Abstract
BACKGROUND
Smell disorders are the most frequent persistent coronavirus disease 2019 (COVID-19) complications.
AIM
To describe the patterns and characteristics of persistent smell and taste disorders in Egyptian patients.
METHODS
Assessment was done to 185 patients (adults = 150, age: 31.41 ± 8.63 years; children = 35; age: 15.66 ± 1.63 years). Otolaryngology and neuropsychiatric evaluations were done. Measurements included: A clinical questionnaire (for smell and taste); sniffin' odor, taste and flavor identification tests and the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS).
RESULTS
Duration of disorders was 11.53 ± 3.97 ms (6-24 ms). Parosmia (n = 119; 64.32%) was developed months after anosmia (3.05 ± 1.87 ms). Objective testing showed anosmia in all, ageusia and flavor loss in 20% (n = 37) and loss of nasal and oral trigeminal sensations in 18% (n = 33) and 20% (n = 37), respectively. Patients had low scoring of sQOD-NS (11.41 ± 3.66). There were no specific differences in other demographics and clinical variables which could distinguish post-COVID-19 smell and taste disorders in children from adults.
CONCLUSION
The course of small and taste disorders are supportive of the nasal and oral neuronal compromises. Post-COVID-19 taste and trigeminal disorders were less frequent compared to smell disorders. Post-COVID-19 flavor disorders were solely dependent on taste and not smell disorders. There were no demographics, clinical variables at onset or specific profile of these disorders in children compared to adults.
Keywords: Post-COVID-19 complications, Anosmia, Ageusia, Trigeminal sensory loss, Parosmia, Quality of life
Core Tip: Smell loss is the most frequent acute manifestation of coronavirus disease 2019 (COVID-19) infection with an estimated prevalence of 40%-86% in adults and 16%-20% in children. Smell disorders (loss or distortion) are also the most frequent long-lasting complications of COVID-19 infection with an estimated prevalence of 20%-40% in adults. Compared to smell, taste disorders are less frequent acute manifestation of viral infection (occurring in 10%-42% of adults) and long-lasting complications of COVID-19 infection. Reports about the prevalence and prognosis of these disorders in children are few or even lacking compared to adults. Also the mechanisms and treatment of these persistent disorders are still challenges. Evidence from experimental studies suggested that injury and degeneration of the neuronal olfactory and gustatory sensory epithelia by severe peripheral viral infection and its immunopathology and the delay or lack of neuronal regeneration might contribute to these disorders. Here, we tried to determine the predictors for persistent disorders and distinguish their differences in children compared to adults.
INTRODUCTION
Several months after the initial description of the severe acute coronavirus disease 2019 (COVID-19) infection, many data emerged about the prevalence of its mild manifestations including smell and taste disorders. The majority of reports about post-COVID-19 smell and taste disorders were devoted for adult population. Meta-analyses studies reported an estimated prevalence of 40%-86% for smell loss and 10.2%-42% for taste loss in adults infected with COVID-19[1]. Smell loss has been found to be the hallmark or the isolated symptom of COVID-19 infection in 25%-44% of adults[2]. Reports about these disorders in children and adolescents as a manifestation of COVID-19 infection are few and their results are highly variable[3-5]. In the meta-analysis study done by Yan et al[4], the authors estimated that the pooled prevalence of post-COVID-19 anosmia, ageusia, anosmia or ageusia and both anosmia and ageusia in children were 16% (95%CI: 8.2-23.8), 9.2% (95%CI: 4.3-14.2), 15.5% (95%CI: 10.3–20.7) and 20.2% (95%CI: 14.1–26.3), respectively. Smell disorders were found to be the most frequent long-lasting or persistent (months to years) COVID-19 complications in approximately 20%-40% of adults[6-8], however, the systematic estimation of the prevalence of these disorder in children is lacking.
Studies reported two main patterns for smell loss (with or without taste loss) as acute manifestations of COVID-19 infection: (1) Sudden smell loss in association with general, systemic or other ear, nose and throat (ENT) viral manifestations. This was the most frequent pattern. It might occur either at the same time with other viral manifestations (22.8%-88.0%)[9,10], after the recovery of other viral manifestations (26.6%-65.4%)[11], or before the onset of other manifestations (11.8%)[9]; and (2) Isolated smell loss without any other viral manifestations (approximately 16%-19.4%)[10].
Studies also reported two prognoses for these disorders. They are: (1) Transient deficits which resolved within days to weeks after onset (mean: Approximately 20 d). This was the most frequent prognosis occurring in 60%-80% of patients[12-14]; and (2) Long-lasting/persistent disorders (deficits and distortions) lasting for months to years. The long-lasting/persistent disorders have been reported to occur in 20%-40% of patients[14].
Studied found that the nose and mouth are the main sites for entry of β-coronaviruses (CoVs) into the body including severe acute respiratory syndrome coronavirus 1[15], Middle east respiratory syndrome coronavirus (MERS-CoV)[16] and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[17,18]. The olfactory epithelial cells, the susentacular, Bowman’s gland, micovillar cells (but not neuronal cells or receptors) are overloaded by the angiotensin-converting enzyme type 2 (ACE2) receptors, which are the targets for viral entry into the body[19]. In golden Syrian hamsters, the researchers observed infection of large proportion of sustentacular cells by SARS-CoV-2 within few days after its instillation, followed by desquamation and infiltration of immune cells into the olfactory epithelium and lamina propria, disorganization of the epithelial structure and loss of cilia necessary for olfactory transduction. The authors also observed partial restoration of the epithelium within approximately 2 wk[17,18]. Authors suggested that the relatively fast recovery (approximately 15 d) observed in the majority of patients (60%-80%) after COVID-19 infection is compatible with the rapid regeneration of the non-neuronal olfactory epithelial cells in hamsters and the partial recovery of the olfactory epithelium within 1-2 wk. However, the mechanisms that cause long-lasting/persistent smell loss and distortion are due to the dysfunction or destruction of the olfactory neurons due to viral infection and the disorganization of the olfactory receptors within the epithelium[20,21].
Post-COVID-19 taste disorders are less frequent compared to smell disorders and their pathogenesis is understudied. In animal models, researchers found that ACE2 and sialic acid receptors are the tongue cells by which SARS-CoV-2 enters the body. Sialic acid is a salivary mucin component. Mucin conveys the molecules of tastants into taste pores to prevent their enzymatic degradation[22]. It has been suggested that SARS-CoV-2 infection could interfere with the transport of tastants and accelerate their degradation.
This work aimed to determine the patterns of post-COVID-19 smell and taste disorders at onset in a cohort of adults and children and quantify the extent of these disorders using validated objective measures. The intra- (within the same group) and inter- (adults vs children) individual variability in demographics, clinical characteristics and quality of life variables, were determined. The inter-individual variability of clinical characteristics within the same family members infected with COVID-19 was also explored. This is the first study in our country which systematically evaluated adults as well as children with post-COVID-19 persistent smell and taste disorders.
MATERIALS AND METHODS
Study design, period and region
This was a cross-sectional observational study carried throughout a period of a year (August 2020 to September 2021). The initial sample size was 320 patients with post-COVID-19 persistent smell and taste disorders. The diagnosis of COVID-19 infection was relied on the high clinical suspicion (i.e. having sudden onset of smell and taste deficits during the locally active COVID-19 pandemic in absence of obvious alternative explanation), and according to the World Health Organization (WHO) interim guidance[23]. Patients were recruited from the out-patient clinic (for care of post-COVID-19 ENT disorders in children and adults) of the Otolaryngology department of Assiut University Hospital (a tertiary referral hospital), Assiut, Egypt. Inclusion criteria: (1) Adults and children with sudden onset of smell loss during the active COVID-19 pandemic in absence of alternative causes; and (2) Persistence of smell disorders (with or without taste disorders) for ≥ 6 mo. Smell disorder was defined as subjective complete or partial smell loss or distortion. Taste disorder was defined as subjective loss or distortion of all (total) or any (partial) of the 5 basic tastes (sweet, salt, sour, bitter and umami). Flavor disorder was defined as subjective loss of aroma of foods and drinks. Exclusion Criteria: (1) Progressive smell and taste loss; (2) An existing cause which could explain the patient’s disorders as otolaryngological conditions (i.e., rhinitis, rhinosinusitis or any nasal disorders), head or nasal surgery or trauma, unusual exposure to toxins, chemicals or metals or regular intake of drugs; and (3) Medical, neurologic or psychiatric diseases which are known causes of smell or taste disorders, e.g. brain tumors (e.g. olfactory groove meningioma, pituitary adenoma with suprasellar extension, etc.), temporal lobe epilepsy, Parkinson’s disease, Alzheimer’s disease, etc.), or vitamin deficiencies (e.g. zinc or thiamine deficiencies).
After application of inclusion and exclusion criteria, 185 patients (adults = 150; Children = 35; males = 89, females = 96) were included for the final statistical analysis. Excluded were 135 adult patients due to presence of diabetes Mellitus (n = 52), rhinosinusitis (n = 50), psychosis (n = 13), allergic rhinitis (n = 15) and Parkinson’s disease (n = 5). The statistical analyses were done to the included 185 patients.
