Abstract
Context
Ketogenic diets (KDs) and low-fat diets (LFD) result in similar weight loss, but have differential cardiometabolic effects on lipids and insulin. Generally, weight loss decreases renin–angiotensin–aldosterone system (RAAS) activity.
Objective
Investigate the effects of KDs with varying sodium content vs LFD on RAAS in overweight and obese adults.
Methods
Twenty-eight participants were randomized 1:1 to a KD + ketone salt supplement (KD + KS) or a KD + placebo (KD + PL) arm with prepared hypocaloric meals. Twelve participants were enrolled in a post hoc LFD arm. Serum renin, aldosterone, and anthropometric and metabolic biomarkers were assessed at 0, 2, 4, and 6 weeks. Linear mixed models with random intercepts were used to compare between group differences controlling for sex and body mass index.
Results
Participants had a median age of 33 years, 51% female, weighed 91.3 kg, with body mass index 30.6 kg/m2. At 6 weeks, weight decreased by 6, 8, and 7 kg on average in the KD + KS, KD + PL, and LFD groups, respectively (P < .05). Aldosterone increased by 88% and 144% in the KD + PL and KD + KS groups, respectively, but did not change in the LFD after 6 weeks while renin decreased across groups. Systolic and diastolic blood pressure did not change in the KD + PL and KD + KS groups. Log ketones were positively associated with aldosterone (P < .001). Aldosterone was not associated with cardiovascular measures including blood pressure and ejection fraction (P > .05).
Conclusion
KD reduced weight and increased aldosterone without worsening cardiometabolic risk factors. Future KD studies are needed to elucidate mechanistic connections between ketones and aldosterone.
Keywords: ketogenic diet, adiposity, ketones, aldosterone, renin
Ketogenic diets (KDs) improve cardiometabolic risk profiles among individuals with obesity, metabolic syndrome, and type 2 diabetes (1), but little is known regarding the effect of the KD on the renin–angiotensin–aldosterone system (RAAS). The RAAS is a central regulator of cardiac output, blood pressure, and sodium and potassium homeostasis (2, 3). Aldosterone, the end effector of the RAAS, activates mineralocorticoid receptors (MRs) causing cardiac inflammation and fibrosis as well as the remodeling of arteries, veins, and capillaries (4). Reducing the activity of the RAAS through angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and MR antagonists is thus 1 of the primary targets in the treatment of hypertension (5) and heart failure (6). Additionally, aldosterone substantially impairs glucose metabolism and trials of ramipril (ACE inhibitor) and valsartan (ARB) were shown to improve glucose control (7). Given the importance of the RAAS in cardiometabolic health, it is imperative to better understand how KDs and circulating concentrations of ketones impact this RAAS axis.
Weight-loss interventions utilizing nonketogenic very low calorie diets (320-1200 kcal/day) or mild caloric restriction (1200 and 1800 kcal/day) are associated with reduced aldosterone and renin concentrations (8-16). However, it is unclear whether the weight loss–induced decrease in RAAS extends to hypocaloric KDs. Since a decrease in circulating sodium triggers the adrenal gland to increase production of aldosterone, any impact of KDs on the RAAS would likely be modulated by its sodium content. Similar to fasting, KDs are associated with decreased insulin stimulation and increased excretion of sodium, a process called the natriuresis of fasting or more broadly the natriuresis of nutritional ketosis. If the additional excretion of sodium is not replaced, a KD leads to decreased circulating volume and associated symptoms (ie, dizziness, fatigue, weakness), and presumably increased adrenal stimulation of aldosterone. Given the large number of studies assessing KDs published in the last several decades, it is surprising that few have measured aldosterone. Vaswani (17) noted that subjects with obesity fed a 12-week KD (800 kcal/day and 3430 mg sodium/day) experienced a significant decline in plasma renin activity while changes in serum aldosterone concentrations were minimal. Urinary sodium excretion was unchanged in that study, possibly indicating that the level of dietary sodium was adequate to prevent a compensatory aldosterone response. Affarah et al fed healthy men 2 different weight maintenance diets with 2300 mg/day sodium that varied in carbohydrate (13% and 52% of energy) in a crossover manner (18). Plasma renin activity was not different between diets, but plasma aldosterone consistently decreased during the transition from the low- to the high-carbohydrate diet. These studies leave many questions unanswered about whether typical weight loss KDs with different sodium contents influence the RAAS in a predictable manner compared with low-fat/high-carbohydrate diets.
In this report, the objective was to examine changes in aldosterone and renin during a 6-week controlled-feeding study testing the effects of adding an exogenous ketone salt to a hypocaloric KD on indices of keto adaption in overweight or obese subjects. The design allowed comparison of 2 precisely controlled matched KDs varying in sodium content (2351 vs 6100 mg/day), and a third group fed an energy- and protein-matched low-fat diet (LFD). We also examined the longitudinal association between change in biological cardiometabolic risk factors with change in aldosterone and ketones. The main hypothesis was that compared with the KD with higher sodium content, the KD with lower sodium was expected have greater increases in aldosterone and that change in cardiometabolic risk factors would be associated with change in aldosterone and ketones.
Materials and Methods
Study Design
This project was part of a larger study that involved a 2-arm randomized controlled feeding trial designed to assess the effects of a KD with an exogenous ketone salt supplement (KD + KS) and a KD with placebo (KD + PL) on multiple outcomes including body composition and measures of cardiometabolic risk (19, 20). Here we focus primarily on the RAAS and related biomarkers. Twenty-eight participants were enrolled and randomized 1:1 to either KD + PL or KD + KS. Two participants in the KD + KS (86% completion rate) and 1 participant in the KD + PL (93% completion rate) did not complete the study. A body mass index-and age-matched group of participants (n = 12) were assigned post hoc to an energy- and protein-matched LFD in order to explore the influence of macronutrient distribution on all variables of interest (100% completion rate).
Our team of dietitians developed a unique, weekly rotational menu that had all the ingredients precisely weighed (±0.1 g) and analyzed for macro/micronutrient profiles with advanced nutrient analysis software (Nutritionist Pro, Axxya Systems, Redmond, WA). Both KDs were developed based on previous standards for well-formulated KDs (21-23) and the LFD was created in accordance with the USDA's Dietary Guidelines for Americans 2015 to 2020. Each menu was designed to provide 75% of estimated energy requirements of weight maintenance. To individualize the menus for all participants, we created a dietary template of 2000 kcal/day, then scaled all the menu items up or down to meet the energy requirements for weight loss. Protein was standardized at 1.5 g/kg reference weight for all participants irrespective of diet. A fraction of the total daily protein provided to KD groups consisted of 2 chocolate or vanilla shakes that contained a combination of whey protein isolate (∼15 g/serving) and fat (high oleic sunflower oil + medium chain fatty acids). A fraction of the fat content in KD groups was provided as 10 g of medium chain triglyceride (MCT) oil (caprylic and capric acid) consumed at breakfast and with the afternoon snack. The KDs provided less than 50 g of carbohydrates per day. The remaining calories consisted of fat emphasizing monounsaturated and saturated sources. The LFD was standardized for protein to match the KD and provided 25% fat (<10% saturated fat) with the remaining calories coming from carbohydrate. The primary goal was to provide at least 32 g of fiber per day, ∼100 g of simple carbohydrates (<25 g of added sugars), and <30 g of added oils/day to meet USDA requirements. Participants were allowed to consume calorie-free/salt-free products in moderation during the entire intervention. The full dietary composition of the 3 diets has been previously published (19) and the macronutrient/micronutrient compositions can be found elsewhere (Table S1 (24)).
The KD + KS received a ketone supplement twice a day. The KS consisted of 50:50 D- and R-β-hydroxybutyrate (βOHB) salts with noncaloric raspberry flavoring. Each serving contained 11.8 g of βOHB, 1874 mg of sodium, 570 mg of calcium, and 57 mg of magnesium (Metagenics, Inc., Aliso Viejo, CA). Each KS dose was dissolved in at least 230 milliliters of water and stirred vigorously to ensure proper mixing, then consumed in <5-minute spans. The first dose was taken in the morning and the second dose was consumed 6 hours later after lunch. The KD + PL and LFD group both received a calorie-free flavored PL to maintain the double-blind nature of the study. The PL was identical in taste to the KS but contained no minerals or βOHB. To ensure a consistent caloric restriction across groups, we decreased the fat content of the KD + KS group by the estimated caloric content of the ketone supplement, which was primarily attributed to βOHB.
