Abstract
Objectives
Carbapenem-resistant Klebsiella pneumoniae (CRKP) infection/colonisation has been reported in hospitals. The clinical characteristics of CRKP infection/colonisation in the intensive care unit (ICU) have received little attention. This study aims to investigate the epidemiology and extent of K. pneumoniae (KP) resistance to carbapenems, the sources of CRKP patients and CRKP isolates, and the risk factors for CRKP infection/colonisation.
Design
Retrospective single-centre study.
Data source
Clinical data were obtained from electronic medical records.
Participants
Patients isolated with KP in the ICU from January 2012 to December 2020.
Main outcome measures
The prevalence and changing trend of CRKP were determined. The extent of KP isolates resistance to carbapenems, the specimen types of KP isolates, and the sources of CRKP patients and CRKP isolates were all examined. The risk factors for CRKP infection/colonisation were also assessed.
Results
The rate of CRKP in KP isolates raised from 11.11% in 2012 to 48.92% in 2020. CRKP isolates were detected in one site in 266 patients (70.56%). The percentage of CRKP isolates not susceptible to imipenem increased from 42.86% in 2012 to 98.53% in 2020. The percentage of CRKP patients from general wards in our hospital and other hospitals gradually converged in 2020 (47.06% vs 52.94%). CRKP isolates were mainly acquired in our ICU (59.68%). Younger age (p=0.018), previous admission (p=0.018), previous ICU stay (p=0.008), prior use of surgical drainage (p=0.012) and gastric tube (p=0.001), and use of carbapenems (p=0.000), tigecycline (p=0.005), β-lactams/β-lactamase inhibitors (p=0.000), fluoroquinolones (p=0.033), and antifungal drugs (p=0.011) within the prior 3 months were independent risk factors for CRKP infection/colonisation.
Conclusions
Overall, the rate of KP isolates resistance to carbapenems increased, and the severity of this resistance significantly increased. Intensive and local infection/colonisation control measures are necessary for ICU patients, especially those with risk factors for CRKP infection/colonisation.
Keywords: epidemiology, adult intensive & critical care, infection control, microbiology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the largest study to explore the clinical characteristics of carbapenem-resistant Klebsiella pneumoniae (CRKP) infection/colonisation in the intensive care unit (ICU).
This study takes into account the extent of K. pneumoniae isolates resistance to carbapenems, as well as the sources of CRKP patients and CRKP isolates in the ICU.
This study spans a long period of 9 years.
The generalisation of our findings to specialised hospitals requires further assessment.
Some information is not available in the electronic medical records, which may have potential effects on the results.
Introduction
Klebsiella pneumoniae (KP) is a gram-negative pathogen that commonly causes nosocomial infections. With the widespread and unreasonable use of antibiotics, particularly carbapenems, the prevalence of carbapenem-resistant K. pneumoniae (CRKP) has increased. CRKP strains have been reported from sporadic cases in the first few years, then endemic outbreaks have been observed.1 2 Now carbapenem resistance has occurred in many countries, and become a worldwide problem.3–5 The China Antimicrobial Surveillance Network (CHINET) has reported that the resistance rates of K. pneumoniae to imipenem have increased progressively from 3.0% in 2005 to 25% in 2018, and meropenem was 2.9% in 2005 and 26.3% in 2018.6 Moreover, the resistance rates vary considerably among regions, hospitals and wards.7 8
Carbapenem-resistant pathogens impose difficulties in selecting the appropriate antimicrobial therapy.9 In the intensive care unit (ICU), CRKP carriers are particularly limited in therapeutic options. Thus, CRKP may evolve to cause considerable clinical problems, including the risk of high mortality, prolonged hospital stay, and heavy economic burden.10–12 In our ICU, we encounter similar issues. K. pneumoniae is one of the most common bacteria detected, with drug-resistant strains prevailing. Consequently, we are interested in monitoring the occurrence and developments of CRKP in our ICU.
