Summary
Patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) often report disrupted and unrefreshing sleep in association with worsened fatigue symptoms. However, the nature and magnitude of sleep architecture alteration in ME/CFS is not known, with studies using objective sleep measures in ME/CFS generating contradictory results. The current manuscript aimed to review and meta-analyse of case-control studies with objective sleep measures in ME/CSF. A search was conducted in PubMed, Scopus, Medline, Google Scholar, and Psychoinfo databases. After review, 24 studies were included in the meta-analysis, including 20 studies with 801 adults (ME/CFS= 426; controls = 375), and 4 studies with 477 adolescents (ME/CFS= 242; controls = 235), who underwent objective measurement of sleep. Adult ME/CFS patients spend longer time in bed, longer sleep onset latency, longer awake time after sleep onset, decreased sleep efficiency, decreased stage 2 sleep, increased Stage 3, and longer rapid eye movement sleep latency. However, adolescent ME/CFS patients had longer time in bed, longer total sleep time, longer sleep onset latency, and reduced sleep efficiency. The meta-analysis results demonstrate that sleep is altered in ME/CFS, with changes seeming to differ between adolescent and adults, and suggesting sympathetic and parasympathetic nervous system alterations in ME/CFS.
Keywords: Sleep, Objective measurements, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), Systematic review, Polysomnography (PSG), Actigraphy Watch
Introduction
Myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is a complex and debilitating condition with poorly understood aetiology affecting between 1% and 2% of people worldwide [1]. Patients with ME/CFS experience periods of severe fatigue lasting at least six months, exacerbated by mental and/or physical exertion which is not alleviated by rest or sleep [2]. Disrupted and unrefreshing sleep is among the most common concerns, affecting up to 95% of ME/CFS patients [3,4]. As a result of such a high prevalence of sleep disturbances, the relationship between ME/CFS and sleep quality has been a significant area of interest in ME/CFS research.
Sleep studies have historically used both subjective and objective instruments to determine sleep quality in ME/CFS. Subjective measurements include self-report measures such as sleep diaries and scales, and structured clinical interviews [5] including Pittsburgh Sleep Quality Index (PSQI) [6,7]. A multidisciplinary review under the National Sleep Foundation investigated objective indicators of sleep quality across a healthy life span [8]. They reached a consensus that sleep latency, number of awakenings > 5 minutes, total awake time after sleep onset, and sleep efficiency are appropriate indicators of healthy sleep quality across the lifespan of a healthy population. However, further investigation of the sleep architecture or nap-related variables was recommended, as they are less clear [8]. To measure sleep quality objectively, studies have used electroencephalography (EEG), polysomnography (PSG), and/or actigraphy [6,7].
EEG is used to record the electrical patterns of brain activity during sleep and can be determine sleep stages as well as cycle functions [9]. PSG has remained the most frequently used technique to evaluate the sleep disturbances in ME/CFS [10], and it measures multiple physiological functions including brain waves, blood oxygen level, heart rate and breathing, eye and leg movements. [10]Using EEG and PSG, the two sleep cycles of non-rapid eye movement and rapid eye movement (REM) sleep can be studied [11], with non-rapid eye movement being further divided into three distinctive stages (i.e. Stage 1, Stage 2, Stage 3) [11]. However, both PSG and EEG require the patient to be at a sleep laboratory or hospital facility. Recent studies have started to use Actigraphy watch to investigate sleep quality in ME/CFS patients [7,12,13], due to their ease of use. In addition, some studies have investigated the correlation between the observed sleep patterns between subjective and objective measures in ME/CFS [14,15].
While the sleep-wake regulation and circadian rhythms can change with gender and age, consistency in these functions is essential for refreshing sleep and overall physical and psychological health [11]. The relationship between good sleep and overall wellbeing is well-established. However, objective measurements of sleep deficits in ME/CFS are inconsistent among studies [4,16] and the exact mechanism that underpins unrefreshing sleep in this disorder remains unclear.
This systematic review and meta-analysis analysed the existing literature on objective sleep measurements in ME/CFS based on the parameters outlined in the National Sleep Foundation’s sleep quality recommendations [8]. To our knowledge, this is the first meta-analysis of the objective sleep literature in ME/CFS to confirm whether sleep deficits in ME/CFS can be objectively measured and to uncover patterns of the deficits that warrant further investigations. We are interested in objective sleep measures to identify the differences in sleep quality between the ME/CFS patients and the health controls in both adults and adolescents. This systematic review and meta-analysis would summarise the sleep quality studies of ME/CFS to specifically address the following questions: (i) What are the objective measurements of sleep quality used in the field of ME/CFS?; (ii) What are the differences in sleep quality between ME/CFS and age-gender matched healthy controls using objective measures?; (iii) What is the difference between adult and adolescent patients with ME/CFS?
Methods
Search strategy and data sources
We conducted a systematic search for relevant articles on sleep outcomes in ME/CFS, using the PECO (Population, Exposure, Comparison (if any) and Outcome) model [17] and following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. Our population was people aged 10–65 years old, and our exposure was ME/CFS, and our outcome was sleep outcomes. The search strategy was developed to identify research articles published from January 1990 to April 2021 in PubMed (NCBI), Scopus, Medline, and PsycInfo electronic databases. The study was prospectively registered on PROSPERO (CRD42021262494), and the search was documented using the PRISMA flow chart. The search fields included the title, abstract, and keywords, using population and exposure conditions as search criteria. The population search terms were “Chronic Fatigue Syndrome,” “Myalgic Encephalomyelitis,” “Encephalomyelitis, Myalgic,” “Chronic Fatigue Syndromes,” and “Fatigue Syndromes, Chronic.” The exposure conditions were “Sleep,” “Monitoring, Sleep,” and “Sleep Monitoring”. This search strategy was used for all above database with modified search terms to fit each database’s specific requirements. The full search terms for each databased were provided in Supplementary Table 1
To ensure we did not miss any relevant studies, we conducted an additional search on Google Scholar on 5/10/2022 using the keywords “chronic fatigue syndrome” and “objective sleep,” limiting the total number of pages of search results to 10. We also reviewed reference lists from previous systematic reviews to identify other relevant studies. After exporting the articles into EndNote, we removed duplicates and checked reference lists of relevant original and review articles for any missed publications.
Study selection and data extraction
We included the studies that satisfied the following inclusion criteria: (i) the article had to be written in English, peer reviewed and contain original data (i.e., was not a review or editorial); (ii) the diagnosis of ME/CFS was based on an accepted criteria including International Consensus Criteria [18], Canadian consensus criteria [19], Institute of Medicine (IOM) [20], London criteria [21], Reeves criteria [22], CDC Fukuda criteria [23], Oxford criteria[24]; (iii) control participants had to be on average age-matched to the ME/CFS participants; (iv) sleep qualities were measured overnight; (v) the sleep quality were measured objectively.
Studies were excluded if: (i) the descriptive statistics (demographics) were not provided for each group; (ii) it was a review or editorial article; (iii) not published in a peer review journal; (iv) poor definition of the ME/CFS groups using unknown ME/CFS definition criteria; or (v) no healthy control group.
Three researchers independently reviewed the research articles (AM, TA, SR) based on the articles’ titles and abstracts. Disagreements were resolved by discussing to a consensus. The characteristics, measurements, and outcomes of the selected studies were extracted into a table template in parallel by two reviewers (AM, TA) to avoid transcription errors. Results were tabulated as mean ± standard deviation (SD) or standard error of the mean where possible.
A set of parameters extracted includes total sleep time (minutes), total bedtime (minutes), awake time after sleep onset (minutes), sleep efficiency (%), sleep latency (minutes), REM sleep (%), REM latency (min), percentage of stage 1 (%), stage 2 (%), stage 3 (%), and slow wave sleep (%) relevant to total sleep time.
Risk of bias
The risk of bias for each study was evaluated using a tool adapted from QUADAS-2, a quality assessment tool of diagnostic accuracy studies. Details of adaptation for observational studies were described in previous studies [25,26]. This study reviewed observational studies; thus, the QUADAS-2 tool was adapted in 4 domains: (i) patient selection, (ii) index test, (iii) control standard, and (iv) control of confounding factors. Each domain was summarised as low risk (no signalling question violated), moderate risk (one signalling question violated), or high risk (more than one signalling questions violated). Studies with high risk in one or more domains were interpreted with caution and relevant to the violations.
The patient selection bias was assessed by: (i) was a random sample of patients enrolled; and (ii) were any inappropriate exclusion criteria for patients avoided. The index test domain was assessed by: (i) did any potential protocol bias exist?; ii) did a pre-specified significance threshold set; and (iii) was a multiple comparison correction performed appropriately. The control standard bias was assessed by: (i) were patients and controls matched for age or were controlled for age in the analysis; (ii) were patients and controls matched for gender (have same gender ratio) or were controlled for gender in the analysis; and (iii) were controls recruited using the same criteria used for patients. The bias in the control of confounding factors was assessed by: (i) were confounding factors identified or screened for?; (ii) were the confounding factors controlled for in the analysis?
Synthesis of results
We extracted the following information from each article: author details, affiliations, country, ME/CFS diagnostic criteria, number of participants, demographics, aim of paper, sleep quality measurement tool, analysis method, other observations, and conclusion. Several outcomes were collected including sleep timing and latency, sleep stages, and circadian rhythm. Studies with one common author were assessed to define if the study sample were the same and would be excluded if they were concluded to be similar.
