Abstract
Alcohol use disorder (AUD) rates have risen dramatically in the United States, resulting in increasing rates of alcohol-associated liver disease (ALD), but many patients struggle to access alcohol use treatment. AUD treatment improves outcomes, including mortality, and represents the most urgent means by which care can be improved for those with liver disease (including ALD and others) and AUD. AUD care for those with liver disease involves 3 steps: detecting alcohol use, diagnosing AUD, and directing patients to alcohol treatment. Detecting alcohol use can involve questioning during the clinical interview, the use of standardized alcohol use surveys, and alcohol biomarkers. Identifying and diagnosing AUD are interview-based processes that should ideally be performed by a trained addiction professional, but nonaddiction clinicians can use surveys to determine the severity of hazardous drinking. Referral to formal AUD treatment should be made, especially where more severe AUD is suspected or identified. Therapeutic modalities are numerous and include different forms of one-on-one psychotherapy, such as motivational enhancement therapy or cognitive behavior therapy, group therapy, community mutual aid societies (such as Alcoholics Anonymous), inpatient addiction treatment, and relapse prevention medications. Finally, integrated care approaches that build strong relationships between addiction professionals and hepatologists or medical providers caring for those with liver disease are crucial to improving care for this population.
INTRODUCTION
Alcohol use disorder (AUD) and alcohol-associated liver disease (ALD) rates have risen dramatically in the United States. Alcohol cessation is the most important aspect of long-term survival for those with ALD, but hepatology providers often find managing alcohol use challenging. In this review, we will examine the changing epidemiology of ALD, including the increases among women and young people. Then, we will present the best ways to detect alcohol use, determine how severe an alcohol use problem your patient has, and review the types of AUD treatment and ways to motivate your patient with ALD to engage in treatment and reduce or stop alcohol use.
EPIDEMIOLOGY
The United States has seen, in recent years, a dramatic increase in ALD rates.1 In the United States, the prevalence of all stages of ALD has been estimated at 8%, but the proportion with cirrhosis has grown from 2.2% to 6.6% from 2001 to 2016.2 Over a 16-year period, mortality for all-cause cirrhosis rose 65%, but these increases were almost entirely due to rises in ALD mortality, especially among young people ages 25–34 and women.3 Globally, increases in both compensated and decompensated alcohol–associated cirrhosis have also been demonstrated, rising from 288 per 100,000 persons to 290 per 100,000 persons from 1990 to 2017 for compensated cirrhosis and 24.3 per 100,000 persons to 30 per 100,000 persons for decompensated cirrhosis over the same timeframe.4
Mortality
As a result, ALD is now the greatest contributor to liver-related mortality in Europe, making up 40%–50% of total liver disease deaths attributed to ALD (though rates vary from country to country).4 A biopsy-based study of long-term mortality in a cohort of Swedish patients with ALD followed showed an overall 5-year mortality for all stages of ALD of 40.9% versus 5.8% in the non-ALD reference population.5 However, even for early-stage alcohol–associated steatosis, mortality was 5.2% versus 0.5% in matched controls, with a higher risk for mortality in those with more advanced ALD (adjusted HR: 6.07, 95% CI, 5.43–6.77 for cirrhosis; adjusted HR: 2.72, 95% CI, 2.2–3.35 for those with steatosis only).5 These dramatic increases in ALD have been amplified and accelerated by the COVID-19 global pandemic, which saw rises in ALD hospitalizations, as well as waitlisting and transplants for acute alcohol-associated hepatitis (AAH) in the United States.6–8
What is driving the increases in ALD?: Risk factors
Although various different factors influence the impact of alcohol use on the development and worsening of liver disease (eg, genetics, sex, comorbid liver disease, and obesity9), the overall dose of alcohol use over time remains the leading causative factor for ALD and increased mortality.10–12 For those with AAH, any consumption of alcohol 6 months following an index AAH hospitalization resulted in increased mortality of at least double for 1–2 drinks per day, increasing up to 6-fold for those consuming 100-g alcohol or more per day.13 Alcohol consumption in those with known cirrhosis increases the risk of first-time ascites, variceal bleeding, and mortality as well.14,15 In addition, while projected increases in obesity and NASH prevalence in the United States will increase liver disease and transplant burden,16 alcohol use in the obese population with or without metabolic syndrome has also been shown to increase rates of liver disease, including decompensation and mortality.17–20 As such, the combined effect of rising obesity and metabolic syndrome rates coupled with rising alcohol consumption in the United States may explain part of the increase in the ALD burden.
Sex differences
In a meta-analysis of studies evaluating alcohol dose and risk of cirrhosis, there were clear sex disparities with women consistently more likely to get cirrhosis at lower drinking levels than men.12 Although as little as a single drink per day (compared with long-term abstainers) was associated with an increased risk of cirrhosis in women [relative risk (RR): 1.40, 95% CI, 1.00–1.97], 5–6 drinks per day were associated with an increased risk in both men and women (pooled RR: 6.26, 95% CI, 2.38–16.50).12 However, not all who drink heavily will go on to develop more advanced ALD, cirrhosis, or AAH. Chronic alcohol use of 20- to 50-g alcohol (~2–5 standard US drinks per day at 14-g alcohol per drink) in women and 60- to 80-g alcohol per day, sometimes termed “heavy drinking” (consuming 4 or more drinks per day for women and 5 or more drinks per day for men21) produces hepatic steatosis (or fatty liver) in 90% or more of drinkers.22,23 Certain drinking patterns have also been identified, which may be more damaging, especially for women. In the Million Woman Study in the United Kingdom, for example, while an overall increased dose of alcohol was associated with an increased risk of cirrhosis, certain drinking patterns, such as drinking with meals, were associated with a lower incidence of cirrhosis (RR: 0.69, 95% CI, 0.62–0.77).24
Unsurprisingly, the dismal epidemiologic trends of increasing ALD have been preceded by similar rises in drinking across nearly all demographics, including all age ranges, races and ethnicities, and both sexes. Although global definitions of moderate drinking differ by country, the National Institutes of Health in the United States defines moderate drinking as anything over 1 drink per day for women or 2 drinks per day for men (Figure 1) although there is increasing suggestion that even this level of alcohol consumption may be unhealthy.25 In the United States, rates of alcohol use have risen across all demographics over the past 15 years though the most pronounced increases have been among women and young people.26,27 In a meta-analysis of several large US databases on alcohol use, past-year alcohol use and binge drinking rates increased for all demographics, with rates increasing most for women.28 In fact, the overall rate of drinking for men remained constant, while nearly the entirety of the increase in overall population rates was provided by increasing rates among women. Reasons for this rise in drinking among women are complex, but may be related to social and cultural factors, and advertising that targets women specifically, associating alcohol use with specific female life experiences (“wine moms” and “mommy juice”) and with friendship, camaraderie, and emotional support.29,30
FIGURE 1.