For residents of long-term acute care hospitals, high levels of CRKP colonisation pressure increased the risk for horizontal transmission.13 CRKP isolates may contaminate the environment, as well as hands, gloves or gowns of ICU staffs, and then spread among the environment, ICU staff and patients.14 In addition, ICU patients are transferred from general wards or other hospitals, and discharged to different locations, which further facilitates the transmission of pathogens. Therefore, patients admitted to the ICU have an increased risk of exposure to multidrug-resistant bacteria, including CRKP.15
In recent years, studies have reported the epidemiology, risk factors and outcomes of CRKP bloodstream infections in the ICU16 17 and hospitals.18–20 Furthermore, various studies have been presented on CRKP infections in the ICU and hospitals,21 22 CRKP colonisation in the ICU23 and CRKP infection/colonisation in the hospital.24 25 Considering the epidemiological and clinical challenges of CRKP isolates in the ICU, it is imperative to monitor CRKP infection and colonisation in the ICU. Moreover, CRKP bloodstream infections have been widely reported, and CRKP infection/colonisation of other sites also need attention.26 Therefore, studies of CRKP infection and colonisation with all specimen types in the ICU are urgently needed. In addition, there are limited data available on the extent of KP isolates resistance to carbapenems, as well as the sources of CRKP patients and CRKP isolates in the ICU.
Therefore, we performed a study to investigate the clinical characteristics of CRKP infection/colonisation in the ICU from 2012 to 2020, including epidemiology, sources of CRKP patients and CRKP isolates, and risk factors for the development of CRKP in KP patients.
Methods
Study design and population
This was a retrospective cohort study in the ICU of Xiangya Hospital, a teaching hospital with 3600 beds in Changsha, China. Subjects were patients with positive KP clinical culture and antimicrobial susceptibility testing during hospitalisation in the ICU from January 2012 to December 2020. Only the first isolate was included in the study, and duplicate isolates of the same patient were not considered.
Clinical data collection and definitions
Patient data were collected via electronic medical records and included the following: demographics (age, sex, bed occupied, admission and discharge dates), comorbidities (hypertension, diabetes, coronary artery disease, hepatitis/cirrhosis, chronic renal insuffciency, malignancy, cerebrovascular disease), previous admission (the ward/hospital where the patient was admitted before this hospitalisation in the ICU), recent events (prior surgery, previous ICU stay), recent invasive procedures (tracheostomy tube, surgical drainage, indwelled central venous catheter, gastric tube, urinary catheter), antibiotic administration 3 months prior to KP isolation (carbapenems, tigecycline, glycopeptides, β-lactams/β-lactamase inhibitors, third-generation/fourth-generation cephalosporins, fluoroquinolones, aminoglycosides, antifungal drugs), microbiological data (specimen types and monitoring time, the antibiotic susceptibility results).
In our hospital, KP isolates were tested for their susceptibility to carbapenems (ertapenem/imipenem/meropenem) and other antimicrobials by bioMerieux VITEK-2 (bioMerieux).27 The MIC was interpreted according to the Clinical and Laboratory Standards Institute breakpoints. Carbapenem-susceptible K. pneumoniae (CSKP) was identified when MIC was ≤1 mg/L for imipenem, meropenem and ≤0.5 mg/L for ertapenem. KP isolates that tested intermediate or resistant to one or more carbapenems were considered as carbapenem-resistant.28 If the first isolate of KP was detected within 48 hours or before admission to the ICU, it was considered as community-acquired or input-acquired based on their previous admission; while if it was detected after 48 hours of ICU hospitalisation, it was considered as ICU acquired.
Statistical analysis
Statistical descriptions were conducted to describe the characteristics of the study population. Continuous variables were described as mean±SD. Univariate logistic regression was conducted to identify the potential factors. Variates were chosen based on previous studies and the professional experience. The ORs and the 95% CIs of each variable were calculated, and variables with a p<0.05 were included in multivariate logistic regression. The multivariate logistic regression used the backward method. A p<0.05 was considered statistically significant. All analyses were carried out by SPSS software (V.22.0, SPSS).
Patient and public involvement
Patients and the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
Epidemiology
From January 2012 to December 2020, a total of 880 unique patients in the ICU were separated with KP isolates (infected or colonised). A total of 377 (42.50%) patients were identified clinical culture with CRKP, while 503 (57.50%) patients with CSKP. The separate rate of CRKP in KP isolates from 2012 to 2020 is shown in online supplemental table A1. CRKP rate was low in 2012 (11.11%) and 2013 (13.04%), but after that, the rate increased rapidly to peak in 2017 (64.35%), then decreased and stabilised at 48.92% in 2020.
