Abstract
Currently, the incidence of metabolic disorders is increasing, setting a challenge to global health. With major advancement in the diagnostic tools and clinical procedures, much has been known in the etiology of metabolic disorders and their corresponding pathophysiologies. In addition, the use of in vitro and in vivo experimental models prior to clinical studies has promoted numerous biomedical breakthroughs, including in the discovery and development of drug candidates to treat metabolic disorders. Indeed, chemicals isolated from natural products have been extensively studied as prospective drug candidates to manage diabetes, obesity, heart-related diseases, and cancer, partly due to their antioxidant and anti-inflammatory properties. Continuous efforts have been made in parallel to improve their bioactivity and bioavailability using selected drug delivery approaches. Here, we provide insights on recent progress in the role of inflammatory-mediated responses on the initiation of metabolic disorders, with particular reference to diabetes mellitus, obesity, heart-related diseases, and cancer. In addition, we discussed the prospective role of natural products in the management of diabetes, obesity, heart-related diseases, and cancers and provide lists of potential biological targets for high throughput screening in drug discovery and development. Lastly, we discussed findings observed in the preclinical and clinical studies prior to identifying suitable approaches on the phytochemical drug delivery systems that are potential to be used in the treatment of metabolic disorders.
1. Introduction
All life forms, including humans, require a highly orchestrated process, is termed as metabolism, to break down the ingested foods to become their simpler elements [254]. In eukaryotes, this process is essential to provide energy required for a species to develop and live. Failure to do so will negatively affect the species survival [322]. Unfortunately, we have witnessed an increasing trend of metabolism-related problems, simplified as metabolic disorders in recent years. People with metabolic disorders may have different characteristics compared to the ones with normal metabolism. Such discrepancy may occur as a result of certain pathological condition that leads to distinct phenotypes [173, 254].
At present, the most predominant metabolic disorders are diabetes mellitus, obesity, heart-related diseases, and cancer [254]. Although much has been known regarding the etiology and pharmacological management of these metabolic disorders, the mechanistic basis is complex and remains to be fully elucidated. Nevertheless, chronic inflammation appears to be one of the key players in the initiation, progression, and transition of the abovementioned metabolic disorders [103,129,225,269]. Stimulation of various pro-inflammatory cytokines in response to the release of endogenous yet danger-associated ligands have been observed to occur in most of the, if not all, metabolic disorders-related condition [129, 131, 269].
Growing evidence indicates that natural products and their bioactive compounds, particularly phytochemicals, can provide various benefits to the human health. Indeed, one of the most focused natural products research areas is the potential application of phytochemicals to treat diabetes, obesity, cardiovascular-related problems, and different types of cancers [254], possibly by targeting the oxidative stress-related pathways and regulatory network of inflammatory process [16,20]. In this review, we discussed a current understanding on the pathophysiology of diabetes, obesity, heart-related diseases, and cancers in correlation with inflammation-mediated induction of metabolic disorders. Furthermore, we later provide a brief and concise discussion on the prospective role of natural products in the management of the diabetes, obesity, heart-related diseases, and cancers by listing the potential biological targets for the phytochemicals and findings observed in the preclinical and clinical studies prior to describing current approaches on the phytochemical drug delivery systems that have been used in the treatment of metabolic disorders.
1.1. Inflammation-mediated induction of metabolic disorders
Survival mechanisms like as metabolic and immunological systems are crucial. Many mechanisms involved in metabolism and immunity, as well as systems that detect nutrients and pathogens, have been conserved across species. Therefore, metabolic control and immunological response are intricately linked, with the health of one depending on the other. The malfunction of this interface has been linked to a variety of chronic metabolic illnesses, including obesity, type 2 diabetes, and cardiovascular disease, and hence can be thought of as a central homeostatic mechanism [130, 158, 164, 225]. As a group, these illnesses pose the greatest danger to the health and well-being of people around the world today.
1.1.1. Implications for the metabolism-inflammation link
The maintenance of metabolic balance depends on insulin, the primary anabolic hormone in animals. Cellular substrates of insulin, including the insulin receptor substrate (IRs) family of proteins, are tyrosine phosphorylated when insulin binds to their receptor. Although changes like serine phosphorylation, regulated by intracellular regulatory pathways, are essential for mediating many of insulin's metabolic actions, they are suppressed under conditions of stress and inflammation [240,289]. People who are overweight, insulin resistant, or have type 2 diabetes also showed this inhibition. Immune mediators, such as cytokines like tumor necrosis factor (TNF)-α, may play a vital regulatory role in systemic glucose homeostasis, as they can initiate the alterations that reduce insulin's efficacy [201]. Insulin signaling is a highly conserved and dominant metabolic route in nutrition and energy homeostasis, and it has been shown that inflammation can contribute to metabolic dysregulation at multiple levels [204,225].
Exploring the connections between immune responses and metabolic regulation has benefited greatly from the discovery platform provided by the identification of the relationship between inflammation and insulin signaling [14, 214]. Nutrients, such as circulating lipids, directly stimulate many of the inflammatory signaling pathways that impede insulin-receptor signaling [164]. Organelle stress caused by nutritional excess and processing errors leads to metabolic stress, which in turn induces further inflammatory pathways. The serine phosphorylation of IRs1 in both circumstances results in the disruption of the insulin signaling system and different metabolic responses due to the activation of kinases such as JUN N-terminal kinase (JNK; also known as maPK8) and Iκb kinase-β (IKKβ). Immune signaling pathways can also activate extracellular-signal-regulated kinase (ERK), ribosomal protein S6 kinase (S6K; also known as RPS6KB1), mammalian target of rapamycin (mTOR; also known as FRAP1), protein kinase C, and glycogen synthase kinase 3, all of which can disrupt the insulin signaling pathway [90,104,289]. It is likely that changes in metabolic responses will be connected to a wide variety of immunological signaling pathways and proteins. Moreover, metabolic signaling pathways might influence the immunological response. The inflammatory response can be dampened, for instance, by turning on nuclear receptors such as peroxisome proliferator-activated receptors (PPARs) and liver X receptors (LXRs). Several other metabolic hormones, including leptin, resistin, and adiponectin, have immunological functions as well [158, 238].
1.1.2. How does inflammation trigger metabolic dysfunction?
-
•
Pattern recognition receptors (PRRs) as metabolic sensors
It is well known that PRRs in the innate immune system may detect foreign molecules (pathogen-associated chemical patterns) and launch a defense response. But it is now known that the ability of PRRs to identify endogenous ligands generated in the obese state is a trigger in obesity-associated inflammation [179]. The Toll-like receptor 4 (TLR4) is the most studied PRR because it responds to free fatty acids (FAs) by producing inflammatory signals and activating the nuclear factor kappa B (NF-κB). Obesity-induced inflammatory activation is prevented in TLR4-deficient mice, and these mice also show resistance to insulin infusion-induced fat gain [152]. Although leukocytes play a role in mediating this effect, there is strong evidence that TLR4 activation in non-hematopoietic cells has direct consequences on the metabolic phenotype [14]. Nearly all members of the TLR family are expressed in adipose tissue, and TLR2-knockout mice are protected from high-fat DIO and insulin resistance, indicating a broad function for TLRs in obesity and its associated morbidities. Mice lacking TLR5 exhibit obesity and insulin resistance due to changes in their gut microbiome, demonstrating that TLRs monitor and control gut microorganisms in a way that contributes to metabolism in addition to FAs [179,238].
Obesity-induced signals are also detected by the Nod-like receptor (NLR) family of PRRs. Leukocytes are directed toward stimuli that activate NLRs in order to limit tissue damage. NLRs are triggered by danger signals from stressed or dying cells. When NLRs are activated, caspase-1 is activated to produce IL-1β and IL-18 in macrophages. When glucose levels remain high for an extended period of time, cells in the pancreas begin to die. Diet-induced obesity (DIO) also induces caspase-1 and IL-1β in adipose tissue, and NLRP3- and caspase-1-deficient mice are resistant to DIO-induced inflammation [130, 158]. Mice lacking NLRP3 exhibit reduced M1 and increased M2 gene expression without quantitative changes in adipose tissue macrophages (ATMs), suggesting that changes in the M1 activation of ATMs underlie this protective effect. Numerous mechanisms may contribute to meta-inflammation, if PRRs can serve as universal dual sensors of pathogenic and endogenous signals pertinent to obesity [225].
-
•
IKKβ and NF-κB
Multiple pathways, some of which may or may not involve the adaptor protein MyD88, are involved in transmitting intracellular signals that are triggered by TLR activation. MyD88−/− mice are more prone to insulin resistance with DIO, although the significance of MyD88-dependent signaling in other metabolic organs remains elusive [158]. When a person is obese, the activation of IKKβ happens downstream of MyD88 and plays a crucial role in inflammation throughout the body, particularly in the liver, myeloid cells, and hypothalamus. Salicylate, an IKKβ inhibitor, is under clinical trials for the treatment of type 2 diabetes, and its insulin-sensitizing effect is likely due to this inhibitor's broad spectrum of activity [54,68]. TLR/IKKβ signals are ultimately translated into NF-κβ-dependent activation of inflammatory gene transcription. DIO induces NF–B expression primarily in adipose tissue and atrial myocytes, as seen by in vivo imaging. One NF-κβ-sensitive gene activated by high-fat diet is Ikke, a protein kinase that appears to play a role in regulating body weight and insulin resistance by inhibiting thermogenesis. There are still questions about how to tell the difference between the metabolic effects of acute and chronic NF-κβ activation, and this highlights the significance of temporal management of NF-κβ activation. Acute exercise in lean individuals, for instance, causes a temporary release of proinflammatory cytokines like IL-6 from muscle NF-κβ [267].
-
•
Role of ceramides and intracellular lipids in inflammation and metabolic processes
There are other implications of TLR4 activation beyond NF-κB activation. The equilibrium between intracellular lipid species like ceramides and sphingolipids may play an important role in both metabolism and inflammation [240]. Saturated FAs propensity to promote insulin resistance is prevented by ceramide synthesis inhibition. TLR4 is required for lipopolysaccharide (LPS) and saturated FA-induced ceramide formation in numerous metabolic organs, including the brain and muscle, where it can block insulin signaling via the Akt pathway. Salicylates lower ceramide levels in the liver, muscle, and hypothalamus, indicating that IKKβ is required for TLR4-mediated ceramide synthesis in metabolic organs [152].
Adiponectin, an adipokine, has been known for a long time to have beneficial effects on a variety of cell types, including increasing insulin sensitivity and decreasing the activity of proinflammatory pathways. Because adiponectin increases ceramidase activity and alters the ratio of ceramides to sphingosine-1-phosphate, control of ceramides may be a mechanism by which adiponectin exerts its effects [130]. Protecting against cardiomyocyte and cell apoptosis suggests that adiponectin's effect on cellular ceramide concentration is significant for numerous organs. It is possible that adiponectin receptor-associated ceramidase activity is not the only factor at play [14]. Adiponectin infusion increased insulin sensitivity in hepatocytes via IRS2 activation, as discovered by Ref. [19]; however this effect was not cell autonomous [19]. This insulin-sensitizing effect was unexpectedly caused by the activation of IL-6 by adiponectin in macrophages, and it occurred substantially independently of the adiponectin receptors R1 and R2.
-
•
JNK and stress
Through upstream pathways shared by IKKβ/NF-κB in response to stress signals including fatty acids (FAs), insulin, hyperglycemia, and inflammatory cytokines, obesity also activates JNK in insulin-responsive tissues. In comparison to other components of inflammatory signaling, the unique role played by JNK in hematopoietic and non-hematopoietic cells in obesity is well characterized [14, 238]. Even though both JNK1 and JNK2 isoforms play a part in metabolic control, JNK1 has a more significant role in DIO protection. Body weight and energy expenditure are regulated by JNK1's actions in nonhematopoietic cells. Inactivating JNK1 in the hypothalamus protects mice against DIO and mimics the lower body weight phenotype found in JNK1-deficient animals. The IKK pathway is also involved in the regulation of hypothalamic signals. Although inhibiting JNK1 in hematopoietic cells does not affect adiposity, it is sufficient to reduce the inflammation brought on by obesity, which has positive metabolic consequences [130].
Activation of JNK1 and IKKβ/NF-kB appear to be tightly linked to ER stress and the downstream activation of the molecular pathways directing the unfolded protein response in a variety of metabolic organs (e.g., hypothalamus and adipose tissue). Obesity is characterized by widespread activation of ER stress signaling components and cascades (ATF6, PERK, IRE-1), and therapeutic suppression of ER stress can correct metabolic abnormalities [142,225]. At the crossroads of ER stress and nutrition is the PRR represented by the double-stranded RNA-dependent protein kinase, which in turn translates these signals into an inflammatory response via the coiled-coil domain of the JNK. More research is required to determine the extent to which ER stress is present in different acute and chronic stress scenarios and how its mechanism coincides with its role in the pathogenesis of atherosclerosis and foam cell biology [246].
1.2. Pathophysiology of metabolic disorders: what we have known so far
1.2.1. Diabetes mellitus
The pathophysiology of diabetes mellitus is closely associated with two essential factors, i.e., insulin levels and the body's ability to utilize this hormone. Insulin is the key determinant responsible for assisting the entry of blood glucose into the cells to be metabolized for yielding energy. Therefore, any conditions affecting the physiological roles of insulin will result in disturbances of glucose levels.
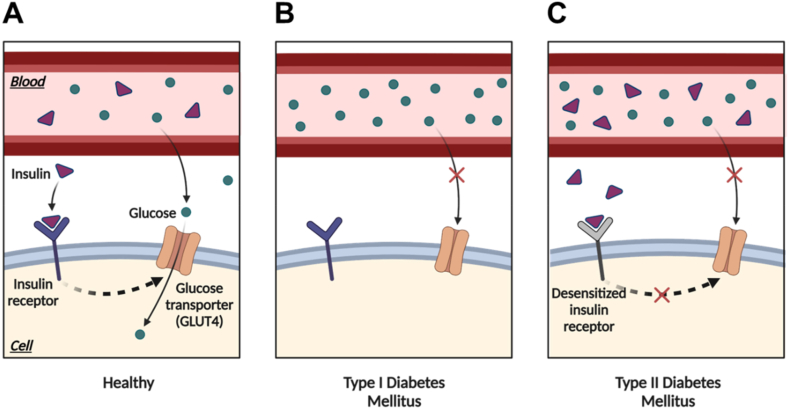
Several types of diabetes mellitus have been introduced; however, the type 1 (T1) and type 2 (T2) diabetes mellitus (DM) seem to be the most recognized types of diabetes. Although both types show different pathogenesis mechanisms, the inability of the insulin to be utilized by the cell to facilitate the entry of the glucose is the main pathophysiological event in both T1DM and T2DM (see Fig. 1).
-
•
Type 1 diabetes mellitus (T1DM)
Fig. 1.
Physiological regulation of glucose in a healthy cell (A) and pathophysiological differences between the T1 (B) and T2 (C) DM. In healthy cells (A), glucose is transported into the cells using GLUT4 in the presence of insulin. When blood glucose levels rise, insulin is released and binds to its receptor on the cell surface. This activates a series of events leading to translocation of GLUT4 transporters to the cell surface. With GLUT4 transporters now present in the membrane, glucose can bind to them and enter the cell. However, in the T1DM (B), the lack of insulin production or absence of insulin prevents the proper translocation of GLUT4 transporters to the cell surface. Without sufficient insulin, GLUT4 remains trapped inside intracellular vesicles, impairing glucose uptake into cells. This leads to elevated blood glucose levels. In T2DM, insulin resistance can disrupt the transport of glucose inside cells using GLUT4. Insulin resistance reduces the effectiveness of insulin in promoting glucose uptake, thus, decreases glucose uptake into cells, leading to elevated blood glucose levels. GLUT4, glucose transporter 4; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
A condition where the function of pancreatic beta cells is disturbed leading to their inability to produce insulin anymore could result in the emergence of T1DM. It is now concluded that the failure of the pancreatic cells to produce insulin is closely linked to a condition called autoimmune disease [132]. Instead of protecting the body from foreign substances, the immune system attacks the other systems, tissues, or cells in the autoimmune disease, including insulin-producing pancreatic beta-cells. When the latter is attacked, their function to produce a proper insulin level is damaged. Consequently, blood glucose level increases significantly leading to the emergence of hyperglycemia manifestation.
Although many cornerstones have been achieved in recent years on the pathophysiological aspects of T1DM, no clear answer could explain the autoimmune condition of this type of diabetes. However, several things related to the involvement of the immune system in the emergence of T1DM have become more evident and are revealed. First, it was found more than three decades ago that the expression of a molecule called human leukocyte antigen (HLA) was relatively higher in diabetic patients [25]. As this molecule is pivotal in regulating the immune response by encoding various related proteins involved in the antigen presentation, any condition altering the expression and function of this molecule may lead to the loss of self-tolerance mechanisms [308].
Secondly, the role of humoral and cellular immunity is significant in the pathogenesis of type 1 diabetes mellitus. As inflammation is inherently involved in the course of the disease, the excessive action of the immune cells, including T lymphocytes and B lymphocytes, is unavoidable [49]. The link between the latter cells and T1DM was established almost 50 years ago when Bottazzo and co-workers demonstrated the presence of autoantibodies for pancreatic islet cells in patients suffering from type 1 diabetes mellitus [31]. More recently, Wilcox and colleagues reported that T lymphocytes also played a significant role in T1DM as these immune cells were the dominant immune cells found in pancreas samples collected from 29 diabetic patients after doing post-mortem analysis [291].
Like other autoimmune diseases, the emergence and progression of T1DM are linked to the time of development. At this point, Eisenbarth published a paper proposing the putative pathological stages of T1DM [72]. In this concept, three previous stages would be experienced by a patient before type 1 diabetes mellitus diagnosis is established. In the first stage, when the mass and function of pancreatic beta cells are still normal, some triggering factors play important roles in activating the self-targeting immune pathway that could attack the beta cells. In stage 2, autoimmunity has been detected as autoantibodies against the beta cells could be observed in this stage. However, at this stage, the individual still has normal blood glucose and insulin levels indicating the reduced mass of the beta cells in the second stage is still sufficient to supply the need for insulin. As time goes by, the next stage is characterized by the significant reduction of the mass and function of the beta cells, leading to hyperglycemia. In the final stage, when the diagnosis of T1DM is established, the lack of beta cell mass is observed resulting in the total dysfunctionality of the cell to produce insulin [72,308].
-
•
Type 2 diabetes mellitus (T2DM)
Unlike T1DM, severe hyperglycemia in the T2DM patient is not primarily caused by the destruction of beta cells. Conversely, this pathogenic condition is induced by the failure of the peripheral tissues and cells to utilize insulin leading to their inability to uptake blood glucose. This condition is known as insulin resistance. As a consequence, hyperglycemia occurs even though the insulin circulating in the blood is at the physiological level. Following this condition, the vicious cycle occurs when the beta cells keep producing insulin because they constantly receive "information" that the circulated glucose level still exceeds the normal level [308]. If this event keeps happening, when the diagnosis of T2DM is established, the beta cells have been in a failed condition to secrete insulin.
Several factors have been proposed to play important roles in regulating the action of the beta cells to produce insulin. One of the relatively new concepts is the role of gut-related hormones (also known as incretins). It has been known that two gut hormones act as a messenger to stimulate insulin secretion after ingesting glucose. However, this mechanism is not fully activated when the supply of glucose is given intravenously. Those hormones are glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) [137]. To maintain blood glucose levels after food consumption, both incretins stimulate insulin production, while only GLP-1 shows the ability to decrease glucagon secretion. It has been demonstrated that in T2DM, the secretion of the incretins, especially GLP-1, is lowered significantly leading to the failure to induce insulin production after food ingestion [66]. Inversely, glucagon level increases facilitating the conversion of glycogen to glucose. Collectively, these events result in the elevation of blood glucose levels.
The role of the kidney in regulating blood glucose levels has also been established. This role is closely linked to kidney function in the reabsorption of glucose in the tubules after passing the filtration in the glomerulus. Approximately 90% glucose reabsorption occurs in the proximal tubules via the action of the sodium-glucose cotransporter 2 (SGLT2) membrane transporter, while the rest is reabsorbed in the descending tubule in the loop of Henle through SGLT1 [92,308]. As an important note, the reabsorption process keeps taking place until the maximum reabsorption capacity is achieved at 200 mg/dL [1]. It has been noticed that this capacity increases in patients suffering from T2DM. As a result, the event of hyperglycemia is exacerbated.
The exact mechanism by which insulin resistance occurs is still blurry. However, the link between insulin resistance and fat accumulation as well as obesity is more explicit. It has been demonstrated that the liver and muscles play a significant role in the emergence of insulin resistance. This role is putatively linked to their capacity to store excessive fats in the body [57,308]. The excessive accumulation of fat in several sites, particularly liver and muscle, has been accepted as one of the determinants involved in initiating reduced insulin sensitivity. Many factors take part in creating the accumulation of fat in those tissues or organs. Still, it is evident that the excessive supply of calories not followed by the proper physical activity, often observed in the state of obesity, plays a significant role. Specifically, fat accumulation in beta cells could destroy their function so that they cannot produce insulin at the physiological level and eventually fail to maintain the level of blood glucose [28,231].
Finally, the genetic aspects also play a role in the pathophysiology of T2DM. Although some sources have mentioned that T2DM does not have a strong pattern of inheritance, some genetical aspects should be observed carefully as family history and genetic predisposition have been known as one of the risk factors of T2DM [82, 299].
1.2.2. Obesity
As various factors, including environmental, social, behavioral, physiological, medical, and genetic factors, contribute to the emergence and persistence of obesity, the pathogenesis of this condition is complex [80]. In terms of environmental factors, a number of lifestyles are modified following the success of controlling infectious diseases that were the main cause of death in the previous centuries followed by multiple technological achievements. For example, the installation of various transportation modes and easy access to electronic and portable devices have minimized physical activities. This condition is exacerbated by easy access to high-calorie foods.
Genetic factors also contribute to the pathogenesis of obesity. Surprisingly, the heritability of body mass index ranges from 40 to 70% [34]. Several monogenic mutations or changes linked to the pathogenesis of obesity have been identified. Of those, deficiency of the leptin and melanocortin-4 receptors attracts more interest. These receptors regulate human energy homeostasis [99,270]. Several studies have demonstrated that in obesity, a deficiency of these proteins is often detected [202, 277].
Genetic and environmental factors play essential roles in influencing various physiological systems responsible for energy homeostasis. One of those systems is the nervous system. Guarino and colleagues proposed the importance of the autonomic nervous system in the pathophysiology of obesity [86]. Specifically, this group underlined the increased sympathetic nervous system activity in obese individuals [86].
