Abstract
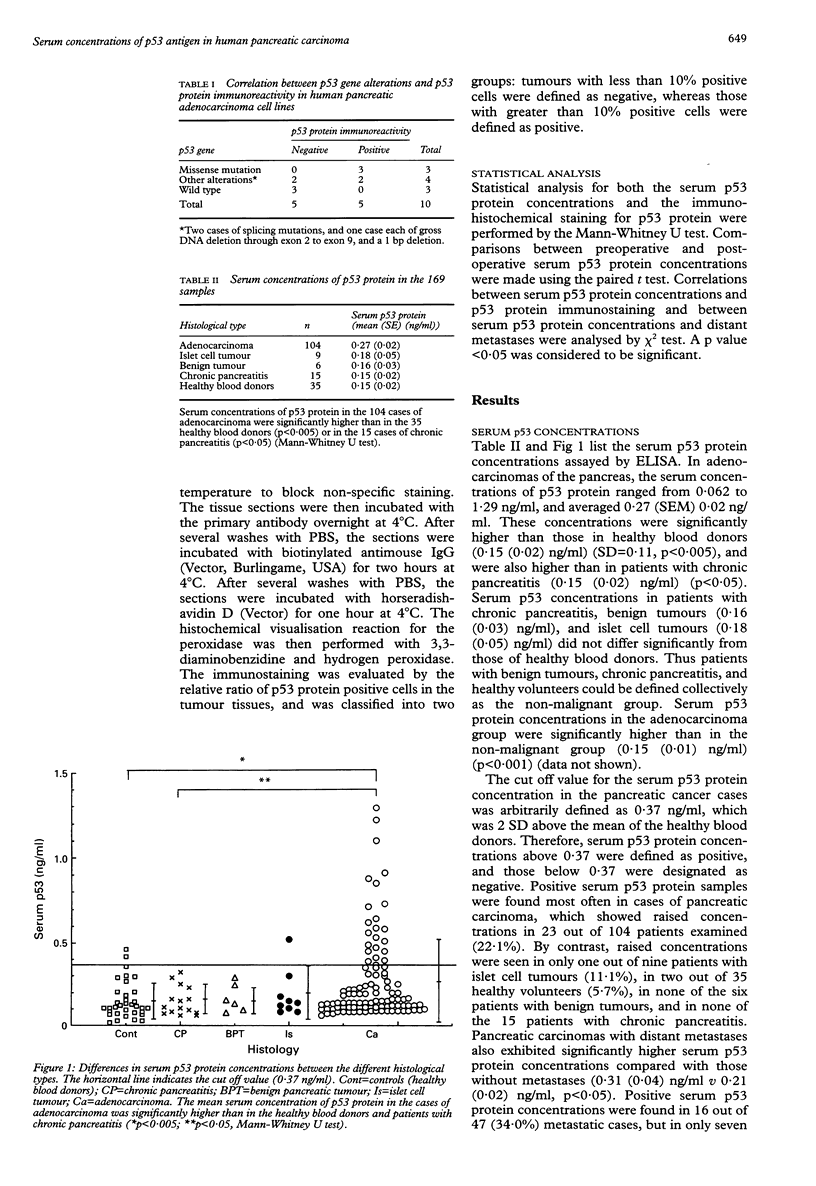
BACKGROUND: Alterations in the p53 gene are often found in pancreatic cancer, and accumulation of the p53 protein has been noted in tumour cells. AIMS: To investigate whether serum p53 protein concentrations could be used as markers for p53 gene mutations in neoplasms of the pancreas. METHODS: Serum p53 protein concentrations were determined by an enzyme linked immunosorbent assay (ELISA) in 104 cases of pancreatic adenocarcinoma, and 61 matched formalin fixed tissue sections were also stained by an anti-p53 DO-7 monoclonal antibody. RESULTS: The mean serum concentration of p53 protein in the adenocarcinoma patients was 0.27 (SEM 0.02) ng/ml, and was significantly higher than in 35 healthy blood donors (0.15 (0.02) ng/ml, SD = 0.11) or in 15 cases of chronic pancreatitis (0.15 (0.02) ng/ml). Adopting an arbitrary cut off value for the serum p53 protein concentration of 0.37 ng/ml, which corresponded to a value 2 SD above the mean value from the healthy blood donors, positive serum p53 protein concentrations were found in 23 out of 104 (22.1%) patients with adenocarcinomas examined, 16 out of 47 (34.0%) patients with carcinomas with distant metastases, but only seven of 57 patients (12.3%) with carcinomas without metastases (p < 0.05). In 11 patients with pancreatic adenocarcinomas, the mean serum p53 protein concentration after tumour resection was 0.21 (0.05) ng/ml, and had decreased compared with the preoperative concentrations (0.25 (0.05) ng/ml) (P < 0.05). There were no significant associations between the serum concentrations of p53 protein and serum concentrations of markers such as CA19-9 or CEA; however, serum concentrations of p53 protein demonstrated a potential role as an additional tumour marker. Immunohistochemical studies disclosed that the p53 protein was expressed in 28 out of 61 pancreatic adenocarcinomas (45.9%). Serum p53 protein concentrations in the positively immunostained cases were significantly higher than in the negatively immunostained cases (0.35 (0.05) ng/ml v 0.15 (0.01) ng/ml; p < 0.005). Furthermore, positive immunostaining for p53 protein was found in eight out of 10 (80%) serum positive p53 protein cases with adenocarcinomas. CONCLUSION: An increase in serum p53 protein concentrations appears during the progression of pancreatic adenocarcinoma and correlates with the accumulation of p53 protein as a result of a mutation of the p53 gene. An analysis of p53 antigen concentrations can detect p53 gene alterations, which could be useful for the selection of treatment regimens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelopoulou K., Diamandis E. P., Sutherland D. J., Kellen J. A., Bunting P. S. Prevalence of serum antibodies against the p53 tumor suppressor gene protein in various cancers. Int J Cancer. 1994 Aug 15;58(4):480–487. doi: 10.1002/ijc.2910580404. [DOI] [PubMed] [Google Scholar]
- Barton C. M., Lemoine N. R. Antisense oligonucleotides directed against p53 have antiproliferative effects unrelated to effects on p53 expression. Br J Cancer. 1995 Mar;71(3):429–437. doi: 10.1038/bjc.1995.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C. M., Staddon S. L., Hughes C. M., Hall P. A., O'Sullivan C., Klöppel G., Theis B., Russell R. C., Neoptolemos J., Williamson R. C. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991 Dec;64(6):1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayever E., Haines K. M., Iversen P. L., Ruddon R. W., Pirruccello S. J., Mountjoy C. P., Arneson M. A., Smith L. J. Selective cytotoxicity to human leukemic myeloblasts produced by oligodeoxyribonucleotide phosphorothioates complementary to p53 nucleotide sequences. Leuk Lymphoma. 1994 Jan;12(3-4):223–231. doi: 10.3109/10428199409059593. [DOI] [PubMed] [Google Scholar]
