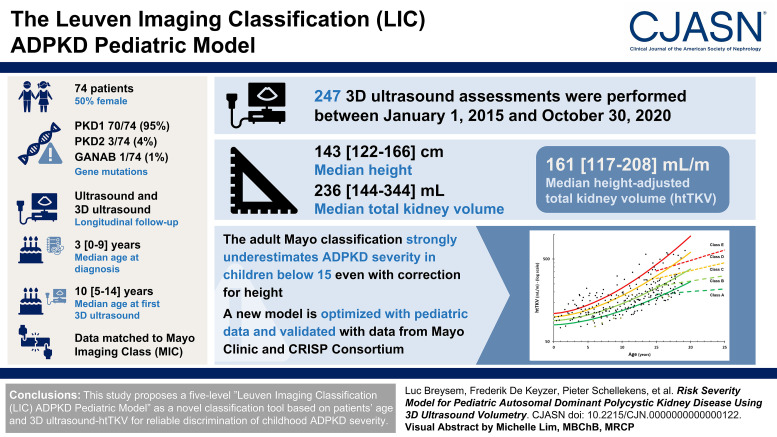
Visual Abstract
Keywords: ADPKD, Kidney volume, Cystic kidney, Pediatrics
Abstract
Background
Height-adjusted total kidney volume (htTKV) measured by imaging defined as Mayo Imaging Class (MIC) is a validated prognostic measure for autosomal dominant polycystic kidney disease (ADPKD) in adults to predict and stratify disease progression. However, no stratification tool is currently available in pediatric ADPKD. Because magnetic resonance imaging and computed tomography in children are difficult, we propose a novel 3D ultrasound-based pediatric Leuven Imaging Classification to complement the MIC.
Methods
A prospective study cohort of 74 patients with genotyped ADPKD (37 female) was followed longitudinally with ultrasound, including 3D ultrasound, and they underwent in total 247 3D ultrasound assessments, with patients' median age (interquartile range [IQR]) at diagnosis of 3 (IQR, 0–9) years and at first 3D ultrasound evaluation of 10 (IQR, 5–14) years. First, data matching was done to the published MIC classification, followed by subsequent optimization of parameters and model type.
Results
PKD1 was confirmed in 70 patients (95%), PKD2 in three (4%), and glucosidase IIα unit only once (1%). Over these 247 evaluations, the median height was 143 (IQR, 122–166) cm and total kidney volume was 236 (IQR, 144–344) ml, leading to an htTKV of 161 (IQR, 117–208) ml/m. Applying the adult Mayo classification in children younger than 15 years strongly underestimated ADPKD severity, even with correction for height. We therefore optimized the model with our pediatric data and eventually validated it with data of young patients from Mayo Clinic and the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease used to establish the MIC.
Conclusions
We proposed a five-level Leuven Imaging Classification ADPKD pediatric model as a novel classification tool on the basis of patients' age and 3D ultrasound-htTKV for reliable discrimination of childhood ADPKD severity.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a hereditary kidney disease mainly caused by PKD1 and PKD2 mutations, morphologically characterized by bilateral kidney cysts of variable diameter, progressively enlarging with time.1 This results in gradual kidney enlargement, progressive parenchymal destruction, and kidney failure, usually beyond the fourth decade.2 It is a clinical and radiological slow progressive disease, with variable phenotypic expression.3,4 Clinical signs, e.g., hypertension or kidney functional decline, are usually more obvious in adults with ADPKD.5–7
Early signs can already be visualized in utero, and later diagnosis is usually made with ultrasound and/or magnetic resonance imaging (MRI), either by screening known ADPKD families, symptoms, or by finding incidental kidney cysts that cannot be categorized as simple kidney cysts.8–11
In children, ultrasound is the initial imaging modality because it is usually well tolerated and easy to perform. Findings range from a normal-sized kidney without cysts and normal parenchyma to an enlarged kidney with innumerable cysts without remaining visible normal parenchyma.4,8,12–15 Imaging diagnostic criteria for ADPKD in adolescents (>15 years) and adults have been defined.16 By ultrasound, cysts <0.5 cm can be seen with a linear or convex probe, applying the highest resolution settings, keeping in mind the restrictions of ultrasound.4,17 If ultrasound is not conclusive, MRI or computed tomography (CT) can still be used for more accurate cyst detection.11 Presently in pediatrics (<15 years), validated diagnostic imaging criteria for ADPKD diagnosis are not yet available. A recent study, however, reports approaches to classify kidney cysts in children and how to differentiate simple kidney cysts with kidney cystic diseases, such as ADPKD.9
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort study reported an inverse correlation between MRI-based kidney volume and function of adolescents (>15 years) and adults with ADPKD. Moreover, baseline total kidney volume (TKV), corrected for patient's height, can predict the timing of future kidney function decline in adulthood, and height-adjusted total kidney volume (htTKV) was found to be an appropriate biomarker.2,18–21 Classification into a five-class severity risk model, on the basis of age and MRI-determined htTKV, is considered a validated stratification tool in adult ADPKD (the Mayo Imaging Classification [MIC]). They found earlier eGFR decline and higher chance of kidney failure with increasing MIC classes, considering that patients can progress faster than expected and move to a higher class.22 In children (<15 years) with ADPKD, there are no dedicated studies investigating the clinical applicability of htTKV for prediction of disease progression or kidney function decline in adulthood.
Because the US Food and Drug Administration and European Medicines Agency have recently accepted TKV as a prognostic biomarker for ADPKD,23 the use of MIC allows selection of high-risk patients for treatment and clinical trials. There is no current cure for ADPKD, but therapeutic trials have been initiated in adults, and tolvaptan has been approved for clinical use.11,24 As therapies are most likely more effective in early disease, the pediatric population is becoming of great interest for ADPKD treatment, especially for disease stratification.7,25,26 Furthermore, models of htTKV growth indicate large differences before age 20, highlighting the need to understand patterns of pediatric htTKV growth.27
The aim of this project was first to evaluate whether htTKV using 3D ultrasound in a genotyped ADPKD pediatric population can be used to develop a risk stratification model to differentiate slow from fast progressors and second to evaluate whether our model can be used as precursor of the MIC adult risk model.
Methods
Ethical Statement
The study was approved by the local ethical board (Ethical Committee Research KU/UZ Leuven, S59500 and S59638) and institutional review boards associated with Mayo Clinic and CRISP and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from both parents and patients when appropriate.
