Summary
Background
Severe COVID-19 is associated with innate immunopathology, and CD14, a proximal activator of innate immunity, has been suggested as a potential therapeutic target.
Methods
We conducted the COVID-19 anti-CD14 Treatment Trial (CaTT), a Phase II randomized, double-blind, placebo-controlled trial at 5 US-sites between April 12, 2021 and November 30, 2021 (NCT04391309). Hospitalized adults with COVID-19 requiring supplemental oxygen (<30 LPM) were randomized 1:1 to receive 4 daily doses of intravenous IC14, an anti-CD14 monoclonal antibody, or placebo. All participants received remdesivir. The primary outcome was time-to-resolution of illness, defined as improvement on the 8-point NIH-Ordinal COVID-19 Scale to category ≤3. Secondary endpoints were safety and exploratory endpoints were pro-inflammatory and antiviral mediators in serum on days 0–5 & 7. The trial was stopped after 40 patients were randomized and treated due to slow enrollment.
Findings
40 participants were randomized and treated with IC14 (n = 20) or placebo (n = 20). The median time-to-recovery was 6 days (95% CI, 5–11) in the IC14 group vs. 5 days (95% CI, 4–10) in the Placebo group (recovery rate ratio: 0.77 (95% CI, 0.40, 1.48) (log-rank p = 0.435). The number of adverse events was similar in each group, and no IC14-attributable secondary infections occurred. In repeated-measures mixed-effects analyses, IC14 treatment increased serum sCD14 concentrations, an expected pharmacodynamic effect. Pre-planned, exploratory analyses suggested that IC14 treatment decreased the trajectories of circulating MIP-1β and TNF-α.
Interpretation
IC14 treatment did not improve time-to-resolution of illness in hypoxemic patients with COVID-19 in this small trial. Results of exploratory analyses suggested IC14 had biologic effects that warrant future clinical investigation.
Funding
National Institute of Allergy and Infectious Diseases.
Keywords: COVID-19, CD14, CD14-blockade, Innate immunity, IC14
Research in context.
Evidence before this study
CD14 is an important proximal activator of innate immunity in response to infection and tissue damage. Prior to this trial, we searched Pubmed for studies published, up to February 2021, using the following terms: “cluster of differentiation 14” OR “CD14” OR “IC14” OR “CD14-blockade” AND “COVID-19” OR “SARS-CoV-2” OR “severe acute respiratory syndrome related coronavirus 2” AND “clinical trial” OR “treatment” OR “RCT”. We did not identify prior publication of a clinical trial investigating CD14 blockade with the monoclonal antibody IC14 in COVID-19. Severe COVID-19 is associated with innate immunopathology and derangements in CD14-pathway specific markers. Animal studies, pre-clinical studies and early phase clinical studies suggest CD14-blockade with a specific monoclonal antibody (IC14) may effectively reduce immunopathology in diseases involving excessive activation of innate immunity, such as bacterial sepsis and viral lung injury. Taken together, CD14 was identified as a potential therapeutic target for the treatment of hospitalized and hypoxemic patients with COVID-19.
Added value of this study
This clinical trial evaluated the potential anti-inflammatory effects of anti-CD14 therapy in hospitalized hypoxemic patients with COVID-19 pneumonia. In this small trial, IC14 treatment had the expected pharmacodynamic effect on sCD14, but did not improve time-to-resolution of illness in hypoxemic patients with COVID-19. Exploratory analyses suggested that IC14 was associated with a decrease in several plausible downstream proinflammatory mediators, particularly in patients with higher O2 requirements. The incidence of adverse events was similar in the IC14 and placebo groups, and no IC14-attributable secondary infections occurred.
Implications of all the available evidence
CD14 blockade with IC14 may warrant additional clinical investigation to determine whether anti-CD14 therapy might improve systemic inflammation and patient centered outcomes in patients with COVID-19 pneumonia.
Introduction
The coronavirus disease (COVID-19) global pandemic has resulted in more than 6 million deaths worldwide (source: COVID-19 Map - Johns Hopkins Coronavirus Resource Center (jhu.edu)). While significant strides have been made to identify and adopt the use of effective immune modifiers in patients with COVID-19 pneumonia and respiratory failure (e.g. dexamethasone,1 tocilizumab,2 and baricitinib3), the mortality of hospitalized patients with progressive respiratory failure remains unacceptably high at approximately 30%.4 In COVID-19, progressive respiratory failure is thought to be driven in part by overly exuberant innate immune activation resulting in deleterious inflammation, acute lung injury with alveolar flooding, and inadequate viral clearance.5, 6, 7
CD14, a proximal point in the innate immune response to infection, has been identified as a potential target to reduce innate immune activation in COVID-19 respiratory failure.8, 9, 10 During infection with SARS-CoV-2, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are recognized by a family of membrane toll-like receptors (TLRs) on macrophages, neutrophils and other innate immune cells.9 Membrane bound (mCD14) and soluble CD14 (sCD14) cooperate with TLRs to facilitate innate immune responses.8 IC14, a chimeric monoclonal antibody, is an investigational anti-CD14 therapeutic. The putative mechanism of action of IC14 in COVID-19 is to block interactions between CD14 and PAMPs and DAMPs, thus attenuating the inflammatory cascade that leads to tissue injury and COVID-19-related lung dysfunction.10
The rationale for investigating CD14 blockade in COVID-19 is supported by comprehensive immune profiling in COVID-19 and prior work investigating the effects of CD14 blockade in other disease states. In COVID-19, an enhanced inflammatory phenotype characterized by activated innate immunity pathways in lung immune cells, an increase in circulating CD14+ monocytes, and a proinflammatory cytokine rich milieu is associated with adverse outcomes.11, 12, 13, 14, 15, 16 Several studies suggest that modulation of this heightened innate immune response may improve clinical outcome. In fact, the effects of successful immune modulating medications in COVID-19, such as baricitinib and dexamethasone, may be mediated by a reduction in innate immune mediators (e.g. IL-6 and TNF-alpha (TNF-α)) that may be affected by targeting CD14.17, 18, 19, 20 Presepsin, an N-terminal fragment of full length CD14 that is generated in the setting of CD14-dependent innate immune activation, and a marker of CD14 signaling,9,21 has been associated with clinical outcomes in observational studies of patients with severe COVID-19.22, 23, 24, 25, 26 In early phase clinical trials in bacterial sepsis and ARDS, IC14 reduced systemic inflammation (e.g. circulating IL-6, TNF-α, MIP-1beta (MIP-1β)) but the trials were not powered to detect patient-centered clinical outcomes.27 In pre-clinical models, CD14-mediated innate immune responses have been shown to drive lung and other organ injury during infection with other respiratory viruses, including: SARS-CoV-1,28 RSV,29 and influenza A.30 These data suggest that CD14 may play a role in amplifying deleterious inflammation in COVID-19 illness. We hypothesized that therapeutic blockade of CD14 with IC14 may dampen immunopathology in patients with COVID-19 and improve clinical outcome.10,14
We conducted a randomized, double-blind, placebo-controlled clinical trial, “COVID-19 anti-CD14 Treatment Trial” (CaTT), to assess the efficacy of CD14 blockade using the IC14 monoclonal antibody in hospitalized hypoxemic patients with COVID-19 pneumonia. The primary objective was to evaluate the effect of IC14 on time to resolution of illness. The key secondary objective was to assess the safety of IC14, and exploratory objectives were to assess the effects of IC14 on downstream pro-inflammatory and antiviral mediators of COVID-19 illness.
