Abstract
We previously demonstrated that increased expression of the SERPINA5 gene is associated with hippocampal vulnerability in Alzheimer’s disease (AD) brains. SERPINA5 was further demonstrated to be a novel tau-binding partner that colocalizes within neurofibrillary tangles. Our goal was to determine whether genetic variants in the SERPINA5 gene contributed to clinicopathologic phenotypes in AD. To screen for SERPINA5 variants, we sequenced 103 autopsy-confirmed young-onset AD cases with a positive family history of cognitive decline. To further assess the frequency of a rare missense variant, SERPINA5 p.E228Q, we screened an additional 1114 neuropathologically diagnosed AD cases. To provide neuropathologic context in AD, we immunohistochemically evaluated SERPINA5 and tau in a SERPINA5 p.E228Q variant carrier and a matched noncarrier. In the initial SERPINA5 screen, we observed 1 individual with a rare missense variant (rs140138746) that resulted in an amino acid change (p.E228Q). In our AD validation cohort, we identified an additional 5 carriers of this variant, resulting in an allelic frequency of 0.0021. There was no significant difference between SERPINA5 p.E228Q carriers and noncarriers in terms of demographic or clinicopathologic characteristics. Although not significant, on average SERPINA5 p.E228Q carriers were 5 years younger at age of disease onset than noncarriers (median: 66 [60–73] vs 71 [63–77] years, P = .351). In addition, SERPINA5 p.E228Q carriers exhibited a longer disease duration than noncarriers that approached significance (median: 12 [10–15]) vs 9 [6–12] years, P = .079). More severe neuronal loss was observed in the locus coeruleus, hippocampus, and amygdala of the SERPINA5 p.E228Q carrier compared to noncarrier, although no significant difference in SERPINA5-immunopositive lesions was observed. Throughout the AD brain in either carrier or noncarrier, areas with early pretangle pathology or burnt-out ghost tangle accumulation did not reveal SERPINA5-immunopositive neurons. Mature tangles and newly formed ghost tangles appeared to correspond well with SERPINA5-immunopositive tangle-bearing neurons. SERPINA5 gene expression was previously associated with disease phenotype; however, our findings suggest that SERPINA5 genetic variants may not be a contributing factor to clinicopathologic differences in AD. SERPINA5-immunopositive neurons appear to undergo a pathologic process that corresponded with specific levels of tangle maturity.
Keywords: Alzheimer’s disease, SERPINA5, tau
1. Introduction
Alzheimer’s disease (AD) is neuropathologically defined by the aberrant accumulation of 2 proteins. These abnormal proteins include amyloid-β that deposits extracellularly to form amyloid-β plaques, and hyperphosphorylated tau that accumulates intracellularly to form neurofibrillary tangles.[1] As the hippocampus can be differentially affected in AD,[2] it is important to understand what mechanisms contribute to this selective vulnerability. Utilizing a unique translational neuropathology approach, our group observed that hippocampal gene expression changes are associated with neuropathologic changes in AD.[3] Using transcriptomic analysis and machine learning, we identified SERPINA5 as the top gene predictive of hippocampal vulnerability. SERPINA5 expression was increased in AD and its expression was positively associated with an advanced tangle maturity marker (i.e., Ab39 that recognizes mature tangles and ghost tangles [4]).[3] Immunofluorescence microscopy showed that SERPINA5 colocalizes with tau in tangles and, interestingly, co-immunoprecipitation experiments on postmortem brain tissue indicated that SERPINA5 interacts with tau in a disease-specific manner. Together, these findings suggest that SERPINA5 plays an important role in the development and progression of AD.
SERPINA5, also known as protein C inhibitor, is a predicted 47-kDa secreted serine protease inhibitor that plays an important role in blood coagulation and thrombosis.[5] It interacts with and inhibits many blood coagulation factors, including activated protein C, thrombin, factor XI, factor Xa, and plasma kallikrein.[5,6] In humans, SERPINA5 was shown to be expressed in many organs, predominantly in the liver[7] but also in the brain.[3] However, in rodents, SERPINA5 is expressed only in the reproductive organs.[8] Genetically, the SERPINA5 gene is positioned on chromosome 14q32.1[9] and consists of 6 exons: 2 untranslated regions and 4 coding regions. As SERPINA5 expression is increased in AD and associated with neuropathology, our objective was to determine if genetic variants in the SERPINA5 gene may be a contributing disease factor.