Study Design and Patients
A prospective longitudinal pediatric cohort, followed yearly in the dedicated ADPKD clinic (University Hospitals Leuven), was included between January 1, 2015, and October 30, 2020. All eligible patients and/or their legal representatives gave their informed consent, and no patients declined to participate. We included patients younger than 19 years with clinical ADPKD diagnosis, defined as the presence of at least one kidney cyst on ultrasound and a confirmed pathogenic ADPKD gene mutation. We excluded patients with another genetically proven cystic kidney disease (i.e., NPHP, PKHD1, and HNF1B). We have included five patients who participated in an ongoing tolvaptan study, but only the evaluations before and after the study to minimize the potential effects of tolvaptan.
All patients received early routine clinical and ultrasound TKV measurement follow-up, with varying number and timing of ultrasonographic follow-ups according to age at diagnosis and inclusion year. Demographical characteristics (age, sex, genotype, reason, and age of diagnosis) were collected at enrollment. Anthropometric data (height, weight, body mass index [BMI], and creatinine values) were collected at enrollment and each follow-up.
We report clinical data of each Leuven Imaging Classification (LIC) subcategory on BMI and hypertension, including absolute systolic/diastolic BP, BP SD, BMI, and BMI SD score, on the basis of the Flemish Growth Charts from 2004.28 Hypertension was defined as mean systolic or diastolic BP above +2 SD for children (<16 years), confirmed on 24-hour ambulatory BP monitoring and/or the use of antihypertensive medication. Adult cutoffs were used for children aged 16 years or older (≥140/90 mm Hg).29,30
The eGFR was calculated with the Chronic Kidney Disease in Children (CKiD) equation (revised/bedside Schwartz equation) for children <18 years old and with the Chronic Kidney Disease Epidemiology Collaboration equation for older patients. We also provided eGFR calculated with the full-age spectrum equation.31–33 All available serum creatinine values (mg/dl) were used to calculate eGFR at the time of TKV evaluation. Serum creatinine (mg/dl) was measured on a Roche Cobas 8000 C702 module using an enzymatic colorimetric method (traceable to the gold standard isotope dilution mass spectrometry method). eGFR slopes per LIC subclass were determined with linear regression analysis on the basis of the longitudinal measurements of eGFR in each subclass, using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA) statistical package for analysis.
The validation cohort included patients with genotyped ADPKD (age <19 years) from Mayo Clinic and CRISP consortium.
Ultrasonographic Evaluation
3D ultrasound volumetry was assessed yearly. To decrease bias, measurements were assessed by a single senior pediatric radiologist as described in a previous study.34
Prediction Model and Statistics
In patients with ADPKD older than 15 years, five age-dependent risk categories with different htTKV progression and correlation with eGFR decline are suggested by the MIC (classes 1A through 1E), using MRI/CT–based volumetry. As previously described, the four cutoff volume growth lines are defined with a two-parameter model on the basis of the equation:
with A=150 ml/m htTKV at age 0 and B=1.5%, 3%, 4.5%, and 6%/year increase in htTKV.22 First, we extrapolated the current adult model to check whether the htTKV severity assessment with 3D ultrasound could be directly used for patients younger than 15 years.
Subsequently, we attempted to improve this model by first tweaking the parameters to better fit our pediatric population and second by optimizing the model itself. In each step, the metric we used for evaluation was a combination of a resulting equal distribution over all severity levels including the longitudinal information of the cases that were scanned multiple times, constrained by a choice of simplicity (number of parameters) of the model. Linear regression analysis was performed to check for htTKV increases over different age subgroups.
We additionally validated our model by incorporating data from the validation cohort including HtTKV of patients with ADPKD aged 15–19 years from the CRISP and Mayo Clinic datasets. Because of the consistent and significant differences found between MRI and 3D ultrasound volumetric measurements,34 we have performed a correction step to convert the MRI values to the corresponding 3D ultrasound (3DUS) values using previously published conversion factors:
Results
Patients
In this longitudinal prospective single-center project, we studied 74 children with genotyped ADPKD (37 males/37 females, from 58 unique families) with a median age (interquartile range [IQR]) at diagnosis of 3 (IQR, 0–9) years and at first 3D ultrasound evaluation of 10 (IQR, 5–14) years; PKD1: n=70 patients (95%), PKD2: n=3 (4%), glucosidase IIα unit: n=1 (1%). The cohort was representative of the described ADPKD phenotype; ADPKD diagnosis was prenatal in seven patients (10%), postnatal incidental finding in 14 (19%), presenting features in four (5%), and active screening because of familial history in 49 (66%) (Table 1). All patients were included in a follow-up regimen with yearly volumetric assessments, leading to 247 evaluations.
Table 1.
Baseline characteristics of participants
| Demographic Data | Number (N=74) |
|---|---|
| Sex (male/female)a | 37/37 |
| Reason and setting of diagnosis | |
| Age at diagnosis, yrb | 3 (0–9) |
| Prenatal diagnosis, n (%)a | 7 (10) |
| Postnatal incidental finding, n (%)a | 14 (19) |
| Family screening, n (%)a | 49 (66) |
| Presenting features, n (%)a | 4 (5) |
| Genetic, n (%)a | |
| PKD1 | 70 (95) |
| PKD2 | 3 (4) |
| GANAB | 1 (1) |
| Anthropometric data at first measurement b | |
| Age at first 3D measurement, yr | 10 (5–14) |
| Body height, cm | 141 (114–163) |
| Weight, kg | 32 (19–52) |
| BMI, kg/m2 | 17 (15–19) |
| BMI, kg/m2, SDS (n=68) | −0.4 (−1.1 to 0.5) |
| Biochemical data at first measurement c | |
| Serum creatinine, mg/dl | 0.5±0.2 (0.2–1.4) |
| eGFR, ml/min per 1.73 m2 − CKiD | 120±33 (48–299) |
| eGFR, ml/min per 1.73 m2 − FAS-age | 112±35 (48–351) |
| Number of TKV measurements per patient, n (%) a | |
| 1 measurement | 10 (14) |
| 2 measurements | 17 (23) |
| 3 measurements | 11 (15) |
| 4 measurements | 16 (22) |
| 5 measurements | 14 (19) |
| 6 measurements | 6 (8) |
GANAB, glucosidase IIα unit; BMI, body mass index; SDS, SD score; CKiD, revised/bedside Schwartz equation; FAS, full-age spectrum; TKV, total kidney volume.
Values are provided as number (%).
Values are provided as median (interquartile range).
Values are provided as mean±SD (range).
Over these 247 evaluations, the median patient age (IQR) was 11 (IQR, 7–15) years, with height 143 (IQR, 122–166) cm, right and left kidney volumes 105 (IQR, 65–146) ml and 125 (IQR, 76–191) ml, totaling a TKV of 236 (IQR, 144–344) ml and an htTKV of 161 (IQR, 117–208) ml/m.
