it may be possible to enrich for tumor-forming cells by sorting a priori58, for the majority there is currently no robust method to separate normal and tumor cell populations prior to seeding into a matrix for culture. An approach to overcome this issue is to take advantage of culture conditions by utilizing selective media that omits certain factors required for growth of normal organoids, as tumor cells gradually lose dependence on those factors during malignant transformation59. Blood contamination, particularly erythrocytes, can also affect organoid generation and matrix stability therefore standard approaches to eliminate these through lysis are typically used60.
Matrix
Following cell isolation, cells are typically seeded into biologically-derived matrices such as Matrigel7,61 or natural ECM such as collagen62, or into synthetic hydrogels3,63,64. Matrigel is mainly comprised of laminin, collagen IV, entactin, perlecan and growth factors, and is similar in composition to the basement membrane62. As a continuation from the above example using intestinal organoids, we will now briefly describe how cells are encapsulated into matrices. Isolated intestinal crypts are first re-suspended into cold Matrigel and pipetted into a pre-warmed low-attachment well-plates for culture. The resulting cell-matrix construct is typically a flat semi-sphere gel. The complete composition of intestinal organoid culture medium have been previously reported30. To passage organoids, the gel is first broken up mechanically by pipetting culture medium to release the encapsulated organoids, dissolved as much as possible using cold medium, PBS or special dissolving solutions and then organoids are mechanically dissociated and pieces are re-encapsulated in Matrigel for culture again. To measure the proliferation rate of the cultured organoids, intestinal organoids are first released from Matrigel using enzymatic digestion. The retrieved cell pellet is then repeatedly washed to remove single cells, and crypts are counted. The expansion rate is calculated as the number of organoids from each well divided by the number initially crypts seeded in Matrigel in that well.
While Matrigel can support organoid culture, the inherently heterogeneous and poorly defined composition of this biologically-derived matrix offers little control over the biochemical and biophysical spatiotemporal cues that are necessary for improving organoid culture. Therefore, other matrices with defined compositions64 have been explored as alternative matrices to Matrigel, such as recombinant human collagen62, fibrin65, or synthetic hydrogels3,63. Natural matrices can be recombinantly produced from proteins or polysaccharides to address the batch-to-batch variability of Matrigel61. On the other hand, synthetic hydrogels have emerged as powerful tools that enable independent manipulation of biochemical and biophysical matrix properties to control organoid features and enhance functionality. The ideal organoid matrix should overall be stress-relaxing and highly dynamic in biochemical and biophysical properties to accommodate or control changes in organoid structure during culture. For example, dynamic hydrogels based on poly (ethylene glycol) (PEG) were recently shown to enable reproducible intestinal organoid formation and demonstrate how hydrogel properties could be tuned to control stemness and differentiation in cultured organoids3. The viscoelastic profile of hydrogels has also been shown to define the mechanical confinement of growing organoids66. In other examples, the activity of adult stem cells can be controlled using PEG hydrogels with photo-degradable moieties67; biomimetic polymers can be modified to incorporate essential ECM signals to generate organoids with tailored features3. Tunable PEG hydrogels can promote intestinal crypt budding68; while dextran-based GMP-compatible hydrogels support expansion of cells for longer passages69. Lastly, microfabricated arrays were recently reported to enable the uniform production of crypt-villi-shaped epithelium70. Even the recent advancements using the synthetic matrix to grow organoids, organoid growth by the synthetic matrix is still less efficient than Matrigel-cultured organoids. There is an unmet demand to develop a better matrix.
Organoids can arise either from round colonies generated by single cells7,35 or, from initial multicellular structures such as intestinal crypts30,63, cell aggregates24 or micropatterned cells26. The aim of the latter approach is to establish a cellular niche which involves other cells of the same or different type from the beginning. To form cell aggregates, the simplest method is to use an ultra-low attachment dish coated with hydrophilic hydrogel71 to prevent cell attachment; subsequent centrifugation can promote aggregate formation, enhancing cell-cell contacts72. The size and compaction of cell aggregates can be tuned and controlled, such as through the use of microwell arrays72,73. Another well-established method to form cell aggregates is the hanging drop method, where aggregates form at the bottom of the drop. In a recent example, droplet microfluidics was used to aggregate murine cholangiocytes to form complex organoids with liver mesenchymal cells74. Droplet microfluidics can print one organoid per well and enable the rapid generation of intra-organoid heterogeneity75. Another example of microfluidics use in organoid research comes from a recent paper, where authors use droplet-based microfluidics to perform better scRNA-seq analysis of intestinal organoid cell identities during various developmental stages, revealing extensive population heterogeneity76. Microwell structures or microfabricated pillar arrays70 have also been developed to enable enhanced uniformity in cell aggregation72,73,77. In one example, microfabricated patterns of Laminin-512 were shown to reproducibly support human pluripotent stem cells to form lumen structures in Matrigel and differentiate into human neural tube-like structures26. These examples, amongst others emerging in the field, illustrate how our knowledge of biomaterials and tissue engineering, can be extrapolated to provide precise control over organoid structure and function.
Soluble factors
Organoid cultures are fundamentally based on our accumulated knowledge of developmental biology14, where soluble cues are presented to cells in a spatiotemporally controlled manner (Soluble factors used to differentiate TDC and iPSC into various tissue types are listed in Supplementary Table 1). Translated to organoid culture, these soluble cues are recapitulated in vitro in the form of biologics, mainly as proteins such as growth factors78, or small-molecule drugs, which can activate or inhibit signaling pathways. While growth factors could be costly and unstable, and many small-molecule drugs can affect multiple pathways resulting in poor reproducibility78, some organoid protocols have combined the use of both biologics and small-molecule drugs79. The use of conditioned medium from engineered cell lines producing biologically active growth factors, e.g., L Wnt-3A, can replace commonly used growth factors, such as Wnt3a ligands80. These conditioned media face a similar issue of batch-to-batch variation and require stringent tests to ensure reproducibility81. Thus, novel sorrugate molecules are starting to arise as potential substitutes to condition medium80,81.
It is critical to consider how and when soluble cues are are added to organoid cultures because soluble cues in vivo are typically presented to cells by the ECM or nearby cells, coordinated in time and space. This is the concept of spatiotemporal presentation. For example, it is now known that fibroblast growth factor (FGF) activity and specificity can be regulated by cell surface heparan sulfate proteoglycans, suggesting how the addition of free FGF into cell culture medium may not recapitulate the way FGF is presented in vivo82. The importance of presenting soluble cues in a spatiotemporally relevant manner is especially important for growing human iPSC into complex structures with multiple cell lineages such as in the case of kidney organoids83. Based on previous studies84, it is known that the ureteric epithelium develops from early migrating presomitic mesoderm cells. To recapitulate this process, the effect of different durations of initial Wnt signaling before the addition of FGF was investigated83. The spatiotemporal presentation of soluble factors can be achieved using different tissue engineering approaches. In one strategy, these growth factors can be encapsulated within nanoparticles, and conjugated onto cell surfaces for controlled release85–88. To mimic how certain growth factors are bound to the ECM in vivo, researchers conjugated polymers with heparin which can bind to growth factors, or conjugated these growth factors to the polymer itself89. Surface tethering can also be achieved with nanotechnologies, such as nano-imprint lithography, electron-beam, and electrospinning, can fabricate substrates with nanopillars, nanopits, or nanochannels, mimicking the basement membrane for 3D organoid culture90. Lastly, microfluidic systems can be leveraged to create miniaturized niches with precise control over mechano-chemical properties91. With directed flow and gradients of gas or small molecules, these systems can finely control environmental parameters within organoids18. In one example, a microfluidic neural tube device was developed to present simultaneous opposing gradients of growth factors to direct neural tube patterning, enabling recapitulation of the in vivo structure92.
Physical cues
Beside biochemical cues, it is also important to consider when and whether it is necessary to provide appropriate physical cues to cultured organoids. Nutrient supply and waste removal, which are diffusion-dependent, become less efficient during organoid growth into larger tissue structures. This is the reason why intestinal organoids need to be fragmented into smaller cell clusters and reseeded regularly7. Inadequate nutrient and waste diffusion is also problematic in brain organoid culture, where the resulting millimeter-sized constructs often exhibit necrosis within the inner core due to nutrient inaccessibility. This problem can be partially resolved using shaking cultures23 93, spinning bioreactors or suspension under continuous agitation23, or in continuously stirred bioreactors93. These bioreactors can monitor pH, temperature, oxygen, and glucose levels to maximize mass transfer while minimizing shear stress. In this regard, perfusable microfluidic chips have also been developed to promote the long-term culture of organoids21,22,94. Lastly, it may be useful to consider providing topographical cues to control organoid culture in vitro; the topography of the substrate is known to modulate cell area, shape, and cell-cell interactions, resulting in biochemical signals that can affect stem cell fate25. Topography-directed morphogenesis has been demonstrated using intestinal organoids grown on soft hydrogels95.
Integrating cues
In contrast to the original self-organizing organoid model, the above described cues can be integrated to confer greater control over organoid morphogenesis. Integration of cues is a commonly employed strategy in the field of tissue engineering to construct tissues in vitro and in vivo96. Ideally, specific physical or chemical cues should be presented in a spatiotemporally, physiologically relevant manner using simple, reproducible and robust methods (Supplementary Table 2). We will illustrate this using the example on intestinal organoids7. Following the identification and isolation of LGR+ intestinal stem cells residing in crypts, biologically-derived Matrigel was leveraged to mimic the laminin-enriched crypt environment, supplemented with exogeneously added growth factors in the cell culture medium. Most intestinal stem cells could survive, proliferate, and form organoids7. However, given the stochastic nature of organoid development, the resulting organoids vary in size, bud numbers and function. In one example of how environmental cues (mechanical and biochemical) were integrated to exert control over organoid morphogenesis, the organoid maturation process was dissected into different stages, and biomaterials engineering was used to identify the optimal mechano-chemical environment at each stage3. It was shown that mechanically dynamic matrices that could switch from high to low stiffness over time, enabled control over the morphogenesis process3. In another example, human neural tube morphogenesis26 was simulated using micropatterning which created mechano-chemical gradients to regulate cell-cell/matrix interactions, and soluble factor presentation to orchestrate morphogenesis.
Besides these examples, other engineering methods have been used in organoid culture to control cell proliferation, differentiation and morphogenesis. A popular bioengineering approaches to reconstruct tissues is bioprinting. Bioprinting is an additive manufacturing technique that enables direct deposition of stem cells, organoids, and biomaterials to fabricate 3D organoid-based tissue structures with controlled cell-matrix structures97. This technology uses ‘bioink’, comprising living organoids encapsulated within a biomaterial, to precisely create 3D biological geometries that mimic that of the native tissue in a layer-by-layer approach. In one recent example of how bioprinting was used to generate large tissue constructs, human small intestinal organoids were used to form self-evolving tissue constructs by a concept called bioprinting-assisted tissue emergence (BATE), where centimeter-scale intestinal tissues were printed sequentially to mimic boundaries in the gastrointestinal tract98. Another bioengineering approach to reconstruct tissues, especially across different tissues and organs, is the use of microfluidic platforms. Microfluidic systems have been used to create miniaturized cellular models comprising organoids that recapitulate critical aspects of organ physiology, termed organ-on-a-chip99 (OoC). OoC devices can potentially incorporate other bioengineering techniques to imitate the key physiological and structural features of organs and tissues. For example, physical constraints were engineered into the organoid environment using synthetic scaffolds to provide physical boundaries100. OoC devices also support co-culture or multi-organ systems. The two-organ system has been simulated using connected chambers containing different tissue constructs to mimic liver injury (with intestinal epithelial cells and liver cells)101 and Type-2 diabetes (with human pancreatic islets and liver spheroids)102. A two-organoid microfluidic device was recently fabricated with multiple readouts using cardiac and hepatic organoids103. Moreover, OoC devices have improved the characteristics of hPSC-derived pancreatic islets104, intestinal105, stomach106, and liver77 organoids by supporting physiological flow rates. Kidney organoids have been cultured in an OoC device which enables fluid flow to simulate shear stress107 and the generation of a vascular network108.
Results
It is important to evaluate whether the cultured organoids recapitulate key aspects of the human tissue or organ. To achieve this, characterization of these organoids is typically performed by assessing organoid cellular composition, structure, functions, and robustness of phenotype. In this section, we discuss commonly employed analytical methods using representative examples.
Validation of organoid composition and structure
Organoid growth is a process of initial cell aggregation, proliferation, migration and differentiation. When assessing whether the organoids have been successfully established, it is critical to first determine whether the organoids contain the desired cell types, and the degree to which the organoids accurately mimic the functions of the corresponding tissue in vivo. To achieve this, both low-throughput gene expression validation and high-throughput whole-genome transcriptome analyses are typically performed. Real-time PCR is often first performed as an easy, fast, and quantitative read-out on marker genes indicating cell identity, including key transcription factors and differentiation markers. Western blotting provides further quantitative information regarding protein abundance, protein degradation, protein-protein interactions, and post-translational modifications, which represent the activities of a specific signaling pathway in a committed cell type. The most common tools used to evaluate organoid composition are immunofluorescence and immunohistochemical imaging using sections or whole-mount, and the specific cell markers antibody staining further elucidates various cell types’ spatial distribution and proportion. A high throughput analysis of single cell RNA-sequencing (scRNA-seq) profiles all the cell types in the organoids, undifferentiated and committed, at the whole-genome transcriptome level. These cell types are then compared to cells freshly isolated from the corresponding tissue or organ to evaluate the degree of similarity for each cell population. Given how it is expected that the in vitro cultured organoids contain different states of immature cells, scRNA-seq can be helpful for determining the extent of organoid heterogeneity in terms of cell differentiation status.
The assessment of organoid morphology is performed to determine whether there is structural similarity between the cultured organoids and corresponding in vivo tissue/organ109. For example, for secretory tissues such as pancreatic islets, the islet-like globular structure should be present within the organoids, and intra-cellular hormone vesicles110 are also expected to be observed. For branching epithelial organoids, such as breast organoids111, distinct branching structures are expected. For intestinal organoids, crypt-like structures100 should be observed. For more complex organoid culture systems comprising incorporated supportive cells, like fibroblast112 and/or endothelial cells107,110, it is expected that the incorporated endothelial cells should form a vascular network107 that recapitulates the endothelial-epithelial interactions around the organoids.