Methods
Data collection: Detailed ENT evaluation was done by the consultant otolaryngologist (Ahmed MAA). Evaluation included routine clinical examination. It also included rhinoscopy and nasal endoscopy. Detailed medical and neuropsychiatric histories and examinations (Hamed SA and Kamal-Eldeen EB) were done. Clinical data were gathered during face to face interviewing. The data included: (1) Demographics: Age, gender, residence, smoking habit, and socioeconomic status. The socioeconomic status was classified according to the Socio-Economic Scale[24] into high, middle, low or very low; (2) COVID-19 infection inquiries included: Manifestations and their severity, investigations, course, recurrence, comorbidities, consequences and previous treatments' for smell and taste disorders. The severity of viral infection manifestations at the onset were classified as: Mild: Slight symptoms, no viral pneumonia or hypoxia and normal chest imaging; moderate: Fever, dry cough, dyspnea and tachypnea and abnormal chest radiology (i.e. manifestation of pneumonia); severe: Either dyspnea, respiratory rate > 30/min, or a PaO2/FiO2 < 300 mmHg or SpO2 < 90% on room air (i.e. manifestations of severe pneumonia and severe respiratory distress); and critical: Which included acute respiratory distress syndrome, respiratory failure, multiorgan failure or sepsis or septic shock [i.e. requirements for admission to the intensive care unit (ICU)][23]; and (3) The patterns and prognoses of COVID-19 infection in contacts (i.e. incubation carriers) were also determined.
A clinically designed questionnaire
It was a questionnaire for clinical data collection and included the questionnaire component (for smell and taste) of the National Health and Nutrition Examination Survey[25]. The answers to questions characterized the individual differences’ in perception and intensity of appetizing aroma and scents; sweetness, saltiness, sourness and bitterness of foods and drinks (which helps to differentiate taste as a gustatory, as opposed to flavor which is a function of retronasal olfaction) and pungent odorants and tastants (i.e. with strong trigeminal compounds; nippy, blazing, hot or irritant as black pepper, mustard, vinegar, ginger, cinnamon, chiles, mint gum and spices). Data were also gathered about the demographics and clinical characteristics of close contacts infected with COVID-19.
The occurrence of anosmia, hyposmia, dysosmia, parosmia, cacosmia, phantosmia, ageusia, dysguesia or distortion of aroma, had been differentiated in the questionnaire. Anosmia is the term used to define the absence or loss of the sense of smell. Hyposmia is the decreased sense of smell. A distortion of the sense of smell is defined as dysosmia. Dysosmia has two types, parosmia and phantosmia. Parosmia refers to a perception of different odor of an odorant than it was in the past. Cacosmia is a type of parosmia in which the odor, which was previously enjoyable, is smelled as repulsive or offensive. Phantosmia is the perception of an odor in absence of actual staff to be smelled. Ageusia refers to the loss of sense of taste. Hypogeusia is a diminished sense of taste. Dysgeusia refers to the distortion of the sense of taste. In this study, we used the term dysgeusia to refer to distortion of taste as well as flavor (or aroma).
Sniffin' odor identification test
Sniffin' odor identification test (SOIT) was used for quantitative assessment of smell (orthonasal olfaction) loss. We used 16 odorants familiar to Egyptians[26]. They were tea, vanilla, coconut, cacao, coriander, cardamom, thyme, fennel, clove, green cumin, garlic, mixed oregano, old Rumi cheese chips, cinnamon, black pepper and ginger. Odorants were kept in opaque containers and arranged in order so that cinnamon, black pepper and ginger were the last to be tested (i.e. three hot smells with strong nasal trigeminal sensory stimulation). The examiner covered the patient's eyes with a blindfold before exposure to the odorants; then asked the patient to smell each substance as often as liked or even to choose from four named odorants options. Each nostril was examined separately and the correct answer received one point. The total score of SOIT equals 16. Normosmia was considered if total SOIT's scoring ranged from 12 to 16, hyposmia if it ranged from 9 to 11, and anosmia if ≤ 8.
Taste identification test
This test was used for quantitative assessment of smell loss. We used sugar (sweet), salt (salty), lemon (sour), old Rumi cheese chips (umami) and coffee (bitter), the five basic tastants. They were kept in opaque containers. Coffee was the last to be tasted as it may alter subsequent taste perceptions if tasted first. The patient's eyes were covered with a blindfold and nostrils were plugged with cotton to block concurrent smell. The examiner put half a teaspoon of a tastant on the patient's hand and allowed him/her to taste as often as liked, then rinsed the mouth with tap water after each tastant's exposure.
Flavor identification test
The same 16 substances of SOIT were used to test flavor (retronasal olfaction) which were tea, vanilla, coconut, cacao, coriander, cardamom, thyme, fennel, clove, green cumin, garlic, mixed oregano, old Rumi cheese chips, cinnamon, black pepper and ginger. Tastants were kept in opaque containers and arranged in order so that cinnamon, black pepper and ginger were the last to be tasted (i.e. tastants with strong oral trigeminal sensory stimulation). The patient's eyes were covered with a blindfold and nostrils were plugged with cotton to block concurrent smell (orthonasal olfaction). The examiner put half tea spoonful from each tastant on the patient’s hand and asked the patient to taste as much substance as often as liked to recognize it. The total score of the test equals 16. Normal flavor was considered if total score ranged from 12 to 16, impaired (partial loss) if it ranged from 9 to 11, and complete loss if ≤ 8.
Psychiatric interviewing
It was done for determination of comorbid psychiatric condition (s) in response to the presence of the chemosensory disorders and for the differentiation between psychiatric symptoms and disorders. The impact of olfactory dysfunction on the quality of life (QoL) of patients has been evaluated by the Arabic translated and validated short version of the Questionnaire of Olfactory Disorders-Negative Statements (sQOD-NS). sQOD-NS is a seven-item questionnaire about the impact of smell disorder on social activities, eating behavior, annoyance and anxiety. The rating scale for each item is ranged from 0 to 3. A total score of 21 indicated no impact on QoL and zero scoring indicated severe impact[27].
Imaging studies
It included computed tomography (CT) on nasal cavities, anterior cranial fossa and sinuses as a part of ENT evaluation and CT of the brain as a part of neurologic evaluation.
Statistical analyses
Data were analyzed with SPSS (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Descriptive statistics were reported as numbers (%) and mean (SD). Comparative statistics (intra- and inter- individuals) between variables for adults and children were performed using independent sample t-test, Chi square test and Mann-Whitney U test. Correlation analyses was done between total scores of sQOD-NS and different demographic and clinical variables (age, gender, duration of disorders, presence or absence of parosmia, presence or absence of taste/flavor loss) using Spearman’s correlation coefficient. The level of statistical significance was set at P < 0.05.
RESULTS
Demographics and patterns of viral infection at onset
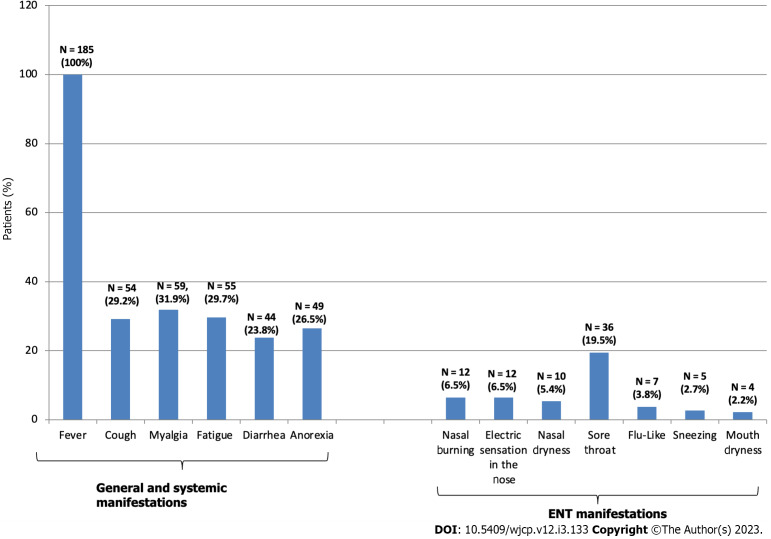
This work included 185 patients. The majority were rural residents (64.86%) and of low socioeconomic status (51.9%) (Figure 1). The duration of persistent disorders at the period of the study ranged from 6 to 24 mo (mean = 11.53 ± 3.97 mo), among were 51.35% (n = 95) had duration of ≥ 12 mo. Patients had normal rhinoscopy, nasal endoscopy and CT on nasal cavities, anterior cranial fossa, and sinuses. None had previous hospital admission due to COVID-19 infection and none received vaccination by the time of the study. Patterns of viral infection at onset were isolated sudden smell (with or without taste loss) (n = 63, 34.5%) or sudden smell loss in concurrent association with systemic and/or ENT manifestations (n = 122, 65.95%). Systemic manifestations were reported in 54.05% (n = 100, or 100/185) and ENT (other than smell or taste disorders) manifestations were reported in 37.84% (n = 70 or 70/185). Fever (> 38 °C) was the most common systemic manifestation (100%). Sore throat was the most common ENT manifestation (n = 36, 51.34% or 36/70) (Figure 2). The duration of recovery of systemic and ENT manifestations varied from 3 to 21 d after onset (mean = 8.11 ± 3.69). The most frequently done investigations after onset for diagnosis of COVID-19 infection were complete blood count (CBC) (n = 130, 70.27%), serology (n = 130, 70.27%) and CT of the chest (particularly for patients with moderate manifestations of viral infection) (n = 32, 17.3%) (Figure 1). Only 43 (23.24%) did reverse transcription polymerase chain reaction (RT-PCR). The majority of patients (78.92%; n = 146) did frequent otolaryngology consultations (at onset and afterward) and received different treatment modalities for months, but none was effective (Figure 1). They included nasal irrigations, particularly patients with cacosmia, (duration: Range: 3–15 d, mean = 8.34 ± 3.86), local steroids (duration: Range: 6–60 d, mean = 18.28 ± 9.94), systemic steroids (duration: Range: 3–30 d, mean = 11.89 ± 6.41), and vitamins and supplements as omega-3 capsule, vitamin B complex (vitamins B1, B2, B6, B9, B12), vitamin C, vitamin E, zinc, selenium, L-carnitine and pentoxifylline tablets (duration: Range: 6–90 d, mean = 41.65 ± 24.04). The most frequently prescribed vitamins and supplements were zinc, omega 3 and vitamin B complex. Some patients (22.70%, n = 42) turned to facebook groups to share tips and vent to subjects with same symptoms. This group practiced sniffing strong odors (as essential oils or scents) or pungent herbs (duration: Range: 30–120 d, mean = 65.50 ± 32.84). However, none provided a beneficial effect. Few (n = 39, 21.08) neither did previous otolaryngology consultation nor received any treatment modalities (Figure 1).