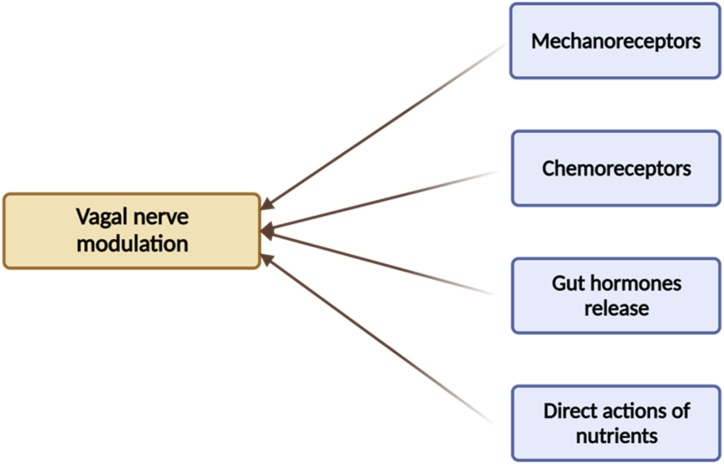
Furthermore, the vagal nerve is also linked to the pathogenesis of obesity as this nerve is the main link bridging the brain and the gut for the modulation of satiety [24]. This nerve receives information from the gut after ingesting process via several ways, i.e., mechanical stimulation, gut hormones release, chemoreceptors activation, and direct actions of some nutritive compounds (Fig. 2) [37, 86, 110]. While the first way is stimulated by gastric distension after feeding, the second way is mediated by various gut hormones. To date, a number of gut hormones have been identified, including cholecystokinin, peptide YY (PYY), pancreatic polypeptide (PP), glucagon-like peptide-1 (GLP-1), ghrelin, insulin, and leptin [86]. Although these hormones play different functions, the final aim is to regulate food intake and gastric emptying.
Fig. 2.
The role of vagal nerve is essential for regulating energy-balance ratio. The action of this nerve is affected by the information received from the mechanical stimulation, gut hormones release, chemoreceptors activation, and direct actions of some nutritive compounds.
Upon receiving the information from the peripheral receptors, vagal nerve projects the information to the complex of area postrema and nucleus of the solitary tract in the brainstem where the information is processed to be further projected to the dorsal motor nucleus [86]. The modulation of this pathway may cause several events associated with the gastric emptying control, absorption rate, and changes in the secretion of the gut hormones [24, 76, 86]. Given the essential role of the vagal nerve, any conditions that can cause disturbances in the action of this nerve in receiving information from the gut could lead to energy-balance dysregulation.
1.2.3. Heart-related diseases
As there are many types of heart diseases with their characteristics and due to space limitations, we do not provide pathophysiological aspects of each type of heart-related disease in this part. We select coronary artery disease (CAD) as the representative.
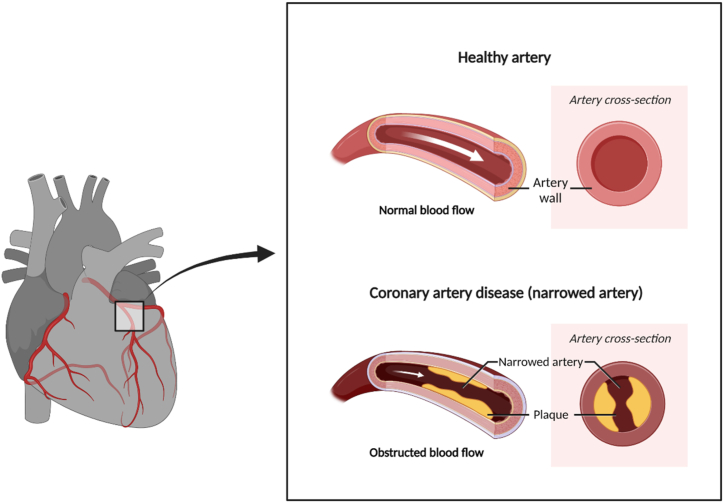
As its name suggests, CAD occurs when there is an obstruction in the coronary arteries. These vessels supply blood to the heart, ensuring the organ gets sufficient oxygen and nutrients. Once these arteries are blocked, the heart will not work correctly as it has no adequate energy to run its function (Fig. 3).
Fig. 3.
The pathogenesis of coronary artery disease. As the coronary artery is blocked, the blood supply needed by the heart would not be sufficient leading to a condition where the insufficiency of oxygen and nutrients occurs.
Although several causes of artery blockage have been listed, atherosclerosis becomes the leading cause of blocking the blood flow in the arteries. Atherosclerosis could be initiated when a low-grade inflammation is detected in the inner layer of the medium-sized arteries, including the coronary [11]. Several risk factors, including hypertension, high blood cholesterol level, diabetes mellitus, and genetics, are known to worsen this condition [11]. Although the pathogenic process in atherosclerosis is considered to be slow, this progression results in the thickening of the intima layer of the coronary occurring gradually [11]. Over time, this pathogenic event is followed by the narrowing process of the artery lumen. However, several factors can shift the slow progression of atherosclerosis to rapid atherosclerotic progression. Those factors are the formation of plaque hemorrhage and the non-occlusive thrombus in the intraluminal area [11].
-
•
Formation of plaque hemorrhage
The thickening of the intima layer of the coronary during atherosclerosis disturbs its blood supply. Therefore, a compensation mechanism is activated where the vessels that originally nourish the outer layer of the arteries grow and supply the intima layer with nutrients and oxygen [85]. Unfortunately, these growing vessels possess thin walls and weak endothelial integrity. Therefore, these vessels are vulnerable to suffer from rupture. Once the rupture occurs, the blood cells experience deposition and subsequently enlarge the plaque size. This condition is exacerbated by the fact that the red cell membrane contains high lipids, making the plaque formed rich in lipids and vulnerable to inflammation [144].
Intriguingly, the arterial lumen does not narrow easily in the initial phase of plaque formation. Several compensations and remodeling mechanisms help the affected artery maintain its lumen diameter. However, when the plaque volume approaches 40%, these mechanisms cannot compensate for the pathological effects that emerge from the formed plaque [85].
-
•
Formation of thrombus
Several major contents of an atherosclerotic plaque have been identified as inflammatory cells, including macrophage foam cells, debris from dead cells, and cholesterol in various forms [151]. These core contents of plaque are formed under the fibrous cap mainly composed of collagen, elastin, and smooth muscle cell. As the luminal side of the cap is lined by only a single layer of endothelial cells, the atherosclerotic plaque is vulnerable to experiencing tears [29]. This vulnerability gets more prominent in the presence of the inflammatory cells-derived foam cells responsible for weakening and thinning the fibrous cap [11].
Once the fibrous cap tears, the plaque core is exposed to the circulated blood, forming the coronary thrombus. The formed thrombus does not necessarily follow the flowing blood direction as other events might also occur, e.g., the thrombus is lysed and incorporated again into the arterial wall. This process is responsible for the further narrowing of the arterial lumen. Following the tear of the fibrous cap, the thrombus can also experience further growth and progress so that a total coronary lumen occlusion could occur [11]. Several factors determine which mechanism would be followed by the formed thrombus, e.g., the size, the volume, and the contents of the plaque [249].
1.2.4. Cancer
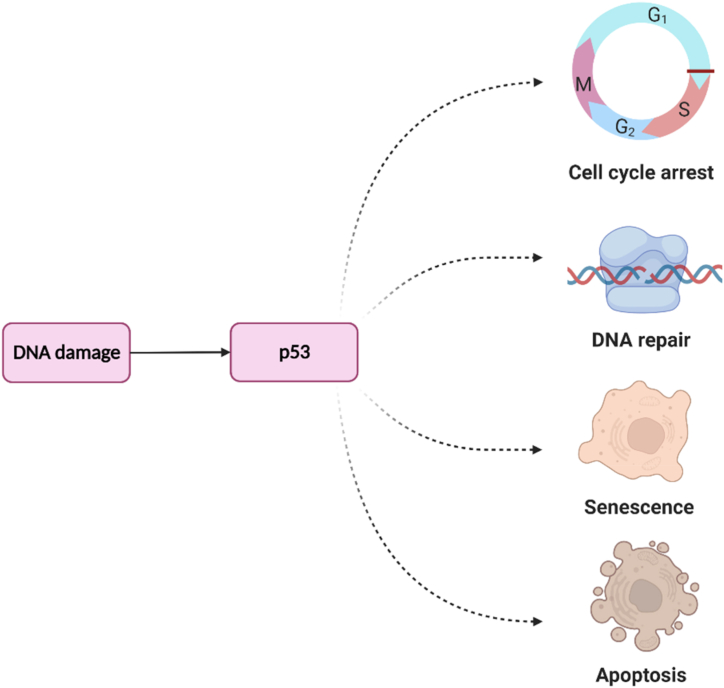
The pathogenesis of cancer is closely linked to DNA damage. As our cells are continuously exposed to various stresses that could lead to damage of DNA, several mechanisms have been developed by our body to mitigate the affected DNA, i.e., cell-cycle arrest, DNA repair mechanism, cellular senescence, and induction of apoptosis (Fig. 4) [156]. These mechanisms are strictly regulated by the p53 family (p53, p63, and p73) appointed as the “guardian of the genome” [199]. At this point, any conditions that destruct the functionalities of the p53 family could lead to the emergence of cancerous events.
Fig. 4.
Main mechanisms by which p53 deals with DNA damage. p53, a tumor suppressor protein, employs multiple mechanisms to address DNA damage. It halts the cell cycle, activates DNA repair, promotes cellular senescence, and induces apoptosis. These processes collectively maintain genomic stability, prevent the propagation of mutations, and inhibit the development and progression of cancer.
More than half of human cancers are linked to missense mutations in the p53 family [186,199]. Of several sites of mutation, the DNA-binding domain (DBD) site seems to be the most vulnerable site for mutation in the family of p53 [199]. In normal conditions, the expression of p53 must be maintained at a very low level. However, when a particular stressor attacks a cell, p53 immediately upregulates the expression of the murine/human double minute 2 (MDM2). Interestingly, MDM2 also has an activity to exert a negative feedback mechanism for p53 so that the expression of p53 is prevented from being higher [156].
As stated above, the mitigation of the damaged DNA carried out by the p53 family consists of apoptosis, cell-cycle arrest, and senescence mechanisms. In the former mechanism, p53 could initiate apoptosis through extrinsic or intrinsic pathways. While the action of the death receptors mediates the former pathway, the latter pathway is associated with the release of cytochrome c to the cytosolic region of mitochondria [219]. In the final stage of both pathways, caspase-3 seems to play a major role in executing the apoptotic events [87,236].
Another strategy for mitigating the damaged DNA is the activation of the cell-cycle arrest. This strategy is essential for evaluating the ongoing process and repairing the damage during the cell cycle. To facilitate and activate the cell cycle process, the role of the cyclin-dependent kinases (CDKs) family is crucial, while the inhibition of this protein family is linked to the termination of cellular duplication which is beneficial for preventing the division of cancer cells. The p53 family regulates the CDKs as p53 could induce the activation of the p21 protein which is responsible for inhibiting the CDKs [156, 199]. Finally, cellular senescence could also be activated by p53 through its action on some genes, e.g., p21, p16-Rb, and BTG2 [148, 199]. This mechanism is pivotal, particularly in diminishing the progression and spread of cancer cells.
Some other mechanisms are also linked to the action of p53 in protecting the cells from the attack of cancerous cells. Some of them are associated with its ability to prevent several events, e.g., cancer migration to other tissues, angiogenesis, oxidative stress, and drug resistance. In addition, p53 could also induce autophagy and promote genome stabilization [26, 98, 199, 304].
1.3. Prospective biological targets for natural products to manage metabolic disorders
To date, the potencies of natural products on tackling metabolic syndromes have been widely explored. Several reasons underlie the efforts carried out for seeking new candidates for those pathologic conditions. Those reasons are from the ineffective existing drugs, the adverse side effects showed by the existing drugs, drug-interaction issues, dosage used for therapy, to the unaffordable price. It is assumed that those drawbacks could be tackled by new drugs developed from natural products. However, excessive exploration on the natural products is linked to the harmful impact on the nature. Therefore, although the nature stores the priceless entities for being developed as a drug, the exploration of the nature should be carried out wisely.
Here we listed several natural compounds that have been reported to show potencies to alleviate diabetes mellitus and obesity (Table 1), heart-related diseases (Table 2), and cancer (Table 3). We equipped the lists with the sources from which the compounds are extracted, the putative mechanism(s) of action of each compound, the models used during the experiments, and the key findings of the studies that we cited. In addition, we also provide a list of natural compounds with protective effect against diabetes mellitus, obesity, heart-related diseases, and cancer by specifically modulating excessive effects of proinflammatory cytokines (Table 4).
Table 1.
Prospective biological targets for natural products to manage diabetes mellitus and obesity.
| No | Source(s) | Compound or extract(s) | Mechanism of action(s) | Experimental model(s) | Key findings | Refs |
|---|---|---|---|---|---|---|
| 1 | Psidium guajava | Triterpenoid (Corosolic acid) | α-glucosidase inhibitor | An in vitro assay of α-glucosidase inhibition | Corosolic acid derived from P. guajava extract exhibited the best inhibition of α-glucosidase among nine triterpenoids isolated with IC50 value of this compound was 1.33 μg/mL. However, the result showed that the extract of P. guajava leaves was more effective than the individual of its compounds. | [42] |
| The ethyl acetate fraction of leaves extract | Modulates advanced glycation end products, Serum fructosamine, and fasting blood glucose levels | An in vivo study using rats induced by streptozotocin to provide diabetic myocardium |
|
[247] | ||
| Ethanol extracts from leaves and bark | α-glucosidase, α-amylase inhibitor; stimulate glucose uptake in muscle; inhibit liver glucose production and triglyceride accumulation in adipocytes | An in vitro study using the cell lines (H4IIE, C2C12, and 3T3-L1) |
|
[22] | ||
| Ethanol extract from leaves | Inhibits glucose absorption | An in vivo study with an alloxan diabetes test method and oral glucose tolerance test in rats | Administration of extract at 1,300 mg/kg BW each day for 14 days lowered blood glucose levels, indicating that ethanol extracts inhibited blood glucose absorption by promoting its antidiabetic agent as an α-glucosidase inhibitor. | [180] | ||
| Methanol extract from leaves | Increases glucose uptake | An in vitro study using glucose uptake in 3T3-L1 cells | The glucose uptake significantly increased by approximately 52% at a concentration of 100 μg/mL of extract. | [45] | ||
| Adipogenesis and lipolysis | An in vitro study using adipogenesis assay, and lipolysis assay in 3T3-L1 cells | Guajava leaves extract (GLE) decreased lipid accumulation during adipocyte differentiation. Lipid content could be reduced by approximately 88%, and the glucose uptake significantly increased by approximately 52% at a concentration of 100 μg/mL GLE. | ||||
| 2 | Ficus tikoua Bur. | n-butanol fraction (NBF) of Ethanol extract | • Stimulates glucose uptake via P13K/AKT and AMPK pathway • α-glucosidase inhibitor |
An In vitro study using 3T3-L1 cells and in vivo experimental models in mice |
|
[282] |
| 3 | Ganoderma resinaceum | Triterpenoid lactones | α-glucosidase inhibitor | An in vitro α-glucosidase inhibitory assay | Compounds 1 and 2 were more potent α-glucosidase inhibitors than acarbose, with IC50 values of 0.75 ± 0.018 mM and 1.64 ± 0.022 mM, respectively. | [44] |
| 4 | Cyclocarya paliurus | Triterpenoid glycosides isolated from leaves ethanol extract | Increase glucose uptake via AMPK/p38 pathways | An in vitro study in 3T3-L1 adipocytes and C2C12 myotubes | Compound 1 significantly enhanced insulin-stimulated glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes. The promising mechanisms of compound 1 in enhancing glucose uptake in cells are upregulating the AMP-activated protein kinase (AMPK)-p38 pathways. | [74] |
| 5 | Cornelian cherry (Cornus mas L.) | Extracts of red and yellow from fruits | Modulate blood glucose levels and marker carbonyl oxidative stress | An in vivo study using rats induced by streptozotocin |
|
[70] |
| 6 | Tiliacora triandra | Ethanol extract | Insulin sensitizer and insulin secretagogue | An in vivo study using diabetic rats induced with high-fat diet (HFD)/streptozotocin (STZ) |
|
[170] |
| 7 | Citrus junos Tanaka or Yuja | Ethanol extract from Yuja peel | Increases glucose uptake via AMPK and PPAR-γ signaling pathways |
|
|
[139] |
| Decreases liver fat contents, triglyceride serum, and total cholesterol levels• | To clarify the antiobesity effect of YPEE, some parameters were measured. Interestingly, aside from regulating the AMPK and PPAR-γ signaling pathways, administration YPEE to high-fat diet groups dramatically decreased body weight, liver fat contents, triglyceride serum, and total cholesterol levels compared to the untreated group. | |||||
| 8 | Syzygium cumini | Aqueous extract from seed | α-amylase and α-glucosidase inhibitor | An in vitro α-amylase and α-glucosidase inhibitory assay | Syzygium cumini kernel phenolic (SCKP) extract offered potential antioxidant activity and antidiabetic as α-amylase and α-glucosidase inhibitor leading to the inhibition of glucose absorption in the intestine. | [168] |
| 9 | Passiflora edulis | Hydroethanolic extract 70% from leaves |
|
|
|
[223] |
| 10 | Glycyrrhiza foetida and Amorpha fruticosa | Amorfrutins | Activate nuclear receptor PPARγ (peroxisome proliferator-activated receptor gamma) |
|
|
[288] |
| 11 | Carapa guianensis | 7-deacetoxy-7-oxogedunin (CG-1) isolated from seeds | Adipogenesis and lipolysis inhibitors |
|
|
[174] |
| 12 | Camellia sinensis, Astrocaryum aculeatum | 8-C-ascorbyl-(−)-epigallocatechin | α-glucosidase and protein tyrosine phosphatase-1B (PTB-1B) inhibitor |
|
|
[314] [167] |
| 13 | Hovenia dulcis Thunberg | Flavonoids | Modulate AKT1 and GSK3β pathways |
|
|
[55] |
| 14 | Leea macrophylla | Ethanol extract from root | Increases insulin secretion, stimulates glucose uptake in the liver, and activates glycogenesis |
|
|
[212] |
| 15 | Fadogia ancylantha (Makoni tea) | Bidesmosidic oleanolic acid saponins | α-amylase, α-glucosidase, and lipase inhibitor |
|
|
[75] |
| 16 | Angelica decursiva | Coumarin-derivatives | α-glucosidase and protein tyrosine phosphatase-1B (PTB-1B) inhibitor |
|
|
[7] |
| 17 | Euonymus alatus (Thunb.) | An in vitro study α-glucosidase and PTB-1B inhibitory assay | Compounds 15, 20, and 23 were potent inhibitors on α-glucosidase with IC50 values of 10.5 ± 0.8, 9.5 ± 0.6, and 9.1 ± 0.5 μM, respectively. Moreover, compounds 6, 7, and 23 were non-competitive inhibitors and vigorously inhibited PTB-1B with IC50 values of 13.7 ± 2.1, 5.6 ± 0.9, 13.7 ± 0.2 μM, respectively. | [120] | ||
| 18 | Viburnum macrocephalum f. keteleeri | Lignans glycosides |
|
[317] | ||
| 19 | Limonium gmelinii (Willd.) Kuntze | Nineteen compounds were isolated from ethyl acetate extract of the roots of Limonium gmelinii (Plumbaginaceae), and compounds 1, 2, 14, and 18 strongly inhibited α-glucosidase with approximately range IC50 less than five μM. The activity of compounds 1–19 remarkably inhibited PTB-1B in the range IC50 of 1.71–50 μM. | [272] | |||
| 20 | Hizikia fusiformis (Harvey) Okamura |
|
[233] | |||
| 21 | Artemisia capillaris | Esculetin, Quercetin, 3,5-Dicaffeoylquinic acid methyl ester• |
Vigorous inhibitory activity of esculetin, quercetin, 3,5-Dicaffeoylquinic acid methyl ester against α-glucosidase was observed with IC50 values of 82.92, 58.93, and 86.95 μM, respectively; and protein tyrosine phosphatase-1B (PTB-1B) of 11.32, 17.40, 24.74, and 36.77 μM, respectively. | [183] | ||
| 22 | – | Hesperidin, naringin | α-glucosidase inhibitor | An in vitro study using p-nitrophenyl- D-glycopyranoside (p-NPG) as the substrate | Hesperidin and naringin possessed antidiabetic activity with remarkable inhibition against α-glucosidase with IC50 of 14.72 and 12.64 nM, respectively. | [261] |
| Increase insulin secretion, decrease blood glucose and HbA1c | An in vivo study in HFD/STZ-induced diabetic rats |
|
[169] | |||
| 23 | Acacia auriculiformis | Extract acetone from bark and empty pod | α-amylase, α-glucosidase inhibitors | An in vitro study using α-amylase and α-glucosidase assay |
|
[230] |
| 25 | – | Phenolic compounds |
|
[262] | ||
| 26 | Chelidonium majus | Chelerythrine | Activates PPAR-γ receptor |
|
Chelerythrine significantly inhibited the CDK5-mediated phosphorylation of PPARγ and exhibited a unique mechanism in modulating glucose uptake and lipid metabolism. | [319] |
| 27 | – | Natural Prenylchalconaringenins and Prenylnaringenins | α-amylase, α-glucosidase inhibitors |
|
|
[253] |
| 28 | Tetracera indica Merr. | Wogonin, norwogonin, and techtochrysin | Increase glucose uptake | An in vitro study in the 3T3-L1 cell |
|
[93] |
| 29 | Oroxylum indium | Flavonoid glycosides, oroxins C and D | α-amylase, α-glucosidase, lipase inhibitors | In vitro study on α-amylase, α-glucosidase, lipase | Oroxins C and D inhibited lipase with IC50 of 190.1 ± 18.2 80.0 ± 9.5 μM, respectively. However, oroxins C significantly inhibited α-amylase two-fold higher than acarbose with IC50 of 210.3 ± 19.1 μM. Similarly, oroxins D with IC50 of 180.4 ± 25.7 μM was more potent in inhibition of α-glucosidase than acarbose. | [155] |
| 30 | Bauhinia forficata Link. | Kaempferitrin | Increase glucose uptake in soleus muscle | An in vivo study in alloxan-induced diabetic rats |
|
[38] |
| 31 | Dillenia indica | Kaempferol | Apoptosis cascade inhibition and increases insulin secretion | An in vitro study caspase-3 activity, intracellular ATP and cAMP, insulin secretion assay using isolated beta cells and human islets |
|
[316] [167] |
| 32 | Hypolepis punctata (Thunb.) Mett. | Pterosin A | Increase glucose uptake via insulin sensitizer | An in vivo study using high-fat diet (HFD)–induced diabetic mice, and a dexamethasone-induced insulin-resistance (IR) mouse model |
|
[106] |
| 33 | Eugenia punicifolia | Aqueous extract from Eugenia punicifolia leaves (EEP) |
|
An in vitro study in 3T3-L1 cells |
|
[162] |
| 34 | Grape | Grape-seed proanthocyanidin extract (GSPE) | Reducing body weight gain, adiposity, and liver steatosis | An in vivo study using cafeteria diet (CAF) high-fat/high-sucrose-induced syndrome metabolic in rats |
|
[243] |
| 35 | Adansonia digitata L. | Hydromethanolic extracts from fruit pulp and leaf | α-amylase, α-glucosidase, pancreatic lipase, and angiotensin-converting enzyme inhibitors | An in vitro enzymatic assay and study in SW-872 human liposarcoma cells |
|
[47] |
| 36 | Garcinia dulcis | G. dulcis rind powder (CGD) |
|
An in vivo study using high fat/carbohydrate diet (HFD) induced metabolic syndrome in rats |
|
[124] |
| 37 | Phaseolus vulgaris L. | Dry extract |
|
An in vivo study using high-fat diet (HFD) induced metabolic syndrome in C57BL/6 mice |
|
[176] |
| 38 | Cuscuta pedicellata | Naringenin, kaempferol, aromadenderin, quercetin, aromadenderin-7-O-b-d- glucoside, taxifolin 7-O-b-d-glucoside |
|
An in vivo study using a high-fat diet (HFD) induced obesity in rats |
|
[175] |
| 39 | Mushrooms: Lentinus edodes and Schizophyllum commune |
Ethanol and hexane extract s | α-amylase, α-glucosidase, and pancreatic lipase inhibitors | An in vitro study using enzyme assays |
|
[300] |
| 40 | Vernonia mesplilfolia Less. | Ethanol and aqueous extracts | The ethanol extract was the most potent in inhibiting α-amylase and pancreatic lipase, with IC50 of 331.16 and 781.72 μg/mL, respectively. On the other hand, the aqueous extract exhibited the most potent α-glucosidase inhibitor with IC50 of 450.88 μg/mL. | [274] |
Table 2.
