Abstract
The mitochondrial uniporter mediates the crucial cellular process of mitochondrial uptake, which regulates cell bioenergetics, intracellular signaling, and cell death initiation. The uniporter contains the pore-forming MCU subunit, an EMRE protein that binds to MCU, and the regulatory MICU1 subunit, which can dimerize with MICU1 or MICU2 and under resting cellular [] occludes the MCU pore. It has been known for decades that spermine, which is ubiquitously present in animal cells, can enhance mitochondrial uptake, but the underlying mechanisms remain unclear. Here, we show that spermine exerts dual modulatory effects on the uniporter. In physiological concentrations of spermine, it enhances uniporter activity by breaking the physical interactions between MCU and the MICU1-containing dimers to allow the uniporter to constitutively take up even in low [] conditions. This potentiation effect does not require MICU2 or the EF-hand motifs in MICU1. When [spermine] rises to millimolar levels, it inhibits the uniporter by targeting the pore region in a MICU-independent manner. The MICU1-dependent spermine potentiation mechanism proposed here, along with our previous finding that cardiac mitochondria have very low MICU1, can explain the puzzling observation in the literature that mitochondria in the heart show no response to spermine.
Introduction
The mitochondrial calcium uniporter (hereafter referred to as the uniporter) is a multi-subunit channel that mediates mitochondrial uptake. When intracellular signals arrive at mitochondria, the uniporter becomes activated to rapidly transport into the mitochondrial matrix to modulate the magnitude and frequency of these signals1,2. The imported can also raise matrix [] to stimulate multiple -dependent dehydrogenases in the TCA cycle and enhance oxidative phosphorylation1, 2. However, entry of too much can induce the mitochondria permeability transition, leading to apoptotic cell death1, 2. Malfunction of the uniporter has been implicated in a wide range of pathologies3, 4, including cardiac ischemia-reperfusion injury, heart failure, neurodegeneration, and cancer metastasis, among others. Considering the uniporter’s critical importance in pathophysiology, it is important to understand how its activity is regulated.
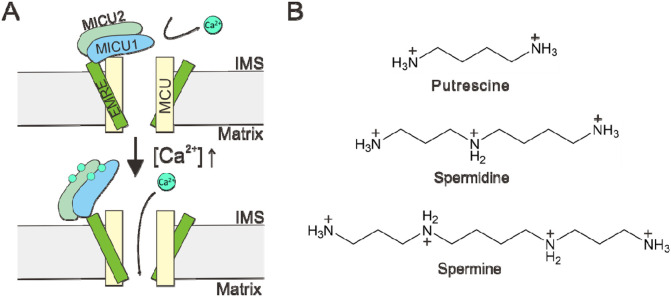
It has been known for decades that the uniporter is regulated by cytoplasmic . Following the discovery of uniporter genes in the early 2010s5-8, extensive studies have established an “occlusion” mechanisms underlying such regulation9-20. As summarized in Fig. 1A, the uniporter is inactive in resting cellular [] (~100 nM). This is because a MICU1 subunit, which can form a homodimer or heterodimerize with MICU2 in the intermembrane space (IMS), blocks the IMS entrance of the uniporter’s pore formed by the MCU subunit. When cytoplasmic signals elevate IMS [], binding to MICU1 would cause MICU1 separation from MCU to open the pore, thus leading to activation of the uniporter. In this -activated state, MICU1 remains bound to an EMRE subunit via electrostatic interactions. This interaction ensures unfailing MICU1 association within the uniporter complex, so that once the signal is over, MICU1 can rapidly block the pore to close the uniporter.
Figure 1. Regulation of the mitochondrial calcium uniporter.
(A) A molecular model underlying -dependent uniporter activation. (B) Chemical structures of common biological polyamines.
Here, we investigate a possible uniporter regulator, spermine, which along with spermidine and putrescine (Fig. 1B) are important biological polyamines ubiquitously present in animal cells, playing critical roles in cell growth, differentiation, protein synthesis, and apoptosis21-23. Spermine, which is a positively charged molecule, is known to modulate the activity of many cation channels by blocking the channel pore. For example, it confers the inward-rectification property of multiple inward-rectifier K+ (Kir) channels and AMPA-/kainate-type glutamate-receptor channels by entering into the pore from the intracellular side to block outward currents upon membrane depolarization24-29. Block of cyclic nucleotide-gated channels30, voltage-gated Na+ channels31, 32, and transient receptor potential channels33, 34 has also been reported.
