Abstract
The effect of meditative movement, which includes yoga, tai chi and qi gong, on breathlessness in advanced disease is unknown. This systematic review aims to comprehensively assess the evidence on the effect of meditative movement on breathlessness (primary outcome), health-related quality of life, exercise capacity, functional performance and psychological symptoms (secondary outcomes) in advanced disease. 11 English and Chinese language databases were searched for relevant trials. Risk of bias was assessed using the Cochrane tool. Standardised mean differences (SMDs) with 95% confidence intervals were computed. 17 trials with 1125 participants (n=815 COPD, n=310 cancer), all with unclear or high risk of bias, were included. Pooled estimates (14 studies, n=671) showed no statistically significant difference in breathlessness between meditative movement and control interventions (SMD (95% CI) 0.10 (−0.15–0.34); Chi2=30.11; I2=57%; p=0.45), irrespective of comparator, intervention or disease category. Similar results were observed for health-related quality of life and exercise capacity. It was not possible to perform a meta-analysis for functional performance and psychological symptoms. In conclusion, in people with advanced COPD or cancer, meditative movement does not improve breathlessness, health-related quality of life or exercise capacity. Methodological limitations lead to low levels of certainty in the results.
Short abstract
Meditative movement is safe but does not improve breathlessness in people with advanced COPD or advanced cancer. Methodological limitations provide uncertainty in the results which future research should address. #PalliPulm #RespiratoryCare https://bit.ly/3ZI9MZH
Introduction
Breathlessness is a common and burdensome symptom in advanced stages of malignant and nonmalignant disease. Prevalence estimates among people living with advanced cancer, COPD and chronic heart failure range from 16 to 77% [1, 2], 56 to 98% [2] and 18 to 88% [2], respectively. A systematic review of interventions for breathlessness in advanced disease highlighted meditative movement as a potential interventional approach to improve this symptom, with research needed to address the paucity of studies in this area [3].
Meditative movement is a category of exercise defined by “some form of movement or body positioning, a focus on breathing, and a cleared or calm state of mind with a goal of deep states of relaxation” [4]. It includes practices of yoga, tai chi and qi gong [4]. Yoga originated in ancient India and is a relaxation and meditation technique based on postures, exercises and breathing techniques [5]. Tai chi originated in ancient China and involves slow, gentle and flowing movements, upper and lower extremity movements, core strengthening, balance techniques, meditative breathing, and body awareness [6]. Qi gong is also an ancient Chinese practice that includes meditation, slow physical movement and controlled breathing [7].
It is postulated that meditative movement may improve breathlessness in advanced disease based on effects seen in nonadvanced cancer and early-stage chronic disease. The mechanisms of the effects include addressing deconditioning and improving cardiopulmonary function [5, 8–12], improving physical activity [13], reducing anxiety and depression [4], a breathing retraining component leading to a reduced respiratory rate [14, 15], and increased tidal breathing and peripheral oxygen saturation levels [15].
To date, systematic reviews of meditative movement or a single practice, e.g. yoga, have been undertaken in people living with cancer [16–25], COPD [8, 10, 11, 12, 18, 22] and chronic heart failure [5, 9, 18, 22]. Although they demonstrated that meditative movement interventions are safe, none of them investigated breathlessness as a primary outcome and of those that did investigate the effect on breathlessness, the results are conflicting [11, 12, 16, 18, 25]. Furthermore, none of the reviews included people with advanced disease as the population of interest. Therefore, the aim of this review is to provide a comprehensive synthesis of the evidence base regarding the effect of meditative movement on breathlessness in advanced disease. The objectives were to determine the effect of meditative movement on 1) breathlessness, 2) secondary outcomes of exercise capacity, functional performance, psychological symptoms and health-related quality of life, and 3) to determine the safety profile of studies involving meditative movement in people with advanced disease.
Methods
The protocol for this systematic review was pre-specified and registered on the PROSPERO international prospective register of systematic reviews (CRD42021271421) on 23 September 2021 and is reported according to the PRISMA 2020 statement [26].
Information sources
We searched the following databases from their inception to the present, without date or language restrictions: Cochrane Database of Systematic Reviews, the Cochrane Library; Cochrane Central Register of Controlled Trials; Medline (Ovid); Embase (Ovid); PsycINFO (Ovid); CINAHL (EBSCO); the Wanfang database; Chinese National Knowledge Infrastructure; and SinoMed. The searches were conducted on 4 January 2022 and repeated on 4 February 2023. The full strategies are shown in the online supplement. In addition, we checked reference lists of reviews and retrieved articles for relevant studies and performed citation searches on key articles. We included randomised controlled trials (RCTs) or randomised crossover trials if separate data for both time periods was presented (we only used the data of the first period for analysis to avoid any potential for carry-over effects). We required full journal publication; where this was not available or where there was insufficient information or data for extraction, we tried to obtain data by contacting the authors.
Eligibility criteria
Articles were eligible for inclusion if they included adult patients (aged ≥18 years) diagnosed with advanced disease(s) with a high prevalence of breathlessness, undergoing yoga, tai chi and/or qi gong in any setting (comparator arm: treatment, standard care or a comparator intervention). We included articles if the majority (≥50%) of participants met the following criteria for advanced disease: 1) patients with advanced local or metastatic cancer (e.g. T (tumour site and size) N (lymph node involvement) M (metastatic spread) classification of malignant tumours state ≥T3 or N≥1 or M≥1) [27]; patients with severe COPD defined as a forced expiratory volume in 1 s predicted of ≤50% according to Global Initiative for Chronic Obstructive Lung Disease criteria [28]; and patients with chronic heart failure and New York Heart Association stage III or IV [29]. If study participants were stratified for these inclusion criteria, we only included the subgroups of interest.
Studies were excluded if they included paediatric populations. Reviews, editorials, case reports and case series were also excluded.
Selection process
Following the database search, conducted by two reviewers (C.M.N. and Y.M./L.J.B.), all identified records were uploaded Endnote™ 20 (Clarivate®, London, UK) then transferred into Covidence (Melbourne, Australia), where duplicates were identified and removed. At the screening and full-text review stage, the title and abstract and full text were respectively translated from Chinese to English by a native Chinese speaker who is undertaking a PhD (in English) at Kings College London (Y.M.). Two reviewers independently screened the records to identify potentially eligible papers by an initial title and abstract screening (C.M.N. and M.M./L.J.B./J.B./Y.M.). Two reviewers (C.M.N. and M.M./L.J.B./J.B./Y.M.) then independently assessed each article in its entirety for eligibility using standardised eligibility criteria. Disagreements and unclear decision were resolved with a third author. We did not anonymise the studies in any way before assessment. Screening and selection of articles was reported according to the PRISMA criteria and flow chart.
Outcome measures
The primary outcome measure was breathlessness measured by self-reported instruments, including but not limited to the Medical Research Council Dyspnoea Scale (MRC), the modified MRC, the Baseline Dyspnoea Index, the Borg Dyspnoea Scale, the Chronic Respiratory Questionnaire – Dyspnoea domain, the Breathlessness Numerical Rating Scale and the Breathlessness Visual Analogue Scale. Other terms for breathlessness such as dyspnoea, shortness of breath and difficulty breathing were accepted.
Secondary outcome measures included health-related quality of life (e.g. European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaires C30 and LC13 (EORTC QLQ-C30; EORTC QLQ-LC13), the Chronic Respiratory Questionnaire (CRQ) and the Kansas City Cardiomyopathy Questionnaire), functional exercise capacity (e.g. 6-min walk test (6MWT) and the incremental shuttle walk test), functional performance measures (e.g. short physical performance battery, 1-min sit-to stand-test, the timed up and go test (TUG) and walking speed tests, e.g. 4-m gait speed), psychological symptoms, i.e. anxiety and depression symptoms (e.g. Hospital Anxiety and Depression Scale (HADS), Generalised Anxiety Disorder Assessment-7 and Public Health Questionnaire-9), and safety (e.g. adverse events and serious adverse events).
Data extraction and management
For data extraction, two reviewers (C.M.N. and L.J.B./Y.M./J.B.) used a pre-piloted, standardised data extraction form and checked for agreement. Differences in data extraction were resolved by reference to original articles, discussion to establish consensus or consultation of a third reviewer (M.M.).
