Abstract
Background:
Ultrasonography is capable of detecting morphological changes in femoral articular cartilage cross-sectional area in response to an acute bout of walking; yet, the response of femoral cartilage cross-sectional area varies between individuals. It is hypothesized that differences in joint kinetics may influence the response of cartilage to a standardized walking protocol. Therefore, the study purpose was to compare internal knee abduction and extension moments between individuals with anterior cruciate ligament reconstruction who demonstrate an acute increase, decrease, or unchanged medial femoral cross-sectional area response following 3,000 steps.
Methods:
The medial femoral cartilage in the anterior cruciate ligament reconstructed limb was assessed with ultrasonography before and immediately following 3,000 steps of treadmill walking. Knee joint moments were calculated in the anterior cruciate ligament reconstructed limb and compared between groups throughout the stance phase of gait using linear regression and functional, mixed effects waveform analyses.
Findings:
No associations between peak knee joint moments and the cross-sectional area response were observed. The group that demonstrated an acute cross-sectional area increase exhibited 1) lower knee abduction moments in early stance in comparison to the group that exhibited a decreased cross-sectional area response; and 2) greater knee extension moments in early stance in comparison to the group with an unchanged cross-sectional area response.
Interpretation:
The propensity of femoral cartilage to acutely increase cross-sectional area in response to walking is consistent with less-dynamic knee abduction and knee extension moment profiles.
Keywords: ACL Reconstruction, Ultrasound, Cartilage Cross-Sectional Area, Knee Abduction Moment, Knee Extension Moment
I. Introduction
Individuals who undergo anterior cruciate ligament reconstruction (ACLR) are at risk for developing post-traumatic osteoarthritis (PTOA) as early as 10 years following reconstruction (Luc et al., 2014). Understanding the femoral cartilage response to loading during activities of daily living is important for determining early alterations in morphology and mechanical tissue properties that may linked to future cartilage disease progression (Eckstein, 2005; Harkey et al., 2017; Pfeiffer et al., 2020; Sutter et al., 2015). Previous work has established that resting morphological cartilage tissue changes occur early post-ACLR and are associated with PTOA disease onset and progression (Eckstein et al., 2015; Frobell, 2011; Iriondo et al., 2021). Assessing the acute femoral cartilage response to loading following ACLR may provide additional information regarding the capacity of the tissue to resist loading, which is important for determining the functional status of the cartilage. Ultrasonography is an easily-accessible and reliable method to assess in vivo femoral cartilage morphology (e.g., thickness, cross-sectional area [CSA]) (Abraham et al., 2011; Iagnocco, 2010; Naredo et al., 2009) and has the capability to assess cartilage morphology before and acutely following a mechanical loading activity in a time-efficient and cost-effective manner.
Previous work demonstrates that uninjured individuals exhibit medial femoral cartilage deformation (e.g. reduction in CSA), as viewed in the axial plane, following 20 minutes of running (Harkey et al., 2017) and after completing 3,000 (Pfeiffer et al., 2020) and 5,000 steps of walking at a self-selected pace (Harkey et al., 2018). However, other studies have demonstrated that not all individuals undergo femoral cartilage CSA deformation following similar acute bouts of activity. Studies assessing the morphological response of femoral cartilage to loading using ultrasonography found subgroups of individuals that exhibited either negligible changes in femoral cartilage CSA, CSA deformation, or demonstrated increased femoral cartilage CSA following a standardized walking task (Evans-Pickett et al., 2022; Pfeiffer et al., 2019). While the response of femoral cartilage CSA to acute loading varies among individuals preforming a similar task, limited research has sought to understand the mechanisms contributing to variability in the acute morphological cartilage response to loading.
Previous researchers have hypothesized that differences in mechanical joint loading may explain heterogeneous, chronic changes in cartilage morphology in the ACLR population (Andriacchi et al., 2009). Persistent aberrant gait biomechanics are common following ACLR (Andriacchi et al., 2004; Davis-Wilson et al., 2020; Kaur et al., 2016). Lesser peak knee extension moments (KEM) have been reported early post-ACLR (H. F. Hart et al., 2016; Slater et al., 2017) and may reflect the inability to regain quadricep strength post-operatively (Lewek et al., 2002; Palmieri-Smith et al., 2016). Knee abduction moments (KAM) are associated with medial compartmental loading (Andriacchi, 1994; Schipplein & Andriacchi, 1991), and the medial compartment has been observed to demonstrate the most pronounced changes in osteoarthritis disease progression (Dearborn et al., 1996; Pelletier et al., 2007). Literature assessing KAM has primarily focused on individuals with idiopathic osteoarthritis and has linked greater KAM to more progressive cartilage disease (Mündermann et al., 2005); yet there is evidence that PTOA may exhibit a unique pathophysiology that differs from idiopathic osteoarthritis. Limited studies have evaluated the relationship between knee kinetics and changes in femoral cartilage morphology; however, previous work by Pamukoff et al. found a weak association between lesser peak KAM and greater resting medial femoral cartilage thickness as assessed by ultrasound in individuals 60.6 ± 24.8 months post-ACLR ( 2018). In the same study, no association between peak KEM and resting medial femoral cartilage thickness was observed (Pamukoff et al., 2018). Different analytical techniques exist to comprehensively study biomechanical changes throughout stance phase (Z. Horton et al., 2021; Park et al., 2017). Less-dynamic KAM profiles as described by Reeves and Bowling (i.e., waveform with lower local maximum peaks and greater local minimum peaks) (2011) and altered KEM profiles described by Davis-Wilson et. al. (lesser KEM surrounding the first peak and greater KEM in late stance; 2020) may lead to more sustained and pathological tissue loading during cyclic activities (i.e., walking) may increase cartilage strain during ambulation (Arokoski et al., 2008; Sophia Fox et al., 2009).
Therefore, the purpose of this study was to first assess the association between peak kinetic variables of interest (KEM and KAM) and the acute CSA response to a 3,000-step treadmill walking task. Next, we compared KEM and KAM throughout the entirety of stance phase between individuals with ACLR who demonstrate an acute increase (CSAI), decrease (CSAD) or unchanged (CSAU) medial femur CSA response following a standardized treadmill walking task. We hypothesized that there would be an association between lesser peak KAM and KEM and an increase in medial femoral CSA. Further we hypothesized that the CSAI group would demonstrate lesser KAM at both the first and second peak and greater KAM at midstance, as compared to the CSAD and CSAU groups. We also hypothesized that the CSAI group would exhibit lower first peak KEM and greater KEM in late stance.
2. Methods
2.1. Study Design
We conducted a cross-sectional analysis at a single visit between 6 and12 months post-ACLR utilizing data from a larger randomized experiment (NCT03035994). All participants were recruited from a local orthopaedic clinic, the surrounding community, recreational clubs, and intercollegiate athletics. Participants were retrospectively assigned to the CSAI, CSAD, or CSAU medial femoral CSA response groups after the cohort completed all study procedures. After a 45-minute unloading period, femoral articular cartilage ultrasonography assessment was conducted before and immediately following a standardized 3,000-step walking protocol during which gait kinematics and kinetics were sampled. Study protocols and recruitment methods were approved by the Biomedical Institutional Review Board and all participants provided written, informed consent.