Interestingly, Nicchitta and Williamson35 reported in 1984 that spermine can enhance the ability of mitochondria to buffer extramitochondrial . In particular, they showed that isolated liver mitochondria, or mitochondria in permeabilized hepatocytes, can only reduce extramitochondrial [] ([]ex) to a steady-state level of 0.5–1 μM. However, adding spermine causes mitochondria to absorb more , thus lowering []ex to 0.2–0.5 μM. It was also shown that spermidine can similarly improve mitochondrial buffering, albeit with ~5-fold lower efficacy and potency, but putrescine is ineffective. These initial observations have since been verified in multiple laboratories36-42.
It has been speculated that spermine enhances mitochondrial buffering by stimulating mitochondrial uptake, which is mediated by the uniporter. However, kinetic analyses have led to puzzling results. Some investigators found that spermine increases the rate of mitochondrial uptake39, but others observed only inhibitory effects36, 37, 43. Moreover, some groups reported more complicated phenomena, with spermine increasing mitochondrial uptake rate when []ex is below 5–10 μM, but reduces the rate at higher []ex35, 40, 41. The research efforts gradually came to a halt in the 1990s, leaving several mechanistic questions unanswered. First, does spermine actually modulate uniporter activity? If yes, what are the underlying molecular mechanisms? Moreover, how do we reconcile the contradictory observations about the kinetics of mitochondrial uptake mentioned above?
We decided to pursue these questions for two reasons. First, from the perspective of pure channel biophysics, we are curious about how spermine potentially enhances uniporter function, instead of just blocking the pore as in the case of other cation channels. Second, spermine regulation of mitochondrial buffering has been considered as physiologically relevant as the half maximal effective concentration (EC50) of spermine, 100–200 μM, falls into the concentration range of free spermine in the cytoplasm44. Thus, a deep understanding of spermine potentiation could advance our knowledge of how cells regulate the all-important processes of cytoplasmic and mitochondrial signaling and homeostasis.
Results
Spermine perturbs MICU1 block of the uniporter
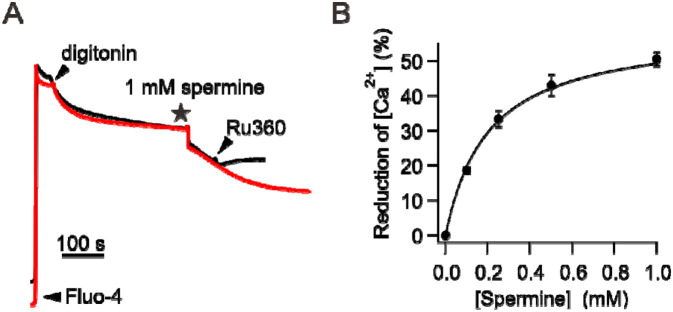
To observe the potentiation of mitochondrial buffering by spermine, wild-type (WT) HEK293 cells were permeabilized with digitonin in the presence of Fluo-4 to monitor changes in []ex. Fig. 2A shows that mitochondria reduce []ex to a steady-state level of 517 ± 27 nM, and adding 1 mM of spermine causes a further reduction of []ex by about 50% (255 ± 8 nM). Experiments relating spermine concentrations to percentage of []ex reduction yielded an EC50 of 201 μM (Fig. 2B). These results are consistent with the original data from Nicchitta and Williamson35. We also show that following spermine addition, increased mitochondrial absorption is indeed mediated by the uniporter, as this process can be abolished by using Ru360 to inhibit the uniporter (black trace, Fig. 2A).
Figure 2. Enhancement of mitochondrial absorption induced by spermine.
(A) Spermine potentiation of mitochondrial uptake. Adding 1 mM spermine causes mitochondria to sequester more . This spermine effect is abolished by adding 100 nM Ru360 as shown in the black trace. (B) Spermine dose-response relationship.
