Abstract
In recent decades, the development of new drugs has become increasingly expensive and inefficient, and the molecular mechanisms of most pharmaceuticals remain poorly understood. In response, computational systems and network medicine tools have emerged to identify potential drug repurposing candidates. However, these tools often require complex installation and lack intuitive visual network mining capabilities. To tackle these challenges, we introduce Drugst.One, a platform that assists specialized computational medicine tools in becoming user-friendly, web-based utilities for drug repurposing. With just three lines of code, Drugst.One turns any systems biology software into an interactive web tool for modeling and analyzing complex protein-drug-disease networks. Demonstrating its broad adaptability, Drugst.One has been successfully integrated with 21 computational systems medicine tools. Available at https://drugst.one, Drugst.One has significant potential for streamlining the drug discovery process, allowing researchers to focus on essential aspects of pharmaceutical treatment research.
Keywords: Drug repurposing, Systems medicine, Interactive network enrichment, Biomedical network exploration, Network integration, Biomedical data analysis, Data visualization
Introduction
In recent years, rapid technological advancements and unmet medical needs have fueled the development of computational tools that leverage systems biology methodologies to decipher complex biomedical data [1]. These tools frequently target the identification of specific proteins or genes in a given disease context, such as marker genes indicative of disease progression [2,3]. The visualization of these results in a biomedical network context can greatly improve their interpretability, allowing us to better understand the underlying disease mechanisms and the interrelationships among the identified entities [4]. This principle applies to a variety of biomedical fields, including oncology [5,6], virology [7], and disease subtype identification and patient stratification through differential gene expression analysis [8,9]. Rendering these intricate cellular processes as graphs aids researchers in tailoring more precise pharmaceutical treatments, minimizing side effects [10,11] and opening prospects for novel therapeutic and diagnostic strategies, such as mechanistic drug repurposing [12].
Key challenges in the development of systems biology platforms include the integration of comprehensive biomedical data and the creation of flexible graphical user interfaces for data analysis, prioritization, and visualization. Stand-alone software such as Cytoscape [13] visualizes biological networks but necessitates local installation for each user. To circumvent this, developers often provide online solutions dependent solely on browser compatibility. However, this presents additional hurdles for researchers who may lack sufficient web-development skills and need to establish and maintain an infrastructure, including a server hosting a database and a website. Beyond network visualization, the collection, harmonization, integration, and incorporation of diverse biomedical data demand a significant time investment [14]. Moreover, the database should be maintained and regularly updated, a chore that is often not addressed by bioinformatics tools that primarily provide a result overview with a limited set of features. Thus, if network exploration is not neglected due to the additional workload, unique solutions are being developed from scratch, resulting in network visualizers and explorers of varying quality [7,8,15].
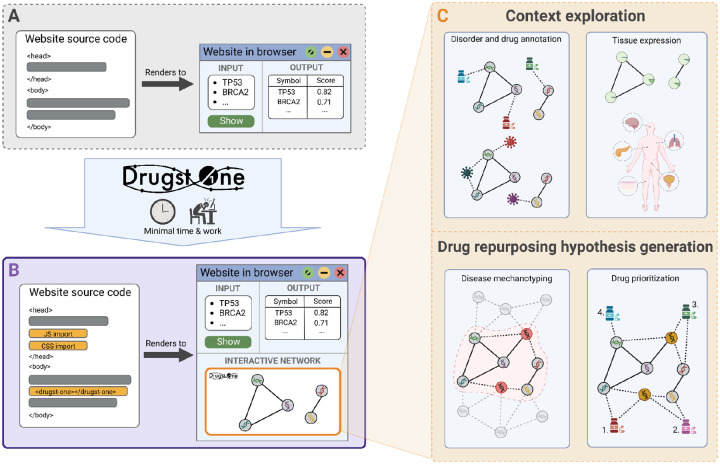
We developed Drugst.One to reduce software engineering overhead, bundle development capacities, and to standardize and simplify network analysis and visual network exploration for biomedical web tools (Figure 1). With minimal programming effort, Drugst.One can turn any gene or protein-based systems biology tool into a powerful online toolkit for network integration and visualization, as well as mechanistic drug repurposing. Drugst.One is a customizable plug-and-play solution for web-application developers in need of a feature-rich network explorer coupled with a biomedical protein-drug-disease network data warehouse. With as little as three lines of code, Drugst.One can be added to any biomedical web tool, highlighting opportunities for drug repurposing and elucidating disease mechanisms. Incorporating multiple state-of-the-art databases (see Supplementary Table 1) to complement visualized data, Drugst.One provides an intuitive interface for applying algorithms for exploratory network analyses, drug target and drug repurposing candidate identification and prioritization. Weekly updates guarantee the relevance of its database for frequently changing data. Currently, Drugst.One is integrated into 21 systems medicine software resources (Table 1), including mirDIP (see Supplementary Figure S3) [16] and WikiPathways [17]. In this article, we describe the functionality of Drugst.One and demonstrate its utility on the basis of two studies – on drug repurposing for inflammatory bowel disease (IBD) and on exploring the smooth muscle cell (SMC) proliferation pathway.