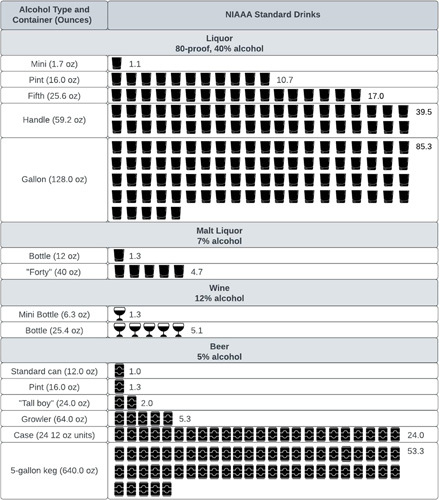
Number of standard drinks in commonly sold alcohol container volumes. Abbreviations: NIAAA, National Institute on Alcohol Abuse and Alcoholism.
DETECTING ALCOHOL USE
Given these considerations, how should we approach alcohol use management in our patients with chronic liver disease? The first step is to find out if your patient is drinking. Detecting alcohol use can be done in 3 primary ways: elicitation during clinical history, using validated surveys, and through alcohol biomarkers.
Quantifying alcohol intake by history
Querying patients about their alcohol use during the history is valuable but comes with some pitfalls. Asking patients how many drinks they have in a day may give an inaccurate answer, even in settings where hepatologists are asking about alcohol use regularly, such as transplant clinics.31 Knowledge of “what’s in a drink” may not be uniform across the population. In several studies of drink estimation, the number of standard drinks actually poured (when instructed to pour only 1 drink) ranged from 1.4 to 2.1 drinks.32 In addition, misconceptions about the harms of different types of alcohol to the liver are common, including that beer is not harmful to the liver, only hard liquor.33 As a result of these misconceptions, patients may underestimate or not disclose their alcohol use or erroneously switch beverages (liquor to beer), thinking that they are eliminating their risk. Alternate styles of questioning may be helpful in overcoming these challenges. For example, instead of asking “how many drinks do you drink in a day?”, asking what the patient drinks and then querying how long it takes him or her to get through a bottle of their drink of choice (eg, a bottle of wine or a fifth of vodka) may produce a more accurate assessment of total dose of alcohol consumed. See Table 1 for ways to approach counseling around alcohol use. Clinicians should be aware of how many standard drinks are in typical bottles and beverage containers to convey this information to patients who may be less aware of the total dose of alcohol that they are truly consuming (Figure 1).
TABLE 1.
Challenges and potential approaches to discussing alcohol and substance use with patients
| Challenges in discussing alcohol and substance use | Potential approaches |
|---|---|
| Inaccurate or imprecise patient estimates of alcohol use | Use a visual chart (Figure 2) to help patients understand how much alcohol is in a single drink Ask what patients drink and how long it takes them to get through the unit of alcohol the commonly purchase [eg, “how long it takes to get through a “fifth” (750 mL) of liquor,” or “how often would you purchase another “pint” (375 mL) because you ran out?”] |
| Stigma and shame | Use first-person plural pronouns to discuss substance matters to reduce confrontational undertones (eg, we, us) Normalize asking about alcohol use (eg, “Doctors commonly ask all their patients about we are doing with alcohol use,” “It is common for all of us to find it difficult to talk about our drinking with our doctor”) Use destigmatizing language to reduce defensiveness and encourage psychological openness (eg, instead of “addict” “substance abuse,” or “alcohol-associated,” say “person with alcohol use disorder” or “patients with alcohol challenges”; use substance use disorder or alcohol use disorder in general)a Normalize AUD care and treatment as an expected part of medical and liver care and of equal importance and relevance. Project reasonable, proportional, and authentic optimism about patients’ potential for improvement |
| Ambivalence about changing alcohol use behaviors | Use a patient-centered, motivational interviewing 34 approach based on the following interview principles (use your OARS): Open-ended questions: for example, “What brings you here today? How do you hope I might be able to help you today?” Affirming: Center on the patient and their successes no matter how small (“you” not “I” language). For example, “You did a really good job of keeping track of your drinking this week!” “Thanks for coming in today. It’s good to see you!” Reflections: Nonjudgmentally reflecting back something that your patient has said (with an emphasis on reflecting back language “change language”). For example, “You increased your alcohol use after your mother died last year, and now you realize it may be time to cut back.” Summarizing: Pull together several things someone told you. Often, reflections and affirmations are in summaries. |
For further suggestions regarding destigmatizing language in alcohol use care, please see.
Responses to alcohol queries may also be influenced by stigma and shame. Alcohol use is highly stigmatized, and patients frequently feel a great deal of shame when confronted with a situation where they may have to disclose their alcohol consumption.35 Added to this, cirrhosis and liver disease themselves are highly stigmatized,36,37 which is largely because cirrhosis is closely associated in the public mind with alcohol use. Stigma and shame operate on many levels in patients with cirrhosis, including social stigma (when others avoid the patient or the patient is treated in a discriminative way due to their liver disease) or self-stigma (as when patients fear disclosing their illness or feel they must self-punish or self-alienate as a result of their liver disease).36 This manifests in clinics for those consuming alcohol as potentially reduced disclosure of alcohol use and lower levels of help-seeking for AUD treatment. Such high levels of stigma can also tragically prevent patients from recognizing their drinking problems or lead patients to decide that they are unworthy or undeserving of help for their alcohol use or, even, for medical care and liver transplantation, often leading to even more substance use. Frequently, the pressures of the medical encounter, particularly when a patient is being evaluated for liver transplantation, place enormous stress on the patient and, with consequences for a slip or relapse so high, may incentivize a lack of candor around alcohol consumption.
Counteracting the stigma associated with alcohol use starts by compassionately and kindly addressing alcohol use as a normal and expected part of the hepatology encounter, a health behavior similar to others discussed with patients, which also contributes to and causes other medical problems (Table 1). Compassionate and respectful inquiry forms a major basis of motivational interviewing (MI), a patient-centered interviewing approach to behavior change that is particularly helpful in those who may be ambivalent about change.34 Stigma related to addiction has persisted for many reasons, but one major reason is the resistance to consider addiction a true disease, as it is currently conceptualized in addiction research.38 Alcohol use alters brain circuitry responsible for stress, mood, and reward,39,40 and susceptibility to addictive substance use arises for a variety of reasons outside the control of the individual, including genetic susceptibility, social and environmental exposures, the frequent co-occurrence of other mood disorders, and traumatic life experiences, to name a few.38
AUDIT-C
A helpful aid to historical questioning of alcohol consumption is the use of validated alcohol use questionnaires. The Alcohol Use Disorders Identification Test (AUDIT) is a validated tool to screen for problem alcohol consumption that is recommended for use.9 It can be programmed in many electronic health records and pushed out to patients ahead of their appointments, whether in person or virtual, allowing for efficient querying of alcohol consumption during the clinical encounter. The AUDIT has 2 versions, a shorter 3-question version called the AUDIT-C and a longer 10-question version (AUDIT).41 For the shorter AUDIT-C, values of 3 or more in women and 4 or more in men are considered positive and suggestive of heavy drinking and need for intervention, while, for the 10-question AUDIT, values of 8–14 suggest hazardous or harmful consumption and values of 15 or more may suggest a moderate to severe AUD.