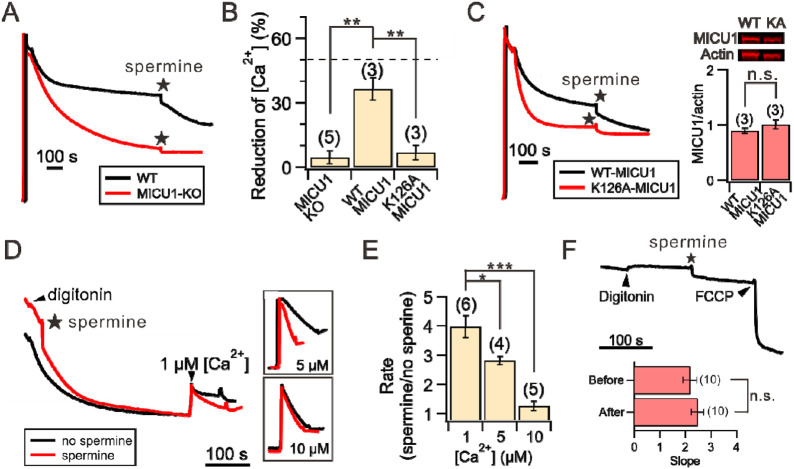
The effect of spermine on []ex is remarkably similar to that caused by MICU1-knockout (KO) — In the absence of MICU1, mitochondria can absorb more to achieve lower []ex because the uniporter would not be blocked by MICU1 when []ex decreases (Fig. 1A). Such similarity led us to hypothesize that spermine exerts its potentiation effect by perturbing MICU1 block of the MCU pore. This hypothesis predicts that spermine would pose no effects on mitochondria absorption when the uniporter is not blocked by MICU1 under the following conditions: (1) the MICU1 gene is deleted (MICU1-KO), (2) WT MICU1 is substituted by a K126A MICU1 mutant that cannot block MCU12, or (3) MICU1 is separated from the MCU pore due to increased []ex. Indeed, we show that MICU1-KO abolishes spermine’s stimulatory effects (Fig. 3A-B), and the phenotype can be restored by expressing WT but not K126A-MICU1 (Fig. 3B-C). Moreover, while spermine increases the rate of uniporter-mediated mitochondrial uptake at 1-μM []ex, the effect becomes smaller at 5-μM []ex, and is completely eliminated when []ex rises to 10 μM (Fig. 3D-E). Altogether, these results demonstrate that spermine enhances mitochondrial buffering by inhibiting MICU1 block of the uniporter. Of note, we observed no effects of spermine on inner mitochondrial membrane (IMM) potentials (Fig. 3F), indicating that spermine does not affect the driving force for influx, a conclusion in line with previous reports35, 42.
Figure 3. Spermine perturbation of MICU1 block.
(A) MICU1 dependence of spermine potentiation effects. (B) A bar chart summarizing the ability of spermine to induce reduction of []ex in MICU1-KO cells (left), or MICU1-KO cells transfected with WT MICU1 (middle) or K126A MICU1 (right). Dashed line: WT HEK cells. (C) Representative traces of spermine effects on MICU1-KO cells transfected with WT or K126A MICU1. The two MICU1 constructs were expressed to similar levels as shown in Western blot images, which were quantified in the bar chart. (D) Spermine-induced acceleration of mitochondrial uptake. Various concentrations of were added to induce net mitochondrial uptake in the presence (red) or absence (black) or spermine. Inlets highlight the traces after adding . (E) Fold-increase of mitochondrial uptake rate in response to spermine. (F) Lack of spermine effects on IMM potentials. The slopes before and after adding spermine are presented in the bar chart. 1 mM spermine was used in the entire figure. *P < 0.05; **P < 0.01; ***P < 0.001; n.s.: no significance.
The mechanisms underlying spermine potentiation
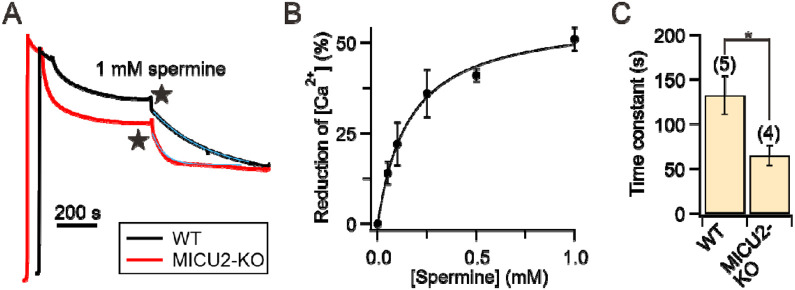
The results above indicate that spermine potentiation requires at least MCU, EMRE, and MICU1 because the effect can be eliminated by (1) MICU1-KO (Fig. 3A-B) or (2) using Ru360 to block influx through a -conducting pore formed by MCU and EMRE (Fig. 2A). As in HEK cells, the uniporter also possesses MICU2, which resides in a MICU1-MICU2 heterodimer (MICU1-2) (Fig. 1A), we asked if MICU2 is necessary for spermine’s actions. Accordingly, we applied spermine to permeabilized MICU2-KO HEK cells, whose uniporters contain a MICU1-MICU1 homodimer (MICU1-1)9, 45. Mitochondria in these cells reduce []ex to 394 ± 43 nM, and 1 mM of spermine causes a further reduction to 211 ± 15 nM (Fig. 4A). The EC50 is 158 μM (Fig. 4B), comparable with that obtained using WT cells (Fig. 2B). We thus conclude that MICU2 is not required for spermine potentiation. Previous studies9, 19 suggest that MICU1-1 separates from MCU more readily than MICU1-2. It is thus anticipated that spermine disrupts MCU block by MICU1-1 more easily than MICU1-2. This is indeed the case, as exponential fit of the []ex reduction time course following spermine addition (blue curves, Fig. 4A) shows that a new steady state is reached ~2-fold faster in MICU2-KO than in WT cells (Fig. 4C).