Figure 1:
Drugst.One enables web developers to add a fully functional network explorer to any website with minimal coding effort (biomedical web tool before (A) and after (B) Drugst.One integration). (C): A network can be explored manually or by using network medicine algorithms to identify disease mechanisms and drug repurposing candidates. Associated diseases and tissue-specific expression are additional information layers to gain insight into the network context.
Table 1.
Systems medicine tools that integrate Drugst.One listed in alphabetical order. Options are ‘Link-out’, referring to a URL based redirect from the tool or website to the Drugst.One standalone page, ‘Plugin’, referring to the integration of the javascript-based plugin into the web tool, and programmatic access using the ‘Python package’.
| Tool | URL | Tool Description | Integration | Integration status |
|---|---|---|---|---|
| BiCoN [8] | https://exbio.wzw.tum.de/bicon/ | Network-constrained patient stratification through biclustering | Plugin | Done |
| DOMINO [21] | http://domino.cs.tau.ac.il/ | Active module identification with improved empirical validation | Link-out | In progress |
| G-Browser | https://exbio.wzw.tum.de/genome-browser/ | An enhanced genome browser plugin that seamlessly integrates data sources and functions for genetics research. | Plugin | Done |
| GraphFusion | https://github.com/CarlosJesusGH/GraphFusion | An intuitive web-based graph analytics, fusion, and visualization tool | Plugin | Done |
| GraphSimViz [22] | https://graphsimviz.net/ | Visualization of diseasomes, drugomes, and drug-disease networks | Plugin | Done |
| HitSeekR [23] | https://exbio.wzw.tum.de/hitseekr/ | User-friendly tool for drug (target) identification in high-throughput screening | Plugin | Done |
| Interactive Enrichment Analysis [24] | https://github.com/gladstone-institutes/Interactive-Enrichment-Analysis/ | Enrichment analysis on multiple public datasets | Link-out | In progress |
| mirDIP [16] | https://ophid.utoronto.ca/mirDIP/ | Integrated microRNA-target data integration portal | Plugin | Done |
| NAViGaTOR [25] | https://ophid.utoronto.ca/navigator/ | Network visualization and analysis software | Link-out | In progress |
| NDEx IQuery [20] | https://www.ndexbio.org/iauery/ | Web tool for pathway and network-based gene set analysis | Plugin | Planned |
| NeEDL - Epistasis Disease Atlas | https://epistasis-disease-atlas.com | Web resource to visualize, investigate, and interpret higher-order genetic interactions of single nucleotide polymorphisms in 18 human heritable diseases. | Link-out | Done |
| NeEDL - R Shiny App | https://hub.docker.com/r/bigdatainbiomedicine/needl | R shiny app to visualize, investigate, and interpret higher-order genetic interactions of single nucleotide polymorphisms on locally computed datasets. | Plugin | Done |
| openPIP [26] | https://github.com/BaderLab/openPIP | Open platform to store and retrieve protein-protein interaction datasets. | Link-out | Done |
| pathDIP [27] | https://ophid.utoronto.ca/pathDIP | Integrated pathway database and pathway enrichment analysis portal | Plugin | In progress |
| Pathway Figure OCR [28] | https://pfocr.wikipathways.org | Platform for browsing pathway information extracted from published figures. | Link-out | Done |
| ProHarMeD | https://proharmed.zbh.uni-hamburg.de/ | Closing the gap between (harmonized) proteomics results and mechanotyping / drug repurposing | Plugin | Done |
| ROBUST-Web [29] | https://robust-web.net/ | ROBUST is a disease module identification tool. | Plugin | Done |
| SCANet | https://pypi.org/proiect/scanet/ | SCANet is an all-in-one package for single-cell profiling covering the whole differential mechanotyping workflow, from inference of trait/cell-type-specific gene co-expression modules to mechanistic drug repurposing candidate prediction. | Python package | Done |
| Seed Connector Algorithm [30] | https://github.com/bwh784/SCA | Identification of network modules by adding a minimal number of edges between the seed nodes. | Link-out | Done |
| UnPaSt | https://unpast.zbh.uni-hamburg.de | Visualizer and context explorer for unsupervised expression data bicluster results. | Plugin | Done |
| WikiPathways [17] | https://wikipathways.org | Platform for browsing and visualizing pathways. | Link-out | Done |
Results
Drugst.One overview
Drugst.One closes the gap between disease mechanism mining and hypothesis generation for drug repurposing. The required input is a list of proteins or genes in HGNC, UniProt, Ensembl, or Entrez ID space. On demand, these entities are integrated into the interactome and automatically annotated with clinically relevant information, e.g., targeting drugs or known disease associations. Exploratory functions allow the visualization of known drug indications and disease associations as well as an overlay for tissue-specific expression information (Figure 1C). For most information-enriching functions, Drugst.One provides several data sources to choose from (Supplementary Notes 3.1). Convenience features for network control (such as enabling the interactive mode or resetting the view) and export are available to assist exploratory analysis further.