Study Participants
Eligible participants were required to be 21-65 years of age with a body mass index of ≥27 to 35 kg/m2. Exclusion criteria included pre-existing gastrointestinal disorders or food allergies; excess alcohol consumption (>14 drinks/week); disease conditions (diabetes, liver, kidney, or other metabolic, or endocrine dysfunction); and use of diabetic medications. Participants who met the inclusion criteria were scheduled for a screening meeting where the study was described in detail and a medical history questionnaire was filled in. Besides body mass index being elevated, participants were otherwise healthy and were not on medications that impact the RAAS system, including ACE inhibitors, ARBs, beta blockers, etc. A full consort diagram has been previously published (19) and a participant flow diagram is shown elsewhere (Fig. S1 (24)). All eligible participants signed an informed consent document approved by the Ohio State University Institutional Review Board (IRB # 2017H0395) and all participants gave written informed consent.
Testing Assessments
All participants reported to the testing facility between 05:00 hours and 07:00 hours for biweekly assessments (PAES Building, 305 Anne and John Glenn Ave, Columbus, OH). Pretesting guidelines instructed participants to consume no caffeine for >12 hours before testing, no food for at least 6 hours, sleep 8 to 10 hours the night before, and abstain from strenuous exercise 48 hours before the visit. Upon arrival, weight and height were measured on a scale and electronic stadiometer (SECA 703 Digital, Hamburg, Germany) calibrated to the nearest ±0.01 kg and ±0.1 cm, respectively. Respiratory quotients (RQs) were calculated via indirect calorimetry using a metabolic cart fitted with a canopy (ParvoMedics TrueOne 2400) (25). Gas exchanges were measured for a total of 25 minutes at 15-second refresh intervals. The RQ was calculated from the running average of the last 5 minutes of continuous cart readings to ensure that the participant was fully rested, and exhaled gases were void of erroneous fluctuations. Blood pressure was measured via auscultation with a manual sphygmomanometer by the same laboratory technician. Seated participants had their first blood pressure recorded from the nondominant arm, followed by a duplicate reading 5 minutes later. Systolic and diastolic duplicates were averaged and recorded as average blood pressure.
Daily fasted βOHB and glucose were monitored in whole capillary blood using a portable ketone and glucose meter (Abbott FreeStyle, Columbus, OH). All blood was drawn before 08:00 hours in the same upright position. Phlebotomy was done via venipuncture in the antecubital fossa using 21G butterfly needles (Eppendorf, Hamburg, Germany). Approximately 20 mL of whole blood was collected in a 10 mL plasma-rated vacuum tube and 10 mL serum separator tube (Eppendorf, Hamburg, Germany). Plasma was inverted 8 times and centrifuged immediately at 1200g at 4 °C for 10 minutes. The serum separator tube was inverted 5 times and set on ice for 30 minutes to ensure proper coagulation of red blood cells before centrifugation. Plasma fractions were aliquoted in individual samples, snap-frozen in liquid nitrogen, and stored at −80 °C for future analyses. The serum separator tube was shipped the same day to an off-campus laboratory (Quest Diagnostics, Chicago, IL) for comprehensive metabolic and lipid panel analyses. Insulin resistance was estimated using homeostasis model assessment for insulin resistance (HOMA-IR) = (fasting plasma glucose [mmol/L] × fasting plasma insulin [mU/mL]) ÷ 22.5% (26). Serum aldosterone and renin were assayed using enzyme-linked immunosorbent assay (27). Serum aldosterone (ng/dL) was assayed with a commercially available colorimetric assay kit with a range of detection between 3.9 pg/mL and 250 pg/mL with sensitivity equal to 4.7 pg/mL (mean coefficient of variation (CV) = 6.5%) (Abcam., Cambridge, UK). Serum direct renin (pg/mL) was analyzed with a commercially available fluorometric assay kit (mean CV = 8.5%) (Abcam., Cambridge, UK). Urine collection methods have been previously reported in Buga et al (19). In brief, participants were provided with a container at each biweekly testing session for a 24-hour urine collection. Containers were pretreated with stabilizers (gentamicin 20 mg, Germall II 1.25 g) to deter disappearance and degradation at room temperature. Upon sample return, samples were stored at −20 °C until analysis (Litholink Corporation, Chicago, IL, USA). A 3T magnetic resonance imaging scan (MAGNETOM Prisma Fit, Siemens Healthineers, Erlangen, Germany) was used to collect information on changes in cardiac function at baseline and at completion of the intervention at the Martha Morehouse Medical Plaza (2050 Kenny Rd, Columbus, OH 43221). Information regarding cardiac function was then assessed using SuiteHeart software (Neosoft, Pewaukee, WI) to quantify ejection fraction. Visceral and subcutaneous adipose tissue data were collected by an individual with more than 5 years of experience using methodology detailed previously (28).
Statistical Analysis
Descriptive statistics were performed to summarize the study population including medians for the continuous variables and frequencies for the categorical variables. The baseline characteristics were stratified by dietary interventions. Differences in baseline characteristics across groups were assessed using Kruskal–Wallis test or Fisher's exact test as appropriate. For the outcomes, linear mixed models with random intercept were used to explore the change of these measures across time by different diet group controlling for sex and body mass index. For each measure, the differences between any 2 diet groups and 95% CI within each time point were reported. These models were adjusted for sex and body mass index because they were factors involved in the selection of the LFD group. Means for each group over time were estimated and displayed graphically with 95% CI. Scatter plots were constructed to graphically illustrate relationships between average estimates for pairs of outcomes. Additional analyses were conducted using a mixed model controlling for time, sex, and body mass index to explore the associations of aldosterone and ketones with inflammatory markers, lipids, glycemic and cardiac measures. All analyses were conducted using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was defined as a 2-sided alpha < .05.
Results
Baseline Characteristics
Baseline participant characteristics were similar across groups including weight, body mass index, systolic and diastolic blood pressures, serum glucose, HOMA-IR, and all measured lipid markers (Table 1). The median age was 33 (interquartile range [IQR] 28, 38) and 51% of participants were women. Median body mass index and baseline weight were 30.6 kg/m2 (IQR 29.9, 31.6) and 91.3 kg (IQR 81, 100.1), respectively. Median systolic blood pressure was 118 mmHg (IQR 111, 124). Compared with the LFD, serum aldosterone and renin were higher (P = .014 and P = .0001, respectively) in the KD groups. Fasting capillary Beta-Hydroxybutyrate (BHB) was below the threshold for nutritional ketosis in all groups at baseline. There were no differences in baseline aldosterone in men and women (P = .5434) (Table S2 (24)).
Table 1.
