Abstract
Chronic musculoskeletal (MSK) pain is one of the most prevalent causes, which lead patients to a physician’s office. The most common disorders affecting MSK structures are osteoarthritis, rheumatoid arthritis, back pain, and myofascial pain syndrome, which are all responsible for major pain and physical disability. Although there are many known management strategies currently in practice, phytotherapeutic compounds have recently begun to rise in the medical community, especially cannabidiol (CBD). This natural, non-intoxicating molecule derived from the cannabis plant has shown interesting results in many preclinical studies and some clinical settings. CBD plays vital roles in human health that go well beyond the classic immunomodulatory, anti-inflammatory, and antinociceptive properties. Recent studies demonstrated that CBD also improves cell proliferation and migration, especially in mesenchymal stem cells (MSCs). The foremost objective of this review article is to discuss the therapeutic potential of CBD in the context of MSK regenerative medicine. Numerous studies listed in the literature indicate that CBD possesses a significant capacity to modulate mammalian tissue to attenuate and reverse the notorious hallmarks of chronic musculoskeletal disorders (MSDs). The most of the research included in this review report common findings like immunomodulation and stimulation of cell activity associated with tissue regeneration, especially in human MSCs. CBD is considered safe and well tolerated as no serious adverse effects were reported. CBD promotes many positive effects which can manage detrimental alterations brought on by chronic MSDs. Since the application of CBD for MSK health is still undergoing expansion, additional randomized clinical trials are warranted to further clarify its efficacy and to understand its cellular mechanisms.
Keywords: Cannabidiol, orthopedics, regenerative medicine, inflammation, exosome
Impact Statement
Cannabidiol (CBD) has gained a lot of significance from orthopedists and sports medicine physicians due to its potential part in the treatment of chronic pain in musculoskeletal (MSK) conditions. CBD plays vital physiological roles in human health that go well beyond immunomodulation, anti-inflammation, and antinociception. Recent investigations show that CBD also enhances cell proliferation and migration, especially in human MSCs. CBD is still relatively new in MSK medicine, and even though new studies are emerging, the clinical application of CBD requires more robust data from clinical trials to further elucidate the mechanisms that contribute to the improvement of MSK structures.
Introduction
Cannabidiol (CBD) is considered as a non-intoxicating product of Cannabis sativa, a plant that contains several organic compounds with various physiological effects. 1 Although this plant contains at least 144 cannabinoids (CBs), the well-studied compounds are Δ9-tetrahydrocannabinol (Δ9-THC), which is notorious for its intoxicating and psychoactive effects, and CBD, for its beneficial effects in biological systems.2,3
CBD was first isolated many decades ago and was initially considered to be biologically inactive since it seemed to impart no considerable effects. 1 It was not until the early 1970s that researchers documented one of the first significant effects of this CB in convulsive crises in mice. 4 With considerable optimism, this was later replicated in a preclinical trial involving epileptic patients in 1980, 5 and since then, continuous research expanding into other fields of medicine has been on the rise.
Cannabis sativa L., in particular, has gained a lot of appreciation from orthopedists and sports medicine physicians due to its potential role in the treatment management of chronic pain in numerous conditions. 6 Parenthetically, osteoarthritis (OA) and tendinopathies are major orthopedic disorders responsible for pain and disability in adult populations. OA, for instance, remains the utmost typical degenerative and progressive joint disease affecting vital musculoskeletal (MSK) structures like cartilage, ligaments, and bone.7,8 Furthermore, the Global Burden of Disease Study 2019 revealed that the number of patients suffering from OA increased globally around 48% from 1990 to 2019, which represents OA as 15th most common cause of number of years lived with disability. 9 This fact is associated with risk factors like the aging and the prevalence of poor metabolic health, especially the obesity.10–12
Previous data have revealed positive insights regarding the application of CBD toward the improvement of pain and functional outcomes in various MSK conditions.13–18 These provide valuable insight considering the heavy socioeconomic burden associated with the chronic MSK pain. The objective of this article is to review and discuss the therapeutic potential of CBD in the context of MSK regenerative medicine.
Pathophysiology of common MSK diseases
It is well-established that chronic MSK pain is the key factor for physical disability in the adult population. 19 The World Health Organization (WHO) estimates that 20–33% (over 1.71 billion individuals) of the global population suffers from chronic MSK pain. 20 This type of disorder is characterized by acute or chronic pain in MSK structures, which involve muscles, tendons, ligaments, bones, and nerves. 21 The most common conditions responsible for visits to a physician’s office are OA, rheumatoid arthritis, myofascial pain syndrome (MPS), and low back and neck pain. 22 Less common incidents are generally accompanying with injuries like of tendon sprains, ligament tears, muscle tears, fractures, and similar damage during sports. 20
If left untreated, these conditions progressively increase suffering, disability, and drug consumption, which subsequently diminish an individual’s quality of life. 23 This also translates to a main community health problem due to significant high expenses for health-care systems and insurance for disability. Advanced age may remain the top variable associated with the increased risk of musculoskeletal disorders (MSDs) and MSK pain; however, these conditions may still unfold at any given age for various reasons. Therefore, every individual is at risk of experiencing MSK pain throughout an entire lifetime. 24 Acute pain can become chronic due to numerous factors. The level of intensity, site, and time of noxious stimuli are dictated by the interplay between mechanical, chemical, and thermal receptors and immune cells. 25 Under standard conditions, noxious stimuli and painful sensations gradually decrease with the progression of healing. However, when intense pain persists, secondary mechanisms are activated in both the peripheral nervous systems (PNSs) and central nervous systems (CNSs). Upregulation of inflammatory mediators sensitizes neurons, diminishing normal functioning and exacerbating pain perception. 25
Although MSK pathologies vary from patient to patient for several different reasons, these conditions often share considerable similarities, especially pain. The pain related with MSDs is often severe; about 25% of adults report pain ranging from 7 to 10 of the visual analog scale (VAS). 19 The pain is typically intense and localized, which may be aggravated depending on certain postures and movements executed by the patient. Body aches, stiffness, and malaise are the most common features of all MSDs. Certain exercises can temporarily improve range of motion, mobility, and pain, depending on the individual’s condition. However, there is a risk of overuse and further injuries to MSK structures if the movements are incorrectly executed. 26
Another major problem in individuals with chronic MSD and pain is fatigue and sleep disorders. MSK pain can disrupt normal sleep cycles as patients can have trouble falling or remaining asleep due to intense pain. 27 Some patients may have difficulty finding an appropriate or comfortable position which may force them to remain in ergonomically poor positions. Sleep deprivation further aggravates their condition and impairs MSK tissue regeneration and maintenance, delaying the healing process.28–30 Another study found that duration is also a significant factor, as both long and short sleep durations have been associated with MSK pain. 31 Adequate sleep is important for the body. The circadian rhythm has been shown to impact stem cell regenerative capacity 32 by improving the stem cell proliferation microenvironment, resulting in stem cell maintenance and controlled division. 33 This is directly tied to melatonin, the “sleep hormone.” In addition to displaying roles of anti-inflammatory, antioxidant, and neuroprotective, it also assists in the regeneration of several tissues beyond the MSK system. 34