Drugst.One originates from the network-based drug repurposing platforms CoVex [7] and CADDIE [5], developed for the application in SARS-CoV-2 and cancer, respectively. While they provide disease-specific information, both tools share underlying principles and algorithms. These tested and published methods form the Drugst.One algorithmic toolkit for more extensive analysis. Module identification algorithms provide means to identify additional potential drug targets from the interactome to enrich the mechanistic context. In a second step, drugs that are directly or indirectly linked can be ranked. This allows the assessment of the compound’s potential to be repurposed using network-based algorithms. Although both steps work automatically, users can infuse their expert knowledge by adjusting input gene sets. Users can choose among seven drug prioritization and drug target identification algorithms to rank small molecules directly or indirectly targeting disease proteins, thus serving as potential drug repurposing candidates (Supplementary Notes 3.2).
Overrepresentation analysis using g:Profiler [18] or functional coherence validation using DIGEST [19] on all loaded proteins in the network can be run with one click. Further, searching for curated pathways containing the same proteins in NDEx IQuery [20] allows for even more interoperability. A full list of projects partnering with the ‘Drugst.One Initiative’ by integrating Drugst.One can be found in Table 1. Collaborators that assisted and provided technologies that helped to build features in Drugst.One can be found in Supplementary Notes 4.
Drugst.One integration and customization
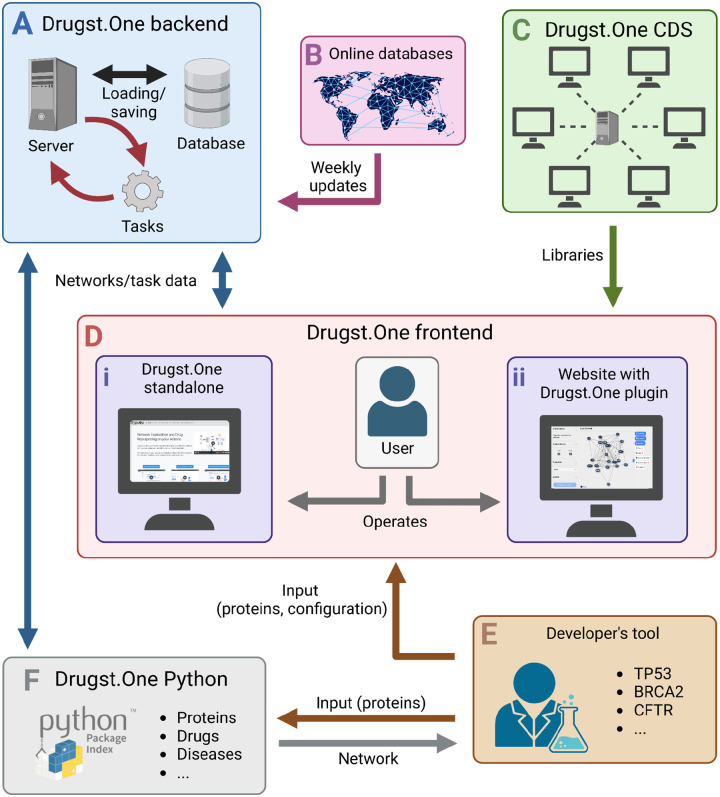
The Drugst.One ecosystem is a multi-component platform consisting of a website, the web plugin, a server, a content delivery system (CDS), and a Python package, as depicted in Figure 2 (for details, see Supplementary Notes 1).
Figure 2:
The Drugst.One ecosystem: The Drugst.One server (A) updates weekly from online databases (B), executes computationally demanding tasks, and provides data to the Drugst.One plugin (D i and D ii). The frontend is loaded from the content delivery system (CDS), (C), receives the network data from the developer integrating Drugst.One (E), and presents it to the user. Drugst.One can also be accessed programmatically through a Python package (F).