Validation of organoid functionality
Evaluation of the functionality includes but is not limited to the generation of mature cells, the formation of the vasculature or neuronal networks26,44, the accurate response to external stimuli, the effective secretion of cytokines or hormones, etc. It should be noted that the organoids should be compared with the freshly isolated tissues as a positive control. Discrete physiological recapitulations were required for different tissues, such as acid secretion in gastric organoids and mucus secretion in intestinal organoids. Various methods of characterization to assess the structure and functions of organoids are listed in Table 1.
Table 1.
Methods used in organoid research to assess/characterise organoid structure and function
| Result characterization methods | Function | Example Applications | Ref. | |
|---|---|---|---|---|
| Organoid structure | Bright field imaging | Qualitative assessment of morphology and viability. Quantitative assessment of organize size and number. | Crypt-like structures for intestinal organoids, islet-like globular structure for pancreatic islet organoids, branching structure for breast or lung organoids, hollow cystic structures for cholangiocyte and cholangiocarcinoma organoids, grape-like structures for hepatocyte and hepatocellular carcinoma organoids | 35–37,42,100,110,111,125,126,132,155,201,203 |
| Immunofluorescent staining | Quantitation viable cells. Edu and Ki67 staining to evaluate the survival and proliferation of the cells in the organoids. Staining of differentially expressed proteins in different layer or geometry. Qualitative and quantitative assessment of cellular composition, spatial distribution, and proportion of different cell types and maturation state of the different subpopulations. | Bile canaliculi can be shown to form between the hepatocytes (identified by markers, such as such as BSEP or MRP2 for bile canaliculi, Albumin or HNF4alpha for hepatocytes)) and in some cases bile ducts can also be formed between the cholangiocytes (identified by markers such as Keratin19 and SOX9) | 36,100,107,110,111,125,126,132,155,201,203 | |
| Transmission and scanning electron microscopy | Characterizing the cellular interaction and ultrastructure of the cells. | Measuring the size and density of mitochondria and cellular secretion, or for hepatocyte organoids glycogen accumulation, Golgi and mitochondria morphology, bile canaluciuli presence. | 7,36,41,42,100,107,109,117,125 | |
| Organoid function | qPCR and single and bulk cell RNA-sequencing | Quantitation of the expression of marker genes, including key transcription factors and differentiation markers, to indicate the cell identity and cellular composition of the organoids. | Single cell RNA-sequencing can profile all cell types in the organoids. Also useful for screening differentially expressed markers under different stimuli. | 35,37,38,41,42,100,107,110,117,125,126,132,201,203 |
| Immunofluorescent imaging | Use of fluorescently labelled dyes to determine specific functions of certain tissues. Transport of Rhodamine123 (substrate of MDR1 on cholangiocytes) or fluorescein diacetate (in the bile canaliculi of hepatocytes) to verify correct cell functionality of organoids | Swelling assays to determine functionality of ion transporters (e.g for CFTR functionality in gut, lung and pancreas organoids), CMFDA ( 5-chloromethylfluorescein diacetate) dyes to determine bile transporter functionality, or dextran-based fluorescent dyes to investigate permeability of epithelium or endothelium. | 36–38,41,100,107,111,114,125,126,132,155,201,203 | |
| Alcian blue (AB) and periodic acid-Schiff (PAS) staining | Detection of neutral and acidic glycoproteins produced by specific cells in organoids | Measurement of mucus secretion ability of the differentiated goblet cells in intestinal organoids | 123,124 | |
| ELISA and colorimetric assays | Secretome quantification in response to external stimuli | Determine c-peptide secretion in pancreas organoids or endogenous production of secretome molecules by organoids as assessment of their maturity. | 35,36,38,41,110,114–116,125,126,132 | |
| Luciferase assays | Luminescent assays for measuring enzyme activity | Various Cytochrome activity for hepatocyte organoids (CYP3A4, CYP1A2, P450). | 35,36,125,126 | |
| Calcium signaling | Characterizing the electrophysiology property of the organoid | Used for organs like the heart, neuron, retina, skeletal muscle and pacncreatic islet. | 27,114,116,125,126 | |
| Implantation | Testing the in vivo function of organoids for cell therapy. | The full potential of the islet organoids in mouse transplantation can be tested by characterization of normalized and stable blood glucose level, normal plasma insulin level and maintained body weight | 35,41,110,114,116,117,125,126,132,155,203 |
TEM – transmission electron microscopy, SEM– scanning electron microscopy, qPCR – quantitative polymerase chain reaction, ELISA – enzyme-linkedimmunosorbent assay
Pancreatic islet organoids
We use the example of mouse pancreatic islet organoids to discuss various characterization and validation methods (FIG. 3). The formation of four endocrine differentiated cell types (β-cell, α-cell, δ-cell, PP-cell) is examined by the expressions of the corresponding hormones, Insulin (Ins), Glucagon (Gcg), Somatostatin (Sst), or Pancreatic polypeptide (Ppy), by immunostaining113,114. To assess the extent of β-cell maturation, the ultrastructural morphometric analysis of insulin secretory granules is performed by immune-gold transmission electron microscopy (TEM) image110. To evaluate the ability of the β-cells in the organoids’ responsiveness to glucose, one performs Glucose-stimulated insulin secretion (GSIS) experiments, for example insulin or C-peptide ELISA assay with cyclic high and low glucose incubation115. As the insulin release is associated with the Ca2+ dynamics, the calcium signaling traces imaging rapidly increased in response to glucose and returned to baseline114,116. To test the full potential of the islet organoids, the in vivo functional evaluation is to ameliorate the hyperglycemic phenotypes of streptozotocin (STZ, a toxic to the insulin-producing β cells of the pancreas in mammals)-induced type 1 diabetic mouse model upon organoid transplantation110,114,117. The characterization parameters include normalized and stable blood glucose level, normal plasma insulin level and maintained body weight.
Fig. 3. Representative results of pancreatic islet organoids validation analysis.
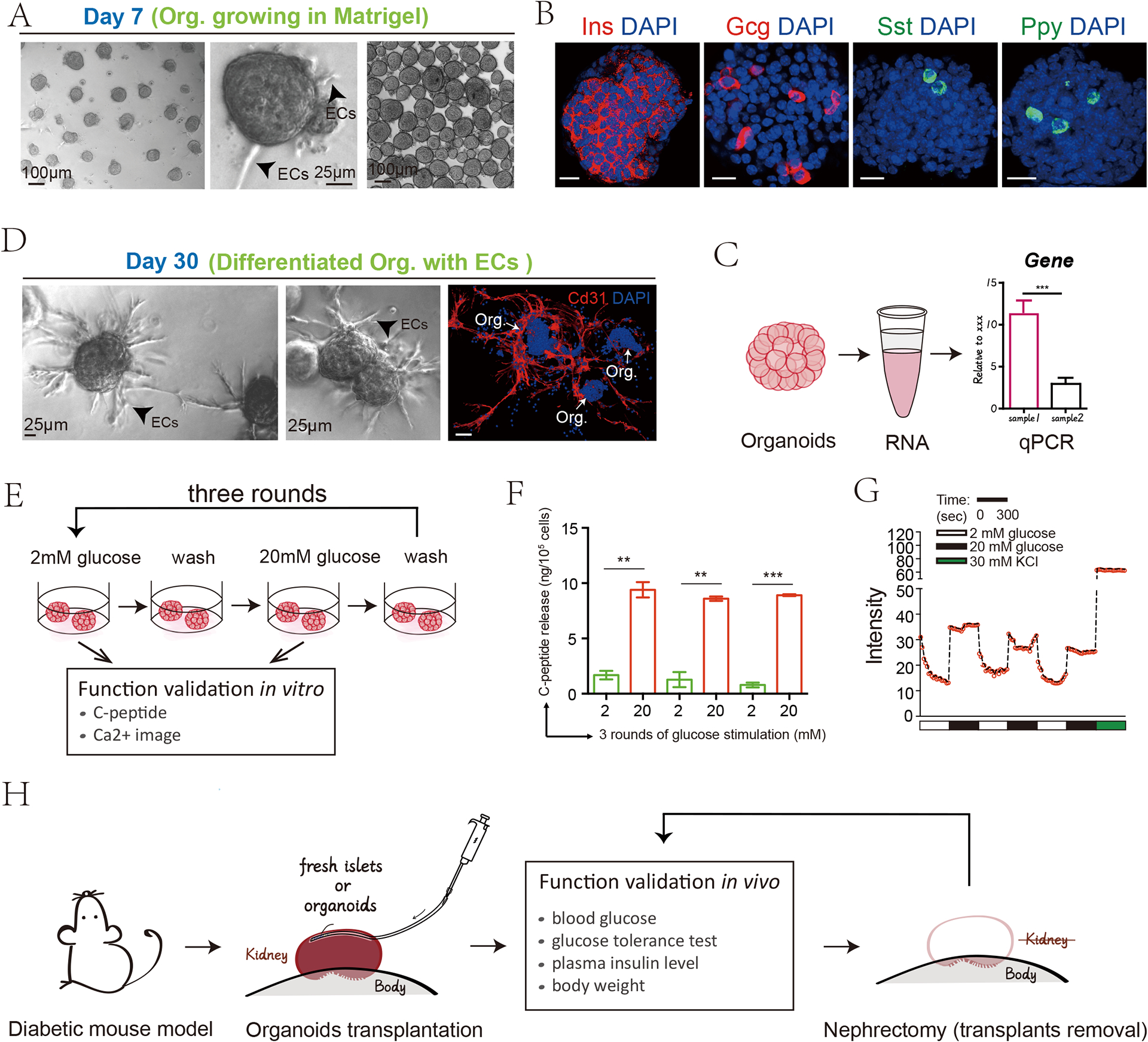
- Representative view of pancreatic islet organoids.
- Cell types and hormones secretion level validation by immunofluorescence or immunohistochemical staining.
- Real-time qPCR analysis for some key transcription factors and differentiation markers.
- The maturation of the organoids can be induced through prolonged culture for a total of 30 days at any passage.
- Schematic of the pancreatic islet organoids function validation in vitro
- Measurement of the secreted C-peptide by ELISA
- Intensity of calcium signalling traces imaging indicating the capability of responding acutely to glucose
- Schematic of the islet organoids function validation in vivo
Intestinal organoids
Intestinal organoids are among the first organoid types successfully established in vitro7,30. Classical crypt-villus-like architecture is regarded as an important symbol of the successful organoid establishment, exhibiting the proper branching morphology with robust multicellular composition and location reflecting the organoids differentiation and maturation level118,119. The expression of Lgr5 marker can anlyze the maintenance of intestinal stem cells by real-time qPCR or by imaging using a Lgr5- fluorescent reporter7,120. The differentiated cells are assessed by the staining of their markers, including Lysozyme (Paneth cells), Villin (enterocytes), Mucin 2 (goblet cells), and Chromogranin A (enteroendocrine cells)7,100,121. These various cell types can also be distinguished by TEM according to their characteristic subcellular structure7,121. Furtherly, functional intestinal organoids exhibit a relatively thick mucus layer due to mucus secretion from mature goblet cells122, which can be detected by Alcian blue (AB) and periodic acid-Schiff (PAS) staining123,124.
Liver organoids
In the case of the liver, both cholangiocyte (ductal cell of the bile duct)35,36 and hepatocyte organoids125,126 have been established, both from mouse and human tissue samples. To assess the organoids, passaging time and proliferation of the organoid cells are measured. Regarding marker expression, specific cholangiocyte (for example Keratin 19, Keratin 7, SOX9) or hepatocyte markers (such as Albumin, HNF4α, MRP4) are assessed both on the RNA (qRT-PCR, scRNA-seq) and protein level (immunofluorescence staining). Often also markers of liver progenitors (like LGR5) are used to assess the differentiation state of the cultured cells. For the assessment of organoid functionality, further markers such as CYP3A4, CYP3A11 for hepatocytes are analysed on the RNA level. Furthermore, Albumin secretion, LDL-uptake, presence of glycogen accumulation assessed with periodic acid-Shiff (PAS) staining, bile acid production and activities of liver enzymes such as CYP3A4 are assessed using chemical assays to confirm hepatocyte function. The ultra-structure of the organoids is also often assessed for typical hepatocyte or cholangiocyte morphology by transmission electron microscopy (TEM). Finally, for an ultimate test of functionality, organoids are often transplanted in FRG immune-deficient mice, to look at integration and functionality of the graft in vivo35,125,126. (FIG. 4)
Figure 4. typical characterization of cancer organoids: liver cancer subtype132.