The principal hallmarks of the most common MSDs include the overly pro-inflammatory and catabolic microenvironments. 35 More specifically, chronic degenerative MSDs such as OA are outlined by a combination of cellular changes and stresses from biochemical and biomechanical, which cause several secondary problems. These then lead to pathological changes including remodeling of the subchondral bone, bone marrow lesions, development of osteophytes along with changes in the joint capsule, synovium, periarticular muscles, ligaments, meniscal tears, and extrusion. 35 Moreover, the improved turnover of subchondral bone in both clinical and preclinical studies have showed the cytokines with other growth factors secreted during pathological progression may involve with and harm the articular cartilage. This can leads to trigger a positive feedback loop due to several failed efforts to repair cartilage and bone tissue, finally leading to OA. 35 To elaborate, synoviocytes and osteoarthritic chondrocytes produce high quantities of matrix metalloproteinases (MMPs), including MMPs (1, 3, 9, and 13). 36 Synoviocytes release pro-inflammatory cytokines (tumor necrosis factor-alpha [TNF-α], interleukin [IL]1β and IL-6) and proteolytic enzymes, major mediators of inflammation and pain. 37 Once the chondrocytes fail to uphold the equilibrium between synthesis and degradation of the extracellular matrix (ECM) components, OA arises. 38 It has been hypothesized that trauma, microfractures, and inflammation can slightly increase proteolytic enzyme activity, generating “wear” particles causes the “wear-and-tear” process. 39 Although macrophages eventually remove microparticles and cellular debris. The absent of these particles devastates the system. This phenomenon leads to much burden to eliminate them. This turn out to be mediators of inflammation and signaling chondrocytes to release catabolic enzymes. 39
Similar processes take place in chronic back pain. Alterations involving the spinal structures, especially the intervertebral disks (IVD), may be linked to variables such as age, trauma, metabolic status, toxic substances, vascular pathology, infections, and genetics.40,41 A healthy IVD is similar to articular cartilage at the cellular and molecular levels. Chondrocyte-like cells synthesize collagen, proteoglycans, and other proteins to form the nucleus-pulposus matrix and then the cartilaginous in vertebral endplate. Fibroblast-like cells produce both collagens (type I and II), which serves to maintain the annulus fibrosus. This is important as type II collagen holds proteoglycan aggregates and forms a matrix with hygroscopic properties. 42 In turn, this imparts the nucleus-pulposus hydrostatic properties, allowing it to house compressive forces and brace the annulus. 41 Nevertheless, the matrix components undergo constant degradation by chondrocyte-secreted enzymes, especially MMPs. This process becomes pathological when the rate of degradation surpasses that of synthesis, disrupting homeostasis and impeding matrix renewal. 43 In response to injury, type 1 macrophages (Mφ1) invade the vertebral landmarks and release pro-inflammatory mediators, promoting the synthesis of MMPs and inhibition of new matrix formation.44–46 Mφ1 are more associated with antimicrobial and pro-inflammatory roles, 47 which explains why they also often secrete superoxide (O2−). This free radical plays a crucial role in signaling; 48 however, it can also degrade hyaluronic acid and proteoglycans, inhibiting the proliferation of chondrocytes. 49 IL-1 and TNF-α are also secreted and contribute to the production of nitric oxide (NO); another free radical that elicits significant physiological functions but is also often associated with degradative effects. 50
MPS is a pain condition, which is originating from sensory, motor, and autonomic symptoms produced by direct or indirect traumatic events, and ergonomic, structural, or systemic factors.51,52 The most distinct feature of MPS is the having a trigger points (TrPs), which are called hyperirritable points present in skeletal muscle/fascia, causing referred local tenderness, pain, and autonomic alterations upon compression.51–53 TrPs can be categorized into active/latent – active TrPs are usually responsible for impulsive pain and tenderness, whereas latent “trigger points” are foci of hyperirritability in skeletal muscles, and are clinically linked with tenderness, local spasms, or stated pain upon palpation.51–53 In addition, they may also be present in many no pain skeletal muscles and undergo activation and conversion into their active form by repeated noxious stimuli. 53 Due to limited or erroneous diagnosis, TrPs can sometimes be interpreted as other similar chronic pain conditions including radiculopathies, fibromyalgia, or tendon and joint diseases. 52 Therefore, medical experts emphasize that a proper MPS diagnosis must confirm the presence of TrPs. 54 Research suggests that they arise from dysfunctions in neuromuscular junctions and the surrounding connective tissue, or as a result of electrical perturbations and excess release of acetylcholine. 52 This process induces depolarization of the muscle fiber postjunctional membrane, resulting in calcium release and uptake. Calcium release promotes muscle ischemia and biosynthesis of pro-inflammatory mediators such as substance P, bradykinin, TNF-α, IL-1β, IL-6, and IL-8.52,55
Similarly, tendon disorders are also extremely common among the general population, albeit rather more frequent in athletes. This pathology also ranges from traumatic injuries to chronic disease processes, much like the pathophysiological progressions described earlier. 56 Tendon injuries are mostly attributed to overuse conditions causing significant physical stress on these tissues, 57 or other intrinsic and extrinsic risk factors such as aging, biological sex, and even poor ergonomics which can contribute to the development of specific tendinopathies.58,59 The production of various biomechanical and biochemical signals generates numerous physiological responses with varying effects. For instance, the activation of specific cell signaling pathways may or may not alter the regular function and composition of ECM. Depending on the given biological fate, certain reactions may subsequently trigger stable or unstable cell proliferation, migration, apoptosis, and morphogenesis. 60 Adequate tendon homeostasis is crucial in dictating the fate of tendons. This process is regulated by mechanical loading, followed by cell activity, neuronal, and cellular regulators. These regulators are secreted locally or distantly and followed via blood circulation and/or nerve supply.61,62 Therefore, impaired biomechanics and cellular dysfunctions may be acknowledged as the major culprits in tendon pathologies. Proper loading of tendons stimulates anabolic responses, such as the increase of collagen mRNA expression and elevated synthesis of these vital proteins.63,64 Collagen synthesis reaches its maximum in 24 h after physical modulation, and its levels are kept high for up to 80 h. 65 However, inadequate rest between activities and excessive loading ultimately forces tenocytes to produce pro-inflammatory molecules. This in turn fragilizes collagen fibrils, leading to the degradation of collagen and elevating the risk of microdamage. 65 The chronic degenerative alterations in tendons are also strongly outlined by increased levels of pro-inflammatory biomarkers such as ILs (18, 15, 6, and 1β) and TNF-α,66,67 along with the activation of the prototypical pro-inflammatory signaling pathway nuclear factor kappa B (NF-κB). 68 In addition, there is also the participation of the innate immune system in the early onset of the disease, especially with the crucial participation of macrophages during inflammation and tissue repair. It is known that signaling pathways can induce the polarization of macrophages into either Mφ1 (pro-inflammatory) or Mφ2 (anti-inflammatory) subtypes.47,69 However, inflammatory mediators such as interferons, NF-κB, and glucocorticoid receptor activation pathways regulate monocyte differentiation and macrophage polarization. 69 This means that overstimulated inflammatory pathways in chronic tendon disorders dictate macrophage polarization, leading to failed, fibrotic healing responses. 70 Under repetitive mechanical stress, transforming growth factor beta (TGF-β) binds to myofibroblasts and differentiates into fibroblasts. 71 Normally, at the end of the healing cascade, myofibroblasts will be free of mechanical stress, and these cells undertake apoptosis. The problem arises once this mechanism fails to activate, as myofibroblasts then trigger a hyperproliferative process leading to fibrosis, another prominent histological feature of tendinopathy. 72 The repetitive unsuccessful healing responses also contribute to the deterioration of the mechanical properties, eventually leading to ruptures. Moreover, the aberrant sprouting and ingrowth of sensory nerve fibers take place after neoangiogenesis; however, since these nerves are not retracted they trigger nociception and increased pain signaling.72,73
Although the described disorders do have their particularities, it seems evident that the aspect of chronic inflammation is frequently presented as a central pathophysiological hallmark in virtually all processes of MSDs.
Conventional approach
There are different ways to approach MSDs, although patients usually decide to visit physiatrists and orthopedic specialists due to various discomforts. 20 To overcome the hurdle, medical doctors must be carefully evaluate the patient to avoid any confusion and also to avoid any misdiagnosis since some MSK conditions can sometimes be intricate and may present an intricate nature.74,75 Comprehensive care involves an initial evaluation of the patient’s status via questionnaires, palpation, and examination of radiological evidence, when possible.