TKV values lay mostly above the normal ranges as described in the literature (Supplemental Figure 1).35
Baseline variables from participants per LIC subcategory are reported in Table 2. BP was missing for five patients. BMI SD is only used beginning at 2 years old, while our cohort included children younger than 2 years and were, therefore, missing for six patients.
Table 2.
Baseline variables from the different Leuven Imaging Classification classes
| LIC Severity | Class A | Class B | Class C | Class D | Class E |
|---|---|---|---|---|---|
| Number of patients | 18 | 16 | 20 | 12 | 8 |
| Sex, M/F | 6/12 | 8/8 | 13/7 | 7/5 | 3/5 |
| Age, yr | |||||
| Median (LQR–UQR) | 11 (7–16) | 12 (8–15) | 7 (4–12) | 10 (4–14) | 6 (4–8) |
| Range | 1.00–18 | 0–18 | 0–17 | 1–19 | 3–15 |
| Systolic BP, mm Hg | |||||
| Median (LQR–UQR) | 103 (89–116) | 104 (86–113) | 100 (87–116) | 108 (92–111) | 96 (93–113) |
| Range | 81–127 | 80–140 | 71–138 | 85–126 | 82–118 |
| Systolic BP, mm Hg, SDS | |||||
| n (%) | 18 (100) | 16 (100) | 18 (90) | 10 (83) | 7 (87.5) |
| Median (LQR–UQR) | 0.00 (−0.70 to 0.50) | 0.00 (−0.68 to 0.57) | 0.00 (−0.70 to 0.60) | 0.00 (−0.70 to 0.50) | −0.05 (−0.73 to 0.50) |
| Range | −4.3 | −2.30 to 2.00 | −2.30 to 2.00 | −2.30 to 2.00 | −2.30 to 2.00 |
| Diastolic BP, mm Hg | |||||
| Median (LQR–UQR) | 55 (52–64) | 59 (54–68) | 56 (51–59) | 60 (60–64) | 54 (50–63) |
| Range | 40–70 | 47–78 | 47–76 | 56–67 | 48–71 |
| Diastolic BP, mm Hg, SDS | |||||
| n (%) | 18 (100) | 16 (100) | −0.18 (90) | 10 (83.3) | 7 (87.5) |
| Median (LQR–UQR) | 0.25 (−0.60 to 0.30) | −0.25 (−0.60 to 0.30) | −0.30 (−0.60 to 0.30) | −0.20 (−0.60 to 0.30) | −0.25 (−0.60 to 0.30) |
| Range | −3.8 | −1.60 to 2.20 | −1.60 to 2.20 | −1.60 to 2.20 | −1.60 to 2.20 |
| Hypertension, n (%) | 3 (16.7) | 1 (6.25) | 2 (10.0) | 3 (25.0) | 1 (12.5) |
| TKV, ml | |||||
| Median (LQR–UQR) | 179 (101–272) | 220 (133–348) | 158 (106–288) | 285 (159–532) | 209 (184–284) |
| Range | 58–371 | 60–508 | 58–630 | 76–901 | 147–706 |
| hTKV, ml/m | |||||
| Median (LQR–UQR) | 124 (89–161) | 146 (112–195) | 126 (103–182) | 193 (124–318) | 180 (164–217) |
| Range | 74–198 | 89–286 | 91–319 | 103–551 | 141–445 |
| Genetics | |||||
| PKD1 % (n) | 19 (14) | 22 (16) | 27 (20) | 15 (11) | 11 (8) |
| Truncating % (n) | 29 (4) | 25 (4) | 15 (3) | 27 (3) | 38 (3) |
| Nontruncating % (n) | 71 (10) | 75 (12) | 85 (17) | 73 (8) | 63 (5) |
| PKD2 % (n) | 5 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Truncating % (n) | 75 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nontruncating % (n) | 25 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| GANAB % (n) | 0 (0) | 0 (0) | 0 (0) | 1.35 (1) | 0 (0) |
| Serum creatinine, mg/dl | |||||
| Median (LQR–UQR) | 0.56 (0.46–0.71) | 0.52 (0.39–0.60) | 0.44 (0.30–0.59) | 0.49 (0.29–0.75) | 0.37 (0.32–0.55) |
| Range | 0.30–0.96 | 0.27–0.99 | 0.18–0.74 | 0.18–1.43 | 0.21–0.65 |
| Height, cm | |||||
| Median (LQR–UQR) | 148 (125–167) | 153 (119–168) | 126 (104–156) | 148 (110–165) | 122 (105–136) |
| Range | 78–188 | 67–198 | 55–198 | 73–177 | 103–159 |
| Weight, kg | |||||
| Median (LQR–UQR) | 32 (22–53) | 41 (19–55) | 25 (16–42) | 35 (21–54) | 24 (17–29) |
| Range | 11–77 | 8–76 | 4–98 | 10–97 | 14–42 |
| BMI, kg/m 2 | |||||
| Median (LQR–UQR) | 16 (14–18) | 18 (15–19) | 17 (15–19) | 18 (17–20) | 16 (14–17) |
| Range | 13–26 | 13–23 | 13–28 | 15–31 | 13–18 |
| BMI, kg/m 2 , SDS | |||||
| n (%) | 18 (100) | 15 (94) | 17 (85) | 11 (92) | 7 (88) |
| Median (LQR–UQR) | −0.4 (−1.1 to 0.5) | −0.6 (−1.2 to 0.5) | −0.4 (−1.1 to 0.5) | −0.3 (−1.1 to 0.6) | −0.5 (−1.1 to 0.7) |
| Range | −2.6 to 2.2 | −2.6 to 2.2 | −2.6 to 2.2 | −2.6 to 2.2 | −2.5 to 2.2 |
| eGFR (CKiD) or CKD-EPI, ml/min per 1.73 m 2 | |||||
| Median (LQR–UQR) | 107 (95–118) | 119 (112–130) | 126 (110–144) | 120 (113–133) | 131 (104–150) |
| Range | 81–129 | 82–155 | 94–166 | 48–299 | 101–205 |
| eGFR (FAS-Age), ml/min per 1.73 m 2 | |||||
| Median (LQR–UQR) | 103 (93–120) | 115 (111–127) | 114 (110–133) | 105 (99–122) | 118 (95–124) |
| Range | 81–131 | 92–161 | 95–156 | 51–363 | 95–165 |
LIC, Leuven Imaging Classification; M, male; F, female; LQR, lower quartile range; UQR, upper quartile range; GANAB, glucosidase IIα unit; SDS, SD score; n, number of patients; TKV, total kidney volume; htTKV, height-corrected total kidney volume; BMI, body mass index; CKiD, revised/bedside Schwartz equation; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration formula; FAS-Age, full-age spectrum–age formula.