Methods
Trial design
CaTT was a multicenter, double-blind, randomized, placebo-controlled trial in patients hospitalized with COVID-19 illness who had hypoxemia and radiographic features consistent with SARS-CoV-2-related pneumonia. The efficacy and safety of treatment with intravenous IC14 vs. placebo were evaluated over a 60-day period. Participants were enrolled from April 12, 2021 to November 30, 2021 at 5 centers in the United States. This clinical trial was conducted under US IND 153196, held by the Division of Allergy, Immunology, and Transplantation (DAIT) within the National Institute of Allergy and Infectious Diseases (NIAID). Medical monitors at DAIT/NIAID and the Vanderbilt Coordinating Center (VCC) oversaw trial safety, and a DSMB chartered by and advisory to DAIT/NIAID reviewed the protocol prior to trial initiation, and reviewed enrollment and safety data at monthly intervals during the trial. The VCC led the implementation of trial procedures including data collection and analyses. Implicit Bioscience Ltd., provided the investigational drug, IC14, and Gilead Science provided remdesivir, but neither company participated in the funding, design, data analysis, or implementation of the trial. The University of Washington Investigational Review Board (IRB) served as the central IRB for the trial (approval #: 00010824), which was registered in ClinicalTrials.gov (NCT04391309).
The trial protocol, which includes a statistical analysis plan, and the study investigators are included in the online Supplement. No significant changes to the protocol were made after the trial began. Study stopping rules and details of safety monitoring and reporting, including pre-specified DSMB reviews are outlined in the study protocol (Supplement, Clinical Trial Protocol, Sections 11.5 and 12, page 40).
Participants
Hospitalized patients were initially screened through the electronic medical record under a waiver of consent (Supplement, Clinical Trial Protocol, Section 8.2, page 32). Hospitalized patients ≥18 years of age were eligible for enrollment if they had SARS-CoV-2 infection confirmed by RT-PCR of a nasal swab specimen, radiologic findings compatible with diagnosis of SARS-CoV-2 pneumonia, and hypoxemia defined by the following: SpO2 ≤ 94% on room air, or requirement for ≥2 L per minute (LPM) O2 per nasal cannula but not more than 30 LPM to maintain SpO2 ≥ 94%. The trial protocol includes a full description of enrollment and exclusion criteria (Supplement, Clinical Trial Protocol, Section 4, Page 24). Informed consent (written or electronic) was obtained from each participant or a legally authorized representative by the site principal investigator or a designee.
Randomization and masking
Participants were randomized in a 1:1 ratio to receive IC14 or placebo. The sponsor, investigators, and participants were blinded to the treatment group. Randomization was stratified by groups of trial site and baseline use of dexamethasone. Computer-generated random block allocation with random block size was performed using an internet-based tool (4G Clinical, Wellesley, MA) by the VCC. Randomized treatment assignment was provided securely to an unmasked pharmacist at each site.
Procedures
The IC14 study drug (4 mg/kg) or an identical appearing placebo (0.9% NaCl) was administered on Day 1, followed by IC14 (2 mg/kg) or placebo on days 2–4. In addition, all participants received remdesivir 200 mg IV × 1 followed by 100 mg daily × 4 days (to complete a 5-day course). Treatment doses were omitted if the participant was discharged from the hospital prior to day 5. Treatment with dexamethasone was allowed at the discretion of the attending physicians, and treatment with other immune modulating medications (e.g. tocilizumab, baricitinib) were permitted per NIH Treatment Guidelines (https://www.covid19treatmentguidelines.nih.gov/) if there was evidence of clinical worsening.
All participants were assessed daily while hospitalized or until hospital discharge. Daily assessments included eight-point ordinal scale score,31 respiratory support, clinical labs, physical exam, imaging, and medications. If discharged, telephone or in-person follow-up was conducted on days 14, 28 and 60. Adverse events (AEs) were recorded daily during hospitalization and at post-discharge follow up visits. On-trial participant information was collected by Research Staff using a secure electronic data collection system (VEEVA Vault CDMS, Pleasanton, CA).
Serum was obtained at trial entry and on days 1 through 5, 7, 14, and 28 for exploratory biomarker, pharmacokinetic and drug antibody measurements. Nasal swabs for measurement of SARS-CoV-2 viral load using a reverse transcription polymerase chain reaction (RT-PCR) assay were obtained at trial entry and on days 4, 7, and 14 as long as participants remained hospitalized.
Outcomes
The primary efficacy outcome was the time to resolution of illness as measured by a relative recovery rate within the first 28 days, with recovery defined as improving to Category 3 or less on the 8-point NIH Ordinal Severity Scale (OSS).31 Secondary clinical outcomes are listed in the trial protocol (Supplement, Clinical Trial Protocol, Section 3.2) and included: change in OSS between trial entry and days 14 and 28, OSS on day 14, all-cause mortality through day 60, proportion alive-and-free of invasive mechanical ventilation through day 28.
Safety of IC14 was assessed by: 1) change in renal, liver, hematologic and coagulation parameters as captured by clinical labs through day 28; 2) cumulative AEs of special interest (AESI) through Day 60, including new infections, infusion reactions, and new eye abnormalities and their relatedness to study-drug administration, 3) cumulative incidence of severe AEs through day 60; 4) viral clearance by SARS-CoV-2 nasopharyngeal RT-PCR. AEs were classified according to the National Cancer Institute’s Common technology for Adverse Events (CTCAE) version 5.32
The exploratory objective was to investigate whether treatment with IC14 had an effect on the serum concentrations of a panel of pre-specified circulating biomarkers, including pathway specific markers (sCD14, presepsin), pro-inflammatory mediators (IL-6, MCP-1, TNF-α, MIP-1β, eotaxin, MCP-4, MDC, TARC, IL-10) elaborated down-stream of CD14 activation that have been shown to track with COVID-19 severity and outcomes, and key anti-viral mediators (IP-10, IFN-gamma (IFN-γ)).7,23,33, 34, 35, 36, 37, 38, 39, 40, 41 Based on previously reported IC14 pharmacodynamic effects, we hypothesized that IC14 would increase circulating sCD14 levels by binding sCD14 and slowing clearance.27 We also hypothesized that IC14 would attenuate levels of presepsin,23 proinflammatory mediators, and anti-viral mediators.
