Abstract
Fluorine-containing compounds comprise twenty to thirty percent of all commercial drugs, and the proportion of fluorinated pharmaceuticals is rapidly growing. While magic angle spinning (MAS) NMR spectroscopy is a popular technique for analysis of solid pharmaceutical compounds, fluorine has been underutilized as a structural probe so far. Here we report a fast (40 kHz) MAS 19F NMR approach for structural characterization of fluorine-containing crystalline pharmaceutical compounds at natural abundance, using the antimalarial fluorine-containing drug mefloquine as an example. We demonstrate the utility of 2D 19F-13C and 19F-19F dipolar-coupling-based correlation experiments for 19F and 13C resonance frequency assignment, which permit identification of crystallographically inequivalent sites. The efficiency of 19F-13C cross-polarization (CP) as well as the effect of 1H and 19F decoupling on spectral resolution and sensitivity were evaluated in a broad range of experimental conditions. We further demonstrate a protocol for measuring accurate interfluorine distances based on 1D DANTE-RFDR experiments combined with multi-spin numerical simulations.
Keywords: fast 19F MAS NMR, 19F-13C heteronuclear correlations, 19F decoupling, F-F RFDR, interfluorine distances, mefloquine
Abstract Graphic:
INTRODUCTION
Fluorinated compounds are becoming increasingly dominant in medicinal chemistry1. Fluorine-containing molecules comprise 20% of all pharmaceuticals and 30% of newly approved drugs2–4, and according to current estimates, over 150 commercial drugs contain fluorine atoms or fluoroalkyl groups, including several of the most-prescribed and/or most-profitable on the market2–4. Structural characterization of pharmaceuticals relies on crystallography and NMR spectroscopy; fluorine NMR is becoming increasingly important in this regard. 19F is a 100% naturally abundant spin 1/2 nucleus and possesses a high gyromagnetic ratio, making it a very attractive probe for NMR applications. 19F resonance frequencies are also exquisitely sensitive to the local structural and electronic environment around the fluorine nuclei, exhibiting a chemical shift range of over 300 ppm5,6.
While 19F NMR has been extensively applied in the analysis of fluorinated organic and biological molecules in soIution7–13, MAS NMR studies remain relatively scarce14–26. This, in part, is due to i) strong dipolar couplings that result in broad lines, requiring high-power 1H and 19F decoupling with specialized hardware, and ii) relatively low sensitivity in heteronuclear polarization transfer experiments in the common regime of magic angle spinning (MAS) frequencies, below 30 kHz. Increasing the MAS frequencies to 40 kHz and above results in significant improvements in sensitivity and resoIution24–26, yielding substantially narrowed 19F lines, even in the absence of 1H decoupling19,20,25. Furthermore, homonuclear 19F-19F recoupling experiments, such as 2D radio frequency driven recoupling (RFDR) and delays alternating with nutation for tailored excitation (DANTE)-RFDR, are effective and can be used for interfluorine distance measurements, including in multi-spin systems15,18,19,24. In particular, long-range 1H-19F and 19F-19F interatomic distances27 together with 19F-13C contacts,13,21,28,29 are indispensable parameters30 in structure elucidations.
Here we report on a fast MAS 19F NMR crystallography approach for structure determination of crystalline fluorine-containing pharmaceutical compounds at natural abundance. This integrated approach was developed by us for structural analysis of active pharmaceutical ingredients and provides detailed information on the 3D structure. Our methodology was established using the antimalarial drug mefloquine31 (Fig. 1) whose X-ray structure is known. Combining 2D homonuclear 19F-19F and heteronuclear 19F-13C dipole-coupling-based experiments with Density Functional Theory (DFT) calculations, complete assignments of 19F and 13C chemical shifts were obtained, revealing three crystallographically inequivalent fluorine sites in the unit cell of mefloquine. We show that at MAS frequencies of 40 kHz and above, low-power double-quantum cross polarization (CP) is optimal for achieving high 19F-13C polarization transfer efficiencies and for recording short- and long-range 19F-13C correlations. The effects and the efficiency of 1H and 19F decoupling on 19F-13C heteronuclear correlation (HETCOR) spectra were evaluated and a comprehensive analysis of multi-spin effects was performed. Magnetization exchange curves recorded in DANTE-RFDR experiments allowed us to extract accurate intra- and intermolecular fluorine-fluorine distances.
Figure 1.
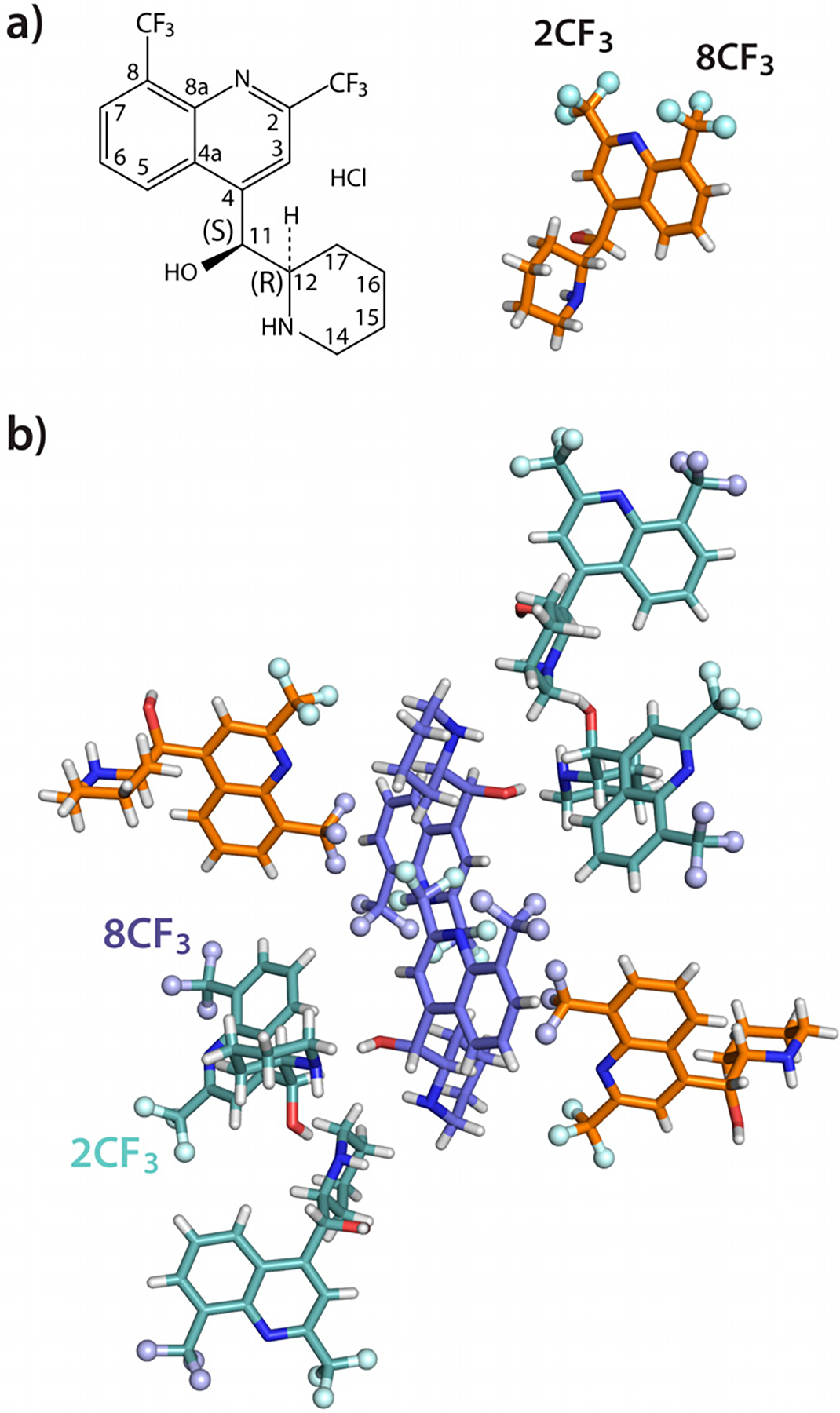
a) Chemical and 3D structure of mefloquine. b) Arrangement of mefloquine molecules in the crystal. The three types of inequivalent molecules are colored in purple, orange and cyan. The fluorine atoms are shown as spheres. The 8CF3 and 2CF3 groups are colored in light purple and light cyan, respectively.
It is well known that polymorphs and solvates can impact the therapeutic performance for certain drugs,32 and patenting new polymorphs after the original drug is a common strategy for extending drug commercial lifecycle.33 The fast MAS 19F NMR crystallography approach, exemplarily demonstrated herein for crystalline mefloquine, allows for quick and efficient characterization of polymorphs. We envision that it will be broadly adopted for analysis of active pharmaceutical ingredients in pharmaceutical formulations of unknown structures.