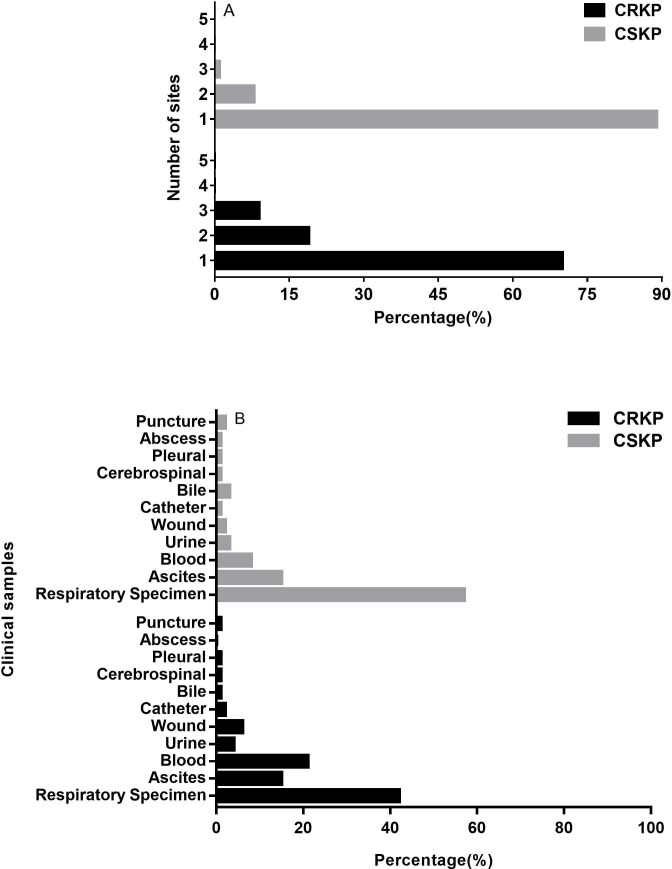
In our study, KP isolates were obtained from various specimen types. 266 (70.56%) CRKP isolates were detected from one site, while 450 (89.46%) CSKP isolates were detected from one site. CRKP isolates could be detected from 2 to 5 sites (19.1%, 9.28%, 0.8% and 0.27%, respectively), and CSKP isolates could be detected from 2 to 4 sites (8.55%, 1.59% and 0.4%, respectively) (figure 1A). In addition, CRKP isolates were mainly isolated from respiratory tract specimens (42.67%), followed by blood (21.05%), and ascites (15.79%). CSKP isolates mainly isolated from respiratory tract specimens (57.22%), followed by ascites (15.32%), blood (8.98%) (figure 1B).
Figure 1.
The number (A) and distribution (B) of Klebsiella pneumoniae isolated sites. CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-susceptible Klebsiella pneumoniae.
Carbapenems non-susceptibility profiles of CRKP isolates
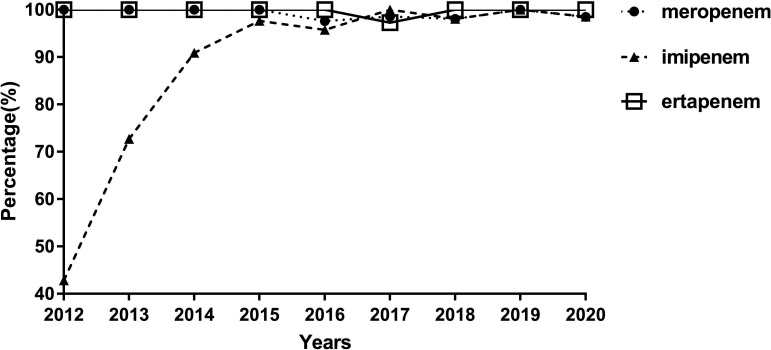
In our hospital, all KP isolates were tested for their susceptibility to at least one carbapenem, and we analysed the resistance of each KP strain against ertapenem, imipenem and meropenem. During the study period of 2012–2020, the rate of CRKP isolates tested not susceptible to imipenem increased from 42.86% to 98.53%, and the non-susceptible rates to meropenem and ertapenem remained steadily close to or reached 100%. Since 2017, the rates of CRKP isolates non-susceptible to meropenem, imipenem, and ertapenem have all approached or reached 100% (figure 2).
Figure 2.
Trends in the prevalence of Klebsiella pneumoniae isolates non-susceptible to imipenem, meropenem and ertapenem.