Extrapolation of the Adult MIC ADPKD Model
First, we extrapolated the MIC model to our pediatric 3D ultrasound TKV data (Figure 1). We demonstrated strong underestimations of the disease severity, especially in the patients younger than 10 years. These would nearly all be classified as class A, and only above 15 years of age, a normal distribution around the severity lines can be seen, which is in line with the original MIC model constraints. Applying this extrapolated model to our dataset caused the following subdivision: 148 (60%), 43 (17%), 25 (10%), 12 (5%), and 19 (8%) patients for each of the five severity grades (1A–1E) in increasing order, respectively. Clearly, although htTKV corrects for some of the height differences in the adult population, it could not correct for all size changes during childhood. Second, because of the use of a single volume (150 ml) at age 0 in the MIC, any volume in young children below this threshold could not be reliably assigned to a severity class.
Figure 1.
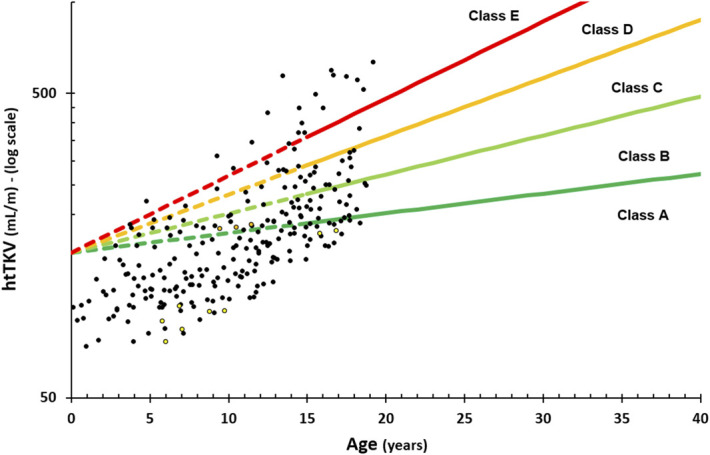
3D ultrasound measurements in our pediatric population (black circles—PKD1, yellow circles—PKD2, and orange circles—glucosidase IIα unit [GANAB]) with the original adult Mayo Imaging Class (MIC) extrapolated to the pediatric range. htTKV, height-adjusted total kidney volume.
Adjusted Mayo ADPKD Model
Optimizing the parameters in the original adult MIC model () with A the starting htTKV at age 0 and B the yearly htTKV % increase led to the following parameters for the four cutoffs, respectively: A=70, 80, 90, and 100 and B=6%, 7.5%, 9%, and 10.5% (Figure 2). This resulted in an improved distribution of patients across the severity scores, specifically 58 (24%), 95 (39%), 45 (18%), 27 (11%), and 22 (9%) for classes A to E, respectively. The choice of different starting volumes for the different cutoffs was made to allow for better assignment into different severity grades of very young children (<10 years of age), which was lacking in the original MIC model.22
Figure 2.
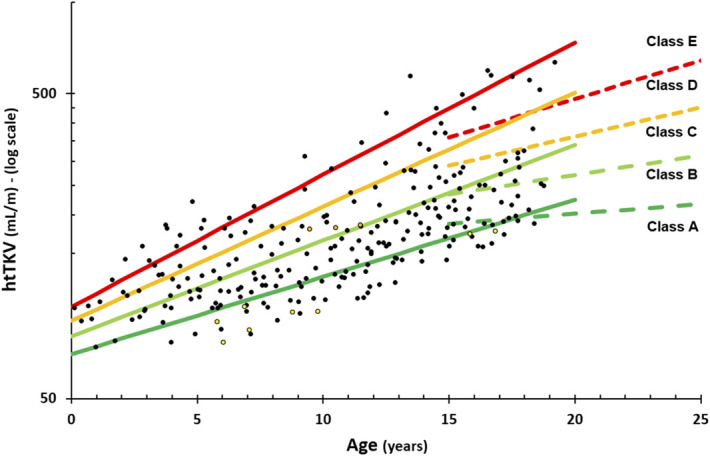
3D ultrasound measurements in our pediatric population (black circles—PKD1, yellow circles—PKD2, and orange circles—GANAB) with the optimized MIC into five severity grades of autosomal dominant polycystic kidney disease (ADPKD). Dashed lines indicate the original adult MIC for reference.
The LIC Pediatric ADPKD Model
Although the adjusted model above fitted the data reasonably well, it did not perform equally well over the entire pediatric age range. Regression analysis of the subgroup of patients aged 0–10 years showed a significant increase (R2=0.074, P = 0.005) with an htTKV increase of 4 ml/m per year. However, when looking at the subgroup of patients aged 10–20 years, the regression analysis found a much stronger growth (R2=0.21, P < 0.001) with an htTKV increase of 20 ml/m per year. To better fit this discrepancy between first and second decade of kidney growth in our ADPKD patient population, we proposed a new model using cutoffs between severity grades of the form htTKV (ml/m)=A×B^(age^1.6) with A=80, 90, 100, and 110 and B=1.01, 1.012, 1.015, and 1.018 (Figure 3). This led to an improved distribution of patients across the severity scores, especially splitting up the previously dominant class B group, to subdivisions of 57 (23%), 67 (27%), 55 (22%), 35 (14%), and 33 (13%) cases for classes A to E, respectively.
Figure 3.