Baseline characteristics of participants by intervention assignment
| Variable | Low-fat diet (n = 12) | Ketogenic diet only group (n = 13) | Ketogenic supplement group (n = 12) | Total (n = 37) | P value |
|---|---|---|---|---|---|
| Age | 32.5 (27.5, 40.5) | 34 (30, 48) | 33 (26, 36) | 33 (28, 38) | .5726 |
| Female | 6 (50%) | 7 (54%) | 6 (50%) | 19 (51%) | .9800 |
| Adiposity | |||||
| Weight (kg) | 90.8 (87.4, 98.3) | 95.4 (89.9, 99.8) | 82.1 (80.7, 102.7) | 91.3 (81.1, 100.1) | .7178 |
| Body mass index (kg/m2) | 30.5 (29.3, 32.9) | 30.6 (29.4, 34.1) | 30.8 (29.9, 31.6) | 30.6 (29.4, 32.7) | .7822 |
| Visceral adipose tissue (cm3)a | 2217.2 (1580.7, 3172) | 2792.2 (1685.6, 3727.2) | 2873.1 (1450.4, 4361.2) | 2323.7 (1568, 3925.8) | .8622 |
| Subcutaneous adipose tissue (cm3)a | 5406.9 (4617.9, 7622.4) | 5403.6 (4112.9, 7408.2) | 4799.9 (3856.9, 6733.7) | 5268.4 (4040, 7384.5) | .7211 |
| Liver fat percentage (%)a | 3 (1.7, 6.7) | 2.7 (2, 4.3) | 3.2 (2.1, 7.4) | 3 (2, 6.7) | .9193 |
| Total fat mass (DXA) (kg) | 79.6 (62.8, 85.1) | 78.9 (65.3, 86) | 68.6 (61.7, 78) | 73.1 (62.6, 83.8) | .4090 |
| Total lean mass (DXA) (kg) | 118.5 (107.8, 134.3) | 110.2 (106, 137.3) | 117.4 (99.9, 150.1) | 114.2 (104.4, 137.3) | .9008 |
| Blood pressure | |||||
| Systolic blood pressure (mmHg) | 118.5 (116.5, 125.8) | 115 (110, 123.5) | 117 (111.5, 120.8) | 117.5 (111, 123.5) | .4855 |
| Diastolic blood pressure | 79.5 (76.8, 87.8) | 78 (72, 84.5) | 73.8 (62.3, 80.8) | 78 (72.5, 84.5) | .0846 |
| RAAS | |||||
| Serum aldosterone (ng/dL) | 8 (5.6, 9.1) | 13.6 (10.2, 16.5) | 13.7 (9.1, 23) | 10.5 (7.5, 16.5) | .0140 |
| Direct renin (pg/mL) | 6709 (5707, 7649) | 13 601 (9365, 15989) | 12 538 (10249, 14106) | 10 161 (7651, 14770) | .0001 |
| Blood metabolic panel | |||||
| Fasted capillary ketones (mmol/L BHB) | 0.1 (0.1, 0.1) | 0.2 (0.1, 0.2) | 0.4 (0.3, 0.5) | 0.2 (0.1, 0.3) | <.0001 |
| Serum glucose (mg/dL)b | 87 (81, 92.5) | 88 (84, 91.5) | 87.5 (80.5, 94.5) | 87.5 (82, 92.5) | .9059 |
| Insulin (mU/mL) | 15.1 (9.9, 19.6) | 11 (7, 12.3) | 9.7 (6.3, 14.8) | 11 (8, 15.8) | .1512 |
| Average capillary glucose (mg/dL) | 102.4 (96.1, 106.7) | 90.5 (85.1, 99) | 94.2 (89.6, 100.1) | 94.6 (90.5, 103.6) | .0444 |
| Creatinine (mg/dL)b | 0.9 (0.8, 1) | 0.8 (0.8, 1) | 0.9 (0.7, 1.1) | 0.9 (0.8, 1) | .7018 |
| Sodium (mmol/L)b | 138.5 (137, 140) | 138 (137, 139.5) | 138.5 (136.5, 140.5) | 138 (137, 140) | .8997 |
| Potassium (mmol/L)c | 4.3 (4.2, 4.7) | 4.3 (4.1, 4.8) | 4.4 (4.3, 4.7) | 4.3 (4.2, 4.7) | . .9234 |
| Blood lipid panel | |||||
| Total cholesterol (mg/dL)b | 185 (165.5, 201.5) | 191 (176.5, 216.5) | 189 (178, 205) | 190 (170, 202.5) | .7996 |
| Triglycerides (mg/dL)b | 110 (79.5, 144) | 113 (68.5, 175) | 80.5 (58.5, 128.5) | 102.5 (70, 136.5) | .4796 |
| High-density lipoprotein Cholesterol (mg/dL)b | 57.5 (46, 66.5) | 52 (42, 63) | 52 (48.5, 70.5) | 52 (47.5, 67) | .9122 |
| Low-density lipoprotein Cholesterol (mg/dL)b | 107 (93.5, 121) | 104 (92.5, 120) | 114 (93, 125.5) | 107.5 (92.5, 125) | .8325 |
| Total cholesterol/high density Lipoprotein ratiob | 3.3 (2.7, 4.1) | 3.4 (2.6, 4.9) | 3.5 (2.7, 4.4) | 3.3 (2.7, 4.6) | .9216 |
| Non-high–density cholesterolb | 129.5 (109, 145) | 132.5 (108, 143) | 137 (113.5, 145) | 132.5 (111, 145) | .8246 |
| Respiratory quotient 25 minutesa | 0.9 (0.9, 1) | 0.8 (0.8, 0.9) | 0.8 (0.8, 0.9) | 0.9 (0.8, 0.9) | .0725 |
| HOMA-IRd | 1.1 (0.7, 1.4) | 0.7 (0.3, 1.2) | 0.7 (0.2, 1) | 0.8 (0.4, 1.3) | .2382 |
Median (Q1, Q3) is used for continuous variables due to non-normality of the data. To assess the differences across treatment groups chi-square test was used for categorical variables and Kruskal–Wallis test was used for continuous variables.
Abbreviations: BHB, Beta-Hydroxybutyrate; DXA, dual X-ray absorptiometry; HOMA-IR, homeostasis model assessment for insulin resistance; RAAS, renin–angiotensin–aldosterone system.
Visceral adipose tissue, subcutaneous adipose tissue, and liver fat percentage are missing 1 participant in the ketogenic supplement group.
Total cholesterol, triglycerides, high density lipoprotein, low-density lipoprotein, serum glucose, creatinine, sodium–ketogenic diet group missing 1 participant.
Baseline potassium missing 2 in the ketogenic diet only group and missing 1 participant in the ketogenic supplement group at baseline.
Log transformed.
Primary Outcomes
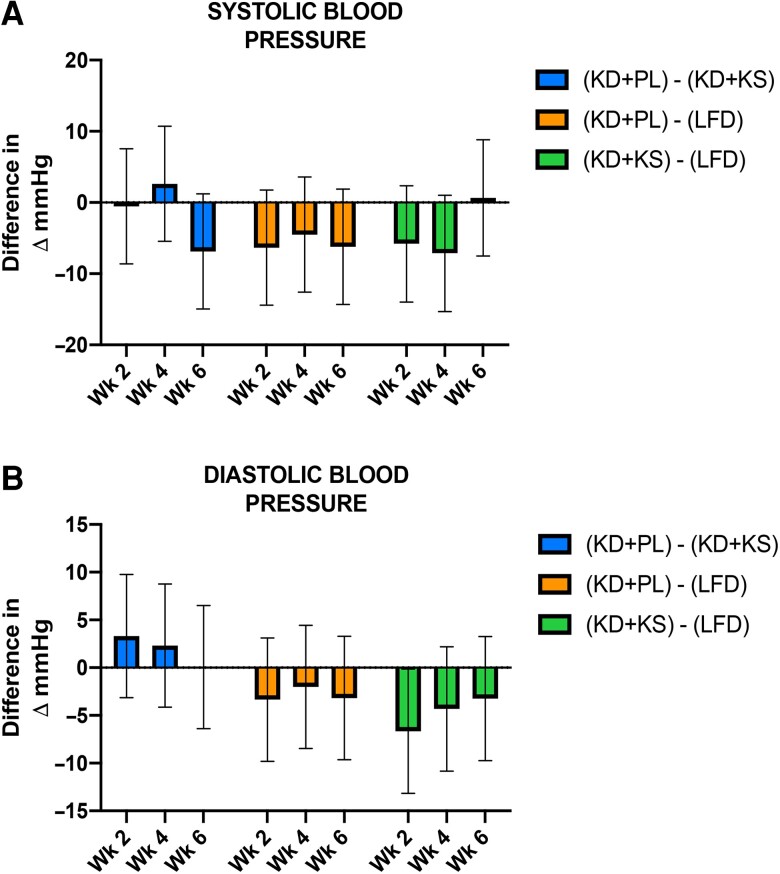
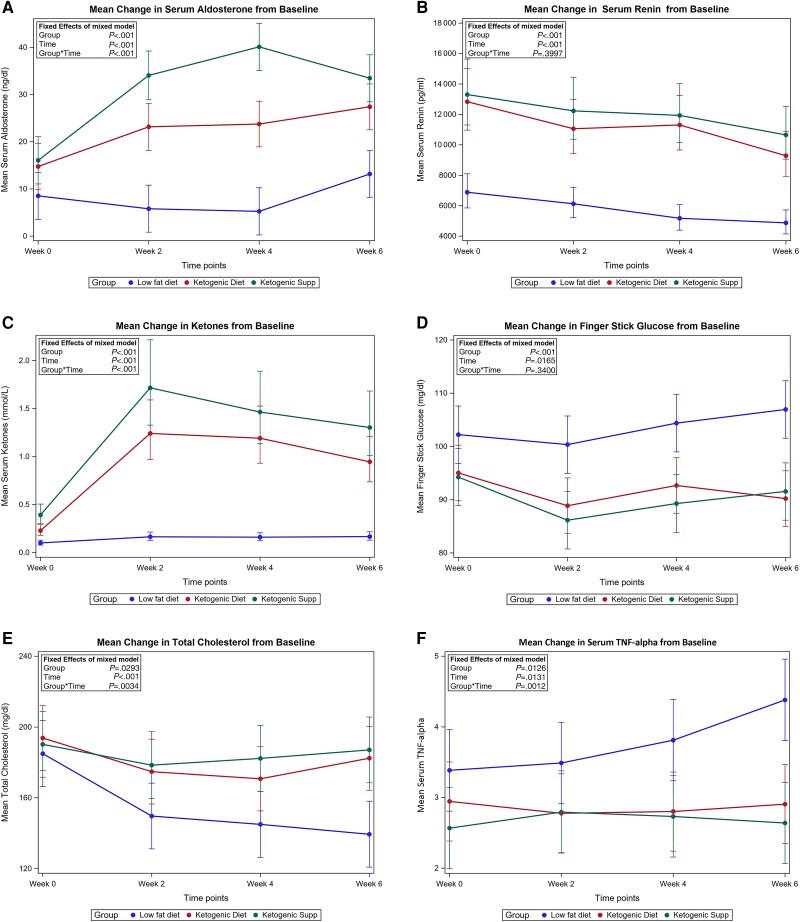
Primary outcomes are shown in Table 2, Figs. 1-3, and elsewhere (Table S3 (24)). There were no differences in weight or fat loss between interventions. The KD + PL and KD + KS groups lost 8.3 and 7.4 kg after 6 weeks, respectively (Table S3 (24) and Fig. 1). Aldosterone more than doubled in the KD + KS group at week 2, peaked at week 4 (182% of baseline levels), and decreased thereafter, reaching a final median serum level of 33.4 ng/dL (144% of baseline levels) at week 6. Similarly, aldosterone increased by 51% at week 2, increased further at week 4, and plateaued thereafter, reaching a final median serum level of 25.6 ng/dL (88% of its baseline levels) at week 6 in the KD + PL group. In contrast, aldosterone decreased initially in the LFD intervention between weeks 2 and 4, but was not statistically different from baseline at week 6 (Table S3 (24)). Changes in aldosterone were statistically different across groups (P < .0001). Renin decreased by 26%, 11%, and 24% in the KD + PL, KD + KS, and LFD groups, respectively. There was no significant difference with respect to renin (P = .3997) (Table 2; Table S3 (24)). Figure 2 shows the mean changes in aldosterone, renin, and ketones from baseline to 6 weeks. Ketones peaked 2 weeks into the intervention and slightly decreased thereafter. Overall, capillary ketones increased 4-fold in the KD + PL group and 2-fold in the KD + KS group following the 6-week intervention (Table S3 (24)). Both the KD + PL and KD + KS groups had 5-fold higher fasted capillary ketones than the LFD but did not differ from each other after accounting for sex and body mass index (Table 2). Systolic and diastolic blood pressure did not change in either KD intervention (Fig. 3). Serum sodium, serum potassium, and urinary potassium did not change from baseline in any groups. Urinary sodium decreased in the KD + PL group but did not change in the KD + KS group after 6 weeks. However, relative to LFD, there was more sodium excretion in the KD + KS group after 6 weeks (Table 2).
