Abstract
Background
Choline, as a neurotransmitter acetylcholine precursor, is reportedly associated with cognitive function. Although there are several cohort and animal studies on choline-containing foods and cognitive function, only a few interventional studies were reported. Egg yolk is a rich source of different choline-containing chemical forms, such as phosphatidylcholine (PC), lysophosphatidylcholine (LPC), and α-glycerophosphocholine (α-GPC). This study aimed to investigate the effect of consuming 300 mg of egg yolk choline per day on cognitive function of Japanese adults.
Methods
A 12-week, randomized, double-blind, placebo-controlled, parallel-group study was conducted in 41 middle-aged and elderly males and females (43.9% female) aged ≥ 60 years and ≤ 80 years without dementia. Participants were randomly assigned to placebo and choline groups. The choline group received a supplement containing egg yolk choline (300 mg/day), and the placebo group received an egg yolk supplement free from choline for 12 weeks. Assessments of Cognitrax, Trail Making Tests (TMT) part A and B, the MOS 36-Item Short-Form Health Survey (SF-36), the Simplified Japanese Version of the WHO-Five Well-Being Index (WHO-5), and plasma choline levels were performed before and 6 and 12 weeks after supplement intake. In the present study, 19 subjects (9 in the placebo group and 10 in the choline group) were excluded due to the violation of the discontinuation criteria or participant compliance, and 41 subjects were analyzed.
Results
The change amount of verbal memory scores and verbal memory test-correct hit (delay) was significantly higher in the choline group than in the placebo group at baseline-6 and baseline-12 weeks. The plasma free choline level was significantly higher in the choline group compared with the placebo group at 6 weeks. Conversely, the choline group showed significantly lower Cognitrax processing speed scores, symbol digit coding testing correct responses, and SF-36 physical quality of life summary scores compared to the placebo group at 6 weeks.
Conclusions
The results suggested that continued 300 mg/day intake of egg yolk choline improved verbal memory, which is a part of cognitive functions. To confirm the observed effects of egg yolk choline, more well-designed and large-scale studies are warranted.
Trial registration
Study protocols were pre-registered in the Clinical Trials Registration System (UMIN-CTR) (UMIN 000045050).
Keywords: Phosphatidylcholine, Egg yolk, Cognitive function, Verbal memory, Clinical trial
Background
Recently, population aging has progressed worldwide. The number of people aged ≥ 60 years is expected to increase by 2 billion till 2050 [1]. Accompanying with this dramatic increase in aged population, the number of people affected by dementia is also increasing currently, with an estimated 132 million in 2050 globally[2]. Conditions that are not dementia but have mild cognitive decline are referred to as mild cognitive impairment (MCI). People with MCI are at risk for conversion to dementia while it has been known to recover to normal cognitive function. Since recovering to normal cognitive function is impossible once the cognitive status of dementia is achieved, it is important to prevent dementia at the stage of MCI before dementia [3–6].
Exercise habituation, smoking cessation, nutritional intervention, and social activity are recommended to reduce the risk of developing dementia [7]. In a study conducted in Japan, one of the foods that are expected to be effective in preventing dementia is chicken eggs, which is listed as one of the foods recommended to increase its consumption in old age [8]. A prospective cohort study in Finland reported that individuals with high egg ingestion have better cognitive function than those with low egg intake [9], suggesting association with egg intake and cognitive function.
PC, one of the choline compound in the egg yolk, is the popular source of choline, a precursor to the neurotransmitter acetylcholine, which is hypothesized to help maintain cognitive function [10, 11]. The amount of acetylcholine in the brain decreases with age due to a lack of the enzyme that converts choline to acetylcholine, resulting in decline of memory. Acetylcholine decrease also causes Alzheimer’s disease, a type of dementia [12]. In animal studies, significant differences were reported in memory and learning abilities between mice fed a free choline-deficient diet and a free choline-enriched diet [13]. Furthermore, administration of PC from hen’s eggs increased brain acetylcholine levels and improved memory and learning abilities [14, 15]. In contrast, intake of lecithin after the progression of Alzheimer’s dementia showed no improved effect on cognitive function, hypothesizing the importance of taking a choline compound before cognitive decline progresses [16].
The choline compounds in egg yolk are composed primarily PC, and its metabolites, LPC and α-GPC . They are collectively called egg yolk choline. Although there are various chemical forms of choline, we focused on egg yolk choline because of the high proportion of PC, the phospholipid form in foods.
Therefore, a 12-week, randomized, double-blind, placebo-controlled, parallel-group study was conducted to investigate the effect of continuous intake of egg yolk choline on cognitive function. Subjects were healthy, middle-aged, and elderly Japanese males and females aged 60 to 80 years without dementia who were concerned or had been pointed out by others about their forgetfulness.
Methods
Subjects
The participants were 60 middle-aged and elderly Japanese males and females aged 60–80 years without dementia who had been made aware of their forgetfulness or had it pointed out by others. Moreover, they were judged not to be ill by a clinical investigator and with 26 points or more on the Mini-Mental State Examination-Japanese (MMSE-J) screening test [17, 18]. Exclusion criteria included the following: a history of psychiatric disorders, cerebrovascular diseases, or other serious diseases; a Geriatric Depression Scale Short Version-Japanese (GDS-S-J) of ≥6 points in the screening test [19]; use of medicines that may affect the central nervous system, foods with health claims to improve cognitive function, and other medicines that may affect the test results; regular use of foods with health claims to improve blood flow or have an antioxidative effect, which may contribute to improving cognitive function; impaired in vision and hearing; impaired daily living activities; excessive smoking and alcohol consumption; extremely irregular dietary habits; allergies to the supplement ingredients; participation in other studies; and those who were judged by the investigator to be inappropriate as participants.
The sample size was determined according to a previous pilot study [20]. The number of samples required was ≥ 39 when type 1 risk α was 0.05 and power 1-β was 80%. The number of participants in this study was 60, 30 per group, to ensure this number of people. Sample size calculations were according to Cancer research and biostatistics statistical tools (https://stattools.crab.org/). The investigator fully explained the purpose and content of the study to the participants and provided written consent based on the participants free will before conducting the study.
Study design
The study was conducted at KSO Inc. (Tokyo, Japan) from August to December 2021, under the supervision of Iwama Y. as the principal investigator with support from a project team composed of a doctor, a clinical laboratory technician, and a nutritionist. This 12-week, randomized, double-blind, placebo-controlled, parallel-group study divided the participants into the placebo and choline groups by the block-randomization method.
The Cognitrax test [21] and Trail Making Tests (TMT) part A and B [22] were performed for the assessment of cognitive function. To assess the quality of life (QOL) of participants, MOS 36-Item Short-Form Health Survey (SF-36) [23, 24] and the Simplified Japanese Version of the WHO-Five Well-Being Index (WHO-5) [25] were performed before and 6 and 12 weeks after intake of capsules. The cognitive function test was performed in a private room with one clinical psychologist per participant and no other interference. Additionally, blood was withdrawn to analyze the plasma choline level at each time point. Dietary surveys with brief-type self-administered diet history questionnaires (BDHQs) were performed [26, 27] and blood and urine samples were taken for safety evaluation before and 12 weeks after intake.
There were no dropouts during the study period, but five participants were excluded due to a substantial change in the living environment during the study period, a violation of study limitations, and a lack of reliability. One participant could not take the 12-weeks test on that day due to illness, and only a safety evaluation was conducted six days later. Furthermore, 14 subjects were excluded because they had 10% or more changes in the clinical test that have been reported to be associated with cognitive function [28, 29], before and after intake and deviated from one or more of the criteria (systolic blood pressure of < 140 mmHg, diastolic blood pressure of < 90 mmHg, pulse rate of < 100 bpm, and LDL-cholesterol of < 140 mg/dL). Thus, the analysis included 41 participants with 20 in the placebo group and 21 in the choline group. The detailed flow diagram of the study is shown in Figure 1.