Publication bias
We used Egger’s test to check for funnel plot asymmetry and detect possible publication bias in sleep measures reporting among studies. However, due to the limited number of studies, the Egger’s method may be biased [27]. As a result, we opted not to divide the studies based on modalities, as some parameters had very few studies using actigraphy. We used MedCalc software (https://www.medcalc.org/download/) to run Egger’s test with a two-tailed hypothesis and a significance level of α = 0.05.
Statistical analysis
All variables were analysed, and Forest Plots were generated using the mean difference method (RevMan 5.4.1) for a pooled mean effect and 95% confidence interval using random effects models [28]. To obtain an estimate of effect sizes for each variable, Forest Plots were re-generated using the standardised mean difference method [28]. Heterogeneity between studies was assessed using the I-squared (I2) statistic as percentage of the effect estimates variability due to heterogeneity [26], interpreted as I2 < 40%: heterogeneity not important, I2 = 40–60%: moderate heterogeneity, I2 = 60–75%: substantial heterogeneity, I2 >75%: considerable heterogeneity [26]. In addition, to evaluate if the PSG and actigraphy are equivalent and identify if they both show similar measures of sleep or not, the studies were sub-divided based on PSG or actigraphy and evaluated separately. All analysis was performed using a random effect model to account for the heterogeneity within the data, with p ≤ 0.05 for significance.
Results
Study selection and characteristics
A total of 2181 records were identified from the different searches performed, with a total of 998 reviewed after removing duplicates (see Fig. 1). A total of 951 articles did not meet the inclusion criteria and were excluded upon screening based on abstract and title. A total of 47 articles were identified to investigate the sleep in ME/CFS [3,12,14,15,27–69]. Twenty-four publications were included in the meta-analysis as they contained both ME/CFS patients and healthy controls [3,29–51], See Supplementary Table 2. The reasons for exclusion for each of the 23 excluded studies were shown in Supplementary Table 3.
Fig. 1.
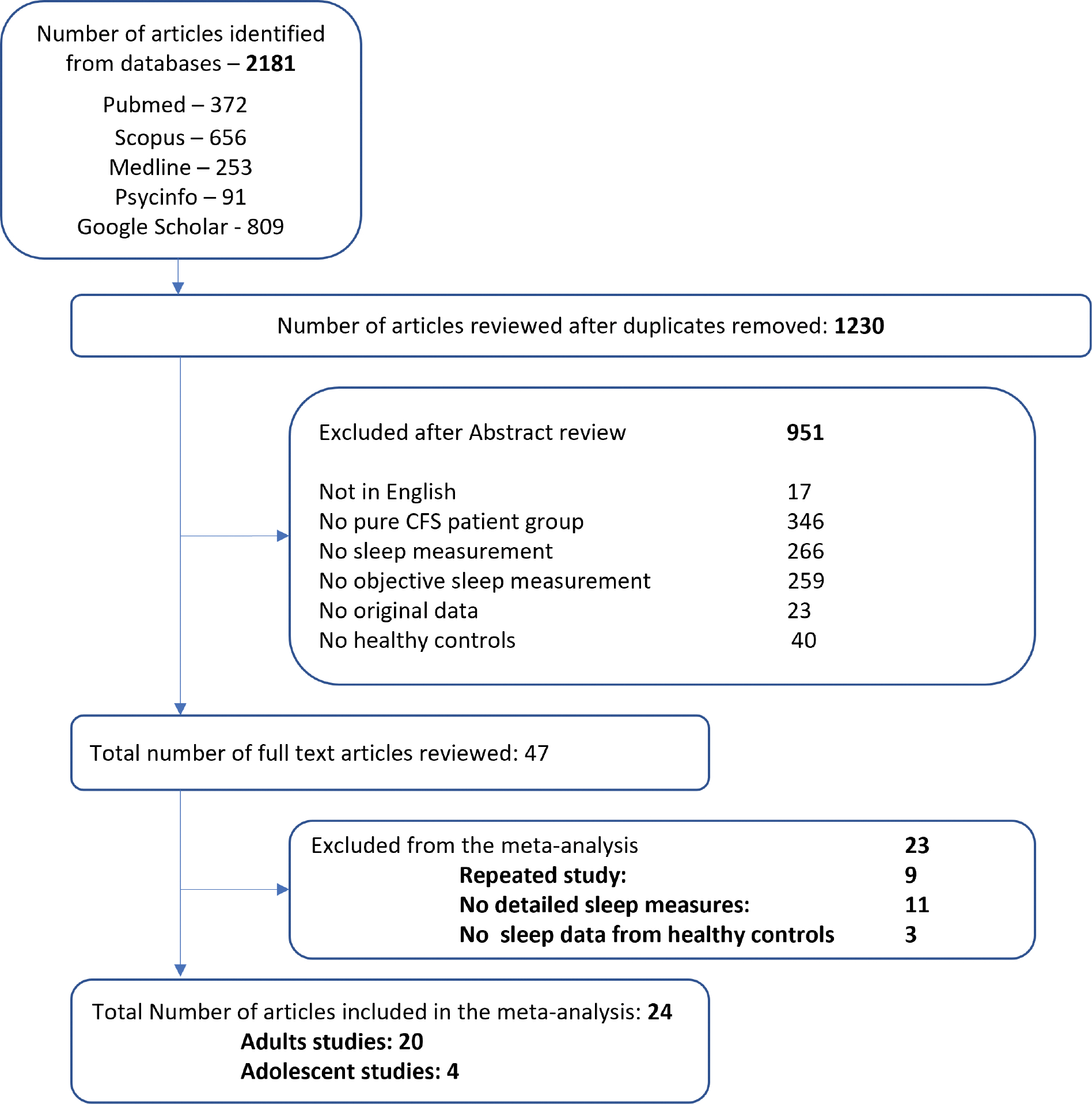
Flow diagram of the systematic review Legend: ME/CFS: Chronic Fatigue Syndrome.
Study characteristics
Studies included in the current review have used different ME/CFS diagnostic criteria. A total of four studies investigated sleep in adolescents with ME/CFS [31–34], while a total of 20 studies investigated the sleep quality in adults with ME/CFS [3,29,30,35–51]. Fukuda criteria were the main diagnostic tool for adult patients with ME/CFS and was used in 17 studies [3,29,35–37,39–49,51,52]. In addition, one study used both Oxford and Fukuda criteria [50], one study used Fukuda and the Canadian criteria [38], while one study used the Institute of Medicine (IOM) criteria [30].
As for studies investigating the sleep measures in adolescents with ME/CFS, one study used Fukuda [32], one study used CFS in children and adolescents: assessment guidelines [34], one study used Paediatric case definition for ME/CFS [31], and one study used case-definition of the Royal College of Paediatrics and Child Health [33].
Participant characteristics for each of the studies included are summarised in Supplementary Table 2. In brief, summary characteristics across all studies with available data report in Supplementary Table 2 for adults (18–65 years old) and adolescents (13–18 years old) with ME/CFS and healthy controls.
Risk of bias results
Risk of bias was assessed for different studies included in the current review. All 24 studies were deemed to be at low risk of bias for the measurement of exposure status (ME/CFS) as this was part of the inclusion criteria for studies. With the adapted QUADAS-2, all 24 out of 24 reviewed articles complied with all signalling questions in the domain of patient selection.
In the domain of index test, 14 articles violated one signalling question, and 5 articles violated two more signalling questions. In the domain of control standard, 10 articles violated one signalling question, and two articles violated two more signalling questions. In the domain of confounding factor control, 15 articles violated one signalling question, and three articles violated two or more signalling questions. The risks of bias of each article are summarized in Supplementary Table 4.
Publication bias
Egger’s test was used to test for the presence of publication bias for reporting sleep issues between studies. In adults, data showed publication bias in sleep variables including total bedtime (p = 0.005), awake after sleep (p = 0.005), sleep latency (p = 0.007), sleep efficiency (p = 0.008). However, no publication bias was observed in total sleep time (p = 0.52), any of the sleep stage (i.e. Stage 1, 2,3, SWS, REM; p > 0.05), or REM Latency (p = 0.79), see Supplementary Figures 1,2.
To test if the bias is the result from using different modalities, we re-ran the analysis using only studies that used PSG as their modality to study sleep, we found that publication bias was still present in total bedtime (p = 0.005) and sleep latency (p = 0.04), with no publication bias in total sleep time (p = 0.28), awake after sleep (p = 0.051), sleep efficiency (p = 0.06), or any of the sleep stages (p > 0.05).
Sleep objective characteristics in ME/CFS adult patients
Studies investigating the ME/CFS patients sleep used different objective sleep tools including PSG and actigraphy watches. Using a random effect model, compared to control groups, adult ME/CFS patients spent 26.63 min longer in bed [95% CI 6.13–47.13 min, p = 0.01; Fig. 2A], spent 7.19 min longer before falling into sleep [95% CI = 4.27–10.11 min, p < 0.0001; Fig. 4A] and spent 17.31 min longer awake time after sleep onset [95% CI = 10.82–23.80 min, p < 0.0001; Supplementary Fig. 3]. Hence, the sleep efficiency was found to reduce in ME/CFS patients of 4.5% [95% CI = 3.29–5.71%, p < 0.0001; Fig. 5A]. There were no significant differences in total sleep time (p = 0.94) (see Fig. 3A).
Fig. 2.

Total time in bed (min) differences between ME/CFS and healthy controls. (A) Fourteen studies reported the differences in adults, showing that ME/CFS patients spent 26.63 minutes longer total time in bed than controls, with no significant differences in the PSG studies, and with 77.21 min longer time spent in bed reported by actigraphy watches. (B) Two studies reported the differences in adolescents with ME/CFS patients as compared to healthy controls, showing the adolescents have 72.04 minutes longer time in bed than, with considerable heterogeneity between the studies. The size of the green squares represents the study weight into the observed meta-analysis results.