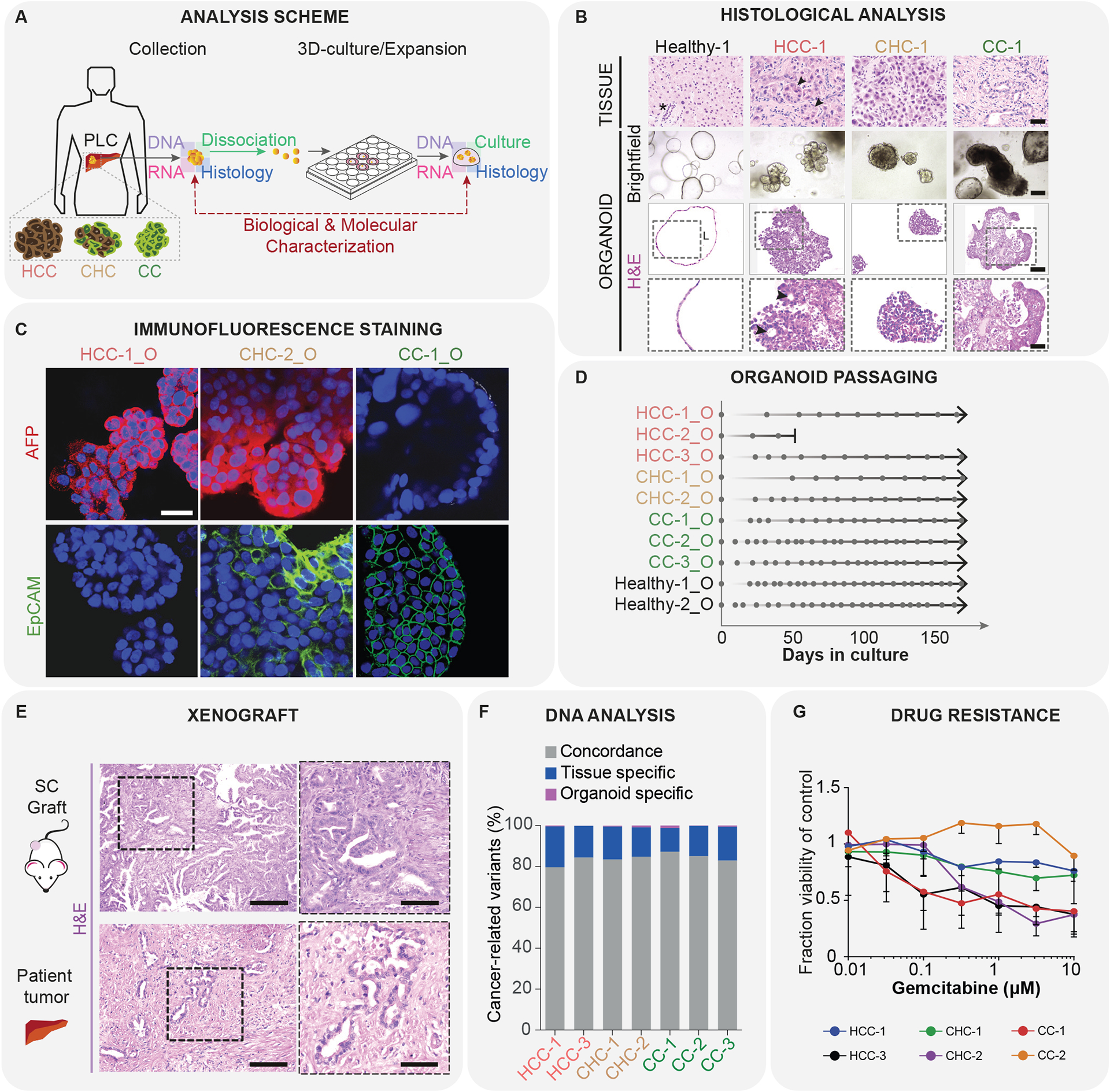
-
Isolation of cells from patient samples and organoid culture; schematic of tissue isolation and processing;HCC, hepatocellular carcinoma; CC, cholangiocarcinoma; CHC, combined HCC/CC tumors.
- Histological analysis of liver cancer samples: top, tissue; middle, organoid brightfield images; bottom, histological H&E staining of organoids; scale bar, middle row, 100 μm; top and bottom rows, 50 μm.
- Analysis of specific marker gene expression: immunofluorescene staining for AFP (hepatocyte/HCC marker; red) and EpCAM (ductal/CC marker; green); blue - DAPI, scale bar, 30 μm.
- Organoid formation efficiency: growth and splitting curves; dot, splitting time point, arrow, continuous expansion.
- Transplantation into immunodeficient mice: xenograft and histopathology analysis, matching to the patient origianal tissue sample; scale bars, left, 125 μm; right, 62.5 μm
- Analysis of genetic changes in the cancer organoids and their concordance to the mutations in the original tumor sample.
- Organoid sensitivity to drugs: IC50 curves for gemcitabine treatment.
Validation of tumor organoids
Patient-derived tumor organoids must maintain the genomic, transcriptomic, morphologic and functional profiles of the tissue of origin. As such, it is important to validate them by comparing to the tissue they are derived from in terms of histology and immunohistochemistry (IHC) profiles16, transcriptomics as well as genomics15,127. Cellular organization, tissue structure and protein expression patterns of tumor organoids can be easily compared to the cancer of origin16. Similarly, organoids are expected to have gene expression profiles similar to the tumor they are established from. This has been shown by RNAseq on many tumor types including bladder cancer128 and esophageal cancer129 amongst others. Lastly, it is important to confirm whether tumor organoids have diverged from the patient tumor at the genomic level. Several studies have shown concordance of organoids and parent tissue in terms of mutations and copy number variations (CNV)15,127. A greater degree of divergence may be attributed to intra-tumor spatial heterogeneity and sampling bias: tumors can be spatially diverse, and organoids generated from a specific portion may lack the representativeness of distant regions130,131. In addition to this topographical issue, culture conditions can contribute to selecting specific clones, possibly altering or reducing clonality over time129.
For this reason, validation of tumor organoids is even more critical when culturing over a long period of time. While some studies have reported that molecular characteristics can be maintained over long-term culture, others have observed tumor evolution after serial passaging129,132. For instance, exome sequencing of liver cancer organoids showed how mutation concordance decreased from 92% for organoids cultured for less than two months to 80% for those grown for over four months132. Colorectal tumor organoids established from microsatellite instable tumors had higher de novo mutations after long-term culture than stable ones59. Some of the differences observed may be attributable to how different clones and subclones adapt and expand in culture. Using deep targeted sequencing of 500 cancer-associated genes, truncal mutations were shown to be retained while subclonal ones are gained or lost, with mutational changes at each passage127. Most mutations and CNVs are are preserved in the late passage of lung cancer organoids, while de novo mutations can be attributed to the sub-clonal expansion of small subsets of cells present in the tumor of origin15. Overall, these studies showed that genetic drift can occur in organoids cultured for extended time, influencing their reliability as models for functional and drug discovery studies133. This reiterates the importance of validation in the context of tumor organoid establishment, characterization and testing. As an example of analysis, crucial steps in characterisation of liver cancer organoids are demonstrated (FIG. 4). Lastly, normal tissue contamination is an issue for establishing tumor organoids and should be addressed when validating patient-derived models. Presence of normal cells can be estimated by histological comparison and IHC as well as sequencing55,134.
Data analysis
Image processing and analyses
ImageJ / Fiji135, CellProfiler136 and other software are used for image processing. The number of organoids formed in a certain period, organoid size (area or volume) and cell number (nuclei number) could measure the cell proliferation rate. Cell proliferation could be quantified by the ratio of EdU or Ki67 positive nuclei using the plugins in the software. Morphological evaluation, for example, the bud formation ratio in the intestine organoid system, is also necessary when quantifying the success rate considering functional maturation. Mean/normalized fluorescence intensity of maturation markers (either by live imaging with knock-in reporters or immunofluorescence staining at the endpoint of the experiment) for different tissue types could be recorded to measure their maturation level. As for lineage tracing purposes, long-term live imaging could be analyzed using software to track the cell behavior. Web resources could be used for pathway analysis and visualization of multi-omics data to generate heatmaps for understanding the differentiation stage of organoid137,138. Other software, like e.g.: Profiler, GSEA, Cytoscape and EnrichmentMap, have been used for biomolecular interaction networks, pathway enrichment analysis and visualization139–142.
Machine learning and Artificial Intelligence data analytics classification of phenotypes
Miniaturization and automation could make it more consistent for high throughput and multidimensional phenotyping of organoids; machine learning and artificial Intelligence-based data analytics showed their superiority in this case. Various software have been developed in recent years, like ilastik143, OrganoidTracer144 and Phindr3D145. Most of them have Fiji or CellProfiler plugins, which makes it possible for users without substantial computational expertise to handle larger data set in a much shorter time. Those software usually contain functions for image segmentation, object classification, morphological characterization, fluorescence intensity quantification, cell counting and tracking for hundreds of images. Good image quality (homogeneous) is a prerequisite for a trustable result, so optimizing the image acquisition process is crucial. At different stages of the data processing and different parts of the data, validation of the accuracy of the trained algorithm is recommended.
Applications
Organoids as engineered cell-based models to recapitulate relevant physiological structures and functions of interest have shown great potential in both basic research and clinical applications.
Tissue regeneration
Tissue-derived organoids can be a potential source of transplantable material for regenerative medicine. Organoids of murine intestines31, livers35,125,126 and pancreas69,146 were successfully transplanted into mice, and the transplanted organoids could restore organ dysfunction. For instance, liver organoids have been generated from hepatocytes isolated from murine livers126. The organoids were injected into mice with a deficiency in fumarylacetoacetate hydrolase (Fah), a defect causing liver injury resulting in short survival (40 days) after treatment with the hepato-protective nitisinone drug is withdrawn35. Organoid engraftment rates were as high as 80%, and the engrafted mice survived more than 100 days after suspension of nitisinone therapy126. Similarly, pancreatic islets cultured in an artificial ECM could be successfully engrafted into either streptozocin-induced or autoimmune-driven diabetic mouse models, rescuing insulin production and reversing hyperglycemia147. Beyond murine organoids, Huch and colleagues successfully engrafted human liver organoids derived from EpCAM+ ductal cells from liver samples into immunodeficient mice with acute liver damage induced35. Human pancreas organoids can be generated from ALDHhigh stem cells and have the potential to produce insulin146 without in vivo evidence yet to confirm engraftment. Sachs and colleagues showed that intestinal organoids could fuse into tubular structures resembling in vivo intestines148,149. A more complicated intestinal organoid can also be generated in an artificial ECM with modulable biodegradability3. While there is a long way for organoids to be used in regenerative medicine, improved protocols to generate normal tissue organoids, good manufacturing practices assuring high reproducibility, grafting, and function can be established150.
Drug discovery and development studies
Technical challenges to rapid and high-throughput screening of 3D organoids have been addressed in recent years and surpassed by different means, including alternative plate design or seeding geometries16,151,152. Phan and colleagues screened 240 targeted agents on organoids derived from patients with ovarian or peritoneal tumors, with results available within a week from surgery. The study included a carcinosarcoma of the ovary, a sporadic ovarian cancer for which no -line therapy was established151. Tumor organoids can be screened to select effective drugs from structurally similar candidates153 and investigate the synergic effects of different drugs for combination therapy154. Non-tumor cells from adjacent normal tissue in biopsies or surgically resected samples can be used to develop control healthy organoids to confirm the tumor-specific efficacy of the tested drugs155. Organoids can be screened with libraries of immune cells engineered to express chimeric antigen receptors (CAR) against different neo-antigens156. Checkpoint inhibitors and other immuno-oncology (IO) drugs can also be screened in tumor organoid models that include immune cells157.
Biomarker research
Since organoids can maintain the genomic profile of the parent tissue158, drug screening can be used to provide connections between genetic mutations and drug responses37,154,159. By screening gastric cancer organoids from seven patients against 37 drugs, Yan and colleagues showed that tumors with ARID1A mutations were sensitive to ATR inhibitors159, supporting a previous study160 and functionally identifying an intervention for a class of mutations that were considered undruggable.
Precision medicine applications
There is growing interest in tailoring cancer therapy to each patient. Precision medicine is often synonymous with genomics161. However, as shown in several clinical trials161–165, only a small proportion of patients (approx. 10–20%) have an actionable alteration that can be coupled to a specific intervention161. In addition, the SHIVA trial has shown how this strategies based on tumor sequencing do not outperform better than the conventional physician’s choice161,162. While the clinical efficacy of genomics precision medicine interventions can be debated, it is unquestionable that most cancer patients have no targetable alteration to guide therapy. Functional precision medicine (FPM) has been proposed as a more direct alternative161,166. FPM is based on testing drug responses on patient tumors to identify effective regimens and does not require any knowledge of a tumor molecular profile or characteristics a priori. Organoids are ideally suited for FPM due to their ease of establishment, similarity with the tissue of origin and tractability158,167 and have been deployed to identify therapeutic leads in a number of tumors168, including rare cancers that tend to be understudied and poorly characterized at the molecular level16,151,169,170. A recent paper by Guillen et al leveraged organoids established from patient-derived xenografts to identify eribulin as therapy for a recurrent triple negative breast cancer patient. Progression-free survival was 3.5 times longer on the eribulin regimen than the previous therapy the patient received171. This case study is indicative of the potential of organoids to impact clinical care.
Source material to establish xenografts
An application of organoids that is becoming more popular involves using them as starting seeding material to generate xenografts with high take rates. These involve using intact organoids15,59, or individual cells after digestion55,172. Matano and colleagues seeded human intestinal organoids transformed by CRISPR-Cas9 to carry mutations in APC, SMAD4, TP53, KRAS, and/or PIK3CA in subcapsular kidney space of immunocompromised mice. They found that benign organoids carrying mutations in 1–2 genes could not form tumors while more malignant ones with mutations in 4–5 genes were tumorigenic173.
Patient-derived organoids can also generate xenografts, confirm their tumorigenic capacity and recapitulate the histology of parental tumors15,55. In a study of colorectal cancer, benign tumor organoids showed no or minimal engraftment in mice. In contrast, colorectal cancer organoids derived from metastases were more invasive than those from primary tumors59. Hubert and colleagues directly compared the morphology of xenografts formed by glioblastoma multiforme cells cultured in vitro either as tumor spheres or organoids. They showed that while cells from tumorspheres displayed a solid growth pattern, organoids were more diffusive, similar to the tumor of origin172. Lee and colleagues showed how organoid lines derived from bladder tumors of lumenal origin transplanted in mice gave rise to tumors with the same lumenal phenotype127.
Infectious diseases
Organoid models are now widely used in research on host-pathogen interactions. For instance, organoids derived from tissues and organs such as intestine174,175, livers176, lungs175,177, oral mucosa178, stomach33,179, have been cocultured with pathogens such as bacteria33,179,180, viruses174,176–178,181 and parasites175. In human intestinal organoids, differentiated intestinal cells are more susceptible to human rotavirus (HRV) infection than undifferentiated ones. Organoids infected with HRV or rotavirus enterotoxin showed lumenal swelling, a characteristic of rotavirus-induced diarrhea181. Human liver organoid was used as a drug screening platform to test anti-HRV drugs and antibodies, identifying mycophenolic acid, IFN-γ and anti-rotavirus-VP7 antibodies capable of blocking rotavirus replication ex vivo176. Organoids can also be used in studies of oncogenic pathogens. Infection of murine gastric organoids by H. pylori showed increased cytosolic β-catenin levels induced by bacterially originated CagA, which led to increased proliferation in infected organoids179.