Fig. 1.
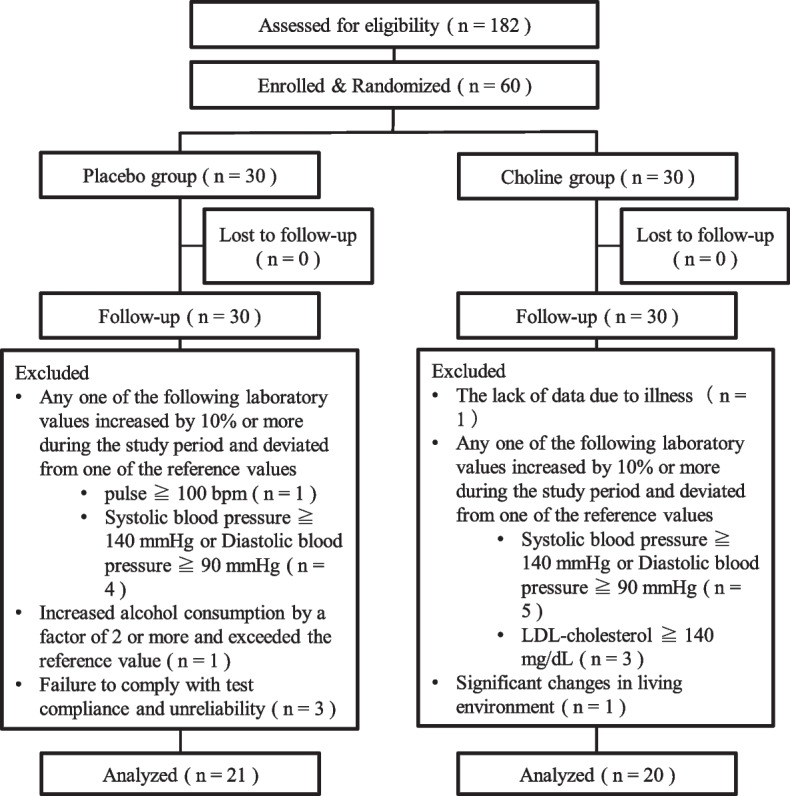
Flow chart
Supplements
The supplements used in this study were soft capsule-shaped and were manufactured by Aliment Industry Co., Ltd. (Yamanashi, Japan). In the previous study, it was reported that 300 mg of egg yolk choline per day maintained verbal memory, one of the typical cognitive functions [20]. Therefore, egg yolk oil, including 300 mg of egg yolk choline, was included (Kewpie Corporation, Tokyo, Japan) in supplements for the choline group, and lecithin-free egg yolk oil was included (Kewpie Corporation, Tokyo, Japan) in supplements for the placebo group. Table 1 shows the nutrient composition of each supplement. Participants consumed seven capsules per day after breakfast. Egg yolk choline is presented in PC equivalent and in theoretical value. The choline value is calculated from egg yolk choline.
Table 1.
Nutrient composition of each capsule (/7 capsule)
| Placebo | Choline | ||
|---|---|---|---|
| Energy | (kcal) | 21 | 22 |
| Protein | (g) | 0.7 | 0.7 |
| Fat | (g) | 1.5 | 1.5 |
| Carbohydrates | (g) | 0.4 | 0.4 |
| Salt equivalent | (g) | 0.0 | 0.0 |
| Egg yolk choline in PC equivalent* | (mg) | 0.0 | 289.6 |
| Choline† | (mg) | 0.0 | 38.8 |
*The value was presented as a theoretical value
†The value presented was calculated from egg yolk choline
Primary endpoint
As the primary endpoint, the Cognitrax test (Health Solution Inc., Tokyo, Japan), a computer-based cognitive test with a Japanese version developed by CNS Vital Signs, LLC. (USA) [21], was administered. Cognitrax comprised 7 test items (verbal memory [VBM], visual memory [VIM], finger tapping [FTT], symbol digit coding [SDC], Stroop test [ST], shifting attention [SAT], and continuous performance [CPT]), and the results were shown as 11 domain scores (composite memory, VBM, VIM, psychomotor speed, reaction time, complex attention, cognitive flexibility, processing speed, executive function, simple attention, and motor speed).
Secondary endpoints
As secondary endpoints, five tests were conducted. All tests other than safety evaluations were conducted before the test and 6 and 12 weeks after intake. Safety evaluations were conducted before the test and 12 weeks after intake.
Trail making test
TMT part A (TMT-A) and part B (TMT-B) were performed to assess attention and executive function. Participants were asked to connect the numbers from 1 to 25 in TMT-A and to connect the numbers from 1 to 13 and the 12 Japanese letters alternatively in TMT-B as early as possible. The time taken to perform the test and the number of errors were assessed.
SF-36
SF-36 consisted of 36 questions to measure eight health concept subscales (physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role, and mental health). Additionally, physical component summary scores (PCS), mental component summary scores, and role/social component summary scores were calculated from eight subscales.
WHO-5
WHO-5 consisted of five questions (cheerful and in good spirits, calm and relaxed, active and vigorous, wake up feeling fresh and rested, things that interest me) to assess the condition in the last 2 weeks, and each score and total score were calculated.
Plasma choline level
Plasma free and fat-soluble choline levels were analyzed, respectively. 100 μL of plasma was mixed with 900 μL of methanol. Following sonication for 10 minutes, the samples were centrifuged at 15,000 rpm for 10 min. at 4°C, and the supernatant was aliquoted. Filter filtration was performed immediately before the analysis, and plasma free choline levels was measured by LC/MS/MS quantitative analysis (Waters, QTRAP4500, 40 °C, A: acetonitrile /B:5 mM ammonium acetate [pH 4.0]), and fat-soluble choline levels were also measured by LC/MS/MS quantitative analysis (BRUKER, micrOTOF-QII, 30°C, A: acetonitrile /B:5 mM ammonium acetate [pH4.0]). The analyses were conducted by the specialist in charge of the analyses of Kewpie Corporation (Tokyo, Japan).
Dietary survey with BDHQs
This was performed to ensure that subjects were not overeating or undereating, which would negatively influence the evaluation of the effects of functional foods.
Safety evaluation
As a safety evaluation, it was performed that, physical measurement (height, weight, and body mass index), physical examination (systolic blood pressure, diastolic blood pressure, and pulse), hematological tests (leukocyte count, erythrocyte count, hematocrit, and platelet count), blood biochemistry tests (total protein, albumin, total bilirubin, indirect bilirubin, alkaline phosphatase, aspartate transaminase, alanine transaminase, L-lactate dehydrogenase, gamma-glutamyl transpeptidase, total cholesterol, triglycerol, high-density lipoprotein-cholesterol, urea-nitrogen, creatinine, sodium, potassium, chloride, blood glucose, and hemoglobin A1C), urinalysis (protein, glucose, and occult blood reaction), and medical interviews. Blood and urine analyses were conducted at BML, Inc. (Saitama, Japan). Blood collection was performed after fasting for 4 h or more. The participants completed daily life diaries to investigate the status of consumption of the supplements and the presence of adverse events.
Ethical approval
The study was conducted after obtaining approval from the Nihonbashi Cardiology Clinic Institutional Review Board (approval number: NJI-021-07-01, approval date: July 27, 2021), in accordance with the principles of the Declaration of Helsinki by the World Medical Association and the ethical guidelines for bioscience and medical research involving human subjects set forth by Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare, and Ministry of Economic, Trade, and Industry. The study protocol was pre-registered with the Clinical Trials Registry System (UMIN-CTR) (UMIN 000045050).