The following data were extracted from included studies: basic study information (first author, year of publication, journal, publication language, country study conducted in, study design); participants (number, disease, disease severity, age, sex, ethnicity); intervention and control (setting, type of intervention(s), type of control, prescription and progression of intervention and/or control, level of supervision, duration and frequency); process outcomes (adherence and completion of allocated intervention); primary and secondary outcome(s) (instrument name, measurement domain, time point of assessment; n at each timepoint; statistical description); and adverse and serious adverse events (total number, number and type in intervention and/or control arm).
Assessment of risk of bias
Two reviewers (C.M.N. and L.J.B./Y.M./J.B.) independently assessed each study for risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions [30]. The reviewers obtained information to aid this assessment from study reports and protocols. Thereafter, they made a judgement as to the level of risk of bias for a specific domain and rated it as low risk, high risk or level of risk unclear and summarised the results in a risk of bias table. The reviewers assessed the following for each study: random sequence generation; allocation concealment; blinding of participants and personnel; incomplete outcome data; selective reporting; and study size. Discrepancies between the reviewers was resolved by discussion involving a third reviewer (M.M.).
Measurement of treatment effect
The mean change from baseline or mean post-intervention values and standard deviation (sd) for each group were recorded. Means were calculated when medians were reported and sds when 95% confidence interval, standard errors or ranges were reported using the guidance in the Cochrane Handbook for Systematic Reviews of Interventions [30]. Using Review Manager 5.4, mean differences (MDs) for outcomes measured using the same instrument and SMDs for outcomes measured using different instruments with 95% CIs were calculated and these data were plotted using forest plots.
Dealing with missing data
Corresponding study authors were contacted if data were missing on study characteristics or outcome measures which would preclude study inclusion or limit the use of a study at further stages of the review. If studies did not report outcomes based on intention-to-treat analyses this was considered as a source of bias during methodological assessment.
Assessment of reporting bias
Publication bias was minimised by searching trials registers for projected and registered studies that had never been published. Authors were contacted for unpublished information if there were such studies registered or some relevant information was missing and could therefore narrow the risk of reporting bias. We planned to assess publication bias using funnel plots when at least 10 studies are available for meta-analysis.
Assessment of heterogeneity and data synthesis
Statistical heterogeneity in each meta-analysis was assessed using the Chi2 test and I2 statistic and used to assess clinical heterogeneity [30]. Where trials demonstrated statistical homogeneity (I2<50% or p>0.10), a meta-analysis was performed using an inverse variable fixed-effect model to estimate the overall direction, size and consistency of the effect of meditative movement on the outcome. Where included trials demonstrated clinical heterogeneity (I2>50% or p<0.10), the random effects model was used. For outcomes where meta-analysis was considered not appropriate, the findings from individual studies were described. A pooled quantitative analysis was performed when trials were clinically homogenous. We extracted group data according to intervention, comparator and outcome. For multi-arm studies with multiple interventions, we considered each intervention separately.
Subgroup analysis and investigation of heterogeneity
Three subgroup analyses were conducted to explore possible sources of heterogeneity: 1) different types of comparator intervention – active comparator interventions (defined as those that included supervised intervention, e.g. pulmonary rehabilitation, education/counselling sessions) may be more effective in improving breathlessness than passive comparator interventions (which was sub-divided into two groups: usual care, e.g. usual pharmacological care, and usual care plus educational information, e.g. usual pharmacological care supplemented with a leaflet on physical activity); 2) diagnosis, as meditative movement may be influenced by disease category; 3) intervention type, as there may be variation in the effect on breathlessness according to meditative movement discipline, e.g. tai chi, yoga or qi gong.
Results
Article selection
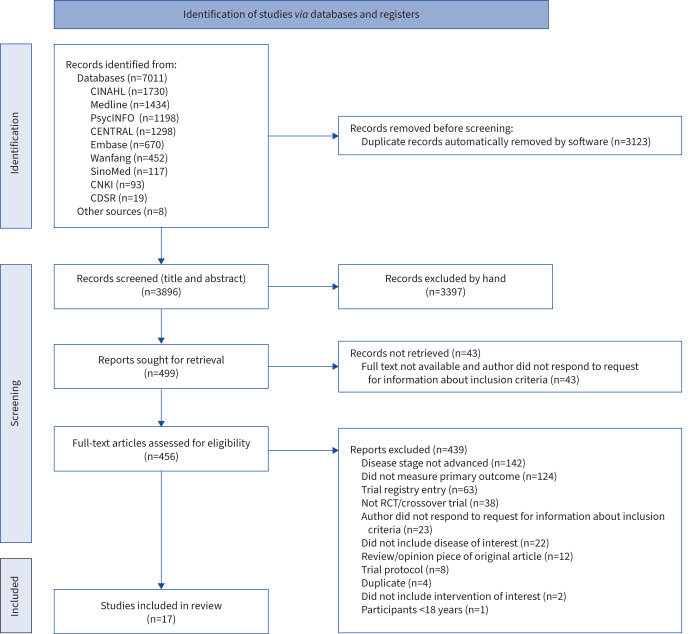
A total of 7019 citations were identified and 3123 duplicates were removed, as depicted in the PRISMA flow diagram (figure 1). In total, 3896 titles and abstracts were screened and 3397 articles were excluded. A total of 499 articles were sought for retrieval, of which 43 were unable to be retrieved. A total of 456 articles underwent full-text review for eligibility. From these, 439 were excluded, leaving 17 unique studies [13, 31–46] The most common reasons for exclusion were failing to meet the review criteria for the proportion of participants with advanced disease (≥50%) and no measure of breathlessness.
FIGURE 1.
PRISMA diagram showing literature search and selection of articles. CDSR: Cochrane Database of Systematic Reviews; CENTRAL: Cochrane Central Register of Controlled Trials; CINAHL: Cumulative Index to Nursing and Allied Health Literature; CNKI: Chinese National Knowledge Infrastructure; RCT: randomised controlled trial.
Characteristics of the included studies
A summary of the study characteristics is shown in table 1. The 17 studies were published between 2009 and 2022, 13 in English [13, 34–44, 46] and four in Chinese languages [31–33, 45]. The trials (of which three were three-arm RCTs [39, 43, 45] and one a randomised crossover trial [36]) were conducted in China (n=7) [13, 31–33, 35, 44, 45], USA (n=4) [34, 39, 40, 42], Hong Kong (n=2) [38, 43], India (n=2) [37, 46], Canada (n=1) [36] and Vietnam (n=1) [41].
TABLE 1.
