Abstract
Bacterial vaginosis (BV) is the most common cause of vaginal discharge and is often associated with other health consequences mainly in pregnant women. BV is described by an imbalance in the vaginal microbiota where strictly and facultative anaerobic bacteria outgrow the lactic acid‐ and hydrogen peroxide‐producing Lactobacillus species. The species involved in BV are capable to grow and form a polymicrobial biofilm in the vaginal epithelium. The treatment of BV is usually performed using broad‐spectrum antibiotics, including metronidazole and clindamycin. However, these conventional treatments are associated with high recurrence rates. The BV polymicrobial biofilm may have an important role on the treatment outcome and is accounted as one of the factors for treatment failure. Other possible reasons for treatment failure include the presence of species resistant to antibiotics or the chance of reinfection after treatment. Therefore, novel strategies to increase the rates of treatment have been studied namely the use of probiotics and prebiotics, acidifying agents, antiseptics, plant‐based products, vaginal microbiota transplantation, and phage endolysins. Although some of them are still in an initial phase of development with very preliminary results, they show great perspectives for application. In this review, we aimed to study the role of the polymicrobial nature of BV in treatment failure and explore a few alternatives for treatment.
Bacterial vaginosis (BV) is the most common cause of vaginal discharge and is often associated with other health consequences mainly in pregnant women. BV aetiology is yet undefined and significant high recurrence rates have been reported. Here we discuss the current and novel therapeutic approaches against BV‐associated biofilm infections.
INTRODUCTION
Bacterial vaginosis (BV) is very common among reproductive‐aged women, affecting around 23% to 29% of women, with a higher prevalence on women at Middle East, North and sub‐Saharan Africa, North America, and South Asia (Javed et al., 2019; Peebles et al., 2019). Among races, BV is more prevalent on black women irrespective of the geographical region (Javed et al., 2019), and less prevalent on white and Asian women (Kenyon et al., 2013; Peebles et al., 2019). BV treatment is usually performed using antibiotics, namely metronidazole and clindamycin (Workowski et al., 2021). Although the treatment shows to be effective in relieving the signs and symptoms of infection, in a long‐term, cases of recurrence are very common (Bradshaw, Morton, et al., 2006). Nowadays, the high rates of BV recurrence observed after treatment are a major problem that leads to physical and psychological distress of women suffering from BV, as well as an increased economic burden in the treatment of patients (Bilardi et al., 2013; Peebles et al., 2019). Several reasons are pointed as an explanation for the high recurrence rates including, (i) the development of bacterial resistance to commonly used antibiotics (Muzny & Sobel, 2022), (ii) the presence of a biofilm in BV that proves difficult in the eradication of infection (Li et al., 2020), (iii) or the reduction of antibiotic bioavailability due to the protective effect of non‐susceptible species (Rosca, Castro, Sousa, França, Vaneechoutte, et al., 2022). These reasons demand for an urgent development of novel therapeutic agents to treat this infection. In this review, we will address how bacterial interactions can affect the BV treatment outcome.
THE VAGINAL MICROBIOME
The vaginal ecosystem is a diverse and dynamic environment where different microorganisms often inhabit in a mutualistic relationship with each other and the host (Chen et al., 2021). Lactobacillus species usually predominate the healthy vaginal microbiome and are acknowledged for being responsible to preserve the health of the vaginal ecosystem through the production of antimicrobial compounds such as lactic acid, hydrogen peroxide, and bacteriocin‐like substances (Amabebe & Anumba, 2018; Borges et al., 2014). The production of lactic acid is directly related to the acidic vaginal environment (pH 3.5‐4.5) that favours Lactobacillus spp. growth and prevents colonization by pathogenic microorganisms (Atassi & Servin, 2010). Moreover, many Lactobacillus spp. are capable to keep pathogens out of the female reproductive tract through the regulation of the host's immune response (Rizzo et al., 2015) or due to inhibition of cells adhesion to the vaginal epithelial cells (Ojala et al., 2014). Different species of Lactobacillus can colonize the healthy vagina, and based on the predominant species that compose the vaginal microbiota, five community state types (CST) have been defined (Ravel et al., 2011). CST I is dominated by L. crispatus, CST II by L. gasseri, CST III by L. iners, and CST V by L. jensenii. Communities in group IV occur in around 27% of women and are characterized by the presence of many anaerobes. Consequently, the vaginal pH may vary in the different groups (Amabebe & Anumba, 2018; Ravel et al., 2011). It has been proposed that changes in the vaginal microbiota and physiology may lead to the reduction of beneficial lactobacilli and the overgrowth of microorganisms that cause dysbiosis (Chen et al., 2021; Rosca et al., 2020). The concept of dysbiosis is still a great source of controversy regarding its definition when applied to characterize a specific state of the microbiota, such as gut microbiota dysbiosis (Brüssow, 2020), and the problems are transversal to the definition of vaginal microbiota dysbiosis (Lev‐Sagie et al., 2022). Several authors have been using vaginal dysbiosis to define the state where Lactobacillus species are not dominant in the vaginal microbiota, and can be associated with some symptoms (van de Wijgert & Jespers, 2017). Therefore, BV is sometimes defined as a state of vaginal dysbiosis.
Microbiologically, BV is characterized by the reduction of healthy Lactobacillus species, that produce hydrogen peroxide and lactic acid, and the overgrowth of facultative and strictly anaerobic bacteria, such as Gardnerella species, Fannyhessea vaginae (previously known as Atopobium vaginae [Nouioui et al., 2018]), Prevotella bivia, Mobiluncus spp., Peptostreptococcus anaerobius, Megasphaera sp., among many others (Fredricks et al., 2005, 2007; Muzny et al., 2018). BV‐related anaerobic species display synergistic interactions during infection and develop a polymicrobial biofilm in the vaginal epithelium (Hardy, Cerca, et al., 2017; Muzny et al., 2019) (Figure 1).
FIGURE 1.
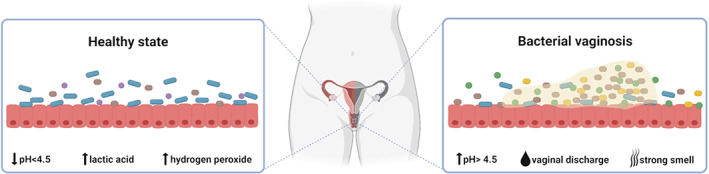
Representation of the vaginal microbiome in health and bacterial vaginosis. The healthy vaginal microbiome is dominated by Lactobacillus species that produce lactic acid and hydrogen peroxide and create an acidic environment. In bacterial vaginosis, the vaginal microbiome is highly colonized by anaerobic species that interact and develop a polymicrobial biofilm on the vaginal epithelium. The most common symptoms of infection are the presence of a vaginal discharge and a strong smell, and an increase of vaginal pH. Figure created with BioRender.com.
CLINICAL DIAGNOSIS AND CONSEQUENCES OF BV
A significant percentage of women with BV do not report symptoms (Koumans et al., 2007). However, in symptomatic women, BV can cause vaginal discomfort (including vaginal or perineal itching and burning), a profuse vaginal discharge (that may have a watery thin consistency, greyish‐white colour), and an unpleasant odour (often described as fishy) (Coudray & Madhivanan, 2020; Jung et al., 2017). BV is also characterized by the increase of vaginal pH and the presence of clue cells on microscopic analysis (Hay, 2014; Swidsinski et al., 2022). Other health consequences, mostly related to pregnancy, have also been associated with BV, such as a higher risk of miscarriages (Soyer Caliskan et al., 2022) and preterm delivery (Mohanty et al., 2022), or a higher risk for pelvic inflammatory disease (Haggerty et al., 2020), increased possibility of acquiring Human Immunodeficiency Virus (Armstrong & Kaul, 2021) and other sexually transmitted infections (Brotman et al., 2010).
The diagnosis of BV is usually performed by the Amsel criteria or the Nugent score (Redelinghuys et al., 2020). The Amsel criteria evaluate the clinical symptoms associated with BV and when three out of four defined criteria are present, a positive diagnosis is obtained (Amsel et al., 1983). In the Nugent score, a microscopic analysis of a vaginal smear is performed and the morphotypes present in the sample are counted and classified according to a semi‐quantitative scale. A score > 7 is indicative of a positive case of BV (Nugent et al., 1991).
Despite the prevalence of BV and its impact on women's health, there is not yet a definitive answer regarding its aetiology. Two main hypotheses have been proposed: the single agent theory and the polymicrobial consortia theory (Jung et al., 2017). There is, however, evidence that supports that key bacterial species, such as Gardnerella spp., are fundamental for triggering BV, but that some secondary anaerobes do contribute to the development of BV (Cerca, 2019). In the next sections, we will address how bacterial interactions can affect the BV treatment outcome.
POLYMICROBIAL NATURE OF BV BIOFILMS
In nature, and also in infectious diseases, bacteria grow predominantly as communities of sessile cells that live in a biofilm (multicellular life phase), rather than as axenic planktonic cultures (unicellular life phase) (Vestby et al., 2020). Biofilms can be defined as structured communities of bacteria attached to a surface and embedded in a self‐produced matrix of extracellular polymeric substances, that provides protection towards adverse environmental stress conditions (Flemming et al., 2016). The formation of the biofilm is a dynamic and complex process that involves multiple interactions between single or multiple bacterial species and the host cells (Luo et al., 2022), which can be controlled by different mechanisms (Liu et al., 2022). To date, the exact process of the polymicrobial biofilm formation in BV remains unknown, although it has been hypothesized that it follows the same route as the formation of oral biofilms, including (i) initial adhesion to the vaginal epithelium, (ii) maturation of the biofilm with the incorporation of diverse anaerobic species and (iii) dispersion of the biofilm by the detachment of aggregates adhered to epithelial cells, also known as clue cells (Jung et al., 2017; Machado & Cerca, 2015; Muzny et al., 2019) (Figure 2).
FIGURE 2.
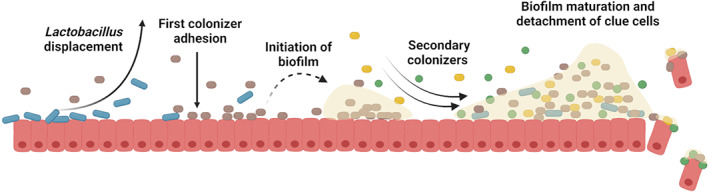
Polymicrobial biofilm development model in bacterial vaginosis. The first colonizer adheres to the vaginal epithelium, causes the displacement of Lactobacillus species, and starts the formation of biofilm. Following colonizers join the formed biofilm and increase its biomass. When the biofilm is mature, epithelial vaginal cells, covered in bacteria forming biofilm, are detached (clue cells). Figure created with BioRender.com.