Prospective biological targets for natural products to manage heart-related diseases.
| No | Source(s) | Compound or extract(s) | Mechanism of action(s) | Experimental model(s) | Key findings | Refs |
|---|---|---|---|---|---|---|
| 1 | Rhizoma coptidis | Berberine (BBR) | Induces the mitophagy-mediated HIF-1a/BNIP3 pathway |
|
|
[323] |
|
|
[318] | ||||
| Modulating AMPK activity in both non-ischemic areas and risk areas of the heart |
|
|
[41] | |||
|
|
[40] | ||||
| 2 | Aralia elata | Total saponins of Aralia elata (Miq) Seem (AS) | Modulate contractile function and intracellular calcium via activation PKCε phosphorylation |
|
AS showed positive effects in treating myocardial ischemia/reperfusion injury by exerting its mechanism to improve coronary blood flow, decrease oxygen consumption and heart workload with several actions, maintain the contraction and relaxation of myocytes, and activate PKCε, a Ca2þ-independent PKC isoform. | [284] |
| Inhibit endoplasmic reticulum stress-related apoptosis | An in vivo study in myocardial I/R injury rats |
|
[285] | |||
| Activate PI3K/Akt pathway and inhibition of MAPKs family | An in vivo study using lipopolysaccharide-induced cardiac dysfunction mice |
|
[43] | |||
| Elatoside C |
|
An in vitro study in hypoxia/reoxygenation (H/R)- induced H9c2 cardiomyocyte injury |
|
[283] | ||
| 3 | Brassica oleracea var. capitata rubra | Anthocyanin |
|
An in vivo study using atherogenic (ATH) diet-induced hypercholesterolemia and related cardiac in rats |
|
[226] |
| 4 | Songling Xuemaikang Capsule (SXC) (Puerariae thomsoni, Pinus massonana, and powdered nacre) | Songling Xuemaikang Capsule (SXC) | Inhibits of cardiac hypertrophy via CaMKIIδ and ERK1/2 pathways |
|
|
[209] |
| 5 | Beta vulgaris | Betanin | Sentrin-specific protease −2 (SENP2) inhibitor | An in-silico study (PDB ID: 1TH0) | Betanin showed low toxicity, high binding energy, and hydrogen bonds to the SENP2 active site with low RMSD. | [255] |
| 6 | Wuwei Yuganzi San (WYS) | Sennoside D, quercetin, and procyanidin B-5,3’-O-gallate | Inhibiting of several crucial protein targets of CHD such as, ADAM17, AKR1C2, ALB, AKT1, and ADH1C | An in-silico study using AutoDock Vina software | The compounds showed binding affinity to protein targets, approximately < -10 kcal/mol, offered the promising therapeutic CHD. | [311] |
| 7 | Allium sativum, Peganum harmala, and Berberis vulgaris | Ethanol extract from A.sativum and P. harmala, and Methanol extract from B. vulgaris | Restoration of left ventricular remodeling, decreasing hs-CRP and NT-ProBNP | An in vivo study using isoproterenol-induced heart failure in rats |
|
[134] |
| 8 | Terminalia arjuna (Roxb.) | Lyophilized aqueous extract of stem bark | The extract modulated ERK/Akt, ER stress marker Grp78, and epigenetic regulator HDAC5. | An in vivo study using isoproterenol-induced cardiac hypertrophy in rats |
|
[147] |
| 9 | Radix salviae Milthiorrhizae | Salvianic acid A (SAA) as a water-soluble fraction | Inhibite L-type calcium channels and decreasing myocardial contractility | An in vivo study using iso-induced myocardial ischemia injury in rats | Low and high doses of SAA inhibited cell shortening by 33.48 ± 0.75%, significantly reduced CK and LDH levels, inhibited L-type calcium channels in a dose-dependent manner, and histopathology of rat hearts were in normal structures. | [248] |
| 10 | Cissampelos pareira | Ethanol extract from root | Antioxidant activity and ameliorating calcineurin activity | An in vivo study using isoproterenol-induced cardiac dysfunction in rats |
|
[244] |
| 11 | Salvia miltiorrhiza | Salvia miltiorrhiza hydrophilic extract (SMHE) | Antioxidant activity | A clinical study in diabetic patients with chronic heart disease (CHD) |
|
[211] |
| 12 | Phyllanthus tenellus | pino- cembrin-7-O-[3′′-O-galloyl-4′′,6′′-(S)-hexahydroxydiphenoyl]- α-D-glucose (P7OG) | Inhibit platelet aggregation, vasorelaxation, protection vascular disorders | An in vitro study using G-6-P, vascular reactivity, aggregation platelet assays. | P7OG greatly inhibited glucose-6-phosphatase, ADP, collagen with IC50 at 17.20, 26, 61 μM, respectively. In addition, P7OG showed remarkably inhibition effect on the G-6-Pase (83%) assayed in intact microsomes. | [73] |
| 13 | Abies alba | Silver fir trunk extract (SFTE) | Antiarrhythmia, vasoralaxan, antioxidant | An in vivo study using ischemic-reperfused isolated heart rats | SFTE significantly decreased lactate dehydrogenase (LDH) release rate, increased coronary flow rate, and restored arrhythmias duration by 80%, compared to untreated group during the reperfusion period. | [65] |
Table 3.
Prospective biological targets for natural products to manage cancer.
| No | Source(s) | Compound or extract(s) | Mechanism of action(s) | Experimental model(s) | Key findings | Refs |
|---|---|---|---|---|---|---|
| 1 | Arthrospira platensis | Aqueous extract |
|
|
|
[256] |
| 2 | Calotropis gigantea | Dichloromethane extract (CGDCM) | Promote apoptosis through the mitochondria-dependent pathway | An in vitro study using human colorectal carcinoma HCT116 (CCL-247, ATCC, USA) and colorectal adenocarcinoma HT-29 (HTB-38, ATCC, USA). |
|
[293] |
| 3 | Bombax buonopozense | Ethanol extract |
|
|
|
[266] |
| 4 | Glycosmis parva | Arborinine | Inhibits the growth of tumor | An in vitro study using adriamycin-resistant SGC-7901 (SGC-7901/ADR) cell line, Vincristine- resistant SGC-7901 (SGC-7901/VCR) cell line, Paclitaxel-resistant MGC803 (MGC/PTX) cell line. |
|
[46] |
| 5 | Moringa oleifera | Soluble extract from leaves |
|
An in vitro study using A549 lung adenocarcinoma cells |
|
[157] |
| 6 | Sponge Hyrtios sp. | Methanol extract | Induces apoptosis via activation p53 and inhibition JNK pathway | An in vitro study using human colorectal carcinoma RKO (CRL-2577) and RKO-E6 (CRL-2578) cells |
|
[126] |
| 7 | Juniperus indica Bertol | The crude extract of the liquid oil | Antiproliferative effect by interfering with Akt/mTOR signaling pathway | An in vitro study using OECM-1 human gingival squamous cancer cells line. | Induces apoptosis via activation p53 and inhibition JNK pathway | [107] |
| 8 | Rhaponticum carthamoides (Willd.) | Methanol extract from root | Induces mitochondrial dysfunction | An in vitro study using leukemia cells (K-562 and CCRF-CEM) and lung adenocarcinoma cells (A549). |
|
[245] |
| 9 | Xanthium strumarium | Chloroform and methanol extracts from fruit | Inhibit autophagy-related (ATG) proteins | An in vitro study using ATG4B cleavage assays. |
|
[39] |
| 10 | Litchi chinensisSonnnerat | n-butyl alcohol extract of Litchi seed (NLS) |
|
An in vitro study using prostate cancer cell lines PC3, DU145, RM1, and C4–2B |
|
[88] |
| 11 | Annona muricata L. | Ethanol extract from leaves |
|
An in vitro study using liver cancer HepG2 cells and colon cancer HCT116 cells |
|
[160] |
| 12 | Neptunia oleracea Lour (water mimosa) | Methanol extract |
|
An in vitro study using jurkat (acute T cell leukemia) and MV-4-11(biphenotypic B myelomonocytic leukemia) cell line. |
|
[27] |
| 13 | Cyanthillium cinereum (L.) | Sesquiterpene lactones |
|
An in vitro study in 786-O cell line, K-562 leukemic cell line, and MCF-7 breast cancer cell line |
|
[60] |
| 14 | Tourneuxia variifolia | Ethyl acetate (EtOAc) and n-butanol (n- BuOH) extracts | Inhibit the activity of HeLa cells | An in vitro study using human cervical adenocarcinoma (HeLa) cell line |
|
[309] |
| 15 | Tapinanthus sp. (Loranthaceae) |
|
Inhibit proliferation | An in vitro study using glioblastoma (U87MG, C6) and prostate (PC-3) cancer cells |
|
[81] |
| 16 | Xylocarpus granatum | Ethyl acetate extract from leaves |
|
An in vitro study using HeLa, T47D, and HT-29 cell line |
|
[53] |
| 17 | Diospyros kaki L. | Total flavonoids from persimmon leaves (FPL) |
|
An in vitro study in prostate cancer PC-3 cells |
|
[62] |
| 18 | Tephroseris kirilowii (Turcz.) Holub. | Isorhamnetin (IH), genkwanin (GN), acacetin (Aca) |
|
|
|
[310] |
| 19 | Artemisia aucheri Boiss. | Methanol extract from leaves |
|
An in vitro study using HT29 colon cancer cells |
|
[6] |
| 20 | Calligonum comosum (L’Her) | Methanol fruit hairs extract (MFH) |
|
An in vitro study using human hepatocarcinoma cells (HepG2) |
|
[9] |
| 21 | Bombax buonopozense | Ethanol extract from stem bark |
|
|
|
[266] |
| 22 | Raphanus sativus L. | Ethanol extract from seed |
|
An in vitro study using oral squamous cell carcinoma (KB and KBCD133+) |
|
[3] |
| 23 | Orobanche crenata | Methanol extract |
|
An in vitro study using hepatocellular carcinoma (HepG2), human prostate cancer (PC3), human breast adenocarcinoma (MCF-7), and human colon carcinoma (HCT-116) |
|
[96] |
Table 4.
Natural products displaying potency as anti-diabetes mellitus, -obesity, -heart-related diseases, and -cancer by specifically modulating excessive effects of proinflammatory cytokines.
| No | Source(s) | Compound or extract(s) | Mechanism of action(s) | Experimental model(s) | Key findings | Refs |
|---|---|---|---|---|---|---|
| Natural products to manage diabetes mellitus and obesity | ||||||
| 1 | Psidium guajava | Total triterpenoids of leaves extract | Inhibitor of proinflammatory cytokines by NF-κB pathway | An in vivo study using rats induced by a high-fat diet and streptozotocin to provide diabetic peripheral neuropathy | Significantly decreased serum blood glucose levels in rats and suppressed the expression of proinflammatory mediators via PI3K and Akt pathways. | (X [287]. |
| Natural products to manage heart-related diseases | ||||||
| 2 | Rhizoma coptidis | Berberine (BBR) | Suppressing NF-κB and JNK signaling pathways |
|
|
[306] |
| 3 | Aralia elata | Total saponins of Aralia elata (Miq) Seem (AS) | Inhibit NF-kB activated by TNF-α and stimulating PI3K/Akt signaling pathway to regulate the pro- and anti-apoptotic | An in vitro study in endothelial Cell Injury induced by TNF-α using human umbilical vein endothelial cell (HUVEC) |
|
[321] |
| 4 | Grape | Oligomerized grape seed proanthocyanidins (GSP) |
|
An in vivo study using an iso-induced cardiac remodeling model in rats |
|
[324] |
| 5 | Salvia miltiorrhiza Bge. and Carthamus tinctorius L. | Danhong injection (DHI) contains 5-hydro- xymethylfurfural, Danshensu, protocatechuic acid, protocatechuic aldehydrate, caffeic acid, rosmarinic acid, lithospermic acid, salvianolic acid B, salvianolic acid A and salvianolic acid C | Anti-cardiac hypertrophic by modulating p38 and NF-κB pathway |
|
DHI suppressed the elevation of P38 phosphorylation and activation NF-κB inhibiting translocation of p65 into the nucleus. The subsequent event is the restoration of cardiac hypertrophy induced by ISO. | [172] |
| 6 | – | Quercetin, luteolin and epigallocatechin gallate |
|
An in vitro study using EA. hy-926 cells |
|
[296] |
| Natural products to manage cancer | ||||||
| 7 | Gynura procumbens | Ethanol supernatant extracts (EEGS) |
|
An in vivo study using nanodiethylnitrosamine (nanoDEN)-induced mouse liver cancer |
|
[313] |
| 8 | Citrullus lanatus (Thunb.) Mansfeld | Lycopene |
|
An in vitro study using adenocarcinoma cell line (A549 CCL-185™) |
|
[61] |
1.4. Natural products for the management of inflammation-related metabolic disorders
The process through which complex macromolecules like proteins, carbohydrates, and lipids are broken down into their constituent parts is called metabolism. When regular metabolic processes are hampered, it can lead to metabolic disorders. Diabetes mellitus, obesity, heart-related syndromes, and cancer are the metabolic disorders that are seen the most frequently [103]. Over the last decade, numerous efforts have been made to include natural products into drug development [18]. More than two-thirds of drug active ingredients are derived from natural sources [189].
1.4.1. Natural products to manage diabetes
A lack of functioning β-cells in the Langerhans islets causes insulin resistance, which in turn causes high blood sugar levels to remain elevated and, eventually, diabetes mellitus [30,117]. "Diabetes Mellitus" is a phrase that was coined from the Greek language. In Greek, the word "Diabetes" means "a passer through," while the word "Mellitus" means "sweet." [239]. When the body stops producing or effectively utilizing insulin, it causes serious problems for the cardiovascular system, the blood vessel system, the eyes, and the kidneys. The prevalence of diabetes is rising rapidly, making it one of the world's leading health concerns. In 2019, the International Diabetes Federation (IDF) predicted that 463 million adults had diabetes; this number is expected to increase to 578 million by 2030 and to 700 million by 2045 [108,222]. In addition, roughly 374 million people worldwide had diabetes in 2017 but did not know it [108]. There are two distinct types of diabetes mellitus: type 1 and type 2. The immune system mistakenly attacks and destroys β-cells in response to environmental triggers such as chemicals [105] and viruses [115], resulting in T1DM. Therefore, exogenous insulin is essential for the management of type 1 diabetes [121]. About 10% of all diabetic patients suffer from this condition, which is particularly common in young people [21]. Unlike type 1, which typically manifests in childhood or adolescence, T2DM (also known as "non-insulin-dependent diabetes") develops in adulthood and is characterized by the body's inefficient use of insulin (known medically as "peripheral tissue resistance") [260]. Sunlight exposure in childhood was found to protect against the onset of T1DM [114]. A healthy lifestyle, including a nourishing food, exercise, increased physical activity, not smoking, and maintaining a moderate body weight, can help reduce the chance of developing T2DM [5, 17, 303]. Though there are medications capable of curing T2DM, including metformin [232], sulfonylurea [113], and insulin [143] are the currently available scientifically proven synthetic anti-diabetic medications. Also, α-glucosidase inhibitors [131], thiazolidinediones [275], glucagon-like peptide-1 receptor agonists [205], pramlintide [101], and dipeptidyl peptidase-4 inhibitors [56] are some of the newer medications with little evidence supporting their use. Therefore, there is a lack of drugs that are both effective and have few unwanted side effects, such as severe hypoglycaemia [112], and in some conditions, they lack safety [200], so it is important to investigate alternative medicines for the management of diabetes. In most cases, the availability, affordability, and safety of alternative medicines would far outweigh their disadvantages [200]. Consistent efforts are being made to investigate diabetes and discover new therapeutic strategies, such as the identification of natural products with anti-diabetic effects [187], due to the disease's high prevalence and the lack of satisfactory treatment options. People with diabetes have used a wide range of alternative treatments to control their condition. Pre-clinical and clinical trials have been conducted on a variety of natural products for the treatment of diabetes.
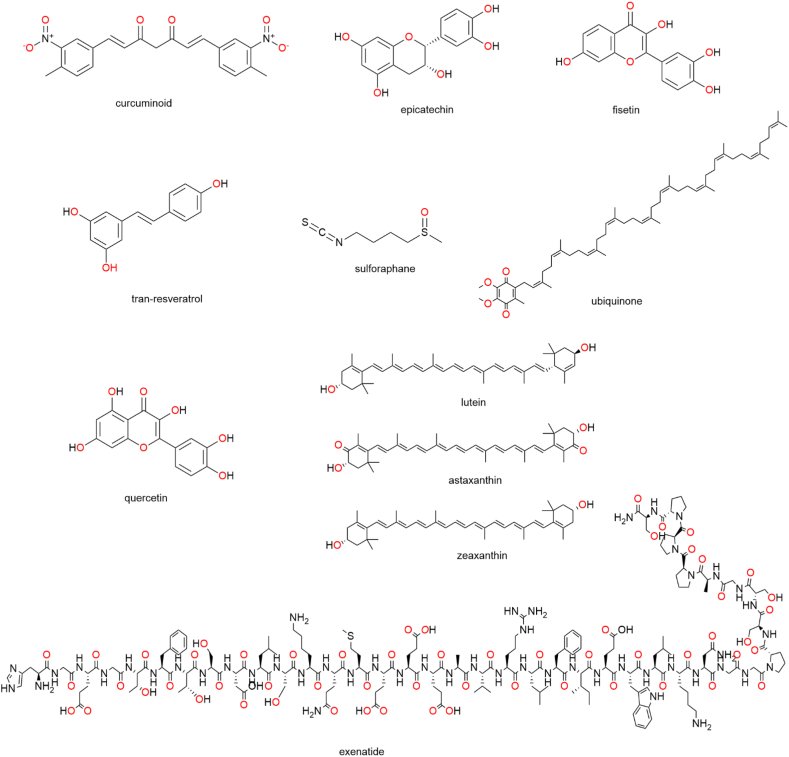
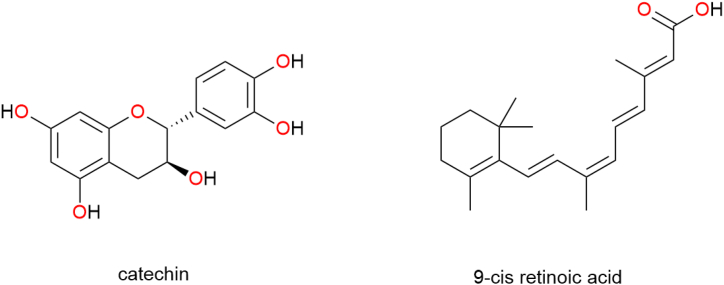
As shown in Table 5 and Fig. 5, many naturally occurring substances have the potential to aid in the control of blood sugar levels in diabetic patients. Mechanisms of anti-diabetic action include the suppression of digestive enzymes like α-glucosidase and α-amylase [216], changes in glucose uptake and the expression of glucose transporters [69], increased insulin secretion and pancreatic β-cell proliferation [150], suppression of insulin resistance [220], and regulation of oxidative stress [109]. Evidenced by the vast quantity of molecules with natural product origins that have undergone clinical trials, natural products remain a promising source for the development of novel therapeutics.
Table 5.
Clinical trials of natural products with anti-diabetic activity (https://clinicaltrials.gov).
| Compound | ClinicalTrials.gov Identifier | Type of study | Characteristics of patients (n) | Dose and time of treatment | Condition | Phase | Additional Refs |
|---|---|---|---|---|---|---|---|
| Curcuminoid | NCT02529982 | randomized, double-blind, placebo-controlled trial | curcumin group (n = 25) meals or placebo group (n = 28) | 1500 mg capsule for 10 weeks | type 2 diabetes | – | [100] |
| Trans-resveratrol | NCT01677611 | randomized, placebo-controlled trial | n = 10 | 500 mg to a maximum of 3 g daily | type 2 diabetes | phase 1 | |
| Resveratrol | NCT01354977 | a placebo-controlled study | resveratrol group (n = 12) or placebo group (n = 8) | 1,000 mg twice daily for 28 days | type 2 diabetes | phase 2 | |
| Quercetin | NCT01839344 | crossover, double-blinded, controlled trial | Quercetin, acarbose and placebo (n total = 19) | 250 mg; oral single dose of 2000 mg | type 2 diabetes | phase 2 | |
| Epicatechin | NCT02330276 | double-blinded randomized | Each dose has n = 4 | epicatechin 10 mg, 30 mg, or 100 mg | pre-diabetes | phase 1 | |
| Sulforaphane | NCT02801448 | randomized, double blind, placebo-controlled trial | sulforaphane group or placebo group; n = 103 | sulforaphane-containing broccoli sprout extracts once daily for 12 weeks | type 2 diabetes | phase 2 | |
| Ubiquinone | NCT02062034 | randomized double-blind placebo-controlled study | ubiquinone group, antioxidant combination group, placebo; n = 40 | 400 mg daily of oral ubiquinone for 24 weeks | non-proliferative diabetic retinopathy, type 2 diabetes | phase 2 | [97] |
| Lutein, astaxanthin, zeaxanthin, vitamin C, vitamin E, zinc copper | NCT03702374 | randomized double-blind placebo-controlled study | antioxidant combination group and placebo; n = 132 | antioxidant combination tablet once a day for 12 months | diabetic retinopathy | phase 3 | [163,184,217] |
| Fisetin | NCT03325322 | randomized double-blind placebo-controlled study | fisetin group and placebo; n = 30 | 20 mg/kg/day, orally for 2 consecutive days | diabetes mellitus, diabetic nephropathies, chronic kidney diseases | phase 2 | |
| Exenatide | NCT02735031 | randomized double-blind placebo-controlled study | exenatide group and placebo; n = 10 | week 1–2: 5 μg twice daily; week 3–6: 10 μg twice daily (if tolerated) | type 1 diabetes, hypoglycemia | phase 2/3 | |
| Exenatide | NCT01876849 | open-label | N = 275 | injection 5mcg or 10 mcg, twice daily | type 2 diabetes | phase 3 |
Fig. 5.
Natural compounds with antidiabetic potential and their chemical structures.