Statistical analysis
Allocation-adjustment factors were age, sex, egg consumption, plasma free choline level, Cognitrax short version total and verbal memory scores, MMSE-J score, and GDS-S-J score. The reason why the verbal memory score was added to the adjustment factor is that the function was confirmed by previous research [20]. The allocation was conducted by the head of statistical analysis, who was not involved in laboratory tests.
The test results are presented as the mean ± standard error of the amount of change. The statistical significance level was set at P <0.05. Statistical analysis was performed using the computer software IBM Statistical Package for the Social Sciences version 28.0 (IBM Japan Co., Ltd. (Tokyo, Japan)). The change amounts of Cognitrax, TMT-A and B, and plasma choline levels were compared between the choline and placebo groups at different time points by a Student’s t-test. Mann-Whitney’s U test was performed to compare SF-36 and WHO-5 between the choline and placebo groups at different time points. To evaluate safety assessments, paired t-test was performed within groups, and student t-test was performed between groups.
Results
Subject characteristics
Participant characteristics are shown in Table 2. No significant differences were found between the two groups. Additionally, the sample consumption rate was 99%.
Table 2.
Participant characteristics
| Placebo | Choline | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | ± | SE | Mean | ± | SE | |||
| Number of subjects | 21 (11: 10) | 20 (12: 8) | ||||||
| (Male: Female) | ||||||||
| Age (y) | 66 | ± | 1.22 | 64.7 | ± | 0.67 | > 0.05 | |
| Height (cm) | 160 | ± | 1.72 | 163 | ± | 1.62 | > 0.05 | |
| Body weight (kg) | 60.1 | ± | 2.29 | 60.4 | ± | 2.64 | > 0.05 | |
| BMI | 23.4 | ± | 0.75 | 22.3 | ± | 0.65 | > 0.05 | |
| Systolic blood pressure (mmHg) | 121 | ± | 3.67 | 125 | ± | 3.47 | > 0.05 | |
| Diastolic blood pressure (mmHg) | 76.7 | ± | 2.67 | 76.8 | ± | 2.81 | > 0.05 | |
| Pulse (/m) | 79.7 | ± | 2.46 | 78.1 | ± | 2.04 | > 0.05 | |
| MMSE-J | 28.8 | ± | 0.21 | 28.9 | ± | 0.24 | > 0.05 | |
| GDS-S-J | 1.33 | ± | 0.3 | 1.7 | ± | 0.35 | > 0.05 | |
| Cognitrax | Verbal memory | 52.7 | ± | 1.11 | 50.9 | ± | 1.42 | > 0.05 |
| Psychomotor speed | 162 | ± | 3.66 | 160 | ± | 5.7 | > 0.05 | |
| Processing Speed | 53 | ± | 1.62 | 55.8 | ± | 1.83 | > 0.05 | |
| Simple Attention | 39.8 | ± | 0.11 | 39.4 | ± | 0.4 | > 0.05 | |
| Motor Speed | 108 | ± | 2.84 | 104 | ± | 4.98 | > 0.05 | |
| Plasma free choline level (μM) | 14.3 | ± | 0.59 | 14.3 | ± | 0.67 | > 0.05 | |
| Egg consumption (n/week) | 2.84 | ± | 0.44 | 3.39 | ± | 0.39 | > 0.05 | |
Mean ± SE of 20 or 21 subjects. The P-values are from the Student’s t-test, comparing values in the placebo and choline groups
Cognitrax
The results of the cognitive function scores and the results of each test score are shown in Table 3 and Table 4, respectively. At 6 and 12 weeks after intake, the verbal memory score and the number of correct hits (delayed) on verbal memory tests were significantly higher in the choline group than in the placebo group. Conversely, at 6 weeks after intake, the processing rate score and correct response on the SDC test were significantly lower in the choline group than in the placebo group.
Table 3.
Effects of egg yolk choline on ⊿ Cognitrax Score in subjects
| group | n | ⊿ 6 weeks | ⊿ 12 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SE | P-value | Mean | ± | SE | P-value | |||
| Composite Memory | Placebo | 21 | − 0.90 | ± | 1.59 | 0.569 | − 0.38 | ± | 1.27 | 0.470 |
| Choline | 20 | 0.40 | ± | 1.61 | 1.10 | ± | 1.59 | |||
| Verbal Memory | Placebo | 21 | − 0.33 | ± | 0.57 | 0.003** | 0.33 | ± | 0.73 | 0.043* |
| Choline | 20 | 3.35 | ± | 1.00 | 2.75 | ± | 0.90 | |||
| Visual Memory | Placebo | 21 | − 0.57 | ± | 1.30 | 0.213 | − 0.71 | ± | 1.07 | 0.550 |
| Choline | 20 | − 2.95 | ± | 1.36 | − 1.65 | ± | 1.13 | |||
| Psychomotor speed | Placebo | 21 | 3.81 | ± | 1.69 | 0.711 | 4.43 | ± | 1.95 | 0.834 |
| Choline | 20 | 2.75 | ± | 2.31 | 3.80 | ± | 2.27 | |||
| Reaction Time | Placebo | 21 | 2.76 | ± | 10.7 | 0.941 | 0.62 | ± | 10.7 | 0.418 |
| Choline | 20 | 1.30 | ± | 16.6 | − 13.15 | ± | 13.1 | |||
| Complex Attention | Placebo | 21 | − 0.81 | ± | 0.68 | 0.894 | − 1.14 | ± | 0.84 | 0.995 |
| Choline | 20 | − 0.95 | ± | 0.81 | − 1.15 | ± | 0.67 | |||
| Cognitive Flexibility | Placebo | 21 | 2.14 | ± | 1.47 | 0.317 | 3.76 | ± | 1.92 | 0.458 |
| Choline | 20 | 4.30 | ± | 1.54 | 5.60 | ± | 1.51 | |||
| Processing Speed | Placebo | 21 | 2.86 | ± | 1.53 | 0.045* | 2.43 | ± | 1.60 | 0.414 |
| Choline | 20 | − 1.40 | ± | 1.37 | 0.85 | ± | 1.01 | |||
| Exclusive Function | Placebo | 21 | 1.57 | ± | 1.58 | 0.175 | 3.57 | ± | 1.82 | 0.325 |
| Choline | 20 | 4.60 | ± | 1.51 | 5.95 | ± | 1.53 | |||
| Simple Attention | Placebo | 21 | − 0.10 | ± | 0.14 | 0.296 | 0.00 | ± | 0.14 | 0.684 |
| Choline | 20 | 0.20 | ± | 0.25 | 0.10 | ± | 0.20 | |||
| Motor Speed | Placebo | 21 | 0.90 | ± | 1.32 | 0.178 | 2.24 | ± | 1.21 | 0.734 |
| Choline | 20 | 3.95 | ± | 1.81 | 3.05 | ± | 2.07 | |||
Mean ± SE of 20 or 21 subjects. The P-values are from the Student’s t-test, comparing values between the placebo and choline groups
*P < 0.05
**P < 0.01
Table 4.