2.2. Participants
All participants were between 16 and 35 years of age and underwent primary, unilateral arthroscopic bone-patellar-tendon bone autograft ACLR. We excluded individuals: 1) with a history of a lower extremity orthopaedic surgery before or after ACLR; 2) who required multi-ligament reconstruction at the time of ACLR; 3) with a body mass index (BMI) >35kg/m2; 4) with radiographic knee OA; or 5) who were pregnant. Relevant demographic information including sex, BMI, months post-ACLR and self-selected walking speed were collected and reported in Table 1. A previous study demonstrated a moderate association (r=0.46) between greater peak KAM and thinner resting medial femoral cartilage thickness after accounting for covariates (Pamukoff et al., 2018). Therefore, we estimate that 38 individuals would be necessary to determine statistical significance if moderate associations (r>0.44) were detected between peak kinetic variables (KEM and KAM) and the acute CSA response to a standardized walking protocol (two tailed alpha= 0.05; 1-ß = 0.8; G*Power Statistical power Analysis Software 3.1). Secondly, with a minimum of 11 (5 gait trials each) participants in each group, we will have the capability to detect statistically significant, between-group differences in the presence of moderate effect sizes (d>0.54) utilizing the functional mixed effects model analysis (two tailed alpha= 0.05; 1-ß = 0.8; G*Power Statistical power Analysis Software 3.1).
TABLE 1.
Demographic Information by Group (Mean ± SD)
| Unchanged CSA (n=17) |
Increased CSA (n=11) |
Decreased CSA (n= 11) |
P value |
|
|---|---|---|---|---|
|
| ||||
| Age | 20.50 ± 5.27 | 19.35 ± 1.89 | 20.74 ± 3.51 | 0.4 4 |
| Body Mass Index (kg/m2) | 24.75 ± 3.50 | 24.39 ± 2.70 | 24.68 ± 3.58 | 0.9 3 |
| Sex (% Female) | 41.12% | 63.64% | 54.55% | N/A |
| Months Post-ACLR | 7.82 ± 2.24 | 8.36 ± 1.96 | 8.36 ± 1.57 | 0.4 5 |
| Walking Speed (m/s) | 1.21 ± 0.07 | 1.21 ± 0.11 | 1.27 ± 0.12 | 0.1 5 |
| Medial Compartment CSA Change Score (mm2) | −0.02 ± 0.83* | 3.21 ± 1.08* | −4.09 ± 1.75* | <0. 01* |
ACLR: Anterior Cruciate Ligament Reconstruction; CSA: Cross-sectional Area; SD: Standard Deviation
Significant Group Difference
2.3. Ultrasound Image Acquisition
All participants rested in a long-sit position with their back against a wall for 45 minutes to offload the femoral articular cartilage. Ultrasonographic images were obtained from the ACLR limb prior to and immediately following the walking condition with the knee in 140° of flexion as verified by a handheld goniometer (Harkey et al., 2017). The treatment table was affixed with a tape measure, and the distance between the wall and the posterior calcaneus was recorded (Harkey et al., 2017) to verify positioning between the baseline and post-walking timepoints. Images were acquired utilizing a LOGIQe ultrasound system (General Electric, Fairfield, Connecticut) by 1 of 2 investigators trained by a musculoskeletal radiologist. A 12-MHz linear probe was aligned transversely over the femur, just superior to the base of the patella and was rotated in the sagittal plane to view the femoral cartilage surface (Harkey, Blackburn, Hackney, et al., 2018; Naredo et al., 2009). Additionally, a transparent grid was added to the ultrasound screen to improve placement reliability of the probe at the post-loading assessment (Harkey, Blackburn, Hackney, et al., 2018; Harkey et al., 2017). Three ultrasonographic images were acquired before and immediately following a 3,000-step treadmill walking task.
A single, blinded investigator performed all manual segmentations of the US images (ImageJ, National Institutes of Health, Bethesda, MD). Previous research has demonstrated strong intra-session and inter-session reliability in processing femoral articular cartilage ultrasound images (Harkey et al., 2017; Pfeiffer et al., 2020). Femoral articular cartilage cross-sectional area (mm2) was determined by segmenting the visible tibial cartilage-bone interface and femoral soft tissue-cartilage interface (Harkey, Blackburn, Hackney, et al., 2018). The horizontal midpoint of the entire femoral articular cartilage CSA was utilized to separate the medial and lateral compartments (Figure 1). The medial femoral CSA of the three segmented images was averaged, and medial compartment CSA change scores were calculated as the CSA measured during the post-loading condition subtracted from the pre-loading CSA measurement. Participants were then classified based on a previously reported minimal detectable change within the medial compartment (±1.68 mm2) (Harkey, Blackburn, Hackney, et al., 2018). Groups were defined as CSAI (CSA change scores >+1.68 mm2), CSAD (< −1.68 mm2) and CSAU (within ±1.68 mm2).
FIGURE 1.
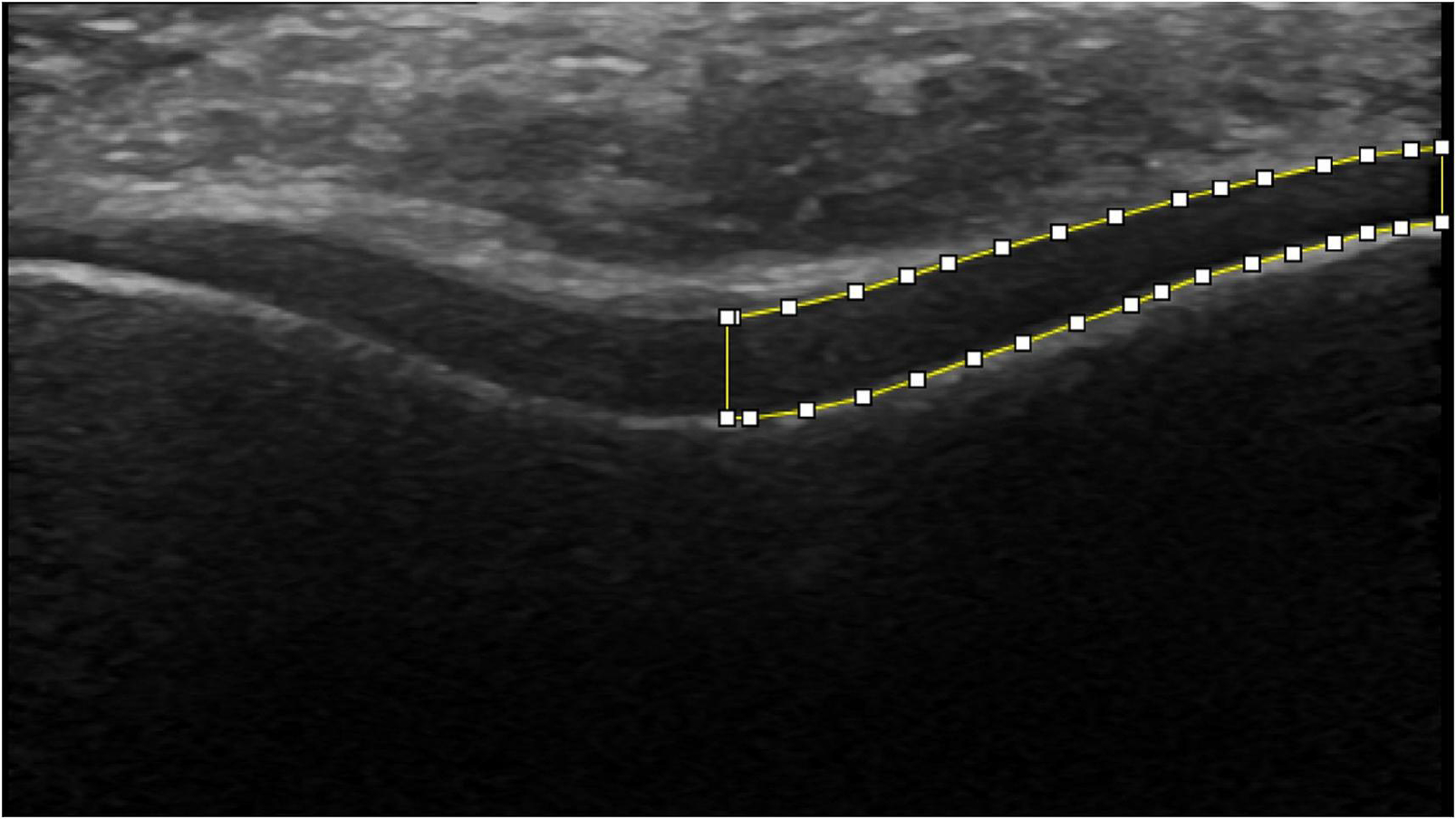
Femoral compartment cross-sectional area (mm2) was determined by segmenting the visible tibial cartilage-bone interface and femoral soft tissue-cartilage interface and separated into the medial compartment as separated by the horizontal midpoint.