Figure 4. Spermine effects on MICU2-KO cells.
(A) Representative traces comparing spermine actions on WT vs. MICU2-KO cells. Blue curves: single exponential fit. (B) Spermine dose-response relationship obtained using MICU2-KO cells. (C) The time constants of spermine-induced []ex reduction.
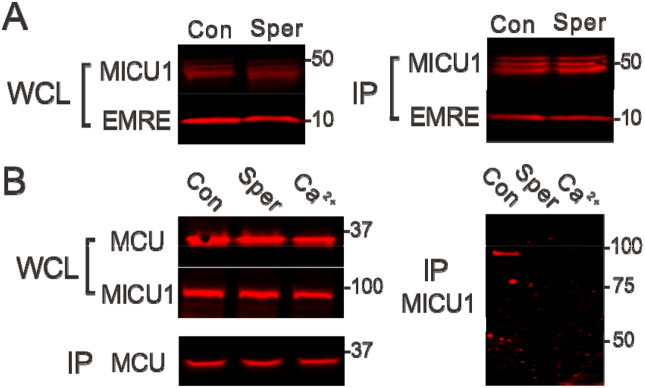
We considered two possible mechanisms about how spermine perturbs MICU1 block. First, spermine prevents MICU1 from binding to MCU to occlude the pore. Second, spermine might disrupt the EMRE-MICU1 interaction, thus facilitating MICU1 dissociation from the uniporter complex15. To test these alternative scenarios, we conducted co-immunoprecipitation (CoIP) experiments examining how spermine affects MCU-MICU1 or EMRE-MICU1 interactions. Results show that MICU1 binding to EMRE is unaffected by 1 mM spermine (Fig. 5A). By contrast, spermine fully breaks complex formation between MCU and the MICU1-1 dimer, recapitulating the effect of adding 10 μM to dislodge MICU1-1 from MCU (Fig. 5B). We thus conclude that spermine enhances mitochondrial absorption by breaking the MCU-MICU1 interactions that block the uniporter.
Figure 5. Perturbation of uniporter subunit interactions.
(A) Spermine effects on MICU1-EMRE interactions. Indicated uniporter subunits were expressed in MICU1-MCU-EMRE-KO cells. MICU1 was used to pull down EMRE. A C463S MICU1 mutant, which cannot form a disulfide-connected MICU1-1 dimer, was used in this experiment, but using WT MICU1 produces similar results that spermine does not affect MICU1 binding to EMRE. (B) Disruption of the MCU-MICU1 complex by spermine. MCU was immobilized to pull down WT MICU1 coexpressed in MICU1-MCU-EMRE-KO cells. The MICU1 band migrates to ~100 kDa as it represents a MICU1-1 dimer connected by an intersubunit disulfide. WCL: whole-cell lysate. IP: proteins obtained after CoIP. Con: 1 mM EGTA. Sper: 1 mM EGTA and 1 mM spermine. : no EGTA and 10 μM added . N = 3 for all experiments.
Where does spermine bind? We fist consider an idea that spermine might bind to MICU1’s two EF-hand binding motifs to drive MICU1 into a conformation that mimics the -bound conformation that cannot block MCU. Accordingly, we tested spermine effects on a MICU1 mutant (ΔEF MICU1) containing 4 mutations (D231A, E242K, D421A, and E423K) to disable both EF hands. However, Fig. 6 shows that 1 mM spermine causes a similar reduction of []ex in MICU1-KO cells expressing WT or ΔEF MICU1. This result thus ruled out the possibility that spermine binds to EF hands. We are currently in the process of identifying the spermine binding site.
Figure 6. The effect of disabling MICU1 EF hands on spermine action.
(A) Representative traces of spermine potentiation on MICU1-KO cells transfected with WT- or EF-hand disabled (ΔEF) MICU1. (B) A bar chart summarizing the reduction of []ex as in (A).
