Abstract
Previous studies demonstrated associations of endogenous sex hormones with diabetes. Less is known about their dynamic relationship with diabetes progression through different stages of the disease, independence of associations, and role of the hypothalamic-pituitary gonadal axis.
The purpose of this analysis was to examine relationships of endogenous sex hormones with incident diabetes, prediabetes, and diabetes traits in 693 postmenopausal women and 1015 men aged 45 to 74 years without diabetes at baseline participating in the Hispanic Community Health Study/Study of Latinos and followed for 6 years. Baseline hormones included estradiol, luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), and, in men, testosterone and bioavailable testosterone. Associations were analyzed using multivariable Poisson and linear regressions. In men, testosterone was inversely associated with conversion from prediabetes to diabetes (incidence rate ratio [IRR] for 1 SD increase in testosterone: 0.821; 95% CI, 0.676, 0.997; P = 0.046), but not conversion from normoglycemia to prediabetes. Estradiol was positively associated with increase in fasting insulin and homeostatic model assessment of insulin resistance. In women, SHBG was inversely associated with change in glycosylated hemoglobin, postload glucose, and conversion from prediabetes to diabetes (IRR = 0.62; 95% CI, 0.44, 0.86, P = 0.005) but not from normoglycemia to prediabetes. Relationships with other hormones varied across glycemic measures. Stronger associations of testosterone and SHBG with transition from prediabetes to diabetes than from normoglycemic to prediabetes suggest they are operative at later stages of diabetes development. Biologic pathways by which sex hormones affect glucose homeostasis await future studies.
Keywords: sex, hormones, diabetes
Currently, 37.3 million adults or 11.3% of the US adult population have diabetes, most of which is type 2 diabetes; an additional 38.0% have prediabetes and are at heightened risk of developing type 2 diabetes (1). Rates are higher in men than women and among Black, American Indian/Alaskan Natives, Hispanic, and Asian adults than non-Hispanic White adults (1). Among Hispanics, there is wide variation in diabetes rates, with those of Mexican and Puerto Rican heritage having higher rates than those of Cuban or South American backgrounds (2). Rates of diabetes increase with age and after menopause in women, suggesting that variations in sex hormones affect the incidence and prevalence of the disease. Hypothesized mechanisms for the postmenopausal increased risk of diabetes are age-related increased weight independent of menopause, changes in body fat distribution with associated loss of estrogens, increased testosterone, and elevated inflammatory cytokines (3). The precise nature of these relationships, however, remains incompletely understood.
Among men, previous studies have shown fairly consistent inverse associations of testosterone (4-29) and its binding protein, sex hormone binding globulin (SHBG) (7, 11, 12, 24-31) with measures of diabetes, prediabetes, and insulin resistance in both cross-sectional and prospective cohort studies (8). In addition, there is evidence that some of the associations may be bidirectional and that their effects may be modified by measures of adiposity (6, 18, 29). Testosterone may affect diabetes risk by altering expression of the insulin signaling intermediate insulin-receptor substrate-1 and glucose transporter type 4 (GLUT4) or by modulating mitochondrial function (32). Studies relating the androgen precursor dehydroepiandrosterone (DHEA) and its sulfated derivative DHEAS with glucose homeostasis have been less consistent, with inverse associations noted in some, but not all, previous studies (33-37).
The effects of estrogens on diabetic traits have been less well studied in epidemiologic analyses. The estrogen receptors (ER) ERα and ERβ affect glucose homeostasis through alterations in insulin sensitivity in the liver and muscle, adipocyte growth, pancreatic β-cell function, and regulation of GLUT4 expression in muscle (38-43). In men, there is some evidence that endogenous estrogens increase risk of diabetes (6, 8, 22) and prediabetes (11, 22). In postmenopausal women, diabetes and insulin resistance have been positively associated with endogenous estrogens (8, 34, 44-46), bioavailable estrogens (47), and testosterone but negatively with SHBG (8, 12, 45, 46, 48). Associations with DHEAS are largely inconsistent (23, 36, 44, 46). The inverse associations of SHBG with diabetes (8) and insulin resistance (49) have been somewhat stronger in postmenopausal women than in men (8, 49). In contrast exogenous hormone replacement therapy, both estrogens alone as well as in combination with progestins, has generally been associated with lower risk of diabetes (50-53), with some evidence of protective effects closer to the beginning of menopause and less of a protective effect or even increased risk afterward (54, 55).
The hypothalamic-pituitary-gonadal axis is a complex regulatory system governing circulating levels of sex steroid hormones (testosterone and estradiol). Regulation of this system includes negative feedback loops involving multiple hormonal layers integrating peripheral and pituitary secreted gonadotropins follicle stimulating hormone (FSH) and luteinizing hormone (LH), and with peripheral hormone bioavailability further regulated by circulating binding proteins (eg, SHBG). There are a few studies examining the effects of pituitary hormones on diabetes or prediabetes, and they generally show inverse associations of markers of insulin resistance, prediabetes, and diabetes with FSH and/or LH (56-59).
Despite the increasing number of studies examining the effects of sex hormones on glucose homeostasis, many questions remain unanswered. It is unclear how sex hormones alter diabetes pathogenesis, whether they act independently or synergistically, the role of the complete hypothalamic-pituitary-gonadal axis, and how the effects of the hormones relate to well-established risk factors such as obesity and metabolically deleterious fat distribution. To the best of our knowledge, there have been no population-based studies of these associations among diverse Hispanic/Latino adults, a group at particularly high risk for diabetes. The Hispanic Community Health Study/Study of Latinos (HCHS/SOL), the largest longitudinal cohort of Hispanic/Latino adults in the United States, noted that among Hispanic/Latino adults aged 45 years and older, the prevalence of diabetes varied from 10.2% for those of South American background to 18.3% for those of Mexican background (60). Baseline examinations, however, did not originally include measurements of endogenous sex hormones. The present ancillary study is nested within the HCHS/SOL cohort and, with the addition of hormones measured from stored serum samples at baseline, examines the effect of baseline sex hormones, and the complex relationships between the hypothalamic-pituitary-gonadal axis and glucose homeostasis, on the development of insulin resistance, prediabetes, and diabetes at the first follow-up visit, on average 6 years after the baseline visit.
Methods
The HCHS/SOL, a prospective cohort of 16 415 adult Latinos with high rates of diabetes (2), offers a unique opportunity to examine the effects of sex hormones on diabetes and prediabetes in a large, diverse prospective cohort with substantial variation in diabetes risk. Details of the HCHS/SOL have been described elsewhere (2, 60, 61). After institutional research committees approved the investigation and consent was obtained from participants, 16 415 Hispanic/Latino adults aged 18 to 74 years were recruited from San Diego, CA; Bronx, NY; Miami, FL; and Chicago, IL, between March 2008 and June 2011 (visit 1). Recruitment was based on a multistage probability sample, with random selection of census blocks followed by random selection of households and oversampling of blocks with higher concentrations of Hispanic/Latino residents, households with Hispanic/Latino surnames, and persons aged 45 to 74 years. Sampling weights were then generated to reflect the probabilities of selection at each stage. Participants included persons of Cuban, Mexican, Puerto Rican, Dominican, Central American, and South American backgrounds.
The baseline examination (visit 1) included questionnaire data on demographic factors, education, income, acculturation, years of residence in the United States, language preference, physical activity, weight change in the past year, smoking and medical history, medication use, reproductive and breast feeding history, two 24-hour dietary recalls, dietary frequency, blood pressure, height, weight, waist/hip ratio, and fasting serum and plasma samples, with measurements that included total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol, triglycerides, fasting glucose, 2-hour postload glucose in the subset of persons with fasting glucose <150 mg/dL, insulin, glycosylated hemoglobin (HbA1c), and high sensitivity C-reactive protein (hsCRP). From 2014 to 2017, the study implemented the 6-year follow-up (visit 2) that included repeat assessment of lifestyle and medical history, height, weight, waist/hip ratio, dietary history, lipids, fasting and postload glucose, fasting insulin, and HbA1c. Before the baseline and follow-up study visits, participants were instructed to refrain from physical activity and smoking, and fast for at least 8 hours with allowances for water and essential medications.
The Persistent Organic Pollutants, Endogenous Hormones and Diabetes in Latinos Ancillary Study selected HCHS/SOL participants aged 45 to 74 years at baseline using a case-cohort study design. To select the random sample, participants were stratified by baseline glucose measurements (1171 with prediabetes at baseline and 1172 with normal baseline glucose measurements) approximately equally divided between men and women aged 45 to 74 years (1146 men and 1197 women), and with only 1 participant per household within each sex and blood glucose subgroup. Participants who transitioned from prediabetes to diabetes during the follow-up period were oversampled to ensure that approximately one-half of those in this category had transitioned to diabetes. Of the 2343 HCHS/SOL ancillary study participants with hormone measurements available, 1708 (73%) were included in the cross-sectional analyses (693 postmenopausal women and 1015 men) and 1621 (69%) in the longitudinal analyses (657 postmenopausal women and 964 men) after excluding pre- or perimenopausal women (based on self-report and hormone measures, described later), relevant medication use (including 10 women taking metformin), and those with missing data (Supplementary Table S1 (62)).
Hormone Measurements
Gonadotropin and sex steroid hormone measurements were conducted on stored serum samples collected at the baseline HCHS/SOL examination. All hormones were measured using a Roche COBAS 6000 chemistry analyzer (Roche Diagnostics, Indianapolis, IN). LH and FSH were measured using an LH or FSH reagent/sandwich immunoassay (Catalog #11732234122, RRID: AB_2800498 and Catalog #11775863122, RRID: AB_2800499, Roche Diagnostics). SHBG was measured using an SHBG reagent/sandwich immunoassay method/electrochemiluminescence assay (Catalog #03052001160, RRID: AB_2891165, Roche Diagnostics). Estradiol was measured using an estradiol III reagent/competitive immunoassay method (Catalog #06656021190, RRID: AB_2905575, Roche Diagnostics). DHEAS was measured using a DHEAS reagent/competitive immunoassay method/electrochemiluminescence assay (Catalog #03000087122, RRID: AB_2909490, Roche Diagnostics).
In male samples, testosterone was measured using a testosterone II reagent/competitive immunoassay/electrochemiluminescence assay (Catalog #05200067160, RRID: AB_2783736 Roche Diagnostics). The lower limits of detection for LH, FSH, SHBG, estradiol, DHEAS, and testosterone were 0.100 mIU/mL, <0.100 mIU/mL, 0.800 nmol/L, 18.4 pmol/L, 0.003 µmol/L, and 2.5 ng/dL, respectively. Interassay coefficients of variation were 3.3% at 11.15 mIU/mL, 3.1% at 17.23 mIU/mL, 3.8% at 19.74 nmol/L, 5.7% at 143.3 pmol/L, 4.6% at 5.43 µmol/L, and 2.2% at 272.5 ng/dL for LH, FSH, SHBG, estradiol, DHEAS, and testosterone, respectively. All assays were performed by the Advanced Research & Diagnostics Laboratory at the University of Minnesota, which is a CAP/CLIA-certified laboratory.
In men, concentrations of testosterone (ng/dL), SHBG (nmol/L), and estradiol (pg/mL) were used to calculate concentrations of unbound (free) testosterone according to the method of Vermeulen et al (63). Limited sample volume and lower testosterone levels precluded measurements of testosterone in women. Because this sample was of postmenopausal women, estradiol was extremely skewed, with 71% below the limit of detection; in women, estradiol was analyzed dichotomously as below or above the limit of detection, rather than as a continuous variable. Using reference values from assays performed at the HCHS/SOL study laboratory and derived from study participants, we dichotomized individual hormone measures among women as low SHBG (< 20 nmol/L), low estradiol (< 18.4 pmol/L), and low DHEAS (<0.26 µmol/L); whereas among men, hormone measures were dichotomized as high LH (>8.6 mIU/mL), high FSH (> 12.4 mIU/mL), low SHBG (<10 nmol/L), high estradiol (>159 pmol/L), low testosterone (<193 ng/dL), and low DHEAS (<0.44 µmol/L).