The sources of KP patients and KP isolates
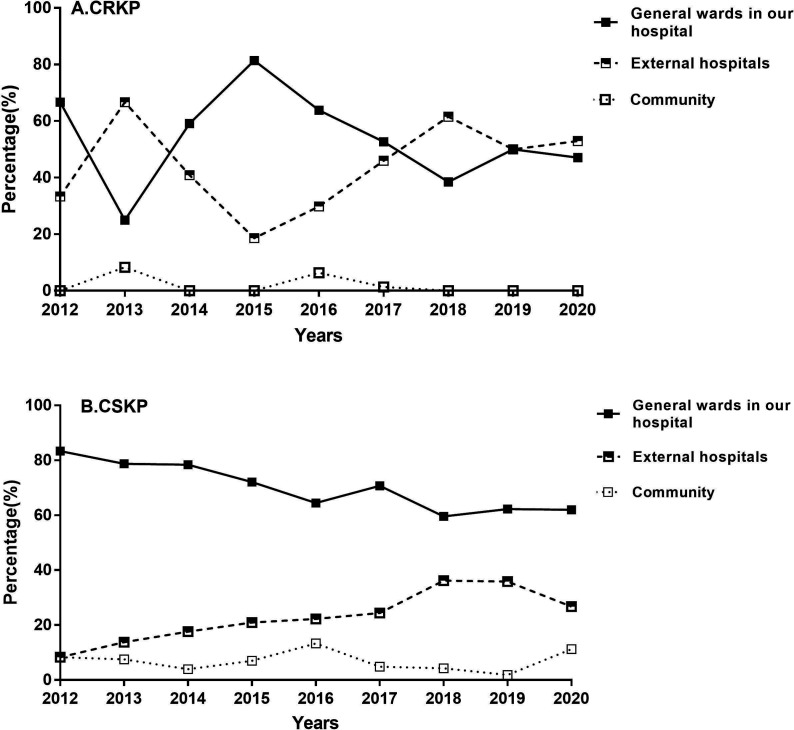
ICU patients were generally transferred from the community, other hospitals or general wards in our hospital. The patients in our study were isolated with KP isolates, and the distribution of patient sources is displayed in figure 3. For CRKP group, patients were mainly from general wards in our hospital and other hospitals (91.67%–100%). Notably, the number of patients from other hospitals and general wards in our hospital went up and down alternately, and gradually converged in the recent 2 years. For CSKP group, patients were mainly from general wards in our hospital (59.57%–83.33%), but the trend was downward.
Figure 3.
Changing trends in the sources of patients with Klebsiella pneumoniae isolates. CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-susceptible Klebsiella pneumoniae.
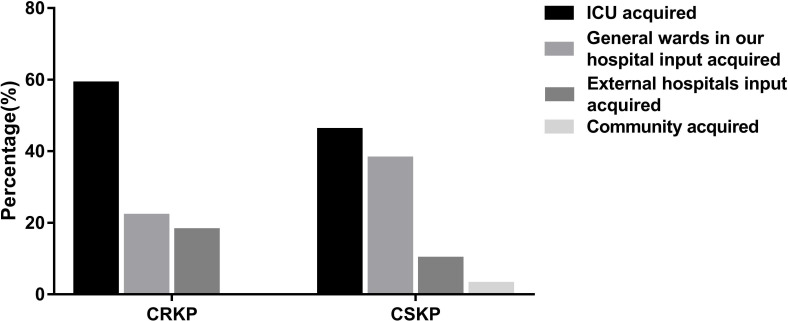
The sources of KP isolates were summarised, including the community input, other hospitals input, general wards of our hospital input and ICU acquired. Sources of CRKP and CSKP isolates are shown in figure 4. For CRKP carriers, CRKP isolates were mainly acquired in our ICU (59.68%), followed by general wards in our hospital (22.02%) and other hospitals (18.30%), and there were no CRKP isolates acquired from the community. For CSKP carriers, CSKP isolates most often originated in the ICU (46.72%) and general wards of our hospital (38.97%), and less frequently in other hospitals (10.93%) and community (3.38%).
Figure 4.
Distribution of the sources of Klebsiella pneumoniae isolates. CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-susceptible Klebsiella pneumoniae; ICU, intensive care unit.
Risk factors for CRKP infection/colonisation
A comparison of baseline characteristics between CRKP and CSKP patients is shown in table 1. In bivariate analysis, there were significant differences in age, previous admission, previous ICU stay and prior use of tracheostomy tube, surgical drainage, indwelled central venous catheter, gastric tube and exposure to carbapenems, tigecycline, glycopeptides, β-lactams/β-lactamase inhibitors, fluoroquinolones, aminoglycosides and antifungal drugs within the prior 3 months were significantly much more in CRKP group.
Table 1.