Fig. 4.

Sleep latency (min) differences between ME/CFS and healthy controls. (A) Seventeen studies reported the differences in adults with overall difference showing that ME/CFS patients took 7.19 min longer to fall to sleep than controls and with a considerable heterogeneity between the studies. (B) Three studies reported non-significant differences in adolescents with overall difference showing ME/CFS patients to have a trend of 27.61 minutes longer time to fall to sleep, with a considerable heterogeneity between the studies. The size of the green squares represents the study weight into the observed meta-analysis results.
Fig. 5.

Sleep efficiency (%) differences between ME/CFS and healthy controls. (A) Eighteen studies reported the differences in adults with overall difference showing that ME/CFS patients had 4.5% less sleep efficiency with a moderate heterogeneity between the studies. Studies that used PSG showed less the sleep efficiency in ME/CFS patients of 4.81%, while studies that used Actigraphy reported reduce sleep efficiency in ME/CFS patients of 3.24%. (B) Two studies reported non-significant differences in adolescents with overall difference showing ME/CFS patients had 4.5% less sleep efficiency when compared to controls, with a considerable heterogeneity between the studies. The size of the green squares represents the study weight into the observed meta-analysis results.
Fig. 3.

Total sleep time (min) differences between ME/CFS and healthy controls. (A) Nineteen studies reported the differences in adults showing that adult ME/CFS patients have no differences in total time with only 0.52 minutes less total sleep time than controls, with a considerable heterogeneity between the studies. However, when subdivide the studies based on modalities used to measure sleep, ME/CFS showed a non-significant 8.41 min less total sleep time when using PSG but demonstrated 45.96 min more total sleep time when using Actigraphy. (B) Four studies reported the differences in adolescents showing that ME/CFS adolescent patients had 54.6 min longer total sleep time than, with a considerable heterogeneity between the studies. The size of the green squares represents the study weight into the observed meta-analysis results.
When we subdivided studies based on the measuring tool used for the sleep investigation, we found that actigraphy showed significantly different results in some of the sleep measures as compared to PSG, mainly in total bedtime (p = 0.002) and total sleep time (p = 0.0004). However, the rest of the sleep measures tend to agree between actigraphy and PSG studies with no significant differences between the two measuring tools in sleep latency, sleep efficiency, awake time after sleep (p > 0.05).
The studies that used Actigraphy showed that compared to control groups, adult ME/CFS patients spent 77.21 min longer time in bed [95% CI 41.82–112.6 min, p < 0.0001; Fig. 2A], 45.96 min longer total sleep time [95% CI = 18.37–73.56 min, p = 0.0001; Fig. 3A], and 3.24% less sleep efficiency [95% CI = 0.42–6.07%, p = 0.02; Fig. 5A]. However, actigraphy showed no significant differences in sleep latency (p = 0.07; Fig. 4A) or wake time after sleep onset (p = 0.13).
The studies that used PSG showed no significant differences between adult ME/CFS patients and controls in total time spent in bed (p = 0.21) and total sleep time (p = 0.17). However, ME/CFS patients were found to spend 7.74 min longer sleep latency [95% CI = 4.29–11.19 min, p < 0.0001; Fig. 4A] and 16.21 min longer awake time after sleep onset [95% CI = 9.57–22.86 min, p < 0.0001; Supplementary Fig. 3], that resulted in 4.81% less sleep efficiency [95% CI = 3.41–6.20%, p < 0.0001; Fig. 5A], as compared to controls. For results summary see Table 1.
Table 1:
Summery of the sleep macro-architecture measurement differences in ME/CFS.
| Adults (Actigraphy) | Adults (PSG) | Adolescents | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sleep macro-architecture measure | Effect | difference | # studies | Effect | difference | # studies | Effect | difference | # studies |
| Total sleep time (mins) | ↑*** | 45.96 | 3 | ↓ | 8.41 | 16 | ↑** | 54.6 | 4 |
| Total Bedtime (mins) | ↑**** | 77.21 | 3 | ↑ | 13.21 | 11 | ↑* | 72.04 | 2 |
| Wake time after sleep onset (mins) | ↑ | 20.64 | 3 | ↑**** | 16.21 | 13 | |||
| Sleep efficiency (%) | ↓* | 3.24 | 4 | ↓***** | 4.81 | 14 | ↓ | 4.53 | 2 |
| Sleep latency (mins) | ↑ | 8.51 | 3 | ↑**** | 7.74 | 14 | ↑ | 27.61 | 3 |
| REM latency (mins) | ↑* | 12.17 | 14 | ||||||
| REM sleep (%) | ↓ | 0.17 | 15 | ||||||
| Stage 1 sleep (N1; %) | ↓ | 0.07 | 15 | ||||||
| Stage 2 sleep (N2; %) | ↓* | 2.53 | 15 | ||||||
| Stage 3 sleep (N3; %) | ↑** | 2.91 | 6 | ||||||
| Slow Wave Sleep (SWS; %) | ↑ | 2.44 | 13 | ||||||
Down and up arrows denote that the outcome is decreased/increased in ME/CFS compared with controls, respectively.
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
p ≤ 0.0001.
Sleep objective characteristics in ME/CFS adolescent patients
In addition, we evaluated sleep differences in adolescents separately from adults, as they tended to differ in the sleep measures of the ME/CFS patients when compared to controls. Only one study used PSG to investigate the sleep in adolescents, making it difficult to subdivide studies based on the modalities. Studies showed adolescent ME/CFS patients would spend 72.04 min longer time in bed [95% CI = 9.2–134.87 min, p = 0.02; Fig. 2B] and 54.6 min longer total sleep time [95% CI = 18.06–91.14 min, p = 0.003; Fig. 3B], as compared to controls. However, there was no significant differences in sleep latency (p = 0.1; Fig. 4B), or sleep efficiency (p = 0.25; Fig. 5B), for more details see Table 1.
Sleep Stages differences in adults
Compared to controls, the ME/CFS group had 2.53% less proportion of stage 2 sleep (N2) relative to the total sleep time [95% CI= 0.36 to 4.7, p = 0.02], while showing 2.91% more stage 3 sleep [N3; 95% CI = 0.68 to 5.15, p = 0.01], and ME/CFS patients had 12.17 minutes delayed onset of the REM sleep compared to controls [95% CI = 0.46 to 23.87 min, p = 0.04]. However, a no significant differences were observed in Stage 1 (N1), REM sleep, or slow wave sleep (SWS) (p > 0.05). See Supplementary Figures 4–6 for forest plots and Table 1 for results summary.
Effect of PSG scoring and adaptation night on the observed differences in sleep measures and sleep stages in adults
To test the effect of other variables on the measured effects, we aimed to investigate the effect of the fatigue severity, psychiatric comorbidities, taking drug treatments, whether the participants included OSA patients, adaptation night for PSG, and PSG scoring methods (R&K vs. AASM) on the meta-analysis findings.
To assess the effect of PSG scoring methods (R&K vs. AASM) on our results, we conducted a subgroup analysis separating studies based on their scoring method. Out of the 16 studies included in our analysis, 8 studies used AASM scoring methods and 8 studies used R&K approach for PSG scoring. Our analysis showed no significant differences between the subgroups (p > 0.05) in terms of sleep measures and stages, except for REM Latency. Specifically, R&K studies did not show significant differences between ME/CFS and healthy controls in REM Latency (p = 0.61), while AASM studies showed significantly longer REM Latency in ME/CFS patients (p = 0.002).
Similarly, We conducted a sub-analysis of the PSG data based on whether the studies used adaptation night as part of their protocol. Out of the total of 16 studies, 10 reported using adaptation night while 6 did not. We found no statistically significant differences between the two sub-groups, except for sleep efficiency (p = 0.01) and sleep stage 2 (p < 0.0001).
The rest of the sub-analyses were not conducted due to the limited number of studies that reported the different variables. For instance, only three studies reported including OSA patients, eleven studies excluded OSA patients, and 10 studies did not explain if they included or excluded OSA patients. This makes it hard to perform such subgroup analysis as we did for PSG scoring. Similarly, limited studies reported the fatigue severity, fatigue durations, psychiatric comorbidities, taking drug treatments in their studies, as shown in Supplementary Table 2.
Discussion
ME/CFS is a complex illness with a constellation of symptoms and comorbidities. Sleep disturbance and unrefreshing sleep are major complaints from patients diagnosed with ME/CFS. To our knowledge, this study is the first meta-analysis of studies examining objective sleep measures in patients with ME/CFS as compared with age-matched controls, using measures derived from traditional sleep macro-architecture (Table 2), and showed significantly altered sleep macrostructure.
A total of 20 studies performed in adults and four studies performed in adolescents were included in the present meta-analysis. PSG studies showed different results from those that used actigraphy watches to investigate the sleep in ME/CFS in total sleep time and total bedtime. In addition, adults with ME/CFS had longer time in bed, less total sleep time, longer time to fall asleep, longer wake time after sleep onset, decreased sleep efficiency, less time spent in sleep N2, longer sleep N3, and longer time to reach REM sleep. In addition, adolescent ME/CFS have longer total time in bed, longer total sleep time, longer time to fall sleep as compared to age-matched controls. However, sleep efficiency, sleep latency, and awakenings after sleep (ordered according to effective size), in adult patients with ME/CFS significantly differ from those in controls, irrespective of the methodology used for the objective investigation of the sleep. All these changes were of small-to-moderate effect-size. These findings suggests that criteria to diagnose sleep problems might differ based on modalities and age. It is also important to note that the available research on sleep in ME/CFS was limited by recruitment decisions, confounding factors, small sample size and non-replicated findings. Future well-designed studies with a published protocols would be required to understand sleep quality in ME/CFS patients.
