Abstract
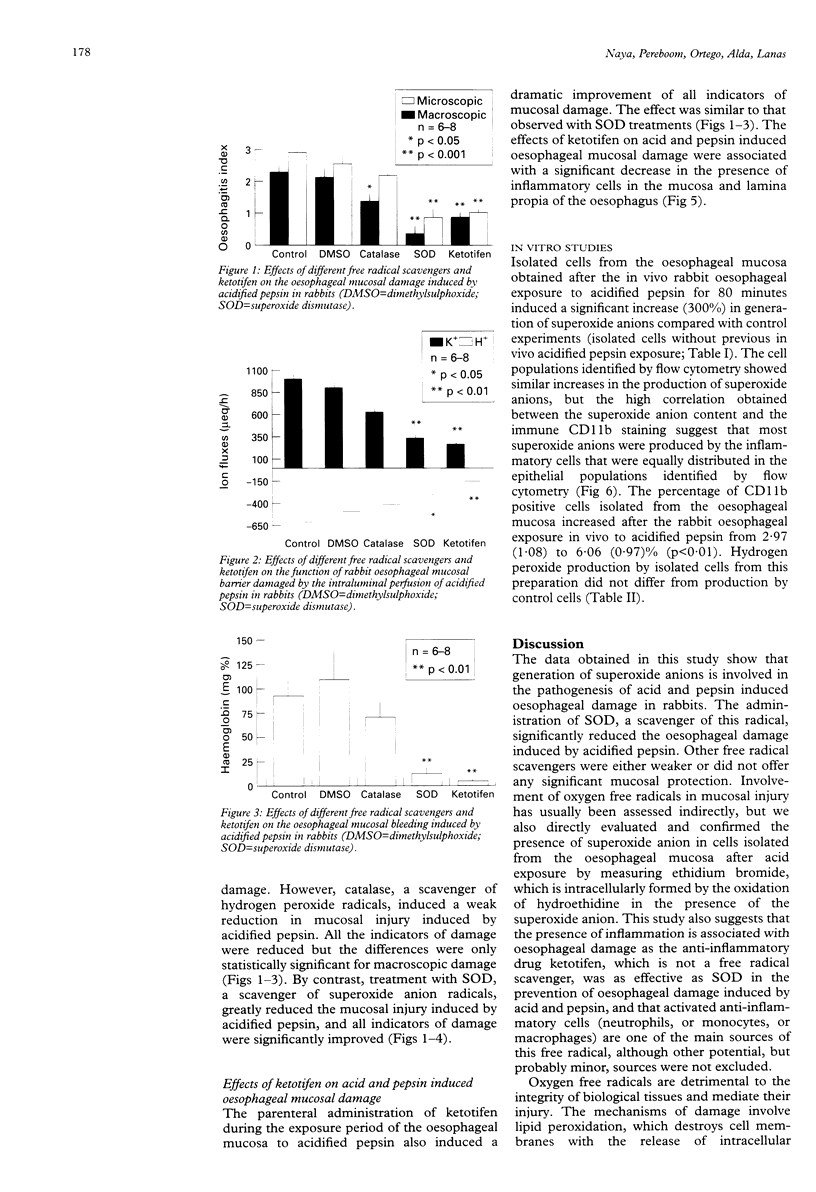
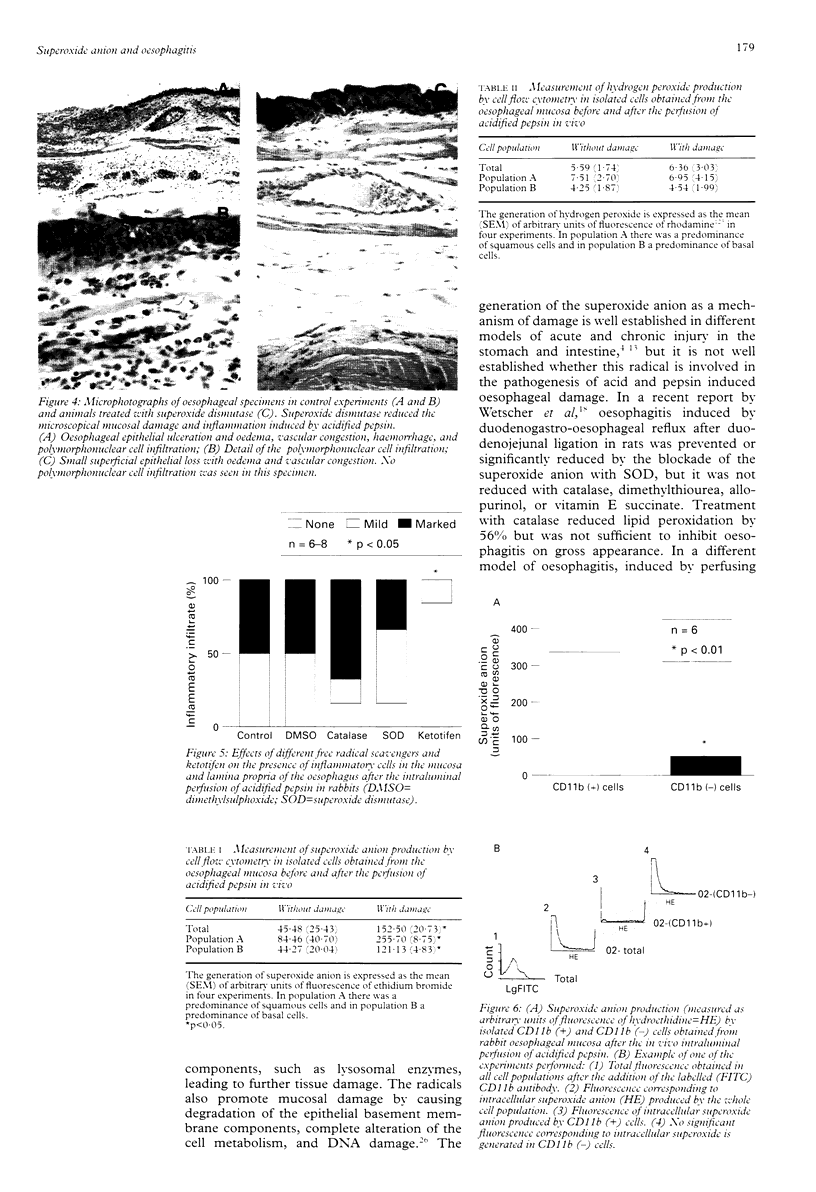
BACKGROUND: Reactive oxygen metabolites have been associated with gastrointestinal injury. OBJECTIVE: To investigate whether mucosal reactive oxygen metabolites are involved in acid and pepsin induced oesophagitis, and if so, which specific metabolites. METHODS: The effects of free radical scavengers and the anti-inflammatory drug ketotifen on rabbit oesophagitis induced by acidified pepsin were studied. Isolated oesophageal cells were obtained before and after oesophageal injury and the generation of superoxide anion and hydrogen peroxide was analysed by flow cytometry. The presence of inflammatory cells was determined by indirect immunofluorescence with a mouse antirabbit CD11b antibody. RESULTS: Of the free radical scavengers tested, superoxide dismutase, which reacts with the superoxide anion, significantly reduced oesophagitis, whereas catalase, which reacts with hydrogen peroxide, had only a mild effect and dimethylsulphoxide had no effect. Ketotifen significantly reduced the inflammation and also prevented the induction of oesophagitis. Isolated cells obtained from the oesophageal mucosa after acidified pepsin exposure generated increased amounts of superoxide anions, which were mainly produced by CD11b positive cells. CONCLUSIONS: Reactive oxygen metabolites, especially superoxide anion, produced by inflammatory cells play a significant part in the genesis of oesophagitis induced by acid and pepsin in rabbits and might be a target for future medical therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay R. L., Dinda P. K., Morris G. P., Paterson W. G. Morphological evidence of mast cell degranulation in an animal model of acid-induced esophageal mucosal injury. Dig Dis Sci. 1995 Aug;40(8):1651–1658. doi: 10.1007/BF02212685. [DOI] [PubMed] [Google Scholar]
- Binnaka T., Yamaguchi T., Hirohara J., Hiramatsu A., Mizuno T., Sameshima Y. Gastric mucosal damage induced in rats by intravenous administration of platelet-activating factor. Scand J Gastroenterol Suppl. 1989;162:67–70. doi: 10.3109/00365528909091127. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Chorev M., Okon E., Rachmilewitz D. Effect of drugs on colonic eicosanoid accumulation in active ulcerative colitis. Scand J Gastroenterol. 1992 Nov;27(11):968–972. doi: 10.3109/00365529209000172. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Okon E., Rachmilewitz D. Ketotifen effectively prevents mucosal damage in experimental colitis. Gut. 1992 Nov;33(11):1498–1503. doi: 10.1136/gut.33.11.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Rachmilewitz D. Ketotifen--old drug, new indication: reduction of gastric mucosal injury. Scand J Gastroenterol. 1993 Mar;28(3):202–204. doi: 10.3109/00365529309096072. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Hojo M., Hamasaki Y., Fujita I., Koga H., Matsumoto S., Miyazaki S. Effects of anti-allergy drugs on fMet-Leu-Phe-stimulated superoxide generation in human neutrophils. Ann Allergy. 1994 Jul;73(1):21–26. [PubMed] [Google Scholar]
- Hossain M., Okubo Y., Sekiguchi M. Effects of various drugs (staurosporine, herbimycin A, ketotifen, theophylline, FK506 and cyclosporin A) on eosinophil viability. Arerugi. 1994 Jun;43(6):711–717. [PubMed] [Google Scholar]
- Khalbuss W. E., Marousis C. G., Subramanyam M., Orlando R. C. Effect of HCl on transmembrane potentials and intracellular pH in rabbit esophageal epithelium. Gastroenterology. 1995 Mar;108(3):662–672. doi: 10.1016/0016-5085(95)90437-9. [DOI] [PubMed] [Google Scholar]
- Kubes P., Kanwar S., Niu X. F., Gaboury J. P. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993 Oct;7(13):1293–1299. doi: 10.1096/fasebj.7.13.8405815. [DOI] [PubMed] [Google Scholar]
- Lanas A. I., Nerín J., Esteva F., Sáinz R. Non-steroidal anti-inflammatory drugs and prostaglandin effects on pepsinogen secretion by dispersed human peptic cells. Gut. 1995 May;36(5):657–663. doi: 10.1136/gut.36.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanas A. I., Sousa F. L., Ortego J., Esteva F., Blas J. M., Soria J., Sáinz R. Aspirin renders the oesophageal mucosa more permeable to acid and pepsin. Eur J Gastroenterol Hepatol. 1995 Nov;7(11):1065–1072. doi: 10.1097/00042737-199511000-00009. [DOI] [PubMed] [Google Scholar]
- Layden T. J., Agnone L. M., Schmidt L. N., Hakim B., Goldstein J. L. Rabbit esophageal cells possess an Na+,H+ antiport. Gastroenterology. 1990 Oct;99(4):909–917. doi: 10.1016/0016-5085(90)90606-2. [DOI] [PubMed] [Google Scholar]
- Lillemoe K. D., Johnson L. F., Harmon J. W. Alkaline esophagitis: a comparison of the ability of components of gastroduodenal contents to injure the rabbit esophagus. Gastroenterology. 1983 Sep;85(3):621–628. [PubMed] [Google Scholar]