RESULTS
19F MAS NMR Spectra: MAS Frequency and Decoupling
19F MAS spectra of mefloquine acquired at MAS frequencies of 10, 40, and 60 kHz are shown in Fig. 2. Very strong signals emerge in only a single scan, and the signal-to-noise ratios (SNR) for spectra acquired with 32 scans at MAS frequencies of 10, 40, and 60 kHz, without and with 1H decoupling, are remarkably high: 307/561, 1215/1655, 1535/2120, respectively. At a MAS frequency of 10 kHz, a manifold of 8 spinning sidebands was observed for each of the two broad peaks with isotropic chemical shifts of 16.2 and 8.8 ppm. The corresponding line widths for the center bands are 922 and 745 Hz in the absence of 1H decoupling. When 1H Spinal-64 decoupling at an RF B1 field of 90 kHz was applied, the lines became somewhat narrower, 843 and 317 Hz, respectively. The upfield resonance exhibited asymmetric lineshape but remained unresolved. The 19F T1 are 1.4 s and 2.5 s for the downfield and upfield peaks, respectively, while 1H T1 are 4.7–5.0 s.
Figure 2.
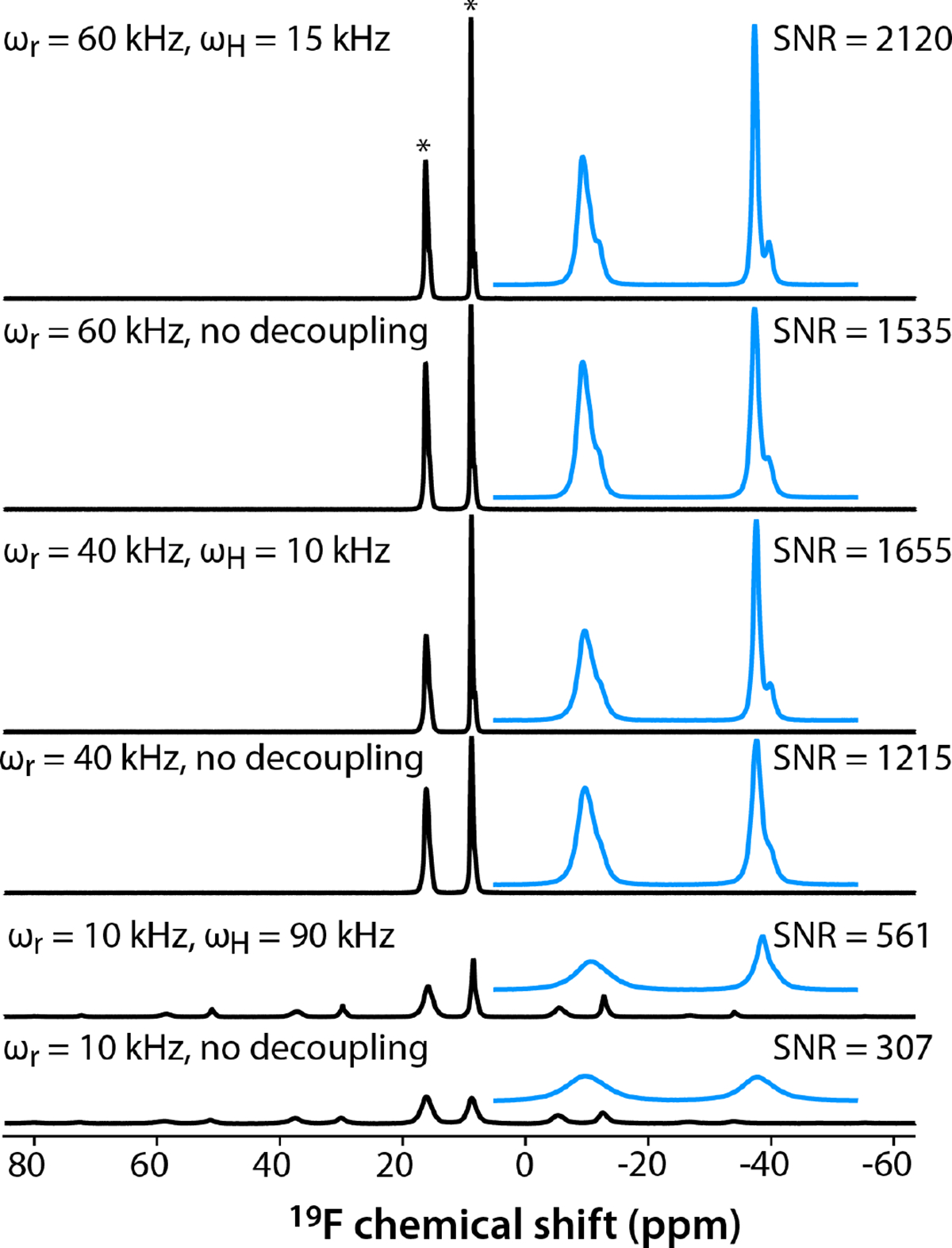
19F MAS spectra of mefloquine acquired at MAS frequencies of 60 kHz (top two traces), 40 kHz (middle two traces), and 10 kHz (bottom two traces), with or without SWf-TPPM 1H decoupling at the RF field strength, as indicated next to each spectrum. The blue traces are expansions around the isotropic peaks (marked with asterisks). The signal-to-noise ratios (SNR) are indicated next to each trace. The spectra were acquired at 11.7 T using a 1.3 mm HFX MAS probe, averaging 32 scans.
The spectra at 40 kHz and 60 kHz provided in Fig. 2 reveal 5 distinct resonances, with isotropic chemical shifts of 16.2 ppm, 15.9 ppm (a shoulder), 15.6 ppm, 8.8 ppm, and 8.2 ppm. Dramatic improvements in resolution are seen when increasing the MAS frequencies to 40 and 60 kHz. At 40 kHz, the line widths of the downfield and upfield resonances are 440 and 230 Hz without decoupling; with 1H SWf-TPPM decoupling at the RF field of 10 kHz, those are 378 and 143 Hz, respectively. At 60 kHz without 1H decoupling, the line widths are 322 and 171 Hz; those in the presence of 15 kHz 1H SWf-TPPM are 300 and 117 Hz, respectively. Taken together, these results unequivocally demonstrate that fast MAS frequencies are critical from both sensitivity and resolution, and that, even at 60 kHz, 1H decoupling is necessary. Conversely, even with high-power (90 kHz) 1H decoupling, the resolution is very poor at 10 kHz MAS and the SNR is 4-fold lower than at 60 kHz MAS.
19F-13C Cross-Polarization: Optimal Conditions for Fast MAS Experiments
1H-13C and 19F-13C CPMAS spectra of mefloquine are shown in Fig. 3. All carbon resonances are detected in the 1H-13C CPMAS spectrum (Fig. 3a) as well as the non-1H decoupled 19F-13C spectrum (Fig. 3b), except for C14, which is farthest away from the two CF3 groups. In the 19F-13C CPMAS experiments, the polarization transfer efficiency between fluorine and the aromatic carbons is similar to that in the 1H-13C CPMAS experiment, with a 0.6 signal-to-noise ratio (SNR) per square root of scans.
Figure 3.