2.4. Gait Biomechanics Collection and Analysis
Prior to data collection, preferred walking speed was determined over a 6m walkway using infrared timing gates (TF100, TracTronix) as previously described and used to set the treadmill speed for the biomechanical assessment (Luc-Harkey et al., 2016, 2018). Participants were outfitted with 26 retroreflective markers and a 3 marker-sacral plate and walked for 3,000 steps on a dual-belt treadmill with 2 178×102 cm force plates (Bertec, Columbus Ohio). An 8-camera, 3D motion capture system (Qualisys, Göteborg, Sweden) collected marker trajectories at 120Hz that were low-pass filtered at 10 Hz (4th order recursive Butterworth). Kinetic data were sampled at 1200Hz and low-pass filtered at 10 Hz (4th order recursive Butterworth). The biomechanical variables of interest, KEM and KAM, was calculated via an inverse dynamics approach (Visual 3D, C-Motion, Germantown, MD; 2020 ×64). Hip, knee and ankle joint centers were calculated as previously described (Davis-Wilson et al., 2020; Evans-Pickett et al., 2020). The final 10 steps of the 3,000-step task were removed to ensure that gait biomechanics were not evaluated during treadmill deceleration; therefore, the first 5 of the final 15 steps were utilized for analysis. All steps were time-normalized and averaged, recognizing stance phase as the time occurring between heel strike (vGRF>20 N) and toe off (vGRF<20 N). Internal KEM and KAM was normalized to the product of height (m) and weight (N) and multiplied by −1 so that greater extension and abduction are recognized as positive. Peak KEM and KAM were derived from the greatest value occurring within the first 50% of stance phase for each of the five steps, with a single average peak KEM and KAM value per participant utilized for analysis. Data extraction, filtering and time normalization (101 data points per stance phase) were performed using Visual 3D (C-Motion, Germantown, MD; 2020 ×64).
2.5.1. Statistical Analyses
Percentages, means, and standard deviations as appropriate, were calculated for participant demographics and reported in Table 1. Between-group differences were assessed using a one-way analysis of variance (ANOVA) for each demographic variable (Table 1).
2.5.2. Linear Regression Models to Predict Change in CSA
Separate, univariate linear regression models were utilized to assess the association between peak KEM and KAM on CSA change scores. Models were individually constructed with peak KEM and KAM as the predictor variables of interest and the CSA change score as the criterion variable. The unstandardized beta coefficients, standard error, and R2 were reported for the primary model. Faster walking speed is associated with greater peak knee kinetics (Chung & Wang, 2010; Davis-Wilson et al., 2020) and sex differences in cartilage thickness 4 years following ACLR have been documented (Pius et al., 2021). Therefore, walking speed and sex were assessed as potential covariates in multiple linear regression models. Peak KEM and KAM were each included as the primary explanatory variable of interest in addition to walking speed and sex, added separately in respective models for CSA change scores. The beta coefficients and change in R2 were reported for the new models in reference to the primary model. A two-sided alpha level of 0.05 was set a priori, and all statistical analyses were performed in R Studio (v 1.3.1056).
2.5.3. Functional Mixed Effect Model
Next, we compared the mean difference of the biomechanical variables of interests (KEM and KAM) for 1) CSAI vs. CSAD 2) CSAI vs. CSAU, and 3) CSAD vs. CSAU. A functional mixed effects Bayesian hierarchical model (Liu & Guo, 2012) comprised of two levels was used to analyze the waveform data at each consecutive 1% of stance phase. Similar to traditional linear mixed effects models (Liu & Guo, 2012), the functional version can be used to control for biases caused by multiple observations per subject. The top level models observed waveforms using penalized B-splines (Lang & Brezger, 2004) with normally distributed errors. The use of a smoothness penalty on the spline coefficients allows for flexible waveform estimation which is robust to overfitting. In the second level, the spline coefficients are modeled using a mixed effects model with subject random effects. Specifically, this level uses dummy variables to model waveform coefficients with a normal distribution centered on their respective group average waveform with adjustments from a subject-specific random effect. Trials (i.e. 5 steps per subject) are treated as replicates within each subject and the subject effects are modeled as random effects. The groups (CSAI, CSAD, and CSAU) constitute the fixed effects in the model. Model parameters are then estimated and 95% confidence intervals (CI) were constructed and plotted around the mean differences. The waveforms were considered statistically different in areas where the CI of the difference did not include zero. We reported the maximum between-group difference in each region of stance phase where the 95% CI did not include zero. Cohen’s d effect sizes were calculated utilizing the group means and pooled standard deviation and associated 95% CI were reported at the percentage of stance that observed the largest between-group difference within statistically significant regions. All calculations were performed in the R programming language (R Core Team, 2021.09.2) using code hosted by the fourth author (W. Z. Horton, 2022).
3. Results:
Thirty-nine participants were classified into CSAI (n=17), CSAD (n=11) and CSAU (n=11) groups. Demographic information and mean CSA change scores are reported by group in Table 1. By design, medial compartment CSA change scores were statistically different between-groups (p<0.01; Table 1). No other statistically significant, between-group differences in demographic variables were observed (Table 1).
Linear Regression Analysis: Peak KEM and KAM
We did not find a statistically significant association between peak KEM (R2=0.001, β=−10.836, p=0.816) and or peak KAM (R2=0.041, β=−93.008, p=0.214) and the change in medial femur CSA. After controlling for walking speed and sex, the association between peak KEM and KAM and the CSA change score remained not statistically significant (Table 2; Table 3).
TABLE 2.
Regression Model Comparison for Average Peak Knee Extension Moment β (Standard Error)
| Predictor Variables | Primary Model |
Model Adjusted for Sex |
Model Adjusted for Speed |
|---|---|---|---|
|
| |||
| Peak Knee Extension Moment | −10.836 (46.238) |
−11.745 (48.638) | −6.965 (45.942) |
| Sex | − | −0.072 (1.033) | − |
| Walking Speed | − | − | −6.509 (5.082) |
|
| |||
| Change in R 2 | − | 0.001 | 0.041 |
| Model p Value | 0.816 | 0.971 | 0.437 |
TABLE 3.
Regression Model Comparison for Average Peak Knee Abduction Moment β (Standard Error)
| Predictor Variables | Primary Model |
Model Adjusted for Sex |
Model Adjusted for Speed |
|---|---|---|---|
|
| |||
| Peak Knee Abduction Moment | −93.008 (73.531) |
−93.360 (74.692) | −85.986 (73.273) |
| Sex | − | 0.072 (0.978) | − |
| Walking Speed | − | − | −6.100 (4.994) |
|
| |||
| Change in R 2 | − | 0.001 | 0.039 |
| Model p Value | 0.214 | 0.466 | 0.223 |
3.1. Functional, Mixed Effects Model Analysis: Comparing KEM by Group
No statistically significant differences in KEM were observed between the CSAI group and the CSAD group [Figure 2A–B]. CSAD exhibited greater KEM at 14–19% [0.004; d=1.15; Figure 2E–F] in comparison to the CSAU group. Greater KEM was observed in the CSAI group in comparison to the CSAU group between 14–16% [0.004; d=1.09; Figure 2C–D].
Figure 2.