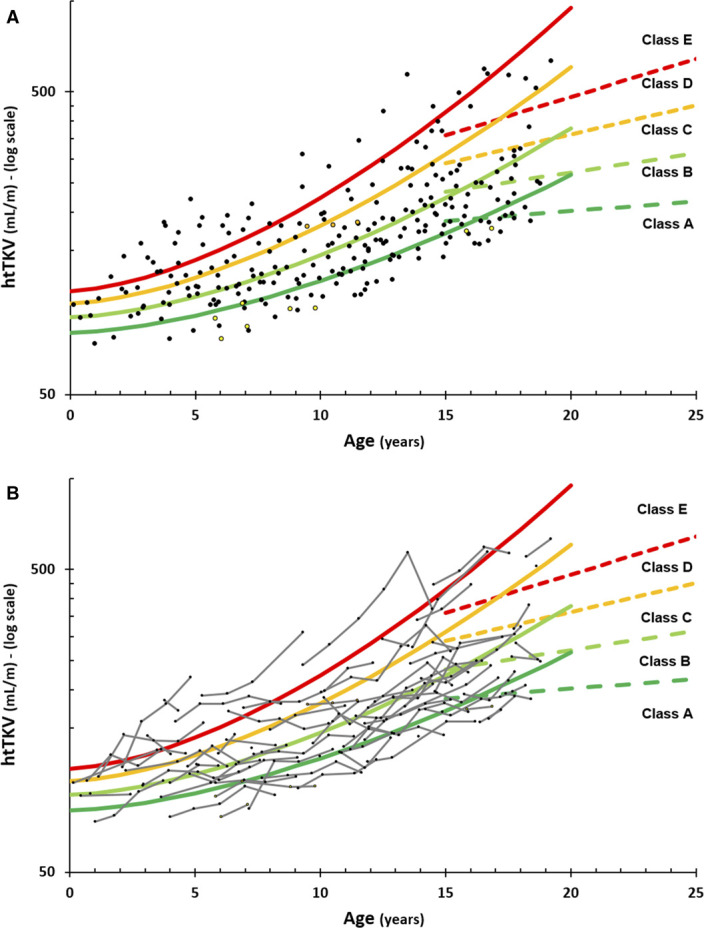
3D ultrasound measurements in our pediatric population (black circles—PKD1, yellow circles—PKD2, and orange circles—GANAB) with the newly Leuven Imaging Classification (LIC) proposed pediatric ADPKD model. LIC (solid lines) and MIC (dashed lines, only up to age 25) models are shown with all cross-sectional data (A) and longitudinal evaluations of each patient (B, small gray lines).
Evaluation of Longitudinal Scans in Our LIC Model
Of 64 patients with longitudinal data, 29 patients (45%) remained in the same class, 26 (41%) changed just one class, and only nine (14%) changed by two classes during the entire follow-up period (Figure 3B). Of those nine patients, six were younger than 5 years at first presentation, where the classification lines were very close together, so a small measurement variability could have resulted in a misclassification. The remaining three cases with two level changes had a long follow-up (at least five evaluations).
eGFR Decline from Each LIC Category
The median creatinine and eGFR values per subcategories are reported in Table 2. Three patients from the cohort had another comorbidity that might influence the eGFR (Charcot-Marie-Tooth disease, Duchenne muscular dystrophy, and brain tumor). Linear regression analysis was performed on the eGFRs of each severity class. Using revised/bedside Schwartz equation in childhood and Chronic Kidney Disease Epidemiology Collaboration during adulthood, we observed an overall decline of −2.47 ml/min per 1.73 m2 per year in the whole cohort. eGFR calculated with full-age spectrum–age equation showed a more moderate decline of −0.22 ml/min per 1.73 m2 per year. Division into the different LIC classes showed a trend toward a more rapid decline of eGFR against age from class A to class E (−0.63, −2.16, −2.32, −2.15, and −4.74 ml/min per 1.73 m2 per year for A through E, respectively) (Supplemental Figure 2).
Validation with Additional Data from the Mayo Clinic and CRISP in the Overlapping/Transition Age Range
The provided dataset, 71 evaluations of 39 patients (21 evaluated once, nine twice, four three times, and five four times), from the Mayo and CRISP group is indicated in Figure 4, combined with both the original adult model and our newly proposed pediatric model. Note that the correction factors of TKV between MRI and 3D ultrasound values have been applied to the Mayo cases for evaluation and visualization where appropriate. For this age range, the included patients were much more homogeneously distributed compared with the MIC adult model, where the young adults are scored as mostly in the highest severity class. Separation into severity categories with the MIC model yielded a distribution of one (1%), eight (11%), 18 (25%), 18 (25%), and 26 (37%) cases, respectively, while our pediatric model distributed the group into five (7%), 21 (30%), 26 (37%), 12 (17%), and seven (10%) cases for severity classes A through E, respectively.
Figure 4.
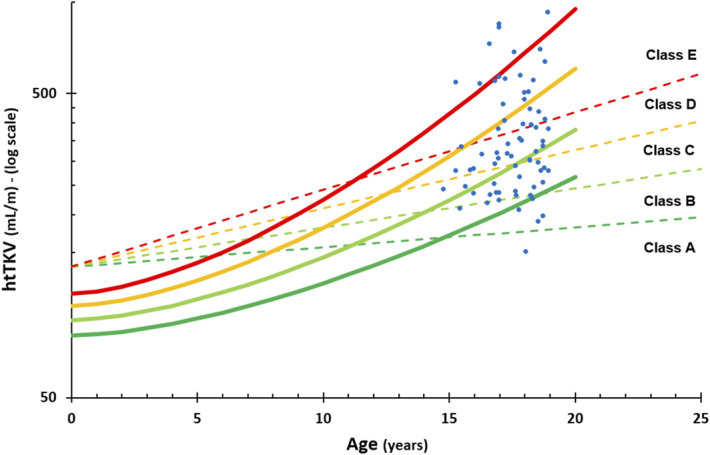
Incorporation of the validation cohort (blue dots) in the Leuven classification pediatric model (full lines), with the MIC model extrapolated to the pediatric age range as comparison (dashed lines).
Discussion
Young patients with ADPKD with early disease stages represent the new target population for future treatment.36 To address the lack of validated biomarkers for disease progression in this age group, we propose the Leuven Imaging Classification (LIC) Pediatric ADPKD Model, a new two-parameter ADPKD risk stratification model applicable to children and adolescents on the basis of htTKV and patients' age, with the incorporation of a validation cohort from Mayo and CRISP consortium. This newly developed model in pediatric ADPKD is based on 3D ultrasound kidney volumetry and better reflects the growth rate of htTKV from birth. Using this risk stratification model in clinical practice as precursor of the MIC adult risk model could allow categorization of ADPKD disease severity from a young age into slow or fast progressors and facilitate selecting patients in clinical trials, permitting decision making about initiating early treatment.