Initially, practitioners may decide to apply conventional treatment methods in attempts to identify and correct risk factors. Common strategies such as rest, administration of pharmacological substances, orthotic treatment, physical rehabilitation, friction massage, nutritional supplements, laser therapy, and dry needling alone or in combination.76,77 However, a major challenge related with these treatments is that oftentimes they only target pain but do not arrest the pathological progression of MSDs. 78 Furthermore, chronic non-steroidal anti-inflammatory drug (NSAID) use, for example, is very concerning. It has been reported that even though it effectively alleviates pain, it can also elevate the risk of acute renal failure, peptic ulcer disease, and myocardial infarction. 79
Non-pharmacological approaches have restricted potential meanwhile they are usually more restricted to physical therapy, nerve ablation, physical aids, education of maintain postural, and weight loss programs. In more complicated cases, however, for instance the end-stage OA, surgical procedures may prove to be unavoidable and, thus, tremendously detrimental to the patient.50,80 To overcome these challenging scenarios, medical doctors have shifted their focus in the direction of CBs, which could be a novel therapeutic compounds and required further studies.
Biological mechanisms of CBD
In recent years, CBD has piqued the interest of researchers due to the unfolding of biological effects beyond its ability to reduce pain. In 2020, Miller et al. 16 showed an in vitro study assessing the CBD role in the activation of the regenerative capacity of stem cells. In their study, the researchers treated human adipose-derived stem cells (ASCs) and bone marrow–derived mesenchymal stem cells (BMSCs) with either low or high levels of CBD and performed migration, proliferation, and wound closure (scratch) assays. Miller and colleagues learned that both ASCs and BMSCs showed a significant cellular migration increase after exposure to CBD. The migration of ASCs increased by 412% with low-dose CBD, and 251% with high-dose CBD compared to control. The migration of BMSCs increased by 298% and 166% with low and high doses of CBD, respectively, compared to control. In regard to proliferation, higher CBD doses were more effective for BMSCs (48% CBD of low-dose and 86% CBD of high-dose), but no statistically significant differences were observed for ASCs. However, in terms of wound closure, ASCs treated with low-dose CBD had a 49% and 78% quicker-wound closure rate at 20 and 44 h, respectively. Overall, at least in this particular setting, CBD priming of human mesenchymal stem cells (MSCs) at both low and high concentrations proved to enhance the three essential pillars in regenerative medicine: migration, proliferation, and wound healing. Such findings are of significant value for regenerative medicine because stem cell therapy alone has also demonstrated optimistic effects in various settings, especially in the autologous MSCs for the treatment of MSDs. 81
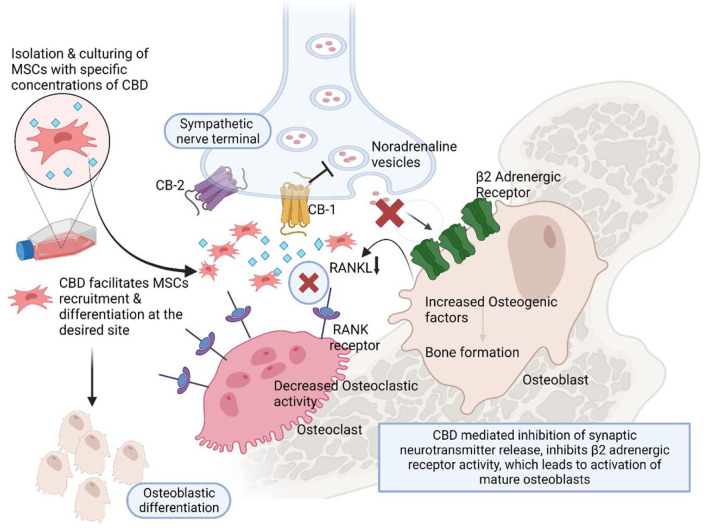
CB receptors also play crucial roles in eliciting positive clinical outcomes. 18 For instance, both the CB-1 and CB-2 receptors, in particular, are commonly present in bone tissue and are known to impart different effects on bone metabolism. 18 Physiologically, the CB-1 receptor controls the growth of the bone during skeletal development and predominantly dictates metabolism of bone by acting on the peripheral sympathetic nerve terminals, inhibiting the production of norepinephrine at sympathetic nerve terminals once it is activated in case of MSK injuries.82,83 This blocks osteoblast β2-adrenergic receptors, subsequently increasing osteoblast activity and differentiation. 83 The reduction in β2-adrenergic receptors stimulation of leads to reducing the formation of osteoclast by decreasing the receptor activator of NF-κB ligand, one of the major proteins responsible for osteoclastogenesis as depicted in Figure 1. 84 To elaborate, preclinical reports showed that number and function of osteoblast were not affected during bone growth in CB-1 receptor-deficient mice; however, during middle age, decrease in the osteoblastogenesis and increase in the adipogenesis occur, which eventually reduced the bone formation during late ages.82,83,85 Furthermore, an in vitro study 15 showed the CB-1 receptor’s activation during the differentiation of osteogenic and displays a functional role in survival of MSCs at acute cell stress. Conversely, as a side note, the administration of Δ9-THC in this in vitro setting imparted negative effects on MSC survival and osteogenesis.
Figure 1.
Biological effects of cannabinoids on osseous tissue through various mechanisms.
Source: Created with Biorender.com.
CB-1 is a presynaptic heteroreceptor located in the presynaptic peripheral and central nerve terminals where pain signals are highly transmitted. Activation of the CB-1 receptor in the presynaptic region inhibits the calcium influx-mediated neurotransmitter release from its vesicles. It inhibits presynaptic transmission, thereby decreasing the beta-adrenergic activity on the mature osteoblasts. Furthermore, it facilitates MSCs recruitment and accelerates osteoblastic differentiation under suitable conditions.
These observations partially illustrate the function of CB-1 receptor stimulation and its role in maintaining the health of bone tissue. This could be of great value for osteoarthritic patients considering that the subchondral bone is significantly affected by the detrimental alterations that take place at the onset of OA. 86
CB-2 receptor is also present in bone-related cells with their precursors albeit at increased levels in comparison to the CB-1 receptor, playing key roles in bone homeostasis. 87 Stimulation of CB-2 receptor positively affects osteoblasts in terms of proliferation and cellular activity since it promotes increases in osteogenic factors (bone sialoprotein, runt-related transcription factor 2, alkaline phosphatase, osteopontin, and osteocalcin), while its effects on osteoclasts remain controversial.18,88 Mice models of CB-2 receptor deficiency reveal dramatic increases in osteoporotic conditions, with decreased number and function of osteoblasts and concomitant increases in osteoclasts.89,90 Interestingly, the lack of CB-2 receptor expression in these knockout mice did not reveal significant physiological alteration of any additional organ systems except for bone.89,90 Other CB receptors, such as TPRV1, TPRV4, GPR119, and GPR55, resemble in structure and bind to both CB-1 and CB-2 receptors but are still under further research in bone metabolism.18,89,90
It is worth noting that a role as the regulator of inflammation has been proposed for the CB-2 receptor. 91 The fact that it is also highly expressed in immune cells suggests the possibility for the endocannabinoid system (ES) involved in immunomodulation. 91 As the name suggests, the ES is regulated by several lipid signaling molecules, better known as endocannabinoids. 92 Anandamide or arachidonoyl ethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) are the two commonly reported endocannabinoids. 92 AEA functions in maintaining the basal endocannabinoid signaling, as it binds to both the receptors (CB-1 and CB-2). 93 When elevated, this molecule acts as a complete agonist for the transient receptor potential vanilloid 1; it mediates the integration of noxious stimuli that cause pain. 94 In contrast, 2-AG is a complete agonist for both the receptors (CB-1 and CB-2). 95
Both the endocannabinoids 2-AG and AEA are known to function on several immune cells via activation of CB-2 receptor. Research shows that 2-AG appears to modulate leukocyte recruitment, migration, chemokine release, and adhesion to fibronectin, illustrating its pro-inflammatory effect. 96 Conversely, AEA downregulates these functions and further dampens the making of NO and the secretion of pro-inflammatory cytokines. 91 There have been reports of AEA in promotion of synthesis of IL-10 (a major anti-inflammatory cytokine).