Laboratory measurements
Serum biomarkers were measured at the University of Washington, Seattle, WA. IL-6, MCP-1, TNF-α, MIP-1β, IP-10, eotaxin, MCP-4, MDC, TARC, IFN-γ, IL-10, IL-8, were measured using electrochemiluminescence immunoassays (Meso Scale Diagnostics, Rockville, MD). sCD14 and presepsin concentrations were measured using enzyme-linked immunosorbent assays (sCD14: R&D Systems, Minneapolis, MN and presepsin: MyBiosource, San Diego, CA). Immunoassay performance characteristics are summarized in Supplemental Table S1.
Sample size and statistical analyses
The trial was designed to detect an improvement in the recovery rate ratio of 1.4 in the IC14 treated group relative to placebo, because a target effect of this magnitude was thought to be clinically important. Using a log-rank test, we determined that a total of 278 recoveries by 28 days would provide 80% power to detect a recovery rate ratio of 1.4 in the IC14-treated group relative to placebo with a two-sided type I error rate of 0.05 using Schoenfeld’s formula.42 We then determined the number of total participants needed to observe this number of events using the Weibull distribution to model the patient recovery experience based on data from Beigel et al.31 To achieve a recovery rate ratio of 1.4 and assuming the placebo arm in this study would have a recovery experience similar to the remdesivir arm in ACTT-1 with a recovery rate of 0.851 by 28 days, the recovery rate in the IC14 group would be 0.931 based on the Weibull distribution. Using these recovery rates at Day 28, we determined that approximately 312 participants would need to be enrolled (Supplement, Clinical Trial Protocol, Section 13.11, Supplemental Table S7, page 52).
The safety and efficacy populations for analyses consisted of all participants who received at least one dose of study medication (modified intent to treat, m-ITT). The primary outcome was evaluated by difference in time-to-recovery between IC14 and placebo groups using the log-rank test with death censored at day 28.43 For the secondary outcomes, differences between IC14 and placebo groups were evaluated by point estimates and 95% confidence intervals for the differences in means (for continuous outcomes) or proportions (for categorical outcomes). Differences in AEs between IC14 and placebo groups were evaluated using point estimates and 95% confidence intervals for the difference in proportion experiencing at least one AE per category. For the exploratory biomarker analyses, we included serial measures from serum collected at several times (days 1 through 5 and 7) in order to evaluate the effect of IC14 on the time-related trajectory of biomarkers, including: 1) CD14 pathway specific markers; 2) down-stream pro-inflammatory mediators; and 3) down-stream antiviral mediators. The analysis used mixed effects models for repeated measures with treatment by time interaction terms and adjustment for baseline day 0 (pre-treatment) measurements. A p-value ≤ 0.05 for the IC14 treatment by time interaction term was considered statistically significant. A sensitivity analysis excluded participants treated with tocilizumab. To test whether IC14 was more biologically active amongst those with more severe lung injury and immune dysregulation, a post hoc exploratory analysis was performed stratifying by median pre-treatment O2 flow rates (2 LPM). Given the small number of participants in the sensitivity and post hoc analyses, we used a p-value threshold of ≤0.20 for the IC14 treatment by time interaction term to detect trends that might be related to the effects of IC14. Due to the exploratory nature of the biomarker analyses and the small trial size, we did not correct for multiple hypothesis testing. Detailed methods for the biomarker analyses are provided in the supplement (Supplement, Supplemental Methods, Biomarker Analyses).
Role of funders
The study was funded by supplements to a cooperative agreement awarded by the National Institute of Allergy and Infectious Disease (NIAID). NIAID project scientists participated collaboratively in study design, data analyses, interpretation, and writing of the report but not in data collection. The manuscript content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health or any other Agency of the United States Government. The authors are solely responsible for the study design, data collection, interpretation, manuscript preparation, and decision to submit the manuscript.
Results
Trial population
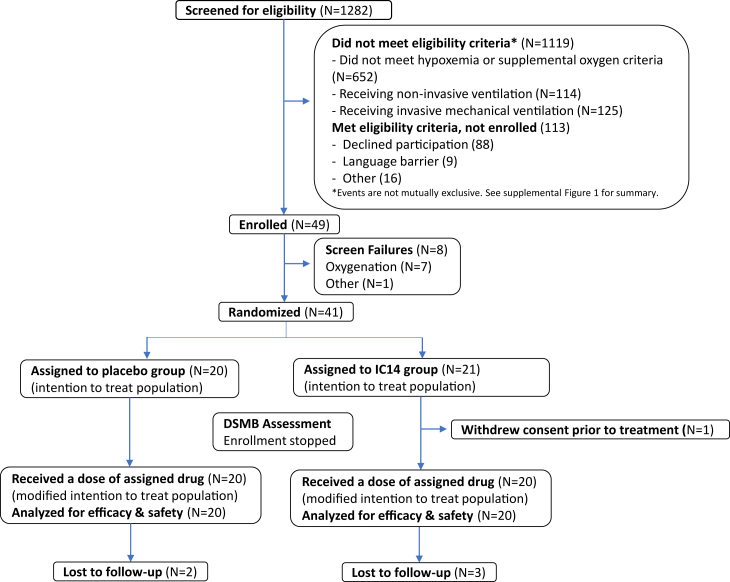
A total of 49 participants were enrolled between April 12, 2021 and December 1, 2021, when the SARS-CoV-2 delta variant was predominant in the US and prior to the emergence of the Omicron variant. Enrollment was closed on December 1, 2021 by the trial sponsor, DAIT/NIAID, based on the recommendation of the Data Safety Monitoring Board (DSMB), because of a slow rate of enrollment, due in large part to the relatively narrow enrollment oxygenation criteria and the evolving epidemiology of COVID-19 illness. A total of 1282 patients were screened for eligibility (Fig. 1 and Supplemental Fig. S1). Of these, 49 patients agreed to participate, signed informed consent and were considered to be enrolled in the trial. Eight of these participants were not randomized because they no longer met eligibility criteria at the time of randomization, including no longer being sufficiently hypoxemic or being too hypoxemic (were using high-flow oxygen >30 LPM). Forty-one participants were randomized and forty received at least one dose of study medication, 20 in the IC14 group and 20 in the placebo group. One participant withdrew consent after randomization before being treated and declined further participation and was not included in the analysis. Therefore, the participants included in the safety and m-ITT populations were the same.
Fig. 1.
Trial CONSORT diagram.