The web plugin can be added to any webpage by importing one JavaScript and one stylesheet file from the https://cdn.drugst.one distribution server, and by adding the ‘drugst-one’ HTML tag to the source code of any system medicine tool’s website (Supplementary Figure S2). Features can be customized to a high degree through JSON configuration strings that are passed as attributes. This includes default states of on/off toggles, the network, and the node and edge groups that define the network style. The plugin is responsive to changes during runtime, allowing developers to add buttons or other controls to the host page, for example switching between networks. For seamless integration of the rendered plugin into any website, styling and coloring are controllable by adding specific CSS variables to the website stylesheet. To assist developers in the integration process, the Drugst.One website provides conclusive documentation of available parameters, features, and styles. It further offers an interactive configuration page at https://drugst.one/playground where configuration options are categorized, and the replication of a configured Drugst.One instance is achieved by simple copy-pasting of the generated code snippets to the developers’ websites. This low-code approach allows bioinformatics researchers to provide the community with an interactive mechanism mining web tool within hours or even minutes instead of days. The lightweight Drugst.One JavaScript library connects to the Drugst.One data warehouse server, which handles all the computationally expensive work like data annotation, mapping, and asynchronous algorithm execution.
Alternatively, a standalone integration of Drugst.One is provided at https://drugst.one/standalone, which can be accessed and customized using URLs or POST-based requests. This way, results from any website or even a command line tool can be redirected to Drugst.One through a simple web service request (Supplementary Notes 2). Detailed documentation about all Drugst.One integration options can be found at https://drugst.one/doc.
Drugst.One integration examples
Drugst.One plugin integration with ROBUST-Web
ROBUST-Web (https://robust-web.net) presents a modified version of ROBUST [31] in an online web interface. It provides a network-based disease module identification algorithm based on prize-collecting Steiner trees that mitigates study bias using edge costs derived from study-attention or bait-usage information. Given a set of seed genes and a PPI network, ROBUST-Web constructs disease modules and passes nodes and edges to the Drugst.One plugin that takes care of result presentation and visualization in an interactive network view. Drugst.One also serves as a network explorer for the analysis of modules by offering an estimation of functional coherence with DIGEST [19], GO enrichment with g:Profiler, or a lookup in NDEx IQuery for identifying pathways with the same participants. Additionally, it adds disease annotations and drug repurposing functions to make the results of ROBUST-Web more actionable and derive hypotheses for follow-up research.
Drugst.One plugin integration with BiCoN
BiCoN [8] is a systems medicine tool for simultaneous patient stratification and disease mechanism identification, i.e., network-based endotyping. BiCoN uses a molecular interaction network as input and identifies two subgroups of patients along with a subnetwork that is enriched for differentially expressed genes between the two groups. These subnetworks can serve as composite biomarkers but may also be enriched for putative drug targets. Since BiCoN also features a web version (https://exbio.wzw.tum.de/bicon), we integrated the Drugst.One plugin for enhancing the result presentation by interactively visualizing the identified subnetworks. This allows users to explore possible drug repurposing candidates targeting the newly identified disease mechanisms, which can subsequently be experimentally validated.
Drugst.One link-out from WikiPathways
WikiPathways [32] is a widely used, community-driven platform for exploring molecular pathways. It allows users to upload, edit, browse, and download a constantly growing pool of pathway datasets. Pathway data can be used to identify and understand key players in metabolism, which is critical for understanding rare or common diseases such as COVID-19 [32]. Thus, pathways allow for the prediction of drug target and drug repurposing candidates and are commonly used in the development of new disease treatments [33]. When inspecting individual pathways on the WikiPathways platform, users now have the option to forward the pathway genes to the Drugst.One standalone version by clicking a ‘Query Drugst.One’ link now provided by WikiPathways, located in the search menu of the ‘Participants’ table. The link redirects the user to the Drugst.One website, visualizing pathway genes, drugs directly targeting them, and offering the complete toolset of Drugst.One. In the following, we give an example of the Drugst.One usage for exploration of the smooth muscle differentiation and proliferation pathway (WP1991).