Table 2.
Difference in changes between selected biomarkers across interventions
| Ketogenic diet: ketogenic supplement | Ketogenic diet: low-fat diet | Ketogenic supplement: low-fat diet | P value for interaction | |
|---|---|---|---|---|
| Variables | Difference (95% CI) | Difference (95% CI) | Difference (95% CI) | |
| Weight (kg) | .0712 | |||
| 2 weeks | −0.01 (−4.96, 4.94) | −3.35 (−8.31, 1.61) | −3.34 (−8.29, 1.62) | |
| 4 weeks | 0.34 (−4.61, 5.29) | −3.00 (−7.96, 1.96) | −3.34 (−8.30, 1.61) | |
| 6 weeks | 0.10 (−4.85, 5.06) | −3.27 (−8.23, 1.69) | −3.37 (−8.33, 1.58) | |
| Serum aldosterone (ng/dL)a | <.0001 | |||
| 2 weeks | −0.52 (−0.94, −0.10) | 1.25 (0.84, 1.67) | 1.77 (1.35, 2.20) | |
| 4 weeks | −0.53 (−0.94, −0.12) | 1.57 (1.16, 1.98) | 2.10 (1.69, 2.52) | |
| 6 weeks | −0.17 (−0.59, 0.24) | 1.05 (0.63, 1.46) | 1.22 (0.81, 1.64) | |
| Renin (pg/mL)a | .3997 | |||
| 2 weeks | −0.10 (−0.33, 0.13) | 0.59 (0.36,0.82) | 0.69 (0.46, 0.92) | |
| 4 weeks | −0.05 (−0.28, 0.17) | 0.78 (0.56,1.01) | 0.84 (0.61, 1.06) | |
| 6 weeks | −0.14 (−0.36, 0.09) | 0.65 (0.42,0.87) | 0.78 (0.55, 1.01) | |
| Fasted capillary ketones (mmol/L BHB)a | <.0001 | |||
| 2 weeks | −0.32 (−0.68, 0.03) | 2.01 (1.65, 2.37) | 2.34 (1.9, 2.70) | |
| 4 weeks | −0.21 (−0.56, 0.15) | 2.00 (1.65, 2.36) | 2.21 (1.85, 2.57) | |
| 6 weeks | −0.32 (−0.68, 0.04) | 1.73 (1.37, 2.09) | 2.05 (1.69, 2.41) | |
| Systolic blood pressure | .5777 | |||
| 2 weeks | −0.54 (−8.62, 7.54) | −6.34 (−14.43, 1.75) | −5.80 (−13.97, 2.36) | |
| 4 weeks | 2.62 (−5.46, 10.70) | −4.51 (−12.60, 3.58) | −7.13 (−15.30, 1.03) | |
| 6 weeks | −6.87 (−14.95, 1.21) | −6.21 (−14.30, 1.88) | 0.66 (−7.51, 8.82) | |
| Diastolic blood pressure | .7002 | |||
| 2 weeks | 3.31 (−3.14, 9.76) | −3.35 (−9.81, 3.11) | −6.66 (−13.16, −0.15) | |
| 4 weeks | 2.31 (−4.14, 8.76) | −2.01 (−8.47, 4.45) | −4.32 (−10.83, 2.18) | |
| 6 weeks | 0.06 (−6.39, 6.52) | −3.18 (−9.64, 3.28) | −3.24 (−9.74, 3.26) | |
| Serum glucose (mg/dL) | .1900 | |||
| 2 weeks | −1.62 (−10.76, 7.51) | −7.87 (−16.87, 10.14) | 0-6.24 (−15.36, 2.87) | |
| 4 weeks | 1.88 (−7.14, 10.90) | −1.62 (−10.64, 7.41) | −3.50 (−12.47, 5.47) | |
| 6 weeks | −3.09 (−11.99, 5.81) | −7.17 (−16.08, 1.74) | −4.08 (−13.05, 4.89) | |
| Serum insulin (mIU/L)a | .7175 | |||
| 2 weeks | −0.05 (−0.48, 0.39) | −0.56 (−0.99, −0.13) | −0.51 (−0.95, −0.07) | |
| 4 weeks | 0.11 (−0.31, 0.54) | −0.36 (−0.79, 0.07) | −0.47 (−0.91, −0.04) | |
| 6 weeks | −0.23 (−0.66, 0.20) | −0.45 (−0.88, −0.02) | −0.22 (−0.65, 0.21) | |
| Serum IL-1ba | .8305 | |||
| 2 weeks | −0.33 (−0.83, 0.18) | 0.21 (−0.28, 0.71) | 0.54 (0.03, 1.05) | |
| 4 weeks | −0.14 (−0.64, 0.36) | 0.19 (−0.31, 0.70) | 0.33 (−0.17, 0.83) | |
| 6 weeks | −0.08 (−0.58, 0.41) | 0.09 (−0.41, 0.58) | 0.17 (−0.33, 0.67) | |
| Serum IL-10a | .2550 | |||
| 2 weeks | 0.34 (−0.11, 0.78) | −0.18 (−0.62, 0.26) | −0.51 (−0.96, −0.07) | |
| 4 weeks | 0.36 (−0.08, 0.80) | −0.52 (−0.96, −0.08) | −0.88 (−1.32, −0.44) | |
| 6 weeks | 0.40 (−0.04, 0.84) | −0.48 (−0.93, −0.04) | −0.89 (−1.33, −0.44) | |
| Serum IL-6a | .0443 | |||
| 2 weeks | −0.20 (−0.71, 0.30) | 0.16 (−0.33, 0.66) | 0.37 (−0.14, 0.87) | |
| 4 weeks | 0.10 (−0.39, 0.60) | −0.09 (−0.59, 0.40) | −0.20 (−0.70, 0.30) | |
| 6 weeks | 0.30 (−0.19, 0.80) | −0.02 (−0.51, 0.48) | −0.32 (−0.82, 0.18) | |
| Serum IL-8a | .4177 | |||
| 2 weeks | −0.17 (−0.48, 0.15) | −0.14 (−0.45, 0.17) | 0.03 (−0.28, 0.35) | |
| 4 weeks | 0.00 (−0.31, 0.32) | −0.08 (−0.38, 0.23) | −0.08 (−0.39, 0.24) | |
| 6 weeks | 0.10 (−0.22, 0.41) | −0.15 (−0.46, 0.16) | −0.24 (−0.56, 0.07) | |
| Serum TNF-α | .0012 | |||
| 2 weeks | −0.02 (−0.83, 0.80) | −0.71 (−1.52, 0.10) | −0.69 (−1.51, 0.12) | |
| 4 weeks | 0.07 (−0.74, 0.88) | −1.01 (−1.82, −0.20) | −1.08 (−1.89, −0.27) | |
| 6 weeks | 0.27 (−0.54, 1.08) | −1.47 (2.28, −0.66) | −1.74 (−2.55, −0.93) | |
| Serum sodium (mmol/L) | .8478 | |||
| 2 weeks | −1.77 (−5.29, 1.76) | −2.82 (−6.28, 0.64) | −1.05 (−4.57, 2.46) | |
| 4 weeks | −0.81 (−4.21, 2.60) | −2.64 (−6.04, 0.77) | −1.83 (−5.27, 1.61) | |
| 6 weeks | 0.39 (−3.02, 3.79) | −1.19 (−4.60, 2.21) | −1.58 (−5.02, 1.86) | |
| Serum potassium (mmol/L) | .1062 | |||
| 2 weeks | 0.09 (−0.25, 0.42) | 0.11 (−0.21, 0.44) | 0.03 (−0.30, 0.35) | |
| 4 weeks | 0.37 (0.02, 0.72) | 0.06 (−0.27, 0.40) | −0.31 (−0.64, 0.03) | |
| 6 weeks | 0.58 (0.24, 0.92) | 0.41 (0.08, 0.74) | −0.17 (−0.50, 0.17) | |
| Urine sodium 24-hour urine | .0057 | |||
| 2 weeks | −124.73 (−177.32, −72.14) | 35.42 (−17.22, 88.06) | 160.15 (108.13, 212.16) | |