Alcohol biomarkers
Alcohol biomarkers are important adjuncts to the history and screening questions about alcohol use and are increasingly used in hepatology care, as recommended by guidelines.9 Alcohol biomarkers are urine, hair, and blood moieties that indicate alcohol use. They come in 2 broad categories: indirect and direct. Indirect biomarkers (ie, AST:ALT ratios, elevated gamma glutamyl-transferase levels, and high mean corpuscular volume) reflect the toxic effects of alcohol on organs, tissues, or body chemistry and do not directly represent alcohol metabolites. By contrast, direct alcohol biomarkers are direct products of ethanol metabolism. These include the blood alcohol level, urine ethyl glucuronide, urine ethyl sulfate, hair ethyl glucuronide, and phosphatidylethanol (PETH). The duration of drinking detected varies by biomarker, ranging from ~12 hours for blood alcohol levels to several months for hair ethyl glucuronide (Table 2), and assay availability also varies by the health system. Urine and hair ethyl glucuronide, urine ethyl sulfate, and phosphatidylethanol have been validated in patients with liver disease, including cirrhosis patients and those both pre-transplant and post-transplant, with varying degrees of sensitivity and specificity (Table 2). Discordance rates for alcohol biomarkers with reported abstinence by history have been estimated between 10% and 30%.42 Principles for using alcohol biomarkers include notifying patients that you will be checking biomarkers, normalizing their use as a regular part of clinical care like other objective markers of health behaviors (eg, hemoglobin A1c, body weight, and serum drug levels), and reassuring patients that use of biomarkers is not intended to “catch” or punish patients but rather to detect slips and relapses to aid in offering appropriate care and supporting patients in re-establishing abstinence. In addition, while dose estimations may be possible with PETH,43 precise dose estimation with most alcohol biomarkers is difficult, so the use of biomarkers to indicate the presence or absence of alcohol use of any amount, followed by a discussion with the patient, is recommended. In addition, false positives and false negatives are found with each biomarker, making knowledge of this critical alongside querying for potential reasons for false testing when using these in the clinic, particularly for high-stakes decisions, such as transplant candidacy.44
TABLE 2.
Detection time and detection accuracy in clinic use among liver disease patients for various alcohol biomarkers
| Test | Source | Detection time | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| Ethyl glucuronide | Urine | 3–5 d | 76–89 | 93–99 | 64–100 | 86–93 |
| Ethyl glucuronide | Hair | Months | 81–100 | 83–98 | 68–95 | 86–100 |
| Ethyl sulfate | Urine | 3–5 d | 82 | 86 | 70 | 93 |
| Phosphatidylethanol | Blood | 2–3 wk | 98–100 | 66–96 | 85 | 100 |
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
Diagnosing AUD
Once alcohol use has been established, determining the severity of a potential alcohol use problem is important, as this can determine what alcohol treatments might be best indicated. Risky drinking is defined, as noted above, as more than 1 drink per day for women or 2 drinks per day for men. Heavy drinking is considered anything greater than 4 drinks per day for women or 5 drinks per day for men, while binge drinking consists of consuming similar amounts as heavy drinking but over a shorter time period, often 2–3 hours. AUD, by contrast, is defined not by the dose of alcohol consumed but by the consequences experienced due to alcohol consumption of whatever amount. Previously called alcohol abuse or dependence or alcohol addiction, AUD is categorized using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition as mild, moderate, or severe based on 11 symptom criteria, which include the accumulation of negative consequences across a range of life categories (Figure 2). To diagnose AUD, the patient should be interviewed by a trained professional with expertise in addiction, such as a social worker, psychologist, or psychiatrist. However, shorter screening surveys, such as the 10-item AUDIT, discussed above, can assess the risk of an AUD using standard risk cutoff scores (≥15 may indicate moderate to severe AUD). Those who score high on the AUDIT could be referred for more formal evaluation.
FIGURE 2.
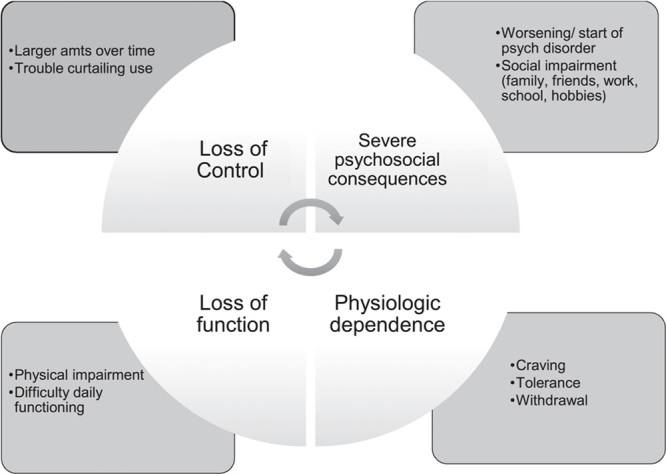
Diagnosis of alcohol use disorder, according to criteria from the Diagnostic and Statistical Manual of Mental Disorders.
ALCOHOL USE TREATMENT
For patients with liver disease and AUD or problem alcohol use, liver and other health-related consequences can be severe. For those with ALD, especially cirrhosis or AAH, the consequences of ongoing drinking, even at low amounts, are high with increases in decompensating events, such as variceal bleeding, ascites, and HE, increased rates of HCC, higher recurrence of cirrhosis and mortality after transplantation, and higher overall liver-related mortality.11–13,45–47 Because of the effect of any alcohol on mortality and hepatic decompensation for those with ALD, particularly for those with cirrhosis or AAH, total abstinence is the recommended goal for all patients with ALD, who are actively drinking in any amount. However, total abstinence is often difficult for patients with ALD to achieve. Elsewhere in the AUD treatment community, harm reduction approaches are frequently used. Harm reduction seeks to move patients toward alcohol consumption goals that the patient sets and which may not include total abstinence.48 Harm reduction, as the name implies, seeks to reduce the harms of substance use without necessarily requiring total abstinence and individualize the approach to alcohol counseling by setting alcohol reduction or cessation goals with our patients, instead of for our patients.
Alcohol use treatment options
Brief interventions
For some patients with more mild AUD symptoms, brief behavioral interventions delivered in the clinic may be beneficial. Such interventions consist of a short, 10–15 minute, in-clinic discussion with a health care provider focused on education about the harms of alcohol use and the benefits of reduction or cessation and patient-centered goal-setting and MI to help promote changes in alcohol use. Although brief interventions are largely not effective in those with more severe AUD, they can be beneficial in those with milder alcohol misuse symptoms and have been tested in several settings, including primary care, inpatient, and emergency departments.49–52 A meta-analysis of brief interventions, most of which were performed in primary care or emergency department settings, found that, compared with minimal to no intervention, patients who received brief interventions consumed less alcohol (mean difference −20 g/week, CI, −29 to −12).53 For those with more severe alcohol misuse, referral to treatment is a critical component of the Screening, Brief Intervention, Referral to Treatment (SBIRT) model. However, interventions to increase referral to treatment have been largely ineffective, showing no increase in treatment utilization in a meta-analysis of referral interventions (RR: 1.08, 95% CI, 0.92−1.28).54,55
Motivational interviewing
MI is a patient-centered communication paradigm helpful in working with less motivated and ambivalent patients toward behavior change. More than a technique, MI is a style and complete approach to patient care that involves a “collaborative conversation style for strengthening a person’s own motivation and commitment to change.”34 The style of MI is to ask, prompt, and guide patients, rather than force or paternalistically direct, and to develop their own internal reasons and motivation for change. Motivational enhancement therapy is a more structured way of providing MI during psychotherapy. A large body of literature, including numerous systematic reviews and meta-analyses, has shown the effectiveness of MI for alcohol reduction and cessation,56–58 including some studies showing effect among those with ALD and other liver diseases.59 One study of integrated cognitive behavior therapy (CBT), motivational enhancement therapy (MET) (which is a standardized method of delivering MI), and comprehensive medical care in ALD patients found that the combination of CBT and MET delivered in 24–48 sessions over 2 years resulted in a 74% increase in abstinence in the intervention group compared with a 48% increase in the control group (p = 0.02).60 Another recent randomized pragmatic trial evaluated the effectiveness of an SBIRT intervention that incorporated MI delivered by the medical provider as a stand-alone treatment versus SBIRT plus formal alcohol treatment (a combination of CBT, MET, and substance abuse treatment delivered by an integrated mental health professional) in a group of hepatitis C patients with alcohol use.61 In this trial, patients in both arms had significant increases in abstinence at 6 months, with no difference between treatment arms (SBIRT-only: 20.5%; SBIRT + alcohol treatment: 23.3%; and the coefficient for intervention effect: 0.65, 95% CI, −0.65, 1.96). In the United States, training for MI is available in many forms including through the Motivational Interviewing Network of Trainers (https://motivationalinterviewing.org) and by means of online courses and educational books.
