Abstract
Background
Atopic dermatitis is a common, chronically recurring inflammatory skin disease. It gives rise to a high disease burden and is of major importance in social medicine.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed, including the current German and European guidelines.
Results
Basic therapy with drug-free topical agents markedly improves the barrier function of the skin. Adults should apply at least 250 g per week. Patient-specific trigger factors such as allergens, stress, microbial pathogens, or skin irritants should be eliminated or avoided. In mild and moderately severe forms, external treatment with topical glucocorticosteroids and topical calcineurin inhibitors usually suffices; proactive therapy is given to patients with frequent recurrences or a long course of disease. Systemic anti-inflammatory treatment with biological agents such as dupilumab and tralokinumab, Janus kinase inhibitors such as baricitinib, upadacitinib, and abrocitinib, or conventional immunosuppressant drugs is indicated particularly in severe cases. The patient should be actively involved in the choice and planning of treatment; the patient’s age and the cutaneous findings should be taken into account. Interdisciplinary patient education yields a sustained benefit.
Conclusion
A combination of baseline therapy, reactive and proactive anti-inflammatory therapy, and systemic therapy as needed is the foundation of successful interdisciplinary treatment for atopic dermatitis.
Atopic dermatitis (atopic eczema, AE, neurodermatitis) is among the more common skin diseases. Its 1-year prevalence in Germany is at least 7% in children (e1) and 4–5% in adults (1). Approximately half of the patients suffer intermittently from moderate to severe atopic dermatitis, which often cannot be adequately treated by external methods alone, especially in adults (e2). The high prevalence, chronic course, and disease burden of atopic dermatitis make it a condition of socioeconomic importance (e2, e3). Both the severe itch and the accompanying stigmatization lead to marked psychosocial comorbidity and distress (2); thus, optimal management is needed, in conformity with the guidelines (3– 5).
Learning goals
Prevalence.
Atopic dermatitis is among the more common skin diseases. Its 1-year prevalence is at least 7% in childhood and 4–5% in adulthood.
Reading this article should enable the reader to:
know the main diagnostic and therapeutic measures for atopic dermatitis;
know the role of allergy, allergy evaluation, individualized basic therapy, and external treatment, both reactive and proactive;
know the indications for systemic treatment, patient education, and psychodermatological intervention.
The need for optimal treatment.
The high prevalence, chronic course, and disease burden of atopic dermatitis make it a disease of socioeconomic importance. The severe pruritus and the accompanying stigmatization lead to marked psychosocial comorbidity and distress. Optimal treatment is needed in conformity with the guidelines.
Clinical features
The skin lesions, which are usually accompanied by severe pruritus, include infiltrated erythema, erythema with erosions caused by scratching, lichenified areas, and pruriginous papules and nodules. The nummular variant of childhood resembles nummular eczema in adults (e4). Atopic dermatitis significantly impairs the quality of life (6).
Minimal manifestations include dry lip inflammation (cheilitis sicca), inflammatory fissures at the corner of the mouth (perlèche), infranasal erosion, infra-auricular tears, retro-auricular intertrigo, fingertip and toe-tip eczema (“atopic winter feet”), nipple eczema, and pityriasis alba.
So-called atopic stigmata are typical skin signs, not pathological in themselves, that indicate an atopic diathesis. These include dry skin, hyperlinearity of the palms and soles, infraorbital double eyelid crease, periorbital halo formation, facial pallor, rarefaction of the lateral portion of the eyebrow, and white dermographism.
Atopic dermatitis is a global problem and one of the more common skin diseases even in low-income countries (7). On highly pigmented skin, the characteristic erythema appears gray (“ashy”) rather than red as in Caucasians (8).
Formal catalogs of diagnostic criteria (Hanifin and Rajka [e5]) or UK-Working Party [e6]) have been incorporated into international guidelines (9).
Rash depending on age at typical sites, pruritus as a leading symptom, and a tendency to IgE-mediated sensitization and diseases in the patient’s own or family history are the main criteria.
The differential diagnosis includes other skin diseases such as infections (e.g., scabies), other forms of eczema (allergic contact dermatitis, irritative-toxic eczema, seborrheic eczema) and, in infants, seborrheic dermatitis (box 1).
Box 1. The differential diagnosis of atopic dermatitis.
-
Eczematous diseases that can resemble atopic dermatitis clinically and histologically
infantile seborrheic eczema*1
irritative contact dermatitis
allergic contact dermatitis
microbial (nummular) eczema
scabies with chronic course
eczema stage of cutaneous T-cell lymphoma
-
Cutaneous inflammation on the hands and feet as differential diagnoses of atopic hand and foot eczema
psoriasis palmoplantaris
tinea manuum and pedum
irritant contact dermatitis
allergic contact dermatitis
-
Immunodeficiency syndromes
hyper-IgE syndrome: autosomal dominant hyper-IgE syndrome (STAT3 defect)*2 and autosomal recessive hyper-IgE syndrome (DOCK8 defect)
Wiskott-Aldrich syndrome
Omenn syndrome
Netherton syndrome
*1 Diaper region typically spared in atopic dermatitis
*2 Autosomal dominant hyper-IgE syndrome, unlike the other immunodeficiency syndromes listed, is usually easily distinguishable on clinical grounds from atopic dermatitis with elevated IgE.
Patients very often have associated diseases including other atopic conditions (asthma, allergic rhinoconjunctivitis), rarely vernal keratoconjunctivitis, giant papillary conjunctivitis, superficial keratitis punctata, atopic keratoconjunctivitis, or otitis externa and media. Food allergies are demonstrable in 30% of children with more severe atopic dermatitis, and immediate (type 1) hypersensitivity to cow‘s milk, hen’s eggs, peanuts, soy, and nuts is common.
Attention deficit—hyperactivity disorder (ADHD) is also associated with atopic dermatitis, possibly because of itch-induced insomnia (e7, e8).
The main diagnostic criteria.
Rash depending on age at typical sites, pruritus as a leading symptom, and a tendency to IgE-mediated sensitization and diseases in the patient’s own or family history are the main criteria.
Depression and anxiety disorders are more common, especially in patients with severe atopic dermatitis. Severe personal distress may lead to reactive depression (6).
Psychological stress can trigger an episode or aggravate the disease (10). Comorbidities such as alopecia areata are much more common in patients with atopic dermatitis; chronic inflammatory bowel disease, rheumatoid arthritis, and obesity are somewhat more common than in the general population.
In contrast, psoriasis, type 1 diabetes, and certain types of cancer are less common (e9). The marked Th2 dominance in atopic dermatitis seems likely to inhibit Th1-induced diseases (11).
Complications of atopic dermatitis
Associated diseases.
Patients very often have associated diseases including other atopic conditions. Food allergies are demonstrable in 30% of children with more severe atopic dermatitis, and immediate (type 1) hypersensitivity to cow’s milk, hen’s eggs, peanuts, soy, and nuts is common.