This is a first effort toward defining different risk classes in pediatric ADPKD on the basis of htTKV, similar to the adult ADPKD MIC. In contrast to the adults, eGFR decline cannot be used in children with ADPKD as a parameter to validate the htTKV progression in the different classes of the model.37 However, we did note a slightly faster decrease in eGFR measurements with increased age in higher severity classes (Supplemental Figure 2). The future aim is to validate this model using a larger longitudinal cohort such as the database of the ADPedKD registry.38
ADPKD is characterized by exponentially growing cysts, increasing kidney volumes already at a young age, and associated secondary inflammation/fibrosis of the kidney parenchyma.39,40 Age-dependent htTKV in adults has become a validated and accepted surrogate prognostic biomarker.2,19,24 However, ADPKD is a disease with significant interindividual variability and kidney function decline related to the type of genetic mutation (PKD1 versus PKD2), although the rates of growth do not differ.18,41 These data illustrate the critical role that the rate of cyst development during childhood plays in the severity of disease and subsequent timing of kidney failure.27
In contrast to the MIC, pediatric htTKV is assessed with ultrasound in our model because it is easy to perform in children, although MRI or CT is more accurate for short-term disease evolution.22,30,42–44
Extrapolating the adult MIC model to our pediatric cohort created a clinically incorrect model as the 0 year htTKV is not realistic. As reported in the literature, normal TKV in children younger than 18 year are below 150 ml, ranging from 15 ml at year 0 to 131 ml at year 17.35 In addition, severity of pediatric ADPKD in age younger than 10 years is strongly underestimated on the basis of the adult MIC model. Adjusting the starting htTKV (to 70–80–90–100 ml) and yearly increase of TKV (to 6%, 7.5%, 9%, 10.5%/year) to the childhood data allowed us to obtain more equally divided risk groups that cover the entire pediatric age range. However, the model did not account for the observed growth acceleration after 10 years of age. We concluded that adapting the two parameters in the MIC formula to pediatric ADPKD was not reliable enough to predict kidney growth toward adolescence. Although we expect a faster growth in normal children around puberty, we do not have enough viable information in the literature for separate TKV adjustments for different growth rates before and after puberty. We therefore proposed a higher exponential formula that better fits both decades of childhood, which is also in accordance with other reports.45
Of the 64 patients with longitudinal data, we found 29 (45%) remained within their class and 26 (41%) changed by only one class during follow-up. This is more than in the adult MIC classification where 87% of cases remained within the same class over a follow-up period of 4 years.22 In adulthood, the absolute htTKV difference between the severity group lines is much larger compared with our pediatric population so that normal measurement variability is less likely to cause a class switch in adults. However, most of our pediatric cases remained close to their original severity estimation, indicating that the model could be a good predictor of adulthood ADPKD disease and would allow prediction of htTKV evolution over time, with some individual variation.
The strength of our study is the availability of a well-characterized and genotyped ADPKD pediatric cohort and a clinically representative population. Moreover, to lower the radiation exposure and procedural discomfort, we have chosen to apply annual 3D ultrasound for kidney volume assessment.22,45 To obtain the most accurate ultrasound volumetric measurements compared with MRI volumetry, we have used the reported stacked contour volumetric measuring technique.44,46–48
To resolve the abrupt transition from our pediatric model to the adult MIC model situated between 15 and 19 years of age, we incorporated a validation cohort using the Mayo and CRISP consortium data. It would be very interesting to extend the data to older ages and to work on a unique model covering the entire age spectrum and ranging from birth until late stages.
Although a clear correlation between BMI and TKV change has been demonstrated in the adult population,49,50 in our cohort, BMI was in the normal limit, and we found no differences in BMI SD between the LIC subcategories. Similarly, we did not find an increase in hypertension within the LIC categories A to E. However, the number of patients per subcategory was too small to make any conclusion.
This study contains some limitations. The current used correction factor from MRI to ultrasound still requires an external validation. Although the genotype of our population is well characterized, it remains a small study cohort of 74 patients. Moreover, there is a small number of patients with PKD2 genotype (4% in the LIC versus 16% in the MIC), the less severe form of ADPKD, and all situated in the lowest class A, which may mean that our population is more severe than the total childhood population. Compared with the adult ADPKD model, we also found a higher variability in severity class for pediatric patients during follow-up, likely due to the small volumetric differences between subsequent severity classes in lower age ranges or individual patient growth differences. This needs to be further investigated in a larger database. Finally, the main limitation of our model is that 3D ultrasound is not in the routine use; however, we reported a correction factor to convert the 3D volumetry to 2D estimation.30 We are also currently expanding our datasets including 2D and kidney length measurements to provide adapted models to be implemented in the future daily practice.
In conclusion, we provided the LIC ADPKD pediatric model, a novel risk stratification tool that nicely followed the kidney growth in children with ADPKD over the first two decades of life, on the basis of 3D ultrasound kidney volumetric measurements. This tool will be used as precursor of the MIC adult risk model to classify ADPKD disease severity in children, allowing the identification of those at risk of becoming fast progressor, which would be ideal candidates for disease-modifying interventions at a young age.
Supplementary Material
Acknowledgments
D. Mekahli is supported by the Research Foundation Flanders (FWO) (G0C8920N) and clinical senior research grant for D. Mekahli (1804123N) and the University Hospitals Leuven. The CRISP study was supported by NIDDK grants: DK056943, DK056956, DK056957, and DK056961. The authors thank the participating children and their parents, as well as the nurses involved in the care of the patients. Thanks also to the CRISP consortium: Vicente E. Torres (Mayo Clinic), Arlene B. Chapman (University of Chicago), Alan Yu (Kansas University Medical Center), Kyongtae Bae (University of Pittsburgh), Frederick F. Rahbari Oskoui (Emory University), Douglas P. Landsittel (Indiana University School of Public Health), William M. Bennett (Legacy Health, Portland), and Michal Mrug (University of Alabama, Birmingham).
Footnotes
Members of CRISP Consortium: A Yu (Kansas Medical Center), AB Chapman (University of Chicago), FF Rahbari Oskoui (Emory University), VE Torres, FT Chebib (Mayo Clinic), DP Landsittel (University of Pittsburgh), WM Bennett (Legacy Health, Portland), M Mrug (University of Alabama, Birmingham), and KT Bae (University of Hong Kong).