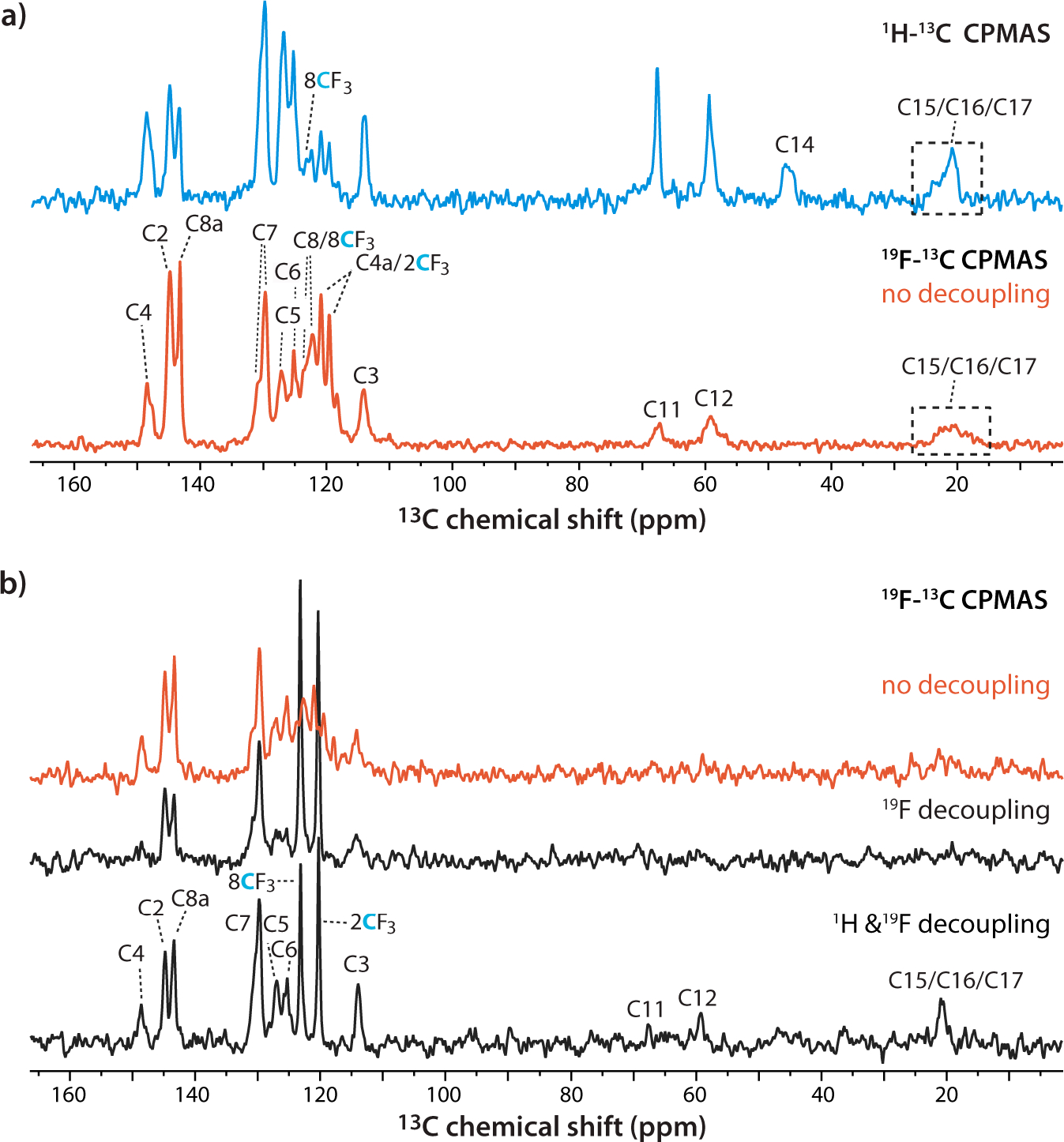
a) 1H-13C and 19F-13C CPMAS spectra of mefloquine. The spectra were acquired at 20.0 T using a 1.9 mm HX MAS probe, averaging 512 scans and 1920 scans and CP contact time of 1.4 and 6 ms, respectively. The 19F-13C spectrum was acquired without decoupling. b) 19F-13C CPMAS spectra of mefloquine without decoupling acquired (top trace), with 19F decoupling (middle trace), and with 1H and 19F decoupling (bottom trace). The spectra were acquired at 16.4 T using a 1.6 mm HFXY MAS probe, averaging 512 scans; the CP contact time was 7 ms. The MAS frequency was 40 kHz in every case. Assignments for individual carbon signals are shown in the different spectra.
Effects and efficiency of 1H and 19F decoupling was evaluated for different decoupling sequences (Fig. 3b–d). For fluorine decoupling, high-power π-pulses were introduced every rotor period34 and proton decoupling employed the low power XiX scheme35, applied simultaneously with fluorine decoupling. Fluorine decoupling (Fig. 3c) increased the signal intensity and narrowed the CF3 carbon resonances, as expected. At the same time, with only fluorine decoupling, non-CF3 carbon signal intensities are reduced by different degrees relative to the 1H-decoupled spectra. When proton and fluorine decoupling is applied simultaneously (Fig. 3d), the spectrum exhibits improved resolution throughout.
19F-13C polarization transfer efficiencies were assessed by recording CPMAS spectra for different Hartmann-Hann matching conditions with radiofrequency (rf) fields varied from 5 kHz to 120 kHz. Both double-quantum (DQ) and zero-quantum (ZQ) conditions were examined. We found that several DQ and ZQ conditions led to efficient CP transfers for 15 kHz or 35 kHz 19F radio frequency (rf) fields. DQ transfers appear to be more efficient than their ZQ counterparts, with the low-power CP transfers considerably more efficient than the high-power conditions (Fig. S1 and Table S1 of the Supporting Information). The highest transfer efficiency was achieved for first-order DQ CP with 15 kHz 19F and 25 kHz 13C rf fields (Fig. S1, Fig. S2a–b).
In addition, 19F-13C CPMAS spectra were recorded with different contact times, to obtain magnetization buildup profiles for different resonances. Contact times of 6 ms or higher are necessary to cross-polarize the aliphatic carbons while intensities for the aromatic carbons reach their maximum at 7 ms and decrease thereafter (Fig. S2c–g and Fig. S3). Overall, our results indicate that it is advantageous to record 19F-13C CPMAS spectra with contact times of both 7 and 10 ms. Setting the contact time to 10 ms in the 2D HETCOR experiments permits detection of cross-peaks corresponding to long-range correlations, as discussed below.
1H-13C and 19F-13C HETCOR: Resonance Assignments and Long-Range Correlations
13C resonances were assigned using 2D 1H-13C and 19F-13C HETCOR spectra and DFT calculations (Fig. S4 of the Supporting Information); all chemical shifts are summarized in Table 2. While most of the 13C signals could be assigned unambiguously based on the calculated frequencies, remaining ambiguities were resolved by analyzing the 2D 1H-13C HETCOR spectra recorded with CP contact times of 0.5 ms and 1.1 ms. Strong peaks present in the 2D HETCOR spectrum recorded with a contact time of 0.5 ms correspond to correlations between carbons and their directly bonded protons, while correlations between carbons and protons separated by two or three bonds appear in the spectrum with 1.1 ms contact time (Fig. 4a). The resonances of the 2CF3 and 8CF3 groups give rise to intense signals and could be assigned with confidence on the basis of the 2D 19F-13C HETCOR spectrum (contact time of 1 ms), despite their partial overlap with those from C8 and C4a (Fig. 4b). All carbon resonance assignments were validated from the contact-time dependencies of 1D 1H-13C and 19F-13C CPMAS spectra (Fig. S3a, Fig. S4b). For instance, carbon resonances of CF3 groups as well as C2, C8a and C7, which are all within three bonds of the 19F atoms, are clearly observed in spectra with 1 ms contact time. In contrast, signals corresponding to carbons distant from the fluorine atoms, are relatively weak (C5 and C6) or missing (C4). Similarly, resonances of aromatic carbons, such as C8a, C8 and C4a, have low intensities in the 1 ms 1H-13C CPMAS spectra. Assignments of C15, C16 and C17 resonances are tentative as the corresponding signals partly overlap. To the best of our knowledge, the 13C and 19F chemical shifts of mefloquine have not been reported to date. For several carbon atoms, including C2, C3, C4, C6 and the carbon of 8CF3 group, several sets of distinct peaks are observed, which correspond to the crystallographically inequivalent positions of these atoms in the unit cell (Fig. 4c). We also note that the application of 1H and 19F decoupling results in significantly improved spectral resolution, consistent with our observations in the 1D 19F-13C and 1H-13C CPMAS experiments (Fig. 4b–d and Fig. S5 of the Supporting Information).
Table 2.
MAS NMR Experimental and DFT Calculated 13C Isotropic Chemical Shifts and Interatomic Distances in Mefloquine
| Carbon atom | δ13C (ppm) MAS NMR | δ13C (ppm) DFT | Distance to 19F (Å) | ||
|---|---|---|---|---|---|
| a | b | 2CF3 | 8CF3 | ||
|
| |||||
| C4 | 147.5 | 148 | 157.2 | 4.5* | 5.2 |
| C2 | 144.3 | 145 | 150.9 | 2.3 | 4.1 |
| C8a | 143.6 | 143.2 | 146.7 | 4.1 | 3 |
| C7 | 129.7 | 134.3 | 6.3 | 3.4 | |
| C5 | 126.8 | 132.4 | 6.2 | 4.9 | |
| C6 | 125.2 | 124.7 | 128.7 | 6.8 | 4.6 |
| 8CF3 | 123.0 | 122.6 | 126.2 | 5 | 1.3 |
| C8 | 122.2# | 126.6 | 5 | 2.4 | |
| C4a | 120.8# | 125.1 | 4.9 | 4.3 | |
| 2CF3 | 120.1 | 122.4 | 1.3 | 4.7 | |
| C3 | 114.3 | 114.1 | 114.2 | 3.4 | 5.2 |
| C11 | 67.7 | 71.7 | 5.8 | 6.7 | |
| C12 | 59.3 | 58.8 | 58.4 | 6.3 | 7 |
| C14 | 47 | 44.9 | 8.5 | 9 | |
| C15 | 23.1, 22.4 | (22.3–23.6) | 24.8 | 8.1 | 9.1 |
| C17 | 21.7, 21.4 | (21.3–22.0) | 21.4 | 5.7 | 7 |
| C16 | 21.0, 20.1 | (19.8–20.9) | 20.3 | 6.8 | 7.7 |
Resonances were assigned on the basis of 19F-13C CPMAS, 1H-13C CPMAS and 19F-13C HETCOR spectra. Correlations detected in 2D FC-HETCOR with the 7.0 ms CP contact time are highlighted in green and those only detected with the 1ms CP contact in light green.
Figure 4.