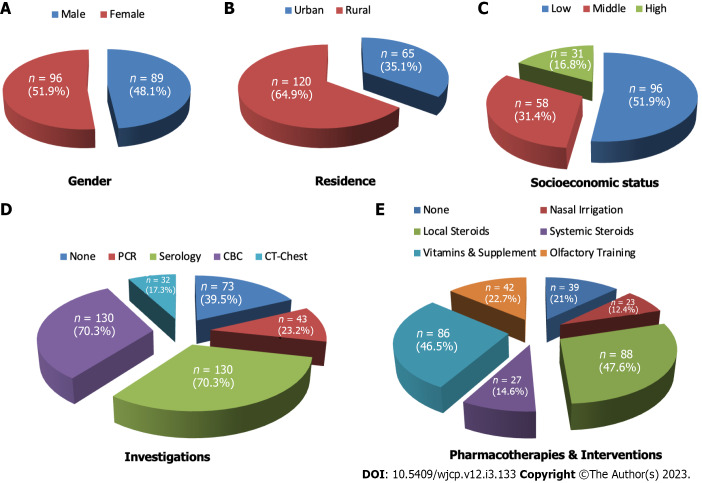
Figure 1.
Demographics, laboratory and treatment characteristics of the studied patients. A: This study included 96 (51.9%) females and 89 (48.1%) males; B: The majority were rural residents (64.9%, n = 120), while 35.1% (n = 65) were urban residents; C: The majority were of low socioeconomic status (51.9%, n = 96), while 31.4% (n = 58) and 16.8% (n = 31) were of middle and high socioeconomic states, respectively; D: Investigations done by patients included serology and complete blood count (70.3%, n = 130, polymerase chain reaction (23.2%) and computed tomography-chest (17.3%, n = 32). While 39.5% (n = 73) did not do any investigations. E: The received pharmacotherapies and interventions to treat chemosensory disorders included local steroids (47.6%, n = 88), vitamins and supplements (46.5%, n = 86), olfactory training (22.7%, n = 42) and systemic steroids (12.4%, n = 23), while 22.7% of the patients (n = 42) did not receive any therapy. PCR: Polymerase chain reaction; CBC: Complete blood count; CT: Computed tomography.
Figure 2.
General, systemic and ear, nose and throat manifestations of viral infection of the studied patients. ENT: Ear, nose and throat.
Characteristics of contacts (incubation carriers)
The majority of patients (n = 108, 58.38%) were the only affected family member, the remaining (n = 77, 41.62%) had ≥ 1 household contact (range: 1–5) who developed systemic and/or ENT manifestations of COVID-19 infection at the same time as the patients or within the previous 2-4 wk before patients' onset. They developed complete recovery of systemic or ENT manifestations within 3 to 30 d (mean = 12.95 ± 5.97) and of smell and taste loss (n = 40, 21.62%) deficits within 2 to 30 d (mean = 5.03 ± 1.10) with the exception of 4 (2 mothers and 2 daughters, included in the study) who developed persistent smell and taste disorders. None of the completely recovered contacts developed distortion of chemosensations. Inter-individual manifestations were present in the same infected family members (mild, moderate or severe infection; systemic or ENT manifestations or both; fever, cough, diarrhea, fatigue, insomnia, uveitis, etc.). A prominent example was a family case cluster of 5 patients who demonstrated highly variable presentations, durations, severities, and progression of COVID-19 infection manifestations. The presented patient (included in the study) was a 20-year old-female with persistent anosmia and ageusia for 14 mo duration and developed parosmia within approximately 3 mo after smell loss. She did not have other viral manifestations at onset. Her infected household contacts were: (1) A 58-year-old father with severe manifestations of viral infection (fever, cough, shortness of breath, myalgia and sore throat) and confirmed diagnosis of severe viral pneumonia due to COVID-19 infection. He was also admitted to ICU for weeks and markedly improved within approximately 30 d; (2) A 52-year old mother with fever, abdominal pain, severe diarrhea and myalgia for 15 d. She did home-isolation and recovered completely within approximately 21 d; (3) A 16-year old brother with moderate infection (fever, cough, sore throat and rhinorrhea or flu-like symptoms). He did self-isolation and completely recovered within about 7-14 d; and (4) A 23-year old brother with mild manifestations of low grade fever, minimal cough, sore throat and reversible smell and taste loss. He recovered completely within 7-10 d.
Manifestations of smell and taste disorders
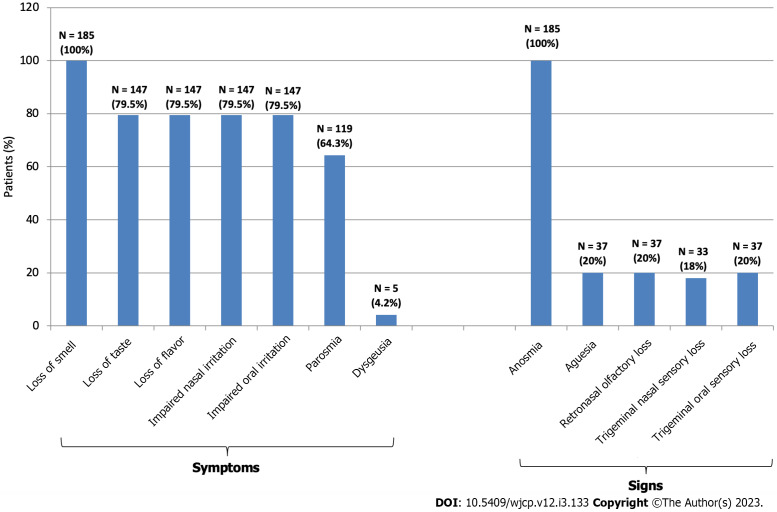
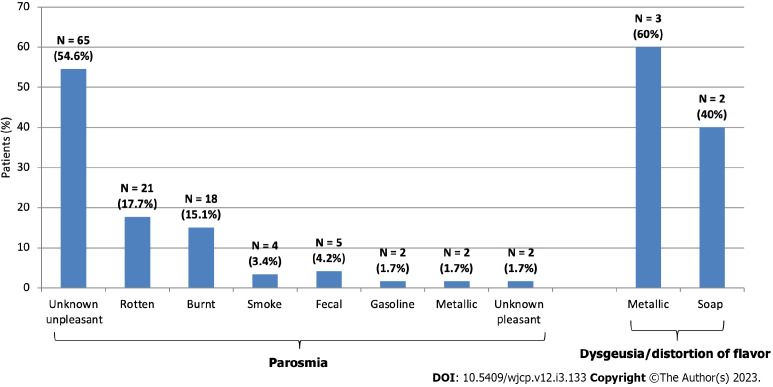
Subjective manifestations: At presentation, all complained of severe smell loss. They firstly noticed the sudden inability to recognize cooking scents or odors. However, taste and flavor (aroma) losses and loss of nasal and mouth irritation by pungent odorants (odors with strong trigeminal stimulation) and tastants were the complaint of 79.46% (n = 147) (Figure 3). Examples of complaints: “I can no longer smell the familiar scent of my favorites from perfumes, food dishes, and drinks”; “I have forgotten what normal tastes and smells are like”, “I changed my eating habits by adding too much sugar or salt to food to try to make it taste better”; “the pungent tastes don't affect me’. One hundred and nineteen (64.32%) also complained of parosmia. The onset of development of parosmia was ≥ 2 mo after smell loss (range: 2–6 mo; mean = 3.05 ± 1.87 mo). Cacosmia was the complaint of 40.34% of patients with parosmia (n = 48 or 48/119). The smells experienced by patients with parosmia included burnt (like burnt hair or burnt leather), rotten (as spoiled or fermented food), gasoline, fecal, smoke or metallic (like copper), or chemical (like sulphur). However, the majority (n = 65, 54.62%) complained of unpleasant unknown odor which was difficult to describe (i.e. new). Examples of complaints: “I can’t stand the scent of my own body sweat’; “smell of food was disgusting, repulsive and intolerable resulting in gastric upset’; ‘even tap water smells putrid’. None had phantosmia. Two experienced pleasant odors (1.68%) which were difficult to describe (i.e. unknown) (Figure 4). The majority of patients with parosmia complained of an aberrant taste with different foods and drinks similar to that being smelled. Few described aberrant tastes for only selective food types (e.g. proteins as meat, cheese, etc. or fruits as cucumber, Guava, etc.). But these distortions of taste/aroma were fluctuant in severity (i.e. not constant), however, five adults developed dysgeusia/distortion of flavor (approximately 1 mo) after improvement of parosmia, among were 3 described a taste/aroma of "metallic" and 2 had "soap' as dominant or the overriding sensation in the mouth for a whole range of foods and drinks including water.