Activation of the ES appears to be triggered in chronic MSK pain. For instance, 2-AG and AEA are present in the synovial fluid of patients (with rheumatoid arthritis), unlike in healthy individuals. 97 The mRNA of both receptors (CB-1 and CB-2) is also found in the synovial tissues of arthritic patients. 97 However, researchers found that the activation of the CB-2 tone blocks the making of MMPs and pro-inflammatory cytokines from fibroblast-like synoviocytes. 98 In addition, this process can also contribute to osteoblast differentiation, 90 and the inhibition of fibroblast-like synoviocytes proliferation, at least in vitro settings. 99 Furthermore, inhibition of CB receptors has reported that endocannabinoids can increase or inhibit the perception of pain. 100 This antinociceptive property exhibited by CB agonists appears to be in accordance with their capacity to shift binding ligands from the receptor of CB, which subsequently impedes cell signaling.
Such findings put forward the CB-2 receptor appears to be a potential therapeutic target for chronic MSK diseases. Its activation in inflammatory diseases, like rheumatoid arthritis, may improve the pathophysiological of the disease, especially in terms of inflammation and bone loss. 91
CBD has been shown to affect the nervous system in different ways, especially in terms of circadian rhythm regulation. 101 Recently, a large retrospective case series 101 revealed that 25–75 mg of CBD generated positive effects on sleep improvement as adjuvant therapy in 72 adults with sleep disorders, without causing serious adverse effects. CBD treatment reduced anxiety scores within the first month in 57 patients, remaining low throughout the study duration. The sleep scores increased in 48 patients within the initial four weeks but varied over time. These observations lie in parallel with previous results showing that CBD has also been shown to reduce wakefulness, which eventually leads to longer sleep duration in patients with insomniacs. 102 Researchers have previously hypothesized that the expression and location of the CB-1 receptor and its interactions with the endocannabinoids in the CNS are in great part responsible for the calming effects experienced by patients, which may facilitate sleep. 103 This is particularly significant because the circadian rhythm regulates about 40% of protein-coding genes in many tissues through mechanisms associated with the clock of central circadian as well as feedback loops of cell-intrinsic autoregulatory. 104 In addition, the circadian rhythm is also responsible for stimulating cellular activity including migration, division, metabolism, and other biological developments. 32 The physiological alterations that take place during sleep provide a favorable microenvironment for migration, proliferation, and differentiation of stem cells. These biological events are established either directly via circadian clock gene expression or indirectly via hormonal activity and cytokine modulation. 32 These observations propose an alternative manner in which CBD may contribute to the amelioration of chronic MSDs by improving sleep, which in turn allows adequate regulation of additional biological processes involved in tissue regeneration and pain relief.
Immunomodulation is also a key component of CBD that further reinforces its potential in regenerative medicine, even more so in autoimmune conditions. In a mouse model of diabetes, 105 researchers reported that treatment of CBD significantly reduces levels of TNF-α and interferon-γ in plasma, which are secreted by peritoneal macrophages, and activated Th1 cells, which are predominantly linked to inflammatory responses. In addition, CBD also upregulates the synthesis of anti-inflammatory cytokines such as IL-4 and IL-10, which are secreted by T helper 2 cells. 105 Interestingly, a parallel study showed that Δ9-THC promoted an antioxidant effect in diabetic mouse model (induced by a streptozotocin), reducing the levels of expression of IL-1 and IL-6 along with malondialdehyde, delaying disease progression overall. 106
These observations can also be interpreted in the context of other MSK conditions, such as diabetic peripheral neuropathy, for instance. This severe MSK condition is significantly associated with the elevated production of major pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) through glial cells in the spinal cord.107,108 Since both the receptors (CB-1 and CB-2) are also expressed in activated microglia cells, 109 CBD therapy may prove to be a feasible alternative in such conditions. To elaborate, Toth et al. 109 compared the degrees of the potency of CBD (CB-2 receptor and GPR55-antagonist) against synthetic CBs SR144528 (CB-2 receptor antagonist), SR141716A (selective CB-1 receptor antagonist), WIN 55 (CB-1 receptor agonist), and nabilone (non-selective CB-1 and CB-2 receptor agonist) for neuropathic pain. The researchers found that only CBD was able to reduce pain by impeding the promotion of microglial cell numbers and their phospho-P38 mitogen-activated protein kinase expression in the spinal cord of the mice dorsa. Another animal study 13 showed that prophylactic administration of CBD stopped joint pain development and nerve damage in osteoarthritic rats. In regard to safety, animal studies show that systemic administration of CBD does not seem to promote adverse effects. 110 The exploratory behavior in rats, for example, is not changes by systemic CBD, which indicates partial central effects without the induction of psychoactive symptoms. 110 These findings hold significant value, given the fact that neuronal homeostasis is also an indispensable factor of MSK integrity and the subsequent pain relief for better quality of life.
The progression of MSDs is by cellular immunity responding to an inflammatory environment. 111 Exosomes and microvesicles are released from all cells including immune cells and which are one key mediator for cell-to-cell communications and microenvironments. 112 A new study showed that CBDs inhibit the release of exosomes and microvesicles.113,114 The effect may also contribute to the beneficial effects of CBD on MSDs.
Future prospects
Regardless of the growing studies, the application of CB products in MSK regenerative medicine is still in its infancy. Although not fully elucidated, CBD demonstrates potent anti-inflammatory, antihyperalgesia, and analgesic properties in numerous MSDs. However, even with optimistic results, it must be emphasized that the majority of the publications are somewhat restricted to preclinical studies. This shows the need for additional investments in translational research as the literature would highly benefit from more clinical trials evaluating not only CBD but also other CB products for chronic MSDs and associated pain.
There are, of course, other challenges that must be addressed with the introduction of CB derivatives in clinical practice. Although CB products have shown the potential to treat orthopedic conditions, they may raise ethical issues for elite athletes. According to the World Anti-Doping Agency (WADA) regulations, CB products are prohibited in competition except for CBD. 115 Unlike the intoxicating Δ9-THC molecule, CBD is no longer banned by WADA and also shown to be safe and well tolerated in humans. 1 Over the years, the prejudice associating CBD with recreational drugs has slowly faded away. Some countries are beginning to review legislation regarding the use of CBD for medicinal purposes with the liberation of without a prescription “nutraceutical” products. 1 In the United States, however, the use of extracted CBD should not be taken casually as it is still considered Schedule I on the list of controlled substances by the US Drug Enforcement Agency.6,116
Another point to consider is the administration of CBD in pregnant women. Although understudied, the Food and Drug Administration agency strongly advises against the use of CB products in general during pregnancy and breastfeeding. 117 These compounds may cross the placental barrier and directly affect fetal development. Potential risks could include disruption in innate and adaptive immune system in the developing fetus. 118
Conclusions
CBD is a natural biological compound derived from the cannabis plant with numerous benefits for several conditions. CBD plays vital physiological roles in human health that go well beyond immunomodulation, anti-inflammation, and antinociception. Recent investigations show that CBD also enhances cell proliferation and migration, especially in MSCs. These features could be of significant value in MSK medicine. Although there is still some controversy in the literature, this non-intoxicating molecule has been considered safe and generally well tolerated in humans. This area is still relatively new in MSK medicine, and even though new studies are emerging, the clinical application of CBD requires more robust data from clinical trials to further elucidate the mechanisms that contribute to the improvement of MSK structures.
Footnotes
Authors’ Contributions: GOMA, VOMA, GSS, SV, MAF, NSB, AM, JVBL, MJ, AN, NJ, LFdF, MGLA, RV, RLR, PG, B-CA, and JFSSL participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript. GOMA and GSS wrote the first draft of the manuscript. RLR and PG acquired the funding. All authors approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1I1A1A01068652 and NRF-2021R1I1A1A01040732).