Spermine also blocks the uniporter
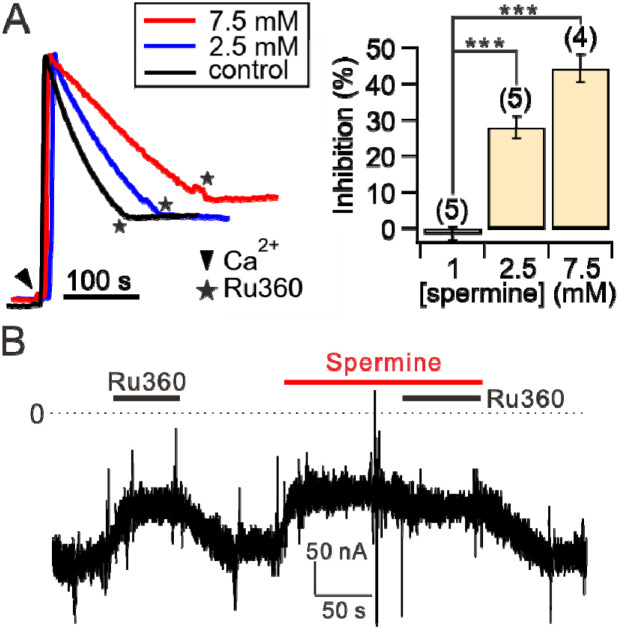
We then sought to examine if spermine also inhibits the uniporter, because spermine is well known for its function of blocking cation channels24, and as it has been shown previously that spermine can suppress mitochondrial uptake under some conditions35-37, 40, 41, 43. To this end, various concentrations of spermine were added to permeabilized WT HEK cells. After []ex reaches a steady state, 10 μM was then added to fully release MICU1 block. When MICU1 block is relieved, spermine loses its potentiation effects (as shown in Fig. 3D-E). Therefore, the initial rate of mitochondrial uptake immediately after adding 10 μM would inform us whether spermine can inhibit the uniporter. Fig. 7A shows that with 1-mM spermine, no inhibition was detectable. However, uniporter uptake is reduced by ~30% and ~45% when [spermine] is increased to 2.5 and 7.5 mM, respectively (Fig. 7A). These results thus demonstrate that spermine also exerts inhibitory effects on the uniporter, albeit with a >10-fold lower potency than the stimulatory effect.
Figure 7. Spermine inhibition of the uniporter.
(A) Spermine inhibition of mitochondrial uptake. The initial rate of mitochondrial uptake in the presence of 1, 2.5, or 7.5 mM spermine was normalized to that in the absence of spermine (control) to calculate the percentage of inhibition as shown in the bar chart. (B) A representative TEVC recording showing spermine inhibition. 100 nM of Ru360 was added first to identify hME-mediated inward current. Applying spermine causes a similar level of current reduction as induced by Ru360, suggesting that spermine fully inhibits the uniporter. Consistently, Ru360 does not further suppress the current when applied in the presence of spermine. Dashed line: 0 current.
To further investigate the mechanisms underlying spermine inhibition, we used two-electrode voltage clamp (TEVC) to assess spermine effects on a human MCU-EMRE fusion protein (hME) expressed in Xenopus oocytes46. We recorded hME-mediated inward currents with voltage clamped at −80 mV in the presence of 20 mM in the perfusion solution. As expected, varying [spermine] from 50 μM to 5 mM produces no potentiation effect (not shown) since no MICU1 is present in the system. By contrast, hME is fully inhibited by low millimolar levels of spermine (Fig. 7B). These results demonstrate that spermine inhibits the uniporter by targeting the transmembrane MCU-EMRE region. Ongoing electrophysiological work assessing voltage dependence of the inhibition could help address the issues of whether spermine acts as a pore blocker and why spermine inhibition appears to be more potent in oocytes than HEK cells.
Discussion
This work demonstrates that spermine potentiates the uniporter by perturbing MICU1 block of MCU, while inhibits the uniporter by targeting MCU-EMRE in an MICU-independent manner. Indeed, observations made decades ago have already hinted about the mechanisms of spermine potentiation. It is well known that as [] increases, the rate of mitochondrial uptake rises along a sigmoidal curve, as increased [] relieves MICU1 block of MCU in a cooperative manner to open the uniporter. Kröner reported that spermine, similar to MICU1-KO9, 16, 47, altered this dose-response from a sigmoidal to a linear relationship40, consistent with spermine abolishing MICU1’s regulatory effect as proposed here.
Our results could help explain some puzzling observations in the literature. It was reported that spermine stimulates mitochondrial uptake in hepatic mitochondria but not cardiac mitochondria40 (but also see another reference42). This observation is consistent with our recent findings that cardiac mitochondria have very low levels of MICU1, leading to a large population of MICU1-free uniporters9. These MICU1-deregulated uniporters exhibit a linear [] dose-response as we and others observed9, 48, and are expected to show no response to spermine. As described in the Introduction, previous studies reported complicated effects of spermine on mitochondrial uptake kinetics. These can be understood in light of spermine’s dual, independent modulatory effects on the uniporter. As the inhibitory effect requires higher [spermine], it explains why multiple groups35, 40, 41 observed spermine potentiation at low [spermine] while the effects become inhibitory as [spermine] rises. Some labs observed only potentiation39 or inhibition36, 37, 43. This could be due to the concentration of spermine used in their experiments falling into the range in which the stimulatory or inhibitory effects dominate.