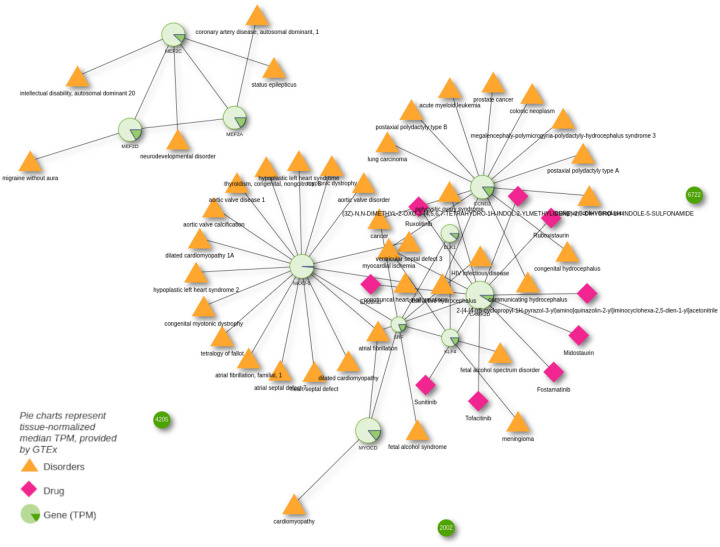
WikiPathway WP1991 describes the mechanism behind smooth muscle cell (SMC) differentiation and proliferation. The WikiPathways web interface now incorporates a button to export the pathway genes into Drugst.One (using the magnifier glass in the table showing the proteins participating in the pathway, state 03.07.23) and visualizing their interactions with drugs in Drugst.One directly. To gain a general overview of the complications (symptoms, comorbidities, etc.) associated with dysfunctional SMC development and their drivers, Drugst.One allows for extending the WikiPathways-exported network by the corresponding disorders and their associated pathway genes. Several disease nodes appear in the network, mainly representing various cardiovascular disorders (CVDs), e.g., cardiomyopathy, coronary artery disease, and aortic valve (Figure 3). The importance of SMCs for proper vascular functionality [34,35] and thus to atherosclerosis, hypertension, myocardial infarction, and other cardiovascular diseases was reported before [36–38]. An isolated subnetwork community of proteins is formed by the three myocyte enhancer factors MEF2A, MEF2C, and MEF2D. Besides their obvious cardiovascular implications, these factors play a role in neurological processes [39]. An impact of SMCs on status epilepticus was shown in mouse models [40], and a connection between migraine and SMC dysfunction was suggested as well [41,42].
Figure 3:
Participants of WikiPathway WP1991 displayed in Drugst.One. Adjacent diseases and drugs are enabled, as well as diseases linked to drugs targeting this smooth muscle cell proliferation and differentiation pathway. Normalized median expression values for ‘Artery - Aorta’ are overlaid as pie charts, where 360° represent the maximum observed transcripts per million (TPM) in the selected tissue and all other TPMs are exponentially scaled.
Drugst.One allows for the projection of (gene) expression data from GTEx on the proteins in the network. The relative expression of these genes appears to be quite high in arteries and organs that have to perform physical motion, like heart, lung, bladder, and skeletal muscles, but with observable fluctuations in the relative expression of genes like CCND2.
With one mouse click, we import drug target information for drug repurposing candidate prediction. Despite SMCs relation to cardiovascular diseases, no corresponding CVD drugs have been identified. Mainly anti-cancer drugs (e.g., sunitinib, erlotinib, midostaurin, and ruxolitinib) targeting calcium/calmodulin-dependent protein kinase II delta (CAMK2D), which is associated with cancer growth [43], are found. Notably, however, CAMK2D also plays a role in calcium signaling, which is essential for the upkeep of SMC function [44,45]. Hence, this may explain the observed cardiovascular side effects of CAMK2D-targeting drugs. According to SIDER [46], sunitinib may cause hypertensive symptoms and corresponding studies suggest that midostaurin has cardiotoxic effects [47].
Algorithms integrated in Drugst.One can extend the search space by looking for indirectly connected drugs. The selection menu offers a function to automatically add all displayed proteins to the selection, serving as the starting point (seeds) of subsequent searches. The harmonic centrality algorithm (see Supplement 3.2.3) was used to extend the network by the ten drugs with the highest score, including indirectly (transitively) connected drugs from the NeDRex database. Through this search, the tyrosine kinase inhibitor nintedanib, which has shown promising effects in pulmonary arterial smooth muscle cells and intestinal smooth muscle cells [48,49], can be identified.
This shows the identification potential of mechanism-associated drugs through the network-based drug repurposing functions Drugst.One incorporates. Whereas before only drugs primarily used in cancer were present through direct association with SMC pathway participants, Drugst.One suggested more relevant options for this context.