Clinical characteristics and risk factors for CRKP infection/colonisation
| CRKP (377) | CSKP (503) | Univariate analysis | Multivariate analysis | |||
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| General characteristics | ||||||
| Male sex | 294 (77.98) | 363 (72.17) | 1.37 (1.00 to 1.87) | 0.050 | ||
| Age (years) | 54.43±15.51 | 57.04±16.17 | 0.87 (0.79 to 0.96) | 0.005 | 0.87 (0.78 to 0.98) | 0.018 |
| Previous admission | ||||||
| No previous admission | 5 (1.33) | 36 (7.16) | 0.72 (0.57 to 0.90) | 0.004 | 0.73 (0.56 to 0.95) | 0.018 |
| Other hospitals | 169 (44.83) | 110 (21.87) | ||||
| Our hospital | 203 (53.85) | 357 (70.97) | ||||
| Recent events | ||||||
| Prior surgery | 214 (56.76) | 289 (57.46) | 0.97 (0.74 to 1.27) | 0.838 | ||
| Previous ICU stay | 111 (29.44) | 65 (12.92) | 2.81 (1.99 to 3.96) | 0.000 | 1.69 (1.15 to 2.47) | 0.008 |
| Invasive procedures | ||||||
| Tracheostomy tube | 154 (40.85) | 155 (30.82) | 1.55 (1.17 to 2.05) | 0.002 | ||
| Surgical drainage | 251 (66.58) | 285 (56.66) | 1.52 (1.16 to 2.01) | 0.003 | 1.50 (1.09 to 2.06) | 0.012 |
| Indwelled central venous catheter | 221 (58.62) | 221 (43.94) | 1.81 (1.38 to 2.37) | 0.000 | ||
| Gastric tube | 278 (73.74) | 288 (57.26) | 2.10 (1.57 to 2.80) | 0.000 | 1.73 (1.25 to 2.39) | 0.001 |
| Urinary catheter | 296 (78.51) | 380 (75.55) | 1.18 (0.86 to 1.63) | 0.302 | ||
| Comorbidities | ||||||
| Hypertension | 128 (33.95) | 155 (30.82) | 1.17 (0.88 to 1.56) | 0.283 | ||
| Diabetes | 60 (15.92) | 66 (13.12) | 1.25 (0.85 to 1.83) | 0.255 | ||
| Coronary artery disease | 39 (10.34) | 61 (12.13) | 0.82 (0.53 to 1.27) | 0.371 | ||
| Hepatitis/cirrhosis | 27 (7.16) | 24 (4.77) | 1.49 (0.82 to 2.71) | 0.187 | ||
| Chronic renal insuffciency | 10 (2.65) | 11 (2.19) | 1.34 (0.53 to 3.41) | 0.538 | ||
| Malignancy | 22 (5.84) | 31 (6.16) | 0.90 (0.51 to 1.59) | 0.719 | ||
| Cerebrovascular disease | 24 (6.37) | 19 (3.78) | 1.73 (0.93 to 3.21) | 0.081 | ||
| Antibiotics used within 3 months | ||||||
| Carbapenems | 250 (66.31) | 174 (34.59) | 3.72 (2.81 to 4.93) | 0.000 | 2.84 (2.07 to 3.89) | 0.000 |
| Tigecycline | 88 (23.34) | 31 (6.16) | 4.64 (3.00 to 7.16) | 0.000 | 2.01 (1.24 to 3.27) | 0.005 |
| Glycopeptides | 105 (27.85) | 95 (18.89) | 1.66 (1.21 to 2.28) | 0.002 | ||
| β-lactams/β-lactamase inhibitors | 277 (73.47) | 284 (56.46) | 2.14 (1.60 to 2.85) | 0.000 | 1.88 (1.35 to 2.63) | 0.000 |
| Third-generation/fourth-generation cephalosporins | 115 (30.5) | 149 (29.62) | 1.04 (0.78 to 1.40) | 0.778 | ||
| Fluoroquinolones | 96 (25.46) | 66 (13.12) | 2.26 (1.60 to 3.20) | 0.000 | 1.54 (1.04 to 2.29) | 0.033 |
| Aminoglycosides | 26 (6.9) | 17 (3.38) | 2.12 (1.13 to 3.96) | 0.019 | ||
| Antifungal drugs | 84 (22.28) | 36 (7.16) | 2.63 (1.84 to 3.76) | 0.000 | 1.66 (1.13 to 2.44) | 0.011 |
CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-susceptible Klebsiella pneumoniae; ICU, intensive care unit.