An important issue that needs to be addressed regards where spermine binds to modulate uniporter activity. We showed that spermine does not contact the EF hands in MICU1. This is not surprising as EF hands are commonly present in other binding proteins, and there have been no reports to date that spermine can interact with these EF hands. This work shows that spermine can inhibit the uniporter in the absence of MICU1 dimers. We speculate that spermine acts as a pore blocker, similar to how it inhibits other cation ion channels. We are conducting further studies to complete our knowledge about spermine regulation of the uniporter complex.
Materials and methods
Cell Culture and Molecular Biology
HEK293 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% FBS in a 5% CO2 incubator at 37 °C. Deletion of uniporter-subunit genes in HEK cells was achieved via CRISPR-Cas9 as described in our previous work15, 49. Site-directed mutagenesis was performed using the QuickChange kit (Agilent), with sequences verified using Sanger sequencing.
CoIP and western blots
To perform CoIP, HEK cells in 10-cm dishes were transfected at 70-80% confluency and used for experiments 2 days after transfection. Cells were lysed in the presence of a protease inhibitor cocktail (Roche cOmplete EDTA-free) in 1 mL of an ice-cold solubilization buffer (SB, 150 mM NaCl, 50 mM HEPES, 4 mM DDM, pH 7.4-NaOH) supplemented with 1 mM EGTA, 1 mM spermine, or 10 μM CaCl2. 10 minutes after cell lysis, the lysate was clarified by spinning down at 13,000 g for 10 min. 100 μL of the lysate was taken out and used as the whole-cell lysate samples in Fig. 5. 25 μL of FLAG-conjugated (Sigma Aldrich, A2220) or 1D4-conjugated (homemade, 50% slurry) resins were then added to the remaining cell lysate and incubated on a tube revolver at 4°C for 1 hour. The beads were collected on a spin column, washed six times with 1-mL SB, and then eluted with 140 μL of 1X SDS loading buffer for the IP samples in Fig. 5.
Protein samples were transferred to low-fluorescence PVDF membranes and were blocked in a Tris-buffered saline (TBS)-based intercept blocking buffer (Li-Cor). The membranes were incubated with primary antibodies diluted in TBST (TBS plus 0.075% Tween-20) at 4 °C overnight. These include: anti-1D4 (produced in house, 0.1 mg/mL), anti-MICU1 (Sigma Aldrich HPA037480, 1:10,000), or anti-FLAG (Sigma Aldrich F1804, 1:10,000) antibodies. After 1-hour incubation with fluorescent secondary antibodies, goat anti-rabbit IRDye 680RD (Li-Cor 92568171, 1:10,000) or goat anti-mouse IRDye 680RD (Li-Cor 925-68070, 1:15,000), at room temperature, the signals were captured using a LI-COR Odyssey CLx imager. Band intensities were quantified using the LI-COR Image Studio software (version 5.2).
Mitochondrial uptake assays
To quantify the stimulation effects of spermine, 2 × 107 HEK cells were washed in 10 mL of a wash buffer (120 mM KCl, 25 mM HEPES, 2 mM KH2PO4, 1 mM MgCl2, 50 mM EGTA, pH 7.2-KOH), and resuspended in 2 mL of a recording buffer (120 mM KCl, 25 mM HEPES, 2 mM KH2PO4, 1 mM MgCl2, 5 mM succinate, pH 7.2-KOH). The sample was placed in a stirred quartz cuvette in a Hitachi F-7100 spectrofluorometer (ex: 494nm, ex-slit: 2.5 nm, em: 516 nm, em-slit: 5.0 nm, sampling frequency: 1 Hz). After adding 0.25 μM of Fluo-4 (Invitrogen, F14200) to monitor []ex, 30 mM of digitonin (Sigma, D141) was added to permeabilize the cells. When []ex reached a steady state, spermine was added to induce further mitochondrial absorption. At the end of the recording, 40 μM of CaCl2 was added to obtain the saturating fluorescence (), followed by adding 500 μM of EGTA to obtain the minimum fluorescence signal (). [ex at a certain fluorescence signal () was calculated using the following equation using a Fluo-4 Kd of 345 nM as provided by the manufacturer:
To measure the rate of mitochondrial calcium uptake rate (Fig. 3D-E and 7A), Calcium Green 5N (Invitrogen, C3737) was used to monitor []ex. Quantification was done by calculating the slope of the first 10 s of fluorescence signal decline after adding .