Sleep macro-architecture results revealed altered sleep in ME/CFS patients
The longer awake time after sleep seems to be a major feature of the disturbed sleep reported by many ME/CFS patients, with studies showing increased number [3,4,53] and duration [3,29,34,38,39,46,50,53] of awakenings in ME/CFS. However, the rate and duration of awakenings could be affected by several factors including the one-night studies, physical capacity, and environmental distractions. For instance, the use of adaptation night prior to recording night, and the flexibility of timing to sleep seem to be necessary for accurate measurement of sleep in CFS [54].
The longer sleep onset latency is another possible feature of disturbed sleep in ME/CFS patients, suggesting difficulties to initiate sleep in ME/CFS patients [38,39,42,44]. Furthermore, the altered parasympathetic activity at sleep onset could also contribute to the longer sleep latency [39,55]. Longer sleep periods and longer napping time could also contribute to the sleep onset latency at night. Togo and colleagues found that ME/CFS patients who reported sleeping during the day to have lower sleep efficiency, longer sleep latency, and more periods of interrupted sleep during night [48]. Although it could be argued that reduced physical exercise could lead to altered sleep in general population [56,57] and subsequently in ME/CFS patients, no association between reduced physical capacity and sleep quality was observed in ME/CFS [35]. Further, ME/CFS patients seem to be more sensitive to the increased sleep latency and wakefulness time.
Can deep sleep (N3) and REM sleep latency be possible indicators for ME/CFS sleep pathology?
The sleep microstructure is another way to investigate the sleep disturbance in ME/CFS patients. Our current systematic review and meta-analysis showed ME/CFS patients spend more time in deep sleep (N3) and take longer to reach REM stage, whereas no changes were observed in the SWS or REM duration in ME/CFS. This suggest that the alterations within deep sleep (stage 3), and longer REM latency would be potential markers to distinguish ME/CFS patients from healthy controls. However, it is important to note that changes in N3 and REM sleep can be observed in other diseases, and single PSG parameter has not been demonstrated to be a biomarker [58], which would require a combination of sleep changes and other measures for diagnosis of ME/CSF.
The longer time spent in deep sleep (N3) period suggesting deep sleep to be critical for the ME/CFS patients diagnosis [40,41,48,49,59]. Le Bon et al. showed only differences in the percentage of N3 sleep in ME/CFS that was associated with lower ultra-slow (0.5–0.8Hz) delta power in ME/CFS, potentially indicating altered neuronal membrane potential or a failure in neural recruitment during sleep in ME/CFS patients [41]. Furthermore, the heart rate variability was reduced during N3 of sleep, which were further correlated with poorer self-reported sleep quality and wellbeing [39], suggesting altered parasympathetic nervous system activity in the ME/CFS compared with control participants. This confirms the presence of an abnormal sleep progression as well as non-REM sleep instability, which is taking place in ME/CFS in association with the fatigue symptoms.
Our meta-analysis revealed delayed REM sleep (i.e. longer REM latency), but with no change in the time spent in REM sleep in the ME/CFS group, which is different between ME/CFS and other sleep disorders. Although functions of the REM sleep remain elusive, it is a unique sleep stage in mammals and birds. The REM sleep is the period of sleep that the learning and memory consolidation are hypothesised to take place. REM sleep is associated with the most highly aroused brain state during sleep and the most vulnerable in individuals with disrupted and non-restorative sleep [60]. In addition, delay in REM sleep onset was suggested to associate with key symptoms of ME/CFS such as brain fog, memory and concentration difficulties [3,60]. This suggests that REM sleep instability may be an objective marker for ME/CFS [61].
Important factors to consider when studying sleep in ME/CFS.
This systematic review observed high heterogeneity in previous findings of the sleep quality in ME/CFS. We postulated that different selection criteria, medication status, the matched control group used, first night recording of PSG versus multiple night studies, as well as home recordings of PSG versus lab setup recording may all have contributed to the heterogeneous results and the potential publication bias in sleep measures.
We only included studies with age limited to the maximum of 65 years old, as it is suggested that the normal aging processes may alter the sleep macro-architecture. Previous studies showed altered sleep patterns in the aged population, including delayed sleep onset, reduced sleep duration, greater sleep fragmentation and increased awakenings with more time in lighter sleep (N1 and N2) and reduced time in deeper sleep (N3) and REM sleep [62,63].
In addition, females seem to report having ME/CFS symptoms including sleep problems more than males, with the literature suggesting more females between 29–35 years to report ME/CFS [64,65]. Some of the included studies only used female participants to account for the gender-differences in sleep [35,38,41,43,48,49,66,67]. Aerenhouts and colleagues studied objectively measured sleep–wake pattern in females and showed only longer sleep time and bedtime in ME/CFS in comparison with controls [35]. Fatt and colleagues demonstrated that the female ME/CFS patients had similar total sleep time, but they spent more time in bed, reduced sleep efficiency, delayed sleep onset, longer wake after sleep onset time, and spent greater percentage of total sleep time in N1 and SWS [39]. Togo and colleagues demonstrated longer total sleep, longer durations of N1, N2, and REM sleep in ME/CFS patients whereas total duration of wakefulness did not differ as compared to controls [49]. Moreover, poor sleep quality was associated with greater pro-inflammatory cytokine and fatigue severity levels in female ME/CFS patients [68]. Therefore, this would suggest the need for larger scale investigations with gender effects specifically investigated to define potential gender differences between studies.
Investigating sleep in twins would be another way to control for the environmental and genetic factors that might lead to sleep problems in ME/CFS patients given the limited information about the genetic component of ME/CFS. Three of the included studies were twin-based [37,51,66], which helped in reducing the confounding genetic and environmental factors, given that there is still limited information available on the genetic component of ME/CFS. Studies showed no differences between the two twin groups on the objective measures of sleep [37,66,69]. However, the ME/CFS twins had more subjective sleep complaints as compared to their healthy twins [51], and with a tendency of higher apnea-hypopnea index, longer REM latency in the ME/CFS twin [66]. Ball et al. reported longer N3 and REM sleep, higher values for the apnea–hypopnea index and apnea–hypopnea arousal index in the ME/CFS twin compared to their unaffected twin [37]. This supports the findings of our current meta-analysis in a controlled genetic and environmental framework. However, future studies involving non-twin siblings, or other unaffected family members, may shed light on other variables linked with sleep disturbances in ME/CFS, including socio-economic, environmental, or other possible factors.
Other comorbidities including fibromyalgia, migraine and irritable bowel syndrome could also contribute to the altered sleep quality. Only limited literature separated these comorbidities from ME/CFS, with only two studies investigating the effect of fibromyalgia comorbidities with ME/CFS [40,48]. These studies showed reduced total sleep time, Stage 1 and REM sleep time, and a greater probability of transition from REM sleep to waking [40], suggesting this could be a specific sleep problem for those with ME/CFS alone. They also showed that shorter duration of N2 sleep could be specific to ME/CFS with fibromyalgia, but not to ME/CFS alone [40]. This supports the need for future studies investigating the differences between ME/CFS alone, Fibromyalgia alone, and comorbid group with descent sample sizes to be able to define possible sleep characteristics that might help differentiate the different groups.
Furthermore, sleep apnea is another condition with overlapping symptoms and high comorbidity in ME/CFS that could account for the sleep issues observed in some ME/CFS patients. In the current study, only three studies reported including sleep apnea patients, eleven studies excluded sleep apnea patients, and 10 studies did not explain if they included or excluded sleep apnea patients. Studies showed that ME/CFS and sleep apnea-hypopnea syndrome had similar sleep duration and efficiency [44,70]. However, sleep latency was longer in sleep apnea patients [70], while higher subjective fatigue and lower sleepiness were observed in ME/CFS compared to sleep apnea patients [70]. The total sleep time did not differ between the ME/CFS, sleep apnea-hypopnea syndrome and control groups [44,45,71]. Other studies suggested ME/CFS to have 49.8% predominant or co-morbid sleep disorder and about 28.7% of ME/CFS patients have obstructive sleep apnea [71,72], with more than 50% of chronic fatigue patients with sleep problems are diagnosed with one or more sleep disorders (e.g. obstructive sleep apnea, periodic limb movement disorder, restless legs syndrome) [71]. Due to the conflicting results within literature on the differences between sleep apnea and ME/CFS, future studies should specifically examine sleep problems and comorbidities with ME/CFS to define diagnostic biomarkers that differentiate ME/CFS patients from sleep apnea patients.
In the current meta-analysis, we observed publication bias which could be attributed to several factors including the limited reporting of confounding variables in some of the studies. For instance, most of the studies did not report on the fatigue severity (n = 15), fatigue duration (n=17), psychiatric comorbidities (n=18), or taking drug treatments (n=13 unknown, n=4 taking medications, n=7 exclude if taking medication) within their protocol which makes it hard to provide conclusive results due to the potential bias. The limited literature in children with variable methods would also potentially contribute to the publication bias [27], as it is suggested that the limited literature would cause publication bias.