Outcome Assessment
All participants had fasting plasma glucose (FPG) and HbA1c measured. FPG was measured in EDTA plasma using a hexokinase enzymatic method (Roche Diagnostics) on a Roche Modular P chemistry analyzer (Roche Diagnostics). HbA1c was measured in EDTA whole blood using a Tosch G7 automated high-performance liquid chromatography analyzer (Tosch Bioscience Inc., South San Francisco, CA). Insulin was measured in serum using a commercial ELISA assay (Mercodia AB, Uppsala, Sweden).
Following the initial fasting blood draw, all participants who did not report taking antihyperglycemic medications, previous diagnosis with diabetes, and/or who had an FPG <150 mg/dL underwent a standard 75-g 2-hour oral glucose tolerance test (OGTT), and 2-hour (postload) plasma glucose was measured. Homeostasis model assessment index of insulin resistance (HOMA-IR) was calculated as the product of fasting glucose (mg/dL) and fasting insulin (mU/L) divided by 405, whereas homeostatic model assessment index of β-cell function (HOMA-B) was calculated as the product of fasting insulin (mU/L) and 360 divided by fasting glucose (mg/dL) minus 63. We defined prediabetes at baseline examination among participants if they met 1 of the following criteria: (1) laboratory tests elevated above normal but outside the diabetic range (fasting time >8 hours and FPG = 100-125 mg/dL [5.6-6.9 mmol/L]; or 2-hour postload OGTT = 140-199 mg/dL [7.8-11.0 mmol/L]; or HbA1c = 5.7%-6.4%), and (2) no self-reported diabetes. Normoglycemia was defined as (1) (fasting time >8 hours and FPG <100 mg/dL [5.6 mmol/L]; (2) 2-hour postload OGTT < 140 mg/dL [7.8 mmol/L]; or HbA1c < 5.7%), and (3) no self-reported diabetes. Diabetes at study visit 2 was classified as meeting 1 or more of the following criteria: (1) elevated fasting glucose (fasting time >8 hours and FPG ≥ 126 mg/dL [7.0 mmol/L] or fasting time ≤8 hours and fasting glucose ≥200 mg/dL [11.1 mmol/L]); or (2) glucose intolerance (2-hour postload OGTT ≥200 mg/dL [11.1 mmol/L]); or (3) elevated HbA1c (HbA1c ≥6.5%); or (4) self-reported diabetes.
For the prospective analysis, we examined progression from normoglycemic at baseline examination to prediabetes, from normoglycemic at baseline examination to prediabetes or diabetes, and from prediabetes at baseline examination to diabetes at study visit 2. We also examined continuous change scores, using change in absolute levels, for the following glycemic measures: HbA1c, fasting glucose, fasting insulin, postload glucose, HOMA-B, and HOMA-IR. Change scores were calculated as the difference in glycemic measurement between study visit 2 and baseline examination.
Covariates
Study participants reported their age, sex (male or female), HCHS/SOL recruitment center (Bronx, NY; Chicago, IL; Miami, FL; or San Diego, CA), educational attainment (less than high school, high school diploma/GED, greater than high school diploma/GED), Hispanic/Latino heritage (Dominican, Central American, Cuban, Mexican, Puerto Rican, South American, more than 1/Other heritage), smoking status (current, former, or never smokers), annual household income ($30 000 or more, less than $30 000, or missing/not reported), health insurance status (currently insured or not currently insured), alcohol consumption (no consumption, low, or high consumption), physical activity level based on the World Health Organization Global Physical Activity Questionnaire (64) (low levels, moderate levels, or high physical activity levels), diet quality score per the Alternative Healthy Eating Index 2010 (65) (range from 0 to 110, with higher scores indicating better diet quality), family history of diabetes, and history of gestational diabetes (only among female participants). Anthropometric measurements of weight (kg), height (cm), hip (cm), and waist circumference (cm) were performed by trained study staff following a standard protocol. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared, whereas waist/hip ratio was calculated as waist circumference in centimeters divided by hip circumference in centimeters. For the longitudinal analysis assessing continuous change scores in glycemic measures, we included change in BMI and waist/hip ratio measurements calculated as BMI at visit 2 minus BMI at baseline and waist/hip ratio at visit 2 minus waist/hip ratio at baseline examination. Acculturation score-Multi-Ethnic Study of Atherosclerosis (66) (range from 0 to 5 with 0 indicating least acculturation and 5 indicating most acculturation) was defined by summing scores from proxy measures of acculturation: nativity and years of residence in the United States and primary language spoken at home.
Participants’ self-reported medication use was recorded by trained study staff. Three blood pressure (BP) measurements were taken on the right arm by a trained technician and the average systolic BP (mmHg) and diastolic BP (mmHg) were obtained. Hypertension was defined as average systolic BP ≥ 140 mmHg, average diastolic BP ≥ 90 mmHg, or self-reported use of antihypertensive medication (67). Among women, menopause status was derived by a series of steps that included evaluation of the participant's age (> 62 years old determined to be postmenopausal), history of bilateral oophorectomy, and menstruation status (among those who reported absence of menses, if FSH was ≥ 25.8 mIU/mL or LH ≥ 7.7 mIU/mL, the participant was determined to be postmenopausal; among those with FSH < 25.8 mIU/mL and LH < 7.7 mIU/mL, the participant was determined to be perimenopausal). If the participant reported current menses or did not answer the question regarding menstruation status and FSH was ≥ 25.8 mIU/mL or LH ≥ 7.7 mIU/mL, the participant was determined to be perimenopausal, and for those with FSH < 25.8 mIU/mL and LH < 7.7 mIU/mL, the participant was determined to be premenopausal. Furthermore, measurements of lipid metabolism and inflammation at baseline examination were included as covariates: hsCRP (mg/L), total cholesterol (mg/dL), triglycerides (mg/dL), HDL cholesterol (mg/dL), and low-density lipoprotein cholesterol (mg/dL). For the longitudinal analysis, we also included information on mean follow-up time for participants calculated from the date of first study visit 1 (baseline examination) to visit 2 (first follow-up examination).
Statistical Analyses
Our analysis accounted for the complex sampling design used to select participants for the HCHS/SOL cohort, with additional sampling weights created to account for the sampling design of this ancillary study. We used statistical methods that included stratification factors, clustering, and weights to adjust for sampling probability and nonresponse, consistent with guidelines established by the HCHS/SOL Statistics and Data Analysis Committee. Statistical analyses and data management were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and STATA (version 17.0, StataCorp, College Station). Because of skewed data we natural log-transformed glycemic measures (fasting insulin, HOMA-B, and HOMA-IR) to approximate a normal distribution. For descriptive analyses, we calculated means (95% CI) for continuous data using SAS PROC SURVEYMEANS and assessed frequencies (percentages) for categorical data using SAS PROC SURVEYFREQ. Differences between groups were evaluated using t tests for continuous data and χ2 tests for categorical data. To examine correlations among hormone concentrations with other covariates, we calculated Spearman correlation coefficients.
Cross-sectional associations of sex hormones with continuous glycemic measures were modeled using multivariable linear regression, whereas cross-sectional associations of sex hormones with prediabetes status were modeled using multivariable logistic regression. Longitudinal associations of sex hormones with diabetes incidence and progression were modeled using Poisson regression to estimate incident rate ratios (IRR), whereas associations with change scores for continuous glycemic measures were modeled using multivariable linear regression. For both the cross-sectional and longitudinal analyses, hormone concentrations were individually modeled as continuous and dichotomous predictors based on cutoffs from laboratory reference ranges, as described previously. To estimate nonlinear dose-response models, we created quintiles of hormone concentrations with quintile 1 as the reference category. For continuous hormone models, we conducted Z-score standardization of hormones using the corresponding sample mean and SD of the hormone measure from the HCHS/SOL ancillary study and present findings as a 1 SD increase in hormone concentration. For exploratory analyses, to evaluate the hypothalamic-pituitary-gonadal axis, we also created the ratio of LH/testosterone, bioavailable estradiol/bioavailable testosterone, and estradiol/testosterone in men, as well as LH/FSH in both men and women. We also mutually controlled SHBG, estradiol, and testosterone for one another in men. For both the cross-sectional and longitudinal analyses, stratification by sex was obtained by using the domain or subpopulation commands in SAS or STATA. The longitudinal analyses examining diabetes incidence and progression were further stratified by baseline prediabetes status.
Covariates were evaluated as potential confounders by constructing a series of models leading to the final model 4 presented in the tables in this paper. For the cross-sectional analyses, level 1 models adjusted for age in years, BMI, waist/hip ratio, acculturation score-Multi-Ethnic Study of Atherosclerosis, HCHS/SOL study sites, Hispanic/Latino background, educational attainment, statin medication use, family history of diabetes, and gestational diabetes (women only). Level 2 models adjusted for covariates in level 1 plus lifestyle factors such as cigarette use, alcohol use, healthy eating index, and physical activity. Level 3 models adjusted for covariates in level 2 plus hypertension, high triglycerides (≥ 200 mg/dL), and low HDL (< 40 mg/dL), whereas the final level 4 models adjusted for covariates in level 3 plus hsCRP (continuous). Moreover, for the longitudinal analysis examining change scores in glycemic measures; level 1 models additionally adjusted for baseline levels of each glycemic measure, prediabetes status at baseline, mean follow-up time between visit 1 and 2, and antidiabetic medication use at follow-up, whereas level 2 models additionally adjusted for change in BMI and waist/hip ratio measurements. Other covariate adjustments for levels 1 through 4 in the longitudinal analysis were consistent with the cross-sectional analysis. Finally, before proceeding to higher level models, we examined model fit using R2 and Akaike Information Criterion for linear and logistic regression models, respectively. Using level 1 models, we examined model fit for associations of untransformed and natural log-transformed hormones with (1) prediabetes and (2) untransformed and natural log-transformed glycemic measures. We also determined whether BMI and waist/hip ratio were independent predictors in both cross-sectional and longitudinal analysis by exploring change in estimates of hormone concentrations adjusted for BMI alone, waist/hip ratio alone, and both BMI and waist/hip ratio included in the model. We found no difference in the main estimates of interest across these alternative approaches and included both BMI and waist/hip ratio in subsequent level 2 through 4 models. Overall results did not substantially vary among the different level models. Therefore, for simplicity, only level 4 models are presented in the tables.
For the longitudinal analysis, to examine subgroups at higher risk, we examined potential effect modification between hormone concentrations and age, baseline obesity, and hsCRP by including a product term (hormone*effect modifier) to level 4 models. Product terms with a P value less than 0.05 were further assessed by stratum specific estimates as follows: baseline overweight or obesity (BMI ≥ 25 vs <25), age (>median vs <median; 52 years and 56 years for males and females, respectively), and CRP (>2 mg/L vs ≤2 mg/L).
In an effort to explore possible mechanisms by which hormones may be operative (decreased muscle glucose uptake represented by increased postload glucose vs impaired suppression of hepatic glucose production represented primarily by increased fasting glucose as well as increased postload glucose) (68), we conducted an exploratory subgroup analysis for transition from prediabetes to diabetes in men and in women using Poisson regression models adjusted for age and BMI at baseline and using time between visits 1 and 2 as an offset. Prediabetes was defined by only elevated fasting plasma glucose (100-125 mg/dL) with normal postload glucose (<140 mg/dL) and by elevated postload glucose (140-199 mg/dL) with normal fasting plasma glucose (<100 mg/dL). Likewise, we examined transition from normoglycemia to prediabetes defined by only elevated fasting plasma glucose, only elevated postload glucose, and both elevated fasting plasma glucose and postload glucose. For this subgroup analysis, we used multinomial logistic regression models adjusting for age and BMI at baseline to examine associations because of limitations of Poisson regression for nominal outcomes.
Results
Hormones were measured in 1197 women and 1146 men. Exclusions are summarized in Supplementary Table S1 (62), with criteria not mutually exclusive. Among women, the largest number of exclusions were for those who were either pre- or perimenopausal status (n = 363), followed by those using exogenous sex steroids (n = 41) or thyroid hormones (n = 90). The largest exclusions for men were for use of 5-alpha reductase inhibitors (n = 31) followed by use of thyroid medications (n = 21). In addition, 23 women and 58 men were excluded because of missing data on covariates. The final numbers included in the analyses in this paper were 693 women and 1015 men.