| 4 weeks | −56.38 (−108.98, −3.79) | −5.25 (−57.89, 47.40) | 51.14 (−0.88, 103.15) | |
| 6 weeks | −79.87 (−134.42, −25.32) | 28.39 (−24.25, 81.04) | 108.26 (54.15, 162.37) | |
| Urine potassium 24-hour urine | .2996 | |||
| 2 weeks | 9.69 (−12.28, 31.66) | −13.04 (−35.03, 8.95) | −22.73 (−44.49, −0.97) | |
| 4 weeks | 11.71 (−10.26, 33.68) | −32.92 (−54.91, −10.93) | −44.63 (−66.39, −22.87) | |
| 6 weeks | 1.16 (−21.71, 24.03) | −19.20 (−41.19, 2.79) | −20.36 (−43.08, 2.36) | |
| Serum creatinine (mg/dL) | .3314 | |||
| 2 weeks | −0.07 (−0.20, 0.07) | −0.14 (−0.28, −0.01) | −0.07 (−0.21, 0.06) | |
| 4 weeks | 0.01 (−0.12, 0.14) | −0.10 (−0.24, 0.03) | −0.11 (−0.25, 0.02) | |
| 6 weeks | −0.01 (−0.15, 0.12) | −0.09 (−0.23, 0.04) | −0.08 (−0.21, 0.05) | |
| Fasted capillary glucose (mg/dL) | .3400 | |||
| 2 weeks | 2.72 (−4.82, 10.26) | −11.47 (−19.02, −3.92) | −14.19 (−21.80, −6.58) | |
| 4 weeks | 3.39 (−4.15, 10.93) | −11.70 (−19.25, −4.15) | −15.10 (−22.71, −7.48) | |
| 6 weeks | −1.33 (−8.87, 6.22) | −16.73 (−24.28, −9.18) | −15.41 (−23.02, −7.80) | |
| Leptina | .6507 | |||
| 2 weeks | 0.44 (−0.49, 1.38) | −0.29 (−1.22, 0.65) | −0.73 (−1.66, 0.21) | |
| 4 weeks | 0.43 (−0.50, 1.36) | −0.35 (−1.29, 0.58) | −0.79 (−1.72, 0.15) | |
| 6 weeks | 0.14 (−0.79, 1.07) | −0.27 (−1.20, 0.66) | −0.41 (−1.34, 0.53) | |
| HDL | .2118 | |||
| 2 weeks | −3.96 (−16.60, 8.68) | −0.05 (−12.65, 12.55) | 3.91 (−8.72, 16.53) | |
| 4 weeks | −1.79 (−14.32, 10.74) | 1.72 (−10.84, 14.27) | 3.51 (−9.05, 16.07) | |
| 6 weeks | 0.92 (−11.61, 13.46) | 6.68 (−5.87, 19.24) | 5.76 (−6.80, 18.32) | |
| LDL | .0023 | |||
| 2 weeks | −1.88 (−24.72, 20.96) | 19.89 (−2.84, 42.62) | 21.77 (−1.04, 44.58) | |
| 4 weeks | −8.37 (−30.97, 14.22) | 22.12 (−0.51, 44.76) | 30.49 (7.83, 53.16) | |
| 6 weeks | −4.46 (−27.05, 18.13) | 34.37 (11.73, 57.00) | 38.83 (16.16, 61.49) | |
| Triglycerides | .3662 | |||
| 2 weeks | 2.07 (−40.37, 44.50) | 24.03 (−18.01, 66.07) | 21.96 (−20.39, 64.32) | |
| 4 weeks | −1.58 (−43.24, 40.09) | 17.65 (−24.08, 59.38) | 19.23 (−22.67, 61.12) | |
| 6 weeks | −1.64 (−43.31, 40.03) | 16.59 (−25.14, 58.32) | 18.23 (−23.67, 60.12) | |
| HOMA-IRa | .5451 | |||
| 2 weeks | −0.02 (−0.49, 0.46) | −0.60 (−1.07, −0.14) | −0.59 (−1.06, −0.12) | |
| 4 weeks | 0.14 (−0.32, 0.61) | −0.38 (−0.85, 0.09) | −0.52 (−0.99, −0.06) | |
| 6 weeks | −0.29 (−0.75, 0.18) | −0.56 (−1.02, −0.09) | −0.27 (−0.74, 0.19) | |
Linear mixed models with random intercept were used to explore the change of these measures across time by different diet group controlling for sex and body mass index. For each measure, the differences of any 2 diet groups and 95% CI within each time point, and P values of interaction term (time × diet group) were reported. Interpretations: The change in aldosterone across time was significantly different across dietary interventions after controlling for sex and body mass index (P < .001). In week 2, the mean of log-aldosterone in the ketogenic diet + placebo group was significantly lower than that in the ketogenic diet + supplement group: diff (95% CI) = −0.52 (−0.94, −0.1).
Abbreviations: BHB, HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance; IL, interleukin; LDL, low-density lipoprotein; TNF, tumor necrosis factor
Variables are log transformed.
Figure 1.
Mean differences in weight loss between groups over 6 weeks. The figure presents the weight loss of the 3 groups in comparison to each other at Weeks 2, 4, and 6. The blue bars represent the differential weight loss of the ketogenic diet + placebo vs ketogenic diet + ketogenic salt.
Figure 3.
Systolic (A) and diastolic (B) blood pressure between groups over 6 weeks. Linear mixed models with random intercept were used to explore the difference in the change in weight, systolic blood pressure, and diastolic blood pressure across time by different diet group controlling for sex and body mass index. For each measure, the differences of any 2-diet group and 95% CI within each time point are displayed.
Figure 2.
(A-F) Mean changes in selected biomarkers by dietary groups over 6 weeks. Means for each group over time were estimated and displayed graphically with 95% CI. The P values were type 3 tests of fixed effects from a mixed model. The most important one is interaction between group and time. If interaction P < .05, then the change of the outcome across time were significant different among diet groups, after controlling for sex and body mass index.