Since the start of the Covid-19 pandemic, several laboratories have investigated the effects of the SARS-Cov-2 virus on organoids. Salahudeen and colleagues generated SARS-Cov-2 infectable distal lung organoids with ACE2-expressing lumenal cells182. SARS-Cov-2 targets goblet cells rather than ciliate ones, in contrast to a previous study in a 2D air-liquid interface model183. Studies in intestinal organoids show that SARS-Cov-2 can also infect enterocytes183,184. Zang and colleagues used CRISPR-Cas9 and drug screenings to show that knock-outs of either TMPRSS2 or TMPRSS4, or treatment with Camostat can reduce viral infection184.
Biology of common and rare diseases
Organoids have demonstrated significant utility in modelling and investigating both common as well as rare diseases arising from various different organs. Cystic fibrosis (CF) is a genetic disorder caused by mutations in the gene CFTR, which expresses a transmembrane chloride and bicarbonate transporter. Over 2,000 mutations have been reported, yet small molecule therapeutics deployed clinically only target a subset of these185,186. Mutations in CFTR cause multi-organ dysfunctions affecting lungs, pancreas, liver and intestine187. CF organoid models of colon188, liver and bile ducts189, rectum190,191, the respiratory system177,192, and the small intestine188,193, have been derived from adult stem cells of relevant organs of either mouse models or patients with applications from investigating the biology of disease to mirroring patients’ drug responses190. Berkers and colleagues used forskolin, a CFTR activator, to induce organoid swelling due to chloride and fluid flux into the lumen191. They demonstrated how reduced swelling of patient-derived rectal organoids correlated with clinical response parameters of patients and could mimic individualized clinical responses to specific therapeutic regimens191. Schwank and colleagues have used CF organoids to investigate gene therapy interventions and were able to rescue the effect of CFTR mutations in intestinal organoids using CRISPR-Cas9193.
Inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease have also been studied with organoid models194. For instance, organoids derived from intestinal epithelial cells of pediatric IBD patients had IBD-specific DNA methylation signatures despite retaining similar morphologies195. Liver organoids were generated to model liver diseases like α-1-antitrypsin deficiency36, Alagille syndrome36 and primary sclerosing cholangitis196. Lung organoids have been established to investigate diseases such as goblet cell metaplasia197.
Reproducibility and data deposition
Heterogeneity and reproducibility
Organoids exhibit heterogeneity and variability between cells forming the organoid (intra-organoid heterogeneity), between organoids in the same dish and between individual patients (inter-organoid heterogeneity). It is valuable to recapitulate the individual-to-individual differences, especially in the context of human diseases. It is also exploited in cancer research to mimic cancer development in a patient-specific manner for personalized medicine127–129,132,155,159,167,198–200. Similarly, the intra-organoid variation reflects the complexity of cellular composition of the tissues. This is advantageous for modeling tissue development or regeneration, with cells forming an organoid encompassing different cellular states, from stem/progenitor to more differentiated cells36,125,201. While these levels of variation and heterogeneity reflect the complexity of biological systems, left uncontrolled, they can jeopardize the reproducibility and robustness of the system.
Significant differences in morphology and functionality or even organoid formation efficiency depend on the different cells-of-origin of the culture or different cells in the organoid as well as different medium composition120,202–204. Regarding the cells of origin, the batch and quality of the iPSC/PSC used to generate the starting culture can influence the variability of the organoid line obtained. Similarly, the different primary cells isolated from different animals or patients also impact the subsequent cultures13. Also, the media is usually optimized for cell proliferation whereas differentiation toward certain cell fates can be largely influenced by the culture conditions, thus increasing variability and heterogeneity. In that regard, a nice example was described by Fujii M et al. who demonstrated how refining of culture conditions could increase the diversity of differentiated cell fates in human small intestinal organoids120. Similarly, undefined ECMs (e.g. Matrigel) suffer from their unknown composition and batch to batch variability, making reproducibility a significant concern13,205. Another important factor becomes apparent when looking into transcriptomic changes during organoid development206 or studying gene expression changes during organoid passages35,202. When organoids mimic development, these changes can lead to confounding effects – the temporal heterogeneity. Therefore, recording the passage and the time in culture is essential to identify sources of variation that affect the results.
To better understand and control heterogeneity, the field has been moving from bulk analysis towards employing single cell approaches, such as scRNAseq, to help delineate the different populations of organoid-forming cells. Many single-cell-resolved datasets are emerging, with several notable examples in more homogeneous organoid production3,70,100,207 and resolving organoid heterogeneity during growth progression204,208. Pooling together different cells and/or organoids for bulk analysis can lead to inaccurate results3,70,100,204,207,208. Therefore, good record-keeping about what was pooled in experiment deems crucial to drawing robust conclusions.
Additionally, it is critical to reduce the sources of variability and heterogeneity as the field grows in complexity. Our efforts to generate more complete organoid structures containing different cellular populations (reviewed in209,210), such as the recently published endothelial and healthy human colon and human colorectal cancer organoids211, murine cholangiocyte and mesenchymal organoids74, and murine pancreatic islet and endothelial organoids110, will require controlling sources of variation derived from incorporating additional cells to an already heterogeneous system. Exerting control over the variation while generating more functionally relevant structures represents a significant challenge for the field.
Finally, significant heterogeneity is also observed following organoid treatments. While this represents a drawback when trying to use organoids as a tool for investigating molecular mechanism, it is beneficial when aiming to model patient responses. In particular, for cancer treatment, several different patient-derived organoid models tested, including but not limited to: pancreas, colorectal and liver cancer all exhibit heterogeneous responses to known chemotherapeutic and anticancer agents49,132,212. This parallels the variability in patient outcomes observed in the clinic and have positioned cancer organoids as potential predictive tool for drug testing (Extensively reviewed in213). This heterogeneity in treatment responses is now being exploited for the treatment of cystic fibrosis patients191, with a large ongoing European multicentre clinical trial study, led by the HIT-Cystic Fibrosis consortium, to identify potential responders214. Similar initiatives are lagging behind for cancer patients, though mostly because of disparities in establishment rates and lengthy timelines (extensively reviewed in215). In that regard, while this manuscript was under revision, the group of Markus Heim reported the use of organoids to inform the treatment of a patient suffering from a rare form of liver cancer. Unfortunately, the treatment was discontinued due to deterioration of the patient’s general condition, preventing the authors to draw conclusions regarding the predictive value of the organoid system for the drug treatment of that patient216.
Minimum reporting standards
To improve the reproducibility and reliability of the results, it is essential to account for the variability between different cell isolations and intra-organoid heterogeneity. While one could consider that at least 3 independent experiments with at least 3 different biological replicates are a minimum requirement, they might not always be sufficient. In fact, the number of organoids to study per biological replicate as well as the number of biological replicates will very much depend on the question asked, the specific experiment and the variability of the phenotype observed. Whenever possible, to calculate the number of independent organoids per biological replicate, sample size calculations similar to the ones used for mouse experiments, that take into account statistical power and variance of the experiment, would be highly recommended. The data should be robust, meaning repeated in at least three independent biological replicates, which refers to independent isolations from different animals or patients. However, replicates and the question asked in each experiment should be determined in advance to avoid hypothesis fishing and increasing replicates to get a significance between conditions217,218. Data should be reported correctly as a mean of each replicate, with each replicates being an average of a similar number of organoids. Recently, several publications have provided guidelines to improve reproducibility, including general biological reporting and accurate statistics219–221. Especially recommended is the use of SuperPlots for data visualization, which show both the mean of each independent biological replicate and the spread of the individual data points222,223. This type of plot is ideal for organoid reporting, as it allows immediate visualization of any potential artifact concerning batch variation. Journal collections that deepen all aspects of statistics used in biological sciences are valuable resources to facilitate accurate reporting224.
Organoids should be characterized in full and not only checked for the expression of markers, but also benchmarking to the tissue of origin. Beside determining if the cells have similar morphology and expression patterns as the tissue-of-origin, showing a similar function (for eample, ELISA for Albumin, bile acid production by liver/hepatocyte organoids) is a must. Whenever possible, results should be normalized against DNA content or structural proteins such as actin, while functional data should be normalised to the total number of cells or area of structures. Improving reporting standards is the first step toward increasing reproducibility, thus enabling the field to move forward.
Data Deposition
Standardized organoid data repositories are currently missing. Only a patient-derived repository225 led by the National Cancer Institute (NCI in the USA) and the Human Cancer Models Initiative (HCMI), a combined international effort of several institutions (Cancer Research UK; NCI; Hubrecht Organoid Technology’s the HUB; and the US Office of Cancer Genomics’s OCG), have started to generate organoid data repositories, but only for cancer organoids (Supplementary Table 3). However, healthy patient-derived organoids and organoid models from non-cancer diseases, both human and murine, are yet to be documented in one database, evidencing the apparent need to fully document, report and deposit all organoid lines generated. The recent launch of the Human Cell Atlas-Organoid initiative, documenting all human-derived organoids226, is the first step in that direction.
On another note, when depositing data from human organoids, important aspects to consider are all ethical regulations and the necessity of obtaining patient consent and keeping patient anonymity. Critical points have been extensively reviewed in227,228.
A consensus in organoid nomenclature is also missing. Aside from a couple of notable examples, where leaders in the intestine, liver and pancreas addressed the nomenclature of gut, hepatic, pancreatic and biliary organoids229,230, the field is waiting for a more clearly-defined nomenclature system. Similarly, there is no repository where to submit organoid data. For transcriptomics-related data, such as gene expression, non-coding RNA, ChIP, genome methylation, high-throughput RT-PCR, SNP arrays, SAGE, protein arrays, Gene Expression Omnibus (GEO) is generally recommended231,232. For code used to analyze organoid datasets, GitHub is recommended (https://github.com). We envision that data and organoid depositories will arise shortly to fill in the presently missing gap in data repositories for organoid work.
Limitations and optimisations
The reproducibility, both morphological and functional, of the obtained 3D organoid systems, remains a major bottleneck. In this section, we will elaborate on the limitations and the recent developments in organoid research, providing a path towards a more optimal pipeline for developing, characterizing, and benchmarking organoid systems.
Limited level of maturity and function
None of the present organoid model systems reproduce the entire physiological repertoire of cell types, maturation level and/or functions of their respective organ; they rather exhibit certain functions of the tissue they predominantly form. The vast majority of tissue-derived organoid models are missing tissue-specific cell types, including niche-specific mesenchyme, immune cells, vascularization, innervation or microbiome. Recently, ductal cell-liver mesenchymal cell co-cultures have been shown to recapitulate part of the liver portal tract architecture74. Specifically challenging is that not all cell types have the same proliferation rate, growth factor requirements, or even requirements for oxygen exposure (hypoxia for vasculature). Pluripotent-stem cell derived organoids are better in recapitulating different cell types and cellular interactions of the developing organ but fail on exhibiting adult-tissue structures and functions, as well as cell maturation. One strategy that helps is in vivo transplantation233. However, this is at expenses of giving up control over the formed tissue constructs. Meanwhile, optimizations on differentiation protocols is to enrich maturation and specific functions of interest.
Another factor contributing to limited maturity and function is the nutrient (in)accessibility and accumulation of dead cells in hollow lumens. This is particularly important for iPSC-derived organoids. As organoids grow in size, the nutrient supply to cells localized in the center of the organoid gets restricted, resulting in cellular death. It is common with organoids that form a more compact structure, such as brain organoids. For tissue-derived organoids forming a hollow cyst (cholangiocyte, pancreas), dead cells will eventually start accumulating in their lumens which cannot be avoided, but can be resolved by mechanical fragmentation of organoids. Constant fragmentation of formed structures prevents carrying out the long-term studies. However, PSC-derived organoids cannot be fragmented and passaged; and new strategies to solve the nutrient accessibility problem are being developed, including the long-term maintenance of brain slices in vitro234.
Limited control of organoid heterogeneity
Once cells form an organoid, we have minimal input in cellular behavior within the organoid. Even in the same experimental settings, the result is often a plethora of phenotypic traits (shape, size, cell composition) rather than a stereotypic culture. Optimizing morphogenic gradients, tissue-specific cell-ECM interaction, and local biochemical and biophysical properties are essential for minimizing batch-to-batch heterogeneity235.
To generate more complex multicellular mature and functional structures, the organoid field has started to create assembloids as demonstrated for human cortico-motor assembloids236. Such effort allows the creation of more complex structures, connecting multiple types of tissues with defined interface such as connecting cerebral cotex, spine and skeletal muscle with neuro-muscular junctions, but at the cost of reproducibility. As recently discussed in another review focused on hepatic, biliary and pancreatic (HBP) organoids230, reproducibility in multi-cellular and multi-tissue organoid systems decreases as it is challenging to coordinate proliferation and differentiation of multiple cell types.
The limited control of intra-organoid heterogeneity is detrimental for high throughput screening applications and makes it difficult for studies requiring high spatiotemporal resolution imaging. Instead of creating more complex organoid systems, simpler models of reduced dimensions to recapitulate the essential tissue structures and functions of interest are gaining momentum. Variants of ECM combinations, micropatterned 2D mono-culture or co-culture237,238, cell sheets239, stacked 3D structures26, and micro-positioned ECM substrates70,240 allow the formation of reproducible tissue structures and functions with a high degree of spatiotemporal control such as stretching241 and osmotic forces242 (FIG. 5).
Fig. 5. Reducing the heterogeneity with complexity reduction.