Other therapeutic modalities
A wide variety of other therapeutic modalities have been shown to be effective in assisting patients in reducing or stopping alcohol use. In addition to inpatient alcohol use treatment (which often combines many of the following modalities into an inpatient, residential setting), these include CBT, 12-step facilitation, contingency management, couples and family therapy, mindfulness-based relapse prevention, contingency management and community reinforcement, and social behavior and network theory (Table 3).
TABLE 3.
Behavioral modalities for treatment of alcohol use
| Behavioral therapy | Provider delivering intervention | Efficacy in ALD | Description |
|---|---|---|---|
| Screening, Brief Intervention, Referral to Treatment (SBIRT) | Any clinician | 20–23% achieve abstinence w/ SBIRT | Providing screening for alcohol use, brief (usually motivationally interviewing themed) discussion on alcohol reduction, and referral to alcohol treatment where indicated. |
| Cognitive behavior therapy (CBT) | Trained MHSAa provider | CBT/MET for 2 y: 74% increase in abstinence vs. 48% in controls | Focuses on modifying dysfunctional thoughts, emotions, and behaviors. In AUD treatment, used to identify cues and triggers for relapse, improve coping strategies, substance-refusal training, increase focus on substance-free activities. |
| Motivational enhancement therapy (MET)/motivational interviewing (MI) | Any clinician | CBT/MET for 2 y: 74% increase in abstinence v 48% in controls | MI and it is more structure version (MET) are widely used, evidence-based approaches for eliciting and strengthening personal motivation to change. Especially helpful for those who are ambivalent about or resistant to positive behavior change. |
| 12-step facilitation | Trained MHSAa provider | Unknown, no data | Therapy modality in which focuses on total alcohol abstinence and regular participation in 12-step/alcohol-associated anonymous meetings |
| Contingency management | Trained MHSAa provider | Unknown, no data | Intervention based on operant conditioning where patients receive an incentive (often financial) in exchange for evidence of reduction in use or abstinence |
MHSA: mental health/substance abuse provider
Abbreviations: ALD, alcohol-associated liver disease; AUD, alcohol use disorder.
Adapted from Leggio and Mellinger.79
Community mutual aid societies
Community mutual aid societies consist of organizations of those with alcohol use problems who come together to assist and support one another in reducing alcohol or achieving and maintaining abstinence. The most well-known is Alcoholics Anonymous (AA), but other societies (such as SMART Recovery, Celebrate Recovery, and Refuge Recovery) also exist and focus on recovery from alcohol use problems using different philosophies and approaches. A recent meta-analysis of AA and 12-step facilitation (a counseling approach based on and supporting the 12-step approach to alcohol cessation) found that, among 1936 participants with alcohol use problems and compared with other treatment modalities, such as CBT or MET, AA participation plus a standardized approach to 12-step facilitation improved rates of continuous abstinence at 12 months (RR: 1.21, 95% CI, 1.03, 1.42) with a consistent effect maintained at 24 and 36 months.62 Although this data does not specifically include ALD patients, AA or other mutual aid societies may be especially appealing given the high availability of these groups, virtual options for meetings, and low/no cost.
Relapse prevention medications
Relapse prevention medications constitute an important part of the AUD treatment armamentarium (Table 4). However, because of a lack of safety data in advanced liver disease patients and unfamiliarity with prescribing among hepatologists, few patients with liver disease and AUD receive medication therapy for AUD.69,70 Among those who do access medication therapy for AUD, outcomes are improved, including reduced incidence and progression of ALD.71 In addition, those few ALD patients who do access psychosocial treatment often find that their addiction providers are uncomfortable prescribing relapse medications, some of which are metabolized by the liver, leaving these patients undertreated.72
TABLE 4.
Relapse prevention medications and potential use in liver disease patients
| Medication | FDA approved | Metabolism and excretion | Starting and effective dose | Liver disease considerations |
|---|---|---|---|---|
| Disulfiram | Yes | M: hepatic E: 70% renal |
250–500 mg daily | Severe, sometimes fatal, DILI and/or acute liver failure requiring transplant. Reports of neuropathy and psychosis. Not recommended for use in liver disease. |
| Naltrexone | Yes | M: hepatic E: mostly renal, 2% fecal |
Start: 25 mg daily Effective dose: 50 mg daily orally; 380 mg intramuscularly monthly. |
Rare potential for hepatotoxicity, some documented elevations in liver enzymes but no known cases of liver failure. Can see drug and metabolite accumulation in advanced cirrhosis (Childs class B or C).63 Suggest oral formulation over intramuscular for those with cirrhosis. Interacts with opioids so ensure patient is not on narcotics before starting. Meta-analysis in AUD patients without liver disease showed moderate efficacy. |
| Acamprosate | Yes | M: no hepatic E: renal |
Start: 333 mg three times daily Effective dose: 666 mg orally 3 times daily |
No evidence of hepatotoxicity. Meta-analysis in AUD patients without known liver disease showed moderate efficacy. |
| Gabapentin | No | M: not hepatic E: 75% renal, 25% fecal |
Start: no clear starting dose recommended Effective dose: 900–1800 mg 3 times daily |
No hepatotoxicity. Theoretical abuse potential. Use with caution for those with HE. Dose reduce in renal failure. For those with chronic kidney disease, lower doses may be as effective. |
| Baclofen | No | M: limited hepatic E: renal |
Start: 5 mg 3 times daily for 3 days, then increase to target dose by 5 mg every 3 day increments Effective dose: 10–20 mg 3 times daily |
No evidence for direct hepatotoxicity but may precipitate HE. Has been tested in randomized trials in ALD patients 64–66 and other observational trials.67,68 Dose reduce in renal failure and avoid administering in end-stage renal disease. |
| Topiramate | No | M: limited hepatic E: renal |
Start: 25–50 mg daily. Increase in 25–50 mg increments weekly to effective dose goal. Effective dose: 300 mg daily |
No evidence for hepatotoxicity but could affect liver function. Could worsen/confound hepatic encephalopathy. Dose reductions in hepatic and renal impairment. Effective dose needed may be lower in those with more advanced liver or kidney disease. |
Abbreviations: ALD, alcohol-associated liver disease; AUD, alcohol use disorder; FDA, Food and Drug Administration.