ORCID iDs: Madhan Jeyaraman  https://orcid.org/0000-0002-9045-9493
https://orcid.org/0000-0002-9045-9493
Naveen Jeyaraman  https://orcid.org/0000-0002-4362-3326
https://orcid.org/0000-0002-4362-3326
Lucas Furtado da Fonseca  https://orcid.org/0000-0001-6497-833X
https://orcid.org/0000-0001-6497-833X
Prakash Gangadaran  https://orcid.org/0000-0002-0658-4604
https://orcid.org/0000-0002-0658-4604
Byeong-Cheol Ahn  https://orcid.org/0000-0001-7700-3929
https://orcid.org/0000-0001-7700-3929
References
- 1.McCartney D, Benson MJ, Desbrow B, Irwin C, Suraev A, McGregor IS. Cannabidiol and sports performance: a narrative review of relevant evidence and recommendations for future research. Sports Med Open 2020;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farré M, Zuardi AW, McGuire PK. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des 2012;18:4966–79 [DOI] [PubMed] [Google Scholar]
- 3.ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. Prog Chem Org Nat Prod 2017;103:1–36 [DOI] [PubMed] [Google Scholar]
- 4.Carlini EA, Leite JR, Tannhauser M, Berardi AC. Letter: Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol 1973;25:664–5 [DOI] [PubMed] [Google Scholar]
- 5.Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 1980;21:175–85 [DOI] [PubMed] [Google Scholar]
- 6.Argueta DA, Ventura CM, Kiven S, Sagi V, Gupta K. A balanced approach for cannabidiol use in chronic pain. Front Pharmacol 2020;11:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramires LC, Jeyaraman M, Muthu S, Shankar AN, Santos GS, da Fonseca LF, Lana JF, Rajendran RL, Gangadaran P, Jogalekar MP, Cardoso AA, Eickhoff A. Application of orthobiologics in Achilles tendinopathy: a review. Life 2022;12:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, March L, Chew M; Lancet Commission on Osteoarthritis. Osteoarthritis in 2020. Lancet 2021;397:1060. [DOI] [PubMed] [Google Scholar]
- 9.Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) Results. 2019. https://ghdx.healthdata.org/gbd-2019
- 10.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res 2017;5:16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer FW, Neogi T, Nevitt MC, Felson DT, Zhu Y, Zhang Y, Lynch JA, Javaid MK, Crema MD, Torner J, Lewis CE, Guermazi A. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthr Cartil 2010;18:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med 2009;8:55–60 [PubMed] [Google Scholar]
- 13.Philpott HT, O’Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017;158:2442–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vela J, Dreyer L, Petersen KK, Arendt-Nielsen L, Duch KS, Kristensen S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: a randomized, double-blind, placebo-controlled trial. Pain 2022;163:1206–14 [DOI] [PubMed] [Google Scholar]
- 15.Gowran A, McKayed K, Campbell VA. The cannabinoid receptor type 1 is essential for mesenchymal stem cell survival and differentiation: implications for bone health. Stem Cells Int 2013;2013:e796715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller HP, Ostrovsky O, De Leo N, Badach J, Lin A, Bonawitz SC, Williamson J, Hakim A. Role of marijuana component (cannabidiol) in induction of regenerative ability of stem cells. Plast Reconstr Surg Glob Open 2020;8:148–9 [Google Scholar]
- 17.Ruhl T, Karthaus N, Kim B-S, Beier JP. The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs. Exp Cell Res 2020;389:111881. [DOI] [PubMed] [Google Scholar]
- 18.Apostu D, Lucaciu O, Mester A, Benea H, Oltean-Dan D, Onisor F, Baciut M, Bran S. Cannabinoids and bone regeneration. Drug Metab Rev 2019;51:65–75 [DOI] [PubMed] [Google Scholar]
- 19.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF, Aboyans V, Abu-Raddad LJ, Ackerman IN, Adamu AA, Adetokunboh O, Afarideh M, Afshin A, Agarwal SK, Aggarwal R, Agrawal A, Agrawal S, Ahmadieh H, Ahmed MB, Aichour MTE, Aichour AN, Aichour I, Aiyar S, Akinyemi RO, Akseer N, Al Lami FH, Alahdab F, Al-Aly Z, Alam K, Alam N, Alam T, Alasfoor D, Alene KA, Ali R, Alizadeh-Navaei R, Alkerwi A, Alla F, Allebeck P, Allen C, Al-Maskari F, Al-Raddadi R, Alsharif U, Alsowaidi S, Altirkawi KA, Amare AT, Amini E, Ammar W, Amoako YA, Andersen HH, Antonio CAT, Anwari P, Ärnlöv J, Artaman A, Aryal KK, Asayesh H, Asgedom SW, Assadi R, Atey TM, Atnafu NT, Atre SR, Avila-Burgos L, Avokphako EFGA, Awasthi A, Bacha U, Badawi A, Balakrishnan K, Banerjee A, Bannick MS, Barac A, Barber RM, Barker-Collo SL, Bärnighausen T, Barquera S, Barregard L, Barrero LH, Basu S, Battista B, Battle KE, Baune BT, Bazargan-Hejazi S, Beardsley J, Bedi N, Beghi E, Béjot Y, Bekele BB, Bell ML, Bennett DA, Bensenor IM, Benson J, Berhane A, Berhe DF, Bernabé E, Betsu BD, Beuran M, Beyene AS, Bhala N, Bhansali A, Bhatt S, Bhutta ZA, Biadgilign S, Bicer BK, Bienhoff K, Bikbov B, Birungi C, Biryukov S, Bisanzio D, Bizuayehu HM, Boneya DJ, Boufous S, Bourne RRA, Brazinova A, Brugha TS, Buchbinder R, Bulto LNB, Bumgarner BR, Butt ZA, Cahuana-Hurtado L, Cameron E, Car M, Carabin H, Carapetis JR, Cárdenas R, Carpenter DO, Carrero JJ, Carter A, Carvalho F, Casey DC, Caso V, Castañeda-Orjuela CA, Castle CD, Catalá-López F, Chang H-Y, Chang J-C, Charlson FJ, Chen H, Chibalabala M, Chibueze CE, Chisumpa VH, Chitheer AA, Christopher DJ, Ciobanu LG, Cirillo M, Colombara D, Cooper C, Cortesi PA, Criqui MH, Crump JA, Dadi AF, Dalal K, Dandona L, Dandona R, das Neves J, Davitoiu DV, de Courten B, De Leo DD, Defo BK, Degenhardt L, Deiparine S, Dellavalle RP, Deribe K, Des Jarlais DC, Dey S, Dharmaratne SD, Dhillon PK, Dicker D, Ding EL, Djalalinia S, Do HP, Dorsey ER, dos Santos KPB, Douwes-Schultz D, Doyle KE, Driscoll TR, Dubey M, Duncan BB, El-Khatib ZZ, Ellerstrand J, Enayati A, Endries AY, Ermakov SP, Erskine HE, Eshrati B, Eskandarieh S, Esteghamati A, Estep K, Fanuel FBB, Farinha CSES, Faro A, Farzadfar F, Fazeli MS, Feigin VL, Fereshtehnejad S-M, Fernandes JC, Ferrari AJ, Feyissa TR, Filip I, Fischer F, Fitzmaurice C, Flaxman AD, Flor LS, Foigt N, Foreman KJ, Franklin RC, Fullman N, Fürst T, Furtado JM, Futran ND, Gakidou E, Ganji M, Garcia-Basteiro AL, Gebre T, Gebrehiwot TT, Geleto A, Gemechu BL, Gesesew HA, Gething PW, Ghajar A, Gibney KB, Gill PS, Gillum RF, Ginawi IAM, Giref AZ, Gishu MD, Giussani G, Godwin WW, Gold AL, Goldberg EM, Gona PN, Goodridge A, Gopalani SV, Goto A, Goulart AC, Griswold M, Gugnani HC, Gupta R, Gupta R, Gupta T, Gupta V, Hafezi-Nejad N, Hailu GB, Hailu AD, Hamadeh RR, Hamidi S, Handal AJ, Hankey GJ, Hanson SW, Hao Y, Harb HL, Hareri HA, Haro JM, Harvey J, Hassanvand MS, Havmoeller R, Hawley C, Hay SI, Hay RJ, Henry NJ, Heredia-Pi IB, Hernandez JM, Heydarpour P, Hoek HW, Hoffman HJ, Horita N, Hosgood HD, Hostiuc S, Hotez PJ, Hoy DG, Htet AS, Hu G, Huang H, Huynh C, Iburg KM, Igumbor EU, Ikeda C, Irvine CMS, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Jassal SK, Javanbakht M, Jayaraman SP, Jeemon P, Jensen PN, Jha V, Jiang G, John D, Johnson SC, Johnson CO, Jonas JB, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kamal R, Kan H, Karam NE, Karch A, Karema CK, Kasaeian A, Kassa GM, Kassaw NA, Kassebaum NJ, Kastor A, Katikireddi SV, Kaul A, Kawakami N, Keiyoro PN, Kengne AP, Keren A, Khader YS, Khalil IA, Khan EA, Khang Y-H, Khosravi A, Khubchandani J, Kiadaliri AA, Kieling C, Kim YJ, Kim D, Kim P, Kimokoti RW, Kinfu Y, Kisa A, Kissimova-Skarbek KA, Kivimaki M, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Kosen S, Koul PA, Koyanagi A, Kravchenko