1.4.2. Natural products as lipid-lowering agents
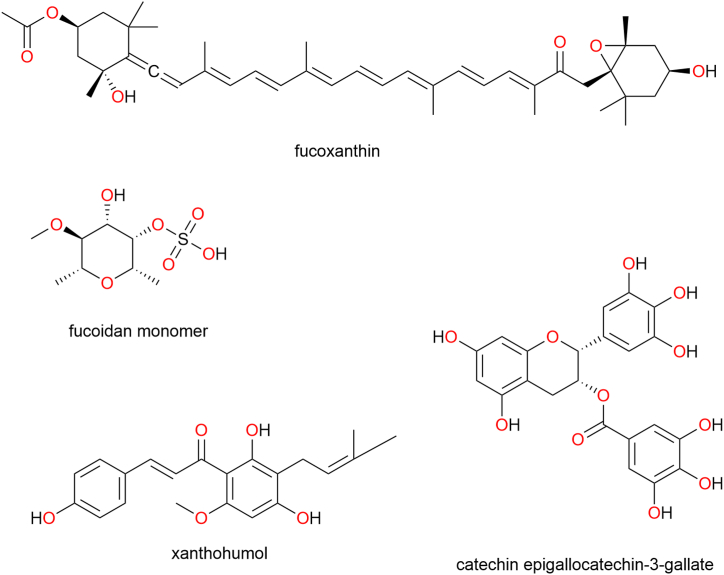
Adipose tissue build-up to an unhealthy degree characterizes obesity [185]. It is one of the world's most serious public health issues, affecting people of all ages and genders and all races [118, 292]. Obesity is typically brought on by an inability to maintain a healthy balance between dietary intake and energy expenditure, which is controlled by a wide range of physiological mechanisms [51]. There was a significant increase from 1980 to 2013 in the global prevalence of overweight, with 36.9% of men and 38.0% of women being overweight that year [182]. 671 million people were found to be obese throughout the world in this survey [182]. BMI values between 25.0 and 29.9 kg/m2 and 30.0 kg/m2 are commonly used to define overweight and obesity, respectively [48,229]. Obesity is the result of a complex interplay between genetic predisposition, the built environment, and individual behavior [273,301]. Many diseases and conditions are linked to obesity, including metabolic syndrome [59], pulmonary diseases [227], dyslipidaemia [58], cancer [36,138], non-alcoholic fatty liver disease [294], hypertension [234], gastrointestinal diseases [79], and diabetes mellitus [10,141]. The rising rates of obesity-related illness and death also place a heavy financial burden on healthcare systems [153]. There are currently available synthetic anti-obesity drugs such as orlistat, a reversible inhibitor of lipase enzymes in the GI tract that can reduce fat absorption [102], and lorcaserin, a serotonin-2C receptor agonist that suppresses appetite and promotes satiety [33]. Therefore, numerous natural products have the clinical potential as lipid-lowering agents for obese and overweight people, as shown in Table 6 and Fig. 6.
Table 6.
Clinical trials of natural products as lipid-lowering agents (https://clinicaltrials.gov).
| Compound | ClinicalTrials.gov Identifier | Type of study | Characteristics of patients (n) | Dose and time of treatment | Condition | Phase | Additional Refs |
|---|---|---|---|---|---|---|---|
| Catechin | NCT00692731 | randomized, double-blind, controlled study | catechin group and control group | 500 mL/day of a beverage providing approximately 625 mg catechins | overweight, obesity | – | [100] |
| Polyphenols | NCT05255367 | open label | n = 26 | Daily consumption of 100 mL of commercial berry and pomegranate juice, 20 g dark chocolate, and 1 green tea for 2 months to see if diet supplementation with (poly)phenol rich foods worked. | overweight, obesity | – | |
| 9-cis retinoic acid of Dunaliella bardawil | NCT00156169 | randomized, double-blind, controlled study | Dunaliella group and control group n = 50 | four Dunaliella capsules, providing 60 mg b-carotene per day after fibrate treatment | low HDL, cholesterol | phase 3 | [23,235] |
| Exenatide | NCT01061775 | open-label | n = 19 | 5mcg twice day for 4 weeks, then 10mcg twice daily for 20 weeks. | hypothalamic obesity | phase 1/2 | [161] |
Fig. 6.
Natural compounds as lipid-lowering agent and their chemical structures.
1.4.3. Natural products to treat heart-related diseases
Diseases of the heart and blood vessels are referred to as cardiovascular diseases (CVD) [78, 198]. The most frequent forms of cardiovascular disease are hypertension [140], coronary artery disease [218], cerebrovascular disease [206], angina pectoris [122], and atherosclerosis [77]. Risk factors for cardiovascular disease can be split into two groups: modifiable and non-modifiable risk factors [178]. Modifiable risk factors include insufficient physical exercise, an unhealthy diet, obesity, and a disordered lipid profile [228]; non-modifiable risk factors include smoking and high blood pressure [128]. There are some personal risk factors that cannot be changed, such as genes, sex, age, or family history [111]. Tobacco usage is associated with an increased risk of cardiovascular disease [145], lung disease [166], and cancer [95]. Numerous studies have demonstrated that CVD risk can be reduced with healthy eating, regular exercise, and smoking cessation [127, 252].
Along with diabetes, cancer, and chronic respiratory illness, cardiovascular disorders are one of the four main non-communicable diseases (NCDs) accounting for serious concerns [32,89,177]. According to the World Health Organization, cardiovascular illnesses were responsible for 17.9 million deaths in 2016, or 44% of all NCD deaths [135, 295]. Thus, CDV constitute the main cause of death around the globe [295]. CDV are currently among the leading causes of death around the world [268]. Current CVD disease treatment strategies make use of a wide range of potent pharmaceutical options. Unfortunately, most of these medications have a poor safety record and cause severe adverse effects [276]. In the search for new drug leads, natural products have long been held in high regard. The potential of several natural products as sources of treatments for cardiovascular diseases is increasingly being recognized [242]. Natural products can contribute numerous advantages to treatment plans via a wide variety of processes. The first step in delaying the beginning and progression of coronary artery disease (CAD) is to prevent the oxidation of LDL cholesterol [257,312], which may be accomplished with the use of products with antioxidant activity. Also, in patients with advanced CAD, antioxidant medications protect against oxidative damage brought on by ischemia/reperfusion [297, 298]. In addition, they boost nitric oxide levels, which benefits cardiovascular and endothelial function [165]. Second, their anti-inflammatory properties aid in protecting against reperfusion injury, atherosclerotic, myocardium hypertrophy, and vascular plaque development [265,278]. Third, the plasma lipids profile can be improved by using some natural products, and these products have powerful anti-atherogenic actions like in resveratrol [192, 213, 215]. It is possible that natural product has curative effects beyond just antioxidant and anti-inflammatory ones, including anti-apoptotic [191], anticoagulant [271], vasodilatory [259], and diuretic [149]. Therefore, numerous natural products have the clinical potential to treat heart-related diseases, as shown in Table 7 and Fig. 7.
Table 7.
Clinical trials of natural products to treat heart-related diseases (https://clinicaltrials.gov).
| Compound | ClinicalTrials.gov Identifier | Type of study | Characteristics of patients (n) | Dose and time of treatment | Condition | Phase | Additional Refs |
|---|---|---|---|---|---|---|---|
| Fucoxanthin and oligo fucoidan | NCT02875392 | randomized, Interventional, placebo-controlled trial | FuciHiQ group (n = 21) or placebo group (n = 21) | FucoHiQ (275 mg Oligo Fucoidan + 275 mg HS Fucoxanthin) 550mg/capsule 6 per day | non-alcoholic Fatty Liver Disease | – | [100] |
| Xanthohumol | NCT01367431 | Observational | 20 mg group, 60 mg group and 180 mg group; n = 48 | one capsule of one of the three doses (20, 60, 180 mg) randomly assigned | heart disease | – | |
| Cocoa polyphenols | NCT00654862 | randomized, Interventional, placebo-controlled trial | 250 mg group, 1000 mg group, placebo; n = 48 | oral administration of capsules with 1000 or 250 mg polyphenols | hypertension | phase 1 | |
| Catechin epigallocatechin-3-gallate (EGCG) | NCT01662232 | randomized, Interventional, placebo-controlled trial | 200 mg group, placebo group; n = 50 | 200 mg EGCG | cardiovascular diseases | – | |
| Exenatide | NCT00650546 | Open label | N = 8 | 5 mcg twice a day titrated to 10 mcg twice a day | nonalcoholic fatty liver disease | phase 2/3 |
Fig. 7.
Natural compounds to treat heart-related diseases and their chemical structures.
1.4.4. Natural products with anticancer activity
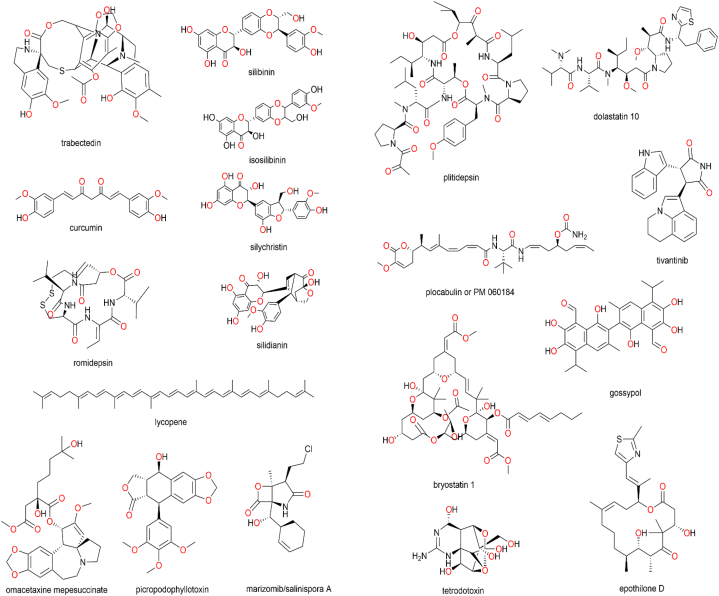
Global cancer registry expansion has stimulated research into potential new treatments that are selectively toxic to cancer cells while being safe for healthy tissue [4]. Previous anticancer medications showed relatively high toxicity not only to the tumor cells, but also to the normal cells of the body portion where the cancer had formed [207]. New anticancer medications are currently being researched both from various sources including marine and terrestrial [50, 71]. Medical practitioners have relied on plants for ages to treat a wide variety of conditions. Some plants are used for their medicinal properties and consumed as part of local folk medicine in many different cultures. As the number of people diagnosed with cancer rises, including breast cancer [290], so does the demand for effective treatments. After being extracted and purified, many different plant-based anticancer drugs are tested on cells (including various cancer cell lines) and experimental animals. The discovery of significant biological activity in many plants with a history of use in traditional medicine has led to their inclusion into mainstream medicine [224]. These compounds can be obtained, for example, through plant extracts. Alternatively, combination of biology, chemistry, and technologies can be used to synthesize plant-based anticancer compounds [251]. There are several kinds of chemicals found in nature (including plants and aquatic creatures) that display anticancer effects, such as diterpenes, quinone, peptides and their cyclic form, alkaloids, purine, sesquiterpene, and macrocyclic polyether. It is generally more cost-effective to obtain these substances from their natural sources than to prepare them synthetically. Moreover, numerous natural products have the clinical potential to cancer, as shown in Table 8 and Fig. 8.
Table 8.
Clinical trials of natural products to as anti-cancer agents (https://clinicaltrials.gov).
| Compound | ClinicalTrials.gov Identifier | Type of study | Characteristics of patients (n) | Dose and time of treatment | Condition | Phase | Additional Refs |
|---|---|---|---|---|---|---|---|
| Trabectedin | NCT01343277 | A multicenter, open- label, randomized, active- controlled, parallel- group | trabectedin group (n = 378) or dacarbazine group (n = 172) | trabectedin Arm: 1.5 mg/m2 as a 24 h IV infusion q3wk. |
advanced liposarcoma, Leiomyosarcoma | phase 3 | [100] |
| Sylmarin (mixture of flavonolignans consisting of silibinin, isosilibinin, silychristin, silidianin) | NCT03130634 | open-label, randomized, comparative, double arm, single center | sylmarin group or control group; n = 70 | during six cycles of FOLFIRI chemotherapy, the patients will take silymarin (150 mg) 3x daily from day 1 to day 7 during one cycle of treatment. | Metastatic, colorectal cancer | phase 4 | |
| Silibin-Phytosome | NCT00487721 | non-Randomized | silibin-phytosome group or control group; n = 12 | 13 g daily, in three divided doses for 2–10 weeks. | prostate cancer | phase 2 | |
| Xanthohumol | NCT02432651 | randomized | 2 mg group, 12 mg group and 24 mg group, placebo; n = 64 | 2/12/24 mg xanthohumol at breakfast, lunch, and dinner for 3 weeks. | oxidative Stress | Phase 1 | |
| Catechin (Sinecatechins 10%) | NCT02029352 | randomized double-blinded | catechin group or placebo group; n = 42 | twice daily (morning and evening) in a thin layer to the tumor including 5 mm of the surrounding skin | carcinoma | Phase 2/3 | |
| Lycopene | NCT00068731 | randomized double-blinded | lycopene group or placebo group; n = 47 | twice daily on days 1–28. Courses repeat every 28 days for at least 4 months | prostate cancer | phase 2 | |
| Catechin epigallocatechin-3-gallate (EGCG) | NCT02577393 | randomized double-blinded | prophylactic EGCG group, therapeutic EGCG group, placebo; n = 83 | 440 lmol/L | lung neoplasms | phase 2 | |
| Curcumin | NCT01740323 | randomized double-blinded | resveratrol group (n = 15) or placebo group (n = 15) | 500 mg BID | breast cancer | phase 2 | |
| Resveratrol | NCT00256334 | randomized, placebo- controlled, double blind | resveratrol group, placebo group; n = 11 | one of four dose cohorts: plant-derived resveratrol tablets at a dose of 80 mg/day, plant-derived resveratrol tablets at a dose of 20 mg/day, Grape Powder (GP) at a dose of 120 g/day, and GP at a dose of 80 g/day. | colon cancer | phase 1 | |
| Resveratrol | NCT00920803 | double-blind, randomized | resveratrol group, placebo group; n = 9 | 5 g once daily for 14 days | neoplasms, colorectal | phase 1 | |
| Resveratrol | NCT00433576 | non-Randomized | n = 20 | STAGE II: Patients receive oral resveratrol on days 1–8. Patients undergo colorectomy on day 9 | aAdenocarcinoma of the Colon Adenocarcinoma of the Rectum Stage I Colon Cancer Stage I Rectal Cancer Stage II Colon Cancer Stage II Rectal Cancer Stage III Colon Cancer Stage III Rectal Cancer | phase 1 | |
| Sulforaphane | NCT00982319 | randomized double-blinded | n = 34 | 100 μmols of sulforaphane dissolved in 150 mL mango juice once a day for 14 days | breast cancer | phase 2 | |
| Romidepsin | NCT00106418 | non-randomized, multicenter, open-label trial | n = 35 | 13 mg/m^2 of romidepsin intravenously over 4 h on Days 1, 8, and 15 of each 28-day cycle | prostate cancer | phase 2 | |
| Romidepsin | NCT01353664 | open-label, single-arm study | n = 19 | same dose, infusion time and frequency used for the last dose of romidepsin given | lung cancer | phase 2 | |
| Omacetaxine mepesuccinate | NCT00375219 | open-label | chronic phase group (n = 62), accelerated phase (n = 20), blast phase (n = 21) | 1.25 mg/m^2 subcutaneously, twice daily for 14 consecutive days every 28 days until response | chronic myeloid leukemia | phase 2 | |
| Picropodophyllotoxin | NCT01466647 | open single-center, explorative | n = 12 | a repeated BID treatment for 14 days, followed by a 7-day observation period for two treatment periods | non-small Cell Lung Cancer | phase 1 | |
| Picropodophyllotoxin | NCT01561456 | open label, randomized, multi-center | n = 100 | oral suspension at 400 mg twice daily for 21 days per cycle | non-small-cell Lung Cancer Squamous Cell Carcinoma Adenocarcinoma of the Lung | phase 2 | |
| Marizomib/salinosporamide A | NCT00396864 | multicenter, open-label study | n = 51 | injection at doses ranging from 0.0125 to 0.8 mg/m2 over 1–10 min on Day 1, Day 8, Day 15 of each 28-day Cycle; 11 dose cohorts during dose-escalation | cancer lymphomas | phase 1 | |
| Plitidepsin | NCT00229203 | non-randomized, multicentre, open-label | plitidepsin group (n = 32) and plitidepsin with dexamethasone (n = 19) | 5 mg/m2, 3-h infusion every 2 weeks | Multiple Myeloma | phase 2 | |
| Plitidepsin | NCT01102426 | non-randomized, multicentre, open-label | plitidepsin+ dexamethasone Group (n = 171), dexamethasone (n = 84) |
5 mg/m2 intravenously (i.v.) over 3 h on Day 1 and 15 every 4 weeks. dexamethasone: 4 mg tablet. 40 mg orally on Day 1, 8, 15 and 22 every four weeks at least 1 h before plitidepsin infusion. | multiple myeloma | phase 3 | |
| Plocabulin/PM 060184 | NCT03427268 | open-label, multicentre study | PM 060184 group (n = 32) | 9.3 mg/m2 PM 060184 i.v. as a 30-min infusion via a central or peripheral venous catheter; It administered on Day 1 and Day 8 q3wk | colorectal cancer | phase 2 | |
| Bryostatin 1 | NCT00003968 | open Label | n = 35 | bryostatin 1 IV over 1 h on days 1, 8, and 15. Treatment continues every 4 weeks in the absence of unacceptable toxicity or disease progresssion. | kidney cancer | phase 2 | |
| Tetrodotoxin | NCT00725114 | multicentre, Randomized, Double-blind, Placebo-controlled, Parallel-design | tetrodotoxin group, placebo group; n = 165 | 30 μg twice daily for 4 days | cancer pain | phase 3 | |
| Tivantinib | NCT01755767 | randomized, double-blind study | tivantinib 240 mg BID Cohort group (n = 28), Placebo Matching 240 mg BID Cohort group (n = 15), Tivantinib 120 mg BID Cohort group (n = 226), Placebo Matching 120 mg BID Cohort group (n = 114) | the dosage of 120/240 mg tablets administered by mouth twice daily (BID), once in the morning and once in the evening, with food, for a total daily dose of 240/480 mg. | hepatocellular carcinoma | phase 3 | |
| Tivantinib | NCT02029157 | randomized double-blind, placebo-controlled | tivantinib 120 mg BID Cohort group (n = 134), Placebo Matching 120 mg BID Cohort group (n = 61) | twice-a-day oral tivantinib (120 mg bid) | liver cancer | phase 3 | [146] |
| Gossypol | NCT00540722 | Open-label | gossypol group (n = 56) | once daily on days 1–21. Treatment repeats every 28 days | glioblastoma | phase 2 | |
| Epothilone D | NCT00077259 | open-label | n = 16–69 | drug IV over 90 min on days 1, 8, and 15. Courses repeat every 28 days | colorectal cancer | phase 2 | |
| Dolastatin 10 | NCT00003677 | open-label | n = 9 | IV bolus once every 21 days. | pancreatic cancer | phase 2 |
Fig. 8.
Natural compounds with anti-cancer activity and their chemical structures.
1.5. Drug delivery approaches for natural products in the management of metabolic disorders
Recently, the use of natural products as the main active agents for the treatment of numerous diseases, including metabolic disorders. Due to the limitation of bioactive compounds the natural products, several drug delivery approaches have been developed to overcome the problems [125, 197, 264, 280]. In this review, we showed numerous approaches containing natural compounds to the treatment of metabolic disorders.
1.5.1. Drug delivery approaches for natural products in the management of diabetes
Grape (Vitis vinifera) has been well-known to possess phenolic compounds, showing antioxidant activities. Gharib and coworkers investigated two major phenolic compounds in the grape, cyanidin and delphinidin as antidiabetic agents in the form of liposomal delivery system [84]. Liposomal system has been widely used to improve the efficacy of many drugs to treat some diseases [35, 94, 116, 133, 221, 302]. In their study, two compounds were incorporated into liposomes using an extrusion technique, showing the entrapment efficiencies of more than 80% for both compounds. It was found that free drugs could reduce the glycation of albumin in vitro study to 30.5% for delphinidin and 46% for cyanidin. Interestingly, following the formulation into liposomal system, the glycation of albumin values was 8.5% for delphinidin and 14.6% for cyanidin. Furthermore, in vivo study showed that the liposomal could exhibit higher anti-glycation efficacy compared to free compounds. In the diabetic mice, the administration of liposomal was able to significantly reduce the albumin and HbA1c glycation rate in comparison to free compounds. Accordingly, this showed the feasibility of the formulation of natural compounds in the improvement of diabetic therapy.
In another study, using similar system, Yücel and co-workers encapsulated a natural compound, resveratrol into two different types of liposomes, PEGylated and non-PEGylated [307]. In their study, the combination of dipalmitoylphosphatidylcholine (DPPC) and cholesterol was used to prepare multi-bilayered particles with size of 215 nm. The diabetic (streptozotocin-induced) pancreatic cell line was treated with resveratrol solution and resveratrol-loaded liposomes for 24 h. The findings showed that insulin concentrations increased, with a greater degree in the liposome formulations treated groups, whereas glucose concentrations decreased. In vitro study, it was found that liposomal formulation could show a significant antioxidant activity in pancreatic cells compared to free solution. Thus, this could show the promising approach in the therapy of diabetes mellitus and associated oxidative stress.
Mao et al. explored the benefit of Echinacea purpurea as antidiabetic agent [171]. It has been reported that the extract of E. purpurea contains numerous phenolic compounds and isobutylamides, exhibiting antidiabetic activity. To further improve the effectiveness, the extract was incorporated into chitosan/silica nanoparticles with particle size of 218 nm, 66.9% of entrapment efficacy and 39.9% of drug loading. Furthermore, it was found that the formulation could reduce the oxidative stress in LC-540 cells with strong antioxidant activity. Importantly, in the in vivo study using diabetes rat models induced by streptozotocin (STZ), the nanoparticles could reduce the glucose blood level to the normal rate, increase the resistance of insulin and the resistance of plasma fibroblast growth factor 21 (FGF 21), compared to the free form.
Another type of nanoparticles, gold nanoparticles were also investigated for their antidiabetic activity. Daisy and team synthetized gold nanoparticles using Cassia fistula stem bark aqueous extract [52]. Gold nanoparticles have been greenly synthetized using many natural compounds [119, 136, 181, 188, 208, 237, 241]. In this study, numerous characterizations were carried out, including ultraviolet–visible spectroscopy, Fourier transform infrared spectroscopy, and scanning electron microscopy to investigate their absorbance pattern, the possible functional groups, the size of the nanoparticles, respectively. Overall, the results showed that the gold nanoparticles prepared from C. fistula stem bark aqueous extract exhibited promising hypoglycemic activity compared to aqueous extract according to the analysis of level of serum glucose, body weight, kidney function evaluation, liver function evaluation, and profile of lipid. It was found that the administration of gold nanoparticles could decrease serum biochemistry parameters in rats with streptozotocin-induced diabetes. Therefore, this showed the potency of gold nanoparticles of C. fistula to improve the diabetic therapy.
1.5.2. Drug delivery approaches for natural products in the management of obesity
The application of drug delivery system containing natural compounds has been also used in the treatment of obesity. One of Ayurvedic medicine, Salacia chinensis, has been reported to show potential pharmacological effects. Gao and team developed gold nanoparticles loading S. chinensis to investigate its anti-obesity activity [83]. The study was conducted in a high-fat diet (HFD) treated obese rats. Initially, the nanoparticles prepared were characterized for their physicochemical parameters. The results showed that the formulation exhibited a spherical shape with crystal form. Essentially, in the in vivo study, the nanoparticles could reduce several obesity parameters in the HFD rats, including the bodyweight changes, resistin, adipose index, inflammatory markers, BMI, leptin, CRI, adiponectin, AI, liver marker enzymes, lipid profile, dan AMPK signaling proteins. Furthermore, the liver histopathological evaluation showed a promising result with the reduction of hepatocyte degradation following the administration of nanoparticles of Salacia chinensis. Using similar approach, Ansari et al. developed gold nanoparticles synthesized using Smilax glabra rhizome [13]. The nanoparticles were 21 nm in size with excellent cell uptake property. It was found that the administration of the nanoparticle in HFD rats showed superior antiobesity activity based on several parameters, including lipid profile, liver markers, hormones like leptin, adiponectin and resistin, as well as histopathological evaluations.