Discussion
Biomedical research generates a wealth of data that could inform the development of novel therapies or treatments. However, despite this potential, a significant portion of the analyses conducted in this field fail to translate into clinical trials, leading to major issues in the effectiveness of public health research [50]. To this end, Drugst.One has the potential to help transform specifically omics-based research results into actionable hypotheses with potential clinical impact. Drugst.One offers a community-driven solution to streamline the knowledge distributed over many online resources for multi-omics analyses and other biomedical tools [51] to turn the results of biomedical analyses into concrete candidate drug targets and drug repurposing hypotheses. Still, we emphasize that the drug target and drug repurposing predictions are merely candidates and supervision with expert knowledge is still required before experimental validation. Drugst.One delivers explainable indications based on established biological data like expression and known disease associations or drug indications, however, the interpretation of their application in the case-specific context is up to the user. Therefore, we designed Drugst.One to be operated with maximal transparency and allow optional user input for every step of the analysis.
With the infrastructure and the resources being provided, Drugst.One helps to find a community-wide solution for standardization and streamlining the visualization of explainable disease modules and their pharmacological implications. Drugst.One provides various interfaces to be highly accessible and customizable by all members of the community while maintaining up-to-date database information and network analysis algorithms. Smooth integration into most biomedical websites and tools is confirmed by 21 resources already integrating Drugst.One. For future developers who wish to customize Drugst.One before its integration, an interactive web interface provides copy-paste-able code for customized plugin integration with their own website. An endpoint for developers who want to link out from any of their websites, apps, or command line tools is provided by Drugst.One as well.
Drugst.One complies with community standards regarding data management as defined by the FAIRness principles [52]. Download links for any data shown in Drugst.One are provided at any step, whether it is a table with drug target and drug candidates or the visualized network with all activated extensions like expression information. Export to current community standards is supported via exporting compatible .graphml files, which can be loaded directly into, e.g., Cytoscape [13]. To further increase reproducibility and interoperability, concrete plans to implement save and export functions of Drugst.One networks to NDEx [53,54] are made.
In summary, Drugst.One offers an important service to the systems medicine research community to tackle the widely recurring problem of web-based disease mechanism mining and drug repurposing candidate prediction by capturing the results of biomedical assays.
Supplementary Material
Acknowledgments
REPO-TRIAL: This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 777111. This publication reflects only the authors’ view and the European Commission is not responsible for any use that may be made of the information it contains.
RePo4EU: This project is funded by the European Union under grant agreement No. 101057619. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them. This work was also partly supported by the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract No. 22.00115.
This work was supported by the German Federal Ministry of Education and Research (BMBF) within the framework of “CLINSPECT-M” (grant FKZ161L0214A). This work was supported by the Technical University Munich – Institute for Advanced Study, funded by the German Excellence Initiative. This work was supported in part by the Intramural Research Programs (IRPs) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 422216132.
JB was partially funded by his VILLUM Young Investigator Grant nr.13154.
This project has received funding from the European Research Council (ERC) Consolidator Grant 770827 and the Spanish State Research Agency AEI 10.13039/501100011033 grant number PID2019-105500GB-I00.
IJ was supported in part by funding from Natural Sciences Research Council (NSERC #203475), Canada Foundation for Innovation (CFI #225404, #30865), Ontario Research Fund (RDI #34876), IBM and Ian Lawson van Toch Fund.
SL has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 965193 for DECIDER.
Images were created with https://www.biorender.com
Conflicts of Interest
JSR reports funding from GSK, Pfizer and Sanofi and fees from Travere Therapeutics and Astex Pharmaceuticals.
References
- 1.Hufsky F, Lamkiewicz K, Almeida A, Aouacheria A, Arighi C, Bateman A, et al. Computational strategies to combat COVID-19: useful tools to accelerate SARS-CoV-2 and coronavirus research. Brief Bioinform. 2021;22: 642–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O’Meara MJ, et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. bioRxiv. 2020. doi: 10.1101/2020.03.22.002386 [DOI] [Google Scholar]