We conducted an analysis to determine whether the PSG scoring method could introduce bias. Our results indicate no significant differences between Rechtschaffen and Kales (R&K) and American Academy of Sleep Medicine (AASM) scoring methods in the meta-analysis results. However, when comparing studies that used adaptation night with those that did not, we found significant differences in sleep efficiency and sleep stage 2. These findings suggest that publication bias in sleep efficiency may be related to not using an adaptation night before collecting sleep measures. Further studies with larger sample sizes and more comprehensive data collection are necessary to fully address these potential factors that could contribute to observed publication bias.
Methodological consideration of sleep studies in ME/CFS patients
Several studies established similarities and differences between subjective and objective measures of sleep quality in the ME/CFS cohort. Studies used different tools to investigate the sleep quality in ME/CFS including objective tools (i.e., EEG, PSG, and actigraphy watches), and subjective tools (i.e., PSQI and sleep diaries). PSG is the most common objective measurement tool of sleep parameters used in 75% (18/24) of the ME/CFS studies analysed in the current study [3,29,30,34,36,37,39–45,48–51,73], whereas only 25% (6/24) included actigraphy [30,49,55,60,61,65] which tends to be more recent studies performed after 2014 investigating the ME/CFS.
Our meta-analysis revealed differences between the observed results between PSG and actigraphy watches, in total sleep time and total bedtime, but no significant difference in sleep efficiency, sleep latency or wakefulness after sleep. In ME/CFS, only one study utilised overnight PSG, actigraphy, and sleep diary simultaneously in 49 ME/CFS patients [14] and showed high correlation between all sleep measurement tools in total sleep time, sleep efficiency, and wake after sleep time [14]. However, sleep latency did not differ when measured by actigraphy watch and did not correlate sleep diary or the PSG [14], with more sleep latency reported in subjective diaries as compared to PSG [6,14,15]. In addition, Previous study showed actigraphy and PSG to agree on all sleep measures except the sleep latency [74], with moderate positive correlations between both actigraphy and PSG for all sleep measures in depressed insomniacs [74]. Therefore, future studies measuring sleep using all three methods in ME/CFS and patients would be required for further investigation of the correlation between measures in ME/CFS patients. Patients also tend to report longer sleep delay in subjective sleep measures but not in objective measure using the actigraphy watch [38], and this was predicative of the next-day’s fatigue level in ME/CFS, suggesting the sleep perception and negative mood on waking might be the key elements for the unrefreshing sleep [7]. These results reflect on the importance of using the objective measures alongside subjective sleep measures, due to the inaccuracy of subjective measures in reflecting the actual sleep time. The subjective measures might reveal other important patterns and/or associations. Therefore, the collection of both objective and subjective measures could help in identifying the possible perception of sleep in ME/CFS patients, which could contribute to their feeling of unrefreshing sleep.
Biological mechanisms causing sleep disturbance in ME/CFS patients
We propose a model to explain the possible biological mechanisms associated with the sleep disturbance in ME/CFS (Fig 6), with two hypotheses addressing how altered sleep in ME/CFS patients compares to healthy controls. The two proposed hypotheses are complementary to each other.
Fig. 6:

Once the blood brain barrier (BBB) allows the infiltration of peripheral pro-inflammatory cytokines to pass through to the brain actively or passively, some pro-inflammatory cytokines play dual-roles as sleep regulators leading to the sleep disturbances. These pro-inflammatory cytokines initiate a positive feedback mechanism that upregulates the activation of microglia resulting in increased pro-inflammatory cytokine production and increased BBB leakage. Furthermore, the unregulated cytokine release could increase the microglial activation and priming causing a chronic pro-inflammatory microenvironment including astrocytes, hypoxia, reactive oxygen species (ROS), elevated cytokine levels, and microglial activation. These chronic proinflammatory environments could cause the alteration of the chemoreceptors in the afferent vagus nerve that has synaptic connection to the nucleus of the solitary tract (NTS) of dorsal brainstem. This causes alteration of the sympathetic and parasympathetic nervous system which could lead to disruption of the sleep, along with other symptoms of ME/CFS.
The first hypothesis is linked to the chronic inflammation associated with the ME/CFS [75–77]. Poor sleep quality has been suggested to have a bi-directional link with the inflammation in both patients and healthy individuals [78,79]. In general, pro-inflammatory cytokines have been suggested to promote sleep, while anti-inflammatory cytokines prevent sleep [80]. Previous investigations in ME/CFS demonstrated elevated pro-inflammatory cytokines levels such as interleukin-6 (IL-6), IL-8, IL-1α, and tumour necrosis factor-alpha (TNF-α), and relatively lower levels of anti-inflammatory cytokines were shown most consistently peripheral in ME/CFS patients [81,82]. However, the findings of studies investigating the cytokines in ME/CFS have failed to define a specific set of biomarkers for ME/CFS, which could be attributed to methodology differences in laboratory procedure, participants definition, sampling time, fatigue phase [83]. The IL-1, IL-6, and TNF were suggested to modulate sleep and the increased pro-inflammatory signalling has been linked with the sleep problems in ME/CFS patients [78,84]. For instance, longer sleep latency was associated with higher circulating IL-1β and IL-6 levels, while shorter sleep duration was associated with greater IL-1β, IL-6 and TNF-α levels [78]. This might possibly suggest that short unrefreshing sleep to be the result of the inflammatory process in patients with chronic fatigue [78,85]. Furthermore, increased IL-1α, increased IL-8, and decreased IL-6 were characteristic for early course of ME/CFS illness, while elevated IL-1α and IL-6 and reduced IL-8 were more abundant pattern in mid and late course ME/CFS subjects [82]. These cytokine markers were shown to result in 75–88% accuracy of ME/CFS classification, suggesting them as robust biomarkers independent of age in screening of ME/CFS patients [82]. Large portion of ME/CFS patients report developing symptoms following viral infection, and the viral infections in cerebral endothelial cells could increase blood brain barrier (BBB) permeability through both direct and immune-mediated damage [86]. Such increases in TNFα and IL-6 have been shown to increase BBB permeability [87,88], leading to increased microglial activity as neuroinflammation response. Previous study used positron emission tomography and found evidence of widespread activation of the brain’s innate immune system [89], that was associated with the severity of neuropsychological symptoms. Therefore, more studies investigating the neuroinflammation markers using positron emission tomography would be appropriate to investigate the underlying neuroinflammation in ME/CFS patients.
The second hypothesis is linked to the impaired sympathetic and parasympathetic autonomic nervous system function. Normal sleep is characterised by changes in sympathetic and parasympathetic activity predominance. Studies showed high heart rate [90,91], higher arterial blood pressure and diastolic blood pressure [91], and reduced heart rate variability [55,90,91] during sleep in ME/CFS. This would suggest vagal modulation of heart rate during sleep in ME/CFS leading to increased activity of the sympathetic nervous system and reduced activity of the parasympathetic nervous system in ME/CFS patients. In addition, unrefreshing sleep in ME/CFS suggests that negative cognitions could initiate autonomic arousal processes during wakefulness extending to sleep periods that cause sympathetic activity during sleep [4]. Furthermore, poor self-reported wellbeing and sleep quality is associated with reduced parasympathetic signalling during deeper sleep, but not other sleep stages, suggesting an autonomic hypervigilance occurs during the deeper sleep [39]. Our previous investigation showed negative correlation of sleep quality with T1-weighted and magnetization transfer T1-weighted spin echo signal intensities in the prefrontal cortex in ME/CFS patients [92], that regulate a wide range of subcortical autonomic centres including the nucleus tractus solitarius and ventrolateral medulla [93]. Another study observed correlations between the autonomic functions (blood pressure and heart rate) and brainstem substructures’ volumes including the vasomotor centre, midbrain reticular formation, limbic nuclei, and hypothalamus [94]. These regions maintain the sympathetic and parasympathetic responses by managing the blood pressure, heart rate, and sleep cycles [95]. Overall, these findings suggest a state of sympathetic autonomic nervous system predominance, nocturnal sympathetic hypervigilance, or neuroendocrine alterations that cause the alterations in the heart rate and heart rate variability in sleep in ME/CFS patients. In addition, this suggests that the nocturnal dysfunction of the cardiac autonomic nervous system in ME/CFS, causing lower parasympathetic tone and higher sympathetic tone in deep sleep, could be a biological marker of unrefreshing sleep in ME/CFS.
Importantly, the chronic proinflammatory environment may cause alterations of the chemoreceptors in the afferent vagus nerve with synaptic connections to the brainstem regions [96]. This may lead to alteration of the sympathetic and parasympathetic nervous system function causing disrupted sleep, along with other symptoms of ME/CFS.
Possible causes of changes in sleep in children and adolescents
The sleep patterns in adult ME/CFS patients have been studied extensively, however there is limited research focused on sleep in paediatric ME/CFS. Therefore, it is important to investigate the sleep behaviour in children and adolescents with ME/CFS given the ongoing developmental changes at this age. In the current meta-analysis, we found significant increase of the total bedtime and total sleep time associated with trend of (but not significant) increased sleep latency and reduced sleep efficiency. The increased total sleep time in adolescents was the opposite to what has been reported in adults studied using PSG, but similar to the actigraphy studies in adults. It is important to note that 3 out of the 4 studies used actigraphy watches to study the sleep in adolescents. However, the observed difference of the total sleep time observed in adolescents was higher than adults when comparing actigraphy studies, with almost 10 minute longer total sleep time in adolescents.