To overcome the bioavailability and solubility of issue of resveratrol as antiobesity agent, Wan and coworkers formulated PLGA nanoparticles loading resveratrol [281]. The nanoparticles were prepared using oil in water emulsion method, producing particles with size of 176.1 nm and zeta potential of −22.6 mV. Moreover, the entrapment efficiency and the drug loading were found to be 97% and 14.9%, respectively with sustained release behavior in the gastrointestinal tract and excellent physical stability profiles. Importantly, compared to free resveratrol, the administration of PLGA nanoparticles showed a better antiobesity activity through lipogenesis, enhancing lipolysis and lowering hepatocellular proliferation. Morover, Andelbaky et al. isolated cellulose nanocrystal from grape and investigated the antiobesity activity [12]. The nanocrystal was isolated using sodium hydroxide and bleached using sulphuric acid. In the rat obesity model, by observing the body weight, the lipid profiles, liver function and kidney function, the nanocellulose showed antiobesity activity compared to the positive control grape seed powder.
Using different administration route, Ariamoghaddam et al. developed nanofibers patches for transdermal delivery of curcumin [15]. Transdermal route has been used to deliver numerous drugs as alternative to the conventional oral route. Several studies have shown that the administration of bioactive compounds via this route could result in better bioavailability compared to other routes [35, 67, 133, 190, [194], [195], [196], 221, 263, 279]. The nanofibers were fabricated using polyvinyl alcohol and gelation, producing formulation with fiber diameter of 200–250 nm and highly reproducible. The effectiveness of transdermal delivery was evaluated by observing the body weight, the level of blood parameters and MRI imaging. It was found that the level of leptin decreased following the transdermal delivery of curcumin using this approach. Importantly, MRI imaging showed the decrease of adipose tissue around 4–7%. Accordingly, this showed that the transdermal delivery could be an alternative delivery route of natural compounds for obesity therapy.
1.5.3. Drug delivery approaches for natural products in the management of heart related diseases
With respect to the application of drug delivery system of natural product in the treatment of heart related diseases, polyphenol has been still widely used. For example, Qi et al. developed self-assembly nanoparticles from several types of polyphenol, namely gallic acid, catechin, tannic acid and epigallocatechin gallate, to prepare functionalized nanoparticles [210]. The four polyphenols have different type of phenolic hydroxyl groups and following optimization process, combined with cyclodextrin, the use of tannic acid to prepare the nanoparticles showed the optimum formulation with potent antioxidant activity. The results showed that the nanoparticles could potentially protect the cells from hypoxic-ischemic injury. In vivo study, following intravenous injection in the ventricular fibrillation cardiac arrest model in rats and myocardial hypertrophy model in mice, the formulation localized in the injured heart. In the two models, the nanoparticles were able to result in significant pharmacological effects. Therefore, this could be a promising system for the treatment of heart-targeting diseases.
Another study highlighted the formulation of zinc oxide nanoparticles containing Artemisia herba-alba leaves’ extract (AHALE) to improve the cardioprotective effect of AHALE [8]. The efficacy study was carried out in myocardial infarction model in male rats induced by isoproterenol. Several parameters were investigated, showing that the administration of the nanoparticles could increase the level of heart markers, lipid profile markers and lipid peroxidation products compared to free AHALE. Moreover, the reduction of the activity of antioxidant activity was found in the animal model following the administration of this approach. In addition, they also investigated the effect of the administration of the nanoparticles before the inducement of isoproterenol and they found that the oxidative stress could be avoided. Therefore, this system could also be used to prevent the heart diseases. With the same purpose, the development of silver nanoparticles from Mentha piperita, stabilized by chitosan was conducted by Wang and team [286]. Silver nanoparticles have been found to show numerous pharmacological effects [181, 193, 286]. In this study, the nanoparticles were found to possess sizes around 5 nm–15 nm with spherical shape. The formulation was administered orally in rats with heart failure model, and it was found that the size of the infarct was significantly reduced and the function of the cardiac was improved, indicated by lower left ventricular end diastolic pressure and raised ± dp/dt(max).
Furthermore, Tan et al. encapsulated total flavonoid extract fromDracocephalum moldavica L. (TFDM) with myocardial protective activity in solid lipid nanoparticles [258]. This study was designed due to the low solubility of the flavonoid compounds in the extract. The nanoparticles were optimized using central composite design, resulting in optimum formulation with size of 104.83 nm, PDI value of 0,201 and zeta potential of −28.7 mV. Importantly, the in vivo studies showed significant higher myocardial protection compared to free extract, according to the area of infarct, histopathological evaluation, cardiac enzyme parameters and serum inflammatory factors.
In terms of another type of heart related disease, Yu and team developed smart delivery containing polyphenol compounds for thrombolytic therapy [305]. The system consisted of thrombin-responsive nanoparticles prepared via noncovalent interactions form tannic acid to cross-link urokinase-type PA (uPA) and a thrombin-cleavable peptide on a sacrificial mesoporous silica template. The results showed that the nanoparticles could hold active uPA. Importantly, in the presence of thrombin, the nanoparticles showed improved the activation of plasminogen, indicating the responsive behavior of the system.
1.5.4. Drug delivery approaches for natural products in the management of cancer
Natural products have long been utilized as medications with pharmacological actives to aid in the treatment of a wide range of medical conditions. Even so, our understanding of their potential as materials remains limited. Natural compounds of small molecular weight extracted from traditional Chinese medicine have been demonstrated to exhibit novel properties in recent years, including the ability to self-assemble into gels (i.e., natural product gels, NPG). However, there is a lack of competence in the application development of these natural compounds, which significantly reduces their practical worth and slows the improvement of natural products in industrial area. Therefore, Zhi et al. used a family of triterpenoid natural compounds with its own ability to self-assemble (gel scaffolds material) for the development of drug delivery systems. Remarkably, these NPG were not only enabled synergistic treatment of cancers via bioactive natural products, but also displayed remarkable self-healing, regulated gelation, good safety, and prolonged release. When it comes to tumor therapy, NPG scaffolds have many advantages than non-bioactive gel scaffolds. These include more tumor inhibition, improved health and body recovery, enhanced immune system, fewer toxic side effects, and increased chances of survival. Constructing NPG scaffolds is a significant step toward the discovery of novel uses for natural products, as it makes full use of these materials in their self-assembled form [320].
Chemoprevention of associated-colorectal cancer (CRC) in patients with inflammatory bowel disease (IBD) has shifted to prioritize anti-inflammatory therapies. Current anti-inflammatory medications used in IBD therapy have not yet been studied enough for their potential chemopreventive effects. For this reason, research exploring novel chemopreventive possibilities is essential, and natural compounds derived from food and complementary and alternative medicine have become attractive resources owing to their multi-component nature and ability to target multiple cancer types. Danggui Decoction (DGD) is a traditional Chinese medicinal formula for the treatment of inflammatory bowel disease (IBD) that includes the ingredients Angelicae sinensis Radix, Zingiberis Rhizoma Recens, and Jujubae Fructus; DGD supercritical fluid extracts (DGDSFE) and DGD polysaccharide extracts (DGDPE) are promising candidates for chemoprevention treatment. To explore this promising activity, Liu and coworkers used extrusion-spheronization and coating technologies to create a multi-unit pellet drug delivery system (MUPDDS) with two separate components: pellets containing DGDSFE for colon targeting and pellets containing DGDPE for peripheral targeting [159]. This MUPDDS was tested for its chemopreventive properties in a rat model of cancer which formerly induced with 1,2-dimethylhydrazine and sodium dextran sulfate. Serum levels of TNF-α, IL-1β, and hepcidin were reduced, while levels of IFN-γ and IL-2 in splenocyte supernatant were elevated, indicating anti-inflammation, iron metabolism regulation, and immune regulation of DGDSFE and DGDPE in MUPDDS, which led to a decrease in tumor incidence, tumor number, and tumor volume after 14 weeks of daily administration. In addition, a comparison with extracts, DGDSFE colon-targeted pellets, and DGDPE pellets showed the feasibility and advantage of MUPDDS in chemoprevention, presenting an encouraging technique to improve the effect of traditional Chinese medicines in cancer prevention.
The use drug delivery containing natural products has also been applied for the treatment of hepatocellular cancer. Patients with unresectable metastatic or recurrent hepatocellular cancer continue to benefit most from the use of combination chemotherapy medication. It is well known that there is also a significant advance in the management of this disease. It has been found that the immunomodulator called lentinan, which has been used in the treatment of cancer, also possesses anti-tumor activities. Lentinan has been shown to inhibit hepatocellular cancer, though the exact processes by which this occurs are not yet understood. In vitro and in vivo studies using HepG2 cells and H22 tumor-bearing mice demonstrated that Lentinan strongly synergizes with oxaliplatin in inhibiting NF-κB, Stat3, and survivin signaling via the mitochondrial route. Additionally, Lentinan reduced oxaliplatin's negative effects. In light of these results, Lentinan was proposed as a promising drug for use in combination with oxaliplatin in the treatment of hepatocellular carcinoma [315].
Many bioactive substances are now collected from nature, particularly those that have anticancer effects. As anticancer, these substances can alter the signaling pathways involved in the cell cycle, decrease interactions between cytoskeleton components, or overexpress antitumoral proteins. To increase pharmacokinetic and pharmacodynamic parameters, these drugs' physicochemical characteristics and targeted delivery effectiveness may be modified. The delivery method of exosomes, which is enhanced by a number of features, has the potential to make them the next generation of transporters for therapeutic compounds. Exosomes are a subtype of cellular vesicles (30–150 nm) derived from membranes that are crucial for intercellular communication. Numerous uses, including medicine delivery, have been developed for these nanovesicles due to their inherent capacity as nanocarriers [2, 91, 123, 154, 250]. Donoso-Quezada and coworkers showed that plant-derived bioactive substances, like saponins and flavonoids from black bean extract, may be integrated into the exosomal structure and taken up by recipient cells in vitro. According to our preliminary research, exosomal formulations of the extract appear to increase the antiproliferative response, adding to our understanding of the characteristics of exosomes as nanocarriers. In the short term, our effort will be focused on extending the in vitro data supporting the increased activity of exosomal formulations, and in the medium term, we will work on the creation of new, more effective methods to create, isolate, and purify exosomes [63].
In order to increase the response in vitro, in another study, they loaded exosomes isolated from various cell lines with saponins and flavonoids from a black bean extract (Phaseolus vulgaris L.) with antiproliferative activity. In order to transfer these chemicals to recipient cells, they demonstrated that exosomes might be loaded with at least three different phytochemicals in a single step. Additionally, they discovered that the exosomal extract has higher bioactivity than that of other formulations of the same extract. Exosomes offer a possible alternative, according to our findings, for enhancing the delivery of complex combinations of bioactive chemicals, such as plant extracts. Therefore, developing novel products for human use with improved nutraceutical characteristics may be one of the future uses for these nanovesicles [64].
Another type of approach used for the cancer treatment is mesoporous silica nanoparticles. Mesoporous silica nanocarriers for drug delivery were developed by Porrang and team from natural materials, including rice and wheat husk [203]. By using acid leaching, the biogenic silica in grain husk was first removed, and it was subsequently transformed into sodium silicate as a silica precursor. Subsequently, using continuous and discrete sol-gel methods, sodium silicate was added to the template mixture to create mesoporous silica nanoparticles. The XRD, FT-IR, BET, and SEM analyses were used to examine the impacts of natural source type and precursor addition method on the morphological and physicochemical features of nanocarriers. Their findings indicated that spherical nanocarriers made of rice husk were more crystalline and had slit-shaped pores. The results also demonstrated that the discrete addition of the precursor improved their hydrophilicity, particle size, and pore size in contrast to continuous addition, most likely as a result of the precursor's high starting concentration in the reaction mixture. Model anticancer drug doxorubicin (DOX) was loaded into the nanocarriers, and the behavior of the drug release was examined at two different pH values (7.4 and 5.4). Due to DOX increased solubility in an acidic environment, the accumulated released drug at pH 5.4 was generally around twice as much as pH 7.4. Additionally, due to their larger pore diameters than continuous mode nanocarriers, discrete mode nanocarriers had higher cumulative released drug concentrations at pH 5.4. On the HFF-2 and MCF-7 cell lines, respectively, the biocompatibility and cytotoxicity of nanocarriers and nanocarriers loaded with DOX were also examined. Additionally, a morphological analysis of the MCF-7 cells was used to assess apoptosis as the mechanism of cell death. The DOX-loaded nanocarriers, particularly discrete mode produced nanocarriers, displayed high-efficiency anticancer action on the MCF-7 cell line within tolerable toxicity limits and apoptosis induction.
2. Concluding remarks and future perspectives
Over the last two decades, tremendous efforts have been made to reveal the mechanistic basis of metabolic disorders and prospective biological targets that are clinically translatable and important in the pharmacological management of the diseases. From numerous experimental findings, we now know that metabolism and immunity are interconnected and serious malfunction in the regulatory networks can result in the development of inflammation-induced metabolic diseases, including diabetes, obesity, cardiovascular diseases, and cancer. Hence, the interconnected biological interface of immune system and metabolism has been suggested to play a tremendous role in the homeostatic mechanism to maintain humans’ health. Nevertheless, despite advancement in the diagnostic tools and clinical procedures to detect the hallmarks of metabolic disorders, and rapid progress in the discovery and development of safe and potent drugs to treat metabolic disorders, the number of FDA-approved drugs to manage metabolic disorders remains low. To improve this number, efforts to utilize natural products and their isolated compounds are expanding.
Metabolic disorders are multifactorial; thus, the use of multiple medications may be required to achieve a proper pharmacological response. To this end, a less-risky use of pharmaceutical preparations, such as natural products, may play a beneficial role. Indeed, it has been widely suggested that natural products have a low risk to elicit dangerous adverse effects and such feature can be safely used in the urge to treat metabolic disorders. However, it is important to note that slow pace of animal and clinical studies to demonstrate the efficacy and safety of natural products has been one of the most unsettling avenues in the scientific efforts to advance biomedical and pharmaceutical research in this field. Therefore, it is crucial to tackle this problem as fast and as decisive as possible to minimize the gap in providing scientific evidence for the benefits of plant-derived phytochemicals in the management of metabolic disorders. Such endeavor shall provide valuable support in the long-term battle against the increasing incidence of metabolic disorders-related diseases.
Data availability statement
Data will be made available on request.
Author contributions
F.N. and J.S-G. designed the outline of the manuscript, F.N., A.F., S.S.M., A.D.P., M.S., and D. C wrote the initial draft, F.N., A.F., S.S.M., A.D.P., M.S., D.C., T.B.E., and J.S-G revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to offer our gratitude to the members of Unhas Fly Research Group (UFRG) for their suggestions during the preparation of the manuscript outline. Research carried out in F.N's lab is supported by Penelitian Fundamental Kolaboratif (PFK) 2023 Grant (No. 00323/UN4.22/PT.01.03/2023) from Hasanuddin University. Funding for open access charge: Universidade de Vigo/CISUG.
Contributor Information
Firzan Nainu, Email: firzannainu@unhas.ac.id.
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
References
- 1.Abdul-Ghani M.A., Defronzo R.A. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr. Pract. 2008;14:782–790. doi: 10.4158/EP.14.6.782. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A.K., Aqil F., Jeyabalan J., Spencer W.A., Beck J., Gachuki B.W., Alhakeem S.S., Oben K., Munagala R., Bondada S., Gupta R.C. Nanomedicine: Nanotechnology, Biology, and Medicine. Elsevier Inc.; 2017. Milk-derived exosomes for oral delivery of paclitaxel. [DOI] [PubMed] [Google Scholar]
- 3.Ahn K., Ji H., Kim H.E., Cho H., Sun Q., Shi S., He Y., Kim B.G., Kim O. Raphanus sativus L. seed extracts induce apoptosis and reduce migration of oral squamous cell carcinoma KB and KB(CD133+)cells by downregulation of β-catenin. Nutr. Cancer. 2020;72:1378–1389. doi: 10.1080/01635581.2019.1684527. [DOI] [PubMed] [Google Scholar]
- 4.Alatrash G., Jakher H., Stafford P.D., Mittendorf E.A. Cancer immunotherapies, their safety and toxicity. Expet Opin. Drug Saf. 2013;12:631–645. doi: 10.1517/14740338.2013.795944. [DOI] [PubMed] [Google Scholar]
- 5.Alberti K.G.M.M., Zimmet P., Shaw J. International diabetes federation: a consensus on type 2 diabetes prevention. Diabet. Med. 2007;24:451–463. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 6.Ali A.N.M., Saeed N., Omear H.A. The anticancer properties of Artemisia aucheri boiss extract on HT29 colon cancer cells. J. Gastrointest. Cancer. 2021;52:113–119. doi: 10.1007/s12029-019-00354-2. [DOI] [PubMed] [Google Scholar]
- 7.Ali M.Y., Jannat S., Jung H.A., Choi J.S. Insulin–mimetic dihydroxanthyletin-type coumarins from angelica decursiva with protein tyrosine phosphatase 1B and α-glucosidase inhibitory activities and docking studies of their molecular mechanisms. Antioxidants. 2021;10:292. doi: 10.3390/antiox10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alshehri M.A. Saudi Journal of Biological Sciences. The Author(s)); 2022. Cardioprotective properties of Artemisia herba alba nanoparticles against heart attack in rats: a study of the antioxidant and hypolipidemic activities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzahrani A.J. Potent antioxidant and anticancer activities of the methanolic extract of Calligonum comosum (L'Her) fruit hairs against human hepatocarcinoma cells. Saudi J. Biol. Sci. 2021;28:5283–5289. doi: 10.1016/j.sjbs.2021.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alzaman N., Ali A. Obesity and diabetes mellitus in the Arab world. J. Taibah Univ. Med. Sci. 2016;11:301–309. [Google Scholar]
- 11.Ambrose J.A., Singh M. 2015. Pathophysiology of Coronary Artery Disease Leading to Acute Coronary Syndromes. F1000prime reports 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andelbaky M.S.M.E., Ibrahim H.S., Hassan M.L., Sayed Z.E. Nanoparticles effects of red grape (Vitis vinifera) seeds and grape seeds powder on obese hyperlipidemic rats. ARC J. Nutr. Growth. 2016;2:1–15. [Google Scholar]
- 13.Ansari S.A., Bari A., Ullah R., Mathanmohun M., Veeraraghavan V.P., Sun Z. Journal of Photochemistry and Photobiology B: Biology. Elsevier; 2019. Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. [DOI] [PubMed] [Google Scholar]
- 14.Antonelli M., Kushner I. It's time to redefine inflammation. Faseb. J. 2017;31:1787–1791. doi: 10.1096/fj.201601326R. [DOI] [PubMed] [Google Scholar]
- 15.Ariamoghaddam A.R., Ebrahimi-Hosseinzadeh B., Hatamian-Zarmi A., Sahraeian R. Materials Science and Engineering C. Elsevier; 2018. In vivo anti-obesity efficacy of curcumin loaded nanofibers transdermal patches in high-fat diet induced obese rats. [DOI] [PubMed] [Google Scholar]
- 16.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astrup A. Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Publ. Health Nutr. 2001;4:499–515. doi: 10.1079/phn2001136. [DOI] [PubMed] [Google Scholar]
- 18.Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awazawa M., Ueki K., Inabe K., Yamauchi T., Kubota N., Kaneko K., Kobayashi M., Iwane A., Sasako T., Okazaki Y., Ohsugi M., Takamoto I., Yamashita S., Asahara H., Akira S., Kasuga M., Kadowaki T. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metabol. 2011;13:401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Baek S.J., Hammock B.D., Hwang I.K., Li Q., Moustaid-Moussa N., Park Y., Safe S., Suh N., Yi S.S., Zeldin D.C., Zhong Q., Bradbury J.A., Edin M.L., Graves J.P., Jung H.Y., Jung Y.H., Kim M.B., Kim W., Lee J., Li H., Moon J.S., Yoo I.D., Yue Y., Lee J.Y., Han H.J. Natural products in the prevention of metabolic diseases: lessons learned from the 20th KAST frontier scientists workshop. Nutrients. 2021;13 doi: 10.3390/nu13061881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balakumar P., Maung-U K., Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016;113:600–609. doi: 10.1016/j.phrs.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Beidokhti M.N., Eid H.M., Villavicencio M.L., Jäger A.K., Lobbens E.S., Rasoanaivo P.R., Mcnair L.M., Haddad P.S., Staerk D. Evaluation of the antidiabetic potential of Psidium guajava L.(Myrtaceae) using assays for α-glucosidase, α-amylase, muscle glucose uptake, liver glucose production, and triglyceride accumulation in adipocytes. J. Ethnopharmacol. 2020;257 doi: 10.1016/j.jep.2020.112877. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Amotz A., Mokady S., Avron M. The β-carotene-rich alga Dunaliella bardawil as a source of retinol in a rat diet. Br. J. Nutr. 1988;59:443–449. doi: 10.1079/bjn19880053. [DOI] [PubMed] [Google Scholar]