Demographic and lifestyle differences at baseline by sex and prediabetes status are presented in Table 1 and Supplementary Tables S2 and S3 (62). Both men and women with prediabetes were somewhat older than those without prediabetes (60.2 vs 58.2, P = 0.099 for women; 56.3 vs 54.0, P = 0.004 for men). Similarly, the percent who were obese (BMI ≥30) was significantly higher in those who had prediabetes (51.9 vs 26.6%, P = 0.002 for women: 34.3% vs 20.5%, P = 0.001 for men). Men, but not women, tended to be born outside the mainland United States (82.9% vs 75.0%, P = 0.04) and to have Spanish rather than English as their preferred language (87.8% vs 78.3%, P = 0.009). Men, but not women, with prediabetes tended to have a lower acculturation scale (1.61 vs 2.15, P < 0001) and to have hypertension (40.2 vs 29.4%, P = 0.01). There were no significant differences by prediabetes status among either men or women in physical activity, family history of diabetes, recruitment center, or education.
Table 1.
Demographic characteristics of postmenopausal women and men by baseline diabetes status
| Women N = 693 | Men N = 1015 | |||||
|---|---|---|---|---|---|---|
| Prediabetic | Normoglycemic | Prediabetic | Normoglycemic | |||
| N = 388 | N = 305 | N = 498 | N = 517 | |||
| n (%) or mean (95% CI) | n (%) or mean (95% CI) | P value | n (%) or mean (95% CI) | n (%) or mean (95% CI) | P value | |
| Age | ||||||
| Age, y | 60.2 (58.2-62.3) | 58.2 (56.8-59.6) | 0.099 | 56.3 (55.2-57.4) | 54.0 (53.0-55.1) | 0.004 |
| Age 45-54 | 135 (26.5) | 137 (33.6) | 0.336 | 269 (44.5) | 335 (60.1) | 0.007 |
| Age 55-64 | 204 (40.4) | 137 (42.1) | 172 (35.1) | 144 (26.6) | ||
| Age 65+ | 49 (33.1) | 31 (24.2) | 57 (20.4) | 38 (13.3) | ||
| Hispanic/Latino background a | ||||||
| Dominican | 46 (9.6) | 33 (9.3) | 0.871 | 46 (9.3) | 40 (7.2) | 0.225 |
| Central American | 49 (7.5) | 23 (4.9) | 0.451 | 38 (5.3) | 47 (9.6) | 0.270 |
| Cuban | 43 (25.6) | 54 (31.9) | 0.455 | 117 (32.5) | 89 (24.0) | 0.062 |
| Mexican (ref) | 148 (33.4) | 115 (30.3) | ref | 174 (29.8) | 196 (34.0) | ref |
| Puerto Rican | 76 (17.5) | 49 (14.9) | 0.878 | 81 (13.4) | 82 (17.1) | 0.689 |
| South American | 21 (3.5) | 24 (5.5) | 0.227 | 28 (3.7) | 53 (5.6) | 0.454 |
| More than 1/Other heritage | 5 (2.9) | 7 (3.3) | 0.786 | 14 (5.9) | 10 (2.6) | 0.088 |
| County of birth | ||||||
| Foreign | 297 (78.0) | 243 (82.9) | 0.321 | 402 (82.9) | 394 (75.0) | 0.040 |
| United States (excluding territories) | 91 (22.0) | 62 (17.1) | 96 (17.1) | 123 (25.0) | ||
| Nativity subscore (MESA) | ||||||
| Non-US born and YRSUS < 10 | 74 (19.81) | 62 (25.5) | 0.015 | 102 (24.9) | 99 (18.5) | 0.000 |
| Non-US born and YRSUS 10-19 | 72 (18.47) | 49 (16.4) | 0.140 | 102 (23.4) | 89 (17.1) | 0.001 |
| Non-US born and YRSUS ≥20 | 211 (51.97) | 171 (54.0) | 0.048 | 253 (44.8) | 248 (47.1) | 0.006 |
| US born (ref) | 31 (9.75) | 23 (4.1) | ref | 41 (7.0) | 81 (17.3) | ref |
| Language preference | ||||||
| Spanish | 340 (91.3) | 270 (87.3) | 0.311 | 437 (87.8) | 414 (78.3) | 0.009 |
| English | 48 (8.7) | 35 (12.7) | 61 (12.2) | 103 (21.8) | ||
| Educational attainment | ||||||
| Less than high school | 193 (41.9) | 124 (38.0) | 0.208 | 196 (39.0) | 169 (29.9) | 0.144 |
| High school diploma/GED | 64 (12.6) | 67 (21.4) | 123 (21.5) | 127 (22.0) | ||
| Greater than high school diploma or GED | 131 (45.6) | 114 (40.6) | 179 (39.5) | 221 (48.1) | ||
| Body mass index | ||||||
| Under/normal weight (BMI < 25) | 46 (13.3) | 72 (26.5) | 0.002 | 76 (20.2) | 137 (30.5) | 0.001 |
| Overweight (25 ≤ BMI < 30) | 133 (34.7) | 129 (46.9) | 219 (45.5) | 261 (49.0) | ||
| Obese (BMI ≥ 30) | 209 (51.9) | 104 (26.6) | 203 (34.3) | 119 (20.5) | ||
| Physical activity level | ||||||
| High | 14 (2.8) | 21 (5.4) | 0.312 | 87 (15.8) | 91 (14.4) | 0.583 |
| Moderate | 159 (40.4) | 135 (46.8) | 231 (43.5) | 241 (47.8) | ||
| Low | 215 (56.8) | 149 (47.8) | 180 (40.7) | 185 (37.8) | ||
| History of gestational diabetes | ||||||
| No | 381 (99.6) | 303 (99.7) | 0.528 | na | na | |
| Yes | 7 (0.5) | 2 (0.3) | na | na | ||
| Hypertension (BP ≥ 140/90 or medication use) | ||||||
| No | 237 (57.9) | 212 (60.0) | 0.748 | 288 (59.8) | 385 (70.6) | 0.012 |
| Yes | 151 (42.1) | 93 (40.0) | 210 (40.2) | 132 (29.4) | ||
| Cigarette use | ||||||
| Never (ref) | 252 (68.0) | 195 (69.1) | ref | 195 (35.4) | 217 (44.3) | ref |
| Former | 78 (17.1) | 61 (16.5) | 0.867 | 167 (37.4) | 179 (31.3) | 0.069 |
| Current | 58 (14.9) | 49 (14.3) | 0.862 | 136 (27.2) | 121 (24.4) | 0.142 |
| Alcohol use b | ||||||
| None | 231 (65.5) | 200 (66.2) | 0.992 | 195 (44.4) | 206 (44.4) | 0.655 |
| Low | 151 (32.9) | 98 (32.4) | 265 (49.4) | 266 (47.8) | ||
| High | 6 (1.5) | 7 (1.4) | 38 (6.2) | 45 (7.8) | ||
| Family history of diabetes | ||||||
| No | 187 (52.2) | 180 (61.4) | 0.239 | 269 (58.4) | 326 (62.5) | 0.382 |
| Yes | 201 (47.8) | 125 (38.6) | 229 (41.6) | 191 (37.6) | ||
| Statin medication use | ||||||
| No | 326 (86.6) | 268 (88.6) | 0.621 | 458 (90.8) | 480 (91.7) | 0.748 |
| Yes | 62 (13.5) | 37 (11.4) | 40 (9.2) | 37 (8.3) | ||
| Recruitment center | ||||||
| Bronx | 105 (29.5) | 71 (28.6) | 0.808 | 114 (27.2) | 100 (25.4) | 0.357 |
| Chicago (ref) | 95 (11.2) | 60 (11.9) | ref | 117 (12.5) | 151 (14.9) | ref |
| Miami | 80 (34.7) | 86 (41.0) | 0.787 | 159 (39.6) | 140 (33.3) | 0.109 |
| San Diego | 108 (24.7) | 88 (18.5) | 0.317 | 108 (20.7) | 126 (26.4) | 0.757 |
| Acculturation score—MESA c | 1.80 (1.57, 2.02) | 1.73 (1.46, 1.99) | 0.649 | 1.61 (1.44, 1.78) | 2.15 (1.95, 2.34) | <0.0001 |
Abbreviations: BMI, body mass index; BP, blood pressure; MESA, Multi-Ethnic Study of Atherosclerosis; YRSUS, Years living in mainland United States.
P value of overall test for Hispanic/Latino background: women (P = 0.684) and men (P = 0.083).
No alcohol use defined as never or former users, low use defined as <7 drinks/wk for women and <14 drinks/wk for men, high-level use defined as at least 7 drinks/wk for women and at least 14 drinks/wk for men.
MESA acculturation score is a summary score based on nativity, language spoken at home, and years of residence in the United States, and ranges from 0 to 5 in 0.5 increments. 0 indicates lowest level of acculturation, 5 the highest.
Correlations among the hormones and selected covariables are presented in Supplementary Table S4 (62). There were particularly strong correlations between FSH and LH in women (r = 0.81), and in men (r = 0.56), as well as between SHBG and testosterone in men (r = 0.68).
Longitudinal: Men
Progression from normoglycemia and from prediabetes
In men, among the 517 who were normoglycemic at baseline, 233 remained normoglycemic, 274 progressed to prediabetes, and 10 progressed to diabetes. Among the 498 men who were prediabetic at baseline, 43 were normoglycemic, 219 remained prediabetic, and 236 progressed to diabetes at follow-up. Results of progression from normoglycemic to prediabetes and to prediabetes plus diabetes as well as results of progression from prediabetes to diabetes are presented in Table 2 and Supplementary Table S5 (62). In men, after controlling for confounders, testosterone was inversely associated (IRR = 0.821; 95% CI, 0.676, 0.997; P = 0.046), whereas low testosterone (IRR = 1.75; 95% CI, 1.18, 2.59; P = 0.005) and the estradiol-to-testosterone ratio (IRR = 2.36; 95% CI, 1.02, 5.45; P = 0.044) were positively associated with conversion from prediabetes to diabetes. SHBG was not associated with conversion from prediabetes to diabetes when analyzed with SHBG as a continuous variable, but it was significantly and inversely associated in the fourth and fifth quintiles (Supplementary Table S5 (62)).
Table 2.
Association of hormones with diabetes progression at visit 2 among men
| Normoglycemic at baseline→ prediabetes or diabetes at follow-up N = 284/517 | Normoglycemic at baseline→ prediabetes at follow-up N = 274/507 | Prediabetic at baseline→ diabetes at follow-up N = 236/498 | ||||
|---|---|---|---|---|---|---|
| IRR (95% CI) | P value | IRR (95% CI) | P value | IRR (95% CI) | P value | |
| LH (mIU/mL) | 1.02 (0.93, 1.12) | 0.667 | 1.02 (0.93, 1.12) | 0.651 | 0.93 (0.80, 1.09) | 0.368 |
| High LH | 1.07 (0.79, 1.44) | 0.670 | 1.05 (0.77, 1.44) | 0.739 | 0.86 (0.57, 1.27) | 0.442 |
| FSH (mIU/mL) | 1.07 (1.00, 1.15) | 0.036 | 1.08 (1.01, 1.15) | 0.032 | 1.03 (0.91, 1.16) | 0.668 |
| High FSH | 1.25 (0.86, 1.81) | 0.242 | 1.26 (0.87, 1.84) | 0.222 | 1.16 (0.75, 1.79) | 0.507 |
| LH/FSH ratio | 0.82 (0.67, 0.99) | 0.037 | 0.81 (0.66, 0.98) | 0.033 | 0.91 (0.69, 1.21) | 0.530 |
| SHBG (nmol/L) | 1.05 (0.96, 1.16) | 0.284 | 1.05 (0.95, 1.16) | 0.329 | 0.85 (0.63, 1.15) | 0.299 |
| E2 (pmol/L) | 1.02 (0.92, 1.13) | 0.735 | 1.02 (0.91, 1.13) | 0.767 | 0.94 (0.81, 1.08) | 0.395 |
| High E2 | 0.86 (0.56, 1.32) | 0.478 | 0.84 (0.53, 1.31) | 0.440 | 0.92 (0.43, 1.95) | 0.826 |
| Bio E2 (pmol/L) | 0.98 (0.87, 1.10) | 0.747 | 0.98 (0.87, 1.11) | 0.760 | 0.97 (0.85, 1.11) | 0.678 |
| T (ng/dL) | 0.97 (0.87, 1.08) | 0.587 | 0.97 (0.87, 1.08) | 0.553 | 0.82 (0.68, 0.997) | 0.046 |
| Low T | 1.52 (1.04, 2.23) | 0.031 | 1.54 (1.04, 2.27) | 0.031 | 1.75 (1.18, 2.59) | 0.005 |
| Bio T (ng/dL) | 0.93 (0.84, 1.03) | 0.152 | 0.93 (0.84, 1.03) | 0.168 | 0.89 (0.76, 1.05) | 0.182 |
| E2/T ratio | 1.01 (0.73, 1.39) | 0.955 | 1.01 (0.73, 1.40) | 0.959 | 2.36 (1.02, 5.45) | 0.044 |
| Bio E2/Bio T ratio | 1.00 (0.85, 1.17) | 0.953 | 0.99 (0.84, 1.17) | 0.941 | 1.46 (0.84, 2.52) | 0.178 |
| LH/T ratio | 0.50 (0.16, 1.59) | 0.240 | 0.50 (0.16, 1.59) | 0.237 | a | |
| DHEAS (µmol/L) | 1.00 (0.87, 1.16) | 0.965 | 1.01 (0.87, 1.17) | 0.891 | 1.04 (0.90, 1.19) | 0.624 |
Model adjusted for age, BMI, waist/hip ratio, Hispanic background, acculturation score-MESA, recruitment site, education, statin medication use, family history of diabetes, cigarette use, alcohol use, physical activity, hypertension, high triglycerides, low HDL, and CRP. Individual continuous hormone concentrations are standardized using the following SDs: LH, 4.00 mIU/mL; FSH, 6.96 mIU/mL; SHBG, 28.65 nmol/L; E2, 40.40 pmol/L; Bio E2, 26.90 pmol/L; T, 169.95 ng/dL; Bio T, 49.11 ng/dL; DHEAS, 2.51 µmol/L. Dichotomized and ratio hormone measures are not standardized.