The relevance of microbial biofilms in BV was first highlighted in 2005, in a fluorescence in situ hybridization study, when Swidsinski and colleagues demonstrated that a polymicrobial biofilm, mainly composed by Gardnerella spp., where F. vaginae was also detected, was present in women with BV (Swidsinski et al., 2005). The same authors later supported their first observations by demonstrating the presence of a polymicrobial biofilm in half of the patients diagnosed with BV (Swidsinski et al., 2013). The presence of both Gardnerella and F. vaginae adhered in a biofilm was later independently confirmed (Hardy et al., 2016). Some features of Gardnerella spp. may explain its higher virulence potential, including the production of vaginolysin and sialidase that cause cell death and exfoliation of the vaginal mucosa, respectively (Garcia et al., 2021; Hardy, Jespers, et al., 2017), the high capacity of adhesion to host cells (Patterson et al., 2010), the ability to displace protective Lactobacilli from vaginal cells (Castro et al., 2015), and the ability to form biofilms on vaginal cells (Jung et al., 2020). Interestingly, the association between Gardnerella and F. vaginae has been pointed out as a very high specific marker for BV diagnosis, wherein F. vaginae is rarely detected in the absence of Gardnerella (Menard et al., 2008; Sehgal et al., 2021). More recently, it has been demonstrated that Gardnerella spp. secretes some undefined compound that is required for F. vaginae to maintain cellular viability in BHI broth (Castro et al., 2020).
Despite the pivotal role of Gardnerella spp. in BV, the roles of other BV‐associated species should not be neglected. Several studies have started assessing the interplay between Gardnerella spp. and other BV‐associated species on biofilms. One of the first studies addressing interactions between BV‐associated bacteria revealed a synergistic effect between Gardnerella spp. and P. bivia when Pybus and colleagues elucidated the symbiotic relationship between these two species by the production of ammonia by P. bivia and amino acids by Gardnerella (Pybus & Onderdonk, 1997).
P. bivia has been often found in BV cases (Datcu et al., 2013; Ravel et al., 2013), but more recently, it has been pointed out that P. bivia might be a possible early colonizer in incident BV (Muzny et al., 2018). Furthermore, more recently, using a mouse model, Gilbert and colleagues demonstrated that the co‐colonization with Gardnerella and P. bivia revealed some clinical aspects characteristic of BV such as high levels of sialidase produced by both bacteria, epithelial exfoliation, and the absence of inflammatory response (Gilbert et al., 2019).
Despite more studies have been performed on F. vaginae or P. bivia interactions with Gardnerella, other BV species have also been found to interact with Gardnerella spp. In fact, we have previously shown that many BV‐associated species can increase the total biofilm biomass in dual (Castro & Cerca, 2015) and triple‐species biofilms mediated by G. vaginalis (Castro et al., 2021). Interestingly, some, but not all BV‐associated species, were able to significantly alter G. vaginalis gene expression, including genes associated with antimicrobial tolerance (Castro et al., 2019).
TREATMENT OF BV AND POSSIBLE OUTCOMES
It has been pointed out that in order to fully understand the pathogenicity of microbes in the host and to improve or develop effective BV treatments without recurrence, it is very important to assess BV‐associated bacterial interactions with secondary BV‐associated microbial species (Cerca, 2019). Perhaps due to the lack of fundamental knowledge regarding BV aetiology, current treatment options for BV are focused on the alleviation of symptoms, which are achieved by the reduction of BV‐associated bacterial load and promotion of normal vaginal microbiota restoration (Sobel & Sobel, 2021; Vodstrcil et al., 2021). The most common antibiotics are metronidazole and clindamycin available in both oral and or topical regimens. Alternative antibiotics include, tinidazole, and secnidazole. The Centers for Disease Control and Prevention (CDC) recommend the use of antibiotics for symptomatic women to relieve vaginal symptoms and signs of infection. Due to the lack of evidence, guidelines from CDC do not recommend screening or treatment of BV in asymptomatic women (Workowski et al., 2021). The treatments for BV are usually well‐tolerated, except for some side effects occurring with the use of metronidazole which can include nausea, abdominal pain, headache, and metallic taste (Brandt et al., 2008). Patients under tinidazole treatment tend to report similar side effects to metronidazole (Armstrong & Wilson, 2009; Menard, 2011), in the same frequency (Schwebke & Desmond, 2011) or even less frequently (Dickey et al., 2009). Clinical cure rates of BV after treatment with antibiotics are reported to vary between 46.8% and 96.2% (Muñoz‐Barreno et al., 2021). However, women often experience several episodes of recurrence (Bradshaw & Sobel, 2016) being reported that around 60% of women experience a new case of BV within a year after treatment (Bradshaw, Morton, et al., 2006). The high rates of recurrence raise the question of whether the currently used treatments are effective or not.
Several hypotheses have been proposed to explain the high recurrence rates in BV, including the increasing development of bacterial resistance to the commonly used antimicrobial agents (Schuyler et al., 2016), as well as the failure to eradicate the biofilm (Swidsinski et al., 2008). BV recurrence can also be a result of reinfection (Ratten et al., 2021) (Figure 3).
FIGURE 3.
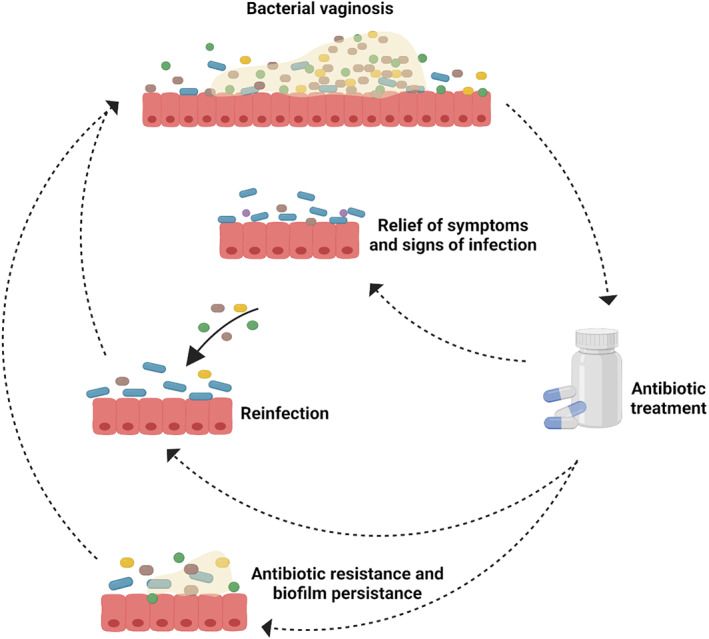
Schematic representation of bacterial vaginosis cure and recurrence after antibiotic treatment. A case of recurrence may occur either by the reinfection with anaerobic pathogens after the antibiotic treatment or caused by the antimicrobial resistance of bacteria and failure of antibiotics in eliminating the biofilm that recovers its capacity to grow and develop the infection. Figure created with BioRender.com.
These clinical observations of high recurrence rates have been partially explored in in vitro antimicrobial susceptibility studies. Antimicrobial resistance is a worldwide problem that has been increasing in the last decades. In the case of BV, some resistance to the commonly used antimicrobial agents has been increasingly reported (Muzny & Sobel, 2022). Table 1 summarizes some of the in vitro studies that have evaluated the response of some BV‐associated bacteria to common antibiotics used in the treatment of BV.
TABLE 1.
In vitro response of BV‐associated bacteria to antimicrobial agents.
| Microorganisms evaluated | Antimicrobial agents used | Results | Conclusion | Reference |
|---|---|---|---|---|
| Gardnerella spp. | Metronidazole | 23 out of 36 of the Gardnerella strains were resistant to metronidazole | A high percentage of Gardnerella isolates were resistant to metronidazole | Simoes et al. (2001) |
| 1059 isolates collected from women with BV | Metronidazole and clindamycin |
Differences in the resistance to clindamycin were observed before and after treatment of women with BV (16% vs 59%). Only six out of 1059 isolates demonstrated metronidazole resistance |
Among the isolates analysed more resistance to clindamycin was detected | Beigi et al. (2004) |
| Gardnerella spp. and Fannyhessea vaginae | Metronidazole and clindamycin | Four out of nine strains of F. vaginae were resistant to metronidazole and all of the strains were susceptible to clindamycin. All strains of Gardnerella were susceptible to metronidazole | Although F. vaginae showed some resistance to metronidazole, all Gardnerella isolates were susceptible | De Backer et al. (2006) |
| Gardnerella spp. and other BV‐associated bacteria | Metronidazole, tinidazole and clindamycin | All bacteria tested were resistant to metronidazole and tinidazole, and 67% of bacteria were resistant to clindamycin | Bacteria were more susceptible to clindamycin than to metronidazole and tinidazole | Alves et al. (2014) |
| Gardnerella spp. | Metronidazole, tinidazole, secnidazole, and clindamycin | Gardnerella isolates were resistant to secnidazole (71.5%), tinidazole (60.3%) and metronidazole (59.8%) | Isolates were highly resistant to metronidazole, tinidazole and secnidazole and only 6.9% was resistant to clindamycin | de Souza et al. (2016) |
| Gardnerella spp. | Metronidazole and clindamycin | Gardnerella isolates were resistant to metronidazole (54.5%) with biofilm‐forming isolates showing a higher resistance. G. vaginalis isolates were more susceptible to clindamycin, with a resistance rate of 27.3% | Gardnerella isolates showed more susceptibility to clindamycin than to metronidazole both in planktonic and biofilm | Li et al. (2020) |
Beigi et al. evaluated the in vitro tolerance of BV‐associated bacteria to metronidazole and clindamycin and concluded that the bacteria were more resistant to clindamycin than to metronidazole (Beigi et al., 2004). When Alves and colleagues tested BV‐associated bacteria in response to metronidazole, clindamycin, and tinidazole, the species showed more resistance to metronidazole and tinidazole (Alves et al., 2014). In the particular case of Gardnerella spp., the majority of the studies reported resistance to metronidazole (de Souza et al., 2016; Li et al., 2020; Simoes et al., 2001). Although some discrepancies have been noted in the available literature, it seems that BV‐associated bacteria show higher levels of resistance to metronidazole than to clindamycin (Muzny & Sobel, 2022).