Characteristics of included trials
| Study, author [ref.], country | Study design | Subjects n | Disease severity | Age (years) | Sex (male) | Intervention | Control |
| Intervention: tai chi; Disease: COPD | |||||||
| Yeh et al. [34], USA | Single-centre, two-arm feasibility RCT | 10 | FEV1 % pred: IG: 53±7% CG: 47±7% |
IG: 65±6 CG: 66±6 |
IG: 3 (60%) CG: 3 (60%) |
Tai chi (based on Master Cheng Man-Ch'ing's Yang-style short form): • Supervised (two tai chi masters), group-based practice in medical centre: 1 h, twice weekly, 12 weeks • Unsupervised home practice with video and diary: three times weekly, 12 weeks. |
Usual care: pharmacological therapy plus general exercise advice as per ACCP COPD guidelines. |
| Yuexia et al. [32], China | Single-centre, two-arm RCT | 24 | FEV1 % pred: IG: 42±14% CG: 44±12% |
IG: 69±8 CG: 73±5 |
NR | Tai chi (24-step simplified tai chi quan for rehabilitation training published by the State Sports General Administration in 1956): • Supervised (two tai chi masters), group-based practice in outpatient setting: 60 min·day−1 for 8 weeks. |
Usual care: pharmacological therapy. |
| Polkey et al. [13], China | Single centre, two-arm RCT | 120 | FEV1 % pred: IG 49±13% CG: 47±15% |
NR | NR | Tai chi (type not described): • Indacterol: 150 μg OD for 26 weeks (commenced 2 weeks before tai chi). • Tai chi: supervised (weeks 1–2 in person, weeks 3–12 video-conference). Group based in outpatient setting: 1 h, five times a week for 12 weeks. Advised to continue tai chi at home or in community group after intervention finished. • Education: not described. |
• Indacterol: 150 μg OD for 26 weeks (commenced 2 weeks before PR). • PR: arm and leg weights (aim 70–80% 1 RM, 2×10 reps, 3×10 reps depending on exercise), hybrid resistance and aerobic exercise (rowing machine), aerobic whole-body exercise (cycle/treadmill: aim Borg 4–6, 60–80% max HR): 1 h exercise, three times per week for 12 weeks plus education. At end of programme, encouraged to be as physically active as possible. • Education: not described. |
| Zhu et al. [44], China | Single-centre, two-arm RCT | 60 | FEV1 % pred: IG: 35±14% CG: 41±16% |
IG: 68±5 CG: 68±7 |
IG: 28 (93%) CG: 29 (97%) |
Tai chi (modified – no further description): • Supervised (qualified tai chi instructor, two research assistants), group-based practice in outpatient setting: 40–50 min, three times per week for 3 months. • Unsupervised home practice with DVD (no further description). |
Self-management handbook. |
| Moy et al. [39], USA | Multi-centre, three-arm pilot RCT | 91 | FEV1 % pred: IG tai chi: 48±18% IG walking: 51±25% CG: 46±18% |
IG tai chi: 70±8 IG walking: 67±7 CG: 71±9 |
IG tai chi: 17 (47%) IG walking: 9 (50%) CG: 27 (73%) |
Tai chi (designed for older, physically limited population) post-PR completion: • Supervised (tai chi instructor), group-based practice in university fitness facility. Weeks 1–12: 2×1-h sessions per week, weeks 13–24: 1×1-h session per week. • Unsupervised home practice with DVD: advised to do 3×30 min·week−1. Concomitant intervention Walking programme (Borg 3–5 and 60% max HR) post-PR completion: • Supervised (trained research co-ordinator), group-based walking programme in university fitness facility. Weeks 1–12: 2×1-h session per week; weeks 13–24: 1×1-h session per week. • Unsupervised home walking and stretching programme with written instructions: advised to do 3×30 min·week−1 plus written instructions on disease self-management. |
Post-PR completion: unsupervised exercise plan (as part of PR routine care post-discharge) and allowed to continue in the PR maintenance programme for 24 weeks. |
| Intervention: tai chi; Disease: cancer | |||||||
| Cheung et al. [43], Hong Kong | Single-centre, three-arm feasibility RCT | 30 | Lung cancer All stage 3 or 4 (no other data provided) |
IG tai chi: 61±7 IG exercise: 61±12 CG: 58±9 |
IG tai chi: 6 (66%) IG exercise: 5 (50%) CG: 5 (46%) |
Tai chi (24-form Yang style): • Supervised (tai chi master) practice in outpatient setting: 60 min, twice weekly for 12 weeks. • Unsupervised home practice: advised to do 3×30 min·week−1 for 12 weeks. After intervention period, encouraged to self-practice five times per week (total 150 min·week−1). Concomitant intervention Exercise programme: • Supervised (two licenced instructors) exercise programme in gym (supervised session: treadmill/cycle ergometer at 50–60% HR reserve, four strengthening exercises (upper and lower limbs, abdomen) at 60% 1 RM×10 reps once per week): 60 min twice weekly for 12 weeks. • Unsupervised home practice: advised to do 90 min aerobic exercise at Borg 3–4 per week for 12 weeks. After intervention encouraged to practice 150 min aerobic exercise/week plus two sets of strengthening exercises ×10 reps. |
Written information on WHO physical activity guidelines (i.e. 150 min physical activity per week) plus exercise log. |
| Intervention: yoga; Disease: COPD | |||||||
| Donesky-Cuenco et al. [42], USA | Single-centre, two-arm pilot RCT | 41 | FEV1 % pred: IG 52±11% CG 44±19% (from completer data: IG n=14, CG n=15) |
IG: 72±7 CG: 68±12 (from completer data: IG n=14, CG n=15) |
IG: 4 (29%) CG: 4 (27%) (from completer data: IG n=14, CG n=15) |
Yoga (Iygengar yoga: asanas (poses) and visama vritti pranayama (timed breathing): • Supervised (yoga instructor) practice in outpatient setting: 1 h, twice per week for 12 weeks. • Unsupervised home practice with video of one yoga class: advised to practice daily. |
Education pamphlet: Living with COPD. |
| Kaminsky et al. [40], USA | Multi-centre, two-arm pilot RCT | 43 | FEV1 % pred: IG: 43±16% CG 42±13% |
IG: 68±7 CG: 68±9 |
IG: 33% CG: 45% |
Yoga (pranayama: Dirgha breath): • Supervised (research co-ordinator trained by yoga instructor) practice (30 min) and education (30 min) based on Living Well with COPD material) in outpatient centre. 12-week intervention: week 1–2, 2×1-hour sessions; week 6, 1-h session. • Unsupervised home practice diary: advised to practice daily building up to 30 min·day−1 for 12 weeks. |
Education: • Supervised (research co-ordinator) education session based on Living Well with COPD material) in outpatient centre. 12-week, intervention: week 1–2, 2×1-h supervised visits; week 6, 1-h supervised visit. |
| Malik et al. [46], India | Single-centre, two-arm pilot RCT | 60 | FEV1 % pred: IG: 47±13% CG: 53±20% |
IG: 61±7 CG: 61±8 |
IG: 28 (93%) CG: 27 (90%) |
Yoga (asana, kriya, pranayama): • Supervised (qualified yoga instructor) practice in outpatient setting: 45 min×2. • Supervised home tele-yoga: 5×45 min·week−1 for 12 weeks. • Usual treatment: counselling for adherence, inhaler technique, nutrition (no further information provided). • Participants visited the outpatient department monthly or as needed (rationale not provided). |
PR: • Supervised (qualified physiotherapist) practice in outpatient setting: 2×45 min. • Supervised home tele-PR: 5×45 min·week−1 for 12 weeks. • Usual treatment: counselling for adherence, inhaler technique, nutrition (no further information provided). • Participants visited the outpatient department monthly or as needed (rationale not provided). |
| Intervention: yoga; Disease: cancer | |||||||
| Hosakote et al. [37], India | Multi-centre, two-arm RCT | 88 | Breast cancer: IG stage 1: 2±5%; stage 2: 11±25%; stage 3: 31±71% CG stage 1: 3±7%; stage 2: 7±16%; stage 3: 34±77% |
NR | IG: 0 (0%) CG: 0 (0%) |
Yoga (asanas (poses), breathing exercises): • Pre- or post-radiotherapy 1:1 supervised (yoga instructor) practice in outpatient setting: 1 h at least three times per week for 6 weeks. • Unsupervised home practice diary: advised to practice four times per week for 6 weeks. |
Brief supportive therapy with education: • 1:1 counselling and education (reinforcing social support, coping preparation) with social worker: 15 min, 3–4 sessions over 6 weeks (every 10 days). |
| Intervention: qi gong; Disease: COPD | |||||||
| Dongxin Zhao et al. [31], China | Single-centre, two-arm RCT | 48 | COPD post-hospitalisation for pneumothorax FEV1 % pred: IG: 31±9% CG: 30±9% |
Whole cohort: 75±8 | NR | Qi gong (liuzijue qi gong): • Supervised (qi gong expert) practice in outpatient setting: 15–30 min, 2–3 times per day for 6 months. • Continuous oxygen therapy: >15 h·day−1. |
Usual care: usual health education (no description). |
| Ng et al. [38], Hong Kong | Single-centre, two-arm RCT | 80 | FEV1 % pred: IG: 37±2% CG: 37±2% |
IG: 72±1 CG: 73±1 |
IG: 93% CG: 85% |