Disulfiram
Disulfiram, or Antabuse, is a Food and Drug Administration (FDA)-approved medication for alcohol relapse prevention. Disulfiram can be, however, highly hepatotoxic and has been linked to instances of liver failure, need for transplant, and death. It should not be used in patients with liver disease.
Acamprosate
Introduced in Europe in 1989, acamprosate is an FDA-approved medication for alcohol relapse prevention, which works by modulating the NMDA receptor, possibly as a partial coagonist.73 Acamprosate has no hepatic metabolism and no reported hepatotoxicity. It is renally metabolized, so the dose must be reduced if the glomerular filtration rate is <60, and administration should be avoided if <30. Acamprosate has been tested widely in multiple studies of patients with AUD but has never been formally tested in liver disease patients exclusively. One of the largest studies of medication-assisted therapy for AUD, the COMBINE study,74 evaluated the impact of 16 weeks of naltrexone or acamprosate with or without combined behavioral intervention and found that, while naltrexone was effective in reducing alcohol use at a dose of 100 mg daily by mouth daily (HR for return to heavy drinking: 0.78 (95% CI, 0.63, 0.97), acamprosate, at a dose of 1000 mg 3 times daily, was not (HR: 0.93 (95% CI, 0.75, 1.16). However, other meta-analyses have found that acamprosate is effective at promoting alcohol reduction and abstinence.75,76 In 1 meta-analysis, acamprosate had a number-needed-to-treat (NNT) for return to any drinking of 12 (8–26) at a dose of 666 mg 3 times daily.75
Naltrexone
Naltrexone is a potent competitive mu-opioid receptor antagonist that has been approved by the FDA for alcohol relapse prevention. Like acamprosate, it has been widely tested in the general AUD population but has not had any formal randomized trials in patients with liver disease. Naltrexone is metabolized by the liver, so concerns have been raised about hepatotoxicity. A small pharmacokinetic study performed in 18 patients with no cirrhosis, compensated cirrhosis (Childs A-B), and decompensated (Childs C) cirrhosis showed that elimination half-life was similar between groups (with slight increase in decompensated cirrhosis), but that the primary drug, naltrexone, and metabolite, 6β-natrexol, responsible for most of the pharmacologic effects, were both more elevated in cirrhosis patients.63 Elevated liver enzymes have been reported with the use of naltrexone, but there have been no reported cases of liver failure or liver-related death due to its use. Naltrexone does seem to be effective in several meta-analyses in the general AUD population. In one large meta-analysis, naltrexone had an NNT of 20 (11–500) for return to any drinking and 12 (8–26) for return to heavy drinking at a dose of 50 mg orally per day. In the COMBINE study,74 naltrexone for 16 weeks was found to be effective at promoting abstinence, with or without combined behavioral interventions (HR for return to heavy drinking: 0.78 (95% CI, 0.63, 0.97). Because of concerns about hepatotoxicity, the use of naltrexone has been limited, but a recent retrospective study in liver disease patients suggests that this concern may be overstated.77 However, a risk/benefit analysis should be undertaken tailored to each patient and considering the severity of AUD, the need for anticraving agents, and the stage of liver disease.
Baclofen
Unlike most other relapse prevention medications, baclofen, a GABA-B receptor agonist, has been tested in patients with advanced ALD, including those with cirrhosis. It is largely metabolized in the kidney, not the liver, so has no reported direct hepatotoxicity. In a small randomized controlled trial of patients with Childs A, B, and C cirrhosis in Italy,64 those randomized to baclofen at a dose of 10 mg 3 times daily had more total abstinence [30/42 (71%) versus 12/42 (29%), p = 0.02] at 12 weeks compared with controls. The study was limited by exclusion criteria, which included HE, the presence of pharmacologic treatment for other mental illnesses, and other substance use (other than tobacco), limitations that may reduce generalizability. Several other observational, uncontrolled studies of baclofen in ALD patients have also been published, which have shown reductions in alcohol consumption and an increase in total abstinence in patients with all stages of ALD, including cirrhosis.67,68,78 However, a randomized trial of baclofen 30 mg daily for 12 weeks in veterans with chronic hepatitis C and active alcohol use showed no difference between placebo and baclofen on abstinence rates.65 A more recent randomized trial, the BacALD trial, examined the effects of baclofen at either 10 mg 3 times daily or 25 mg 3 times daily for 12 weeks in a cohort of patients with and without ALD.66 Abstinence rates were similar between the baclofen 30- and 75-mg groups (21% and 23% respectively, p = 0.15) with improvement over placebo (10%). Critically, more patients experienced adverse events in the baclofen 75-mg group, with 51% reporting significant sedation or drowsiness. For those with cirrhosis, precipitation of HE is a concern, and thus, baclofen should be used cautiously in those with cirrhosis and any history of HE. It should not be used in those with ongoing or poorly controlled HE. Importantly, all of these studies, including the randomized baclofen trials, included some form of behavioral therapy alongside baclofen administration. Also, given their metabolism and excretion by the kidney, baclofen should be avoided in those with end-stage renal disease.
Other medications
Other non-FDA–approved medications for potential use include topiramate, gabapentin, and varenicline. Topiramate is an antiepileptic medication, which acts by decreasing dopaminergic activity and enhancing GABA action, and is FDA-approved for the treatment of epilepsy, migraines, and (when combined with phentermine) obesity.79 It is not extensively metabolized and is cleared renally, but clearance can be reduced by hepatic insufficiency. Topiramate has been shown in several clinical trials to reduce alcohol consumption and achieve alcohol abstinence, but caution is warranted, particularly in those with any degree of hepatic encephalopathy, as some patients have reported side effects, including mental status changes.80–82 Gabapentin is a well-known, widely used medication for neuropathy and nonopioid pain relief that also has evidence in the general AUD population for some benefits in promoting abstinence, improving alcohol withdrawal symptoms, and reducing cravings.83–86 Gabapentin modulates GABA and glutamate antagonism, and has no known hepatotoxicity. It is renally cleared and, in advanced liver disease patients with varying renal function, caution must be used given the risk of altered mental status. Any medication that sedates a patient should raise caution for causing or worsening HE and must be carefully weighed against its benefits and followed closely. Gabapentin may also be particularly helpful in reducing alcohol use in those with alcohol withdrawal symptoms and has been shown in retrospective studies to reduce inpatient benzodiazepine use for alcohol withdrawal and shorten the length of stay in those admitted for alcohol intoxication and withdrawal.84,87,88 Consideration can be given to starting this medication inpatient for patients experiencing mild withdrawal and then continuing it into the outpatient setting for additional anticraving benefits. Varenicline, a nicotinic acetylcholine receptor partial agonist, is FDA-approved for tobacco cessation and has some evidence to suggest that those with both AUD and tobacco cessation saw improved alcohol-associated outcomes as well when on varenicline.89–92 Varenicline is less well-studied for AUD than gabapentin or topiramate, however, and caution in use is warranted for those with renal disease. For all relapse medication use in liver disease, a thorough understanding of metabolism and side effects is necessary to appropriately weigh risks and benefits before prescribing.