Effects of egg yolk choline on ⊿ Cognitrax test score in subjects
| group | n | ⊿ 6 weeks | ⊿ 12 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SE | P-value | Mean | ± | SE | P-value | ||||
| Verbal Memory Test (VBM) | Correct Hits (immediate) | Placebo | 21 | − 0.14 | ± | 0.43 | 0.213 | 0.24 | ± | 0.37 | 0.736 |
| Choline | 20 | 0.55 | ± | 0.34 | 0.40 | ± | 0.30 | ||||
| Correct Passes (immediate) | Placebo | 21 | − 0.05 | ± | 0.16 | 0.221 | 0.00 | ± | 0.17 | 0.241 | |
| Choline | 20 | 0.30 | ± | 0.23 | 0.35 | ± | 0.24 | ||||
|
Correct Hits (delay) |
Placebo | 21 | − 0.10 | ± | 0.37 | 0.015* | − 0.24 | ± | 0.54 | 0.046* | |
| Choline | 20 | 1.95 | ± | 0.72 | 1.60 | ± | 0.72 | ||||
| Correct Passes (delay) | Placebo | 21 | − 0.05 | ± | 0.26 | 0.131 | 0.33 | ± | 0.16 | 0.801 | |
| Choline | 20 | 0.55 | ± | 0.29 | 0.40 | ± | 0.21 | ||||
| Visual Memory Test (VIM) | Correct Hits (immediate) | Placebo | 21 | − 0.48 | ± | 0.55 | 0.707 | − 0.10 | ± | 0.44 | 0.22 |
| Choline | 20 | − 0.75 | ± | 0.46 | − 0.85 | ± | 0.42 | ||||
| Correct Passes (immediate) | Placebo | 21 | − 0.24 | ± | 0.62 | 0.423 | 0.14 | ± | 0.49 | 0.478 | |
| Choline | 20 | − 0.85 | ± | 0.42 | − 0.35 | ± | 0.48 | ||||
| Correct Hits (delay) | Placebo | 21 | 0.00 | ± | 0.48 | 0.349 | − 0.43 | ± | 0.45 | 0.803 | |
| Choline | 20 | − 0.75 | ± | 0.64 | − 0.25 | ± | 0.56 | ||||
| Correct Passes (delay) | Placebo | 21 | 0.14 | ± | 0.49 | 0.378 | − 0.33 | ± | 0.58 | 0.868 | |
| Choline | 20 | − 0.60 | ± | 0.68 | − 0.20 | ± | 0.55 | ||||
| Finger Tapping Test (FTT) | Right Tap Average | Placebo | 21 | 0.90 | ± | 1.04 | 0.275 | 1.76 | ± | 0.87 | 0.838 |
| Choline | 20 | 2.55 | ± | 1.06 | 2.05 | ± | 1.11 | ||||
| Left Tap Average | Placebo | 21 | 0.00 | ± | 0.48 | 0.144 | 0.48 | ± | 0.54 | 0.664 | |
| Choline | 20 | 1.40 | ± | 0.82 | 1.00 | ± | 1.06 | ||||
| Symbol Digit Coding Test (SDC) | Correct Responses | Placebo | 21 | 2.90 | ± | 1.21 | 0.021* | 2.19 | ± | 1.33 | 0.382 |
| Choline | 20 | − 1.20 | ± | 1.19 | 0.75 | ± | 0.91 | ||||
| Errors | Placebo | 21 | 0.05 | ± | 0.38 | 0.762 | − 0.24 | ± | 0.38 | 0.772 | |
| Choline | 20 | 0.20 | ± | 0.32 | − 0.10 | ± | 0.27 | ||||
| Stroop Test (ST) | Simple reaction time | Placebo | 21 | − 0.95 | ± | 8.76 | 0.549 | − 5.81 | ± | 6.10 | 0.880 |
| Choline | 20 | − 16.0 | ± | 23.15 | − 9.40 | ± | 22.74 | ||||
| Complex Reaction Time | Placebo | 21 | 0.38 | ± | 11.46 | 0.606 | − 1.67 | ± | 13.46 | 0.481 | |
| Choline | 20 | 12.1 | ± | 19.3 | − 14.6 | ± | 12.2 | ||||
| Stroop Reaction Time | Placebo | 21 | 5.38 | ± | 14.51 | 0.509 | 3.14 | ± | 13.18 | 0.524 | |
| Choline | 20 | − 9.80 | ± | 17.66 | − 11.55 | ± | 18.93 | ||||
| Stroop Commission Errors | Placebo | 21 | − 0.57 | ± | 0.36 | 0.058 | − 0.19 | ± | 0.26 | 0.103 | |
| Choline | 20 | 0.30 | ± | 0.25 | 0.35 | ± | 0.20 | ||||
| Shifting Attention Test (SAT) | Correct Responses | Placebo | 21 | 1.24 | ± | 0.94 | 0.104 | 2.62 | ± | 1.15 | 0.222 |
| Choline | 20 | 3.55 | ± | 1.03 | 4.55 | ± | 1.05 | ||||
| Errors | Placebo | 21 | − 0.33 | ± | 0.74 | 0.465 | − 0.95 | ± | 0.75 | 0.645 | |
| Choline | 20 | − 1.05 | ± | 0.62 | − 1.40 | ± | 0.60 | ||||
| Correct Responses Time | Placebo | 21 | − 26.67 | ± | 21.16 | 0.323 | − 40.19 | ± | 23.1 | 0.123 | |
| Choline | 20 | − 59.65 | ± | 25.44 | − 90.90 | ± | 22.28 | ||||
| Continuous Performance Test (CPT) | Correct Responses | Placebo | 21 | − 0.10 | ± | 0.07 | 0.259 | − 0.05 | ± | 0.05 | 0.427 |
| Choline | 20 | 0.10 | ± | 0.16 | 0.05 | ± | 0.11 | ||||
| Commission Errors | Placebo | 21 | 0.10 | ± | 0.07 | 0.259 | 0.05 | ± | 0.05 | 0.427 | |
| Choline | 20 | − 0.10 | ± | 0.16 | − 0.05 | ± | 0.11 | ||||
| Errors | Placebo | 21 | 0.00 | ± | 0.10 | 0.527 | − 0.05 | ± | 0.13 | 0.990 | |
| Choline | 20 | − 0.10 | ± | 0.12 | − 0.05 | ± | 0.14 | ||||
| choice reaction time | Placebo | 21 | 8.19 | ± | 5.34 | 0.857 | − 2.57 | ± | 5.25 | 0.675 | |
| Choline | 20 | 6.60 | ± | 7.00 | 1.60 | ± | 8.48 | ||||
Mean ± SE of 20 or 21 subjects
*statistical significance by Student’s t-test for between-group at P < 0.05
TMT
The results of the TMT scores are shown in Table 5. No significant differences could be identified at any time point during the study period.
Table 5.
Effects of egg yolk choline on ⊿TMT test scores in subjects
| group | n | ⊿ 6 weeks | ⊿ 12 weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SE | P-value | Mean | ± | SE | P-value | ||||
| TMT-A | Time (sec) | Placebo | 21 | − 5.24 | ± | 1.48 | 0.183 | − 4.67 | ± | 1.68 | 0.415 |
| Choline | 20 | − 2.20 | ± | 1.7 | − 6.80 | ± | 1.99 | ||||
| Error | Placebo | 21 | − 0.14 | ± | 0.1 | 0.106 | − 0.10 | ± | 0.1 | 0.681 | |
| Choline | 20 | 0.05 | ± | 0.05 | − 0.05 | ± | 0.05 | ||||
| TMT-B | Time (sec) | Placebo | 21 | − 15.67 | ± | 6.63 | 0.070 | − 18.62 | ± | 6.64 | 0.134 |
| Choline | 20 | − 0.60 | ± | 4.49 | − 6.95 | ± | 3.55 | ||||
| Error | Placebo | 21 | − 0.19 | ± | 0.23 | 0.474 | − 0.05 | ± | 0.28 | 0.871 | |
| Choline | 20 | 0.05 | ± | 0.25 | − 0.10 | ± | 0.14 | ||||
Mean ± SE of 20 or 21 subjects. The P-values are from the Student’s t-test, comparing values between the placebo and choline groups
Plasma choline concentration
The results of plasma choline levels are shown in Table 6. Free choline levels were significantly higher in the choline group compared with the placebo group at 6 weeks after ingestion. The choline group remained at a high level after 12 weeks of ingestion, though not significant. No significant changes were observed in fat-soluble choline concentrations between groups.