M, Krishnaswami S, Krohn KJ, Kumar GA, Kumar P, Kumar S, Kyu HH, Lal DK, Lalloo R, Lambert N, Lan Q, Larsson A, Lavados PM, Leasher JL, Lee PH, Lee J-T, Leigh J, Leshargie CT, Leung J, Leung R, Levi M, Li Y, Li Y, Li Kappe D, Liang X, Liben ML, Lim SS, Linn S, Liu PY, Liu A, Liu S, Liu Y, Lodha R, Logroscino G, London SJ, Looker KJ, Lopez AD, Lorkowski S, Lotufo PA, Low N, Lozano R, Lucas TCD, Macarayan ERK, Magdy Abd El Razek H, Magdy Abd El Razek M, Mahdavi M, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malhotra R, Malta DC, Mamun AA, Manguerra H, Manhertz T, Mantilla A, Mantovani LG, Mapoma CC, Marczak LB, Martinez-Raga J, Martins-Melo FR, Martopullo I, März W, Mathur MR, Mazidi M, McAlinden C, McGaughey M, McGrath JJ, McKee M, McNellan C, Mehata S, Mehndiratta MM, Mekonnen TC, Memiah P, Memish ZA, Mendoza W, Mengistie MA, Mengistu DT, Mensah GA, Meretoja TJ, Meretoja A, Mezgebe HB, Micha R, Millear A, Miller TR, Mills EJ, Mirarefin M, Mirrakhimov EM, Misganaw A, Mishra SR, Mitchell PB, Mohammad KA, Mohammadi A, Mohammed KE, Mohammed S, Mohanty SK, Mokdad AH, Mollenkopf SK, Monasta L, Montico M, Moradi-Lakeh M, Moraga P, Mori R, Morozoff C, Morrison SD, Moses M, Mountjoy-Venning C, Mruts KB, Mueller UO, Muller K, Murdoch ME, Murthy GVS, Musa KI, Nachega JB, Nagel G, Naghavi M, Naheed A, Naidoo KS, Naldi L, Nangia V, Natarajan G, Negasa DE, Negoi RI, Negoi I, Newton CR, Ngunjiri JW, Nguyen TH, Nguyen QL, Nguyen CT, Nguyen G, Nguyen M, Nichols E, Ningrum DNA, Nolte S, Nong VM, Norrving B, Noubiap JJN, O’Donnell MJ, Ogbo FA, Oh I-H, Okoro A, Oladimeji O, Olagunju TO, Olagunju AT, Olsen HE, Olusanya BO, Olusanya JO, Ong K, Opio JN, Oren E, Ortiz A, Osgood-Zimmerman A, Osman M, Owolabi MO, Pa M, Pacella RE, Pana A, Panda BK, Papachristou C, Park E-K, Parry CD, Parsaeian M, Patten SB, Patton GC, Paulson K, Pearce N, Pereira DM, Perico N, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Pigott DM, Pillay JD, Pinho C, Plass D, Pletcher MA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Prasad N, Purcell C, Qorbani M, Quansah R, Quintanilla BPA, Rabiee RHS, Radfar A, Rafay A, Rahimi K, Rahimi-Movaghar A, Rahimi-Movaghar V, Rahman MHU, Rahman M, Rai RK, Rajsic S, Ram U, Ranabhat CL, Rankin Z, Rao PC, Rao PV, Rawaf S, Ray SE, Reiner RC, Reinig N, Reitsma MB, Remuzzi G, Renzaho AMN, Resnikoff S, Rezaei S, Ribeiro AL, Ronfani L, Roshandel G, Roth GA, Roy A, Rubagotti E, Ruhago GM, Saadat S, Sadat N, Safdarian M, Safi S, Safiri S, Sagar R, Sahathevan R, Salama J, Saleem HOB, Salomon JA, Salvi SS, Samy AM, Sanabria JR, Santomauro D, Santos IS, Santos JV, Santric Milicevic MM, Sartorius B, Satpathy M, Sawhney M, Saxena S, Schmidt MI, Schneider IJC, Schöttker B, Schwebel DC, Schwendicke F, Seedat S, Sepanlou SG, Servan-Mori EE, Setegn T, Shackelford KA, Shaheen A, Shaikh MA, Shamsipour M, Shariful Islam SM, Sharma J, Sharma R, She J, Shi P, Shields C, Shifa GT, Shigematsu M, Shinohara Y, Shiri R, Shirkoohi R, Shirude S, Shishani K, Shrime MG, Sibai AM, Sigfusdottir ID, Silva DAS, Silva JP, Silveira DGA, Singh JA, Singh NP, Sinha DN, Skiadaresi E, Skirbekk V, Slepak EL, Sligar A, Smith DL, Smith M, Sobaih BHA, Sobngwi E, Sorensen RJD, Sousa TCM, Sposato LA, Sreeramareddy CT, Srinivasan V, Stanaway JD, Stathopoulou V, Steel N, Stein MB, Stein DJ, Steiner TJ, Steiner C, Steinke S, Stokes MA, Stovner LJ, Strub B, Subart M, Sufiyan MB, Sunguya BF, Sur PJ, Swaminathan S, Sykes BL, Sylte DO, Tabarés-Seisdedos R, Taffere GR, Takala JS, Tandon N, Tavakkoli M, Taveira N, Taylor HR, Tehrani-Banihashemi A, Tekelab T, Terkawi AS, Tesfaye DJ, Tesssema B, Thamsuwan O, Thomas KE, Thrift AG, Tiruye TY, Tobe-Gai R, Tollanes MC, Tonelli M, Topor-Madry R, Tortajada M, Touvier M, Tran BX, Tripathi S, Troeger C, Truelsen T, Tsoi D, Tuem KB, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Uneke CJ, Updike R, Uthman OA, Uzochukwu BSC, van Boven JFM, Varughese S, Vasankari T, Venkatesh S, Venketasubramanian N, Vidavalur R, Violante FS, Vladimirov SK, Vlassov VV, Vollset SE, Wadilo F, Wakayo T, Wang Y-P, Weaver M, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, Whiteford HA, Wijeratne T, Wiysonge CS, Wolfe CDA, Woodbrook R, Woolf AD, Workicho A, Xavier D, Xu G, Yadgir S, Yaghoubi M, Yakob B, Yan LL, Yano Y, Ye P, Yimam HH, Yip P, Yonemoto N, Yoon S-J, Yotebieng M, Younis MZ, Zaidi Z, Zaki MES, Zegeye EA, Zenebe ZM, Zhang X, Zhou M, Zipkin B, Zodpey S, Zuhlke LJ, Murray CJL. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390: 1211–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Tallawy SN, Nalamasu R, Salem GI, LeQuang JAK, Pergolizzi JV, Christo PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther 2021;10:181–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith E, Hoy DG, Cross M, Vos T, Naghavi M, Buchbinder R, Woolf AD, March L.The global burden of other musculoskeletal disorders: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:1462–9 [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc KE, LeBlanc LL. Musculoskeletal disorders. Prim Care 2010;37:389–406 [DOI] [PubMed] [Google Scholar]
- 23.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 2011;25:173–83 [DOI] [PubMed] [Google Scholar]
- 24.Babatunde OO, Jordan JL, Van der Windt DA, Hill JC, Foster NE, Protheroe J. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS ONE 2017;12:e0178621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voscopoulos C, Lema M. When does acute pain become chronic? Br J Anaesth 2010;105:i69–85 [DOI] [PubMed] [Google Scholar]
- 26.Russell IJ. Future perspectives in generalised musculoskeletal pain syndromes. Best Pract Res Clin Rheumatol 2011;25:321–31 [DOI] [PubMed] [Google Scholar]
- 27.Dieppe P. Chronic musculoskeletal pain. BMJ 2013;346:f3146 [DOI] [PubMed] [Google Scholar]
- 28.Shamim T. Musculoskeletal disorders due to poor ergonomic practice in dentistry. Saudi J Med Med Sci 2017;5:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Yao H, Su W, He Y, Cheng K, Wang Y, Peng W, Li P. Sleep deprivation worsened oral ulcers and delayed healing process in an experimental rat model. Life Sci 2019;232:116594. [DOI] [PubMed] [Google Scholar]
- 30.Mônico-Neto M, Dáttilo M, Ribeiro DA, Lee KS, de Mello MT, Tufik S, Antunes HKM. REM sleep deprivation impairs muscle regeneration in rats. Growth Factors 2017;35:12–8 [DOI] [PubMed] [Google Scholar]
- 31.Chun MY, Cho B-J, Yoo SH, Oh B, Kang J-S, Yeon C. Association between sleep duration and musculoskeletal pain. Medicine (Baltimore) 2018;97:e13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkhenany H, AlOkda A, El-Badawy A, El-Badri N. Tissue regeneration: impact of sleep on stem cell regenerative capacity. Life Sciences 2018;214:51–61 [DOI] [PubMed] [Google Scholar]
- 33.Circadian clocks: from stem cells to tissue homeostasis and regeneration. EMBO Rep 2018;19:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majidinia M, Reiter RJ, Shakouri SK, Mohebbi I, Rastegar M, Kaviani M, Darband SG, Jahanban-Esfahlan R, Nabavi SM, Yousefi B. The multiple functions of melatonin in regenerative medicine. Ageing Res Rev 2018;45:33–52 [DOI] [PubMed] [Google Scholar]
- 35.Lana JF, Macedo A, Ingrao ILG, Huber SC, Santos GS, Santana MHA. Leukocyte-rich PRP for knee osteoarthritis: current concepts. J Clin Orthop Trauma 2019;10:S179–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan G-H, Tanaka M, Masuko-Hongo K, Shibakawa A, Kato T, Nishioka K, Nakamura H. Characterization of cells from pannus-like tissue over articular cartilage of advanced osteoarthritis. Osteoarthr Cartil 2004;12:38–45 [DOI] [PubMed] [Google Scholar]