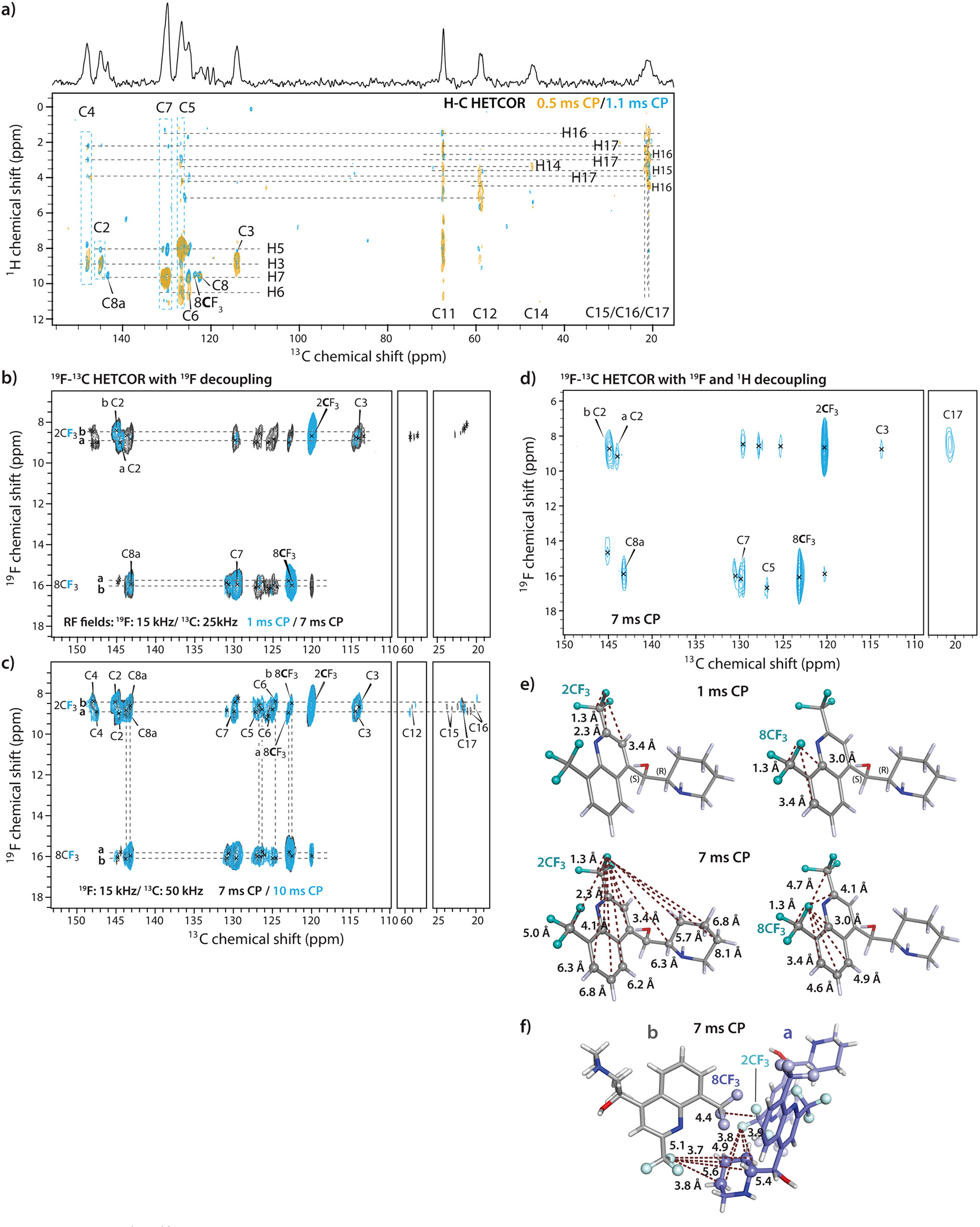
a) 2D 1H-13C HETCOR spectra acquired with CP contact times of 0.5 ms (yellow) and 1.1 ms (blue). b)-d) 2D 19F-13C HETCOR spectra acquired with CP contact times of 1ms and 7 ms (b), 7 ms and 10 ms with 19F decoupling (c), and with a CP contact time of 7 ms with 1H and 19F decoupling (d). The rf fields for 19F and 13C were 15 kHz and 25 kHz (b) or 50 kHz (c), respectively. The spectrum shown in d) was acquired at 16.4 T with 8 scans. All other spectra were acquired at 20.0 T with 256 scans. The MAS frequency was 40 kHz. Carbon assignments are indicated. e) Short- and long-range intramolecular 19F-13C distances in the mefloquine crystal structure consistent with correlations in the HETCOR spectra for 1ms and 7 ms contact times. f) Intermolecular 19F-13C distances < 6 Å in the crystal lattice.
The 19F-13C multiple-bond correlations were extracted from a series of 19F-13C HETCOR spectra acquired with different contact times. Resonance assignments labeled in Fig. 4b–d were made based on the following considerations: correlations to C3, C4 and the aliphatic carbons are only observed for the 19F signal at 8.8 ppm; therefore, this signal is assigned to the 2CF3 group, which is close to the aliphatic carbons. By exclusion, the 19F signal at 15.9 ppm is therefore assigned to the 8CF3 group. This assignment is consistent with the DFT calculations, which indicate that the 2CF3 group is downfield-shifted and that the 19F chemical shift difference between the 2CF3 and 8CF3 groups is 8.1 ppm.
To unequivocally distinguish between short- and long-range 19F-13C correlations, we carefully examined the 2D HETCOR spectra acquired with different CP contact times. As can be noted, 19F-13C correlations corresponding to atom pairs separated by 3 or more bonds are only detected when the CP contact time is 7 ms or longer (Fig. 4c and Fig. S5 of the Supporting Information), while 19F-13C correlations involving directly bonded carbons and carbons within 3 bonds of the fluorine are observed with contact time as short as 1 ms. To observe fluorine correlations with the piperidine ring carbons, such as C12 and C15–17, contact times of 9 ms or longer are necessary. Overall, 19F-13C correlations, corresponding to intra- and intermolecular distances of as long as 6.8 Å were observed. All experimental correlations described above are consistent with the structure of mefloquine and map unambiguously to specific distances (Fig. 4e,f).
19F-19F RFDR: Assignment of Resonances from Crystallographically Inequivalent Sites
At room temperature, the fluorine atoms of the CF3 groups are expected to give rise to a single 19F resonance due to motional averaging36,37. However, multiple 19F chemical shifts were observed for carbon and fluorine atoms of the 2CF3 and 8CF3 moieties in the 19F-13C HETCOR spectra (Fig. 4b–d), also consistent with the 1D 19F MAS spectra (Fig. 2). The presence of several distinct fluorine signals for each trifluoromethyl group can be explained by the crystal structure of mefloquine: there are three molecules in the unit cell, which result in inequivalent atom positions for the CF3 groups. To confirm this interpretation, we performed 1D 19F DANTE excitation experiments38,39, where selective DANTE irradiation was applied at different 19F frequencies (Fig. 5a–d). When DANTE pulses were applied on the 8CF3 resonance at 15.9 ppm (Fig. 5c) and 2CF3 at 8.8 ppm (Fig. 5d), multiple resolved resonances can be seen at each of the two positions, with resolution increasing with increased DANTE interpulse delay from 2 to 4 rotor cycles. With DANTE selective excitation, at least two signals for the 2CF3 moiety and three for the 8CF3 moiety were observed. The line widths of the three 8CF3 resonances are 0.42 ppm, 0.35 ppm and 0.67 ppm, and the overall width of the 8CF3 peak in the absence of the DANTE excitation is 1.13 ppm.
Figure 5.