Cutaneous infections are the most common type of complication of atopic dermatitis. Only a few pathogens are responsible; the clinical features are generally characteristic (box 2). Staphylococcus aureus is the most common pathogen; it causes both colonization and infection (12). Severe infections are treated systemically, milder ones with topical antiseptic agents. Long-term continuous prophylaxis with antiseptic-containing emollients or topical antibiotics is not recommended (5). Antibacterially coated silver textiles are of highly variable quality (e10). Colonization with Malassezia species is of importance in the head and neck variant of atopic dermatitis; topical antifungal therapy may be considered (5, 13, 14, e11).
Box 2. The treatment of infectious complications of atopic dermatitis.
-
Bacterial infections (mostly impetiginization, Staphylococcus aureus)
topical antiseptics in externally applied preparations, bath solutions, and textiles (e.g., octenidine, triclosan, chlorhexidine in externally applied preparations; silver in externally applied preparations or textiles)
systemic antibiotics for extensive lesions
-
Viral infections
mechanical ablation with fine forceps, curretage (eczema molluscatum)
chemical ablation (eczema molluscatum, verrucae vulgares)
systemic acyclovir or valacyclovir (eczema herpeticatum)
-
mycotic infections (dermatophytes [tinea], Malassezia species [head-neck-shoulder variant of atopic dermatitis])
antifungal agents (for example, cyclopirox, azole antifungals, usually topical)
Complications of atopic dermatitis.
Cutaneous infections are the most common type of complication of atopic dermatitis. Staphylococcus aureus is the most common pathogen; it causes both colonization and infection
Disseminated infection with herpes simplex virus, known as eczema herpeticatum, is a dermatologic emergency. If clinically suspected, this potentially life-threatening disease should be treated immediately with systemically administered acyclovir (15). The risk of developing eczema herpeticatum is multiplied in patients with severe, untreated atopic dermatitis and in those with the IgE-associated (extrinsic) subtype (16). Disseminated coxsackie virus infection (eczema coxsackium) may take a similar course on the skin (17). Other viral dermatoses, such as molluscum contagiosum or common warts (verruca vulgaris), may occur in disseminated form in patients with atopic dermatitis (eczema molluscatum, eczema verrucatum). Pseudomonas aeruginosa infections play only a minor role among the complications of atopic dermatitis.
Genetics and pathogenesis
Atopic dermatitis arises on the background of an inherited predisposition (diathesis) and is precipitated by environmental and lifestyle factors (18). Associated factors that are well documented include living in an urban environment and regions with low ultraviolet exposure and a dry climate, a a “western” diet, small family size, high educational level, and frequent exposure to antibiotics during pregnancy and the first year of life, yet the effects of these factors are small (e2). Over 30 genomic regions show robust associations with atopic dermatitis. They mainly contain genes with known roles in the structure and function of the epidermis, or in immune mechanisms (e12). Null mutations with loss of function in the pro-filaggrin (FLG) gene result in a lack of functional filaggrin peptides in the outer epidermis, leading to a complex skin barrier defect. Approximately 10% of the population carry a single FLG mutation and exhibit generalized skin dryness, palm hyperlinearity, and a threefold increased risk of atopic dermatitis (e10). The cytokine gene cluster, in which gene variants and epigenetic mechanisms influence expression of the type 2 cytokines IL-4, IL-5, and IL-13, is another risk locus (e14, e15). Skin barrier dysfunction and predominantly T-cell-mediated cutaneous inflammation are the central molecular and immune mechanisms of atopic dermatitis (e16).
Barrier dysfunction is characterized by reduced diversity of the skin microbiome and frequent colonization with Staphylococcus aureus, as well as dryness, altered lipid composition, and increased permeability of the epidermis. Pro-inflammatory, barrier-destabilizing, and pruritogenic mediators are present at higher levels in the eczematous lesions. Because of the dominance of type 2 cytokines, this is considered a type 2 inflammation.
Genetics and pathogenesis.
Atopic dermatitis arises on the background of an inherited predisposition (diathesis) and is precipitated by environmental and lifestyle factors.
Diagnosis, differential diagnoses, triggers
Atopic dermatitis is diagnosed on clinical grounds. Pruritus is a mandatory diagnostic criterion. Along with the symptoms and signs, the patient’s history must include information on the age of onset and time course of the condition, the personal and family history of atopy, and food allergies. A whole-body examination is needed to evaluate the typical distribution pattern of the eczematous rash. Skin biopsy for the histopathological evaluation of potential differential diagnoses is rarely indicated (19); the main differential diagnoses are listed in Box 1 (e17).
If atopic dermatitis is suspected, the potential psychosomatic, allergic, or environmental triggers should be identified (ebox). The importance of these triggers varies widely across individuals, and their avoidance is a component of the personalized treatment plan. The role of dietary factors is often overestimated, particularly in childhood; rather, acute and chronic skin irritations and cold temperatures should always be considered as potential triggers of skin barrier dysfunction (20). Infections and vaccinations can also aggravate atopic dermatitis, but children and adults with atopic dermatitis should nevertheless be vaccinated as usual, as recommended by the STIKO (German Standing Committee on Vaccination). Neither infection nor vaccination against SARS-CoV-2 increases the risk of developing atopic dermatitis (21). In acute exacerbations, it is recommended to defer vaccination until the skin condition stabilizes, if possible.
eBox. Psychosocial aspects.
depressed mood
shame and disgust
insomnia
social anxiety
stigmatization
suicidal ideation
compulsive scratching
Allergological evaluation for atopic dermatitis
The significance of allergic reactions for the course of atopic dermatitis must be investigated on an individual basis. 80% of patients have an IgE-mediated hypersensitivity to common foods or inhaled allergens such as pollen, animal hair, or house dust mites (3). Allergy testing (by skin prick test or in vitro test) is particularly indicated in patients with a history of immediate-type reactions in addition to atopic dermatitis, or in those who have delayed eczematous reactions a few hours after contact with an allergen. Specific IgG measurement is of no value in the diagnostic evaluation of suspected allergies and should be abandoned.
The diagnosis of atopic dermatitis.
Atopic dermatitis is diagnosed on clinical grounds. Pruritus is a mandatory diagnostic criterion. Along with the symptoms and signs, the patient’s history must include information on the age of onset and time course of the condition, the personal and family history of atopy, and food allergies.
Allergological evaluation in atopic dermatitis.
The significance of allergic reactions for the course of atopic dermatitis must be investigated on an individual basis. 80% of patients have an IgE-mediated hypersensitivity to common foods or inhaled allergens such as pollen, animal hair, or house dust mites.