Figure 3.
Manifestations of smell, taste, flavor and trigeminal sensory disorders of the studied patients.
Figure 4.
Types of parosmia and dysgeusia/distortion of flavor in the studied patients.
Objective manifestations
Objective testing revealed that participants had anosmia (SOIT scoring ≤ 8). True ageusia was found in only 20% (n = 37) (Figure 4) Patients with ageusia also had flavor (retronasal olfaction) loss (n = 37, 20%). Loss of all type of taste sensation (i.e. complete or total ageusia) was the most frequent (n = 21, 56.76% or 21/37), however, few (n = 16, 43.24% or 16/37) had selective (or partial) ageusia (salt and sweet = 3, 18.75% or 3/16); bitter and sour = 6, 37.5% or 6/16; umami = 7, 43.75% or 7/16). Losses of nasal and oral trigeminal sensations were found in 18% (n = 33) and 20% (n = 37) of patients, respectively (Figure 4).
Consequences of smell and taste disorders
Consequences of smell and taste disorders on patients were anorexia (n = 36; 19.46%) (particularly with cacosmia), anxiety symptoms (n = 21; 11.35%), insomnia (n = 12; 6.49%) and headache (n = 10; 5.41%). None had psychiatric disorders. The results of sQOD-NS are shown in Table 1. Patients had low total score (11.41 ± 3.66) of quality of life due to these disorders. All were afraid of permanent loss of smell and taste. None did self-isolation after onset and the problems did not restrict the patient’s social activities. Loss of appetite and even anorexia were reported by some patients particularly those with cacosmia but none developed significant weight loss.
Table 1.
Results of the questionnaire of olfactory disorders-negative statements
|
|
sQOD-NS items
|
Patients
|
Adults
|
Children
|
P value
|
| 1 | Changes in my sense of smell isolate me socially | 0.0–3.0 (2.84 ± 0.57) | 3 | 0.0–3.0 (2.17 ± 1.10) | 0.0001 |
| 2 | The problems with my sense of smell have a negative impact on my daily social activities | 0.0–3.0 (2.84 ± 0.57) | 3 | 0.0–3.0 (2.17 ± 1.10) | 0.0001 |
| 3 | The problems with my sense of smell make me more irritable | 0.0–3.0 (1.19 ± 1.03) | 0.0–3.0 (1.67 ± 0.99) | 0.0–3.0 (1.31 ± 1.180) | 0.497 |
| 4 | Because of the problems with my sense of smell, I eat out less | 0.0–3.0 (1.34 ± 1.06) | 0.0–3.0 (1.18 ± 1.01) | 1.0–3.0 (2.09 ± 0.85) | 0.0001 |
| 5 | Because of the problems with my sense of smell, I eat less than before (loss of appetite) | 0.0–3.0 (1.66 ± 1.28) | 0.0–3.0 (2.00 ± 1.03) | 0.0–3.0 (2.00 ± 1.03) | 0.0001 |
| 6 | Because of the problems with my sense of smell, I have to make more effort to relax | 0.0–3.0 (1.49 ± 1.11) | 0.0–3.0 (1.35 ± 1.12) | 0.0–3.0 (1.31 ± 1.180) | 0.065 |
| 7 | I’m afraid I’ll never be able to get used to the problems with my sense of smell | 0 | 0 | 0 | - |
| Total score of sQOD-NS | 2.0–18.0 (11.41 ± 3.66) | 6.0–18.8 (11.49 ± 3.37) | 2.0–18.0 (11.06 ± 4.74) | 0.515 |
sQOD-NS: Questionnaire of olfactory disorders-negative statements.
Comparative results between adults vs children
For each group (adults or children), no significant differences had been identified in relation to gender. No significant differences have been identified between adults and children in other demographics (with the exception that the majority of adults were rural residents), patterns of COVID-19 infection at onset and manifestations of disorders apart of the significantly lower frequency of children with loss of nasal trigeminal sensation compared to adults (P = 0.025) (Table 2). At presentation and compared to adults, the disorders had a moderate impact on many children daily routines and they caused restriction of food intake, lack of appetite or even anorexia, however, there was no significant difference in the total score of sQOD-NS for children compared to adults (Table 1).
Table 2.
Comparative results of adults versus children with smell and taste disorders, n (%)
|
Characteristics
|
Adults (n = 150)
|
Child (n = 35)
|
P value
|
| Age | 20–50 (31.41 ± 8.63) | 12–19 (15.66 ± 1.63) | - |
| Gender | 0.952 | ||
| Male | 72 (48) | 17 (48.6) | |
| Female | 78 (52) | 18 (51.4) | |
| Residence | 0.009 | ||
| Urban | 46 (30.7) | 19 (54.3) | |
| Rural | 104 (69.3) | 16 (45.7) | |
| Socio-economic status | 0.589 | ||
| Low | 79 (52.7) | 17 (48.6) | |
| Middle | 47 (31.3) | 11 (31.4) | |
| High | 24 (16) | 7 (20) | |
| Classification of patients | 0.077 | ||
| Mild COVID-19 infection | 123 (82) | 30 (85.7) | |
| - Minimal systemic manifestations | 72 (58.54) | 18 (51.43) | |
| - Isolated smell and taste loss | 51 (41.46) | 12 (34.29) | |
| Moderate COVID-19 infection | 27 (18) | 5 (14.3) | |
| Patterns of smell loss at onset (with or without taste loss) | 0.593 | ||
| Concurrent association with systemic and/or ENT viral manifestations at onset | 99 (66) | 23 (65.7) | |
| Isolated loss | 51 (34) | 12 (34.3) | |
| Systemic manifestations | 82 (54.67) | 18 (51.43) | 0.897 |
| Fever | 82 (100) | 18 (100) | |
| Cough | 42 (51.22) | 12 (66.67) | |
| Myalgia | 49 (59.76) | 10 (55.56) | |
| Fatigue | 45 (54.88) | 10 (55.56) | |
| Diarrhea | 34 (41.46) | 10 (55.56) | |
| Anorexia | 31 (37.80) | 18 (100) | |
| ENT manifestations | 56 (53.33) | 14 (40) | 0.989 |
| Nasal burning | 9 (16.07) | 3 (21.43) | |
| Electric like sensation in the nose | 9 (16.07) | 3 (21.43) | |
| Nasal dryness | 8 (14.29) | 2 (14.29) | |
| Sore throat | 29 (51.79) | 7 (50) | |
| Flu-like | 5 (8.93) | 2 (14.29) | |
| Sneezing | 5 (8.93) | 0 | |
| Mouth dryness | 4 (7.14) | 0 | |
| Duration of recovery of general, respiratory, gastrointestinal and other ENT manifestations; days | 4–30 (12.55 ± 6.04) | 5–15 (7.13 ± 2.69) | 0.082 |
| Previous treatment trials for smell and taste disorders | 0.335 | ||
| None | 29 (19.3) | 10 (28.57) | |
| Nasal irrigation | 19 (12.67) | 4 (11.43) | |
| Local steroids | 65 (43.33) | 23 (65.71) | |
| Systemic steroids | 14 (9.33) | 13 (37.14) | |
| Vitamins and supplements | 69 (43.33) | 17 (48.57) | |
| Olfactory training | 25 (16.67) | 17 (48.57) | |
| Consequences of sensory disorders | 0.516 | ||
| Headache | 6 (4) | 4 (11.43) | |
| Insomnia | 10 (6.67) | 2 (5.71) | |
| Anxiety | 16 (10.67) | 5 (14.29) | |
| Anorexia | 31 (20.67) | 5 (14.29) | |
| Recurrence of COVID-19 infection with smell loss | 3 (2) | - | |
| Duration of disorders at presentation | 6–24 (11.43 ± 3.87) | 6–24 (11.96 ± 4.41) | 0.515 |
| < 12 mo | 77 (51.33) | 13 (52.38) | 0.131 |
| ≥ 12 mo | 73 (48.67) | 22 (62.86) | |
| Onset of parosmia after smell loss | 2–6 (3.60 ± 1.52) | 1–5 (2.81 ± 1.47) | 0.442 |
| Parosmia | 98 (65.33) | 21 (60) | 0.284 |
| Types of parosmia | 42 (42.86) | 6 (28.57) | 0.709 |
| Unknown (unpleasant) | 52 (53.06) | 13 (52.38) | |
| Rotten | 17 (17.35) | 4 (19.05) | |
| Burnt | 15 (15.31) | 3 (14.29) | |
| Smoke | 3 (3.06) | 1 (4.76) | |
| Fecal | 5 (5.10) | - | |
| Gasoline | 2 (2.04) | - | |
| Metallic | 2 (2.04) | - | |
| Unknown (pleasant) | 2 (2.04) | - | |
| Dysgeusia | 5 (3.33) | - | |
| Types of dysgeusia | |||
| Metallic | 3 (60) | - | |
| Soap | 2 (40) | - | |
| Objective manifestations | |||
| Anosmia (orthonasal olfactory loss) | 150 (100) | 35 (100) | - |
| Ageusia | 30 (20) | 7 (20) | 0.918 |
| Flavor loss (retronasal olfactory loss) | 30 (20) | 7 (20) | 0.596 |
| Nasal trigeminal sensory loss | 30 (20) | 3 (8.57) | 0.025 |
| Oral trigeminal sensory loss | 30 (20) | 7 (20) | 0.918 |
Data are presented as number (%) and range (mean ± SD).