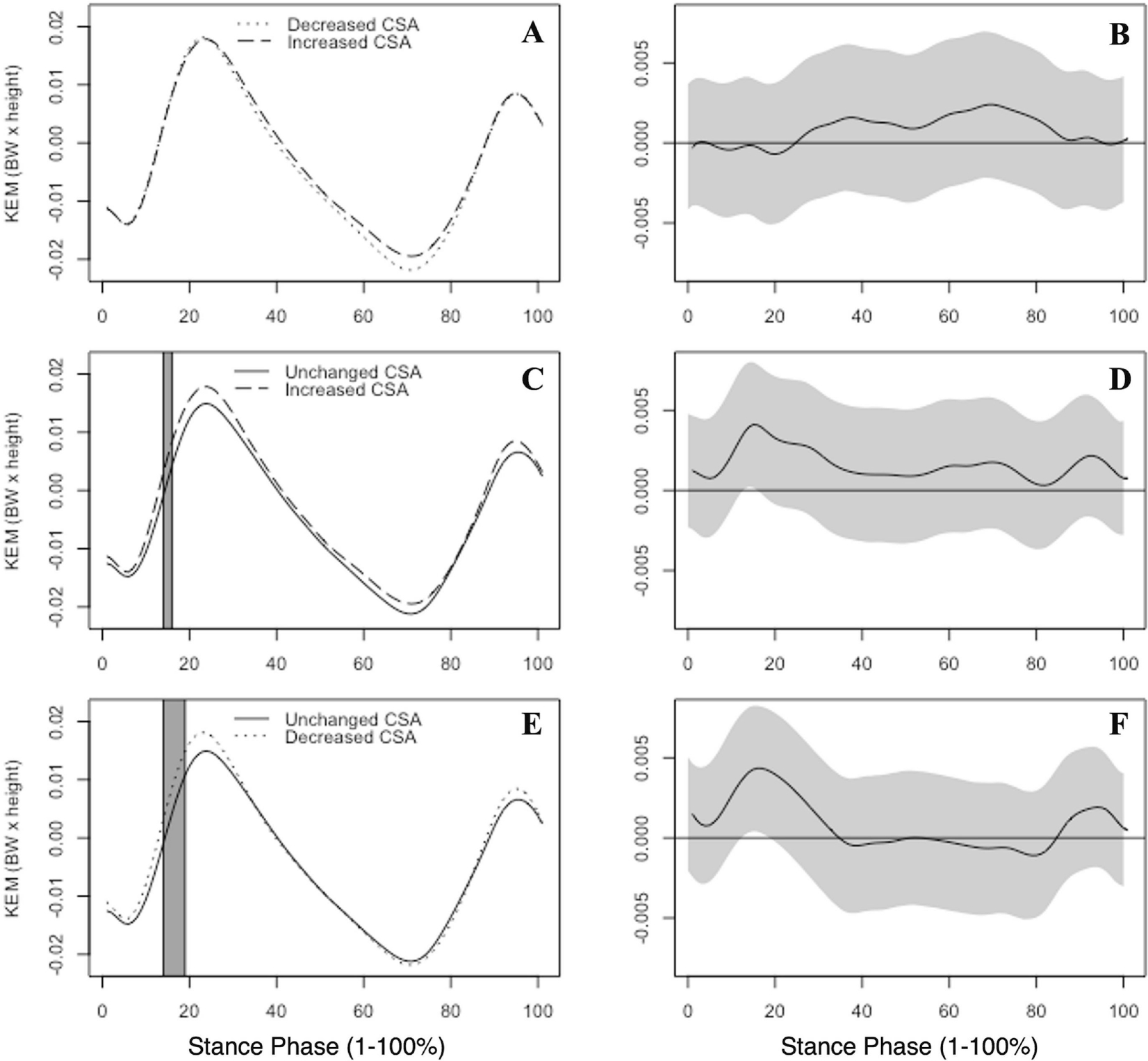
Group mean internal knee extension moments (KEM), normalized by body weight (BW) and height, are illustrated. KEM is reported by group between the increased and decreased CSA groups (A), unchanged and increased CSA groups (C) and the unchanged and decreased CSA groups (E) across the entirety of stance phase (1–100%). Gray boxes indicate areas where the 95% confidence intervals did not include zero. Corresponding KEM mean differences with associated 95% confidence intervals (grey bands) between groups are shown at right (B,D,F). Positive values indicate greater internal KEM.
3.2. Functional Mixed Effects Model Analysis: Comparing KAM by Group
The CSAI group displayed lesser KAM between 14–20% of the stance phase in comparison to the CSAD group [−0.0079 d=−1.79; Figure 3A–B]. The CSAD group exhibited greater KAM between 16–18% of stance [0.005; d=1.10; Figure 3E–F] in comparison to the CSAU group. Additionally, no differences in KAM were observed between the CSAI and the CSAU groups [Figure 3C–D].
Figure 3.
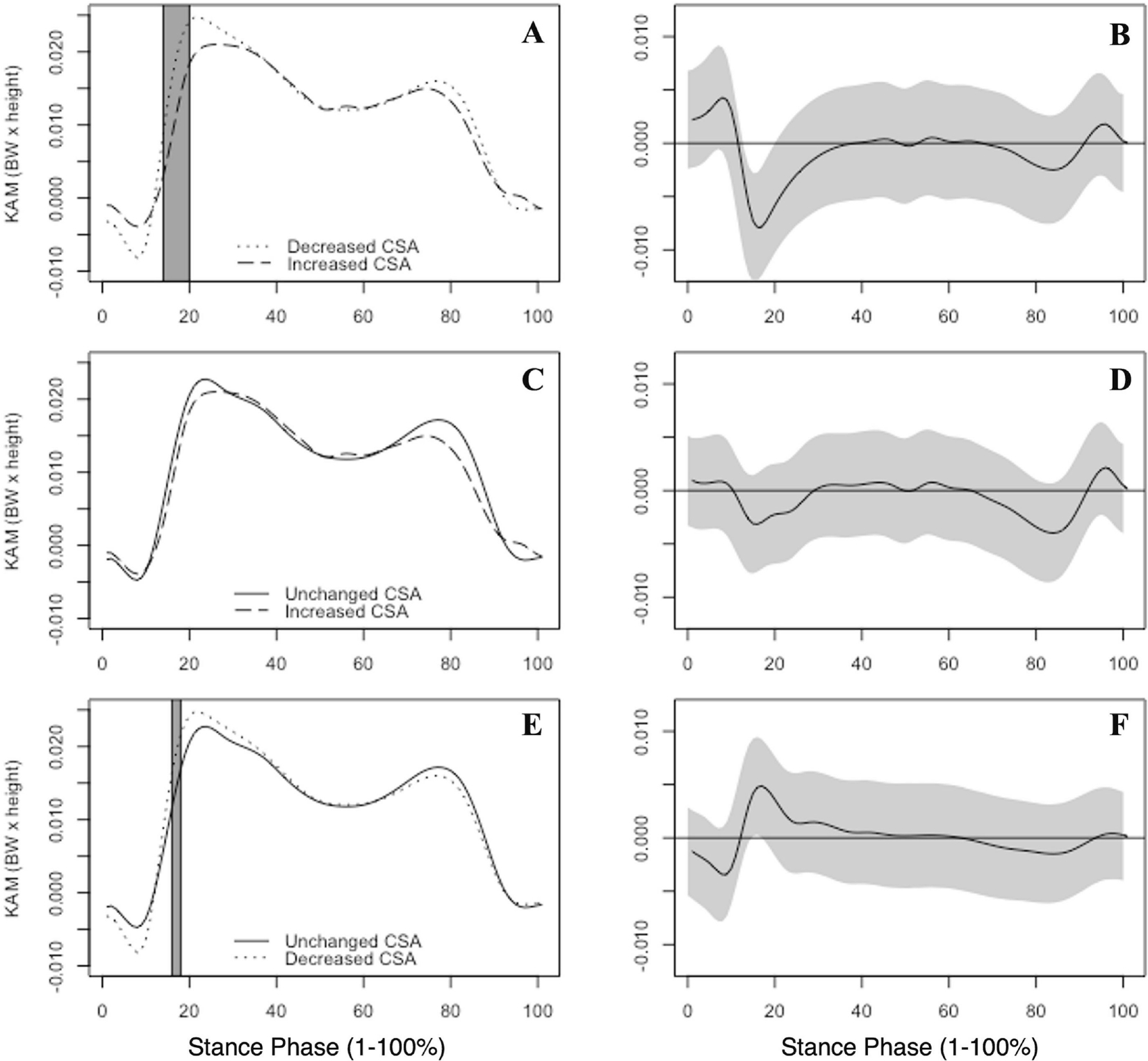
Group mean internal knee abduction moments (KAM), normalized by body weight (BW) and height, are illustrated. KAM is reported by group between the increased and decreased CSA groups (A), unchanged and increased CSA groups (C) and the unchanged and decreased CSA groups (E) across the entirety of stance phase (1–100%). Gray boxes indicate areas where the 95% confidence intervals did not include zero. Corresponding KAM mean differences with associated 95% confidence intervals (grey bands) between groups are shown at right (B,D,F). Positive values indicate greater internal KAM.