- 37.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6:625–35 [DOI] [PubMed] [Google Scholar]
- 38.Heijink A, Gomoll AH, Madry H, Drobnič M, Filardo G, Espregueira-Mendes J, Van Dijk CN. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2012;20:423–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Peng Z, Vasilev K, Ketheesan N. Investigation of wear particles generated in human knee joints using atomic force microscopy. Tribol Lett 2013;51:161–70 [Google Scholar]
- 40.Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimaging Clin N Am 2007;17:87–103 [DOI] [PubMed] [Google Scholar]
- 41.Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration. J Bone Joint Surg Br Vol 2008;90 –B:1261–70 [DOI] [PubMed] [Google Scholar]
- 42.Eyre DR, Matsui Y, Wu J-J. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans 2002;30:844–8 [DOI] [PubMed] [Google Scholar]
- 43.Tokuhara CK, Santesso MR, Oliveira GSN, de Ventura TM, da S, Doyama JT, Zambuzzi WF, Oliveira RC, de. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J Appl Oral Sci 2019;27:e20180596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva AJ, Ferreira JR, Cunha C, Corte-Real JV, Bessa-Gonçalves M, Barbosa MA, Santos SG, Gonçalves RM. Macrophages down-regulate gene expression of intervertebral disc degenerative markers under a pro-inflammatory microenvironment. Front Immunol 2019;10:1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu P, Li J, Fujino M, Zhuang J, Li X-K. Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediators Inflamm 2013;2013:701970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflammation 2021;18:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lana JF, Huber SC, Purita J, Tambeli CH, Santos GS, Paulus C, Annichino-Bizzacchi JM. Leukocyte rich PRP versus leukocyte-poor PRP – the role of monocyte/macrophage function in the healing cascade. J Clin Orthop Trauma 2019;10:S7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiste RC, Freitas M, Mercadante AZ, Fernandes E. Superoxide anion radical: generation and detection in cellular and non-cellular systems. Curr Med Chem 2015;22:4234–56 [DOI] [PubMed] [Google Scholar]
- 49.Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, Miyamoto K, Ninomiya K, Suzuki T, Iwasaki R, Yagi M, Takaishi H, Toyama Y, Suda T. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med 2007;204:1613–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Setti T, Arab MGL, Santos GS, Alkass N, Andrade MAP, Lana JFSD. The protective role of glutathione in osteoarthritis. J Clin Orthop Trauma 2021;15:145–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai MJ, Saini V, Saini S. Myofascial pain syndrome: a treatment review. Pain Ther 2013;2:21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urits I, Charipova K, Gress K, Schaaf AL, Gupta S, Kiernan HC, Choi PE, Jung JW, Cornett E, Kaye AD, Viswanath O. Treatment and management of myofascial pain syndrome. Best Pract Res Clin Anaesthesiol 2020;34:427–48 [DOI] [PubMed] [Google Scholar]
- 53.Celik D, Mutlu EK. Clinical implication of latent myofascial trigger point. Curr Pain Headache Rep 2013;17:353. [DOI] [PubMed] [Google Scholar]
- 54.Gerber LH, Sikdar S, Aredo JV, Armstrong K, Rosenberger WF, Shao H, Shah JP. Beneficial effects of dry needling for treatment of chronic myofascial pain persist for 6 weeks after treatment completion. PM R 2017;9:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther 2008;12:371–84 [DOI] [PubMed] [Google Scholar]
- 56.Loiacono C, Palermi S, Massa B, Belviso I, Romano V, Di Gregorio A, Sirico F, Sacco AM. Tendinopathy: pathophysiology, therapeutic options, and role of nutraceutics. A narrative literature review. Medicina 2019;55:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med 2003;22:675–92 [DOI] [PubMed] [Google Scholar]
- 58.Albers IS, Zwerver J, Diercks RL, Dekker JH, Van den Akker-Scheek I. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC Musculoskelet Disord 2016;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morton S, Williams S, Valle X, Diaz-Cueli D, Malliaras P, Morrissey D. Patellar tendinopathy and potential risk factors: an international database of cases and controls. Clin J Sport Med 2017;27:468–74 [DOI] [PubMed] [Google Scholar]
- 60.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ackermann PW. Neuronal regulation of tendon homoeostasis. Int J Exp Pathol 2013;94:271–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 2012;21:228–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzzoni V, Selistre-de-Araújo H, de Cássia Marqueti R. Tendon remodeling in response to resistance training, anabolic androgenic steroids and aging. Cells 2018;7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magnusson SP, Kjaer M. The impact of loading, unloading, ageing and injury on the human tendon. J Physiol 2019;597:1283–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snedeker JG, Foolen J. Tendon injury and repair – a perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomaterialia 2017;63:18–36 [DOI] [PubMed] [Google Scholar]
- 66.Morita W, Dakin SG, Snelling SJB, Carr AJ. Cytokines in tendon disease: a systematic review. Bone Joint Res 2017;6:656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang C, Chen Y, Huang J, Zhao K, Chen X, Yin Z, Heng BC, Chen W, Shen W. The roles of inflammatory mediators and immunocytes in tendinopathy. J Orthop Transl 2018;14:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM, Thomopoulos S. Targeting the NF-kB signaling pathway in chronic tendon disease. Sci Transl Med 2019;11:eaav4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orekhov AN, Orekhova VA, Nikiforov NG, Myasoedova1 VA, Grechko AV, Romanenko EB, Zhang D, Chistiakov DA. Monocyte differentiation and macrophage polarization. Vessel Plus 2019;3:10 [Google Scholar]
- 70.Arvind V, Huang AH. Reparative and maladaptive inflammation in tendon healing. Front Bioeng Biotechnol 2021;9:719047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaji DA, Howell KL, Balic Z, Hubmacher D, Huang AH. Tgfβ signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. eLife 2020;9:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ackermann PW, Renström P. Tendinopathy in sport. Sports Health 2012;4:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schubert TEO, Weidler C, Lerch K, Hofstädter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis 2005;64:1083–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Man G, Mologhianu G. Osteoarthritis pathogenesis – a complex process that involves the entire joint. J Med Life 2014;7:37–41 [PMC free article] [PubMed] [Google Scholar]
- 75.Saunders CJ, Jalali Sefid Dashti M, Gamieldien J. Semantic interrogation of a multi knowledge domain ontological model of tendinopathy identifies four strong candidate risk genes. Sci Rep 2016;6:19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med 2002;36:239–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott A, Huisman E, Khan K. Conservative treatment of chronic Achilles tendinopathy. CMAJ 2011;183:1159–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hafsi K, McKay J, Li J, Lana JF, Macedo A, Santos GS, Murrell WD. Nutritional, metabolic and genetic considerations to optimise regenerative medicine outcome for knee osteoarthritis. J Clin Orthop Trauma 2019;10:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marcum ZA, Hanlon JT. Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care 2010;18:24–7 [PMC free article] [PubMed] [Google Scholar]