Qi gong (baduanjin eight movement routine adapted for COPD) post-PR completion: • Supervised (trained therapist) practice in respiratory care hospital: 4×45 min at PR sessions 9, 10, 11 and 12. • Unsupervised home qi gong (at least once per day, 4 times per week for 6 months), walking programme (30 min·day−1 for 6 months) and diary. |
Breathing and walking programme post-PR completion: • Supervised (instructor not described) breathing training (pursed lip, co-ordinated breathing) in respiratory care hospital: 4×45 min at PR sessions, 9, 10, 11 and 12. • Unsupervised home walking programme: 30 min·day−1 for 6 months. |
| Xiao et al. [35], China | Multi-centre, two-arm RCT | 126 | FEV1 % pred: IG: 42±5% CG: 41±4% |
IG: 72±2 CG: 71±1 |
IG: 92% CG: 94% |
Qi gong (liuzijue qi gong adjusted based on physical condition) alongside PR programme (12 outpatient sessions): • Supervised (trained therapist) practice in respiratory care hospital with ambulatory rehabilitation programme: 4×45 min (time period not described). • Unsupervised home practice (12–15 min, four times per week) plus walking programme (30 min·day−1) for 6 months. Provided with audio-visual home learning package, training log. |
Breathing and walking programme alongside PR programme (12 outpatient sessions): • Supervised (instructor not described) breathing training (pursed lip, co-ordinated breathing) in respiratory care hospital with ambulatory rehab programme: 44×45 min (time period not described). Unclear if programme was performed during or after PR. • Unsupervised home walking programme: 30 min·day−1 for 6 months. |
| Siqin et al. [33], China | Single-centre, two-arm RCT | 60 | FEV1 % pred: IG: 40±13% CG: 47±12% |
IG: 63±5 CG: 64±6 |
IG: 23 (82%) CG: 19 (66%) |
Qi Gong (liuzijue qi gong): • Supervised (medical staff) group-based practice in outpatient setting: 30 min, five times per week for 3 months. |
Usual care: pharmacological therapy according to COPD guidelines. |
| Yanchan et al. [45], China | Single-centre, three-arm RCT | 52 | Whole cohort: GOLD stage 3–4 |
IG qi gong: 70±9 IG breathing: 68±7 CG: 69±7 |
IG qi gong: 18 (95%) IG breathing: 13 (93%) CG: 18 (95%) |
Qi gong (baduanjin qi gong): • Supervised (qi gong expert) practice in outpatient setting: 20–30 min, ≥5 times per week for 12 weeks. Concomitant intervention Breathing exercises: • Supervised (qi gong expert) breathing exercises (pursed lip, abdominal and deep breathing) in outpatient setting: 20–30 min, ≥5 times per week for 12 weeks. |
Usual care: pharmacological therapy. |
| Intervention: qi gong; Disease: cancer | |||||||
| Vanderbyl et al. [36], Canada | Single-centre, two-arm randomised crossover trial | 36 | Advanced nonsmall cell lung or gastro-intestinal cancer at stage 3/4: IG: 11±100% CG: 13±100% (only completer data reported) |
IG: 66±12 CG: 64±8 (only completer data reported) |
IG: 7 (64%) CG 7 (54%) (only completer data reported) |
Qi gong (guolin qi gong: walking programme with co-ordinated arm movements and “in in out” breathing pattern): • Supervised (not described), group-based practice in outpatient setting: 12×45 min over 6 weeks. • Unsupervised home practice: advised to practice daily for 1 h. Followed by a 2-week wash-out period. |
Exercise programme: • Supervised (not described) endurance and strength training delivered 1:1/group “as appropriate” (cardiovascular and resistance exercise: 60–70% max HR or 2–4 METs; frequency and intensity calibrated individual's ability to progress): 12×45 min over 6 weeks. • Unsupervised home walking: advised to walk daily for 1 h and not to practice qi gong. Followed by a 2-week wash-out period. |
| Molassiotis et al. [41], Vietnam | Single-centre, randomised, parallel group, wait-list controlled trial | 156 | Lung cancer: IG stage 1: 4±5%; stage 2: 4±5%; stage 3: 23±30%; stage 4: 47±60% CG stage 1: 0±0%; stage 2: 6±8%; stage 3: 23±30%; stage 4: 49±63% |
Whole group: 57±9 | Whole group: 116 (74%) |
Qi gong (seven postures): • Supervised (qi gong master), group-based practice in outpatient setting. Weeks 1–2: 90 min twice per week. • Semi-supervised home practice supplemented with weekly telephone call (researcher), DVD, diary. Weeks 3–6: advised to practice 30 min·day−1, five times per week. • Unsupervised home practice supplemented with DVD, diary: advised to do 30 min·day−1, five times per week for 6 weeks. |
Usual care (commenced qi gong after end of study). |
Data are presented as mean±sd or n (%), unless otherwise stated. ACCP: American College of Chest Physicians; CG: control group; FEV1: forced expiratory volume in 1 s; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HR: heart rate; IG: intervention group; max: maximum; MET: metabolic equivalent; NR: not reported; OD: once daily; PR: pulmonary rehabilitation; RCT: randomised controlled trial; Reps: repetitions; RM: repetition maximum; WHO: World Health Organization.
The studies included 1125 participants with COPD (studies: n=13; combined sample size: n=815) or cancer (studies: n=4; sample size: n=310; cancer type: lung [41, 43], nonsmall cell lung or gastro-intestinal [36] and breast cancer [37]). No study included participants with chronic heart failure. The mean age of participants ranged from 57 to 75 years. 14 [13, 31–33, 35–38, 40, 41, 43–46] and three studies [13, 31, 32] did not report ethnicity and sex, respectively. Across those that did, the majority of participants were male (70%) and white (74%).
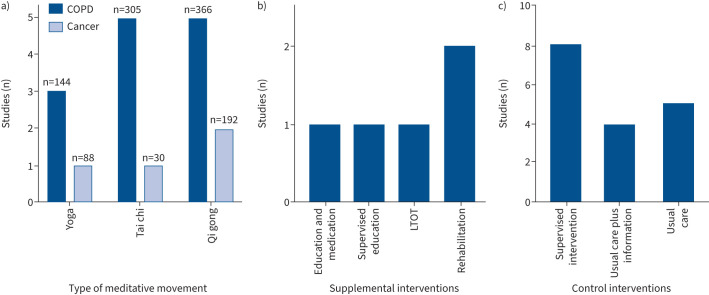
Figure 2 provides an overview of the types of meditative movement, supplemental and control interventions. Briefly, meditative movement interventions included yoga [37, 40, 42, 46], tai chi [13, 32, 34, 39, 43, 44] and qi gong [31, 33, 35, 36, 38, 41, 45]). In five studies, meditative movement was supplemented by an additional intervention [13, 31, 35, 38, 40]. The control interventions included eight active (supervised intervention) [13, 35–40, 46] and nine passive comparator interventions, which were sub-divided into five usual care [31–33, 41, 45] and four usual care supplemented with educational information [34, 42–44]. For the three-arm RCTs, the concomitant interventions were supervised walking (n=1) [39], supervised exercise programme (n=1) [43] and supervised breathing sessions (n=1) [45].
FIGURE 2.
Number of studies according to a) the type of meditative movement (number on top of bar indicates the number of participants), b) supplemental interventions and c) control interventions. LTOT: long-term oxygen therapy.
Regarding the meditative movement interventions, in 15 studies [31–45] it was delivered in person and in two, in person and via videoconference [13, 46]. 12 studies involved a combination of supervised and unsupervised instruction [13, 35–45] and five involved supervision only [31–33, 45, 46]. 11 [13, 32–36, 38–41, 44] were group-based interventions, one was delivered one-to-one [37] and it was unclear how five were delivered [31, 42, 43, 45, 46]. The median (range) in duration of intervention was 14 (6–24) weeks. The content of the meditative movement interventions was heterogenous: yoga (Iygengar and Visama Vritti pranayama (n=1) [42], Dirgha breath (n=1) [40], intervention type not described (n=2) [37, 46]); tai chi (Master Cheng Man-Ch'ing's Yang-style short form (n=1) [34], simplified tai chi quan (n=1) [32], tai chi modified for older people (n=1) [39], 24-form Yang style (n=1) [43], not described (n=2) [13, 44]); and qi gong (seven-posture (n=1) [41], Guolin qi gong (n=1) [36], Baduanjin qi gong (n=2) [38, 45], Liuzijue qi gong (n=3) [31, 33, 35]). The mean (range) intervention adherence rate was 82 (22–100) %. The mean (range) completion rates for the intervention and control groups were 79 (58–100) % and 89 (71–100) %, respectively.
Risk of bias assessment
A total of 13 studies [13, 32, 33, 36–38, 40–46] were at overall unclear risk of bias and four [31, 34, 35, 39] were at high risk of bias (supplementary figures S1 and S2). No studies were at low risk of bias. Random sequence generation was used in 10 studies [34, 36–41, 43, 44] and allocation concealment described in seven [34, 36, 38, 39, 41, 43, 46]. Blinding of participants was not possible due to the nature of the intervention but blinding of outcome assessors was only reported in nine studies [34–36, 38–41, 43, 46]. There were incomplete outcome data reported in 11 studies [13, 31–35, 39, 40, 44–46] and selective reporting in three [33, 34, 45]. None of the studies met the criteria for low risk of bias for study size.