- Berrozpe G., Schaeffer J., Peinado M. A., Real F. X., Perucho M. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer. 1994 Jul 15;58(2):185–191. doi: 10.1002/ijc.2910580207. [DOI] [PubMed] [Google Scholar]
- Berrozpe G., Schaeffer J., Peinado M. A., Real F. X., Perucho M. Comparative analysis of mutations in the p53 and K-ras genes in pancreatic cancer. Int J Cancer. 1994 Jul 15;58(2):185–191. doi: 10.1002/ijc.2910580207. [DOI] [PubMed] [Google Scholar]
- Bi S., Lanza F., Goldman J. M. The involvement of "tumor suppressor" p53 in normal and chronic myelogenous leukemia hemopoiesis. Cancer Res. 1994 Jan 15;54(2):582–586. [PubMed] [Google Scholar]
- Boschman C. R., Stryker S., Reddy J. K., Rao M. S. Expression of p53 protein in precursor lesions and adenocarcinoma of human pancreas. Am J Pathol. 1994 Dec;145(6):1291–1295. [PMC free article] [PubMed] [Google Scholar]
- Casey G., Yamanaka Y., Friess H., Kobrin M. S., Lopez M. E., Buchler M., Beger H. G., Korc M. p53 mutations are common in pancreatic cancer and are absent in chronic pancreatitis. Cancer Lett. 1993 May 14;69(3):151–160. doi: 10.1016/0304-3835(93)90168-9. [DOI] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Hruban R. H., Goodman S. N., Polak M., van den Berg F. M., Allison D. C., Cameron J. L., Offerhaus G. J. Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol. 1994 Jun;101(6):684–688. doi: 10.1093/ajcp/101.6.684. [DOI] [PubMed] [Google Scholar]
- Fan S., el-Deiry W. S., Bae I., Freeman J., Jondle D., Bhatia K., Fornace A. J., Jr, Magrath I., Kohn K. W., O'Connor P. M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994 Nov 15;54(22):5824–5830. [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattani A. M., Mandeli J., Bruckner H. W. Tumor markers in patients with pancreatic carcinoma. Cancer. 1996 Jul 1;78(1):57–62. doi: 10.1002/(SICI)1097-0142(19960701)78:1<57::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Laurent-Puig P., Lubin R., Semhoun-Ducloux S., Pelletier G., Fourre C., Ducreux M., Briantais M. J., Buffet C., Soussi T. Antibodies against p53 protein in serum of patients with benign or malignant pancreatic and biliary diseases. Gut. 1995 Mar;36(3):455–458. doi: 10.1136/gut.36.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Rush M., Charalambous D., Rode J. Immunohistochemical demonstration of the p53 tumour suppressor gene product in cancer of the pancreas and chronic pancreatitis. J Gastroenterol Hepatol. 1993 Sep-Oct;8(5):465–469. doi: 10.1111/j.1440-1746.1993.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Lubin R., Zalcman G., Bouchet L., Trédanel J., Legros Y., Cazals D., Hirsch A., Soussi T. Serum p53 antibodies as early markers of lung cancer. Nat Med. 1995 Jul;1(7):701–702. doi: 10.1038/nm0795-701. [DOI] [PubMed] [Google Scholar]
- Lundin J., Nordling S., von Boguslawsky K., Roberts P. J., Haglund C. Prognostic value of immunohistochemical expression of p53 in patients with pancreatic cancer. Oncology. 1996 Mar-Apr;53(2):104–111. doi: 10.1159/000227545. [DOI] [PubMed] [Google Scholar]