Abbreviations: Bio E2, bioavailable estradiol; Bio T, bioavailable testosterone; BMI, body mass index; CRP, C-reactive protein; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; HDL, high-density lipoprotein; IRR, incident rate radio; LH, luteinizing hormone; MESA, Multi-Ethnic Study of Atherosclerosis; SHBG, sex hormone binding globulin; T, testosterone.
Estimate unstable.
Low testosterone was also positively associated with conversion from normoglycemic to prediabetes (IRR = 1.54; 95% CI, 1.04, 2.27; P = 0.031). Testosterone was not significantly associated with conversion from normoglycemic to prediabetes when analyzed as a continuous variable; however, each of quintiles 2, 3, and 5 had point estimates suggesting reduced risk vs quintile 1 (Supplementary Table S5 (62)). FSH was also positively associated (IRR = 1.08; 95% CI, 1.01, 1.15; P = 0.032) and the ratio of LH/FSH was inversely associated (IRR = 0.81; 95% CI, 0.66, 0.98; P = 0.033) with conversion from normoglycemic to prediabetes. DHEAS was not associated with conversion to either prediabetes or diabetes (Table 2).
Changes in measures of glucose homeostasis from baseline to follow-up
Estradiol was positively associated with change in fasting insulin (β = 1.17; 95% CI, 0.31, 2.02; P = 0.008) and change in HOMA-IR (β = 0.39, 95% CI, 0.06, 0.72; P = 0.020) (Table 3), relationships that remained significant after controlling for SHBG (Supplementary Table S6 (62)). Similarly, bioavailable estradiol was significantly associated with change in fasting insulin (β = 0.75; 95% CI, 0.16, 1.33; P = 0.012) and change in HOMA-IR (β = 0.23; 95% CI, 0.03, 0.44; P = 0.027). High estradiol, however, was inversely associated with postload glucose (β = −23.48; 95% CI, −43.57, −3.39; P = 0.022) (Table 4). The total estradiol-to-testosterone and bioavailable estradiol-to-bioavailable testosterone ratios were inversely related to change in HOMA-B (estradiol/testosterone: β = −39.74; 95% CI, −75.85, −3.65; P = 0.031; BioE2/BioT: β = −19.09; 95% CI, −34.11, −4.06; P = 0.013) (Table 3). Testosterone was positively associated with change in HOMA-B (β = 7.01; 95% CI, 0.85, 13.16; P = 0.026), an association that remained significant after control for SHBG (Supplementary Table S6). Testosterone was not significantly associated with change in fasting glucose alone; however, it was inversely associated with change in fasting glucose controlling for SHBG (Supplementary Table S6 (62)). FSH was positively associated with change in fasting glucose (β = 1.25; 95% CI, 0.03, 2.48; P = 0.045) (Table 4). There were no significant associations of DHEAS or SHBG with any of the measures of glucose homeostasis.
Table 3.
Association of hormones with prospective change in continuous insulin and HOMA outcomes
| Fasting insulin change scorea | HOMA-B change scoreb | HOMA-IR change scorec | ||||
|---|---|---|---|---|---|---|
| β (95% CI)a | P value | β (95% CI)b | P value | β (95% CI)c | P value | |
|
Women
LH |
−0.30 (−1.18, 0.58) |
0.502 |
4.51 (−3.35, 12.37) |
0.259 |
−0.13 (−0.41, 0.15) |
0.371 |
| FSH | −0.86 (−1.73, 0.02) | 0.054 | 0.17 (−8.90, 9.25) | 0.970 | −0.30 (−0.56, −0.03) | 0.027 |
| LH/FSH ratio | −0.47 (−3.65, 2.71) | 0.771 | 13.83 (−17.01, 44.66) | 0.378 | −0.20 (−1.14, 0.75) | 0.684 |
| SHBG | −1.18 (−2.48, 0.13) | 0.077 | −7.48 (−16.75, 1.79) | 0.113 | −0.36 (−0.78, 0.06) | 0.095 |
| Low E2 | 0.89 (−1.81, 3.59) | 0.518 | 5.02 (−13.06, 23.11) | 0.585 | 0.27 (−0.59, 1.13) | 0.540 |
| DHEAS | 0.01 (−0.85, 0.87) | 0.988 | 1.76 (−8.91, 12.42) | 0.746 | 0.01 (−0.24, 0.25) | 0.957 |
| Men | ||||||
| LH | 0.37 (−0.54, 1.27) | 0.426 | −3.23 (−8.26, 1.79) | 0.206 | 0.17 (−0.17, 0.51) | 0.323 |
| High LH | 0.27 (−1.66, 2.20) | 0.783 | −12.94 (−26.34, 0.46) | 0.058 | 0.25 (−0.44, 0.94) | 0.476 |
| FSH | 0.50 (−0.46, 1.46) | 0.306 | −3.69 (−9.11, 1.74) | 0.182 | 0.23 (−0.12, 0.57) | 0.193 |
| High FSH | 2.54 (−0.93, 6.01) | 0.151 | −7.33 (−26.20, 11.55) | 0.446 | 1.05 (−0.22, 2.31) | 0.104 |
| LH/FSH ratio | −0.11 (−1.23, 1.01) | 0.847 | 1.93 (−6.94, 10.79) | 0.669 | −0.05 (−0.43, 0.33) | 0.796 |
| SHBG | 1.24 (−0.18, 2.66) | 0.086 | 1.85 (−4.59, 8.29) | 0.573 | 0.45 (−0.10, 1.00) | 0.112 |
| E2 | 1.17 (0.31, 2.02) | 0.008d | 3.08 (−2.42, 8.58) | 0.271 | 0.39 (0.06, 0.72) | 0.020d |
| High E2 | 3.10 (−2.11, 8.31) | 0.242 | 9.83 (−7.78, 27.44) | 0.273 | 1.18 (−0.91, 3.26) | 0.267 |
| Bio E2 | 0.75 (0.16, 1.33) | 0.012 | 1.99 (−3.93, 7.92) | 0.509 | 0.23 (0.03, 0.44) | 0.027 |
| T | 0.78 (−0.08, 1.63) | 0.075 | 7.01 (0.85, 13.16) | 0.026d | 0.24 (−0.07, 0.54) | 0.125 |
| Low T | −0.68 (−4.00, 2.64) | 0.689 | −26.93 (−64.21, 10.36) | 0.156 | −0.21 (−1.36, 0.93) | 0.715 |
| Bio T | 0.04 (−0.64, 0.72) | 0.914 | 4.87 (−1.19, 10.92) | 0.115 | −0.03 (−0.26, 0.20) | 0.827 |
| E2/T ratio | 0.84 (−1.36, 3.05) | 0.452 | −39.74 (−75.83, −3.65) | 0.031 | 0.38 (−0.32, 1.08) | 0.284 |
| Bio E2/Bio T ratio | 0.49 (−0.64, 1.61) | 0.399 | −19.09 (−34.11, −4.06) | 0.013 | 0.22 (−0.14, 0.58) | 0.239 |
| LH/T | −1.35 (−3.55, 0.84) | 0.227 | e | −0.03 (−0.78, 0.71) | 0.929 | |
| DHEAS | −0.43 (−1.18, 0.31) | 0.254 | −3.83 (−9.93, 2.28) | 0.218 | −0.13 (−0.37, 0.12) | 0.318 |
Model adjusted for baseline diabetes status, time between visits, diabetes medication at follow-up, age, BMI, waist-hip ratio, acculturation score, recruitment site, Hispanic background, education, statin use, family history of diabetes, gestational diabetes (women only), cigarette use, alcohol use, physical activity, BMI change (visit 1-visit 2), waist/hip ratio change (visit 1-visit 2), hypertension, high triglycerides, low HDL, CRP. Individual continuous hormone concentrations are standardized using the following standard deviations for men: LH, 4.00 mIU/mL; FSH, 6.96 mIU/mL; SHBG, 28.65 nmol/L; E2, 40.40 pmol/L; Bio E2, 26.90 pmol/L; T, 169.95 ng/dL; Bio T, 49.11 ng/dL; DHEAS, 2.51 µmol/L. Individual continuous hormone concentrations are standardized using the following standard deviations for women: LH, 11.73 mIU/mL; FSH, 24.85 mIU/mL; SHBG 29.24 nmol/L; DHEAS, 1.35 µmol/L. Dichotomized and ratio hormone measures are not standardized.
Abbreviations: Bio E2, bioavailable estradiol; Bio T, bioavailable testosterone; BMI, body mass index; CRP, C-reactive protein; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; HDL, high-density lipoprotein; HOMA-B, homeostatic model assessment index of β-cell function; HOMA-IR, homeostatic model assessment index of insulin resistance; LH, luteinizing hormone; SHBG, sex hormone binding globulin; T, testosterone.
Adjusted for baseline fasting insulin.
Adjusted for baseline HOMA-B.
Adjusted for baseline HOMA-IR.
Significant (P < 0.05) after adjustment for SHBG.
Estimate unstable.
Table 4.