In the multivariate analysis, younger age (p=0.018), previous admission (p=0.018), previous ICU stay (p=0.008), prior use of surgical drainage (p=0.012) and gastric tube (p=0.001), and use of carbapenems (p=0.000), tigecycline (p=0.005), β-lactams/β-lactamase inhibitors (p=0.000), fluoroquinolones (p=0.033), and antifungal drugs (p=0.011) within the prior 3 months were risk factors significantly associated with CRKP infection/colonisation.
Discussion
In this study, we reported clinical characteristics of CRKP infection/colonisation over a period of 9 years. Many findings in our study were the first reported. This study observed a high incidence and growth trend of CRKP in KP isolates, and since 2017, the rates of CRKP isolates non-susceptible to meropenem, imipenem and ertapenem have all approached or reached 100%. An important finding of this study was that CRKP isolates were mainly acquired in the ICU rather than input acquired. This suggests that more attention should be given to identifying the possible routes and effective interventions for localised acquisition.
Despite the high prevalence of CRKP in the hospital, few studies explored changing trend of the resistance rate of K. pneumoniae to carbapenems in the ICU. We found a study from an ICU in Southern Italy that carbapenem resistant K. pneumoniae rates rose from 0% in 2008 to 59.2% in 2013.29 In China, multicentre data from the CHINET described that the resistance change of K. pneumoniae to meropenem increased from 2.9% in 2005 to 26.3% in 2018.6 Considering the trends in China and abroad, we predicted that the resistance rate of K. pneumoniae to carbapenems would increase in our ICU. As expected, we found an impressive increase in CRKP numbers and rates from 2012 to 2020. Specifically, the carbapenem resistance rate increased from 11.11% in 2012 to 64.35% in 2017, and then the resistance rate decreased and stabilised at 48.92% in 2020. It may be attributed to the implementation of antimicrobial stewardship in our hospital.
Koppe et al30 reported 99.9% of the K. pneumoniae isolates were tested against at least one carbapenem in hospitals in Germany. In our study, all K. pneumoniae isolates were tested against at least one carbapenem. We discovered that the rate of CRKP isolates that were not susceptible to imipenem increased significantly from 42.86% to 98.53%. Meanwhile the non-susceptible rates to meropenem and ertapenem remained steadily close to or reached 100%. The results clearly indicate the severity of KP resistance to carbapenems in the ICU, which poses a much more difficult challenge for the treatment of CRKP infections.
It is noteworthy that specimen types may be associated with CRKP and CSKP. Our findings have practical implication for predicting CRKP according to specimen types. When K. pneumoniae specimens were isolated from the bloodstream, urinary, wound and catheter tip, the rates of CRKP were higher than those of CSKP. Although bloodstream infection with CRKP was the most commonly reported, the number of respiratory specimen was higher than the total of other specimen types. This suggests that pulmonary infection/colonisation with K. pneumoniae should not be ignored.
For patients isolated with K. pneumoniae in the ICU, their sources were not reported before. We found that the incidence of CRKP patients from other hospitals had increased to 52.94%, exceeding the rate of patients from our hospital. The trend of CSKP patients from our hospital was downward. It indicated that antibiotic resistance control measures in our hospital were progressive. Special attention should be paid to patients who transferred to the ICU from other hospitals.
In the study, we found that CRKP isolates were mainly acquired in the ICU. It confirmed ICU admission was an important risk factor for acquiring CRKP. It demonstrated that CRKP isolates may be acquired through horizontal transmission during ICU hospitalisation. Previous studies have reported interventions to reduce transmission of carbapenem-resistant Enterobacteriaceae.31 32 Knowledge of local prevalence rate of CRKP and tailored surveillance actions based on local circumstance are crucial.
In our study, we observed that younger age, previous admission, previous ICU stay, prior use of surgical drainage and gastric tube, and use of carbapenems, tigecycline, β-lactams/β-lactamase inhibitors, fluoroquinolones, and antifungal drugs within the prior 3 months were risk factors for ICU KP patients isolated with CRKP. Interestingly, we found the median age of CRKP patients was younger than that of CSKP patients, which was consistent with the earlier studies.24 25 Although we cannot explain this finding, it is possible that it constitutes a risk factor, and further studies are required to confirm the finding. For invasive procedures, prior use of surgical drainage and gastric tube were not reported as independent risk factors before. This may be due to the different types of patients in these studies or differences in sample size. Antimicrobial exposure was reported as a potential risk factor for CRKP infection/colonisation. Although antibiotics identified in these studies and antimicrobial exposure time vary,13 24 25 local and practical antibiotic stewardship measures are necessary.