Analysis (adults versus children) was done using nonparametric tests: Independent Samples Mann-Whitney U test. Criteria: Alpha = 0.05, CI level = 95. COVID-19: Coronavirus disease 2019; ENT: Ear, nose and throat.
DISCUSSION
Studies which provided information about the prevalence, patterns and prognoses of post-COVID-19 smell and taste disorders are mainly from Europe and Asia. The majority were based on subjective self-evaluation or questionnaire completion of adult patients. The majority evaluated patients after short periods of follow-ups. Also little is known about the prevalence of these disorders in children. There are few reports which estimated the true frequency of these disorders after direct medical examination and comprehensive objective testing of patients over long periods (≥ 1 year) of follow-ups[7,8,28,29]. We provided the first detailed characterization of a cohort of Egyptian adults and children who developed persistent smell, taste and aroma disorders after COVID-19 infection.
Despite the increasing number of publications of post-COVID-19 complications, there is no consensus definition for “persistent post-COVID-19 smell disorders”, because of the wide variability of the periods of follow-up of patients since the onset of acute viral manifestations. In adults, some used these terms if the duration of disorders exceeded a month while many suggested longer durations equal or more than 3 or 6 mo. They also suggested other terms for these complications as “Long-lasting COVID-19 disorders” and “Chronic COVID-19 disorders”[6,9,12,14]. In this study, we included participants with duration of disorders of at least 6 mo since onset of acute manifestations, among were 52.43% of the patients (n = 97) had duration of disorders for ≥ 12 mo and 6 patients had the disorders for 24 mo. Fortunato et al[28] reported persistent smell dysfunction in 70% of patients at one year after the onset of COVID-19 infection. Boscolo-Rizzo et al[7] reported persistent smell dysfunction in 46% of patients by objective testing after a median of 401 d after the onset of COVID-19 infection. Lechien et al[8] followed 171 patients with post-COVID-19 smell disorders for 24 mo and reported a prevalence of olfactory dysfunction to range from 2.9% to 29.8%. For children, the definition of persistent post-COVID-19 smell disorders has been suggested in March 2022 by the Delphi process (which involved both researchers and family advocates) as manifestations that are lasting in a child for ≥ 3mo after the acute viral manifestations and cannot be explained by other known etiologies of smell disorders[29]. To our knowledge, there are only two reports for evaluation of smell and taste disorders in children[5,30]. In the study of Elvan-Tuz et al[30], the authors reported that in 8.4% of children, anosmia did not regress after a month since the onset of COVID-19 infection. In the study of Buonsenso et al[5], the authors found that 1.7% (13/784) of children had persistent smell disorders at a mean of 3mo after the onset of COVID-19 infection.
In this study, it is obvious that the number of adults with persistent disorders was 2.9 higher than children. We could not provide a prevalence of the disorders in patients with previous COVID-19 infection in spite of the fact that the patients were recruited from the Otolaryngology out-patient clinic of the university hospital (a tertiary referral hospital). This is because the healthcare service in our country is patchy and distributed between private clinics and governmental and university hospitals’ clinics. Patients were mainly referred from specialist otolaryngologists to the university hospital for the purpose of comprehensive evaluation. We did not find gender difference in the development of smell disorders after COVID-19 infection which is consistent with most of the previous reports. Few found potential gender difference with higher susceptibility for females to develop smell and taste disorders compared to males[31].
The most frequently done investigations (in approximately 70%) by the patients at onset were CBC, quick serology testing (IgM/IgG), CT of the chest (17%) and RT-PCR, particularly in patients with moderate manifestations. However, 17.3% did not do any investigations particularly those with isolated disorders. It has been indicated that the sudden onset of chemosensory dysfunctions in absence of a cause to explain these conditions in the presence of active local pandemic are often the main warning manifestations and the strongest predictors of COVID-19 infection[10,11]. Studies reported that people with loss of smell and taste had six-fold higher odds of being infected with COVID-19 positive and loss of smell and taste were associated with 10-fold higher odds of COVID-19 diagnosis[31-33]. The followings are the possible reasons for paucity of investigations at onset: (1) The presence of household members and intimately contacted friends at work places who experienced similar manifestations and diagnosed as having COVID-19 infection; (2) The information from the social media that smell and taste loss after COVID-19 infection are transient conditions in the majority of patients, i.e. disappear within weeks or few months (1-3 mo)[1,2,6,12]; (3) The reluctance to do investigations because of the minimal systemic manifestations or isolated disorders; (4) Polymerase chain reaction (PCR), serology and chest CT impose significant constraints on medical healthcare services in many parts of the world especially with limited infrastructure and resources and poor socioeconomic status of patients; and (5) Many healthcare providers are also reluctant to ask for investigations particularly if there was a delay for several weeks or months between the onset of chemosensory manifestations and the patient’s realization of the olfactory evaluations. It has been indicated that the viral load is significantly reduced after 14 d from onset and the estimated false-negative PCR rates for SARS-CoV-2 are high[33].
In this study, participants who had infected household family members distinguished the heterogeneity in phenotypes and prognoses of smell and taste losses encountered in their contacts[34]. It has also been previously reported that COVID-19 exhibits wide variation in duration, severity, and progression of symptoms, even within same family (i.e. a familial cluster of COVID-19 infection)[10,11]. It is interesting to mention that none of the participants, who were the only affected family members (~60%) did self-isolation or limited their social activities or transmitted the viral infection to other close contacts at the acute stage. Therefore, the questions are: Whether minimal/mild manifestations or isolated deficits at onset are highly contagious or not?, and, whether self-quarantine is a requirement as previously announced by the WHO and the ENT UK or not? More data are required to prove these issues. The announced recommendations by the WHO and the ENT UK for self-isolation for those with de novo loss or smell or taste during the active pandemic of COVID-19 was not clear compared to the recommendation for persons in intimate contact with patients having active COVID-19 infection (which is self-isolation for ≥ 2 wk). Some suggested self-isolation for ≥ 7d for those with de novo loss or smell or taste[10,11].
In this study, most of the participants (82%) had mild systemic or ENT manifestations at onset, among were 41.5% had isolated deficits in absence of any other viral manifestations. Many studies indicated that mild viral manifestations at onset could predict the prognosis of persistent smell disorders[9]. It has been suggested that the site and dosage of the initial viral burden, along with the effectiveness of the host's innate immune response to SARS-CoV-2 infection are important variables which potentially determine the spread of the virus within an individual and therefore the clinical course of infection, i.e. the high viral load in the upper airway could be correlated with reduction of the overwhelming host’s immune response resulting in a less severe general and systemic viral infection[35]. However, persistent smell disorders have also been reported in patients with severe viral infection diagnosis at onset[1,2,6,12].
At presentation, all patients complained of severe deficits in smell and the majority (approximately 80%) complained of associated taste loss, loss of aroma perception when eating foods and drinking beverages and loss of smell and taste of pungent substances. While objective testing showed that all had anosmia (orthonasal olfactory loss), 20% had true ageusia, flavor loss (retronasal olfactory loss) and oral trigeminal chemosensory loss and 18% had nasal trigeminal chemosensory loss. This mismatch between subjective and objective findings is not surprising. This also can explain the wide variability in the estimated prevalence of these disorders (i.e. over estimation bias) as the majority of reports relied on subjective or self-reported manifestations. In general, it is often difficult to distinguish between taste and smell and the belief that taste loss is a secondary result of smell loss is true to a great extent. This usually reflects loss of flavor perception as a function of smell and not true loss of taste. Also there is an intimate functional correlation between the flavor and taste systems and the intimate central connections between the olfactory and trigeminal systems and the overlapping activations in the piriform cortex, the ventral insula, and the frontal cortex[36]. The majority of patients with ageusia (56.8%) had total loss of sensation for the 5 basic types (salt, sweet, bitter, sour and umami) (i.e. complete ageusia), while, 43.24% had specific losses (or partial ageusia) particularly for umami[6]. Previous studies reported true complete or partial ageusia due to COVID-19 infection in 10.2%-42%. Some authors also found selective loss for sweet and bitter tastes[1,9,37]. Schwab et al[37] followed more than 400 patients infected with COVID-19 for 4 mo and reported little or no recovery from aguesia in patients who did not regained normal taste after approximately 2 mo. The prevalence of true nasal sensory (trigeminal) loss has been estimated to be 18%-20% with COVID-19 infection and the true oral trigeminal sensory loss was reported in 20%-33%[38].
We observed that none of the patients reported aguesia or flavor loss or trigeminal sensory loss in absence of anosmia. Flavor and loss of oral trigeminal sensation were dependent on aguesia and not anosmia. Discoveries found that orally sourced odors share processing circuitry with taste internal odors to produce flavor preferences[39,40]. Furthermore, the oral trigeminal sensation is considered a part of flavor perception[41]. The occurrence of taste, flavor and trigeminal sensory losses at lower frequencies and the differential taste loss in some patients, support that viral infection could involve taste and trigeminal tissues independent to smell[40,42].