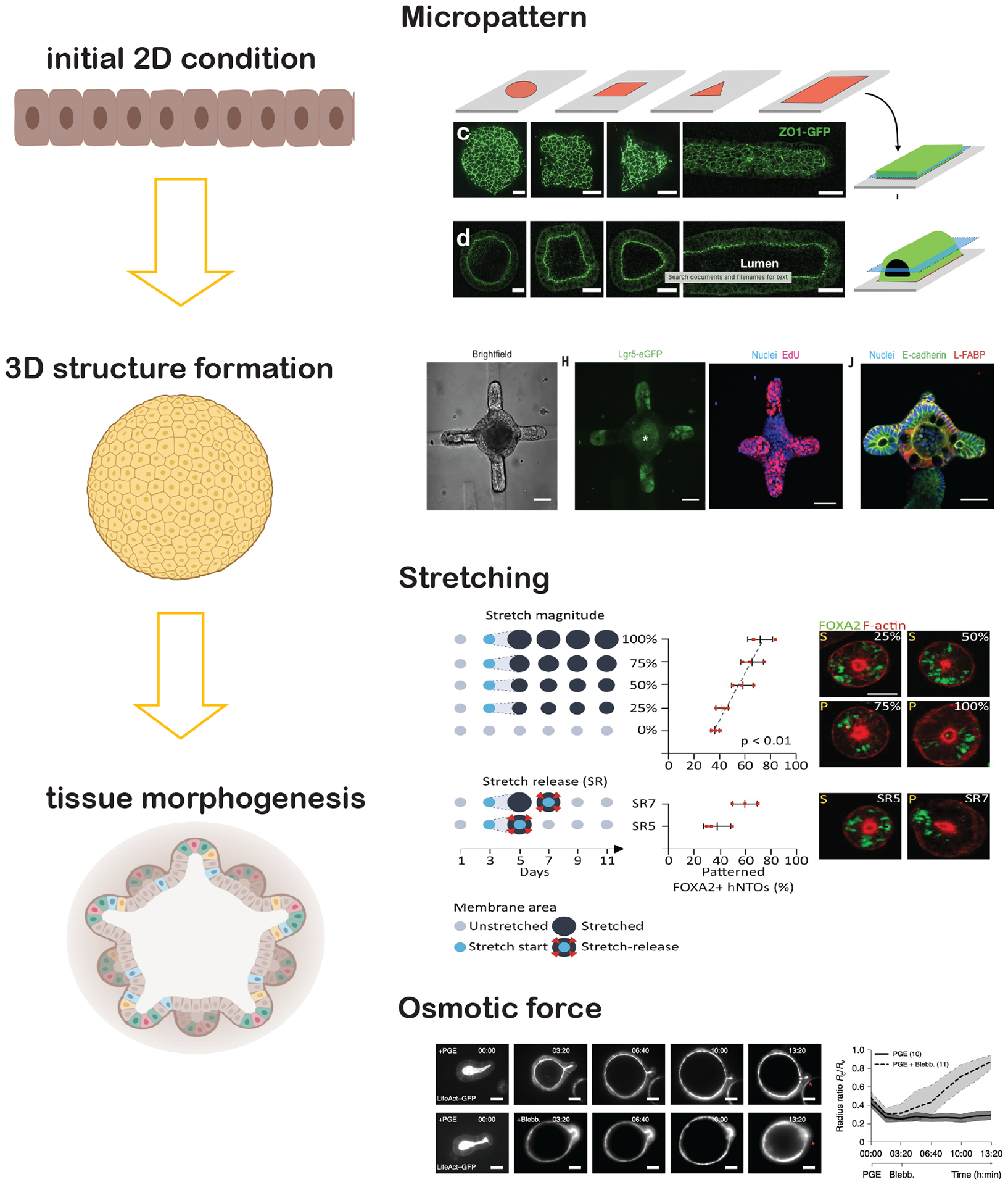
Simpler models of reduced dimensions to recapitulate the essential tissue structures and functions of interest are gaining momentum. Micropatterned 2D mono- or co-culture allow the formation of reproducible initial 2D condition which can further form the initial 3D structure26,70. Then a high degree of spatiotemporal control, such as stretching241 and osmotic forces242 can be applied to direct certain tissue morphogenesis.
Optimizing ECM composition
Engineering methods have been implemented to optimize these limitations (FIG. 6). There are two main paths to overcome the use of non-specific ECM such as Matrigel: one is the use of synthetic matrices with more complete control over composition and stiffness, and the other is to take decellularized tissue and create tissue-specific matrices205,243. There are significant efforts to identify chemically-defined, GMP-compatible ECMs that enable the growth and the long-term expansion of human organoids. In that regard, some advances were shown with human pancreas organoids, intestinal organoids and colorectal cancer organoids, which could grow in a dextran-based fully defined ECM; however, they would not expand long term3,69.
Fig. 6. Side-by-side comparison of the current limitations for organoid culture and approaches to overcome them.
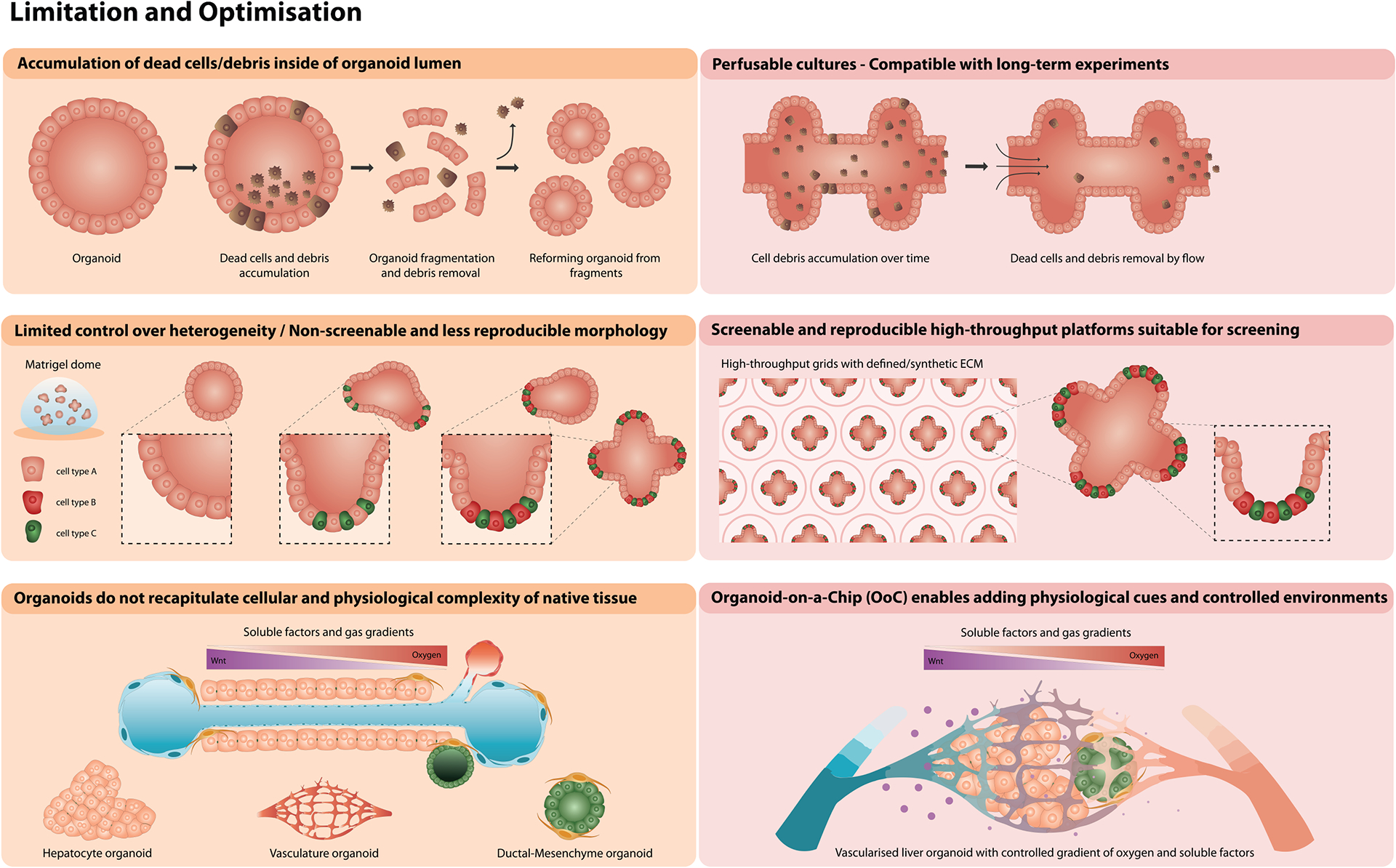
Top panel: Accummulation of dead cells and cell debris inside of cystic organoid lumina (left) has been overcome by (right) designing a perfusable open-end structures that use inducible flow to wash out cell debris, which are compatible with long-term experiments.
Middle panel: Organoids grown in Matrigel domes display high-variability of cell heterogeneity and morphology (left) which can be overcome by utilising (right) grids with patterned synthetic ECM which provide cues for cell differentiation. These platforms are additionally compatible with high-throughput screenings.
Bottom panel: Single-cell type derived organoids do not recapitulate the cellular and physiological complexity of native tissue (left), but (right) combining organoids with organ-on-chip (OoC) as novel technology would enable creating controlled micro-envirorment, suitable for multiple cell types
Organoids meet organs-on-a-chip
It has been shown that by maximizing the mass transfer and minimizing the shear stress in the perfusive soluble microenvironment, cells grown in organ-on-a-chip set-ups upregulate their functions, getting a step closer to a native tissue94,244,245. A more recent example shows how the presence of fluid flow enhances kidney organoids’ maturation and favors their vascularization in vitro107. Nikolaev et al. engineered physical constraints into the organoid environment and intestinal cells when provided with boundaries through engineered scaffolds self-organized in crypts of the same size. At the same time, they overcame the inaccessibility of cystic organoids and clearance of cell debris by creating a perfusable culture of mini-intestines where cells are arranged to form tube-shaped epithelia and similar spatial arrangement as the in vivo tissue100.
Outlook
Moving forward, the trend is to develop more complex models that recapitulate in vivo structure and function as faithfully as possible, in terms of the recapitulating cell types over time, tissue architecture, measurable molecular events and phenotypic functions. Rather than focusing exclusively on the most prominent markers or functional assays, architectural benchmarking of native tissue should also be performed. In the example of hepatocyte organoids125, hepatocyte functions are preserved, yet the liver tissue architecture does not match the native tissue where hepatocytes are arranged in cords. Similarly, organoids like pancreatic or colon cancer organoids grow isotropically, forming a cyst instead of the tubular structure they would form in their native tissue. To derive more complex functions, organoids with multi-cellular and multi-tissue structures will be important, especially in the context of studying cell-cell interactions246. Along this vein, assembloids and organs-on-chips are also becoming increasingly complex and more broadly adopted.
On the other hand, the engineer’s (cell as a machine) approach28,247 is to pursue simpler reductionist models defined by the minimal functional modules (MFMs) that drive a complex cellular or tissue FOI, to study the mechanobiological causation in development or repair, or to develop a robust system for high throughput screening. The basic premise is that a complex biological function is executed by coordinated operation of a limited number of functional modules, each described by a small set of molecules, and chemical reactions driving physical attribute changes of mesoscale (sub-cellular or inter-cellular tissue/multi-cellular) structures associated with FOI in the specific spatiotemporal stage/phase/step. For example bile canaliculi in liver exhibit hourly cycles of expansion and contraction. To study the causative contraction events with high resolution, only regions of adjacent hepatocytes forming the bile canaliculi are directly studied in the context of the entire regulatory machinery of adjacent hepatocytes27,248. One can choose to create a much larger structure involving cholangiocytes than the one operated by the MFMs but the model will be noisy and costly. Each functional module is coupled to another and can be modeled together or independently at different length scale. Simple reductionist models have been useful for high resolution mechanistic understanding of tissue morphogenetic events such as defects29,240,249.
Geometrically constraining the size of the initial 2D seeding pattern and 3D formation by micropatterning and supporting 3D cell growth using Matrigel, Karzburn et al., induced tissue-like neural tube morphogenesis and produced highly reproducible neural tubes. They identified the mechanisms of neural tube folding and modeled neural tube defects26. They focused on neural tube formation and characterizing a selected subset of relevant molecular events. In another example, symmetry breaking in a uniform sphere of cells and the emergence of a Paneth cell is a critical event in the early stage of intestinal organoid formation. The mechanism was unknown until Serra et al. showed that it is caused by transient activation of mechanotransducer YAP1, which induces NOTCH-DLL1 lateral inhibition events204. Gjorevski et al. translated this knowledge to control YAP1 activation by applying geometrical constraints in hydrogel scaffolds and producing a uniform and reproducible intestinal microtissues3.
Organoids can be constrained by reducing the 3rd dimension in a 2.5D culture. 2.5D is 3D culture with restricted 3rd dimension. Typical examples are culturing cells on curved or patterned surfaces, flattened or constrained cellular construct250, and overlaying ECM on a flat cell monolayer at high confluency which would pull the cells upward, forcing more cell-cell interactions to adopt 3D cell morphology. For example, hepatocytes in a collagen sandwich have sufficient contact area to attain polarity and form a bile canalicular lumen that contracts in the same periodic cycles as in vivo, although missing the 3D network, and lunes are wider and cholestatic compared to the native tissue. This cell-based model enables high-resolution dissection of the mechanism of bile canaliculi contraction into steps and an understanding of the molecular machinery regulating the phase transitions27,251. 2.5D culture reduces the depth-driven variabilities of a typical organoid: diffusional constraints in the hypoxic core, limited accessibility for drugs/transfection agents, and impeded imaging transparency250. We would also envision more engineered organoid models based on CRISPR-edited cells for disease modeling, even though these cells and models are synthetic.
Beside these technological advances on creating more physiologically relevant, robust and easier to use organoid models, we shall see greater impact in applications. In the last two decades, while there have been discussions to replace animal testing, these efforts have not panned out in the form of concrete actions. However, this is rapidly changing with regulatory hardstops now established on multiple fronts252. Organoids that can recapitulate the complex physiological functions in vivo has also boosted confidence that the new alternative methods are now viable options. There will be more extrapolation of animal research findings in human organoids to better understand human biology and pathophysiology. We envision widespread adoption of organoids as cell sources for cell therapy, regenerative medicine, in vitro diagnostics, and drug discovery.