Our study has several limitations. First, our hospital is a tertiary hospital that admits a variety of critically ill patients, and the sources of patients and other epidemiology data may not be suitable for specialised hospitals. Second, as a retrospective analysis, clinical data were obtained from electronic medical records, and missing information may have potential effects on the results. Nevertheless, the sample size of our study was not small, and did not hamper the statistical power of our analysis.
Conclusions
Overall, the rate of KP isolates resistance to carbapenems increased, and the severity of this resistance significantly increased. Intensive and local infection/colonisation control measures are necessary for ICU patients, especially those with risk factors for CRKP infection/colonisation.
bmjopen-2022-065786supp001.pdf (25.4KB, pdf)
Supplementary Material
Acknowledgments
The authors thank all of the study participants.
Footnotes
Contributors: PW and TY contributed to the concept and design of the study. XZ managed the data collection. PW and BZ analysed the data. PW wrote the initial draft. FW submitted and revised the article. PW acts as guarantor for the final manuscript. All authors read and approved the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Ethics Committee of Xiangya Hospital Central South University (2018091076).
References
- 1.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of Carbapenems. Antimicrob Agents Chemother 2014;58:2322–8. 10.1128/AAC.02166-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cristina ML, Alicino C, Sartini M, et al. Epidemiology, management, and outcome of Carbapenem-resistant Klebsiella pneumoniae bloodstream infections in hospitals within the same Endemic metropolitan area. J Infect Public Health 2018;11:171–7. 10.1016/j.jiph.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 3.Mularoni A, Martucci G, Douradinha B, et al. Epidemiology and successful containment of a Carbapenem-resistant Enterobacteriaceae outbreak in a Southern Italian transplant Institute. Transpl Infect Dis 2019;21:e13119. 10.1111/tid.13119 [DOI] [PubMed] [Google Scholar]
- 4.Lee C-R, Lee JH, Park KS, et al. Global dissemination of Carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol 2016;7:895. 10.3389/fmicb.2016.00895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducomble T, Faucheux S, Helbig U, et al. Large hospital outbreak of KPC-2-producing Klebsiella pneumoniae: investigating mortality and the impact of screening for KPC-2 with polymerase chain reaction. J Hosp Infect 2015;89:179–85. 10.1016/j.jhin.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 6.Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis 2019;38:2275–81. 10.1007/s10096-019-03673-1 [DOI] [PubMed] [Google Scholar]
- 7.Hu F-P, Guo Y, Zhu D-M, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect 2016;22:S9–14. 10.1016/j.cmi.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Liu C, Shen Z, et al. Prevalence, risk factors and molecular epidemiology of Carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008-2018. Emerg Microbes Infect 2020;9:1771–9. 10.1080/22221751.2020.1799721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi Y. Treatment options for Carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis 2019;69:S565–75. 10.1093/cid/ciz830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed NK, Alkhawaja S, Azam NFAEM, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in a tertiary care center in the Kingdom of Bahrain. J Lab Physicians 2019;11:111–7. 10.4103/JLP.JLP_101_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-David D, Kordevani R, Keller N, et al. Outcome of Carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 2012;18:54–60. 10.1111/j.1469-0691.2011.03478.x [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Qiao F, Zhang Y, et al. In-hospital medical costs of infections caused by Carbapenem-resistant Klebsiella pneumoniae. Clin Infect Dis 2018;67:S225–30. 10.1093/cid/ciy642 [DOI] [PubMed] [Google Scholar]
- 13.Mills JP, Talati NJ, Alby K, et al. The epidemiology of Carbapenem-resistant Klebsiella pneumoniae Colonization and infection among long-term acute care hospital residents. Infect Control Hosp Epidemiol 2016;37:55–60. 10.1017/ice.2015.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Z, Zhou Y, Du M, et al. Prospective investigation of Carbapenem-resistant Klebsiella pneumonia transmission among the staff, environment and patients in five major intensive care units, Beijing. J Hosp Infect 2019;101:150–7. 10.1016/j.jhin.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 15.Mularoni A, Bertani A, Vizzini G, et al. Outcome of transplantation using organs from donors infected or colonized with carbapenem-resistant gram-negative bacteria. Am J Transplant 2015;15:2674–82. 10.1111/ajt.13317 [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Wang J-feng, Xu W-lan, et al. Clinical and molecular characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bloodstream infections in the intensive care unit. Antimicrob Resist Infect Control 2017;6:102. 10.1186/s13756-017-0256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadimitriou-Olivgeris M, Fligou F, Bartzavali C, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis 2017;36:1125–31. 10.1007/s10096-017-2899-6 [DOI] [PubMed] [Google Scholar]