2. Methods
2.1. Study samples for the discovery cohort
The study design is cross-sectional in nature, as we specifically evaluated the frequency of SERPINA5 genetic variants in an autopsied series. The Florida autopsied multi-ethnic cohort[10] is a set of 2809 autopsied brains based at the Mayo Clinic in Jacksonville, Florida, and is derived from the State of Florida brain bank, a component of the Alzheimer’s Disease Initiative supported by the State of Florida Department of Elder Affairs (https://elderaffairs.org/programs-services/bureau-of-elder-rights/alzheimers-disease-initiative/). The Florida autopsied multi-ethnic cohort was queried for neuropathologically diagnosed AD cases with an age at onset younger than 65 (i.e., young-onset AD) and a positive family history of cognitive decline. Further inclusion/exclusion criteria can be found in Supplemental Digital Content, http://links.lww.com/MD/J134. We identified 103 young-onset AD cases with a positive family history of cognitive decline to perform initial screening. Demographically, the cohort consisted of 44/103 (43%) females and 59/103 (57%) males. Age at onset ranged from 43 to 65 years, and age at death ranged from 54 to 86 years. Age at onset is calculated as the number of years from birth until the individual first reported symptoms of cognitive impairment based upon retrospective evaluation of medical records. Disease duration is the time elapsed in years from symptom onset until death. All brains and DNA samples were acquired with appropriate ethical approval and informed consent from individuals and/or their legal guardians. The research performed on postmortem samples was approved by the Mayo Clinic Research Executive Committee, and all methods were carried out in accordance with relevant guidelines and regulations.
2.2. Genetic screening
To undertake genetic screening of the 103 cases, DNA was extracted from frozen brain tissues using standard protocols. To determine the presence of SERPINA5 genetic variants, we sequenced each case for all 6 exons of SERPINA5 (RefSeq: NM_000624.6). DNA fragments were PCR-amplified by Apex products and 7 primer sets (see Table S1, Supplemental Digital Content, http://links.lww.com/MD/J135) using a 60 to 50 Touchdown program. The subsequent PCR products were purified using the Agencourt AMPure system (Agencourt Bioscience Corporation, Beverly, MA). Sequencing was performed with the Big Dye Terminator v3.1 kit (Applied Biosystems, Foster City, CA) and purified with Agencourt CleanSEQ (Agencourt Bioscience Corporation). Sequences were run on an ABI3730 DNA-analyzer (Applied Biosystems) and subsequently analyzed with Sequencher software (Gene Codes, Ann Arbor, MI). A copy number assay for SERPINA5 (Hs02961512_cn) was also performed.
2.3. Rare variant frequency validation cohort
To determine the frequency of the SERPINA5 p.E228Q variant, we performed a TaqMan genotyping assay (C_162935990_10, rs140138746) on a cohort of 1114 autopsy-confirmed AD cases. Genotypes were generated and analyzed on Applied Biosystems QuantStudio 7 real-time PCR instruments and software (Applied Biosystems). Demographically, the cohort consisted of 617/1114 (55%) females and 497/1114 (45%) males. The age at death ranged from 48 to 102 years. Whole genome sequencing data from 838 individuals from the Mayo Clinic Biobank Study were utilized as control cohort, as previously described.[11] Briefly, 1000 ng DNA from each sample was subject to standard library preparation protocol using NEBNext DNA Library Prep Master Mix Set for Illumina (New England BioLabs Inc., Ipswich, MA). Each sample was then sequenced on 1 lane of Illumina HiSeq X instrument using v2 flow cells and reagents targeting 30 × genomic coverage. Data was then processed using the Mayo Genome GPS v4.0 pipeline, mapping to the GRCh38 build. Extensive quality control was performed as previously described. The combined annotation dependent depletion (CADD) score for the p.E228Q variant was obtained from https://cadd.gs.washington.edu (CADD model GRCh37-v1.6).[12]
2.4. Immunohistochemistry
Standardized neuropathologic procedures were performed by a single board-certified neuropathologist as previously defined[10] and further described in Supplemental Digital Content, http://links.lww.com/MD/J134. To complement genetic screen results, we selected a SERPINA5 p.E228Q variant carrier and noncarrier for visual comparison of histologic changes and tau accumulation. We matched for age at death (carrier = 75 years vs noncarrier = 76 years), sex (both males), disease duration (16 years vs 17 years), Braak tangle stage (both VI), and presence of TDP-43 co-pathology. Immunohistochemistry was performed on 9 brain regions that included the entorhinal cortex, limbic structures (amygdala and 2 hippocampal subsectors [CA1 and subiculum]), association cortices (superior temporal, inferior parietal, and middle frontal), and primary cortices (visual and motor). These corticolimbic brain regions and the pons (locus coeruleus) were stained with the following commercially available antibodies: SERPINA5 (R&D, MAB1266, 1:100); AT8 (Thermo, MN1020, 1:2500, which recognizes “earlier” levels of tangle maturity [pretangles and mature tangles]); and 2E9 (Novus, NBP2-25162, 1:100,000, which recognizes more “advanced” levels of tangle maturity [mature tangles and ghost tangles]). The stained slides were digitally scanned at 20x magnification on an Aperio AT2 scanner (Leica Biosystems, Buffalo Grove, IL). ImageScope x64 software v12.4.2.700 (Leica Biosystems, Buffalo Grove, IL) was used for photomicrograph capture. Routine hematoxylin and eosin stained slides were also digitized and used to demonstrate neuronal loss.