Secondary Outcomes
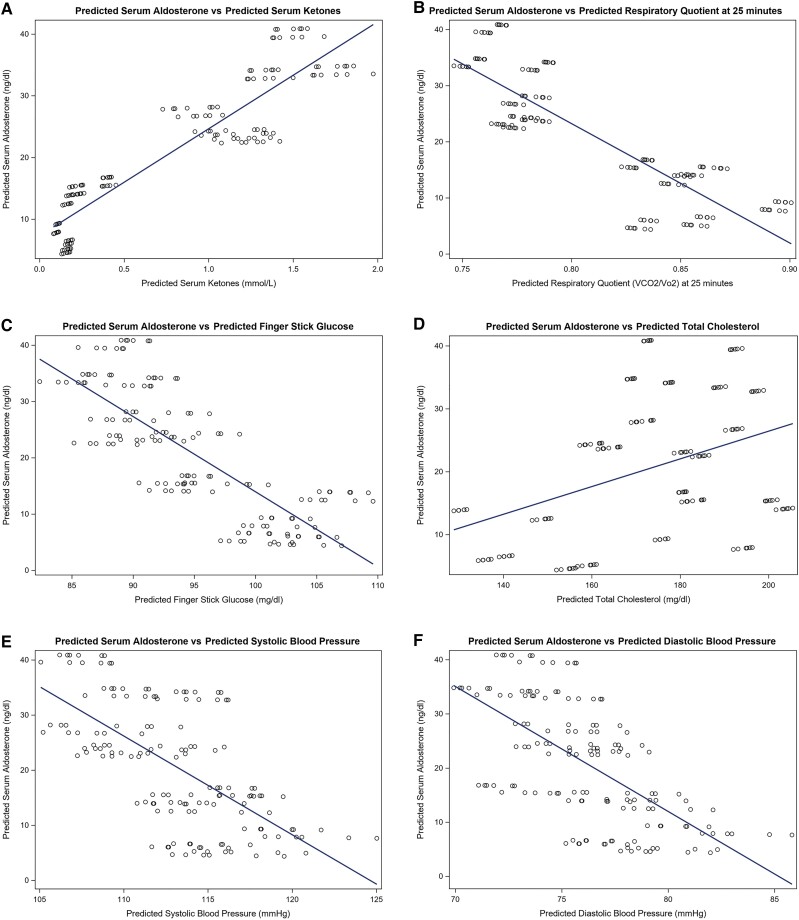
Markers of cardiometabolic risk improved or were steady over the course of the intervention among participants on the KD. Insulin and HOMA-IR decreased in both the KD + PL and KD + KS groups from baseline while interleukin (IL)-1β, IL-10 IL-6, IL-8, serum creatinine, and tumor necrosis factor (TNF)-α did not change after the 6-week intervention. Similar results were observed in the LFD group (Table S2 (24)). However, TNF-α increased from baseline in the LFD and the change was significantly different from the KD + KS and KD + PL groups (P = .0012). The longitudinal associations of markers of interest with aldosterone and ketones are explored in Table 3. Significant negative associations existed between RQ-25 (P = .019) and glucose (P = .004) with log-aldosterone and positive associations between total cholesterol (P = .006) and log-aldosterone. Predicted means reflecting these associations are shown graphically in Fig. 4A-4F. After adjusting for time, sex, and body mass index, log-aldosterone (β = .31, CI 0.15, 0.46, P < .001) and log-renin (β = .47, CI 0.08, 0.86, P = .0183) were positively associated with log-ketones, but a negative association existed between insulin (β = −0.42, CI −0.61, −0.23, P < .0001) and log-ketones. Furthermore, TNF-α (β = −.89, CI −1.37, −0.41, P < .001), log-leptin (β = −.30, CI −0.45, −0.15, P < .001), and log-HOMA-IR (β = −.399 CI −0.577, −0.221, P < .001) were negatively associated with log-ketones. Cardiovascular risk factors such as systolic blood pressure (P = .78), diastolic blood pressure (P = .44), ejection fraction (P = .80), and log-HOMA-IR (P = .332) were not associated with log-aldosterone (Table 3).
Table 3.
Longitudinal association between biological markers with log-aldosterone and log-ketones
| Measurement | Log-aldosterone | Log-ketones | ||
|---|---|---|---|---|
| Beta (95% CI) | P value | Beta (95% CI) | P value | |
| Log-interleukin-10 | −0.195 (−0.436, 0.046) | .112 | −0.240 (−0.467, −0.013) | .038 |
| Log-interleukin-6 | 0.120 (−0.052, 0.291) | .169 | −0.166 (−0.318, −0.014) | .032 |
| Log-interleukin-8 | −0.215 (−0.539, 0.110) | .193 | −0.087 (−0.380, 0.207) | .560 |
| Log-interleukin-1β | −0.011 (−0.184, 0.162) | .902 | 0.004 (−0.151, 0.159) | .961 |
| Log-insulin | −0.051 (−0.277, 0.176) | .657 | −0.421 (−0.613, −0.229) | <.001 |
| Log-MCP-1 | −0.006 (−0.604, 0.593) | .985 | 0.276 (−0.310, 0.862) | .354 |
| Log-TNF-α | −0.377 (−0.878, 0.123) | .138 | −0.893 (−1.371, −0.415) | <.001 |
| RQ (25 minutes) | −2.304 (−4.214, −0.393) | .019 | −3.548 (−5.195, −1.901) | <.001 |
| Glucose (average) | −0.016 (−0.027, −0.005) | .004 | −0.023 (−0.032, −0.013) | <.001 |
| Total cholesterol | 0.006 (0.002, 0.010) | .006 | 0.003 (−0.001, 0.007) | .112 |
| Systolic blood pressure | 0.001 (−0.009, 0.012) | .781 | −0.007 (−0.016, 0.002) | .146 |
| Diastolic blood pressure | 0.006 (−0.009, 0.020) | .435 | −0.010 (−0.023, 0.003) | .114 |
| Ejection fraction | 0.007 (−0.016, 0.03) | .5604 | −0.003 (−0.027, 0.021) | .8058 |
| Log-renin | 0.841 (0.487, 1.194) | <.001 | 0.470 (0.081, 0.859) | .018 |
| Log-aldosterone | —- | − | 0.30 5 (0.152, 0.458) | <.001 |
| Log-leptin | −0.059 (−0.216, 0.099) | .463 | −0.297 (−0.447, −0.148) | <.001 |
| Log-HOMA-IR | −0.105 (−0.317, 0.108) | .332 | −0.399 (−0.577, −0.221) | <.001 |
Linear mixed models with random intercept were used to explore the longitudinal associations between each measure with log-aldosterone, log-ketones, or log-leptin, controlling for time, sex, and body mass index. Beta coefficient and 95% CI were reported. Interpretations: There was a positive association between log-aldosterone and log-ketones (P = .0001). A 1% percent change in aldosterone will result in [ −1] × 100 = 0.3% increase in ketones. There was a positive association between total cholesterol and log-aldosterone (P = .0062), a 1 unit increase in total cholesterol will result in (−1) × 100 = 1% increase in aldosterone.
Abbreviations: MCP-1, monocyte chemoattractant protein-1; TNF-α , tumor necrosis factor-α; RQ, respiratory quotient.
Figure 4.
(A-F) Predicted relationships between serum aldosterone and selected biomarkers over 6 weeks. Scatter plots were constructed to graphically illustrate relationships between average estimates for pairs of outcomes, for example there was a positive association between predicted Aldosterone with predicted ketones.
Discussion
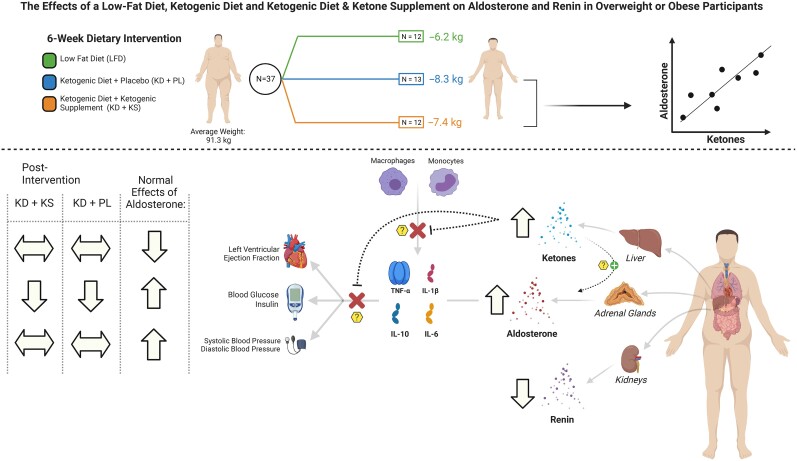
In this prospective, controlled feeding study, contrary to our hypothesis, serum aldosterone increased by 88% among obese/overweight individuals randomized to a hypocaloric KD with a βOHB salt PL and 144% among those ingesting a mineral containing racemic βOHB supplement twice daily. Aldosterone did not change in the LFD group despite similar weight loss between groups. Aldosterone concentrations were inversely related to renin, which strongly suggests independent renin activation. Exploratory analyses revealed no adverse impact of either iteration of the KD intervention on important clinical markers of cardiometabolic risk. After controlling for time effects, sex, and body mass index, ketone concentrations were positively associated with aldosterone (P < .0001), suggesting a potential new role of ketones on the RAAS axis. The novel findings are summarized in Fig. 5.
Figure 5.
The effects of a low-fat diet, ketogenic diet, and ketogenic diet and ketone supplement on aldosterone and renin in overweight or obese participants. Figure summarizes the effects of a low-fat diet, ketogenic diet and ketogenic diet + ketone supplement on aldosterone, renin, inflammation and cardiovascular risk factors among overweight or obese participants.