BV recurrence can also be attributed to reinfection with anaerobic bacteria after the treatment (Eschenbach, 2007). However, the causes of reinfection are not clear, but some behavioural practices after the treatment of BV may have an effect. For instance, Bradshaw and colleagues found higher recurrence rates associated with having the same sexual partner before and after the treatment, and the inconsistency of condom use (Bradshaw et al., 2013). Schwebke and Desmond have earlier observed similar findings, wherein the consistent use of condoms reduced the recurrence by 50% (Schwebke & Desmond, 2007). More recently, in a pilot study, Plummer and colleagues found that women whose partners were treated for BV were likely to show low levels of recurrence after 3 weeks (Plummer et al., 2018) or 12 weeks post‐treatment (Plummer, Vodstrcil, et al., 2021). This led some authors to suggest that the exclusive treatment of women with BV may be a limited therapeutic option and will not prevent the appearance of recurrent cases (Vodstrcil et al., 2021). The high levels of recurrence can also be explained by other factors, including the failure to recolonize the vaginal epithelium with Lactobacillus spp. after treatment (Hemmerling et al., 2010), genetic and immune factors (Muzny et al., 2020). In the next section, we will focus on the role of BV‐associated biofilm on the treatment failure.
THE IMPACT OF BIOFILM AND POLYMICROBIAL INTERACTIONS ON ANTIMICROBIAL TOLERANCE
Biofilms are known to provide increased tolerance to antimicrobial agents explained by several factors including, the slow diffusion of antimicrobials through the biofilm (Khan et al., 2021), the inability of the antibiotic to reach the cells at deep layers of the biofilm (Tseng et al., 2013), the reduced metabolic activity of the cells (Gaio & Cerca, 2019; Wood, 2017), and the presence of antibiotic resistant bacteria within the biofilm (Michaelis & Grohmann, 2023).
When we focus the attention on the polymicrobial nature of BV we find some studies that indicate that the presence of two or more species associated with BV leads to higher tolerance to antimicrobial treatments. Swidsinski and colleagues showed that the presence of biofilm made difficult the treatment of BV when they reported that a biofilm mainly composed by Gardnerella spp. and F. vaginae was temporally suppressed during treatment with metronidazole for seven days, but regained its activity after treatment cessation (Swidsinski et al., 2008). In vivo evidence of likely bacterial interactions or bacterial associations during BV that can lead to increased antimicrobial tolerance has been reported. Bradshaw and colleagues conducted a large cohort study of 139 women infected with BV that were treated with oral metronidazole (400 mg, twice daily for 7 days) (Bradshaw, Tabrizi, et al., 2006). Participants were scheduled for follow‐up over the course of 12 months to examine the recurrence of F. vaginae and Gardnerella spp. Their results showed that although F. vaginae was not present in all women with BV relapses, recurrence rates were significantly higher when these two species were simultaneously present (83% recurrence rate vs 38% recurrence when only Gardnerella spp. was present). These authors suggested that F. vaginae association with Gardnerella was pivotal in BV treatment failure. Very recently, we compared the antimicrobial tolerance of single‐species versus triple‐species biofilms containing G. vaginalis, F. vaginae, and P. anaerobius and found a synergistic effect on the triple‐species biofilms that led to a significant increase in metronidazole tolerance (Rosca, Castro, Sousa, França, Vaneechoutte, et al., 2022). This was the first functional evidence of BV‐associated synergy that led to antimicrobial therapy failure. Taking also into consideration other infections caused by polymicrobial biofilms, it is now evident that microbial interactions do enhance antimicrobial tolerance (DeLeon et al., 2014; Perez et al., 2014), but further research is needed to provide a better mechanistic insight underlying this effect. The presence of naturally resistant BV‐associated bacteria in the polymicrobial biofilm, such as F. vaginae, is likely to be the dominant mechanism to explain the BV recurrence cases (Vodstrcil et al., 2021).
PROMISING ALTERNATIVE STRATEGIES FOR BV TREATMENT
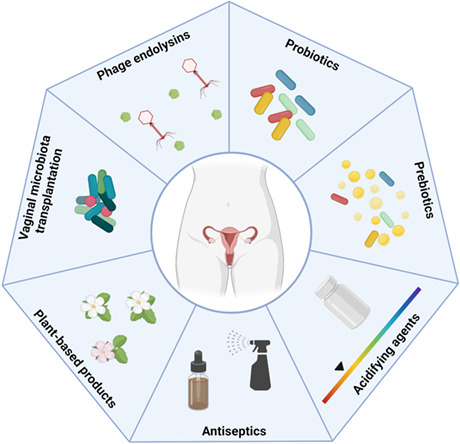
In an attempt to overcome BV treatment failure and relapses of infection, new strategies of treatment have been pursued in recent years (Figure 4).
FIGURE 4.
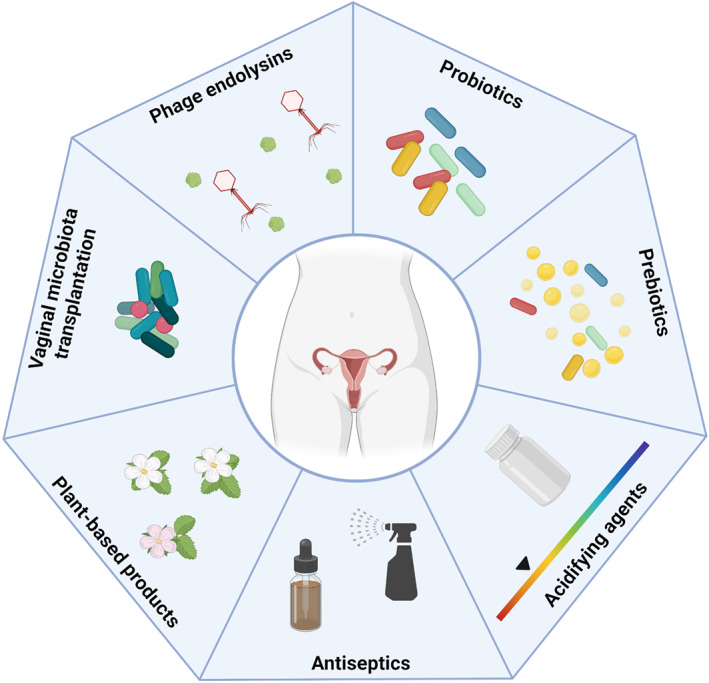
Alternative treatments for bacterial vaginosis. New strategies to treat this infection include the use of probiotics, prebiotics, acidifying agents, antiseptics, plant‐based products, vaginal microbiome transplantation, and phage endolysins. Figure created with BioRender.com.
Probiotics
The administration of oral and vaginal probiotics has been one of the most recommended non‐antibiotic therapies for BV, with some positive clinical outcomes (Joseph et al., 2021). The focus on Lactobacillus as protective strains is based on their phenotypic surface properties (aggregation, adhesion, and biofilm formation) and ability to produce lactic acid and hydrogen peroxide (Amabebe & Anumba, 2018). However, a few years ago, a study reported that the physiologic concentrations of hydrogen peroxide were not able to inactivate the activity of BV‐associated bacteria, although lactic acid at physiologic concentrations did have activity against all BV‐associated bacteria tested (O'Hanlon et al., 2011).
Despite these limitations, several clinical trials have described the probiotic potential against BV, with a single‐ or multi‐strain administration, applied intravaginally or orally, and the interest in using probiotics for the treatment of BV is old. Three decades ago, Hallen and colleagues conducted the first study aiming the treatment of BV only with probiotics. Women were randomly assigned to receive treatment with L. acidophilus and 57% of women showed a significant improvement in vaginal wet smear results (Hallén et al., 1992). A more recent in vitro study reported that Lactobacillus species were able to inhibit the formation of Gardnerella biofilm and reduce the biofilm biomass. The percentage of reduction in biofilm formation was greater with the use of L. rhamnosus (32.7% ± 1.9%, and 29.4% ± 2.7%) than with L. casei (12.6% ± 0.7%, and 0.5% ± 1.6%), at a 24 h and 48 h pre‐formed biofilm, respectively (He et al., 2021).
On the other hand, the first study that administered oral probiotics dates back to 2012, where the authors reported a significant reduction in vaginal pH after receiving oral probiotic yogurt (100 g, twice daily for 1 week) compared to orally administered clindamycin (300 mg, twice a daily for 1 week). Since 80% of individuals from the probiotic group and 84% of subjects in the clindamycin group had a complete symptomatic cure, Hantoushzadeh and colleagues concluded that probiotic and antibiotic treatments were equally effective (Hantoushzadeh et al., 2012). More recently, a randomized controlled cross‐over study whereby patients took one capsule containing three sub‐strains of L. crispatus (109 CFU/strain, once daily for 1 week) reported significant reductions in the Nugent score and Gardnerella spp. counts (Rostok et al., 2019). However, the effect of the administration of probiotics for the treatment of BV seems to be controversial. A study combining the recommended first‐line therapies of oral metronidazole (400 mg, twice daily for 7 days), with a vaginal intervention of either 2% clindamycin cream (one applicator for 7 days) or vaginal L. acidophilus probiotic (1 × 107 CFU, single pessary for 12 days) did not reduce 6‐month BV recurrence (Bradshaw et al., 2012). A new exploratory study evaluated the application of probiotic vaginal capsules containing L. gasseri and L. rhamnosus at 1 × 108 CFU/capsule. The patients with BV received either oral antibiotics (cefixime, doxycycline, and metronidazole) for 7 days or a combination of antibiotics followed by probiotics administration once daily for 30 days followed by once a week until day 190. The results showed that in women treated with the combination of antibiotics and probiotics, a higher number of the Lactobacillus species administered was detected in the vaginal microbiome; however, no differences in the recurrence of BV after 6 months in women treated only with antibiotics or antibiotics and probiotics were observed (Marcotte et al., 2019).
Prebiotics
Prebiotics are a source of nutrients for specific species and favour the growth of beneficial microorganisms and are another alternative that has been studied in the treatment of BV (Vieira‐Baptista et al., 2022). Collins and colleagues evaluated a set of prebiotics namely, lactitol, lactulose, raffinose, and oligofructose and their activity to stimulate Lactobacillus and BV‐associated bacteria. Lactulose was found to be the most promising prebiotic as it was highly specific to promote lactobacilli growth and not stimulate BV‐associated bacteria (Collins et al., 2018). The use of maltose gel was evaluated in the vaginal microbiota in an animal model (Rhesus Macaque) that is normally colonized by anaerobic BV‐related bacteria. The prebiotic maltose induced the proliferation of Lactobacillus, resulting in the inhibition of BV‐associated bacteria in the vaginal environment (Zhang et al., 2020).