2.5. Statistical analysis
To compare the demographic and clinicopathologic characteristics of SERPINA5 p.E228Q variant carriers (n = 6) to noncarriers (n = 1211), Sigma Plot Version 13 (Systat Software, San Jose, CA) was used to perform statistical analyses. We performed nonparametric analyses using the Mann-Whitney rank sum test to make between-group comparisons for continuous (age at death, age at onset, disease duration) and ordinal variables (Braak tangle stage, Thal amyloid phase). P values < .05 were considered statistically significant, and all statistical tests were 2-sided. Data shown as median (25th, 75th percentile).
3. Results
3.1. Genetic analyses of SERPINA5
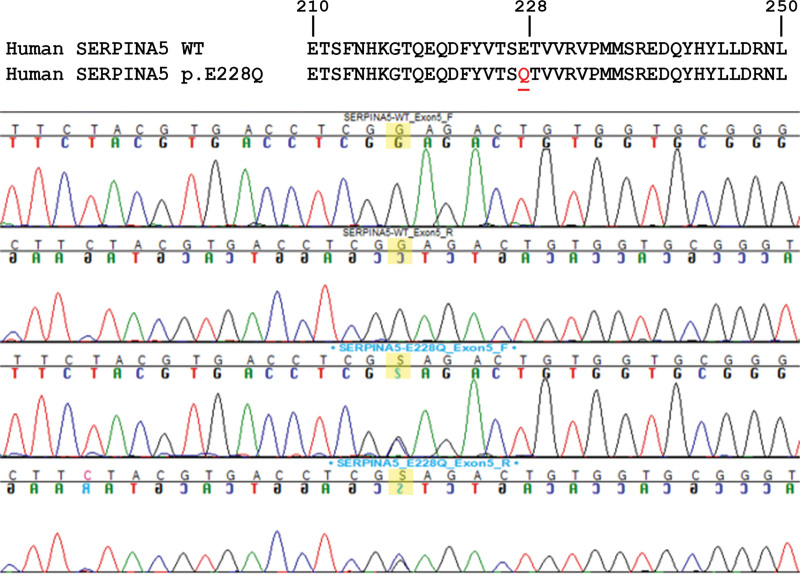
In our young-onset AD cohort with a positive family history of cognitive decline, we observed no copy number variants in the SERPINA5 gene. Common variants in SERPINA5 were observed at comparable frequencies to publically available online genomic variant database and Mayo Clinic Biobank controls (Table 1). In addition, we observed 1 individual with a rare missense variant in exon 5 of the SERPINA5 gene, resulting in a C > G transversion (Chr14: 95056440 G > C; p.Glu228Gln; dbSNP ID: rs140138746) (Fig. 1). The allelic frequency of this variant within our initial screening cohort was 0.0049. For the remainder of this paper, this rare missense variant will be referred to as SERPINA5 p.E228Q.
Table 1.
Common variants in SERPINA5 observed in our cohort of young-onset Alzheimer’s disease with positive family history of cognitive decline, their population allelic frequency in our cohort, our Mayo Clinic Biobank control cohort, and their population allelic frequency in gnom AD.
| dbSNP ID | Position | Allelic frequency in cohort | Allelic frequency in controls | Allelic frequency in gnom AD |
|---|---|---|---|---|
| SERPINA5 Exon 1 | ||||
| rs2144076 | Chr14:95047686 C > T | 0.2330 | 0.2136 | 0.2222 |
| rs8019792 | Chr14:95047810 A > G | 0.3252 | 0.3258 | 0.3065 |
| rs8020223 | Chr14:95047837 G > A | 0.3252 | 0.3258 | 0.3046 |
| rs8019810 | Chr14:95047847 A > C | 0.3252 | 0.3258 | 0.3045 |
| SERPINA5 Exon 3 | ||||
| rs6113 | Chr14:95053849 T > C | 0.0922 | 0.1026 | 0.1517 |
| rs6118 | Chr14:95053863 C > T | 0.0922 | 0.1026 | 0.1523 |
| rs6115 | Chr14:95053890 G > A | 0.6456 | 0.6587 | 0.6132 |
| rs6111 | Chr14:95053891 C > T | 0.1019 | 0.1026 | 0.1507 |
| rs6119 | Chr14:95054012 A > G | 0.0874 | 0.1020 | 0.1498 |
| rs6112 | Chr14:95054176 T > C | 0.6214 | 0.6587 | 0.6130 |
| SERPINA5 Exon 6 | ||||
| rs6109 | Chr14:95058360 A > G | 0.6796 | 0.7082 | 0.6512 |
| rs6116 | Chr14:95058462 A > C | 0.4660 | 0.4785 | 0.3916 |
| rs6108 | Chr14:95058631 A > T | 0.5971 | 0.6038 | 0.5323 |
Reference assembly: GRCh37. Genomic database used to obtain allelic frequency: gnom AD v2.1.1[13].