Association of hormones with prospective change in continuous glucose outcomes
| HbA1c change scorea | Fasting glucose change scoreb | Postload glucose change scorec,d | ||||
|---|---|---|---|---|---|---|
| β (95% CI)a | P value | β (95% CI)b | P value | β (95% CI)c | P value | |
|
Women
LH |
−0.02 (−0.04, 0.01) |
0.129 |
−1.05 (−1.92, −0.19) |
0.017 |
−1.73 (−5.53, 2.06) |
0.369 |
| FSH | −0.019 (−0.042, 0.004) | 0.108 | −1.13 (−1.99, −0.27) | 0.011 | −2.34 (−6.48, 1.79) | 0.265 |
| LH/FSH ratio | −0.002 (−0.137, 0.133) | 0.976 | −1.37 (−4.79, 2.04) | 0.430 | −9.03 (−27.47, 9.41) | 0.336 |
| SHBG | −0.04 (−0.06, −0.01) | 0.002 | −0.72 (−1.68, 0.25) | 0.145 | −6.58 (−9.90, −3.27) | 0.0001 |
| Low E2 | −0.059 (−0.116, −0.002) | 0.042 | 0.88 (−1.46, 3.22) | 0.460 | 0.58 (−8.83, 9.99) | 0.904 |
| DHEAS | −0.01 (−0.04, 0.02) | 0.435 | 0.16 (−0.78, 1.11) | 0.733 | −0.32 (−4.03, 3.40) | 0.867 |
| Men | ||||||
| LH | −0.01 (−0.04, 0.03) | 0.660 | 1.02 (−0.17, 2.21) | 0.093 | −3.29 (−7.19, 0.61) | 0.098 |
| High LH | −0.01 (−0.13, 0.10) | 0.793 | 2.44 (−0.69, 5.56) | 0.127 | 2.59 (−8.16, 13.34) | 0.636 |
| FSH | 0.003 (−0.037, 0.042) | 0.897 | 1.25 (0.03, 2.48) | 0.045 | −3.26 (−8.41, 1.88) | 0.213 |
| High FSH | 0.01 (−0.13, 0.15) | 0.882 | 3.71 (−1.05, 8.47) | 0.127 | −9.05 (−24.20, 6.10) | 0.241 |
| LH/FSH ratio | 0.01 (−0.05, 0.06) | 0.792 | 0.27 (−1.87, 2.41) | 0.804 | 5.69 (−0.86, 12.24) | 0.088 |
| SHBG | −0.02 (−0.06, 0.02) | 0.357 | 0.29 (−1.30, 1.88) | 0.721 | −3.62 (−7.68, 0.43) | 0.080 |
| E2 | −0.03 (−0.07, 0.01) | 0.097 | 0.14 (−1.22, 1.50) | 0.839 | −1.91 (−5.61, 1.79) | 0.310 |
| High E2 | 0.005 (−0.172, 0.181) | 0.960 | 0.90 (−4.00, 5.80) | 0.718 | −23.48 (−43.57, −3.39) | 0.022 |
| Bio E2 | −0.03 (−0.06, 0.01) | 0.154 | 0.10 (−1.30, 1.51) | 0.884 | −0.46 (−4.79, 3.87) | 0.835 |
| T | −0.02 (−0.05, 0.02) | 0.356 | −0.78 (−2.12, 0.56) | 0.251e | −2.10 (−6.46, 2.26) | 0.345 |
| Low T | 0.02 (−0.34, 0.38) | 0.908 | 2.05 (−8.12, 12.22) | 0.693 | 23.31 (−5.58, 52.19) | 0.113 |
| Bio T | 0.004 (−0.034, 0.041) | 0.850 | −0.54 (−1.80, 0.73) | 0.406 | 0.10 (−4.70, 4.90) | 0.969 |
| E2/T ratio | −0.03 (−0.21, 0.15) | 0.777 | 3.55 (−1.83, 8.94) | 0.195 | 9.57 (−13.74, 32.87) | 0.420 |
| Bio E2/Bio T ratio | −0.01 (−0.09, 0.07) | 0.789 | 1.56 (−0.69, 3.81) | 0.173 | 0.92 (−8.29, 10.14) | 0.844 |
| LH/T | 0.15 (−0.01, 0.31) | 0.060 | f | f | ||
| DHEAS | −0.03 (−0.10, 0.05) | 0.494 | −0.23 (−2.28, 1.82) | 0.825 | 2.80 (−2.62, 8.23) | 0.310 |
Model adjusted for baseline diabetes status, time between visits, diabetes medication at follow-up, age, BMI, waist/hip ratio, acculturation score, recruitment site, Hispanic background, education, statin use, family history of diabetes, gestational diabetes (women only), cigarette use, alcohol use, physical activity, BMI change (visit 1-visit 2), waist-hip ratio change (visit 1-visit 2), hypertension, high triglycerides, low HDL, CRP. Individual continuous hormone concentrations are standardized using the following SDs for men: LH, 4.00 mIU/mL; FSH, 6.96 mIU/mL; SHBG, 28.65 nmol/L; E2, 40.40 pmol/L; Bio E2, 26.90 pmol/L; T, 169.95 ng/dL; Bio T, 49.11 ng/dL; DHEAS, 2.51 µmol/L. Individual continuous hormone concentrations are standardized using the following SDs for women: LH, 11.73 mIU/mL; FSH, 24.85 mIU/mL; SHBG 29.24 nmol/L; DHEAS, 1.35 µmol/L. Dichotomized and ratio hormone measures are not standardized.
Abbreviations: Bio E2, bioavailable estradiol; Bio T, bioavailable testosterone; BMI, body mass index; CRP, C-reactive protein; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LH, luteinizing hormone; SHBG, sex hormone binding globulin; T, testosterone.
Adjusted for baseline HbA1c.
Adjusted for baseline fasting glucose.
Adjusted for baseline postload glucose.
Women, N = 518; men, N = 832.
Significant (P < 0.05) after adjustment for SHBG.
Estimate unstable.
Longitudinal: Women
Progression from normoglycemia and from prediabetes
In women, among the 305 who were normoglycemic at baseline, 141 remained normoglycemic, 157 progressed to prediabetes, and 7 progressed to diabetes. Among the 388 women who were prediabetic at baseline, 25 were normoglycemic, 174 remained prediabetic, and 189 progressed to diabetes at follow-up. Results of progression from normoglycemic to prediabetes and to prediabetes plus diabetes as well as results of progression from prediabetes to diabetes are presented in Table 5 and Supplementary Table S7 (62).
Table. 5.
Association of hormones with diabetes progression at visit 2 among women
| Normoglycemic at baseline → prediabetes or diabetes at follow-up, N = 164/305 | Normoglycemic at baseline → prediabetes at follow-up, N = 157/298 | Prediabetic at baseline → diabetes at follow-up, N = 189/388 | ||||
|---|---|---|---|---|---|---|
| IRR (95% CI) | P value | IRR (95% CI) | P value | IRR (95% CI) | P value | |
| LH (mIU/mL) | 0.93 (0.82, 1.05) | 0.230 | 0.92 (0.81, 1.05) | 0.214 | 0.77 (0.54, 1.10) | 0.151 |
| FSH (mIU/mL) | 0.92 (0.79, 1.06) | 0.250 | 0.91 (0.78, 1.06) | 0.232 | 0.77 (0.58, 1.04) | 0.086 |
| LH/FSH ratio | 1.12 (0.69, 1.81) | 0.657 | 1.15 (0.71, 1.86) | 0.580 | 0.40 (0.07, 2.27) | 0.300 |
| SHBG (nmol/L) | 0.94 (0.81, 1.09) | 0.404 | 0.95 (0.81, 1.10) | 0.466 | 0.62 (0.44, 0.86) | 0.005 |
| Low E2 | 0.81 (0.59, 1.10) | 0.174 | 0.79 (0.57, 1.08) | 0.132 | 1.25 (0.75, 2.09) | 0.391 |
| DHEAS (µmol/L) | 0.89 (0.75, 1.05) | 0.169 | 0.89 (0.74, 1.06) | 0.191 | 0.79 (0.61, 1.02) | 0.076 |
Model adjusted for age, BMI, waist to hip ratio, Hispanic background, acculturation score-MESA, recruitment site, education, statin medication use, family history of diabetes, gestational diabetes, cigarette use, alcohol use, physical activity, hypertension, high triglycerides, low HDL, and CRP. Individual continuous hormone concentrations are standardized using the following standard deviations: LH, 11.73 mIU/mL; FSH, 24.85 mIU/mL; SHBG 29.24 nmol/L; DHEAS, 1.35 µmol/L. Dichotomized and ratio hormone measures are not standardized.
Abbreviations: BMI, body mass index; CRP, C-reactive protein; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; HDL, high-density lipoprotein; IRR, incident rate ratio; LH, luteinizing hormone; MESA, Multi-Ethnic Study of Atherosclerosis; SHBG, sex hormone binding globulin.
SHBG was inversely associated with conversion from prediabetes to diabetes (IRR = 0.62; 95% CI, 0.44, 0.86; P = 0.005) but not from normoglycemic to prediabetes after controlling for potential covariates (Table 5). LH was inversely associated with conversion from prediabetes to diabetes in quintiles 2, 3, and 4 (Supplementary Table S7 (62)), but not when analyzed as a continuous variable. There were no significant associations with conversion from normoglycemic to prediabetes.
Changes in measures of glucose homeostasis from baseline to follow-up
SHBG was inversely associated with change in HbA1c (β = −0.04; 95% CI, −0.06, −0.01; P = 0.002) and postload glucose (β = −6.58; 95% CI, −9.90, −3.27; P < 0.0001) (Table 4). Low estradiol was inversely associated with change in HbA1c (β = −0.059; 95% CI, −0.116, −0.002; P = 0.042). LH and FSH were inversely associated with change in fasting glucose (LH: β = −1.05; 95% CI, −1.92, −0.19; P = 0.017; and FSH, β = −1.13; 95% CI, −1.99, −0.27; P = 0.011). FSH was inversely associated with change in HOMA-IR (β = −0.30; 95% CI, −0.56, −0.03; P = 0.027) (Table 3). There was no significant association of DHEAS with any of the diabetes endpoints.
Effect Modification
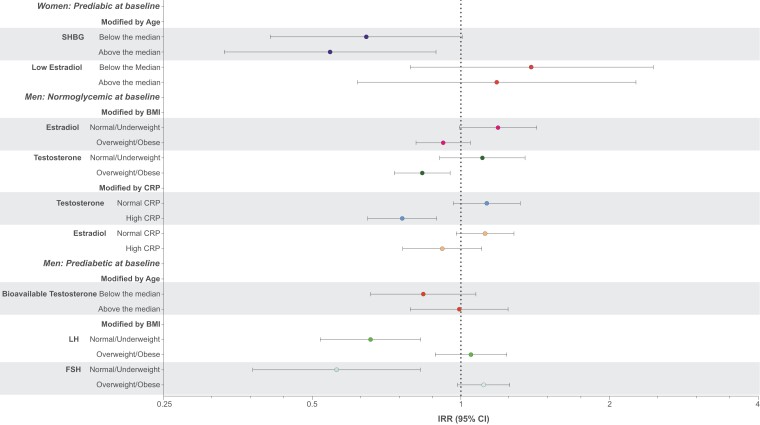
Associations of hormones with prospective measures of glucose homeostasis by baseline overweight/obesity vs normal/underweight (BMI ≥ 25 vs <25), age (>median vs <median), and CRP (>2 mg/L vs <2 mg/L) are shown in Supplementary Tables S8 and S9 (62) and in Fig. 1 (for those with interaction terms P < 0.05)
Figure 1.
Effect modification by age, body mass index, and C-reactive protein on association between standardized hormones and diabetes progression.
BMI
In women, change in HOMA-IR and fasting insulin were inversely related to LH only in women who were overweight or obese. In men, change in HbA1c was inversely associated with testosterone and bioavailable testosterone, change in HOMA-B was inversely associated with SHBG, and change in LH and FSH were inversely associated with the transition from prediabetes to diabetes but only in those who were not overweight or obese. Change in fasting insulin was positively associated with E2, bioavailable E2, and testosterone; SHBG was positively associated with change in HOMA-B; and testosterone was inversely associated with the transition from normal to prediabetes in those who were overweight or obese.
Age
In women, SHBG was inversely associated with the transition from prediabetes to diabetes, with the association significant only in those above the median age (aged 56 years for women and 52 years for men). SHBG was also inversely associated with change in fasting insulin and HOMA-B, only in women above the median age. In men, change in HbA1c was positively associated with FSH, and estradiol and bioavailable estradiol were positively associated with change in HOMA-B in those below the median age.
CRP
In women, change in HbA1c was inversely associated with low E2 among those with normal CRP. In men, testosterone was inversely associated with conversion from normoglycemia to prediabetes and bioavailable testosterone was inversely associated with change in postload glucose only in those with high CRP.
Separation of Hormonal Impact on Transitions From Normoglycemia to Prediabetes and From Prediabetes to Diabetes Using 2 Definitions of Prediabetes
Prediabetes can arise from dysfunction in multiple metabolic tissues, resulting in unique physiological signatures. To identify possible mechanisms of hormonal action on the development of diabetes and prediabetes, we defined prediabetes by fasting glucose without other abnormal glucose parameters and by postload glucose without other abnormal glucose parameters.