Discussion:
Although peak KEM and KAM were not associated with the change in CSA following an acute bout of walking, we did detect differences in kinetic profiles between the CSAI, CSAD, and CSAU groups when assessing KEM and KAM throughout the entirety of stance phase. Both the CSAI and CSAD groups demonstrated greater KEM in early stance in comparison to the CSAU group. Consistent with our hypothesis, the CSAI group demonstrated generally lower KAM in early stance compared to the CSAD group. The CSAI group exhibited lower KAM values in early and late stance in comparison to the CSAU group; however, the differences were not statistically significant. The CSAI group visually exhibited a less-dynamic KAM waveform throughout stance compared to the CSAU and CSAD groups. The present study identifies differences in internal knee kinetics during early stance phase between ACLR individuals who demonstrate ultrasound-assessed changes in the medial femur CSA response following an acute loading activity. The current study, which utilized blinded ultrasound assessors, supports the hypothesis that the response of articular cartilage to loading is linked to differences in gait biomechanical profiles (Andriacchi et al., 2009). The study further supports the rationale of an interplay between limb-level kinetics and the acute response of medial femoral cartilage to loading. Previous research has successfully demonstrated the ability to acutely modify knee kinetics throughout stance phase by cuing an increase in peak vertical ground reaction force (Evans-Pickett et al., 2020). Therefore, it is logical to suggest, that future biomechanical interventions using similar methods could evaluate the effect of modifying limb-level kinetic profiles on the acute response of cartilage to loading in order to determine the mechanistic effect of knee joint kinetics on the acute response of cartilage to loading.
4.1. An increased cross-sectional area response may indicate underlying pathology
Previous work has demonstrated that uninjured individuals typically exhibit decreased femoral CSA following 3000 steps of walking (Pfeiffer et al., 2020), suggesting that an increase in femoral cartilage CSA following loading may reflect a pathological cartilage response following ACL injury (Frobell, 2011; Guilak et al., 2018; Harkey et al., 2018). While resting femoral cartilage thinning is a marker of more advanced PTOA progression (Arnoldi et al., 2011; Faber et al., 1999; Frobell, 2011), resting cartilage thickening has been reported in the first several years following ACL injury and ACLR. An MRI-based study found resting articular cartilage thickness increases in the first two years following ACL injury (Frobell, 2011), which is hypothesized to reflect increased fluid composition of the tissue (Bertrand & Held, 2017). Similarly, a study assessing cartilage morphology with ultrasonography found greater resting femoral articular cartilage thickness in individuals who were 37 ± 27 months post-ACLR compared to both the contralateral limb and uninjured controls (Harkey, Blackburn, Nissman, et al., 2018). Increased water content within the tissue may reflect loss of proteoglycans and increased tissue permeability, which may impair the capacity of the cartilage to adequately attenuate load (Nissinen et al., 2021; Pastrama et al., 2019; Sophia Fox et al., 2009). While we did not conduct radiographic assessments of PTOA prevalence in our cohort, based on these previous data, we can hypothesize that the CSAI group identified in our cohort exhibited a more pathological response to loading compared to the CSAD groups.
4.2. Knee kinetics following ACLR
The CSAI group exhibited greater KEM in comparison to both the CSAD and CSAU groups in early stance. Although not assessed in the present study, a stiffened knee gait (i.e. reduced knee flexion excursion) in addition to greater KEM in the first 50% of stance may reflect a compensatory strategy to overcome persistent quadricep strength deficits post-ACLR (Lewek et al., 2002; Palmieri-Smith & Thomas, 2009). Previous research has not found associations between peak KEM in the first half of stance phase and MRI-estimates of cartilage composition (Pfeiffer, Spang, et al., 2019); however, the between-group differences in KEM in the present study were observed prior to the first KEM peak. Therefore, it is possible that KEM during the load acceptance phase may be important to understand early PTOA development. The CSAI group demonstrated KAM profiles with lesser magnitudes prior to the first KAM peak in comparison to the other two groups and exhibited statistically lower KAM magnitudes in comparison to the CSAD group, which may be a biomechanical marker linked to underlying PTOA development. Previous work has suggested that lesser limb-level loading and frontal plane knee kinetics during walking gait is associated with deleterious cartilage changes in the medial compartment of the femur (Pfeiffer, Spang, et al., 2019; Pietrosimone et al., 2017). Specifically, lesser peak KAM is associated with greater concentrations of matrix metalloproteinase-3, a plasma marker of cartilage degeneration, interleukin 6, a pro-inflammatory cytokine at 6 months post-ACLR (Pietrosimone et al., 2017), and greater T1rho relaxation times, an MRI estimate of cartilage proteoglycan density, within the first year post-ACLR (Pfeiffer, et al., 2019). In a separate cohorts, peak KAM was shown to further decrease with disease progression following ACLR (Erhart-Hledik et al., 2018; Wellsandt et al., 2016), highlighting the need to intervene early post-ACLR. Individuals with less-dynamic KAM profiles associated with MRI measures of lower femoral cartilage proteoglycan density 6 months post-ACLR yet increase KAM to magnitudes similar to uninjured controls by 12 months post-ACLR (Evans-Pickett, Lisee, et al., 2022), suggesting that lesser peak frontal plane knee kinetics early post-ACLR are time-dependent and linked to deleterious changes in cartilage composition. Therefore, less-dynamic KEM and KAM in the CSAI group may characterize a subgroup of individuals that would benefit from targeted gait interventions to improve the dynamic nature of the kinetic waveforms following ACLR.
4.3. Limitations
This study did not determine the mechanisms linking less-dynamic kinetic waveforms to increased femoral cartilage CSA. It should be noted that we only measured changes in femoral cartilage morphology in the anterior femur, thereby changes measured in the anterior cartilage may be subject to altered cartilage mechanics at other locations on the femoral surface. It is possible that less-dynamic kinetic waveforms cause portions of the anterior femur to be loaded in a more sustained manner causing greater cartilage strain due to the time-dependent, viscoelastic nature of the tissue (Arokoski et al., 2008). Although speculative, it is possible that increased tissue strain in more posterior or central portions of the femoral cartilage may cause greater water permeability in the anterior portions of the cartilage creating an increase in anterior femoral cartilage size. However, the present study was cross-sectional in nature and the causality of the relationship between knee kinetics and the cartilage response to load cannot be gleaned from our experiment. It is possible the CSAI group exhibited altered cartilage physiology that caused an increased morphological response to any load and decreasing KAM in early stance and increasing KEM in early stance was a protective response to prevent excessive loading of the tissue. Further, dual-belt treadmill walking can alter spatiotemporal aspects of gait and frontal plane kinetics (Altman, 2012) and cannot be directly compared to overground walking (Dewig et al., 2022). Future studies are needed to understand the mechanistic effect of modifying KEM and KAM on the articular cartilage response to loading.