AD = Alzheimer’s disease.
Figure 1.
(Top) Protein sequence of the resulting amino acid change in SERPINA5 (p.E228Q). (Bottom) Chromatogram showing the forward and reverse sequence derived from a noncarrier compared to that from an individual with the rare missense variant observed in exon 5 of the SERPINA5 gene, a G > C transversion.
To further assess SERPINA5 p.E228Q variant frequency, we screened 1114 neuropathologically diagnosed AD cases. In this validation cohort, we further identified 5 individuals with the SERPINA5 p.E228Q variant, resulting in an allelic frequency of 0.0021 within the validation cohort that was similar to its population allelic frequency of 0.0048 in the Mayo Clinic Biobank controls and 0.0048 in gnomAD v2.1.1.[13] The CADD score for this variant was 10.89 and thus predicted to be pathogenic, scoring within the top 10% most deleterious substitutions.
3.2. Demographic and clinicopathologic characteristics of SERPINA5 p.E228Q carriers and noncarriers
Table 2 shows the demographic and clinicopathologic characteristics of the 6 SERPINA5 p.E228Q variant carriers and 1211 noncarriers from our combined discovery and validation cohorts. Although statistical significance was not reached in the analyses, possibly due to the small sample size, we observed some interesting trends. SERPINA5 p.E228Q carriers were on average 5 years younger at age of disease onset than noncarriers (median: 66 [60–73] vs 71 [63–77] years, P = .351). In addition, SERPINA5 p.E228Q carriers exhibited a longer disease duration than noncarriers (median: 12 [10–15]) vs 9 [6–12] years, P = .079). There were no dramatic differences in age at death (P = .682), Braak tangle stage (P = .978), or Thal amyloid phase (P = .266) between SERPINA5 p.E228Q carriers and noncarriers.
Table 2.
Comparison of demographic and clinicopathologic characteristics of noncarriers and carriers of the SERPINA5 p.E228Q variant from our combined discovery and validation cohorts.
| Characteristic | SERPINA5 p.E228Q | Overall P value | |
|---|---|---|---|
| Carriers (n = 6) | noncarriers (n = 1211) | ||
| Age at death, yr | 79 (74, 85) | 81 (75, 87) | .682 |
| Age at onset, yr | 66 (60, 73) | 71 (63, 77) | .351 |
| Disease duration, yr | 12 (10, 15) | 9 (6, 12) | .079 |
| Braak tangle stage | VI (IV, VI) | VI (V, VI) | .978 |
| Thal amyloid phase | 5 (5, 5) | 5 (5, 5) | .266 |
Data shown as median (25th, 75th percentile). Statistical analysis was performed using the Mann-Whitney Rank Sum Test.
AD = Alzheimer’s disease.
Further characteristics of the 6 SERPINA5 p.E228Q carriers are summarized in Table 3. Among the 6 variant carriers, 4 were males (67%) and 2 were females (23%). Family history of cognitive deficits was described in 2 (23%) carriers: father, brother, and sister in case #1 and mother in case #3. Lewy body pathology was observed in 5 carriers (83%), and significant cerebrovascular disease was found in 3 carriers (50%).
Table 3.
Complete characteristics of each individual SERPINA5 p.E228Q variant carrier in our series.
| ID | Neuropathologic diagnosis | Sex | Age at death (yr) | FHx of cognitive deficits | Brain weight (grams) | Lewy body pathology | TDP-43 pathology | Cerebrovascular disease |
|---|---|---|---|---|---|---|---|---|
| 1* | AD/VaD/ALB | M | 75 | Yes | 1020 | ALB | Yes | Yes |
| 2 | AD/VaD | F | 72 | No | 920 | None | No | Yes |
| 3 | AD/TLBD/HpScl | M | 82 | Yes | 1040 | TLBD | Yes | No |
| 4 | AD/ALB | F | 89 | No | 960 | ALB | No | Yes |
| 5 | AD/ALB | M | 86 | No | 1100 | ALB | Yes | No |
| 6 | AD/ALB/HpScl/VaD | M | 86 | No | 1300 | ALB | Yes | Yes |
AD = Alzheimer’s disease, ALB = amygdala predominant Lewy bodies, FHx = family history, HpScl = hippocampal sclerosis of a TDP-43 etiology, TLBD = transitional Lewy body disease.