In women, the inverse association of SHBG with transition from prediabetes to diabetes was not significant when prediabetes was defined by fasting glucose nor when it was defined by postload glucose (Supplementary Table S10 (62)). In men, the inverse associations of testosterone, bioavailable testosterone, and LH with transition from prediabetes to diabetes were significant, whereas associations of SHBG and estradiol were of borderline significance but only when prediabetes was defined by postload glucose. There were no significant associations of hormones with transition from normoglycemic to prediabetes (Supplementary Table S11 (62)) in either sex using either fasting or postload glucose, except for the ratio of LH/testosterone in men, which was inversely associated with transition to prediabetes when prediabetes was defined by fasting glucose alone.
Cross-sectional: Men
In men at baseline, after controlling for potential confounders, SHBG was inversely associated with prevalent prediabetes vs normoglycemia, fasting glucose, and HbA1c; and positively associated with HOMA-B (Supplementary Tables S12 and S13 (62)), but only the association with HOMA-B remained significant after controlling for testosterone (Supplementary Table S6 (62)). Testosterone was inversely associated with prediabetes, HbA1c, postload insulin, postload glucose, and HOMA-B, and bioavailable testosterone was inversely associated with fasting insulin, HOMA-IR, and HOMA-B, whereas low testosterone was positively associated with HbA1c and HOMA-B. The associations of testosterone with fasting insulin, HOMA-B, postload insulin, and postload glucose remained significant on controlling for SHBG (Supplementary Table S6 (62)). Associations of other hormones with measures of glucose homeostasis were variable.
Cross-sectional: Women
In women, SHBG was inversely associated with HbA1c, fasting glucose, fasting insulin, postload insulin, postload glucose, HOMA-IR, and HOMA-B (Supplementary Tables S12 and S13 (62)). Cross-sectional and longitudinal results are summarized in Tables 6–9.
Table 6.
Summary of cross-sectional and longitudinal associations of hormones with continuous diabetes outcomes among women
| HbA1c | Fasting glucose | Postload glucose | Fasting insulin | Postload insulin | HOMA-B | HOMA-IR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Longitudinal | Baseline | Longitudinal | Baseline | Longitudinal | Baseline | Longitudinal | Baseline | Longitudinala | Baseline | Longitudinal | Baseline | Longitudinal | |
| LH | — | — | — | ↓ | — | — | — | — | — | ND | — | — | — | — |
| FSH | — | — | — | ↓ | — | — | — | — | — | ND | — | — | — | ↓ |
| LH/FSH Ratio | ↓ | — | — | — | — | — | — | — | — | ND | — | — | — | — |
| SHBG | ↓ | ↓ | ↓ | — | ↓ | ↓ | ↓ | — | ↓ | ND | ↓ | — | ↓ | — |
| Low SHBGb | — | ND | — | ND | ↑ | ND | — | ND | — | ND | — | ND | — | ND |
| Low E2 | — | ↓ | — | — | — | — | — | — | — | ND | — | — | — | — |
| DHEAS | — | — | — | — | — | — | — | — | — | ND | — | — | — | — |
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; HbA1c, hemoglobin A1c; HOMA-B, homeostatic model assessment index of β-cell function; HOMA-IR, homeostatic model assessment index of insulin resistance; LH, luteinizing hormone; ND, not done; SHBG, sex hormone binding globulin.
— Nonsignificant findings.
↑ Significant positive association after adjustment.
↓ Significant inverse association after adjustment.
a Post-Load Insulin was not measured at follow-up visit.
b Low SHBG was not modeled in the longitudinal sample because of a low sample size.
Table 9.
Summary of cross-sectional and longitudinal associations of hormones with diabetes status among men
| Prediabetes vs normoglycemic at baseline | Normoglycemic at baseline → prediabetes or diabetes at follow-up | Normoglycemic at baseline → prediabetes at follow-up | Prediabetic at baseline → diabetes at follow-up | |
|---|---|---|---|---|
| LH | — | — | — | — |
| High LH | — | — | — | — |
| FSH | — | ↑ | ↑ | — |
| High FSH | — | — | — | — |
| LH/FSH ratio | — | ↓ | ↓ | — |
| SHBG | ↓ | — | — | ↓a |
| E2 | — | — | — | — |
| High E2 | — | — | — | — |
| Bio E2 | — | — | — | — |
| T | ↓ | ↓a | ↓a | ↓ |
| Low T | — | ↑ | ↑ | ↑ |
| Bio T | — | — | — | — |
| E2/T ratio | — | — | — | ↑ |
| Bio E2/Bio T ratio | — | — | — | — |
| LH/T ratio | — | — | — | — |
| DHEAS | — | — | — | — |
Abbreviations: Bio E2, bioavailable estradiol; Bio T, bioavailable testosterone; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone binding globulin; T, testosterone.
– Nonsignificant findings.
↑ Significant positive association after adjustment.
↓ Significant inverse association after adjustment.
a Association determined from modeling hormone quintiles, indicating possible threshold effect.
Table 7.
Summary of cross-sectional and longitudinal associations of hormones with diabetes status among women
| Prediabetes vs normoglycemic at baseline | Normoglycemic at baseline → prediabetes or diabetes at follow-up | Normoglycemic at baseline → prediabetes at follow-up | Prediabetic at baseline → diabetes at follow-up | |
|---|---|---|---|---|
| LH | — | — | — | ↓a |
| FSH | — | — | — | — |
| LH/FSH ratio | — | — | — | — |
| SHBG | — | — | — | ↓ |
| Low E2 | — | — | — | — |
| DHEAS | — | — | — | — |
Abbreviations: DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone binding globulin.
– Nonsignificant findings.
↓ Significant inverse association after adjustment.
a Association determined from modeling hormone quintiles.
Table 8.
Summary of cross-sectional and longitudinal associations of hormones with continuous diabetes outcomes among men
| HbA1c | Fasting glucose | Postload glucose | Fasting insulin | Postload insulin | HOMA-B | HOMA-IR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Longitudinal | Baseline | Longitudinal | Baseline | Longitudinal | Baseline | Longitudinal | Baseline | Longitudinala | Baseline | Longitudinal | Baseline | Longitudinal | |
| LH | — | — | — | — | — | — | — | — | — | ND | — | — | — | — |
| High LH | — | — | ↑ | — | — | — | — | — | — | ND | — | — | — | — |
| FSH | — | — | — | ↑ | ↑ | — | — | — | — | ND | — | — | — | — |
| High FSH | — | — | — | — | — | — | — | — | — | ND | — | — | — | — |
| LH/FSH Ratio | — | — | — | — | — | — | — | — | — | ND | — | — | — | — |
| SHBG | ↓ | — | ↓ | — | — | — | — | — | — | ND | ↑ | — | — | — |
| E2 | — | — | — | — | — | — | — | ↑ | — | ND | — | — | — | ↑ |
| High E2 | — | — | — | — | ↑ | ↓ | — | — | — | ND | — | — | — | — |
| Bio E2 | — | — | — | — | — | — | — | ↑ | — | ND | — | — | — | ↑ |
| T | ↓ | — | — | — | ↓ | — | — | — | ↓ | ND | ↓ | ↑ | — | — |
| Low T | ↑ | — | — | — | — | — | — | — | — | ND | ↑ | — | — | — |
| Bio T | — | — | — | — | — | — | ↓ | — | — | ND | ↓ | — | ↓ | — |
| E2/T Ratio | — | — | — | — | — | — | — | — | — | ND | — | ↓ | — | — |
| Bio E2/Bio T Ratio | — | — | — | — | — | — | — | — | — | ND | ↑ | ↓ | — | — |
| LH/T Ratio | — | — | — | — | — | — | ↓ | — | — | ND | ↑ | — | ↓ | — |
| DHEAS | — | — | ↑ | — | — | — | — | — | — | ND | ↓ | — | — | — |
Abbreviations: Bio E2, bioavailable estradiol; Bio T, bioavailable testosterone; DHEAS, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle stimulating hormone; HbA1c, hemoglobin A1c; HOMA-B, homeostatic model assessment index of β-cell function; HOMA-IR, homeostatic model assessment index of insulin resistance; LH, luteinizing hormone; ND, not done; SHBG, sex hormone binding globulin; T, testosterone.
– Nonsignificant findings.
↑ Significant positive association after adjustment.
↓ Significant inverse association after adjustment.
a Pos-Load Insulin was not measured at follow-up visit.
Discussion
The strongest associations with glycemic traits in this study were the inverse associations of testosterone and bioavailable testosterone in men and SHBG in women, findings that are consistent with previous literature summarized below. These associations were significant after controlling for demographic and lifestyle factors as well as systemic inflammation as measured by CRP levels. Although there is evidence of effect modification by age, BMI, and CRP, associations in stratified analyses were not consistent. In addition, there was some evidence for estradiol having adverse effects, more in men than women, although the results varied across the various glycemic traits. Results for pituitary hormones also generally are consistent with protective effects of testosterone and adverse effects of estradiol with negative feedback on the pituitary, although results again are not consistent across glycemic traits.
A novel aspect of this study was the ability to examine associations at different stages of development of diabetes, including transition from normoglycemia to prediabetes and from prediabetes to diabetes. The stronger associations of testosterone among men and SHBG among women with transition from prediabetes to diabetes than from normoglycemia to prediabetes, suggest that testosterone and SHBG are operative later in the stages of diabetes development. Also, consistent with later effects is the positive association of testosterone with change in HOMA-B, without associations with other measures of glucose homeostasis, in longitudinal analyses. The inverse associations in men of testosterone, bioavailable testosterone, and LH with transition from prediabetes defined by postload glucose, but not when it was defined by fasting glucose, suggests that the effect may be primarily through effects on muscle glucose uptake, although effects on postprandial regulation of hepatic glucose production also may be involved (68). Estradiol was not related to glycemic transition in men, nor was low estradiol associated with transition in women. There were, however, positive associations in men of estradiol with changes in insulin levels and HOMA-IR, but not with changes in other measures of glucose homeostasis, suggesting an effect at an early stage of diabetes development.
Previous Literature
SHBG
This study confirms inverse associations of SHBG with selected measures of glucose regulation that have been noted elsewhere (7-9, 11, 12, 17-31), with some studies finding more consistent associations in women than men (8, 49, 69). Among women in our study, some associations with measures of glucose persisted in both cross-sectional and longitudinal analyses, in which there were inverse associations with conversion from prediabetes to diabetes, but not from normoglycemic to prediabetes, as well as with changes in HbA1c and postload glucose. In men, however, there were inverse associations in cross-sectional analyses, but in longitudinal analyses, there was no significant association in conversion from normoglycemic to prediabetes, nor were there any with changes in any of the continuous variables. The association with conversion from prediabetes to diabetes with SHBG in the higher quintiles, but not when analyzed as a continuous variable, suggests the possibility of a threshold effect. The loss of associations in the longitudinal analyses also raises the possibility of reverse causality in cross-sectional studies, which is biologically plausible because insulin decreases SHBG formation in the liver (69). However, the prospective inverse associations in several measures of glucose homeostasis in women are consistent with SHBG being protective late in the development of the disease. Genetic studies have identified 2 single nucleotide polymorphisms related to both SHBG and diabetes (rs6259 and rs6257) (31, 27, 69). Others have found that SHBG is anti-inflammatory and lipolytic (70), and that it may activate a specific high-affinity receptor in the plasma membrane (69).
Testosterone
In general, associations of testosterone, low testosterone, and bioavailable testosterone with diabetic traits in men persisted in cross-sectional and longitudinal analyses. The results are similar to those in previous studies, which found consistent inverse associations of diabetic traits with total testosterone (4-29). Some (4, 5, 23, 29), but not all (22), studies also found associations with bioavailable testosterone. In longitudinal analyses in this study, testosterone was inversely, whereas low testosterone and the ratio of estradiol/testosterone were positively, associated with conversion from prediabetes to diabetes. Low testosterone, however, was also positively associated with conversion from normoglycemia to prediabetes. The longitudinal associations in this study suggest that the results are not the result of reverse causation with insulin decreasing testosterone, similar to findings in a previous investigation (4). However, the association of testosterone with conversion from normoglycemic to prediabetes, in selected quintiles, but not when analyzed as a continuous variable, suggests a possible threshold effect. The lack of some associations after controlling for SHBG may be due to collinearity of testosterone and SHBG and is consistent with some previous studies (71, 72).