The mere fact of sensitivity to a certain type of food does not imply a need for abstinence, or for treatment; only clinically relevant food allergies of the immediate type, or very marked late-type reactions, are an indication for the targeted elimination of the allergen. In case of doubt, provocative tests should be carried out under appropriate medical supervision (3). Unspecific diets are of unproven efficacy and are not recommended in the guidelines (3). In persistent atopic dermatitis and hypersensitivity to house dust mite allergens, hypoallergenic mattress covers („encasings“) and the frequent washing of pillows and comforters are recommended (19). What to do in case of sensitization to pet allergens must be decided on an individual basis (clinical relevance, symptom severity).
Food sensitivity.
Food sensitivity does not imply a need for abstinence or treatment; only clinically relevant food allergies of the immediate type, or very marked late-type reactions, are an indication for the targeted elimination of the allergen. In case of doubt, provocative tests should be carried out under medical supervision
Patch testing with contact allergens (e.g., external substances) is recommended for the additional demonstration of allergic contact dermatitis, which is hard to distinguish from concomitant atopic dermatitis on clinical grounds alone (3). The same is true in overt cases of hand eczema; here, substances in occupational use can lead to skin irritation or specific sensitization (22) (box 4).
Box 4. Selected differential diagnoses of atopic dermatitis in childhood*.
-
seborrheic eczema
often arises before age 3 months
flexor surfaces also affected (e.g., diaper area, groin region)
-
scabies
typical predilection sites (e.g. palmoplantar in infants)
papulovesicles and nodules, demonstration of mites (reflected light microscopy)
-
hyper-IgE syndrome
frequently recurring skin infections, “cold abscesses,” recurrent pneumonia
chronic Candida infection (nails, mucosa)
-
Langerhans cell histiocytosis
disseminated papules and crusts, also on the hairy scalp
in further course, erosions or ulcerations on flexor surfaces of large joints
-
Netherton syndrome
erythroderma, characteristic scaling (ichthyosis linearis circumflexa)
sparse and short hair (bamboo hair), failure to thrive, multiple allergies
-
Omenn syndrome
ichthyosiform erythroderma, alopecia
lymphadenopathy, hepatosplenomegaly, dystrophy, severe immune deficiency
-
Wiskott-Aldrich syndrome
atopic-dermatitis-like rash in boys
petechiae, skin hemorrhages, recurrent infections
-
allergic contact eczema
eczema in allergen-exposed areas
crescendo reaction
-
dermatomycoses
erythematous plaques with prominent edges, often with pustules
often, fine lamellar scaling
-
lichen ruber planus
polygonal, markedly pruritic papules and plaques
Wickham striae, potentially also on the oral mucosa
-
psoriasis vulgaris
erythematosquamous plaques, nail dystrophy/chromia
predilection sites: hairline, umbilical, hairy scalp, rima ani
*modified from: Ott, Kopp, Lange. Kinderallergologie in Klinik und Praxis, Springer Verlag 2014
Topical treatment
Patch testing.
Epicutaneous testing with contact allergens (e.g., external substances) is recommended for the additional demonstration of allergic contact dermatitis, which is hard to distinguish from concomitant atopic dermatitis on clinical grounds alone.
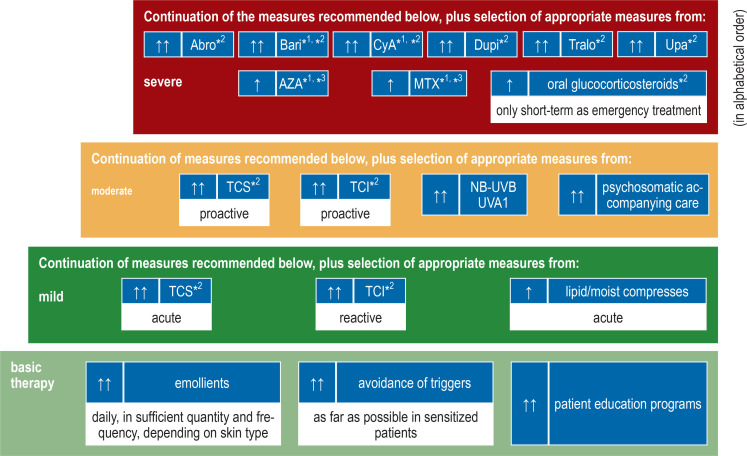
Disruption of the epidermal barrier (perceived as „dry skin“) is a major characteristic of atopic dermatitis, and basic skin barrier therapy thus plays a central role in treatment, whatever the degree of severity of the condition (9), in order to remedy the impaired keratinization, decreased water binding, and abnormal lipid composition. For this purpose, hydrophilic, lipid-containing topical preparations are used; most contain key components of the natural epidermal lipids (cholesterol, ceramides, free fatty acids). Unfortunately, in practice this treatment often goes by the name of ”skin care,” falsely suggesting that it is something different from, or less important than, a medical therapeutic measure. We therefore prefer the term “basic therapy” (figure).
Figure.
Stepwise plan for the treatment of atopic dermatitis in adults
– also antiseptic, antibiotic, antiviral, or antifungal therapy for infections
– Ccheck compliance and diagnosis if treatment is not sufficiently effective
*1see (4, 5) for important limitations and definitions, as well as stepwise treatment schedule for adolescents and children,
*2 approved indication,*3 off-label treatment
↑↑ dark green) strong recommendation for use / ↑ (light green) weak recommendation for use
Abro, abrocitinib; AZA,azathioprine; Bari, baricitinib; CyA,cyclosporin; Dupi, dupilumab; MTX, methotrexate; TCI, topical calcineurin inhibitors; TCS, topical corticosteroids; Tralo, tralokinumab; Upa, upadacitinib; UVA1, ultraviolet A1; NB-UVB, narrow-spectrum ultraviolet B
Lipids are supplied in the long term with emollients. These also contain moisturizers (humectants) and occludent agents that impede the evaporation of water. Transepidermal water loss is increased in atopic dermatitis (e18). Appropriate topical treatment with emollients can lessen the need for anti-inflammatory drugs such as glucocorticoids (23). Basic therapy also includes appropriate skin cleansing: strong alkaline detergents and irritating measures are contraindicated (3).
Practical aspects
Emollients are sometimes poorly tolerated when used on markedly inflamed lesions during flare-ups of atopic dermatitis. In such cases, anti-inflammatory treatment should be given first (5).
It is important to choose the right type of preparation (water-in-oil versus oil-in-water) according to the affected body region, the acuity of the disease process, the time of year, and individual features. Acute lesions tend to require hydrophilic preparations, while chronic lesions require lipophilic ones. Lipophilic products should be used on extensor surfaces; flexor and intertriginous zones should never be treated with excessively fatty substances. Hydrophilic preparations are preferred in the summer, lipophilic ones in the winter.
