Abstract
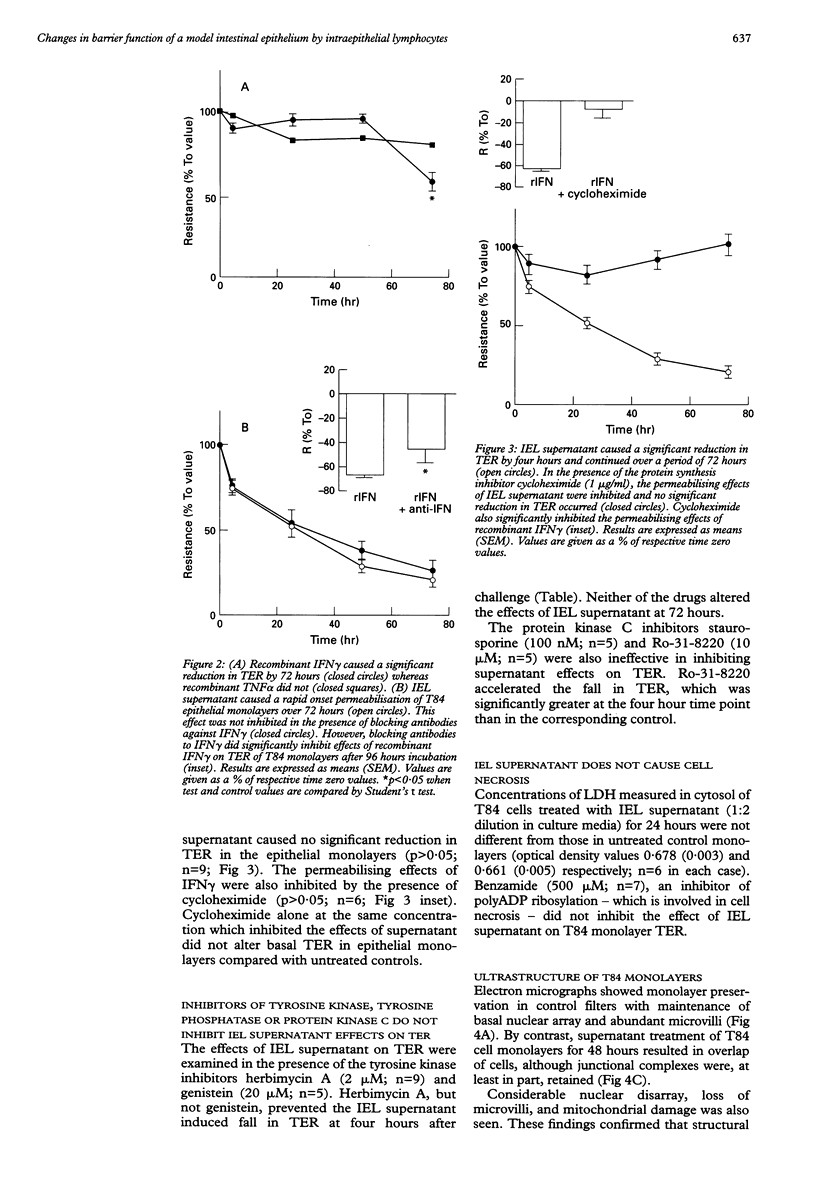
BACKGROUND: Elements of the mucosal immune system may play an important part in regulating epithelial barrier function in the intestinal tract. Intraepithelial lymphocytes (IELs) represent a subtype of immunocyte which is strategically placed to regulate epithelial function at most mucosal sites. AIMS AND METHODS: An IEL derived cell line (SC1) was used to examine its effects on the model epithelium T84--a tumour derived cell line which retains the phenotype of colonic crypt cells. Transepithelial electrical resistance (TER) was used as a marker of epithelial integrity. RESULTS: Coculture of T84 cells with SC1 produced a significant fall in TER as did exposure of T84 monolayers to IEL derived supernatant. Recombinant interferon-gamma (rIFN gamma) also reduced TER in T84 monolayers. Cycloheximide prevented the effects of IEL supernatant and of rIFN gamma on TER. The fall in TER in response to rIFN gamma was attenuated by blocking antibodies, which did not alter the fall in resistance induced by IEL supernatant. Fractions of IEL supernatant, separated on the basis of size, evoked temporally distinct changes in TER. Ultrastructural studies support the hypothesis that the slow onset but severe fall in TER indicates catastrophic effects on the monolayer. The more rapid onset fall in TER was not associated with gross changes in monolayer morphology. Reduction of TER by IEL supernatant was not influenced by inhibitors of tyrosine phosphatase or of protein kinase C. Although herbimycin did reduce the rapid onset change in TER, the tyrosine kinase inhibitor genistein did not alter responses to IEL supernatant. CONCLUSIONS: Mucosal T cells may influence barrier function by a process involving new protein synthesis by epithelial cells. This model may have relevance in some inflammatory conditions of the gastrointestinal tract.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. B., Planchon S. M., Roche J. K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993 Mar 15;150(6):2356–2363. [PubMed] [Google Scholar]
- Agarwal S., Piesco N. P. Poly ADP-ribosylation of a 90-kDa protein is involved in TNF-alpha-mediated cytotoxicity. J Immunol. 1994 Jul 15;153(2):473–481. [PubMed] [Google Scholar]
- Balk S. P., Ebert E. C., Blumenthal R. L., McDermott F. V., Wucherpfennig K. W., Landau S. B., Blumberg R. S. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991 Sep 20;253(5026):1411–1415. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- Barrett K. E. Positive and negative regulation of chloride secretion in T84 cells. Am J Physiol. 1993 Oct;265(4 Pt 1):C859–C868. doi: 10.1152/ajpcell.1993.265.4.C859. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Kett K., Krajci P., Kvale D., Rognum T. O., Scott H., Sollid L. M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989 Dec;97(6):1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- Castro G. A., Arntzen C. J. Immunophysiology of the gut: a research frontier for integrative studies of the common mucosal immune system. Am J Physiol. 1993 Oct;265(4 Pt 1):G599–G610. doi: 10.1152/ajpgi.1993.265.4.G599. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Bègue B., Gagnon J., Meo T. The human intraepithelial lymphocyte marker HML-1 is an integrin consisting of a beta 7 subunit associated with a distinctive alpha chain. Eur J Immunol. 1992 Jan;22(1):273–277. doi: 10.1002/eji.1830220140. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Parkos C. A., Delp C., Arnaout M. A., Madara J. L. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J Cell Biol. 1993 Feb;120(3):785–798. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan S. P., Parkos C. A., Matthews J. B., D'Andrea L., Awtrey C. S., Lichtman A. H., Delp-Archer C., Madara J. L. Interferon-gamma induces a cell surface phenotype switch on T84 intestinal epithelial cells. Am J Physiol. 1994 Aug;267(2 Pt 1):C402–C410. doi: 10.1152/ajpcell.1994.267.2.C402. [DOI] [PubMed] [Google Scholar]