- Matsumoto T., Moriguchi R., Yamada H. Role of polymorphonuclear leucocytes and oxygen-derived free radicals in the formation of gastric lesions induced by HCl/ethanol, and a possible mechanism of protection by anti-ulcer polysaccharide. J Pharm Pharmacol. 1993 Jun;45(6):535–539. doi: 10.1111/j.2042-7158.1993.tb05594.x. [DOI] [PubMed] [Google Scholar]
- NORRIS H. T., ZAMCHECK N., GOTTLIEB L. S. The presence and distribution of mast cells in the human gastrointestinal tract at autopsy. Gastroenterology. 1963 Apr;44:448–455. [PubMed] [Google Scholar]
- Nakamura K., Aoike A., Rokutan K., Hosokawa T., Koyama K., Kawai K. The role of oxygen radicals in the pathogenesis of gastric mucosal lesions induced in mice by feeding-restriction stress. Scand J Gastroenterol Suppl. 1989;162:47–50. doi: 10.3109/00365528909091122. [DOI] [PubMed] [Google Scholar]
- Olyaee M., Sontag S., Salman W., Schnell T., Mobarhan S., Eiznhamer D., Keshavarzian A. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut. 1995 Aug;37(2):168–173. doi: 10.1136/gut.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phull P. S., Green C. J., Jacyna M. R. A radical view of the stomach: the role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995 Mar;7(3):265–274. [PubMed] [Google Scholar]
- Pothoulakis C., Karmeli F., Kelly C. P., Eliakim R., Joshi M. A., O'Keane C. J., Castagliuolo I., LaMont J. T., Rachmilewitz D. Ketotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileum. Gastroenterology. 1993 Sep;105(3):701–707. doi: 10.1016/0016-5085(93)90886-h. [DOI] [PubMed] [Google Scholar]
- Rothe G., Oser A., Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988 Jul;75(7):354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- Rothe G., Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J Leukoc Biol. 1990 May;47(5):440–448. [PubMed] [Google Scholar]
- Sasaki H., Higashiyama H., Kumada K., Morimoto T., Takeuchi E., Ozawa K. Evaluating microscopic arterial reconstruction: a comparison of laser-assisted versus conventional vascular anastomosis in 70% hepatectomized rat model. Eur Surg Res. 1994;26(1):35–45. doi: 10.1159/000129316. [DOI] [PubMed] [Google Scholar]