Results of synthesis
Primary outcome: breathlessness
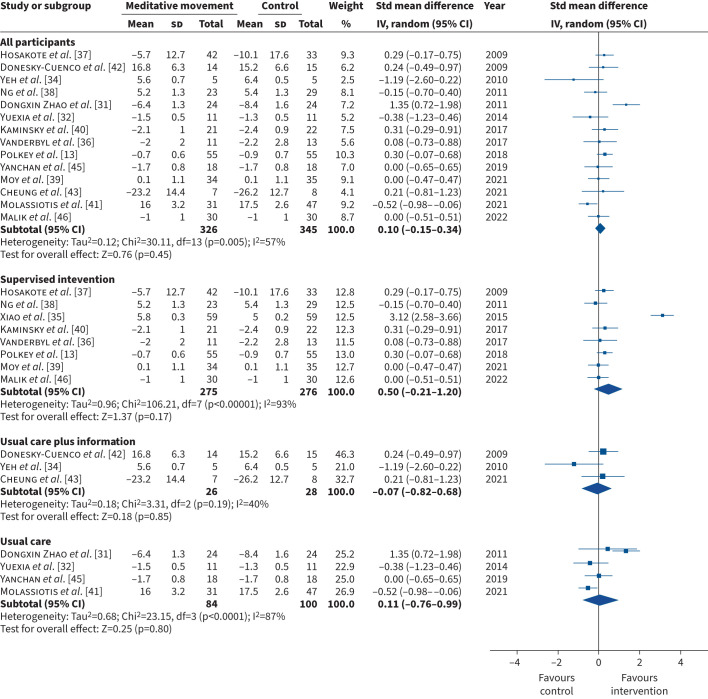
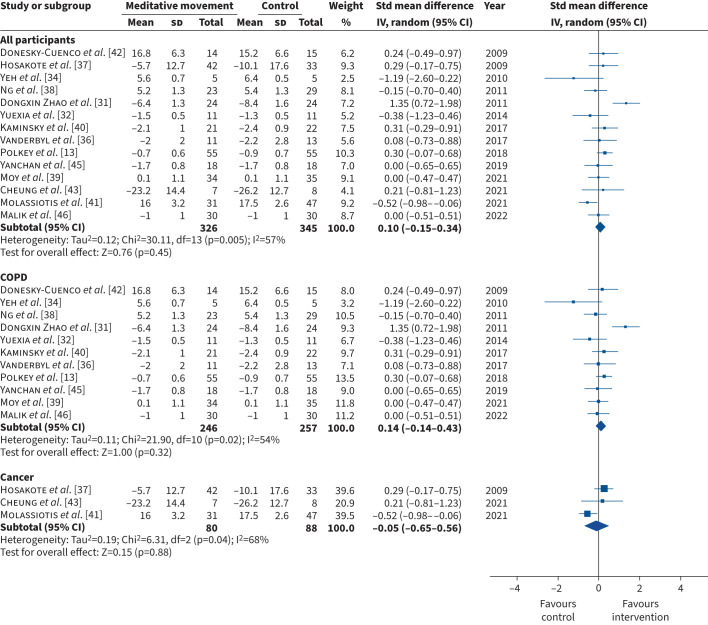
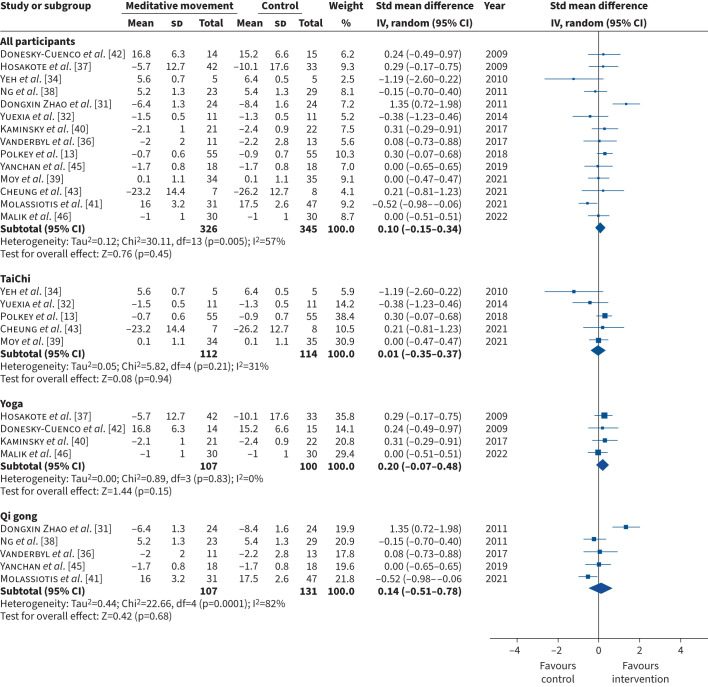
All 17 studies assessed at least one measure of breathlessness. The outcome measures included mMRC (n=7) [13, 32, 33, 40, 44–46], CRQ-D (n=5) [34, 35, 38, 39, 42], EORTC QLQ-LC13 (n=1) [43], EORTC QLQ-C30 Dyspnoea (n=1) [37], Borg scale (n=1) [31], 11-point Likert scale – dyspnoea (n=1) [36] and the Cancer Dyspnoea Scale (n=1) [41]. In two studies, data were presented in a format that could not be retrieved for meta-analysis: one study did not report the number of participants who attended the post-intervention assessment [44] and one did not report the standard deviation of the mean [33]. When we performed the meta-analysis, data from one study was a major outlier [35]. We contacted the authors to confirm whether the data reported in the study was accurate but did not receive a response. We therefore excluded this study from the meta-analysis but report the data in the supplementary file (figures S3, S4 and S5). Thus, we included 14 studies (n=671) in the meta-analysis. The pooled estimate showed no statistically significant difference in the mean difference in breathlessness for meditative movement compared with the control intervention (SMD (95% CI) 0.10 (−0.15–0.34); Chi2=30.11; I2=57%; p=0.45) (figures 3, 4, 5). Sub-group analysis according to comparator arm (supervised intervention, usual care plus information, usual care) (figure 3), disease category (COPD, cancer) (figure 4) and intervention (tai chi, yoga, qi gong) (figure 5) demonstrated similar results. The funnel plots, which are symmetrical, are in the supplementary file (figures S6, S7 and S8).
FIGURE 3.
Forest plot comparing meditative movement and control intervention for the primary outcome – breathlessness – for all participants, active comparator and passive comparator. Std: standardised; IV: inverse variance; df: degree of freedom.
FIGURE 4.
Forest plot comparing meditative movement and control intervention for the primary outcome – breathlessness – for all participants, people with COPD and people with cancer. Std: standardised; IV: inverse variance; df: degree of freedom.
FIGURE 5.
Forest plot comparing meditative movement and control intervention for the primary outcome – breathlessness – for all participants and according to intervention type (tai chi, yoga, qi gong). Std: standardised; IV: inverse variance; df: degree of freedom.
Secondary outcome: health-related quality of life
A total of 13 studies [13, 34–44, 46] assessed at least one measure of health-related quality of life. The outcome measures included Short-Form 36 (n=3) [35, 38, 42], St George's Respiratory Questionnaire (n=3) [13, 40, 46], CRQ (n=2) [34, 39], EORTC QLQ-C30 Functional (n=2) [41, 43], EORTC QLQ-C30 Psychological (n=1) [37], COPD Assessment Test (n=1) [44], Functional Assessment of Cancer Therapy–General (n=1) [36]. In two studies, data were presented in a format that could not be retrieved for meta-analysis, as the number of participants who attended the post-intervention assessment were not reported [13, 44]. As described in the previous section, the study with outlier data was excluded but reported in supplementary figure S9. Thus, we included 10 studies (n=454) in the meta-analysis. The pooled estimate did not show a statistically significant difference in the mean difference in health-related quality of life in meditative movement compared with the control intervention (SMD (95% CI) 0.16 (−0.03–0.35); Chi2=15.35; I2=41%; p=0.08) (supplementary figures S10 and S11).