The main consideration, however, is quantity: the most common mistake in topical basic therapy is to use too little of it. The so-called fingertip unit or FTU (e19) corresponds to a strand of ointment, 5 mm in diameter, that fits on the tip of an adult’s index finger (approximately 0.5 g). This amount suffices for two adult palms. Adolescents and adults need at least 250 g of emollient per week (5).
Safety
The use of emollients is safe. Possible complications due to superinfection are avoided by removal with a wooden spatula (“No fingers in the container!”). Contact eczema induced by components of the emollient ointment is rare and can be diagnosed with patch testing (e20) (box 4).
Topical treatment.
Basic skin barrier therapy thus plays a central role in treatment, whatever the degree of severity of the condition, in order to remedy the impaired keratinization, decreased water binding, and abnormal lipid composition.
Anti-inflammatory treatment
Topical glucocorticosteroids remain the main anti-inflammatory drugs for the treatment of atopic dermatitis. Preparations are selected according to their potency and therapeutic index (TIX) (e21).
These drugs are generally applied once daily. Moderately potent topical glucocorticosteroids (e.g., prednicarbate 0.25%, hydrocortisone butyrate 0.1%) usually suffice for older children, adolescents, and adults; diluted preparations (e.g., prednicarbate 0.08%) are appropriate for infants (3, e22). High- or very-high-potency glucocorticosteroids (class 3, or exceptionally class 4 in adults) may be indicated for the short-term treatment of marked, refractory, lichenified eczematous lesions from school age onward. Wet wraps enhance the effect of topical glucocorticosteroids. This should be done only for a short period of time and under medical supervision. Proactive treatment, defined as the long-term, twice-weekly interval therapy of recurrence-prone areas with topical anti-inflammatory drugs after the visible lesions have healed, lessens the risk of recurrence and lowers the glucocorticoid requirement without increasing the risk of side effects such as skin atrophy (23). There have been controlled trials of tacrolimus, fluticasone propionate, and methylprednisolone aceponate. Tacrolimus ointment is currently the only preparation approved for proactive treatment.
Safety
Treatment with topical glucocorticosteroids is problematic on the face, in intertriginous areas, the scrotum, and, in infants and young children, the capillitium as well. In these areas, only low- or intermediate-potency topical glucocorticosteroids should be used, and for no more than a few days (3).
Topical calcineurin inhibitors (tacrolimus and pimecrolimus) have been approved since 2002 for the anti-inflammatory treatment of atopic dermatitis. Even their prolonged use does not cause skin thinning, steroid-induced rosacea, or perioral dermatitis (3). Common side effects include transient warmth or a burning sensation, but not more frequent bacterial skin infections. The risk of viral infections appears to be slightly elevated (16).
Topical calcineurin inhibitors do not increase the risk of basal-cell carcinoma or lymphoma (5, e23). Out of basic considerations of safety, effective sun protection is recommended when topical calcineurin inhibitors are used (16).
Anti-inflammatory treatment.
Topical glucocorticosteroids remain the main anti-inflammatory drugs for the treatment of atopic dermatitis. Preparations are selected according to their potency and therapeutic index.
Narrow-spectrum UVB and UVA are recommended for ultraviolet light therapy in atopic dermatitis (5). The potential role of topical Janus kinase (JAK) inhibitors, phosphodiesterase inhibitors, and recently introduced tar preparations has yet to be determined.
Systemic treatment
Three substance classes are now available for systemic anti-inflammatory treatment: conventional immunosuppressants, biologic agents, and JAK inhibitors (3, 25) (figure).
Systemic glucocorticosteroids should be used only in exceptional cases and for short periods (from a few days to three weeks) to treat an acute flare (4). Cyclosporine is approved for the short- and medium-term treatment of severe atopic dermatitis in patients aged 16 and older and should not be used for more than two years, preferably as interval therapy every few months. Methotrexate or azathioprine can be used off label in individual cases for longer-term immunosuppression (e24).
Dupilumab and tralokinumab are monoclonal antibodies for subcutaneous injection (e25). Dupilumab binds the alpha subunit of the IL-4 receptor, blocks IL-4 and IL-13 signaling pathways, and is approved for use from age 6 onward (box 3). At least 75% improvement in clinical scores is achieved after 3–4 months of treatment by approximately 50% of patients on monotherapy and 70% of those taking dupilumab in combination with topical glucocorticosteroids (e33, e34). The most common side effects are local reactions at the injection site and ocular symptoms (especially conjunctivitis), which are usually mild and transient (26). Tralokinumab binds IL-13 and is currently approved for the treatment of moderate to severe atopic dermatitis from age 12 onward. In phase 3 trials, at least 75% improvement in clinical scores was achieved by approximately 30% of patients after 16 weeks of monotherapy, and by 56% after treatment with trakolinumab in combination with a topical class 3 glucocorticosteroid (27, 28).
Box 3. Anti-inflammatory systemic treatment.
-
Short-term/interval treatment
cyclosporine
-
Long-term/permanent treatment
biologic agents: dupilumab (anti-IL4/IL13Ra), tralokinumab (anti-IL13)
JAK inhibitors: Abrocitinib (JAK-1-selective), baricitibib (JAK-1/JAK2-selective), upadacitinib (JAK-1-selective)
-
Systemic treatment for children and adolescents
dupilumab (from age 6)
upadacitinib (from age 12)
tralokinumab (from age 12)
The main side effects are injection-site reactions and conjunctivitis (e26). No laboratory tests are required before or during treatment with dupilumab and tralokinumab (e27, e28).
Systemic corticosteroids.
These should be used only exceptionally and for short periods to treat acute flares. Cyclosporine is approved for short- and medium-term treatment of severe atopic dermatitis in patients aged 16 and older. It should not be used for more than two years, preferably as interval therapy every few months.
Three JAK inhibitors—baricitinib, upadacitinib, and abrocitinib—have been approved to date for the treatment of moderate to severe atopic dermatitis in adults; upadacitinib is also approved for children aged 12 and above (4). Janus kinases transduce intracellular signals from cytokine receptors on the cell surface. Depending on their dose and selectivity, JAK inhibitors can act more broadly than antibodies. Baricitinib inhibits JAK1 and JAK2 equally; upadacitinib and abrocitinib are more selective for JAK1, the preferred target of the newer JAK inhibitors for atopic dermatitis. JAK inhibitors are administered orally; they have a short half-life and a rapid onset of action (e29). Response rates for at least 75% improvement in clinical scores in monotherapy are approximately 35% for baricitinib, 60% for abrocitinib, and 75% for upadacitinib (at the highest dose in each case) (29– 31). In comparative studies, more patients displayed clinically relevant improvement in the first days to weeks of treatment with abrocitinib or upadacitinib compared to dupilumab, but the outcomes became increasingly similar the longer the drugs were continued (e30, e31). The side effect profile of JAK inhibitors depends on the particular agent and is more complex and broader than that of biologic agents (e32). Side effects reported in studies of the use of JAK inhibitors to treat atopic dermatitis include an increased frequency of upper respiratory tract infections, herpes simplex, and varicella zoster reactivation. Patients at risk should, therefore, be vaccinated against herpes zoster. Transient nausea has been described more frequently with abrocitinib, transient acneiform skin manifestations with upadacitinib (4). Regular laboratory testing is needed, according to the manufacturers’ recommendations. An increased incidence of thromboembolic events, cardiovascular disease, cancer or serious infections has not been observed in patients with atopic dermatitis. Nevertheless, to minimize the risk of serious adverse events, JAK inhibitors should not be used in persons over age 65, persons at increased risk of serious cardiovascular problems or cancer, or current or past smokers, unless there is no good alternative treatment (e35). Before treatment with JAK inhibitors is started, latent infections such as tuberculosis and hepatitis, marked renal or hepatic dysfunction, and pregnancy must be ruled out (4). Women of childbearing age must use effective contraception while being treated with JAK inhibitors.