Such difference could be attributed to the difference in sleep between adults and adolescents as adolescents tend to sleep more [97]. However, it is also reported that adolescents sleep an average of 9 hours and 20 minutes and awoke spontaneously during early adolescence, while with increasing age adolescents tend to sleep the same amount of time but without awoke spontaneously before the end of the sleep window [98,99]. Furthermore, no differences in the actual sleep duration and sleep latency between young adults (23 years old) and adolescents (14 years old) [100]. These studies make it difficult to derive definitive conclusions on the differences between adults and adolescents. In addition, the various criteria used for the definition of ME/CFS in adolescents were different from those used in adults. This may be a cause of bias in the adolescent findings, hence future studies with similar criteria applied in both adolescents and adults would be needed to address this.
Strengths, limitations, and future directions
This systematic review and meta-analysis was registered on PROSPERO and is the first study to evaluate the studies with objective sleep measurements of sleep macro-architecture and micro-architecture in ME/CFS and age-matched controls. Our findings are reliable and valid as we only included studies that used the worldwide well-established ME/CFS diagnostic methods.
However, there are several limitations that must be acknowledged. Firstly, several studies were not included because of the limited information available about the sleep which limited their input in the meta-analyses. Other studies had no controls in their sample making it difficult to compare their findings with controls in our meta-analysis. A further limitation is the increased heterogeneity within the studies that might influence the results. We tried to investigate the possible sources of heterogeneity by splitting studies based on age (i.e. adults vs. Adolescents), and methodology used in monitoring sleep (i.e. PSG vs. Actigraphy). However, we were unable to investigate the potential publication bias since several studies had missing information and most of these studies did not document the study protocol previously. Thirdly, the studies included with adolescent participants with and without ME/CFS were limited, which might cause the findings to be less reliable. Additional studies are required to reduce this effect. Fourthly, there is a wide range of accepted international criteria for the definition of ME/CFS patients. In addition, these studies have limited information about the post-exertional malaise in the patients and controls, now regarded as essential for the diagnosis for ME/CFS.
Furthermore, the effects of psychiatric comorbidities and/or treatments are not controlled for in the current meta-analysis, therefore, future studies with more detailed information on history would be required. In addition, the adaptation night for PSG, and PSG scoring methods used in studies with PSG would be needed. Only few studies reported performing sleep study for at least nights [36,37,101], to habituate the participants to the setup of the PSG, however, most studies do not report having habituation night. Therefore, studies using PSG would need to have a habituation night to familiarize the participant to have accurate measures.
Overall, no cause-and-effect inferences can be drawn based on the sleep deficits observed in ME/CFS relative to age-matched controls, and future longitudinal and randomised controlled trials are required to robustly demonstrate causal links. Future studies could employ multimodal imaging to examine the associations between the imaging findings and the topographical sleep to explore the mechanisms within ME/CFS. In addition, researchers could establish taskforces to develop guidelines for pooling large sleep and cognition dataset internationally with standardised EEG signal analysis approaches in ME/CFS patients.
Conclusion:
Our analyses confirms that there is an array of increased sleep disturbance in patients with ME/CFS when compared to age-matched controls by meta-analysis of data from 801 adults and 477 adolescents. The findings showed longer sleep latency, reduced sleep efficiency, increased longer REM latency, longer bedtime, and altered sleep microstructure. These changes to sleep are exacerbated in ME/CFS, which also differ between adults and adolescents with ME/CFS.
Practice Points:
There are significant alterations in sleep macrostructure in ME/CFS patients.
Methods used to study sleep in ME/CFS could result in different observations in total sleep time and total bedtime.
Changes in sleep in adults are different from adolescents, suggesting that neurodevelopment plays a key role in these changes.
Sleep alterations were suggested being associated with sympathetic and parasympathetic nervous system alterations in ME/CFS.
Research Agenda
Future research should,
Investigate the role of chronic inflammation in the distrusted sleep in ME/CFS.
Employ neuroimaging methodology to examine the link between sleep and neurobiological biomarkers of ME/CFS.
Perform comprehensive phenotyping of patients in studies with large cohorts of age- and gender-matched populations to examine the role of coexisting sleep disorders on the fatigue situation.
Perform large studies to determine the age effect on the sleep measures in the ME/CFS patients.
Deliver targeted treatments (device, pharmacological) to normalize sleep alterations and assess cognitive and physical recovery and long-term trajectories.
Establish a taskforce to develop guidelines for pooling large multimodality sleep data internationally in ME/CFS.
Supplementary Material
Acknowledgement
This research was sponsored by The Australian National Health and Medical Research Council (NHMRC) Ideas Grant (GNT1184219) and Mason Research Foundation grants (MAS2018F00019, MAS2018F00024). VDC was partially supported by the National Institutes of Health grant (2112455) and by National Institutes of Health grant (R01MH118695).
Abbreviations
- CDC
Centre for Disease Control
- CI
Confidence Interval
- EEG
Electroencephalography
- I2
I-squared heterogeneity measure
- ME/CFS
Myalgic encephalomyelitis/Chronic Fatigue Syndrome
- N1
Sleep Stage 1
- N2
Sleep Stage 2
- N3
Sleep Stage 3
- PRISMA
Preferred Reporting Items for systematic Reviews and Meta analyses
- PSG
Polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- REM
Rapid eye movement
- SD
Standard Deviation
- SWS
Slow Wave Sleep
Footnotes
Competing interests
The authors report no competing interests.
Consent for publication
No individual information was used in the preparation of any of the figures. All data used in the preparation of the manuscript were of group levels, without any individual images or personal information used in the preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med 2020;18. 10.1186/s12967-020-02269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rowe PC, Underhill RA, Friedman KJ, Gurwitt A, Medow MS, Schwartz MS, et al. Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: A primer. Front Pediatr 2017;5:121. 10.3389/fped.2017.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gotts ZM, Deary V, Newton JL, Ellis JG. A comparative polysomnography analysis of sleep in healthy controls and patients with chronic fatigue syndrome. Fatigue 2016;4:80–93. 10.1080/21641846.2016.1167470. [DOI] [Google Scholar]
- [4].Jackson ML, Bruck D. Sleep abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: A review. Journal of Clinical Sleep Medicine 2012;8:719–28. 10.5664/jcsm.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang L, Zhao Z-X. Objective and subjective measures for sleep disorders. Neurosci Bull 2008;23:236–40. 10.1007/s12264-007-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tobback E, Mariman AN, Hanoulle IP, Delesie LM, Vogelaers DP, Pevernagie DA. Polysomnographic and multiple sleep latency testing data in a large sample of patients with chronic fatigue syndrome and their relationship with subjective scores. Fatigue 2016;4:94–103. 10.1080/21641846.2015.1106176. [DOI] [Google Scholar]
- [7].Russell C, Wearden AJ, Fairclough G, Emsley RA, Kyle SD. Subjective but not actigraphy-defined sleep predicts next-day fatigue in chronic fatigue syndrome: A prospective daily diary study. Sleep 2016;39:937–44. 10.5665/sleep.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ, et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health 2017;3:6–19. 10.1016/j.sleh.2016.11.006. [DOI] [PubMed] [Google Scholar]
- [9].Campbell IG. EEG recording and analysis for sleep research. Curr Protoc Neurosci 2009. 10.1002/0471142301.ns1002s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rundo JV, Downey R. Polysomnography. In: Levin KH, Chauvel P, editors. Handb Clin Neurol, vol. 160, Elsevier; 2019, p. 381–92. 10.1016/B978-0-444-64032-1.00025-4. [DOI] [PubMed] [Google Scholar]
- [11].Harding K, Feldman M. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. J Am Acad Child Adolesc Psychiatry, vol. 47, 2008, p. 473–4. 10.1097/01.chi.0000270812.55636.3b. [DOI] [Google Scholar]
- [12].Ohinata J, Suzuki N, Araki A, Takahashi S, Fujieda K, Tanaka H. Actigraphic assessment of sleep disorders in children with chronic fatigue syndrome. Brain Dev 2008;30:329–33. 10.1016/j.braindev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- [13].Kawabata M, Ueno T, Tomita J, Kawatani J, Tomoda A, Kume S, et al. Temporal organization of rest defined by actigraphy data in healthy and childhood chronic fatigue syndrome children. BMC Psychiatry 2013;13:281. 10.1186/1471-244X-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Creti L, Libman E, Baltzan M, Rizzo D, Bailes S, Fichten CS. Impaired sleep in chronic fatigue syndrome: How is it best measured? J Health Psychol 2010;15:596–607. 10.1177/1359105309355336. [DOI] [PubMed] [Google Scholar]