Lactoferrin is another prebiotic that has been studied. Otsuki and Imai reported the use of lactoferrin (vaginal suppositories 150 mg/day and oral tablets 700 mg/day) in six women with a history of pregnancy losses or preterm delivery and refractory BV. After one month of lactoferrin administration, Lactobacillus became dominant in the vaginal microbiota and those who were pregnant had a normal delivery without complications (Otsuki & Imai, 2017). Prebiotics have also been tested in combination with antibiotics. Very recently was studied the antimicrobial activity of bovine lactoferrin alone or in combination with metronidazole or clindamycin against G. vaginalis isolates, and the results showed inhibition of Gardnerella growth in a dose‐dependent effect and the combination with clindamycin resulted in a synergistic effect (Pino et al., 2022).
A group of patients with BV was treated with metronidazole (250 mg tablets, 3 per day) plus a prebiotic vaginal gel (5 mg vaginal gel daily) for 7 days, and the study showed that in the treatment group the symptoms of infection were lower than in the group that did not receive the prebiotic gel (Hakimi et al., 2018). A very recent review analysed the studies using probiotics/prebiotics in combination with antibiotics for BV treatment and concluded that the combined therapy is more effective in reducing BV recurrence than the antibiotics alone (Afifirad et al., 2022).
Acidifying agents
Lactic acid is one of the options studied for the treatment of BV due to its antimicrobial activities against BV‐associated bacteria and capacity to restore optimal conditions for Lactobacillus. Several over‐the‐counter products are available, although the use of these products is not recommended on guidelines (Plummer, Bradshaw, et al., 2021). On an early study, the use of a lactic acid gel (225 mg, for 7 days) was as effective as oral metronidazole (500 mg, twice daily for 7 days) in the treatment of patients with BV. Furthermore, the combination of the lactic acid gel with metronidazole had better results than the metronidazole alone and promotes Lactobacillus colonization of the vaginal microbiome (Decena et al., 2006). Lactic acid pessaries were tested following treatment with metronidazole (2 g oral, single dose) to assess the efficacy to eliminate the BV biofilm. After treatment with metronidazole, the majority of women with BV was free of symptoms and the presence of biofilm was only detected in 27.3% of cases at visit 2 (7 to 28 days after), where they initiated the lactic acid treatment for 3 weeks (twice per week). At visit 3 the percentage of patients with biofilm reduced to 18.2%, however at visit 4 (end of treatment) the number increased to 36.4% and the recurrence rates were high (Gottschick et al., 2017). More recently, Armstrong‐Buisseret and colleagues conducted a large controlled trial to compare whether intravaginal lactic acid gel (5 mL, once daily for 7 days) is better than oral metronidazole (400 mg, twice daily for 7 days) for BV treatment. Primary outcome data were available for 409 participants (204 with metronidazole and 205 with lactic acid gel arm). BV symptoms resolution at week 2 was higher with metronidazole (70%) than with lactic acid gel (47%). Likewise, microbiological resolution of BV at week 2 was higher with metronidazole (59/77, 77%) than with lactic acid gel (31/73, 42%), although more side effects were reported in the metronidazole group. However, a follow‐up of 6 months post‐treatment revealed similar recurrence ratios among treatments in participants who had initial resolution (metronidazole: 51/72, 71%; lactic acid gel: 32/46, 70%) (Armstrong‐Buisseret et al., 2022).
Boric acid has been used for decades to treat vaginal infections and has been reported for the treatment of BV, but still with limited data available (Powell et al., 2019). In an early clinical study for the treatment of recurrent BV, patients were treated with oral nitroimidazole for 7 days, followed by boric acid for 21 days (intravaginal, 600 mg/day) and metronidazole gel twice weekly for 16 weeks, if in remission. The study showed that the percentage of cure was 87% after 12 weeks but dropped to 65%, 28 weeks after treatment (Reichman et al., 2009). More recently, Marrazzo and colleagues used the TOL‐463 boric acid‐based vaginal gel (2 g insert or 5 g gel, once daily for 7 days) for the treatment of BV, reporting a 50%–59% early clinical cure rate and considered this strategy as effective and safe for the treatment of BV (Marrazzo et al., 2019).
The combination of conventional antibiotic therapy with boric acid was tested recently as an approach to treat recurrent BV. The regimen consisted of oral nitroimidazole (500 mg, oral) twice a day for 7 days with simultaneous vaginal boric acid 600 mg daily for 30 days, followed by 0.75% metronidazole vaginal gel twice weekly for 5 months. After 30 days of treatment, only one patient remained with symptoms and was diagnosed with refractory BV. After 5 months of a maintenance regimen, 21 of 69 patients had BV and after 6 months of therapy discontinuation, 9 of 29 women developed BV. Overall, 20 women remained free of BV for one year (Surapaneni et al., 2021).
A very recent study used acid electrolyzed water, containing 6% of HCl, against Gardnerella spp. This new product showed an antibacterial effect by inhibiting the growth of Gardnerella species and had better antimicrobial activity than metronidazole. Also, it had a negligible effect on L. acidophilus. Furthermore, vaginal samples were collected from women with BV and the new treatment was able to eliminate all the microbial viability in the cultured samples (Zhao et al., 2022).
Antiseptics
Antiseptics include a large group of different compounds, such as benzydamine (Boselli et al., 2012), chlorhexidine (Mirzaeei et al., 2021), dequalinium chloride (Mendling et al., 2016), octenidine (Swidsinski et al., 2015), polyhexamethylene biguanide (Koban et al., 2012), povidone iodine (Wewalka et al., 2002), that have been tested against vaginal infections for several years. They have a large spectrum of activity and generally act by the disruption of the cell membrane and very little evidence regarding antimicrobial resistance to these compounds is reported. Chlorhexidine was recently used in a clinical trial for the treatment of patients with BV and compared with metronidazole (250 mg tablets, twice a day) for 5 days. Patients treated with chlorhexidine vaginal gel reported high satisfaction scores than those treated with oral metronidazole. The improvement of symptoms was 100% in both groups however, more patients treated with chlorhexidine reported side effects (Mirzaeei et al., 2021).
Dequalinium chloride is one of the antiseptics more studied for the treatment of BV and has demonstrated good efficacy in the treatment of BV (Mendling et al., 2016). Weissenbacher and colleagues compared the treatment of BV using dequalinium chloride (10 mg vaginal tablets) and clindamycin vaginal cream (2%) in a randomized clinical trial. They reported that the two different treatments had equal efficacy and clinical cure rates 1 week after treatment were similar (Weissenbacher et al., 2012).
Very recently a study showed the potential of dequalinium chloride to disrupt Gardnerella biofilms and this compound was able to reduce the metabolism and the biomass of Gardnerella biofilms (Gaspar et al., 2021). A recent study reported the application of dequalinium chloride (100 mg vaginal tablet) for 6 days in a total of 573 patients diagnosed with BV. After treatment, around 85% of patients reported the alleviation of symptoms within 4–6 weeks (Antoni Vives et al., 2022).
Octenidine hydrochloride/phenoxyethanol was compared with metronidazole (500 mg vaginal tablets) treatment for 7 days and the authors found that the two therapies resulted in similar rates of treatment, and a longer period of treatment with octenidine (7 days vs 14 days) resulted in a significantly higher percentage of patients treated (Mikic & Budakov, 2010). However, in a more recent study, Swidsinskii and colleagues demonstrated that, despite the high rates of cure after 7 days of treatment with octenidine and the capacity to eliminate the Gardnerella biofilm from patients with BV, after 6 months the relapse rates of infection were about 66% and the biofilm was again detected. Moreover, repeated and prolonged periods of treatment with octenidine led to an increase in bacterial resistance (Swidsinski et al., 2015).
Plant‐based natural products
Natural products have been used for several years against pathogenic microorganisms associated with different infections (Kim et al., 2022). Plant‐based natural products represent a rising and effective approach to treat BV infection, as their mechanisms of action may prevent bacteria from developing antimicrobial resistance, which represents a great advantage compared to antibiotics (Palmeira‐de‐Oliveira et al., 2013).
Early in 1991, Blackwell reported the first therapeutic success after the application of tea tree oil to cure BV (Blackwell, 1991). From then on, several essential oils and their major components have been studied for the treatment of vaginal infections (Falconi‐McCahill, 2019). One of the first studies showing the anti‐biofilm potential of thymol, a small hydrophobic molecule present in thyme essential oil, demonstrated in vitro inhibitory effect on both newly formed and mature Gardnerella spp. biofilms (Braga et al., 2010). Artemisia princeps Pamp. essential oil was found to have a great effect in inhibiting the growth of Gardnerella, as well as some of its major components (Trinh et al., 2011). More recently, the effect of single compounds from T. capitata was tested against Gardnerella species biofilms, showing great activity in inhibiting the biofilm culturability (Sousa et al., 2022). T. capitata oil was also tested in a six‐species polymicrobial biofilm associated with BV reducing the biofilm biomass and culturability (Rosca, Castro, Sousa, França, Cavaleiro, et al., 2022).
Myrtus communis, Berberis vulgaris or metronidazole vaginal gel were used on 120 women with BV and the groups receiving M. communis or B. vulgaris had a better response than metronidazole and no cases of recurrence were reported, whereas in the group treated with metronidazole 30% of women experienced relapse (Masoudi et al., 2016). In a randomized study, 80 women with BV received a treatment of metronidazole or a Calendula officinalis vaginal cream. After treatment, both groups reported no symptoms of BV and no side effects were described (Pazhohideh et al., 2018).
The combinations of essential oils with antibiotics have also been assessed for the treatment of BV. Extract from M. communis was combined with metronidazole in a vaginal gel for the treatment of women with BV. Treatment with metronidazole and extract combination was effective in treating BV and patients did not show reinfection within 3 weeks after treatment, although 12% of the patients treated only with metronidazole showed reinfection (Masoudi et al., 2017).
Taken together, these studies strengthened the importance of exploring essential oils and their main constituents as alternative approaches to treat BV, but also support the idea that bacteria can interact synergistically when co‐incubated, enhancing tolerance to antimicrobial therapy and causing high clinical recurrence rates of BV.
Vaginal microbiota transplantation approach
Vaginal microbiota transplantation (VMT) is another novel promising approach to treat dysbiosis whereby researchers aim to “reset” the vaginal microbiome to a healthy state, by direct inoculating vaginal discharge from a healthy donor into the vagina of a woman with BV (DeLong, Zulfiqar, et al., 2019; Lev‐Sagie et al., 2019). A first exploratory study involving the intervention of VMT for symptomatic, intractable, and recurrent BV has recently published important findings (Lev‐Sagie et al., 2019). Lev‐Sagie et al. recruited five patients with recurrent BV for VMT procedures; four of them were associated with full long‐term remission during follow‐up (at 5 to 21 months after VMT), with a reconstituted Lactobacillus‐dominated vaginal microbiome, significant improvement of symptoms, and normalization of Amsel criteria. The authors also pointed out that remission may require repeated VMT sessions to elicit a long‐standing clinical response. Safety is an important issue in VTM, considering the risk of contracting infectious microorganisms or agents when the aim is to treat a disease, particularly in immunocompromised recipients. Other works have addressed how studies evolving VMT should be conducted and some protocols regarding the design, methodology, and reproducibility of VMT trials are available (DeLong, Bensouda, et al., 2019; Yockey et al., 2022).