- Lundin J., Roberts P. J., Kuusela P., Haglund C. The prognostic value of preoperative serum levels of CA 19-9 and CEA in patients with pancreatic cancer. Br J Cancer. 1994 Mar;69(3):515–519. doi: 10.1038/bjc.1994.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. C., Neugut A. I., Garbowski G., Forde K. A., Treat M., Smith S., Carney W. P., Brandt-Rauf P. W. Levels of p53 antigen in the plasma of patients with adenomas and carcinomas of the colon. Cancer Lett. 1995 May 8;91(2):235–240. doi: 10.1016/0304-3835(95)03744-h. [DOI] [PubMed] [Google Scholar]
- Luo J. C., Zehab R., Anttila S., Ridanpaa M., Husgafvel-Pursiainen K., Vainio H., Carney W., De Vivo I., Milling C., Brandt-Rauf P. W. Detection of serum p53 protein in lung cancer patients. J Occup Med. 1994 Feb;36(2):155–160. doi: 10.1097/00043764-199402000-00010. [DOI] [PubMed] [Google Scholar]
- Luo J. C., Zehab R., Anttila S., Ridanpaa M., Husgafvel-Pursiainen K., Vainio H., Carney W., De Vivo I., Milling C., Brandt-Rauf P. W. Detection of serum p53 protein in lung cancer patients. J Occup Med. 1994 Feb;36(2):155–160. doi: 10.1097/00043764-199402000-00010. [DOI] [PubMed] [Google Scholar]
- Nakamori S., Yashima K., Murakami Y., Ishikawa O., Ohigashi H., Imaoka S., Yaegashi S., Konishi Y., Sekiya T. Association of p53 gene mutations with short survival in pancreatic adenocarcinoma. Jpn J Cancer Res. 1995 Feb;86(2):174–181. doi: 10.1111/j.1349-7006.1995.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegata N. S., Sessa F., Renault B., Bonato M., Leone B. E., Solcia E., Ranzani G. N. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994 Mar 15;54(6):1556–1560. [PubMed] [Google Scholar]
- Ruggeri B., Zhang S. Y., Caamano J., DiRado M., Flynn S. D., Klein-Szanto A. J. Human pancreatic carcinomas and cell lines reveal frequent and multiple alterations in the p53 and Rb-1 tumor-suppressor genes. Oncogene. 1992 Aug;7(8):1503–1511. [PubMed] [Google Scholar]
- Scarpa A., Capelli P., Mukai K., Zamboni G., Oda T., Iacono C., Hirohashi S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993 May;142(5):1534–1543. [PMC free article] [PubMed] [Google Scholar]
- Schlichtholz B., Legros Y., Gillet D., Gaillard C., Marty M., Lane D., Calvo F., Soussi T. The immune response to p53 in breast cancer patients is directed against immunodominant epitopes unrelated to the mutational hot spot. Cancer Res. 1992 Nov 15;52(22):6380–6384. [PubMed] [Google Scholar]
- Suwa H., Yoshimura T., Yamaguchi N., Kanehira K., Manabe T., Imamura M., Hiai H., Fukumoto M. K-ras and p53 alterations in genomic DNA and transcripts of human pancreatic adenocarcinoma cell lines. Jpn J Cancer Res. 1994 Oct;85(10):1005–1014. doi: 10.1111/j.1349-7006.1994.tb02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- Vojtesek B., Fisher C. J., Barnes D. M., Lane D. P. Comparison between p53 staining in tissue sections and p53 proteins levels measured by an ELISA technique. Br J Cancer. 1993 Jun;67(6):1254–1258. doi: 10.1038/bjc.1993.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw A. L., Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992 Feb 13;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Yamanaka Y., Friess H., Buchler M., Korc M. p53 expression in human pancreatic cancer correlates with enhanced biological aggressiveness. Anticancer Res. 1994 Nov-Dec;14(6B):2477–2483. [PubMed] [Google Scholar]
- Zhang S. Y., Ruggeri B., Agarwal P., Sorling A. F., Obara T., Ura H., Namiki M., Klein-Szanto A. J. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994 Feb;118(2):150–154. [PubMed] [Google Scholar]