Atopic dermatitis in childhood
Atopic dermatitis is the most common inflammatory skin disease, and one of the more common chronic diseases, of childhood and adolescence (e1). In infants and young children, chronic, severe atopic dermatitis is associated with a risk of failure to thrive. Consideration must be given to rarer conditions resembling atopic dermatitis and to differential diagnoses associated with dystrophy, which may be life-threatening.
Increased risk of herpes zoster.
Side effects reported in studies of the use of JAK inhibitors to treat atopic dermatitis include an increased frequency of upper respiratory tract infections, herpes simplex, and varicella zoster reactivation. Patients at risk should, therefore, be vaccinated against herpes zoster.
The typical cutaneous manifestations of atopic dermatitis often arise at the age of three months or later. Their sites of predilection change over time:
infants: cheeks, hairy scalp (capillitium), extensor surfaces of the limbs;
toddlers and schoolchildren: flexor surfaces (elbows, popliteal region, neck);
adolescents, adults: hand and foot eczema as well.
Most children with atopic dermatitis have difficulty falling asleep and sleeping through the night, leading to daytime sleepiness. In more than 80% of cases, acute exacerbations disturb the nocturnal sleep of parents and siblings as well. In the authors’ view, children with mild atopic dermatitis do not need allergy testing if they respond well to standard treatment with an otherwise unremarkable allergy history.
Parents often interpret the clinical features of atopic dermatitis as an allergy requiring further investigation. Nevertheless, undirected allergy screening should not be performed. Especially in children, very high total IgE values or allergen-specific IgE antibody titers of no clinical relevance are often found. This must be explained to the parents in detail in order to prevent senseless abstinence measures. Of course, allergy testing is indicated if the history is suggestive of IgE-mediated triggers.
Psychosocial aspects and education programs
Sleep disturbance in children with atopic dermatitis.
Most children with atopic dermatitis have difficulty falling asleep and sleeping through the night, leading to daytime sleepiness. In more than 80% of cases, acute exacerbations disturb the nocturnal sleep of parents and siblings as well.
Therapeutic patient education programs.
Educational programs are recommended as a part of basic therapy in all guidelines worldwide, and many randomized trials have shown their efficacy. Outpatient or inpatient psychotherapy is recommended when psychological factors are obvious triggers of the condition.
Psychological comorbidity is evident in atopic dermatitis. One-quarter of patients suffer from depression, social anxiety, stigmatization, and negative coping with the itch-scratch cycle. The risk of suicide is increased (e36). To prevent psychosocial problems, possible comorbidity should be addressed early, and patients should be made aware of the availability of offerings such as patient education programs and self-help groups. In Germany, atopic dermatitis education programs are available from a working group for patient education in atopic dermatitis (Arbeitsgemeinschaft Neurodermitis-Schulung, AGNES).
Patient education is for persons of all ages suffering from atopic dermatitis. For children up to 6 years of age, there is parental training; for those aged 7–12, there is joint training for the patient and his/her parents; adolescents (13– 18) receive education without their parents, and there is a separate patient education program for adult patients. Patient education comprises six interactive sessions, of two hours each, in which participants are given comprehensive information about atopic dermatitis, enabling them to cope with the disease (32, 33). Information on topical and systemic treatment, basic therapy, dressings, nutritional aspects, psychological factors, relaxation training, and stress management is provided. Habit-reversal techniques, i.e., alternatives to scratching, have been particularly successful.
Educational programs are recommended as a part of basic therapy in all guidelines worldwide, and many randomized trials have shown their efficacy (3). Outpatient or inpatient psychotherapy is recommended when psychological factors are obvious triggers of the condition. The distressing nocturnal itch almost always impairs sleep, promoting the development of depression and social withdrawal. Techniques for stress reduction, such as progressive muscle relaxation, are useful in addition to dermatologic therapy. Stress has been shown to be a provoking factor both in animal experiments and in patients with atopic dermatitis. This was associated with changes in proinflammatory neuropeptides in the skin (box 4).
Perspectives
Over the past eight years, our understanding of the pathophysiology of atopic dermatitis has markedly improved. Many new drugs, especially systemic drugs such as JAK inhibitors and biologic agents, are already available in pharmacies. Nevertheless, reactive and proactive external therapy with emollients, topical glucocorticosteroids, and topical calcineurin inhibitors is still of central importance. Patients with atopic dermatitis should be actively involved in treatment planning and selection. If indicated, interdisciplinary patient education should be offered.
Box 5. Guideline-based intervention for atopic dermatitis*1: evidence level (EL)*2 und recommendation grade (RG)*3 of the procedures listed, according to the European guideline on atopic dermatitis (4, 5).
-
Avoidance of trigger factors
measures to reduce house dust mites (EL 2b, RG B)
attempt to reduce pollen on the skin in case of sensitization (EL-, RG D)
avoidance of relevant contact allergens in case of positive patch tests (EL-, RG D)
targeted elimination diet in case of proven clinical relevance of food (immediate-type allergy and/or eczema exacerbation) (EL 2b, RG B)
-
Treatment
regular use of basic therapeutic agents (topically applied preparations that do not contain drugs) (EL 3b, RG C)
use of topical glucocorticosteroids (TCS) and/or topical calcineurin antagonists (TCI) (EL 1b, RG A)
recurrence prophylaxis by proactive treatment with topical TCS or TCI (EL 1b, RG A)
preferential use of calcineurin inhibitors (TCI) in areas more prone to side effects from corticosteroids (face, intertriginous areas) (EL 1b, RG A)
no use of antihistamines (H1R antagonists) (EL 1b, RG A)
no use of oral antibiotics in the absence of superinfection (EL 1b, RG A)
use of systemic antibiotics in case of bacterial superinfection (EL 2b, RG B)
topical antiseptics in case of evidence of superinfection (EL 4, RG C)
antifungal treatment for head and neck dermatitis (EL 2b, RG B)
use of antiseptic textiles (EL 2b, RG B)
no use of topical antibiotics (EL -, RG D)
antiviral systemic therapy (e.g., acyclovir) for eczema herpeticatum (EL 4, RG D)
phototherapy: narrow-band UVB (311 nm) rather than broad-band UVB (EL 1 a, RG A)
equal efficacy of medium-dose UVA-1 (50 J/cm 2) and UVB 311 nm (EL 1 b, RG A)
systemic glucocorticosteroids only short-term, especially in adults; unfavorable risk/benefit profile for longer treatment (EL-, RG D)
use of cyclosporine in severely affected adults (EL 1a, RG A).