INTEGRATED CARE FOR ALCOHOL USE AND LIVER DISEASE
Patients with liver disease and comorbid AUD frequently do not receive adequate treatment for their alcohol use problems, in part because of discomfort among hepatologists and addiction specialists regarding prescribing relapse prevention medications in liver disease patients and due to inadequate referrals to AUD treatment.69,70,72 However, improving outcomes for patients with AUD and liver disease requires integrating alcohol use and liver disease treatment across multiple domains of care.93 Integrated care is a well-established idea that focuses on care, which is team-driven, population-focused, measurement-guided, evidence-based, and improves quality.93 For those with the most advanced liver disease (cirrhosis and/or alcoholic hepatitis) and AUD, integrated multidisciplinary clinics and inpatient consult teams have shown promise in improving outcomes.94,95 Integrated care is, however, a spectrum, ranging from incorporating routine alcohol questioning with AUDIT or other surveys all the way to fully integrated, colocated ALD clinics. Local resources for creating a fully integrated ALD clinic may be limited, but no matter where a provider practices, integrating alcohol use care with liver care is feasible at some level. Although achieving improved outcomes for patients with AUD and liver disease, integrated care models also require strong interprofessional relationships based on mutual respect, shared knowledge and goals, and strong communication practices focused on frequent, timely, accurate, and problem-solving communication.93,96–101 Although a full discussion of how to implement integrated care for AUD and liver disease is beyond the scope of this article, additional, more detailed information on the implementation of integrated care can be found in the reference list.93,94,96–99 Nonetheless, clinicians should integrate alcohol use and liver disease as much as possible to improve outcomes for patients.
CONCLUSIONS
Management of AUD and liver disease requires integrating alcohol use detection, diagnosis of severity of AUD, and direction to treatment, which often means referral to higher levels of care, into the standard hepatology practice. At the bedside, the concerned liver clinician can provide compassionate alcohol detection (through interviews, surveys, and alcohol biomarkers) in the context of a MI approach that gently and kindly centers the patient’s concerns and respectfully recognizes the ambivalence that patients may feel when asked to reduce or stop alcohol use. The use of relapse prevention medications is recommended and can be a powerful adjunct to behavioral therapies to help patients stop alcohol use. Clinicians should feel empowered to start such medications and monitor for effectiveness and side effects. Only when AUD care is integrated into liver care, even in the most foundational of ways, such as routinely asking about alcohol use and utilizing the AUDIT screen, we can begin to turn the tide on alcohol’s impact on liver disease.
Supplementary Material
Acknowledgments
CONFLICTS OF INTEREST
Gerald Winder consults for Alexion. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AA, Alcoholics Anonymous; AAH, acute alcohol-associated hepatitis; ALD, alcohol-associated liver disease; AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; CBT, cognitive behavior therapy; FDA, Food and Drug Administration; MET, motivational enhancement therapy; MI, motivational interviewing; NIAAA, National Institute on Alcohol Abuse and Alcoholism; NNT, number-needed-to-treat; NPV, negative predictive value; PETH, phosphatidylethanol; PPV, positive predictive value; SBIRT, Screening, Brief Intervention, Referral to Treatment.
Jessica L. Mellinger was supported by NIAAA K23 023666.
Contributor Information
Jessica L. Mellinger, Email: jmelling@med.umich.edu.
Anne C. Fernandez, Email: acfernan@med.umich.edu.
G. Scott Winder, Email: gwinder@med.umich.edu.
REFERENCES
- 1.Organization WH. Global Status Report on Alcohol. 2018.
- 2.Dang K, Hirode G, Singal AK, Sundaram V, Wong RJ. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020;115:96–104. [DOI] [PubMed] [Google Scholar]
- 3.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagström H, Thiele M, Roelstraete B, Söderling J, Ludvigsson JF. Mortality in biopsy-proven alcohol-related liver disease: a population-based nationwide cohort study of 3453 patients. Gut. 2021;70:170–9. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MS, Valbuena VSM, Brown CS, Waits SA, Sonnenday CJ, Englesbe M, et al. Association of COVID-19 with new waiting list registrations and liver transplantation for alcoholic hepatitis in the United States. JAMA Netw Open. 2021;4:e2131132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholankeril G, Goli K, Rana A, Hernaez R, Podboy A, Jalal P, et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatology. 2021;74:3316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damjanovska S, Karb DB, Cohen SM. Increasing prevalence and racial disparity of alcohol-related gastrointestinal and liver disease during the COVID-19 pandemic: a population-based national study. J Clin Gastroenterol. 2022;57:185–188. [DOI] [PubMed] [Google Scholar]
- 9.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–33. [DOI] [PubMed] [Google Scholar]
- 10.Parker R, Aithal GP, Becker U, Gleeson D, Masson S, Wyatt JI, et al. Natural history of histologically proven alcohol-related liver disease: a systematic review. J Hepatol. 2019;71:586–93. [DOI] [PubMed] [Google Scholar]
- 11.Rehm J, Taylor B, Mohapatra S, Irving H, BALIUNAS D, PATRA J, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29:437–445. [DOI] [PubMed] [Google Scholar]
- 12.Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114:1574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louvet A, Labreuche J, Artru F, Bouthors A, Rolland B, Saffers P, et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: a prospective study. Hepatology. 2017;66:1464–73. [DOI] [PubMed] [Google Scholar]
- 14.Jepsen P, Ott P, Andersen PK, Sørensen HT, Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–82. [DOI] [PubMed] [Google Scholar]
- 15.Jepsen P, Ott P, Andersen PK, Vilstrup H. The clinical course of alcoholic cirrhosis: effects of hepatic metabolic capacity, alcohol consumption, and hyponatremia--a historical cohort study. BMC Res Notes. 2012;5:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh ND, Marrero WJ, Wang J, Steuer J, Tapper EB, Konerman M, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology. 2019;70:487–95. [DOI] [PubMed] [Google Scholar]
- 17.Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Åberg F, Helenius‐Hietala J, Puukka P, Färkkilä M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67:2141–9. [DOI] [PubMed] [Google Scholar]
- 19.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput J. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–11. [DOI] [PubMed] [Google Scholar]
- 20.Peeraphatdit T, Ahn JC, Choi DH, Allen AM, Simonetto DA, Kamath PS, et al. A cohort study examining the interaction of alcohol consumption and obesity in hepatic steatosis and mortality. Mayo Clin Proc. 2020;95:2612–2620. [DOI] [PubMed] [Google Scholar]
- 21.Mellinger J, Winder GS, Fernandez AC. Measuring the alcohol in alcohol-associated liver disease: choices and challenges for clinical research. Hepatology. 2021;73:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis. 2012;16:659–66. [DOI] [PubMed] [Google Scholar]