Table 6.
Effects of egg yolk choline on plasma choline levels in subjects
| group | n | ⊿ 6 weeks | ⊿ 12 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SE | P-value | Mean | ± | SE | P-value | |||
| Plasma free choline level (μM) | Placebo | 21 | 0.34 | ± | 0.51 | 0.039* | 0.04 | ± | 0.58 | 0.415 |
| Choline | 20 | 1.80 | ± | 0.46 | 1.06 | ± | 0.65 | |||
| Plasma fat-soluble choline level (μM) | Placebo | 21 | − 0.14 | ± | 0.10 | 0.106 | 87.9 | ± | 68.8 | 0.681 |
| Choline | 20 | 0.05 | ± | 0.05 | 117.5 | ± | 42.1 | |||
Mean ± SE of 20 or 21 subjects
*statistical significance by Student’s t-test for between-group at P < 0.05
QOL survey
SF-36 revealed significantly lower PCS 12 weeks post-ingestion in the choline group compared with the placebo group (1.88 ± 1.1vs. −1.76 ± 1.19; P = 0.029). No significant differences were found in WHO-5 among all parameters (data not shown).
Dietary survey with BDHQs
The results of the dietary survey with BDHQs are shown in Table 7. At 0 week, the placebo group consumed significantly less protein than the choline group. At 12 weeks, the fat intake in the placebo group was significantly lower than at 0 week.
Table 7.
Dietary survey with BDHQs
| group | n | 0 week | 12 weeks | P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SE | P-value1 | Mean | ± | SE | P-value1 | ||||
| Energy | Placebo | 21 | 1864 | ± | 143 | 0.122 | 1664 | ± | 130 | 0.426 | 0.090 |
| Choline | 20 | 1571 | ± | 117 | 1526 | ± | 110 | 0.585 | |||
| Protein | Placebo | 21 | 74.9 | ± | 7.2 | 0.048* | 68.1 | ± | 6.9 | 0.276 | 0.095 |
| Choline | 20 | 58.0 | ± | 3.9 | 59.1 | ± | 4.0 | 0.737 | |||
| Fat | Placebo | 21 | 60.3 | ± | 5.2 | 0.152 | 52.2 | ± | 4.6 | 0.642 | 0.004† |
| Choline | 20 | 50.6 | ± | 4.0 | 49.3 | ± | 4.0 | 0.637 | |||
| Carbohydrates | Placebo | 21 | 233 | ± | 15.3 | 0.280 | 215 | ± | 15.2 | 0.402 | 0.370 |
| Choline | 20 | 206 | ± | 18.7 | 196 | ± | 15.9 | 0.518 | |||
| Salt equivalent | Placebo | 21 | 11.5 | ± | 1.1 | 0.068 | 10.5 | ± | 0.9 | 0.436 | 0.111 |
| Choline | 20 | 9.3 | ± | 0.5 | 9.7 | ± | 0.6 | 0.448 | |||
Mean ± SE of 20 or 21 subjects
*statistical significance by Student’s t-test for between-group at P < 0.05
†statistical significance by Paired t-test for within-group at P < 0.05
Safety evaluation
The results of the blood and urine analyses are shown in Table 8. Any changes were within the normal range, although significant changes were observed in some parameters. In physical measurement, there were no significant changes (Date not shown). The investigator judged that continuous egg yolk choline ingestion at 300 mg for 12 weeks had no safety problems.
Table 8.
Blood and urine analyses parameter
| Group | 0 week | 12-week | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | ± | SE | n | Mean | ± | SE | ||||
| Hematological Tests | WBC (/μL) | Placebo | 30 | 5197 | ± | 195 | 30 | 5137 | ± | 221 | |
| Choline | 30 | 5187 | ± | 186 | 30 | 4917 | ± | 185 | |||
| RBC (× 104/μL) | Placebo | 30 | 457 | ± | 7 | 30 | 456 | ± | 7 | ||
| Choline | 30 | 458 | ± | 8 | 30 | 464 | ± | 8 | |||
| Hemoglobin (g/dL) | Placebo | 30 | 14.2 | ± | 0.2 | 30 | 14.3 | ± | 0.2 | ||
| Choline | 30 | 14.1 | ± | 0.3 | 30 | 14.3 | ± | 0.2 | |||
| Hematocrit (%) | Placebo | 30 | 43.9 | ± | 0.6 | 30 | 44.6 | ± | 0.6 | ||
| Choline | 30 | 43.6 | ± | 0.7 | 30 | 45.0 | ± | 0.7 | |||
| Platelet Count (× 104/μL) | Placebo | 30 | 23.2 | ± | 1.0 | 30 | 23.6 | ± | 0.8 | ||
| Choline | 30 | 24.8 | ± | 1.0 | 30 | 26.2 | ± | 1.0 | |||
| MCV (fL) | Placebo | 30 | 96.2 | ± | 0.8 | 30 | 98.0 | ± | 0.7 | ||
| Choline | 30 | 95.4 | ± | 0.7 | 30 | 97.2 | ± | 0.8 | |||
| MCH (pg) | Placebo | 30 | 31.1 | ± | 0.3 | 30 | 31.4 | ± | 0.3 | ||
| Choline | 30 | 30.7 | ± | 0.3 | 30 | 30.8 | ± | 0.2 | |||
| MCHC (%) | Placebo | 30 | 32.3 | ± | 0.2 | 30 | 32.0 | ± | 0.2 | ||
| Choline | 30 | 32.2 | ± | 0.1 | 30 | 31.7 | ± | 0.1 | |||
| NEUT (%) | Placebo | 30 | 54.9 | ± | 1.4 | * | 30 | 56.3 | ± | 1.5 | |
| Choline | 30 | 59.4 | ± | 1.4 | 30 | 58.5 | ± | 1.2 | |||
| LYMPH (%) | Placebo | 30 | 36.2 | ± | 1.4 | * | 30 | 34.8 | ± | 1.3 | |
| Choline | 30 | 31.8 | ± | 1.2 | 30 | 32.5 | ± | 1.0 | |||
| MONO (%) | Placebo | 30 | 5.61 | ± | 0.14 | 30 | 5.76 | ± | 0.19 | ||
| Choline | 30 | 5.51 | ± | 0.25 | 30 | 5.59 | ± | 0.28 | |||
| EOSINO (%) | Placebo | 30 | 2.55 | ± | 0.33 | 30 | 2.43 | ± | 0.38 | ||
| Choline | 30 | 2.61 | ± | 0.32 | 30 | 2.71 | ± | 0.28 | |||
| BASO (%) | Placebo | 30 | 0.770 | ± | 0.078 | 30 | 0.730 | ± | 0.059 | ||
| Choline | 30 | 0.693 | ± | 0.057 | 30 | 0.733 | ± | 0.056 | |||
| Blood Biochemistry Tests | AST(GOT) (U/L) | Placebo | 30 | 22.1 | ± | 0.81 | 30 | 25.1 | ± | 1.51 | |
| Choline | 30 | 21.8 | ± | 0.78 | 30 | 23.6 | ± | 1.00 | |||
| ALT(GPT) (U/L) | Placebo | 30 | 19.6 | ± | 1.37 | 30 | 20.3 | ± | 1.38 | ||
| Choline | 30 | 17.6 | ± | 1.33 | 30 | 18.3 | ± | 1.48 | |||
| LD(LDH) (U/L) | Placebo | 30 | 202 | ± | 4 | 30 | 205 | ± | 5 | ||
| Choline | 30 | 193 | ± | 4 | 30 | 204 | ± | 5 | |||
| Total Bilirubin (mg/dL) | Placebo | 30 | 0.913 | ± | 0.052 | 30 | 0.877 | ± | 0.044 | ||
| Choline | 30 | 0.890 | ± | 0.041 | 30 | 0.847 | ± | 0.046 | |||
| ALP (U/L) | Placebo | 30 | 69.7 | ± | 3.1 | 30 | 71.0 | ± | 3.1 | ||
| Choline | 30 | 76.3 | ± | 4.7 | 30 | 77.1 | ± | 4.2 | |||
| γ-GT (γ-GTP) (U/L) | Placebo | 30 | 27.8 | ± | 2.9 | 30 | 26.6 | ± | 2.5 | ||
| Choline | 30 | 24.6 | ± | 2.9 | 30 | 25.0 | ± | 3.4 | |||
| Glucose (mg/dL) | Placebo | 30 | 91.7 | ± | 1.8 | 30 | 89.5 | ± | 1.4 | ||
| Choline | 30 | 89.0 | ± | 1.7 | 30 | 89.8 | ± | 1.9 | |||
| HbA1c (%) | Placebo | 30 | 5.49 | ± | 0.05 | 30 | 5.46 | ± | 0.06 | ||