The general relationship between adiposity and aldosterone has been well elucidated. The 2008 Primary Aldosteronism Prevalence in Italy (PAPY) study reported a positive association between plasma aldosterone and body mass index in obese patients with essential hypertension (29). Aldosterone may be elevated independent of renin in severe obesity. This finding was not established in mildly obese or lean controls, which suggests that the synthesis of aldosterone may be decoupled from the classic RAAS pathway in obesity (30). Given these aforementioned associations and the finding that adipocytes are able to independently synthesize aldosterone, levels would be expected to decrease following weight loss (31).
Evidence from numerous weight loss interventions align with these expectations. Interventions that produce weight loss have consistently resulted in reductions in renin and aldosterone despite considerable heterogeneity in caloric intakes, salt intakes, and macronutrient profiles (8-10, 12-14, 16, 32). To our knowledge, only 1 other study had previously investigated the effects of weight loss in a low/very low carbohydrate dietary intervention. Among 8 participants with obesity assigned to an 800-kcal diet with either 70 g or 10 g of carbohydrates, plasma renin decreased while upright serum aldosterone increased from 30 ng/dL at baseline to 42 ng/dL at week 12 (17). The ∼40% increase in aldosterone was less than in both KD interventions in the present study. Additionally, in contrast to the aforementioned investigation, the increase in aldosterone was validated through rigorous statistical methodology. Similarly, however, the observed rise in aldosterone occurred despite a high sodium intake (3430 mg/day). The findings from this study corroborate this phenomenon, which was especially noteworthy in the KD + KS group. These participants consumed a total of more than 6000 mg sodium/day, which may be necessary to compensate for the natriuretic effect of KDs, but which represents approximately 3 times the daily upper limit recommended by the USDA dietary guidelines. The increase in aldosterone in a high sodium environment is surprising given that aldosterone is suppressed by high sodium loads under normal physiology. In support of these findings, in a study with larger reductions in body weight (∼20 kg) and caloric restriction (320 kcal/day), those with a higher salt intake (2750 mg) experienced a 5 ng/dL greater reduction in aldosterone compared with those with a lower salt intake (920 mg) (8). These findings further support the notion that changes to RAAS physiology become pronounced in a state of ketosis or during carbohydrate restriction.
The findings from the current study show alterations in RAAS homeostasis in response to the KD, findings made more surprising in the context of the absence of adverse changes to the cardiometabolic risk profile. Aldosterone is a known risk factor for hypertension. In the Framingham Offspring Study, individuals in the highest quartile compared with the lowest quartile of aldosterone at baseline had a 61% higher risk of developing hypertension over a 4-year period (33). Additionally, aldosterone is associated with insulin resistance (34). Given its relationship with these aforementioned risks, the elevation in aldosterone in those on a KD could be concerning from a cardiometabolic perspective. However, participants in the KD arms did not experience increases in blood pressure throughout the 6-week intervention. Furthermore, despite elevated aldosterone levels in the KD + PL and KD + KS groups, there was no evidence of any deleterious effects on cardiovascular health for these groups. In addition, all groups experienced a significant reduction in blood glucose across the study intervention. These findings indicate that, despite high aldosterone and sodium consumption, detrimental change in cardiometabolic risk factors including blood pressure and glucose did not occur in the KD groups.
Aldosterone can also adversely impact the vascular system, independent of blood pressure, through inflammation (35, 36). Aldosterone induces inflammation directly in cardiac tissue leading to fibrosis and remodeling; however, this effect is prevented by MR antagonist (37). Furthermore, it has been recently reported that the NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome is necessary for the development of aldosterone-induced vascular dysfunction (38). Mice receiving infusions of aldosterone experience a significant increase in vascular cell adhesion protein-1, intracellular adhesion molecule-1, and systolic blood pressure. These effects are negated in NLRP3 knockout mice who experience no increase in the aforementioned parameters compared with controls (38).
Interestingly, βOHB has been shown to directly inhibit the NLRP3 inflammasome in vitro through an unknown mechanism that is independent of UCP2, Sirt2, GPR109RA, or the magnitude of histone acetylation in macrophages (39). A terminal product of the NLRP3 inflammasome cascade is IL-1β. This proinflammatory cytokine is largely produced by macrophages and monocytes and plays an important role in the development of atherosclerosis by promoting the expression of adhesion molecules and by increasing endothelin-mediated vasoconstriction (40, 41). A recent study demonstrated that sodium-glucose cotransporter-2 inhibitors significantly suppress NLRP3 inflammasome activation and subsequent secretion of IL-1β in human macrophages from patients with type 2 diabetes and cardiovascular disease. This effect may be mediated via increased serum ketone levels and decreased serum levels of insulin (42). Blocking IL-1β with a monoclonal antibody leads to a significant reduction in major adverse cardiovascular events among patients with a prior myocardial infarction and high levels of proinflammatory biomarkers (43). This may partially explain the cardiovascular benefits observed in both the CANVAS trial and EMPA-REG OUTCOME trial (44, 45). Participants in the KD + PL and KD + KS groups experienced no significant change in IL-1β, which may indicate that these individuals were at least partially protected from the effects of aldosterone on the vascular system. Similar to our previous work with KDs (46) increases in TNF-α, another inflammatory cytokine (47), were also blunted with KD compared with LFD in the current study. IL-6, an inflammatory cytokine associated with higher cardiovascular risk through vascular inflammation, vascular stiffness, and endothelial dysfunction that promotes hypertension (48), also did not increase with KD. The absence of increases in inflammatory cytokines alongside increases in aldosterone among those on the KD is reassuring from a cardiometabolic perspective. The data indicate that the 2 major sources of cardiovascular risk stemming from aldosterone elevations (hypertension and inflammation) are inhibited in the context of a KD, potentially due to elevated chronic βOHB concentrations.
Our study has several strengths. First, participants received customized meals tailored to their respective dietary assignment with individual caloric and protein needs allowing for a highly accurate and controlled feeding study. Second, the study benefited from optimal compliance of participants as reflected in the 95% completion rate in the study. Finally, participants underwent comprehensive biomarker and imaging assessments throughout the study period enabling the assessment of the intervention at various points in time. The study should be interpreted in light of some limitations. All participants were non-Hispanic White; hence the findings need to be evaluated in other racial/ethnic groups to assess the impact of race and ethnicity on outcomes. The 6-week intervention was chosen due to successful metabolic changes observed in previous KD studies, and this intervention did in fact elicit positive responses. However, 6 weeks may not be sufficient to elicit pronounced cardiac responses in some people. While the majority of baseline characteristics of participants were similar, those in the KD + PL and KD + KS groups had significantly higher renin and aldosterone levels than the low-fat group at baseline, although the majority of the analyses performed focused on change from baseline. Urine was collected 24 hours after intervention, thus there is no true baseline for urine markers. Furthermore, though participants in the LFD group were matched for body mass index and age with KD participants, they were recruited post hoc and thus were not randomized. Although we did not see unexpected sex differences at baseline and adjusted for sex in all models and did not find significant 2-way interactions, future larger studies should be performed to confirm the lack of sex differences shown in this study.
Conclusion
Individuals consuming a well-formulated KD with varying levels of sodium experienced a statistically and biologically significant increase in aldosterone and decrease in renin over 6 weeks. Exploratory analyses revealed that βOHB concentration was positively associated with aldosterone, a novel finding adding to the extant literature. Despite a substantial increase in aldosterone, individuals in the KD groups experienced no adverse changes in cardiometabolic risk factors. These findings suggest that ketones may have a cardioprotective effect, an important line of future investigation.
Abbreviations
- βOHB
β-hydroxybutyrate
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- CV
coefficient of variation
- DXA
dual energy X-ray absorptiometry
- HOMA-IR
homeostasis model assessment for insulin resistance
- IL
interleukin
- KD
ketogenic diet
- KD + KS
KD + ketone salt supplement
- KD + PL
KD + placebo
- LFD
low-fat diet
- MCT
medium chain triglyceride
- MR
mineralocorticoid receptor
- RAAS
renin–angiotensin–aldosterone system
- RQ
respiratory quotient
- TNF
tumor necrosis factor
Contributor Information
Paul Belany, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University College of Medicine, Columbus, OH 43210, USA.
Madison L Kackley, Department of Human Sciences, The Ohio State University, Columbus, OH 43210, USA.
Songzhu Zhao, Department of Human Sciences, The Ohio State University, Columbus, OH 43210, USA.
Bjorn Kluwe, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University College of Medicine, Columbus, OH 43210, USA.
Alex Buga, Department of Biomedical Informatics and Center for Biostatistics, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA.
Christopher D Crabtree, Department of Human Sciences, The Ohio State University, Columbus, OH 43210, USA.
Divya Nedungadi, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University College of Medicine, Columbus, OH 43210, USA.
David Kline, Department of Biomedical Informatics and Center for Biostatistics, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA.