- 18.Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control 2016;5:48. 10.1186/s13756-016-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y-T, Su C-F, Chuang C, et al. Appropriate treatment for bloodstream infections due to Carbapenem-resistant Klebsiella pneumoniae and Escherichia coli: A nationwide multicenter study in Taiwan. Open Forum Infect Dis 2019;6:ofy336. 10.1093/ofid/ofy336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Prete R, Ronga L, Addati G, et al. Trends in Klebsiella pneumoniae strains isolated from the bloodstream in a teaching hospital in southern Italy. Infez Med 2019;27:17–25. [PubMed] [Google Scholar]
- 21.Li Y, Shen H, Zhu C, et al. Carbapenem-resistant Klebsiella pneumoniae infections among ICU admission patients in central China: prevalence and prediction model. Biomed Res Int 2019;2019:9767313. 10.1155/2019/9767313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cienfuegos-Gallet AV, Ocampo de Los Ríos AM, Sierra Viana P, et al. Risk factors and survival of patients infected with Carbapenem-resistant Klebsiella pneumoniae in a KPC Endemic setting: a case-control and cohort study. BMC Infect Dis 2019;19:830. 10.1186/s12879-019-4461-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin X, Wu S, Hao M, et al. The colonization of carbapenem-resistant Klebsiella pneumoniae: epidemiology, resistance mechanisms, and risk factors in patients admitted to intensive care units in China. J Infect Dis 2020;221:S206–14. 10.1093/infdis/jiz622 [DOI] [PubMed] [Google Scholar]
- 24.Jiao Y, Qin Y, Liu J, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization and predictors of mortality: a retrospective study. Pathog Glob Health 2015;109:68–74. 10.1179/2047773215Y.0000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kofteridis DP, Valachis A, Dimopoulou D, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection/colonization: a case-case-control study. J Infect Chemother 2014;20:293–7. 10.1016/j.jiac.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 26.Ripabelli G, Salzo A, Mariano A, et al. Healthcare-associated infections point prevalence survey and antimicrobials use in acute care hospitals (PPS 2016-2017) and long-term care facilities (HALT-3): a comprehensive report of the first experience in Molise region, central Italy, and targeted intervention strategies. J Infect Public Health 2019;12:509–15. 10.1016/j.jiph.2019.01.060 [DOI] [PubMed] [Google Scholar]
- 27.Lan Y, Zhou M, Jian Z, et al. Prevalence of Pks Gene cluster and characteristics of Klebsiella pneumoniae-induced bloodstream infections. J Clin Lab Anal 2019;33:e22838. 10.1002/jcla.22838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng X, Yang J, Duan J, et al. Assessing molecular epidemiology of Carbapenem-resistant Klebsiella pneumoniae (CR-KP) with MLST and MALDI-TOF in central China. Sci Rep 2019;9:2271. 10.1038/s41598-018-38295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agodi A, Barchitta M, Quattrocchi A, et al. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter Baumannii resistance indicators in an intensive care unit of Southern Italy, 2008-2013. Antimicrob Resist Infect Control 2015;4:43. 10.1186/s13756-015-0087-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koppe U, von Laer A, Kroll LE, et al. Carbapenem non-susceptibility of Klebsiella pneumoniae isolates in hospitals from 2011 to 2016, data from the German antimicrobial resistance surveillance (ARS). Antimicrob Resist Infect Control 2018;7:71. 10.1186/s13756-018-0362-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayden MK, Lin MY, Lolans K, et al. Prevention of Colonization and infection by Klebsiella pneumoniae Carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015;60:1153–61. 10.1093/cid/ciu1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth DJA, Khader K, Slayton RB, et al. The potential for interventions in a long-term acute care hospital to reduce transmission of carbapenem-resistant Enterobacteriaceae in affiliated healthcare facilities. Clin Infect Dis 2017;65:581–7. 10.1093/cid/cix370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065786supp001.pdf (25.4KB, pdf)
Data Availability Statement
Data are available on reasonable request.