| Choline | 30 | 5.51 | ± | 0.04 | 30 | 5.48 | ± | 0.05 | |||
| Total Cholesterol (mg/dL) | Placebo | 30 | 225 | ± | 6 | 30 | 228 | ± | 6 | ||
| Choline | 30 | 222 | ± | 6 | 30 | 230 | ± | 6 | |||
| LDL-Cholesterol (mg/dL) | Placebo | 30 | 132 | ± | 5 | 30 | 133 | ± | 5 | ||
| Choline | 30 | 130 | ± | 6 | 30 | 133 | ± | 5 | |||
| HDL-Cholesterol (mg/dL) | Placebo | 30 | 71.9 | ± | 4.1 | 30 | 78.5 | ± | 4.3 | ||
| Choline | 30 | 69.5 | ± | 3.4 | 30 | 77.4 | ± | 4.1 | |||
| Triglyceride (mg/dL) | Placebo | 30 | 107 | ± | 15 | 30 | 85.4 | ± | 10.0 | ||
| Choline | 30 | 104 | ± | 12 | 30 | 102 | ± | 14 | |||
| Phospholipid (mg/dL) | Placebo | 30 | 242 | ± | 6 | 30 | 243 | ± | 6 | ||
| Choline | 30 | 241 | ± | 5 | 30 | 248 | ± | 6 | |||
| Total Protein (g/dL) | Placebo | 30 | 7.35 | ± | 0.07 | 30 | 7.25 | ± | 0.08 | ||
| Choline | 30 | 7.23 | ± | 0.07 | 30 | 7.22 | ± | 0.07 | |||
| Albumin (g/dL) | Placebo | 30 | 4.39 | ± | 0.04 | 30 | 4.40 | ± | 0.04 | ||
| Choline | 30 | 4.35 | ± | 0.05 | 30 | 4.39 | ± | 0.04 | |||
| BUN (UN) (mg/dL) | Placebo | 30 | 15.8 | ± | 0.57 | 30 | 16.2 | ± | 0.76 | ||
| Choline | 30 | 15.1 | ± | 0.70 | 30 | 16.1 | ± | 0.65 | |||
| Creatinine (mg/dL) | Placebo | 30 | 0.847 | ± | 0.027 | 30 | 0.834 | ± | 0.028 | ||
| Choline | 30 | 0.791 | ± | 0.025 | 30 | 0.781 | ± | 0.026 | |||
| Uric Acid (mg/dL) | Placebo | 30 | 5.48 | ± | 0.21 | 30 | 5.42 | ± | 0.20 | ||
| Choline | 30 | 5.55 | ± | 0.24 | 30 | 5.42 | ± | 0.23 | |||
| Na (mEq/L) | Placebo | 30 | 141 | ± | 0 | 30 | 140 | ± | 0 | ||
| Choline | 30 | 141 | ± | 0 | 30 | 141 | ± | 0 | |||
| Cl (mEq/L) | Placebo | 30 | 103 | ± | 0 | 30 | 103 | ± | 0 | ||
| Choline | 30 | 104 | ± | 0 | 30 | 103 | ± | 0 | |||
| K (mEq/L) | Placebo | 30 | 4.25 | ± | 0.06 | 30 | 4.18 | ± | 0.05 | ||
| Choline | 30 | 4.21 | ± | 0.05 | 30 | 4.27 | ± | 0.05 | |||
| Ca (mg/dL) | Placebo | 30 | 9.47 | ± | 0.06 | 30 | 9.32 | ± | 0.04 | ||
| Choline | 30 | 9.48 | ± | 0.05 | 30 | 9.40 | ± | 0.05 | |||
| Urinalysis | Acidity (pH) | Placebo | 30 | 6.30 | ± | 0.14 | 30 | 6.27 | ± | 0.13 | |
| Choline | 30 | 6.10 | ± | 0.12 | 30 | 6.15 | ± | 0.14 | |||
| Specific Gravity | Placebo | 30 | 1.01 | ± | 0.00 | 30 | 1.02 | ± | 0.00 | ||
| Choline | 30 | 1.02 | ± | 0.00 | 30 | 1.02 | ± | 0.00 | |||
Mean ± SE of 30 subject
*statistical significance by Student’s t-test for between-group at P < 0.05
†statistical significance by Paired t-test for within-group at P < 0.05
Discussion
This study evaluated the effects of continuous egg yolk choline ingestion at 300 mg on cognitive function in middle-aged and older adults who were aware of healthy forgetfulness but not dementia or who had been indicated by others for forgetfulness. The results showed that there was a significant improvement in the choline group compared with the placebo group in verbal memory ability, a part of the cognitive function.
In this test, plasma choline concentration was measured. Previous studies revealed that phosphatidylcholine ingestion increases plasma free choline and brain acetylcholine levels [14, 15]. Conversely, low plasma free choline levels are associated with low cognitive function, suggesting that plasma free choline levels and cognitive function are closely related [30]. In the present study, ingestion of egg yolk choline also increased plasma free choline levels, suggesting contribution to the improvement of verbal memory. Moreover, it was challenging to look at the other neurotransmitters due to the limited blood samples. Further future investigations will be required to uncover the potential mechanisms of action, for instance, plasma levels.
The ingested egg yolk choline is well absorbed in the small intestine by a complex mechanism. PC is degraded to LPC by pancreatic phospholipase A2 in the small intestine [31], and a portion of LPC is further degraded to α-GPC by lysophospholipase [32]. Most of the LPC is absorbed in the small intestine, resynthesized to PC and transported as chylomicrons and then derived to the liver via the lymphatics [33]. On the contrary, α-GPC is also absorbed in the small intestine and transported via portal vein to the liver [34]. Egg yolk choline transported to the liver is released into the blood as free choline and transported to the brain [34]. It has also been reported that part of orally ingested egg yolk choline is hydrolyzed to free choline in the small intestine [35]. Free choline is believed to be absorbed by sodium-dependent transporters and transferred to circulation [36]. Choline transported to the brain is thought to cross the blood–brain barrier and reach to brain cells via the choline transporter CHT1 or CHT2 [33]. After reaching the neurons, choline and acetyl-CoA are converted into the neurotransmitter acetylcholine by choline acetyltransferase (acetyl-CoA: choline O-acetyltransferase; ChAT, EC 2.3.1.6). Acetylcholine released extracellularly at nerve terminals is implicated in memory and learning functions in the brain by stimulating muscarinic and nicotinic acetylcholine receptors [37, 38]. In the present study, egg yolk choline-containing PC, LPC, and α-GPC were ingested, and plasma free choline levels were confirmed to be elevated, suggesting that egg yolk choline served as an acetylcholine precursor in the brain, resulting in increased acetylcholine levels and the maintenance and improvement of verbal memory abilities involving acetylcholine receptors. No change was found in plasma fat-soluble choline levels, which were thought to be strongly influenced by blood phospholipid variations. However, the pharmacokinetics of plasma free and fat-soluble choline remained to be clarified.