- 80.Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res 2018;11:2189–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Labusca L, Zugun-Eloae F, Mashayekhi K. Stem cells for the treatment of musculoskeletal pain. World J Stem Cells 2015;7:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Idris AI, Ralston SH. Role of cannabinoids in the regulation of bone remodeling. Front Endocrinol (Lausanne) 2012;3:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, Bari M, Guzmán M, Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res 2013;52:633–50 [DOI] [PubMed] [Google Scholar]
- 84.Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F. β2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology 2011;152:1412–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Idris AI, Sophocleous A, Landao-Bassonga E, Canals M, Milligan G, Baker D, van’t Hof RJ, Ralston SH. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab 2009;10:139–47 [DOI] [PubMed] [Google Scholar]
- 86.Azzini GOM, Santos GS, Visoni SBC, Azzini VOM, Santos RG, dos Huber SC, Lana JF. Metabolic syndrome and subchondral bone alterations: the rise of osteoarthritis – a review. J Clin Orthop Trauma 2020;11:S849–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whyte L, Ford L, Ridge S, Cameron G, Rogers M, Ross R. Cannabinoids and bone: endocannabinoids modulate human osteoclast function in vitro. Br J Pharmacol 2012;165:2584–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qian H, Zhao Y, Peng Y, Han C, Li S, Huo N, Ding Y, Duan Y, Xiong L, Sang H. Activation of cannabinoid receptor CB2 regulates osteogenic and osteoclastogenic gene expression in human periodontal ligament cells. J Periodontal Res 2010;45:504–11 [DOI] [PubMed] [Google Scholar]
- 89.Idris AI. Cannabinoid receptors as target for treatment of osteoporosis: a tale of two therapies. Curr Neuropharmacol 2010;8:243–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sophocleous A, Landao-Bassonga E, Van’t Hof RJ, Idris AI, Ralston SH. The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology 2011;152:2141–9 [DOI] [PubMed] [Google Scholar]
- 91.Turcotte C, Blanchet M-R, Laviolette M, Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci 2016;73:4449–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barrie N, Manolios N. The endocannabinoid system in pain and inflammation: its relevance to rheumatic disease. Eur J Rheumatol 2017;4:210–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol 2000;57:1045–50 [PubMed] [Google Scholar]
- 94.Starowicz K, Przewlocka B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos Trans R Soc Lond B Biol Sci 2012;367:3286–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, Suhara Y, Takayama H, Waku K. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem 2000;275:605–12 [DOI] [PubMed] [Google Scholar]
- 96.Larose M-C, Turcotte C, Chouinard F, Ferland C, Martin C, Provost V, Laviolette M, Flamand N. Mechanisms of human eosinophil migration induced by the combination of IL-5 and the endocannabinoid 2-arachidonoyl-glycerol. J Allergy Clin Immunol 2014;133:1480–2, 1482.e1–3 [DOI] [PubMed] [Google Scholar]
- 97.Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 2008;10:R43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fukuda S, Kohsaka H, Takayasu A, Yokoyama W, Miyabe C, Miyabe Y, Harigai M, Miyasaka N, Nanki T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. BMC Musculoskelet Disord 2014;15:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gui H, Liu X, Wang Z-W, He D-Y, Su D-F, Dai S-M. Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatology (Oxford) 2014;53:802–9 [DOI] [PubMed] [Google Scholar]
- 100.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999;283:401–4 [DOI] [PubMed] [Google Scholar]
- 101.Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in anxiety and sleep: a large case series. Perm J 2019;23:18–041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crippa JA, de S, Zuardi AW, Garrido GEJ, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JEC, McGuire PK, Busatto GF. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacol 2004;29:417–26 [DOI] [PubMed] [Google Scholar]
- 103.Kendall DA, Yudowski GA. Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front Cell Neurosci 2017;10:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paatela E, Munson D, Kikyo N. Circadian regulation in tissue regeneration. Int J Mol Sci 2019;20:2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R, Gallily R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008;54:244–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vella RK, Jackson DJ, Fenning AS. Δ9-Tetrahydrocannabinol prevents cardiovascular dysfunction in STZ-diabetic Wistar-Kyoto rats. Biomed Res Int 2017;2017:7974149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain 2009;13:807–11 [DOI] [PubMed] [Google Scholar]
- 108.Coelho MA, Jeyaraman M, Jeyaraman N, Rajendran RL, Sugano AA, Mosaner T, Santos GS, Bizinotto Lana JV, Lana AVSD, da Fonseca LF, Domingues RB, Gangadaran P, Ahn B-C, Lana JFSD. Application of Sygen® in diabetic peripheral neuropathies – a review of biological interactions. Bioengineering 2022;9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toth CC, Jedrzejewski NM, Ellis CL, Frey WH. Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain 2010;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL, Westlund KN. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 2016;20:936–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res 2020;38:253–7 [DOI] [PubMed] [Google Scholar]
- 112.Gangadaran P, Rajendran RL, Kwack MH, Jeyaraman M, Hong CM, Sung YK, Ahn B-C. Application of cell-derived extracellular vesicles and engineered nanovesicles for hair growth: from mechanisms to therapeutics. Front Cell Dev Biol 2022;10:963278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, Inal JM, Bell JD, Lange S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol 2018;9:889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kosgodage US, Uysal-Onganer P, MacLatchy A, Mould R, Nunn AV, Guy GW, Kraev I, Chatterton NP, Thomas EL, Inal JM, Bell JD, Lange S. Cannabidiol affects extracellular vesicle release, miR21 and miR126, and reduces prohibitin protein in glioblastoma multiforme cells. Transl Oncol 2019;12:513–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mareck U, Fusshöller G, Geyer H, Huestis MA, Scheiff AB, Thevis M. Preliminary data on the potential for unintentional antidoping rule violations by permitted cannabidiol (CBD) use. Drug Test Anal 2021;13:539–49 [DOI] [PubMed] [Google Scholar]
- 116.Drug scheduling, https://www.dea.gov/drug-information/drug-scheduling (accessed 15 November 2022)
- 117.US Food & Drug Administration. What you should know about using cannabis, including CBD, when pregnant or breastfeeding. https://www.fda.gov/consumers/consumer-updates/what-you-should-know-about-using-cannabis-including-cbd-when-pregnant-or-breastfeeding (2020, accessed 25 July 2022)
- 118.Dong C, Chen J, Harrington A, Vinod KY, Hegde ML, Hegde VL. Cannabinoid exposure during pregnancy and its impact on immune function. Cell Mol Life Sci 2019;76:729–43 [DOI] [PMC free article] [PubMed] [Google Scholar]