Identified in discovery cohort.
3.3. Immunohistochemical description of the SERPINA5 p.E228Q variant
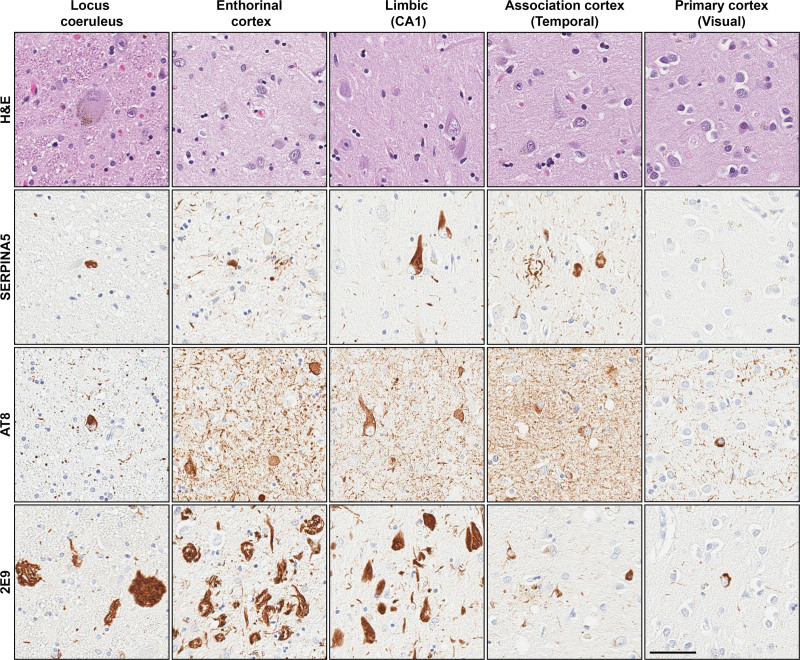
Immunohistochemical staining of SERPINA5 and 2 tau markers that recognize predominantly more pretangles/mature tangles (AT8) or mature tangles/ghost tangles (2E9) are shown in Figure 2 and Figure S1, Supplemental Digital Content, http://links.lww.com/MD/J136. The SERPINA5 p.E228Q carrier (Case #1 in Table 3) had SERPINA5-immunopositive lesions throughout the brain. Representative neuropathologic images of the carrier and noncarrier are shown in Figure 2 and Figure S1, Supplemental Digital Content, http://links.lww.com/MD/J136, respectively. According to the microscopic investigation of the routine Hematoxylin and eosin-stained tissue sections, both the carrier and noncarrier had severe neuronal loss and burnt-out ghost tangle pathology in the entorhinal cortex. SERPINA5 burden in the entorhinal cortex of the carrier was comprised of more neuropil thread pathology than was observed in the noncarrier. The amygdala and hippocampal subsectors (CA1 and subiculum) of the carrier showed severe neuronal loss compared to moderate neuronal loss observed in the noncarrier. Neuropil threads and SERPINA5-immunopositive neurons forming tangles were observed in pyramidal neurons across limbic structures in both cases. These SERPINA5-positive tangles were predominantly found in layer V with more SERPINA5-positive neuropil threads and neuritic plaques found in the upper layers of the superior temporal, inferior parietal, and middle frontal cortices. The primary visual and primary motor cortex showed no detectable neuronal loss and only a few SERPINA5-positive tangles. As the locus coeruleus is considered to be affected earliest by tau pathology in AD,[14] we additionally examined SERPINA5 in stained sections of the pons. Severe neuronal loss was observed in the locus coeruleus of the carrier compared to moderate neuronal loss in the noncarrier. Although extensive 2E9-positive ghost tangles were observed in the locus coeruleus in both cases, we only observed sparse SERPINA5-positive tangles.
Figure 2.
Photomicrographs of a 75-year-old male SERPINA5 p.E228Q carrier neuropathologically diagnosed with Alzheimer’s disease. Representative images of the locus coeruleus, entorhinal cortex, limbic structures (CA1 hippocampal subsector), association cortices (superior temporal), and primary cortices (visual) are shown. Case characteristics – disease duration = 6 years, Braak tangle stage = VI, Thal phase = 5, and TDP-43 positive. Immunohistochemistry–SERPINA5 and tau (early tangle marker: AT8, advanced tangle marker: 2E9). Scale bar–50μm.