- 3.Zolotareva O, Kleine M. A Survey of Gene Prioritization Tools for Mendelian and Complex Human Diseases. J Integr Bioinform. 2019;16. doi: 10.1515/jib-2018-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hütter CVR, Sin C, Müller F, Menche J. Network cartographs for interpretable visualizations. Nature Computational Science. 2022;2: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartung M, Anastasi E, Mamdouh ZM, Nogales C, Schmidt HHHW, Baumbach J, et al. Cancer driver drug interaction explorer. Nucleic Acids Res. 2022;50: W138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadegh S, Skelton J, Anastasi E, Bernett J, Blumenthal DB, Galindez G, et al. Network medicine for disease module identification and drug repurposing with the NeDRex platform. Nat Commun. 2021;12: 6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadegh S, Matschinske J, Blumenthal DB, Galindez G, Kacprowski T, List M, et al. Exploring the SARS-CoV-2 virus-host-drug interactome for drug repurposing. Nat Commun. 2020;11: 3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazareva O, Canzar S, Yuan K, Baumbach J, Blumenthal DB, Tieri P, et al. BiCoN: Network-constrained biclustering of patients and omics data. Bioinformatics. 2020. doi: 10.1093/bioinformatics/btaa1076 [DOI] [PubMed] [Google Scholar]
- 9.Zolotareva O, Khakabimamaghani S, Isaeva OI, Chervontseva Z, Savchik A, Ester M. Identification of Differentially Expressed Gene Modules in Heterogeneous Diseases. doi: 10.1101/2020.04.23.055004 [DOI] [PubMed] [Google Scholar]
- 10.Nelissen E, van Hagen BTJ, Argyrousi EK, van Goethem NP, Heckman PRA, Paes D, et al. Soluble guanylate cyclase stimulator riociguat improves spatial memory in mice via peripheral mechanisms. Neurosci Lett. 2022;788: 136840. [DOI] [PubMed] [Google Scholar]
- 11.Casas AI, Hassan AA, Larsen SJ, Gomez-Rangel V, Elbatreek M, Kleikers PWM, et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc Natl Acad Sci U S A. 2019;116: 7129–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh K-I, Cusick ME, Valle D, Childs B, Vidal M, Barabási A-L. The human disease network. Proc Natl Acad Sci U S A. 2007;104: 8685–8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobentanzer S, Aloy P, Baumbach J, Bohar B, Carey VJ, Charoentong P, et al. Democratizing knowledge representation with BioCypher. Nat Biotechnol. 2023. doi: 10.1038/s41587-023-01848-y [DOI] [PubMed] [Google Scholar]
- 15.Levi H, Elkon R, Shamir R. DOMINO: a network-based active module identification algorithm with reduced rate of false calls. Mol Syst Biol. 2021;17: e9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauschild A-C, Pastrello C, Ekaputeri GKA, Bethune-Waddell D, Abovsky M, Ahmed Z, et al. MirDIP 5.2: tissue context annotation and novel microRNA curation. Nucleic Acids Res. 2023;51: D217–D225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pico AR, Kelder T, van Iersel MP, Hanspers K, Conklin BR, Evelo C. WikiPathways: pathway editing for the people. PLoS Biol. 2008;6: e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47: W191–W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamowicz K, Maier A, Baumbach J, Blumenthal DB. Online in silico validation of disease and gene sets, clusterings or subnetworks with DIGEST. Brief Bioinform. 2022;23. doi: 10.1093/bib/bbac247 [DOI] [PubMed] [Google Scholar]
- 20.Pillich RT, Chen J, Churas C, Fong D, Ideker T, Liu SN, et al. NDEx IQuery: a multi-method network gene set analysis leveraging the Network Data Exchange. bioRxiv. 2022. p. 2022.10.24.513552. doi: 10.1101/2022.10.24.513552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levi H, Rahmanian N, Elkon R, Shamir R. The DOMINO web-server for active module identification analysis. Bioinformatics. 2022;38: 2364–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadegh S, Skelton J, Anastasi E, Maier A, Adamowicz K, Möller A, et al. Lacking mechanistic disease definitions and corresponding association data hamper progress in network medicine and beyond. Nat Commun. 2023;14: 1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.List M, Schmidt S, Christiansen H, Rehmsmeier M, Tan Q, Mollenhauer J, et al. Comprehensive analysis of high-throughput screens with HiTSeekR. Nucleic Acids Res. 2016;44: 6639–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Interactive-Enrichment-Analysis: A set of Shiny apps to provide interactive enrichment analysis and exploration of results. Github; Available: https://github.com/gladstone-institutes/Interactive-Enrichment-Analysis
- 25.Brown KR, Otasek D, Ali M, McGuffin MJ, Xie W, Devani B, et al. NAViGaTOR: Network Analysis, Visualization and Graphing Toronto. Bioinformatics. 2009;25: 3327–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmy M, Mee M, Ranjan A, Hao T, Vidal M, Calderwood MA, et al. OpenPIP: An Open-source Platform for Hosting, Visualizing and Analyzing Protein Interaction Data. J Mol Biol. 2022;434: 167603. [DOI] [PubMed] [Google Scholar]