Secondary outcome: exercise capacity
A total of 14 studies [13, 31, 32, 34–36, 38–40, 42–46] assessed exercise capacity using 6MWT. As described in the previous section, two studies [13, 44] were excluded as data were presented in a format that count not be retrieved for meta-analysis and one excluded because of outlier data [35] (data are presented in supplementary figure S12). Thus, we included 11 studies (n=407) in the meta-analysis. The pooled estimate did not show a statistically significant difference in the mean difference in exercise capacity in meditative movement compared with the control intervention (MD (95% CI) 17.250 (−6.06–41.06); Chi2=35.09; I2=72%; p=0.15) (supplementary figures S13 and S14).
Secondary outcome: functional performance
Only three studies [34, 36, 43] assessed functional performance using a variety of outcome measures, therefore a meta-analysis was not performed. The outcome measures included TUG (n=2) [34, 43] 30-s sit-to-stand test (n=1) [43], walking speed over 50 feet (n=1) [36] and two-repetition sit-to-stand test (n=1) [36]. There was no significant within- or between-group difference in TUG [34, 43] 30-s sit-to-stand test [43] or walking speed over 50 feet [36]. Although there was a significant within-group improvement in two-repetition sit-to-stand test in the meditative movement group, the between-group difference was not significant [36].
Secondary outcome: psychological symptoms
Only seven studies [34, 36, 39, 41–43, 46] assessed psychological symptoms using a variety of outcome measures, therefore a meta-analysis was not performed. Of these studies, one assessed anxiety symptoms only Depression Anxiety Stress Scale21 – Anxiety [41]), two assessed depression symptoms only (Centre for Epidemiological Studies – Depression scale (CES-D) [34, 39]) and four assessed both (HADS (n=2) [36, 43], State Anxiety Inventory – Anxiety and CES-D (n=1) [42], PHQ-9 and GAD-7 (n=1) [46]). All of the studies, except one [46] reported no significant within- or between-group differences in psychological symptoms.
Secondary outcome: safety
Adverse and serious adverse events are reported in supplementary table S1. A total of 10 (63%) [13, 32, 34, 35, 39, 40, 42, 43, 45, 46] and 11 (69%) [13, 32, 34–36, 38, 40–43, 46] studies collected data on adverse and serious adverse events, respectively, in both the intervention and control groups. The overall number (% of study population) of adverse and serious adverse events were 81 (14%) and 32 (5%), respectively. Two studies that reported 11 adverse [40, 42] and two serious adverse events [36, 40] in total, did not report the number of events according to study arm. Excluding these studies, there were (number (% of intervention/control arm population)) 44 (19%) and 26 (11%) adverse events and 16 (6%) and 15 (5%) serious adverse events in the meditative movement and control arms, respectively. However, none appeared to be related to the intervention.
Discussion
Summary of findings
This systematic review identified 17 studies involving 1125 people with advanced COPD or cancer from six countries. The meditative movement interventions included tai chi, yoga and qi gong and their component parts were heterogenous. All studies were determined to have a high or unclear risk of bias. The effect of meditative movement on breathlessness in people with advanced disease was compared to a control intervention, with additional sub-group analysis according to control intervention, disease category and intervention type. The effect of meditative movement on secondary outcome measures (health-related quality of life, exercise capacity, functional performance, mood and safety) was explored, but there were only sufficient data to conduct a meta-analysis of health-related quality of life and exercise capacity. Overall, no statistically significant improvement in breathlessness, health-related quality of life, exercise capacity, functional performance or mood was found. There was incomplete reporting of safety outcomes. The overall number (% of study population) of adverse and serious adverse events were 81 (14%) and 32 (5%), respectively. Although there were a higher proportion of adverse events in the intervention arm, none appeared to be related to a meditative movement intervention.
Strengths and limitations
This is the largest systematic review to investigate the effect of meditative movement in people with advanced disease. Strengths of this review include the comprehensive literature search, encompassing Chinese language databases and the inclusion of studies published in languages other than English, namely Chinese. We employed a rigorous methodology following a protocol published on PROSPERO (CRD42021271421). Reviewers worked independently in pairs and ensured that all titles and abstracts were duplicate-screened, and disagreements resolved in discussion with separate reviewer. Although we searched for a number of advanced diseases, the included studies only recruited people with COPD or cancer and there was a preponderance for the former, so the findings are not generalisable to other advanced diseases. All of the included studies were categorised as unclear or high risk of bias and despite using a random effects model when I2>50% for the meta-analysis, statistical heterogeneity was high for breathlessness (including all sub-group analyses) and exercise capacity, therefore the results should be interpreted with caution. We were unable to include all 17 retrieved studies in the meta-analysis because of missing outcome data. In addition, we excluded one study from the meta-analysis because the data was a major outlier and the authors did not clarify whether the data was accurate. This provides confidence in our results. Nonetheless, the meta-analysis of breathlessness included 14 studies involving 671 participants. The mMRC scale was the most common outcome measure used to assess breathlessness. Although it is valid and widely used in advanced COPD and advanced cancer, it is a blunt outcome measure that may not be sufficiently sensitive to changes in breathlessness experience. We were unable to perform a meta-analysis on functional performance or psychological symptoms due to the paucity of studies measuring these outcomes.
Interpretation of findings in the context of published literature
This meta-analysis demonstrates that meditative movement does not improve breathlessness in advanced COPD or cancer. As this is the first meta-analysis to evaluate meditative movement in advanced disease, it is not possible to compare the results to previous literature in this population. A systematic review of nonpharmacological interventions for breathlessness outcomes in advanced disease did not synthesise data on meditative movement due to lack of study homogeneity [3]. Of note, systematic reviews that investigated meditative movement in nonadvanced diseases only included breathlessness as a secondary outcome measure and the number of included studies is small. However, similar to this review, there was heterogeneity in both the meditative movement and control interventions. Cramer et al. [11] (three studies, n=140) and Chen et al. (three studies, n=367) [18] reported that yoga and tai chi, respectively, did not improve breathlessness in people with COPD, and Van Vu et al. [25] (two studies, n=82) reported that qi gong did not improve breathlessness in people with lung cancer. In contrast, Wu et al. [12] (two studies, n=48) reported a significant improvement in breathlessness favouring the control group, not meditative movement, in people with COPD, and Buffart et al. [16] reported that there were insufficient data to investigate the effect of yoga on breathlessness in people with cancer or cancer survivors. Our large and comprehensive meta-analysis confirms that meditative movement does not improve breathlessness outcomes in people advanced COPD and cancer.
The reasons why meditative movement does not improve breathlessness in advanced disease may be multifactorial. As noted in the limitation section, the most common measurement of breathlessness, the mMRC, may not be sufficiently sensitive to detect changes in breathlessness.
The meditative movement interventions were heterogenous, even within single practices, e.g. different types of yoga, variation in breathing and physically active components, and in many studies were offered alongside other interventions, e.g. education; therefore, it is challenging to evaluate the effect on breathlessness. Of the 17 studies included in this review, one involved breathing exercises only [40] whereas the remaining studies included both breathing and exercise components. Owing to limitations in the intervention description and lack of objective measurement to quantify intervention energy expenditure, it is not possible to understand the extent balance the of breathing and/or exercise the interventions involved. Nonetheless, meditative movement interventions are hypothesised to influence breathing through a number of mechanisms including addressing deconditioning and breathing retraining, i.e. both breathing and exercise mechanisms. Furthermore, sub-group analysis according to intervention type demonstrated no statistically significant difference in breathlessness compared to control intervention.
In addition, the comparator interventions were heterogenous and ranged from usual care to supervised exercise interventions, which renders investigation of the specific effect of meditative movement on breathlessness difficult. However, sub-group analysis according to control intervention demonstrated no statistically significant difference on the effect of meditative movement on breathlessness. Of note, there was a trend for improvement in breathlessness favouring meditative movement compared to supervised control interventions. This trend was not evident in the usual care plus information or usual care control interventions. This may be because of the small number of studies and sample sizes in these groups compared to the supervised control intervention group (supervised intervention: eight studies, n=551; usual care plus information: three studies, n=54; usual care: four studies, n=184).
Meditative movement did not improve health-related quality of life, exercise capacity or psychological symptoms. Published literature on the effect of meditative movement on health-related quality of life is conflicting. Seven systematic reviews involving people with nonadvanced diseases (study range: n=2–9; sample size range: n=59–708) [5, 8, 12, 16, 17, 19, 47] report that meditative movement, or individual interventions (e.g. tai chi, yoga, qi gong), improve health-related quality of life in people with cancer (active cancer, cancer survivors), heart failure and COPD. In contrast, three reviews (study range: n=3–11; sample size range: n=91–788) report that meditative movement [8], tai chi [18] and yoga [11] did not improve this outcome. Irrespective of the outcome of the review, there was heterogeneity in the meditative movement and control interventions.