4. Discussion
In this study of 103 young-onset AD cases with a positive family history of cognitive decline, we observed 13 common variants in the SERPINA5 gene at a similar frequency to that previously reported.[13] In addition, we observed 1 individual with a rare missense variant, SERPINA5 p.E228Q (rs140138746). To further investigate the frequency of this variant, we screened an additional 1114 neuropathologically diagnosed AD cases and observed 5 individuals carrying this variant (<1%). This revealed an allelic frequency in AD (0.0021) comparable to that observed in the general population (0.0026).
Although the discovery cohort of young-onset AD cases with a positive family history was used to identify SERPINA5 p.E228Q variant carriers, age was not used to select the validation cohort. Despite this, we continued to see a nonsignificant pattern of median age at onset of 66 years in carriers, which was 5 years younger than that in the noncarriers in our cohort. Previous studies have indicated that disease duration in AD dementia ranges between 3 to 10 years.[15] Interestingly, SERPINA5 p.E228Q carriers were found to have a longer than-average disease duration of 12 years, which approached significance as this was 3 years longer than that of noncarriers.
Glutamic acid (E) and glutamine (Q) amino acids are structurally quite similar except they differ in their side group by having either an -OH or an -NH2, respectively, connected to the carbonyl group of the side chain. This difference is highlighted in red in Figure S2, Supplemental Digital Content, http://links.lww.com/MD/J137. Based on their structural similarities, one may not expect there to be a noticeable steric change in the protein. This may partly explain why the CADD score predicted the variant to be pathogenic, but global neuropathology measures (i.e., Braak tangle stage[16] and Thal amyloid phase[17]) were not found to differ between SERPINA5 p.E228Q carriers and noncarriers. We observed a high frequency of amygdala predominant Lewy body pathology in 4/6 (67%) of the SERPINA5 p.E228Q carriers. Lewy body pathology exists concurrently with AD pathology, occurring most frequently in the olfactory bulb and amygdala as compared to the substantia nigra, that is more commonly seen in Parkinson disease.[18] Studies have shown that Lewy body pathology is detected in 60% of AD cases,[19] and amygdala predominant Lewy bodies are detected in 18%.[20] The trend toward longer disease duration may account for the greater frequency of amygdala predominant Lewy bodies, as it is more commonly observed in severe end-stage AD brains.
We additionally sought to immunohistochemically evaluate SERPINA5 and tau immunopositivity across 9 different brain regions in a SERPINA5 pE228Q carrier and matched noncarrier. Regions involved in Braak tangle staging[16] were specifically chosen to assess SERPINA5 immunopositivity in the context of spatiotemporal progression of tau. To examine tangle changes, we used 2 antibodies (AT8 and 2E9) to encompass the dynamic lifespan of tangle maturity.[4] Although unable to test the hypothesis in the current study, our recent data suggests SERPINA5 acts as a “tipping point” in the tangle maturation process that could contribute to associated neurodegeneration.[3] The “tipping point” phenomenon refers to an increased abundance of SERPINA5-immunopositive mature tangles in the AD brain as compared to SERPINA5-immunopositive pretangles, suggesting that SERPINA5 expedites the tangle maturation process.[7] In the current study, both the SERPINA5 pE228Q carrier and noncarrier had SERPINA5-immunopositive lesions throughout the brain that corresponded well with mature tangles. Interestingly, in areas of extensive ghost tangle accumulation, SERPINA5 lesions were uncommon in either case, suggesting a loss of SERPINA5 expression in dead neurons.
There are several limitations to this study. First, we did not sequence the promoter and regulatory regions of the SERPINA5 gene. It is well established that variants in these regions can affect gene expression and contribute to disease.[21] Therefore, regulatory region variants could be contributing to the increased expression of SERPINA5 that we observed in AD, although more thorough sequencing must be undertaken before such a conclusion can be made. In addition, it will be important to sequence more cases for SERPINA5 variants. Future directions include screening cases of other tauopathies, such as primary age-related tauopathy, corticobasal degeneration, and progressive supranuclear palsy, which will assist in determining whether SERPINA5 variants contribute to the development of tau pathology in general. Furthermore, neuropathologic analysis was performed on a single SERPINA5 p.E228Q variant carrier with an age-matched control. Therefore, any observations must be considered carefully. Together, these findings suggest that although SERPINA5 gene and protein expression may differ in AD compared to that in healthy controls, SERPINA5 genetic variants likely do not influence disease phenotype.
Acknowledgments
We are grateful to Virginia Phillips, Ariston Librero, Jo Landino, and Monica Castanedes Casey for histologic support; and Jessica Tranovich, Sabrina Rothberg, and Kelsey Caetano-Anolles for programmatic support. We are especially grateful to the patients and their families for the kind gift of brain donation. We thank the Mayo Clinic Center of Individualized Medicine for collection and sequencing of the Mayo Clinic Biobank samples.
Author contributions
Conceptualization: Billie J. Matchett, Sarah J. Lincoln, Melissa E. Murray.