The present study links in vivo ultrasound morphology assessments with gait biomechanical variables, specifically KEM and KAM, following an acute bout of walking. We acknowledge that the present study was not without limitation. Current methods of biomechanical analyses are limited in the ability to account for the individual contributions of muscles surrounding the knee joint. Quadriceps and hip muscular weakness occur early-post ACLR and can become chronic in this population (J. M. Hart et al., 2010; Hurley et al., 1994; Palmieri-Smith & Thomas, 2009; Petersen et al., 2014; Thomas et al., 2013). Future research would benefit from utilizing advanced joint contact force modeling techniques to understand the compartmental loads linked to different articular cartilage responses to acute loading after ACLR. Further, while ultrasonography is capable of detecting immediate morphological changes within the articular cartilage after dynamic activity, ultrasonography is only able to assess the anterior femoral cartilage regions and is limited by the probe’s field of view. Other analysis techniques, such as average thickness measurements, could provide additional information regarding acute changes in cartilage morphology following a standardized walking protocol. Quantitative MRI is more appropriate in assessing the articular cartilage response to loading across multiple regions of the tissue (Sutter et al., 2015). Further, although understanding the magnitude of discrete biomechanical variables are valuable, discrete analyses are limited in the ability to account for loading profiles throughout the entirety of stance phase. Therefore, the purpose of categorizing individuals into groups (i.e. CSAI, CSAU, CSAD) was to facilitate the ability to conduct a between-group functional mixed effects model analysis. The categorization of participants into different groups was not conducted arbitrarily but was rather based on a previously published minimally detectable change value (Harkey, Blackburn, Hackney, et al., 2018). There are limitations to characterizing individuals into different groups based on any cutoff threshold as it cannot be assumed that all participants within each group exhibited the same changes in cartilage CSA following loading nor does our analysis suggest the exact point by which cartilage morphology differs. Future research is needed to fully understand the full continuum of changes in the magnitude of the cartilage CSA following acute loading and further grasp the clinical implications of each group’s CSA response to dynamic activities (i.e. walking). Further, we focused on the medial femoral compartment in the ACLR-limb in our analysis, as compositional changes within the medial femoral compartment is most commonly associated with early knee osteoarthritis development (Wise et al., 2012). Previous research identified the associations between peak frontal and sagittal plane kinetic variables and resting medial femoral cartilage thickness were modulated by the presence of concomitant meniscal injury (Pamukoff et al., 2018) and future research should consider the effect of meniscal injury on the acute response of the medial femoral cartilage to loading. Our study did not include an uninjured control group; therefore, it is unclear if KAM differed in our ACLR cohort compared to uninjured controls. A study of uninjured individuals of similar age (21 ± 4 yrs), reported a peak KAM of 0.024 ± 0.01 during treadmill walking (Dewig et al., 2022), which is consistent with both the CSAU (0.023) and CSAD groups (0.025), but not the CSAI group in our study, who demonstrated a lower peak KAM (0.021). However, previous research found that the majority of uninjured individuals displayed acute decreases in femoral CSA following a 3,000 step walking task (Pfeiffer et al., 2020), suggesting the response of the CSAD group may be most closely aligned with uninjured controls.
5. Conclusion
ACLR individuals who exhibit an acute increase in anterior medial femoral articular cartilage CSA following 3,000 steps of walking demonstrate lower KAM and greater KEM in early stance. The propensity of femoral cartilage to acutely increase in CSA in response to walking is consistent with less-dynamic knee kinetic profiles.
Highlights.
Variability in the cartilage morphological response is observed after walking
Cartilage swelling post-walking is linked to less-dynamic knee kinetics
An increased medial femoral cartilage morphological response may be pathological
Acknowledgments:
Research reported in this article was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R21 AR074094;1R03AR066840-01A1), North Carolina Translational and Clinical Sciences Institute, and National Athletic Trainers Association Research and Education Foundation (NATAREF NIA 0001).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AM, Goff I, Pearce MS, Francis RM, & Birrell F (2011). Reliability and validity of ultrasound imaging of features of knee osteoarthritis in the community. BMC Musculoskeletal Disorders, 12(1), 70. 10.1186/1471-2474-12-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP (1994). Dynamics of Knee Malalignment. Orthopedic Clinics of North America, 25(3), 395–403. 10.1016/S0030-5898(20)31924-6 [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Koo S, & Scanlan SF (2009). Gait Mechanics Influence Healthy Cartilage Morphology and Osteoarthritis of the Knee. The Journal of Bone and Joint Surgery. American Volume., 91(Suppl 1), Article Suppl 1. 10.2106/JBJS.H.01408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander E, Dyrby CO, & Koo S (2004). A Framework for the in Vivo Pathometchanics of Osteoarthritis at the Knee (No. 3). 32(3), Article 3. [DOI] [PubMed] [Google Scholar]
- Arnoldi A, Weckbach S, Nussbickel C, Horng A, Nöbauer I, Zysk S, Reiser M, & Glaser C (2011). MRT-basierte Knorpelvolumetrie nach Kreuzbandersatzplastik in Korrelation mit qualitativen Gelenkveränderungen und dem klinischen Outcome. Gibt es Hinweise auf frühzeitige posttraumatische degenerative Veränderungen? RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren, 183(12), 1138–1144. 10.1055/s-0031-1281685 [DOI] [PubMed] [Google Scholar]
- Arokoski JPA, Jurvelin JS, Väätäinen U, & Helminen HJ (2008). Normal and pathological adaptations of articular cartilage to joint loading (No. 10). 10, Article 10. [DOI] [PubMed] [Google Scholar]
- Bertrand J, & Held A (2017). Role of Proteoglycans in Osteoarthritis. In Grässel S & Aszódi A (Eds.), Cartilage: Volume 2: Pathophysiology (pp. 63–80). Springer International Publishing. 10.1007/978-3-319-45803-8_4 [DOI] [Google Scholar]
- Chung M-J, & Wang M-JJ (2010). The change of gait parameters during walking at different percentage of preferred walking speed for healthy adults aged 20–60 years. Gait & Posture, 31(1), 131–135. 10.1016/j.gaitpost.2009.09.013 [DOI] [PubMed] [Google Scholar]
- Davis-Wilson HC, Pfeiffer SJ, Johnston CD, Seeley MK, Harkey MS, Blackburn JT, Fockler RP, Spang JT, & Pietrosimone B (2020). Bilateral Gait 6 and 12 Months Post–Anterior Cruciate Ligament Reconstruction Compared with Controls. Medicine & Science in Sports & Exercise, 52(4), Article 4. 10.1249/MSS.0000000000002208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearborn JT, Eakin CL, & Skinner HB (1996). Medial compartment arthrosis of the knee. American Journal of Orthopedics (Belle Mead, N.J.), 25(1), 18–26. [PubMed] [Google Scholar]