Exercise capacity and psychological symptoms are known to influence breathlessness; therefore, it follows that there was no effect on this outcome. The results of qualitative interviews [24] of one of the studies included in the review [39] stated that participants with COPD who underwent tai chi reported experiencing changes in their breathing including increased awareness of and capacity for regulating breathing. This may suggest that the outcome measures used to assess breathlessness in this review did not capture these experiences, e.g. breathlessness when performing activities of daily living, and/or that there was no carry-over of these changes outside meditative movement practice.
Regarding exercise capacity, previous systematic reviews in populations including nonadvanced COPD, heart failure and cancer (active cancer, cancer survivors) provide conflicting results. Four reviews, involving heterogenous meditative movement and control interventions (study range: n=2–14; sample range: n=59–1054) reported that meditative movement and yoga improve exercise capacity [5, 8, 10, 11] whereas two involving meditative movement and tai chi (study range: n=2–8; sample size range: n=348–644) reported no improvement [12, 18]. Meditative movement may not improve exercise capacity in advanced disease because the interventions may not have been sufficiently challenging to result in physiological adaptations to exercise.
It was not possible to perform meta-analysis of the effect of meditative movement on functional performance and psychological symptoms, but the reviewed literature suggests that there may not be an effect on these measures in advanced COPD or cancer. Only one systematic review has evaluated functional performance measure in nonadvanced disease and reported that tai chi did not improve this outcome in people with osteoarthritis or heart failure [18]. The literature on psychological outcomes is contradictory and predominantly relates to cancer. Three reviews report that qi gong [19], yoga [16] and tai chi [23] improve depression in cancer (active cancer, cancer survivors) whereas one reports that tai chi does not improve this outcome in people with chronic conditions [18]. Only one review investigated anxiety as an individual domain in people with cancer and reported that qi gong did not improve this outcome [19]. The conflicting results may be explained by the disease state, meditative movement and control intervention heterogeneity, as well as measurement heterogeneity. For example, one review reported that tai chi improves the mental health domains of quality of life in cancer survivors [17], whereas another reported that meditative movement does not improve psychosocial outcomes in men with cancer [19].
Although the data were incomplete, this review demonstrates that meditative movement interventions are safe in people with advanced disease, which is similar to data reported in nonadvanced populations [9, 11, 17, 20, 22, 23, 24].
Implications for clinical practice and research
The results of this review suggest that meditative movement, although a safe intervention, should not routinely be offered to people with advanced COPD or cancer for the purpose of improving breathlessness, health-related quality of life, exercise capacity, functional performance or psychological symptoms. There is a paucity of studies that have investigated functional performance (and none of these evaluated functional performance of activities of daily living) and psychological symptoms, especially in diseases other than cancer, which should be evaluated in future research.
Future research should explore the synergistic effect of meditative movement and other evidence-based interventions that improve breathlessness on this outcome, the effect on meditative movement on outcomes such as activities of daily living and physical activity, and the role of meditative movement in other advanced diseases, e.g. chronic heart failure.
Points for clinical practice and questions for future research
Meditative movement is safe but does not improve breathlessness, exercise capacity or health-related quality of life or in advanced COPD or advanced cancer. Future research should explore the synergistic effect of meditative movement and other evidence-based interventions that improve breathlessness in advanced disease.
The interpretation of this research is limited by bias and wide heterogeneity in study populations, interventions, comparator interventions and outcome measures, leading to low levels of certainty in the results.
Future research should investigate the broader effects of meditative movement on functional performance, psychological symptoms, physical activity and activities of daily living in advanced diseases.
Conclusion
Our findings suggest that meditative movement does not improve breathlessness in people with advanced COPD or advanced cancer, irrespective of disease category, intervention or comparator group. However, limitations including unclear or high risk of bias and heterogeneity in study populations, interventions, comparators and outcome measures limit the strength of conclusions. Future research should address these limitations.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0243-2022.SUPPLEMENT (1MB, pdf)
Acknowledgements
We would like to thank Dr Neil O’Connell (Brunel University London, Co-ordinating editor for the Cochrane Pain, Palliative and Supportive Care group) for his advice on outlier data.
Provenance: Submitted article, peer reviewed.
Conflict of interest: C.M. Nolan reports personal fees from Novartis, consultancy work (not reimbursed) with Vicore Pharma and grants from the National Institute for Health and Care Research outside the submitted work. W.D-C. Man reports grants from the National Institute for Health and Care Research (NIHR), outside the submitted work. M. Maddocks reports grants from NIHR, outside the submitted work. The remaining authors have nothing to disclose.
Support statement: L.J. Brighton is funded by an Economic and Social Research Council Post-Doctoral Fellowship (ES/X005259/1). Y. Mo is funded by King's-China Scholarship Council (K-CSC 202106370016). I.J. Higginson, M. Maddocks, J. Bayly and L.J. Brighton are supported by the NIHR Applied Research Collaboration South London (NIHR ARC South London) at King's College Hospital NHS Foundation Trust. M. Maddocks is supported by a National Institute for Health and Care Research (NIHR) Career Development Fellowship (CDF-2017–10–009) and the NIHR Applied Research Collaboration South London. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, NIHR or the Department of Health and Social Care.
References
- 1.Mercadante S, Aielli F, Adile C, et al. Epidemiology and characteristics of episodic breathlessness in advanced cancer patients: an observational study. J Pain Symptom Manage 2016; 51: 17–24. doi: 10.1016/j.jpainsymman.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 2.Moens K, Higginson IJ, Harding R, et al. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage 2014; 48: 660–677. doi: 10.1016/j.jpainsymman.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 3.Bausewein C, Booth S, Gysels M, et al. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev 2008; 2: CD005623. doi: 10.1002/14651858.CD005623.pub2 [DOI] [PubMed] [Google Scholar]
- 4.Larkey L, Jahnke R, Etnier J, et al. Meditative movement as a category of exercise: implications for research. J Phys Act Health 2009; 6: 230–238. doi: 10.1123/jpah.6.2.230 [DOI] [PubMed] [Google Scholar]
- 5.Gomes-Neto M, Rodrigues E Jr, Silva W Jr, et al. Effects of yoga in patients with chronic heart failure: a meta-analysis. Arq Bras Cardiol 2014; 103: 433–439. doi: 10.5935/abc.20140149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan C, Lai J, Chen S. Tai chi chuan: an ancient wisdom on exercise and health promotion. Sports Med 2002; 32: 217–224. doi: 10.2165/00007256-200232040-00001 [DOI] [PubMed] [Google Scholar]
- 7.Oh B, Butow P, Mullan B, et al. A critical review of the effects of medical qigong on quality of life, immune function, and survival in cancer patients. Integr Cancer Ther 2012; 11: 101–110. doi: 10.1177/1534735411413268 [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Zhou R, Wang T, et al. Effects of traditional mind–body movement therapy on chronic cardiopulmonary dyspnoea: a systematic review and meta-analysis. Thorax 2023; 78: 69–75. doi: 10.1136/thoraxjnl-2021-218030 [DOI] [PubMed] [Google Scholar]