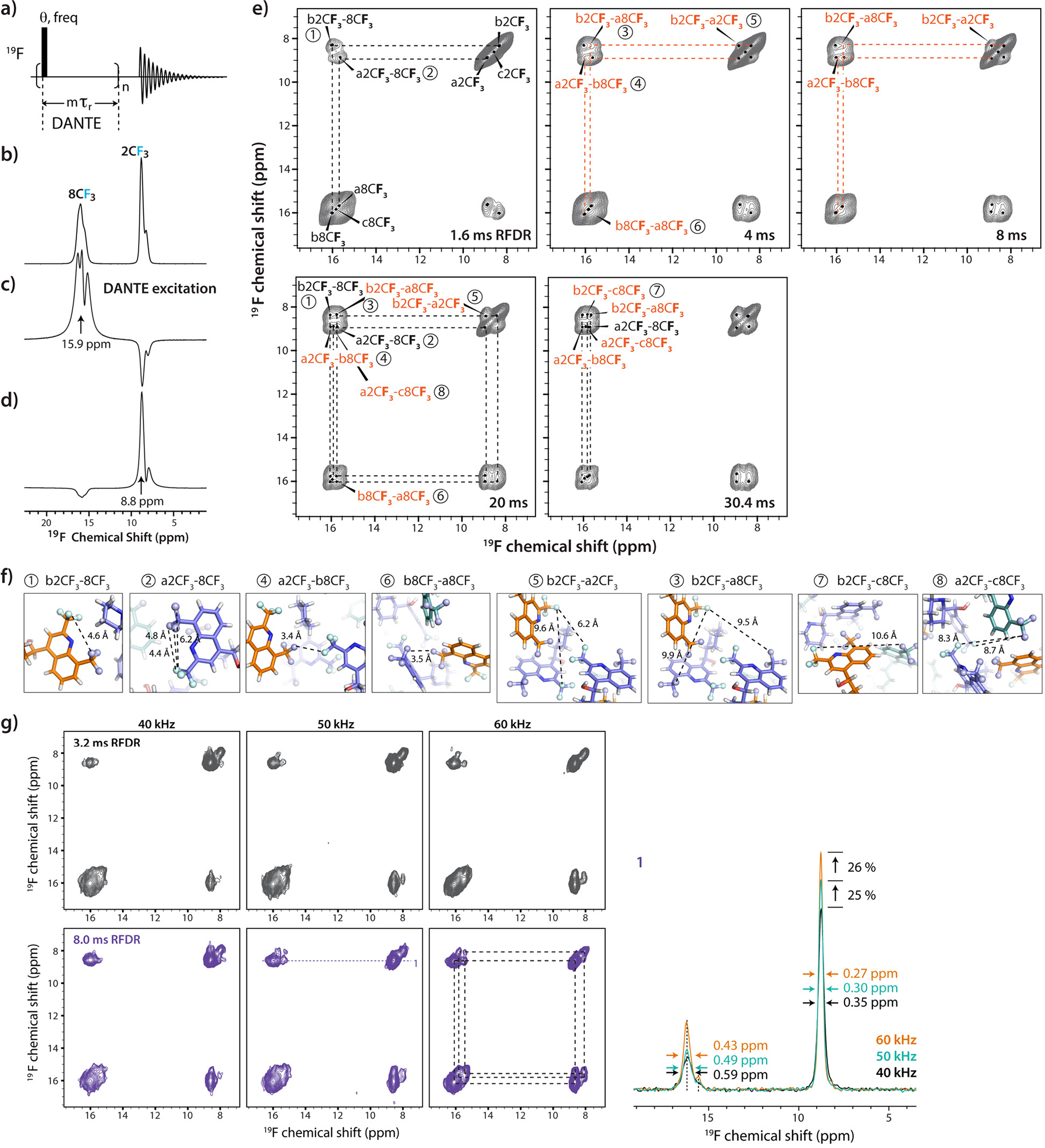
a)-d) Pulse sequence (a), 1D 19F MAS NMR spectrum (b) with 19F DANTE selective excitation of the 8CF3 resonance at 15.9 ppm (c) and 2CF3 resonance at 8.8 ppm (d). Spectra were acquired at 20.0 T, with a MAS frequency of 40 kHz, averaging 16 scans and a DANTE interpulse delay of 4 rotor periods. e) 2D 19F-19F RFDR spectra with increased mixing times from 1.6 ms to 30.4 ms. Intramolecular and intermolecular correlations are shown in black and orange, respectively. f) The intra- and intermolecular interfluorine distances in crystal structure of mefloquine. The notation for each 19F-19F distance is identical to the corresponding correlation 2D 19F-19F RFDR spectra. The fluorine atoms in CF3 groups are shown in light cyan. g) Left panels: 2D 19F-19F RFDR spectra acquired at the MAS frequencies of 40 kHz (left), 50 kHz (middle), and 60 kHz (right). The RFDR mixing times were 3.2 ms (gray) and 8.0 ms (dark purple). Right panel: the 1D traces of the 2D spectra with 8 ms RFDR mixing extracted at 8.61 ppm for MAS frequencies of 40 kHz (cyan), 50 kHz (black) and 60 kHz (orange). The peak widths are indicated in the slices.
In addition, 2D 19F-19F RFDR spectra were acquired with RFDR mixing times ranging from 1.6 ms to 30.4 ms, resulting in multiple resolved 19F signals for each CF3 moiety and multiple sets of cross peaks between 2CF3 and 8CF3 groups (Fig. 5e). Interestingly, the RFDR buildup profiles for the cross peaks are very different. For example, cross peaks labeled “1” and “2” in the spectra were detected with mixing times as short as 1.6 ms, whereas other signals only appear at longer mixing times. To corroborate the presence of these multiple 19F signals in the RFDR spectra, the experiments were performed at MAS frequencies of 40 kHz, 50 kHz and 60 kHz with the RFDR mixing times set to 3.2 ms and 8.0 ms. As shown in Fig. 5g, the spectral resolution increases considerably with the MAS frequency, and the individual peaks become well resolved at 60 kHz. The line widths for the diagonal peaks are 0.35, 0.30, and 0.27 ppm at 40, 50, and 60 kHz, respectively. Furthermore, as a result of enhanced transfer efficiencies at higher spinning frequencies, the peak intensities increase by approximately 25% with every 10 kHz increase in the MAS frequency.
To assign the individual 19F resonances to inequivalent CF3 groups in the unit cell, DFT calculations were performed for the cluster of 8 molecules shown in Fig. 1b. The resulting calculated shifts for molecules a, b and c are distinct (Table 1), consistent with the experimental findings. The assignments of 19F chemical shifts belonging to inequivalent groups and the corresponding intra-and intermolecular 19F-19F correlations are consistent with the observed relative cross peak intensities in the RFDR spectra, acquired with different mixing times (Fig. 5e) and the buildup profiles for the different cross peak intensities (Fig. 5f). Specifically, intramolecular correlations between the 2CF3 and 8CF3 moieties are detected at a mixing time as low as 1.6 ms, whereas correlations for medium- and long-range intermolecular contacts appear only at mixing times of 4.0 ms or longer. Intermolecular 8CF3-8CF3 correlations between inequivalent CF3 groups are strong at the 1.6 ms mixing time, consistent with the short 3.5 Å distances.
Table 1.
MAS NMR Experimental and DFT Calculated 19F Isotropic Chemical Shifts for Mefloquine
| Crystallographically inequivalent molecules | Functional group | δiso (ppm) MAS NMR | δiso (ppm) DFT |
|---|---|---|---|
|
| |||
| a | 2CF3 | 8.8 | 7.4 |
| a | 8CF3 | 15.6 | 15.5 |
| b | 2CF3 | 8.2 | 1.5 |
| b | 8CF3 | 16.1 | 16.1 |
| c | 2CF3 | 8.6 | 1.6 |
| c | 8CF3 | 15.8 | 14.9 |
Taken together, these above findings indicate that 19F chemical shifts are very sensitive to the slight differences in local environments, allowing for the observation and assignment of inequivalent fluorine positions.
Measurement of Accurate Interfluorine Distances
We determined 19F-19F distances using 19F DANTE-RFDR magnetization exchange profiles. The original version of the experiment by McDermott and coworkers15 was modified such that a DANTE excitation pulse train was applied on either 2CF3 or 8CF3. followed by a non-selective homonuclear mixing using RFDR (Fig. 6a). The resulting DANTE-RFDR spectra are shown in Fig. 6b,c and Fig. S8 of the Supporting Information. Increased intensity for the CF3 signals is clearly observed for increasing RFDR mixing times from 0.8 ms to 16 ms, while without RFDR mixing, no intensity buildup is observed.
Figure 6.