The processing speed score and SDC test used to calculate the rate of processing efficiency were significantly higher in the placebo group after 6 weeks, although these parameters have been reported to be a function related to cholinergic nerves [39]. However, since this effect disappeared after 12 weeks, the possibility suggests that temporary factors caused fluctuations. The PCS score of SF-36 was lower in the choline group than in the placebo group. In this context, it is possible that the result was affected by the reduction of the amount of physical activity, because this study was conducted in the spreading phase of the COVID-19 infection [40]. Regarding the dietary survey, blood analyses, and urine analyses, significant differences were shown in some parameters. But these mean scores were within the recommended daily intake or normal range. In addition, there were no adverse events attributed to the supplement intake during the study period.
Soybean is also a well-known source of phospholipids, but egg yolk phospholipids have a high level of PC of approximately 80% compared with an approximately 30% in soybean [41]. In addition, soybean phospholipids contain more phosphatidylethanolamine and phosphatidylinositol than egg yolk phospholipids. In this context, since ethanolamine, a breakdown product of phosphatidylethanolamine, inhibits the passage of choline through the blood–brain barrier [42], choline intake as egg yolk phospholipids would be more effective for cognitive function [43]. Moreover, since free choline is readily degraded to trimethylamine by intestinal bacteria in the upper small intestine before absorption compared with PC, administration of choline as phosphatidylcholine is recommended [44, 45].
Strength and limitation
The appearance of significant differences in VBM score (at 6 weeks: P = 0.003; at 12 weeks: P = 0.043) and the number of correct hits (delayed) on VBM tests (at 6 weeks: P = 0.015; at 12 weeks: P = 0.046) were the strengths of this study. However, this study has some limitations. The first is that no significant differences were observed in cognitive function other than VBM. Secondly, subjects were Japanese old-age people who had a healthy diet from a global perspective. Similar observations have been reported in studies of non-Japanese populations [46], but the proportions of choline sources and egg yolk ingested, differs among populations of different the countries [47]. Neither is choline listed in the dietary reference intake information in Japan, nor is the data for choline given in the Japanese food tables. In addition, there is a single nucleotide polymorphism that modulates choline requirements, and the expression of this gene is known to vary by race [48]. However, the change in choline requirement due to this gene’s expression is related to organ dysfunction, and the effect on cognitive function has not been reported. Therefore, ingesting egg yolk choline was considered effective in maintaining cognitive function regardless of race. Finally, people with dementia or depression didn’t participate and the effects on dementia or depression remain unclear.
In the future, to assess the effects of choline intake more accurately, dietary choline intake will need to be investigated in Japan, or intervention tests will need to be conducted in the area where choline is listed in the dietary reference intake information. Its effect on different age groups or populations other than the Japanese are yet to be elucidated.
Conclusions
This study showed that in middle-aged and older Japanese males and females, ingestion of 300 mg/day egg yolk choline increased plasma free choline levels and improved verbal memory, a part of cognitive function. It has been proposed that the consumption of egg yolk choline could potentially maintain cognitive function in individuals without dementia. However, it remains unclear that whether the intake of egg yolk choline could prevent or reduce the incidence of dementia. Nevertheless, it has been suggested that incorporating regular consumption of egg yolk choline into the diet is an effective strategy for maintaining brain function in adults who are free of dementia.
Acknowledgements
We gratefully acknowledge the individuals who participated in this test. We also thank Enago (www.enago.jp) for the English language review.
Abbreviations
- MCI
Mild cognitive impairment
- PC
Phosphatidylcholine
- LPC
Lysophosphatidylcholine
- MMSE-J
Mini-Mental State Examination-Japanese
- GDS-S-J
Geriatric Depression Scale Short Version-Japanese
- TMT
Trail Making Test
- SF-36
36-Item Short-Form Health Survey
- WHO-5
WHO-Five well-being index
- QOL
Quality of life
- BDHQs
Brief-type self-administered diet history questionnaires
- VBM
Verbal memory
- PCS
Physical component summary scores
- QOL
Quality of life
- SDC
Symbol digit coding
Authors’ contributions
S.Y., N.K., W.W., K.S., M.K., Y.T., and R.M. conceived the study concept and design. M.K., Y.T., and R.M. as principal investigator was responsible for study logistics, data acquisition. Y.S. and N.K. prepared the manuscript. S.Y., N.K., K.S., Y.T., and Y.I. were responsible for conducting the trial, and data collection. S.Y., N.K., Y.T., and R.M. carried out the statistical analysis. Y.I. contributed to the test as clinical investigator. Y.M. and M.S. supervised the study design and commented on the manuscript. All authors reviewed the manuscript.
Funding
This research and was funded by Kewpie Corporation (Tokyo, Japan).
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Declarations
Ethics approval and consent to participate
The study was conducted after obtaining approval from the Nihonbashi Cardiology Clinic Institutional Review Board (approval number: NJI-021-07-01, approval date: July 27, 2021) and registering with the University Hospital Medical Information Network (UMIN) Center, (UMIN 000045050; registration date: August 4, 2021) following the Declaration of Helsinki (the World Medical Association) and the ethical guidelines for bioscience and medical research in the spirit for human subjects (Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labor and Welfare, and Ministry of Economic, Trade, and Industry).
Consent for publication
Not applicable.
Competing interests
S.Y., N.K., W.W., K.S., Y.T., M.K., and R.M. are employees of Kewpie Corporation. The remaining authors have no other conflicts of interest to report in this work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. World population prospects 2022. New York, department of economic and social affairs population division. United Nations; 2022.
- 2.World Health Organization. Global action plan on the public health response to dementia 2017–2025. Geneva, mental health and substance use. World Health Organization; 2017.