- 23.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62(suppl 1):S38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson RF, Hermon C, Liu B, Green J, Reeves GK, Beral V, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Health. 2019;4:e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryazka D, Reitsma MB, Griswold MG, Abate KH, Abbafati C, Abbasi-Kangevari M, et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 2022;400:185–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, et al. Trends in adult alcohol use and binge drinking in the early 21st-century United States: a meta-analysis of 6 National Survey Series. Alcohol Clin Exp Res. 2018;42:1939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson AM, Meadows BR, Emslie C, Lyons A, Sumnall HR. ‘Pretty in Pink’ and ‘Girl Power’: an analysis of the targeting and representation of women in alcohol brand marketing on Facebook and Instagram. Int J Drug Policy. 2022;101:103547. [DOI] [PubMed] [Google Scholar]
- 30.Adams RS, Ledingham E, Keyes KM. Have we overlooked the influence of “wine-mom” culture on alcohol consumption among mothers? Addict Behav. 2022;124:107119. [DOI] [PubMed] [Google Scholar]
- 31.Donnadieu-Rigole H, Olive L, Nalpas B, Winter A, Ursic-Bedoya J, Faure S, et al. Follow-up of alcohol consumption after liver transplantation: interest of an addiction team. Alcohol Clin Exp Res. 2017;41:165–70. [DOI] [PubMed] [Google Scholar]
- 32.Kirouac M, Kruger E, Wilson AD, Hallgren KA, Witkiewitz K. Consumption outcomes in clinical trials of alcohol use disorder treatment: consideration of standard drink misestimation. Am J Drug Alcohol Abuse. 2019;45:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellinger JL, Scott Winder G, DeJonckheere M, Fontana RJ, Volk ML, Lok ASF, et al. Misconceptions, preferences and barriers to alcohol use disorder treatment in alcohol-related cirrhosis. J Subst Abuse Treat. 2018;91:20–7. [DOI] [PubMed] [Google Scholar]
- 34.MWaR S. Motivational Interviewing: Helping People Change. The Guilford Press; 2013. [Google Scholar]
- 35.Schomerus G, Leonhard A, Manthey J, Morris J, Neufeld M, Kilian C, et al. The stigma of alcohol-related liver disease and its impact on healthcare. J Hepatol. 2022;77:516–24. [DOI] [PubMed] [Google Scholar]
- 36.Shabanloei R, Ebrahimi H, Ahmadi F, Mohammadi E, Dolatkhah R. Stigma in cirrhotic patients: a qualitative study. Gastroenterol Nurs May-. 2016;39:216–26. [DOI] [PubMed] [Google Scholar]
- 37.Vaughn-Sandler V, Sherman C, Aronsohn A, Volk ML. Consequences of perceived stigma among patients with cirrhosis. Dig Dis Sci. 2014;59:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkow ND. Stigma and the toll of addiction. N Engl J Med. 2020;382:1289–90. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Koob G. Brain disease model of addiction: why is it so controversial. Lancet Psychiatry. 2015;2:677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volkow ND, Michaelides M, Baler R. The neuroscience of drug reward and addiction. Physiol Rev. 2019;99:2115–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 42.Webzell I, Ball D, Bell J, Sherwood RA, Marsh A, O'Grady JG, et al. Substance use by liver transplant candidates: an anonymous urinalysis study. Liver Transpl. 2011;17:1200–04. [DOI] [PubMed] [Google Scholar]
- 43.Simon TW. Providing context for phosphatidylethanol as a biomarker of alcohol consumption with a pharmacokinetic model. Regul Toxicol Pharmacol. 2018;94:163–71. [DOI] [PubMed] [Google Scholar]
- 44.Bhavsar‐Burke I, Guardiola JJ, Holden JH. Is blood transfusion a means of false positive phosphatidylethanol testing. Liver Transpl. 2022;28:138–9. [DOI] [PubMed] [Google Scholar]
- 45.Louvet A, Bourcier V, Archambeaud I, d’Alteroche L, Chaffaut C, Oberti F, et al. Low alcohol consumption influences outcomes in individuals with alcohol-related compensated cirrhosis in a French multicenter cohort. J Hepatol. 2022;78.501–512. [DOI] [PubMed] [Google Scholar]
- 46.Lucey MR, Connor JT, Boyer TD, Henderson JM, Rikkers LF. Alcohol consumption by cirrhotic subjects: patterns of use and effects on liver function. Am J Gastroenterol. 2008;103:1698–706. [DOI] [PubMed] [Google Scholar]
- 47.Tapper EB, Hunink MGM, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One. 2016;11:e0147237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27:867–886. [DOI] [PubMed] [Google Scholar]
- 49.Saitz R, Palfai TP, Cheng DM, Horton NJ, Freedner N, Dukes K, et al. Brief intervention for medical inpatients with unhealthy alcohol use: a randomized, controlled trial. Ann Intern Med. 2007;146:167–76. [DOI] [PubMed] [Google Scholar]
- 50.Saitz R. Alcohol screening and brief intervention in primary care: absence of evidence for efficacy in people with dependence or very heavy drinking. Drug Alcohol Rev. 2010;29:631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saitz R, Palfai TP, Cheng DM, Horton NJ, Dukes K, Kraemer KL, et al. Some medical inpatients with unhealthy alcohol use may benefit from brief intervention. J Stud Alcohol Drugs. 2009;70:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol Jan-. 2014;49:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaner EF, Beyer FR, Muirhead C, Campbell F, Pienaar ED, Bertholet N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2018;2018:CD004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glass JE, Hamilton AM, Powell BJ, Perron BE, Brown RT, Ilgen MA. Specialty substance use disorder services following brief alcohol intervention: a meta-analysis of randomized controlled trials. Addiction. 2015;110:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilgen MA, Price AM, Burnett-Zeigler I, Perron B, Islam K, Bohnert ASB, et al. Longitudinal predictors of addictions treatment utilization in treatment-naive adults with alcohol use disorders. Drug Alcohol Depend. 2011;113:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klimas J, Tobin H, Field CA, O'Gorman CS, Glynn LG, Keenan E, et al. Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users. Cochrane Database Syst Rev. 2014;12:CD009269. [DOI] [PubMed] [Google Scholar]
- 57.Foxcroft DR, Coombes L, Wood S, Allen D, Almeida Santimano NM. Motivational interviewing for alcohol misuse in young adults. Cochrane Database Syst Rev. 2014;8:CD007025. [DOI] [PubMed] [Google Scholar]
- 58.Kohler S, Hofmann A. Can motivational interviewing in emergency care reduce alcohol consumption in young people? A systematic review and meta-analysis. Alcohol Alcohol. 2015;50:107–17. [DOI] [PubMed] [Google Scholar]
- 59.Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review. Clin Gastroenterol Hepatol. 2016;14:191–202 e1-4; quiz e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willenbring ML, Olson DH. A randomized trial of integrated outpatient treatment for medically ill alcoholic men. Arch Intern Med. 1999;159:1946–52. [DOI] [PubMed] [Google Scholar]
- 61.Proeschold‐Bell RJ, Evon DM, Yao J, Niedzwiecki D, Makarushka C, Keefe KA, et al. A randomized controlled trial of an integrated alcohol reduction intervention in patients with hepatitis C infection. Hepatology. 2020;71:1894–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly JF, Humphreys K, Ferri M. Alcoholics anonymous and other 12-step programs for alcohol use disorder. Cochrane Database Syst Rev. 2020;3:CD012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bertolotti M, Ferrari A, Vitale G, Stefani M, Trenti T, Loria P, et al. Effect of liver cirrhosis on the systemic availability of naltrexone in humans. J Hepatol. Sep 1997;27:505–11. [DOI] [PubMed] [Google Scholar]