Contributor Information
Collaborators: A Yu, AB Chapman, FF Rahbari Oskoui, VE Torres, FT Chebib, DP Landsittel, WM Bennett, M Mrug, and KT Bae
Disclosures
B. Bammens reports consultancy agreements with Baxter and Otsuka; consultancy honoraria from Baxter and Otsuka; speaker's fees from Baxter; and membership with BVN (Belgian Society of Nephrology), NBVN (Nederlandstalige Belgische Vereniging voor Nefrologie), and ISPD (International Society of Peritoneal Dialysis). B. Bammens reports that the Department of Nephrology, Dialysis and Renal Transplantation receives research support from Amgen, Astellas, Nipro, Otsuka, Roche, and Vifor Pharma. F. De Keyzer reports employment with and honoraria from UZ Leuven. P.C. Harris reports employment with Mayo Clinic; consultancy agreements with Mitobridge, Otsuka, Regulus, and Vertex; research funding from Acceleron, Jemincare, Navitor, and Otsuka Pharmaceuticals; and patents or royalties from Amgen, Bayer, Genzyme, GlaxoSmithKline, Millipore, Mitobridge, and Vertex. M.V. Irazabal reports employment with Mayo Clinic and royalties from Mayo Imaging Classification. D. Mekahli reports consultancy for Otsuka Pharmaceuticals and Reata as a representative of the University Hospital of Leuven and the KU Leuven University. D. Mekahli received educational grants from Otsuka Pharmaceuticals paid to the University Hospital of Leuven and KU Leuven University, Leuven, Belgium, all outside the submitted work. D. Mekahli serves on an advisory board of Galapagos, Otsuka Pharmaceuticals, Reata, and Sanofi Genzyme as a representative of the University Hospital of Leuven and KU Leuven. P. Schellekens reports research funding from Research Foundation Flanders (FWO) (G0C8920N) (1804123N). All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Bert Bammens, Luc Breysem, Angélique Dachy, Frederik De Keyzer, Stephanie De Rechter, Peter C. Harris, Maria V. Irazabal, Peter Janssens, Djalila Mekahli, Pieter Schellekens, Chantal Van Ongeval, Rudi Vennekens.
Data curation: Luc Breysem, Frederik De Keyzer, Peter C. Harris, Djalila Mekahli.
Formal analysis: Luc Breysem, Frederik De Keyzer, Djalila Mekahli.
Investigation: Djalila Mekahli.
Methodology: Bert Bammens, Luc Breysem, Angélique Dachy, Frederik De Keyzer, Stephanie De Rechter, Peter C. Harris, Maria V. Irazabal, Peter Janssens, Djalila Mekahli, Pieter Schellekens, Chantal Van Ongeval, Rudi Vennekens.
Validation: Luc Breysem.
Writing – original draft: Luc Breysem, Frederik De Keyzer, Djalila Mekahli.
Writing – review & editing: Bert Bammens, Luc Breysem, Angélique Dachy, Frederik De Keyzer, Stephanie De Rechter, Peter C. Harris, Maria V. Irazabal, Peter Janssens, Djalila Mekahli, Pieter Schellekens, Chantal Van Ongeval, Rudi Vennekens.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B645.
Supplemental Figure 1. Comparison of the TKV in our pediatric dataset (blue dots) with the published normal TKV ranges (orange line [mean] with 95% confidence interval [green—red lines]) by Leung et al.35
Supplemental Figure 2. Evolution of eGFR as a function of age with linear regression analysis per subclass of the LIC model.
References
- 1.Chung EM, Conran RM, Schroeder JW, Rohena-Quinquilla IR, Rooks VJ. From the radiologic pathology archives: pediatric polycystic kidney disease and other ciliopathies: radiologic-pathologic correlation. Radiographics. 2014;34(1):155–178. doi: 10.1148/rg.341135179 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1(1):148–157. doi: 10.2215/CJN.00330705 [DOI] [PubMed] [Google Scholar]
- 3.Bergmann C. Early and severe polycystic kidney disease and related ciliopathies: an emerging field of interest. Nephron. 2019;141(1):50–60. doi: 10.1159/000493532 [DOI] [PubMed] [Google Scholar]
- 4.Pei Y, Hwang YH, Conklin J, et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;26(3):746–753. doi: 10.1681/ASN.2014030297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7(10):556–566. doi: 10.1038/nrneph.2011.109 [DOI] [PubMed] [Google Scholar]
- 6.Cadnapaphornchai MA. Autosomal dominant polycystic kidney disease in children. Curr Opin Pediatr. 2015;27(2):193–200. doi: 10.1097/MOP.0000000000000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rechter S, Breysem L, Mekahli D. Is autosomal dominant polycystic kidney disease becoming a pediatric disorder? Front Pediatr. 2017;5:272. doi: 10.3389/fped.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avni FE, Hall M. Renal cystic diseases in children: new concepts. Pediatr Radiol. 2010;40(6):939–946. doi: 10.1007/s00247-010-1599-5 [DOI] [PubMed] [Google Scholar]
- 9.Gimpel C, Avni FE, Bergmann C, et al. Perinatal diagnosis, management, and follow-up of cystic renal diseases a clinical practice recommendation with systematic literature reviews. JAMA Pediatr. 2018;172(1):74–86. doi: 10.1001/jamapediatrics.2017.3938 [DOI] [PubMed] [Google Scholar]
- 10.Gimpel C, Avni EF, Breysem L, et al. Imaging of kidney cysts and cystic kidney diseases in children: an international working group consensus statement. Radiology. 2019;290(3):769–782. doi: 10.1148/radiol.2018181243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149–168. doi: 10.1038/ki.2009.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avni FE, Garel C, Cassart M, D'Haene N, Hall M, Riccabona M. Imaging and classification of congenital cystic renal diseases. AJR Am J Roentgenol. 2012;198(5):1004–1013. doi: 10.2214/AJR.11.8083 [DOI] [PubMed] [Google Scholar]
- 13.Riccabona M, Avni FE, Damasio MB, et al. ESPR Uroradiology Task Force and ESUR Paediatric Working Group—imaging recommendations in paediatric uroradiology, part V: childhood cystic kidney disease, childhood renal transplantation and contrast-enhanced ultrasonography in children. Pediatr Radiol. 2012;42(10):1275–1283. doi: 10.1007/s00247-012-2436-9 [DOI] [PubMed] [Google Scholar]
- 14.Dillman JR, Trout AT, Smith EA, Towbin AJ. Hereditary renal cystic disorders: imaging of the kidneys and beyond. Radiographics. 2017;37(3):924–946. doi: 10.1148/rg.2017160148 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Blumenfeld JD, Prince MR. MRI in autosomal dominant polycystic kidney disease. J Magn Reson Imaging. 2019;50(1):41–51. doi: 10.1002/jmri.26627 [DOI] [PubMed] [Google Scholar]
- 16.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–212. doi: 10.1681/ASN.2008050507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed B, Nobakht E, Dadgar S, et al. Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2010;56(1):50–56. doi: 10.1053/j.ajkd.2010.02.349 [DOI] [PubMed] [Google Scholar]