Whether testosterone's effects are independent of its effects on body composition, including abdominal fat content, is not clear. Testosterone decreases fat mass and, conversely, weight loss increases testosterone levels (72). In some studies, relationships were no longer significant after controlling for waist circumference or body fat (6, 11, 18). Others have found effect modification, with effects stronger among obese men (29), consistent with the inverse association in our study, with the transition from normal to prediabetes only in overweight/obese men. The results are not consistent, however, with the inverse association of testosterone with change in HbA1c in men who were not overweight/obese and the positive association of testosterone with change in fasting insulin in overweight/obese men in the current study. In our study, the inverse associations of testosterone with measures of glucose homeostasis in men remained after controlling for BMI and waist circumference, suggesting an independent biologic pathway, potentially including direct effects on muscle.
DHEAS
Similar to the current study, in which we found a few inconsistent associations with DHEAS on cross-sectional analyses, but no associations with DHEAS in longitudinal analyses, previous literature relating the androgen precursor DHEA and its sulfated hormone DHEAS to glucose homeostasis has been inconsistent in observational (33-37, 44, 46) and intervention studies (73-75). There is biologic plausibility to a protective effect of DHEA, which has been shown to increase glucose uptake in human adipocytes by stimulating GLUT4 and GLUT1 translocation to the plasma membrane (76).
Estrogens
The role of estrogens in the development of diabetes is unclear. In men, there is evidence that endogenous estrogens increase risk or diabetes and prediabetes in some (6, 8, 11, 18) but not all (17, 23) studies. In postmenopausal women, diabetes has been positively associated with endogenous estrogens (8, 34, 44-46) and bioavailable estrogens (46, 47). In contrast, exogenous postmenopausal hormones, both estrogens alone as well as those in combination with progestins, have generally been associated with lower risk of diabetes (50-53). Estrogen receptors ERα and ERβ, as well as a more recently discovered membrane estrogen receptor, GPR30, affect glucose homeostasis, in part through GLUT4 regulation (38-43, 77) with effects potentially dose dependent (77). Activation of ERα induces insulin biosynthesis and pancreatic β-cell survival (42) and may protect against glucose intolerance by controlling inflammation and body weight (78). ERβ and the ERα/ERβ ratio could also affect glucose homeostasis through alterations in peroxisome proliferator-activated receptor γ signaling, with studies in ovariectomized rats showing that early, but not late, replacement with estrogens prevents oxidative stress through changes in the ERα/ERβ ratio (78).
In our longitudinal analyses, low estradiol in women was inversely associated with change in HbA1c, consistent with previous studies (6, 8, 34, 44, 45, 47). In men, on cross-sectional analyses, high estradiol was positively associated with postload glucose, whereas on longitudinal analyses, estradiol was positively associated with change in fasting insulin and HOMA-IR, and high estradiol was inversely associated with postload glucose. There were no associations of estradiol with other measures of glucose homeostasis. Levels of estradiol in these postmenopausal women, however, were low, with 71% of subjects having levels below the lower limit of detection (18.4 pmol/L). Furthermore, we did not have measurements of estrone, which is converted from androgens in adipose tissue and is the predominant estrogen in postmenopausal women.
Gonadotropins
The few studies that have examined associations of pituitary hormones with glucose homeostasis have more consistently found inverse associations of FSH and or LH with measures of diabetes and prediabetes in women (56-59) than in men (19, 57). Hypothesized mechanisms include negative pituitary feedback from elevated peripheral estrogens and/or obesity associated with increased inflammation (58).
In women in this study, the ratio of LH/FSH, but not LH or FSH individually, was inversely related to HbA1c in cross-sectional analyses. On longitudinal analyses, consistent with previous studies, both LH and FSH were inversely associated with change in fasting glucose and FSH was inversely associated with change in HOMA-IR. LH was also inversely associated with conversion from prediabetes to diabetes in selected quintiles, but not when analyzed as a continuous variable. The inverse associations with pituitary hormones are consistent with increased effects of estrogens on glycemic measures and negative feedback on pituitary hormones.
Among men in our study, cross-sectional analyses revealed that high LH was positively associated with fasting glucose and FSH was positively associated with postload glucose, consistent with low testosterone increasing the risk of diabetes and higher LH reflecting pituitary feedback in response to low testosterone. In longitudinal analyses, FSH was positively related to and the ratio of LH/FSH was inversely related to conversion from normal to prediabetes, whereas FSH was positively related to change in fasting glucose.
Strengths and Limitations
This is the largest population-based study in Hispanic/Latino adults of relationships of endogenous sex hormones with longitudinal measures of glucose homeostasis. It provides a unique opportunity to examine effects of endogenous hormones on the development of glucose homeostasis at various stages of diabetes development. The results, in identifying stages of development of diabetes at which sex hormones may be operative, will assist, not only in understanding the pathogenesis of the disease, but also in identifying individuals who might be at future risk and amenable to intervention at different stages of the disease, as well as the utility of interventions that modulate endogenous sex hormones. Other strengths include careful classification of menopause status using hormones and clinical data, robust identification and exclusion of participants taking medications that affect hormone levels, assessment of multiple steady-state and provocative measures of glucose homeostasis, and complete follow-up of the subgroup examined in this study.
There are, however, some limitations. The results cannot be generalized to other racial and ethnic groups. Levels of estrogens in these postmenopausal women were too low and the percent of estradiol below the limit of detection (71%) too high to fully assess the relationships of estradiol and bioavailable estradiol with glucose homeostasis. In addition, stored serum volumes were inadequate for measurement of testosterone in women. Overall, but not specific, diabetes medication usage was available for visit 2; therefore, analyses examining change in continuous outcomes may not adequately control for differential medication effects. Hormone levels, as well as use of steroid hormone and hormone modifying medications, were obtained only at baseline. Although the numbers within sex-stratified groups were large, power is limited for some subgroup analyses, including by Hispanic/Latino background, prediabetes subtype, effect modification, and more detailed examination of nonlinear associations. In addition, it is possible that our analyses overadjust for CRP, lipids, and BMI if they are in the causal pathway, highlighting the need for further investigation of mediation pathways. The large number of comparisons present a risk of type II error and the analyses are therefore exploratory. Inferences rest on biologic plausibility as well as consistency of results with previous literature and among associations shown in this study.
Conclusions
Inverse associations of SHBG and testosterone in men and SHBG in women with measures of glucose homeostasis in this population-based study of Hispanic/Latino adults are consistent with previous literature. The stronger associations with transition from prediabetes to diabetes than from normoglycemic to prediabetes suggest that testosterone and SHBG are operative at later stages of development of diabetes. There are some suggestions that estradiol in men may increase risk of insulin resistance; however, biologic pathways by which endogenous sex hormones affect glucose homeostasis await future studies.
Abbreviations
- BMI
body mass index
- BP
blood pressure
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- ER
estrogen receptor
- FPG
fasting blood glucose
- FSH
follicle stimulating hormone
- GLUT4
glucose transporter type 4
- HbA1c
glycosylated hemoglobin
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- HDL
high-density lipoprotein
- HOMA-B
homeostatic model assessment index of β-cell function
- HOMA-IR
homeostatic model assessment index of insulin resistance
- hsCRP
high-sensitivity C-reactive protein
- IRR
incident rate ratio
- LDL
low-density lipoprotein
- LH
luteinizing hormone
- OGTT
oral glucose tolerance test
- SHBG
sex hormone binding globulin
Contributor Information
Victoria Persky, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Chibuzor Abasilim, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Konstantina Tsintsifas, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Tessa Day, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Robert M Sargis, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Illinois Chicago and Medical Service, Jesse Brown VA Medical Center, Chicago, IL, USA.
Martha L Daviglus, Institute for Minority Health Research, University of Illinois Chicago, Chicago, IL, USA.
Jianwen Cai, Department of Biostatistics, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sally Freels, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Terry Unterman, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Illinois Chicago and Medical Service, Jesse Brown VA Medical Center, Chicago, IL, USA.
Noel Chavez, Division of Community Health Sciences, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Robert Kaplan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Carmen R Isasi, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Amber Pirzada, Institute for Minority Health Research, University of Illinois Chicago, Chicago, IL, USA.
Michelle L Meyer, Department of Emergency Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Gregory A Talavera, Department of Psychology, San Diego State University, San Diego, CA, USA.
Bharat Thyagarajan, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
Brandilyn A Peters, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Jessica M Madrigal, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Arielle Grieco, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Mary E Turyk, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois Chicago, Chicago, IL, USA.
Funding
This work was supported by grant no. R01ES025159 with the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), which was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001l/N01-HC-65233), University of Miami (HHSN268201300004l/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002l/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003l/NO1-HC-65236/NO1-HC-65236 Northwestern University), and San Diego State University (HHSN268201300005l/N01-HC-65237). The following institutes/centers/offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute of Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and NIH Institution-Office of Dietary Supplements. V.P., M.E.T., and R.M.S. were also supported by the National Institute of Environmental Health Sciences through the ChicAgo Center for Health and EnvironmenT (CACHET) (P30ES027792). J.M. and K.T. received training supported by the National Institute for Occupational Safety and Health Education Research Center at UIC (T42OH008672).