- 64.Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–22. [DOI] [PubMed] [Google Scholar]
- 65.Hauser P, Fuller B, Ho SB, Thuras P, Kern S, Dieperink E. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112:1173–83. [DOI] [PubMed] [Google Scholar]
- 66.Morley KC, Baillie A, Fraser I, Furneaux-Bate A, Dore G, Roberts M, et al. Baclofen in the treatment of alcohol dependence with or without liver disease: multisite, randomised, double-blind, placebo-controlled trial. Br J Psychiatry. 2018;212:362–9. [DOI] [PubMed] [Google Scholar]
- 67.Barrault C, Lison H, Roudot-Thoraval F, Garioud A, Costentin C, Béhar V, et al. One year of baclofen in 100 patients with or without cirrhosis: a French real-life experience. Eur J Gastroenterol Hepatol. 2017;29:1155–60. [DOI] [PubMed] [Google Scholar]
- 68.Barrault C, Alqallaf S, Lison H, Lamote-Chaouche I, Bourcier V, Laugier J, et al. Baclofen combined with psychosocial care is useful and safe in alcohol-related cirrhosis patients: a real-life multicenter study. Alcohol Alcohol. 2022;58.117–124. [DOI] [PubMed] [Google Scholar]
- 69.Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, et al. Gender disparities in alcohol use disorder treatment among privately insured patients with alcohol-associated cirrhosis. Alcohol Clin Exp Res. 2019;43:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogal S, Youk A, Zhang H, Gellad WF, Fine MJ, Good CB, et al. Impact of alcohol use disorder treatment on clinical outcomes among patients with cirrhosis. Hepatology. 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vannier AGL, Shay JES, Fomin V, Patel SJ, Schaefer E, Goodman RP, et al. Incidence and progression of alcohol-associated liver disease after medical therapy for alcohol use disorder. JAMA Netw Open. 2022;5:e2213014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Im GY, Mellinger JL, Winters A, Aby ES, Lominadze Z, Rice J, et al. Provider attitudes and practices for alcohol screening, treatment, and education in patients with liver disease: a survey from the American Association for the Study of Liver Diseases Alcohol-Associated Liver Disease Special Interest Group. Clin Gastroenterol Hepatol. 2020;19.2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mason B, Heyser C. Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets. 2010;9:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. [DOI] [PubMed] [Google Scholar]
- 75.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–900. [DOI] [PubMed] [Google Scholar]
- 76.Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction. 2015;110:920–30. [DOI] [PubMed] [Google Scholar]
- 77.Ayyala D, Bottyan T, Tien C, Pimienta M, Yoo J, Stager K, et al. Naltrexone for alcohol use disorder: hepatic safety in patients with and without liver disease. Hepatol Commun. 2022;6:3433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamini D, Lee SH, Avanesyan A, Walter M, Runyon B. Utilization of baclofen in maintenance of alcohol abstinence in patients with alcohol dependence and alcoholic hepatitis with or without cirrhosis. Alcohol Alcohol Jul-Aug. 2014;49:453–6. [DOI] [PubMed] [Google Scholar]
- 79.Leggio L, Mellinger JL. Alcohol use disorder in community management of chronic liver diseases. Hepatology. 2022;77:1006–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38:1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartwell EE, Feinn R, Witkiewitz K, Pond T, Kranzler HR. World Health Organization risk drinking levels as a treatment outcome measure in topiramate trials. Alcohol Clin Exp Res. 2021;45:1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Intern Med. 2008;168:1188–99. [DOI] [PubMed] [Google Scholar]
- 83.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Anton RF, Latham P, Voronin K, Book S, Hoffman M, Prisciandaro J, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180:728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Falk DE, Ryan ML, Fertig JB, Devine EG, Cruz R, Brown ES, et al. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res. 2019;43:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laska EM, Siegel CE, Lin Z, Bogenschutz M, Marmar CR. Gabapentin enacarbil extended-release versus placebo: a likely responder reanalysis of a randomized clinical trial. Alcohol Clin Exp Res. 2020;44:1875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leung E, Ngo DH, Espinoza JA, Jr, Beal LL, Chang C, Baris DA, et al. A retrospective study of the adjunctive use of gabapentin with benzodiazepines for the treatment of benzodiazepine withdrawal. J Psychiatr Pract. 2022;28:310–8. [DOI] [PubMed] [Google Scholar]
- 88.Smith JT, Sage M, Szeto H, Myers LC, Lu Y, Martinez A, et al. Outcomes after implementation of a benzodiazepine-sparing alcohol withdrawal order set in an integrated health care system. JAMA Netw Open. 2022;5:e220158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Bejczy A, Löf E, Walther L, Guterstam J, Hammarberg A, Asanovska G, et al. Varenicline for treatment of alcohol dependence: a randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2015;39:2189–99. [DOI] [PubMed] [Google Scholar]
- 90.Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med Jul-. 2013;7:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKee SA, Harrison ELR, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl). 2012;223:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winder GS, Fernandez AC, Mellinger JL. Integrated care of alcohol-related liver disease. J Clin Exp Hepatol Jul-Aug. 2022;12:1069–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mellinger JL, Winder GS, Fernandez AC, Klevering K, Johnson A, Asefah H, et al. Feasibility and early experience of a novel multidisciplinary alcohol-associated liver disease clinic. J Subst Abuse Treat. 2021;130:108396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fomin V, Marshall C, Tsai S, Goodman R, Schaefer E, Luther J. Creation of an inpatient alcohol liver service improves early liver disease detection in patients with alcohol use disorder. Clin Gastroenterol Hepatol. 2022;S1542-3565:00382. [DOI] [PubMed] [Google Scholar]
- 96.Winder GS, Clifton EG, Fernandez AC, Mellinger JL. Interprofessional teamwork is the foundation of effective psychosocial work in organ transplantation. Gen Hosp Psychiatry. 2021;69:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Winder GS, Clifton EG, Mellinger JL. Substance use disorders in organ transplantation: perennial challenges and interprofessional opportunities. Curr Opin Organ Transplant. 2022;27:495–500. [DOI] [PubMed] [Google Scholar]
- 98.Winder GS, Clifton EG, Perumalswami P, Mellinger JL. The art of interprofessional psychosocial communication: optimizing patient interfaces with psychiatric specialists in liver transplantation. Transplant Rev (Orlando). 2022;36:100728. [DOI] [PubMed] [Google Scholar]
- 99.Winder GS, Fernandez AC, Klevering K, Mellinger JL. Confronting the crisis of comorbid alcohol use disorder and alcohol-related liver disease with a novel multidisciplinary clinic. Psychosomatics. 2020;61:238–53. [DOI] [PubMed] [Google Scholar]
- 100.Gittell JH, Fairfield KM, Bierbaum B, Head W, Jackson R, Kelly M, et al. Impact of relational coordination on quality of care, postoperative pain and functioning, and length of stay: a nine-hospital study of surgical patients. Med Care. 2000;38:807–19. [DOI] [PubMed] [Google Scholar]
- 101.Havens DS, Vasey J, Gittell JH, Lin WT. Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18:926–37. [DOI] [PubMed] [Google Scholar]