- 18.Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73(1):108–116. doi: 10.1038/sj.ki.5002624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7(3):479–486. doi: 10.2215/CJN.09500911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangri N, Hougen I, Alam A, Perrone R, McFarlane P, Pei Y. Total kidney volume as a biomarker of disease progression in autosomal dominant polycystic kidney disease. Can J Kidney Health Dis. 2017;4:205435811769335. doi: 10.1177/2054358117693355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu ASL, Shen C, Landsittel DP, et al.; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP). Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in autosomal dominant polycystic kidney disease. Kidney Int. 2018;93(3):691–699. doi: 10.1016/j.kint.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irazabal MV, Rangel LJ, Bergstralh EJ, et al.; CRISP Investigators. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160–172. doi: 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erratum, Perrone RD, Mouksassi M-S, et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. 2018;23(4):442–450. doi: 10.1016/j.ekir.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrone RD, Mouksassi MS, Romero K, et al. Total kidney volume is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep. 2017;2(3):442–450. doi: 10.1016/j.ekir.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Rechter S, Bammens B, Schaefer F, Liebau MC, Mekahli D. Unmet needs and challenges for follow-up and treatment of autosomal dominant polycystic kidney disease: the paediatric perspective. Clin Kidney J. 2018;11(suppl 1):i14–i26. doi: 10.1093/ckj/sfy088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer F, Mekahli D, Emma F, et al. Tolvaptan use in children and adolescents with autosomal dominant polycystic kidney disease: rationale and design of a two-part, randomized, double-blind, placebo-controlled trial. Eur J Pediatr. 2019;178(7):1013–1021. doi: 10.1007/s00431-019-03384-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavu S, Vaughan LE, Senum SR, et al. The value of genotypic and imaging information to predict functional and structural outcomes in ADPKD. JCI Insight. 2020;5(15):e138724. doi: 10.1172/jci.insight.138724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roelants M, Hauspie R, Hoppenbrouwers K. References for growth and pubertal development from birth to 21 years in Flanders, Belgium. Ann Human Biol. 2009;36(6):680–94. doi: 10.3109/03014460903049074 [DOI] [PubMed] [Google Scholar]
- 29.Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34(10):1887–1920. doi: 10.1097/HJH.0000000000001039 [DOI] [PubMed] [Google Scholar]
- 30.Massella L, Mekahli D, Paripović D, et al. Prevalence of hypertension in children with early stages ADPKD. Clin J Am Soc Nephrol. 2018;13(6):874–883. doi: 10.2215/CJN.11401017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant. 2014;29(5):1082–1091. doi: 10.1093/ndt/gft277 [DOI] [PubMed] [Google Scholar]
- 32.Pottel H, Björk J, Courbebaisse M, et al. Development and validation of a modifed full age spectrum creatinine based equation to estimate glomerular filtration rate. A cross-sectional analysis of pooled data. Ann Int Med. 2021;174(2):183–191. doi: 10.7326/M20-4366 [DOI] [PubMed] [Google Scholar]
- 33.Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant. 2017;32(3):497–507. doi: 10.1093/ndt/gfw425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breysem L, de Rechter S, de Keyzer F, et al. 3DUS as an alternative to MRI for measuring renal volume in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol. 2018;33(5):827–835. doi: 10.1007/s00467-017-3862-6 [DOI] [PubMed] [Google Scholar]
- 35.Leung VYF, Chu WCW, Yeung CK, et al. Nomograms of total renal volume, urinary bladder volume and bladder wall thickness index in 3,376 children with a normal urinary tract. Pediatr Radiol. 2007;37(2):181–188. doi: 10.1007/s00247-006-0376-y [DOI] [PubMed] [Google Scholar]
- 36.Gimpel C, Bergmann C, Mekahli D. The wind of change in the management of autosomal dominant polycystic kidney disease in childhood. Pediatr Nephrol. 2022;37(3):473–487. doi: 10.1007/s00467-021-04974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mekahli D, Womack H, Dahl NK. Perspectives on drug development in early ADPKD. Clin J Am Soc Nephrol. 2022;17(10):1555–1558. doi: 10.2215/CJN.05190422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Rechter S, Bockenhauer D, Guay-Woodford LM, et al. A global online platform on the management of children with ADPKD. Kidney Int Rep. 2019;4(9):1271–1284. doi: 10.1016/j.ekir.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman AB, Guay-Woodford LM, Grantham JJ, et al.; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64(3):1035–1045. doi: 10.1046/j.1523-1755.2003.00185.x [DOI] [PubMed] [Google Scholar]
- 40.Brun M, Maugey-Laulom B, Eurin D, Didier F, Avni EF. Prenatal sonographic patterns in autosomal dominant polycystic kidney disease: a multicenter study. Ultrasound Obstet Gynecol. 2004;24(1):55–61. doi: 10.1002/uog.1098 [DOI] [PubMed] [Google Scholar]
- 41.Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2006;17(11):3013–3019. doi: 10.1681/ASN.2006080835 [DOI] [PubMed] [Google Scholar]
- 42.Bae KT, Shi T, Tao C, et al. ; HALT PKD Consortium. Expanded imaging classification of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2020;31(7):1640–1651. doi: 10.1681/ASN.2019101121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Neill WC, Robbin ML, Bae KT, et al. Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: the Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis. 2005;46(6):1058–1064. doi: 10.1053/j.ajkd.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 44.Benz EG, Hartung EA. Predictors of progression in autosomal dominant and autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2021;36(9):2639–2658. doi: 10.1007/s00467-020-04869-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW. Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol. 2011;6(2):369–376. doi: 10.2215/CJN.03780410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhutani H, Smith V, Rahbari-Oskoui F, et al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int. 2015;88(1):146–151. doi: 10.1038/ki.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam A, Dahl NK, Lipschutz JH, et al. Total kidney volume in autosomal dominant polycystic kidney disease: a biomarker of disease progression and therapeutic efficacy. Am J Kidney Dis. 2015;66(4):564–576. doi: 10.1053/j.ajkd.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 48.Chebib FT, Torres VE. Assessing risk of rapid progression in autosomal dominant polycystic kidney disease and special considerations for disease-modifying therapy. Am J Kidney Dis. 2021;78(2):282–292. doi: 10.1053/j.ajkd.2020.12.020 [DOI] [PubMed] [Google Scholar]
- 49.Nowak KL, You Z, Gitomer B, et al. Overweight and obesity are predictors of disease progression in early ADPKD. J Am Soc Nephrol. 2018;29(12):2879–2889. doi: 10.1681/ASN.2017070819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nowak KL, Steele C, Gitomer B, Wang W, Ouyang J, Chonchol MB. Overweight and obesity and progression of ADPKD. Clin J Am Soc Nephrol. 2021;16(6):908–915. doi: 10.2215/CJN.16871020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study are available in this article.