Disclaimer Statement:
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the US Department of Health and Human Services; or the National Institute for Occupational Safety and Health. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosure
R.M.S. has received honoraria from the American Medical Forum for lectures and from CVS/Health for an advisory committee, neither of which are related to the current manuscript.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on request.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report website. Accessed May 31, 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2. Daviglus ML, Talavera GA, Avilés-Santa ML, et al. . Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karvonen-Gutierrez CA, Park SK, Kim C. Diabetes and menopause. Curr Diab Reps. 2016;16(4):20. [DOI] [PubMed] [Google Scholar]
- 4. Ottarsdottir K, Nilsson AG, Hellgren M, Lindblad U, Daka B. The association between serum testosterone and insulin resistance: a longitudinal study. Endocr Connect. 2018;7(12):1491‐1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selvin E, Feinleib M, Zhang L, et al. . Androgens and diabetes in men: results from the third national health and nutrition examination survey (NHANES III). Diabetes Care. 2007;30(2):234‐238. [DOI] [PubMed] [Google Scholar]
- 6. Mather KJ, Kim C, Christophi CA, et al. . Steroid sex hormones, sex hormone–binding globulin, and diabetes incidence in the diabetes prevention program. The J Clin EndocrinolMetab. 2015;100(10):3778‐3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Svartberg J, Jenssen T, Sundsfjord J, Jorde R. The associations of endogenous testosterone and sex hormone-binding globulin with glycosylated hemoglobin levels, in community dwelling men. The Tromsø study. Diabetes Metab. 2004;30(1):29‐34. [DOI] [PubMed] [Google Scholar]
- 8. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes. JAMA. 2006;295(11):1288‐1299. [DOI] [PubMed] [Google Scholar]
- 9. Atlantis E, Fahey P, Martin S, et al. . Predictive value of serum testosterone for type 2 diabetes risk assessment in men. BMC Endocre Disords. 2016;16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atlantis E, Lange K, Martin S, et al. . Testosterone and modifiable risk factors associated with diabetes in men. Maturitas. 2011;68(3):279‐285. [DOI] [PubMed] [Google Scholar]
- 11. Arthur R, Rohrmann S, Møller H, et al. . Pre-diabetes and serum sex steroid hormones among US men. Andrology. 2017;5(1):49‐57. [DOI] [PubMed] [Google Scholar]
- 12. O’Reilly MW, Glisic M, Kumarendran B, et al. . Serum testosterone, sex hormone-binding globulin and sex-specific risk of incident type 2 diabetes in a retrospective primary care cohort. Clin Endocrinol (Oxf). 2019;90(1):145‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karakas M, Schäfer S, Appelbaum S, et al. . Testosterone levels and type 2 diabetes—no correlation with age, differential predictive value in men and women. Biomolecules. 2018;8(3):76. Published 2018 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Minooee S, Ramezani Tehrani F, Rahmati M, et al. . The association between serum total testosterone and progression of hyperglycemia: a 15-year prospective cohort study. Andrology. 2019;7(2):148‐155. [DOI] [PubMed] [Google Scholar]
- 15. Akishita M, Fukai S, Hashimoto M, et al. . Association of low testosterone with metabolic syndrome and its components in middle-aged Japanese men. Hypertens Res. 2010;33(6):587‐591. [DOI] [PubMed] [Google Scholar]
- 16. Gyawali P, Martin SA, Heilbronn LK, et al. . The role of sex hormone-binding globulin (SHBG), testosterone, and other sex steroids, on the development of type 2 diabetes in a cohort of community-dwelling middle-aged to elderly men. Acta Diabetol. 2018;55(8):861‐872. [DOI] [PubMed] [Google Scholar]
- 17. Antonio L, Wu FCW, O’Neill TW, et al. . Associations between sex steroids and the development of metabolic syndrome: a longitudinal study in European men. J Clin Endocrino Metab. 2015;100(4):1396‐1404. [DOI] [PubMed] [Google Scholar]
- 18. Vikan T, Schirmer H, Njølstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol. 2010;162(4):747‐754. [DOI] [PubMed] [Google Scholar]
- 19. Holmboe SA, Jensen TK, Linneberg A, et al. . Low testosterone: a risk marker rather than a risk factor for type 2 diabetes. J Clin Endocrinol Metab. 2016;101(8):3180‐3190. [DOI] [PubMed] [Google Scholar]
- 20. Kalme T, Seppälä M, Qiao Q, et al. . Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinoly Metab. 2005;90(3):1550‐1556. [DOI] [PubMed] [Google Scholar]
- 21. Brand JS, Wareham NJ, Dowsett M, et al. . Associations of endogenous testosterone and SHBG with glycated haemoglobin in middle-aged and older men. Clin Endocrinol (Oxf). 2011;74(5):572‐578. [DOI] [PubMed] [Google Scholar]
- 22. Colangelo LA, Ouyang P, Liu K, et al. . Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(6):1049‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90(5):2618‐2623. [DOI] [PubMed] [Google Scholar]
- 24. Svartberg J, Schirmer H, Wilsgaard T, et al. . Single-nucleotide polymorphism, rs1799941 in the sex hormone-binding globulin (SHBG) gene, related to both serum testosterone and SHBG levels and the risk of myocardial infarction, type 2 diabetes, cancer and mortality in men: the Tromsø study. Andrology. 2014;2(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 25. Laaksonen DE, Niskanen L, Punnonen K, et al. . Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5):1036‐1041. [DOI] [PubMed] [Google Scholar]
- 26. Liu Q, Zhao Y, Gu Y, et al. . The association of age-related differences in serum total testosterone and sex hormone-binding globulin levels with the prevalence of diabetes. Arch Gerontol Geriatr. 2020;88:104040. [DOI] [PubMed] [Google Scholar]
- 27. Zhu H, Wang N, Han B, et al. . Low sex hormone-binding globulin levels associate with prediabetes in Chinese men independent of total testosterone. PLoS One. 2016;11(9):e0162004. Published 2016 Sept 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li N, Huang C, Lan B, et al. . Association of gonadal hormones and sex hormone binding globulin with risk of diabetes: a cohort study in middle-aged and elderly Chinese males. Int J Clin Pract. 2021;75(5):e14008. [DOI] [PubMed] [Google Scholar]
- 29. Li J, Lai H, Chen S, Zhu H, Lai S. Interaction of sex steroid hormones and obesity on insulin resistance and type 2 diabetes in men: the third national health and nutrition examination survey. J Diabetes Complications. 2017;31(2):318‐327. [DOI] [PubMed] [Google Scholar]
- 30. Ottarsdottir K, Hellgren M, Bock D, Nilsson AG, Daka B. Longitudinal associations between sex hormone-binding globulin and insulin resistance. Endocr Connect. 2020;9(5):418‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding EL, Song Y, Manson JE, et al. . Sex hormone–binding globulin and risk of type 2 diabetes in women and men. New Engl J Med. 2009;361(12):1152‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinoly. 2009;5(12):673‐681. [DOI] [PubMed] [Google Scholar]
- 33. Phillips AC, Carroll D, Gale CR, Lord JM, Arlt W, Batty GD. Cortisol, DHEAS, their ratio and the metabolic syndrome: evidence from the Vietnam experience study. Eur J Endocrinol. 2010;162(5):919‐923. [DOI] [PubMed] [Google Scholar]
- 34. Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076‐2084. [DOI] [PubMed] [Google Scholar]
- 35. Brahimaj A, Muka T, Kavousi M, Laven JSE, Dehghan A, Franco OH. Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: the Rotterdam study. Diabetologia. 2017;60(1):98‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu J, Zhang A, Yang S, et al. . Combined effects of sex hormone-binding globulin and sex hormones on risk of incident type 2 diabetes. J Diabetes. 2016;8(4):508‐515. [DOI] [PubMed] [Google Scholar]
- 37. Veronese N, Trevisan C, De Rui M, et al. . Serum dehydroepiandrosterone sulfate and risk for type 2 diabetes in older men and women: the pro. V.A study. Can J Diabetes. 2016;40(2):158‐163. [DOI] [PubMed] [Google Scholar]
- 38. Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. 2011;22(1):24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ropero AB, Alonso-Magdalena P, Quesada I, Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73(9-10):874‐879. [DOI] [PubMed] [Google Scholar]
- 40. Barros RPA, Machado UF, Warner M, Gustafsson J-A. Muscle GLUT4 regulation by estrogen receptors ERbeta and ER. Proc Natl Acad Sci USA. 2006;103(5):1605‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med. 2006;12(9):425‐431. [DOI] [PubMed] [Google Scholar]
- 42. Nadal A, Alonso-Magdalena P, Soriano S, Quesada I, Ropero AB. The pancreatic β-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol. 2009;304(1-2):63‐68. [DOI] [PubMed] [Google Scholar]
- 43. Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur Jf Nutr. 2012;51(7):861‐870. [DOI] [PubMed] [Google Scholar]
- 44. Golden SH, Dobs AS, Vaidya D, et al. . Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92(4):1289‐1295. [DOI] [PubMed] [Google Scholar]
- 45. Muka T, Nano J, Jaspers L, et al. . Associations of steroid sex hormones and sex hormone–binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes. 2017;66(3):577‐586. [DOI] [PubMed] [Google Scholar]
- 46. Kalyani RR, Franco M, Dobs AS, et al. . The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clini Endocrinol Metab. 2009;94(11):4127‐4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23(7):912‐918. [DOI] [PubMed] [Google Scholar]
- 48. Fenske B, Kische H, Gross S, et al. . Endogenous androgens and sex hormone–binding globulin in women and risk of metabolic syndrome and type 2 diabetes. J Clin Endocrinol Metab. 2015;100(12):4595‐4603. [DOI] [PubMed] [Google Scholar]
- 49. Wallace IR, Mckinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol (Oxf). 2013;78(3):321‐329. [DOI] [PubMed] [Google Scholar]
- 50. Bonds DE, Lasser N, Qi L, et al. . The effect of conjugated equine oestrogen on diabetes incidence: the Women's Health Initiative randomised trial. Diabetologia. 2006;49(3):459‐468. [DOI] [PubMed] [Google Scholar]
- 51. Pentti K, Tuppurainen MT, Honkanen R, et al. . Hormone therapy protects from diabetes: the Kuopio osteoporosis risk factor and prevention study. EurJ Endocrinoy. 2009;160(6):979‐983. [DOI] [PubMed] [Google Scholar]
- 52. Margolis KL, Bonds DE, Rodabough RJ, et al. . Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative hormone trial. Diabetologia. 2004;47(7):1175‐1187. [DOI] [PubMed] [Google Scholar]
- 53. Espeland MA, Hogan PE, Fineberg SE, et al. . Effect of postmenopausal hormone therapy on glucose and insulin concentrations. Diabetes Care. 1998;21(10):1589‐1595. [DOI] [PubMed] [Google Scholar]
- 54. Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinoly Metab. 2015;100(12):4456‐4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Pelt RE, Schwartz RS, Kohrt WM. Insulin secretion and clearance after subacute estradiol administration in postmenopausal women. J Clin Endocrinol Metab. 2008;93(2):484‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stefanska A, Cembrowska P, Kubacka J, Kuligowska-Prusinska M, Sypniewska G. Gonadotropins and their association with the risk of prediabetes and type 2 diabetes in middle-aged postmenopausal women. Dis Markers. 2019;2019:2384069. Published 2019 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brismar K, Nilsson SE. Interrelations and associations of serum levels of steroids and pituitary hormones with markers of insulin resistance, inflammatory activity, and renal function in men and women aged >70 years in an 8-year longitudinal study of opposite-sex twins. Gend Med. 2009;6(Suppl 1):123‐136. [DOI] [PubMed] [Google Scholar]
- 58. Wang N, Kuang L, Han B, et al. . Follicle-stimulating hormone associates with prediabetes and diabetes in postmenopausal women. Acta Diabetol. 2016;53(2):227‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bertone-Johnson ER, Virtanen JK, Niskanen L, et al. . Association of follicle-stimulating hormone levels and risk of type 2 diabetes in older postmenopausal women. Menopause. 2017;24(7):796‐802. [DOI] [PubMed] [Google Scholar]
- 60. Schneiderman N, Llabre M, Cowie CC, et al. . Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233‐2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. . Design and implementation of the Hispanic community health study/study of Latinos. Ann Epidemiol. 2010;20(8):629‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Persky V, Abasilim C, Tsintsifas K, et al. . Data from “Sex Hormones and Diabetes in 45-74-Year-Old Men and Postmenopausal Women: The Hispanic community Health Study” Deposited Revision in INDIGO November 3, 2022. DOI: 10.25417/uic.21438045; https://figshare.com/s/d3c74f5beba57ac9955f [DOI] [PMC free article] [PubMed]
- 63. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666‐3672. [DOI] [PubMed] [Google Scholar]
- 64. Hoos T, Espinoza N, Marshall S, Arredondo EM. Validity of the global physical activity questionnaire (GPAQ) in adult Latinas. J Phys ActHealth. 2012;9(5):698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kandula NR, Diez-Roux AV, Chan C, et al. . Association of acculturation levels and prevalence of diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2008;31(8):1621‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elfassy T, Zeki Al Hazzouri A, Cai J, et al. . Incidence of hypertension among US Hispanics/L: the Hispanic community health study/study of Latinos, 2008 to 2017. J Am Heart Assoc. 2020;9(12):e015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu H, Zhou D, Jia W, Guo Z. Locating the source of hyperglycemia: liver versus muscle. J Diabetes. 2012;4(1):30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Le TN, Nestler JE, Strauss JF, Wickham EP. Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. 2012;23(1):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yamazaki H, Kushiyama A, Sakoda H, et al. . Protective effect of sex hormone-binding globulin against metabolic syndrome: in vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflammn. 2018;2018:3062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rajala UM, Keinanen-Kiukaanniemi SM, Hirsso PK, et al. . Associations of total testosterone and sex hormone-binding globulin levels with insulin sensitivity in middle-aged Finnish men. Diabetes Care. 2007;30(4):e13. [DOI] [PubMed] [Google Scholar]
- 72. Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):247‐256. [DOI] [PubMed] [Google Scholar]
- 73. Dhatariya K, Bigelow ML, Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes. 2005;54(3):765‐769. [DOI] [PubMed] [Google Scholar]
- 74. Lasco A, Frisina N, Morabito N, et al. . Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol. 2001;145(4):457‐461. [DOI] [PubMed] [Google Scholar]
- 75. Basu R, Dalla Man C, Campioni M, et al. . Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007;56(3):753‐766. [DOI] [PubMed] [Google Scholar]
- 76. Perrini S, Natalicchio A, Laviola L, et al. . Dehydroepiandrosterone stimulates glucose uptake in human and murine adipocytes by inducing GLUT1 and GLUT4 translocation to the plasma membrane. Diabetes. 2004;53(1):41‐52. [DOI] [PubMed] [Google Scholar]
- 77. Ropero AB, Pang Y, Alonso-Magdalena P, Thomas P, Nadal A. Role of ERβ and GPR30 in the endocrine pancreas: a matter of estrogen dose. Steroids. 2012;77(10):951‐958. [DOI] [PubMed] [Google Scholar]
- 78. Clegg D, Hevener AL, Moreau KL, et al. . Sex hormones and cardiometabolic health: role of estrogen and estrogen receptors. Endocrinology. 2017;158(5):1095‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on request.