Data curation: Sarah J. Lincoln, Kelly M. Hinkle, Jacqueline Helminger, Patrick W. Johnson, Michael G. Heckman, Joseph S. Reddy, Minerva M. Carrasquillo, Ranjan Duara, Neill R. Graff-Radford, Cyril Pottier, Nilüfer Ertekin-Taner, Owen A. Ross, Rosa Rademakers.
Formal analysis: Billie J. Matchett, Sarah J. Lincoln, Matt Baker, Daniel P. Wickland.
Funding acquisition: Ranjan Duara, Neill R. Graff-Radford, Dennis W. Dickson, Melissa E. Murray.
Investigation: Billie J. Matchett, Sarah J. Lincoln, Nikoleta Tamvaka, Sydney A. Labuzan, Tiffany N. Hicks Sirmans, Christina M. Moloney, Kelly M. Hinkle, Daniel P. Wickland, Janisse Cabrera-Rodriguez, Jacqueline Helminger, Minerva M. Carrasquillo, Cyril Pottier, Melissa E. Murray
Methodology: Billie J. Matchett, Sarah J. Lincoln, Matt Baker, Nikoleta Tamvaka, Sydney A. Labuzan, Tiffany N. Hicks Sirmans, Christina M. Moloney, Kelly M. Hinkle, Daniel P. Wickland, Janisse Cabrera-Rodriguez, Jacqueline Helminger, Dennis W. Dickson.
Project administration: Melissa E. Murray.
Resources: Steven G. Younkin, Ranjan Duara, Neill R. Graff-Radford, Nilüfer Ertekin-Taner, Owen A. Ross, Rosa Rademakers, Dennis W. Dickson, Melissa E. Murray.
Software: Janisse Cabrera-Rodriguez.
Supervision: Sarah J. Lincoln, Steven G. Younkin, Owen A. Ross, Rosa Rademakers, Dennis W. Dickson, Melissa E. Murray.
Validation: Tiffany N. Hicks Sirmans, Christina M. Moloney, Kelly M. Hinkle, Daniel P. Wickland, Patrick W. Johnson, Michael G. Heckman, Joseph S. Reddy, Steven G. Younkin, Minerva M. Carrasquillo, Cyril Pottier, Nilüfer Ertekin-Taner, Owen A. Ross, Rosa Rademakers.
Visualization: Sydney A. Labuzan, Tiffany N. Hicks Sirmans, Janisse Cabrera-Rodriguez, Owen A. Ross
Writing – original draft: Billie J. Matchett, Melissa E. Murray.
Writing – review & editing: Billie J. Matchett, Sarah J. Lincoln, Matt Baker, Nikoleta Tamvaka, Sydney A. Labuzan, Tiffany N. Hicks Sirmans, Christina M. Moloney, Kelly M. Hinkle, Daniel P. Wickland, Janisse Cabrera-Rodriguez, Jacqueline Helminger, Patrick W. Johnson, Michael G. Heckman, Joseph S. Reddy, Steven G. Younkin, Minerva M. Carrasquillo, Ranjan Duara, Neill R. Graff-Radford, Cyril Pottier, Nilüfer Ertekin-Taner, Owen A. Ross, Rosa Rademakers, Dennis W. Dickson, Melissa E. Murray.
Supplementary Material
Abbreviations:
- AD
- Alzheimer’s disease
- CADD
- combined annotation dependent depletion
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
The investigators are supported by grants from the Alzheimer’s Association (AARG-17-533458); National Institute on Aging (R01-AG054449, R01-AG075802, U19-AG069701, U01-AG57195, P30-AG062677); the Florida Department of Health, and the Ed and Ethel Moore Alzheimer’s Disease Research Program (8AZ06, 20a22); and a kind gift from David and Frances Strawn. This research was supported by a New Vision Research Investigator Award.
All authors have reviewed the contents of the manuscript being submitted, approved its contents, and consented to publication.
How to cite this article: Matchett BJ, Lincoln SJ, Baker M, Tamvaka N, Labuzan SA, Hicks Sirmans TN, Moloney CM, Helminger J, Hinkle KM, Cabrera-Rodriguez J, Wickland DP, Johnson PW, Heckman MG, Reddy JS, Younkin SG, Carrasquillo MM, Duara R, Graff-Radford NR, Pottier C, Ertekin-Taner N, Ross OA, Rademakers R, Dickson DW, Murray ME. The SERPINA5 coding variant E228Q does not contribute to clinicopathologic characteristics in Alzheimer’s disease: A cross-sectional study. Medicine 2023;102:24(e34017).
Contributor Information
Billie J. Matchett, Email: billie.matchett@postgrad.manchester.ac.uk.