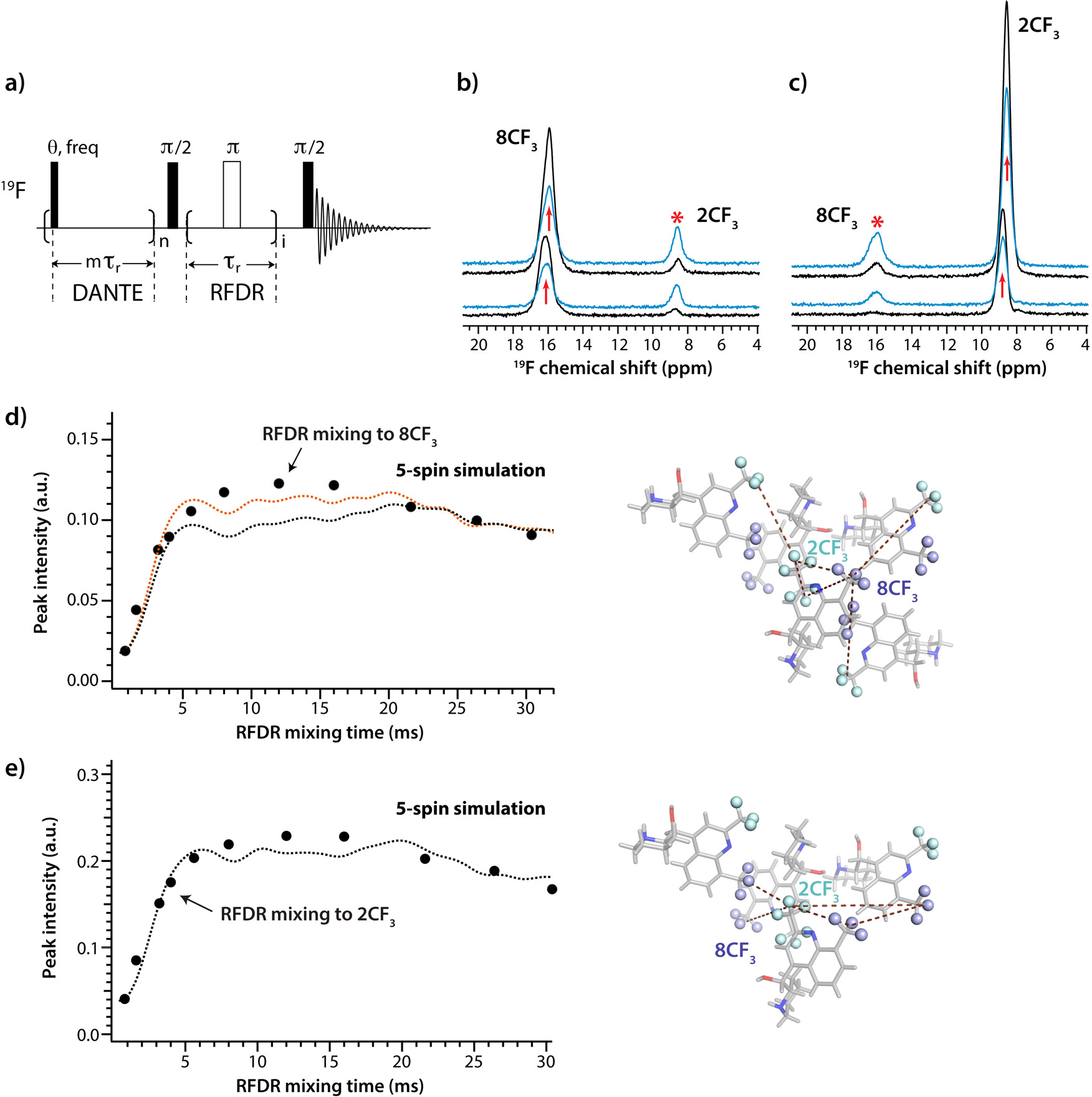
a) Pulse sequence for the 1D RFDR experiment with 19F DANTE-excitation. b),c) 1D 19F-19F DANTE-RFDR spectra with DANTE 90° selective excitation applied to the 19F resonances of 2CF3 (b) and 8CF3 (c), respectively. Spectra acquired with RFDR mixing times of 1.6 ms and 8.0 ms are shown in black and blue, respectively. The position of the DANTE excitation is shown with arrows and the resonances to which the magnetization was transferred by asterisks. d),e) Left: Experimental and simulated 19F-19F DANTE-RFDR magnetization exchange curves for the 8CF3 (d) and 2CF3 (e) resonances. The experimental data points are shown as black circles, the simulated curves, as dashed lines. In d), the 2CF3 spins were excited by DANTE pulses and magnetization was transferred to the 8CF3 spins during RFDR mixing period. In e), DANTE excitation was applied to the 8CF3 resonances and magnetization was transferred to the 2CF3 groups. Errors in the data points as defined by the standard deviation of the noise in a region of over 10 ppm are smaller than the size of the circles. The RMSDs of the simulated DANTE-RFDR magnetization exchange curves (dashed lines) are 0.008 (orange, the 2CF3 — 2CF3 distance is 7.2 Å) and 0.014 (black, the 2CF3 — 2CF3 distance is 7.4 Å) for 8CF3 (d) and 0.014 for 2CF3 (e). Right: Sets of interfluorine distances used in the 5-spin simulations, see also Table 3.
19F-19F DANTE-RFDR magnetization exchange profiles (Fig. 6d,e) are clearly dominated by multi-spin effects, similar to our recent findings for crystalline difluorobenzoic acids24. Numerical simulations were performed to extract interfluorine distances. In order to account for multi-spin effects, we constructed a large number of 2-spin, 3-spin, 4-spin, 5-spin, and 6-spin systems using combinations of the interfluorine distances from the crystal structure as a set of starting values, which were then varied to assess the resulting error (Fig. S9–10 of the Supporting Information). Clearly, 5 spins are necessary and sufficient to reproduce the experimental magnetization exchange curves (Fig. S9). Analysis of the simulated magnetization exchange profiles for different distance combinations showed that the interfluorine distance between the spin excited by the DANTE sequence and the spin to which the magnetization is transferred dominate the exchange profiles. This is clearly seen from the comparison of the simulation of a 2-spin system with a 2CF3-8CF3 distance of 5.4 Å and that of a 5-spin system with four different 2CF3-8CF3 distances (with the shortest one being 5.4 Å) and one additional 2CF3-2CF3 distance (Fig. S10b). The shortest distance determines the initial buildup rate while the addition of other longer distances influences the detailed oscillation profile after the first intensity maximum. Taking longer distances into account distinctly improves the agreement of the fit with the experimental curve. The overall accuracy of the interfluorine distances extracted from DANTE-RFDR experiments is 0.1–0.2 Å for distances shorter than 7.0 Å and 0.2–0.4 Å for distances in the 7.0 – 11.0 Å range, similar to our recent findings for difluorobenzoic acids24.
We also carried out simulations using several 5-spin systems in which the intramolecular distance between 2CF3 and 8CF3 groups as well as several intermolecular distances were included. Excellent agreement is observed between the simulated and experimental curves if intra- and intermolecular 19F-19F distances shorter than 11 Å are taken into account (Fig. 6d–e). The best agreement for the 2CF3 exchange curve is observed for a 2CF3-8CF3 intramolecular distance of 5.5 6, and intermolecular distances of 8.0 Å, 9.9 Å and 10.5 Å, with an added 8CF3-8CF3 distance of 7.0 Å included as well. Likewise, the best agreement for 8CF3 group was obtained with identical 2CF3-8CF3 distances and two additional intermolecular 2CF3-2CF3 distances of 7.4 Å and 10.6 Å. Remarkably, the 2CF3-2CF3 distance determined by the simulation is 7.2 6, only 0.2 6 different from the actual distance of 7.4 Å in the X-ray crystal structure. Overall, all distances determined by the simulations are consistent with 19F-19F distances within the cluster of CF3 groups in the crystal lattice.
DISCUSSION
While DANTE-RFDR protocols for accurate interfluorine distance measurements have been applied here for mefloquine, whose X-ray structure is known, it is important to point out that the present 19F fast MAS NMR crystallography approach is applicable for analysis of any crystalline fluorinated solid of unknown structure. By integrating the information from 19F-19F DANTE-RFDR magnetization exchange curves with 19F, 13C, and 1H chemical shifts and various internuclear correlations, structures can be determined without the need for single crystal diffraction data, as has been shown for other systems40. In our earlier study, we have demonstrated that an unbiased grid search could be successfully applied to derive distance distributions from the experimental 19F-19F DANTE-RFDR data in 2,5-difIuorobenzoic acid, and that five-spin systems are sufficient to reproduce the magnetization exchange profiles for many organic crystals with extensive coupling networks24. In principle, the experiments discussed in this work can also be applied to amorphous systems, albeit with likely lower information content due to their associated structural heterogeneity and broader line widths.
Magnetic field strength is an important consideration for the 19F fast MAS NMR experiments described here, given that the 19F chemical shift range and chemical shift anisotropy (CSA) are proportional to the field strength. We see clear benefits of higher magnetic fields for fast MAS 19F spectroscopy: going from 11.7 T to 16.4 T to 19.9 T in experiments on mefloquine results in increased sensitivity and resolution. Naturally, higher fields are generally advantageous when multiple fluorine sites with similar isotropic chemical shifts are present in the system. This is certainly seen for mefloquine, investigated here, but applies also to fluorinated tryptophans,25 difluorobenzoic acids24 as well as HIV-1 CA protein assemblies labeled with 5F-Trp residues,26 which we reported on previously. At this juncture it is important to note is that for many fluorinated organic moieties the 19F CSA lie in the 40–80 ppm range, which can be efficiently averaged out upon spinning at MAS frequencies exceeding 40 kHz, even at high magnetic fields (19.9 T).
As to the choice of MAS frequency, as observed here for mefloquine and in previous studies,24–26 spinning frequencies of at least 40 kHz are required to obtain well-resolved spectra in the absence of decoupling, with further gains in sensitivity and resolution seen at 60 kHz MAS. As shown here, spinning frequencies of 40 kHz and higher were critical for acquiring high-resolution/high-sensitivity 19F-13C/13C-19F and 19F-1H/1H-19F HETCOR spectra of mefloquine. Even further gains in sensitivity and resolution are anticipated at ultrafast MAS frequencies of 111 kHz and above.
CONCLUSIONS
A fast MAS 19F NMR approach for the structural characterization of fluorine-containing natural abundance pharmaceutical compounds is presented. 19F-13C HETCOR and 19F-19F RFDR experiments together with DFT calculations readily permit assignments and identification of inequivalent sites in the crystal. Accurate interfluorine distances are obtained from DANTE-RFDR magnetization exchange profiles and multi-spin numerical simulations. The NMR crystallography approach presented here can be extended to pharmaceuticals of unknown structures and is broadly applicable to organic and biological molecules, including crystalline organic compounds, peptides and proteins as well as protein assemblies possessing long-range order, such as assemblies of virus proteins and amyloid fibrils.