Demographics and baseline characteristics were similar in the two treatment groups (Table 1). Mean age was 54.5 (SD ± 14.7) in the IC14 group and 47.1 (SD ± 13.6) in the placebo group, 11 (55%) participants in the IC14 group and 13 (65%) participants in the placebo group were male, and 4 (20%) participants in each group were Hispanic or Latino. At randomization, 18 (90%) participants in the IC14 group and 19 (95%) participants in the placebo group were treated by nasal cannula (OSS = 5) with a mean baseline O2 flow rate of 3.8 LPM (SD ± 2.4) in the IC14 group and 3.9 LPM (SD ± 2.0) in the placebo group. Study drug administration was also similar between groups (Supplemental Table S2). Treatment with other immunomodulatory medications was similar between groups. Most participants received dexamethasone (IC14: n = 18 (90%) vs. placebo: n = 19 (95%)) (Table 1), whereas ≤10% of participants received tocilizumab or baricitinib (Supplemental Table S3).
Table 1.
Baseline characteristics of the patient population (modified intention to treat population).
| Placebo (N = 20) | IC14 (N = 20) | |
|---|---|---|
| Age | 48.0 (36.5–54.5) | 53.0 (48.0–66.2) |
| Gender | ||
| Male | 13 (65%) | 11 (55%) |
| Female | 7 (35%) | 9 (45%) |
| Ethnicity | ||
| Hispanic or Latino | 4 (20%) | 4 (20%) |
| Not Hispanic or Latino | 9 (45%) | 10 (50%) |
| Unknown | 7 (35%) | 6 (30%) |
| Race | ||
| White | 15 (75%) | 15 (75%) |
| Black or African American | 1 (5%) | 0 (0%) |
| Asian | 0 (0%) | 3 (15%) |
| American Indian or Alaska Native | 0 (0%) | 0 (0%) |
| Native Hawaiian or Other Pacific Islander | 1 (5%) | 0 (0%) |
| Other | 1 (5%) | 0 (0%) |
| Unknown | 2 (10%) | 2 (10%) |
| Weight (kg) | 98.5 ± 25.0 | 96.0 ± 24.0 |
| Height (cm) | 170.6 ± 12.4 | 164.6 ± 24.5 |
| Eight-point ordinal scale | ||
| 4 | 4 (20%) | 1 (5%) |
| 5 | 14 (70%) | 18 (90%) |
| 6 | 2 (10%) | 1 (5%) |
| Sequential organ failure assessment | 2 (1–2) | 2 (1–3) |
| SpO2 (%) | 93.0 ± 2.3 (N = 19) | 93.8 ± 2.9 (N = 20) |
| O2 flow (LPM) | 3.9 ± 2.0 (N = 14) | 3.8 ± 2.4 (N = 19) |
| Systolic blood pressure (mmHg) | 125.1 ± 16.8 | 124.0 ± 15.7 |
| Diastolic blood pressure (mmHg) | 76.7 ± 11.8 | 73.6 ± 9.3 |
| Heart rate (beats/min) | 84.4 ± 13.7 | 79.8 ± 11.5 |
| Temperature (°C) | 36.9 ± 0.9 | 36.7 ± 0.8 |
| Respiratory rate (breaths/min) | 20.8 ± 3.1 | 21.6 ± 5.8 |
| CXR opacities | ||
| Bilateral | 19 (95%) | 19 (95%) |
| Unilateral | 1 (5%) | 1 (5%) |
| Treatment with corticosteroids | ||
| Yes | 19 (95%) | 18 (90%) |
| No | 1 (5%) | 2 (10%) |
| Use of remdesivir prior to enrollment | ||
| Yes | 11 (55%) | 12 (60%) |
| No | 9 (45%) | 8 (40%) |
| Study site | ||
| University of Washington- Harborview | 5 (25%) | 5 (25%) |
| University of Washington- Montlake | 0 (0%) | 4 (20%) |
| Swedish Medical Center | 2 (10%) | 2 (10%) |
| Virginia Mason Medical Center | 2 (10%) | 0 (0%) |
| Sarasota Memorial Healthcare System | 11 (55%) | 9 (45%) |
Mean ± SD or Median (IQR) presented for continuous variables.
Frequency (proportion) presented for categorical variables.
NIH Ordinal Scale: 1) Not hospitalized, no limitations; 2) Not hospitalized, limitation on activities and/or requiring home oxygen; 3) Hospitalized, not requiring supplemental oxygen—no longer requires ongoing medical care; 4) Hospitalized, not requiring supplemental oxygen—requiring ongoing medical care; 5) Hospitalized, requiring supplemental oxygen; 6) Hospitalized, on non-invasive ventilation or high-flow oxygen devices; 7) Hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); 8) Death.
Clinical efficacy
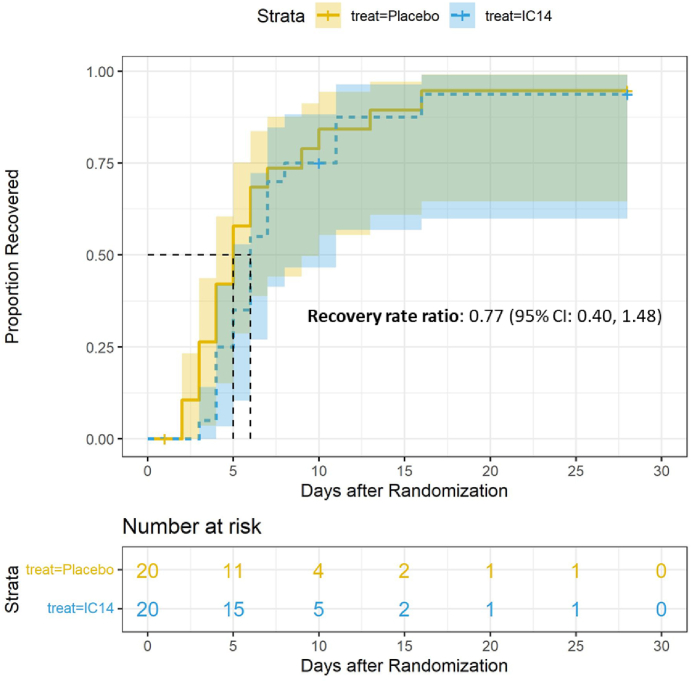
There was no statistically significant difference in time to recovery between groups (Fig. 2). The median time to recovery was 6 days (95% CI, 5–11 days (N = 20)) in the IC14 group vs. 5 days (95% CI, 4–10 days (N = 20)) in the Placebo group, and the recovery rate ratio was estimated to be 0.77 (95% CI is 0.40–1.48) (log-rank p = 0.435). Because of the small study size, analysis of the primary outcome was not stratified by baseline receipt of dexamethasone and groups of study sites. With regard to secondary endpoints, there were very few events of clinical progression (e.g. worsening of respiratory failure, initiation of mechanical ventilation, or death) (Table 2). Only one participant in each group progressed to mechanical ventilation, and each of these participants died. The change in ordinal scale between enrollment and day 14 and day 28 was not statistically different in the IC14 group as compared with the placebo group (Day 14: IC14: −3.1 ± 1.6 (N = 17) vs. placebo: −2.8 ± 1.6 (N = 18); mean difference: −0.23 (95% CI, −1.3, 0.84); Day 28: IC14: −3.7 ± 0.6 (N = 15) vs. placebo: −3.3 ± 1.6 (N = 16); mean difference: −0.35 (95% CI, −1.30, 0.35)) (Table 2).