Phage endolysins
Endolysins are bacteriophage‐encoded peptidoglycan hydrolases. The endolysins are produced at the end of the lytic cycle of phage‐bacteria infection and have an activity in cleaving the peptidoglycan cell wall of bacteria. Endolysins have several applications and are pointed out as a great alternative to the use of antibiotics (Fischetti, 2018).
The use of endolysins as an alternative for BV treatment is still in an initial phase. Landlinger and colleagues were the first to report an engineered phage endolysin with activity against Gardnerella – the PM‐477. This endolysin showed to be highly specific for Gardnerella, being able to reduce the in vitro viability of the cells and to disrupt the biofilm of Gardnerella in vaginal samples from patients with BV, without harming beneficial vaginal microbiota (Landlinger et al., 2021). The same authors recently reported that bacteria do not develop resistance to PM‐477 endolysin after 25 rounds of passing and the endolysin was effective against Gardnerella strains resistant to metronidazole. The endolysins reduced the viability of Gardnerella biofilm cells at lower concentrations than metronidazole and clindamycin (Landlinger et al., 2022).
However promising, when testing PM‐477 against dual‐species biofilms of G. vaginalis and F. vaginae or P. bivia, the biofilm could not be eradicated, as no activity was observed against F. vaginae and P. bivia (Castro et al., 2022). This was not surprising, as endolysins are often very specific to a single species (Rahman et al., 2021). Due to its high efficacy on biofilms, endolysins might be a good option as adjuvant therapy as already observed in some studies that the combination of endolysin with common antibiotics can exert a synergistic effect in the antimicrobial activity (Letrado et al., 2018).
CONCLUDING REMARKS
The treatment of BV with antibiotics still has satisfactory results, however the high recurrence rates that have been observed after treatment raise concerns about conventional therapy. The polymicrobial nature of BV makes this a particularly difficult infection to treat. Similar to other polymicrobial infections, the presence of more than one species leads to a decrease in the antimicrobial efficacy to kill bacteria and eliminate the biofilm. Studies regarding the interactions between BV‐associated bacteria are still scarce and the major focus has been on Gardnerella spp., F. vaginae, and P. bivia. However, as a polymicrobial infection, conclusions should be taken cautiously since other species can have important effects on the treatment outcome. The alternatives for the treatment of BV presented in this review, despite being a potential alternative, still have limited data available and are not currently recommended as therapy.
AUTHOR CONTRIBUTIONS
Lúcia G. V. Sousa: Writing – original draft (lead). Sofia A. Pereira: Writing – original draft (supporting). Nuno Cerca: Conceptualization (lead); supervision (lead); writing – review and editing (lead).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest in this work.
ACKNOWLEDGMENTS
LGVS is supported by FCT with the individual grant 2020.04912.BD. SAP is supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020.
Sousa, L.G.V. , Pereira, S.A. & Cerca, N. (2023) Fighting polymicrobial biofilms in bacterial vaginosis. Microbial Biotechnology, 16, 1423–1437. Available from: 10.1111/1751-7915.14261
REFERENCES
- Afifirad, R. , Darb Emamie, A. , Golmoradi Zadeh, R. , Asadollahi, P. , Ghanavati, R. & Darbandi, A. (2022) Effects of pro/prebiotics alone over pro/prebiotics combined with conventional antibiotic therapy to treat bacterial vaginosis: a systematic review. International Journal of Clinical Practice, 2022, 4774783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, P. , Castro, J. , Sousa, C. , Cereija, T.B. & Cerca, N. (2014) Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. The Journal of Infectious Diseases, 210, 593–596. [DOI] [PubMed] [Google Scholar]
- Amabebe, E. & Anumba, D.O.C. (2018) The vaginal microenvironment: the physiologic role of Lactobacilli . Frontiers in Medicine, 5, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsel, R. , Totten, P.A. , Spiegel, C.A. , Chen, K.C.S. , Eschenbach, D. & Holmes, K.K. (1983) Nonspecific vaginitis. The American Journal of Medicine, 74, 14–22. [DOI] [PubMed] [Google Scholar]
- Antoni Vives, J. , Cancelo, M.J. , Losada, M.Á. & Doménech, A. (2022) Dequalinium chloride use in adult Spanish women with bacterial vaginosis: an observational study. Journal of Obstetrics and Gynaecology, 42, 103–109. [DOI] [PubMed] [Google Scholar]
- Armstrong, E. & Kaul, R. (2021) Beyond bacterial vaginosis: vaginal lactobacilli and HIV risk. Microbiome, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, N.R. & Wilson, J.D. (2009) Tinidazole in the treatment of bacterial vaginosis. International Journal of Women's Health, 1, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong‐Buisseret, L. , Brittain, C. , Kai, J. , David, M. , Anstey Watkins, J. , Ozolins, M. et al. (2022) Lactic acid gel versus metronidazole for recurrent bacterial vaginosis in women aged 16 years and over: the VITA RCT. Health technology assessment, 26, 1–170. [DOI] [PubMed] [Google Scholar]
- Atassi, F. & Servin, A.L. (2010) Individual and co‐operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis‐ass. FEMS Microbiology Letters, 304, 29–38. [DOI] [PubMed] [Google Scholar]
- Beigi, R.H. , Austin, M.N. , Meyn, L.A. , Krohn, M.A. & Hillier, S.L. (2004) Antimicrobial resistance associated with the treatment of bacterial vaginosis. American Journal of Obstetrics and Gynecology, 191, 1124–1129. [DOI] [PubMed] [Google Scholar]
- Bilardi, J.E. , Walker, S. , Temple‐Smith, M. , McNair, R. , Mooney‐Somers, J. , Bellhouse, C. et al. (2013) The burden of bacterial vaginosis: women's experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One, 8, e74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell, A.L. (1991) Tea tree oil and anaerobic (bacterial) vaginosis. Lancet (London, England)., 337, 300. [DOI] [PubMed] [Google Scholar]
- Borges, S. , Silva, J. & Teixeira, P. (2014) The role of lactobacilli and probiotics in maintaining vaginal health. Archives of Gynecology and Obstetrics, 289, 479–489. [DOI] [PubMed] [Google Scholar]
- Boselli, F. , Petrella, E. , Campedelli, A. , Muzi, M. , Rullo, V. , Ascione, L. et al. (2012) Efficacy and tolerability of Fitostimoline (vaginal cream, ovules, and vaginal washing) and of benzydamine hydrochloride (Tantum Rosa vaginal cream and vaginal washing) in the topical treatment of symptoms of bacterial vaginosis. ISRN Obstetrics and Gynecology, 2012, 183403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, C.S. , Morton, A.N. , Hocking, J. , Garland, S.M. , Morris, M.B. , Moss, L.M. et al. (2006) High recurrence rates of bacterial vaginosis over the course of 12 months after Oral metronidazole therapy and factors associated with recurrence. The Journal of Infectious Diseases, 193, 1478–1486. [DOI] [PubMed] [Google Scholar]
- Bradshaw, C.S. , Pirotta, M. , de Guingand, D. , Hocking, J.S. , Morton, A.N. , Garland, S.M. et al. (2012) Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo‐controlled double‐blind trial. PLoS One, 7, e34540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, C.S. & Sobel, J.D. (2016) Current treatment of bacterial vaginosis—limitations and need for innovation. The Journal of Infectious Diseases, 214, S14–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw, C.S. , Tabrizi, S.N. , Fairley, C.K. , Morton, A.N. , Rudland, E. & Garland, S.M. (2006) The Association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. The Journal of Infectious Diseases, 194, 828–836. [DOI] [PubMed] [Google Scholar]
- Bradshaw, C.S. , Vodstrcil, L.A. , Hocking, J.S. , Law, M. , Pirotta, M. , Garland, S.M. et al. (2013) Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clinical Infectious Diseases, 56, 777–786. [DOI] [PubMed] [Google Scholar]
- Braga, P.C. , Dal Sasso, M. , Culici, M. & Spallino, A. (2010) Inhibitory activity of thymol on native and mature Gardnerella vaginalis biofilms: In vitro study. Arzneimittel‐Forschung/Drug Research, 60, 675–681. [DOI] [PubMed] [Google Scholar]
- Brandt, M. , Abels, C. , May, T. , Lohmann, K. , Schmidts‐Winkler, I. & Hoyme, U.B. (2008) Intravaginally applied metronidazole is as effective as orally applied in the treatment of bacterial vaginosis, but exhibits significantly less side effects. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 141, 158–162. [DOI] [PubMed] [Google Scholar]
- Brotman, R.M. , Klebanoff, M.A. , Nansel, T.R. , Yu, K.F. , Andrews, W.W. , Zhang, J. et al. (2010) Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and Trichomonal genital infection. The Journal of Infectious Diseases, 202, 1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow, H. (2020) Problems with the concept of gut microbiota dysbiosis. Microbial Biotechnology, 13, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, J. , Alves, P. , Sousa, C. , Cereija, T. , França, Â. , Jefferson, K.K. et al. (2015) Using an in‐vitro biofilm model to assess the virulence potential of bacterial vaginosis or non‐bacterial vaginosis Gardnerella vaginalis isolates. Scientific Reports, 5, 11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, J. & Cerca, N. (2015) BV and non‐BV associated Gardnerella vaginalis establish similar synergistic interactions with other BV‐associated microorganisms in dual‐species biofilms. Anaerobe, 36, 56–59. [DOI] [PubMed] [Google Scholar]