- 24.Berthoud H.R. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neuro Gastroenterol. Motil. 2008;20(1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertrams J. The HLA association of insulin-dependent (type I) diabetes mellitus. Behring Inst. Mitt. 1984:89–99. [PubMed] [Google Scholar]
- 26.Beyfuss K., Hood D.A. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018;23:100–117. doi: 10.1080/13510002.2017.1416773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhumireddy A., Nellore K., Alapati K.S. Anticancer activity of Neptunia oleracea methanolic extracts. Nat. Prod. Res. 2022;36:1053–1057. doi: 10.1080/14786419.2020.1844693. [DOI] [PubMed] [Google Scholar]
- 28.Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bom M.J., Heijden D.J.V.D., Kedhi E., Heyden J.V.D., Meuwissen M., Knaapen P., Timmer S.a.J., Royen N.V. Early detection and treatment of the vulnerable coronary plaque. Circulation: Cardiov. Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005973. [DOI] [PubMed] [Google Scholar]
- 30.Bosco D., Armanet M., Morel P., Niclauss N., Sgroi A., Muller Y.D., Giovannoni L., Parnaud G., Berney T. Unique arrangement of α-and β-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottazzo G.F., Florin-Christensen A., Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 32.Boutayeb A. Handbook of Disease Burdens and Quality of Life Measures. 2010. The burden of communicable and non-communicable diseases in developing countries; p. 531. [Google Scholar]
- 33.Brashier D.B.S., Sharma A.K., Dahiya N., Singh S.K., Khadka A. Lorcaserin: a novel antiobesity drug. J. Pharmacol. Pharmacother. 2014;5:175–178. doi: 10.4103/0976-500X.130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bray M.S., Loos R.J., Mccaffery J.M., Ling C., Franks P.W., Weinstock G.M., Snyder M.P., Vassy J.L., Agurs‐Collins T., Group C.W. NIH working group report—using genomic information to guide weight management: from universal to precision treatment. Obesity. 2016;24:14–22. doi: 10.1002/oby.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai W., Liu J., Zheng L., Xu Z., Chen J., Zhong J., Song Z., Xu X., Chen S., Jiao C., Guo J., Yi Y., Zhang Y. International Journal of Biological Macromolecules. Elsevier B.V.; 2020. Study on the anti-infection ability of vancomycin cationic liposome combined with polylactide fracture internal fixator. [DOI] [PubMed] [Google Scholar]
- 36.Calle E.E., Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 37.Capasso R., Izzo A. Gastrointestinal regulation of food intake: general aspects and focus on anandamide and oleoylethanolamide. J. Neuroendocrinol. 2008;20:39–46. doi: 10.1111/j.1365-2826.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 38.Cazarolli L.H., Pereira D.F., Kappel V.D., Folador P., Figueiredo Mdos S., Pizzolatti M.G., Silva F.R. Insulin signaling: a potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur. J. Pharmacol. 2013;712:1–7. doi: 10.1016/j.ejphar.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Chang H.W., Liu P.F., Tsai W.L., Hu W.H., Hu Y.C., Yang H.C., Lin W.Y., Weng J.R., Shu C.W. Xanthium strumarium fruit extract inhibits ATG4B and diminishes the proliferation and metastatic characteristics of colorectal cancer cells. Toxins. 2019;11 doi: 10.3390/toxins11060313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang W., Li K., Guan F., Yao F., Yu Y., Zhang M., Hatch G.M., Chen L. Berberine pretreatment confers cardioprotection against ischemia-reperfusion injury in a rat model of type 2 diabetes. J. Cardiovasc. Pharmacol. Therapeut. 2016;21:486–494. doi: 10.1177/1074248415627873. [DOI] [PubMed] [Google Scholar]
- 41.Chang W., Zhang M., Li J., Meng Z., Xiao D., Wei S., Chen L., Wang C., Hatch G.M. Berberine attenuates ischemia-reperfusion injury via regulation of adenosine-5'-monophosphate kinase activity in both non-ischemic and ischemic areas of the rat heart. Cardiovasc. Drugs Ther. 2012;26:467–478. doi: 10.1007/s10557-012-6422-0. [DOI] [PubMed] [Google Scholar]
- 42.Chao I.-C., Chen Y., Gao M.-H., Lin L.-G., Zhang X.-Q., Ye W.-C., Zhang Q.-W. Simultaneous determination of α-glucosidase inhibitory triterpenoids in Psidium guajava using HPLC–DAD–ELSD and pressurized liquid extraction. Molecules. 2020;25:1278. doi: 10.3390/molecules25061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R.-C., Wang J., Yu Y.-L., Sun G.-B., Sun X.-B. Protective effect of total saponins of Aralia elata (Miq) Seem on lipopolysaccharide-induced cardiac dysfunction via down-regulation of inflammatory signaling in mice. RSC Adv. 2015;5:22560–22569. [Google Scholar]
- 44.Chen X.-Q., Lin L.-G., Zhao J., Chen L.-X., Tang Y.-P., Luo D.-L., Li S.-P. Isolation, structural elucidation, and α-glucosidase inhibitory activities of triterpenoid lactones and their relevant biogenetic constituents from Ganoderma resinaceum. Molecules. 2018;23:1391. doi: 10.3390/molecules23061391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi E., Baek S., Baek K., Kim H.-K. Psidium guajava L. leaf extract inhibits adipocyte differentiation and improves insulin sensitivity in 3T3-L1 cells. Nutr. Res. Prac. 2021;15:568–578. doi: 10.4162/nrp.2021.15.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu Y., Xiao Z., Jing N., Yan W., Wang S., Ma B., Zhang J., Li Y. Arborinine, a potential LSD1 inhibitor, inhibits epithelial-mesenchymal transition of SGC-7901 cells and adriamycin-resistant gastric cancer SGC-7901/ADR cells. Invest. N. Drugs. 2021;39:627–635. doi: 10.1007/s10637-020-01016-y. [DOI] [PubMed] [Google Scholar]
- 47.Cicolari S., Dacrema M., Tsetegho Sokeng A.J., Xiao J., Atchan Nwakiban A.P., Di Giovanni C., Santarcangelo C., Magni P., Daglia M. Hydromethanolic extracts from Adansonia digitata L. Edible parts positively modulate pathophysiological mechanisms related to the metabolic syndrome. Molecules. 2020;25:2858. doi: 10.3390/molecules25122858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claessen H., Brenner H., Drath C., Arndt V. Repeated measures of body mass index and risk of health related outcomes. Eur. J. Epidemiol. 2012;27:215–224. doi: 10.1007/s10654-012-9669-7. [DOI] [PubMed] [Google Scholar]
- 49.Clark M., Kroger C.J., Tisch R.M. Type 1 diabetes: a chronic anti-self-inflammatory response. Front. Immunol. 2017;8:1898. doi: 10.3389/fimmu.2017.01898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa-Lotufo L.V., Colepicolo P., Pupo M.T., Palma M.S. Bioprospecting macroalgae, marine and terrestrial invertebrates & their associated microbiota. Biota Neotropica. 2022;22 [Google Scholar]
- 51.Covassin N., Singh P., Mccrady-Spitzer S.K., St Louis E.K., Calvin A.D., Levine J.A., Somers V.K. Effects of experimental sleep restriction on energy intake, energy expenditure, and visceral obesity. J. Am. Coll. Cardiol. 2022;79:1254–1265. doi: 10.1016/j.jacc.2022.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daisy P., Saipriya K. International Journal of Nanomedicine. 2012. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darmadi J., Batubara R.R., Himawan S., Azizah N.N., Audah H.K., Arsianti A., Kurniawaty E., Ismail I.S., Batubara I., Audah K.A. Evaluation of Indonesian mangrove Xylocarpus granatum leaves ethyl acetate extract as potential anticancer drug. Sci. Rep. 2021;11:6080. doi: 10.1038/s41598-021-85383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das U.N. Metabolic syndrome X: an inflammatory condition? Curr. Hypertens. Rep. 2004;6:66–73. doi: 10.1007/s11906-004-0014-8. [DOI] [PubMed] [Google Scholar]
- 55.De Godoi R.S., Almerão M.P., Da Silva F.R. In silico evaluation of the antidiabetic activity of natural compounds from Hovenia dulcis Thunberg. J. Herb. Med. 2021;28 [Google Scholar]
- 56.Deacon C.F. Dipeptidyl peptidase‐4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metabol. 2011;13:7–18. doi: 10.1111/j.1463-1326.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 57.Defronzo R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. (ix) [DOI] [PubMed] [Google Scholar]
- 58.Denke M.A. Connections between obesity and dyslipidaemia. Curr. Opin. Lipidol. 2001;12:625–628. doi: 10.1097/00041433-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Després J.-P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 60.Dharani J., Ravi S. Isolation of sesquiterpene lactones and the antioxidant and anticancer activities of crude extracts from Cyanthillium cinereum. Chem. Nat. Compd. 2022;58:40–46. [Google Scholar]
- 61.Di Sano C., Lazzara V., Durante M., D'anna C., Bonura A., Dino P., Uasuf C.G., Pace E., Lenucci M.S., Bruno A. The protective anticancer effect of natural lycopene supercritical CO(2) watermelon extracts in adenocarcinoma lung cancer cells. Antioxidants. 2022;11 doi: 10.3390/antiox11061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding Y., Ren K., Dong H., Song F., Chen J., Guo Y., Liu Y., Tao W., Zhang Y. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017;275:210–217. doi: 10.1016/j.cbi.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Donoso-Quezada J., Guajardo-Flores D., González-Valdez J. Exosomes as nanocarriers for the delivery of bioactive compounds from black bean extract with antiproliferative activity in cancer cell lines. Mater. Today Proc. 2019 Elsevier Ltd. [Google Scholar]
- 64.Donoso-Quezada J., Guajardo-Flores D., González-Valdez J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomed. Pharmacother. 2020;131:110771. doi: 10.1016/j.biopha.2020.110771. [DOI] [PubMed] [Google Scholar]
- 65.Drevenšek G., Lunder M., Benković E.T., Štrukelj B., Kreft S. Cardioprotective effects of silver fir (Abies alba) extract in ischemic-reperfused isolated rat hearts. Food Nutr. Res. 2016;60 doi: 10.3402/fnr.v60.29623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drucker D.J. The biology of incretin hormones. Cell Metabol. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Dubey V., Mishra D., Dutta T., Nahar M., Saraf D.K., Jain N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control Release. 2007;123:148–154. doi: 10.1016/j.jconrel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Dulloo A.G., Montani J.P. Body composition, inflammation and thermogenesis in pathways to obesity and the metabolic syndrome: an overview. Obes. Rev. 2012;13(2):1–5. doi: 10.1111/j.1467-789X.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 69.Dwyer D.S., Pinkofsky H.B., Liu Y., Bradley R.J. Antipsychotic drugs affect glucose uptake and the expression of glucose transporters in PC12 cells. Progress Neuro-Psychopharmacol. Biol. Psychiatry. 1999;23:69–80. doi: 10.1016/s0278-5846(98)00092-x. [DOI] [PubMed] [Google Scholar]
- 70.Dzydzan O., Bila I., Kucharska A.Z., Brodyak I., Sybirna N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019;10:6459–6472. doi: 10.1039/c9fo00515c. [DOI] [PubMed] [Google Scholar]
- 71.Eghtedari M., Porzani S.J., Nowruzi B. Anticancer potential of natural peptides from terrestrial and marine environments: a review. Phytochem. Lett. 2021;42:87–103. [Google Scholar]
- 72.Eisenbarth G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 73.Estrada O., Di Giulio C., Dorta-Ledezma R., Gonzalez-Mujica F., Motta N., Zea E., Cupitra N., Contreras W., Narvaez-Sanchez R., Calderón J.C. A compound isolated from Phyllanthus tenellus demonstrates metabolic and vascular effects in vitro. Planta Med. 2020;86:78–84. doi: 10.1055/a-1019-9401. [DOI] [PubMed] [Google Scholar]
- 74.Fang Z.-J., Shen S.-N., Wang J.-M., Wu Y.-J., Zhou C.-X., Mo J.-X., Lin L.-G., Gan L.-S. Triterpenoids from Cyclocarya paliurus that enhance glucose uptake in 3T3-L1 adipocytes. Molecules. 2019;24:187. doi: 10.3390/molecules24010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng Z.L., Wu S.P., Li W.H., Guo T.T., Liu Q.C. Concise synthesis and antidiabetic effect of three natural triterpenoid saponins isolated from Fadogia ancylantha (Makoni tea) Helv. Chim. Acta. 2015;98:1254–1266. [Google Scholar]
- 76.Forster E.R., Green T., Elliot M., Bremner A., Dockray G.J. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am. J. Physiol. 1990;258:G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- 77.Frostegård J. SLE, atherosclerosis and cardiovascular disease. J. Intern. Med. 2005;257:485–495. doi: 10.1111/j.1365-2796.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 78.Fuchs F.D., Whelton P.K. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujimoto A., Hoteya S., Iizuka T., Ogawa O., Mitani T., Kuroki Y., Matsui A., Nakamura M., Kikuchi D., Yamashita S. Obesity and gastrointestinal diseases. Gastroenterol. Res. Practice. 2013;2013:760574. doi: 10.1155/2013/760574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gadde K.M., Martin C.K., Berthoud H.-R., Heymsfield S.B. Obesity: pathophysiology and management. J. Am. Coll. Cardiol. 2018;71:69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gade I.S., Chadeneau C., Richard T.S., Atchade A.T., Talla E., Seite P., Vannier B., Guillard J., Laurent S., Henoumont C., Nwabo A.H.K., Muller J.M. A new flavonoid glycoside from Tapinanthus sp. (Loranthaceae) and evaluation of anticancer activity of extract and some isolated compounds. Nat. Prod. Res. 2022;36:4085–4093. doi: 10.1080/14786419.2021.1963243. [DOI] [PubMed] [Google Scholar]
- 82.Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B., Ostolaza H., Martín C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao L., Hu Y., Hu D., Li Y., Yang S., Dong X., Alharbi S.A., Liu H. Anti-obesity activity of gold nanoparticles synthesized from Salacia chinensis modulates the biochemical alterations in high-fat diet-induced obese rat model via AMPK signaling pathway. Arab. J. Chem. 2020;13:6589–6597. [Google Scholar]
- 84.Gharib A., Faezizadeh Z., Godarzee M. Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms. Planta Med. 2013;79:1599–1604. doi: 10.1055/s-0033-1350908. [DOI] [PubMed] [Google Scholar]
- 85.Glagov S., Weisenberg E., Zarins C.K., Stankunavicius R., Kolettis G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 86.Guarino D., Nannipieri M., Iervasi G., Taddei S., Bruno R.M. The role of the autonomic nervous system in the pathophysiology of obesity. Front. Physiol. 2017;8:665. doi: 10.3389/fphys.2017.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guicciardi M.E., Gores G.J. Life and death by death receptors. Faseb. J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guo H., Luo H., Yuan H., Xia Y., Shu P., Huang X., Lu Y., Liu X., Keller E.T., Sun D., Deng J., Zhang J. Litchi seed extracts diminish prostate cancer progression via induction of apoptosis and attenuation of EMT through Akt/GSK-3β signaling. Sci. Rep. 2017;7 doi: 10.1038/srep41656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Habib S.H., Saha S.J.D., Research M.S.C. Burden of non-communicable disease: global overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2010;4:41–47. [Google Scholar]
- 90.Haffner S.M. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006;97:3a–11a. doi: 10.1016/j.amjcard.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 91.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., Patel T., Piroyan A., Sokolsky M., Kabanov A.V., Batrakova E.V. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasan F.M., Alsahli M., Gerich J.E. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res. Clin. Pract. 2014;104:297–322. doi: 10.1016/j.diabres.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 93.Hasan M., Ahmed Q.U., Soad S.Z.M., Latip J., Taher M., Syafiq T.M.F., Sarian M.N., Alhassan A.M., Zakaria Z.A. Flavonoids from Tetracera indica Merr. induce adipogenesis and exert glucose uptake activities in 3T3-L1 adipocyte cells. BMC Compl. Alternative Med. 2017;17:1–14. doi: 10.1186/s12906-017-1929-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He H., Lu Y., Qi J., Zhao W., Dong X., Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm. Sin. B. 2018;8:97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hecht, S.S.J.N.R.C. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 96.Hegazy M.G., Imam A.M., Abdelghany B.E. Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumour Biol. 2020;42 doi: 10.1177/1010428320918685. [DOI] [PubMed] [Google Scholar]
- 97.Hernández-Ojeda J., Cardona-Muñoz E.G., Román-Pintos L.M., Troyo-Sanromán R., Ortiz-Lazareno P.C., Cárdenas-Meza M.A., Pascoe-González S., Miranda-Díaz A.G. The effect of ubiquinone in diabetic polyneuropathy: a randomized double-blind placebo-controlled study. J. Diabetes Complicat. 2012;26:352–358. doi: 10.1016/j.jdiacomp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Hientz K., Mohr A., Bhakta-Guha D., Efferth T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget. 2017;8:8921. doi: 10.18632/oncotarget.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hinney A., Volckmar A.L., Knoll N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog. Mol. Biol. Transl. Sci. 2013;114:147–191. doi: 10.1016/B978-0-12-386933-3.00005-4. [DOI] [PubMed] [Google Scholar]
- 100.Hodaei H., Adibian M., Nikpayam O., Hedayati M., Sohrab G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol. Metab. Syndrome. 2019;11:1–8. doi: 10.1186/s13098-019-0437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hollander P.A., Levy P., Fineman M.S., Maggs D.G., Shen L.Z., Strobel S.A., Weyer C., Kolterman O.G. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- 102.Holmbäck U., Grudén S., Litorp H., Willhems D., Kuusk S., Alderborn G., Söderhäll A., Forslund A. Effects of a novel weight‐loss combination product containing orlistat and acarbose on obesity: a randomized, placebo‐controlled trial. Obesity. 2022;30:2222–2232. doi: 10.1002/oby.23557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 104.Hotamisligil G.S., Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Howard S.G. Developmental exposure to endocrine disrupting chemicals and type 1 diabetes mellitus. Front. Endocrinol. 2018;9:513. doi: 10.3389/fendo.2018.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsu F.L., Huang C.F., Chen Y.W., Yen Y.P., Wu C.T., Uang B.J., Yang R.S., Liu S.H. Antidiabetic effects of pterosin A, a small-molecular-weight natural product, on diabetic mouse models. Diabetes. 2013;62:628–638. doi: 10.2337/db12-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang X.F., Chang K.F., Lee S.C., Li C.Y., Liao H.H., Hsieh M.C., Tsai N.M. Extract of juniperus indica bertol synergizes with cisplatin to inhibit oral cancer cell growth via repression of cell cycle progression and activation of the caspase cascade. Molecules. 2020;25 doi: 10.3390/molecules25122746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.IDF . 2015. "Diabetes Atlas ". (Brussels, Belgium: International Diabetes Federation) [Google Scholar]
- 109.Ighodaro O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 110.Ikramuddin S., Blackstone R.P., Brancatisano A., Toouli J., Shah S.N., Wolfe B.M., Fujioka K., Maher J.W., Swain J., Que F.G. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915–922. doi: 10.1001/jama.2014.10540. [DOI] [PubMed] [Google Scholar]
- 111.Imes C.C., Lewis F.M. Family history of cardiovascular disease (CVD), perceived CVD risk, and health-related behavior: a review of the literature. J. Cardiovasc. Nurs. 2014;29:108. doi: 10.1097/JCN.0b013e31827db5eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inkster B., Zammitt N.N., Frier B.M. Drug-induced hypoglycaemia in type 2 diabetes. Expet Opin. Drug Saf. 2012;11:597–614. doi: 10.1517/14740338.2012.694424. [DOI] [PubMed] [Google Scholar]
- 113.Islam N., Ayele H.T., Yu O.H.Y., Douros A., Filion K.B. Sulfonylureas and the risk of ventricular arrhythmias among people with type 2 diabetes: a systematic review of observational studies. Clin. Pharmacol. Ther. 2022;111:1248–1257. doi: 10.1002/cpt.2570. [DOI] [PubMed] [Google Scholar]
- 114.Jacobsen R., Frederiksen P., Heitmann B.L. Exposure to sunshine early in life prevented development of type 1 diabetes in Danish boys. J. Pediatr. Endocrinol. Metabol. 2016;29:417–424. doi: 10.1515/jpem-2015-0393. [DOI] [PubMed] [Google Scholar]
- 115.Jaidane H., Hober D. Role of coxsackievirus B4 in the pathogenesis of type 1 diabetes. Diabetes Metabol. 2008;34:537–548. doi: 10.1016/j.diabet.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 116.Jain A., Jain S.K. In vitro release kinetics model fitting of liposomes: an insight. Chem. Phys. Lipids. 2016;201:28–40. doi: 10.1016/j.chemphyslip.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 117.Jain C., Bilekova S., Lickert H. Targeting pancreatic β cells for diabetes treatment. Nature Metabol. 2022:1–12. doi: 10.1038/s42255-022-00618-5. [DOI] [PubMed] [Google Scholar]
- 118.James W.P.T., Gill T. Obesity–introduction: history and the scale of the problem worldwide. Clinical Obesity Adults Children. 2022:1–16. [Google Scholar]
- 119.Jannathul Firdhouse M., Lalitha P. Biogenic green synthesis of gold nanoparticles and their applications – a review of promising properties. Inorg. Chem. Commun. 2022;143:109800. [Google Scholar]
- 120.Jeong S.Y., Nguyen P.H., Zhao B.T., Ali M.Y., Choi J.S., Min B.S., Woo M.H. Chemical constituents of Euonymus alatus (Thunb.) Sieb. and their PTP1B and α‐glucosidase inhibitory activities. Phytother Res. 2015;29:1540–1548. doi: 10.1002/ptr.5411. [DOI] [PubMed] [Google Scholar]
- 121.Jerkins T., Bell D.S.H. Development of exogenous insulin antibody syndrome in a patient with newly diagnosed type 1 diabetes successfully treated with oral immunosuppressive monotherapy. Diabetes Therapy. 2021;12:2795–2799. doi: 10.1007/s13300-021-01129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jespersen L., Hvelplund A., Abildstrøm S.Z., Pedersen F., Galatius S., Madsen J.K., Jørgensen E., Kelbæk H., Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 123.Jiang X.C., Gao J.Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017;521:167–175. doi: 10.1016/j.ijpharm.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 124.John O.D., Mouatt P., Majzoub M.E., Thomas T., Panchal S.K., Brown L. Physiological and metabolic effects of yellow mangosteen (Garcinia dulcis) rind in rats with diet-induced metabolic syndrome. Int. J. Mol. Sci. 2019;21:272. doi: 10.3390/ijms21010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ju Ho P., Jun Sung J., Ki Cheon K., Jin Tae H. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine. 2018;43:110–119. doi: 10.1016/j.phymed.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 126.Jung I.L. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaminsky L.A., German C., Imboden M., Ozemek C., Peterman J.E., Brubaker P.H.J.P.I.C.D. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2021;70:8–15. doi: 10.1016/j.pcad.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 128.Kannel W.B., Vasan R.S. Is age really a non-modifiable cardiovascular risk factor? Am. J. Cardiol. 2009;104:1307–1310. doi: 10.1016/j.amjcard.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Karam B.S., Chavez-Moreno A., Koh W., Akar J.G., Akar F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017;16:120. doi: 10.1186/s12933-017-0604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karra P., Winn M., Pauleck S., Bulsiewicz-Jacobsen A., Peterson L., Coletta A., Doherty J., Ulrich C.M., Summers S.A., Gunter M., Hardikar S., Playdon M.C. Metabolic dysfunction and obesity-related cancer: beyond obesity and metabolic syndrome. Obesity. 2022;30:1323–1334. doi: 10.1002/oby.23444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kashtoh H., Baek K.-H. Recent updates on phytoconstituent alpha-glucosidase inhibitors: an approach towards the treatment of type two diabetes. Plants. 2022;11:2722. doi: 10.3390/plants11202722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katsarou A., Gudbjörnsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J., Jacobsen L.M., Schatz D.A., Lernmark Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Prim. 2017;3:1–17. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 133.Kawamura K., Kadowaki N., Suzuki R., Udagawa S., Kasaoka S., Utoguchi N., Kitawaki T., Sugimoto N., Okada N., Maruyama K., Uchiyama T. Dendritic cells that endocytosed antigen-containing IgG-liposomes elicit effective antitumor immunity. J. Immunother. 2006;29:165–174. doi: 10.1097/01.cji.0000190169.61416.f5. [DOI] [PubMed] [Google Scholar]