- [15].Majer M, Jones JF, Unger ER, Youngblood LS, Decker MJ, Gurbaxani B, et al. Perception versus polysomnographic assessment of sleep in CFS and non-fatigued control subjects: Results from a population-based study. BMC Neurol 2007;7:40. 10.1186/1471-2377-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Snodgrass K, Harvey A, Scheinberg A, Knight S. Sleep disturbances in pediatric chronic fatigue syndrome: A review of current research. Journal of Clinical Sleep Medicine 2015;11:757–64. 10.5664/jcsm.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018;121:1027–31. 10.1016/J.ENVINT.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carruthers BM, van de Sande MI, de Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med 2011;270:327–38. 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carruthers BM, Jain AK, de Meirleir KL, Peterson DL, Klimas NG, Lemer AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr 2003;11:7–115. 10.1300/J092v11n01_02. [DOI] [Google Scholar]
- [20].Clayton EW. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. vol. 313. Washington, District of Columbia: The National Academies Press; 2015. 10.1001/jama.2015.1346. [DOI] [Google Scholar]
- [21].Goudsmit EM, Shepherd C, Dancey CP, Howes S. ME: Chronic fatigue syndrome or a distinct clinical entity? Health Psychology Update 2009;18:26–33. [Google Scholar]
- [22].Reeves WC, Wagner D, Nisenbaum R, Jones JF, Gurbaxani B, Solomon L, et al. Chronic fatigue syndrome - A clinically empirical approach to its definition and study. BMC Med 2005;3. 10.1186/1741-7015-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med 1994;121:953–9. 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- [24].Sharpe MC. A report - Chronic fatigue syndrome: Guidelines for research. J R Soc Med 1991;84:118–21. 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shan ZY, Barnden LR, Kwiatek RA, Bhuta S, Hermens DF, Lagopoulos J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J Transl Med 2020;18. 10.1186/s12967-020-02506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].D’Rozario AL, Chapman JL, Phillips CL, Palmer JR, Hoyos CM, Mowszowski L, et al. Objective measurement of sleep in mild cognitive impairment: A systematic review and meta-analysis. Sleep Med Rev 2020;52:101308. 10.1016/j.smrv.2020.101308. [DOI] [PubMed] [Google Scholar]
- [27].Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/BMJ.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2019. 10.1002/9781119536604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sharpley A, Clements A, Hawton K, Sharpe M. Do patients with “pure” chronic fatigue syndrome have abnormal sleep? Psychosom Med 1997;59:592–6. 10.1097/00006842-199711000-00006. [DOI] [PubMed] [Google Scholar]
- [30].Orjatsalo M, Alakuijala A, Partinen M. Autonomic Nervous System Functioning Related to Nocturnal Sleep in Patients With Chronic Fatigue Syndrome Compared to Tired Controls. Journal of Clinical Sleep Medicine 2018;14:163–71. 10.5664/jcsm.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Josev EK, Jackson ML, Bei B, Trinder J, Harvey A, Clarke C, et al. Sleep Quality in Adolescents With Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). Journal of Clinical Sleep Medicine 2017;13:1057–66. 10.5664/jcsm.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nijhof SL, Rutten JMTM, Uiterwaal CSPM, Bleijenberg G, Kimpen JLL, Putte EM van de. The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology 2014;42:199–206. 10.1016/j.psyneuen.2014.01.017. [DOI] [PubMed] [Google Scholar]
- [33].Pedersen M, Ekstedt M, Småstuen MC, Wyller VB, Sulheim D, Fagermoen E, et al. Sleep–wake rhythm disturbances and perceived sleep in adolescent chronic fatigue syndrome. J Sleep Res 2017;26:595–601. 10.1111/jsr.12547. [DOI] [PubMed] [Google Scholar]
- [34].Stores G, Fry A, Crawford C. Sleep abnormalities demonstrated by home polysomnography in teenagers with chronic fatigue syndrome. J Psychosom Res 1998;45:85–91. 10.1016/S0022-3999(98)00024-5. [DOI] [PubMed] [Google Scholar]
- [35].Aerenhouts D, Ickmans K, Clarys P, Zinzen E, Meersdom G, Lambrecht L, et al. Sleep characteristics, exercise capacity and physical activity in patients with chronic fatigue syndrome. Disabil Rehabil 2015;37:2044–50. 10.3109/09638288.2014.993093. [DOI] [PubMed] [Google Scholar]
- [36].Armitage R, Landis C, Hoffmann R, Lentz M, Watson NF, Goldberg J, et al. The impact of a 4-hour sleep delay on slow wave activity in twins discordant for chronic fatigue syndrome. Sleep 2007;30:657–62. 10.1093/sleep/30.5.657. [DOI] [PubMed] [Google Scholar]
- [37].Ball N, Buchwald DS, Schmidt D, Goldberg J, Ashton S, Armitage R. Monozygotic twins discordant for chronic fatigue syndrome: Objective measures of sleep. J Psychosom Res 2004;56:207–12. 10.1016/S0022-3999(03)00598-1. [DOI] [PubMed] [Google Scholar]
- [38].Cambras T, Castro-Marrero J, Zaragoza MC, Díez-Noguera A, Alegre J. Circadian rhythm abnormalities and autonomic dysfunction in patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. PLoS One 2018;13:e0198106. 10.1371/journal.pone.0198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fatt SJ, Beilharz JE, Joubert M, Wilson C, Lloyd AR, Vollmer-Conna U, et al. Parasympathetic activity is reduced during slow-wave sleep, but not resting wakefulness, in patients with chronic fatigue syndrome. Journal of Clinical Sleep Medicine 2020;16:19–28. 10.5664/JCSM.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kishi A, Natelson BH, Togo F, Struzik ZR, Rapoport DM, Yamamoto Y. Sleep-stage dynamics in patients with chronic fatigue syndrome with or without fibromyalgia. Sleep 2011;34:1551–60. 10.5665/sleep.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].le Bon O, Neu D, Berquin Y, Lanquart JP, Hoffmann R, Mairesse O, et al. Ultra-Slow delta power in chronic fatigue syndrome. Psychiatry Res 2012;200:742–7. 10.1016/j.psychres.2012.06.027. [DOI] [PubMed] [Google Scholar]
- [42].Libman E, Creti L, Baltzan M, Rizzo D, Fichten CS, Bailes S. Sleep apnea and psychological functioning in chronic fatigue syndrome. J Health Psychol 2009;14:1251–67. 10.1177/1359105309344895. [DOI] [PubMed] [Google Scholar]
- [43].Neu D, Mairesse O, Hoffmann G, Dris A, Lambrecht LJ, Linkowski P, et al. Sleep quality perception in the chronic fatigue syndrome: Correlations with sleep efficiency, affective symptoms and intensity of fatigue. Neuropsychobiology 2007;56:40–6. 10.1159/000110727. [DOI] [PubMed] [Google Scholar]
- [44].Neu D, Cappeliez B, Hoffmann G, Verbanck P, Linkowski P, le Bon O. High slow-wave sleep and low-light sleep: Chronic fatigue syndrome is not likely to be a primary sleep disorder. Journal of Clinical Neurophysiology 2009;26:207–12. 10.1097/WNP.0b013e3181a1841b. [DOI] [PubMed] [Google Scholar]
- [45].Neu D, Mairesse O, Montana X, Gilson M, Corazza F, Lefevre N, et al. Dimensions of pure chronic fatigue: psychophysical, cognitive and biological correlates in the chronic fatigue syndrome. Eur J Appl Physiol 2014;114:1841–51. 10.1007/s00421-014-2910-1. [DOI] [PubMed] [Google Scholar]
- [46].Neu D, Mairesse O, Verbanck P, Linkowski P, le Bon O. Non-REM sleep EEG power distribution in fatigue and sleepiness. J Psychosom Res 2014;76:286–91. 10.1016/j.jpsychores.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [47].Rahman K, Burton A, Galbraith S, Lloyd A, Vollmer-Conna U. Sleep-wake behavior in chronic fatigue syndrome. Sleep 2011;34:671–8. 10.1093/sleep/34.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Togo F, Natelson BH, Cherniack NS, FitzGibbons J, Garcon C, Rapoport DM. Sleep structure and sleepiness in chronic fatigue syndrome with or without coexisting fibromyalgia. Arthritis Res Ther 2008;10:R56. 10.1186/ar2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Togo F, Natelson BH. Heart rate variability during sleep and subsequent sleepiness in patients with chronic fatigue syndrome. Auton Neurosci 2013;176:85–90. 10.1016/j.autneu.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vassallo CM, Feldman E, Peto T, Castell L, Sharpley AL, Cowen PJ. Decreased tryptophan availability but normal post-synaptic 5-HT 2c receptor sensitivity in chronic fatigue syndrome. Psychol Med 2001;31:585–91. 10.1017/S0033291701003580. [DOI] [PubMed] [Google Scholar]
- [51].Watson NF, Kapur V, Arguelles LM, Goldberg J, Schmidt DF, Armitage R, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep 2003;26:324–8. 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- [52].Joustra ML, Zijlema WL, Rosmalen JGM, Janssens KAM. Physical activity and sleep in chronic fatigue syndrome and fibromyalgia syndrome: Associations with symptom severity in the general population cohort lifelines. Pain Res Manag 2018;2018. 10.1155/2018/5801510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fischler B, Hoffmann G, Cluydts R, Kaufman L, de Meirleir K. Sleep anomalies in the chronic fatigue syndrome. Neuropsychobiology 1997;35:115–22. 10.1159/000119331. [DOI] [PubMed] [Google Scholar]
- [54].Reeves WC, Heim C, Maloney EM, Youngblood LS, Unger ER, Decker MJ, et al. Sleep characteristics of persons with chronic fatigue syndrome and non-fatigued controls: Results from a population-based study. BMC Neurol 2006;6:41. 10.1186/1471-2377-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Burton AR, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp Brain Res 2010;204:71–8. 10.1007/s00221-010-2296-1. [DOI] [PubMed] [Google Scholar]
- [56].Fuller C, Lehman E, Hicks S, Novick MB. Bedtime Use of Technology and Associated Sleep Problems in Children. Glob Pediatr Health 2017;4. 10.1177/2333794X17736972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Williamson AA, Mindell JA. Cumulative socio-demographic risk factors and sleep outcomes in early childhood. Sleep 2020;43:1–13. 10.1093/SLEEP/ZSZ233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang Y, Ren R, Yang L, Zhang H, Shi Y, Vitiello M v., et al. Patterns of polysomnography parameters in 27 neuropsychiatric diseases: an umbrella review. Psychol Med 2022:1–21. 10.1017/S0033291722001581. [DOI] [PubMed] [Google Scholar]