Fig. 2.
Kaplan–Meier curve for time to clinical improvement in the patient population. Time to clinical improvement was defined as improving to Category 3 or less on the 8-point Ordinal Scale (OSS). Patients were censored for death at day 28.95% confidence intervals are provided around the Kaplan–Meier estimate. The recovery rate ratio and 95% confidence interval are displayed. Modified intention to treat population.
Table 2.
Analysis of secondary clinical efficacy outcomes.
| Placebo, N = 20 | IC14, N = 20 | Difference in mean or proportion (95% CI) | |
|---|---|---|---|
| Days alive and free of acute respiratory failure through day 28 | |||
| 0 days | 1 (5%) | 1 (5%) | |
| 24 days | 0 (0%) | 1 (5%) | |
| 27 days | 1 (5%) | 0 (0%) | |
| 28 days | 18 (90%) | 18 (90%) | 0.00 (−0.21 to 0.21) |
| Alive and free of acute respiratory failure through day 28 | |||
| Yes | 18 (90%) | 18 (90%) | 0.00 (−0.21 to 0.21) |
| No | 2 (10%) | 2 (10%) | |
| Change in the ordinal scale from baseline to days 14 | −2.8 ± 1.6 (N = 18) | −3.1 ± 1.6 (N = 17) | −0.23 (−1.30, 0.84) |
| Change in the ordinal scale from baseline to days 28 | −3.3 ± 1.6 (N = 16) | −3.7 ± 0.6 (N = 15) | −0.35 (−1.30, 0.35) |
| Ordinal scale value on day 14 | 2.0 ± 1.6 (N = 18) | 1.9 ± 1.6 (N = 17) | −0.06 (−1.10, 0.98) |
| All-cause mortality through days 28 | |||
| Alive | 20 (100%) | 19 (95%) | 0.50 (−0.12, 0.24) |
| Died | 0 (0%) | 1 (5%) | |
| All-cause mortality through days 60 | |||
| Alive | 19 (95%) | 19 (95%) | 0.00 (−0.19, 0.19) |
| Died | 1 (5%) | 1 (5%) | |
| Days alive and free of invasive mechanical ventilation through day 28 | |||
| 0 days | 1 (5%) | 1 (5) | |
| 28 days | 19 (95%) | 19 (95%) | 0.00 (−0.19, 0.19) |
| Alive and free of invasive mechanical ventilation through day 28 | |||
| Yes | 19 (95%) | 19 (95%) | 0.00 (−0.19, 0.19) |
| No | 1 (5%) | 1 (5%) | |
| Alive and discharged from the hospital through day 28 | |||
| Yes | 19 (95%) | 19 (95%) | 0.00 (−0.19, 0.19) |
| No | 1 (5%) | 1 (5%) | |
| Begin corticosteroid therapy for worsening COVID-19 illness after randomization | |||
| Yes | 1 (5%) | 1 (5%) | |
| No | 19 (95%) | 19 (95%) | 0.00 (−0.19, 0.19) |
Modified intention to treat population.
Mean ± SD is presented for continuous variables.
Frequency (proportion) is presented for categorical variables.
95% confidence intervals are constructed using a bootstrap approach for continuous variables and the Wilson score method for categorical variables.
Safety
The AEs were balanced between treatment groups (Table 3). Only two AESI occurred in the IC14 group. Neither was severe and neither was judged to be “related” to the study drug (Table 3 and Supplemental Table S4). There were no secondary infections in the IC14 group (Table 3). The incidence of all AEs through day 60 was similar in both treatment groups (IC14: 17 participants with 39 AEs vs. placebo: 13 participants with 44 AEs). All AEs occurred after randomization and on or after the date of study drug initiation. One participant in each group had a fatal, grade 5, AE; both died of worsening respiratory failure (Table 3). In the placebo group, the fatal AE was thought by the investigator to be “possibly related” to the study treatment, and in the IC14 group the fatal AE was thought to be “not related” to the study treatment (Supplemental Table S5). There was no difference in nasopharyngeal SARS-CoV-2 viral load over time between the IC14 and placebo groups (Supplemental Table S6).
Table 3.
Summary of Adverse Events through day 60.
| Placebo (N = 20) |
IC14 (N = 20) |
Difference in proportions (95% CI) | |||
|---|---|---|---|---|---|
| # Participants | # AEs | # Participants | # AEs | ||
| Cumulative adverse events by severity grade | |||||
| Toxicity Grade | |||||
| Mild (Grade 1) | 10 | 28 | 12 | 21 | 0.10 (−0.19, 0.37) |
| Moderate (Grade 2) | 2 | 4 | 4 | 4 | 0.10 (−0.13, 0.33) |
| Severe (Grade 3) | 5 | 10 | 9 | 12 | 0.20 (−0.09, 0.45) |
| Life threatening (Grade 4) | 1 | 1 | 1 | 1 | 0.00 (−0.19, 0.19) |
| Fatal (Grade 5) | 1 | 1 | 1 | 1 | 0.00 (−0.19, 0.19) |
| Total | 13 | 44 | 17 | 39 | 0.29 (−0.07, 0.44) |
| Cumulative adverse events of special interest (AESI) | |||||
| Eye disorders | |||||
| Eye pain | 0 | 0 | 1 | 1 | 0.05 (−0.12, 0.24) |
| Infections and infestations | |||||
| Epstein–Barr virus infection | 1 | 1 | 0 | 0 | −0.05 (−0.24, 0.12) |
| Perianal abscess | 1 | 1 | 0 | 0 | −0.05 (−0.24, 0.12) |
| Investigations | |||||
| Sputum abnormal | 0 | 0 | 1 | 1 | 0.05 (−0.12, 0.24) |
| Skin and subcutaneous tissue disorders | |||||
| Localized maculopapular rash | 1 | 2 | 0 | 0 | −0.05 (−0.24, 0.12) |
| Thrombocytopenia or serious bleeding | 0 | 0 | 0 | 0 | 0.00 (−0.16, 0.16) |
| Hepatobiliary disorders | 0 | 0 | 0 | 0 | 0.00 (−0.16, 0.16) |
| Acute renal failure | 0 | 0 | 0 | 0 | 0.00 (−0.16, 0.16) |
| Total | 2 | 4 | 2 | 2 | 0.00 (−0.21, 0.21) |
Modified intention to treat population.