Guy Brock, Department of Biomedical Informatics and Center for Biostatistics, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA.
Orlando P Simonetti, Department of Radiology, Davis Heart & Lung Research Institute, The Ohio State University, Columbus, OH 43210, USA; Division of Cardiovascular Medicine, Department of Internal Medicine, The Ohio State University, Columbus, OH 43210, USA; Department of Radiology, Wexner Medical Center, The Ohio State University, Columbus, OH 43210, USA.
Jeff S Volek, Department of Human Sciences, The Ohio State University, Columbus, OH 43210, USA.
Joshua J Joseph, Division of Endocrinology, Diabetes and Metabolism, The Ohio State University College of Medicine, Columbus, OH 43210, USA.
Funding
Grants or fellowships: This study was supported by a grant from Metagenics Inc to the Ohio State University. The magnetic resonance imaging for this project was funded by the National Heart Lung and Blood Institute (HHS – NIH, R01HL161618 OPS). Preparation of this manuscript was supported by National Institute of Diabetes and Digestive and Kidney Diseases (K23DK117041, JJJ) of the National Institutes of Health and The Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program ID# 76236. The project described was supported by Award Number Grant UL1TR002733 (GB) from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences, National Institutes of Diabetes, Digestive and Kidney Diseases, the National Institutes of Health or the U.S. Department of Health and Human Services.
Disclosures
J.V. receives royalties for low-carbohydrate nutrition books; is founder, consultant, and stockholder of Virta Health, Inc. and is a member of the advisory boards for Simply Good Foods. OS receives research funding support from The Robert F. Wolfe and Edgar T. Wolfe Foundation and from Siemens Healthineers. The remaining authors have nothing to declare.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Gershuni VM, Yan SL, Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. 2018;7(3):97‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6(5):261. [DOI] [PubMed] [Google Scholar]
- 3. Montani JP, Mizelle HL, Adair TH, Guyton AC. Regulation of cardiac output during aldosterone-induced hypertension. J Hypertens Suppl. 1989;7(6):S206‐S207. [DOI] [PubMed] [Google Scholar]
- 4. Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 6. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2017;70(6):776‐803. [DOI] [PubMed] [Google Scholar]
- 7. Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304(16):930‐933. [DOI] [PubMed] [Google Scholar]
- 9. Engeli S, Böhnke J, Gorzelniak K, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45(3):356‐362. [DOI] [PubMed] [Google Scholar]
- 10. Ho JT, Keogh JB, Bornstein SR, et al. Moderate weight loss reduces renin and aldosterone but does not influence basal or stimulated pituitary-adrenal axis function. Horm Metab Res. 2007;39(9):694‐699. [DOI] [PubMed] [Google Scholar]
- 11. Cooper JN, Fried L, Tepper P, et al. Changes in serum aldosterone are associated with changes in obesity-related factors in normotensive overweight and obese young adults. Hypertens Res. 2013;36(10):895‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dall’Asta C, Vedani P, Manunta P, et al. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis. 2009;19(2):110‐114. [DOI] [PubMed] [Google Scholar]
- 13. Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7(4):355‐362. [DOI] [PubMed] [Google Scholar]
- 14. Harp JB, Henry SA, DiGirolamo M. Dietary weight loss decreases serum angiotensin-converting enzyme activity in obese adults. Obes Res. 2002;10(10):985‐990. [DOI] [PubMed] [Google Scholar]
- 15. Lin P-H, Allen JD, Li Y-J, Yu M, Lien LF, Svetkey LP. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab. 2012;2012:472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rocchini AP, Katch VL, Grekin R, Moorehead C, Anderson J. Role for aldosterone in blood pressure regulation of obese adolescents. Am J Cardiol. 1986;57(8):613‐618. [DOI] [PubMed] [Google Scholar]
- 17. Vaswani AN. Effect of weight reduction on the renin-aldosterone axis. J Am Coll Nutr. 1985;4(2):225‐231. [DOI] [PubMed] [Google Scholar]
- 18. Affarah HB, Hall WD, Heymsfield SB, Kutner M, Wells JO, Tuttle EP. High-carbohydrate diet: antinatriuretic and blood pressure response in normal men. Am J Clin Nutr. 1986;44(3):341‐348. [DOI] [PubMed] [Google Scholar]
- 19. Buga A, Kackley ML, Crabtree CD, et al. The effects of a 6-week controlled, hypocaloric ketogenic diet, with and without exogenous ketone salts, on body composition responses. Front Nutr. 2021;8:618520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crabtree CD, Kackley ML, Buga A, et al. Comparison of ketogenic diets with and without ketone salts versus a low-fat diet: liver fat responses in overweight adults. Nutrients. 2021;13(3):966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hyde PN, Sapper TN, Crabtree CD, et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight. 2019;4(12):e128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volk BM, Kunces LJ, Freidenreich DJ, et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One. 2014;9(11):e113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belany P, Kackley ML, Zhao S, et al. Effects of hypocaloric low-fat, ketogenic, and ketogenic and ketone supplement diets on aldosterone and renin. Figshare. Deposited November 16, 2022. Doi: 10.6084/M9.FIGSHARE.21569226.V1. [DOI] [PMC free article] [PubMed]
- 25. Welch WA, Strath SJ, Swartz AM. Congruent validity and reliability of two metabolic systems to measure resting metabolic rate. Int J Sports Med. 2015; 36(5):414‐418. [DOI] [PubMed] [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. [DOI] [PubMed] [Google Scholar]
- 27. Brown JM, Auchus RJ, Honzel B, Luther JM, Yozamp N, Vaidya A. Recalibrating interpretations of aldosterone assays across the physiologic range: immunoassay and liquid chromatography-tandem mass spectrometry measurements under multiple controlled conditions. J Endocr Soc. 2022;6(6):bvac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crabtree CD, LaFountain RA, Hyde PN, et al. Quantification of human central adipose tissue depots: an anatomically matched comparison between DXA and MRI. Tomography. 2019;5(4):358‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rossi GP, Belfiore A, Bernini G, et al. Body mass index predicts plasma aldosterone concentrations in overweight-obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93(7):2566‐2571. [DOI] [PubMed] [Google Scholar]
- 30. Andronico G, Cottone S, Mangano MT, et al. Insulin, renin-aldosterone system and blood pressure in obese people. Int J Obes Relat Metab Disord. 2001;25(2):239‐242. [DOI] [PubMed] [Google Scholar]
- 31. Briones AM, Nguyen Dinh Cat A, Callera Glaucia E, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways. Hypertension. 2012;59(5):1069‐1078. [DOI] [PubMed] [Google Scholar]
- 32. Sowers JR, Nyby M, Stern N, et al. Blood pressure and hormone changes associated with weight reduction in the obese. Hypertension. 1982;4(5):686‐691. [DOI] [PubMed] [Google Scholar]
- 33. Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351(1):33‐41. [DOI] [PubMed] [Google Scholar]
- 34. Joseph JJ, Echouffo Tcheugui JB, Effoe VS, Hsueh WA, Allison MA, Golden SH. Renin-angiotensin-aldosterone system, glucose metabolism and incident type 2 diabetes mellitus: MESA. J Am Heart Assoc. 2018;7(17):e009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev. 2005;10(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 36. Maron BA, Leopold JA. Mineralocorticoid receptor antagonists and endothelial function. Curr Opin Investig Drugs. 2008;9(9):963‐969. [PMC free article] [PubMed] [Google Scholar]
- 37. Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51(2):161‐167. [DOI] [PubMed] [Google Scholar]
- 38. Bruder-Nascimento T, Ferreira NS, Zanotto CZ, et al. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation. 2016;134(23):1866‐1880. [DOI] [PubMed] [Google Scholar]
- 39. Youm Y-H, Nguyen KY, Grant RW, et al. Ketone body β-hydroxybutyrate blocks the NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andrzejczak D, Górska D. Influence of enalapril, quinapril and losartan on lipopolysaccharide (LPS)-induced serum concentrations of TNF-a, IL-1b, IL-6 in spontaneously hypertensive rats (SHR). Pharmacol Rep. 2007;59(4):437‐446. [PubMed] [Google Scholar]
- 41. Kahlenberg JM, Dubyak GR. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1β processing and release. J Leukoc Biol. 2004;76(3):676‐684. [DOI] [PubMed] [Google Scholar]
- 42. Kim SR, Lee S-G, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119‐1131. [DOI] [PubMed] [Google Scholar]
- 44. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 45. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 46. Forsythe CE, Phinney SD, Fernandez ML, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43(1):65‐77. [DOI] [PubMed] [Google Scholar]
- 47. Bradham W. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53(4):822‐830. [DOI] [PubMed] [Google Scholar]
- 48. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448‐457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.