- [59].le Bon O, Neu D, Valente F, Linkowski P. Paradoxical NREMS distribution in “pure” chronic fatigue patients a comparison with sleep apnea-hypopnea patients and healthy control subjects. J Chronic Fatigue Syndr 2008;14:45–59. 10.1300/J092v14n02_05. [DOI] [Google Scholar]
- [60].Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep 2004;27:285–91. 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- [61].Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. REM sleep instability - A new pathway for insomnia? Pharmacopsychiatry 2012;45:167–76. 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- [62].Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int 2006;23:461–74. 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- [63].Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med 1999;31:130–40. 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- [64].Cleare AJ, Reid S, Chalder T, Hotopf M, Wessely S. Chronic fatigue syndrome. BMJ Clin Evid 2015;2015. [PMC free article] [PubMed] [Google Scholar]
- [65].Working Group convened under the auspices of the Royal Australasian College of Physicians. Chronic fatigue syndrome. Clinical practice guidelines. Medical Journal of Australia 2002;176:S23–56. [Google Scholar]
- [66].Armitage R, Landis C, Hoffmann R, Lentz M, Watson N, Goldberg J, et al. Power spectral analysis of sleep EEG in twins discordant for chronic fatigue syndrome. J Psychosom Res 2009;66:51–7. 10.1016/j.jpsychores.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kishi A, Struzik ZR, Natelson BH, Togo F, Yamamoto Y. Dynamics of sleep stage transitions in healthy humans and patients with chronic fatigue syndrome. Am J Physiol Regul Integr Comp Physiol 2008;294:R1980–1987. 10.1152/ajpregu.00925.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Milrad SF, Hall DL, Jutagir DR, Lattie EG, Ironson GH, Wohlgemuth W, et al. Poor Sleep Quality is Associated with Greater Circulating Pro-Inflammatory Cytokines and Severity and Frequency of Chronic Fatigue Syndrome/ Myalgic Encephalomyelitis (CFS/ME) Symptoms in Women. J Neuroimmunol 2017;303:43. 10.1016/J.JNEUROIM.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Watson NF, Jacobsen C, Goldberg J, Kapur V, Buchwald D. Subjective and objective sleepiness in monozygotic twins discordant for chronic fatigue syndrome. Sleep 2004;27:973–7. 10.1093/sleep/27.5.973. [DOI] [PubMed] [Google Scholar]
- [70].Neu D, Hoffmann G, Moutrier R, Verbanck P, Linkowski P, le Bon O. Are patients with chronic fatigue syndrome just “tired” or also “sleepy”? J Sleep Res 2008;17:427–31. 10.1111/j.1365-2869.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- [71].Pajediene E, Bileviciute-Ljungar I, Friberg D. Sleep patterns among patients with chronic fatigue: A polysomnography-based study. Clinical Respiratory Journal 2018;12:1389–97. 10.1111/crj.12667. [DOI] [PubMed] [Google Scholar]
- [72].Mariman A, Delesie L, Tobback E, Hanoulle I, Sermijn E, Vermeir P, et al. Undiagnosed and comorbid disorders in patients with presumed chronic fatigue syndrome. J Psychosom Res 2013;75:491–6. 10.1016/j.jpsychores.2013.07.010. [DOI] [PubMed] [Google Scholar]
- [73].Neu D, Mairesse O, Verbanck P, le Bon O. Slow wave sleep in the chronically fatigued: Power spectra distribution patterns in chronic fatigue syndrome and primary insomnia. Clinical Neurophysiology 2015;126:1926–33. 10.1016/j.clinph.2014.12.016. [DOI] [PubMed] [Google Scholar]
- [74].Mccall C, Mccall WV. Comparison of Actigraphy with Polysomnography and Sleep Logs in Depressed Insomniacs. J Sleep Res 2012;21:122. 10.1111/J.1365-2869.2011.00917.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Komaroff AL. Inflammation correlates with symptoms in chronic fatigue syndrome. Proc Natl Acad Sci U S A 2017;114:8914–6. 10.1073/pnas.1712475114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].VanElzakker MB, Brumfield SA, Lara Mejia PS. Neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A critical review of research methods. Front Neurol 2019;10:1033. 10.3389/fneur.2018.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jonsjö MA, Olsson GL, Wicksell RK, Alving K, Holmström L, Andreasson A. The role of low-grade inflammation in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) - associations with symptoms. Psychoneuroendocrinology 2020;113. 10.1016/J.PSYNEUEN.2019.104578. [DOI] [PubMed] [Google Scholar]
- [78].Milrad SF, Hall DL, Jutagir DR, Lattie EG, Ironson GH, Wohlgemuth W, et al. Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J Neuroimmunol 2017;303:43–50. 10.1016/j.jneuroim.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, et al. Bidirectional communication between the brain and the immune system: Implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation 2007;13:357–74. 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- [80].Krueger J The Role of Cytokines in Sleep Regulation. Curr Pharm Des 2008;14:3408–16. 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med 2009;7:96. 10.1186/1479-5876-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Russell L, Broderick G, Taylor R, Fernandes H, Harvey J, Barnes Z, et al. Illness progression in chronic fatigue syndrome: A shifting immune baseline. BMC Immunol 2016;17:1–11. 10.1186/s12865-016-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mensah FKF, Bansal AS, Ford B, Cambridge G. Le syndrome de fatigue chronique et le système immunitaire : où en sommes-nous maintenant ? Neurophysiologie Clinique 2017;47:131–8. 10.1016/j.neucli.2017.02.002. [DOI] [PubMed] [Google Scholar]
- [84].Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 2002;51:887–92. 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- [85].Clevenger L, Schrepf A, Christensen D, DeGeest K, Bender D, Ahmed A, et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behav Immun 2012;26:1037–44. 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Soilu-Hänninen M, Erälinna JP, Hukkanen V, Röyttä M, Salmi AA, Salonen R. Semliki Forest virus infects mouse brain endothelial cells and causes blood-brain barrier damage. J Virol 1994;68:6291–8. 10.1128/jvi.68.10.6291-6298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ding Z, Liu J, Lin R, Hou XH. Experimental pancreatitis results in increased blood-brain barrier permeability in rats: A potential role of MCP-1. J Dig Dis 2012;13:179–85. 10.1111/j.1751-2980.2011.00568.x. [DOI] [PubMed] [Google Scholar]
- [88].Farkas G, Márton J, Nagy Z, Mándi Y, Takács T, Deli MA, et al. Experimental acute pancreatitis results in increased blood-brain barrier permeability in the rat: A potential role for tumor necrosis factor and interleukin 6. Neurosci Lett 1998;242:147–50. 10.1016/S0304-3940(98)00060-3. [DOI] [PubMed] [Google Scholar]
- [89].Nakatomi Y, Mizuno K, Ishii A, Wada Y, Tanaka M, Tazawa S, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An11C-(R)-PK11195 PET study. Journal of Nuclear Medicine 2014;55:945–50. 10.2967/jnumed.113.131045. [DOI] [PubMed] [Google Scholar]
- [90].Boneva RS, Decker MJ, Maloney EM, Lin JM, Jones JF, Helgason HG, et al. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: A population-based study. Auton Neurosci 2007;137:94–101. 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [91].Hurum H, Sulheim D, Thaulow E, Wyller VB. Elevated nocturnal blood pressure and heart rate in adolescent chronic fatigue syndrome. Acta Paediatrica, International Journal of Paediatrics 2011;100:289–92. 10.1111/j.1651-2227.2010.02073.x. [DOI] [PubMed] [Google Scholar]
- [92].Shan ZY, Kwiatek R, Burnet R, del Fante P, Staines DR, Marshall-Gradisnik SM, et al. Medial prefrontal cortex deficits correlate with unrefreshing sleep in patients with chronic fatigue syndrome. NMR Biomed 2017;30. 10.1002/nbm.3757. [DOI] [PubMed] [Google Scholar]
- [93].Gabbott PLA, Warner T, Busby SJ. Catecholaminergic neurons in medullary nuclei are among the post-synaptic targets of descending projections from infralimbic area 25 of the rat medial prefrontal cortex. Neuroscience 2007;144:623–35. 10.1016/j.neuroscience.2006.09.048. [DOI] [PubMed] [Google Scholar]
- [94].Barnden LR, Kwiatek R, Crouch B, Burnet R, del Fante P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. Neuroimage Clin 2016;11:530–7. 10.1016/j.nicl.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kimmerly DS, Wong SW, Salzer D, Menon R, Shoemaker JK. Forebrain regions associated with postexercise differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol Heart Circ Physiol 2007;293:299–306. 10.1152/ajpheart.00044.2007. [DOI] [PubMed] [Google Scholar]
- [96].Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry 2018;9. 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep Deprivation in Adolescents and Adults: Changes in Affect. Emotion 2010;10:831–41. 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Carskadon MA. Sleep in Adolescents: The Perfect Storm. Pediatr Clin North Am 2011;58:637–47. 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): A standard measure of sleepiness. Sleep 1987;9:519–24. 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- [100].Verma P, Dubey R, Rani S, Malik S. Sleep Difference between Adolescents and Young Adults. J Adv Med Med Res 2021:352–9. 10.9734/jammr/2020/v32i2430826. [DOI] [Google Scholar]
- [101].Togo F, Natelson BH, Cherniack NS, Klapholz M, Rapoport DM, Cook DB. Sleep is not disrupted by exercise in patients with chronic fatigue syndromes. Med Sci Sports Exerc 2010;42:16–22. 10.1249/MSS.0b013e3181b11bc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