- Deem R. L., Shanahan F., Targan S. R. Triggered human mucosal T cells release tumour necrosis factor-alpha and interferon-gamma which kill human colonic epithelial cells. Clin Exp Immunol. 1991 Jan;83(1):79–84. doi: 10.1111/j.1365-2249.1991.tb05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Fan X. J., Chua A., Shahi C. N., McDevitt J., Keeling P. W., Kelleher D. Gastric T lymphocyte responses to Helicobacter pylori in patients with H pylori colonisation. Gut. 1994 Oct;35(10):1379–1384. doi: 10.1136/gut.35.10.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa H., Li P. M., Yamamoto C., Murakami Y., Mizuno S., Uehara Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem Pharmacol. 1991 Oct 9;42(9):1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- Graber M., June C. H., Samelson L. E., Weiss A. The protein tyrosine kinase inhibitor herbimycin A, but not genistein, specifically inhibits signal transduction by the T cell antigen receptor. Int Immunol. 1992 Nov;4(11):1201–1210. doi: 10.1093/intimm/4.11.1201. [DOI] [PubMed] [Google Scholar]
- Hecht G., Robinson B., Koutsouris A. Reversible disassembly of an intestinal epithelial monolayer by prolonged exposure to phorbol ester. Am J Physiol. 1994 Feb;266(2 Pt 1):G214–G221. doi: 10.1152/ajpgi.1994.266.2.G214. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cells: a local immune mechanism? Scand J Immunol. 1989 Oct;30(4):499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cells: a local immune mechanism? Scand J Immunol. 1989 Oct;30(4):499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Jarry A., Cerf-Bensussan N., Brousse N., Selz F., Guy-Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3- lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol. 1990 May;20(5):1097–1103. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- Kaoutzani P., Colgan S. P., Cepek K. L., Burkard P. G., Carlson S., Delp-Archer C., Brenner M. B., Madara J. L. Reconstitution of cultured intestinal epithelial monolayers with a mucosal-derived T lymphocyte cell line. Modulation of epithelial phenotype dependent on lymphocyte-basolateral membrane apposition. J Clin Invest. 1994 Aug;94(2):788–796. doi: 10.1172/JCI117398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher D., Murphy A., Lynch S., O'Farrelly C. Adhesion molecules utilized in binding of intraepithelial lymphocytes to human enterocytes. Eur J Immunol. 1994 Apr;24(4):1013–1016. doi: 10.1002/eji.1830240437. [DOI] [PubMed] [Google Scholar]
- Klein J. R. Advances in intestinal T-cell development and function. Immunol Today. 1995 Jul;16(7):322–324. doi: 10.1016/0167-5699(95)80145-6. [DOI] [PubMed] [Google Scholar]
- Klein J. R., Kagnoff M. F. Spontaneous in vitro evolution of lytic specificity of cytotoxic T lymphocyte clones isolated from murine intestinal epithelium. J Immunol. 1987 Jan 1;138(1):58–62. [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud N., O'Connell M. A., Stinson J., Goggins M. G., Weir D. G., Kelleher D. Tumour necrosis factor-alpha and microalbuminuria in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995 Mar;7(3):215–219. [PubMed] [Google Scholar]
- Mahon T. M., O'Neill L. A. Studies into the effect of the tyrosine kinase inhibitor herbimycin A on NF-kappa B activation in T lymphocytes. Evidence for covalent modification of the p50 subunit. J Biol Chem. 1995 Dec 1;270(48):28557–28564. doi: 10.1074/jbc.270.48.28557. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R., Grant S. G. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991 Oct 10;353(6344):558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K. Tumor promoter-induced changes in the permeability of epithelial cell tight junctions. Cell. 1981 Jan;23(1):95–103. doi: 10.1016/0092-8674(81)90274-9. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Beagley K. W., McGhee J. R., Kiyono H. Cytokine synthesis by intestinal intraepithelial lymphocytes. Both gamma/delta T cell receptor-positive and alpha/beta T cell receptor-positive T cells in the G1 phase of cell cycle produce IFN-gamma and IL-5. J Immunol. 1993 Jan 1;150(1):106–114. [PubMed] [Google Scholar]
- Yanagihara N., Tachikawa E., Izumi F., Yasugawa S., Yamamoto H., Miyamoto E. Staurosporine: an effective inhibitor for Ca2+/calmodulin-dependent protein kinase II. J Neurochem. 1991 Jan;56(1):294–298. doi: 10.1111/j.1471-4159.1991.tb02595.x. [DOI] [PubMed] [Google Scholar]