- Schweitzer E. J., Bass B. L., Johnson L. F., Harmon J. W. Sucralfate prevents experimental peptic esophagitis in rabbits. Gastroenterology. 1985 Mar;88(3):611–619. doi: 10.1016/0016-5085(85)90128-3. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Takada H., Hai K., Kiriyama H., Mawet E., Komatsu Y., Niwa Y., Matsumura M., Shiina S., Kawase T. Effect of anti-allergic agents on chemotaxis of neutrophils by stimulation of chemotactic factor released from hepatocytes exposed to ethanol. Dig Dis Sci. 1994 Jul;39(7):1569–1575. doi: 10.1007/BF02088066. [DOI] [PubMed] [Google Scholar]
- Stein B. E., Schwartzman M. L., Carroll M. A., Stahl R. E., Rosenthal W. S. Role of arachidonic acid metabolites in acid-pepsin injury to rabbit esophagus. Gastroenterology. 1989 Aug;97(2):278–283. doi: 10.1016/0016-5085(89)90062-0. [DOI] [PubMed] [Google Scholar]
- Stein H. J., Esplugues J., Whittle B. J., Bauerfeind P., Hinder R. A., Blum A. L. Direct cytotoxic effect of oxygen radicals on the gastric mucosa. Surgery. 1989 Aug;106(2):318–324. [PubMed] [Google Scholar]
- Tarnasky P. R., Livingston E. H., Jacobs K. M., Zimmerman B. J., Guth P. H., Garrick T. R. Role of oxyradicals in cold water immersion restraint-induced gastric mucosal injury in the rat. Dig Dis Sci. 1990 Feb;35(2):173–177. doi: 10.1007/BF01536759. [DOI] [PubMed] [Google Scholar]
- Tay H. P., Chaparala R. C., Harmon J. W., Huesken J., Saini N., Hakki F. Z., Schweitzer E. J. Bismuth subsalicylate reduces peptic injury of the oesophagus in rabbits. Gut. 1990 Jan;31(1):11–16. doi: 10.1136/gut.31.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano A., Hiraishi H., Ota S., Shiga J., Sugimoto T. Role of superoxide and hydroxyl radicals in rat gastric mucosal injury induced by ethanol. Gastroenterol Jpn. 1989 Oct;24(5):488–493. doi: 10.1007/BF02773874. [DOI] [PubMed] [Google Scholar]
- Trevethick M. A., Clayton N. M., Strong P., Harman I. W. Do infiltrating neutrophils contribute to the pathogenesis of indomethacin induced ulceration of the rat gastric antrum? Gut. 1993 Feb;34(2):156–160. doi: 10.1136/gut.34.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Arfors K. E., McKnight G. W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Wetscher G. J., Hinder P. R., Bagchi D., Perdikis G., Redmond E. J., Glaser K., Adrian T. E., Hinder R. A. Free radical scavengers prevent reflux esophagitis in rats. Dig Dis Sci. 1995 Jun;40(6):1292–1296. doi: 10.1007/BF02065541. [DOI] [PubMed] [Google Scholar]
- Yasue N., Guth P. H. Role of exogenous acid and retransfusion in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1988 May;94(5 Pt 1):1135–1143. doi: 10.1016/0016-5085(88)90004-2. [DOI] [PubMed] [Google Scholar]