Differences in proportions of participants experiencing at least 1 AE are presented.
95% CI is constructed using Wilson score method.
Exploratory biomarkers
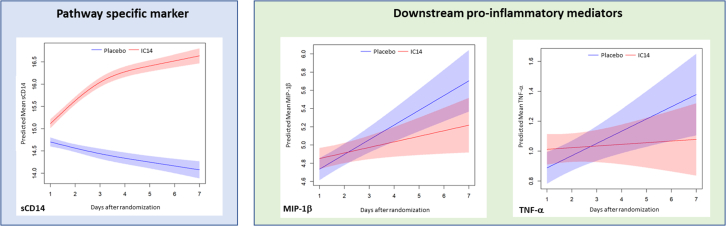
Exploratory serum biomarkers by trial day and treatment arm are summarized in Supplemental Table S7. First, we evaluated the effects of IC14 on CD14 pathway-specific markers. IC14 treatment caused a marked increase in the concentration trajectory of sCD14 consistent with the known effect of the IC14 antibody binding to circulating sCD14 (Fig. 3 and Supplemental Table S8). IC14 treatment did not cause a statistically significant change in the trajectory of the serum presepsin concentration (Supplemental Table S8).
Fig. 3.
IC14 effects on the serum concentration trajectories of serum sCD14, a pathway specific marker, and TNF-α and MIP-1β, both pro-inflammatory innate immune mediators. Graphs representative of IC14 effects on biomarkers measured in serum on days 1–5 & 7 adjusting for baseline levels using mixed effects modelling for repeated measures with a time by treatment interaction term. The curves display the predicted means of natural log transformed biomarker concentrations in picograms per milliliter (ln pg/mL) and 95% CIs. Biomarkers with differences in concentration trajectories over time were selected using a p-value threshold of <0.05 for the treatment by time interaction term. All treated patients with available biomarker measurements were included in these analyses (IC14 N = 19; Placebo N = 18). Biomarkers that are not displayed because they did not meet the criterion of p ≤ 0.05 for the treatment by time interaction term included: presepsin, IL-6, MCP-1, eotaxin, MCP-4, MDC, TARC, IFN-γ, IP-10, IL-10, and IL-8.
Next, we evaluated the effects of IC14 on downstream pro-inflammatory mediators and anti-viral mediators. IC14 was associated with a statistically significant reduction in the trajectories of serum TNF-α and MIP-1β (Fig. 3, Supplemental Table S8). IC14 did not have statistically significant effects on the serum concentration trajectories of either IL-6 or MCP-1 (Supplemental Table S8). We performed a sensitivity analysis removing those participants treated with tocilizumab (n = 3) given its known effects on circulating inflammatory biomarkers such as IL-6 and found that the effects of IC14 on MIP-1β and TNF-α remained (Supplemental Fig. S2 and Table S9). Additionally, in this sensitivity analysis, we observed a trend suggesting IC14 attenuated the trajectories of presepsin and MCP-1 (Supplemental Fig. S2 and Table S9). No statistically significant effects of IC14 on anti-viral mediators were seen.
We then conducted a post-hoc analysis testing whether the effects of IC14 on circulating biomarker concentrations might be different between groups with high or low supplemental oxygen needs at baseline as dichotomized by median baseline oxygen flow (Supplemental Fig. S3 and Table S10). Within each stratum, O2 flow rates were comparable between treatment groups (Supplemental Table S11). Amongst participants with higher baseline O2 flow rates, treatment with IC14 was associated with an increase in the trajectory of sCD14, a decrease in the trajectory of presepsin, and a decrease in the trajectory of TNF-α and MIP-1β. We also found that among participants with high pretreatment O2 flow rates, treatment with IC14 was associated with a decrease in the trajectory of IP-10, a relationship not observed for anti-viral mediators in the trial population at large. Amongst participants with low O2 flow rates, IC14 treatment was not associated with a change in the trajectory of presepsin or pro-inflammatory mediators. However, treatment with IC14 was associated with an increase in the trajectory of IP-10 in this group.
Discussion
The CaTT trial was designed to test whether CD14-blockade with the chimeric monoclonal antibody IC14 would reduce the severity of illness and markers of systemic inflammation in COVID-19 pneumonia. The trial was stopped early after randomization and treatment of 40 participants because of slow recruitment. This was well below the intended trial size of 300, so no conclusions can be drawn regarding clinical efficacy. IC14 did not improve time to resolution of illness in hospitalized, hypoxemic patients with COVID-19, however, inferences are limited by lack of statistical power. In exploratory biomarker analyses, there was evidence of relevant biological effects. Notably, IC14 had a predictable effect on the circulating concentration of sCD14, and IC14 appeared to attenuate the circulating concentrations of TNF-α and MIP-1β, two pro-inflammatory mediators associated with the severity of COVID-19 illness. Taken together, the results suggest that IC14 treatment may reduce deleterious systemic inflammation in COVID-19 pneumonia.
Although an innate immune response is appropriate in the setting of infection and/or damaged tissue, overly exuberant, persistent and dysregulated innate immune signaling may have a role in the pathogenesis of lung dysfunction in COVID-19 pneumonia as well as lung injury from a variety of other primary stimuli.9,10 In COVID-19 and other forms of lung injury, DAMPs elaborated in response to tissue injury may be the primary drivers of on-going innate immune activation.44,45 The putative mechanism of action of IC14 is to block interactions between CD14 and circulating PAMPs and DAMPs thereby reducing innate immune activation.10 In this study, the observed attenuation of serum MIP-1β and TNF-α, with IC14 treatment is consistent with these proposed effects (Fig. 3 and Supplemental Table S8). There were no statistically significant effects of IC14 on the concentration trajectories of either IL-6 or MCP-1, although high variance of these biomarkers may have limited the ability to detect differences (Supplemental Table S8).