MATERIALS AND METHODS
Chemicals
Natural abundance mefloquine hydrochloride was purchased from Acros Organics and used without further recrystallization. For MAS NMR experiments, sample amounts were as follows: 3 mg (1.3 mm rotor for measurements at 11.7 T), 3.7 mg (1.3 mm rotor for measurements at 14.1 T), 9.5 mg (1.6 mm rotor for measurements at 16.4 T), and 13.5 (1.9 mm rotor for measurements at 19.9 T).
MAS NMR spectroscopy
19F and 13C-detected experiments were performed on a 20.0 T narrow bore Bruker AVANCE III spectrometer outfitted with a 1.9 mm HX MAS probe. The Larmor frequencies were 850.4 MHz for 1H, 800.1 MHz for 19F and 213.8 MHz for 13C. For all 19F-detected experiments, the 1H channel was tuned to 19F. All MAS NMR spectra were acquired at a MAS frequency of 40 kHz maintained within ± 10 Hz by Bruker MAS III controller. The sample temperature was calibrated with KBr as an external temperature sensor and was maintained at 12.0±0.3 °C by a Bruker variable temperature controller. Typical 90° pulse lengths were 1.5 μs for 1H, 1.1 is for 19F and 3.0 μs for 13C. 19F chemical shifts were referenced externally with respect to those of trifluoroacetic acid (100 μM solution in 25 mM sodium phosphate buffer, pH 6.5) used as an external reference (0 ppm), which relates to other commonly used reference standards as: neat trifluoroacetic acid (−2.8 ppm), trichloro-fluoro-methane (73.55 ppm), Teflon (−48.45 ppm). 13C chemical shifts were referenced externally to adamantane.
The 19F-13C cross-polarization was performed with a linear amplitude ramp of 70–100 % on 13C and the center of ramp was Hartmann-Hahn matched at the first or second spinning sideband; the carrier frequency on 13C was set to 100 ppm. For optimization of 19F-13C CP, 19F rf fields of 15, 25, 30, 35, 45 and 55 kHz were applied, and Hartmann-Hahn matched at 1~3 times of the spinning frequency (vr). Zero quantum (ZQ) or double quantum (DQ) CP was matched with the 19F rf field fixed while the 13C rf field was systematically varied over a range of 0 kHz to 75 kHz. 19F-13C CPMAS spectra were acquired with 512 scans and CP contact times varied systematically from 1.0 ms to 10.0 ms; the rf fields were 15 kHz for 19F and 25 kHz (DQ-CP) or 55 kHz (ZQ-CP) for 13C. For 2D 19F-13C HETCOR experiments, the CP contact times were 1.0, 7.0 and 10.0 ms; both DQ-CP and ZQ-CP conditions were used; 38 complex points were acquired in t2 dimension. The carrier frequencies in 13C were set to 100.0 ppm. In several experiments, π-pulse 19F decoupling at RF field of 208 kHz was applied during evolution in 13C dimension. A recycle delay of 6.0 s was used for all experiments.
For 1H-13C CPMAS experiments, the 1H-13C cross polarization was performed with a linear ramp; the 1H and 13C RF fields were at 13 kHz and 28 kHz, respectively; the typical CP contact times were 0.5–1.4 ms. 2D 1H-13C HETCOR spectra with 0.5 ms and 1.1 ms CP contact were acquired with 448 and 384 transients, respectively; 80 complex points were collected in the indirect dimension.
2D 19F-19F RFDR spectra were acquired without decoupling with RFDR mixing times of 1.6 ms, 4 ms, 8 ms, 12 ms, 20 ms and 30.4 ms. The typical length of the RFDR π pulse was 8.3 μs and a XY-16 phase cycle was used during the RFDR mixing. For each 19F-19F spectrum, the data were collected with 120 complex points in t2 dimension using States-TPPI phase sensitive detection; 16 transients were averaged for each FID. The 1D 19F DANTE spectra were acquired with 16 scans; 22 0.1-μs DANTE pulses were applied at 8.8 ppm and 15.9 ppm for selective irradiation of 2CF3 and 8CF3 signals, respectively. The DANTE interpulse delay was set to 4 rotor cycles. The recycle delay was 5.0 s.
19F-detected single pulse excitation spectra were also acquired on a 11.7 T wide bore Bruker AVANCE III spectrometer outfitted with a 1.3 mm HFX MAS probe. The Larmor frequencies were 500.13 MHz for 1H and 470.59 MHz for 19F. The MAS frequencies were 10, 40, and 60 kHz maintained within ±10 Hz by Bruker MAS III controller. Spinal-6441 (10 kHz MAS) or swept-frequency two-pulse phase modulation (SWf-TPPM)42 heteronuclear decoupling sequences were applied with the 1H RF field strengths of 90 kHz, 15 kHz, and 10 kHz for the MAS frequencies of 10 kHz, 40 kHz, and 60 kHz, respectively. 19F 90° pulse length was 2.45 μs. The recycle delay was 6.0 s.
Additional 2D 19F-19F RFDR spectra were recorded 14.1 T, on a Magnex narrow-bore magnet interfaced with a Bruker AVIII HD spectrometer, and outfitted with a 1.3 mm Bruker HCN MAS probe. The H channel was tuned to the 19F Larmor frequency of 564.35 MHz and the typical 19F 90° pulse length was 3.3 μs for. 19F-19F RFDR spectra were recorded for MAS frequencies of 40 kHz, 50 kHz and 60 kHz with RFDR mixing times of 3.2 ms and 8.0 ms, 16 transients were averaged and the recycle delay was 2.0 s. The pulse length for the DANTE selective excitation pulses was 0.1 μs. The interpulse delay was set to 2 rotor cycles. The DANTE-RFDR magnetization exchange curves were recorded with RFDR mixing times of 0.8, 1.6, 3.2, 4.0, 5.6, 8.0, 12.0, 16.0, 21.6, 26.4, and 30.4 ms; (XY8)14 phase cycle43 was applied during the RFDR mixing.
Supplemental 19F-13C CPMAS and HETCOR NMR spectra were acquired on a 16.4 T Bruker spectrometer equipped with a PhoenixNMR 1.6 mm HFX MAS probe at a MAS frequency of 40 kHz. The Larmor frequencies were 700.1 MHz for 1H, 658.8 MHz for 19F and 176.0 MHz for 13C. The typical 90° pulse lengths were 2.5 μs for 1H, 2.0 μs for 19F, and 1.97 μs for 13C. The 19F-13C CP contact time was 7.0 ms. 1H and 19F decoupling was applied simultaneously during the t2 evolution in the 13C dimension. 1H decoupling used low-power XiX35 with an rf field of 12.5 kHz. For 19F decoupling, a π-pulse with the rf field of 125 kHz was applied every rotor period. A spin echo 1H π-pulse was applied in the center of the t1 evolution in the 19F dimension to refocus the 1H offset and heteronuclear coupling. The recycle delay was 3 s.
All spectra were processed in TopSpin 4.0 and analyzed with Sparky44 and Mnova.
Numerical simulations
The DANTE-RFDR magnetization exchange curves were simulated using SIMPSON45 (version 3.1.0). In the multi-spin simulation, the magnetization exchange was followed starting from the non-selectively irradiated spin and the evolution of the spin to which the magnetization is transferred was performed from l2x to −l1z. The experimental decay curves of the signals that are selectively excited by the DANTE pulse are scaled to 1 and the experimental buildup curves of the nonselective signals are scaled to 0. The simulated DANTE-RFDR exchange curves were rescaled to match the experimental intensities. The example simulation script is present in the Supporting Information.
DFT calculations
19F and 13C magnetic shielding tensor calculations were carried out in Gaussian 09 (Revision D.01)46. Molecular clusters of mefloquine comprising 8 molecules were generated from the crystal structures by Pymol47. All-atom geometry optimizations were performed using a M06 functional with the cc-pVTZ basis set and geometry-optimized models were used for magnetic shielding tensor calculations at the same level of theory. The chemical shifts were referenced by converting absolute magnetic shielding constants, σ, into absolute chemical shifts, using the relation δi = σref – σi with the value of σref determined by linear regression between calculated and experimental shifts48.
Supplementary Material
Table 3.