- Castro, J. , Machado, D. & Cerca, N. (2019) Unveiling the role of Gardnerella vaginalis in polymicrobial bacterial vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. The ISME Journal, 13, 1306–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, J. , Rosca, A.S. , Cools, P. , Vaneechoutte, M. & Cerca, N. (2020) Gardnerella vaginalis enhances Atopobium vaginae viability in an in vitro model. Frontiers in Cellular and Infection Microbiology, 10, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, J. , Rosca, A.S. , Muzny, C.A. & Cerca, N. (2021) Atopobium vaginae and Prevotella bivia are able to incorporate and influence gene expression in a pre‐formed Gardnerella vaginalis biofilm. Pathogens, 10, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, J. , Sousa, L.G.V. , França, Â. , Tisakova, L.P. , Corsini, L. & Cerca, N. (2022) Exploiting the anti‐biofilm effect of the engineered phage Endolysin PM‐477 to disrupt In vitro single‐ and dual‐species biofilms of vaginal pathogens associated with bacterial vaginosis. Antibiotics, 11, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerca, N. (2019) Could targeting neighboring bacterial populations help combat bacterial vaginosis? Future Microbiology, 14, 365–368. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Lu, Y. , Chen, T. & Li, R. (2021) The female vaginal microbiome in health and bacterial vaginosis. Frontiers in Cellular and Infection Microbiology, 11, 631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, S.L. , McMillan, A. , Seney, S. , van der Veer, C. , Kort, R. , Sumarah, M.W. et al. (2018) Promising prebiotic candidate established by evaluation of lactitol, lactulose, raffinose, and oligofructose for maintenance of a Lactobacillus‐dominated vaginal microbiota. Applied and Environmental Microbiology, 84, e02200‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudray, M.S. & Madhivanan, P. (2020) Bacterial vaginosis‐a brief synopsis of the literature. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 245, 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datcu, R. , Gesink, D. , Mulvad, G. , Montgomery‐Andersen, R. , Rink, E. , Koch, A. et al. (2013) Vaginal microbiome in women from Greenland assessed by microscopy and quantitative PCR. BMC Infectious Diseases, 13, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer, E. , Verhelst, R. , Verstraelen, H. , Claeys, G. , Verschraegen, G. , Temmerman, M. et al. (2006) Antibiotic susceptibility of Atopobium vaginae . BMC Infectious Diseases, 6, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, D.M.K. , Diniz, C.G. , Filho, D.S.C. , de Oliveira, L.M.A. , Coelho, D.M. , Talha, L.d.S. et al. (2016) Antimicrobial susceptibility and vaginolysin in Gardnerella vaginalis from healthy and bacterial vaginosis diagnosed women. Journal of Infection in Developing Countries, 10, 913–919. [DOI] [PubMed] [Google Scholar]
- Decena, D.C.D. , Co, J.T. , Manalastas, R.M. , Palaypayon, E.P. , Padolina, C.S. , Sison, J.M. et al. (2006) Metronidazole with Lactacyd vaginal gel in bacterial vaginosis. The Journal of Obstetrics and Gynaecology Research, 32, 243–251. [DOI] [PubMed] [Google Scholar]
- DeLeon, S. , Clinton, A. , Fowler, H. , Everett, J. , Horswill, A.R. & Rumbaugh, K.P. (2014) Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an In vitro wound model. Infection and Immunity, 82, 4718–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, K. , Bensouda, S. , Zulfiqar, F. , Zierden, H.C. , Hoang, T.M. , Abraham, A.G. et al. (2019) Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Frontiers in Cellular and Infection Microbiology, 9, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong, K. , Zulfiqar, F. , Hoffmann, D.E. , Tarzian, A.J. & Ensign, L.M. (2019) Vaginal microbiota transplantation: the next frontier. The Journal of Law, Medicine & Ethics, 47, 555–567. [DOI] [PubMed] [Google Scholar]
- Dickey, L.J. , Nailor, M.D. & Sobel, J.D. (2009) Guidelines for the treatment of bacterial vaginosis: focus on tinidazole. Therapeutics and Clinical Risk Management, 5, 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenbach, D.A. (2007) Bacterial vaginosis: resistance, recurrence, and/or reinfection? Clinical Infectious Diseases, 44, 220–221. [DOI] [PubMed] [Google Scholar]
- Falconi‐McCahill, A. (2019) Bacterial vaginosis: a clinical update with a focus on complementary and alternative therapies. Journal of Midwifery & Women's Health, 64, 578–591. [DOI] [PubMed] [Google Scholar]
- Fischetti, V.A. (2018) Development of phage Lysins as novel therapeutics: a historical perspective. Viruses, 10, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming, H.C. , Wingender, J. , Szewzyk, U. , Steinberg, P. , Rice, S.A. & Kjelleberg, S. (2016) Biofilms: an emergent form of bacterial life. Nature Reviews Microbiology, 14, 563–575. [DOI] [PubMed] [Google Scholar]
- Fredricks, D.N. , Fiedler, T.L. & Marrazzo, J.M. (2005) Molecular identification of bacteria associated with bacterial vaginosis. The New England Journal of Medicine, 353, 1899–1911. [DOI] [PubMed] [Google Scholar]
- Fredricks, D.N. , Fiedler, T.L. , Thomas, K.K. , Oakley, B.B. & Marrazzo, J.M. (2007) Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. Journal of Clinical Microbiology, 45, 3270–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaio, V. & Cerca, N. (2019) Cells released from S. epidermidis biofilms present increased antibiotic tolerance to multiple antibiotics. PeerJ, 7, e6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, E.M. , Serrano, M.G. , Edupuganti, L. , Edwards, D.J. , Buck, G.A. & Jefferson, K.K. (2021) Sequence comparison of vaginolysin from different Gardnerella species. Pathogens, 10, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar, C. , Rolo, J. , Cerca, N. , Palmeira‐De‐oliveira, R. , Martinez‐De‐oliveira, J. & Palmeira‐De‐oliveira, A. (2021) Dequalinium chloride effectively disrupts bacterial vaginosis (BV) Gardnerella Spp. biofilms. Pathogens, 10, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, N.M. , Lewis, W.G. , Li, G. , Sojka, D.K. , Lubin, J.B. & Lewis, A.L. (2019) Gardnerella vaginalis and Prevotella bivia trigger distinct and overlapping phenotypes in a mouse model of bacterial vaginosis. The Journal of Infectious Diseases, 220, 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschick, C. , Deng, Z.L. , Vital, M. , Masur, C. , Abels, C. , Pieper, D.H. et al. (2017) Treatment of biofilms in bacterial vaginosis by an amphoteric tenside pessary‐clinical study and microbiota analysis. Microbiome, 5, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty, C.L. , Ness, R.B. , Totten, P.A. , Farooq, F. , Tang, G. , Ko, D. et al. (2020) Presence and concentrations of select bacterial vaginosis‐associated bacteria are associated with increased risk of pelvic inflammatory disease. Sexually Transmitted Diseases, 47, 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi, S. , Farhan, F. , Farshbaf‐Khalili, A. , Dehghan, P. , Javadzadeh, Y. , Abbasalizadeh, S. et al. (2018) The effect of prebiotic vaginal gel with adjuvant oral metronidazole tablets on treatment and recurrence of bacterial vaginosis: a triple‐blind randomized controlled study. Archives of Gynecology and Obstetrics, 297, 109–116. [DOI] [PubMed] [Google Scholar]
- Hallén, A. , Jarstrand, C. & Påhlson, C. (1992) Treatment of bacterial vaginosis with Lactobacilli . Sexually Transmitted Diseases, 19, 146–148. [DOI] [PubMed] [Google Scholar]
- Hantoushzadeh, S. , Golshahi, F. , Javadian, P. , Khazardoost, S. , Aram, S. , Hashemi, S. et al. (2012) Comparative efficacy of probiotic yoghurt and clindamycin in treatment of bacterial vaginosis in pregnant women: a randomized clinical trial. Journal of Maternal‐Fetal and Neonatal Medicine, 25, 1021–1024. [DOI] [PubMed] [Google Scholar]
- Hardy, L. , Cerca, N. , Jespers, V. , Vaneechoutte, M. & Crucitti, T. (2017) Bacterial biofilms in the vagina. Research in Microbiology, 168, 865–874. [DOI] [PubMed] [Google Scholar]
- Hardy, L. , Jespers, V. , Abdellati, S. , De Baetselier, I. , Mwambarangwe, L. , Musengamana, V. et al. (2016) A fruitful alliance: the synergy between Atopobium vaginae and Gardnerella vaginalis in bacterial vaginosis‐associated biofilm. Sexually Transmitted Infections, 92, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, L. , Jespers, V. , Van den Bulck, M. , Buyze, J. , Mwambarangwe, L. , Musengamana, V. et al. (2017) The presence of the putative Gardnerella vaginalis sialidase a gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS One, 12, e0172522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, P. (2014) Bacterial vaginosis. Medicine (Baltimore), 42, 359–363. [Google Scholar]
- He, Y. , Na, R. , Niu, X. , Xiao, B. & Yang, H. (2021) Lactobacillus rhamnosus and Lactobacillus casei affect various stages of Gardnerella species biofilm formation. Frontiers in Cellular and Infection Microbiology, 11, 568178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerling, A. , Harrison, W. , Schroeder, A. , Park, J. , Korn, A. , Shiboski, S. et al. (2010) Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV‐05 in women with bacterial vaginosis. Sexually Transmitted Diseases, 37, 745–750. [DOI] [PubMed] [Google Scholar]
- Javed, A. , Parvaiz, F. & Manzoor, S. (2019) Bacterial vaginosis: an insight into the prevalence, alternative treatments regimen and it's associated resistance patterns. Microbial Pathogenesis, 127, 21–30. [DOI] [PubMed] [Google Scholar]
- Joseph, R.J. , Ser, H.L. , Kuai, Y.H. , Tan, L.T.H. , Arasoo, V.J.T. , Letchumanan, V. et al. (2021) Finding a balance in the vaginal microbiome: how do we treat and prevent the occurrence of bacterial vaginosis? Antibiotics, 10, 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H. , Ehlers, M.M. , Peters, R.P.H. , Lombaard, H. , Redelinghuys, M.J. , Bezuidenhoudt, J.E. et al. (2020) Growth forms of Gardnerella spp. and Lactobacillus spp. on vaginal cells. Frontiers in Cellular and Infection Microbiology, 10, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, H.‐S. , Ehlers, M.M. , Lombaard, H. , Redelinghuys, M.J. & Kock, M.M. (2017) Etiology of bacterial vaginosis and polymicrobial biofilm formation. Critical Reviews in Microbiology, 43, 651–667. [DOI] [PubMed] [Google Scholar]
- Kenyon, C. , Colebunders, R. & Crucitti, T. (2013) The global epidemiology of bacterial vaginosis: a systematic review. American Journal of Obstetrics and Gynecology, 209, 505–523. [DOI] [PubMed] [Google Scholar]
- Khan, J. , Tarar, S.M. , Gul, I. , Nawaz, U. & Arshad, M. (2021) Challenges of antibiotic resistance biofilms and potential combating strategies: a review. 3 Biotech, 11, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.G. , Lee, J.H. , Park, S. , Kim, S. & Lee, J. (2022) Inhibition of polymicrobial biofilm formation by saw palmetto oil, lauric acid and myristic acid. Microbial Biotechnology, 15, 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban, I. , Bender, C.P. , Assadian, O. , Kramer, A. & Hübner, N.O. (2012) Clinical use of the antiseptic polihexanide for genital tract infections. Skin Pharmacology and Physiology, 25, 298–304. [DOI] [PubMed] [Google Scholar]