- Dewig DR, Mills HR, Evans-Pickett A, Pietrosimone BG, & Troy Blackburn J (2022). Aberrant gait biomechanics in individuals with ACL reconstruction are magnified during treadmill walking. Journal of Biomechanics, 134, 110989. 10.1016/j.jbiomech.2022.110989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F (2005). In vivo cartilage deformation after different types of activity and its dependence on physical training status. Annals of the Rheumatic Diseases, 64(2), 291–295. 10.1136/ard.2004.022400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, Wirth W, Lohmander LS, Hudelmaier MI, & Frobell RB (2015). Five-Year Followup of Knee Joint Cartilage Thickness Changes After Acute Rupture of the Anterior Cruciate Ligament: Cartilage Change at Five Years after ACL Rupture. Arthritis & Rheumatology, 67(1), 152–161. 10.1002/art.38881 [DOI] [PubMed] [Google Scholar]
- Erhart-Hledik JC, Chu CR, Asay JL, & Andriacchi TP (2018). Longitudinal changes in knee gait mechanics between 2 and 8 years after anterior cruciate ligament reconstruction: LONGITUDINAL GAIT CHANGES AFTER ACLR. Journal of Orthopaedic Research®, 36(5), 1478–1486. 10.1002/jor.23770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Pickett A, Davis-Wilson HC, Johnston CD, Blackburn JT, Hackney AC, & Pietrosimone B (2022). Immediate Effects of Walking with a Knee Brace following Anterior Cruciate Ligament Reconstruction: A Biomechanical, Biochemical, and Structural Approach. Journal of Athletic Training. 10.4085/1062-6050-0700.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Pickett A, Davis-Wilson HC, Luc-Harkey BA, Blackburn JT, Franz JR, Padua DA, Seeley MK, & Pietrosimone B (2020). Biomechanical effects of manipulating peak vertical ground reaction force throughout gait in individuals 6–12 months after anterior cruciate ligament reconstruction. Clinical Biomechanics, 76, 105014. 10.1016/j.clinbiomech.2020.105014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Pickett A, Lisee C, Horton WZ, Lalush D, Nissman D, Blackburn JT, Spang JT, & Pietrosimone B (2022). Worse Tibiofemoral Cartilage Composition is Associated with Insufficient Gait Kinetics Following ACL Reconstruction. Medicine & Science in Sports & Exercise, Publish Ahead of Print. 10.1249/MSS.0000000000002969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber KJ, Dill JR, Amendola A, Thain L, Spouge A, & Fowler PJ (1999). Occult osteochondral lesions after anterior cruciate ligament rupture. Six-year magnetic resonance imaging follow-up study. The American Journal of Sports Medicine, 27(4), 489–494. 10.1177/03635465990270041301 [DOI] [PubMed] [Google Scholar]
- Farrokhi S, O’Connell M, & Fitzgerald GK (2015). Altered gait biomechanics and increased knee-specific impairments in patients with coexisting tibiofemoral and patellofemoral osteoarthritis. Gait & Posture, 41(1), 81–85. 10.1016/j.gaitpost.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobell RB (2011). Change in Cartilage Thickness, Posttraumatic Bone Marrow Lesions, and Joint Fluid Volumes After Acute ACL Disruption: A Two-Year Prospective MRI Study of Sixty-one Subjects. The Journal of Bone & Joint Surgery, 93(12), 1096–1103. 10.2106/JBJS.J.00929 [DOI] [PubMed] [Google Scholar]
- Guilak F, Nims RJ, Dicks A, Wu C-L, & Meulenbelt I (2018). Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biology, 71–72, 40–50. 10.1016/j.matbio.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkey MS, Blackburn JT, Davis H, Sierra-Arévalo L, Nissman D, & Pietrosimone B (2017). Ultrasonographic assessment of medial femoral cartilage deformation acutely following walking and running. Osteoarthritis and Cartilage, 25(6), 907–913. 10.1016/j.joca.2016.12.026 [DOI] [PubMed] [Google Scholar]
- Harkey MS, Blackburn JT, Hackney AC, Lewek MD, Schmitz RJ, Nissman D, & Pietrosimone B (2018). Comprehensively Assessing the Acute Femoral Cartilage Response and Recovery after Walking and Drop-Landing: An Ultrasonographic Study. Ultrasound in Medicine & Biology, 44(2), 311–320. 10.1016/j.ultrasmedbio.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Harkey MS, Blackburn JT, Nissman D, Davis H, Durrington I, Rizk C, Kuismanen A, & Pietrosimone B (2018). Ultrasonographic Assessment of Femoral Cartilage in Individuals With Anterior Cruciate Ligament Reconstruction: A Case-Control Study. Journal of Athletic Training, 53(11), 1082–1088. 10.4085/1062-6050-376-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, Machotka Z, & Crossley KM (2016). Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: A systematic review and meta-analysis. British Journal of Sports Medicine, 50(10), Article 10. 10.1136/bjsports-2015-094797 [DOI] [PubMed] [Google Scholar]
- Hart JM, Pietrosimone B, Hertel J, & Ingersoll CD (2010). Quadriceps Activation Following Knee Injuries: A Systematic Review. Journal of Athletic Training, 45(1), Article 1. 10.4085/1062-6050-45.1.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton WZ (2022). FunctionalMixedEffects.Rpkg (v1.2.0). GitHub. https://github.com/wzhorton/FunctionalMixedEffects.Rpkg [Google Scholar]
- Horton Z, Page G, Reese CS, Lepley L, & White M (2021). Template Priors in Bayesian Curve Registration. 63(4), 487–499. [Google Scholar]
- Hurley MV, Jones DW, & Newham DJ (1994). Arthrogenic Quadriceps Inhibition and Rehabilitation of Patients with Extensive Traumatic Knee Injuries. Clinical Science, 86(3), Article 3. 10.1042/cs0860305 [DOI] [PubMed] [Google Scholar]
- Iagnocco A (2010). Imaging the joint in osteoarthritis: A place for ultrasound? Best Practice & Research Clinical Rheumatology, 24(1), 27–38. 10.1016/j.berh.2009.08.012 [DOI] [PubMed] [Google Scholar]
- Iriondo C, Liu F, Calivà F, Kamat S, Majumdar S, & Pedoia V (2021). Towards understanding mechanistic subgroups of osteoarthritis: 8-year cartilage thickness trajectory analysis. Journal of Orthopaedic Research, 39(6), 1305–1317. 10.1002/jor.24849 [DOI] [PubMed] [Google Scholar]
- Kaur M, Ribeiro DC, Theis J-C, Webster KE, & Sole G (2016). Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sports Medicine, 46(12), Article 12. 10.1007/s40279-016-0510-4 [DOI] [PubMed] [Google Scholar]
- Lang S, & Brezger A (2004). Bayesian P-Splines. Journal of Computational and Graphical Statistics, 13(1), 183–212. 10.1198/1061860043010 [DOI] [Google Scholar]
- Lewek M, Rudolph K, Axe M, & Snyder-Mackler L (2002). The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clinical Biomechanics, 17(1), Article 1. 10.1016/S0268-0033(01)00097-3 [DOI] [PubMed] [Google Scholar]
- Liu Z, & Guo W (2012). Functional mixed effects models: Functional mixed effects models. Wiley Interdisciplinary Reviews: Computational Statistics, 4(6), 527–534. 10.1002/wics.1226 [DOI] [Google Scholar]
- Luc B, Gribble PA, & Pietrosimone BG (2014). Osteoarthritis Prevalence Following Anterior Cruciate Ligament Reconstruction: A Systematic Review and Numbers-Needed-to-Treat Analysis. Journal of Athletic Training, 49(6), Article 6. 10.4085/1062-6050-49.3.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luc-Harkey BA, Franz JR, Hackney AC, Blackburn JT, Padua DA, & Pietrosimone B (2018). Lesser lower extremity mechanical loading associates with a greater increase in serum cartilage oligomeric matrix protein following walking in individuals with anterior cruciate ligament reconstruction. Clinical Biomechanics (Bristol, Avon), 60, 13–19. 10.1016/j.clinbiomech.2018.09.024 [DOI] [PubMed] [Google Scholar]