Supplementary Material
Acknowledgement
This work was supported in parts by grants from CAS (XDA16020200 to Y.A.Z.), the National Key Research, Development Program of China (2020YFA0509002 to Y.A.Z.), NIH grants (R01CA240718, R01CA244729 and R01CA264248 to A.So) and funding from Institute of Bioengineering and Bioimaging, Biomedical Research Council, Agency for Science, Technology and Research (A*STAR), A*STAR, (Project Number IAF (H18/01/a0/017); SMART CAMP; The Institute for Digital Medicine (WisDM); and Mechanobiology Institute of Singapore (R-714-106-004-135) to H.Y.
Glossary terms:
- Stem cell niche
A specific tissue microenvironment where stem cells both reside and receive stimuli that regulate cell fate
- Extracellular matrix (ECM)
A large network composed of an array of (glycol)proteins and other macromolecules that provides structural and mechano-chemical support to cells and tissues.
- Induced pluripotent stem cell (iPSC)
Immature cells that are generated from an adult (mature) cell and that have regained the capacity to differentiate into any type of cell in the body
- Tissue-derived cells (TDC)
Adult or fetal cells derived from tissues, either stem/progenitor cells or differentiated cells
- Organoid
A self-organized three-dimensional tissue that is typically derived from stem cells (pluripotent, fetal, or adult), or even differentiated cells and which mimics the key functional, structural, and biological complexity of an organ
- Perfusion culture
A perfusion cell culture process involves the constant feeding of fresh media and removal of spent media and product
- Organ-on-a-chip (also known as micro-physiological systems) (OoC/MPS)
Systems containing engineered or natural miniature tissue constracts grown inside microfluidic chip
- Passage (also known as splitting)
It represents the subculture of organoids by either creating smaller organoid fragments or single cells using mechanical dissociation or enzymatic digestion
- Engraftment rate
Degree of organoid retention in a host tissue after transplantation
- Assembloids
Complex 3-dimensional structures combining several separately pre-generated cellular compartments/entities
- Functional module
A structured design part of a system with inputs, processing, and outputs.
- Functional precision medicine
A strategy whereby live tumor cells from individuals affected from a specific disease are directly perturbed with drugs to provide immediately translatable, personalized information to guide therapy
- Copy number variations
A phenomenon in which sections of the genome are repeated and the number of repeats in the genome varies between individuals
Footnotes
Declaration of competing interests:
MH is inventor in several patents on organoid technology. A.So and LL are inventors on a patent on organoid technology. A.So is a founder and owner of Icona BioDx. HY is inventor in several patents on cell-based models. The remaining authors declare no competing interests.
References
- 1.Zakrzewski W, Dobrzynski M, Szymonowicz M & Rybak Z Stem cells: past, present, and future. Stem Cell Res Ther 10, 68, doi: 10.1186/s13287-019-1165-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voog J & Jones DL Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115, doi: 10.1016/j.stem.2010.01.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gjorevski N et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564, doi: 10.1038/nature20168 (2016). [DOI] [PubMed] [Google Scholar]; This manuscript describes that there are different requirements for mechanical cues at different stages of intestinal organoid formation.
- 4.Yi SA, Zhang Y, Rathnam C, Pongkulapa T & Lee KB Bioengineering Approaches for the Advanced Organoid Research. Adv Mater 33, e2007949, doi: 10.1002/adma.202007949 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orkin R et al. A murine tumor producing a matrix of basement membrane. The Journal of experimental medicine 145, 204–220 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li ML et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A 84, 136–140, doi: 10.1073/pnas.84.1.136 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]; This demonstrated that Matrigel could support in vitro 3D culture and cell functions.
- 7.Sato T et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265, doi: 10.1038/nature07935 (2009). [DOI] [PubMed] [Google Scholar]; This showed the stem cells’ potential to recapitulate native tissue-like structures and functions.
- 8.Huch M & Koo B-K Modeling mouse and human development using organoid cultures. Development 142, 3113–3125, doi: 10.1242/dev.118570 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Lancaster MA & Knoblich JA Generation of cerebral organoids from human pluripotent stem cells. Nature Protocols 9, 2329–2340, doi: 10.1038/nprot.2014.158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simian M & Bissell MJ Organoids: A historical perspective of thinking in three dimensions. J Cell Biol 216, 31–40, doi: 10.1083/jcb.201610056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.in Definitions of Biomaterials for the Twenty-First Century (eds Williams David & Zhang Xingdong) 257–267 (Elsevier, 2019). [Google Scholar]
- 12.Reis RL 2nd Consensus conference on definitions on biomaterials science. J Tissue Eng Regen Med 14, 561–562, doi: 10.1002/term.3016 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Hofer M & Lutolf MP Engineering organoids. Nat Rev Mater, 1–19, doi: 10.1038/s41578-021-00279-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review describes current limitations of organoid culture and proposed engineering approaches to address these limitations.
- 14.Clevers H Modeling Development and Disease with Organoids. Cell 165, 1586–1597, doi: 10.1016/j.cell.2016.05.082 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Kim M et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun 10, 3991, doi: 10.1038/s41467-019-11867-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work established personalized lung cancer organoids and normal bronchial organoids from patient tissues.
- 16.Al Shihabi A et al. Personalized chordoma organoids for drug discovery studies. Sci Adv 8, eabl3674, doi: 10.1126/sciadv.abl3674 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first publication showing patient-derived tumor organoids can be established for rare, indolent cancers and maintain the original tissue’s morphologic and functional profiles.
- 17.Warmflash A, Sorre B, Etoc F, Siggia ED & Brivanlou AH A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods 11, 847–854, doi: 10.1038/nmeth.3016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Liu Z & Pang Y Concentration gradient generation methods based on microfluidic systems. RSC Advances 7, 29966–29984, doi: 10.1039/c7ra04494a (2017). [DOI] [Google Scholar]
- 19.Kumar A, Placone JK & Engler AJ Understanding the extracellular forces that determine cell fate and maintenance. Development 144, 4261–4270, doi: 10.1242/dev.158469 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White CR & Frangos JA The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci 362, 1459–1467, doi: 10.1098/rstb.2007.2128 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vatine GD et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 24, 995–1005 e1006, doi: 10.1016/j.stem.2019.05.011 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Teng Y, Zhao Z, Tasnim F, Huang X & Yu H A scalable and sensitive steatosis chip with long-term perfusion of in situ differentiated HepaRG organoids. Biomaterials 275, 120904, doi: 10.1016/j.biomaterials.2021.120904 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Qian X et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladoux B & Mege RM Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol 18, 743–757, doi: 10.1038/nrm.2017.98 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Metavarayuth K, Sitasuwan P, Zhao X, Lin Y & Wang Q Influence of Surface Topographical Cues on the Differentiation of Mesenchymal Stem Cells in Vitro. ACS Biomater Sci Eng 2, 142–151, doi: 10.1021/acsbiomaterials.5b00377 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Karzbrun E et al. Human neural tube morphogenesis in vitro by geometric constraints. Nature 599, 268–272, doi: 10.1038/s41586-021-04026-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; A chip-based culture system that enables self-organization of micropatterned stem cells into precise three-dimensional cell-fate patterns and organ shapes.
- 27.Gupta K et al. Bile canaliculi contract autonomously by releasing calcium into hepatocytes via mechanosensitive calcium channel. Biomaterials 259, 120283, doi: 10.1016/j.biomaterials.2020.120283 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Sheetz M & Yu H The Cell as a Machine. (Cambridge University Press, 2018). [Google Scholar]; This book explains the rationale of mechanobiology and the approaches to dissect complex biological functions.
- 29.Saw TB et al. Topological defects in epithelia govern cell death and extrusion. Nature 544, 212–216, doi: 10.1038/nature21718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato T et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Yui S et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med 18, 618–623, doi: 10.1038/nm.2695 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Barker N et al. Lgr5+ ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell stem cell 6, 25–36 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Bartfeld S et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136 e126, doi: 10.1053/j.gastro.2014.09.042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aihara E et al. Characterization of stem/progenitor cell cycle using murine circumvallate papilla taste bud organoid. Scientific reports 5, 1–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huch M et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250, doi: 10.1038/nature11826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the first liver organoid cultures from adult mouse liver tissue, where cells can be expanded long term to form liver organoids even from single cell.
- 36.Huch M et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312, doi: 10.1016/j.cell.2014.11.050 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broutier L et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 11, 1724–1743, doi: 10.1038/nprot.2016.097 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Takebe T et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484, doi: 10.1038/nature12271 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Greggio C et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140, 4452–4462, doi: 10.1242/dev.096628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first fetal tissue organoid culture system that recapitualtes pancreas progenitor development
- 40.Huch M et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32, 2708–2721, doi: 10.1038/emboj.2013.204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first pancreas organoids from adult tissue
- 41.Manzar GS, Kim EM & Zavazava N Demethylation of induced pluripotent stem cells from type 1 diabetic patients enhances differentiation into functional pancreatic β cells. The Journal of biological chemistry 292, 14066–14079, doi: 10.1074/jbc.M117.784280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drakhlis L et al. Human heart-forming organoids recapitulate early heart and foregut development. Nature Biotechnology, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koehler KR, Mikosz AM, Molosh AI, Patel D & Hashino E Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500, 217–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first inner ear organoids developed from iPSCs
- 44.Lee J et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beers J et al. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc 7, 2029–2040, doi: 10.1038/nprot.2012.130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu P, Zhang W, Xin H & Deng G Single Cell Isolation and Analysis. Front Cell Dev Biol 4, 116, doi: 10.3389/fcell.2016.00116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aronowitz JA, Lockhart RA & Hakakian CS Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 4, 713, doi: 10.1186/s40064-015-1509-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaipl US et al. Cooperation between C1q and DNase I in the clearance of necrotic cell-derived chromatin. Arthritis Rheum 50, 640–649, doi: 10.1002/art.20034 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Vlachogiannis G et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926, doi: 10.1126/science.aao2774 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopper O et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nature Medicine 25, 838–849, doi: 10.1038/s41591-019-0422-6 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Tiriac H et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointestinal Endoscopy 87, 1474–1480, doi: 10.1016/j.gie.2017.12.032 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao D et al. Organoid Cultures Derived from Patients with Advanced Prostate Cancer. Cell 159, 176–187, doi: 10.1016/j.cell.2014.08.016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seino T et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 22, 454–467.e456, doi: 10.1016/j.stem.2017.12.009 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Mazzocchi A et al. Pleural Effusion Aspirate for Use in 3D Lung Cancer Modeling and Chemotherapy Screening. ACS Biomaterials Science & Engineering 5, 1937–1943, doi: 10.1021/acsbiomaterials.8b01356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohmussaar K et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 28, 1380–1396 e1386, doi: 10.1016/j.stem.2021.03.012 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Sartini S & Soragni A Cervical organoids go viral. Cell Stem Cell 28, 1337–1338, doi: 10.1016/j.stem.2021.07.007 (2021). [DOI] [PubMed] [Google Scholar]
- 57.De Angelis ML et al. An organoid model of colorectal circulating tumor cells with stem cell features, hybrid EMT state and distinctive therapy response profile. Journal of Experimental & Clinical Cancer Research 41, 86, doi: 10.1186/s13046-022-02263-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergin CJ & Benoit YD Protocol for serial organoid formation assay using primary colorectal cancer tissues to evaluate cancer stem cell activity. STAR Protocols 3, 101218, doi: 10.1016/j.xpro.2022.101218 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujii M et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell 18, 827–838, doi: 10.1016/j.stem.2016.04.003 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Nguyen HTL & Soragni A Patient-Derived Tumor Organoid Rings for Histologic Characterization and High-Throughput Screening. STAR Protocols 1, 100056, doi: 10.1016/j.xpro.2020.100056 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aisenbrey EA & Murphy WL Synthetic alternatives to Matrigel. Nature Reviews Materials 5, 539–551 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng H, Poovaiah N, Forrester M, Cochran E & Wang Q Ex vivo culture of primary intestinal stem cells in collagen gels and foams. ACS Biomaterials Science & Engineering 1, 37–42 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Cruz-Acuña R et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nature cell biology 19, 1326–1335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kratochvil MJ et al. Engineered materials for organoid systems. Nature Reviews Materials 4, 606–622 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Broguiere N et al. Growth of epithelial organoids in a defined hydrogel. Advanced Materials 30, 1801621 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ & Shenoy VB Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chrisnandy A, Blondel D, Rezakhani S, Broguiere N & Lutolf MP Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nature Materials, 1–9 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Georgakopoulos N et al. Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev Biol 20, 4, doi: 10.1186/s12861-020-0209-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gjorevski N et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021, doi:doi: 10.1126/science.aaw9021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valamehr B et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proceedings of the National Academy of Sciences 105, 14459–14464, doi: 10.1073/pnas.0807235105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandenberg N et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nature biomedical engineering 4, 863–874 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Czerniecki SM et al. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell stem cell 22, 929–940. e924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cordero-Espinoza L et al. Dynamic cell contacts between periportal mesenchyme and ductal epithelium act as a rheostat for liver cell proliferation. Cell stem cell 28, 1907–1921. e1908 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang S et al. An automated organoid platform with inter-organoid homogeneity and inter-patient heterogeneity. Cell Reports Medicine 1, 100161 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bues J et al. Deterministic scRNA-seq captures variation in intestinal crypt and organoid composition. Nat Methods 19, 323–330, doi: 10.1038/s41592-021-01391-1 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Wang Y et al. In situ differentiation and generation of functional liver organoids from human iPSCs in a 3D perfusable chip system. Lab on a Chip 18, 3606–3616 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Goonoo N & Bhaw-Luximon A Mimicking growth factors: role of small molecule scaffold additives in promoting tissue regeneration and repair. RSC Advances 9, 18124–18146, doi: 10.1039/c9ra02765c (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siller R, Greenhough S, Naumovska E & Sullivan GJ Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Reports 4, 939–952, doi: 10.1016/j.stemcr.2015.04.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janda CY et al. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 545, 234–237, doi: 10.1038/nature22306 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miao Y et al. Next-Generation Surrogate Wnts Support Organoid Growth and Deconvolute Frizzled Pleiotropy In Vivo. Cell Stem Cell 27, 840–851 e846, doi: 10.1016/j.stem.2020.07.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ornitz DM FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22, 108–112, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 83.Takasato M et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568, doi: 10.1038/nature15695 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Mae SI et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4, 1367, doi: 10.1038/ncomms2378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Q et al. Non-genetic engineering of cells for drug delivery and cell-based therapy. Advanced drug delivery reviews 91, 125–140 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Peng H, Wang C, Xu X, Yu C & Wang Q An intestinal Trojan horse for gene delivery. Nanoscale 7, 4354–4360 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Davoudi Z et al. Intestinal organoids containing poly (lactic‐co‐glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. Journal of biomedical materials research Part A 106, 876–886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davoudi Z et al. Gut Organoid as a New Platform to Study Alginate and Chitosan Mediated PLGA Nanoparticles for Drug Delivery. Marine drugs 19, 282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gjorevski N, Ranga A & Lutolf MP Bioengineering approaches to guide stem cell-based organogenesis. Development 141, 1794–1804 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Dalby MJ, Gadegaard N & Oreffo RO Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nature materials 13, 558–569 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Crane GM, Jeffery E & Morrison SJ Adult haematopoietic stem cell niches. Nat Rev Immunol 17, 573–590, doi: 10.1038/nri.2017.53 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Demers CJ et al. Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development 143, 1884–1892, doi: 10.1242/dev.126847 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fluri DA et al. Derivation, expansion and differentiation of induced pluripotent stem cells in continuous suspension cultures. Nature methods 9, 509–516, doi: 10.1038/nmeth.1939 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huh D et al. Reconstituting organ-level lung functions on a chip. Science 328, 1662–1668, doi: 10.1126/science.1188302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perez-Gonzalez C et al. Mechanical compartmentalization of the intestinal organoid enables crypt folding and collective cell migration. Nat Cell Biol 23, 745–757, doi: 10.1038/s41556-021-00699-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Z-Z et al. Orchestrated biomechanical, structural, and biochemical stimuli for engineering anisotropic meniscus. Science Translational Medicine 11, eaao0750, doi: 10.1126/scitranslmed.aao0750 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Murphy SV & Atala A 3D bioprinting of tissues and organs. Nature biotechnology 32, 773–785 (2014). [DOI] [PubMed] [Google Scholar]
- 98.Brassard JA, Nikolaev M, Hübscher T, Hofer M & Lutolf MP Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nature Materials 20, 22–29 (2021). [DOI] [PubMed] [Google Scholar]
- 99.Park SE, Georgescu A & Huh D Organoids-on-a-chip. Science 364, 960–965 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nikolaev M et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578, doi: 10.1038/s41586-020-2724-8 (2020). [DOI] [PubMed] [Google Scholar]; This study shoed an intestinal organoid with a similar spatial arrangement of crypt- and villus-like domains to that in vivo.