exceptional use of cyclosporine in severely affected children and adolescents (EL 2b, RG B)
use of dupilumab in moderately to severely affected children, adolescents, and adults (EL 1a, RG A)
use of tralokinumab in moderately to severely affected adolescents and adults (EL 1a, RG A)
use of baricitinib in moderately to severely affected adults (EL 1a, RG A)
use of upadacitinib in moderately to severely affected adolescents and adults (EL 1a, RG A)
use of abrocitinib in moderately to severely affected adults (EL 1a)*4
use of azathioprine in severely affected patients (EL 1b, RG A)
use of mycophenolate in severely affected patients (EL 4, EC C)
use of methotrexate in severely affected patients (EL 4, RG C)
adjuvant use of psychotherapy, especially behavioral therapies (EL 3b, RG B)
psychological interventions as an essential and helpful part of therapeutic patient education (EL 1a, RG A)
use of age-adapted interdisciplinary therapeutic patient education in small groups (EL 1a, RG A)
*1 Not all recommendations presented in the text have been evaluated in the European guideline. In particular, at the time the European guideline was written, abrocitinib had not yet been approved for the treatment of atopic dermatitis, so no consensus recommendations regarding this drug included. In contrast, the EDF guideline on atopic dermatitis does include additional recommendations that are not reproduced here in their entirety because of space limitations.
*2 EL = evidence level, as defined in (10): 1a, meta-analysis of randomized, controlled trials; 1b, randomized, controlled trial; 2a, systematic review of cohort studies; 2b, cohort study or low-quality randomized controlled trial; 3a, systematic review of case-control studies; 3b, case-control study; 4, low-quality case series or cohort study
*3 RG = recommendation grade, as defined in (e24): A; 1a, 1b; B; 2a, 2b, 3a, 3b; C, 4; D, expert opinion
*4 Abrocitinib is now approved for adults with severe atopic dermatitis on the basis of good evidence from controlled trials (30).
CME credit for this unit can be obtained via cme.aerzteblatt.de until 30 March 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the 1-year prevalence of atopic dermatitis among children in Germany?
7%
14%
21%
28%
35%
Question 2
What immunosuppressant drug is approved for short-term and interval therapy of atopic dermatitis in adults?
mycophenolate mofetil
methotrexate
cyclosporine
azathioprine
acitretin
Question 3
What topical drug is licensed for the proactive treatment of atopic dermatitis?
fluticasone propionate cream
tacrolimus ointment
prednicarbate ointment
crisaborole cream
mometasone furoate cream
Question 4
What immune-modulating drug is approved for the systemic treatment of atopic dermatitis for children aged 6 years and above, as well as for adults?
dupilumab
baricitinib
tralokinumab
upadacitinib
abrocitinib
Question 5
What percentage of children with sevre atopic dermatitis also have food allergies?
10%
20%
30%
40%
50%
Question 6
What mental illness is frequently associated with atopic dermatitis?
alcohol abuse
borderline syndrome
autism spectrum disorder
dyslexia
attention deficit—hyperactivity disorder
Question 7
What kinase is the preferred target of newly approved drugs for atopic dermatitis?
Janus kinase
tyrosine kinase
pyruvate kinase
Bruton tyrosine kinase
glucokinase
Question 8
According to the text, what relaxation technique is useful alongside dermatologic treatment for patients with atopic dermatitis?
yoga
jogging
progressive muscle relaxation
oil massages
catathymic image experience
Question 9
What psychosocial intervention for patients with atopic dermatitis is recommended in all guidelines as a component of basic treatment?
sleep training
education programs
autogenic training
psychoanalysis
hypnotherapy
Question 10
What pathogen plays a minor role among the complications of atopic dermatitis?
Verucca vulgaris
Pseudomonas aeruginosa
Staphylococcus aureus
Herpes simplex
Molluscum contagiosum
Further information on CME.
Participation in the CME certification program is possible only via the Internet: cme.aerzteblatt.de. This unit can be accessed until 30 March 2024. Submissions by letter, e-mail, or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed with the “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in“Meine Daten”, and consent must be given for results to be communicated. The 15-digit EFN can be found on the CMEcard (8027XXXXXXXXXXX).
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
AW has served as a paid consultant for AbbVie, Aileens, Almirall, BMS, Galapagos, Galderma, GSK, Hans Karrer, Janssen, Leo Pharma, Eli Lilly, L’Oréal, MedImmune, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi-Aventis, and UCB. He has received payment for continuing medical education presentations from AbbVie, Almirall, Beiersdorf, Bioderma, BMS, Galderma, Glenmark, GSK, Hans Karrer, Janssen, Leo Pharma, Eli Lilly, L’Oréal, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi-Aventis, and UCB. He has received reimbursement for expenses related to participation in scientific meetings from AbbVie, Eli Lilly, Leo Pharma, and Pierre Fabre. He serves on advisory boards for AbbVie, Aileens, Almirall, BMS, Galderma, GSK, Janssen, Leo Pharma, Eli Lilly, L’Oréal, MedImmune, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Sanofi-Aventis, and UCB. He has received writing support for publications from AbbVie, Almirall, Beiersdorf, BMS, Galderma, GSK, Leo Pharma, Eli Lilly, L’Oréal, MedImmune, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Santen, and Sanofi-Aventis.
SW has served as a paid consultant for AbbVie, Almirall, Boehringer, Eli Lilly, Galderma, Leo Pharma, Pfizer, Sanofi, and Regeneron. He has received lecture honoraria from AbbVie, Almirall, Eli Lilly, Leo Pharma, Novartis, Pfizer, and Sanofi, reimbursement of travel expenses from AbbVie, Galderma, and Sanofi, and third-party research support from La Roche-Posay, Leo Pharma, Pfizer, and Sanofi.
TW has served as a paid consultant for AbbVie, Allmiral, LEO, Lilly, Novartis, Pfizer, and Regeneron/Sanofi. He has received research support from AbbVie, Beiersdorf, LEO, Novartis, Phadia/Thermo Fischer, and Regeneron/Sanofi, and lecture honoraria from AbbVie, Allmiral, Galderma, Janssen, Leo, Lilly, Meda/mylan, Novartis, Pfizer, Phadia/Thermo Fischer, and Regeneron/Sanofi.