Sets of Interfluorine Distances for the 5-spin Simulations and in the X-ray Crystal Structure
| Type of Interfluorine Distances | Simulation | X-ray |
|---|---|---|
|
| ||
| 2CF3 – 8CF3 | 5.5 Å | 5.5 Å |
| 8.0 Å | 8.0 Å | |
| 9.9 Å | 9.9 Å | |
| 10.5 Å | 10.5 Å | |
|
| ||
| 2CF3 – 2CF3 (Simulation for 8CF3 resonance) | 7.4 Å / 7.2 Å | 7.4 Å |
| 10.6 Å | ||
|
| ||
| 8CF3 – 8CF3 (Simulation for 2CF3 resonance) | 7.0 Å | 7.0 Å |
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (NSF Grant CHE-1708773 to AMG and TP) and by the National Institutes of Health (NIH Grant PSOAI150481, Technology Development Project 2) and is a contribution from the Pittsburgh Center for HIV Protein Interactions. We acknowledge the support of the National Science Foundation (NSF grant CHE-0959496) for the acquisition of the 850 MHz NMR spectrometer and of the National Institutes of Health (NIH Grant P30GM110758) for the support of core instrumentation infrastructure at the University of Delaware.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPORTING INFORMATION
1D 19F-13C CPMAS spectra of mefloquine acquired at various Hartmann-Hahn matching conditions; 13C chemical shift assignments from DFT calculations and 1H-13C CPMAS spectra; 2D 19F-13C HETCOR spectra acquired at various conditions; 2D 19F-19F RFDR spectrum and representative 1D traces; 19F spectra with selective DANTE excitation and inversion pulses applied at different frequencies; 1D 19F DANTE-RFDR spectra acquired with various RFDR mixing times; experimental and simulated 19F DANTE-RFDR magnetization exchange curves for 2CF3 and 8CF3 groups; SNR for different 19F-13C CP conditions; intra- and intermolecular 19F-19F distances in the crystal structure; an example script for DANTE-RFDR multispin simulations. This information is available online at http://pubs.acs.org.
REFERENCES
- (1).Purser S; Moore PR; Swallow S; Gouverneur V Chem. Soc. Rev. 2008, 37, 320–330. [DOI] [PubMed] [Google Scholar]
- (2).Zhou Y; Wang J; Gu Z; Wang S; Zhu W; Luis Acena J; Soloshonok VA; Izawa K; Liu H Chem. Rev. 2016, 116, 422–518. [DOI] [PubMed] [Google Scholar]
- (3).Mei HB; Han JL; Fustero S; Medio-Simon M; Sedgwick DM; Santi C; Ruzziconi R; Soloshonok VA Chem. - Eur. J. 2019, 25, 11797–11819. [DOI] [PubMed] [Google Scholar]
- (4).Inoue M; Sumii Y; Shibata N ACS Omega 2020, 5, 10633–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gakh YG; Gakh AA; Gronenborn AM Magn. Reson. Chem. 2000, 38, 551–558. [Google Scholar]
- (6).Gerig JT httDS://Www.biODhvsics.oro/Portals/0/BPSAssets/Articles/oerio.Ddf 2001.
- (7).Zhao Y; Markopoulos G; Swager TM J. Am. Chem. Soc. 2014, 136, 10683–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Tengel T; Fex T; Emtenas H; Almqvist F; Sethson I; Kihlberg J Org. Biomol. Chem. 2004, 2, 725–731. [DOI] [PubMed] [Google Scholar]
- (9).Norton RS; Leung EWW; Chandrashekaran IR; MacRaild CA Molecules 2016, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lu MM; Ishima R; Polenova T; Gronenborn AM J. Biomol. NMR 2019, 73, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Matei E; Gronenborn AM Angewandte Chemie (International ed. in English) 2016, 55, 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sharaf NG; Gronenborn AM Methods in enzymology 2015, 565, 67–95. [DOI] [PubMed] [Google Scholar]
- (13).Boeszoermenyi A; Chhabra S; Dubey A; Radeva DL; Burdzhiev NT; Chanev CD; Petrov OI; Gelev VM; Zhang M; Anklin C; Kovacs H; Wagner G; Kuprov I; Takeuchi K; Arthanari H Nat. Methods 2019, 16, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Danielson MA; Falke JJ Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 163–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Gilchrist ML; Monde K; Tomita Y; Iwashita T; Nakanishi K; McDermott AE J. Magn. Reson. 2001, 152, 1–6. [DOI] [PubMed] [Google Scholar]
- (16).Lu X; Skomski D; Thompson KC; McNevin MJ; Xu W; Su Y Anal. Chem. 2019, 9f, 6217–6224. [DOI] [PubMed] [Google Scholar]
- (17).Kozorog M; Sani M-A; Separovic F; Anderluh G Chemistry — A European Journal 2018, 24, 14220–14225. [DOI] [PubMed] [Google Scholar]
- (18).Roos M; Mandala VS; Hong MJ Phys. Chem. B 2018, f22, 9302–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Roos M; Wang T; Shcherbakov AA; Hong MJ Phys. Chem. B 2018, 122, 2900–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Shcherbakov AA; Hong MJ Biomol. NMR 2018, 71, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shcherbakov AA; Roos M; Kwon B; Hong MJ Biomol. NMR 2020, 74, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Su YC; DeGrado WF; Hong MJ Am. Chem. Soc. 2010, 132, 9197–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Su YC; Doherty T; Waring AJ; Puchala P; Hong M Biochemistry 2009, 48, 4587–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Fritz M; Kraus J; Quinn CM; Yap GPA; Struppe J; Sergeyev IV; Gronenborn AM; Polenova TJ Phys. Chem. B 2019, 123, 10680–10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lu M; Sarkar S; Wang M; Kraus J; Fritz M; Quinn CM; Bai S; Holmes ST; Dybowski C; Yap GPA; Struppe J; Sergeyev IV; Maas W; Gronenborn AM; Polenova TJ Phys. Chem. B 2018, 122, 6148–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang M; Lu M; Fritz MP; Quinn CM; Byeon I-JL; Byeon C-H; Struppe J; Maas W; Gronenborn AM; Polenova T Angew. Chem. Int. Ed. 2018, 57, 16375–16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wi S; Sinha N; Hong MJ Am. Chem. Soc. 2004, 126, 12754–12755. [DOI] [PubMed] [Google Scholar]
- (28).Abraham A; Crull G Mol. Pharmaceutics 2014, 11, 3754–3759. [DOI] [PubMed] [Google Scholar]
- (29).Lu X; Huang C; Li M; Skomski D; Xu W; Yu L; Byrn SR; Templeton AC; Su YJ Phys. Chem. B 2020, 124, 5271–5283. [DOI] [PubMed] [Google Scholar]
- (30).Lu M; Wang M; Sergeyev IV; Quinn CM; Struppe J; Rosay M; Maas W; Gronenborn AM; Polenova TJ Am. Chem. Soc. 2019, 141, 5681–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Karle JM; Karle IL Antimicrob. Agents Chemother. 2002, 46, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Censi R; Di Martino P Molecules 2015, 20, 18759–18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Gupta H; Kumar S; Roy SK; Gaud RS J Pharm Bioallied Sci 2010, 2, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Liu SF; Schmidt-Rohr K Macromolecules 2001, 34, 8416–8418. [Google Scholar]
- (35).Ernst M; Samoson A; Meier BH J. Magn. Reson. 2003, 163, 332–339. [DOI] [PubMed] [Google Scholar]
- (36).Grage SL; Ulrich AS J. Magn. Reson. 2000, 146, 81–88. [DOI] [PubMed] [Google Scholar]
- (37).Wang XL; Mallory FB; Mallory CW; Beckmann PA; Rheingold AL; Francl MM J. Phys. Chem. A 2006, 110, 3954–3960. [DOI] [PubMed] [Google Scholar]
- (38).Bodenhausen G; Freeman R; Morris GA J. Magn. Reson. 1976, 23, 171–175. [Google Scholar]
- (39).Morris GA; Freeman RJ Magn. Reson. 1978, 29, 433–462. [Google Scholar]
- (40).Martineau C; Senker J; Taulelle F Annual Repods on NMR Spectroscopy 2014, 82, 1–57. [Google Scholar]
- (41).Fung BM; Khitrin AK; Ermolaev K J Magn Reson 2000, 142, 97–101. [DOI] [PubMed] [Google Scholar]
- (42).Augustine C; Kurur ND J. Magn. Reson. 2011, 209, 156–160. [DOI] [PubMed] [Google Scholar]
- (43).Shen M; Hu B; Lafon O; Trebosc J; Chen Q; Amoureux J-PJ Magn. Reson. 2012, 223, 107–119. [DOI] [PubMed] [Google Scholar]
- (44).Goddard TD; Kneller DG University of California, San Francisco. [Google Scholar]
- (45).Bak M; Rasmussen JT; Nielsen NC J. Magn. Reson. 2000, f47, 296–330. [DOI] [PubMed] [Google Scholar]
- (46).Frisch MJ; Trucks GW; Schlegel HB; Scuseria GE; Robb MA; Cheeseman JR; Scalmani G; Barone V; Mennucci B; Petersson GA; Nakatsuji H; Caricato M; Li X; Hratchian HP; Izmaylov AF; Bloino J; Zheng G; Sonnenberg JL; Hada M; Ehara M, et al. Gaussian, Inc., Wallingford CT 2009. [Google Scholar]
- (47).Schrodinger, LLC.
- (48).Baias M; Dumez J-N; Svensson PH; Schantz S; Day GM; Emsley LJ Am. Chem. Soc. 2013, 135, 17501–17507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