- 101.Esch MB, Mahler GJ, Stokol T & Shuler ML Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab on a Chip 14, 3081–3092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bauer S et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Scientific reports 7, 1–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang YS et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proceedings of the National Academy of Sciences 114, E2293–E2302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao T et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab on a Chip 19, 948–958 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Workman MJ et al. Enhanced utilization of induced pluripotent stem cell–derived human intestinal organoids using microengineered chips. Cellular and molecular gastroenterology and hepatology 5, 669–677. e662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee KK et al. Human stomach-on-a-chip with luminal flow and peristaltic-like motility. Lab on a Chip 18, 3079–3085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Homan KA et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16, 255–262, doi: 10.1038/s41592-019-0325-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports an microfluidic model to culture kidney organoids in vitro and showed flow enhenced vascularization.
- 108.Grebenyuk S & Ranga A Engineering Organoid Vascularization. Front Bioeng Biotechnol 7, 39, doi: 10.3389/fbioe.2019.00039 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Below CR et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat Mater 21, 110–119, doi: 10.1038/s41563-021-01085-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang D et al. Long-Term Expansion of Pancreatic Islet Organoids from Resident Procr(+) Progenitors. Cell 180, 1198–1211 e1119, doi: 10.1016/j.cell.2020.02.048 (2020). [DOI] [PubMed] [Google Scholar]; This study indentified a new stem cell population with the potential to expand into organoids for long term culture and transplantation.
- 111.Wang J et al. Endothelial Wnts control mammary epithelial patterning via fibroblast signaling. Cell reports 34, 108897, doi: 10.1016/j.celrep.2021.108897 (2021). [DOI] [PubMed] [Google Scholar]
- 112.Tuveson D & Clevers H Cancer modeling meets human organoid technology. Science 364, 952–955, doi: 10.1126/science.aaw6985 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Friedlander MSH, Nguyen VM, Kim SK & Bevacqua RJ Pancreatic Pseudoislets: An Organoid Archetype for Metabolism Research. Diabetes 70, 1051–1060, doi: 10.2337/db20-1115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J et al. Isolation of mouse pancreatic islet Procr(+) progenitors and long-term expansion of islet organoids in vitro. Nat Protoc, doi: 10.1038/s41596-022-00683-w (2022). [DOI] [PubMed] [Google Scholar]
- 115.Ma X et al. Human expandable pancreatic progenitor-derived β cells ameliorate diabetes. Sci Adv 8, eabk1826, doi: 10.1126/sciadv.abk1826 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pagliuca FW et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439, doi: 10.1016/j.cell.2014.09.040 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yoshihara E et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586, 606–611, doi: 10.1038/s41586-020-2631-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Date S & Sato T Mini-gut organoids: reconstitution of the stem cell niche. Annual review of cell and developmental biology 31, 269–289, doi: 10.1146/annurev-cellbio-100814-125218 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Rahmani S, Breyner NM, Su HM, Verdu EF & Didar TF Intestinal organoids: A new paradigm for engineering intestinal epithelium in vitro. Biomaterials 194, 195–214, doi: 10.1016/j.biomaterials.2018.12.006 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Fujii M et al. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 23, 787–793 e786, doi: 10.1016/j.stem.2018.11.016 (2018). [DOI] [PubMed] [Google Scholar]
- 121.Qu M et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell research 31, 259–271, doi: 10.1038/s41422-020-00453-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakamura T Recent progress in organoid culture to model intestinal epithelial barrier functions. International immunology 31, 13–21, doi: 10.1093/intimm/dxy065 (2019). [DOI] [PubMed] [Google Scholar]
- 123.Jung KB et al. Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat Commun 9, 3039, doi: 10.1038/s41467-018-05450-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Son YS et al. Maturation of human intestinal organoids in vitro facilitates colonization by commensal lactobacilli by reinforcing the mucus layer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 34, 9899–9910, doi: 10.1096/fj.202000063R (2020). [DOI] [PubMed] [Google Scholar]
- 125.Hu H et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 175, 1591–1606 e1519, doi: 10.1016/j.cell.2018.11.013 (2018). [DOI] [PubMed] [Google Scholar]; This study showed the establishment of hepatocyte organoid cultures from mouse and human fetal tissue.
- 126.Peng WC et al. Inflammatory Cytokine TNFalpha Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell 175, 1607–1619 e1615, doi: 10.1016/j.cell.2018.11.012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed the establishment of hepatocyte organoid cultures for engraftment.
- 127.Lee SH et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 173, 515–528 e517, doi: 10.1016/j.cell.2018.03.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu L et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clinical & Translational Immunology 10, e1248, doi: 10.1002/cti2.1248 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li X et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nature Communications 9, 2983, doi: 10.1038/s41467-018-05190-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Honkala A, Malhotra SV, Kummar S & Junttila MR Harnessing the predictive power of preclinical models for oncology drug development. Nature Reviews Drug Discovery 21, 99–114, doi: 10.1038/s41573-021-00301-6 (2022). [DOI] [PubMed] [Google Scholar]
- 131.Gerlinger M et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. New England Journal of Medicine 366, 883–892, doi: 10.1056/NEJMoa1113205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Broutier L et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 23, 1424–1435, doi: 10.1038/nm.4438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed the first liver cancer organoid models from patient material.
- 133.Lo YH, Karlsson K & Kuo CJ Applications of Organoids for Cancer Biology and Precision Medicine. Nat Cancer 1, 761–773, doi: 10.1038/s43018-020-0102-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dijkstra KK et al. Challenges in Establishing Pure Lung Cancer Organoids Limit Their Utility for Personalized Medicine. Cell reports 31, 107588, doi: 10.1016/j.celrep.2020.107588 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Schindelin J et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682, doi: 10.1038/nmeth.2019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McQuin C et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol 16, e2005970, doi: 10.1371/journal.pbio.2005970 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lindeboom RG et al. Integrative multi-omics analysis of intestinal organoid differentiation. Mol Syst Biol 14, e8227, doi: 10.15252/msb.20188227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hernández-de-Diego R et al. PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data. Nucleic Acids Res 46, W503–w509, doi: 10.1093/nar/gky466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Raudvere U et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res 47, W191–w198, doi: 10.1093/nar/gkz369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Powers RK, Goodspeed A, Pielke-Lombardo H, Tan AC & Costello JC GSEA-InContext: identifying novel and common patterns in expression experiments. Bioinformatics 34, i555–i564, doi: 10.1093/bioinformatics/bty271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shannon P et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504, doi: 10.1101/gr.1239303 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reimand J et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc 14, 482–517, doi: 10.1038/s41596-018-0103-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Berg S et al. ilastik: interactive machine learning for (bio)image analysis. Nat Methods 16, 1226–1232, doi: 10.1038/s41592-019-0582-9 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Kok RNU et al. OrganoidTracker: Efficient cell tracking using machine learning and manual error correction. PLoS One 15, e0240802, doi: 10.1371/journal.pone.0240802 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mergenthaler P et al. Rapid 3D phenotypic analysis of neurons and organoids using data-driven cell segmentation-free machine learning. PLoS Comput Biol 17, e1008630, doi: 10.1371/journal.pcbi.1008630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Loomans CJM et al. Expansion of Adult Human Pancreatic Tissue Yields Organoids Harboring Progenitor Cells with Endocrine Differentiation Potential. Stem Cell Reports 10, 712–724, doi: 10.1016/j.stemcr.2018.02.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Elizondo DM et al. Pancreatic islets seeded in a novel bioscaffold forms an organoid to rescue insulin production and reverse hyperglycemia in models of type 1 diabetes. Scientific Reports 10, 4362, doi: 10.1038/s41598-020-60947-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meran L et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nature Medicine 26, 1593–1601, doi: 10.1038/s41591-020-1024-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sugimoto S et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 592, 99–104, doi: 10.1038/s41586-021-03247-2 (2021). [DOI] [PubMed] [Google Scholar]
- 150.Vives J & Batlle-Morera L The challenge of developing human 3D organoids into medicines. Stem Cell Research & Therapy 11, 72, doi: 10.1186/s13287-020-1586-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Phan N et al. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Communications Biology 2, 78, doi: 10.1038/s42003-019-0305-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tebon PJ et al. High-speed live cell interferometry for screening bioprinted organoids. bioRxiv, doi: 10.1101/2021.10.03.462896 (2021). [DOI] [Google Scholar]
- 153.Chen J et al. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Therapy 28, 112–125, doi: 10.1038/s41417-020-0190-y (2021). [DOI] [PubMed] [Google Scholar]
- 154.Verissimo CS et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife 5, doi: 10.7554/eLife.18489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nuciforo S et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell reports 24, 1363–1376, doi: 10.1016/j.celrep.2018.07.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schnalzger TE et al. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J 38, 100928, doi: 10.15252/embj.2018100928 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neal JT et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 175, 1972–1988 e1916, doi: 10.1016/j.cell.2018.11.021 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weeber F et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A 112, 13308–13311, doi: 10.1073/pnas.1516689112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yan HHN et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell 23, 882–897 e811, doi: 10.1016/j.stem.2018.09.016 (2018). [DOI] [PubMed] [Google Scholar]
- 160.Williamson CT et al. ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun 7, 13837, doi: 10.1038/ncomms13837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Letai A Functional precision cancer medicine—moving beyond pure genomics. Nature Medicine 23, 1028–1035, doi: 10.1038/nm.4389 (2017). [DOI] [PubMed] [Google Scholar]; This review disccused about the current stage of precision cancer and suggest the future applications.