IMM depolarization essay
2 × 107 HEK cells were incubated with 40 nM of TMRM (Invitrogen, T668) in Tyrode’s solution (130 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 20 mM HEPES, pH 7.8-NaOH) at 37 °C for 30 min. Cells were washed and pelleted down using 10 mL of a Mg2+-free wash buffer (120 mM KCl, 2 mM K2HPO4, 50 μM EGTA, 25 mM HEPES, pH 7.2-KOH) and resuspended using a 2 mL Mg2+-free recording buffer (120 mM KCl, 2 mM K2HPO4, 5 mM succinate, 25 mM HEPES, pH 7.6-KOH). The sample was loaded in a stirred quartz cuvette in a Hitachi F-7100 spectrofluorometer (excitation: 573 nm; excitation slit: 5 nm; emission: 590 nm; emission slit: 5 nm; sampling rate: 1 Hz). After the cells were permeabilized by 30 mM of digitonin, 1 mM of spermine was added to the cuvette, followed by adding 1 μg/mL of FCCP to completely collapse the IMM potential. The rate of IMM depolarization was quantified by normalizing the slope before or after adding spermine to the total TMRM signal.
Electrophysiology
TEVC was performed as described before46. Briefly, stage V-VI oocytes were injected with 40 ng of hME cRNA and incubated in 18°C in a ND96 solution (96 mM NaCl, 2 mM KCl, 2 mM CaCl2, 0.5 mM MgCl2, 5 mM HEPES, pH 7.4-NaOH). Recordings were performed 2–3 days after cRNA injections. Signals were measured using the Oocyte Clamp OC-725B system (Warner). Voltage and current electrodes were filled with 3 M KCl to have a resistance of 0.5–1 MΩ. All recordings were performed in a Ca-20 solution (70 mM NaCl, 2 mM KCl, 0.5 mM MgCl2, 20 mM CaCl2, 5 mM HEPES, pH 7.4-NaOH).
Statistics
Statistical analysis was performed using two-tailed t test with significance defined as P < 0.05. We performed at least three independent repeats in all experiments. Data are presented as means ± S.E.M. Electrophysiology and mitochondrial flux data were analyzed using Igor Pro 8 (WaveMetrics). All figures were prepared using CorelDRAW.
Acknowledgements
This work is supported by the NIH grant R01-GM129345.
Footnotes
Competing Interest Statement
The authors declare no competing interests.
References
- 1.Kamer KJ, Mootha VK. The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol 16, 545–553 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13, 566–578 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev 102, 893–992 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 19, 713–730 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Sancak Y, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perocchi F, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467, 291–296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai CW, et al. Mechanisms and significance of tissue-specific MICU regulation of the mitochondrial calcium uniporter complex. Mol Cell 82, 3661–3676 e3668 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Structural insights into the Ca(2+)-dependent gating of the human mitochondrial calcium uniporter. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Jacewicz A, Delgado BD, Baradaran R, Long SB. Structures reveal gatekeeping of the mitochondrial Ca(2+) uniporter by MICU1-MICU2. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan M, et al. Structure and mechanism of the mitochondrial Ca(2+) uniporter holocomplex. Nature 582, 129–133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips CB, Tsai CW, Tsai MF. The conserved aspartate ring of MCU mediates MICU1 binding and regulation in the mitochondrial calcium uniporter complex. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paillard M, Csordas G, Huang KT, Varnai P, Joseph SK, Hajnoczky G. MICU1 Interacts with the D-Ring of the MCU Pore to Control Its Ca(2+) Flux and Sensitivity to Ru360. Mol Cell 72, 778–785 e773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai MF, et al. Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csordas G, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab 17, 976–987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallilankaraman K, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 151, 630–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai CW, et al. Evidence supporting the MICU1 occlusion mechanism and against the potentiation model in the mitochondrial calcium uniporter complex. Proc Natl Acad Sci U S A 120, e2217665120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne R, Hoff H, Roskowski A, Foskett JK. MICU2 Restricts Spatial Crosstalk between InsP(3)R and MCU Channels by Regulating Threshold and Gain of MICU1-Mediated Inhibition and Activation of MCU. Cell Rep 21, 3141–3154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Keuren AM, Tsai CW, Balderas E, Rodriguez MX, Chaudhuri D, Tsai MF. Mechanisms of EMRE-Dependent MCU Opening in the Mitochondrial Calcium Uniporter Complex. Cell Rep 33, 108486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casero RA Jr., Murray Stewart T, Pegg AE. Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer 18, 681–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 35, 55–91 (1995). [DOI] [PubMed] [Google Scholar]