Overall, the patients enrolled in this trial were only moderately ill and experienced low rates of progression to mechanical ventilation and death. Indeed, the levels of circulating inflammatory mediators in the CaTT trial were globally lower than those seen in patients requiring higher levels of respiratory support.23,46 Prior studies in COVID-19 pneumonia suggest that anti-inflammatory therapies such as dexamethasone and baricitinib are more likely to show benefit in patients with more severe respiratory disease. We conducted a post-hoc analysis stratified by higher vs. lower baseline oxygen flow rates in order to explore potential differences in the association between IC14 treatment and biomarker trends. Although the sample size was small, the effects of IC14 treatment on inflammatory mediators were more pronounced among participants who required higher oxygen flow rates at baseline. In patients with higher pre-treatment O2 flow rates, IC14 treatment attenuated more inflammatory mediators, including TNF-α, MIP-1β, and IP-10, as well as presepsin, a CD14 pathway-specific marker (Supplemental Fig. S3 and Table S10). In contrast, in patients with lower pre-treatment O2 flow rates, IC14 was not associated with attenuation of pro-inflammatory mediators or presepsin, and IC14 was associated with a relatively higher IP-10 trajectory. The attenuation of IP-10 associated with IC14 in the high oxygen groups suggests a potentially beneficial biologic effect that may be reversed when oxygen needs are lower. However, this interpretation is made with caution and future work will need to clarify how IC14 affects IP-10 levels. Presepsin is an N-terminal fragment of full length CD14 that is generated in the setting of CD14-dependent innate immune activation.21 We and others have shown strong associations between high circulating levels of presepsin and clinical outcomes (e.g. death and ventilator-free days) in severe COVID-19 and in non-COVID-19 respiratory failure.23, 24, 25 These findings suggest that the beneficial effects of IC14 on COVID-19-induced CD14 activation and inflammation may be most prominent in patients with more severe lung injury. Additional support for this assertion comes from a recently-reported multi-center phase II platform trial of treatments for COVID-19 that included an arm testing IC14.47 The IC14 arm was larger than CaTT (IC14 n = 67, concurrent controls n = 76) and enrolled patients that were more severely hypoxemic. While the improvement in recovery did not reach the pre-specified threshold for efficacy, there was a trend towards improved mortality (HR for death; 0.86 (95% CI 0.48–1.51). While conclusions based on these post-hoc, secondary, and exploratory analyses are tentative, taken together they suggest that future trials of IC14 should include populations with more severe lung injury who are likely to have a higher degree of CD14-attributable immunopathology.
The adverse event profiles were similar in the IC14 and placebo groups. Because CD14 is proximally positioned in the host response to pathogens, there is a theoretical concern that IC14 treatment may increase the risk of new infections and/or delay resolution of secondary infections. However, there were no secondary infections in the IC14 group and there was no difference in SARS-CoV-2 viral clearance between groups as assessed by serial nasal RT-PCR. While we note that the sample size was very small and underpowered to detect infrequent adverse effects, the absence of secondary infections could reflect the fact that blockade of CD14 should not be globally immunosuppressive as it would not affect alternative host innate immune pathways that can initiate responses to infection (e.g. complement, RIG-1, RNA sensing TLRs).8,9,27
This trial has several limitations. Most importantly, the premature termination of the trial resulted in a small sample size that was underpowered to detect clinically meaningful differences in the primary endpoint of time to resolution of COVID-19 illness. Second, our enrollment criteria resulted in a trial population that had relatively mild respiratory disease and hypoxemia who rarely worsened, further hindering our ability to detect differences in the primary outcome and exploratory inflammatory responses. To facilitate recruitment in a future study, the inclusion criteria should be modified to include all patients with NIH respiratory ordinal score 6 or lower, (i.e. hospitalized, needing oxygen support, but not on mechanical ventilation). This would expand the enrolled population to include those on higher levels of supplemental oxygen, including those on high flow nasal oxygen and non-invasive ventilation. Third, because dexamethasone treatment became standard-of-care as the CaTT trial was being initiated, most participants were treated with dexamethasone which may have resulted in an overall reduction in inflammation and reduced the ability to detect differences between groups in circulating inflammatory biomarkers. Fourth, due to the small trial size, we were unable to describe the precise time at which IC14 maximally attenuated inflammatory mediators. Finally, the CaTT trial enrolled patients when the SARS-CoV-2 delta variant was predominant, potentially limiting generalizability.
Conclusions
Blockade of CD14 with the monoclonal antibody IC14 did not improve time to resolution of illness in hospitalized, hypoxemic patients with COVID-19, though inferences about clinical efficacy are limited by the small sample size and the high rate of clinical recovery. Pharmacodynamic results and observed differences in circulating inflammatory markers suggest that additional clinical investigation is warranted to determine whether IC14 might improve systemic inflammation and clinical outcomes in COVID-19 pneumonia.
Contributors
TRM, MWW, PMB, WHS, and CY contributed to the conception and design of the trial. EMB, UM, CM, DSM, PF, KV, EDM and FLM were involved in the acquisition of data. HN, TRM, MWW, PMB, FLM, WHS, and CY were involved in data analysis and interpretation. HN and MWW verified the underlying data. HN, TRM, MWW, PMB, FLM, EMB, UM, CM, DSM, JGD, JG, PF, KV, CY, WHS, and EDM contributed to the development of the paper and provided critical review. All authors approved the final version for submission.
Data sharing statement
De-identified participant data will be made available through ImmPort.org.
Declaration of interests
TRM reported: 1) receiving fees from Novartis Pharmaceuticals for the topic of COVID-19 related ARDS, 2) receiving fees from Quantum Leap Healthcare Collaborative as a member of the clinical safety monitoring committee for another trial in COVID, 3) providing unpaid consulting for Implicit Bioscience Ltd for use of the IC14 anti-CD14 monoclonal antibody in ARDS and COVID-19 illness. JGD reported receiving a fee from RMEI: Medical Education for the topic of severe COVID-19 management outside the submitted work. No other disclosures were reported.
Acknowledgements
The authors thank the trial site staff and the patients who volunteered to participate in the trial. Special thanks to Vanderbilt Coordinating Center staff: Christa Stoughton, Krista Vermillion, Sheri Dixon, JaMario Ayers, Jessica Collins, Jessica Marlin; National Institute of Allergy and Infectious Diseases staff: Tatyana Vaysman, Katherine Thompson; Immune Tolerance Network staff: Mary Roy; Central Lab at University of Washington staff: Sana W. Sakr PhD; Richard Ulevitch, PhD, (Scripps Research) for helpful scientific discussions; Research coordinators at the study sites including: Swedish Hospital System: Julie Wallick; Virginia Mason Medical Center: Kate Duran; Sarasota Memorial Healthcare System: Primary Research Coordinator Kaitlyn Meek, BSN and the Sarasota Memorial Research Institute; University of Washington: Carolyn Brager. This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) Immune Tolerance Network Grant #UM1AI109565.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104667.