JR has received payment for serving on the advisory boards of AbbVie, Sanofi, Allergika, and Viatris.
The remaining authors state that they have no conflict of interest.
References
- 1.Zietze HA, Cabral C, Theobald K, et al. [Epidemiology and treatment of adult patients with atopic dermatitis: analysis of longitudinal data of the statutory health insurance scheme] Hautarzt. 2021;72:963–974. doi: 10.1007/s00105-021-04859-5. [DOI] [PubMed] [Google Scholar]
- 2.Kage P, Zarnowski J, Simon JC, Treudler R. Atopic dermatitis and psychosocial comorbidities—What’s new? Allergol Select. 2020;4:86–96. doi: 10.5414/ALX02174E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34:2717–2744. doi: 10.1111/jdv.16892. [DOI] [PubMed] [Google Scholar]
- 4.Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I. J Eur Acad Dermatol Venereol. 2022;36:1409–1431. doi: 10.1111/jdv.18345. [DOI] [PubMed] [Google Scholar]
- 5.Wollenberg A, Kinberger M, Arents B, et al. European guideline EuroGuiDerm) on atopic eczema—part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36:1904–1926. doi: 10.1111/jdv.18429. [DOI] [PubMed] [Google Scholar]
- 6.Ring J, Zink A, Arents BWM, et al. Atopic eczema: burden of disease and individual suffering—results from a large EU study in adults. J Eur Acad Dermatol Venereol. 2019;33:1331–1340. doi: 10.1111/jdv.15634. [DOI] [PubMed] [Google Scholar]
- 7.Suaini NHA, Tan CPT, Loo EXL, Tham EH. Global differences in atopic dermatitis. Pediatr Allergy Immunol. 2021;32:23–33. doi: 10.1111/pai.13335. [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Grendelmeier P, Takaoka R, Ahogo KC, et al. Position statement on atopic dermatitis in Sub-Saharan Africa: current status and roadmap. Eur Acad Dermatol Venereol. 2019;33:2019–2028. doi: 10.1111/jdv.15972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. doi: 10.1111/jdv.14888. [DOI] [PubMed] [Google Scholar]
- 10.Gieler U, Schoof S, Gieler T, Scheewe S, Schut C, Kupfer J. Atopic eczema and stress among single parents and families: an empirical study of 96 mothers. Acta Derm Venereol. 2017;97:42–46. doi: 10.2340/00015555-2457. [DOI] [PubMed] [Google Scholar]
- 11.Eyerich S, Onken AT, Weidinger S, et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med. 2011;365:231–238. doi: 10.1056/NEJMoa1104200. [DOI] [PubMed] [Google Scholar]
- 12.Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid-Grendelmeier P, Scheynius A, Crameri R. The role of sensitization to Malassezia sympodialis in atopic eczema. Chem Immunol Allergy. 2006;91:98–109. doi: 10.1159/000090246. [DOI] [PubMed] [Google Scholar]
- 14.Alexander H, Paller AS, Traidl-Hoffmann C, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol. 2020;182:1331–1342. doi: 10.1111/bjd.18643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollenberg A. Eczema herpeticum. Chem Immunol Allergy. 2012;96:89–95. doi: 10.1159/000331892. [DOI] [PubMed] [Google Scholar]
- 16.Seegraber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2020;34:1074–1079. doi: 10.1111/jdv.16090. [DOI] [PubMed] [Google Scholar]
- 17.Neri I, Dondi A, Wollenberg A, et al. Atypical forms of hand, foot, and mouth disease: a prospective study of 47 Italian children. Pediatr Dermatol. 2016;33:429–437. doi: 10.1111/pde.12871. [DOI] [PubMed] [Google Scholar]
- 18.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 19.Werfel T, Heratizadeh A, Aberer W, et al. S2k guideline on diagnosis and treatment of atopic dermatitis—short version. J Dtsch Dermatol Ges. 2016;14:92–106. doi: 10.1111/ddg.12871. [DOI] [PubMed] [Google Scholar]
- 20.Traidl S, Lang C, Schmid-Grendelmeier P, Werfel T, Heratizadeh A. Comprehensive approach: current status on patient education in atopic dermatitis and other allergic diseases. Handb Exp Pharmacol. 2022;268:487–500. doi: 10.1007/164_2021_488. [DOI] [PubMed] [Google Scholar]
- 21.Buhl T, Beissert S, Gaffal E, et al. COVID-19 and implications for dermatological and allergological diseases. J Dtsch Dermatol Ges. 2020;18:815–824. doi: 10.1111/ddg.14195. [DOI] [PubMed] [Google Scholar]
- 22.Thyssen JP, Schuttelaar MLA, Alfonso JH, et al. Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Dermatitis. 2022;86:357–378. doi: 10.1111/cod.14035. [DOI] [PubMed] [Google Scholar]
- 23.Wollenberg A, Frank R, Kroth J, Ruzicka T. Proactive therapy of atopic eczema–an evidence-based concept with a behavioral background. J Dtsch Dermatol Ges. 2009;7:117–121. doi: 10.1111/j.1610-0387.2008.06772.x. [DOI] [PubMed] [Google Scholar]
- 24.Dinkloh A, Worm M, Geier J, Schnuch A, Wollenberg A. Contact sensitization in patients with suspected cosmetic intolerance: results of the IVDK 2006-2011. J Eur Acad Dermatol Venereol. 2015;29:1071–1081. doi: 10.1111/jdv.12750. [DOI] [PubMed] [Google Scholar]
- 25.Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta-analysis. Allergy. 2021;76:1053–1076. doi: 10.1111/all.14631. [DOI] [PubMed] [Google Scholar]
- 26.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019;181:459–473. doi: 10.1111/bjd.17869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184:437–449. doi: 10.1111/bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450–463. doi: 10.1111/bjd.19573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242–255. doi: 10.1111/bjd.18898. [DOI] [PubMed] [Google Scholar]
- 30.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 31.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 32.Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933–938. doi: 10.1136/bmj.332.7547.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heratizadeh A, Werfel T, Wollenberg A, et al. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845–853 e3. doi: 10.1016/j.jaci.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Ersser SJ, Cowdell F, Latter S, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD004054.pub3. CD004054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Thamm R, Poethko-Müller C, Hüther A, Thamm M. Allergische Erkrankungen bei Kindern und Jugendlichen in Deutschland—Querschnittergebnisse aus KiGGS Welle 2 und Trends. J Health Monit. 2018;3:3–18. [Google Scholar]
- E2.Siegels D, Haufe E, Heinrich L, et al. Status report on the atopic dermatitis registry TREATgermany. Allergol Select. 2021;5:274–286. doi: 10.5414/ALX02262E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014;69:3–16. doi: 10.1111/all.12270. [DOI] [PubMed] [Google Scholar]