- 9.Metin ZG, Ejem D, Dionne-Odom JN, et al. Mind–body interventions for individuals with heart failure: a systematic review of randomized trials. J Card Fail 2018; 24: 186–201. doi: 10.1016/j.cardfail.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Pan L, Hu Q, et al. Effects of yoga training in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Thorac Dis 2014; 6: 795–802. doi: 10.3978/j.issn.2072-1439.2014.06.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer H, Haller H, Klose P, et al. The risks and benefits of yoga for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Clin Rehabil 2019; 33: 1847–1862. doi: 10.1177/0269215519860551 [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Lin Z, Weng H, et al. Effectiveness of meditative movement on COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2018; 13: 1239–1250. doi: 10.2147/COPD.S159042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polkey MI, Qiu Z, Zhou L, et al. Tai Chi and pulmonary rehabilitation compared for treatment-naive patients with COPD: a randomized controlled trial. Chest 2018; 153: 1116–1124. doi: 10.1016/j.chest.2018.01.053 [DOI] [PubMed] [Google Scholar]
- 14.Singh V, Wisniewski A, Britton J, et al. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet 1990; 335: 1381–1383. doi: 10.1016/0140-6736(90)91254-8 [DOI] [PubMed] [Google Scholar]
- 15.Pomidori L, Campigotto F, Amatya TM, et al. Efficacy and tolerability of yoga breathing in patients with chronic obstructive pulmonary disease: a pilot study. J Cardiopulm Rehabil Prev 2009; 29: 133–137. doi: 10.1097/HCR.0b013e31819a0227 [DOI] [PubMed] [Google Scholar]
- 16.Buffart LM, van Uffelen JGZ, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2012; 12: 559. doi: 10.1186/1471-2407-12-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni X, Chan RJ, Yates P, et al. The effects of tai chi on quality of life of cancer survivors: a systematic review and meta-analysis. Support Care Cancer 2019; 27: 3701–3716. doi: 10.1007/s00520-019-04911-0 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Hunt MA, Campbell KL, et al. The effect of tai chi on four chronic conditions—cancer, osteoarthritis, heart failure and chronic obstructive pulmonary disease: a systematic review and meta-analyses. Br J Sports Med 2016; 50: 397–407. doi: 10.1136/bjsports-2014-094388 [DOI] [PubMed] [Google Scholar]
- 19.Ford CG, Vowles KE, Smith BW, et al. Mindfulness and meditative movement interventions for men living with cancer: a meta-analysis. Ann Behav Med 2020; 54: 360–373. doi: 10.1093/abm/kaz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder H, Parlour L, Jenkins V. Randomised controlled trials of yoga interventions for women with breast cancer: a systematic literature review. Support Care Cancer 2012; 20: 3055–3064. doi: 10.1007/s00520-012-1611-8 [DOI] [PubMed] [Google Scholar]
- 21.Klein PJ, Schneider R, Rhoads CJ. Qigong in cancer care: a systematic review and construct analysis of effective qigong therapy. Support Care Cancer 2016; 24: 3209–3222. doi: 10.1007/s00520-016-3201-7 [DOI] [PubMed] [Google Scholar]
- 22.Li G, Yuan H, Zhang W. Effects of tai chi on health related quality of life in patients with chronic conditions: a systematic review of randomized controlled trials. Complement Ther Med 2014; 22: 743–755. doi: 10.1016/j.ctim.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 23.Wayne PM, Lee MS, Novakowski J, et al. Tai chi and qigong for cancer-related symptoms and quality of life: a systematic review and meta-analysis. J Cancer Surviv 2018; 12: 256–267. doi: 10.1007/s11764-017-0665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng Y, Luo T, Xie H, et al. Health benefits of qigong or tai chi for cancer patients: a systematic review and meta-analyses. Complement Ther Med 2014; 22: 173–186. doi: 10.1016/j.ctim.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 25.Van Vu D, Molassiotis A, Ching SSY, et al. Effects of qigong on symptom management in cancer patients: a systematic review. Complement Ther Clin Pract 2017; 29: 111–121. doi: 10.1016/j.ctcp.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Park HY, Suh CH, Woo S, et al. Quality reporting of systematic review and meta-analysis according to PRISMA 2020 guidelines: results from recently published papers in the Korean Journal of Radiology. Korean J Radiol 2022; 23: 355–369. doi: 10.3348/kjr.2021.0808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Hoboken, John Wiley & Sons, 2017. [Google Scholar]
- 28.Gómez FP, Rodriguez-Roisin R. Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for chronic obstructive pulmonary disease. Curr Opin Pulm Med 2002; 8: 81–86. doi: 10.1097/00063198-200203000-00001 [DOI] [PubMed] [Google Scholar]
- 29.New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th Edn. Boston, Little, Brown & Co, 1994. [Google Scholar]
- 30.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, John Wiley & Sons, 2019. [Google Scholar]
- 31.Dongxin Zhao NZ. Rehabilitation effect of liu zi jue breathing exercise combined with oxygen therapy on COPD complicated with spontaneous pneumothorax. Chin Contem Med 2011; 18: 156–157. doi: 10.1097/MD.0000000000027344 [DOI] [Google Scholar]
- 32.Yuexia W, Jiaci M, Donghai C, et al. Effect of taijiquan on quality of life and BODE index in patients with chronic obstructive pulmonary disease. Chin J Rehabil Med 2014; 29: 745–747. [Google Scholar]
- 33.Siqin J, Gongwen L, Kejian S, et al. Clinical study on the intervention of Liu Zi Jue in stable chronic obstructive pulmonary disease patients. West Chin Med 2019; 4: 111–114. [Google Scholar]
- 34.Yeh GY, Roberts DH, Wayne PM, et al. Tai chi exercise for patients with chronic obstructive pulmonary disease: a pilot study. Respir Care 2010; 55: 1475–1482. [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao C, Zhuang Y. Efficacy of liuzijue qigong in individuals with chronic obstructive pulmonary disease in remission. J Am Geriatr Soc 2015; 63: 1420–1425. doi: 10.1111/jgs.13478 [DOI] [PubMed] [Google Scholar]
- 36.Vanderbyl BL, Mayer MJ, Nash C, et al. A comparison of the effects of medical qigong and standard exercise therapy on symptoms and quality of life in patients with advanced cancer. Support Care Cancer 2017; 25: 1749–1758. doi: 10.1007/s00520-017-3579-x [DOI] [PubMed] [Google Scholar]
- 37.Hosakote VS, Rao MR, Nagendra RH, et al. Effects of yoga on symptom management in breast cancer patients: a randomized controlled trial. Int J Yoga 2009; 2: 73–79. doi: 10.4103/0973-6131.60048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng BH, Tsang HW, Jones AY, et al. Functional and psychosocial effects of health qigong in patients with COPD: a randomized controlled trial. J Altern Complement Med 2011; 17: 243–251. doi: 10.1089/acm.2010.0215 [DOI] [PubMed] [Google Scholar]
- 39.Moy ML, Wayne PM, Litrownik D, et al. Long-term exercise after pulmonary rehabilitation (LEAP): a pilot randomised controlled trial of tai chi in COPD. ERJ Open Res 2021; 7: 00025-2021. doi: 10.1183/23120541.00025-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaminsky DA, Guntupalli KK, Lippmann J, et al. Effect of yoga breathing (pranayama) on exercise tolerance in patients with chronic obstructive pulmonary disease: a randomized, controlled trial. J Altern Complement Med 2017; 23: 696–704. doi: 10.1089/acm.2017.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molassiotis A, Vu DV, Ching SSY. The effectiveness of qigong in managing a cluster of symptoms (breathlessness–fatigue–anxiety) in patients with lung cancer: a randomized controlled trial. Integr Cancer Ther 2021; 20: 15347354211008253. doi: 10.1177/15347354211008253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donesky-Cuenco D, Nguyen HQ, Paul S, et al. Yoga therapy decreases dyspnea-related distress and improves functional performance in people with chronic obstructive pulmonary disease: a pilot study. J Altern Complement Med 2009; 15: 225–234. doi: 10.1089/acm.2008.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung DST, Takemura N, Lam TC, et al. Feasibility of aerobic exercise and tai-chi interventions in advanced lung cancer patients: a randomized controlled trial. Integr Cancer Ther 2021; 20: 15347354211033352. doi: 10.1177/15347354211033352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu S, Shi K, Yan J, et al. A modified 6-form tai chi for patients with COPD. Complement Ther Med 2018; 39: 36–42. doi: 10.1016/j.ctim.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 45.Yanchan Z. Clinical study of respiratory baduanjin in patients with chronic obstructive pulmonary disease. Guangzhou University of Chinese Medicine; 2019. https://d.wanfangdata.com.cn/thesis/ChJUaGVzaXNOZXdTMjAyMzAxMTISCFkzNjU4NDI1Ggg5cGFzemg4Ng%3D%3D [Google Scholar]
- 46.Malik S, Dua R, Krishnan AS, et al. Exercise capacity in patients with chronic obstructive pulmonary disease treated with tele-yoga versus tele-pulmonary rehabilitation: a pilot validation study. Cureus 2022; 14: e30994. doi: 10.7759/cureus.30994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilliam EA, Kilgore KL, Liu Y, et al. Managing the experience of breathlessness with tai chi: a qualitative analysis from a randomized controlled trial in COPD. Respir Med 2021; 184: 106463. doi: 10.1016/j.rmed.2021.106463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0243-2022.SUPPLEMENT (1MB, pdf)