- Koumans, E.H. , Sternberg, M. , Bruce, C. , McQuillan, G. , Kendrick, J. , Sutton, M. et al. (2007) The prevalence of bacterial vaginosis in the United States, 2001‐2004; associations with symptoms, sexual behaviors, and reproductive health. Sexually Transmitted Diseases, 34, 864–869. [DOI] [PubMed] [Google Scholar]
- Landlinger, C. , Oberbauer, V. , Tisakova, L.P. , Schwebs, T. , Berdaguer, R. , Van Simaey, L. et al. (2022) Preclinical data on the Gardnerella‐specific Endolysin PM‐477 indicate its potential to improve the treatment of bacterial vaginosis through enhanced biofilm removal and avoidance of resistance. Antimicrobial Agents and Chemotherapy, 66, e02319‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landlinger, C. , Tisakova, L. , Oberbauer, V. , Schwebs, T. , Muhammad, A. , Latka, A. et al. (2021) Engineered phage Endolysin eliminates Gardnerella biofilm without damaging beneficial bacteria in bacterial vaginosis ex vivo. Pathogens, 10, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letrado, P. , Corsini, B. , DÍez‐Martínez, R. , Bustamante, N. , Yuste, J.E. & García, P. (2018) Bactericidal synergism between antibiotics and phage endolysin Cpl‐711 to kill multidrug‐resistant pneumococcus. Future Microbiology, 13, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev‐Sagie, A. , De Seta, F. , Verstraelen, H. , Ventolini, G. , Lonnee‐Hoffmann, R. & Vieira‐Baptista, P. (2022) The vaginal microbiome: II. Vaginal dysbiotic conditions. Journal of Lower Genital Tract Disease, 26, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev‐Sagie, A. , Goldman‐Wohl, D. , Cohen, Y. , Dori‐Bachash, M. , Leshem, A. , Mor, U. et al. (2019) Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nature Medicine, 25, 1500–1504. [DOI] [PubMed] [Google Scholar]
- Li, T. , Zhang, Z. , Wang, F. , He, Y. , Zong, X. , Bai, H. et al. (2020) Antimicrobial susceptibility testing of metronidazole and clindamycin against Gardnerella vaginalis in planktonic and biofilm formation. Canadian Journal of Infectious Diseases and Medical Microbiology, 2020, 1361825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Cao, B. , Yang, L. & Gu, J.D. (2022) Biofilm control by interfering with c‐di‐GMP metabolism and signaling. Biotechnology Advances, 56, 107915. [DOI] [PubMed] [Google Scholar]
- Luo, A. , Wang, F. , Sun, D. , Liu, X. & Xin, B. (2022) Formation, development, and cross‐species interactions in biofilms. Frontiers in Microbiology, 12, 3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, A. & Cerca, N. (2015) Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. The Journal of Infectious Diseases, 212, 1856–1861. [DOI] [PubMed] [Google Scholar]
- Marcotte, H. , Larsson, P.G. , Andersen, K.K. , Zuo, F. , Mikkelsen, L.S. , Brandsborg, E. et al. (2019) An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infectious Diseases, 19, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrazzo, J.M. , Dombrowski, J.C. , Wierzbicki, M.R. , Perlowski, C. , Pontius, A. , Dithmer, D. et al. (2019) Safety and efficacy of a novel vaginal anti‐infective, TOL‐463, in the treatment of bacterial vaginosis and vulvovaginal candidiasis: a randomized, single‐blind, phase 2, controlled trial. Clinical Infectious Diseases, 68, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi, M. , Miraj, S. & Rafieian‐Kopaei, M. (2016) Comparison of the effects of Myrtus communis L, Berberis vulgaris and metronidazole vaginal gel alone for the treatment of bacterial vaginosis. Journal of Clinical and Diagnostic Research, 10, QC04–QC07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi, M. , Rafieian Kopaei, M. & Miraj, S. (2017) A comparison of the efficacy of metronidazole vaginal gel and Myrtus (Myrtus communis) extract combination and metronidazole vaginal gel alone in the treatment of recurrent bacterial vaginosis. Avicenna Journal of Phytomedicine, 7, 129–136. [PMC free article] [PubMed] [Google Scholar]
- Menard, J. , Fenollar, F. , Henry, M. , Bretelle, F. & Raoult, D. (2008) Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clinical Infectious Diseases, 47, 33–43. [DOI] [PubMed] [Google Scholar]
- Menard, J.P. (2011) Antibacterial treatment of bacterial vaginosis: current and emerging therapies. International Journal of Women's Health, 3, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendling, W. , Weissenbacher, E.R. , Gerber, S. , Prasauskas, V. & Grob, P. (2016) Use of locally delivered dequalinium chloride in the treatment of vaginal infections: a review. Archives of Gynecology and Obstetrics, 293, 469–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis, C. & Grohmann, E. (2023) Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics, 12, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic, A.N. & Budakov, D. (2010) Comparison of local metronidazole and a local antiseptic in the treatment of bacterial vaginosis. Archives of Gynecology and Obstetrics, 282, 43–47. [DOI] [PubMed] [Google Scholar]
- Mirzaeei, S. , Zangeneh, M. , Veisi, F. , Parsa, S. & Hematti, M. (2021) Chlorhexidine, clotrimazole, metronidazole and combination therapy in the treatment of vaginal infections. Journal of Medicine and Life, 14, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty, T. , Doke, P.P. & Khuroo, S.R. (2022) Effect of bacterial vaginosis on preterm birth: a meta‐analysis. Archives of Gynecology and Obstetrics. Online ahead of print. 10.1007/s00404-022-06817-5 [DOI] [PubMed] [Google Scholar]
- Muñoz‐Barreno, A. , Cabezas‐Mera, F. , Tejera, E. & Machado, A. (2021) Comparative effectiveness of treatments for bacterial vaginosis: a network meta‐analysis. Antibiotics, 10, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny, C.A. , Blanchard, E. , Taylor, C.M. , Aaron, K.J. , Talluri, R. , Griswold, M.E. et al. (2018) Identification of key bacteria involved in the induction of incident bacterial vaginosis: a prospective study. The Journal of Infectious Diseases, 218, 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny, C.A. , Łaniewski, P. , Schwebke, J.R. & Herbst‐Kralovetz, M.M. (2020) Host‐vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Current Opinion in Infectious Diseases, 33, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny, C.A. & Sobel, J.D. (2022) The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment. Antibiotics, 11, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny, C.A. , Taylor, C.M. , Swords, W.E. , Tamhane, A. , Chattopadhyay, D. , Cerca, N. et al. (2019) An updated conceptual model on the pathogenesis of bacterial vaginosis. The Journal of Infectious Diseases, 220, 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouioui, I. , Carro, L. , García‐López, M. , Meier‐Kolthoff, J.P. , Woyke, T. , Kyrpides, N.C. et al. (2018) Genome‐based taxonomic classification of the phylum actinobacteria. Frontiers in Microbiology, 9, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent, R.P. , Krohn, M.A. & Hillier, S.L. (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology, 29, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon, D.E. , Moench, T.R. & Cone, R.A. (2011) In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infectious Diseases, 11, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala, T. , Kankainen, M. , Castro, J. , Cerca, N. , Edelman, S. , Westerlund‐Wikström, B. et al. (2014) Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis . BMC Genomics, 15, 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki, K. & Imai, N. (2017) Effects of lactoferrin in 6 patients with refractory bacterial vaginosis. Biochemistry and Cell Biology, 95, 31–33. [DOI] [PubMed] [Google Scholar]
- Palmeira‐de‐Oliveira, A. , Silva, B.M. , Palmeira‐de‐Oliveira, R. , Martinez‐de‐Oliveira, J. & Salgueiro, L. (2013) Are plant extracts a potential therapeutic approach for genital infections? Current Medicinal Chemistry, 20, 2914–2928. [DOI] [PubMed] [Google Scholar]
- Patterson, J.L. , Stull‐Lane, A. , Girerd, P.H. & Jefferson, K.K. (2010) Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial‐vaginosis‐associated anaerobes. Microbiology, 156, 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazhohideh, Z. , Mohammadi, S. , Bahrami, N. , Mojab, F. , Abedi, P. & Maraghi, E. (2018) The effect of Calendula officinalis versus metronidazole on bacterial vaginosis in women: a double‐blind randomized controlled trial. Journal of Advanced Pharmaceutical Technology & Research, 9, 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles, K. , Velloza, J. , Balkus, J.E. , McClelland, R.S. & Barnabas, R.V. (2019) High global burden and costs of bacterial vaginosis: a systematic review and meta‐analysis. Sexually Transmitted Diseases, 46, 304–311. [DOI] [PubMed] [Google Scholar]
- Perez, A.C. , Pang, B. , King, L.B. , Tan, L. , Murrah, K.A. , Reimche, J.L. et al. (2014) Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathogens and Disease, 70, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino, A. , Mazza, T. , Matthews, M.A.H. , Castellana, S. , Caggia, C. , Randazzo, C.L. et al. (2022) Antimicrobial activity of bovine lactoferrin against Gardnerella species clinical isolates. Frontiers in Microbiology, 13, 1000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer, E.L. , Bradshaw, C.S. , Doyle, M. , Fairley, C.K. , Murray, G.L. , Bateson, D. et al. (2021) Lactic acid‐containing products for bacterial vaginosis and their impact on the vaginal microbiota: a systematic review. PLoS One, 16, e0246953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer, E.L. , Vodstrcil, L.A. , Danielewski, J.A. , Murray, G.L. , Fairley, C.K. , Garland, S.M. et al. (2018) Combined oral and topical antimicrobial therapy for male partners of women with bacterial vaginosis: acceptability, tolerability and impact on the genital microbiota of couples—a pilot study. PLoS One, 13, e0190199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer, E.L. , Vodstrcil, L.A. , Doyle, M. , Danielewski, J.A. , Murray, G.L. , Fehler, G. et al. (2021) A prospective, open‐label pilot study of concurrent male partner treatment for bacterial vaginosis. mBio, 12, e0232321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, A. , Ghanem, K.G. , Rogers, L. , Zinalabedini, A. , Brotman, R.M. , Zenilman, J. et al. (2019) Clinicians' use of intravaginal boric acid maintenance therapy for recurrent vulvovaginal candidiasis and bacterial vaginosis. Sexually Transmitted Diseases, 46, 810–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus, V. & Onderdonk, A.B. (1997) Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. The Journal of Infectious Diseases, 175, 406–413. [DOI] [PubMed] [Google Scholar]