In this study, rhinorrhea was a less frequent nasal (15% or 7/46) and ENT manifestation (10% or 7/70) at onset of viral infection. This is consistent with previous reports which found that the prevalence of rhinorrhea and nasal congestion was 4%-25% in patients infected with COVID-19 prior to experiencing anosmia[9,43]. We also observed that patients clearly distinguished their current conditions from the previously encountered fluctuation in the severity of smell and taste losses associated with runny nose, post-nasal discharge and congested and blocked nose associated with heavy cold or influenza. They mentioned that "previous nasal manifestations due to flu or influenza always resolve within days, and never affected taste of non-odorant tastants as sugar and salt".
In this study, parosmia was also the complaint of 64.3% (n = 119) of patients. They developed parosmia after anomia by several months[9,44]. Recently, Ferreli et al[43] reported that reported persistent smell dysfunction in 15.1% and 13.1% at 12 and 18 mo after the onset of COVID-19 infection based on a self-reported smell score. They also observed that 23.1% of patients developed parosmia at 18 mo after the onset of COVID-19 infection. We observed that many patients believed that parosmia was a manifestation of smell recovery. Parosmia and particularly cacosmia were also the dominant cause for repeated otolaryngology consultations and the use of nasal douches as a trial to reduce the intensity of the repulsive odors. In this study, the majority of patients with parosmia reported fluctuation in the experience of the same aberrant odor in the oral cavity with different foods and drinks (dysgeusia/distortion of aroma). However, five patients reported dominance of metallic (n = 3) or soap (n = 2) perception as baseline for every food or drink including tap water. They also mentioned that the abnormal sensation in the oral cavity became more apparent, intense and repulsive after reduction of the intensity of parosmia. Dysgeusia, the distortion of taste after COVID-19 infection, has been reported previously[45]. Vaira et al[44] reported anosmia and aguesia in 77% (67/87), anosmia only in 2.3% (2/87) and dysgeusia in 20% (18/87) of patients after COVID-19 infection. Distortion of aroma was reported in approximately 12% of patients after COVID-19 infection[41].
In this study, we did not identified apparent difference in other different demographic and clinical variables between adults and children with persistent disorders. In the literature, studies which addressed president smell and taste disorders in children are scare or poorly addressed[3-5]. This might be due to difficulty to apply objective testing or lack of standardized objective testing for smell and taste for children. In this study, symptoms of anxiety, insomnia, anorexia and headache were prominent among children compared to adults. They were often distressed by the impairments which hinder the enjoyment of food and hygiene problems related to body odor. It has been reported that the loss of the feeling of the depth and complexity of foods may result in anorexia, depression and anxiety. Deficits of olfaction disrupt the joy of eating and drinking and associated with the dangers of inability to know about the surrounding (e.g. environmental toxins as leaking gas or spoiled food). Parosmia as the constant repulsive odor is more distressing than loss of smell in many patients.
In this study, psychiatric evaluation at presentation and determination of quality of life in relation to smell disorders using sQOD-NS revealed lower performances and poor enjoyment of life particularly being afraid of development of permanent disorders. Smell and taste are essential for everyday life and psychological wellbeing. Many studies from Europe and Asia reported the adverse impact of long-lasting smell disorders on quality of life, personal-social functioning and mental health[45,46]. In a crosssectional study by Bagheri et al[46] on 10069 participants responded to an online checklist which evaluated the sense of smell and taste. The results indicated a significant correlation between anosmia and SARSCoV2 infection, decreased taste sensation, and decreased quality of life. In the cross-sectional survey of Abdelhafiz et al[47], the researchers enrolled 502 participants from Egyptians infected with COVID-19; the authors reported high prevalence of mental health symptoms among healthcare workers. Overall, 77.3%, 69.5%, 79.3%, and 83.1% of all participants reported symptoms of, anxiety, insomnia, depression, and stress, respectively. There was no particular assessment of quality of life due to chemosensory disorders in the same patients.
The detailed mechanisms for the persistent smell and taste disorders after COVID-19 infection are still understudied. There are evidences which support that injury of the neuroepithelium is the cause of persistent olfactory disorders after COVID-19 infection[48-50], they include: (1) Previous studies found that the olfactory neuronal epithelium requires 1-3 mo or longer to regenerate, restore the odorant receptor mapping, restore the olfactory receptor connections and rewire the axons within the olfactory system after injury compared to non-neuronal olfactory epithelium (which have susentacular, Bowman’s and microvillar cells) which requires 1-3 wk for recovery after degeneration[21]. Zazhytska et al[50] found that in infected golden hamsters, SARS-CoV-2 caused severe, persistent and widespread disruption of mature olfactory receptors’ nuclear genomic architecture. It also caused down regulation of olfactory receptors and olfactory sensory neuronal signaling genes for odor perception. The authors observed delayed olfactory neuronal transcription than susentacular cells and persistent disruption of olfactory receptor’s layer even after their restoration; (2) Studies indicated that parosmia is a step towards regeneration after olfactory receptors’ or neuronal degeneration (i.e. step toward recovery) and the unpleasant smell will disappear with full recovery[20,21]; and (3) Studies suggested that the disturbance of the spatially organized pattern of the olfactory receptors within the epithelium following viral infection, scars and gliosis are the causes of lost odor (i.e. anosmia) and distortion of smell sensation (i.e. parosmia)[20,21,51]. Earlier in pandemic, some authors suggested that nasal dryness which is attributed to the injury of the mucin producing cells (i.e. Bowman's gland) by viral pathology, is the cause of aberrant nasal smell (or parosmia) because they reported high frequency of nasal dryness among their patients. Lechien et al[52] reported nasal dryness in ≥ 60% of patients. They suggested that, this could be attributed to the damage of the mucous secreting olfactory cells (Bowman’s glands) which provide constant flow of mucous to the surface of the nasal epithelium to trap and dissolve odorous substances for the neurons. The findings of this and other studies do not support this suggestion because: Firstly, nasal dryness is an acute manifestation in some patients which recovered shortly within days to weeks like other ENT or systemic manifestations. In this study, only 5.4% (n = 10) had nasal dryness. Secondly, parosmia developed after recovery of acute manifestations by months. Thirdly, none of the contacts with transient smell loss developed parosmia.
The cause of nasal trigeminal sensory loss could be due to viral infection and damage to brush cells (or their feeding blood vessels). Brush cells are the microvilli columnar cells in the basal surface and in contact with afferent nerve endings of the trigeminal nerve responsible for transduction of nasal general sensation[53].
The mechanisms of taste disorders after COVID-19 infection are less studied compared to smell disorders. In animal models, researchers found that the family of CoVs, including MERS-CoV and SARS-COV-2, potentially use multiple entry oral receptors, making taste bud cells being highly susceptible for SARS-CoV-2 infections. These sites include ACE2, sialic acid and toll like receptors (TLRs)[54,55]. The tongue taste buds express ACE2 and sialic acid[55,56]. Sialic acid is a salivary mucin component. Mucin conveys the molecules of tastants into taste pores to prevent their premature enzymatic degradation[22]. Therefore, infection with SARS-CoV-2 could interfere with glycoproteins mediated transport of tastants and accelerated the degradation of the gustatory particles which contribute to loss of taste. The main function of the TLRs is to recognize the common structural components of microorganisms and activate the endogenous inflammatory immune system. In-situ models of direct binding of coronavirus spike protein of SARS-CoV-2 with TLR 1, 4 and 6, have support the specific roles of these TLRs in severe inflammation of the tongue tissue[57].
We believe that this study have strengths: (1) Up to our knowledge, this is the first study done in Egypt to explore the patterns at onset and characteristics of children as well as adults with post-COVID-19 persistent smell and taste disorders. In general, the evaluations of these disorders in children are limited or even lacking; and (2) The measures used for objective evaluation of smell, taste and flavor sensations are validated and reliable and can be generalized for evaluation of functional recovery overtime of smell and taste disorders in Egyptians. However, this study has limitations: (1) It may appear that patients with persistent disorders have severe deficits (i.e. anosmia or ageusia). This might be due to the recruitment of patients from a tertiary referral hospital (a University Hospital). It is possible that patients with persistent less severe disorders (i.e. hyposmia or hypogeusia) were reluctant to seek medical advice or might visited other available healthcare services (e.g. private clinics); and (2) The number of included children is lower than adults. This could be due to lower frequency of these disorders in children compared to adults. Future studies are required to determine the prevalence of these disorders in pediatric population.
CONCLUSION
This study indicates that children can develop persistent smell and taste disorders due to COVID-19 infection similar to adults. The reported course of small and taste disorders are supportive of the peripheral neuronal compromise. True taste and trigeminal chemosensory disorders could be a complication of COVID-19 infection but with lower frequency compared to smell disorders and also might occur independent to smell disorders. Post-COVID-19 retronasal olfactory (flavor) disorders were solely dependent on taste and not on smell disorders. Smell and taste disorders significantly impair quality of life of patients particularly being afraid of permanent disorders. There were no clinical variables which characterize smell and taste disorders in children from adult patients.