Appendix ASupplementary data
References
- 1.RECOVERY Collaborative Group. Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The REMAP-CAP Investigators. Gordon A.C., Mouncey P.R., et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalil A.C., Patterson T.F., Mehta A.K., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criner G.J., Lang F.M., Gottlieb R.L., et al. Anti-granulocyte-macrophage colony-stimulating factor monoclonal antibody gimsilumab for COVID-19 pneumonia: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2022;205(11):1290–1299. doi: 10.1164/rccm.202108-1859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan M., Liu Y., Zhou R., et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadjadj J., Yatim N., Barnabei L., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Gioia M., Zanoni I. Toll-like receptor co-receptors as master regulators of the immune response. Mol Immunol. 2015;63(2):143–152. doi: 10.1016/j.molimm.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Mabrey F.L., Morrell E.D., Wurfel M.M. TLRs in COVID-19: how they drive immunopathology and the rationale for modulation. Innate Immun. 2021;27(7–8):503–513. doi: 10.1177/17534259211051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin T.R., Wurfel M.M., Zanoni I., Ulevitch R. Targeting innate immunity by blocking CD14: novel approach to control inflammation and organ dysfunction in COVID-19 illness. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z., Ren L., Zhang L., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z., Ren L., Zhang L., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhaskar S., Sinha A., Banach M., et al. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648. https://www.frontiersin.org/articles/10.3389/fimmu.2020.01648/full?report=reader [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen W., Su W., Tang H., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6(1):1–18. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang D., Guo R., Lei L., et al. Frontline science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol. 2021;109(1):13–22. doi: 10.1002/JLB.4HI0720-470R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Brabander J., Duijvelaar E., Schippers J.R., et al. Immunomodulation and endothelial barrier protection mediate the association between oral imatinib and mortality in hospitalised COVID-19 patients. Eur Respir J. 2022;60(6) doi: 10.1183/13993003.00780-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Brabander J., Michels E.H.A., van Linge C.C.A., et al. Association between dexamethasone treatment and the host response in COVID-19 patients admitted to the general ward. Respir Res. 2022;23(1):145. doi: 10.1186/s12931-022-02060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronte V., Ugel S., Tinazzi E., et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130(12):6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mabrey F.L., Bhatraju P.K., Morrell E.D., et al. Plasma interleukin-6 predicts clinical decline after completion of dexamethasone therapy in severe COVID-19. Crit Care Explor. 2022;4(12) doi: 10.1097/CCE.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai Y., Mizugishi K., Nonomura K., Naitoh K., Takaori-Kondo A., Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21(8):564–569. doi: 10.1016/j.jiac.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Bowman E.R., Cameron C.M.A., Avery A., et al. Levels of soluble CD14 and tumor necrosis factor receptors 1 and 2 may be predictive of death in severe coronavirus disease 2019 (COVID-19) J Infect Dis. 2020;223(5):805–810. doi: 10.1093/infdis/jiaa744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabrey F.L., Morrell E.D., Bhatraju P.K., et al. Plasma soluble CD14 subtype levels are associated with clinical outcomes in critically ill subjects with coronavirus disease 2019. Crit Care Explor. 2021;3(12) doi: 10.1097/CCE.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukada A., Kitagawa Y., Matsuoka M., et al. Presepsin as a predictive biomarker of severity in COVID-19: a case series. J Med Virol. 2021;93(1):99–101. doi: 10.1002/jmv.26164. https://onlinelibrary.wiley.com/doi/abs/10.1002/jmv.26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaninotto M., Mion M.M., Cosma C., Rinaldi D., Plebani M. Presepsin in risk stratification of SARS-CoV-2 patients. Clin Chim Acta. 2020;507:161–163. doi: 10.1016/j.cca.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messner C.B., Demichev V., Wendisch D., et al. Clinical classifiers of COVID-19 infection from novel ultra-high-throughput proteomics. Infectious Dis (except HIV/AIDS) 2020 doi: 10.1016/j.cels.2020.05.012. http://medrxiv.org/lookup/doi/10.1101/2020.04.27.20081810 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axtelle T., Pribble J. An overview of clinical studies in healthy subjects and patients with severe sepsis with IC14, a CD14-specific chimeric monoclonal antibody. J Endotoxin Res. 2003;9(6):385–389. doi: 10.1179/096805103225003321. [DOI] [PubMed] [Google Scholar]
- 28.Imai Y., Kuba K., Neely G.G., et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurt-Jones E.A., Popova L., Kwinn L., et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1(5):398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 30.Pauligk C., Nain M., Reiling N., Gemsa D., Kaufmann A. CD14 is required for influenza A virus-induced cytokine and chemokine production. Immunobiology. 2004;209(1–2):3–10. doi: 10.1016/j.imbio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Common terminology criteria for adverse events (CTCAE) Vol. 147. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017. [Google Scholar]
- 33.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik P., Patel U., Mehta D., et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26(3):107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venet F., Cour M., Rimmelé T., et al. Longitudinal assessment of IFN-I activity and immune profile in critically ill COVID-19 patients with acute respiratory distress syndrome. Crit Care. 2021;25:140. doi: 10.1186/s13054-021-03558-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon S., Crowley G., Liu M., Nolan A. Biomarkers of COVID-19, a longitudinal and retrospective assessment of a NYC 1st wave cohort. Am J Respir Crit Care Med. 2021;203 doi: 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2626. [DOI] [Google Scholar]
- 38.Del Valle D.M., Kim-Schulze S., Huang H.H., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bivona G., Agnello L., Ciaccio M. Biomarkers for prognosis and treatment response in COVID-19 patients. Ann Lab Med. 2021;41(6):540–548. doi: 10.3343/alm.2021.41.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwamura A.P.D., Tavares da Silva M.R., Hümmelgen A.L., et al. Immunity and inflammatory biomarkers in COVID-19: a systematic review. Rev Med Virol. 2021;31(4) doi: 10.1002/rmv.2199. [DOI] [PubMed] [Google Scholar]
- 41.Berzuini C., Hannan C., King A., et al. Value of dynamic clinical and biomarker data for mortality risk prediction in COVID-19: a multicentre retrospective cohort study. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-041983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenfeld D.A. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. [PubMed] [Google Scholar]
- 43.Koletsi D., Pandis N. Survival analysis, part 2: Kaplan-Meier method and the log-rank test. Am J Orthod Dentofacial Orthop. 2017;152(4):569–571. doi: 10.1016/j.ajodo.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland K.R., Steinberg K.P., Maunder R.J., Milberg J.A., Allen D.L., Hudson L.D. Pulmonary infection during the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152(2):550–556. doi: 10.1164/ajrccm.152.2.7633706. [DOI] [PubMed] [Google Scholar]
- 45.Brégeon F., Papazian L., Delpierre S., et al. Role of proinflammatory activity contained in gastric juice from intensive care unit patients to induce lung injury in a rabbit aspiration model. Crit Care Med. 2008;36(12):3205–3212. doi: 10.1097/CCM.0b013e31818f0e20. https://pubmed.ncbi.nlm.nih.gov/18936704/ [DOI] [PubMed] [Google Scholar]
- 46.Bhatraju P.K., Morrell E.D., Zelnick L., et al. Comparison of host endothelial, epithelial and inflammatory response in ICU patients with and without COVID-19: a prospective observational cohort study. Crit Care. 2021;25(1):148. doi: 10.1186/s13054-021-03547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.I-SPY COVID Consortium Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial. eClinicalMedicine. 2023;58 doi: 10.1016/j.eclinm.2023.101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.