- 162.Le Tourneau C et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. The Lancet Oncology 16, 1324–1334, doi: 10.1016/S1470-2045(15)00188-6 (2015). [DOI] [PubMed] [Google Scholar]
- 163.Meric-Bernstam F et al. Feasibility of Large-Scale Genomic Testing to Facilitate Enrollment Onto Genomically Matched Clinical Trials. Journal of Clinical Oncology 33, 2753–2762, doi: 10.1200/JCO.2014.60.4165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sholl LM et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 1, doi: 10.1172/jci.insight.87062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schwaederle M et al. On the Road to Precision Cancer Medicine: Analysis of Genomic Biomarker Actionability in 439 Patients. Molecular Cancer Therapeutics 14, 1488–1494, doi: 10.1158/1535-7163.MCT-14-1061 (2015). [DOI] [PubMed] [Google Scholar]
- 166.Friedman AA, Letai A, Fisher DE & Flaherty KT Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer 15, 747–756, doi: 10.1038/nrc4015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van de Wetering M et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945, doi: 10.1016/j.cell.2015.03.053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu R, Zhou X, Wang S & Trinkle C Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacology & Therapeutics 218, 107668, doi: 10.1016/j.pharmthera.2020.107668 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kondo T Current status and perspectives of patient-derived rare cancer models. Human Cell 33, 919–929, doi: 10.1007/s13577-020-00391-1 (2020). [DOI] [PubMed] [Google Scholar]
- 170.Kawasaki K et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell 183, 1420–1435.e1421, doi: 10.1016/j.cell.2020.10.023 (2020). [DOI] [PubMed] [Google Scholar]
- 171.Guillen KP et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nature Cancer 3, 232–250, doi: 10.1038/s43018-022-00337-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hubert CG et al. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res 76, 2465–2477, doi: 10.1158/0008-5472.CAN-15-2402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Matano M et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 21, 256–262, doi: 10.1038/nm.3802 (2015). [DOI] [PubMed] [Google Scholar]
- 174.Ettayebi K et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393, doi: 10.1126/science.aaf5211 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Heo I et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat Microbiol 3, 814–823, doi: 10.1038/s41564-018-0177-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen S et al. Rotavirus Infection and Cytopathogenesis in Human Biliary Organoids Potentially Recapitulate Biliary Atresia Development. mBio 11, doi: 10.1128/mBio.01968-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sachs N et al. Long-term expanding human airway organoids for disease modeling. EMBO J 38, 100300, doi: 10.15252/embj.2018100300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Driehuis E et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov 9, 852–871, doi: 10.1158/2159-8290.CD-18-1522 (2019). [DOI] [PubMed] [Google Scholar]
- 179.Wroblewski LE et al. Helicobacter pylori targets cancer-associated apical-junctional constituents in gastroids and gastric epithelial cells. Gut 64, 720–730, doi: 10.1136/gutjnl-2014-307650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Co JY et al. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell reports 26, 2509–2520 e2504, doi: 10.1016/j.celrep.2019.01.108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saxena K et al. Human Intestinal Enteroids: a New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology. J Virol 90, 43–56, doi: 10.1128/JVI.01930-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salahudeen AA et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 588, 670–675, doi: 10.1038/s41586-020-3014-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lamers MM et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54, doi: 10.1126/science.abc1669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zang R et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 5, 3582, doi: 10.1126/sciimmunol.abc3582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Van Goor F et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 108, 18843–18848, doi: 10.1073/pnas.1105787108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Van Goor F et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 106, 18825–18830, doi: 10.1073/pnas.0904709106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ratjen F et al. Cystic fibrosis. Nat Rev Dis Primers 1, 15010, doi: 10.1038/nrdp.2015.10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dekkers JF et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19, 939–945, doi: 10.1038/nm.3201 (2013). [DOI] [PubMed] [Google Scholar]
- 189.Verstegen MMA et al. Human extrahepatic and intrahepatic cholangiocyte organoids show region-specific differentiation potential and model cystic fibrosis-related bile duct disease. Sci Rep 10, 21900, doi: 10.1038/s41598-020-79082-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dekkers J et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Science Translational Medicine 8, 344ra384–344ra384, doi: 10.1126/scitranslmed.aad8278 (2016). [DOI] [PubMed] [Google Scholar]
- 191.Berkers G et al. Rectal Organoids Enable Personalized Treatment of Cystic Fibrosis. Cell reports 26, 1701–1708 e1703, doi: 10.1016/j.celrep.2019.01.068 (2019). [DOI] [PubMed] [Google Scholar]
- 192.Brewington JJ et al. Detection of CFTR function and modulation in primary human nasal cell spheroids. J Cyst Fibros 17, 26–33, doi: 10.1016/j.jcf.2017.06.010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schwank G et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658, doi: 10.1016/j.stem.2013.11.002 (2013). [DOI] [PubMed] [Google Scholar]
- 194.Boye T, Steenholdt C, Jensen K & Nielsen O Molecular Manipulations and Intestinal Stem Cell-Derived Organoids in Inflammatory Bowel Disease. Stem Cells 40, doi: 10.1093/stmcls/sxac014 (2022). [DOI] [PubMed] [Google Scholar]
- 195.Howell K et al. DNA Methylation and Transcription Patterns in Intestinal Epithelial Cells From Pediatric Patients With Inflammatory Bowel Diseases Differentiate Disease Subtypes and Associate With Outcome. Gastroenterology 154, doi: 10.1053/j.gastro.2017.10.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Soroka C et al. Bile‐Derived Organoids From Patients With Primary Sclerosing Cholangitis Recapitulate Their Inflammatory Immune Profile. Hepatology 70, doi: 10.1002/hep.30470 (2018). [DOI] [PubMed] [Google Scholar]
- 197.Danahay H et al. Notch2 Is Required for Inflammatory Cytokine-Driven Goblet Cell Metaplasia in the Lung. Cell reports 10, 239–252, doi: 10.1016/j.celrep.2014.12.017 (2015). [DOI] [PubMed] [Google Scholar]
- 198.Seidlitz T et al. Mouse Models of Human Gastric Cancer Subtypes With Stomach-Specific CreERT2-Mediated Pathway Alterations. Gastroenterology 157, 1599–1614 e1592, doi: 10.1053/j.gastro.2019.09.026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sachs N et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 172, 373–386.e310, doi: 10.1016/j.cell.2017.11.010 (2018). [DOI] [PubMed] [Google Scholar]
- 200.Boj SF et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338, doi: 10.1016/j.cell.2014.12.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rimland CA et al. Regional Differences in Human Biliary Tissues and Corresponding In Vitro-Derived Organoids. Hepatology 73, 247–267, doi: 10.1002/hep.31252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sampaziotis F et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med 23, 954–963, doi: 10.1038/nm.4360 (2017). [DOI] [PubMed] [Google Scholar]
- 203.Sampaziotis F et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 371, 839–846, doi: 10.1126/science.aaz6964 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Serra D et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 569, 66–72, doi: 10.1038/s41586-019-1146-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kozlowski MT, Crook CJ & Ku HT Towards organoid culture without Matrigel. Commun Biol 4, 1387, doi: 10.1038/s42003-021-02910-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aloia L et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat Cell Biol 21, 1321–1333, doi: 10.1038/s41556-019-0402-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang Y et al. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 128, 44–55, doi: 10.1016/j.biomaterials.2017.03.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lukonin I et al. Phenotypic landscape of intestinal organoid regeneration. Nature 586, 275–280, doi: 10.1038/s41586-020-2776-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kretzschmar K & Clevers H Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev Cell 38, 590–600, doi: 10.1016/j.devcel.2016.08.014 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Kim J, Koo BK & Knoblich JA Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21, 571–584, doi: 10.1038/s41580-020-0259-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Palikuqi B et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 585, 426–432, doi: 10.1038/s41586-020-2712-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tiriac H et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic CancerPancreatic Cancer Organoids Parallel Patient Response. Cancer discovery 8, 1112–1129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wensink GE et al. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis Oncol 5, 30, doi: 10.1038/s41698-021-00168-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.van Mourik P et al. Rationale and design of the HIT-CF organoid study: stratifying cystic fibrosis patients based on intestinal organoid response to different CFTR-modulators. Translational Medicine Communications 5, doi: 10.1186/s41231-020-00060-3 (2020). [DOI] [Google Scholar]
- 215.Foo MA et al. Clinical translation of patient-derived tumour organoids- bottlenecks and strategies. Biomark Res 10, 10, doi: 10.1186/s40364-022-00356-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Meier MA et al. Patient-derived tumor organoids for personalized medicine in a patient with rare hepatocellular carcinoma with neuroendocrine differentiation: a case report. Commun Med (Lond) 2, 80, doi: 10.1038/s43856-022-00150-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Blainey P, Krzywinski M & Altman N Points of significance: replication. Nat Methods 11, 879–880, doi: 10.1038/nmeth.3091 (2014). [DOI] [PubMed] [Google Scholar]
- 218.Pollard DA, Pollard TD & Pollard KS Empowering statistical methods for cellular and molecular biologists. Mol Biol Cell 30, 1359–1368, doi: 10.1091/mbc.E15-02-0076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Krzywinski M & Altman N Significance, P values and t-tests. Nat Methods 10, 1041–1042, doi: 10.1038/nmeth.2698 (2013). [DOI] [PubMed] [Google Scholar]
- 220.Krzywinski M & Altman N Points of significance: error bars. Nat Methods 10, 921–922, doi: 10.1038/nmeth.2659 (2013). [DOI] [PubMed] [Google Scholar]
- 221.Nuzzo R Scientific method: statistical errors. Nature 506, 150–152, doi: 10.1038/506150a (2014). [DOI] [PubMed] [Google Scholar]
- 222.Weissgerber TL, Milic NM, Winham SJ & Garovic VD Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol 13, e1002128, doi: 10.1371/journal.pbio.1002128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lord SJ, Velle KB, Mullins RD & Fritz-Laylin LK SuperPlots: Communicating reproducibility and variability in cell biology. J Cell Biol 219, doi: 10.1083/jcb.202001064 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nature. Statistics for Biologists, <https://www.nature.com/collections/qghhqm/ > (2017).
- 225.Institute, N. C. NCI Patient-Derived Models Repository (PDMR), <https://pdmr.cancer.gov/ > (
- 226.Atlas, H. C. HCA Organoid, <https://hca-organoid.eu/> (2020).
- 227.Bredenoord AL, Clevers H & Knoblich JA Human tissues in a dish: The research and ethical implications of organoid technology. Science 355, eaaf9414, doi: 10.1126/science.aaf9414 (2017). [DOI] [PubMed] [Google Scholar]
- 228.Fuhr A, Kurtz A, Hiepen C & Müller S Organoids as Miniature Twins—Challenges for Comparability and Need for Data Standardization and Access. Organoids 1, 28–36, doi: 10.3390/organoids1010003 (2022). [DOI] [Google Scholar]
- 229.Stelzner M et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol 302, G1359–1363, doi: 10.1152/ajpgi.00493.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Marsee A et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832, doi: 10.1016/j.stem.2021.04.005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Barrett T et al. NCBI GEO: archive for functional genomics data sets--10 years on. Nucleic Acids Res 39, D1005–1010, doi: 10.1093/nar/gkq1184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Edgar R, Domrachev M & Lash AE Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30, 207–210, doi: 10.1093/nar/30.1.207 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Takebe T & Wells JM Organoids by design. Science 364, 956–959, doi: 10.1126/science.aaw7567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xiang Z et al. Long-term maintenance of mature hippocampal slices in vitro. J Neurosci Methods 98, 145–154, doi: 10.1016/s0165-0270(00)00197-7 (2000). [DOI] [PubMed] [Google Scholar]
- 235.Takebe T, Zhang B & Radisic M Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell 21, 297–300, doi: 10.1016/j.stem.2017.08.016 (2017). [DOI] [PubMed] [Google Scholar]
- 236.Andersen J et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 183, 1913–1929 e1926, doi: 10.1016/j.cell.2020.11.017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Toh YC, Xing J & Yu H Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials 50, 87–97, doi: 10.1016/j.biomaterials.2015.01.019 (2015). [DOI] [PubMed] [Google Scholar]
- 238.Toh YC, Blagovic K, Yu H & Voldman J Spatially organized in vitro models instruct asymmetric stem cell differentiation. Integr Biol (Camb) 3, 1179–1187, doi: 10.1039/c1ib00113b (2011). [DOI] [PubMed] [Google Scholar]
- 239.Efremov AK et al. Application of piconewton forces to individual filopodia reveals mechanosensory role of L-type Ca2+ channels. Biomaterials 284, 121477, doi: 10.1016/j.biomaterials.2022.121477 (2022). [DOI] [PubMed] [Google Scholar]
- 240.Li Q et al. Extracellular matrix scaffolding guides lumen elongation by inducing anisotropic intercellular mechanical tension. Nat Cell Biol 18, 311–318, doi: 10.1038/ncb3310 (2016). [DOI] [PubMed] [Google Scholar]
- 241.Abdel Fattah AR et al. Actuation enhances patterning in human neural tube organoids. Nat Commun 12, 3192, doi: 10.1038/s41467-021-22952-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yang Q et al. Cell fate coordinates mechano-osmotic forces in intestinal crypt formation. Nat Cell Biol 23, 733–744, doi: 10.1038/s41556-021-00700-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rezakhani S, Gjorevski N & Lutolf MP Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials 276, 121020, doi: 10.1016/j.biomaterials.2021.121020 (2021). [DOI] [PubMed] [Google Scholar]
- 244.Zhang C, Zhao Z, Abdul Rahim NA, van Noort D & Yu H Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 9, 3185–3192, doi: 10.1039/b915147h (2009). [DOI] [PubMed] [Google Scholar]
- 245.Toh YC et al. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip 7, 302–309, doi: 10.1039/b614872g (2007). [DOI] [PubMed] [Google Scholar]
- 246.Koike H et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature 574, 112–116, doi: 10.1038/s41586-019-1598-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Thomas JD, Lee T & Suh NP A function-based framework for understanding biological systems. Annu Rev Biophys Biomol Struct 33, 75–93, doi: 10.1146/annurev.biophys.33.110502.132654 (2004). [DOI] [PubMed] [Google Scholar]
- 248.Ghallab A et al. Bile Microinfarcts in Cholestasis Are Initiated by Rupture of the Apical Hepatocyte Membrane and Cause Shunting of Bile to Sinusoidal Blood. Hepatology (Baltimore, Md.) 69, 666–683, doi: 10.1002/hep.30213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang Y et al. Biomimetic niches reveal the minimal cues to trigger apical lumen formation in single hepatocytes. Nature Materials 19, 1026–1035, doi: 10.1038/s41563-020-0662-3 (2020). [DOI] [PubMed] [Google Scholar]
- 250.Baptista D, Teixeira L, van Blitterswijk C, Giselbrecht S & Truckenmuller R Overlooked? Underestimated? Effects of Substrate Curvature on Cell Behavior. Trends Biotechnol 37, 838–854, doi: 10.1016/j.tibtech.2019.01.006 (2019). [DOI] [PubMed] [Google Scholar]
- 251.Gupta K et al. Actomyosin contractility drives bile regurgitation as an early response during obstructive cholestasis. J Hepatol 66, 1231–1240, doi: 10.1016/j.jhep.2017.01.026 (2017). [DOI] [PubMed] [Google Scholar]
- 252.Grimm D U.S. EPA to eliminate all mammal testing by 2035, <https://www.science.org/content/article/us-epa-eliminate-all-mammal-testing-2035> (2019).
- 253.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells (1978). [PubMed] [Google Scholar]; This study introduced the concept of stem cell niche.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