- 134.Keihanian F., Moohebati M., Saeidinia A., Mohajeri S.A., Madaeni S. Therapeutic effects of medicinal plants on isoproterenol-induced heart failure in rats. Biomed. Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111101. [DOI] [PubMed] [Google Scholar]
- 135.Khaltaev N., Axelrod S.J.C.D. Countrywide cardiovascular disease prevention and control in 49 countries with different socio‐economic status. Chronic Dis. Transl. Med. 2022;8:296–304. doi: 10.1002/cdt3.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kharey P., Goel M., Husain Z., Gupta R., Sharma D.M.M., Palani I.A., Gupta S. Green synthesis of biocompatible superparamagnetic iron oxide-gold composite nanoparticles for magnetic resonance imaging, hyperthermia and photothermal therapeutic applications. Mater. Chem. Phys. 2023;293:126859. [Google Scholar]
- 137.Kieffer T.J. Gastro-intestinal hormones GIP and GLP-1. Ann. Endocrinol. 2004;65:13–21. doi: 10.1016/s0003-4266(04)95625-9. [DOI] [PubMed] [Google Scholar]
- 138.Kim M., Lee C., Park J. Extracellular matrix remodeling facilitates obesity-associated cancer progression. Trends Cell. Biol. 2022;32:825–834. doi: 10.1016/j.tcb.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 139.Kim S.H., Hur H.J., Yang H.J., Kim H.J., Kim M.J., Park J.H., Sung M.J., Kim M.S., Kwon D.Y., Hwang J.-T. Citrus junos tanaka peel extract exerts antidiabetic effects via AMPK and PPAR-both in vitro and in vivo in mice fed a high-fat diet. Evid. base Compl. Alternative Med. 2013;2013:921012. doi: 10.1155/2013/921012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kjeldsen S.E. Hypertension and cardiovascular risk: general aspects. Pharmacol. Res. 2018;129:95–99. doi: 10.1016/j.phrs.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 141.Kleinert M., Clemmensen C., Hofmann S.M., Moore M.C., Renner S., Woods S.C., Huypens P., Beckers J., De Angelis M.H., Schürmann A. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018;14:140–162. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 142.Klöting N., Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014;15:277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 143.Koh H.-C.E., Cao C., Mittendorfer B. Insulin clearance in obesity and type 2 diabetes. Int. J. Mol. Sci. 2022;23:596. doi: 10.3390/ijms23020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kolodgie F.D., Gold H.K., Burke A.P., Fowler D.R., Kruth H.S., Weber D.K., Farb A., Guerrero L.J., Hayase M., Kutys R., Narula J., Finn A.V., Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 145.Kondo T., Nakano Y., Adachi S., Murohara T. Effects of tobacco smoking on cardiovascular disease. Circ. J. 2019;83:1980–1985. doi: 10.1253/circj.CJ-19-0323. [DOI] [PubMed] [Google Scholar]
- 146.Kudo M., Morimoto M., Moriguchi M., Izumi N., Takayama T., Yoshiji H., Hino K., Oikawa T., Chiba T., Motomura K. A randomized, double‐blind, placebo‐controlled, phase 3 study of tivantinib in Japanese patients with MET‐high hepatocellular carcinoma. Cancer Sci. 2020;111:3759–3769. doi: 10.1111/cas.14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumar S., Jahangir Alam M., Prabhakar P., Ahmad S., Maulik S.K., Sharma M., Goswami S.K. Proteomic analysis of the protective effects of aqueous bark extract of Terminalia arjuna (Roxb.) on isoproterenol-induced cardiac hypertrophy in rats. J. Ethnopharmacol. 2017;198:98–108. doi: 10.1016/j.jep.2016.12.050. [DOI] [PubMed] [Google Scholar]
- 148.Kumari R., Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Laranja S.M., Bergamaschi C.M., Schor N. Evaluation of acute administration of natural products with potential diuretic effects, in humans. Mem. Inst. Oswaldo Cruz. 1991;86:237–240. doi: 10.1590/s0074-02761991000600053. [DOI] [PubMed] [Google Scholar]
- 150.Lee D., Hwang B.S., Choi P., Kim T., Kim Y., Song B.G., Yamabe N., Hwang G.S., Kang K.S., Ham J. Hypoxylonol F isolated from Annulohypoxylon annulatum improves insulin secretion by regulating pancreatic β-cell metabolism. Biomolecules. 2019;9:335. doi: 10.3390/biom9080335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee R.T., Libby P. The unstable atheroma. Arterioscler. Thromb. Vasc. Biol. 1997;17:1859–1867. doi: 10.1161/01.atv.17.10.1859. [DOI] [PubMed] [Google Scholar]
- 152.Lee Y.S., Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35:307–328. doi: 10.1101/gad.346312.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lehnert T., Sonntag D., Konnopka A., Riedel-Heller S., König H.-H. Economic costs of overweight and obesity. Best Pract. Res. Clin. Endocrinol. Metabol. 2013;27:105–115. doi: 10.1016/j.beem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Li D., Yao S., Zhou Z., Shi J., Huang Z., Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydrate Res. 2020;493:108032. doi: 10.1016/j.carres.2020.108032. [DOI] [PubMed] [Google Scholar]
- 155.Li G., Wang G., Tong Y., Zhu J., Yun T., Ye X., Li F., Yuan S., Liu Q. Concise synthesis and antidiabetic activity of natural flavonoid glycosides, oroxins C and D, isolated from the seeds of Oroxylum indium. J. Chem. Res. 2020;45:68–75. [Google Scholar]
- 156.Li X.-L., Zhou J., Chen Z.-R., Chng W.-J. P53 mutations in colorectal cancer-molecular pathogenesis and pharmacological reactivation. World J. Gastroenterol. 2015;21:84. doi: 10.3748/wjg.v21.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lim H.K., Bae W., Lee H.S., Jung J. Anticancer activity of marine sponge Hyrtios sp. extract in human colorectal carcinoma RKO cells with different p53 status. BioMed Res. Int. 2014 doi: 10.1155/2014/413575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lim S., Kim J.W., Targher G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metabol. 2021;32:500–514. doi: 10.1016/j.tem.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 159.Liu J., Hao D., Guo Z., Yu L., Li T., Mei K., Li X., Chen J., Wu Q. Multi-unit pellet drug delivery system of Danggui Decoction extracts for chemoprevention of IBD-associated colorectal cancer in rats. J. Drug Deliv. Sci. Technol. 2022;77:103884. [Google Scholar]
- 160.Liu N., Yang H.L., Wang P., Lu Y.C., Yang Y.J., Wang L., Lee S.C. Functional proteomic analysis revels that the ethanol extract of Annona muricata L. induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J. Ethnopharmacol. 2016;189:210–217. doi: 10.1016/j.jep.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 161.Lomenick J.P., Buchowski M.S., Shoemaker A.H. A 52‐week pilot study of the effects of exenatide on body weight in patients with hypothalamic obesity. Obesity. 2016;24:1222–1225. doi: 10.1002/oby.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lopes Galeno D.M., Carvalho R.P., Boleti A.P., Lima A.S., Oliveira De Almeida P.D., Pacheco C.C., Pereira De Souza T., Lima E.S. Extract from Eugenia punicifolia is an antioxidant and inhibits enzymes related to metabolic syndrome. Appl. Biochem. Biotechnol. 2014;172:311–324. doi: 10.1007/s12010-013-0520-8. [DOI] [PubMed] [Google Scholar]
- 163.López-Contreras A.K., Martínez-Ruiz M.G., Olvera-Montaño C., Robles-Rivera R.R., Arévalo-Simental D.E., Castellanos-González J.A., Hernández-Chávez A., Huerta-Olvera S.G., Cardona-Muñoz E.G., Rodríguez-Carrizalez A.D. Importance of the use of oxidative stress biomarkers and inflammatory profile in aqueous and vitreous humor in diabetic retinopathy. Antioxidants. 2020;9:891. doi: 10.3390/antiox9090891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 166.Macmurdo M., Lopez R., Udeh B.L., Zein J. Beyond tobacco–the secondary impact of substance misuse in chronic obstructive lung disease. J. Asthma. 2022;59:223–229. doi: 10.1080/02770903.2020.1847932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Madariaga-Mazón A., Naveja J.J., Medina-Franco J.L., Noriega-Colima K.O., Martinez-Mayorga K. DiaNat-DB: a molecular database of antidiabetic compounds from medicinal plants. RSC Adv. 2021;11:5172–5178. doi: 10.1039/d0ra10453a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mahindrakar K.V., Rathod V.K. Antidiabetic potential evaluation of aqueous extract of waste Syzygium cumini seed kernel’s by in vitro α-amylase and α-glucosidase inhibition. Prep. Biochem. Biotechnol. 2021;51:589–598. doi: 10.1080/10826068.2020.1839908. [DOI] [PubMed] [Google Scholar]
- 169.Mahmoud A.M., Ashour M.B., Abdel-Moneim A., Ahmed O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012;26:483–490. doi: 10.1016/j.jdiacomp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 170.Makinde E.A., Radenahmad N., Adekoya A.E., Olatunji O.J. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin‐induced diabetes in rats. J. Food Biochem. 2020;44 doi: 10.1111/jfbc.13239. [DOI] [PubMed] [Google Scholar]
- 171.Mao C.F., Zhang X.R., Johnson A., He J.L., Kong Z.L. Modulation of diabetes mellitus-induced male rat reproductive dysfunction with micro-nanoencapsulated Echinacea purpurea ethanol extract. Biomed Res. Int. 2018;2018:4237354. doi: 10.1155/2018/4237354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mao H.P., Wang X.Y., Gao Y.H., Chang Y.X., Chen L., Niu Z.C., Ai J.Q., Gao X.M. Danhong injection attenuates isoproterenol-induced cardiac hypertrophy by regulating p38 and NF-κb pathway. J. Ethnopharmacol. 2016;186:20–29. doi: 10.1016/j.jep.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 173.Mathew H., Farr O.M., Mantzoros C.S. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65:73–80. doi: 10.1016/j.metabol.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Matsumoto C., Koike A., Tanaka R., Fujimori K. A limonoid, 7-deacetoxy-7-oxogedunin (CG-1) from andiroba (Carapa guianensis, Meliaceae) lowers the accumulation of intracellular lipids in adipocytes via suppression of IRS-1/Akt-mediated glucose uptake and a decrease in GLUT4 expression. Molecules. 2019;24:1668. doi: 10.3390/molecules24091668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mehanna E.T., El-Sayed N.M., Ibrahim A.K., Ahmed S.A., Abo-Elmatty D.M. Isolated compounds from Cuscuta pedicellata ameliorate oxidative stress and upregulate expression of some energy regulatory genes in high fat diet induced obesity in rats. Biomed. Pharmacother. 2018;108:1253–1258. doi: 10.1016/j.biopha.2018.09.126. [DOI] [PubMed] [Google Scholar]
- 176.Micheli L., Lucarini E., Trallori E., Avagliano C., De Caro C., Russo R., Calignano A., Ghelardini C., Pacini A., Di Cesare Mannelli L. Phaseolus vulgaris L. extract: alpha-amylase inhibition against metabolic syndrome in mice. Nutrients. 2019;11:1778. doi: 10.3390/nu11081778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Misganaw, A., Mariam, D.H., Ali, A., Araya, T.J.J.O.H. Epidemiology of major non-communicable diseases in Ethiopia: a systematic review. J. Health Popul. Nutr. 2014;32:1. [PMC free article] [PubMed] [Google Scholar]
- 178.Mohammadnezhad M., Mangum T., May W., Lucas J.J., Ailson S. Common modifiable and non-modifiable risk factors of cardiovascular disease (CVD) among pacific countries. World J. Cardiovasc. Surg. 2016;6:153. [Google Scholar]
- 179.Monteiro R., Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010;2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Musdja M.Y., Mahendra F., Musir A. IOP Conference Series: Earth and Environmental Science. IOP Publishing); 2017. Anti-hyperglycemic effect and glucose tolerance of guajava (Psidium guajava L.) leaf ethanol extract in diabetic rats. [Google Scholar]
- 181.Nadaf S.J., Jadhav N.R., Naikwadi H.S., Savekar P.L., Sapkal I.D., Kambli M.M., Desai I.A. Green synthesis of gold and silver nanoparticles: updates on research, patents, and future prospects. OpenNano. 2022;8:100076. [Google Scholar]
- 182.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nurul Islam M., Jung H.A., Sohn H.S., Kim H.M., Choi J.S. Potent α-glucosidase and protein tyrosine phosphatase 1B inhibitors from Artemisia capillaris. Arch Pharm. Res. (Seoul) 2013;36:542–552. doi: 10.1007/s12272-013-0069-7. [DOI] [PubMed] [Google Scholar]
- 184.Olvera-Montano C., Castellanos-Gonzalez J.A., Navarro-Partida J., Cardona-Munoz E.G., Lopez-Contreras A.K., Roman-Pintos L.M., Robles-Rivera R.R., Rodriguez-Carrizalez A.D. Oxidative stress as the main target in diabetic retinopathy pathophysiology. J. Diabetes Res. 2019;2019:8562408. doi: 10.1155/2019/8562408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ortega‐Loubon C., Fernández‐Molina M., Singh G., Correa R. Obesity and its cardiovascular effects. Diabetes/Metabolism Res. Rev. 2019;35:e3135. doi: 10.1002/dmrr.3135. [DOI] [PubMed] [Google Scholar]
- 186.Ozaki T., Nakagawara A. Role of p53 in cell death and human cancers. Cancers. 2011;3:994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park J., Jang H.-J. Anti-diabetic effects of natural products an overview of therapeutic strategies. Mol. Cellular Toxicol. 2017;13:1–20. [Google Scholar]
- 188.Parthiban A., Sachithanandam V., Sarangapany S., Misra R., Muthukrishnan P., Jeyakumar T.C., Purvaja R., Ramesh R. Green synthesis of gold nanoparticles using quercetin biomolecule from mangrove plant, Ceriops tagal: assessment of antiproliferative properties, cellular uptake and DFT studies. J. Mol. Struct. 2023;1272:134167. [Google Scholar]
- 189.Patridge E., Gareiss P., Kinch M.S., Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov. Today. 2016;21:204–207. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 190.Paudel K.S., Milewski M., Swadley C.L., Brogden N.K., Ghosh P., Stinchcomb A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010;1:109–131. doi: 10.4155/tde.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pellecchia M., Reed J.C. Inhibition of anti-apoptotic Bcl-2 family proteins by natural polyphenols new avenues for cancer chemoprevention and chemotherapy. Curr. Pharmaceut. Des. 2004;10:1387–1398. doi: 10.2174/1381612043384880. [DOI] [PubMed] [Google Scholar]
- 192.Penson P.E., Banach M. Natural compounds as anti-atherogenic agents: clinical evidence for improved cardiovascular outcomes. Atherosclerosis. 2021;316:58–65. doi: 10.1016/j.atherosclerosis.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 193.Permana A.D., Anjani Q.K., Sartini Utomo E., Volpe-Zanutto F., Paredes A.J., Evary Y.M., Mardikasari S.A., Pratama M.R., Tuany I.N., Donnelly R.F. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C. 2021;120:111786. doi: 10.1016/j.msec.2020.111786. [DOI] [PubMed] [Google Scholar]
- 194.Permana A.D., Nainu F., Moffatt K., Larrañeta E., Donnelly R.F. Recent advances in combination of microneedles and nanomedicines for lymphatic targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021;13:e1690. doi: 10.1002/wnan.1690. [DOI] [PubMed] [Google Scholar]
- 195.Permana A.D., Paredes A.J., Volpe-Zanutto F., Anjani Q.K., Utomo E., Donnelly R.F. Dissolving microneedle-mediated dermal delivery of itraconazole nanocrystals for improved treatment of cutaneous candidiasis. Eur. J. Pharm. Biopharm. 2020;154:50–61. doi: 10.1016/j.ejpb.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 196.Permana A.D., Paredes A.J., Zanutto F.V., Amir M.N., Ismail I., Bahar M.A., Sumarheni Palma S.D., Donnelly R.F. Albendazole nanocrystal-based dissolving microneedles with improved pharmacokinetic performance for enhanced treatment of cystic echinococcosis. ACS Appl. Mater. Interfaces. 2021;13:38745–38760. doi: 10.1021/acsami.1c11179. [DOI] [PubMed] [Google Scholar]
- 197.Permana A.D., Utami R.N., Courtenay A.J., Manggau M.A., Donnelly R.F., Rahman L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis: an approach for enhanced both dissolution behaviour in biorelevant media and skin retention profiles. J. Photochem. Photobiol. B Biol. 2020;205:111846. doi: 10.1016/j.jphotobiol.2020.111846. [DOI] [PubMed] [Google Scholar]
- 198.Perret-Guillaume C., Joly L., Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog. Cardiovasc. Dis. 2009;52:6–10. doi: 10.1016/j.pcad.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 199.Pflaum J., Schlosser S., Müller M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Philippe J., Raccah D. Treating type 2 diabetes: how safe are current therapeutic agents? Int. J. Clin. Pract. 2009;63:321–332. doi: 10.1111/j.1742-1241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 201.Picchi A., Gao X., Belmadani S., Potter B.J., Focardi M., Chilian W.M., Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 202.Pigeyre M., Yazdi F.T., Kaur Y., Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. 2016;130:943–986. doi: 10.1042/CS20160136. [DOI] [PubMed] [Google Scholar]
- 203.Porrang S., Rahemi N., Davaran S., Mahdavi M., Hassanzadeh B. Preparation and in-vitro evaluation of mesoporous biogenic silica nanoparticles obtained from rice and wheat husk as a biocompatible carrier for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2021;163:105866. doi: 10.1016/j.ejps.2021.105866. [DOI] [PubMed] [Google Scholar]
- 204.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr. Rev. 2007;65:S152–S156. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 205.Pradhan R., Yin H., Yu O., Azoulay L. Glucagon-like peptide 1 receptor agonists and sodium–glucose cotransporter 2 inhibitors and risk of nonalcoholic fatty liver disease among patients with type 2 diabetes. Diabetes Care. 2022;45:819–829. doi: 10.2337/dc21-1953. [DOI] [PubMed] [Google Scholar]
- 206.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pratt W.B. Oxford University Press; USA: 1994. The Anticancer Drugs. [Google Scholar]
- 208.Prema P., Boobalan T., Arun A., Rameshkumar K., Suresh Babu R., Veeramanikandan V., Nguyen V.H., Balaji P. Green tea extract mediated biogenic synthesis of gold nanoparticles with potent anti-proliferative effect against PC-3 human prostate cancer cells. Mater. Lett. 2022;306:130882. [Google Scholar]
- 209.Qi J., Tan Y., Fan D., Pan W., Yu J., Xu W., Wu J., Zhang M. Songling Xuemaikang Capsule inhibits isoproterenol-induced cardiac hypertrophy via CaMKIIδ and ERK1/2 pathways. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112660. [DOI] [PubMed] [Google Scholar]
- 210.Qi Y., Li J., Nie Q., Gao M., Yang Q., Li Z., Li Q., Han S., Ding J., Li Y., Zhang J. Polyphenol-assisted facile assembly of bioactive nanoparticles for targeted therapy of heart diseases. Biomaterials. 2021;275:120952. doi: 10.1016/j.biomaterials.2021.120952. [DOI] [PubMed] [Google Scholar]
- 211.Qian S., Wang S., Fan P., Huo D., Dai L., Qian Q. Effect of Salvia miltiorrhiza hydrophilic extract on the endothelial biomarkers in diabetic patients with chronic artery disease. Phytother Res. 2012;26:1575–1578. doi: 10.1002/ptr.4611. [DOI] [PubMed] [Google Scholar]
- 212.Rahman M., Uddin M., Babteen N.A., Alnajeebi A.M., Zakaria Z.A., Aboelenin S.M. Natural compounds from hatikana extract potentiate antidiabetic actions as displayed by in vivo assays and verified by network pharmacological tools. BioMed Res. Int. 2021;2021:6978450. doi: 10.1155/2021/6978450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ramprasath V.R., Jones P.J.H. Anti-atherogenic effects of resveratrol. Eur. J. Clin. Nutr. 2010;64:660–668. doi: 10.1038/ejcn.2010.77. [DOI] [PubMed] [Google Scholar]
- 214.Reilly M.P., Rader D.J. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 215.Riccioni, G., Gammone, M.A., Tettamanti, G., Bergante, S., Pluchinotta, F.R., D’orazio, N.J.I.J.O.F.S. Resveratrol and anti-atherogenic effects. Nutrition. 2015;66:603–610. doi: 10.3109/09637486.2015.1077796. [DOI] [PubMed] [Google Scholar]
- 216.Riyaphan J., Pham D.-C., Leong M.K., Weng C.-F. Silico approaches to identify polyphenol compounds as α-glucosidase and α-amylase inhibitors against type-II diabetes. Biomolecules. 2021;11:1877. doi: 10.3390/biom11121877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Robles-Rivera R.R., Castellanos-González J.A., Olvera-Montaño C., Flores-Martin R.A., López-Contreras A.K., Arevalo-Simental D.E., Cardona-Muñoz E.G., Roman-Pintos L.M., Rodríguez-Carrizalez A.D. Adjuvant therapies in diabetic retinopathy as an early approach to delay its progression: the importance of oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2020;2020:3096470. doi: 10.1155/2020/3096470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Romero-Corral A., Montori V.M., Somers V.K., Korinek J., Thomas R.J., Allison T.G., Mookadam F., Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 219.Ryan K.M., Phillips A.C., Vousden K.H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 2001;13:332–337. doi: 10.1016/s0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 220.Saadeldeen F.S.A., Niu Y., Wang H., Zhou L., Meng L., Chen S., Sun-Waterhouse D., Waterhouse G.I.N., Liu Z., Kang W. Natural products: regulating glucose metabolism and improving insulin resistance. Food Sci. Hum. Wellness. 2020;9:214–228. [Google Scholar]
- 221.Sacha M., Faucon L., Hamon E., Ly I., Haltner-Ukomadu E. Ex vivo transdermal absorption of a liposome formulation of diclofenac. Biomed. Pharmacother. 2019;111:785–790. doi: 10.1016/j.biopha.2018.12.079. [DOI] [PubMed] [Google Scholar]
- 222.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., Colagiuri S., Guariguata L., Motala A.A., Ogurtsova K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res. Clin. Pract. 2019;157 doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 223.Salles B.C.C., Da Silva M.A., Taniguthi L., Ferreira J.N., Da Rocha C.Q., Vilegas W., Dias P.H., Pennacchi P.C., Da Silveira Duarte S.M., Rodrigues M.R. Passiflora edulis leaf extract: evidence of antidiabetic and antiplatelet effects in rats. Biol. Pharm. Bull. 2020;43:169–174. doi: 10.1248/bpb.b18-00952. [DOI] [PubMed] [Google Scholar]
- 224.Salmon J.W. 2022. Alternative Medicines: Popular and Policy Perspectives. Taylor & Francis. [Google Scholar]
- 225.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sankhari J.M., Thounaojam M.C., Jadeja R.N., Devkar R.V., Ramachandran A.V. Anthocyanin-rich red cabbage (Brassica oleracea L.) extract attenuates cardiac and hepatic oxidative stress in rats fed an atherogenic diet. J. Sci. Food Agric. 2012;92:1688–1693. doi: 10.1002/jsfa.5532. [DOI] [PubMed] [Google Scholar]
- 227.Santamaria F., Montella S., Pietrobelli A. Obesity and pulmonary disease: unanswered questions. Obes. Rev. 2012;13:822–833. doi: 10.1111/j.1467-789X.2012.01008.x. [DOI] [PubMed] [Google Scholar]
- 228.Saravane D., Feve B., Frances Y., Corruble E., Lancon C., Chanson P., Maison P., Terra J.L., Azorin J.M. Drawing up guidelines for the attendance of physical health of patients with severe mental illness. L'encephale. 2009;35:330–339. doi: 10.1016/j.encep.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 229.Sarry El Din A., Hassan N., El-Masry S., Al-Tohamy M. Neck circumference as a simple screening measure for identifying Egyptian overweight and obese adults. Macedonian J. Med. Sci. 2013;6:232–237. [Google Scholar]