- Luc-Harkey BA, Harkey MS, Stanley LE, Blackburn JT, Padua DA, & Pietrosimone B (2016). Sagittal plane kinematics predict kinetics during walking gait in individuals with anterior cruciate ligament reconstruction. Clinical Biomechanics, 39, 9–13. 10.1016/j.clinbiomech.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Mündermann A, Dyrby CO, & Andriacchi TP (2005). Secondary gait changes in patients with medial compartment knee osteoarthritis: Increased load at the ankle, knee, and hip during walking. Arthritis & Rheumatism, 52(9), 2835–2844. 10.1002/art.21262 [DOI] [PubMed] [Google Scholar]
- Naredo E, Acebes C, Moller I, Canillas F, de Agustin JJ, de Miguel E, Filippucci E, Iagnocco A, Moragues C, Tuneu R, Uson J, Garrido J, Delgado-Baeza E, & Saenz-Navarro I (2009). Ultrasound validity in the measurement of knee cartilage thickness. Annals of the Rheumatic Diseases, 68(8), 1322–1327. 10.1136/ard.2008.090738 [DOI] [PubMed] [Google Scholar]
- Nissinen MT, Hänninen N, Prakash M, Mäkelä JTA, Nissi MJ, Töyräs J, Nieminen MT, Korhonen RK, & Tanska P (2021). Functional and structural properties of human patellar articular cartilage in osteoarthritis. Journal of Biomechanics, 126, 110634. 10.1016/j.jbiomech.2021.110634 [DOI] [PubMed] [Google Scholar]
- Palmieri-Smith RM, & Thomas AC (2009). A Neuromuscular Mechanism of Posttraumatic Osteoarthritis Associated with ACL Injury. Exercise and Sport Sciences Reviews, 37(3), Article 3. 10.1097/JES.0b013e3181aa6669 [DOI] [PubMed] [Google Scholar]
- Palmieri-Smith RM, Wojtys EM, & Potter HG (2016). Early Cartilage Changes After Anterior Cruciate Ligament Injury: Evaluation With Imaging and Serum Biomarkers—A Pilot Study. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 32(7), 1309–1318. 10.1016/j.arthro.2015.12.045 [DOI] [PubMed] [Google Scholar]
- Pamukoff DN, Montgomery MM, Holmes SC, Moffit TJ, Garcia SA, & Vakula MN (2018). Association between gait mechanics and ultrasonographic measures of femoral cartilage thickness in individuals with ACL reconstruction. Gait & Posture, 65, 221–227. 10.1016/j.gaitpost.2018.07.174 [DOI] [PubMed] [Google Scholar]
- Park J, Seeley MK, Francom D, Reese CS, & Hopkins JT (2017). Functional vs. Traditional Analysis in Biomechanical Gait Data: An Alternative Statistical Approach. Journal of Human Kinetics, 60(1), 39–49. 10.1515/hukin-2017-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrama M-I, Ortiz AC, Zevenbergen L, Famaey N, Gsell W, Neu CP, Himmelreich U, & Jonkers I (2019). Combined enzymatic degradation of proteoglycans and collagen significantly alters intratissue strains in articular cartilage during cyclic compression. Journal of the Mechanical Behavior of Biomedical Materials, 98, 383–394. 10.1016/j.jmbbm.2019.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J-P, Raynauld J-P, Berthiaume M-J, Abram F, Choquette D, Haraoui B, Beary JF, Cline GA, Meyer JM, & Martel-Pelletier J (2007). Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: A longitudinal study. Arthritis Research & Therapy, 9(4), R74. 10.1186/ar2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen W, Taheri P, Forkel P, & Zantop T (2014). Return to play following ACL reconstruction: A systematic review about strength deficits. Archives of Orthopaedic and Trauma Surgery, 134(10), 1417–1428. 10.1007/s00402-014-1992-x [DOI] [PubMed] [Google Scholar]
- Pfeiffer SJ, Davis-Wilson HC, Pexa B, Szymczak J, Wistreich C, Sorensen R, Wikstrom EA, Blackburn JT, & Pietrosimone B (2020). Assessing Step Count–Dependent Changes in Femoral Articular Cartilage Using Ultrasound. Journal of Ultrasound in Medicine, 39(5), 957–965. 10.1002/jum.15180 [DOI] [PubMed] [Google Scholar]
- Pfeiffer SJ, Spang J, Nissman D, Lalush D, Wallace K, Harkey MS, Pietrosimone LS, Schmitz R, Schwartz T, Blackburn T, & Pietrosimone B (2019). Gait Mechanics and T1ρ MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction: Medicine & Science in Sports & Exercise, 51(4), Article 4. 10.1249/MSS.0000000000001834 [DOI] [PubMed] [Google Scholar]
- Pfeiffer SJ, Valentine JA, Goodwin JS, Nissman DB, Blackburn T, & Pietrosimone B (2019). Effects of a knee valgus unloader brace on medial femoral articular cartilage deformation following walking in varus-aligned individuals. The Knee, 26(5), 1067–1072. 10.1016/j.knee.2019.06.014 [DOI] [PubMed] [Google Scholar]
- Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, Luc-Harkey BA, Ulici V, Marshall SW, Jordan JM, & Spang JT (2017). Biochemical Markers of Cartilage Metabolism are Associated with Walking Biomechanics Six-Months Following Anterior Cruciate Ligament Reconstruction. Journal of Orthopaedic Research : Official Publication of the Orthopaedic Research Society, 35(10), 2288–2297. 10.1002/jor.23534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pius AK, Beynnon BD, Fiorentino N, Gardner-Morse M, Vacek PM, DeSarno M, Failla M, Slauterbeck JR, Sturnick DR, Argentieri EC, & Tourville TW (2021). Articular cartilage thickness changes differ between males and females 4 years following anterior cruciate ligament reconstruction. Journal of Orthopaedic Research, jor.25142. 10.1002/jor.25142 [DOI] [PubMed] [Google Scholar]
- Reeves ND, & Bowling FL (2011). Conservative biomechanical strategies for knee osteoarthritis. Nature Reviews Rheumatology, 7(2), 113–122. 10.1038/nrrheum.2010.212 [DOI] [PubMed] [Google Scholar]
- Schipplein OD, & Andriacchi TP (1991). Interaction between active and passive knee stabilizers during level walking. Journal of Orthopaedic Research, 9(1), 113–119. 10.1002/jor.1100090114 [DOI] [PubMed] [Google Scholar]
- Slater LV, Hart JM, Kelly AR, & Kuenze CM (2017). Progressive Changes in Walking Kinematics and Kinetics After Anterior Cruciate Ligament Injury and Reconstruction: A Review and Meta-Analysis. Journal of Athletic Training, 52(9), 847–860. 10.4085/1062-6050-52.6.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophia Fox AJ, Bedi A, & Rodeo SA (2009). The Basic Science of Articular Cartilage. Sports Health, 1(6), Article 6. 10.1177/1941738109350438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter EG, Widmyer MR, Utturkar GM, Spritzer CE, Garrett WE, & DeFrate LE (2015). In Vivo Measurement of Localized Tibiofemoral Cartilage Strains in Response to Dynamic Activity. The American Journal of Sports Medicine, 43(2), 370–376. 10.1177/0363546514559821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AC, Villwock M, Wojtys EM, & Palmieri-Smith RM (2013). Lower Extremity Muscle Strength After Anterior Cruciate Ligament Injury and Reconstruction. Journal of Athletic Training, 48(5), 610–620. 10.4085/1062-6050-48.3.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, & Snyder-Mackler L (2016). Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. The American Journal of Sports Medicine, 44(1), Article 1. 10.1177/0363546515608475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise BL, Niu J, Yang M, Lane NE, Harvey W, Felson DT, Hietpas J, Nevitt M, Sharma L, Torner J, Lewis CE, & Zhang Y (2012). Patterns of Compartment Involvement in Tibiofemoral Osteoarthritis in Men and Women and in Caucasians and African Americans: The Multicenter Osteoarthritis Study. Arthritis Care & Research, 64(6), 847–852. 10.1002/acr.21606 [DOI] [PMC free article] [PubMed] [Google Scholar]