- 23.Tabor CW, Tabor H. Polyamines. Annu Rev Biochem 53, 749–790 (1984). [DOI] [PubMed] [Google Scholar]
- 24.Nichols CG, Lee SJ. Polyamines and potassium channels: A 25-year romance. J Biol Chem 293, 18779–18788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams K. Interactions of polyamines with ion channels. Biochem J 325 ( Pt 2), 289–297 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372, 366–369 (1994). [DOI] [PubMed] [Google Scholar]
- 27.Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+ channels. Science 266, 1068–1072 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15, 453–462 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Fakler B, Brandle U, Glowatzki E, Weidemann S, Zenner HP, Ruppersberg JP. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell 80, 149–154 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Lu Z, Ding L. Blockade of a retinal cGMP-gated channel by polyamines. J Gen Physiol 113, 35–43 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleidervish IA, Libman L, Katz E, Gutnick MJ. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc Natl Acad Sci U S A 105, 18994–18999 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CJ, Moczydlowski E. Cytoplasmic polyamines as permeant blockers and modulators of the voltage-gated sodium channel. Biophys J 80, 1262–1279 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the -activated cation channel TRPM4b. Pflugers Arch 448, 70–75 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kerschbaum HH, Kozak JA, Cahalan MD. Polyvalent cations as permeant probes of MIC and TRPM7 pores. Biophys J 84, 2293–2305 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicchitta CV, Williamson JR. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem 259, 12978–12983 (1984). [PubMed] [Google Scholar]
- 36.Rustenbeck I, Eggers G, Reiter H, Munster W, Lenzen S. Polyamine modulation of mitochondrial calcium transport. I. Stimulatory and inhibitory effects of aliphatic polyamines, aminoglucosides and other polyamine analogues on mitochondrial calcium uptake. Biochem Pharmacol 56, 977–985 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Lenzen S, Munster W, Rustenbeck I. Dual effect of spermine on mitochondrial transport. Biochem J 286 ( Pt 2), 597–602 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenzen S, Rustenbeck I. Effects of IP3, spermine, and Mg2+ on regulation of transport by endoplasmic reticulum and mitochondria in permeabilized pancreatic islets. Diabetes 40, 323–326 (1991). [DOI] [PubMed] [Google Scholar]
- 39.Rottenberg H, Marbach M. Regulation of transport in brain mitochondria. I. The mechanism of spermine enhancement of uptake and retention. Biochim Biophys Acta 1016, 77–86 (1990). [DOI] [PubMed] [Google Scholar]
- 40.Kroner H. Spermine, another specific allosteric activator of calcium uptake in rat liver mitochondria. Arch Biochem Biophys 267, 205–210 (1988). [DOI] [PubMed] [Google Scholar]
- 41.Jensen JR, Lynch G, Baudry M. Polyamines stimulate mitochondrial calcium transport in rat brain. J Neurochem 48, 765–772 (1987). [DOI] [PubMed] [Google Scholar]
- 42.Lenzen S, Hickethier R, Panten U. Interactions between spermine and Mg2+ on mitochondrial transport. J Biol Chem 261, 16478–16483 (1986). [PubMed] [Google Scholar]
- 43.Akerman KE. Effect of Mg2+ and spermine on the kinetics of transport in rat-liver mitochondria. J Bioenerg Biomembr 9, 65–72 (1977). [DOI] [PubMed] [Google Scholar]
- 44.Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem 266, 20803–20809 (1991). [PubMed] [Google Scholar]
- 45.Patron M, et al. MICU1 and MICU2 finely tune the mitochondrial uniporter by exerting opposite effects on MCU activity. Mol Cell 53, 726–737 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai CW, Tsai MF. Electrical recordings of the mitochondrial calcium uniporter in Xenopus oocytes. J Gen Physiol 150, 1035–1043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne R, Hoff H, Roskowski A, Foskett JK. MICU2 Restricts Spatial Crosstalk between InsP3R and MCU Channels by Regulating Threshold and Gain of MICU1-Mediated Inhibition and Activation of MCU. Cell Rep 21, 3141–3154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wescott AP, Kao JPY, Lederer WJ, Boyman L. Voltage-energized Calcium-sensitive ATP Production by Mitochondria. Nat Metab 1, 975–984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai CW, et al. Proteolytic control of the mitochondrial calcium uniporter complex. Proc Natl Acad Sci U S A 114, 4388–4393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]