- 230.Sathya A., Siddhuraju P. Role of phenolics as antioxidants, biomolecule protectors and as anti–diabetic factors–Evaluation on bark and empty pods of Acacia auriculiformis. Asian Pac. J. Tropical Med. 2012;5:757–765. doi: 10.1016/S1995-7645(12)60139-4. [DOI] [PubMed] [Google Scholar]
- 231.Sattar N., Gill J.M. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. doi: 10.1186/s12916-014-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schernthaner G., Brand K., Bailey C.J. Metformin and the heart: update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism. 2022;130:155160. doi: 10.1016/j.metabol.2022.155160. [DOI] [PubMed] [Google Scholar]
- 233.Seong S.H., Nguyen D.H., Wagle A., Woo M.H., Jung H.A., Choi J.S. Experimental and computational study to reveal the potential of non-polar constituents from hizikia fusiformis as dual protein tyrosine phosphatase 1B and α-glucosidase inhibitors. Mar. Drugs. 2019;17:302. doi: 10.3390/md17050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Seravalle G., Grassi G. Obesity and hypertension. Pharmacol. Res. 2017;122:1–7. doi: 10.1016/j.phrs.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 235.Shaish A., Harari A., Hananshvili L., Cohen H., Bitzur R., Luvish T., Ulman E., Golan M., Ben-Amotz A., Gavish D. 9-cis β-carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis. 2006;189:215–221. doi: 10.1016/j.atherosclerosis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 236.Shakeri R., Kheirollahi A., Davoodi J. Apaf-1: regulation and function in cell death. Biochimie. 2017;135:111–125. doi: 10.1016/j.biochi.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 237.Shanmugam P. Green route synthesis of alpinia calcarata functionalized gold nanoparticles for nonlinear optical applications. Heliyon. 2022;8:e10409. doi: 10.1016/j.heliyon.2022.e10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sharma P. Inflammation and the metabolic syndrome. Indian J. Clin. Biochem. 2011;26:317–318. doi: 10.1007/s12291-011-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sharma V.K., Singh T.G., Dhiman S., Garg N. Probiotic Research in Therapeutics. Springer; 2022. Mechanisms of beneficial effects of probiotics in diabetes mellitus; pp. 97–124. [Google Scholar]
- 240.Shehzad A., Ha T., Subhan F., Lee Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011;50:151–161. doi: 10.1007/s00394-011-0188-1. [DOI] [PubMed] [Google Scholar]
- 241.Shin M., Yang S., Kwak H.W., Lee K.H. Synthesis of gold nanoparticles using silk sericin as a green reducing and capping agent. Eur. Polym. J. 2022;164:110960. [Google Scholar]
- 242.Shukla, S.K., Gupta, S., Ojha, S.K., and Sharma, S.B.J.N.P.R. Cardiovascular friendly natural products: a promising approach in the management of CVD. Nat. Prod. Res. 2010;24:873–898. doi: 10.1080/14786410903417378. [DOI] [PubMed] [Google Scholar]
- 243.Sierra-Cruz M., Miguéns-Gómez A., Grau-Bové C., Rodríguez-Gallego E., Blay M., Pinent M., Ardévol A., Terra X., Beltrán-Debón R. Grape-seed proanthocyanidin extract reverts obesity-related metabolic derangements in aged female rats. Nutrients. 2021;13:2059. doi: 10.3390/nu13062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Singh B.K., Pillai K.K., Kohli K., Haque S.E. Effect of Cissampelos pareira root extract on isoproterenol-induced cardiac dysfunction. J. Nat. Med. 2013;67:51–60. doi: 10.1007/s11418-012-0643-1. [DOI] [PubMed] [Google Scholar]
- 245.Skała E., Synowiec E., Kowalczyk T., Śliwiński T., Sitarek P. Rhaponticum carthamoides transformed root extract has potent anticancer activity in human leukemia and lung adenocarcinoma cell lines. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/8198652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sohail M.U., Yassine H.M., Sohail A., Thani A.a.A. Impact of physical exercise on gut microbiome, inflammation, and the pathobiology of metabolic disorders. Rev. Diabet. Stud. 2019;15:35–48. doi: 10.1900/RDS.2019.15.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Soman S., Rajamanickam C., Rauf A.A., Indira M. Beneficial effects of Psidium guajava leaf extract on diabetic myocardium. Exp. Toxicol. Pathol. 2013;65:91–95. doi: 10.1016/j.etp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 248.Song Q., Chu X., Zhang X., Bao Y., Zhang Y., Guo H., Liu Y., Liu H., Zhang J., Zhang Y., Chu L. Mechanisms underlying the cardioprotective effect of Salvianic acid A against isoproterenol-induced myocardial ischemia injury in rats: possible involvement of L-type calcium channels and myocardial contractility. J. Ethnopharmacol. 2016;189:157–164. doi: 10.1016/j.jep.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 249.Srikanth S., Ambrose J.A. Pathophysiology of coronary thrombus formation and adverse consequences of thrombus during PCI. Curr. Cardiol. Rev. 2012;8:168–176. doi: 10.2174/157340312803217247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Srinivasan S., Vannberg F.O., Dixon J.B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 2016;6:24436. doi: 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Srivastava V., Negi A.S., Kumar J.K., Gupta M.M., Khanuja S.P.S. Plant-based anticancer molecules: a chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005;13:5892–5908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 252.Steptoe A., Wardle J., Cui W., Bellisle F., Zotti A.-M., Baranyai R., Sanderman R. Trends in smoking, diet, physical exercise, and attitudes toward health in European university students from 13 countries, 1990–2000. Prev. Med. 2002;35:97–104. doi: 10.1006/pmed.2002.1048. [DOI] [PubMed] [Google Scholar]
- 253.Sun H., Wang D., Song X., Zhang Y., Ding W., Peng X., Zhang X., Li Y., Ma Y., Wang R., Yu P. Natural prenylchalconaringenins and prenylnaringenins as antidiabetic agents: α-glucosidase and α-amylase inhibition and in vivo antihyperglycemic and antihyperlipidemic effects. J. Agric. Food Chem. 2017;65:1574–1581. doi: 10.1021/acs.jafc.6b05445. [DOI] [PubMed] [Google Scholar]
- 254.Tabatabaei-Malazy O., Larijani B., Abdollahi M. Targeting metabolic disorders by natural products. J. Diabetes Metab. Disord. 2015;14:57. doi: 10.1186/s40200-015-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Taghvaei S., Sabouni F., Minuchehr Z. Identification of natural products as SENP2 inhibitors for targeted therapy in heart failure. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.817990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tajvidi E., Nahavandizadeh N., Pournaderi M., Pourrashid A.Z., Bossaghzadeh F., Khoshnood Z. Study the antioxidant effects of blue-green algae Spirulina extract on ROS and MDA production in human lung cancer cells. Biochem. Biophys. Rep. 2021;28 doi: 10.1016/j.bbrep.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Talebi S., Bagherniya M., Atkin S.L., Askari G., Orafai H.M., Sahebkar A. The beneficial effects of nutraceuticals and natural products on small dense LDL levels, LDL particle number and LDL particle size: a clinical review. Lipids Health Dis. 2020;19:1–21. doi: 10.1186/s12944-020-01250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tan M.E., He C.H., Jiang W., Zeng C., Yu N., Huang W., Gao Z.G., Xing J.G. Development of solid lipid nanoparticles containing total flavonoid extract from Dracocephalum moldavica L. And their therapeutic effect against myocardial ischemia-reperfusion injury in rats. Int. J. Nanomed. 2017;12:3253–3265. doi: 10.2147/IJN.S131893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tang F., Yan H.-L., Wang L.-X., Xu J.-F., Peng C., Ao H., Tan Y.-Z. Review of natural resources with vasodilation: traditional medicinal plants, natural products, and their mechanism and clinical efficacy. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.627458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tasali E., Leproult R., Ehrmann D.A., Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc. Natl. Acad. Sci. USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Taslimi P., Caglayan C., Gulcin İ. The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α‐glycosidase enzymes: an antidiabetic, anticholinergic, and antiepileptic study. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21995. [DOI] [PubMed] [Google Scholar]
- 262.Taslimi P., Gulçin İ. Antidiabetic potential: in vitro inhibition effects of some natural phenolic compounds on α‐glycosidase and α‐amylase enzymes. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21956. [DOI] [PubMed] [Google Scholar]
- 263.Tekko I.A., Permana A.D., Vora L., Hatahet T., Mccarthy H.O., Donnelly R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020;152:105469. doi: 10.1016/j.ejps.2020.105469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Telange D.R., Arunt A., Patil A., Anilm Pethe B., Fegade H.A., Anand S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017;108:36–49. doi: 10.1016/j.ejps.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 265.Tengattini S., Reiter R.J., Tan D.X., Terron M.P., Rodella L.F., Rezzani R. Cardiovascular diseases: protective effects of melatonin. J. Pineal Res. 2008;44:16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 266.Tilaoui M., Achibat H., Lébri M., Lagou S., Ait Mouse H., Zazouli S., Hafid A., Zyad A., Khouili M. Phytochemical screening, antioxidant and in vitro anticancer activities of Bombax buonopozense stem bark extracts. Biotechnol. Biotechnol. Equip. 2021;35:1662–1668. [Google Scholar]
- 267.Tilg H., Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Timmis A., Vardas P., Townsend N., Torbica A., Katus H., De Smedt D., Gale C.P., Maggioni A.P., Petersen S.E., Huculeci R. European Society of Cardiology: cardiovascular disease statistics 2021. Eur. Heart J. 2022;43:716–799. doi: 10.1093/eurheartj/ehab892. [DOI] [PubMed] [Google Scholar]
- 269.Todoric J., Karin M. The fire within: cell-autonomous mechanisms in inflammation-driven cancer. Cancer Cell. 2019;35:714–720. doi: 10.1016/j.ccell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 270.Triantafyllou G.A., Paschou S.A., Mantzoros C.S. Leptin and hormones: energy homeostasis. Endocrinol. Metab. Clin. N. Am. 2016;45:633–645. doi: 10.1016/j.ecl.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 271.Tsai H.-H., Lin H.-W., Lu Y.-H., Chen Y.-L., Mahady G.B. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tuohongerbieke A., Li J., Sabir G., Xin X., Hu M., Duan X., Liu L., Tang D., Zhu J., Aisa H.A. Lignanamides from the roots of Limonium gmelinii (Willd.) Kuntze and their anti-diabetic, cytotoxic and anti-inflammatory activities. Phytochemistry. 2021;184 doi: 10.1016/j.phytochem.2020.112648. [DOI] [PubMed] [Google Scholar]
- 273.Tur J.A., Martinez J.A. Guide and advances on childhood obesity determinants: setting the research agenda. Obes. Rev. 2022;23 doi: 10.1111/obr.13379. [DOI] [PubMed] [Google Scholar]
- 274.Unuofin J.O., Otunola G.A., Afolayan A.J. Inhibition of key enzymes linked to obesity and cytotoxic activities of whole plant extracts of Vernonia mesplilfolia Less. Processes. 2019;7:841. [Google Scholar]
- 275.Vallo J., Arbas R., Basilio J.E., Cayabyab I., Miranda C.N., Santos M., Isabel P., Legaspi L.F., Tiongco R.E. Association of the Pro12Ala gene polymorphism with treatment response to thiazolidinediones in patients with type 2 diabetes: a meta-analysis. Int. J. Diabetes Dev. Ctries. 2022:1–8. [Google Scholar]
- 276.Van Bergen E.D.P., Monnikhof M., Lafeber F., Schutgens R.E.G., Mastbergen S.C., Van Vulpen L.F.D. The fear for adverse bleeding and cardiovascular events in hemophilia patients using (non-) selective non-steroidal anti-inflammatory drugs: a systematic review reporting on safety. Blood Rev. 2022 doi: 10.1016/j.blre.2022.100987. [DOI] [PubMed] [Google Scholar]
- 277.Van Der Klaauw A.A., Farooqi I.S. The hunger genes: pathways to obesity. Cell. 2015;161:119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 278.Villarreal-Molina M.T., Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94:2143–2149. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 279.Volpe-Zanutto F., Ferreira L., Permana A.D., Kirkby M., Paredes A.J., Vora L., Bonfanti A., Charlie-Silva I., Raposo C., Figueiredo M.C., Souza I.M.O., Brisibe A., Costa F.D., Donnelly R.F., Foglio M.A. Artemether and lumefantrine dissolving microneedle patches with improved pharmacokinetic performance and antimalarial efficacy in mice infected with Plasmodium yoelii. J. Control. Release. 2021;333:298–315. doi: 10.1016/j.jconrel.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 280.Vu H.T.H., Hook S.M., Siqueira S.D., Müllertz A., Rades T., Mcdowell A. Are phytosomes a superior nanodelivery system for the antioxidant rutin? Int. J. Pharm. 2018;548:82–91. doi: 10.1016/j.ijpharm.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 281.Wan S., Zhang L., Quan Y., Wei K. Resveratrol-loaded PLGA nanoparticles: enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. Open. Sci. 2018;5:181457. doi: 10.1098/rsos.181457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang H., Zhang K., Chen X., Han M., Lu J., Zhang Y. Vitro and in vivo evaluation of antidiabetic properties and mechanisms of Ficus tikoua bur. Nutrients. 2022;14:4413. doi: 10.3390/nu14204413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang M., Meng X.B., Yu Y.L., Sun G.B., Xu X.D., Zhang X.P., Dong X., Ye J.X., Xu H.B., Sun Y.F., Sun X.B. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis. 2014;19:1727–1735. doi: 10.1007/s10495-014-1039-3. [DOI] [PubMed] [Google Scholar]
- 284.Wang M., Xu X., Xu H., Wen F., Zhang X., Sun H., Yao F., Sun G., Sun X. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J. Ethnopharmacol. 2014;155:240–247. doi: 10.1016/j.jep.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 285.Wang R., Yang M., Wang M., Liu X., Xu H., Xu X., Sun G., Sun X. Total saponins of aralia elata (miq) seem alleviate calcium homeostasis imbalance and endoplasmic reticulum stress-related apoptosis induced by myocardial ischemia/reperfusion injury. Cell. Physiol. Biochem. 2018;50:28–40. doi: 10.1159/000493954. [DOI] [PubMed] [Google Scholar]
- 286.Wang W., Ma P., Zhao Q., Goorani S. Beneficial properties of the biosynthesized silver/chitosan nanoparticles mediated by Mentha piperita in rats with heart failure following myocardial infarction. Inorg. Chem. Commun. 2022;141:109581. [Google Scholar]
- 287.Wang X., Ye K., Lv Y., Wei S., Li X., Ma J., Zhang X., Ye C. Ameliorative effect and underlying mechanisms of total triterpenoids from Psidium guajava Linn (myrtaceae) leaf on high-fat streptozotocin-induced diabetic peripheral neuropathy in rats. Trop. J. Pharmaceut. Res. 2016;15:327–333. [Google Scholar]
- 288.Weidner C., De Groot J.C., Prasad A., Freiwald A., Quedenau C., Kliem M., Witzke A., Kodelja V., Han C.-T., Giegold S. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA. 2012;109:7257–7262. doi: 10.1073/pnas.1116971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Welty F.K., Alfaddagh A., Elajami T.K. Targeting inflammation in metabolic syndrome. Transl. Res. 2016;167:257–280. doi: 10.1016/j.trsl.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wilkinson L., Gathani T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022;95 doi: 10.1259/bjr.20211033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Willcox A., Richardson S.J., Bone A.J., Foulis A.K., Morgan N.G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Current Obesit. Reports. 2015;4:363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 293.Winitchaikul T., Sawong S., Surangkul D., Srikummool M., Somran J., Pekthong D., Kamonlakorn K., Nangngam P., Parhira S., Srisawang P. Calotropis gigantea stem bark extract induced apoptosis related to ROS and ATP production in colon cancer cells. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wong R.J., Ahmed A. Obesity and non-alcoholic fatty liver disease: disparate associations among Asian populations. World J. Hepatol. 2014;6:263. doi: 10.4254/wjh.v6.i5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.World Health O. 2018. Noncommunicable Diseases Country Profiles. [Google Scholar]
- 296.Wu J., Xu X., Li Y., Kou J., Huang F., Liu B., Liu K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014;745:59–68. doi: 10.1016/j.ejphar.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 297.Wu L., Xiong X., Wu X., Ye Y., Jian Z., Zhi Z., Gu L. Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front. Mol. Neurosci. 2020;13:28. doi: 10.3389/fnmol.2020.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wu P.-F., Zhang Z., Wang F., Chen J.-G. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010;31:1523–1531. doi: 10.1038/aps.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wu Y., Ding Y., Tanaka Y., Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014;11:1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wunjuntuk K., Ahmad M., Techakriengkrai T., Chunhom R., Jaraspermsuk E., Chaisri A., Kiwwongngam R., Wuttimongkolkul S., Charoenkiatkul S. Proximate composition, dietary fibre, beta-glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. J. Food Compos. Anal. 2022;105 [Google Scholar]
- 301.Würfel M., Breitfeld J., Gebhard C., Scholz M., Baber R., Riedel-Heller S.G., Blüher M., Stumvoll M., Kovacs P., Tönjes A. Interplay between adipose tissue secreted proteins, eating behavior and obesity. Eur. J. Nutr. 2022;61:885–899. doi: 10.1007/s00394-021-02687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yan Z., Wang F., Wen Z., Zhan C., Feng L., Liu Y., Wei X., Xie C., Lu W. Journal of Controlled Release. Elsevier B.V.; 2012. LyP-1-conjugated PEGylated liposomes: a carrier system for targeted therapy of lymphatic metastatic tumor. [DOI] [PubMed] [Google Scholar]
- 303.Yang J., Qian F., Chavarro J.E., Ley S.H., Tobias D.K., Yeung E., Hinkle S.N., Bao W., Li M., Liu A. Modifiable risk factors and long term risk of type 2 diabetes among individuals with a history of gestational diabetes mellitus: prospective cohort study. BMJ. 2022;378 doi: 10.1136/bmj-2022-070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yeo C.Q.X., Alexander I., Lin Z., Lim S., Aning O.A., Kumar R., Sangthongpitag K., Pendharkar V., Ho V.H., Cheok C.F. p53 maintains genomic stability by preventing interference between transcription and replication. Cell Rep. 2016;15:132–146. doi: 10.1016/j.celrep.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 305.Yu H., Palazzolo J.S., Zhou J., Hu Y., Niego B.E., Pan S., Ju Y., Wang T.Y., Lin Z., Hagemeyer C.E., Caruso F. Bioresponsive polyphenol-based nanoparticles as thrombolytic drug carriers. ACS Appl. Mater. Interfaces. 2022;14:3740–3751. doi: 10.1021/acsami.1c19820. [DOI] [PubMed] [Google Scholar]
- 306.Yu Y., Zhang M., Hu Y., Zhao Y., Teng F., Lv X., Li J., Zhang Y., Hatch G.M., Chen L. Increased bioavailable berberine protects against myocardial ischemia reperfusion injury through attenuation of NFκB and JNK signaling pathways. Int. Heart J. 2018;59:1378–1388. doi: 10.1536/ihj.17-458. [DOI] [PubMed] [Google Scholar]
- 307.Yücel Ç., Karatoprak G.Ş., Aktaş Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J. Nanosci. Nanotechnol. 2018;18:3856–3864. doi: 10.1166/jnn.2018.15247. [DOI] [PubMed] [Google Scholar]
- 308.Zaccardi F., Webb D.R., Yates T., Davies M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad. Med. 2016;92:63–69. doi: 10.1136/postgradmedj-2015-133281. [DOI] [PubMed] [Google Scholar]
- 309.Zerrouki S., Mezhoud S., Yilmaz M.A., Sahin Yaglioglu A., Bakir D., Demirtas I., Mekkiou R. LC/MS-MS Analyses and in vitro anticancer activity of Tourneuxia variifolia extracts. Nat. Prod. Res. 2022;36:4506–4510. doi: 10.1080/14786419.2021.1986818. [DOI] [PubMed] [Google Scholar]
- 310.Zhang H.W., Hu J.J., Fu R.Q., Liu X., Zhang Y.H., Li J., Liu L., Li Y.N., Deng Q., Luo Q.S., Ouyang Q., Gao N. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhang Q., Guo Y., Zhang D. Network pharmacology integrated with molecular docking elucidates the mechanism of wuwei yuganzi san for the treatment of coronary heart disease. Nat. Prod. Commun. 2022;17 [Google Scholar]
- 312.Zhang S., Li L., Chen W., Xu S., Feng X., Zhang L. Natural products: the role and mechanism in low‐density lipoprotein oxidation and atherosclerosis. Phytother Res. 2021;35:2945–2967. doi: 10.1002/ptr.7002. [DOI] [PubMed] [Google Scholar]
- 313.Zhang T., Gu H.W., Gao J.X., Li Y.S., Tang H.B. Ethanol supernatant extracts of Gynura procumbens could treat nanodiethylnitrosamine-induced mouse liver cancer by interfering with inflammatory factors for the tumor microenvironment. J. Ethnopharmacol. 2022;285 doi: 10.1016/j.jep.2021.114917. [DOI] [PubMed] [Google Scholar]
- 314.Zhang Y., Chen M., Tao Y., Chu B., Ma Y., Lu K., Sun H. Natural 8-C-ascorbyl-(−)-epigallocatechin as antidiabetic agent: α-glucosidase and PTP-1B signaling pathway dual regulators. Fitoterapia. 2022;162 doi: 10.1016/j.fitote.2022.105263. [DOI] [PubMed] [Google Scholar]
- 315.Zhang Y., Li Q., Wang J., Cheng F., Huang X., Cheng Y., Wang K. Polysaccharide from Lentinus edodes combined with oxaliplatin possesses the synergy and attenuation effect in hepatocellular carcinoma. Cancer Lett. 2016;377:117–125. doi: 10.1016/j.canlet.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 316.Zhang Y., Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011;670:325–332. doi: 10.1016/j.ejphar.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 317.Zhao C.-C., Chen J., Shao J.-H., Zhang X.-H., Gu W.-Y., Shen J., Liu Y. Lignan constituents from the fruits of viburnum macrocephalum f. keteleeri and their α-amylase, α-glucosidase, and protein tyrosine phosphatase 1B inhibitory activities. J. Agric. Food Chem. 2020;68:11151–11160. doi: 10.1021/acs.jafc.0c03353. [DOI] [PubMed] [Google Scholar]
- 318.Zhao G.L., Yu L.M., Gao W.L., Duan W.X., Jiang B., Liu X.D., Zhang B., Liu Z.H., Zhai M.E., Jin Z.X., Yu S.Q., Wang Y. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016;37:354–367. doi: 10.1038/aps.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zheng W., Qiu L., Wang R., Feng X., Han Y., Zhu Y., Chen D., Liu Y., Jin L., Li Y. Selective targeting of PPARγ by the natural product chelerythrine with a unique binding mode and improved antidiabetic potency. Sci. Rep. 2015;5:1–12. doi: 10.1038/srep12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zhi K., Wang J., Zhao H., Yang X. Self-assembled small molecule natural product gel for drug delivery: a breakthrough in new application of small molecule natural products. Acta Pharm. Sin. B. 2020;10:913–927. doi: 10.1016/j.apsb.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhou P., Xie W., Luo Y., Lu S., Dai Z., Wang R., Sun G., Sun X. Protective effects of total saponins of Aralia elata (Miq.) on endothelial cell injury induced by TNF-α via modulation of the PI3K/Akt and NF-κB signalling pathways. Int. J. Mol. Sci. 2018;20:36. doi: 10.3390/ijms20010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhu J., Thompson C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019;20:436–450. doi: 10.1038/s41580-019-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhu N., Li J., Li Y., Zhang Y., Du Q., Hao P., Li J., Cao X., Li L. Berberine protects against simulated ischemia/reperfusion injury-induced H9C2 cardiomyocytes apoptosis in vitro and myocardial ischemia/reperfusion-induced apoptosis in vivo by regulating the mitophagy-mediated HIF-1α/BNIP3 pathway. Front. Pharmacol. 2020;11:367. doi: 10.3389/fphar.2020.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Zuo Y.M., Wang X.H., Gao S., Zhang Y. Oligomerized grape seed proanthocyanidins ameliorates isoproterenol-induced cardiac remodeling in rats: role of oxidative stress. Phytother Res. 2011;25:732–739. doi: 10.1002/ptr.3331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.