- 27.Rahmati S, Abovsky M, Pastrello C, Kotlyar M, Lu R, Cumbaa CA, et al. pathDIP 4: an extended pathway annotations and enrichment analysis resource for human, model organisms and domesticated species. Nucleic Acids Res. 2020;48: D479–D488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanspers K, Riutta A, Summer-Kutmon M, Pico AR. Pathway information extracted from 25 years of pathway figures. Genome Biol. 2020;21: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar S, Lucchetta M, Maier A, Abdrabbou MM, Baumbach J, List M, et al. Online bias-aware disease module mining with ROBUST-Web. Bioinformatics. 2023. doi: 10.1093/bioinformatics/btad345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R-S, Loscalzo J. Network-Based Disease Module Discovery by a Novel Seed Connector Algorithm with Pathobiological Implications. J Mol Biol. 2018;430: 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernett J, Krupke D, Sadegh S, Baumbach J, Fekete SP, Kacprowski T, et al. Robust disease module mining via enumeration of diverse prize-collecting Steiner trees. Bioinformatics. 2022. doi: 10.1093/bioinformatics/btab876 [DOI] [PubMed] [Google Scholar]
- 32.Martens M, Ammar A, Riutta A, Waagmeester A, Slenter DN, Hanspers K, et al. WikiPathways: connecting communities. Nucleic Acids Res. 2021;49: D613–D621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11: 790–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steucke KE, Tracy PV, Hald ES, Hall JL, Alford PW. Vascular smooth muscle cell functional contractility depends on extracellular mechanical properties. J Biomech. 2015;48: 3044–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaminon A, Reesink K, Kroon A, Schurgers L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int J Mol Sci. 2019;20. doi: 10.3390/ijms20225694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhuge Y, Zhang J, Qian F, Wen Z, Niu C, Xu K, et al. Role of smooth muscle cells in Cardiovascular Disease. Int J Biol Sci. 2020;16: 2741–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M, Gomez D. Smooth Muscle Cell Phenotypic Diversity. Arterioscler Thromb Vasc Biol. 2019;39: 1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allahverdian S, Chaabane C, Boukais K, Francis GA, Bochaton-Piallat M-L. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 2018;114: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhary R, Agarwal V, Kaushik AS, Rehman M. Involvement of myocyte enhancer factor 2c in the pathogenesis of autism spectrum disorder. Heliyon. 2021;7: e06854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cozart MA, Phelan KD, Wu H, Mu S, Birnbaumer L, Rusch NJ, et al. Vascular smooth muscle TRPC3 channels facilitate the inverse hemodynamic response during status epilepticus. Sci Rep. 2020;10: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Napoli R, Guardasole V, Zarra E, Matarazzo M, D’Anna C, Saccà F, et al. Vascular smooth muscle cell dysfunction in patients with migraine. Neurology. 2009;72: 2111–2114. [DOI] [PubMed] [Google Scholar]
- 42.Napoli R, Guardasole V, Zarra E, De Sena A, Saccà F, Ruvolo A, et al. Migraine attack restores the response of vascular smooth muscle cells to nitric oxide but not to norepinephrine. World Journal of Cardiology. 2013. p. 375. doi: 10.4330/wjc.v5.i10.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Q, Li Z. The dysregulated expression and functional effect of CaMK2 in cancer. Cancer Cell Int. 2021;21: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol. 2011;3: a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adelstein RS, Sellers JR. Effects of calcium on vascular smooth muscle contraction. Am J Cardiol. 1987;59: 4B–10B. [DOI] [PubMed] [Google Scholar]
- 46.Kuhn M, Letunic I, Jensen LJ, Bork P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016;44: D1075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giudice V, Vecchione C, Selleri C. Cardiotoxicity of Novel Targeted Hematological Therapies. Life. 2020;10. doi: 10.3390/life10120344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsutsumi T, Nagaoka T, Yoshida T, Wang L, Kuriyama S, Suzuki Y, et al. Nintedanib ameliorates experimental pulmonary arterial hypertension via inhibition of endothelial mesenchymal transition and smooth muscle cell proliferation. PLoS One. 2019;14: e0214697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kataria J, Kerr J, Lourenssen SR, Blennerhassett MG. Nintedanib regulates intestinal smooth muscle hyperplasia and phenotype in vitro and in TNBS colitis in vivo. Sci Rep. 2022;12: 10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drolet BC, Lorenzi NM. Translational research: understanding the continuum from bench to bedside. Transl Res. 2011;157: 1–5. [DOI] [PubMed] [Google Scholar]
- 51.Luo J, Wu M, Gopukumar D, Zhao Y. Big Data Application in Biomedical Research and Health Care: A Literature Review. Biomed Inform Insights. 2016;8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson MD, Dumontier M, Aalbersberg IJJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3: 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pratt D, Chen J, Pillich R, Rynkov V, Gary A, Demchak B, et al. NDEx 2.0: A Clearinghouse for Research on Cancer Pathways. Cancer Res. 2017;77: e58–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pillich RT, Chen J, Churas C, Liu S, Ono K, Otasek D, et al. NDEx: Accessing Network Models and Streamlining Network Biology Workflows. Curr Protoc. 2021;1: e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.