Sarah J. Lincoln, Email: lincoln.sarah@mayo.edu.
Matt Baker, Email: baker.matt@mayo.edu.
Nikoleta Tamvaka, Email: Tamvaka.Nikoleta@mayo.edu.
Sydney A. Labuzan, Email: kmr9up@virginia.edu.
Tiffany N. Hicks Sirmans, Email: Sirmans.Tiffany@mayo.edu.
Christina M. Moloney, Email: moloney.christina@mayo.edu.
Jacqueline Helminger, Email: helminger.jacqueline@gmail.com.
Kelly M. Hinkle, Email: ross.kelly@mayo.edu.
Janisse Cabrera-Rodriguez, Email: janisse.cabrera1@gmail.com.
Daniel P. Wickland, Email: wickland.daniel@mayo.edu.
Patrick W. Johnson, Email: Johnson.Patrick3@mayo.edu.
Michael G. Heckman, Email: Heckman.Michael@mayo.edu.
Joseph S. Reddy, Email: Reddy.Joseph@mayo.edu.
Steven G. Younkin, Email: younkin.steven@mayo.edu.
Minerva M. Carrasquillo, Email: Carrasquillo.Minerva@mayo.edu.
Ranjan Duara, Email: Ranjan.duara@msmc.com.
Neill R. Graff-Radford, Email: graffradford.neill@mayo.edu.
Cyril Pottier, Email: pottier.cyril@mayo.edu.
Nilüfer Ertekin-Taner, Email: Taner.Nilufer@mayo.edu.
Owen A. Ross, Email: Ross.Owen@mayo.edu.
Rosa Rademakers, Email: rosa.rademakers@uantwerpen.vib.be.
Dennis W. Dickson, Email: dickson.dennis@mayo.edu.
References
- [1].Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murray ME, Graff-Radford NR, Ross OA, et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Crist AM, Hinkle KM, Wang X, et al. Transcriptomic analysis to identify genes associated with selective hippocampal vulnerability in Alzheimer’s disease. Nat Commun. 2021;12:2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Moloney CM, Lowe VJ, Murray ME. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: a clinicopathologic perspective for biomarker research. Alzheimers Dement. 2021;17:1554–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Suzuki K, Nishioka J, Hashimoto S. Protein C inhibitor. purification from human plasma and characterization. J Biol Chem. 1983;258:163–8. [PubMed] [Google Scholar]
- [6].Huntington JA. Thrombin inhibition by the serpins. J Thromb Haemost. 2013;11(Suppl 1):254–64. [DOI] [PubMed] [Google Scholar]
- [7].Suzuki K. The multi-functional serpin, protein C inhibitor: beyond thrombosis and hemostasis. J Thromb Haemost. 2008;6:2017–26. [DOI] [PubMed] [Google Scholar]
- [8].Zechmeister-Machhart M, Hufnagl P, Uhrin P, et al. Molecular cloning and sequence analysis of the mouse protein C inhibitor gene. Gene. 1997;186:61–6. [DOI] [PubMed] [Google Scholar]
- [9].Billingsley GD, Walter MA, Hammond GL, et al. Physical mapping of four serpin genes: alpha 1-antitrypsin, alpha 1-antichymotrypsin, corticosteroid-binding globulin, and protein C inhibitor, within a 280-kb region on chromosome I4q32.1. Am J Hum Genet. 1993;52:343–53. [PMC free article] [PubMed] [Google Scholar]
- [10].Santos OA, Pedraza O, Lucas JA, et al. Ethnoracial differences in Alzheimer’s disease from the Florida autopsied multi-ethnic (FLAME) cohort. Alzheimers Dement. 2019;15:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pottier C, Ren Y, Perkerson RB, 3rd, et al. Genome-wide analyses as part of the international FTLD-TDP whole-genome sequencing consortium reveals novel disease risk factors and increases support for immune dysfunction in FTLD. Acta Neuropathol. 2019;137:879–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rentzsch P, Witten D, Cooper GM, et al. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2018;47:D886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Braak H, Thal DR, Ghebremedhin E, et al. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9. [DOI] [PubMed] [Google Scholar]
- [15].Wattmo C, Londos E, Minthon L. Risk factors that affect life expectancy in Alzheimer’s disease: a 15-year follow-up. Dement Geriatr Cogn Disord. 2014;38:286–99. [DOI] [PubMed] [Google Scholar]
- [16].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- [17].Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. [DOI] [PubMed] [Google Scholar]
- [18].DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Uchikado H, Lin WL, DeLucia MW, et al. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rojano E, Seoane P, Ranea JAG, et al. Regulatory variants: from detection to predicting impact. Brief Bioinform. 2019;20:1639–54. [DOI] [PMC free article] [PubMed] [Google Scholar]