ARTICLE HIGHLIGHTS
Research background
Smell loss with or without taste loss is the most frequent acute manifestation of coronavirus disease 2019 (COVID-19) infection with an estimated prevalence of 16%-20% in children and 40%-86% in adults. Smell disorders are also the most frequent complications of COVID-19 infection with an estimated prevalence of approximately 20%-40% of adults. This indicates that COVID-19 has high affinity to olfactory sensory epithelium (and to less extent gustatory sensory epithelium) compared to other parts of the body. Data from patients and their contacts showed that it is impossible to predict the prognosis of smell loss (i.e. none of the demographics, acute manifestations and severity at onset were predictors for the development of persistent disorders). It has been indicated that the mechanisms of transient smell and taste deficits due to COVID-19 infection are different from that of long-lasting/persistent disorders. It has been suggested that injury of the non-neuronal olfactory epithelial cells by viral infection and their rapid regeneration (within days to weeks) are the causes of transient smell deficits because these cells are important for the health of the neuronal cells. However, lasting smell disorders are due to injury of the neuronal olfactory epithelial cells and disorganization of the receptors within the epithelium, because these cells require months to regenerate after injury and restore olfactory epithelium function. The mechanisms of taste disorders after COVID-19 infection are less understood compared to smell disorders but suggested to be due to injury of the gustatory sensory epithelium by viral infection and disturbed salivary milieu which is necessary for the function of the gustatory neurons.
Research motivation
The research hotspots include determination of (1) The patterns of smell and taste disorders at onset; (2) The course of these disorders till presentation; and (3) Whether or not there is/are distinguished features for children with these disorders which differentiate them from adult patients with the same disorders.
Research objectives
This study aimed to systematically evaluate patients with post-COVID-19 infection persistent smell and taste disorders because related studies from many areas of the world including our country are lacking. The descriptive characteristics of patients at onset and presentation and predictors for the development of these disorders were determined in children and adult populations.
Research methods
Data collection which included a clinical questionnaire (for smell and taste); objective testing which included the sniffin' odor, taste and flavor identification tests and the Questionnaire of Olfactory Disorders-Negative Statements for determination of quality of life in response to these disorders.
Research results
This study included 185 patients (adults = 150, age: 31.41 ± 8.63 years; children = 35; age: 15.66 ± 1.63 years) from both gender and had post-COVID-19 infection smell and taste disorders. The duration of the disorders till the time of presentation ranged from 6 to 24 mo (mean: 11.53 ± 3.97 mo) with nearly half of the patients had duration of at least a year. Parosmia was a frequent manifestation (64.32%) in patients with persistent anosmia and was developed after months from onset (3.05 ± 1.87 ms). Total or partial true ageusi, retronasal olfactory loss (or flavor) and trigeminal chemosensory loss were present in 18%-20% of patients. There were no significant differences in patterns at onset and clinical variables at onset and at presentation between children and adults with persistent disorders. These disorders significantly lower quality of life of patients.
Research conclusions
Persistent smell, taste and trigeminal chemosensory disorders are frequent post-COVID-19 complications in children similar to adults. There were no demographics, clinical variables at onset or specific profile of these disorders which distinguish children from adults’ patients.
Research perspectives
Future studies are needed to: (1) Determine the prevalence of these disorders in children and in adults from understudied populations (e.g. Africans); (2) Determine the relationship between smell and taste disorders due to different COVID-19 variants (alpha, delta or omicron). In the literature, there were no relevant studies which stratified recovery according to COVID-19 variants. Here, the period of patients’ recruitment in this study indicated a high possibility of being infected with the wild types of the virus; and (3) Understand the detailed cellular and molecular pathogenic aspects underlying long-lasting/persistent chemosensory disorders after COVID-19 infection.
Footnotes
Institutional review board statement: The protocol of the study was in accordance to the revised Helsinki Declaration (2013) and approved by the medical research ethics committees of the Faculty of Medicine, Assiut University, Assiut, Egypt, No. AUH_SARS-CoV2_SAH/2019.
Informed consent statement: All study participants, or their guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: February 27, 2023
First decision: March 15, 2023
Article in press: April 20, 2023
Specialty type: Pediatrics
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H, South Korea; Suvvari TK, India S-Editor: Li L L-Editor: A P-Editor: Zhang XD
Contributor Information
Sherifa Ahmed Hamed, Department of Neurology and Psychiatry, Assiut University, Faculty of Medicine, Assiut 71516, Egypt. hamedsherifa@aun.edu.eg.
Eman Bahaa Kamal-Eldeen, Department of Pediatrics, Assiut University, Faculty of Medicine, Assiut 71516, Egypt.
Mohamed Azzam Abdel-Razek Ahmed, Department of ENT, Assiut University, Faculty of Medicine, Assiut 71516, Egypt.
Data sharing statement
No additional data are available.
References
- 1.Koyama S, Ueha R, Kondo K. Loss of Smell and Taste in Patients With Suspected COVID-19: Analyses of Patients' Reports on Social Media. J Med Internet Res. 2021;23:e26459. doi: 10.2196/26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomberg B, Mohn KG, Brokstad KA, Zhou F, Linchausen DW, Hansen BA, Lartey S, Onyango TB, Kuwelker K, Sævik M, Bartsch H, Tøndel C, Kittang BR Bergen COVID-19 Research Group, Cox RJ, Langeland N. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27:1607–1613. doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roge I, Smane L, Kivite-Urtane A, Pucuka Z, Racko I, Klavina L, Pavare J. Comparison of Persistent Symptoms After COVID-19 and Other Non-SARS-CoV-2 Infections in Children. Front Pediatr. 2021;9:752385. doi: 10.3389/fped.2021.752385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan Q, Qiu D, Liu X, Guo X, Hu Y. Prevalence of Smell or Taste Dysfunction Among Children With COVID-19 Infection: A Systematic Review and Meta-Analysis. Front Pediatr. 2021;9:686600. doi: 10.3389/fped.2021.686600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonsenso D, Martino L, Morello R, De Rose C, Valentini P. Chronic Olfactory Dysfunction in Children with Long COVID: A Retrospective Study. Children (Basel) 2022;9 doi: 10.3390/children9081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niklassen AS, Draf J, Huart C, Hintschich C, Bocksberger S, Trecca EMC, Klimek L, Le Bon SD, Altundag A, Hummel T. COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre study on Smell and Taste. Laryngoscope. 2021;131:1095–1100. doi: 10.1002/lary.29383. [DOI] [PubMed] [Google Scholar]
- 7.Boscolo-Rizzo P, Hummel T, Hopkins C, Dibattista M, Menini A, Spinato G, Fabbris C, Emanuelli E, D'Alessandro A, Marzolino R, Zanelli E, Cancellieri E, Cargnelutti K, Fadda S, Borsetto D, Vaira LA, Gardenal N, Polesel J, Tirelli G. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post-COVID-19 patients: a matched case-control study with one-year follow-up using a comprehensive psychophysical evaluation. Rhinology. 2021;59:517–527. doi: 10.4193/Rhin21.249. [DOI] [PubMed] [Google Scholar]
- 8.Lechien JR, Vaira LA, Saussez S. Prevalence and 24-month recovery of olfactory dysfunction in COVID-19 patients: A multicentre prospective study. J Intern Med. 2023;293:82–90. doi: 10.1111/joim.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechien JR, Chiesa-Estomba CM, Beckers E, Mustin V, Ducarme M, Journe F, Marchant A, Jouffe L, Barillari MR, Cammaroto G, Circiu MP, Hans S, Saussez S. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. 2021;290:451–461. doi: 10.1111/joim.13209. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Otolaryngology–Head and Neck Surgery. Anosmia as a potential marker of COVID-19 infection - an update. Apr 1, 2020 [cited 6 June 2020]. Available from: https://www.entnet.org/COVID-19/anosmia/
- 11.American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) Anosmia Reporting tool. 2020. [cited 28 September 2021]. Available from: https://www.entnet.org/content/reporting-tool-patients-anosmia-related-COVID-19 .
- 12.Chapurin N, Totten DJ, Chaballout B, Brennan J, Dennis S, Lubner R, Chowdhury NI, Turner JH, Trone T, Chandra RK. Differential olfactory outcomes in COVID-19: A large healthcare system population study. Int Forum Allergy Rhinol. 2022;12:108–111. doi: 10.1002/alr.22870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biadsee A, Dagan O, Ormianer Z, Kassem F, Masarwa S, Biadsee A. Eight-month follow-up of olfactory and gustatory dysfunctions in recovered COVID-19 patients. Am J Otolaryngol. 2021;42:103065. doi: 10.1016/j.amjoto.2021.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estiri H, Strasser ZH, Brat GA, Semenov YR Consortium for Characterization of COVID-19 by EHR (4CE), Patel CJ, Murphy SN. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med. 2021;19:249. doi: 10.1186/s12916-021-02115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasinka M, Plenta I, Pavlovic M, Izakovic V, Kaiserová E, Páleníková O, Stubna J. [Complications of immunosuppresive therapy in chronic nephropathies in children] Cesk Pediatr. 1976;31:122–126. [PubMed] [Google Scholar]
- 16.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 17.Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Ar Gouilh M, Lesellier S, Servat A, Wasniewski M, Picard-Meyer E, Monchatre-Leroy E, Volmer R, Rampin O, Le Goffic R, Marianneau P, Meunier N. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 2020;89:579–586. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, Kaewpreedee P, Perera RAPM, Poon LLM, Nicholls JM, Peiris M, Yen HL. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–838. doi: 10.1038/s41586-020-2342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee KK, Costanzo RM. Changes in odor quality discrimination following recovery from olfactory nerve transection. Chem Senses. 1998;23:513–519. doi: 10.1093/chemse/23.5.513. [DOI] [PubMed] [Google Scholar]
- 21.Iwema CL, Fang H, Kurtz DB, Youngentob SL, Schwob JE. Odorant receptor expression patterns are restored in lesion-recovered rat olfactory epithelium. J Neurosci. 2004;24:356–369. doi: 10.1523/JNEUROSCI.1219-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pushpass RG, Pellicciotta N, Kelly C, Proctor G, Carpenter GH. Reduced Salivary Mucin Binding and Glycosylation in Older Adults Influences Taste in an In Vitro Cell Model. Nutrients. 2019;11 doi: 10.3390/nu11102280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance. Jan 17, 2020. [cited 3 April 2023]. Available from: https://www.who.int/publications-detail/Laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 .