- Rahman, M.U. , Wang, W. , Sun, Q. , Shah, J.A. , Li, C. , Sun, Y. et al. (2021) Endolysin, a promising solution against antimicrobial resistance. Antibiotics, 10, 1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratten, L.K. , Plummer, E.L. , Murray, G.L. , Danielewski, J. , Fairley, C.K. , Garland, S.M. et al. (2021) Sex is associated with the persistence of non‐optimal vaginal microbiota following treatment for bacterial vaginosis: a prospective cohort study. BJOG: An International Journal of Obstetrics & Gynaecology, 128, 756–767. [DOI] [PubMed] [Google Scholar]
- Ravel, J. , Brotman, R.M. , Gajer, P. , Ma, B. , Nandy, M. , Fadrosh, D.W. et al. (2013) Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome, 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel, J. , Gajer, P. , Abdo, Z. , Schneider, G.M. , Koenig, S.S.K. , McCulle, S.L. et al. (2011) Vaginal microbiome of reproductive‐age women. Proceedings of the National Academy of Sciences, 108, 4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelinghuys, M.J. , Geldenhuys, J. , Jung, H. & Kock, M.M. (2020) Bacterial vaginosis: current diagnostic avenues and future opportunities. Frontiers in Cellular and Infection Microbiology, 10, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman, O. , Akins, R. & Sobel, J.D. (2009) Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sexually Transmitted Diseases, 36, 732–734. [DOI] [PubMed] [Google Scholar]
- Rizzo, A. , Fiorentino, M. , Buommino, E. , Donnarumma, G. , Losacco, A. & Bevilacqua, N. (2015) Lactobacillus crispatus mediates anti‐inflammatory cytokine interleukin‐10 induction in response to Chlamydia trachomatis infection in vitro. International Journal of Medical Microbiology, 305, 815–827. [DOI] [PubMed] [Google Scholar]
- Rosca, A.S. , Castro, J. , Sousa, L.G.V. & Cerca, N. (2020) Gardnerella and vaginal health: the truth is out there. FEMS Microbiology Reviews, 44, 73–105. [DOI] [PubMed] [Google Scholar]
- Rosca, A.S. , Castro, J. , Sousa, L.G.V. , França, A. , Cavaleiro, C. , Salgueiro, L. et al. (2022) Six bacterial vaginosis‐associated species can form an in vitro and ex vivo polymicrobial biofilm that is susceptible to Thymbra capitata essential oil. Frontiers in Cellular and Infection Microbiology, 12, 824860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca, A.S. , Castro, J. , Sousa, L.G.V. , França, A. , Vaneechoutte, M. & Cerca, N. (2022) In vitro interactions within a biofilm containing three species found in bacterial vaginosis (BV) support the higher antimicrobial tolerance associated with BV recurrence. The Journal of Antimicrobial Chemotherapy, 77, 2183–2190. [DOI] [PubMed] [Google Scholar]
- Rostok, M. , Hütt, P. , Rööp, T. , Smidt, I. , Štšepetova, J. , Salumets, A. et al. (2019) Potential vaginal probiotics: safety, tolerability and preliminary effectiveness. Beneficial Microbes, 10, 385–393. [DOI] [PubMed] [Google Scholar]
- Schuyler, J.A. , Mordechai, E. , Adelson, M.E. , Sobel, J.D. , Gygax, S.E. & Hilbert, D.W. (2016) Identification of intrinsically metronidazole‐resistant clades of Gardnerella vaginalis . Diagnostic Microbiology and Infectious Disease, 84, 1–3. [DOI] [PubMed] [Google Scholar]
- Schwebke, J.R. & Desmond, R.A. (2007) A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clinical Infectious Diseases, 44, 213–219. [DOI] [PubMed] [Google Scholar]
- Schwebke, J.R. & Desmond, R.A. (2011) Tinidazole vs metronidazole for the treatment of bacterial vaginosis. American Journal of Obstetrics and Gynecology, 204, 211.e1–211.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal, P.G. , Dadwal, R. , Sharma, B. , Sehgal, A. , Bagga, R. , Chopra, S. et al. (2021) Detection of co‐infection of Gardnerella vaginalis and Atopobium vaginae using qualitative PCR: a better predictor of bacterial vaginosis. Anaerobe, 69, 102343. [DOI] [PubMed] [Google Scholar]
- Simoes, J.A. , Aroutcheva, A. , Heimler, I. , Shott, S. & Faro, S. (2001) Bacteriocin susceptibility of Gardnerella vaginalis and its relationship to biotype, genotype, and metronidazole susceptibility. American Journal of Obstetrics and Gynecology, 185, 1186–1190. [DOI] [PubMed] [Google Scholar]
- Sobel, J.D. & Sobel, R. (2021) Current and emerging pharmacotherapy for recurrent bacterial vaginosis. Expert Opinion on Pharmacotherapy, 22, 1593–1600. [DOI] [PubMed] [Google Scholar]
- Sousa, L.G.V. , Castro, J. , Cavaleiro, C. , Salgueiro, L. , Tomás, M. , Palmeira‐Oliveira, R. et al. (2022) Synergistic effects of carvacrol, α‐terpinene, γ‐terpinene, ρ‐cymene and linalool against Gardnerella species. Scientific Reports, 12, 4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyer Caliskan, C. , Yurtcu, N. , Celik, S. , Sezer, O. , Kilic, S.S. & Cetin, A. (2022) Derangements of vaginal and cervical canal microbiota determined with real‐time PCR in women with recurrent miscarriages. Journal of Obstetrics and Gynaecology, 42, 2105–2114. [DOI] [PubMed] [Google Scholar]
- Surapaneni, S. , Akins, R. & Sobel, J.D. (2021) Recurrent bacterial vaginosis: an unmet therapeutic challenge. Experience with a combination pharmacotherapy long‐term suppressive regimen. Sexually Transmitted Diseases, 48, 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski, A. , Loening‐Baucke, V. , Swidsinski, S. , Sobel, J.D. , Dörffel, Y. & Guschin, A. (2022) Clue cells and pseudo clue cells in different morphotypes of bacterial vaginosis. Frontiers in Cellular and Infection Microbiology, 12, 905739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski, A. , Loening‐Baucke, V. , Swidsinski, S. & Verstraelen, H. (2015) Polymicrobial Gardnerella biofilm resists repeated intravaginal antiseptic treatment in a subset of women with bacterial vaginosis: a preliminary report. Archives of Gynecology and Obstetrics, 291, 605–609. [DOI] [PubMed] [Google Scholar]
- Swidsinski, A. , Mendling, W. , Loening‐Baucke, V. , Ladhoff, A. , Swidsinski, S. , Hale, L.P. et al. (2005) Adherent biofilms in bacterial vaginosis. Obstetrics and Gynecology, 106, 1013–1023. [DOI] [PubMed] [Google Scholar]
- Swidsinski, A. , Mendling, W. , Loening‐Baucke, V. , Swidsinski, S. , Dörffel, Y. , Scholze, J. et al. (2008) An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. American Journal of Obstetrics and Gynecology, 198, 97.e1–97.e6. [DOI] [PubMed] [Google Scholar]
- Swidsinski, A. , Verstraelen, H. , Loening‐Baucke, V. , Swidsinski, S. , Mendling, W. & Halwani, Z. (2013) Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS One, 8, e53997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh, H.‐T. , Lee, I.‐A. , Hyun, Y.‐J. & Kim, D.‐H. (2011) Artemisia princeps Pamp. essential oil and its constituents eucalyptol and α‐terpineol ameliorate bacterial vaginosis and vulvovaginal candidiasis in mice by inhibiting bacterial growth and NF‐κB activation. Planta Medica, 77, 1996–2002. [DOI] [PubMed] [Google Scholar]
- Tseng, B.S. , Zhang, W. , Harrison, J.J. , Quach, T.P. , Song, J.L. , Penterman, J. et al. (2013) The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environmental Microbiology, 15, 2865–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wijgert, J.H.H.M. & Jespers, V. (2017) The global health impact of vaginal dysbiosis. Research in Microbiology, 168, 859–864. [DOI] [PubMed] [Google Scholar]
- Vestby, L.K. , Grønseth, T. , Simm, R. & Nesse, L.L. (2020) Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics, 9, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira‐Baptista, P. , De Seta, F. , Verstraelen, H. , Ventolini, G. , Lonnee‐Hoffmann, R. & Lev‐Sagie, A. (2022) The vaginal microbiome: V. therapeutic modalities of vaginal microbiome engineering and research challenges. Journal of Lower Genital Tract Disease, 26, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodstrcil, L.A. , Muzny, C.A. , Plummer, E.L. , Sobel, J.D. & Bradshaw, C.S. (2021) Bacterial vaginosis: drivers of recurrence and challenges and opportunities in partner treatment. BMC Medicine, 19, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher, E.R. , Donders, G. , Unzeitig, V. , Martinez De Tejada, B. , Gerber, S. , Halaška, M. et al. (2012) A comparison of dequalinium chloride vaginal tablets (fluomizin®) and clindamycin vaginal cream in the treatment of bacterial vaginosis: a single‐blind, randomized clinical trial of efficacy and safety fluomizin study group. Gynecologic and Obstetric Investigation, 73, 8–15. [DOI] [PubMed] [Google Scholar]
- Wewalka, G. , Stary, A. , Bosse, B. , Duerr, H.E. & Reimer, K. (2002) Efficacy of povidone‐iodine vaginal suppositories in the treatment of bacterial vaginosis. Dermatology, 204, 79–85. [DOI] [PubMed] [Google Scholar]
- Wood, T.K. (2017) Strategies for combating persister cell and biofilm infections. Microbial Biotechnology, 10, 1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workowski, K.A. , Bachmann, L.H. , Chan, P.A. , Johnston, C.M. , Muzny, C.A. , Park, I. et al. (2021) Sexually transmitted infections treatment guidelines, 2021. MMWR Recommendations and Reports, 70, 1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey, L.J. , Hussain, F.A. , Bergerat, A. , Reissis, A. , Worrall, D. , Xu, J. et al. (2022) Screening and characterization of vaginal fluid donations for vaginal microbiota transplantation. Scientific Reports, 12, 17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q.Q. , Liu, Z.H. , Liu, L.L. , Hu, G. , Lei, G.L. , Wang, Y. et al. (2020) Prebiotic maltose gel can promote the vaginal microbiota from BV‐related bacteria dominant to Lactobacillus in rhesus macaque. Frontiers in Microbiology, 11, 594065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Chen, Y. , Gao, L. , Huang, J. , Yang, X. , Pei, L. et al. (2022) Acidic electrolyzed water inhibits the viability of Gardnerella spp. via oxidative stress response. Frontiers in Medicine, 9, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]