- 3.Gabryelewicz T, Styczynska M, Luczywek E, Barczak A, Pfeffer A, Androsiuk W, et al. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22:563–567. doi: 10.1002/gps.1716. [DOI] [PubMed] [Google Scholar]
- 4.Brodaty H, Heffernan M, Kochan NA, Draper B, Trollor JN, Reppermund S, et al. Mild cognitive impairment in a community sample: the Sydney memory and ageing study. Alzheimers Dement. 2013;9:310–317. doi: 10.1016/j.jalz.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia-meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 6.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Risk reduction of cognitive decline and dementia [WHO guidelines] Geneva: Department of Mental Health and Substance Use; World Health Organization; 2019. [PubMed] [Google Scholar]
- 8.Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr. 2013;97:1076–82. doi: 10.3945/ajcn.112.045575. [DOI] [PubMed] [Google Scholar]
- 9.Ylilauri MP, Voutilainen S, Lönnroos E, Mursu J, Virtanen HE, Koskinen TT, et al. Association of dietary cholesterol and egg intakes with the risk of incident dementia or Alzheimer disease: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2017;105:476–484. doi: 10.3945/ajcn.116.146753. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo T. The importance of the choline compound and influence on exercise function. Oleoscience. 2020;20:157–162. doi: 10.5650/oleoscience.20.157. [DOI] [Google Scholar]
- 11.López-Sobaler AM, Lorenzo Mora AM. Salas González MªD, Peral Suárez Á, Aparicio A, Ortega RMª. Importancia de la colina en la función cognitiva [Importance of choline in cognitive function]. Nutr Hosp. 2021;37:18–23. [DOI] [PubMed]
- 12.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 13.Bartus RT, Dean RL, Goas JA, Lippa AS. Age-related changes in passive avoidance retention: modulation with dietary choline. Science. 1980;209:301–303. doi: 10.1126/science.7384805. [DOI] [PubMed] [Google Scholar]
- 14.Masuda Y. Hen’s egg phosphatidylcholine combined with vitamin B12 improved memory dysfunction in brain. Nippon Suisan Gakkaishi. 2002;68:719–722. doi: 10.2331/suisan.68.719. [DOI] [Google Scholar]
- 15.Chung SY, Moriyama T, Uezu E, Uezu K, Hirata R, Yohena N, et al. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J Nutr. 1995;125:1484–1489. doi: 10.1093/jn/125.6.1484. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Flicker L. Lecithin for dementia and cognitive impairment. Cochrane Database Syst Rev. 2003;3:CD001015. doi: 10.1002/14651858.CD001015. [DOI] [PubMed] [Google Scholar]
- 17.Sugishita M, Hemmi I, Takeuchi T. Reexamination of the validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J) Jpn J Cogn Neurosci. 2016;18:168–183. [Google Scholar]
- 18.Sugishita M, Koshizuka Y, Sudou S, Sugishita K, Hemmi I, Karasawa H, et al. The validity and reliabilitu of the japanese version of the mini-mental state examination (MMSE-J) with the original procedure of the attention and calculation task (2001) Jpn J Cogn Neurosci. 2018;20:91–110. [Google Scholar]
- 19.Sugishita M, Asada T. Development of the Geriatric Depression Scale - Short Version-Japanese (GDS-S-J) J Cogn Neurosci. 2009;11:87–90. [Google Scholar]
- 20.Kawada N, Yamashita S, Kimura M, Iwama Y, Miura Y, Sugano M, et al. Determining the minimum effective dose of egg yolk choline for improving cognitive function in healthy middle-aged and older japanese: a randomized double-blind placebo-controlled pilot study. (in press) [DOI] [PMC free article] [PubMed]
- 21.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery. CNS Vital Signs Arch Clin Neuropsychol. 2006;21:623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Hirota C, Watanabe M, Tanimoto Y, Kono R, Higuchi Y, Kono K. A cross-sectional study on the relationship between the Trail Making Test and mobility-related functions in community-dwelling elderly. Nihon Ronen Igakkai Zasshi. 2008;45:647–654. doi: 10.3143/geriatrics.45.647. [DOI] [PubMed] [Google Scholar]
- 23.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/S0895-4356(98)00095-X. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara S, Ware JE, Jr, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol. 1998;51:1045–1053. doi: 10.1016/S0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 25.Awata S, Bech P, Yoshida S, Hirai M, Suzuki S, Yamashita M, et al. Reliability and validity of the Japanese version of the World Health Organization-Five Well-Being Index in the context of detecting depression in diabetic patients. Psychiatry Clin Neurosci. 2007;61:112–119. doi: 10.1111/j.1440-1819.2007.01619.x. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, Notsu A, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14:1200–1211. doi: 10.1017/S1368980011000504. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol. 2012;22:151–159. doi: 10.2188/jea.JE20110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s’ disease in later life: longitudinal, population-based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwagami M, Qizilbash N, Gregson J, Douglas I, Johnson M, Pearce N, et al. Blood cholesterol and risk of dementia in more than 1·8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2021;2:e498–e506. doi: 10.1016/S2666-7568(21)00150-1. [DOI] [PubMed] [Google Scholar]
- 30.Nurk E, Refsum H, Bjelland I, Drevon CA, Tell GS, Ueland PM, et al. Plasma free choline, betaine and cognitive performance: the Hordaland Health Study. Br J Nutr. 2013;109:511–519. doi: 10.1017/S0007114512001249. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda I, Kato M. Mechanisms of intestinal cholesterol absorption Oleoscience. 2012;12:107–114. [Google Scholar]
- 32.Murakami M. New insights into the biological roles of the phospholipase A2 superfamily. J Jpn Biochem Soc. 2018;90:348–360. [Google Scholar]
- 33.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Hibino H. Preparation of deacylated phospholipid: glycerophosphocholine and central nervous system activated functional development. Oleoscience. 2007;7:399–411. doi: 10.5650/oleoscience.7.399. [DOI] [Google Scholar]
- 35.Tso P, Fujimoto K. The absorption and transport of lipids by the small intestine. Brain Res Bull. 1991;27:477–482. doi: 10.1016/0361-9230(91)90145-A. [DOI] [PubMed] [Google Scholar]
- 36.Inazu M. Functional expression of choline transporters in the blood-brain barrier. Nutrients. 2019;11:2265. doi: 10.3390/nu11102265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro FM, Black SA, Prado VF, Rylett RJ, Ferguson SS, Prado MA. The “ins” and “outs” of the high-affinity choline transporter CHT1. J Neurochem. 2006;97:1–12. doi: 10.1111/j.1471-4159.2006.03695.x. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer's disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J. 2018;32:2172–2180. doi: 10.1096/fj.201700692RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada M, Kimura Y, Ishiyama D, Otobe Y, Suzuki M, Koyama S, et al. Effect of the COVID-19 epidemic on physical activity in community-dwelling older adults in japan: a cross-sectional online survey. J Nutr Health Aging. 2020;24:948–950. doi: 10.1007/s12603-020-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narabe H. Phospholipids commercial manufacture and future direction. J Jpn Oil Chem Soc. 1992;41:897–902. doi: 10.5650/jos1956.41.897. [DOI] [Google Scholar]
- 42.Zelinski TA, Choy PC. Ethanolamine inhibits choline uptake in the isolated hamster heart. Biochim Biophys Acta. 1984;794:326–332. doi: 10.1016/0005-2760(84)90163-2. [DOI] [PubMed] [Google Scholar]
- 43.Wurtman RJ, Hirsch MJ, Growdon JH. Lecithin consumption raises serum-free-choline levels. Lancet. 1977;2:68–69. doi: 10.1016/S0140-6736(77)90067-8. [DOI] [PubMed] [Google Scholar]
- 44.Sugano M, Matsuoka R. Nutritional viewpoints on eggs and cholesterol. Foods. 2021;10:494. doi: 10.3390/foods10030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirouchi B, Fukuda A, Akasaka T. Unlike glycerophosphocholine or choline chloride, dietary phosphatidylcholine does not increase plasma trimethylamine-n-oxide levels in sprague-dawley rats. Metabolites. 2022;12:64. doi: 10.3390/metabo12010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchman AL, Sohel M, Brown M, Jenden DJ, Ahn C, Roch M, et al. Verbal and visual memory improve after choline supplementation in long-term total parenteral nutrition: a pilot study. JPEN J Parenter Enteral Nutr. 2001;25:30–35. doi: 10.1177/014860710102500130. [DOI] [PubMed] [Google Scholar]
- 47.Shim E, Park E. Choline intake and its dietary reference values in Korea and other countries: a review. Nutr Res Pract. 2022;16:S126–S133. doi: 10.4162/nrp.2022.16.S1.S126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.da Costa KA, Corbin KD, Niculescu MD, Galanko JA, Zeisel SH. Identification of new genetic polymorphisms that alter the dietary requirement for choline and vary in their distribution across ethnic and racial groups. FASEB J. 2014;28:2970–2978. doi: 10.1096/fj.14-249557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article.