- 24.El-Gilany A, El-Wehady A, El-Wasify M. Updating and validation of the socioeconomic status scale for health research in Egypt. East Mediterr Health J. 2012;18:962–968. doi: 10.26719/2012.18.9.962. [DOI] [PubMed] [Google Scholar]
- 25.Rawal S, Hoffman HJ, Honda M, Huedo-Medin TB, Duffy VB. The Taste and Smell Protocol in the 2011-2014 US National Health and Nutrition Examination Survey (NHANES): Test-Retest Reliability and Validity Testing. Chemosens Percept. 2015;8:138–148. doi: 10.1007/s12078-015-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oleszkiewicz A, Taut M, Sorokowska A, Radwan A, Kamel R, Hummel T. Development of the Arabic version of the "Sniffin' Sticks" odor identification test. Eur Arch Otorhinolaryngol. 2016;273:1179–1184. doi: 10.1007/s00405-015-3718-2. [DOI] [PubMed] [Google Scholar]
- 27.Mattos JL, Edwards C, Schlosser RJ, Hyer M, Mace JC, Smith TL, Soler ZM. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9:1144–1150. doi: 10.1002/alr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortunato F, Martinelli D, Iannelli G, Milazzo M, Farina U, Di Matteo G, De Nittis R, Ascatigno L, Cassano M, Lopalco PL, Prato R. Self-reported olfactory and gustatory dysfunctions in COVID-19 patients: a 1-year follow-up study in Foggia district, Italy. BMC Infect Dis. 2022;22:77. doi: 10.1186/s12879-022-07052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephenson T, Allin B, Nugawela MD, Rojas N, Dalrymple E, Pinto Pereira S, Soni M, Knight M, Cheung EY, Heyman I CLoCk Consortium, Shafran R. Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child. 2022;107:674–680. doi: 10.1136/archdischild-2021-323624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elvan-Tuz A, Karadag-Oncel E, Kiran S, Kanik-Yuksek S, Gulhan B, Hacimustafaoglu M, Ozdem-Alatas S, Kuyucu N, Ozdemir H, Egil O, Elmas-Bozdemir S, Polat M, Bursal-Duramaz B, Cem E, Apaydin G, Teksam O TURK-COVID-19-Anosmia Study Group; LAS VEGAS study investigators, PROVE Network and the Clinical Trial Network of the European Society of Anaesthesiology. Prevalence of Anosmia in 10.157 Pediatric COVID-19 Cases: Multicenter Study from Turkey. Pediatr Infect Dis J. 2022;41:473–477. doi: 10.1097/INF.0000000000003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, Sorokowska A. Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol. 2019;10:242. doi: 10.3389/fpsyg.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pecoraro V, Negro A, Pirotti T, Trenti T. Estimate false-negative RT-PCR rates for SARS-CoV-2. A systematic review and meta-analysis. Eur J Clin Invest. 2022;52:e13706. doi: 10.1111/eci.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song WL, Zou N, Guan WH, Pan JL, Xu W. Clinical characteristics of COVID-19 in family clusters: a systematic review. World J Pediatr. 2021;17:355–363. doi: 10.1007/s12519-021-00434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilyicheva TN, Netesov SV, Gureyev VN. COVID-19, Influenza, and Other Acute Respiratory Viral Infections: Etiology, Immunopathogenesis, Diagnosis, and Treatment. Part I. COVID-19 and Influenza. Mol Gen Microbiol Virol. 2022;37:1–9. doi: 10.3103/S0891416822010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cain WS, Murphy CL. Interaction between chemoreceptive modalities of odour and irritation. Nature. 1980;284:255–257. doi: 10.1038/284255a0. [DOI] [PubMed] [Google Scholar]
- 37.Schwab J, Jensen CD, Fjaeldstad AW. Sustained Chemosensory Dysfunction during the COVID-19 Pandemic. ORL J Otorhinolaryngol Relat Spec. 2021;83:209–218. doi: 10.1159/000515132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, Kaur R, Liuzza MT, Pepino MY, Schöpf V, Pereda-Loth V, Olsson SB, Gerkin RC, Rohlfs Domínguez P, Albayay J, Farruggia MC, Bhutani S, Fjaeldstad AW, Kumar R, Menini A, Bensafi M, Sandell M, Konstantinidis I, Di Pizio A, Genovese F, Öztürk L, Thomas-Danguin T, Frasnelli J, Boesveldt S, Saatci Ö, Saraiva LR, Lin C, Golebiowski J, Hwang LD, Ozdener MH, Guàrdia MD, Laudamiel C, Ritchie M, Havlícek J, Pierron D, Roura E, Navarro M, Nolden AA, Lim J, Whitcroft KL, Colquitt LR, Ferdenzi C, Brindha EV, Altundag A, Macchi A, Nunez-Parra A, Patel ZM, Fiorucci S, Philpott CM, Smith BC, Lundström JN, Mucignat C, Parker JK, van den Brink M, Schmuker M, Fischmeister FPS, Heinbockel T, Shields VDC, Faraji F, Santamaría E, Fredborg WEA, Morini G, Olofsson JK, Jalessi M, Karni N, D'Errico A, Alizadeh R, Pellegrino R, Meyer P, Huart C, Chen B, Soler GM, Alwashahi MK, Welge-Lüssen A, Freiherr J, de Groot JHB, Klein H, Okamoto M, Singh PB, Hsieh JW GCCR Group Author, Reed DR, Hummel T, Munger SD, Hayes JE. More Than Smell-COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chem Senses. 2020;45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blankenship ML, Grigorova M, Katz DB, Maier JX. Retronasal Odor Perception Requires Taste Cortex, but Orthonasal Does Not. Curr Biol. 2019;29:62–69.e3. doi: 10.1016/j.cub.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, Lin C, Joseph PV, Reed DR. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chem Senses. 2022;47 doi: 10.1093/chemse/bjac001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Chen B, Akshita J, Han P, Thaploo D, Kitzler HH, Hummel T. Aberrancies of Brain Network Structures in Patients with Anosmia. Brain Topogr. 2020;33:403–411. doi: 10.1007/s10548-020-00769-2. [DOI] [PubMed] [Google Scholar]
- 43.Ferreli F, Gaino F, Russo E, Di Bari M, Rossi V, De Virgilio A, Di Stadio A, Spriano G, Mercante G. Long-term olfactory dysfunction in COVID-19 patients: 18-month follow-up study. Int Forum Allergy Rhinol. 2022;12:1078–1080. doi: 10.1002/alr.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, Cucurullo M, Salzano FA, Saussez S, Boscolo-Rizzo P, Biglioli F, De Riu G. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol. 2020;134:703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O, Huang E, Zuo QK. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486:90–111. doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagheri S, Ghobadimoghadam S. Safety and Health Protection of Health Care Workers during the COVID-19 Pandemic. Int J Community Based Nurs Midwifery. 2020;8:362–363. doi: 10.30476/IJCBNM.2020.86066.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelhafiz AS, Ali A, Maaly AM, Mahgoub MA, Ziady HH, Sultan EA. Predictors of post-COVID symptoms in Egyptian patients: Drugs used in COVID-19 treatment are incriminated. PLoS One. 2022;17:e0266175. doi: 10.1371/journal.pone.0266175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maier EC, Saxena A, Alsina B, Bronner ME, Whitfield TT. Sensational placodes: neurogenesis in the otic and olfactory systems. Dev Biol. 2014;389:50–67. doi: 10.1016/j.ydbio.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo PM. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, McArthur NG, Moeller R, Uhl S, Omer AD, Gottesman ME, Firestein S, Gong Q, Canoll PD, Goldman JE, Roussos P, tenOever BR, Jonathan B Overdevest, Lomvardas S. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell. 2022;185:1052–1064.e12. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murai A, Iwata R, Fujimoto S, Aihara S, Tsuboi A, Muroyama Y, Saito T, Nishizaki K, Imai T. Distorted Coarse Axon Targeting and Reduced Dendrite Connectivity Underlie Dysosmia after Olfactory Axon Injury. eNeuro. 2016;3 doi: 10.1523/ENEURO.0242-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Genovese F, Tizzano M. Microvillous cells in the olfactory epithelium express elements of the solitary chemosensory cell transduction signaling cascade. PLoS One. 2018;13:e0202754. doi: 10.1371/journal.pone.0202754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gadanec LK, McSweeney KR, Qaradakhi T, Ali B, Zulli A, Apostolopoulos V. Can SARS-CoV-2 Virus Use Multiple Receptors to Enter Host Cells? Int J Mol Sci. 2021;22 doi: 10.3390/ijms22030992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun XL. The role of cell surface sialic acids for SARS-CoV-2 infection. Glycobiology. 2021;31:1245–1253. doi: 10.1093/glycob/cwab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol. 2020;92:2105–2113. doi: 10.1002/jmv.25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.