- E4.Boehner A, Neuhauser R, Zink A, Ring J. Figurierte Erytheme—Aktueller Stand und diagnostisches Vorgehen. J Dtsch Dermatol Ges. 2021;19:963–972. doi: 10.1111/ddg.14450_g. [DOI] [PubMed] [Google Scholar]
- E5.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) Suppl. 1980;92:44–47. [Google Scholar]
- E6.Williams HC, Burney PG, Pembroke AC, Hay RJ. The UK. Working Party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406–416. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- E7.Romanos M, Gerlach M, Warnke A, Schmitt J. Association of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J Epidemiol Community Health. 2010;64:269–273. doi: 10.1136/jech.2009.093534. [DOI] [PubMed] [Google Scholar]
- E8.Schmitt J, Apfelbacher C, Heinrich J, Weidinger S, Romanos M. [Association of atopic eczema and attention-deficit/hyperactivity disorder—meta-analysis of epidemiologic studies] Z Kinder Jugendpsychiatr Psychother. 2013;41:35–42; quiz -4. doi: 10.1024/1422-4917/a000208. [DOI] [PubMed] [Google Scholar]
- E9.Bozek A, Jarzab J, Mielnik M, Bogacz A, Kozlowska R, Mangold D. Can atopy have a protective effect against cancer? PLoS One. 2020;15 doi: 10.1371/journal.pone.0226950. e0226950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Srour J, Berg E, Mahltig B, Smolik T, Wollenberg A. Evaluation of antimicrobial textiles for atopic dermatitis. J Eur Acad Dermatol Venereol. 2019;33:384–390. doi: 10.1111/jdv.15123. [DOI] [PubMed] [Google Scholar]
- E11.Mayser P, Kupfer J, Nemetz D, et al. Treatment of head and neck dermatitis with ciclopiroxolamine cream—results of a double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2006 19:153–158. doi: 10.1159/000092596. [DOI] [PubMed] [Google Scholar]
- E12.Paternoster L, Standl M, Waage J, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. 2015;47:1449–1456. doi: 10.1038/ng.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–1327. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- E14.Lee GR, Fields PE, Griffin TJ, Flavell RA. Regulation of the Th2 cytokine locus by a locus control region. Immunity. 2003;19:145–153. doi: 10.1016/s1074-7613(03)00179-1. [DOI] [PubMed] [Google Scholar]
- E15.Kretschmer A, Möller G, Lee H, et al. A common atopy-associated variant in the Th2 cytokine locus control region impacts transcriptional regulation and alters SMAD3 and SP1 binding. Allergy. 2014;69:632–642. doi: 10.1111/all.12394. [DOI] [PubMed] [Google Scholar]
- E16.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4 1. doi: 10.1038/s41572-018-0001-z. [DOI] [PubMed] [Google Scholar]
- E17.Ott H. Guidance for assessment of erythroderma in neonates and infants for the pediatric immunologist. Pediatr Allergy Immunol. 2019;30:259–268. doi: 10.1111/pai.13032. [DOI] [PubMed] [Google Scholar]
- E18.Werfel T, Heratizadeh A, Niebuhr M, et al. Exacerbation of atopic dermatitis on grass pollen exposure in an environmental challenge chamber. J Allergy Clin Immunol. 2015;136:96–103 e9. doi: 10.1016/j.jaci.2015.04.015. [DOI] [PubMed] [Google Scholar]
- E19.van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen A, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev. 2017;2 CD012119. doi: 10.1002/14651858.CD012119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Åkerström U, Reitamo S, Langeland T, et al. Comparison of moisturizing creams for the prevention of atopic dermatitis relapse: a randomized double-blind controlled multicentre clinical trial. Acta Derm Venereol. 2015;95:587–592. doi: 10.2340/00015555-2051. [DOI] [PubMed] [Google Scholar]
- E21.Luger T, Loske KD, Elsner P, et al. Topische Dermatotherapie mit Glukokortikoiden—Therapeutischer Index. J Dtsch Dermatol Ges. 2004;2:629–634. doi: 10.1046/j.1439-0353.2004.03626.x. [DOI] [PubMed] [Google Scholar]
- E22.Wirén K, Frithiof H, Sjoqvist C, Loden M. Enhancement of bioavailability by lowering of fat content in topical formulations. Br J Dermatol. 2009;160:552–556. doi: 10.1111/j.1365-2133.2008.08981.x. [DOI] [PubMed] [Google Scholar]
- E23.Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta-analysis. Allergy. 2021;76:1053–1076. doi: 10.1111/all.14631. [DOI] [PubMed] [Google Scholar]
- E24.Werfel T, Heratizadeh A, Aberer W, et al. Update „systemic treatment of atopic dermatitis“ of the S2k-guideline on atopic dermatitis. J Dtsch Dermatol Ges. 2021;19:151–168. doi: 10.1111/ddg.14371. [DOI] [PubMed] [Google Scholar]
- E25.Ratchataswan T, Banzon TM, Thyssen JP, Weidinger S, Guttman-Yassky E, Phipatanakul W. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053–1065. doi: 10.1016/j.jaip.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Wollenberg A, Beck LA, de Bruin Weller M, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. 2022;186:453–465. doi: 10.1111/bjd.20810. [DOI] [PubMed] [Google Scholar]
- E27.Beck LA, Thaci D, Deleuran M, et al. Laboratory safety of dupilumab for up to 3 years in adults with moderate-to-severe atopic dermatitis: results from an open-label extension study. J Dermatolog Treat. 2022;33:1608–1616. doi: 10.1080/09546634.2020.1871463. [DOI] [PubMed] [Google Scholar]
- E28.Wollenberg A, Beck LA, Blauvelt A, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS) Br J Dermatol. 2020;182:1120–1135. doi: 10.1111/bjd.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927–940. doi: 10.1016/j.jaci.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E31.Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- E32.Wood H, Chandler A, Nezamololama N, Papp K, Gooderham MJ. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746–754. doi: 10.1111/ijd.15853. [DOI] [PubMed] [Google Scholar]
- E33.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44–56. doi: 10.1001/jamadermatol.2019.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282–1293. doi: 10.1016/j.jaad.2020.06.054. [DOI] [PubMed] [Google Scholar]
- E35.Patel KR, Immaneni S, Singam V, Rastogi S, Silverberg JI. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis. J Am Acad Dermatol. 2019 80:402–410. doi: 10.1016/j.jaad.2018.08.063. [DOI] [PubMed] [Google Scholar]
- E36.Bundesinstitut für Arzneimittel und Medizinprodukte. Januskinase-Inhibitoren: Behandlung von Entzündungskrankheiten. www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/DE/RV_STP/g-l/januskinase.html (last accessed on 9 January 2023) [Google Scholar]